Introduction
Retinal neovascularization (RNV) is the growth of
new capillaries sprouting from the retinal veins and extending
along the vitreous surface of the retina, which leads to vitreous
hemorrhage, retinal detachment and even blindness (1–4).
RNV plays an important role in many ocular diseases, such as
proliferative diabetic retinopathy (PDR), retinal vein occlusion
and retinopathy of prematurity (ROP). However, the pathological
mechanisms involved in the formation and development of RNV remain
unknown and still need to be further studied.
Slit-Robo signaling consists of three Slit (Slit1-3)
secreted proteins and their four corresponding receptors
[roundabout, axon guidance receptor, homologs 1–4 (Robo1-4)].
Slit-Robo signaling was first noted as acting as a repulsive cue in
axonal guidance (5,6). Subsequently, the roles of Slit-Robo
in organogenesis (7), neuronal
migration (8) and tumor
development (9–12) have been explored. Great progress
has been made in studying the role of Robo1 signaling in
angiogenesis, but the role of Robo1 in angiogenesis is still not
fully understood. Han and Zhang demonstrated that Robo1 is involved
in inhibiting corneal neovascularization (13). However, there is also evidence
that Robo1 is pro-angiogenic: Wang et al (14) have reported that Robo1 mediates
human umbilical vein endothelial cell (HUVEC) migration upon Slit2
stimulation, indicating that Slit2-Robo1 signaling promotes cancer
angiogenesis. A previous study by Zhou et al (15) found that Robo1 was expressed in
fibrovascular membranes (FVMs), demonstrating that Robo1
contributes to the development of diabetic retinopathy. The role of
Slit-Robo signaling in RNV of oxygen-induced retinopathy (OIR) is
researched in the present study.
MicroRNAs (miRNAs or miRs), small non-coding RNAs
which are 21–24 nucleotides in length, act as important regulators
of gene expression in mammals; several mammalian miRNAs have been
identified as being located in the intronic regions of
protein-encoding genes (host genes), termed intronic miRNAs
(16). Intronic miRNAs can
modulate the function of their host gene as the majority of them
are coordinately expressed with the host genes (17,18). One example of endogenous RNA
interference is when miRNAs negatively regulate gene translation by
pairing with the 3′-UTR of a specific mRNA (19), and are involved in many functional
regulatory processes (20–22).
Many miRNAs have been found to play key roles in ocular
neovascularization. Intraocular injection of certain miRNAs, such
as miR-31 (21), miR-150
(23), miR-184 (23), miR-126 (24) and miR-410, has been shown to
significantly inhibit retinal and choroidal neovascularization
(25). miRNA-218 is an important
intronic miRNA encoded by the Slit2 and Slit3 genes, which directly
represses the expression of Robo1, Robo2 and contributes to retinal
angiogenesis in mouse embryos (26). In order to investigate the effects
of miR-218 on oxygen-induced RNV, a mouse model of OIR was
established in the present study.
Materials and methods
Cell culture and transfection
Mouse retinal vascular endothelial cells were
purchased from PriCells Biomedical Technology Co., Ltd. (Wuhan,
China), and were cultured in DMEM supplemented with 10%
heat-inactivated fetal bovine serum, 100 U/ml penicillin, and 100
mg/l streptomycin (all from Gibco Life Technologies, Carlsbad, CA,
USA) under conditions with 5% CO2, at 37°C, in a
humidified incubator.
Cells in the exponential growth phase were seeded in
6-well plates (Corning Incorporated, Corning, NY, USA) at a density
of 5×104 cells/ml. The cells were transfected with
miR-218 mimic, miR-218 inhibitor, and the negative control (all
from GenePharma, Shanghai, China) using Lipofectamine 2000
(Invitrogen, Carlsbad, CA, USA) at a 5:1 volume/mass ratio of
reagent to oligodeoxynucleotide in serum-free M199 for 6 h. After
transfection, the cells were incubated in complete DMEM.
Transfection of the miR-218 mimics, miR-218 inhibitor and the
negative control was performed using 1.0 μg of DNA per
transfection. All transfections were performed according to the
manufacturer's instructions.
siRNA design
Robo1 siRNA (sense, 5′-CGGGAAAGGCCG
AGGAACAAAGGCAGC-3′ and antisense, 5′-GCUGCCUUU
GUUCCUCGGCCUUUCCCG-3′) was synthesized by Guangzhou RiboBio Co.,
Ltd. (Guangzhou, China).
Plasmid construction
miR-218 was amplified using the following primers:
miR-218 forward, 5′-TTCTGAGGATCCGTGGAGGCACCTTTTCCATA-3′ and
reverse, 5′-ATTCTAAGATCTTTCACAGCTAGTCACACAATGG-3′. Plasmid vector
pCDH-CMV-miR-218 was constructed by inserting a pri-miR-218 PCR
fragment into the plasmid vector pCDH-CMV-MCS-EF1-Puro (Spiral
Biotech, Inc., Norwood, MA, USA) through EcoRI and
NotI digestion. The pCDH-CMV-mock vector was used as the
negative control.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cells and retinal
tissues using TRIzol reagent (Invitrogen). cDNA was synthesized,
according to the manufacturer's instructions, with a cDNA synthesis
kit (Takara Bio, Inc., Otsu, Japan). qPCR was undertaken using
SYBR-Green.
The primer sequences were as follows: miR-218,
5′-TTG TGCTTGATCTAACCATGT-3′; miR-218-1, 5′-CCATGGAA
CGTCACGCAGC-3′; miR-218-2, 5′-GCGGAAAGCACCGTGCTC-3′; Slit2 forward,
5′-GCGCGTCTGGTGTGAAT GAA-3′ and reverse,
5′-CACAGTGGCACCAGGAGCAT-3′; Slit3 forward,
5′-TGGAAATACGCCTAGAACAG-3′ and reverse, 5′-ACCAGCGACGTGAGTGAT-3′;
Robo1 forward, 5′-GAGGTAGCTATACTACGGGATGAC-3′ and reverse,
5′-CAGATGTAGTAGCCGACATCAGAC-3′; U6 forward,
5′-CGCTTCGGCAGCACATATAC-3′ and reverse,
5′-AAAATATGGAACGCTTCACGA-3′.
Scratch wound assay
Cell migration ability was assessed using a scratch
wound assay. Transfected cells were cultured in 6-well plates. When
cells reached 90% confluence, a scratch wound was created using a
pipette tip. Wound edges were photographed with a Nikon Eclipse
TE20000-U (Nikon, Tokyo, Japan) and scratch widths were analyzed
using ImageJ software (NIH). Each assay was completed in
triplicate.
Mouse model of OIR
Neonatal CB57BL/6J mice were obtained from the
Animal Institute of Chinese Academy of Medical Sciences.
Neovascularization was induced as described by Kong et al
(27). Briefly, at postnatal day
7 (P7), CB57BL/6J mice were exposed to hypoxic conditions (75%
O2) for 5 days (P12) and then returned to room air in
order to induce RNV.
The present study was approved by the Ethics
Committee of Tianjin Eye Hospital (Heping, China). All animal
experiments were conducted in accordance with the ARVO Statement
for the Use of Animals in Ophthalmic and Vision Research and the
Guide for the Care and Use of Laboratory Animals.
Intravitreal injection
Plasmids were packed using Lipof ectamine 2000
(Invitrogen) in accordance with the manufacturer's instructions.
Twelve-day-old mice were anesthetized by intraperitoneal injection
of ketamine hydrochloride (30 mg/kg). Each animal received
intravitreous injections of 0.4 μl (2 μg)
pCDH-CMV-218 in one eye and 0.4 μl (2 μg) control
plasmid [plasmid vector pCDH-CMV-MCS-EF1-Puro (Spiral Biotech,
Inc.) without an insertion of miR-218] in the contra-lateral eye as
the negative control. Intravitreal injections were performed at
11:00 a.m., and were administered to 1-mm posterior to the limbus
of the eye using a 10-μl Hamilton syringe fitted with a 32G
needle.
Western blotting
Total proteins of cells and retinas were isolated
and separated on 10% SDS-PAGE gels (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Western blotting was performed according to
standard protocols. α-tubulin was used as a loading control. The
antibodies anti-Robo1 (ab7279), anti-Slit2 (ab7665), anti-Slit3
(ab11018) were from Abcam, and anti-α-tubulin (T5168) was from
Sigma-Aldrich (St. Louis, MO, USA). Bands were quantified using
ImageJ software (NIH).
Fluorescein angiography
On P17, mice (n=6/group) were anesthetized and
perfused with fluorescein via retro-orbital injection of 2.5 mg/50
μl of FITC-dextran (Sigma-Aldrich), as previously described
(28). Eyes were enucleated and
fixed with 4% paraformaldehyde in PBS for 1 h. Retinas were then
separated from the eyecup. Four incisions were made, and the retina
was flat-mounted on a gelatin-coated slide. The vasculature was
then examined under a fluorescence microscope (Nikon Eclipse
TE20000-U). Images were analyzed using Photoshop 8.0 software
(Adobe Systems, San Jose, CA, USA). Neovascularization was
calculated as a ratio: the number of pixels in neovascular area to
the total number of pixels in the retina.
Quantification of RNV
At p17, the eyes of the mice (n=6/group) were
enucleated and fixed with 10% formaldehyde and embedded in
paraffin. Sagittal sections (6-μm-thick) were made through
the cornea parallel to the optic nerve, and then they were stained
with hematoxylin and eosin (H&E). The nuclei of vascular cells
on the vitreal side of the retina were counted under a light
microscope. Ten non-continuous sections from each eye were
examined, and numbers of cells were averaged in each group. The
average number of pre-retinal vascular nuclei was compared.
Statistical analysis
Data are expressed as the means ± standard deviation
(SD) and were analyzed using SPSS 11.5 (SPSS, Inc., Chicago, IL,
USA). To compare multiple sets of data, one-way analysis of
variance (ANOVA) was used. For paired data sets, the LSD t-test was
used. Bivariate correlations were calculated by Spearman's rank
correlation coefficients. A P-value <0.05 was considered to
indicate a statistically significant difference.
Results
Inhibition of cell migration by miR-218
is mediated by Robo1
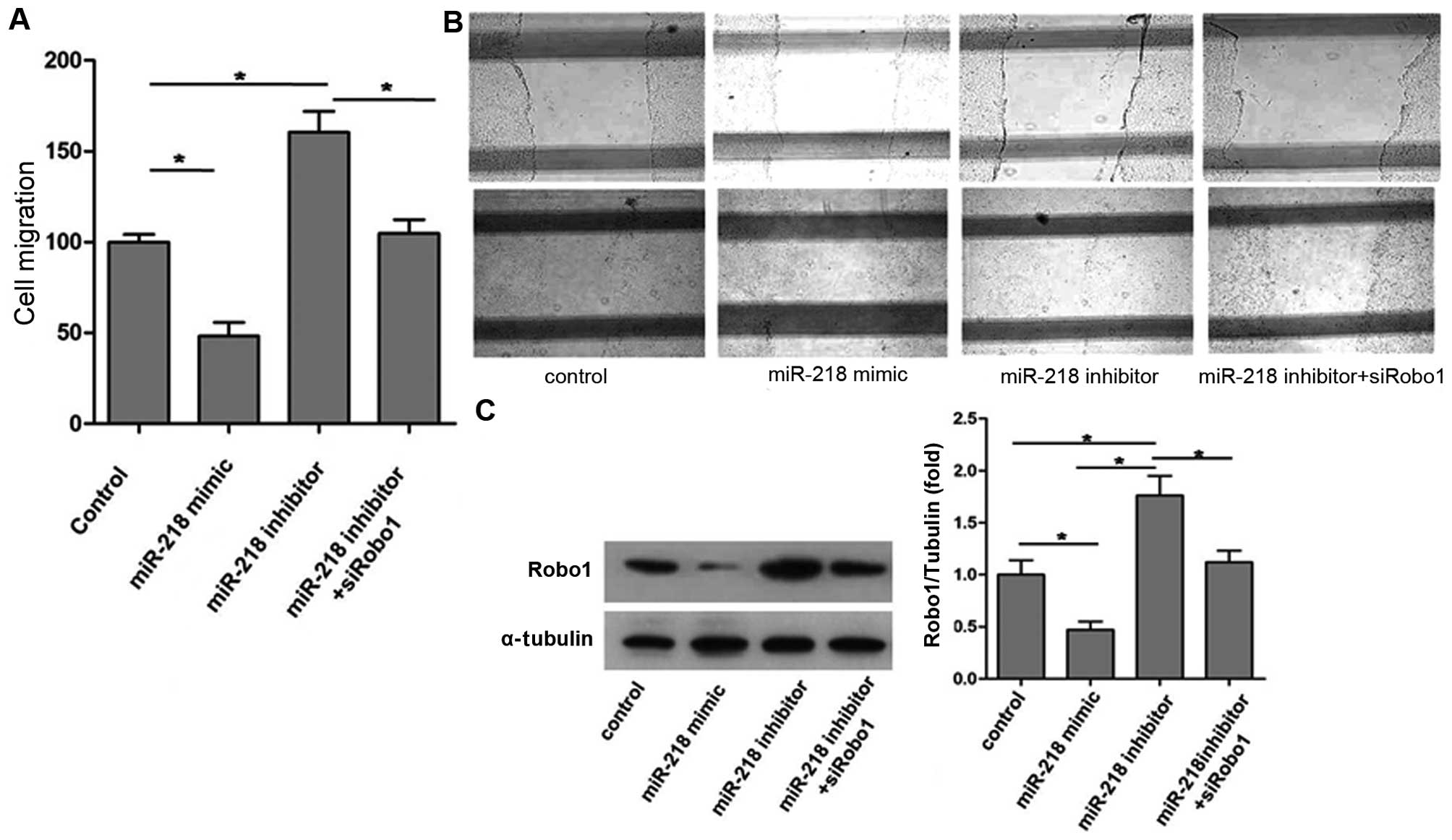
The cell migration ability of each group is shown in
Fig. 1: overexpression of miR-218
caused by miR-218 mimic significantly reduced endothelial cell (EC)
migration, whereas inhibition of miR-218 expression using the
miR-218 inhibitor markedly promoted EC migration (Fig. 1A and B).
Robo1 expression of each group is shown in Fig. 1C. The results indicate that
upregulation of miR-218 expression not only reduced EC migration
but also decreased Robo1 expression. Conversely, downregulation of
miR-218 expression using the miR-218 inhibitor increased Robo1
expression and, as was noted previously, promoted EC mobility.
However, as shown in Fig. 1A,
miR-218 inhibitor did not promote the migratory ability of ECs
after Robo1 knockdown by siRobo1. These observations suggest that
miR-218 suppresses EC migration by inhibiting Robo1 expression.
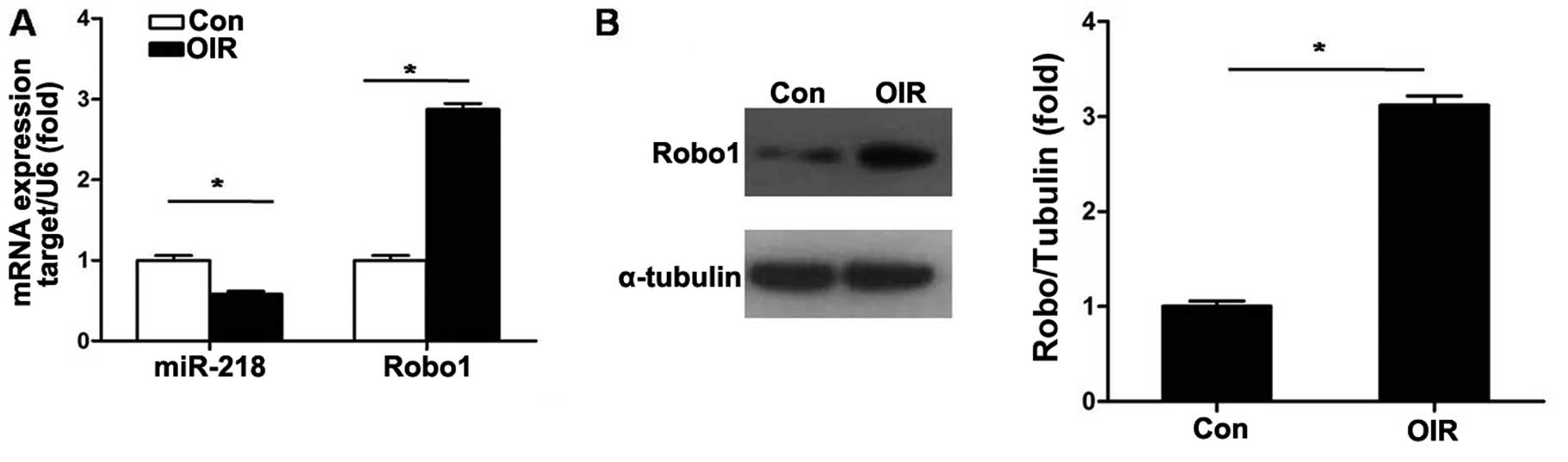
miR-218 and Robo1 expression in RNV of
mice with OIR
The expression level of miR-218 in the retinas of
OIR mice was detected. As shown in Fig. 2A, RT-qPCR results demonstrated
that the expression level of miR-218 was significantly decreased
(P<0.05) in retinas of mice with OIR at P17. We then compared
mRNA and protein expression levels of Robo1 in retinas of mice with
OIR with those of the control mice. The mRNA and protein levels of
Robo1 were markedly upregulated at P17 in mice with OIR (Fig. 2).
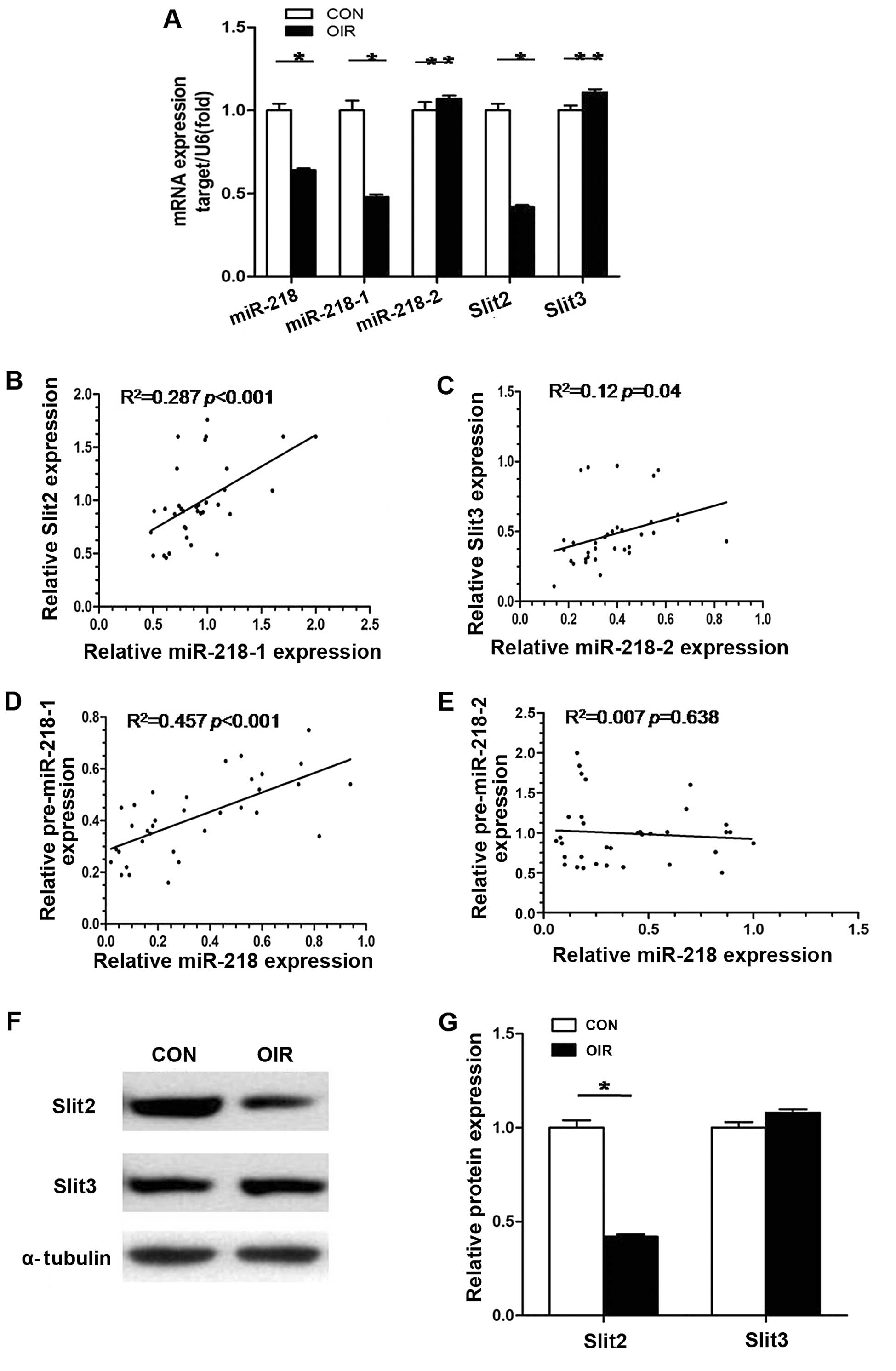
Role of miR-218 coding genes (miR-218-1
and miR-218-2) and corresponding host genes (Slit2 and Slit3)
To determine which miR-218 coding gene is involved
in downregulation of miR-218 in mice with OIR, we evaluated
miR-218-1, miR-218-2, miR-218, Slit2 mRNA and Slit3 mRNA expression
in retinas of mice with OIR by RT-qPCR. As shown in Fig. 3B–E, statistical analysis revealed
that a positive correlation existed between miR-218-1 and Slit2,
miR-218-2 and Slit3, as well as miR-218-1 and miR-218. There was no
correlation, however, between miR-218-2 and miR-218. This meant
that in the retinas of the mice with OIR, miR-218-1 and miR-218-2
were co-transcribed with their host gene respectively and that
downregulation of miR-218 was caused by decreased expression of
miR-218-1, not miR-218-2. When mRNA levels of Slit2 and Slit3 in
retinas from control mice and mice with OIR were compared, mRNA
expression of Slit2 in retinas of mice with OIR was 0.48-fold less
(P<0.05) than in the retinas of the normal control mice; mRNA
expression of Slit3 in retinas from mice with OIR was 1.12-fold
greater than in the control mice although this was not
statistically significant (P>0.05) (Fig. 3A). Similar results were also
observed in protein expression levels of Slit2 and Slit3 (Fig. 3F and G). The results suggest that
decreased expression of miR-218-1 and its host gene Slit2 is
involved in RNV in OIR.
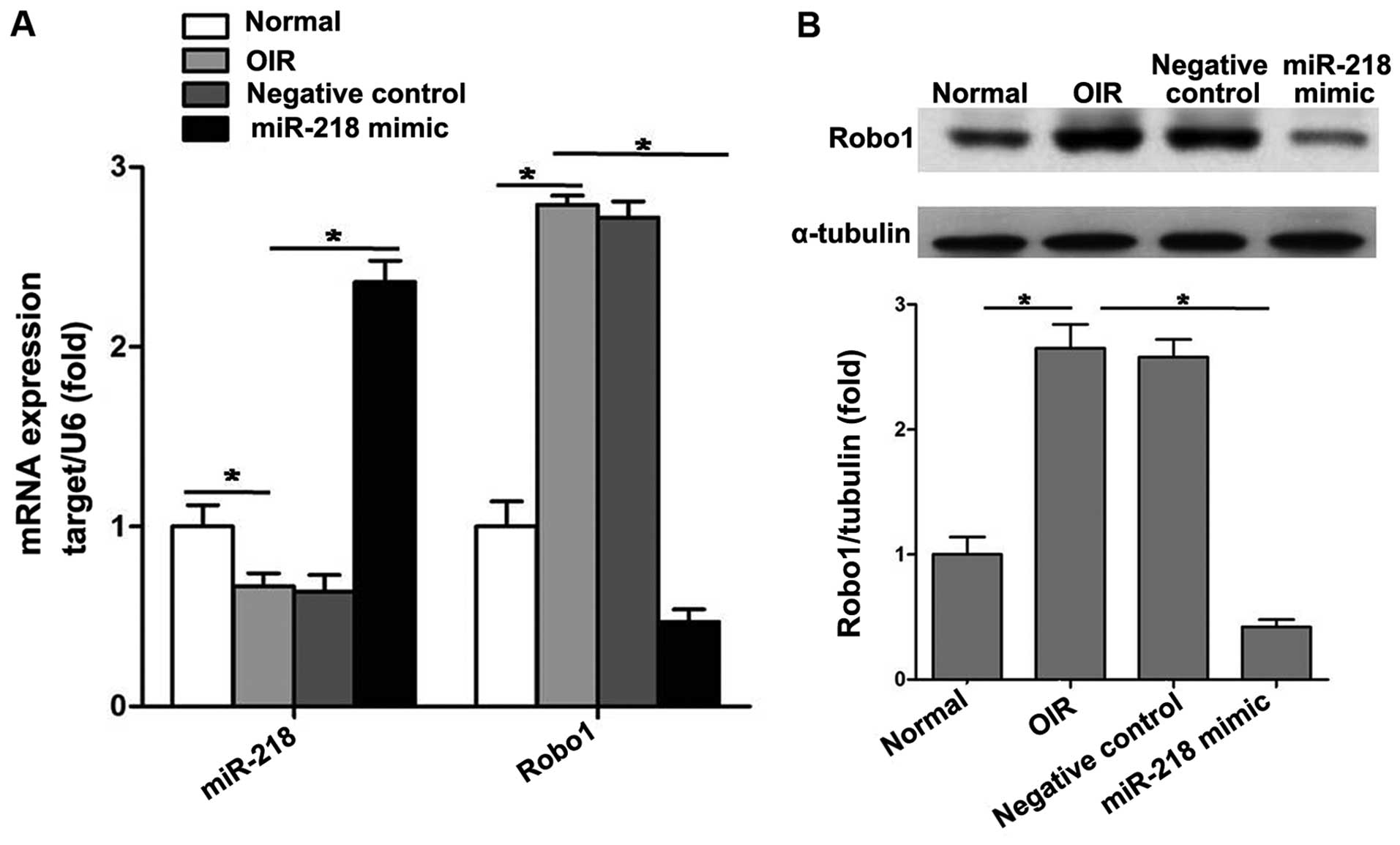
miR-218 inhibits RNV through suppressing
Robo1 expression
A plasmid pCDH-CMV-miR-218 expressing miR-218 and a
negative control plasmid pCDH-CMV-mock expressing mock sequence
were constructed. Intravitreal injection of plasmids was performed
at P12.
At P17, we analyzed mRNA levels of miR-218 and Robo1
after intravitreal injection to verify whether plasmids reached the
retina. RT-qPCR results showed that pCDH-CMV-miR-218 significantly
increased the expression of miR-218 and reduced that of Robo1 in
retinas (Fig. 4A). Western
blotting indicated that intravitreal injection of pCDH-CMV-miR-218
significantly decreased the protein level of Robo1 in the retinas
of mice with OIR (Fig. 4B).
However, pCDH-CMV-mock had almost no effect on the expression of
miR-218 and Robo1.
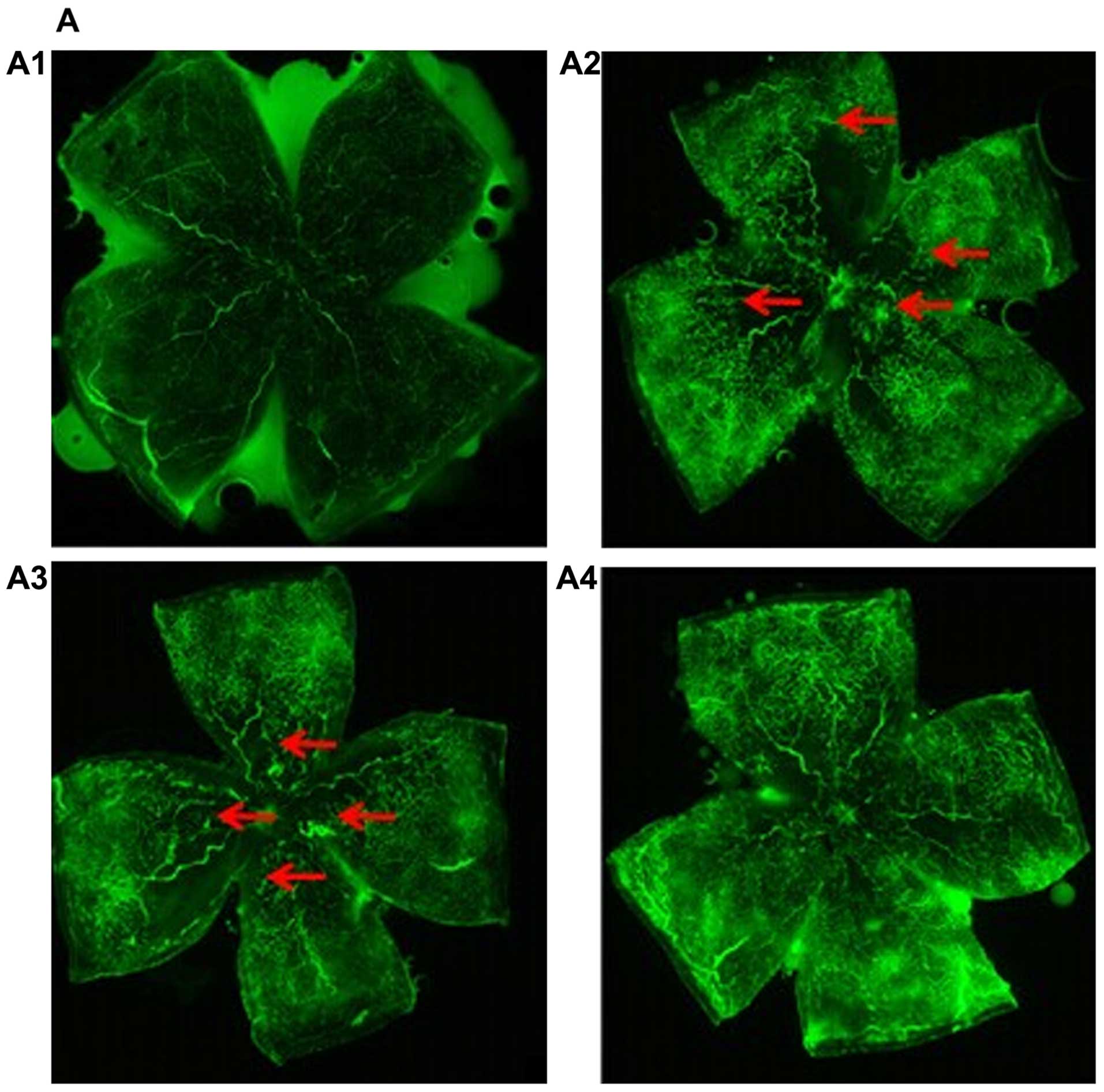
To examine the effects of miR-218 on RNV, we
evaluated the retinal vasculature at P17 by fluorescein angiography
in the flat-mounted retinas. In retinas of normal mice we noted a
mature capillary network that extended from the optic to the
periphery (Fig. 5A1). The retinas
of mice in the OIR and pCDH-CMV-mock groups displayed more
neovascular tufts (Fig. 5A2 and
A3). By contrast, the retinas of the pCDH-CMV-miR-218 group
exhibited lesser neovascularization (Fig. A4). The RNV was quantified by
measuring areas of new blood tufts in the whole mounted retina. The
results showed that a large number of neovascular tufts appeared in
the OIR group [neovascularization (NV), 38.9% of whole retina] and
pCDH-CMV-mock group (NV, 36.8% of whole retina). However, compared
to the OIR group, the neovascularized area was significantly
decreased in the pCDH-CMV-miR-218 group (NV, 18.6% of whole retina)
(Fig. 5B). No neovascularization
was observed in the normal group. These results revealed that
miR-218 exerts an anti-neovascularizing effect on RNV in mice with
OIR.
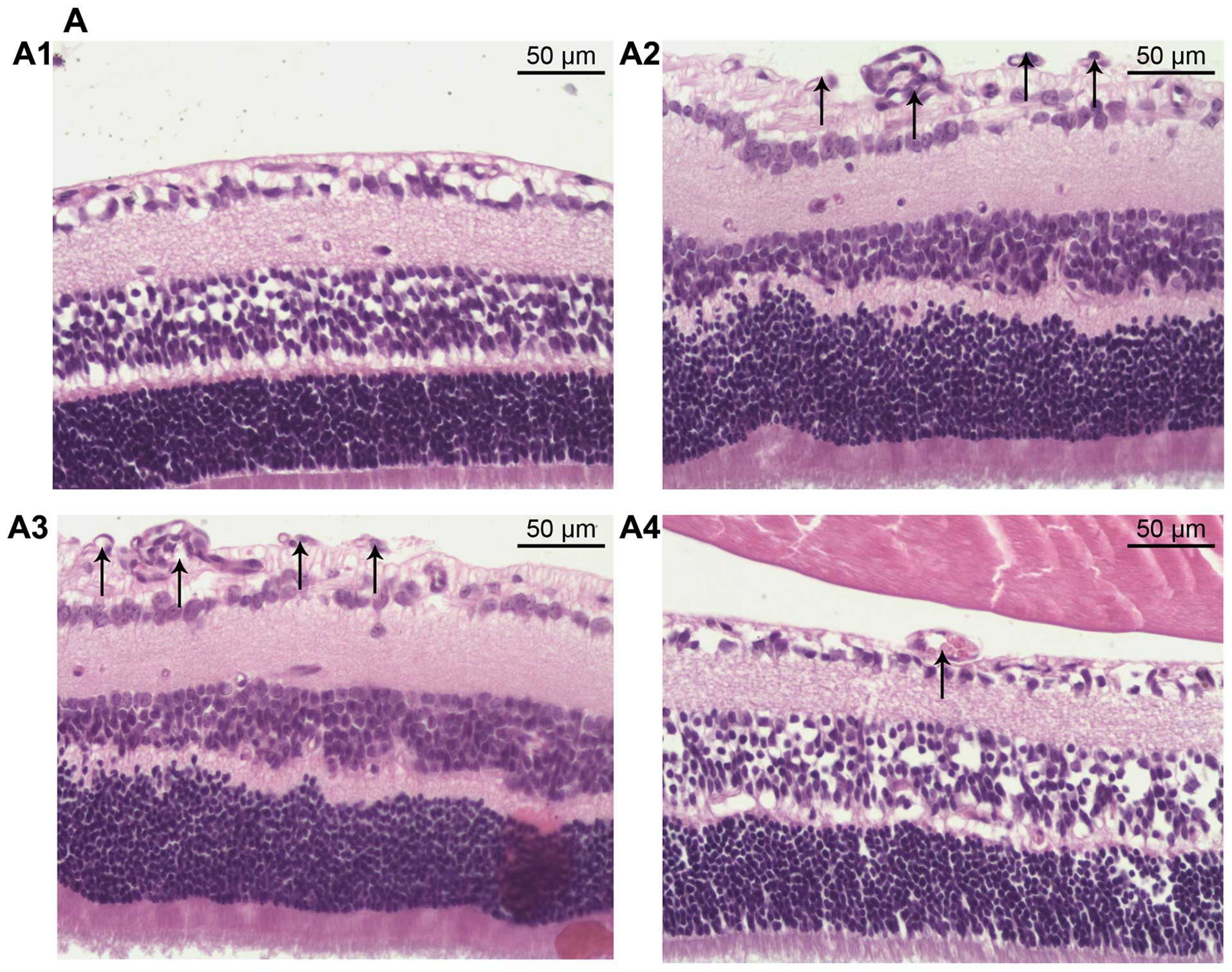
To further study the inhibitory effect of miR-218 on
angiogenesis, histological analysis was performed. We counted the
vascular cell nuclei which broke through the inner limiting
membrane (ILM), a marked feature of OIR (29). There were no neovascular nuclei in
the normal group (Fig. 6A1), and
the average number of vascular cell nuclei was significantly
increased in the OIR group and pCDH-CMV-mock group, 61.48±6.92 and
58.98±6.48, respectively (Fig. 6A2
and A3). However, in the pCDH-CMV-miR-218 group we noted less
neovascularization (Fig. 6A4)
(12.64±1.42 pre-retinal cells/section), indicating that restoration
of miR-218 played an inhibitory role in RNV in OIR (Fig. 6B).
Taken together, these results indicate that miR-218
inhibited retinal angiogenesis in OIR by suppressing Robo1
expression.
Discussion
Robo1, a member of the Robo receptor family, is
involved in signaling in the nervous system (30). However, Robo1 plays a positive
role in the migration of endothelial cells. Previous research using
monkey choroidal retinal endothelial cells (31) and HUVECs (14) has demonstrated that decreased
Robo1 markedly inhibited EC migration, and this conclusion was
further confirmed using mouse retinal endothelial cell in this
study.
In the present study, we noted that in vitro
cell migration was markedly suppressed when the miR-218 mimic was
transfected into ECs. Moreover, the expression of Robo1 decreased
in cells. Additionally, a negative expression pattern was noted
when miR-218-inhibitor was transfected into ECs: Robo1 expression
increased with the decrease of miR-218 expression, and EC migration
increased significantly. However, miR-218 inhibitor and siRobo1 had
almost no effect on EC migration. Our results indicated that the
inhibitory effect of miR-218 on EC migration was mediated by
Robo1.
The data presented in the present study indicate
that mRNA and protein expression levels of Robo1 were significantly
elevated in retinas of mice with OIR. This result was consistent
with that of a previous study (31) and substantiated the potential role
of Robo1 in RNV. Retinal vascular formation was also observed,
suggesting that there was a significant positive correlation
between Robo1 overexpression and RNV. Investigation of tumors
indicated that Robo1 play a part in angiogenesis (14). Previous studies have suggested
that Robo1 is involved in ocular neovascularization (31,32). Genetic evidence provided by Rama
et al supported a negative role for Robo1 in ocular
pathological neovascularization (33). Our results also verified this
point. In the present study, we found that retinal
neovascularization and endothelial cells breaking into the inner
nuclear layer in the retinas were both significantly reduced
following the administration of pCDH-CMV-miR-218. Therefore, we
suggest that downregulation of Robo1 significantly suppresses
RNV.
In our study, a positive correlation between
pre-miR-218 and their host gene was observed, demonstrating that
miR-218-1 and miR-218-2 co-express with their host genes during RNV
in OIR. Furtherore, a significant correlation between the
expression of miR-218 and miR-218-1 was observed, indicating that
the downregulation of miR-218-1 leads to the downregulation of
miR-218. In addition, the expression of miR-218-1 and its host
gene, Slit2 (not miR-218-2 and Slit3), were concomitantly
downregulated during RNV in mice with OIR, indicating that
miR-218-1 and Slit2 are involved in RNV by negatively regulating
retinal angiogenesis.
As an intronic miRNA, miR-218 is involved in
ligand/receptor signaling. In brief, miR-218 is located in and is
co-expressed with its host gene, Slit, whose receptor gene, Robo1,
is one of the targets of miR-218. Thus, Slit-miR-218-Robo signaling
is established. Ligand/receptor signaling transduction mediated by
intronic miRNAs has also demonstrated in other studies. miR-338,
also an intronic miRNA, was shown to silence the target gene, which
was antagonistic to its host gene, AATK (37). Tie et al (34) pointed out that miR-218 mediated
Slit/Robo signaling, forming a negative feedback circuit,
inhibiting gastric cancer metastasis. The Slit-miR-218-Robo
regulatory network (26) was also
found to be essential for the normal vascularization of the retina.
In our study, RNV in mice with OIR was shown to be regulated by the
Slit-miR-218-Robo axis. We thus speculated that the regulation of
Slit-miR-218-Robo signaling may provide a means of suppressing RNV
by preventing the overactivation of ligand/receptor signaling.
In the mice with OIR, we noted that the expression
of miR-218 significantly decreased, and the expression of Robo1
increased. The restoration of miR-218 inhibited retinal
angiogenesis, suggesting that miR-218 is an important regulator of
RNV. Our results also suggest that miR-218 inhibits RNV through
mediating the downregulation of Robo1 expression.
In conclusion, the Slit-miR-218-Robo axis plays a
role in retinal angiogenesis. Slit2 interacts with Robo1 to inhibit
RNV mediated by miR-218. miR-218 may thus be a therapeutic
candidate for the targeted treatment of RNV
Acknowledgments
This study was supported by the Natural Science
Foundation of Tianjin City (no. 13JCYBJC22900).
References
|
1
|
Ishida S, Usui T, Yamashiro K, Kaji Y,
Amano S, Ogura Y, Hida T, Oguchi Y, Ambati J, Miller JW, et al:
VEGF164-mediated inflammation is required for pathological, but not
physiological, ischemia-induced retinal neovascularization. J Exp
Med. 198:483–489. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Avunduk AM, Cetinkaya K, Kapicioğlu Z and
Kaya C: The effect of posterior vitreous detachment on the
prognosis of branch retinal vein occlusion. Acta Ophthalmol Scand.
75:441–442. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moravski CJ, Kelly DJ, Cooper ME, Gilbert
RE, Bertram JF, Shahinfar S, Skinner SL and Wilkinson-Berka JL:
Retinal neovascularization is prevented by blockade of the
renin-angio-tensin system. Hypertension. 36:1099–1104. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Campochiaro PA: Ocular neovascularization.
J Mol Med Berl. 91:311–321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brose K, Bland KS, Wang KH, Arnott D,
Henzel W, Goodman CS, Tessier-Lavigne M and Kidd T: Slit proteins
bind Robo receptors and have an evolutionarily conserved role in
repulsive axon guidance. Cell. 96:795–806. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dickson BJ: Molecular mechanisms of axon
guidance. Science. 298:1959–1964. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu J, Zhang L, Wang D, Shen H, Jiang M,
Mei P, Hayden PS, Sedor JR and Hu H: Congenital diaphragmatic
hernia, kidney agenesis and cardiac defects associated with
Slit3-deficiency in mice. Mech Dev. 120:1059–1070. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cariboni A, Andrews WD, Memi F, Ypsilanti
AR, Zelina P, Chedotal A and Parnavelas JG: Slit2 and Robo3
modulate the migration of GnRH-secreting neurons. Development.
139:3326–3331. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kryczek I, Wei S, Keller E, Liu R and Zou
W: Stroma-derived factor (SDF-1/CXCL12) and human tumor
pathogenesis. Am J Physiol Cell Physiol. 292:C987–C995. 2007.
View Article : Google Scholar
|
|
10
|
Stella MC, Trusolino L and Comoglio PM:
The Slit/Robo system suppresses hepatocyte growth factor-dependent
invasion and morphogenesis. Mol Biol Cell. 20:642–657. 2009.
View Article : Google Scholar :
|
|
11
|
Bauer K, Dowejko A, Bosserhoff AK,
Reichert TE and Bauer R: Slit-2 facilitates interaction of
P-cadherin with Robo-3 and inhibits cell migration in an oral
squamous cell carcinoma cell line. Carcinogenesis. 32:935–943.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schubert T, Denk AE, Ruedel A, Kaufmann S,
Hustert E, Bastone P and Bosserhoff AK: Fragments of SLIT3 inhibit
cellular migration. Int J Mol Med. 30:1133–1137. 2012.PubMed/NCBI
|
|
13
|
Han X and Zhang MC: Potential
anti-angiogenic role of Slit2 in corneal neovascularization. Exp
Eye Res. 90:742–749. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang B, Xiao Y, Ding BB, Zhang N, Yuan X,
Gui L, Qian KX, Duan S, Chen Z, Rao Y and Geng JG: Induction of
tumor angio-genesis by Slit-Robo signaling and inhibition of cancer
growth by blocking Robo activity. Cancer Cell. 4:19–29. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou W, Yu W, Xie W, Huang L, Xu Y and Li
X: The role of SLIT-ROBO signaling in proliferative diabetic
retinopathy and retinal pigment epithelial cells. Mol Vis.
17:1526–1536. 2011.PubMed/NCBI
|
|
16
|
Kim VN, Han J and Siomi MC: Biogenesis of
small RNAs in animals. Nat Rev Mol Cell Biol. 10:126–139. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim YK and Kim VN: Processing of intronic
microRNAs. EMBO J. 26:775–783. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
van Rooij E, Quiat D, Johnson BA,
Sutherland LB, Qi X, Richardson JA, Kelm RJ Jr and Olson EN: A
family of microRNAs encoded by myosin genes governs myosin
expression and muscle performance. Dev Cell. 17:662–673. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sayed D and Abdellatif M: MicroRNAs in
development and disease. Physiol Rev. 91:827–887. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kasinski AL and Slack FJ: Epigenetics and
genetics MicroRNAs en route to the clinic: progress in validating
and targeting microRNAs for cancer therapy. Nat Rev Cancer.
11:849–864. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fabian MR, Sonenberg N and Filipowicz W:
Regulation of mRNA translation and stability by microRNAs. Annu Rev
Biochem. 79:351–379. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shen J, Yang X, Xie B, Chen Y, Swaim M,
Hackett SF and Campochiaro PA: MicroRNAs regulate ocular
neovascularization. Mol Ther. 16:1208–1216. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bai Y, Bai X, Wang Z, Zhang X, Ruan C and
Miao J: Micro- RNA-126 inhibits ischemia-induced retinal
neovascularization via regulating angiogenic growth factors. Exp
Mol Pathol. 91:471–477. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen N, Wang J, Hu Y, Cui B, Li W, Xu G,
Liu L and Liu S: MicroRNA-410 reduces the expression of vascular
endothelial growth factor and inhibits oxygen-induced retinal
neovascularization. PLoS One. 9:e956652014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Small EM, Sutherland LB, Rajagopalan KN,
Wang S and Olson EN: MicroRNA-218 regulates vascular patterning by
modulation of Slit-Robo signaling. Circ Res. 107:1336–1344. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kong YC, Sun B, Zhao KX, Han M and Wang
YC: Small interference RNA targeting vascular endothelial growth
factor gene effectively attenuates retinal neovascularization in
mice model. Chin Med J (Engl). 126:1440–1444. 2013.
|
|
28
|
Li S, Li T, Luo Y, Yu H, Sun Y, Zhou H,
Liang X, Huang J and Tang S: Retro-orbital injection of
FITC-dextran is an effective and economical method for observing
mouse retinal vessels. Mol Vis. 17:3566–3573. 2011.
|
|
29
|
Smith LE: Pathogenesis of retinopathy of
prematurity. Acta Paediatr Suppl. 91:26–28. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kidd T, Bland KS and Goodman CS: Slit is
the midline repellent for the robo receptor in Drosophila. Cell.
96:785–794. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang L, Xu Y, Yu W, Li X, Liqun C, He X
and Peiying H: Robo1: a potential role in ocular angiogenesis.
34:1019–1029. 2009.
|
|
32
|
Huang L, Yu W, Li X, Niu L, Li K and Li J:
Robo1/robo4: different expression patterns in retinal development.
Exp Eye Res. 88:583–588. 2009. View Article : Google Scholar
|
|
33
|
Rama N, Dubrac A, Mathivet T, Ní
Chárthaigh RA, Genet G, Cristofaro B, Pibouin-Fragner L, Ma L,
Eichmann A and Chédotal A: Slit2 signaling through Robo1 and Robo2
is required for retinal neovascularization. Nat Med. 21:483–491.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tie J, Pan Y, Zhao L, Wu K, Liu J, Sun S,
Guo X, Wang B, Gang Y, Zhang Y, et al: MiR-218 inhibits invasion
and metastasis of gastric cancer by targeting the Robo1 receptor.
PLoS Genet. 6:e10008792010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fish JE, Wythe JD, Xiao T, Bruneau BG,
Stainier DY, Srivastava D and Woo S: A Slit/miR-218/Robo regulatory
loop is required during heart tube formation in zebrafish.
Development. 138:1409–1419. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Alajez NM, Lenarduzzi M, Ito E, Hui AB,
Shi W, Bruce J, Yue S, Huang SH, Xu W, Waldron J, et al: MiR-218
suppresses nasopha-ryngeal cancer progression through
downregulation of survivin and the SLIT2-ROBO1 pathway. Cancer Res.
71:2381–2391. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Barik S: An intronic microRNA silences
genes that are functionally antagonistic to its host gene. Nucleic
Acids Res. 36:5232–5241. 2008. View Article : Google Scholar : PubMed/NCBI
|