Introduction
Bone marrow-derived mesenchymal stem cells (BM-MSCs)
have previously been shown to participate in the regeneration
(1,2) and homeostasis of various tissues
(3). Diverse factors, including
stromal cell-derived factor-1 (SDF-1) (4), transforming growth factor-β (TGF-β)
(5,6), platelet-derived growth factor (PDGF)
(7) and substance P (SP)
(8,9), induce the migration of BM-MSCs to
regeneration sites in order to accelerate the wound healing
process. Furthermore, the migration of BM-MSCs plays an important
role in the establishment of the tumor microenvironment (10,11).
Although the migration of BM-MSCs is essential to
many important biological processes, the cellular and molecular
mechanisms responsible for BM-MSC migration were not fully
understood. Several intracellular signaling pathways have been
suggested to act as mediators of BM-MSC migration, including
protein kinase B (Akt) and the mitogen-activated protein
kinase/extracellular signal-regulated kinase 1/2 (MAPK/ERK 1/2)
signaling pathway. These pathways are among the most important
signaling pathways that control the growth of various types of
cells including BM-MSCs (12,13). Moreover, previous research has
suggested that they are also important for the migration of
BM-MSCs, as it has been shown that the pharmacological regulation
of Akt activity affects BM-MSCs migration (14), and SDF-1 promotes the migration of
human BM-MSCs through signal transducer and activator of
transcription 3 (STAT3) and ERKs (15).
Integrin-based focal adhesions are an essential part
of cell-extracellular matrix interactions, which are regulated to
enable cell migration (16). The
focal adhesion kinase (FAK) forms physical and functional complexes
with other proteins inside the focal adhesions in order to control
cell migration in response to various extracellular stimuli
(17,18). The Src tyrosine kinase also
interacts functionally with proteins of these complexes and
regulates the turnover of focal adhesions that directly control
cell migration (16). The
important role which FAK plays has been shown in relation to the
PDGF-mediated migration of BM-MSCs (7).
Cell-cell interactions are also important in cell
migration (16). The cadherin
family mediates these intercellular interactions through adherens
junctions (19). Importantly, the
requirement of N-cadherin for cell migration has been demonstrated
in cancer cells (20), mammary
epithelial cells (21) and the
neural crest (22).
Previous research has demonstrated that SP, an
11-amino-acid neuropeptide involved in pain perception, enhances
the migration of BM-MSCs to participate in tissue regeneration and
wound repair, and that SP upregulated the expression of matrix
metalloproteinases (MMPs) in BM-MSCs (9). However, the mechanisms of
SP-mediated migration of BM-MSCs were unknown. Thus, in the present
study we decided to examine the cellular and molecular mechanisms
that induce the migration of BM-MSCs in response to SP in
vitro using the BM-derived MSC-like cell line ST2.
Materials and methods
Cell culture
The ST2 cell line was purchased from Riken Cell Bank
(Tsukuba, Japan). Cells were cultured at 37°C in a humidified
incubator containing 5% CO2 in RPMI 1640 supplemented
with 10% heat-inactivated fetal bovine serum (FBS) and 1%
penicillin/streptomycin (P/S) (all from Invitrogen, Carlsbad, CA,
USA). When they reached 80% confluence, the cells were harvested
using 0.25% trypsin/EDTA (Invitrogen) and sub-cultured at a ratio
of 1:3–1:4. The medium was changed every 3–4 days. Cells at passage
5–8 were used for experiments.
SP
SP, which we used to induce the mobilization of
BM-MSCs, was purchased from EMD Millipore (San Diego, CA, USA) and
was prepared with 5% acetic acid (Sigma-Aldrich, St. Louis, MO,
USA).
Migration assay
Millicell culture plate inserts (EMD Millipore),
8-µm pore size, were coated with type I collagen (0.5
µg/ml; Nitta Gelatin NA Inc., Osaka, Japan) and allowed to
dry overnight. After washing the inserts three times with
phosphate-buffered saline (PBS), 2.5×104 ST2 cells were
seeded on the upper chamber of each insert suspended in normal
growth media. Cells were incubated for 5–6 h and medium was then
changed for Dulbecco's modified Eagle's medium (DMEM; GE Healthcare
Life Sciences, Buckinghamshire, UK) including 2% FBS and 1% P/S.
After overnight incubation, 300 nM SP was added to the lower
chamber of each well. When required, the cells were pre-treated for
30 min with the following antagonists: the NK1 antagonist RP 67580
(10 µM; Tocris Bioscience, Bristol, UK); the Src inhibitor
PP2 (1 µM); the PI3K inhibitor LY294002 (10 µM); the
MAPK/ERK kinase (MEK) inhibitor PD98059 (10 µM) (all from
EMD Millipore) mouse IgG (40 µg/ml; Jackson Immuno Research,
Pennsylvania, PA, USA) or monoclonal anti-N-cadherin antibody
(clone GC-4, 40 µg/ml, Cat. no. C3865; Sigma-Aldrich). After
12 h incubation, the inserts were fixed using 4% paraformaldehyde
in PBS for 10 min at room temperature and stained with hematoxylin
solution (Sigma-Aldrich) for 30 min. The ST2 cells that remained on
the upper chamber membrane were removed with cotton swabs.
Micrographic images of the lower chamber membrane were obtained
using a light microscope (Nikon Eclipse TS100; Nikon, Tokyo, Japan)
and the number of cells on each image was counted using Adobe
Photoshop CS6 (Adobe Systems, Inc., San Jose, CA, USA).
Western blot analysis
The ST2 cells were seeded on 6-well plates with
normal growth medium and then left to attach for 6 h. The cells
were serum starved for 16–18 h with serum-free DMEM (GE Healthcare
Life Sciences) and then treated with 300 nM SP at different time
points. When required, the cells were exposed to inhibitors for 30
min. To obtain the cell lysate, the cells were rinsed twice with
ice-cold PBS and incubated with 400 µl 2X SDS buffer [100 mM
Tris-Cl (pH 6.8), 4% (w/v) SDS, 0.2% (w/v) bromophenol blue, 20%
glycerol, 200 mM β-mercaptoethanol] for 5 min at room temperature.
The cell lysate was collected and denatured at 92°C for 10 min.
Protein samples were subjected to 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Separated
proteins were transferred onto nitrocellulose membranes (Whatman,
Dassel, Germany). The membranes were subsequently incubated with
primary antibodies against phosphorylated (p-)p44/42 MAPK (ERKs)
(Thr202/Tyr204; 1:1,000, Cat. no. 4370), p-Akt (Ser473; 1:4,000,
Cat. no. 4060S) (both from Cell Signaling Technology, Danvers, MA,
USA), p-FAK (Y397; 1:1,000, Cat. no. 4803) (Abcam, Cambridge, UK),
or p-p38 MAPK (Thr180/Tyr182; 1:1,000, Cat. no. 4511) (Cell
Signaling Technology). Subsequently, target proteins were detected
with horseradish peroxidase (HRP)-conjugated secondary antibodies
and an enhanced chemiluminiscence reagent (Millipore, Billerica,
MA, USA). Band densities were measured using ImageJ software
(National Institutes of Health, Bethesda, MD, USA). The membranes
were then re-blotted with antibody against α-tubulin (1:20,000,
Cat. no. T5168; Sigma-Aldrich) when required after stripping.
Immunocytochemical analysis
For immunofluorescence staining, cells were seeded
on type 1 collagen (1.5 µg/ml; Nitta Gelatin NA Inc.) coated
coverslips and left to attach for 6 h. After 18 h serum starvation,
cells were treated with 300 nM SP for 30 min. The cells were fixed
with 4% paraformaldehyde in PBS for 10 min on ice. Following
washing with 0.1% Triton X-100 (USB Corp., Cleveland, OH, USA),
blocking solution (5% non-fat milk in PBS with 0.1% Triton X-100)
was added for 30 min at room temperature. The cells were then
incubated with Alexa Fluor 546 phalloidin (1:1,000, cat. no.
A22283; Invitrogen) for 30 min. After washing three times with 1%
non-fat milk in PBS with 0.1% Triton X-100, the samples were
mounted using ProLong Gold antifade mounting solution with
4′,6-diamidino-2-phenylindole (DAPI; Invitrogen) and left to dry
overnight before observation. Images were then captured using a
Zeiss LSM 700 confocal microscope (Zeiss, Oberkochen, Germany). The
percentage of cells presenting with ruffled edges was determined in
10 random images per each experimental group. Three independent
experiments were performed for the analysis.
Statistical analysis
Data are presented as the means ± standard
deviation. The unpaired Student's t-test was applied to evaluate
differences between two groups. A p-value <0.05 was considered
to indicate a statistically significant difference. All statistical
analyses were performed using GraphPad version 5.01 software
(GraphPad Software, San Diego, CA, USA) (http://www.graphpad.com).
Results
SP enhances the migration of ST2
cells
A previous study showed that SP induces the
mobilization of BM-MSCs in vivo (9). However, the mechanisms involved in
the SP-mediated migration of BM-MSCs had not previously been
elucidated. We showed in our previous study that SP enhances the
migration potential of the BM-derived MSC-like cell line ST2 in a
wound healing migration assay (23). In order to investigate the
mechanisms involved in the SP-mediated migration of BM-MSCs, we
examined the effects of SP on the migration of ST2 cells in
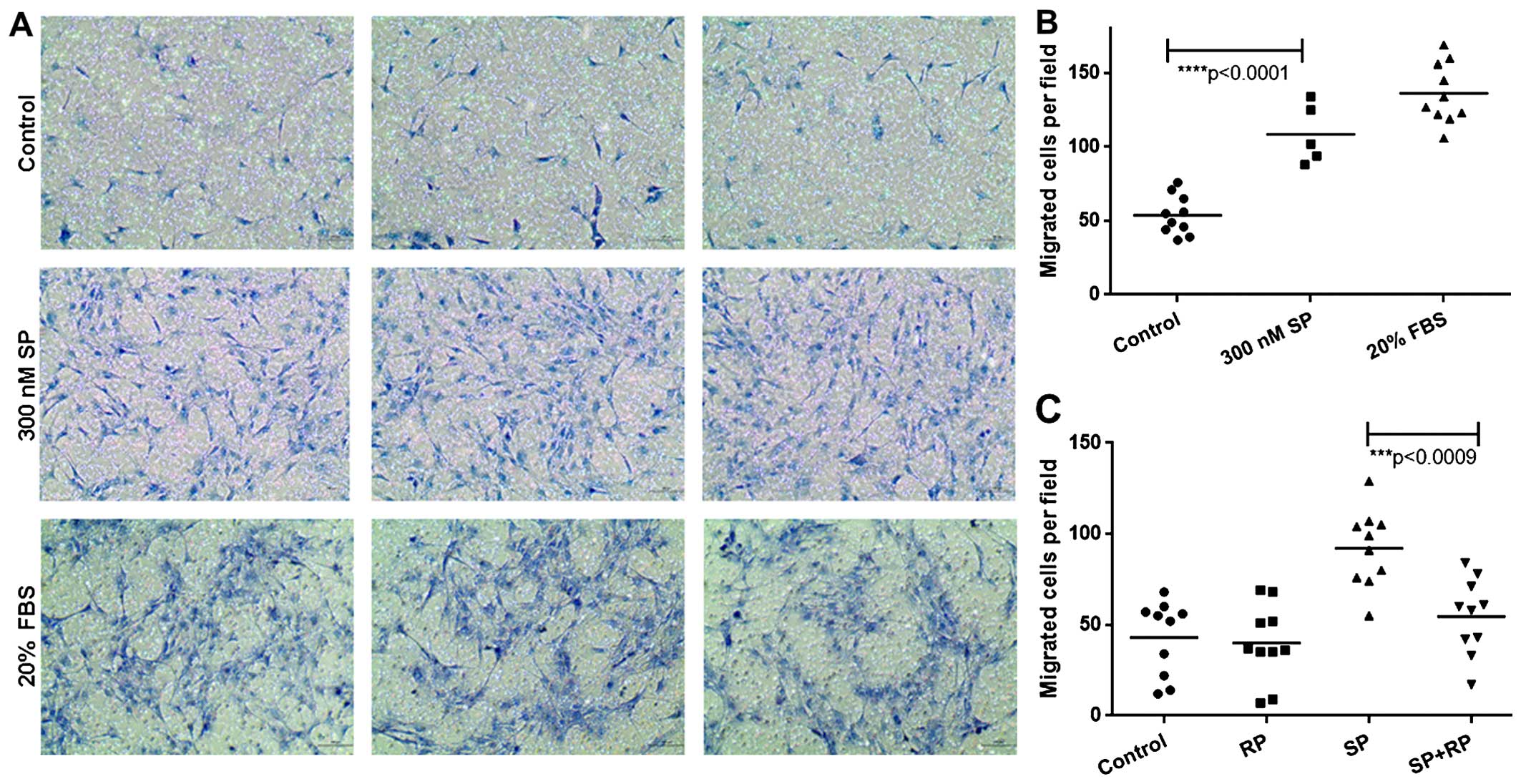
vitro using a Millicell migration assay. We observed that SP
induced the migration of ST2 cells, as the number of migrated cells
was similar to that of the positive control, 20% FBS (Fig. 1A and B). We confirmed the specific
effect of SP on the chemotactic migration of ST2 cells by Millicell
migration assay using the antagonist for SP receptor neurokinin-1
(NK-1), RP 67580. Pre-treating ST2 cells with RP 67580 blocked
their migration in response to SP (Fig. 1C). These data demonstrate that SP
induces the chemotactic migration of ST2 cells. These findings
allowed us to use this cell line to further investigate the
mechanisms involved.
SP induces the activation of ERKs, Akt,
and FAK in ST2 cells
Extracellular signaling through membrane receptors
activates several intracellular signaling pathways, which results
in changes in cell motility and cell migration. Different pathways
have been implicated in the mobilization of BM-MSCs in response to
various chemoattractants and growth factors (24). Certain studies have shown that the
mitogen-activated protein/extracellular signal-regulated kinase 1/2
(MAPK/ERK 1/2) signaling pathway is involved in the expression of a
wide variety of genes controlling migration and in the migration of
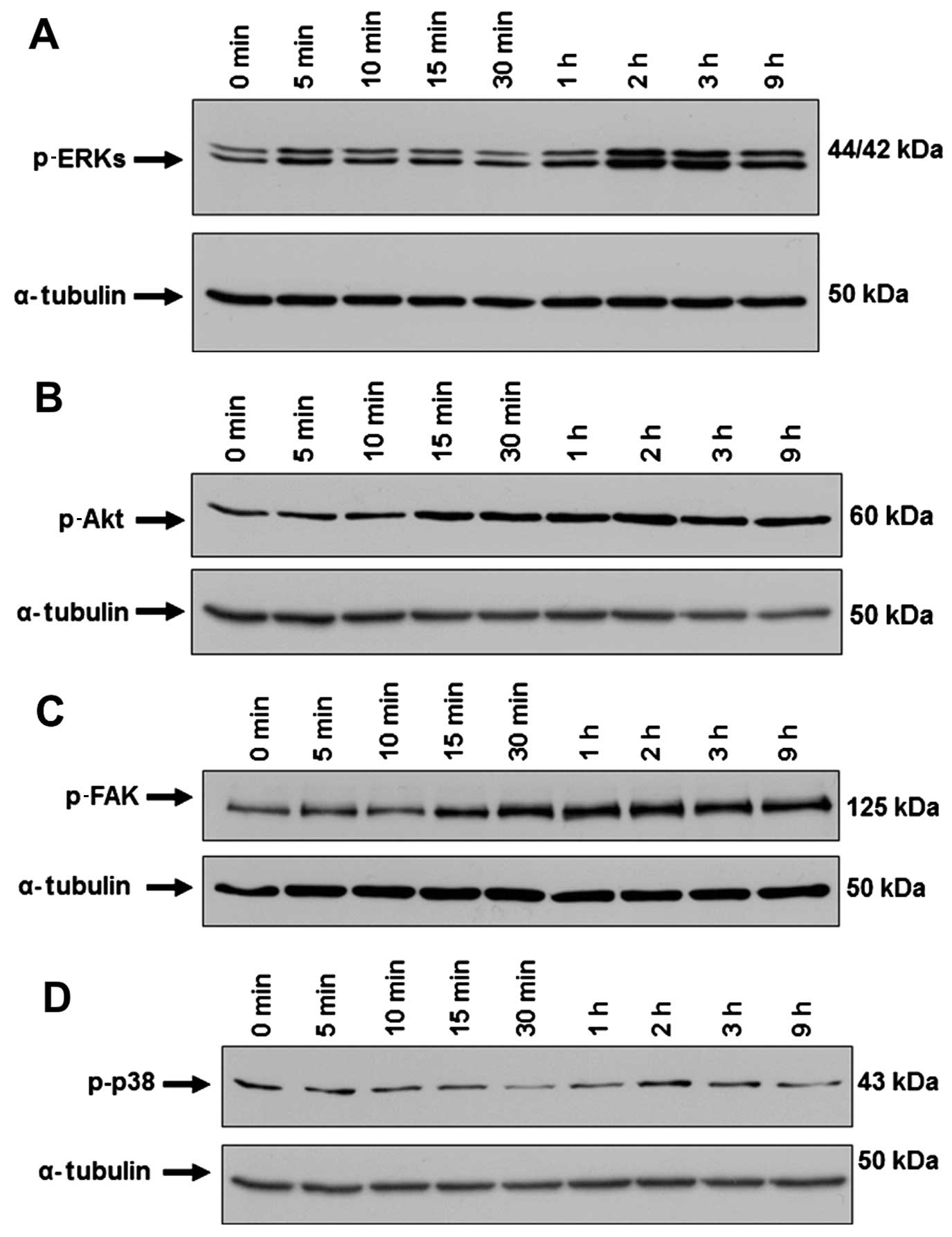
MSCs (25,26). We found that SP induced the
activation of ERKs (Fig. 2A). It
has previously been reported that the phosphoinositide 3-kinase
(PI3K)/Akt signaling pathway is involved in both the SDF-1-
(27) and the basic fibroblast
growth factor (bFGF)-induced migration of MSCs (28,29). In the present study, we observed
that SP induced the activation of Akt in ST2 cells, which started 2
h after SP treatment and persisted up to 9 h (Fig. 2B). Integrin signaling through FAK
has previously been shown to promote cell migration (30). Activation of FAK results in the
reorganization of actin filaments and the cytoskeleton, thus
inducing MSCs migration (30).
The level of p-FAK increased after SP treatment in ST2 cells
(Fig. 2C). Studies have suggested
that the p38 MAPK pathway participates in the tumor necrosis factor
(TNF)-α-induced migration of MSCs (31). However, we did not observe any
significant change in the level of p-p38 in the ST2 cells after SP
treatment (Fig. 2D). Taken
together, these data suggest that the intracellular signaling of
ERKs, Akt and FAK but not p38 contributes to the SP-mediated
migration of ST2 cells.
Inhibition of ERKs, Akt or Src kinase
blocks the migration of ST2 cells in response to SP
To evaluate whether the activation of ERKs in the
ST2 cells after SP treatment is required for the migration of ST2
cells in response to SP, we pretreated ST2 cells with the MEK
inhibitor, PD98059. Inhibition of p-ERKs by PD98059 significantly
reduced the migration of ST2 cells in response to SP, compared with
the SP-treated group (Fig. 3A and
B). Thus, the activation of the ERK pathway is required for the
migration of ST2 cells in response to SP.
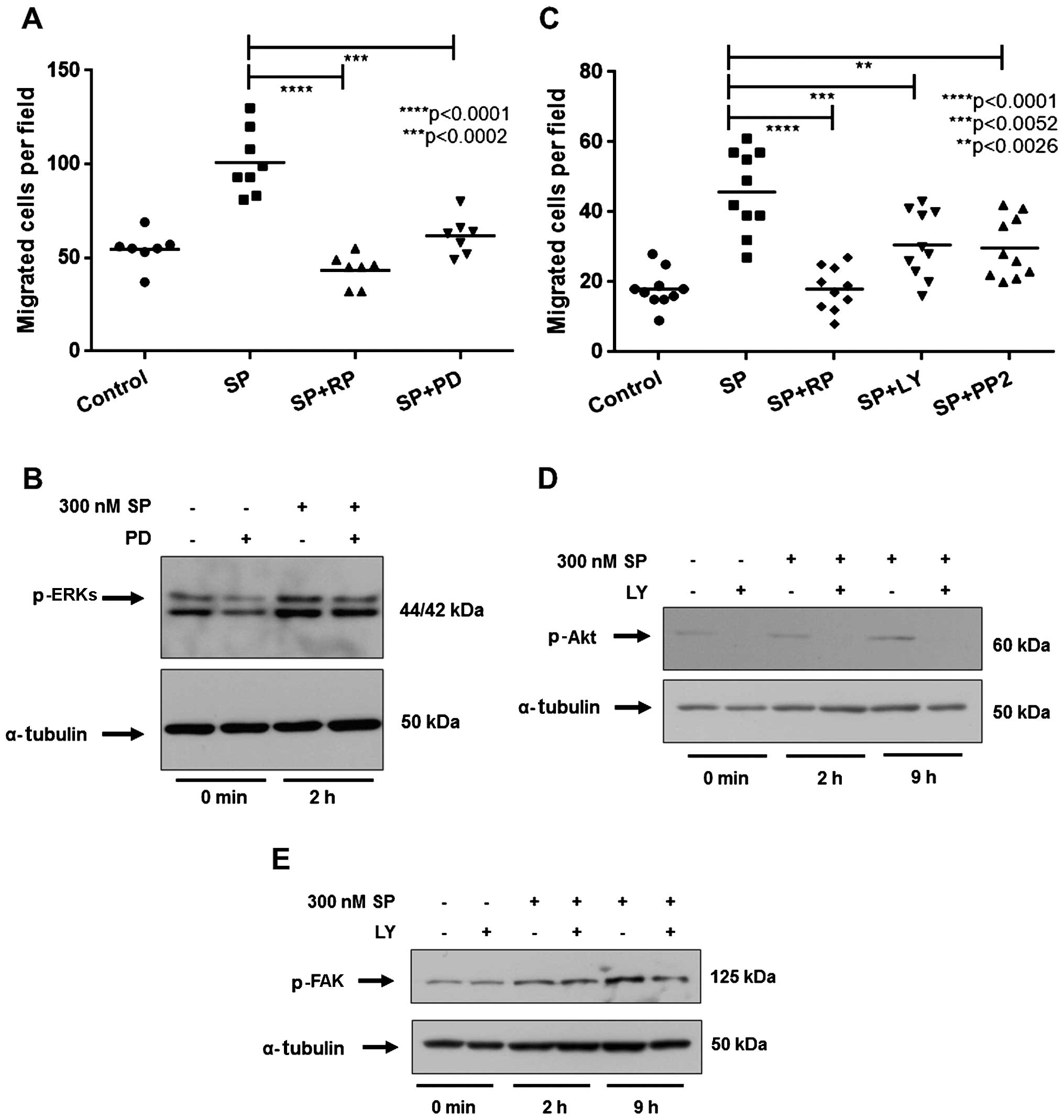 | Figure 3Inhibition of the mitogen-activated
protein kinase (MAPK) extracellular signal-regulated kinase (ERK),
Akt or Src pathway impairs the migration of ST2 cells induced by
substance P (SP). (A and C) The number of migrated ST2 cells in
each experimental group. ST2 cells were treated for 30 min with
inhibitors prior to treatment with 300 nM SP and exposure to (A)
antagonist for SP receptor neurokinin-1 (RP67580; 10 µM, RP)
or MAPK/ERK kinase (MEK) inhibitor, PD98059 (10 µM, PD), (C)
phosphoinositide 3-kinase (PI3K) inhibitor, LY294002 (10 µM,
LY), or Src kinase inhibitor, PP2 (1 µM). Control cells were
treated with the appropriate amount of SP solvent (5% acetic acid).
(B, D and E) Western blot analysis for the levels of (B) p-ERKs,
(D) p-Akt or (E) p-FAK following exposure to PD or LY inhibitors
and treatment with 300 nM SP. Inhibitors were administered at the
concentrations shown for 30 min prior to 300 nM SP treatment for
the indicated times. α-tubulin was used as an internal control.
p-values were calculated using the Student's t-test. |
We subsequently investigated the role of the Akt
pathway in the SP-induced migration of ST2 cells. We noted that the
PI3K inhibitor LY294002 significantly decreased the migration of
ST2 cells in comparison to SP-treated cells (Fig. 3C) as well as the level of p-Akt
(Fig. 3D). Therefore, we suggest
it is likely that Akt activation is necessary for the SP-mediated
migration of ST2 cells. Additionally, we demonstrated that the Src
kinase inhibitor PP2 also blocked the migration of ST2 cells in
response to SP (Fig. 3C). This
suggests that Src kinase plays an important role in the SP-mediated
migration of ST2 cells. Importantly, as shown in Fig. 3E, we observed that LY294002
reduced the SP-induced increase in p-FAK, thus suggesting that Akt
acts upstream of FAK to induce ST2 migration in response to SP.
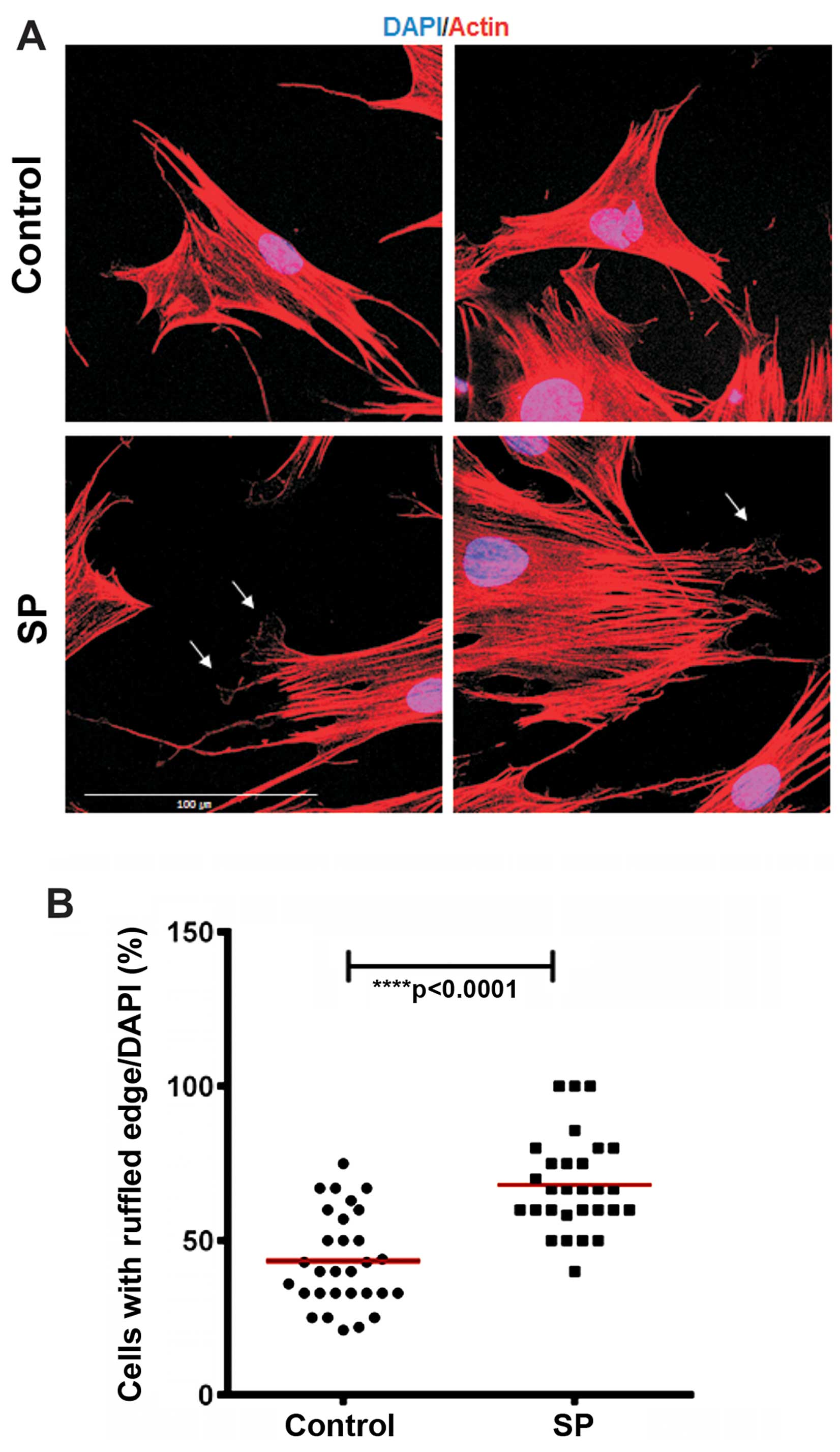
SP treatment increases membrane ruffling
on ST2 cells
Cell movement is driven by a continuous
reorganization and turnover of the actin cytoskeleton that causes
the formation of protrusive structures in the cell membrane at the
leading edge such as filopodia, lamellipodia, ruffles and podosomes
(32,33). In order to evaluate whether SP
induces changes in the actin cytoskeleton, we performed actin
immunostaining of the ST2 cells after 30 min of SP treatment. We
noted that SP increased the number of the ST2 cells that exhibited
ruffled membrane structures, compared with the control group
(Fig. 4). These data suggest that
SP modulates the actin dynamics to induce the migration of ST2
cells.
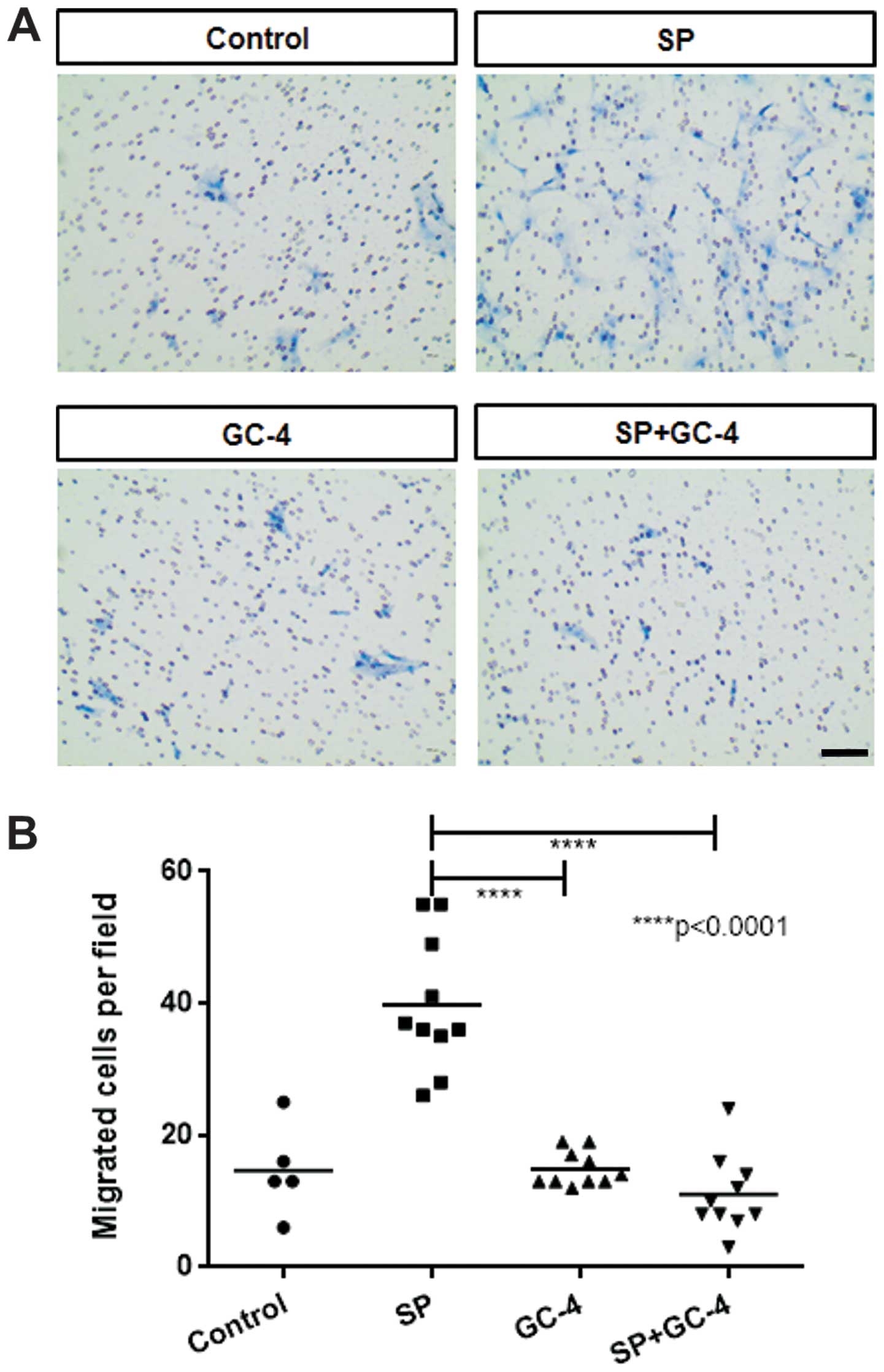
N-cadherin is required for SP-mediated
migration of ST2 cells
Several studies have demonstrated that the cell
adhesion molecule N-cadherin is involved in the migration of
various cell types under several developmental and stimulatory
conditions (20–22). We sought to determine whether
N-cadherin is necessary for the SP-mediated migration of ST2 cells.
In ST2 cells exposed to the N-cadherin functional blocking antibody
(GC-4), a decrease in SP-induced chemotactic migration was noted
(Fig. 5), even though SP did not
markedly affect the mRNA or protein levels of N-cadherin in the ST2
cells (data not shown). Thus, our data suggest that N-cadherin is
required for the migration of ST2 cells in response to SP.
Discussion
To the best of our knowledge, the present study is
the first to demonstrate that SP induces the migration of the
BM-MSC-like cell line, ST2 cells, through N-cadherin as well as by
activating ERKs and Akt. Our results also suggest that FAK and Src
kinase are involved in the migration of ST2 cells in response to
SP. However, in the present study we did not show how SP activates
these important signaling pathways and regulates N-cadherin
function in order to enhance the migration of ST2 cells in response
to SP. Nonetheless, our findings suggest the cellular and molecular
mechanisms responsible for the SP-mediated migration of
BM-MSCs.
A previous study has shown that SP increases the
activity of ERKs and the cellular proliferation of human BM-MSCs
(9). However, that study did not
demonstrate whether ERK activity is required for the increase in
cellular proliferation which is mediated by SP treatment.
Interestingly, even though in the present study we observed an
increase in ERK after SP treatment, our previous study showed that
SP does not induce the proliferation of ST2 cells (23). According to the present data, ERK
activity seems to mediate the migration of ST2 cells in response to
SP. Indeed, we noted that inhibiting ERK actvity blocked the
SP-mediated migration of the ST2 cells to a similar extent as the
antagonist of SP receptor (NK-1), RP 67580. On the contrary, we
have previously reported that SP induces the proliferation of OP9
cells, another BM-derived MSC-like cell line, but ERK is not
activated (23). Therefore, it is
likely that the activation of ERKs in response to SP contributes to
the cellular migration of BM-MSCs, not to their cellular
proliferation.
Previous studies have shown that the phosphorylation
of FAK at Tyr 397 induces cellular migration and that this
phosphorylation is upregulated through PI3K/Akt (34,35). The results of our present study
showed that exposure of ST2 cells to the inhibitor of PI3K,
LY294002, blocked the activation of Akt and the migration of the
ST2 cells in response to SP. Importantly, LY294002 inhibited the
phosphorylation of FAK induced by SP treatment. Therefore, we
suggest that PI3K/Akt acts upstream of FAK, regulating the cellular
migration of ST2 cells in response to SP.
Surprisingly, we found that N-cadherin mediated the
cellular migration of the ST2 cells in response to SP. The
impairment of N-cadherin-mediated intercellular interactions by
exposing the ST2 cells to N-cadherin specific blocking antibody
(clone GC-4), as previously described (36), inhibited SP-mediated migration of
ST2 cells. However, we did not observe any changes in the mRNA and
protein levels of N-cadherin following SP treatment (data not
shown). We hypothesize that SP induces changes in the cellular
localization of N-cadherin and that these changes, rather than its
expression level, are involved in the regulation of cellular
migration by N-cadherin. It has previously been demonstrated that
neural crest cells undergo chemotactic migration towards SDF-1 via
the polarized activity of Rac1 that is dependent on
N-cadherin-mediated intercellular interactions (22). It is likely that N-cadherin
regulates Rac1 activity in ST2 cells in order to induce the
chemotactic migration in response to SP. Further studies are thus
necessary to elucidate how SP modulates N-cadherin and how
N-cadherin promotes the migration of ST2 cells in response to
SP.
This study revealed the molecular and cellular
mechanisms that mediate the migration of the BM-derived MSC-like
cell line ST2 in response to SP. Further research is thus warranted
to confirm the involvement of such mechanisms in BM-MSC migration
mediated by SP, and to examine their specific roles and
interactions which enhance the migration of BM-MSCs.
Acknowledgments
This study was supported by the Korean Health
Technology R&D Project, Ministry of Health and Welfare,
Republic of Korea (HI13C1479), the Basic Science Research Program
through the National Research Foundation of Korea (NRF) funded by
the Ministry of Education (NRF-2012R1A1A2042265), and the Bio and
Medical Technology Development Program of the National Research
Foundation (NRF) funded by the Ministry of Science, ICT and Future
Planning (NRF-2012M3A9C6050485).
References
|
1
|
Ko IK, Lee SJ, Atala A and Yoo JJ: In situ
tissue regeneration through host stem cell recruitment. Exp Mol
Med. 45:e572013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rennert RC, Sorkin M, Garg RK and Gurtner
GC: Stem cell recruitment after injury: lessons for regenerative
medicine. Regen Med. 7:833–850. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Williams AR and Hare JM: Mesenchymal stem
cells: biology, pathophysiology, translational findings, and
therapeutic implications for cardiac disease. Circ Res.
109:923–940. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lau TT and Wang DA: Stromal cell-derived
factor-1 (SDF-1): homing factor for engineered regenerative
medicine. Expert Opin Biol Ther. 11:189–197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wan M, Li C, Zhen G, Jiao K, He W, Jia X,
Wang W, Shi C, Xing Q, Chen YF, et al: Injury-activated
transforming growth factor β controls mobilization of mesenchymal
stem cells for tissue remodeling. Stem Cells. 30:2498–2511. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang F, Tsai S, Kato K, Yamanouchi D,
Wang C, Rafii S, Liu B and Kent KC: Transforming growth factor-beta
promotes recruitment of bone marrow cells and bone marrow-derived
mesenchymal stem cells through stimulation of MCP-1 production in
vascular smooth muscle cells. J Biol Chem. 284:17564–17574. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Veevers-Lowe J, Ball SG, Shuttleworth A
and Kielty CM: Mesenchymal stem cell migration is regulated by
fibronectin through α5β1-integrin-mediated activation of PDGFR-β
and potentiation of growth factor signals. J Cell Sci.
124:1288–1300. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hong HS, Kim Y, Yoon KJ and Son Y: A new
paradigm for stem cell therapy: substance-P as a stem
cell-stimulating agent. Arch Pharm Res. 34:2003–2006. 2011.
View Article : Google Scholar
|
|
9
|
Hong HS, Lee J, Lee E, Kwon YS, Lee E, Ahn
W, Jiang MH, Kim JC and Son Y: A new role of substance P as an
injury-inducible messenger for mobilization of CD29(+) stromal-like
cells. Nat Med. 15:425–435. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bergfeld SA and DeClerck YA: Bone
marrow-derived mesenchymal stem cells and the tumor
microenvironment. Cancer Metastasis Rev. 29:249–261. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mishra PJ, Mishra PJ, Glod JW and Banerjee
D: Mesenchymal stem cells: flip side of the coin. Cancer Res.
69:1255–1258. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gharibi B, Ghuman MS and Hughes FJ: Akt-
and Erk-mediated regulation of proliferation and differentiation
during PDGFRβ-induced MSC self-renewal. J Cell Mol Med.
16:2789–2801. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rodrigues M, Griffith LG and Wells A:
Growth factor regulation of proliferation and survival of
multipotential stromal cells. Stem Cell Res Ther. 1:322010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bulj Z, Duchi S, Bevilacqua A, Gherardi A,
Dozza B, Piccinini F, Adalgisa Mariani G, Lucarelli E, Giannini S,
Donati D and Marmiroli S: Protein kinase B/AKT isoform 2 drives
migration of human mesenchymal stem cells. Int J Oncol. 42:118–126.
2013.
|
|
15
|
Gao H, Priebe W, Glod J and Banerjee D:
Activation of signal transducers and activators of transcription 3
and focal adhesion kinase by stromal cell-derived factor 1 is
required for migration of human mesenchymal stem cells in response
to tumor cell-conditioned medium. Stem Cells. 27:857–865. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huttenlocher A and Horwitz AR: Integrins
in cell migration. Cold Spring Harb Perspect Biol. 3:a0050742011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sieg DJ, Hauck CR, Ilic D, Klingbeil CK,
Schaefer E, Damsky CH and Schlaepfer DD: FAK integrates
growth-factor and integrin signals to promote cell migration. Nat
Cell Biol. 2:249–256. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sieg DJ, Hauck CR and Schlaepfer DD:
Required role of focal adhesion kinase (FAK) for
integrin-stimulated cell migration. J Cell Sci. 112:2677–2691.
1999.PubMed/NCBI
|
|
19
|
Gumbiner BM: Regulation of
cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol.
6:622–634. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Suyama K, Shapiro I, Guttman M and Hazan
RB: A signaling pathway leading to metastasis is controlled by
N-cadherin and the FGF receptor. Cancer Cell. 2:301–314. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park KS, Dubon MJ and Gumbiner BM:
N-cadherin mediates the migration of MCF-10A cells undergoing bone
morphogenetic protein 4-mediated epithelial mesenchymal transition.
Tumour Biol. 36:3549–3556. 2015. View Article : Google Scholar
|
|
22
|
Theveneau E, Marchant L, Kuriyama S, Gull
M, Moepps B, Parsons M and Mayor R: Collective chemotaxis requires
contact-dependent cell polarity. Dev Cell. 19:39–53. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dubon MJ and Park KS: Substance P enhances
the proliferation and migration potential of murine bone
marrow-derived mesenchymal stem cell-like cell lines. Exp Ther Med.
9:1185–1191. 2015.PubMed/NCBI
|
|
24
|
Li L and Jiang J: Regulatory factors of
mesenchymal stem cell migration into injured tissues and their
signal transduction mechanisms. Front Med. 5:33–39. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Alsayed Y, Ngo H, Runnels J, Leleu X,
Singha UK, Pitsillides CM, Spencer JA, Kimlinger T, Ghobrial JM,
Jia X, et al: Mechanisms of regulation of CXCR4/SDF-1
(CXCL12)-dependent migration and homing in multiple myeloma. Blood.
109:2708–2717. 2007.
|
|
26
|
Colston JT, de la Rosa SD and Freeman GL:
Impact of brief oxidant stress on primary adult cardiac
fibroblasts. Biochem Biophys Res Commun. 316:256–262. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang JF, Park IW and Groopman JE: Stromal
cell-derived factor-1alpha stimulates tyrosine phosphorylation of
multiple focal adhesion proteins and induces migration of
hematopoietic progenitor cells: roles of phosphoinositide-3 kinase
and protein kinase C. Blood. 95:2505–2513. 2000.
|
|
28
|
Zha YH, He JF, Mei YW, Yin T and Mao L:
Zinc-finger transcription factor snail accelerates survival,
migration and expression of matrix metalloproteinase-2 in human
bone mesenchymal stem cells. Cell Biol Int. 31:1089–1096. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schmidt A, Ladage D, Schinköthe T,
Klausmann U, Ulrichs C, Klinz FJ, Brixius K, Arnhold S, Desai B,
Mehlhorn U, et al: Basic fibroblast growth factor controls
migration in human mesenchymal stem cells. Stem Cells.
24:1750–1758. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao X and Guan JL: Focal adhesion kinase
and its signaling pathways in cell migration and angiogenesis. Adv
Drug Deliv Rev. 63:610–615. 2011. View Article : Google Scholar :
|
|
31
|
Fu X, Han B, Cai S, Lei Y, Sun T and Sheng
Z: Migration of bone marrow-derived mesenchymal stem cells induced
by tumor necrosis factor-alpha and its possible role in wound
healing. Wound Repair Regen. 17:185–191. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bailly M and Condeelis J: Cell motility:
insights from the backstage. Nat Cell Biol. 4:E292–E294. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pollard TD and Borisy GG: Cellular
motility driven by assembly and disassembly of actin filaments.
Cell. 112:453–465. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Higuchi M, Kihara R, Okazaki T, Aoki I,
Suetsugu S and Gotoh Y: Akt1 promotes focal adhesion disassembly
and cell motility through phosphorylation of FAK in growth
factor-stimulated cells. J Cell Sci. 126:745–755. 2013. View Article : Google Scholar
|
|
35
|
Turecková J, Vojtechová M, Krausová M,
Sloncová E and Korínek V: Focal adhesion kinase functions as an Akt
downstream target in migration of colorectal cancer cells. Transl
Oncol. 2:281–290. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li G, Satyamoorthy K and Herlyn M:
N-cadherin-mediated intercellular interactions promote survival and
migration of melanoma cells. Cancer Res. 61:3819–3825.
2001.PubMed/NCBI
|