Introduction
A number of studies have been performed using stem
cells in order to treat muscle-related diseases in which the
organization of muscle tissue is adversely affected by congenital
hereditary muscle defects, loss of muscle mass, trauma or tumor
removal (1–5). Previous studies have reported the
transplantation of muscle stem cell-derived myoblasts or myogenic
cells in models of muscle injury (6–8).
However, the main issue which researchers confront is the fact that
it is difficult to obtain the required numbers of cells for
transplantation to a site of injury due to the cultivation period
required in order to generate muscle stem cells. Several groups
have reported the differentiation of embryonic stem (ES) cells and
induced pluripotent stem (iPS) cells into myogenic cells (4,9,10).
However, there are major obstacles to the clinical use of ES and
iPS cells, including teratoma formation and the rejection of
transplanted cells by the immune system. Compared with these cells,
mesenchymal stem cells (MSCs), which have the ability to regulate
the immune system and do not form teratomas, may be isolated from
various sources, including bone marrow (11), adipose tissue (12), umbilical cord blood (13), amniotic fluid (14), the placenta (15), dental pulp (16), the tonsils (17) and urine (1). For these reasons, MSCs have been
recognized as a valuable source of cells which may be used to
propagate myogenic cells according to the protocols for
differentiation. Several studies examining the differentiation of
MSCs into myogenic cells have been reported the use of MSCs derived
from adipose tissue, bone marrow, the placenta, amniotic fluid,
umbilical cord blood and urine (1,2,11–14,18).
The tonsils are a newly identified source of MSCs
which may have potential therapeutic applications. Tonsil-derived
MSCs (T-MSCs) readily differentiate into cells of the mesodermal
lineage, including fat, cartilage and bone cells, and into cells of
the endodermal lineage, including hepatocytes (19–21). T-MSCs also exhibit similar
immunosuppressive properties to bone marrow-derived MSCs and
adipose tissue-derived MSCs (22,23). As tonsillar tissues are discarded
after surgery, the isolation of stem cells from these discarded
tissues is also a valuable means of recycling human tissue for stem
cell therapy (23,24).
Skeletal muscle (SKM) possesses the ability to grow
in response to increased workload or to repair itself in the case
of injury. The postnatal growth, repair and maintenance of muscle
fibers depend on a population of muscle stem cells (25) that are located beneath the basal
membrane of muscle fibers. However, an extensive muscle injury may
prevent complete regeneration, particularly in terms of functional
recovery. Severe lesions associated with the loss of healthy
muscular tissue and the development of fibrous scar tissue, as well
as irreversible muscular atrophy following long-term peripheral
nerve injury are examples of situations in which regeneration is
limited (5). As an alternative
approach to the regeneration of damaged SKM, and considered to be
the optimal treatment for certain traumatic or degenerative
diseases (26), the
transplantation of T-MSC-derived myogenic cells is a suitable
method for limiting the atrophy of the affected muscles, and may
even lead to myocyte regeneration and reduced motor deficits.
In the present study, we demonstrated that T-MSCs
may differentiate into myogenic cells in vitro and that the
transplantation of the myoblasts and myocytes generated from human
T-MSCs mediates the recovery of muscle function following injury
in vivo. Immunocytochemistry, reverse
transcription-polymerase chain reaction (RT-PCR), and western blot
analysis confirmed the development of T-MSC-derived myogenic cells
in vitro. Furthermore, the in situ transplantation of
T-MSCs into mice with a partial myectomy of the right gastrocnemius
muscle, led to enhanced muscle function, as demonstrated by gait
assessment (footprint analysis). These results suggest that human
tonsils are a promising source of stem cells and that T-MSCs may be
used to promote the regeneration of SKM following injury.
Materials and methods
Ethics statement
The Institutional Review Board of Ewha Womans
University, Mokdong Hospital (Seoul, Korea) approved all the
experimental procedures used in this study (approval no.
ECT-11-53-02). Informed written consent was obtained from each
patient and/or their legal representatives prior to obtaining the
tissue samples. Animal care and experimental procedures were
approved by the Institutional Animal Care and Use Committee at Ewha
Womans University School of Medicine (ESM no. 14-0285), and all
experiments were performed in accordance with approved guidelines
and regulations, namely the guidelines of the Korean Ministry of
Health and Welfare, the Animal Care Guidelines of the Ewha Womans
University School of Medicine, and the National Research Council
(US) Guide for the Care and Use of Laboratory Animals (27).
Animals
Seven-week-old male C57BL/6 mice (n=40; weighing,
21–24 g; Dae-Han Biolink Co, Ltd, Eumseong, Korea) housed at 21±2°C
and 55±5% humidity under a 12 h light/dark cycle, and supplied with
food and water ad libitum were used for all the experiments.
The mice were fed an autoclaved diet and also provided with water
ad libitum. All the mice were treated in accordance with the
above-mentioned guidelines. A minimum of 10 age-matched mice were
used for each group. The animals were sacrificed by CO2
inhalation.
Isolation of T-MSCs
The isolation of T-MSCs from tonsillar tissue was
performed as previously described (17,28). Briefly, tonsillar tissues were
collected from patients during tonsillectomy, and subsequently
minced and digested in Dulbecco's modified Eagle's medium (DMEM)
containing 210 U/ml collagenase type I (both from Invitrogen,
Carlsbad, CA, USA) and DNase (10 µg/ml, Sigma-Aldrich, St.
Louis, MO, USA). After the cells were passed through a cell
strainer (BD Biosciences, San Jose, CA, USA), mononuclear cells
were obtained by Ficoll-Paque (GE Healthcare, Chalfont St. Giles,
UK) density gradient centrifugation. The cells were cultured for 48
h at 37°C in low-glucose DMEM containing 10% fetal bovine serum
(FBS; Invitrogen) and 1% penicillin/streptomycin (Sigma-Aldrich) in
a humidified chamber with 5% CO2. This was followed by
the removal of non-adherent cells and the T-MSCs were cultured in
fresh medium. These freshly cultured cells were expanded over 3–5
passages, a process which took approximately 4 weeks.
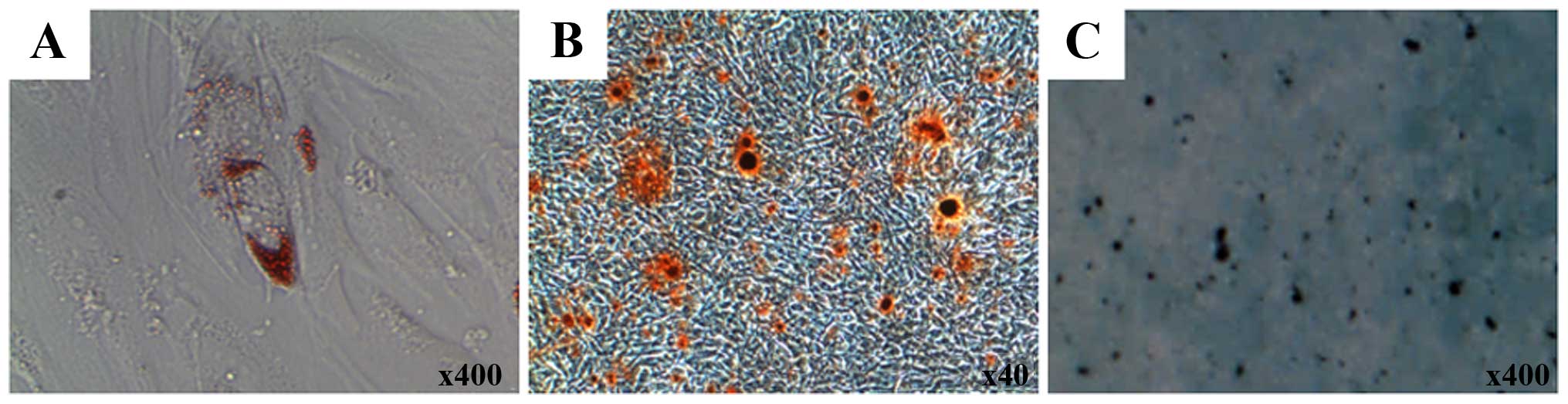
Adipogenic, osteogenic and chondrogenic
differentiation of T-MSCs
The mesodermal differentiation of T-MSCs was induced
as previously described (17)
with minor modifications. Briefly, to induce adipogenic
differentiation, the T-MSCs were cultured in commercially available
adipogenic medium (Invitrogen) for 3 weeks. Subsequently, the cells
were washed twice with phosphate-buffered saline (PBS), fixed in 4%
paraformaldehyde (PFA) for 15 min at room temperature, then washed
with PBS and stained with 2% Oil Red O (Sigma-Aldrich) for 1 h at
room temperature. The T-MSCs were washed again with PBS. The
intracellular lipid droplets were visualized under a microscope
(IX2-SLP; Olympus, Tokyo, Japan). To quantify lipid accumulation,
Oil Red O deposited in the cells was eluted with 100% isopropanol
for 10 min and the absorbance of the eluting solution was measured
at a wavelength of 540 nm using an ELISA microplate reader
(BN03269, VersaMax; Molecular Devices, San Jose, CA, USA).
To induce osteogenic differentiation, the T-MSCs
were cultured in commercially available osteogenic medium
(Invitrogen) for 3 weeks. Thereafter, the cells were washed twice
with PBS, fixed in 4% PFA for 15 min at room temperature and
stained with 2% Alizarin Red S (Sigma-Aldrich) for 1 h. After
rinsing the cells 2 more times with PBS, the extracellular matrix
calcification was visualized under a phase-contrast microscope
(IX2-SLP; Olympus). To quantify calcium deposition, the cells were
incubated with 10% cetylpyridinium chloride for 10 min to extract
the Alizarin Red S. The eluate was collected and absorbance was
measured at a wavelength of 570 nm using an ELISA microplate reader
(BN03269, VersaMax; Molecular Devices).
To induce chondrogenic differentiation, the T-MSCs
were stimulated for 3 weeks in commercially available
chondrogenesis-inducing medium (Invitrogen). Thereafter, the cells
were rinsed with PBS and fixed in 4% PFA for 15 min at room
temperature. After washing, the cells were stained with 1% Alcian
blue (Sigma-Aldrich) for 1 h at room temperature, and the excess
dye was removed. Subsequently, the cells were rinsed again with 0.1
N HCl, and the chondrogenic cells were visualized under a
phase-contrast microscope (IX2-SLP; Olympus). To quantify the
intensity of Alcian blue staining, the cells were solubilized with
400 µl of 1% SDS. The absorbance was read at a wavelength of
605 nm.
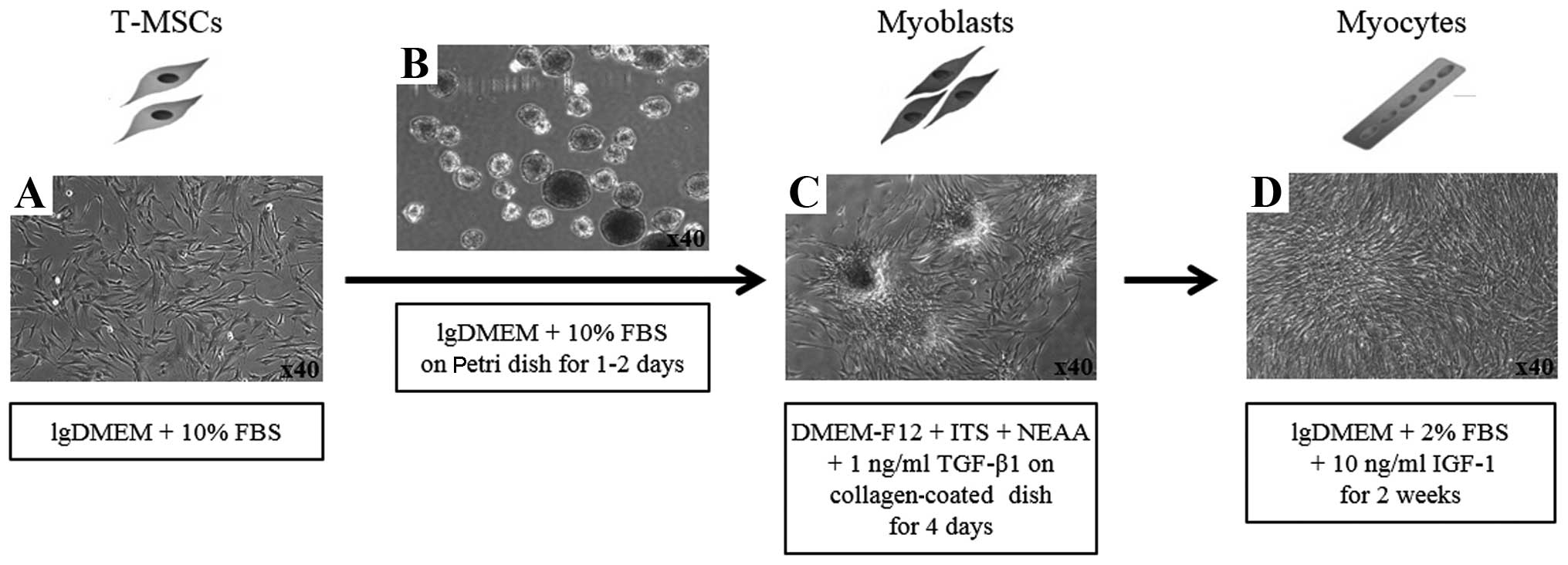
Myogenic differentiation
To induce the myogenic differentiation of the
T-MSCs, 3–4×106 cells were plated in a 15-cm Petri dish
in low-glucose DMEM supplemented with 10% FBS. At 1–3 days, the
cells spontaneously aggregated to form spheres 50–100 µm in
diameter. Once the spheres had formed, the medium was replaced with
DMEM/nutrient mixture F-12 (DMEM/F-12; Invitrogen) supplemented
with 1 ng/ml transforming growth factor-β (TGF-β; R&D Systems,
Minneapolis, MN, USA), non-essential amino acids (NEAA; Invitrogen)
and insulin-transferrin-selenium (ITS; Gibco Life Technologies,
Grand Island, NY, USA) for a further 4 days in order to allow
differentiation into myoblasts. The T-MSCs grew out of the spheres
when transferred to a collagen-coated dish in the above mentioned
myoblast differentiation medium, and formed a rosette-like spread.
To induce terminal differentiation into myocytes, the myoblasts
were cultured for 2 weeks in myogenic induction medium, which
consisted of low-glucose DMEM containing 10 ng/ml insulin-like
growth factor 1 (IGF1; R&D Systems) and 2% FBS (Fig. 2D).
RT-PCR
Total RNA was extracted from the cells using an
RNeasy Mini kit (Qiagen, Germantown, MD, USA). Complementary DNA
(cDNA) was synthesized using SuperScript II (Invitrogen) and
oligo-(dT)20 primers at 42°C for 1 h followed by incubation at 72°C
for 15 min. Target sequences from the cDNA were amplified using
premixed kits (Bioneer, Daejeon, Korea) under the following
conditions: initial denaturation at 95°C for 5 min followed by 35
cycles of denaturation at 95°C for 30 sec, annealing at 45–60°C for
45 sec and extension at 72°C for 44 sec. Normalized amounts of
products were separated on a 1.5% agarose gel and visualized by
ethidium bromide staining. The sequences of the forward and reverse
primers used were as follows: Krüppel-like factor 4 (Klf4)
forward, 5′-CCCGATCAGATGCAGCCGCAAGTC-3′ and reverse,
5′-CTGGCTGGGCTCCTTCCCTCATCG-3′; Rex1 forward,
5′-CAGATCCTAAACAGCTCGCA-3′ and reverse, 5′-GCGTACGCAAATTAAAGTCC-3′;
activin forward, 5′-AGAGCGACCTCACAGCCGTGCTGG-3′ and reverse,
5′-CCGAGGTAGTGCCGTTGACCGACCT-3′; paired box 7 (Pax7)
forward, 5′-CACTGTGACCGAAGCACTGT-3′ and reverse,
5′-GTCAGGTTCCGACTCCACAT-3′; myogenic factor 6 (Myf6)
forward, 5′-AGGAACCCAGACCGAAAAGT-3′ and reverse,
5′-TTGAACATGGCACAAAAGGA-3′; myogenin forward,
5′-GTCTTCGCCGGGCATCCTTG-3′ and reverse,
5′-GAGCTGGGGCATACACGAGGGG-3′; dystrophin forward,
5′-ACCACCTCTGACCCTACACG-3′ and reverse, 5′-GCAATGTGTCCTCAGCAGAA-3′;
and glyceraldehyde 3-phosphate dehydrogenase (GAPDH)
forward, 5′-TGGTATCGTGGAAGGACTCA-3′ and reverse,
5′-CCTGCTTCACCACCTTCTTG-3′.
Immunocytochemistry
The cells grown on coverslips were fixed in 4% (v/v)
PFA (Sigma-Aldrich) for 15 min at room temperature or overnight at
4°C. After rinsing in PBS, the fixed cells were permeabilized and
non-specific epitopes were blocked using 2% bovine serum albumin
(Bovogen Biologicals, East Keilor, VIC, Australia) in 0.1%
Tween-20/PBS, followed by incubation in the diluted primary
antibody for 1 h at room temperature or overnight at 4°C. Following
3 washes in PBS, the samples were incubated for 1 h at room
temperature with secondary antibodies diluted in PBS. The prepared
samples were then mounted using Vectashield mounting medium
containing 4′,6-diamidino-2-phenylindole (DAPI; Vector
Laboratories, Burlingame, CA, USA) and images were captured under a
fluorescence microscope (Nikon Corp., Tokyo, Japan). The
manufacturers and catalog numbers (Cat. no.) of the antibodies
employed were as follows: mouse anti-CD34 (Cat. no. SC-74499; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA), rabbit anti-Pax7 (Cat.
no. ab187339; Abcam, Cambridge, UK), mouse anti-desmin (Cat. no.
D1033; Sigma-Aldrich), rabbit anti-dystrophin (Cat. no. ab15277;
Abcam), mouse anti-myosin heavy chain (MHC, Cat. no. MAB4470;
R&D Systems), rabbit anti-α-actinin (Cat. no. PA5-17308; Thermo
Fisher Scientific, Scoresby, VIC, Australia), rabbit anti-troponin
I type 1 (TNNI1; Cat. no. NBP1-90923; Novus Biologicals, Littleton,
CO, USA), mouse anti-myogenin (Cat. no. ab1835; Abcam) (primary
antibodies), and tetramethylrhodamine (TRITC)-conjugated Alexa-568
goat anti-mouse IgG (Cat. no. A-11031), fluorescein isothinocyanate
(FITC)-conjugated Alexa-568 goat anti-mouse IgG (Cat. no. A-11004),
and TRITC-conjugated Alexa-568 goat anti-rabbit IgG (Cat. no.
A-11011) (all from Life Technologies) (secondary antibodies).
Western blot analysis
The protein concentrations were determined using
Bradford assay reagent (Bio-Rad Laboratories, Hercules, CA, USA)
after lysing the cells in Pro-Prep buffer (iNtRON Biotechnology,
Seongnam, Korea) supplemented with phosphatase inhibitor cocktail
solution (Dawinbio, Hanam, Korea). The cells were washed with
ice-cold PBS and exposed to Pro-Prep buffer supplemented with
phosphatase inhibitor cocktail solution for 30 min on ice.
Insoluble material was removed by centrifugation at 12,000 × g for
10 min at 4°C. The proteins (30–80 µg) were separated by
7.5–13.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
and transferred onto polyvinylidene fluoride or nitrocellulose
membranes (Millipore, Billerica, MA, USA). The membranes were
blocked with 5% skim milk in Tris-buffered saline containing 0.1%
Tween-20 (TBST) for 2 h at room temperature. The blots were then
incubated with primary antibodies overnight at 4°C. The antibodies
used for western blot analysis were rabbit anti-α-actinin (Cat. no.
PA5-17308) (both from Thermo Fisher Scientific), mouse anti-desmin
(Cat. no. D1033) and mouse anti-α-SMA (Cat. no. A2547) (both from
Sigma-Aldrich), rabbit anti-TNNI1 (Cat. no. NBP1-90923; Novus
Biologicals), and mouse anti-myogenin (Cat. no. ab1835; Abcam). The
blots were washed 3 times for 5 min with TBST and then incubated
with horseradish peroxidase-labeled secondary antibody for 1 h at
room temperature. Goat anti-mouse IgG (1:2,500, Cat. no. SC-2005;
Santa Cruz Biotechnology) and goat anti-rabbit IgG (1:2,500, Cat.
no. 7074; Cell Signaling Technology, Beverley, MA, USA) were used
as the secondary antibodies. After additional washes, signals were
detected using a WESTSAVE Gold western blot detection kit (Young In
Frontier Co., Ltd., Seoul, Korea). The protein signals were
visualized by exposing the membranes to a luminescent image
analyzer (LAS-3000; Fujifilm, Tokyo, Japan). The level of
expression of each protein was normalized to that of GAPDH
(Sigma-Aldrich). The results were quantified using ImageJ software
(1.48 V; Wayne Rasband, NIH, USA).
Transplantation of T-MSCs into mice
The surgeries were performed under general
anesthesia using a mixture of Zoletil 50 (Virbac, Carros, France)
and Rompun (Bayer Korea, Seoul, Korea), at a 3:1 ratio,
administered 1 ml/kg intraperitoneally. To establish a model of
myectomy, we removed a 0.5×1.0 cm fraction (40–60 mg) of the
gastrocnemius muscle from each mouse in order to create a defect,
as previously described (5). This
was accomplished by lacerating the lateral side of the right muscle
with a no. 9 scalpel blade. Forty-eight hours after inducing muscle
injury, 1×106 T-MSCs/T-MSC-derived myocytes in PBS (100
µl each) or PBS alone (100 µl, used as the vehicle)
were injected intramuscularly into the midpoint of the damaged part
of the gastrocnemius muscle. The PBS-treated mice served as the
vehicle-treated group (vehicle). A group of normal (uninjured) mice
was also used as a control. In total, there were 5 groups with 8
mice/group: the normal group, the injured group, the
vehicle-treated group, the T-MSC-injected group and the
T-MSC-myocyte-injected group. The mice were sacrificed in order to
obtain tissues for immunohistochemical analysis at 48 h, 7 days,
and at 4 and 8 weeks post-transplantation.
Immunohistochemistry
For immunohistochemistry, mouse gastrocnemius
muscles were fixed in 10% formaldehyde. Following approximately 24
h of fixation at 4°C, the muscles were washed in PBS at room
temperature. The washed muscles were dehydrated in a graded ethanol
series, cleared in xylene, and embedded in paraffin wax. The blocks
were sectioned into 5-µm thick serial sections. The
sectioned tissues were placed onto a microscope slide. Non-specific
epitopes were blocked using 3% bovine serum albumin in 0.1% Triton
X-100/PBS followed by incubation with the appropriate primary
antibody for 1 h at room temperature. Following 3 washes in 0.1%
Triton X-100/PBS, the samples were incubated with secondary
antibodies for 1 h at room temperature or at 4°C overnight. The
tissues were mounted using Vectashield mounting medium containing
DAPI (Vector Laboratories) and images were captured under a
fluorescence microscope (Nikon Corp.). The manufacturers and
catalog numbers of the antibodies employed were as follows: rabbit
anti-dystrophin (Cat. no. ab15277; Abcam), mouse anti-α-SMA (Cat.
no. A2547; Sigma-Aldrich), rabbit anti-TNNI1 (Cat. no. NBP1-90923;
Novus Biologicals) (primary antibodies), Alexa-568 goat anti-mouse
IgG (Cat. no. A-11031), and Alexa-568 goat anti-rabbit IgG (Cat.
no. A-11057) (both from Life Technologies) (secondary
antibodies).
Gait assessment by footprint
analysis
The bottom of each hind foot of each mouse was
coated with non-toxic ink, and the mouse was allowed to walk
through a small tunnel on white paper. Stride length (distance
between the 2 rear paw prints) was measured as previously described
(29,30) at 1, 2, 3, 4 and 8 weeks after
transplantation. The stride lengths of the mice in the normal,
injured, vehicle-treated and T-MSC-myocyte-transplanted groups were
then compared using an unpaired Duncan's test.
Morphological assessment of
regeneration
Photographic images were obtained of the
gastrocnemius muscle from mice in the injured group and the
transplantation groups using a camera (Galaxy Note 2 SHV-E250S
camera; Samsung, Seoul, Korea) and stored as 8 megapixel
Back-illuminated sensor. The criteria for the assessment of
morphological regeneration was the disappearance of any sign of
injury and that the wound was filled with muscle.
Statistical Analysis
The results are presented as the means ± standard
error of the mean (SEM). Statistical comparisons were performed
using Duncan's test with GraphPad Prism software 5.01 (GraphPad
Software, Inc., San Diego, CA, USA) to identify significant
differences between groups. A P-value <0.01–0.05 was considered
to indicate a statistically significant difference. All the
experiments were performed at least 3 times.
Results
Myogenic cells derived from T-MSCs
It has been previously reported that T-MSCs possess
the characteristics of MSCs (17,21). In our study, T-MSCs were found to
differentiate into 3 different cell types, namely adipocytes,
osteocytes and chondrocytes (Fig.
1). To induce myogenic differentiation, the T-MSCs (Fig. 2A) were allowed to form spheres of
approximately 50–100 µm in diameter on the Petri dish
(Fig. 2B). The T-MSCs grew out of
the spheres when transferred to a collagen-coated dish in replating
medium, and formed a rosette-like spread (Fig. 2C). The plated cells were cultured
for 2 weeks to allow terminal differentiation into myocytes
(Fig. 2D). The cultivation of the
cells in vitro for up to 2 weeks in low-glucose DMEM
containing 10 ng/ml IGF1 and 2% FBS altered the morphology of the
myoblasts; they underwent fusion with one another to generate
nascent myotubes (Fig. 2D).
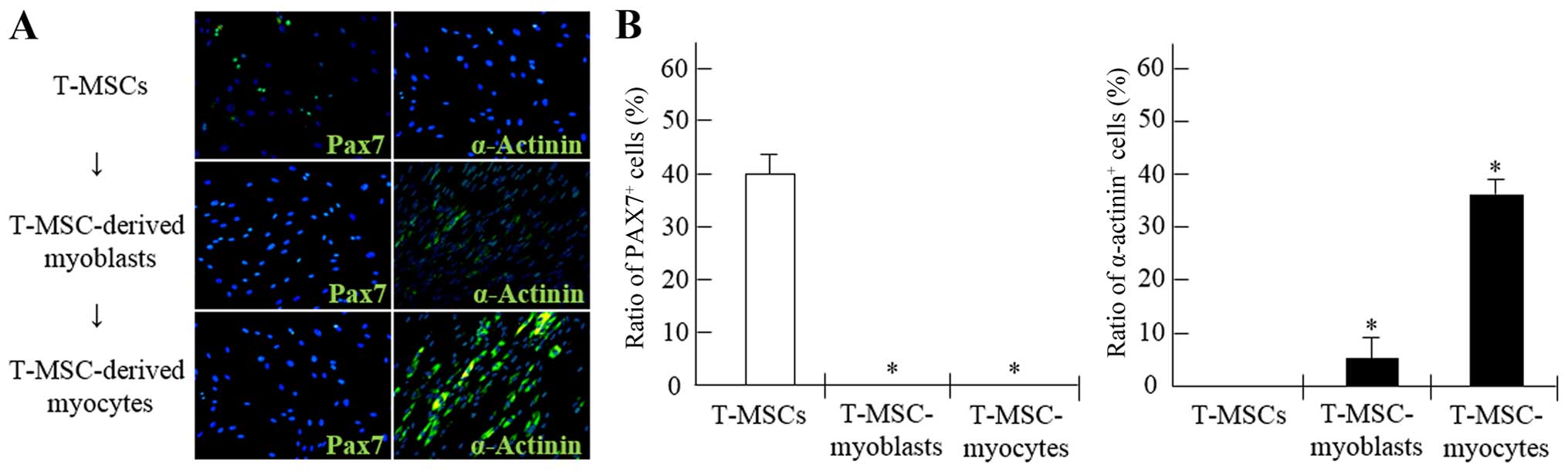
Quantitative analyses of 4 slides were performed at each step
during the process of myogenic differentiations, the Pax7 and
α-actinin positive cells were counted, respectively.
T-MSCs express muscle-related genes under
conditions that induce myogenic differentiation
We performed RT-PCR to determine whether T-MSCs are
capable of expressing myogenic markers and of undergoing myogenic
differentiation in vitro. Myogenesis is accompanied by the
induction of myogenic markers, including myogenin and dystrophin,
at the expense of pluripotency-coupled genes (31–33), suggesting that the T-MSCs have
differentiated into myogenic cells. In particular, we found that
Pax7 and Myf6, which play a role in myogenesis
through the regulation of muscle precursor cell proliferation
(34), were already expressed in
the undifferentiated T-MSCs (Fig.
3A). These results show that T-MSCs possess characteristics of
myogenic precursor cells. The mRNA expression levels of pluripotent
markers, namely Klf4, Rex1, and activin were
upregulated in the undifferentiated T-MSCs and myoblasts, which
have a greater ability to proliferate than myogenic cells. Western
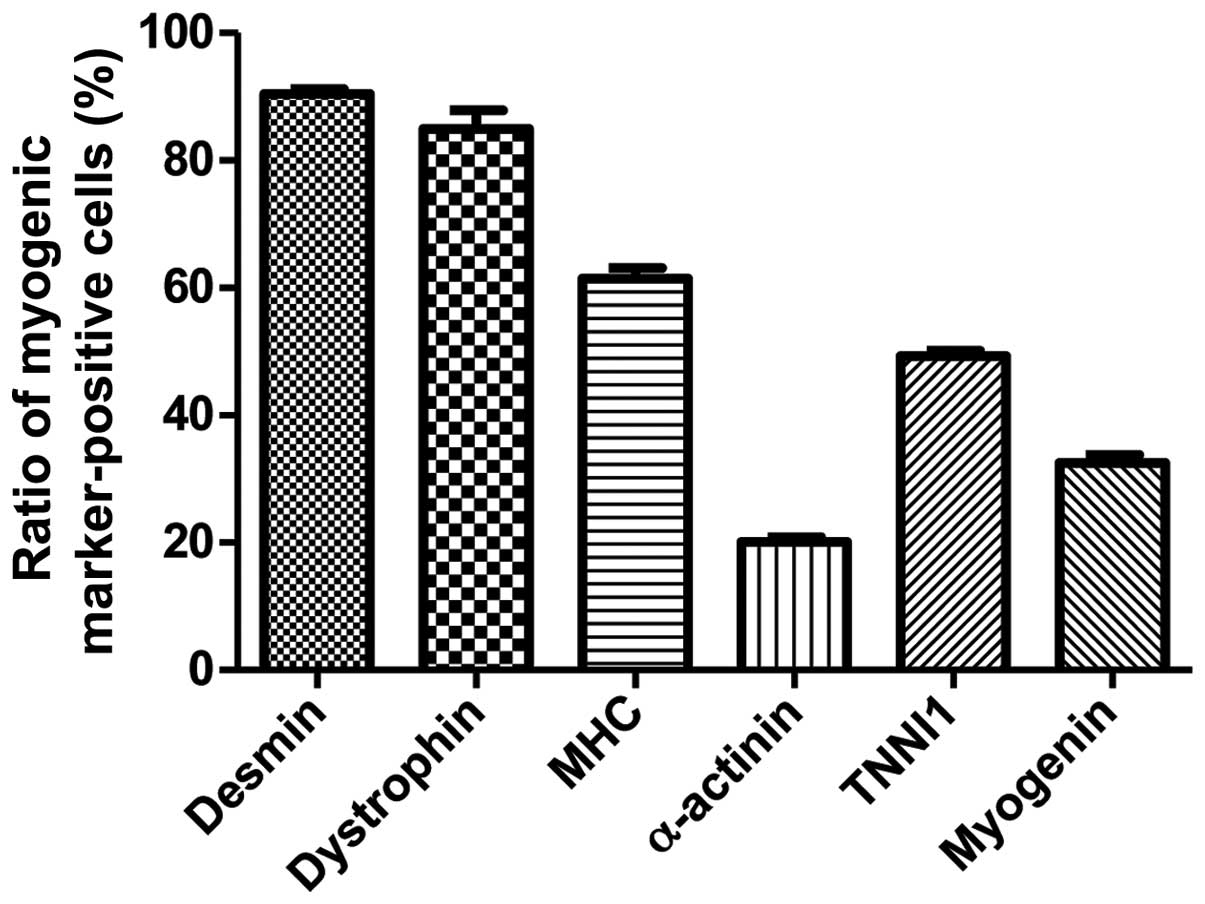
blot analysis revealed higher expression levels of desmin and α-SMA
at the differentiation stage (Fig.
3B; lanes b and c). The expression of α-actinin was similar at
all stages of differentiation (Fig.
3B). The expression of the skeletal myogenic markers, TNNI1 and
myogenin, was similar in the myoblasts and the myocytes (Fig. 3B; lanes b and c). Consistent with
these results, immunostaining of the multinucleated structures
revealed the presence of desmin, α-actinin, TNNI1 and myogenin
(Fig. 4). These results indicate
that T-MSCs possess the characteristics of myogenic precursor cells
and also possess a high capacity to undergo myogenic
differentiation.
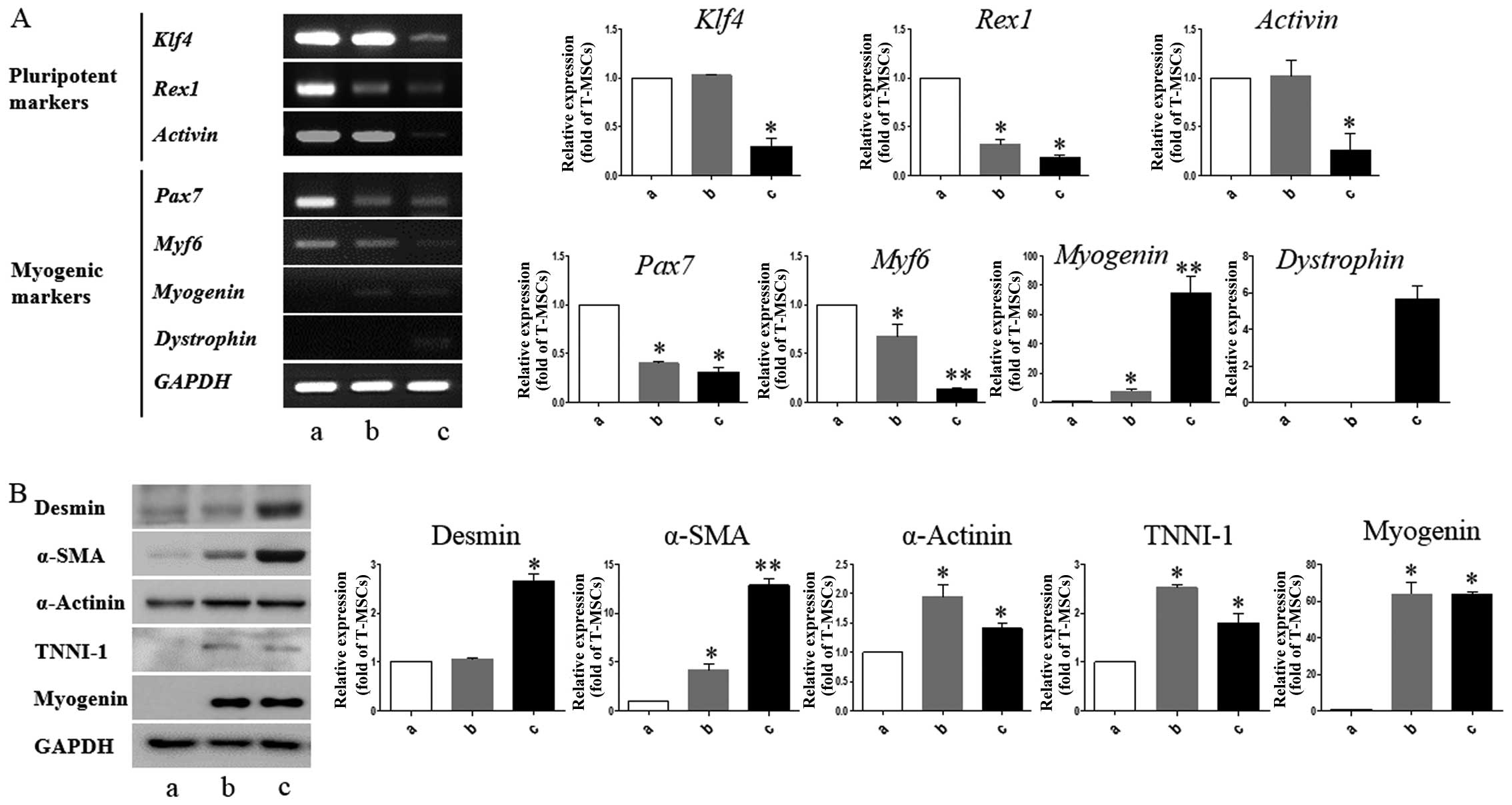 | Figure 3Detection of myogenic markers in
tonsil-derived mesenchymal stem cell (T-MSC)-derived myogenic
cells. (A) Determination of the mRNA expression levels of
pluripotent and myogenic markers. mRNA was isolated from (lane a)
undifferentiated T-MSCs, (lane b) T-MSC-derived myoblasts cultured
in replating medium as rosette-like spread spheres, and (lane c)
terminally differentiated T-MSC-derived myocytes and the cells were
examined by RT-PCR. (B) Protein expression levels of myogenic
markers in T-MSCs during the process of myogenic induction (lane a,
T-MSCs; lane b, T-MSC-derived myoblasts; lane c, T-MSC-derived
myocytes). The levels of GAPDH were measured as a loading control.
Band intensities were quantified using ImageJ software. Data are
the means ± SEM of experiments performed in triplicate.
*P<0.05; **P<0.01. Klf4, Krüppel-like
factor 4; Pax7, paired box 7; Myf6, myogenic factor 6; α-SMA,
α-smooth muscle actin; TNNI1, troponin I type 1; GAPDH,
glyceraldehyde 3-phosphate dehydrogenase. |
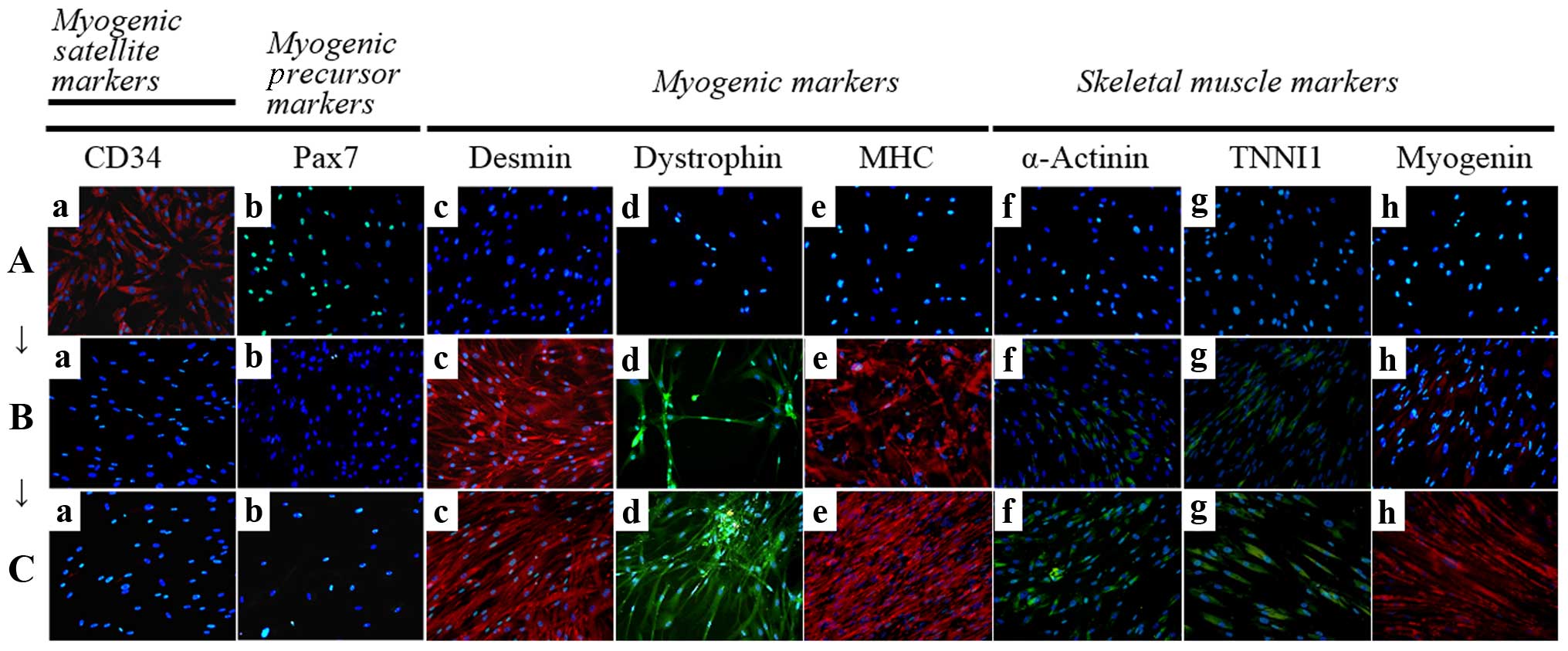 | Figure 4Immunocytochemistry for the detection
of myogenic markers. The expression of muscle-related proteins in
the tonsil-derived mesenchymal stem cells (T-MSCs) during the
process of myogenic induction (T-MSCs; T-MSC-derived myoblasts;
T-MSC-derived myocytes) was evaluated by immunostaining using
antibodies against myogenic satellite cells (panel a, CD34; red),
precursors [panel b, paired box 7 (Pax7); green], differentiated
myogenic cells [panel c, desmin (red); panel d, dystrophin (green);
panel e, myosin heavy chain (MHC; red)], skeletal myogenic cells
[panel f, α-actinin (green); panel g, troponin I type 1 (TNNI1;
green); panel h, myogenin (red)]. The cells were counterstained
with DAPI (blue). The samples were analyzed under a fluorescence
microscope using appropriate filters. (A) Undifferentiated T-MSCs
expressed (panel a) CD34 and (panel b) Pax7, but no other markers
of myogenic cells. (B) Following differentiation into myoblasts,
80–90% of cells expressed (panel c) desmin, (panel d) dystrophin,
and (panel e) MHC and 20–50% of cells expressed skeletal muscle
(SKM) markers including (panel f) α-actinin, (panel g) TNNI1, and
(panel h) myogenin. (Panel a) CD34 and (panel b) Pax7 were not
expressed at any stage of the differentiation process from
T-MSC-derived myoblasts into T-MSC-derived myocytes. (C)
T-MSC-derived myocytes exhibited an increased expression of (panels
c to h) myogenic and SKM cell markers compared with T-MSC-derived
myoblasts and formed multinucleated myotubes. FITC, fluorescein
isothiocyanate; TRITC, tetramethylrhodamine isothiocyanate.
Original magnification, ×200. |
Detection of myogenic markers by
immunostaining in vitro
To determine the phenotypes of the cells within the
spheres and of the T-MSCs, and to measure the innate ability of the
MSCs to differentiate into SKM cells, we replated the spheres onto
coverslips in order to allow differentiation into myoblasts and
skeletal myogenic cells and sequentially cultured them in myogenic
differentiation medium (Fig. 2).
These cells were then fixed and labeled with antibodies against
markers of myogenic satellite cells (CD34), precursors (Pax7),
differentiated myogenic cells (desmin, dystrophin and MHC) and
skeletal myogenic cells (α-actinin, TNNI1 and myogenin). Although
CD34 (red) and Pax7 (green) were expressed in the undifferentiated
T-MSCs (Fig. 4A, panels a and b),
the markers of myogenic cells and skeletal myocytes were not. These
results indicate that T-MSCs possess pre-existing myogenic
characteristics. Following differentiation into myoblasts, 80–90%
of cells expressed desmin (red), dystrophin (green) and MHC (red)
(Fig. 4B, panels c to e and
Fig. 5) and 20–50% of cells
expressed SKM markers [(α-actinin (green), TNNI1 (green) and
myogenin (red)] (Fig. 4B, panels
f to h and Fig. 5). By contrast,
the markers of myogenic satellite and precursor cells, CD34 and
Pax7, were not expressed during the differentiation of
T-MSC-derived myoblasts to T-MSC-derived myocytes (Fig. 4B and C, panels a and b).
Furthermore, the T-MSC-derived myocytes exhibited an increased
expression of the myogenic and the SKM markers compared with the
T-MSC-derived myoblasts or the undifferentiated T-MSCs (Fig. 4A–C, panels c to h).
Alterations occurring within the myogenic
cell population during differentiation in vitro
To demonstrate the potential of T-MSCs for skeletal
myogenic differentiation, the number of Pax7+ and
α-actinin+ cells was counted at each stage of the
differentiation process (Fig. 6).
The proportion of Pax7+ cells among the myogenic
satellite and precursor cells was 39.9% prior to differentiation,
whereas the proportion of Pax7+ cells among the
T-MSC-derived myoblasts and myocytes was <1% (Fig. 6A and B). Moreover, the percentages
of cells containing the SKM structural protein,
α-actinin+, were 5.2 and 34.5% in the myoblasts and
myocytes, respectively. However, the proportion of
α-actinin+ cells in the undifferentiated T-MSCs was
<1% (Fig. 6A and B).
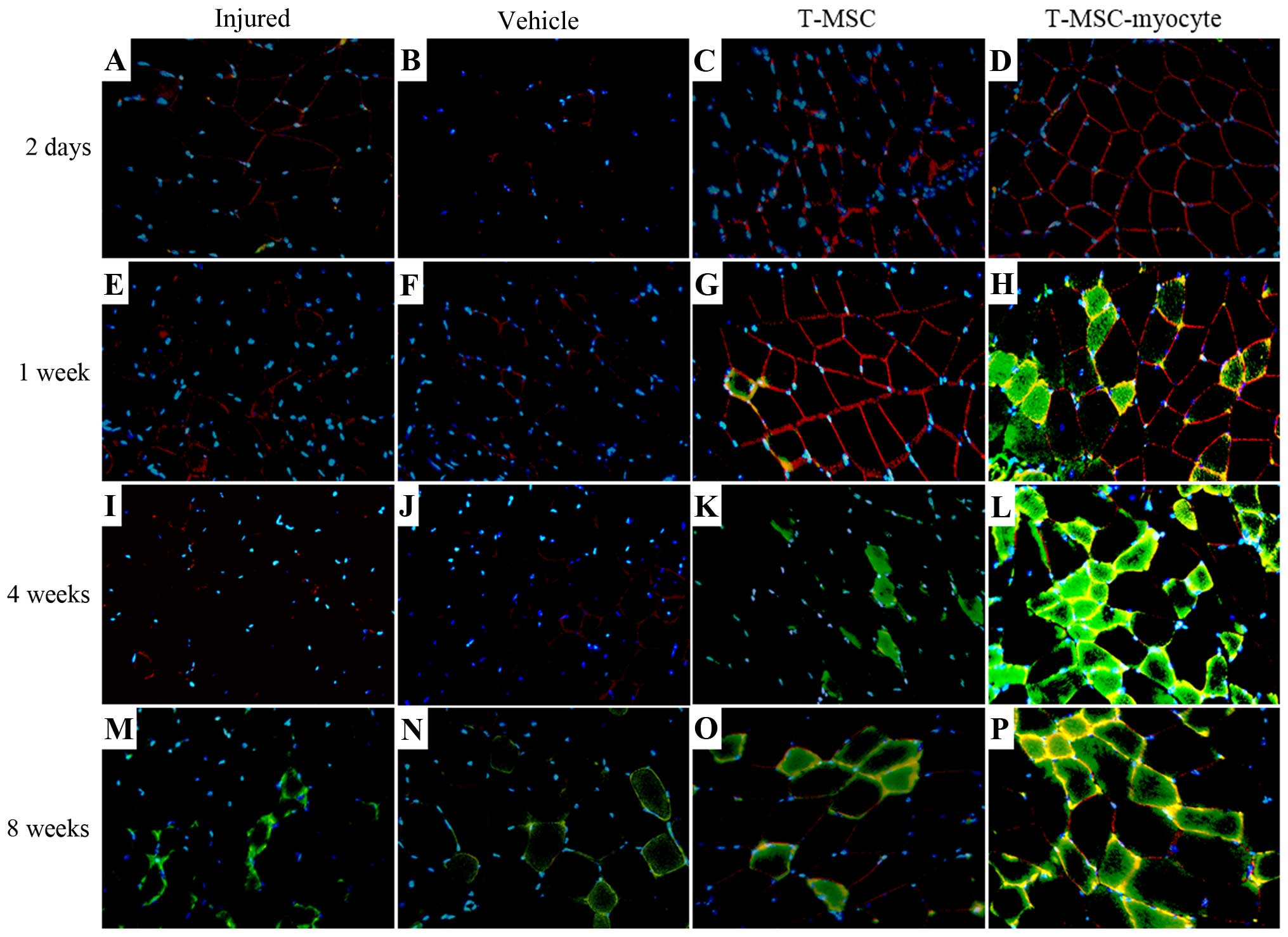
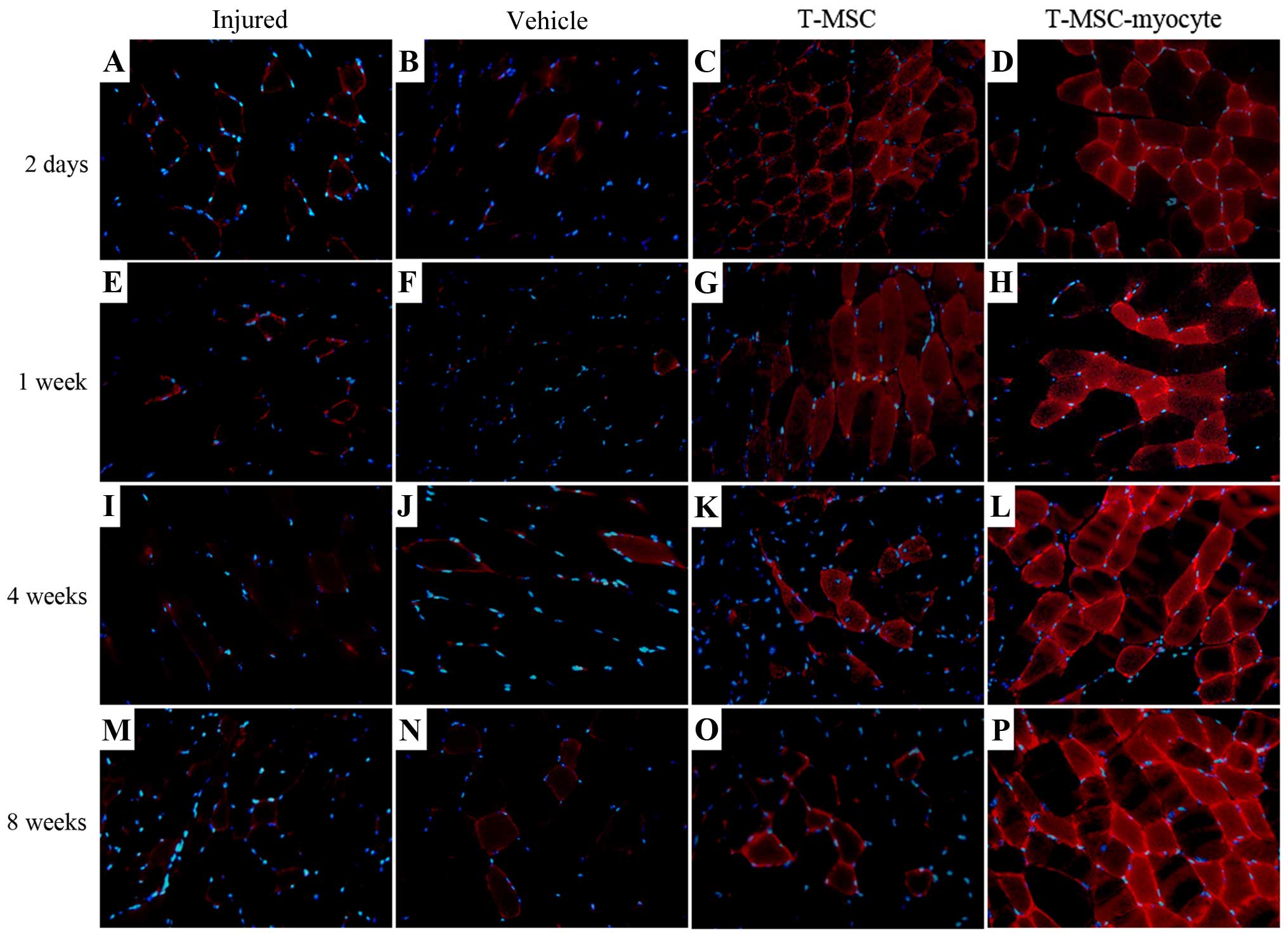
T-MSCs participate in SKM regeneration in
vivo
We analyzed the effects of T-MSC engraftment in
order to assess the regeneration of injured muscle in mice 2 days,
1 week, 4 and 8 weeks post-transplantation. The muscles of the
injured mice and the PBS-injected (vehicle-treated) control mice
exhibited no expression of α-SMA (green) at 2 days to 4 weeks
(Fig. 7A, B, E, F, I and J);
however, α-SMA was expressed at a low level at 8 weeks (Fig. 7M and N) post-transplantation. A
high expression of α-SMA was observed at 1–8 weeks in the muscles
of mice injected with the T-MSC-derived myocytes (Fig. 7H, L and P), whereas the expression
of α-SMA in the T-MSC-injected muscles was low at 4–8 weeks
(Fig. 7K and O)
post-transplantation. There was a minimal expression of dystrophin
(red) in the muscles of the injured mice and the vehicle-treated
mice (Fig. 7A, B, E, F, I, J, M and
N), whereas dystrophin expression was detected as early as 2
days post-transplantation in the muscles of mice injected with
T-MSCs and T-MSC-derived myocytes (Fig. 7C, D, G, H, L, O and P). Dystrophin
is a protein located between the sarcolemma that supports muscle
fiber strength, and the absence of dystrophin reduces muscle
stiffness (35). These results
demonstrated that the T-MSC-derived myocytes enhanced the
generation of newly formed myofibers, which expressed α-SMA and
dystrophin from 1 week post-transplantation. In addition, the
expression of TNNI1 (red), the human SKM troponin gene, was
increased post-transplantation concomitantly with the regeneration
process (Fig. 8C, D, G, H, K, L, O
and P). Particularly, more intense staining for TNNI1 was
observed post-transplantation with the T-MSC-derived myocytes than
with the T-MSCs (Fig. 8D, H, L and
P). Similarly to α-SMA and dystrophin, TNNI1 was minimally
expressed in the muscles of the injured and the vehicle-treated
mice (Fig. 8A, B, E, F, I, J, M and
N).
Attenuation of motor deficits following
the transplantation of T-MSC-derived myocytes in mice with a
partial myectomy of the gastrocnemius muscle
To determine whether the engraftment of
T-MSC-derived myoblasts or myocytes ameliorates muscle function
following injury, we measured the stride length of mice subsequent
to a partial myectomy of the right gastrocnemius muscle and
T-MSC-derived myogenic cell engraftment. Gait assessment was
carried out by footprint analysis, as described in the Materials
and methods at 1, 2, 3, 4 and 8 weeks post-transplantation
(Fig. 9). The stride distances
were markedly reduced in the injured and PBS-injected
(vehicle-treated) mice at 1 week after the myectomy. The stride
lengths of the mice injected with the T-MSC-derived myocytes were
significantly increased at 2, 3, 4 and 8 weeks post-transplantation
(P<0.01–0.05). No such improvement in stride distance was
observed in the injured/vehicle-treated animals. These results
indicated that the engraftment of T-MSC-derived myogenic cells
improved the functional ability of the mice with a partial myectomy
of the right gastrocnemius muscle.
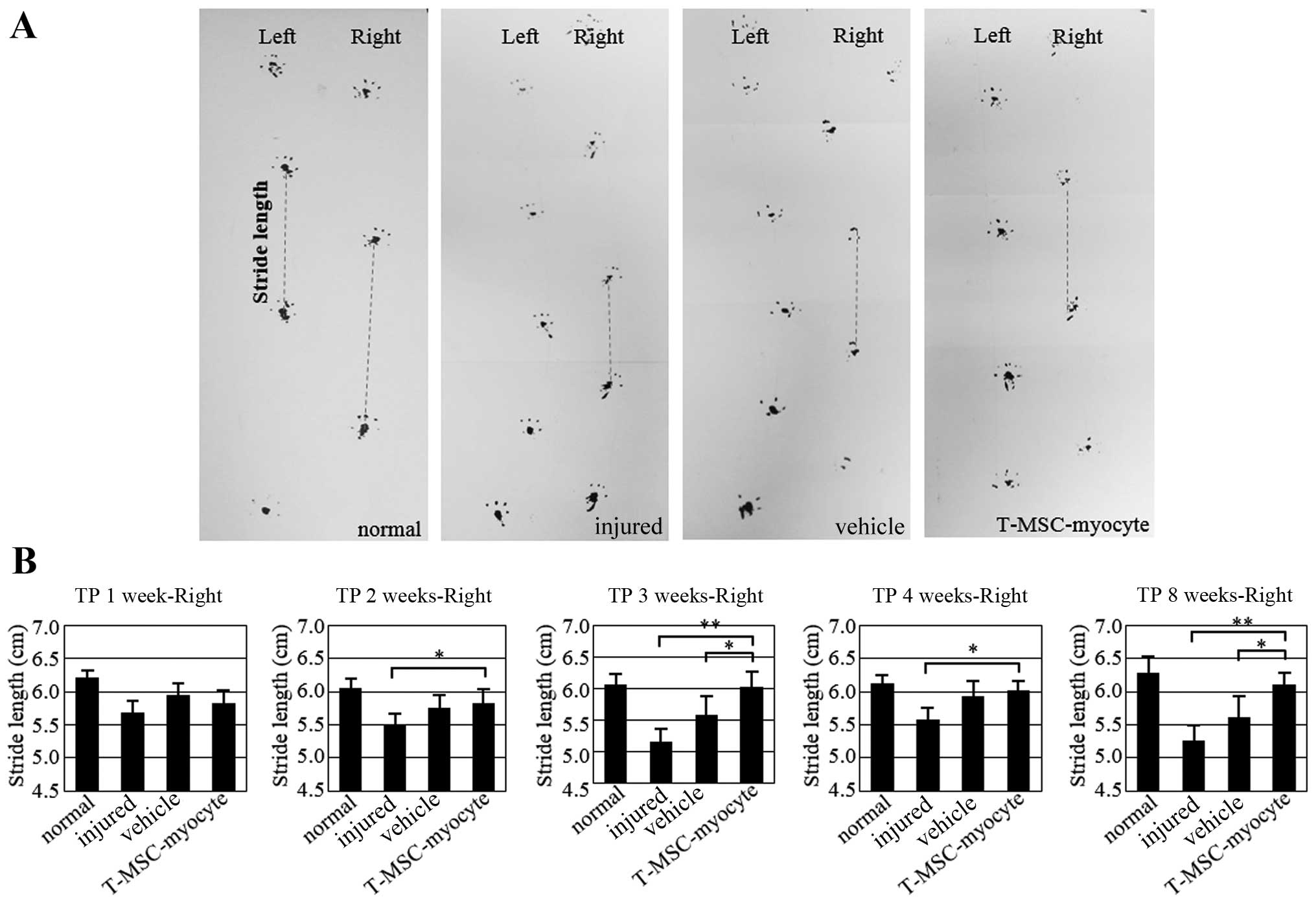 | Figure 9Assessment of gait by footprint
analysis. (A) Parameters measured in footprint analysis are shown,
with dotted lines representing the direction of progression.
Footprinting of normal (n=8) and injured (n=8), sham (n=8), and
myocyte-injected mice (n=8) evaluated at 1, 2, 3, 4 and 8 weeks
post-transplantation for the measurement of stride length (cm). (B)
Histograms represent differences in stride length among the normal,
injured and vehicle-treated mice as well as mice injected with
T-MSC-derived myocytes. Graphs represent the average of multiple
tests from three independent experiments, *P<0.05,
**P<0.01. TP, transplantation. |
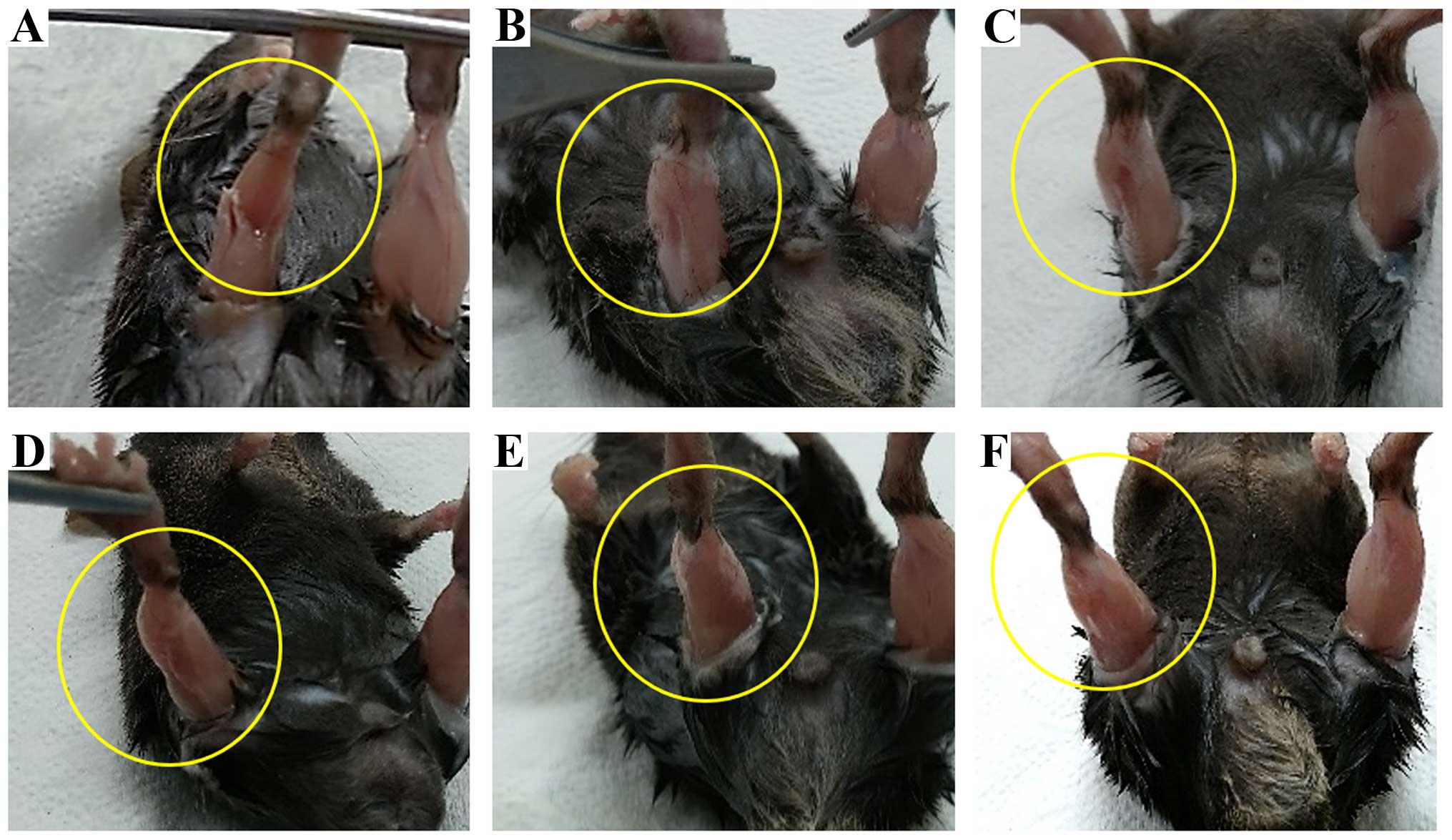
Restoration of SKM by engraftment of the
T-MSC-derived myogenic cells
We analyzed the effects of T-MSC engraftment in mice
to determine whether it elicited the morphological regeneration of
the injured right muscle at 10 weeks post-transplantation. The
muscles from the injured and the vehicle-treated mice (Fig. 10B and C) exhibited signs of
injury at 10 weeks, whereas the damage at 1 week was severe in the
injured mice (Fig. 10A). By
contrast, no sign of injury was apparent in the engrafted mice
(Fig. 10D–F) at 10 weeks
post-transplantation. Uniquely, the shape of the gastrocnemius
muscle mass was restored in the T-MSC-injected mice (Fig. 10D); the stride length of the
T-MSC-injected mice was not measured due to no enhancement of
regeneration in the immunostaining. These changes in the shape of
the gastrocnemius muscle confirmed the results of immunostaining
and the gait assessment analysis, demonstrating that the
T-MSC-derived myogenic cells promoted the regeneration of SKM.
Discussion
Adult stem cells derived from older individuals have
a reduced ability to proliferate, migrate and secrete cytokines or
growth factors than stem cells derived from young individuals
(18,36). Therefore, there is a need to
obtain cells from alternative sources, such as the tonsils which
are removed from younger individuals and are subsequently
discarded. Recent studies have also demonstrated that T-MSCs not
only readily differentiate into mesodermal tissue cells, including
adipocytes, osteocytes and chondrocytes (17,21), but also into hepatocytes (20), endothelial cells (24) and dermal fibroblasts (37). T-MSCs may differentiate into cells
of all 3 germ layers, namely the endoderm, mesoderm and ectoderm.
In this study, we demonstrated that undifferentiated T-MSCs possess
pre-existing characteristics of myosatellite cells and a high
capacity to differentiate into skeletal myocytes. For these
reasons, we suggest that T-MSCs are a more suitable source of cells
for myogenesis and the regeneration of SKM than other types of
MSCs.
TGF-β profoundly influences the differentiation of
many cell types of mesenchymal origin, including preadipocytes
(38,39), osteoblasts (40) and myoblasts (41). In previous studies on cultured
myoblasts, TGF-β was shown to inhibit the expression of
muscle-specific genes, as well as myotube formation without
affecting cell proliferation (42,43). Due to these findings, in our
study, 1 ng/ml TGF-β was only added to the medium in order to
induce the differentiation of the cells into myoblasts (Fig. 2C). During vertebrate
embryogenesis, mesodermal progenitors give rise to distinct
mesenchymal lineages, including skeletal myocytes, osteocytes,
chondrocytes and adipocytes. The commitment and subsequent
differentiation of an MSC toward a particular lineage is regulated
by the coordinated action of several extracellular signals, some of
which, for example IGF1, are shared by adipocytes and myocytes and
may promote the production of one or the other cell type (44). Insulin may also bind to the IGF1
receptor and IGF1 may bind to the insulin receptor; furthermore,
IGF1/insulin hybrid receptors are present in SKM. However, insulin
is particularly important in glucose homeostasis, whereas IGF1 is
principally involved in muscle growth (45). The systemic administration of IGF1
results in increased muscle protein content and reduced protein
degradation (46). The
stimulatory effect of IGF1 on the proliferation of myofibroblasts
and the deposition of extracellular matrix may interfere with the
ability of this growth factor, even at high concentrations, to
promote muscle healing following injury (47). In this study, 10 ng/ml IGF1 was
used in the myogenic induction medium in order to induce terminal
myogenic differentiation (Fig.
2D).
To induce the myogenic differentiation of T-MSCs,
two steps of differentiation procedures, differentiation into
myoblasts and myocytes, were used with different media,
respectively. The expression of myogenic markers, such as myogenin,
dystrophin, α-actinin, and TNNI1 indicated that a substantial
proportion of the T-MSCs in the culture were committed to the
myogenic differentiation pathway. The T-MSC-derived myoblasts fused
to form partial myotubes in vitro. As the T-MSC derivatives
were shown to express myogenic markers both before (as myoblasts)
and after their incorporation into myotubes, we assume that the
inductive signals exist both outside and inside myotubes. In
addition, when these cells were transplanted, they supported a
complete process of myogenesis, allowing the regeneration of
myofibers. Following transplantation, only a small percentage of
T-MSC derivatives expressed human-specific markers such as human
nuclei (HN; data not shown). The reason for the lack of HN
expression in the majority of T-MSC derivatives remains
unclear.
To examine the process of muscle regeneration in a
controlled and reproducible way, it was necessary to develop an
experimental model of muscle injury (26). A number of studies using various
experimental models of injury, including the injection of myotoxic
agents (9), crush injury
(48), ischemia (12), denervation (49) and muscular dystrophy (13), have demonstrated the unique
ability of SKM to regenerate, irrespective of the precise method
used to induce the initial injury (50). In this study, we performed a
surgical myectomy to establish a novel mouse model of SKM injury in
order to mimic a severe loss of muscle mass.
As evidenced by this study, the T-MSC-derived
myogenic cells were capable of restoring injured SKM tissue and
regenerating the muscle. The defect was either repaired or a
gradual remodeling occurred, such that the original defect area was
difficult to define by 10 weeks post-transplantation. However,
repair of the injury was apparent in the mice transplanted with
T-MSC-derived cells (Fig. 10D–F)
at 10 weeks post-transplantation. In another study using
MyoD-transduced human amniotic fluid stem cells which were
transplanted into the injured tibialis anterior muscle of mice, the
muscle tissue area was found to be larger than that of injured
untreated mice or or PBS-injected control mice (14). Furthermore, Merritt et al
(51) demonstrated the ability of
a muscle extracellular matrix to support muscle and blood vessel
regeneration; however, but full recovery of function does not occur
after 42 days (51). In our
study, we demonstrated that the transplantation of T-MSC-derived
myogenic cells promoted the regeneration of the injured SKM.
However, further studies are warranted to determine whether
functional recovery also occurs and the number and size of the
myofibers also needs to be measured to fully determine the
functional outcome.
A number of studies have exploited ES/iPS cells and
muscle stem cell therapy in order to promote SKM regeneration
(4,6,7,9,10).
Although ES/iPS cells have potential, they are associated with
inherent limitations, including histocompatibility and ethical
concerns. Moreover, although muscle stem cells may be isolated from
adult and prenatal tissue, the number of cells that may be
harvested is limited. Therefore, adult stem cells, such as MSCs are
a suitable source of cells for stem cell therapy, and may be used
to promote the regeneration of damaged SKM. In a number of studies,
stem cells derived from bone marrow, adipose tissue, the placenta,
amniotic fluid, umbilical cord blood and urine have shown potential
to promote SKM regeneration (1,11–14,18). However, the degree of regeneration
obtained when using these cells may be insufficient when the injury
or damage is severe (1,11–14,18). T-MSCs may therefore be an
excellent choice due to their availability and their myogenic
differentiation capacity. Our data demonstrated that T-MSCs have a
remarkable capacity for efficient SKM regeneration at 8 weeks
post-transplantation, as demonstrated by a gait assessment
test.
In conclusion, in this study, we demonstrated that
myoblasts may be derived from human T-MSCs and can differentiate
into myocytes in vitro. This myogenic differentiation was
accompanied by the ability to induce an improvement in stride
length of mice with injured SKM, as shown by a gait assessment
test. T-MSCs have myogenic potential and may thus promote muscle
regeneration through either direct de novo muscle
differentiation or by a paracrine mechanism. The functional
improvements afforded by the T-MSC-derived myogenic cells are
potentially useful in the treatment of human SKM injuries and for
damage caused by other degenerative disorders, including congenital
defects, trauma, or tumor removal.
Acknowledgments
This study was supported by grant no. HI12C0135 from
the Korean Health Technology R&D Project, Ministry of Health
and Welfare, Republic of Korea and RP-Grant 2014 from Ewha Womans
University.
References
|
1
|
Chen W, Xie M, Yang B, Bharadwaj S, Song
L, Liu G, Yi S, Ye G, Atala A and Zhang Y: Skeletal myogenic
differentiation of human urine-derived cells as a potential source
for skeletal muscle regeneration. J Tissue Eng Regen Med. Jun
19–2014.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Desai VD, Hsia HC and Schwarzbauer JE:
Reversible modulation of myofibroblast differentiation in
adipose-derived mesenchymal stem cells. PLoS One. 9:e868652014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gunetti M, Tomasi S, Giammò A, Boido M,
Rustichelli D, Mareschi K, Errichiello E, Parola M, Ferrero I,
Fagioli F, et al: Myogenic potential of whole bone marrow
mesenchymal stem cells in vitro and in vivo for usage in urinary
incontinence. PLoS One. 7:e455382012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hosoyama T, McGivern JV, Van Dyke JM,
Ebert AD and Suzuki M: Derivation of myogenic progenitors directly
from human pluripotent stem cells using a sphere-based culture.
Stem Cells Transl Med. 3:564–574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pereira T, Gärtner A, Amorim I,
Armada-da-Silva P, Gomes R, Pereira C, França ML, Morais DM,
Rodrigues MA, Lopes MA, et al: Biomaterials and stem cell therapies
for injuries associated to skeletal muscular tissues. Advances in
Biomaterials Science and Biomedical Applications. Pignatello R:
InTech; Rijeka: pp. 329–355. 2013
|
|
6
|
Kang JS and Krauss RS: Muscle stem cells
in developmental and regenerative myogenesis. Curr Opin Clin Nutr
Metab Care. 13:243–248. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Motohashi N, Asakura Y and Asakura A:
Isolation, culture, and transplantation of muscle satellite cells.
J Vis Exp. 86:e508462014.
|
|
8
|
Shi X and Garry DJ: Muscle stem cells in
development, regeneration, and disease. Genes Dev. 20:1692–1708.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Darabi R, Gehlbach K, Bachoo RM, Kamath S,
Osawa M, Kamm KE, Kyba M and Perlingeiro RC: Functional skeletal
muscle regeneration from differentiating embryonic stem cells. Nat
Med. 14:134–143. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mizuno Y, Chang H, Umeda K, Niwa A, Iwasa
T, Awaya T, Fukada S, Yamamoto H, Yamanaka S, Nakahata T and Heike
T: Generation of skeletal muscle stem/progenitor cells from murine
induced pluripotent stem cells. FASEB J. 24:2245–2253. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dezawa M, Ishikawa H, Itokazu Y, Yoshihara
T, Hoshino M, Takeda S, Ide C and Nabeshima Y: Bone marrow stromal
cells generate muscle cells and repair muscle degeneration.
Science. 309:314–317. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Di Rocco G, Iachininoto MG, Tritarelli A,
Straino S, Zacheo A, Germani A, Crea F and Capogrossi MC: Myogenic
potential of adipose-tissue-derived cells. J Cell Sci.
119:2945–2952. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nunes VA, Cavaçana N, Canovas M, Strauss
BE and Zatz M: Stem cells from umbilical cord blood differentiate
into myotubes and express dystrophin in vitro only after exposure
to in vivo muscle environment. Biol Cell. 99:185–196. 2007.
View Article : Google Scholar
|
|
14
|
Kim JA, Shon YH, Lim JO, Yoo JJ, Shin HI
and Park EK: MYOD mediates skeletal myogenic differentiation of
human amniotic fluid stem cells and regeneration of muscle injury.
Stem Cell Res Ther. 4:1472013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park S, Kim E, Koh SE, Maeng S, Lee WD,
Lim J, Shim I and Lee YJ: Dopaminergic differentiation of neural
progenitors derived from placental mesenchymal stem cells in the
brains of Parkinson's disease model rats and alleviation of
asymmetric rotational behavior. Brain Res. 1466:158–166. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kerkis I, Kerkis A, Dozortsev D,
Stukart-Parsons GC, Gomes Massironi SM, Pereira LV, Caplan AI and
Cerruti HF: Isolation and characterization of a population of
immature dental pulp stem cells expressing OCT-4 and other
embryonic stem cell markers. Cells Tissues Organs. 184:105–116.
2006. View Article : Google Scholar
|
|
17
|
Ryu KH, Cho KA, Park HS, Kim JY, Woo SY,
Jo I, Choi YH, Park YM, Jung SC, Chung SM, et al: Tonsil-derived
mesenchymal stromal cells: evaluation of biologic, immunologic and
genetic factors for successful banking. Cytotherapy. 14:1193–1202.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ting CH, Ho PJ and Yen BL: Age-related
decreases of serum-response factor levels in human mesenchymal stem
cells are involved in skeletal muscle differentiation and
engraftment capacity. Stem Cells Dev. 23:1206–1216. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Djouad F, Jackson WM, Bobick BE, Janjanin
S, Song Y, Huang GT and Tuan RS: Activin A expression regulates
multipotency of mesenchymal progenitor cells. Stem Cell Res Ther.
1:112010. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park M, Kim YH, Woo SY, Lee HJ, Yu Y, Kim
HS, Park YS, Jo I, Park JW, Jung SC, et al: Tonsil-derived
mesenchymal stem cells ameliorate CCI4-induced liver fibrosis in
mice via autophagy activation. Sci Rep. 5:86162015. View Article : Google Scholar
|
|
21
|
Yu Y, Park YS, Kim HS, Kim HY, Jin YM,
Jung SC, Ryu KH and Jo I: Characterization of long-term in vitro
culture-related alterations of human tonsil-derived mesenchymal
stem cells: role for CCN1 in replicative senescence-associated
increase in osteogenic differentiation. J Anat. 225:510–518. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park M, Kim YH, Ryu JH, Woo SY and Ryu KH:
Immune suppressive effects of tonsil-derived mesenchymal stem cells
on mouse bone-marrow-derived dendritic cells. Stem Cells Int.
2015:1065402015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ryu KH, Kim SY, Kim YR, Woo SY, Sung SH,
Kim HS, Jung SC, Jo I and Park JW: Tonsil-derived mesenchymal stem
cells alleviate concanavalin A-induced acute liver injury. Exp Cell
Res. 326:143–154. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park YS, Hwang S, Jin YM, Yu Y, Jung SA,
Jung SC, Ryu KH, Kim HS and Jo I: CCN1 secreted by tonsil-derived
mesenchymal stem cells promotes endothelial cell angiogenesis via
integrin αvβ3 and AMPK. J Cell Physiol.
230:140–149. 2015. View Article : Google Scholar
|
|
25
|
Mauro A: Satellite cell of skeletal muscle
fibers. J Biophys Biochem Cytol. 9:493–495. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chargé SBP and Rudnicki MA: Cellular and
molecular regulation of muscle regeneration. Physiol Rev.
84:209–238. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guide for the Care and Use of Laboratory
Animals. 8th edition. National Research Council. The National
Academies Press; Washington, DC: 2011
|
|
28
|
Cho KA, Kim JY, Kim HS, Ryu KH and Woo SY:
Tonsil-derived mesenchymal progenitor cells acquire a follicular
dendritic cell phenotype under cytokine stimulation. Cytokine.
59:211–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
D'Hooge R, Hartmann D, Manil J, Colin F,
Gieselmann V and De Deyn PP: Neuromotor alterations and cerebellar
deficits in aged arylsulfatase A-deficient transgenic mice.
Neurosci Lett. 273:93–96. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fernagut PO, Diguet E, Labattu B and Tison
F: A simple method to measure stride length as an index of
nigrostriatal dysfunction in mice. J Neurosci Methods. 113:123–130.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Beattie GM, Lopez AD, Bucay N, Hinton A,
Firpo MT, King CC and Hayek A: Activin A maintains pluripotency of
human embryonic stem cells in the absence of feeder layers. Stem
Cells. 23:489–495. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Carpenter MK, Rosler ES, Fisk GJ,
Brandenberger R, Ares X, Miura T, Lucero M and Rao MS: Properties
of four human embryonic stem cell lines maintained in a feeder-free
culture system. Dev Dyn. 229:243–258. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nakatake Y, Fukui N, Iwamatsu Y, Masui S,
Takahashi K, Yagi R, Yagi K, Miyazaki J, Matoba R, Ko MS and Niwa
H: Klf4 cooperates with Oct3/4 and Sox2 to activate the Lefty1 core
promoter in embryonic stem cells. Mol Cell Biol. 26:7772–7782.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ropka-Molik K, Eckert R and Piórkowska K:
The expression pattern of myogenic regulatory factors MyoD, Myf6
and Pax7 in postnatal porcine skeletal muscles. Gene Expr Patterns.
11:79–83. 2011. View Article : Google Scholar
|
|
35
|
García-Pelagio KP, Bloch RJ, Ortega A and
González-Serratos H: Biomechanics of the sarcolemma and costameres
in single skeletal muscle fibers from normal and dystrophin-null
mice. J Muscle Res Cell Motil. 31:323–336. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hass R, Kasper C, Böhm S and Jacobs R:
Different populations and sources of human mesenchymal stem cells
(MSC): a comparison of adult and neonatal tissue-derived MSC. Cell
Commun Signal. 9:122011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Böttcher-Haberzeth S, Biedermann T, Klar
AS, Pontiggia L, Rac J, Nadal D, Schiestl C, Reichmann E and Meuli
M: Tissue engineering of skin: human tonsil-derived mesenchymal
cells can function as dermal fibroblasts. Pediatr Surg Int.
30:213–222. 2014. View Article : Google Scholar
|
|
38
|
Choy L, Skillington J and Derynck R: Roles
of autocrine TGF-beta receptor and Smad signaling in adipocyte
differentiation. J Cell Biol. 149:667–682. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ignotz RA and Massagué J: Type beta
transforming growth factor controls the adipogenic differentiation
of 3T3 fibroblasts. Proc Natl Acad Sci USA. 82:8530–8534. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Centrella M, Horowitz MC, Wozney JM and
McCarthy TL: Transforming growth factor-beta gene family members
and bone. Endocr Rev. 15:27–39. 1994.PubMed/NCBI
|
|
41
|
Olson EN: Proto-oncogenes in the
regulatory circuit for myogenesis. Semin Cell Biol. 3:127–136.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Massagué J, Cheifetz S, Endo T and
Nadal-Ginard B: Type beta transforming growth factor is an
inhibitor of myogenic differentiation. Proc Natl Acad Sci USA.
83:8206–8210. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Olson EN, Sternberg E, Hu JS, Spizz G and
Wilcox C: Regulation of myogenic differentiation by type beta
transforming growth factor. J Cell Biol. 103:1799–1805. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sordella R, Jiang W, Chen GC, Curto M and
Settleman J: Modulation of Rho GTPase signaling regulates a switch
between adipogenesis and myogenesis. Cell. 113:147–158. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Schiaffino S and Mammucari C: Regulation
of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights
from genetic models. Skelet Muscle. 1:42011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zdanowicz MM, Moyse J, Wingertzahn MA,
O'Connor M, Teichberg S and Slonim AE: Effect of insulin-like
growth factor I in murine muscular dystrophy. Endocrinology.
136:4880–4886. 1995.PubMed/NCBI
|
|
47
|
Jones JI and Clemmons DR: Insulin-like
growth factors and their binding proteins: biological actions.
Endocr Rev. 16:3–34. 1995.PubMed/NCBI
|
|
48
|
Bassaglia Y and Gautron J: Fast and slow
rat muscles degenerate and regenerate differently after whole crush
injury. J Muscle Res Cell Motil. 16:420–429. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dedkov EI, Kostrominova TY, Borisov AB and
Carlson BM: Reparative myogenesis in long-term denervated skeletal
muscles of adult rats results in a reduction of the satellite cell
population. Anat Rec. 263:139–154. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Politi PK, Havaki S, Manta P and Lyritis
G: Bupivacaine-induced regeneration of rat soleus muscle:
ultrastructural and immunohistochemical aspects. Ultrastruct
Pathol. 30:461–469. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Merritt EK, Hammers DW, Tierney M, Suggs
LJ, Walters TJ and Farrar RP: Functional assessment of skeletal
muscle regeneration utilizing homologous extracellular matrix as
scaffolding. Tissue Eng Part A. 16:1395–1405. 2010. View Article : Google Scholar
|