Introduction
Endothelial dysfunction has been implicated in the
pathogenesis of many cardiovascular diseases, including thrombotic
disorders (1,2). Oxidative stress plays an important
role in endothelial dysfunction, which results in apoptosis of
endothelial cells, destruction of vascular barrier integrity,
increased endothelial permeability, platelet aggregation and
generation of cytokines, consequently promoting thrombotic diseases
(3,4). Thus, protecting endothelial cells
against apoptosis is likely to be a beneficial intervention
strategy for thrombotic diseases.
Flavonoids are polyphenolic compounds that are
widespread in many plants, and exert various biochemical and
pharmacological effects (5).
Hyperin (quercetin-3-O-galactoside) is considered the major
bioactive flavonoid component in the medicinal herb, Apocynum
venetum L., which has been used extensively for the treatment
of hypertension in Chinese medicine. Previous studeis have
suggested that hyperin plays various biological roles, including
anti-inflammatory (6,7), cytoprotective (8) and anti-ischemic roles (9). Our previous study revealed that
hyperin protects cells from H2O2-induced
injury (10). However, its
underlying mode of action has not yet been elucidated.
Isobaric tags for relative and absolute quantitation
(iTRAQ) (11) is a method used to
screen the entire proteome within the detectable dynamic range for
qualitative and quantitative differences in cell protein expression
before and after drug treatment. Compared to other proteomic
technologies, it has many advantages, including a high throughput
and compatibility with various sample types. The technique has been
shown to be suitable for the identification of lower abundance
proteins such as transcription factors (12), which makes it applicable for
investigating molecular mechanisms and discovering drug
targets.
In the present study, iTRAQ-based proteomic analysis
was applied to investigate the effect of hyperin against
H2O2-induced injury in human
endothelium-derived EA.hy926 cells, and to elucidate the potential
protective mechanism of hyperin in oxidative stress-induced
injury.
Materials and methods
Chemicals and reagents
Hyperin was purchased from the National Institutes
for Food and Drug Control (Beijing, China); its chemical structure
is shown in Fig. 1. Dulbecco's
modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were
purchased from Gibco (Grand Island, NY, USA). iTRAQ reagent was
obtained from Applied Biosystems Life Technologies (Foster City,
CA, USA). Antibodies against BH3-interacting domain death agonist
(Bid; BS1819), myeloid cell leukemia-1 (Mcl-1; BS1220), β-actin
(AP0060), Fas (BS6430) and FasL (BS1122) were obtained from
Bioworld Technology, Inc., (Nanjing, China). Antibody against
truncated tBID (tBID; ab10640) was purchased from Abcam (Cambridge,
UK). Antibodies against caspase-3 (#9664), caspase-8 (#8592) and
caspase-9 (#9509) were purchased from Cell Signaling Technology,
Inc. (Beverly, MA, USA). Other reagents were obtained from
Sigma-Aldrich (St. Louis, MO, USA). H2O2 was
freshly prepared for each experiment from a 3% stock solution.
Cell culture and treatments
EA.hy926 cells were purchased from the Cell Bank of
the Chinese Academy of Sciences (Beijing, China) and were cultured
in DMEM supplemented with heat-inactivated FBS (10%), 100 U/ml
penicillin, and 100 g/ml streptomycin. The cells were incubated in
a humidified incubator aerated with 5% CO2 at 37°C.
Hyperin was dissolved in dimethyl sulfoxide (DMSO), and the DMSO
content in all groups was <0.1%. Prior to treatment, the cells
were incubated with serum-free medium for 24 h and randomly
assigned to three groups: a 'control group', an
'H2O2-exposed group', and a 'hyperin-treated
group'. The cells in the hyperin group were treated with designated
concentrations of hyperin for 24 h prior to 200 μmol/l
H2O2 exposure for 4 h in fresh medium.
Cell viability assay
Cell viability was evaluated using
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. Briefly, EA.hy926 cells (in logarithmic phase) were seeded
into 96-well plates (1×104 cells/well) and cultured for
24 h. The medium was then replaced with fresh medium for the
different treatments. Each concentration of reagent was added to
the culture fluid of six parallel wells and a blank well was used
as a control. Subsequently, 10 μl of 5 mg/ml MTT in
phosphate-buffered saline (PBS) was added to each well and the
cells were further incubated for 4 h. The culture medium was then
carefully removed and DMSO (100 μl/well) was added to
dissolve the formazan precipitate. The plates were shaken for 10
min. Optical density was read at 570 nm (490 nm as reference) on a
universal microplate reader (Model 680; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The viability of the EA.hy926 cells in each
well was expressed as a percentage of the viable control cells.
Protein extraction and labeling with
iTRAQ reagents
The cells of each experimental group were collected
by centrifugation (138 × g for 5 min) and washed twice with PBS.
Protein extracts were prepared using lysis buffer (8 mol/l urea, 30
mmol/l HEPES, 1 mmol/l PMSF, 2 mmol/l EDTA, 10 mmol/l DTT). The
protein concentration was estimated using a Bradford assay. iTRAQ
labeling was performed according to the manufacturer's instructions
(Applied Biosystems/MDS SCIEX, Toronto, ON, Canada). Briefly, 100
μg of each protein sample was reduced, alkylated and
subjected to trypsin hydrolysis. Each sample was labeled separately
with the isobaric tags as follows: control group (114 tags),
H2O2 group (115 tags), and hyperin group (116
tags). All labeled peptides were pooled and dried in a SpeedVac
(Thermo Fisher Scientific, Waltham, MA, USA.
High-pH reversed-phase
chromatography
The combined iTRAQ-labeled samples were dissolved in
200 μl buffer A [25% acetonitrile (ACN), 10 mmol/l
KH2PO4, pH 3.0, with phosphoric acid]. The
proteins were separated on a Luna SCX column (4.6×250 mm, 100-Å
pore size; Phenomenex, Inc., Torrance, CA, USA) with buffers A and
B (buffer b: 25% ACN, 2 mol KCl, 10 mmol/l
KH2PO4, pH 3.0, with phosphoric acid) at a
flow rate of 400 nl/min. A solvent gradient system was used as
follows: 0–35 min, 0% B; 35–36 min, 0–5% B; 36–56 min, 5–30% B;
56–61 min, 30–50% B; 61–66 min, 50% B; 66–71 min, 50–100% B; 71–81
min, 100% B. Elution was monitored by measuring absorbance at 214
nm and fractions were collected every 1 min. The collected eluents
were lyophilized to powder and resuspended in 0.1% (v/v)
trifluoroacetic acid (TFA; 40 μl) for further desalting and
concentration using a Strata-X C18 cartridge (Phenomenex, Torrance,
CA, USA). The desalted samples were lyophilized to powder.
Liquid chromatography-tandem mass
spectrometry (LC-MS/MS)
In this study, iTRAQ-labeled samples were
redissolved in 6 μl eluent buffer A [0.1% formic acid (FA)
in water, v/v]. Each of the fractions was analyzed three times
using a Q-Exactive Orbitrap mass spectrometer (Thermo Fisher
Scientific). A flow rate of 400 nl/min was used for protein
separation on a C18 capillary column (Michrom Bioresources, Inc.,
Auburn, CA, USA). A solvent gradient system was used: 0–10 min, 5%
B (0.1% FA in ACN); 10–40 min, 5–30% B; 40–45 min, 30–60% B; 45–48
min, 60–80% B; 48–55 min, 80% B; 55–58 min, 80–5% B; 58–65 min, 5%
B. A full MS scan (350–2,000 m/z range) was acquired in the
Orbitrap at a mass resolution of 70,000. A maximum of 10 precursors
per cycle were then chosen for fragmentation by high-energy
collision dissociation (HCD) in the C-trap in linear trap
quadrupole with an isolation width of 3.0 m/z. Precursor ion
activation was performed with an isolation width of 2.5 Da. The ion
transfer tube temperature and spray voltage were 320°C and 1.8 kV,
respectively.
Protein analysis
For protein identification, MS/MS spectra were
analyzed using Proteome Discoverer software v. 1.3 (PD; Thermo
Fisher Scientific). The precursor ion mass range was set at
350–6,000 Da. The minimum number of peaks in a spectrum was set to
10, and the threshold for the S/N ratio was set to 1.5. Next, the
MS spectra were searched using Mascot 2.3.0 (Matrix Science,
London, UK) with precursor mass tolerance at 15 ppm, fragment ion
mass tolerance at 20 mmu, trypsin enzyme with 1 miscleavage, methyl
methanethiosulfonate of cysteine and iTRAQ 8-plex of lysine and the
NH2-terminus as fixed modifications, and deamidation of
asparagine and glutamine, oxidation of methionine and iTRAQ 8-plex
of tyrosine as variable modifications. Protein identifications were
accepted at 95% or higher probability and contained at least two
identified peptides with a false discovery rate (FDR) <1%. The
peptides were quantified using PD software. Tagged samples were
normalized by comparing median protein ratios for the reference
channel. Protein quantitative ratios were calculated from the
median of all peptide ratios. The proteins with a relative
expression of >1.2 or <0.8, and with P<0.05 to ensure up-
and downregulation authenticity, were chosen for further analysis.
Protein sequences and functional information were retrieved from
the UniProt databases (http://www.uniprot.org/). For further analysis,
functional annotation analysis of altered proteins was carried out
using DAVID annotation software (http://david.abcc.ncifcrf.gov/) and the KEGG database
(http://www.genome.jp/kegg/) by importing
GenInfo (GI) numbers.
Western blot analysis
Following treatment with various concentrations of
hyperin and/or H2O2 as described above, the
EA.hy926 cells were harvested and washed with PBS. Protein extracts
were prepared with RIPA buffer (Beyotime Institute of
Biotechnology, Shanghai, China). Protein concentration was
estimated using a bicinchoninic acid (BCA) protein assay kit
(Sangon Biotech, Shanghai, China). For western blot analysis, equal
amounts of protein (50 μg) were separated on 12% sodium
dodecyl (SDS)-polyacrylamide gels and electrotransferred onto
nitrocellulose membranes (PALL Gelman Laboratory, Ann Arbor, MI,
USA) that were blocked in 5% non-fat milk. The membranes were
incubated overnight at 4°C with primary antibodies. After three
washes with Tris-buffered saline containing 0.05% Tween-20 (TBS-T),
the membranes were incubated for 1 h with horseradish peroxidase
(HRP)-conjugated goat anti-rabbit IgG secondary antibody at room
temperature. The bands were visualized using an ECL detection kit
(CoWin Biotech, Beijing, China). Quantification of the bands was
performed by densitometric analysis using the Adobe Photoshop 7.0.1
software (Adobe Systems, Inc., San Jose, CA, USA).
Flow cytometric analysis
Quantitative detection of apoptotic cells and
analysis of cell cycle distribution in the cultures were undertaken
using flow cytometry. The EA.hy926 cells treated with different
concentrations of hyperin and/or exposed to 200 μmol/l
H2O2 were collected by centrifugation (138 ×
g for 5 min) and cell density was adjusted to 1×105
cells/ml. The cells were washed twice with cold PBS and centrifuged
(138 × g for 5 min). The pellets were fixed overnight in pre-cooled
70% (v/v) ethanol at 4°C, and then washed with cold PBS. The cells
were suspended in 1 ml of propidium iodide solution (20 mg/ml)
supplemented with 0.25 mg/ml RNase A and 0.1% (v/v) Triton X-100,
and incubated on ice for 30 min in the dark. The samples were
analyzed with a FACSCalibur flow cytometer (BD Biosciences, San
Jose, CA, USA).
Statistical analysis
Each experiment was performed at least 3 times. All
data are expressed as the means ± SD. Statistical analysis was
performed using a Student's t-test and SPSS 18.0 software (SPSS
Inc., Chicago, IL, USA). A P-value <0.05 was considered to
indicate a statistically significant difference.
Results
Hyperin protects EA.hy926 cells against
H2O2-induced cell death
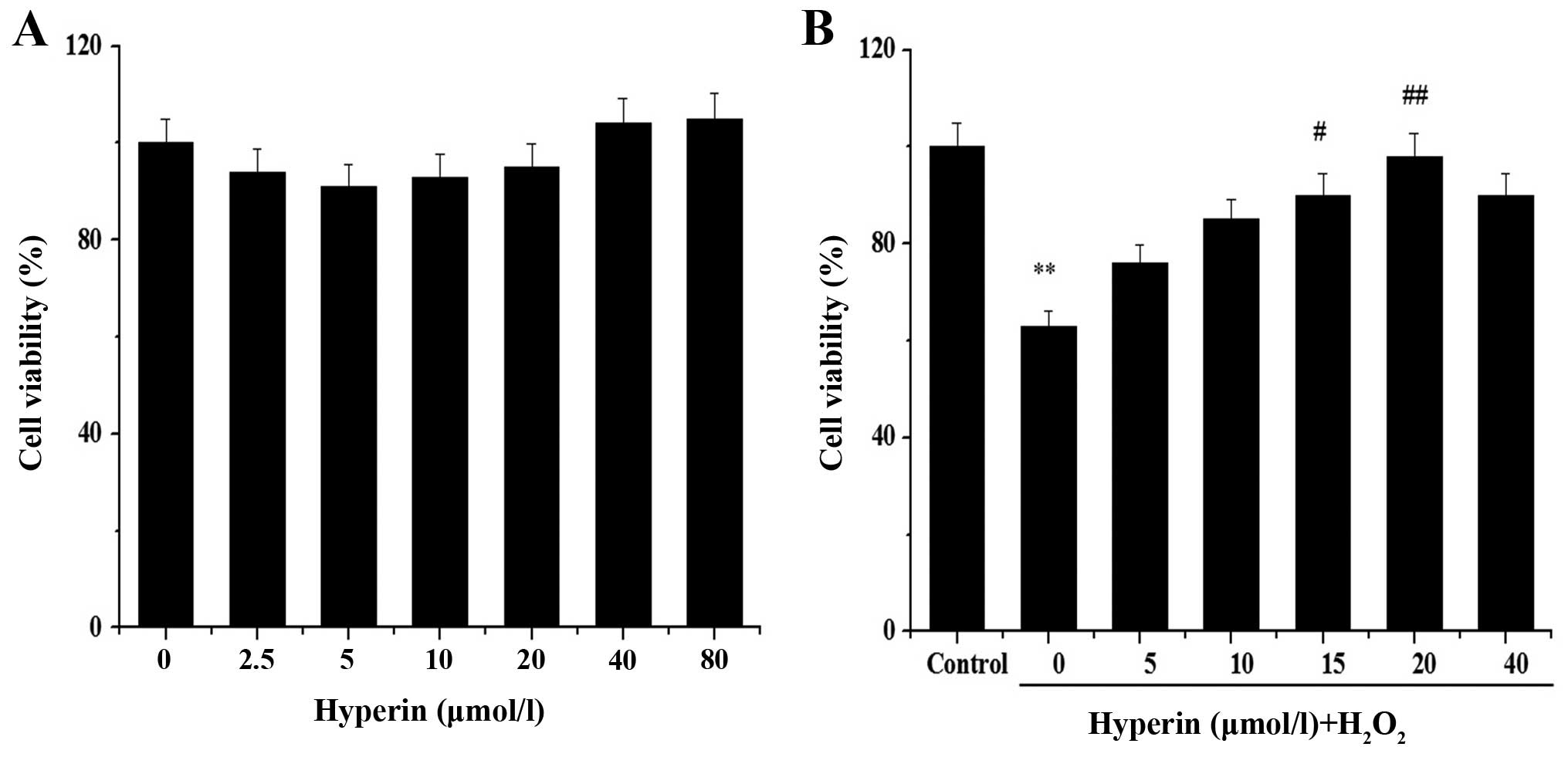
We assessed whether hyperin protects EA.hy926 cells
against the effect of H2O2 by MTT assay. No
obvious cytotoxicity in untreated cells was noted, nor was obvious
cytoxicity noted in cells treated with hyperin at concentrations in
the range of 2.5–80 μmol/l (Fig. 2A). We also noted that hyperin
exerted a protective effect on H2O2-injured
cells in a dose-dependent manner (Fig. 2B). When the cells were treated
with 20 μmol/l hyperin, cell viability was restored to 98%.
Therefore, this concentration was selected for subsequent proteomic
analysis.
Hyperin protects EA.hy926 cells against
H2O2-induced changes in the proteome
A total of 3,640 proteins were identified using
iTRAQ, of which 250 were altered by H2O2
(data not shown); the 250 proteins were functionally classified
into various relevant categories such as transition metal
ion-binding, zinc ion-binding and transferase activity, which are
mainly involved in regulating cellular component organization,
growth, cytoskeleton organization, and response to stimulus, using
the NCBI online database and DAVID software platform; the most
significantly up- and downregulated proteins are shown in Fig. 3. Further analysis of the pathways
and networks revealed that the regulated proteins were mainly
involved in apoptosis, inositol phosphate metabolism and vitamin B6
metabolism.
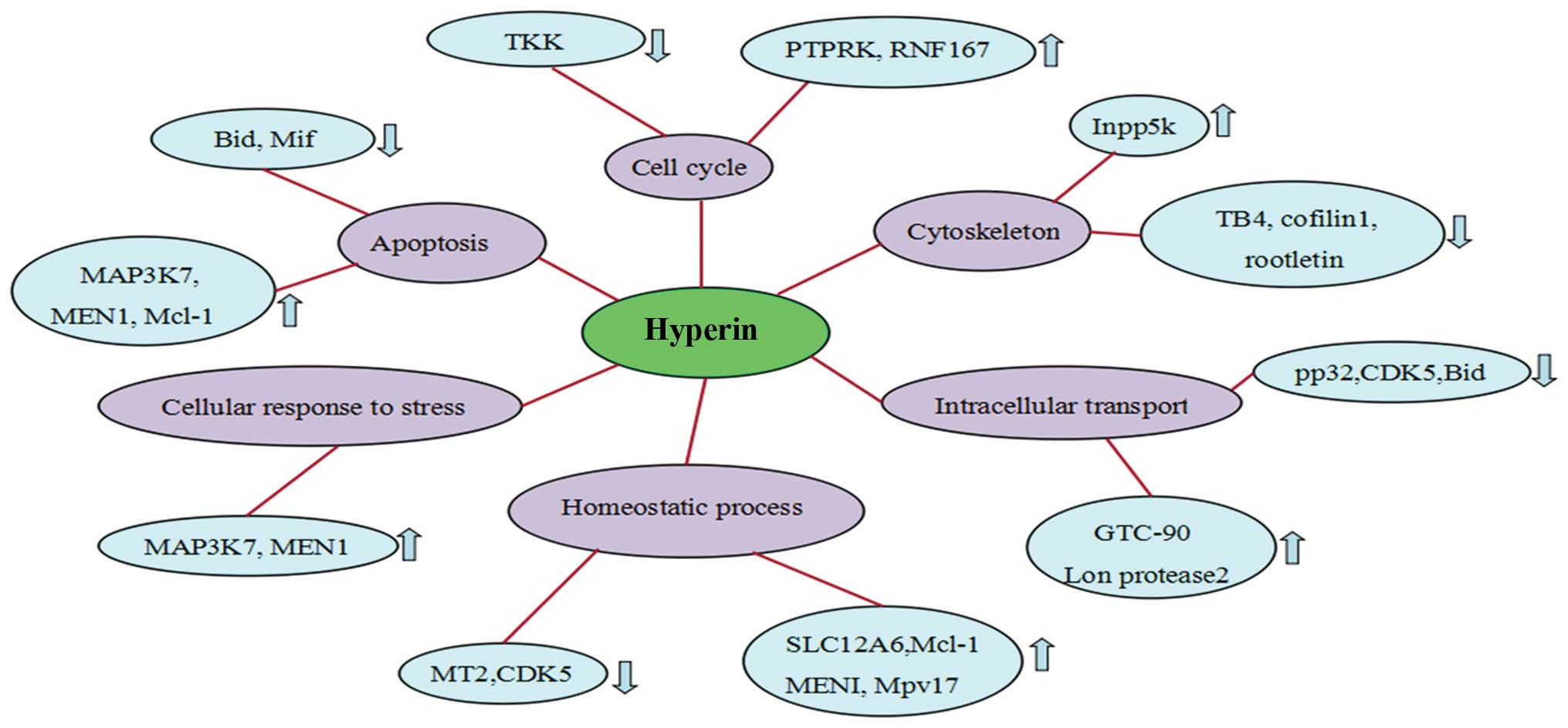
Following treatment with hyperin, of the 250
proteins that exhibited altered expression upon
H2O2 exposure, 52 revealed a tendency towards
restoration of the expression levels (Table I). Compared with the
H2O2 group, 28 proteins were downregulated
and 24 were upregulated. These proteins were associated with
apoptosis, cell cycle and cytoskeleton organization.
 | Table IList of differentially expressed
proteins. |
Table I
List of differentially expressed
proteins.
| Accessiona | Description | MW [kDa]b | Calc. pIc | 115/114d | 116/115e |
|---|
| Q5TZA2 | Rootletin | 228.4 | 5.5 | 4.464 | 0.244 |
| P68402 | Platelet-activating
factor acetylhydrolase IB subunit β | 25.6 | 5.92 | 1.767 | 0.610 |
| P33981 | Dual specificity
protein kinase TTK | 97 | 8.16 | 1.733 | 0.647 |
| Q6NSJ5 | Leucine-rich
repeat-containing protein 8E | 90.2 | 6.96 | 1.653 | 0.555 |
| O60518 | Ran-binding protein
6 | 124.6 | 5.01 | 1.639 | 0.603 |
| Q6PJG6 | BRCA1-associated
ATM activator 1 | 88.1 | 5.27 | 1.462 | 0.801 |
| P55957 | BH3-interacting
domain death agonist | 22 | 5.44 | 1.416 | 0.691 |
| Q9BVK2 | Probable dolichyl
pyrophosphate Glc1Man9GlcNAc2 α-1,3-glucosyltransferase | 60 | 9.14 | 1.416 | 0.691 |
| Q8IUC8 | Polypeptide
N-acetylgalactosaminyltransferase 13 | 64 | 6.83 | 1.385 | 0.791 |
| P02795 |
Metallothionein-2 | 6 | 7.83 | 1.361 | 0.685 |
| P04732 |
Metallothionein-1E | 6 | 7.96 | 1.294 | 0.798 |
| Q71U36 | Tubulin α-1A
chain | 50.1 | 5.06 | 1.290 | 0.654 |
| P62328 | Thymosin β-4 | 5 | 5.06 | 1.271 | 0.780 |
| P51858 | Hepatoma-derived
growth factor | 26.8 | 4.73 | 1.263 | 0.817 |
| P20962 | Parathymosin | 11.5 | 4.16 | 1.261 | 0.736 |
| P14174 | Macrophage
migration inhibitory factor | 12.5 | 7.88 | 1.241 | 0.808 |
| P23528 | Cofilin-1 | 18.5 | 8.09 | 1.236 | 0.790 |
| Q92688 | Acidic leucine-rich
nuclear phosphoprotein 32 family member B | 28.8 | 4.06 | 1.232 | 0.810 |
| O60232 | Sjoegren
syndrome/scleroderma autoantigen 1 | 21.5 | 5.24 | 1.230 | 0.717 |
| Q3MJ13 | WD
repeat-containing protein 72 | 123.3 | 6.67 | 1.230 | 0.821 |
| Q3YEC7 | Rab-like protein
6 | 79.5 | 5.22 | 1.230 | 0.783 |
| P39687 | Acidic leucine-rich
nuclear phosphoprotein 32 family member A | 28.6 | 4.09 | 1.220 | 0.768 |
| Q00535 | Cyclin-dependent
kinase 5 | 33.3 | 7.66 | 1.218 | 0.755 |
| Q15147 |
1-Phosphatidylinositol 4,5-bisphosphate
phosphodiesterase β-4 | 134.4 | 6.9 | 1.217 | 0.729 |
| P58546 | Myotrophin | 12.9 | 5.52 | 1.209 | 0.769 |
| Q8NCW5 | NAD(P)H-hydrate
epimerase | 31.7 | 7.66 | 1.206 | 0.807 |
| P00338 | L-lactate
dehydrogenase A chain | 36.7 | 8.27 | 1.202 | 0.798 |
| O43318 | Mitogen-activated
protein kinase kinase kinase 7 | 67.2 | 7.11 | 0.833 | 1.244 |
| Q9H6Y7 | E3
ubiquitin-protein ligase RNF167 | 38.3 | 5.63 | 0.824 | 1.233 |
| Q04756 | Hepatocyte growth
factor activator | 70.6 | 7.24 | 0.820 | 1.284 |
| O00255 | Menin | 68 | 6.55 | 0.811 | 1.243 |
| Q9UP83 | Conserved
oligomeric Golgi complex subunit 5 | 92.7 | 6.6 | 0.806 | 1.224 |
| Q8IWE4 | DCN1-like protein
3 | 34.3 | 5.12 | 0.796 | 1.280 |
| Q9UBR2 | Cathepsin Z | 33.8 | 7.11 | 0.794 | 1.219 |
| P0CG39 | POTE ankyrin domain
family member J | 117.3 | 5.97 | 0.783 | 1.311 |
| Q9Y676 | 28S ribosomal
protein S18b, mitochondrial | 29.4 | 9.38 | 0.779 | 1.259 |
| Q9UMR5 | Lysosomal
thioesterase PPT2 | 34.2 | 6.33 | 0.769 | 1.227 |
| Q9BT40 | Inositol
polyphosphate 5-phosphatase K | 51.1 | 6.54 | 0.747 | 1.200 |
| P42356 |
Phosphatidylinositol 4-kinase α | 231.2 | 6.87 | 0.733 | 1.404 |
| P39210 | Protein Mpv17 | 19.7 | 9.47 | 0.730 | 1.201 |
| Q9H330 | Transmembrane
protein 245 | 100.9 | 8.87 | 0.713 | 1.369 |
| Q68CQ4 | Digestive organ
expansion factor homolog | 37.3 | 5.66 | 0.705 | 1.234 |
| Q9P2K3 | REST corepressor
3 | 87 | 5.88 | 0.692 | 1.428 |
| Q6PJF5 | Inactive rhomboid
protein 2 | 55.5 | 8.27 | 0.676 | 1.349 |
| Q8NF91 | Nesprin-1 | 96.6 | 8.82 | 0.657 | 1.522 |
| Q9UHW9 | Solute carrier
family 12 member 6 | 1010.5 | 5.53 | 0.611 | 1.366 |
| Q63HN8 | E3
ubiquitin-protein ligase RNF213 | 127.5 | 7.08 | 0.604 | 1.515 |
| Q86UB9 | Transmembrane
protein 135 | 591 | 6.48 | 0.587 | 1.958 |
| Q92560 | Ubiquitin
carboxyl-terminal hydrolase BAP1 | 52.3 | 9.45 | 0.453 | 1.938 |
| Q86WA8 | Lon protease
homolog 2, peroxisomal | 80.3 | 6.84 | 0.442 | 1.352 |
| Q07820 | Induced myeloid
leukemia cell differentiation protein Mcl-1 | 94.6 | 7.3 | 0.408 | 2.215 |
| Q8TE02 | Elongator complex
protein 5 | 34.8 | 4.97 | 0.772 | 1.217 |
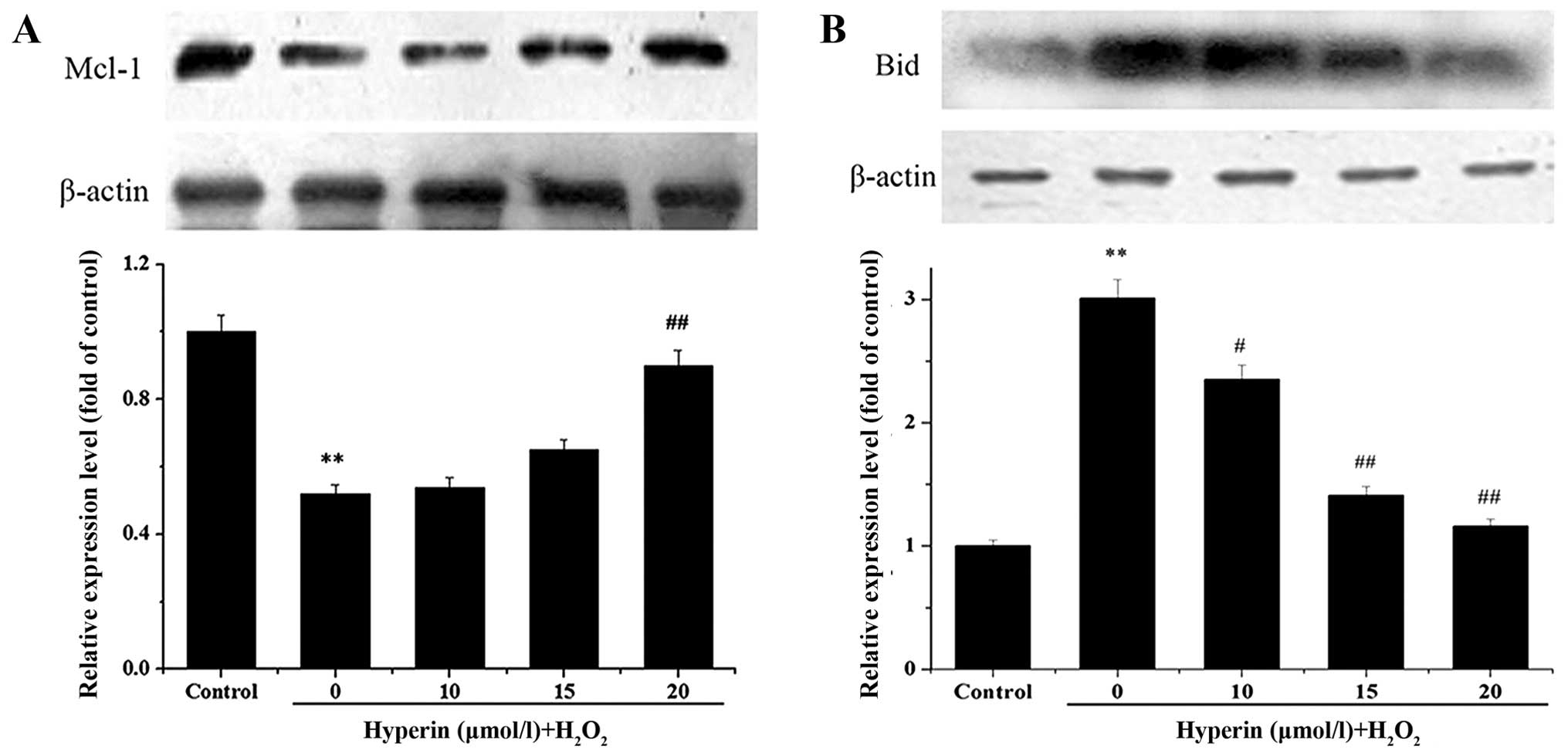
Bid and Mcl-1 protein expression
An examination of Bid and Mcl-1 expression after the
iTRAQ experiment demonstrated marked changes. Therefore, the
results were validated by western blot analysis, as shown in
Fig. 4. Compared with the
H2O2-exposed group, Bid expression in the
hyperin-treated groups was significantly decreased, while Mcl-1
expression was significantly increased. In both cases, the effect
was dose-dependent. The results were in agreement with those of the
proteomics analysis.
Hyperin protects EA.hy926 cells against
H2O2-induced cell cycle arrest
We further analyzed the effect of
H2O2 and hyperin on apoptosis and cell cycle
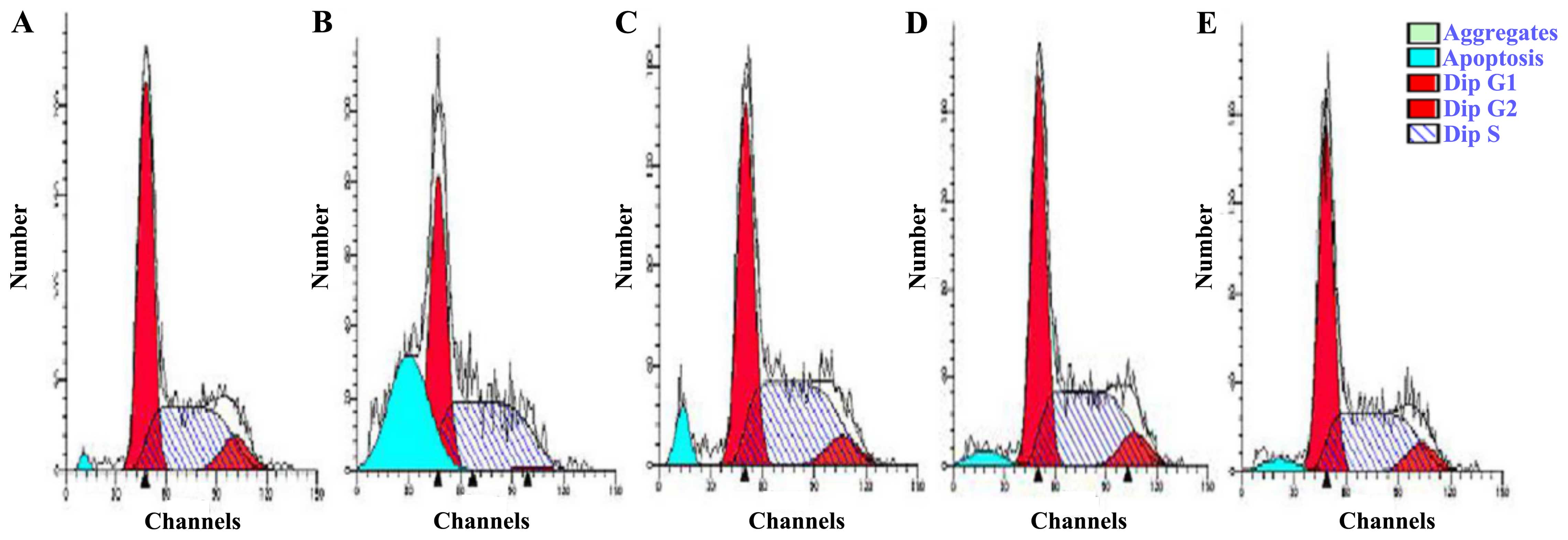
distribution by flow cytometry. The group exposed to 200
μmol/l H2O2 exhibited a higher rate of
apoptosis (30.83%) than the control group (1.32%) (Fig. 5). Treatment with hyperin at 10, 15
or 20 μmol/l resulted in decreased accumulation of apoptotic
cells at the sub-G peak compared with that in the
H2O2-exposed group. The percentage of cells
in the G0/G1 phase was increased in the
H2O2-exposed group; however, it decreased in
the hyperin group in a dose-independent manner. The results
indicated that the cells were blocked in the G0/G1 phase following
H2O2 treatment and that hyperin reduced the
number of cells in cycle arrest, thereby reducing damage.
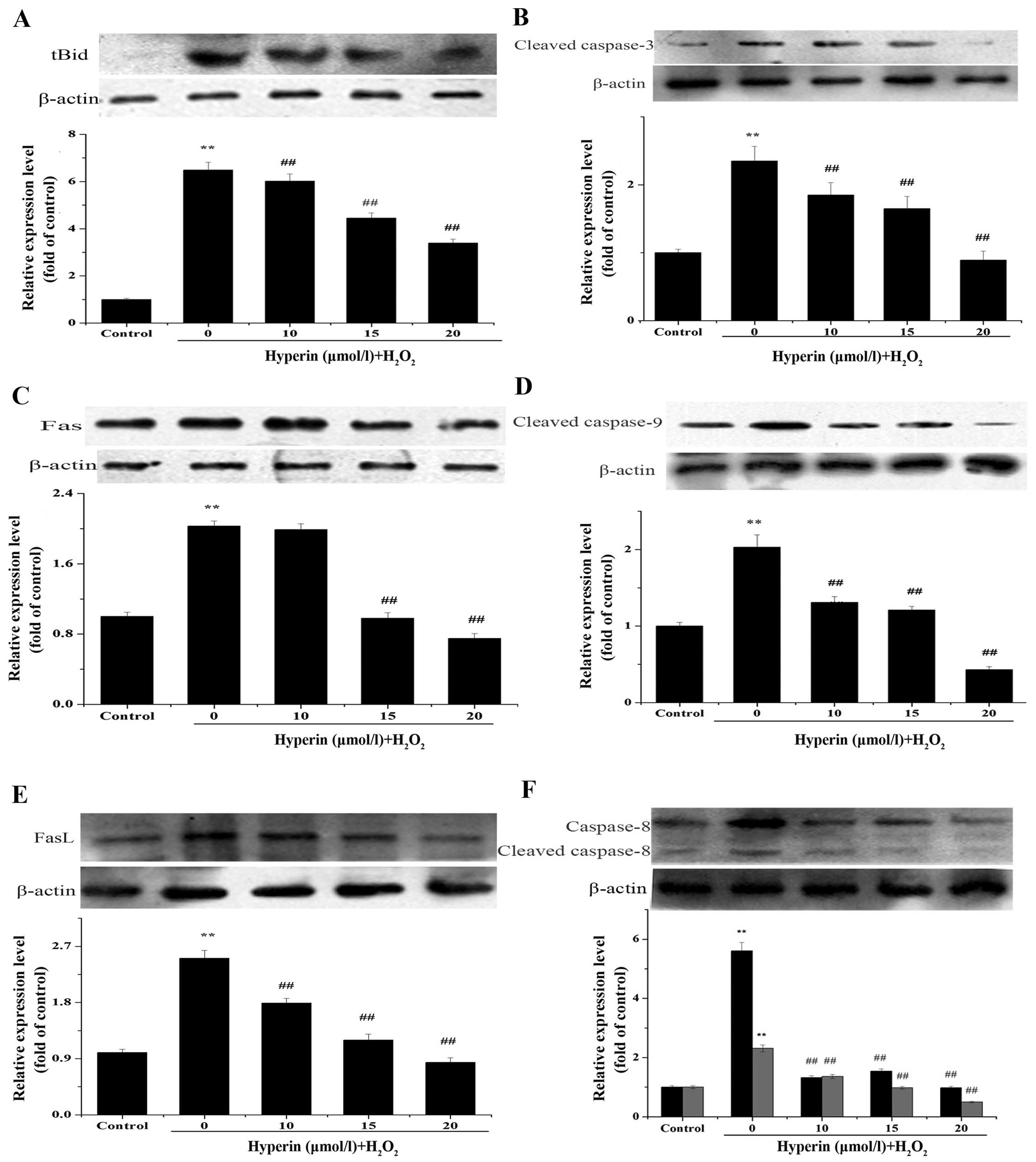
Expression of tBid, Fas, FasL, cleaved
caspase-3, -8 and -9
Fas, FasL, cleaved caspase-3, -8, -9 play important
roles in apoptosis. tBid is the cleaved form of Bid and is involved
in the mitochondrial apoptotic pathway (22,23). The expression of these proteins
was assayed by western blot analysis to clarify the role of the
Bid- and Mcl-1-mediated apoptosis mechanism in
H2O2-injured EA.hy926 cells and the effect of
hyperin. As shown in Fig. 6A–F,
hyperin significantly decreased the relative expression of these
proteins compared to that in the H2O2 group,
in a dose-dependent manner.
Discussion
In our previous study (10), we found that hyperin exerted
protective effects against H2O2-induced cell
injury and cell cycle arrest, and decreased the accumulation of
apoptotic cells at the sub-G peak, as was also demonstrated in the
present study. In this study we aimed to further investigate the
mode of action of hyperin using iTRAQ-based proteomic analysis. A
total of 3,640 proteins were identified, of which 250 were found to
be altered by H2O2; after treatment with
hyperin, 52 of these proteins exhibited a tendency towards normal
expression (see Table I). These
proteins were associated with multiple biological processes
including apoptosis, cell cycle, and cytoskeleton organization. The
results of the MTT assay and flow cytometric analysis revealed that
hyperin protects endothelial cells from cell apoptosis and death
induced by H2O2. Therefore, we focused on the
effect of hyperin on apoptosis. The functional roles of the
proteins with altered expression levels following treatment with
hyperin, which were found to be associated with apoptosis by iTRAQ
analysis, are briefly discussed below.
In the present study, mitogen-activated protein
kinase kinase kinase 7 (MAP3K7) and receptor-type tyrosine-protein
phosphatase-kappa (PTPRK) expression was upregulated following
hyperin treatment (Fig. 3),
indicating that hyperin inhibits H2O2-induced
apoptosis in the EA.hy926 cells. MAP3K7 acts as an essential
component of the MAPK signal transduction pathway, which plays an
important role in the oxidative stress response (13). Also, it is a crucial modulator of
angiogenesis, and it has been demonstrated that MAP3K7 deletion is
marked by TNF-dependent endothelial cell death and vessel
regression (14). PTPRK regulates
various processes, including cell growth, tumor invasion, cell
cycle, and metastasis. Previous research has found that knockdown
of PTPRK resulted in increased apoptosis through the c-Jun
N-terminal kinase (JNK) pathway (15).
By contrast, the expression of macrophage migration
inhibitory factor (Mif), and cofilin 1 (CFL1) in response to
H2O2 was downregulated in hyperin-treated
cells (Fig. 3). Mif is an
inflammatory cytokine with chemokine-like functions, which has the
capacity to induce apoptosis and cell dysfunction (16). Schumacher et al have
reported that an increased level of Mif is associated with
thrombosis (17), and that it
plays a pivotal role in regulating platelet survival and thrombotic
potential (18). CFL1 plays an
important role in the regulation of cell morphology and
cytoskeletal organization. A previous study has implied that
mitochondrial translocation of CFL1 plays a crucial role in the
promotion of apoptosis (19). Our
findings suggested that the downregulation of Mif and CFL1
participates in the antithrombotic effect of hyperin.
iTRAQ proteomic analysis revealed marked changes in
the expression of the anti-apoptotic protein, Mcl-1, and the
pro-apoptotic protein, Bid. However, the mechanism underlying Bid-
and Mcl-1-mediated apoptosis remains unclear and requires further
study.
Mcl-1 is an outer mitochondrial membrane-bound
protein of the Bcl-2 family that has a BH3-like domain, and plays
an important role in the anti-apoptotic process (20). It has been shown to inhibit cell
death by suppressing the release of cytochrome c from the
mitochondria, through binding and sequestering the pro-apoptotic
proteins, Bak and Bax, on the outer mitochondrial membrane in
EA.hy926 cells (21). By
contrast, Bid is the only pro-apoptotic protein with a BH3 domain
and is involved in the Fas and TNF signaling pathways (22). tBid is known to facilitate Bax
translocation to the mitochondria, triggering the release of
cytochrome c from the mitochondria (23). In the present study, we found for
the first time to the best of our knowledge, that hyperin induces
an increase in the level of Mcl-1 and a decrease in Bid, in
response to H2O2, in a dose-dependent manner.
Furthermore, similar to Bid expression, the levels of the
apoptosis-related proteins tBid, Fas, FasL, cleaved caspase-8, -9
and -3 induced by H2O2 exposure were
significantly decreased after treatment with hyperin. These data
are in agreement with previous reseacrh which demonstrated that the
apoptotic pathway is initiated by Fas and FasL, with sequential
activation of the initiator caspase-8 and Bid in the cells damaged
by H2O2 (24). Bid is then cleaved into tBid,
resulting in the release of cytochrome c from mitochondria
into the cytosol. Cytochrome c binds to apoptotic protease
activating factor-1 (Apaf-1) and then recruits caspase-9 to form
the apoptosome complex. This complex results in the activation of
caspase-3 and execution of cell death (25). This process is regulated by
anti-apoptotic proteins such as Mcl-1 and Bcl-2, and proapoptotic
proteins such as tBid (26,27). Our present findings indicated that
hyperin blocks the Bid- and Mcl-1-mediated apoptotic pathways that
are involved in H2O2-induced apoptosis in
EA.hy926 cells.
In conclusion, we systematically examined and
compared the proteomic profiles of untreated,
H2O2-exposed, and hyperin pre-treated
EA.hy926 cells for the first time, to the best of our knowledge.
The results demonstrate that hyperin effectively prevents
H2O2-induced cell injury through regulation
of the Mcl-1- and Bid-mediated anti-apoptotic mechanism. Our
findings suggest that hyperin is a promising candidate for use in
the treatment of thrombotic diseases.
Acknowledgments
This study was financially supported by the National
Natural Science Foundation of China (grant nos. 81274132 and
81172938). We are grateful to Editage for providing editorial
assistance.
References
|
1
|
Versari D, Daghini E, Virdis A, Ghiadoni L
and Taddei S: Endothelial dysfunction as a target for prevention of
cardiovascular disease. Diabetes Care. 32(Suppl 2): S314–S321.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Triggle CR, Samuel SM, Ravishankar S,
Marei I, Arunachalam G and Ding H: The endothelium: influencing
vascular smooth muscle in many ways. Can J Physiol Pharmacol.
90:713–738. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Choy JC, Granville DJ, Hunt DW and McManus
BM: Endothelial cell apoptosis: biochemical characteristics and
potential implications for atherosclerosis. J Mol Cell Cardiol.
33:1673–1690. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aird WC: Endothelium and haemostasis.
Hamostaseologie. 35:11–16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Middleton E Jr, Kandaswami C and
Theoharides TC: The effects of plant flavonoids on mammalian cells:
implications for inflammation, heart disease, and cancer. Pharmacol
Rev. 52:673–751. 2000.PubMed/NCBI
|
|
6
|
Wang WQ, Ma CG and Xu SY: Protective
effect of hyperin against myocardial ischemia and reperfusion
apoptosis. Acta Pharmacol Sin. 17:341–344. 1996.
|
|
7
|
Bernatoniene J, Trumbeckaite S, Majiene D,
Baniene R, Baliutyte G, Savickas A and Toleikis A: The effect of
crataegus fruit extract and some of its flavonoids on mitochondrial
oxidative phosphorylation in the heart. Phytother Res.
23:1701–1707. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li ZL, Liu JC, Hu J, Li XQ, Wang SW, Yi DH
and Zhao MG: Protective effects of hyperoside against human
umbilical vein endothelial cell damage induced by hydrogen
peroxide. J Ethnopharmacol. 139:388–394. 2012. View Article : Google Scholar
|
|
9
|
Müller WE, Singer A, Wonnemann M, Hafner
U, Rolli M and Schäfer C: Hyperforin represents the
neurotransmitter reuptake inhibiting constituent of hypericum
extract. Pharmacopsychiatry. 31(Suppl 1): 16–21. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hao XL: Study on the substance bases of
anti-thrombi activity and the mechanism of anti-apoptosis of human
umbilical vein endothelial cells of total flavonoids from Folium
Apocyni Veneti. Ph.D thesis. Shanxi Medical University; 2009, In
Chinese.
|
|
11
|
Ross PL, Huang YN, Marchese JN, Williamson
B, Parker K, Hattan S, Khainovski N, Pillai S, Dey S, Daniels S, et
al: Multiplexed protein quantitation in Saccharomyces cerevisiae
using amine-reactive isobaric tagging reagents. Mol Cell
Proteomics. 3:1154–1169. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aggarwal K, Choe LH and Lee KH: Shotgun
proteomics using the iTRAQ isobaric tags. Brief Funct Genomics
Proteomics. 5:112–120. 2006. View Article : Google Scholar
|
|
13
|
Lim D, Roh JY, Eom HJ, Choi JY, Hyun J and
Choi J: Oxidative stress-related PMK-1 P38 MAPK activation as a
mechanism for toxicity of silver nanoparticles to reproduction in
the nematode Caenorhabditis elegans. Environ Toxicol Chem.
31:585–592. 2012. View
Article : Google Scholar
|
|
14
|
Morioka S, Inagaki M, Komatsu Y, Mishina
Y, Matsumoto K and Ninomiya-Tsuji J: TAK1 kinase signaling
regulates embryonic angiogenesis by modulating endothelial cell
survival and migration. Blood. 120:3846–3857. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun PH, Ye L, Mason MD and Jiang WG:
Receptor-like protein tyrosine phosphatase κ negatively regulates
the apoptosis of prostate cancer cells via the JNK pathway. Int J
Oncol. 43:1560–1568. 2013.PubMed/NCBI
|
|
16
|
Stojanovic I, Saksida T, Timotijevic G,
Sandler S and Stosic-Grujicic S: Macrophage migration inhibitory
factor (MIF) enhances palmitic acid- and glucose-induced murine
beta cell dysfunction and destruction in vitro. Growth Factors.
30:385–393. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schumacher E, Vigh E, Molnár V, Kenyeres
P, Fehér G, Késmárky G, Tóth K and Garai J: Thrombosis preventive
potential of chicory coffee consumption: a clinical study.
Phytother Res. 25:744–748. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chatterjee M, Borst O, Walker B, Fotinos
A, Vogel S, Seizer P, Mack A, Alampour-Rajabi S, Rath D, Geisler T,
et al: Macrophage migration inhibitory factor limits
activation-induced apoptosis of platelets via CXCR7-dependent Akt
signaling. Circ Res. 115:939–949. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tang Q, Ji Q, Tang Y, Chen T, Pan G, Hu S,
Bao Y, Peng W and Yin P: Mitochondrial translocation of cofilin-1
promotes apoptosis of gastric cancer BGC-823 cells induced by
ursolic acid. Tumour Biol. 35:2451–2459. 2014. View Article : Google Scholar
|
|
20
|
Yang T, Kozopas KM and Craig RW: The
intracellular distribution and pattern of expression of Mcl-1
overlap with, but are not identical to, those of Bcl-2. J Cell
Biol. 128:1173–1184. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shimazu T, Degenhardt K, Nur-E-Kamal A,
Zhang J, Yoshida T, Zhang Y, Mathew R, White E and Inouye M:
NBK/BIK antagonizes MCL-1 and BCL-XL and activates BAK-mediated
apoptosis in response to protein synthesis inhibition. Genes Dev.
21:929–941. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Luo X, Budihardjo I, Zou H, Slaughter C
and Wang X: Bid, a Bcl2 interacting protein, mediates cytochrome c
release from mitochondria in response to activation of cell surface
death receptors. Cell. 94:481–490. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li H, Zhu H, Xu CJ and Yuan J: Cleavage of
BID by caspase 8 mediates the mitochondrial damage in the Fas
pathway of apoptosis. Cell. 94:491–501. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Inagaki M, Omori E, Kim JY, Komatsu Y,
Scott G, Ray MK, Yamada G, Matsumoto K, Mishina Y and
Ninomiya-Tsuji J: TAK1-binding protein 1, TAB1, mediates osmotic
stress-induced TAK1 activation but is dispensable for TAK1-mediated
cytokine signaling. J Biol Chem. 283:33080–33086. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chun KH, Benbrook DM, Berlin KD, Hong WK
and Lotan R: The synthetic heteroarotinoid SHetA2 induces apoptosis
in squamous carcinoma cells through a receptor-independent and
mitochondria-dependent pathway. Cancer Res. 63:3826–3832.
2003.PubMed/NCBI
|
|
26
|
Gogvadze V, Orrenius S and Zhivotovsky B:
Multiple pathways of cytochrome c release from mitochondria in
apoptosis. Biochim Biophys Acta. 1757:639–647. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yin XM: Signal transduction mediated by
Bid, a pro-death Bcl-2 family proteins, connects the death receptor
and mitochondria apoptosis pathways. Cell Res. 10:161–167. 2000.
View Article : Google Scholar : PubMed/NCBI
|