Introduction
Reactive oxygen species (ROS), such as hydrogen
peroxide (H2O2), are generated during normal
cellular metabolism, and they play critical roles in the signal
transmission mechanism (1,2).
However, H2O2 also exerts genotoxicological
effects, as it produces new free radicals and causes damage to the
main cellular components (3,4).
Moreover, the excess production of ROS increases oxidative damage,
leading to cellular dysfunction and cell death (5,6).
Oxidative stress is mainly caused by neurodegenerative disorders,
including dementia, which has attracted significant attention; as
brain cells are damaged, the interaction between neurons is
inhibited, resulting in disability of memory and cognitive
functions (7,8). Glial cells, which outnumber neurons
in the brain, are non-neuronal cells that maintain homeostasis as
well as supporting and protecting neurons in the central nervous
system (CNS) (9). Therefore, the
attenuation of oxidative stress and the inhibition of apoptosis in
glial cells are critical for protection from neurodegenerative
disorders. Thioredoxin reductase 1 (TrxR1) is a nicotinamide
adenine dinucleotide phosphate (NADPH)-dependent oxidoreductase
that decreases the active site of cytosolic thioredoxin-1 (Trx1)
from the disulfide form to the biologically active dithiol form
(10). Trx1 contributes to
antioxidative activity by donating electrons to peroxiredoxins for
the reduction of H2O2 (11,12).
In addition, the heme oxygenase (HO) pathway has
been reported to be active in the CNS and to operate as an
underlying protective mechanism of cells exposed to an oxidizing
agent (13). Moreover, the
enhancement of HO-1 protein expression has been associated with
protection against stress conditions, such as oxidative stress
(14). HO-1 gene expression is
mainly regulated by the nuclear factor-erythroid 2-related factor 2
(Nrf2)-antioxidant response element (ARE) pathway, and the
induction of this enzyme protects cells against oxidative
stress-induced damage and apoptosis (15,16). The Kelch-like ECH-associated
protein-1 (Keap1) controls Nrf2 activation and nuclear accumulation
by binding to the Nrf2 protein and targeting it for proteosomal
degradation (17). Nrf2 is
released from Keap1 repression under conditions of oxidative
stress, and it translocates to the nucleus where it increases
several antioxidant genes, such as HO-1 and TrxR1 (15,18,19). Thus, we suggest that the
activation of Nrf2 is crucial for the cytoprotective mechanism
against oxidative stress.
Baicalein, a flavonoid originally obtained from the
roots of a traditional Chinese herb, Scutellaria baicalensis
Georgi, has been widely used in the treatment of inflammation,
hypertension, cardiovascular disease, bacterial infection and
cancer (20,21). Moreover, the neuroprotective
effects of baicalein have been demonstrated in several experimental
models, such as models of Alzheimer's disease (22,23), ischemic stroke (24,25), and Parkinson's disease (26,27). However, an association between the
Nrf2 pathway with the neuroprotective role of baicalein against
oxidative stress in glial cells has not previously been
demonstrated. Thus, in the present study, we investigated the
neuroprotective effect exerted by baicalein and also its mechanisms
which protect against H2O2-induced neuronal
damage in a model using C6 glial cells.
Materials and methods
Reagents and antibodies
In the present study, Dulbecco's modified Eagle's
medium (DMEM), fetal bovine serum (FBS), and antibiotics
(penicillin and streptomycin) were all purchased from Welgene, Inc.
(Daegu, Korea). Baicalein (5,6,7-trihydroxyflavone; purity 98%),
H2O2,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
propidium iodide (PI),
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazo-lylcarbocyanine-iodide
(JC-1), zinc protoporphyrin (ZnPP) IX and auranofin were all
purchased from Sigma Chemical Co. (St. Louis, MO, USA);
2′,7′-dichlorofluorescein diacetate (DCFH-DA) was obtained from
Molecular Probes, Inc. (Eugene, OR, USA); primary antibody against
phosphorylated histone variant H2A.X (p-γH2A.X; #9718) was obtained
from Cell Signaling Technology, Inc. (Danvers, MA, USA); β-actin
(sc-1616), poly(ADP-ribose) polymerase (PARP; sc-7150), X-linked
inhibitor of apoptosis protein (XIAP; sc-11426), Nrf2 (sc-13032),
Keap1 (sc-15246), HO-1 (sc-7696) and TrxR1 (sc-28321) antibodies
were all purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz,
CA, USA). The secondary antibodies against goat anti-rabbit IgG-HRP
(sc-2004), goat anti-mouse IgG-HRP (sc-2005) and bovine anti-goat
IgG-HRP (sc-2350) were all purchased from Santa Cruz Biotechnology,
Inc.
Cell culture and treatment
C6 glial cells were obtained from the American Type
Culture Collection (ATCC; Manassas, VA, USA) and maintained in DMEM
supplemented with 10% heat-inactivated FBS and antibiotics (100
µg/ml streptomycin, 100 U/ml penicillin) at 37°C in a
humidified incubator in an atmosphere of 5% CO2 in air.
Baicalein was dissolved in dimethyl sulfoxide (DMSO) as a stock
solution at 100 mM, which was then diluted with DMEM to the desired
concentration prior to use. H2O2 was diluted
in DMEM to a final concentration of 0.5 mM.
Cell viability assay
C6 cells were seeded in 6-well plates at a density
of 2x105 cells/well. After incubation for 24 h, the
cells were pretreated with various concentrations of baicalein for
1 h prior to incubation in the absence or presence of
H2O2 for 24 h. An MTT working solution (0.5
mg/ml) was added to the culture plates and incubated for 3 h at
3°C. The culture supernatant was removed from the wells, and DMSO
was added to dissolve the formazan crystals completely. The
absorbance of each well was measured at 540 nm using a microplate
reader (Dynatech Laboratories, Chantilly VA, USA). The effect of
baicalein on cell growth was assessed as the percentage of cell
viability, in which the vehicle-treated cells (0.05% DMSO) were
considered 100% viable.
Comet assay (single-cell gel
electrophoresis)
The cell suspension was mixed with 0.5% low melting
agarose (LMA) at 37°C, and the mixture was spread on fully frosted
microscopic slides pre-coated with 1% normal melting agarose. After
solidification of the agarose, the slides were covered with 0.5%
LMA and then immersed in a lysis solution (2.5 M NaCl, 100 mM
Na-ethylenediaminetetraacetic acid (EDTA), 10 mM Tris, 1% Triton
X-100, and 10% DMSO, pH 10.0) for 30 min at 4°C. The slides were
then placed in a CometAssay Electrophoresis System Starter Kit
(KORMED, Seongnam, Korea) containing 300 mM NaOH and 10 mM Na-EDTA
(pH 13.00) for 40 min to allow for DNA unwinding and examination of
alkali-labile damage. An electrical field was then applied (300 mA,
20 V) for 20 min at 4°C to draw the negatively charged DNA toward
the anode. After electrophoresis, the slides were washed three
times for 5 min at 4°C in a neutralizing buffer (0.4 M Tris, pH
7.5). The slides were then stained with 20 µg/ml PI and
observed using a fluorescence microscope (Carl Zeiss, Inc.,
Oberkochen, Germany). The images were also analyzed using an image
analysis system (Komet 5.5; Kinetic Imaging, Liverpool, UK) to
evaluate the degree of DNA damage. The tail length and the tail
moment were used as measures of the extent of DNA damage. One
hundred cells were randomly selected from one sample (two slides
were made for one sample, 50 randomly selected cells per slide) and
then measured. We calculated the values of the mean tail length for
each sample and the percentage values of the cells in five ranges
of tail length: undamaged cells without a tail, cells with a tiny
tail, cells with a dim tail (28), cells with a clear tail, and only
tail. The ranges of tail length and tail moment were divided
arbitrarily in the present study.
Measurement of intracellular ROS
To assess the generated ROS, the cells were
incubated with 10 µM DCFH-DA for 20 min at room temperature
in the dark to monitor ROS production. ROS levels in the cells were
monitored with a flow cytometer (Becton-Dickinson, San Jose, CA,
USA) using CellQuest Pro software, as previously described
(29).
Measurement of apoptosis
For quantitative assessment of the induced cell
apoptotic rate, a fluorescein-conjugated Annexin V (Annexin V-FITC)
staining assay was performed according to the manufacturer's
instructions (BD Biosciences Pharmingen, San Jose, CA, USA).
Briefly, the cells in each sample were stained with 5 µl
Annexin V-FITC and 5 µl PI. After incubation for 15 min at
room temperature in the dark, the degree of apoptosis was
quantified by flow cytometer as a percentage of the Annexin
V-positive and PI-negative cells (Becton-Dickinson) using CellQuest
Pro software (29).
Measurement of mitochondrial membrane
potential (MMP; ΔΨm)
The mitochondrial transmembrane electrochemical
gradient was measured using JC-1 staining. Briefly, the cells were
collected and incubated with 10 µM JC-1 solution for 15 min
at 37°C in the dark. The fluorescence intensity of the red/green
ratio was quantified by flow cytometer (Becton-Dickinson) using
CellQuest Pro software, as previusly described (30).
Protein extraction and western blot
analysis
After removing the media, the cells were washed with
ice-cold phosphate-buffered saline (PBS) and gently lysed for 20
min in an ice-cold lysis buffer (40 mM Tris, pH 8.0, 120 mM, NaCl,
0.5% nonidet-P40, 0.1 mM sodium orthovanadate, 2 µg/ml
leupeptin, and 100 µg/ml phenymethylsulfonyl fluoride). The
supernatants were collected, and the protein concentrations were
determined using a Bio-Rad protein assay kit (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). For western blot analysis, equal amounts
of protein extracts were denatured by boiling at 95°C for 5 min in
a sample buffer [0.5 M Tris-HCl, pH 6.8, 4% sodium dodecyl sulfate
(SDS), 20% glycerol, 0.1% bromophenol blue and 10%
β-mercaptoethanol] at a ratio of 1:1. The samples were stored at
−80°C or immediately used for western blot analysis. Aliquots
containing 30 µg of total protein were separated by
denaturing SDS-polyacrylamide gel electrophoresis and transferring
electrophoretically to nitrocellulose membranes (Amersham
Biosciences, Arlington Heights, IL, USA). The membranes were then
blocked with 5% skim milk and incubated overnight at 4°C with
primary antibodies, probed with enzyme-linked secondary antibodies
for 1 h at room temperature, and detected using an enhanced
chemiluminescence (ECL) detection system (both from Amersham
Biosciences).
Small interfering RNA (siRNA)
transfection
Nrf2 siRNA and control siRNA were both purchased
from Santa Cruz Biotechnology, Inc. The siRNAs were transfected
into C6 cells according to the manufacturer's instructions using
Lipofectamine® RNAiMAX transfection reagent (Invitrogen,
Carlsbad, CA, USA). The cells were seeded in 6-well culture plates
for transfection and incubated with 50 nM control or Nrf2 siRNA for
24 h in serum-free OPTI-MEM media (Invitrogen). After 24 h
transfection, the cells were incubated under the experimental
conditions.
Statistical analysis
Data are expressed as the means ± standard error of
the mean (SEM). The comparison between groups was undertaken using
ANOVA, and significance was analyzed using Duncan's multiple range
test. A P-value <0.05 was considered to indicate a statistically
significant difference.
Results
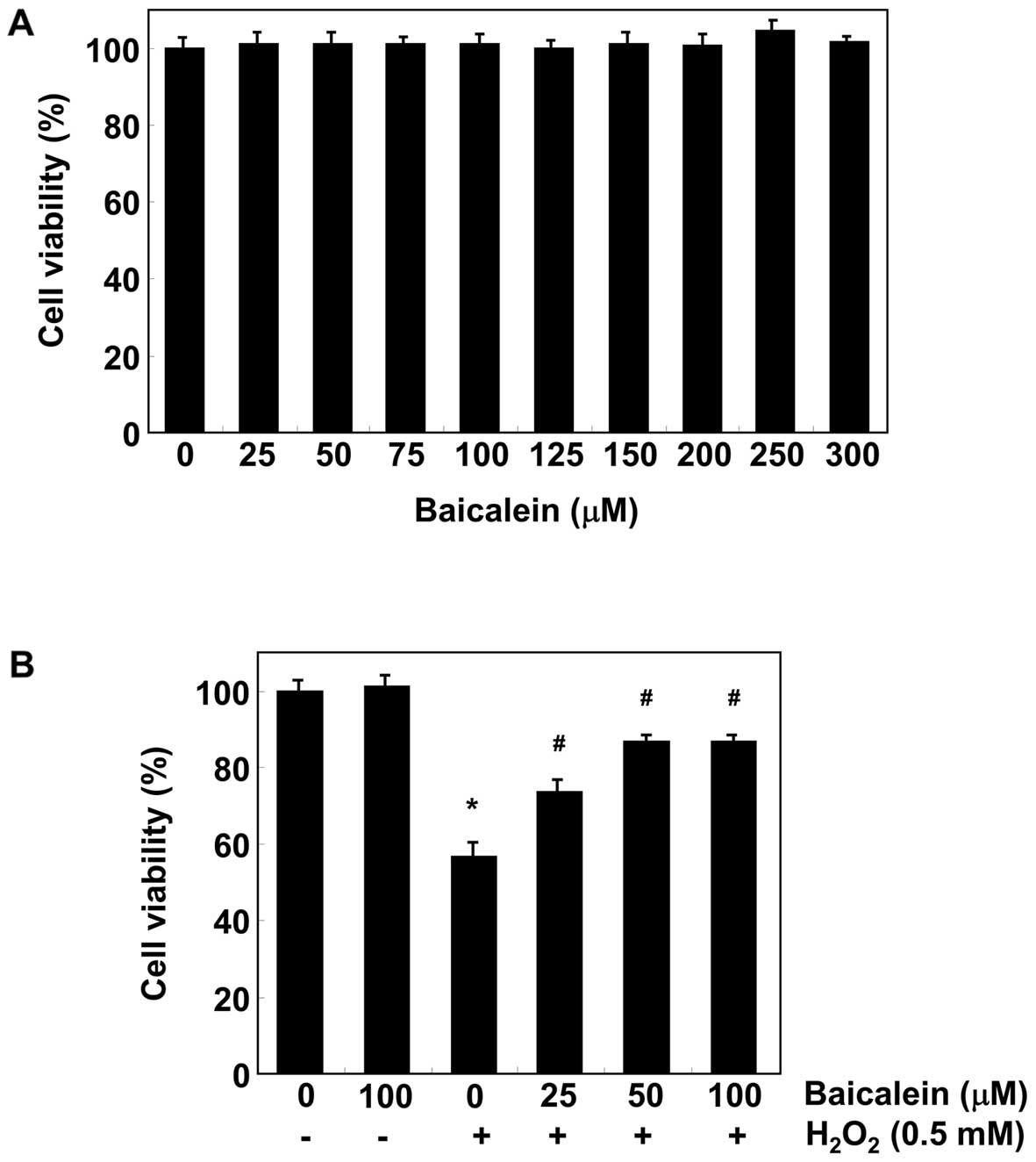
Baicalein prevents
H2O2-induced growth inhibition in C6
cells
We first determined the effect of baicalein on the
viability of C6 cells using the MTT assay. As shown in Fig. 1A, the results demonstrated that
baicalein (25–300 µM) alone for 24 h had no detectable
effect on C6 cell survival. To determine the protective effects of
baicalein on H2O2-induced cytotoxicity in C6
cells, the cells were pre-treated with baicalein for 1 h and
exposed to H2O2 for an additional 24 h. As
shown in Fig. 1B, the treatment
of C6 cells with 0.5 mM H2O2 for 24 h
resulted in approximately a 43% loss of cellular viability compared
with the control cells. However, the cytotoxic effect of
H2O2 was blocked by pretreating cells with
baicalein (25–100 µM).
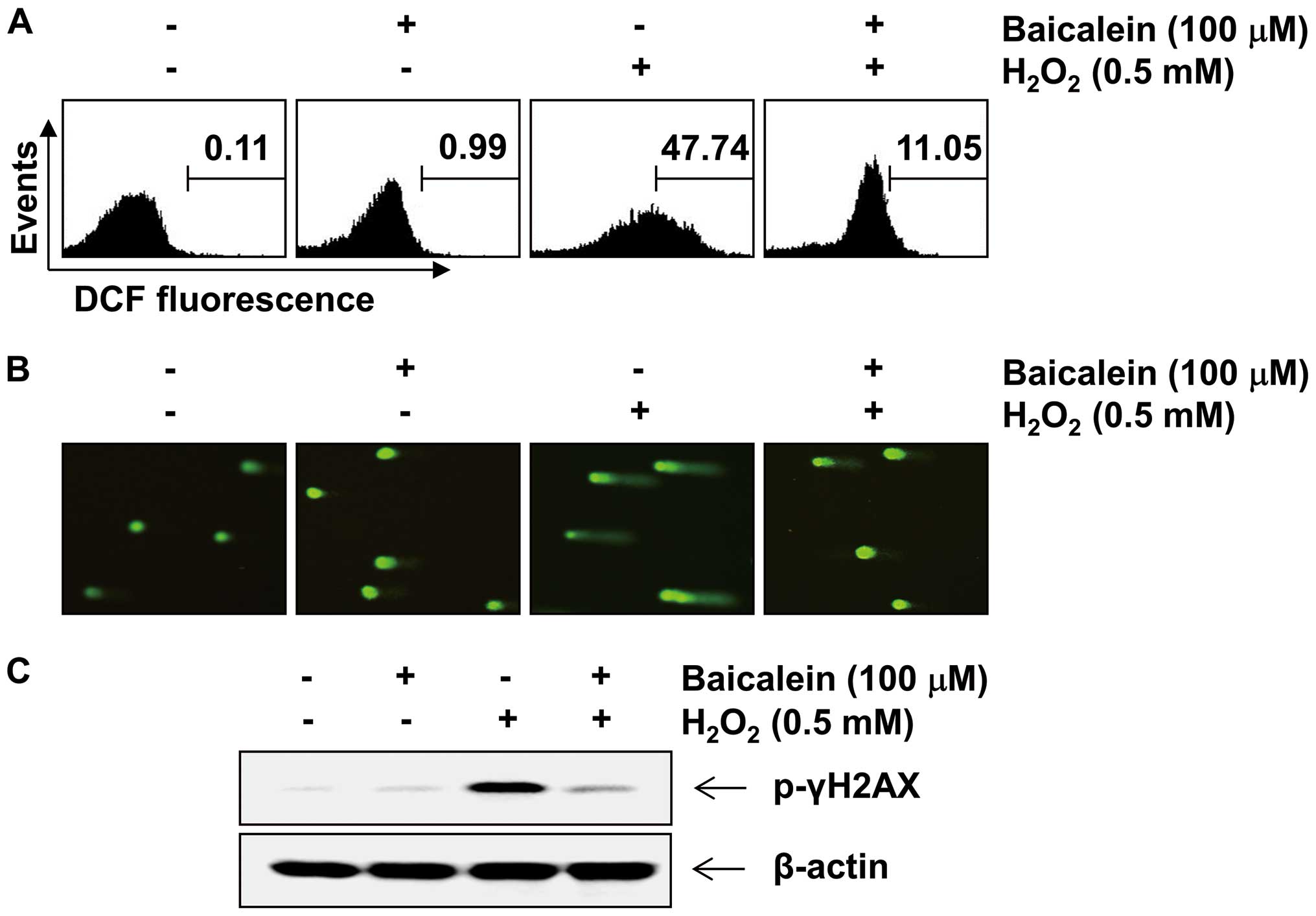
Baicalein attenuates
H2O2-induced ROS generation and DNA damage in
C6 cells
To examine the inhibitory effect of baicalein on
H2O2-induced ROS production, C6 cells were
stimulated with 0.5 mM H2O2 for 30 min in the
presence and absence of baicalein, and the intracellular ROS level
was then determined. As expected, increased ROS generation was
detected in cells after stimulation with H2O2
alone (Fig. 2A). However,
pretreatment with baicalein significantly reduced
H2O2-induced ROS production. We further
examined the effects of baicalein on DNA damage caused by
H2O2 using a Comet assay and western blot
analysis. As indicated in Fig.
2B, treatment with H2O2 alone
significantly increased the number of DNA breaks, resulting in an
increase in fluorescence intensity in the tails of the comet-like
structures, which was associated with an increase in the tail
length and tail moment (Table I).
However, these phenomena were prevented by pretreatment with
baicalein. In addition, the western blot analysis revealed that the
level of p-γH2A.X, a classic marker of DNA double-strand break
formation (31), in C6 cells
treated with H2O2 alone was markedly
increased. However, pretreatment with baicalein was found to
inhibit the increase in p-γH2A.X expression caused by treatment
with H2O2 (Fig.
2C).
 | Table IPreventive effect exerted by
baicalein on H2O2-induced DNA damage in C6
cells (means ± SEM). |
Table I
Preventive effect exerted by
baicalein on H2O2-induced DNA damage in C6
cells (means ± SEM).
| Compounds | Scored cells | Tail moment | Tail length |
|---|
| Control | 100 | 2.27±0.81 | 64.4±4.97 |
| Baicalein (100
µM) | 100 | 1.50±1.23 | 53.16±8.13 |
|
H2O2 (0.5 mM) | 100 | 42.09±4.34 | 156.55±9.04 |
|
Baicalein+H2O2 | 100 | 4.75±1.72 | 97.66±6.21 |
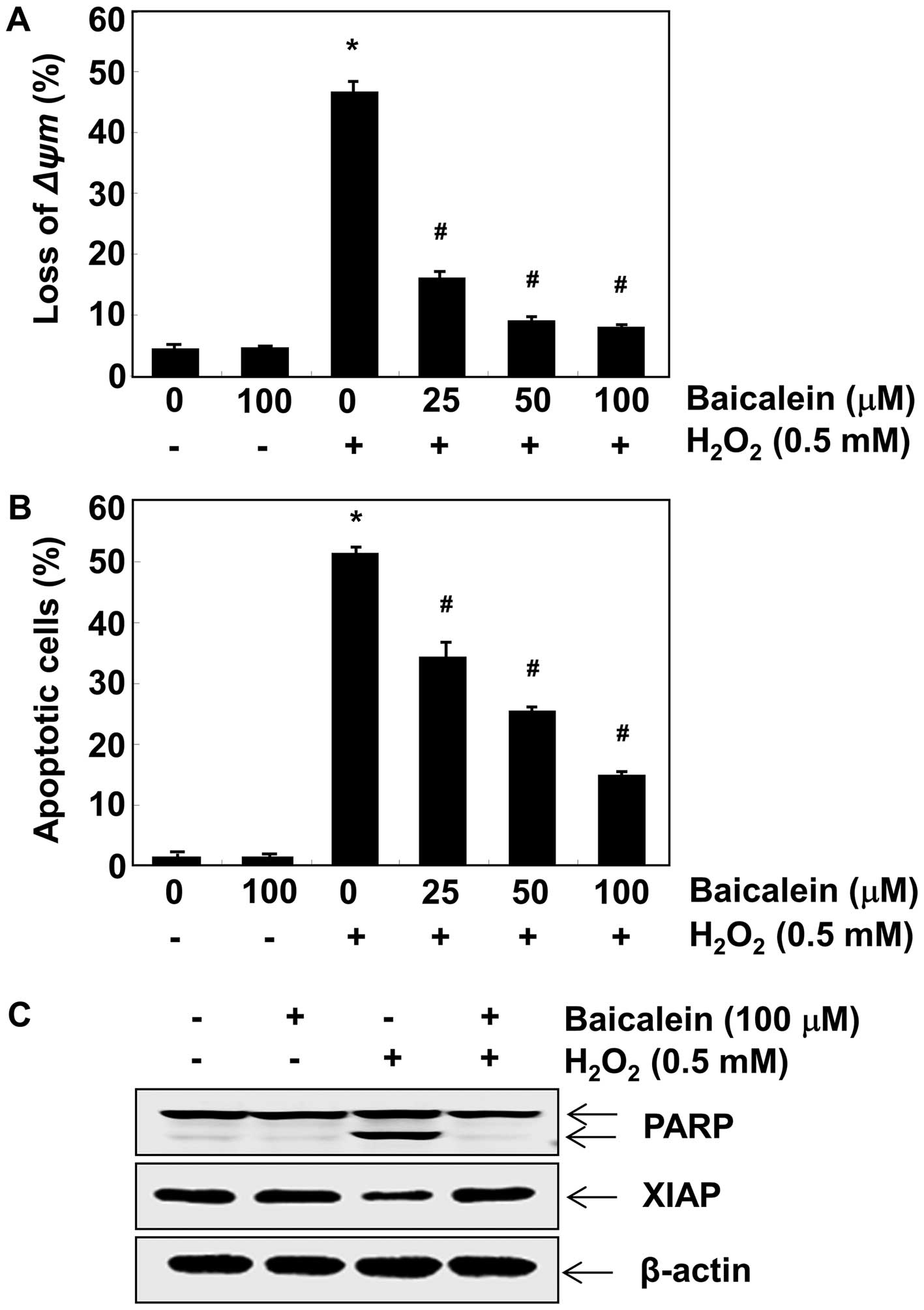
Baicalein reduces
H2O2-induced loss of MMP and apoptosis in C6
cells
As mitochondrial permeability is critical for the
oxidative stress-induced apoptotic pathway (32), we evaluated the effect of
baicalein on the MMP of C6 cells using flow cytometry. After
incubation with H2O2 alone, the loss of MMP
was markedly increased compared to the untreated control (Fig. 3A), which indicated mitochondrial
damage and dysfunction. By contrast, pretreatment with baicalein
effectively prevented the loss of MMP induced by
H2O2 in a concentration-dependent manner. We
also examined the ability of baicalein to protect against
H2O2-triggered C6 cell apoptosis using
Annexin V/PI-staining. The flow cytometry results indicated that
the percentage of apoptotic cells treated with 0.5 mM
H2O2 was approximately 51% (Fig. 3B), which was significantly reduced
by pretreatment with baicalein. Furthermore, we determined the
effects of baicalein on the expression of apoptosis-related
proteins PARP and XIAP. As illustrated in Fig. 3C, the degradation of PARP and the
downregulation of XIAP were observed in
H2O2-treated C6 cells. However, pretreatment
with baicalein effectively protected against these changes.
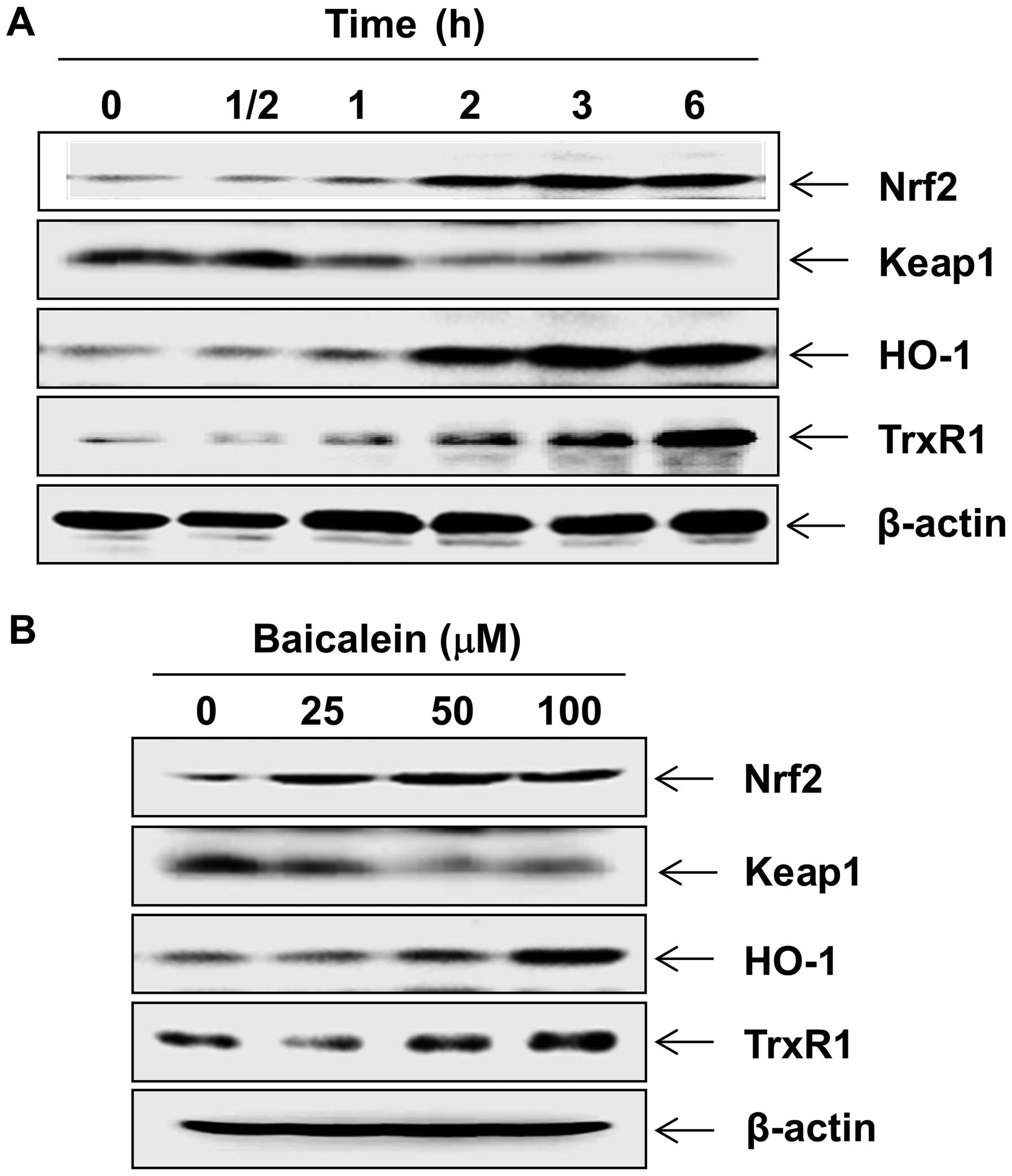
Baicalein upregulates Nrf2, HO-1 and
TrxR1 expression in C6 cells
The fact that Nrf2 signaling regulates cellular
antioxidant response has been well documented (33). We sought to determine whether
signaling was associated with baicalein-mediated neuroprotection.
Western blot analysis indicated that treating C6 cells with
baicalein induced the expression of Nrf2 protein in a
concentration- and time-dependent manner, which was associated with
the downregulation of Keap1 (Fig.
4). Concomitant with the induction of Nrf2, the levels of HO-1
and TrxR1 were also markedly increased after treatment with
baicalein in C6 cells.
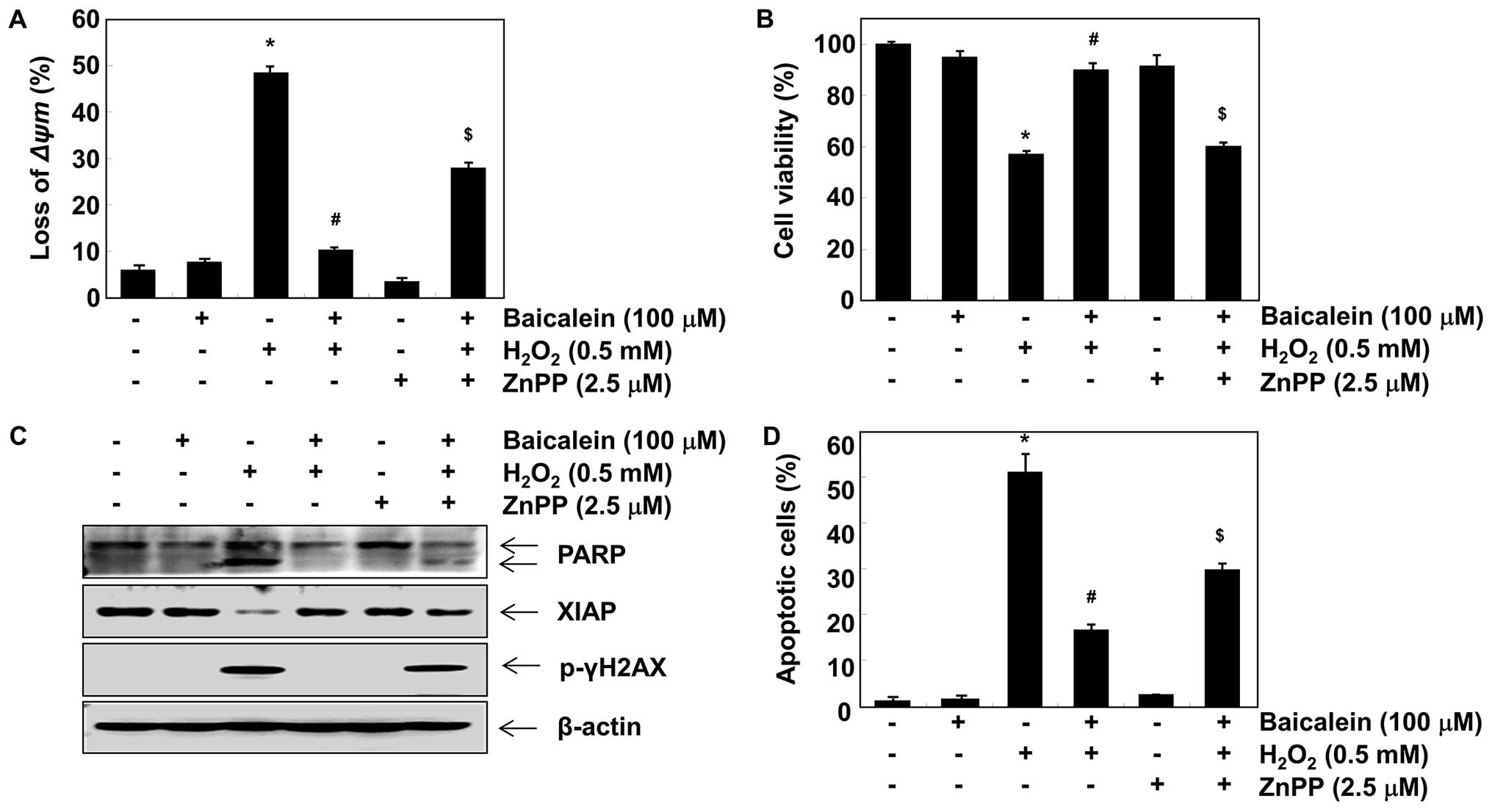
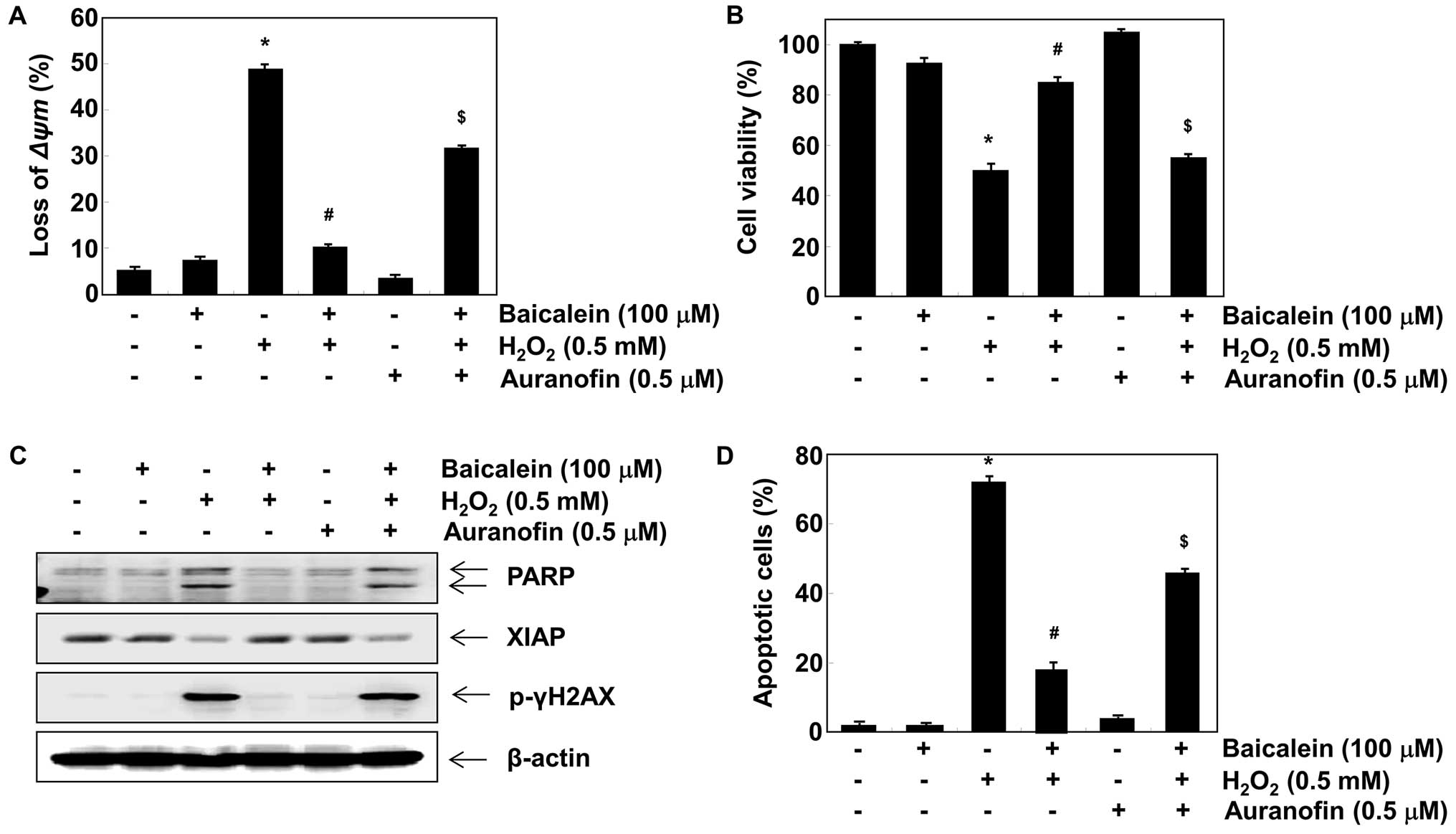
Induction of HO-1 and TrxR1 is involved
in the protective effect exerted by baicalein against
H2O2 treatment in C6 cells
In order to investigate the role of HO-1 induction
in the baicalein-mediated neuroprotective effects exerted against
oxidative stress, we inhibited HO-1 activity using ZnPP, a specific
inhibitor of HO-1. As shown in Fig.
5A and B, in the presence of ZnPP, the protective effects of
baicalein on H2O2-induced loss of MMP and
reduction of cell viability were significantly attenuated.
Furthermore, ZnPP blocked the protection provided by baicalein
against H2O2-induced degradation of PARP,
phosphorylation of γH2A.X, and downregulation of XIAP as well as
apoptosis (Fig. 5C and D). To
determine whether the protective effect of baicalein was related to
its inductive effect on TrxR1 expression, we blocked TrxR1 activity
using auranofin, a selective TrxR1 inhibitor. The results indicated
that auranofin also reversed the inhibition of loss of MMP and
apoptotic activity caused by baicalein in
H2O2-stimulated C6 cells (Fig. 6).
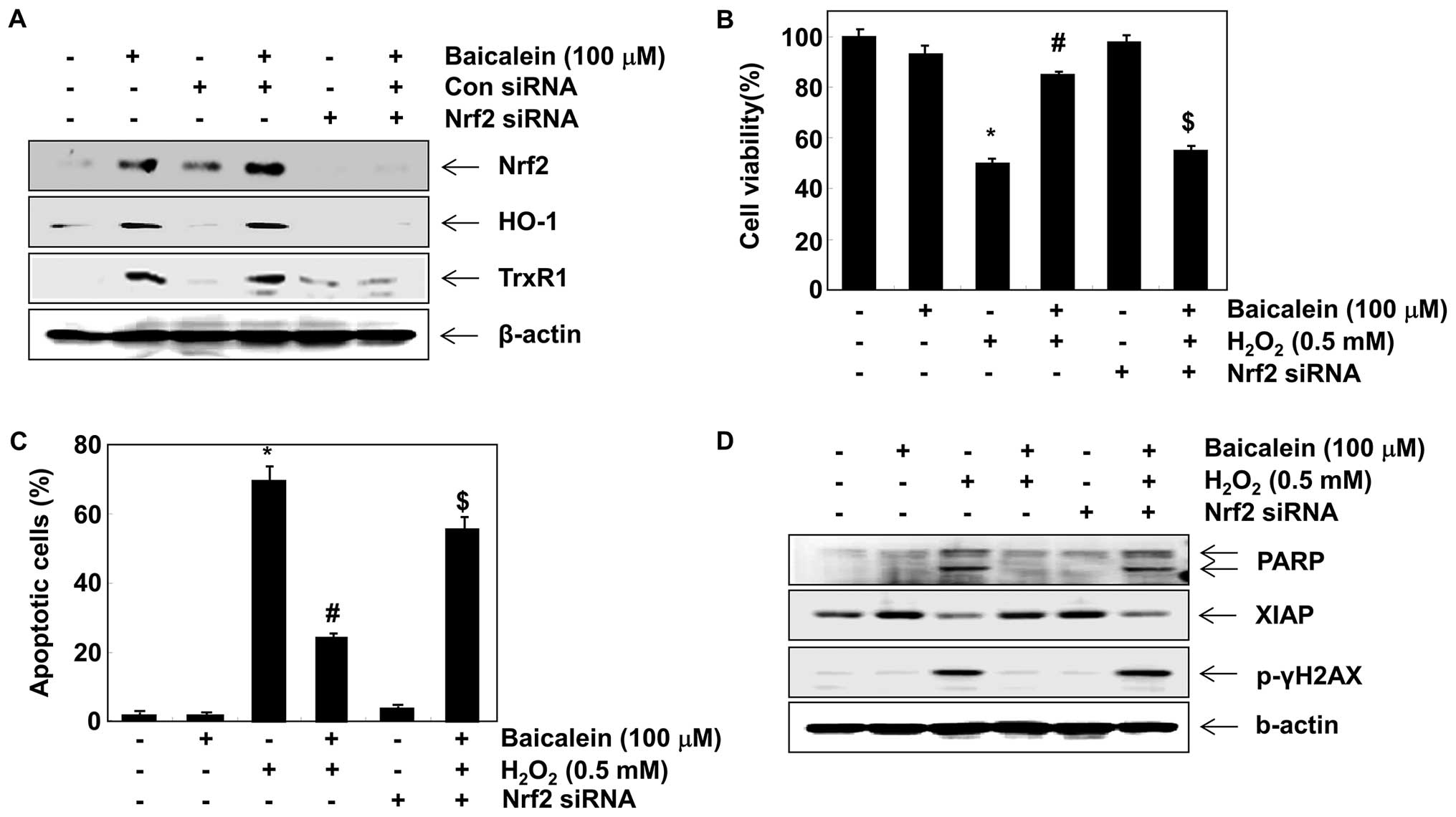
Baicalein upregulates HO-1 and TrxR1
expression via Nrf2 activation in C6 cells
It has previously been reported that HO-1 and TrxR1
are regulated through the Nrf2 cascade (19,34–36). We developed an Nrf2 gene knockdown
model using siRNA transfection to demonstrate the contribution of
Nrf2 signaling to the negative effects of baicalein on
H2O2-induced cytotoxicity. Western blot
analysis indicated that Nrf2 siRNA reduced the baicalein-induced
expression of Nrf2 compared with untransfected control and control
siRNA-transfected cells) (Fig.
7A). The baicalein-induced expression of HO-1 and TrxR1 was
also blocked by Nrf2 siRNA, which is evidence that the augmentation
of HO-1 and TrxR1 was mediated by Nrf2. Furthermore, Nrf2 siRNA
significantly attenuated the protective effects of baicalein
against the H2O2-induced reduction of cell
viability and apoptosis (Fig. 7B and
C), which was associated with the disappearance of the
potential of baicalein to protect against
H2O2-induced PARP degradation, γH2A.X
phosphorylation and XIAP reduction (Fig. 7D).
Discussion
The excessive production of ROS, which causes
oxidative damage to proteins, lipids and DNA, is one of the most
prominent factors related to neurodegeneration (37). The mitochondrial electron
transport system is a major source of intracellular ROS generation
(32), whereby the mitochondria
play a pivotal role in the process of ROS-mediated cell death.
Moreover, H2O2 directly induces mitochondrial
dysfunction, followed by a rapid efflux of intracellular ROS, which
increases the permeabilization and depolarization of the
mitochondrial membrane. This event likely facilitates the rapid
disruption of MMP and the release of apoptosis-inducing factors
that activate the caspase-dependent signaling cascades in the
induction of apoptosis (38).
Therefore, the search for functional food or bioactive compounds
that act against oxidative stress is critical for the prevention
and cure of neurodegenerative disorders. The purpose of the present
study was to determine whether baicalein blocked
H2O2-induced oxidative stress in C6 cells or
not. The results demonstrated that treatment of C6 cells with
H2O2 caused the marked intracellular
accumulation of ROS and the loss of MMP, and further inhibited cell
survival, leading to apoptosis. However, when the C6 cells were
pretreated with baicalein, the H2O2-induced
generation of ROS, loss of MMP, reduction of cell viability, and
apoptosis were significantly attenuated, as previously reported in
studies on other neuron-like cells (27). Thus, we presume that baicalein
improves mitochondrial function through eliminating the
overproduction of ROS induced by H2O2 and
thereby reducing H2O2-induced apoptosis. In
addition, our results showed that H2O2
treatment increased DNA tail moment and length in the comet assay
and expression of p-γH2A.X, which are widely used markers for the
detection of DNA damage (31).
However, in the present study, both events were abolished by
baicalein, indicating that it protected against
H2O2-induced apoptosis in the C6 cells by
reducing the amount of DNA damage caused by the destructive impact
of oxidative stress in the C6 cells.
Apoptosis, which is programmed cell death, is a
tightly regulated cell suicide response that facilitates the
correct development and homeostasis of multicellular organisms; in
mammalian cells, two major apoptotic pathways (the cell death
receptor-mediated and mitochondrial-mediated apoptotic pathways)
have been studied (39). Previous
research has indicated that the impairment of MMP and ROS
generation is closely linked to the initiation of caspase-dependent
apoptotic signaling in many types of cells (5). Caspases, which are a family of
cysteine acid proteases, are the central regulators of the
execution of cell death in response to various apoptotic stimuli
(40). In the final stage of
apoptosis, both pathways induce the activation of executioner
caspases, such as caspase-3 and -7, which results in the
degradation of substrate proteins, such as PARP, a biochemical
hallmark of cell apoptosis (39).
The activation of caspases may also be regulated by a variety of
proteins, including members of the inhibitor of apoptosis proteins
(IAP) family, which promote cell survival after a wide variety of
apoptotic stimuli elicited via intrinsic and extrinsic pathways
through selectively binding with caspases, thus inhibiting caspase
activity and apoptosis (39).
Moreover, previous research has indicated that
H2O2 induces apoptosis through the activation
of caspases in addition to the inhibition of IAP family proteins
(41). In the present study,
western blot analyses revealed that baicalein effectively blocked
the H2O2-induced cleavage of PARP, a
downstream target protein of the activated caspase-3 in C6 cells.
Under the same conditions, baicalein also rescued the
H2O2-induced downregulation of XIAP, a
representative member of the IAP family, compared with the control.
Although further molecular studies are needed, our findings
indicate that baicalein potentially prevents
H2O2-induced apoptosis through the
inactivation of caspase cascades in C6 cells.
Previous research has suggested that Nrf2, a master
cellular sensor for oxidative stress, and its repressor, Keap1,
play indispensable roles in protecting a variety of tissues from a
wide array of toxic insults, including oxidative stress. Under
normal conditions, Nrf2 is inactive and bound in the cytosol by
Keap1 (35). The dissociation of
Nrf2 from Keap1 is a prerequisite for nuclear translocation, and
the subsequent DNA binding of Nrf2 is necessary to regulate the
inducible expression of cytoprotective phase II enzymes (15,18). In the present study, we found that
baicalein increased Nrf2 protein expression and decreased Keap1 in
a concentration- and time-dependent manner in C6 cells. These
results are consistent with those of previous studies (42–44), and were associated with the
induction of HO-1 and TrxR1. However, the inhibition of HO-1
function using ZnPP significantly weakened the inhibitory effects
of baicalein on H2O2-induced MMP loss, growth
inhibition, and apoptosis by blocking PARP cleavage, XIAP
inhibition, and γH2A.X phosphorylation. Moreover, pre-treatment
with a TrxR1 inhibitor also markedly abrogated the protective
effects of baicalein against H2O2-induced
oxidative stress. In addition, the knockdown of Nrf2 by
Nrf2-targeted siRNA completely cancelled out baicalein-induced HO-1
and TrxR1 expression, suggesting that Nrf2 is a critical upstream
regulator of the baicalein-mediated induction of HO-1 and TrxR1.
Furthermore, the removal of Nrf2 also halted the baicalein-induced
restoration of H2O2-mediated growth
inhibition and the apoptosis of C6 cells. These results suggest
that the Nrf2-dependent induction of HO-1 and TrxR1 by baicalein,
at least in part, may participate in the protection against
oxidative stress in C6 cells.
In conclusion, the results of our present study
clearly demonstrated that baicalein exerted a protective effect
against H2O2-induced DNA damage, growth
inhibition, and apoptosis in C6 cells. Baicalein also successfully
suppressed the accumulation of intracellular ROS, leading to
substantial regain of MMP, at least in part, through the activation
of Nrf2 signaling and the induction of HO-1 and TrxR1. These
findings suggest that baicalein has a potential neuroprotective
value as an antioxidant agent.
Acknowledgments
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) grant funded by the Korea Government (no.
2015R1A2A2A01004633) and the High Value-added Food Technology
Development Program (314043–3), Ministry of Agriculture, Food and
Rural Affairs.
References
|
1
|
Dringen R, Kussmaul L and Hamprecht B:
Detoxification of exogenous hydrogen peroxide and organic
hydroperoxides by cultured astroglial cells assessed by microtiter
plate assay. Brain Res Brain Res Protoc. 2:223–228. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dringen R, Pawlowski PG and Hirrlinger J:
Peroxide detoxification by brain cells. J Neurosci Res. 79:157–165.
2005. View Article : Google Scholar
|
|
3
|
Forman HJ: Use and abuse of exogenous
H2O2 in studies of signal transduction. Free
Radic Biol Med. 42:926–932. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Halliwell B: Oxidative stress and
neurodegeneration: where are we now? J Neurochem. 97:1634–1658.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ray PD, Huang BW and Tsuji Y: Reactive
oxygen species (ROS) homeostasis and redox regulation in cellular
signaling. Cell Signal. 24:981–990. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ott M, Gogvadze V, Orrenius S and
Zhivotovsky B: Mitochondria, oxidative stress and cell death.
Apoptosis. 12:913–922. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Coyle JT and Puttfarcken P: Oxidative
stress, glutamate, and neurodegenerative disorders. Science.
262:689–695. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tuppo EE and Arias HR: The role of
inflammation in Alzheimer's disease. Int J Biochem Cell Biol.
37:289–305. 2005. View Article : Google Scholar
|
|
9
|
Minghetti L, Polazzi E, Nicolini A and
Levi G: Opposite regulation of prostaglandin E2 synthesis by
transforming growth factor-beta1 and interleukin 10 in activated
microglial cultures. J Neuroimmunol. 82:31–39. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arnér ES: Focus on mammalian thioredoxin
reductases - important selenoproteins with versatile functions.
Biochim Biophys Acta. 1790:495–526. 2009. View Article : Google Scholar
|
|
11
|
Nakamura T, Nakamura H, Hoshino T, Ueda S,
Wada H and Yodoi J: Redox regulation of lung inflammation by
thioredoxin. Antioxid Redox Signal. 7:60–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rhee SG, Chae HZ and Kim K:
Peroxiredoxins: a historical overview and speculative preview of
novel mechanisms and emerging concepts in cell signaling. Free
Radic Biol Med. 38:1543–1552. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Scapagnini G, Butterfield DA, Colombrita
C, Sultana R, Pascale A and Calabrese V: Ethyl ferulate, a
lipophilic polyphenol, induces HO-1 and protects rat neurons
against oxidative stress. Antioxid Redox Signal. 6:811–818. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sakata Y, Zhuang H, Kwansa H, Koehler RC
and Doré S: Resveratrol protects against experimental stroke:
putative neuroprotective role of heme oxygenase 1. Exp Neurol.
224:325–329. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chapple SJ, Siow RC and Mann GE: Crosstalk
between Nrf2 and the proteasome: therapeutic potential of Nrf2
inducers in vascular disease and aging. Int J Biochem Cell Biol.
44:1315–1320. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jeong WS, Jun M and Kong AN: Nrf2: a
potential molecular target for cancer chemoprevention by natural
compounds. Antioxid Redox Signal. 8:99–106. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kensler TW, Wakabayashi N and Biswal S:
Cell survival responses to environmental stresses via the
Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 47:89–116.
2007. View Article : Google Scholar
|
|
18
|
Chen XL and Kunsch C: Induction of
cytoprotective genes through Nrf2/antioxidant response element
pathway: a new therapeutic approach for the treatment of
inflammatory diseases. Curr Pharm Des. 10:879–891. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brigelius-Flohé R, Müller M, Lippmann D
and Kipp AP: The yin and yang of nrf2-regulated selenoproteins in
carcinogenesis. Int J Cell Biol. 2012:4861472012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ciesielska E, Gwardys A and Metodiewa D:
Anticancer, antiradical and antioxidative actions of novel Antoksyd
S and its major components, baicalin and baicalein. Anticancer Res.
22:2885–2891. 2002.
|
|
21
|
Li-Weber M: New therapeutic aspects of
flavones: the anticancer properties of Scutellaria and its main
active constituents Wogonin, Baicalein and Baicalin. Cancer Treat
Rev. 35:57–68. 2009. View Article : Google Scholar
|
|
22
|
Lebeau A, Esclaire F, Rostène W and
Pélaprat D: Baicalein protects cortical neurons from beta-amyloid
25–35 induced toxicity. Neuroreport. 12:2199–2202. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang SY, Wang HH, Chi CW, Chen CF and Liao
JF: Effects of baicalein on beta-amyloid peptide-(25–35)-induced
amnesia in mice. Eur J Pharmacol. 506:55–61. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu C, Wu J, Xu K, Cai F, Gu J, Ma L and
Chen J: Neuroprotection by baicalein in ischemic brain injury
involves PTEN/AKT pathway. J Neurochem. 112:1500–1512. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xue X, Qu XJ, Yang Y, Sheng XH, Cheng F,
Jiang EN, Wang JH, Bu W and Liu ZP: Baicalin attenuates focal
cerebral ischemic reperfusion injury through inhibition of nuclear
factor κB p65 activation. Biochem Biophys Res Commun. 403:398–404.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang M, Porat-Shliom Y, Pei Z, Cheng Y,
Xiang L, Sommers K, Li Q, Gillardon F, Hengerer B, Berlinicke C, et
al: Baicalein reduces E46K alpha-synuclein aggregation in vitro and
protects cells against E46K alpha-synuclein toxicity in cell models
of familiar Parkinsonism. J Neurochem. 114:419–429. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee HJ, Noh YH, Lee DY, Kim YS, Kim KY,
Chung YH, Lee WB and Kim SS: Baicalein attenuates
6-hydroxydopamine-induced neurotoxicity in SH-SY5Y cells. Eur J
Cell Biol. 84:897–905. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Osman AG, Mekkawy IA, Verreth J, Wuertz S,
Kloas W and Kirschbaum F: Monitoring of DNA breakage in embryonic
stages of the African catfish Clarias gariepinus (Burchell, 1822)
after exposure to lead nitrate using alkaline comet assay. Environ
Toxicol. 23:679–687. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim YS, Li XF, Kang KH, Ryu B and Kim SK:
Stigmasterol isolated from marine microalgae Navicula incerta
induces apoptosis in human hepatoma HepG2 cells. BMB Rep.
47:433–438. 2014. View Article : Google Scholar :
|
|
30
|
Song JL, Choi JH, Seo JH, Kil JH and Park
KY: Antioxidative effects of fermented sesame sauce against
hydrogen peroxide-induced oxidative damage in LLC-PK1 porcine renal
tubule cells. Nutr Res Pract. 8:138–145. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rogakou EP, Pilch DR, Orr AH, Ivanova VS
and Bonner WM: DNA double-stranded breaks induce histone H2AX
phosphorylation on serine 139. J Biol Chem. 273:5858–5868. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu Y, Adachi M, Zhao S, Hareyama M, Koong
AC, Luo D, Rando TA, Imai K and Shinomura Y: Preventing oxidative
stress: a new role for XBP1. Cell Death Differ. 16:847–857. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
DeNicola GM, Karreth FA, Humpton TJ,
Gopinathan A, Wei C, Frese K, Mangal D, Yu KH, Yeo CJ, Calhoun ES,
et al: Oncogene-induced Nrf2 transcription promotes ROS
detoxification and tumorigenesis. Nature. 475:106–109. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Satoh T, Okamoto SI, Cui J, Watanabe Y,
Furuta K, Suzuki M, Tohyama K and Lipton SA: Activation of the
Keap1/Nrf2 pathway for neuroprotection by electrophilic phase II
inducers. Proc Natl Acad Sci USA. 103:768–773. 2006. View Article : Google Scholar
|
|
35
|
Cebula M, Schmidt EE and Arnér ES: TrxR1
as a potent regulator of the Nrf2-Keap1 response system. Antioxid
Redox Signal. 23:823–853. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Espinosa C, Pérez-Llamas F, Guardiola FA,
Esteban MA, Arnao MB, Zamora S and López-Jiménez JA: Molecular
mechanisms by which white tea prevents oxidative stress. J Physiol
Biochem. 70:891–900. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Peterson LJ and Flood PM: Oxidative stress
and microglial cells in Parkinson's disease. Mediators Inflamm.
2012:4012642012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Choi IY, Lee SJ, Ju C, Nam W, Kim HC, Ko
KH and Kim WK: Protection by a manganese porphyrin of endogenous
peroxy-nitrite-induced death of glial cells via inhibition of
mitochondrial transmembrane potential decrease. Glia. 31:155–164.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mahata B, Biswas S, Rayman P, Chahlavi A,
Ko J, Bhattacharjee A, Li YT, Li Y, Das T, Sa G, et al: GBM derived
gangliosides induce T cell apoptosis through activation of the
caspase cascade involving both the extrinsic and the intrinsic
pathway. PLoS One. 10:e01344252015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
McIlwain DR, Berger T and Mak TW: Caspase
functions in cell death and disease. Cold Spring Harb Perspect
Biol. 5:a0086562013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wei M, Zhang M, Adams A and Duan Y: JNK
and AKT/GSK3β signaling pathways converge to regulate periodontal
ligament cell survival involving XIAP. Biochem Biophys Res Commun.
448:485–491. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Havermann S, Rohrig R, Chovolou Y, Humpf
HU and Wätjen W: Molecular effects of baicalein in Hct116 cells and
Caenorhabditis elegans: activation of the Nrf2 signaling pathway
and prolongation of lifespan. J Agric Food Chem. 61:2158–2164.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Qin S, Deng F, Wu W, Jiang L, Yamashiro T,
Yano S and Hou DX: Baicalein modulates Nrf2/Keap1 system in both
Keap1-dependent and Keap1-independent mechanisms. Arch Biochem
Biophys. 559:53–61. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yeh CH, Ma KH, Liu PS, Kuo JK and Chueh
SH: Baicalein decreases hydrogen peroxide-induced damage to
NG108-15 cells via upregulation of Nrf2. J Cell Physiol.
230:1840–1851. 2015. View Article : Google Scholar : PubMed/NCBI
|