Introduction
The incidence of thyroid cancer has been increased
over the past few years (1–3).
Thyroid nodules are one of most prevalent thyroid diseases,
detectable by cervical echography in between 50 and 67% of healthy
individuals (4). A confirmation
study is required to verify the diagnosis as only 5% of these
thyroid nodules are malignant (4).
Usually, diagnosing thyroid nodules as benign or
malignant is performed by cytological evaluation (5). For this purpose, fine-needle
aspiration (FNA) is the most commonly used sample extraction
technique, since it is rapid, inexpensive and simple, as shown by
Knezević-Usaj et al (6).
Subsequent cytological analysis following FNA provides four
possible results: non-diagnostic, positive to malignancy or
suspicious, indeterminate and benign cytology (7). Indeterminate FNA cytological results
are obtained in between 15 to 30% of cases (8–11).
Moreover, only between 5 and 15% of cases are malignant,
particularly in those with indeterminate cytological results
(11). Consequently, patients
with FNA indeterminate cytological results undergo diagnostic
surgery, even though this has been proven to be unecessary in
>50% of cases where patients are later found to have benign
disease (5,12).
Microarray-based gene expression profiling studies
of thyroid nodules have proposed molecular markers (11,13,14). However, it has been demonstrated
in other types of cancer that some biomarkers identified in one
cohort may fail to reproduce similar results with a high degree of
accuracy in other cohorts (15,16). For example, the accuracy of the
Afirma® genomic test for FNA thyroid samples, which is
based on the expression of >170 genes and one of the most
extensively studied, has been confirmed by some studies (17–20); however, it has been seriously
questioned by more recent investigations in terms of its
sensitivity, cost-effectiveness, or its ability to complement tests
with a high specificity such as the BRAF mutation test (21–24).
Given that those patients with thyroid nodules of
indeterminate FNA cytology may undergo unnecessary surgical
intervention, and that previously proposed molecular biomarkers
cannot be used in these cases, or that the accuracy of a currently
available test has been questioned, a molecular, robust, biomarker
for FNA, that is simple, and cost-effective, is still required for
clinical investigations and practice. In contrast to other authors,
in this study, we propose a molecular biomarker designed
specifically from FNA indeterminate thyroid samples identified by a
bioinformatics approach, which has been validated in six datasets,
including four from other authors. We demonstrate that the accuracy
of the proposed biomarker is superior to other previously proposed
biomarkers for thyroid tumors. The proposed biomarker is composed
of 15 genes and has the potential to be easily implemented into
clinical practice using common and cost-effective
real-time-polymerase chain reaction (RT-PCR) assays.
Data collection methods
Datasets and processing
We used six gene expression micro-array datasets
from five different authors (Table
I), which we obtained from large microarray repositories. The
main inclusion criteria were that the number of samples was >40
and that the study contained histopathological diagnoses. To
compare the results from the different datasets and microarray
platforms, we transformed the gene expression data to a uniform
distribution between 0 and 1, where 0 represents the lowest and 1
the highest expression. Multiple probes assigned to the same gene
were averaged if they were correlated using a Pearson coefficient
of ≥0.7. The probe with the highest expression was used if
duplicate symbols remained. To facilitate future biomarker
measurements in clinical practice using RT-PCR, which may use an
internal control for normalization (25), we transformed the original
Alexander dataset of 173 genes (11), which represent the previously
identified Afirma® test, to a dataset of all
combinations of gene-by-gene expression differences. This generated
a dataset of 2,850 gene expression differences. In preliminary
experiments, we observed that differences allowed better prediction
than the raw expression measure (data not shown), which is
consistent with other observations, where pairs of genes are more
accurate predictors than separate genes, as shown by Grate
(26).
 | Table ICharacteristics of datasets used. |
Table I
Characteristics of datasets used.
| Authors/(Refs.)
dataset/(use) | ID/Platform | Sample
characteristics | No. of
benign/malignant samples | Diagnosis |
|---|
| Alexander et
al (11) indeterminate
(training set) | GSE34289 | 265 Indeterminate
FNA: | 180/85 | FNA cytology |
| Affymetrix | 180 Benign after
surgery (B) | | |
| Afirma-T (custom)
173 probes | 85 Malignant after
surgery | | |
| Giordano et
al (13) (test set) | GSE27155 | 89
Adenomas/carcinomas: | 17/72 | Surgical
pathology |
| Affymetrix | 10 Follicular
adenomas (B) | | |
| HG_U133A 22,283
probes | 7 Oncocytic
adenomas (B) | | |
| 13 Follicular
carcinomas | | |
| 8 Oncocytic
carcinomas | | |
| 51 Papillary
carcinomas | | |
| Borup et al
(14) (test set) | E-MEXP-2442 | 69
Adenomas/carcinomas: | 45/24 | Surgical
pathology |
| Affymetrix | 22 Follicular
adenomas (B) | | |
| HG U133 Plus 2.0
54,613 probes | 12 Microfollicular
adenomas (B) | | |
| 9 Nodular goiters
(B) | | |
| 18 Follicular
carcinomas | | |
| 4 Anaplastic
carcinomas | | |
| 2 Papillary
carcinomas | | |
| 2 Normal (B) | | |
| Alexander et
al (11) determinate (test
set) | GSE34289 | 99 Determinate
FNA: | 44/55 | FNA |
| Affymetrix | 44 Benign after
surgery (B) | | cytology |
| Afirma-T (custom)
173 probes | 55 Malignant after
surgery | | |
| TCGA (test set)
(https://tcga-data.nci.nih.gov/tcga/) | Illumina
Hi-Seq | 547 Thyroid
samples: | 57/490 | Surgical
pathology |
| RNA-Seq | 490 Papillary
cancers | | |
| 20,500 probes | 57 Benign tissues
(B) | | |
| Tomás et al
(51) | GSE33630 | 105 Thyroid
tumor/non-tumor: | 45/60 | International |
| Dom et al
(52) (test set) | Affymetrix | 11 Anaplastic
carcinomas | | Pathology Panel of
the Chernobyl Tissue Bank |
| HG_U133 Plus 2.0
54,675 probes | 49 Papillary
carcinomas
45 Patient-matched non-tumor controls (B) | | |
Biomarker identification
To the best of our knowledge, the Alexander dataset
is the only data providing details of cytologically indeterminate
thyroid samples [Alexander et al (11)]; therefore, we used this 'training'
dataset as a gold standard in order to identify the biomarker. To
discover combinations of gene differences that together yield the
optimal classification of malignant and non-malignant samples, we
used GALGO, a genetic algorithm for feature selection (27). GALGO is a feature selection
approach based on genetic algorithms coupled with a classifier.
Briefly, GALGO first generates a population of random combinations
of features. Each combination of this population is evaluated using
the accuracy of a classifier and the selected features. The genetic
algorithm then selects those combinations with higher accuracy,
which are subsequently re-combined and changed replacing a gene
difference with another. The process is repeated until a predefined
number of cycles yields a highly accurate feature combination.
Since this process is stochastic, the specific features may change;
thus, GALGO typically performs this procedure multiple times.
Subsequently, a representative feature combination is selected
based on the number of times each feature is present in the highly
accurate combinations and a forward selection procedure. In this
study, as proposed by GALGO tutorials (http://bioinformatica.mty.itesm.mx/GALGO), we used 300
combinations having five features to select the representative
biomarker. For the classes, we used benign and malignant cytology
as the sample class. For classification, we used the nearest
centroid (NC) method shown in the study by Dabney (28). The NC method is based on centers
per gene per class estimated as the mean of the gene expression
values from the samples of the same class. The samples were
classified as the class with the minimum Euclidean distance. The
GALGO tutorial has further details of the genetic algorithm and the
NC classifier (27). This
procedure was performed using the subset of the Alexander dataset
(GSE34289) that corresponded to indeterminate FNA cytology and
post-surgery determinate, which is composed of 188 samples, 131 as
benign and 57 as malignant. We used only 57 randomly selected
benign samples for training to balance the number of benign samples
with malignant samples.
Biomarker evaluation
To evaluate the performance of the proposed
biomarkers and those proposed by other authors, we used an NC
classifier learning the parameters from the gene expression
measurements of their corresponding datasets. Given that the
Alexander dataset is the only available data providing details of
indeterminate thyroid samples (11), we used this dataset as the gold
standard to evaluate the performance of biomarkers in the
undetermined samples. For the cytologically-determined samples, we
used the other five datasets shown in Table I as Test datasets.
Results
Previously proposed thyroid cancer
biomarkers are not robust
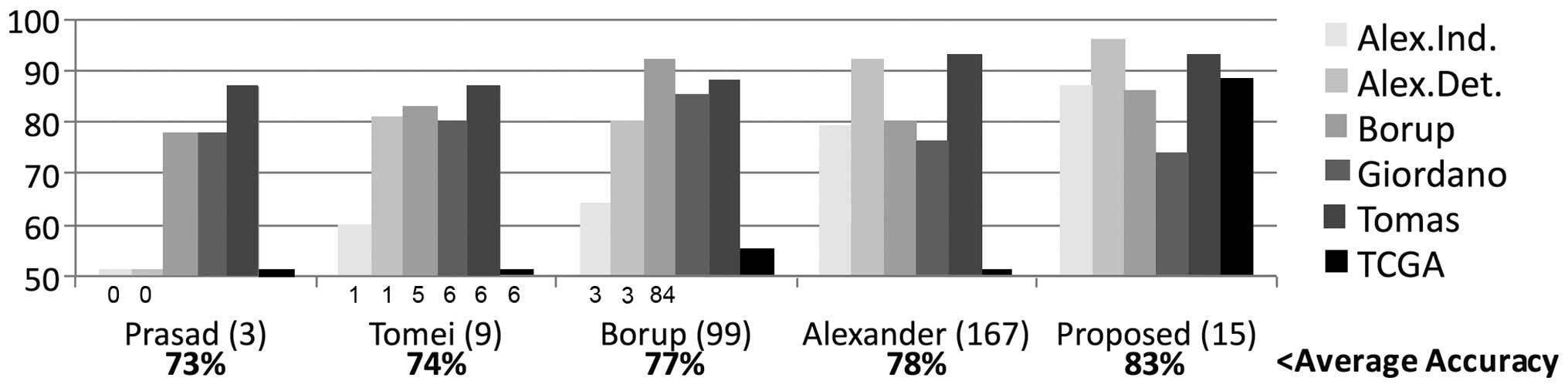
To predict thyroid tumor malignancy, we compared the
accuracy of four previously described biomarkers involving between
3 and 167 genes (10,11,14,29) evaluated in six datasets using an
NC classifier. The average accuracy ranged between 73 and 78%
(Fig. 1). However, we observed
some issues. Firstly, none of these four biomarkers accurately
predicted The Cancer Genome Atlas (TCGA) subtypes; the maximum was
55%. Secondly, the accuracies evaluated in the 265 indeterminate
FNA samples (Alexander dataset) were poor. Thirdly, two of the
biomarkers needed almost 100 genes or more, which would generate
technical and economic difficulties in clinical practice.
Identification of a highly accurate and
robust 15-gene biomarker
The application of previously proposed biomarkers
was associated with several concerns: low accuracy, lack of
robustness, need to screen of a high number of genes, as well as
poor performance when used to analyze indeterminate samples. These
biomarkers were all identified through the study of thyroid samples
with a definitive cytological diagnosis. Therefore, we specifically
selected thyroid tumors with indeterminate cytology from the
Alexander GSE34289 dataset (11).
To facilitate measurements in a clinical laboratory using RT-PCR
and to improve accuracy, we used all combinations of gene
differences (26,30–32) instead of the 173 gene expression
profiles in GSE34289. To select a low number of genes, we used a
multivariate search to identify optimal combinations (27). This strategy is based on genetic
algorithms coupled with an NC classifier. Finally, to validate the
proposed biomarker in silico, we used five additional
data-sets (Table I).
The average accuracy (83%) of the proposed biomarker
was superior to the other biomarkers (Fig. 1). The proposed biomarker was the
most accurate in four of the six datasets, including the TCGA
dataset and the cytologically indeterminate samples; it was also
highly competitive in the remaining two datasets [Borup (14) and Giordano (13)]. The biomarker identified using
GALGO was based on 15 gene differences covering 15 genes
(CCND1, CLDN16, CPE, LRP1B,
MAGI3, MAPK6, MATN2, MPPED2,
PFKFB2, PYGL, PTPRE, SEMA3D,
SERGEF, SLC4A4 and TIMP1). This signature
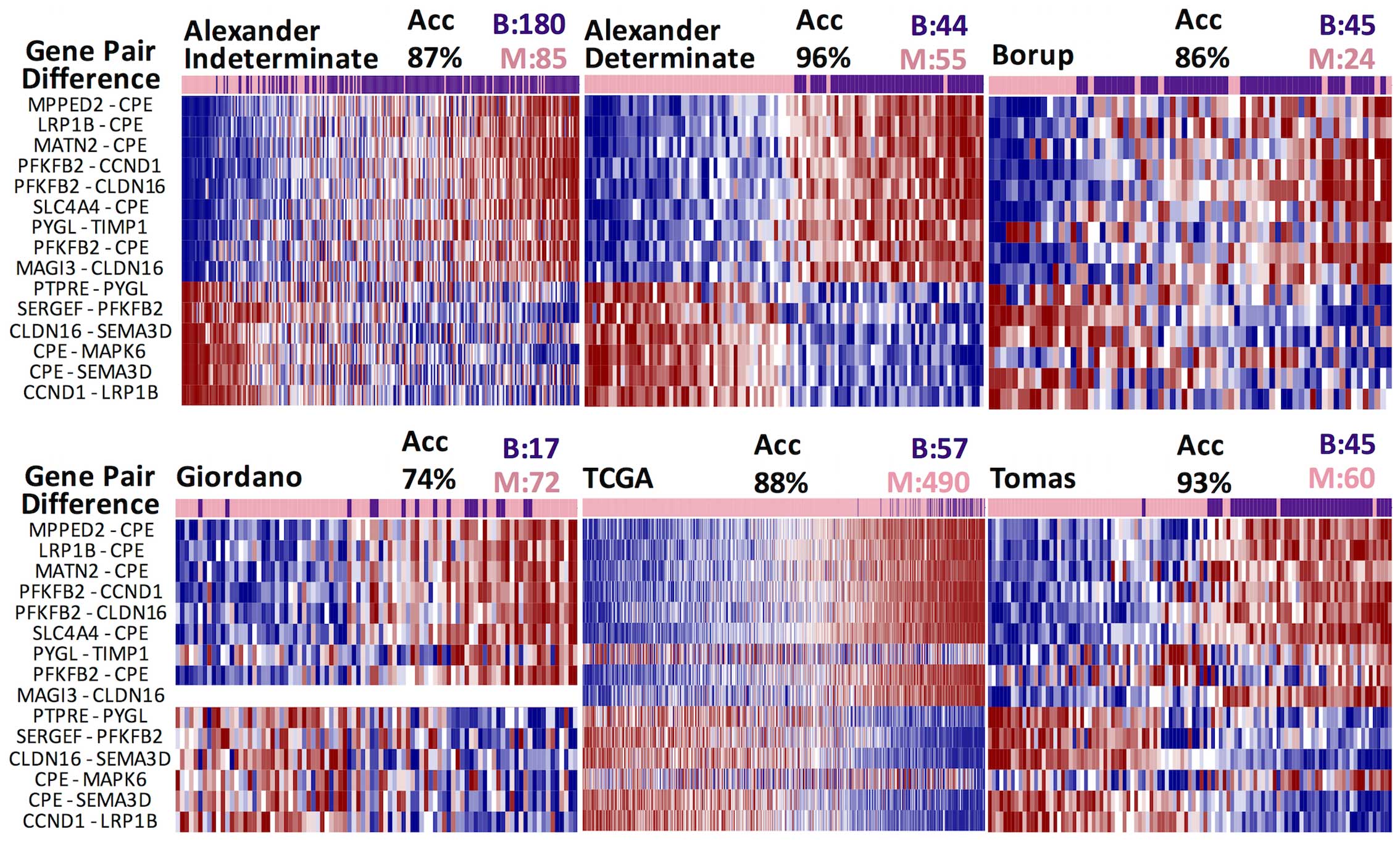
seems to be preserved across the six datasets, exhibiting clear
differences between malignant and benign samples (Fig. 2). Twelve of these gene differences
were statistically altered in four, five or six datasets (Table II). We also tested the signature
across available strata. We observed a high degree of accuracy
which was independent of gender, age, tumor size and ethnicity
(Table III).
 | Table IIDifferences in centroids between
benign and malignant samples across datasets. |
Table II
Differences in centroids between
benign and malignant samples across datasets.
| Gene
difference | Giordano
| Borup
| Alex. Ind
| Alex. Det
| TCGA
| Tomás
| Highlyb significant |
|---|
| Diff | p-valuea | Diff | p-valuea | Diff | p-valuea | Diff | p-valuea | Diff | p-valuea | B | p-valuea |
|---|
| MPPED2 -
CPE | 0.89 | 5 | 4.15 | 6 | 0.35 | 10 | 3.67 | 20 | −0.05 | 8 | 0.29 | 20 | 6 |
| LRP1B -
CPE | 0.45 | 2 | 2.91 | 10 | 0.39 | 13 | 3.53 | 23 | −0.09 | 31 | −0.26 | 32 | 5 |
| PYGL -
TIMP1 | 0.29 | 2 | 0.98 | 2 | 0.19 | 12 | 2.61 | 19 | 0.17 | 14 | −0.26 | NS | 3 |
| SLC4A4 -
CPE | 0.73 | 3 | 3.19 | 4 | 0.29 | 8 | 3.14 | 18 | −0.1 | 20 | −0.21 | 28 | 6 |
| PFKFB2 -
CLDN16 | 0.66 | 6 | 0.94 | NS | 0.44 | 13 | 4.47 | 19 | 0.17 | 37 | 0.00 | 15 | 5 |
| MATN2 -
CPE | 0.66 | 3 | 1.53 | 2 | 0.21 | 6 | 2.55 | 13 | 0.24 | 13 | 0.01 | 6 | 5 |
| MAGI3 -
CLDN16 | – | – | 1.18 | 2 | 0.51 | 12 | 3.44 | 19 | 0.08 | 15 | 0.33 | 20 | 4 |
| PFKFB2
-CCND1 | 0.25 | NS | −0.22 | NS | 0.17 | 8 | 2.04 | 19 | 0.18 | 15 | −0.35 | 10 | 4 |
| PFKFB2 -
CPE | −0.65 | NS | −1.23 | 2 | 0.13 | 2 | 2.13 | 9 | −0.11 | 23 | −0.47 | NS | 2 |
| SERGEF -
PFKFB2 | 0.04 | NS | 0.44 | NS | −0.14 | 7 | −1.06 | 7 | −0.19 | 14 | 0.26 | 3 | 4 |
| CPE -
MAPK6 | −0.26 | NS | 0.22 | NS | −0.4 | 10 | −4.7 | 24 | 0.09 | 26 | 0.03 | 4 | 4 |
| CPE -
SEMA3D | −0.34 | NS | −1.17 | 2 | −0.38 | 9 | −4.06 | 17 | −0.01 | 2 | 0.47 | 21 | 3 |
| CCND1 -
LRP1B | −0.5 | 5 | −2.66 | 9 | −0.41 | 15 | −3.43 | 26 | −0.1 | 13 | 0.14 | 35 | 6 |
| PTPRE -
PYGL | −0.37 | 7 | −0.4 | NS | −0.1 | 8 | −0.96 | 9 | −0.07 | 14 | 0.08 | 6 | 5 |
| CLDN16 -
SEMA3D | −0.79 | 11 | −2.08 | 3 | −0.67 | 12 | −5.8 | 18 | −0.25 | 41 | −0.0 | 29 | 6 |
 | Table IIIAccuracy of the biomarker in groups
of samples. |
Table III
Accuracy of the biomarker in groups
of samples.
| Authors/(Refs.)
Dataset | Groups | Samples | Accuracy |
|---|
| Alexander et
al (11) (indeterminate) | Data available | 265 | 0.82 |
| Male | 61 | 0.82 |
| Female | 204 | 0.82 |
| Age ≤47 | 103 | 0.79 |
| Age >47 | 162 | 0.83 |
| Alexander et
al (11) (determinate) | Data available | 102 | 0.94 |
| Male | 27 | 0.91 |
| Female | 75 | 0.94 |
| Age ≤47 | 38 | 0.91 |
| Age >47 | 64 | 0.95 |
| Borup et al
(14) | Data available | 69 | 0.86 |
| Male | 23 | 0.71 |
| Female | 46 | 0.89 |
| TCGA (https://tcga-data.nci.nih.gov/tcga/) | Data available | 555 | 0.88 |
| Male | 151 | 0.89 |
| Female | 404 | 0.87 |
| Tumor size ≤3
cm | 323 | 0.90 |
| Tumor size >3
cm | 196 | 0.86 |
| Asian | 54 | 0.94 |
|
African-American | 25 | 0.86 |
| Latino | 42 | 0.94 |
| Caucasian | 330 | 0.90 |
| Tomás et al
(51) | Data available | 105 | 0.93 |
| Dom et al
(52) | (no strata
available) | – | – |
| Giordano et
al (13) | Data available | 89 | 0.74 |
| (no strata
available) | – | – |
Genes in biomarker play important roles
in cancer
Remarkably, the majority of the 15 genes that
compose the biomarker have been previously associated with cancer.
CLDN16 has been shown to be elevated in patients with
thyroid papillary cancer (33).
LRP1B inactivation has been shown to influence the tumor
environment, thereby increasing the growth and invasiveness of
thyroid cancer cells (34).
SLC4A4 is expressed in low levels in papillary thyroid
carcinoma (35). TIMP1, an
inhibitor of the metalloproteinases in the extracellular matrix
(36), has been shown to be
highly expressed in thyroid cancer (37,38). CCND1, which is involved in
the inactivation of the retinoblastoma (RB) protein, as well as in
the G1-S phase transition within the cell cycle, has been shown to
be associated with many tumors (39), including thyroid papillary
carcinomas and follicular adenomas and carcinomas, as shown by
Seybt et al (40).
SEMA3D, a semaphorin that guides migrating cells during
developmental morphogenesis and in adult tissues (41), has been shown to have
anti-tumorigenic properties (42). The expression levels of
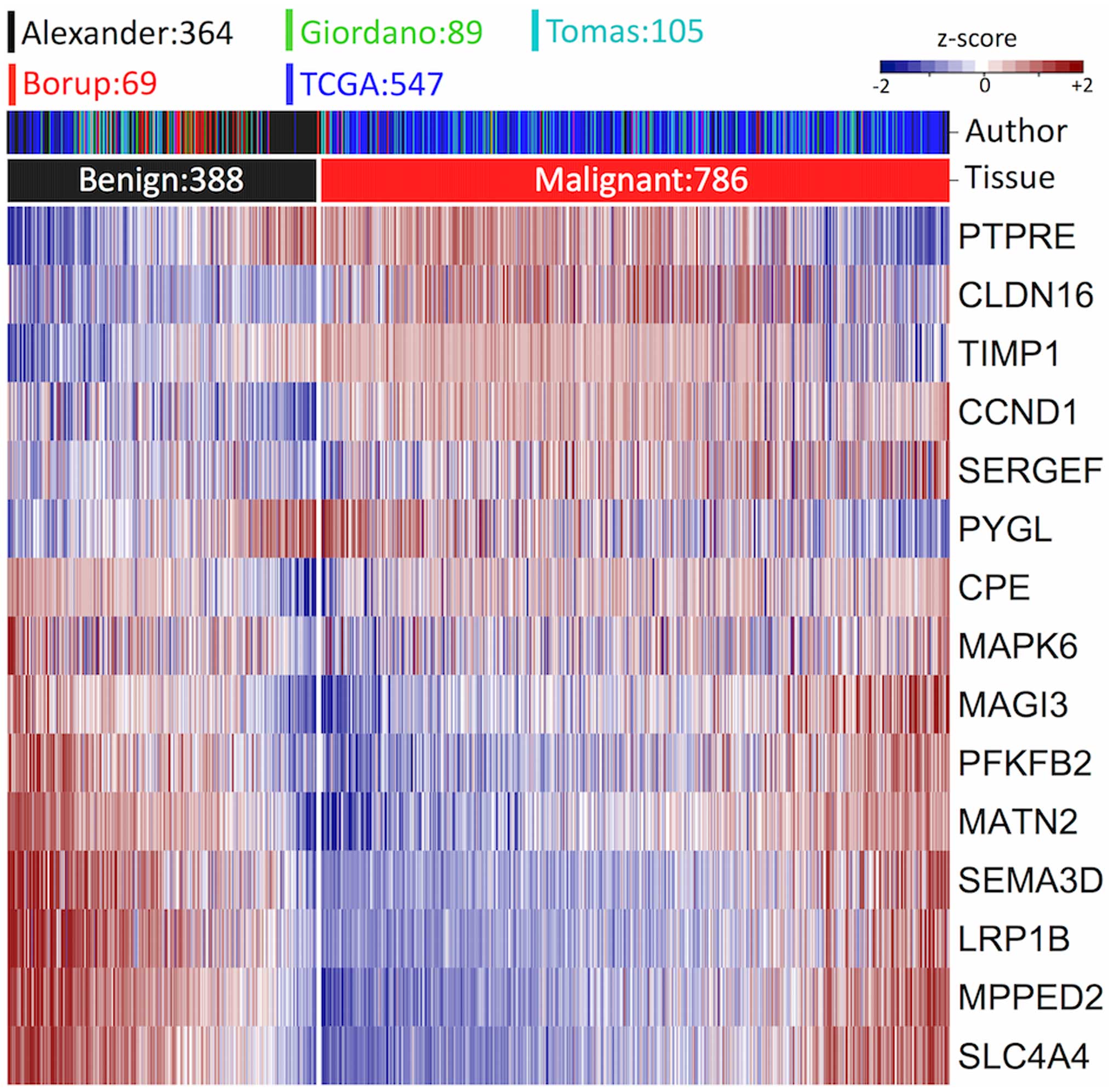
CLDN16, LRP1B, SLC4A4, TIMP1,
CCND1 and SEMA3D across subtypes in the six datasets
analyzed in the present study were consistent with the findings of
the above-mentioned studies (Fig.
3). PFKFB2, which is involved in the control of
glycolysis, has been shown to be highly expressed in patients with
papillary thyroid cancer aged >40 years compared with younger
patients (43). However, in the
present study, PFKFB2 appeared to be more highly expressed
in the benign tumors across the datasets. CPE mutations have
been related to deficiencies in thyrotropin-releasing hormone
(44), suggesting that it plays
important roles in the thyroid gland. CPE has been shown to
be associated with tumor growth and metastases in pheocromocytomas
and others types of cancer (45).
In this study, we found a consistently high expression of
CPE in malignant tumors; however, CPE expression
levels varied in the benign samples. MATN2 and MPPED2
seem to be highly correlated (r=0.7 in benign samples in Alexander
dataset) and highly expressed in the benign thyroid gland (Fig. 3). It is well known that the former
is involved in the formation of filamentous networks in the
extracellular matrix and the latter displays low
metallophosphoesterase activity. MPPED2 has been proposed to
play an important role in neuroblastoma tumorigenesis (46) and the increased expression of this
gene has been shown to be associated with a good prognosis
(46), which is consistent with
the higher expression observed in the benign thyroid tumors in the
present study. By contrast, MATN2 overexpression has been
observed in pilocytic astrocytoma (47). MAPK6 is a member of the
Ser/Thr protein kinase family that has been found to be associated
with tumor invasion in lung cancer (48). Polymorphisms in MAGI3 and
PYGL have been associated with various disorders,
MAGI3 with hypothyroidism (49) and PYGL with relapse in
leukemia (50). This literature
review of the involved genes suggests that the majority play or may
play an important role in thyroid tumors.
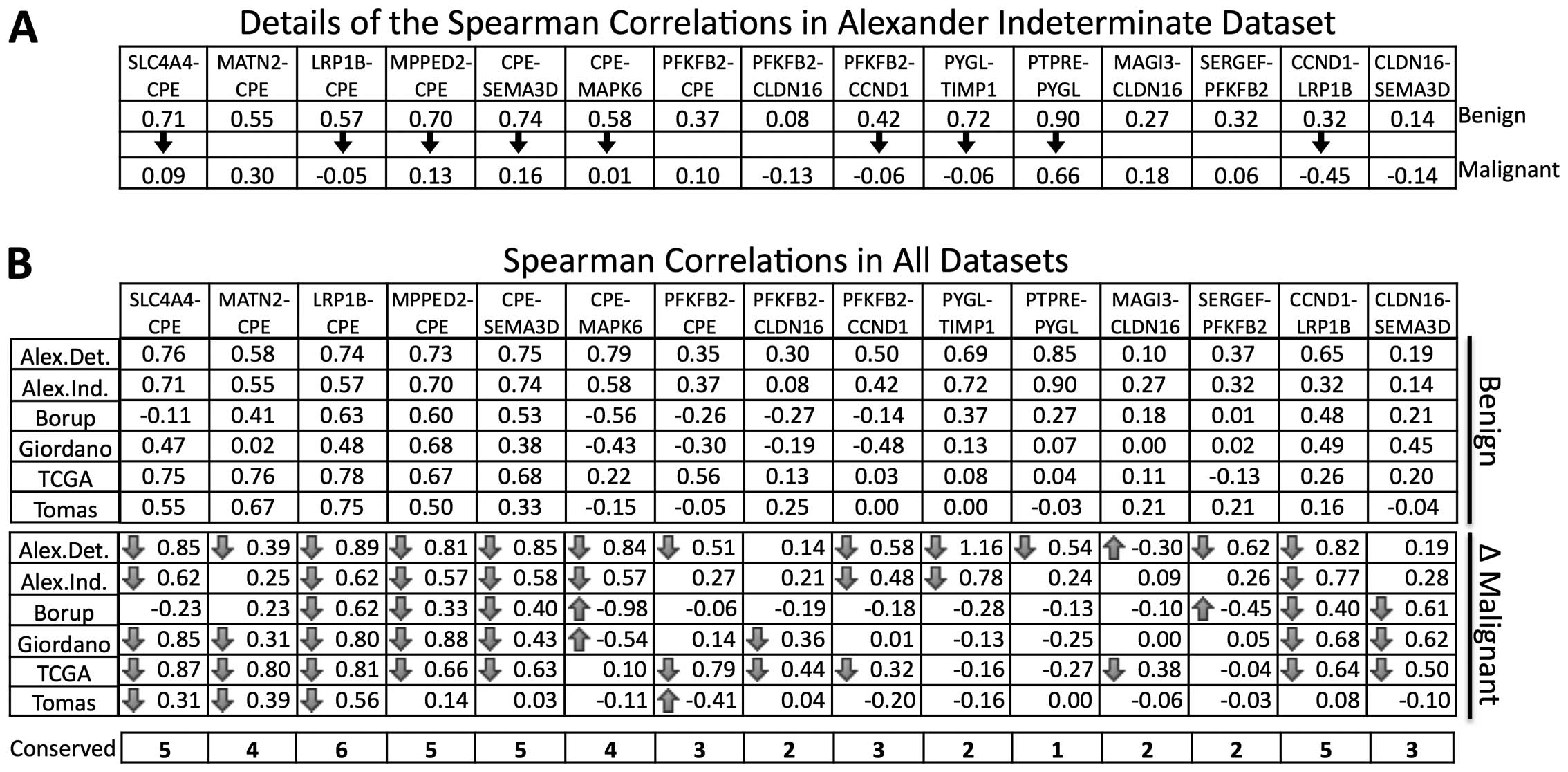
Alterations in correlation coefficients
characterize differences in gene expression
Eight genes are included in only one difference and
seven in more than one difference (Fig. 2). Notably, the CPE gene was
found in seven gene differences indicating an important
contribution within the biomarker. We observed a higher correlation
of genes combined with CPE in the benign samples compared
with those correlations in the malignant tumor samples from the
Alexander indeterminate dataset (Fig.
4A). By contrast, a higher correlation between the malignant
samples was not observed. A similar analysis of all gene pairs
across the datasets confirmed this trend (Fig. 4B).
Discussion
Previously proposed biomarkers were not robust
across datasets or indeterminate FNA samples when evaluated under
similar conditions in silico. This may be due to
characteristics of the samples, microarray technology, or the
methodology used for biomarker identification. Whereas other
studies have focused on determinate samples in order to identify
biomarkers (10,11,13,14,29,51,52), we specifically used indeterminate
samples as the training set, and therefore, we captured the
particular expression signatures in these samples. We then showed
that the signatures were also conserved in five studies of
determinate tumors, which validates the proposed signature. For
this purpose, we used gene expression differences between pairs of
genes and a multivariate search methodology. Notably, the proposed
biomarker is more compact and more accurate than other previously
proposed biomarkers.
The proposed biomarker was found to be robust when
evaluated in other databases and across patient characteristics
(tumor size, age, gender and ethnicity). These results suggest that
differences in expression are independent of the cohort,
methodology, genomic technology and particular characteristics of
the cohort and thus, it is highly likely to represent true
biological alterations. In the Giordano (13) and Borup (14) databases, the biomarker was capable
of classifying, with high accuracy, many cellular types of thyroid
cancer. In the case of determinate FNA samples in the Alexander
database (11), the performance
of the biomarker was higher (96%) than that of the indeterminate
ones (87%) even though the latter was used to identify the
biomarker. This result suggests that indeterminate samples may
contain transitional stages between benign and malignant
subtypes.
The differences in gene expression allow for the
easier measurement in widely used technologies, such as RT-PCR,
thereby facilitating implementation in clinical practice.
Surprisingly, most of the gene pairs in differences were highly
correlated in the benign tumors and poorly correlated in the
malignant tumors. This concurs with observations in prostate
(53), colon, lung, pancreatic,
cervical and gastric cancers (54) where the tumor correlation
distribution is different than in normal counterparts, generally
sharper around zero. Notably, these results suggest that
differences in correlations may be an important characteristic of
tumor transformation, which may be exploited for biomarker
identification, cancer prognosis and gene targeting. We
hypothesized that these differences between malignant and
non-maligant samples were important for the multivariate search and
the classifier to select the genes involved in the proposed
biomarker. This may explain the high number of occurrences of the
CPE gene, which showed the largest differences in correlation
coefficients (ten in benign and one in malignant samples).
From the 15 genes identified in our biomarker, which
is a subset of those from the study by Alexander et al
(11), none are similar to those
proposed by Prasad et al (HMGA2, MRC2, and
SFN) (55) and Tomei et
al (KIT, C21orf4, PDK3/Hs.296031,
DDI2, CDH1, LSM7 and TC1) (29), and only two (MATN2 and
MPPED2) are included in the genes from the study by Borup
et al (14). Thus, the
proposed signature combined with the use of gene differences and a
NC classifier appears to be distinctive.
The contribution of the multivariate search was
important since the other methodologies tested, such as PAM-R
(56) and support vector machine
recursive feature elimination (SVM-RFE) (57), generated lower accuracies (65 and
75%, respectively) or higher numbers of gene differences (85 and
15, respectively). Besides the multivariate search, we believe that
the use of the gene-pair difference was an important factor which
enabled us to identify the highly accurate marker. We then showed
that the gene-pair difference may also associated with the
difference in correlation coefficients between genes and tumor
subtypes. Nevertheless, this approach is almost prohibited in large
datasets since a dataset of 20,000 genes would generate 200 million
differences combinations. Thus, the use of the 173 genes (or a
stringent gene filter) was also a critical factor.
As the proposed biomarker was only tested in
silico, a validation study is warranted to confirm the
potential use of this biomarker in clinical practice. Although we
aim to explore this line of research in the near future, the
availability of the proposed biomarker and the methodology used may
encourage other research groups to test the biomarker or to design
better ones.
In conclusion, the proposed biomarker is composed of
15 gene differences involving 15 genes. The majority of the genes
have been associated with cancer and some specifically with thyroid
cancer in the research literature. Our analysis suggests that the
proposed biomarker is more accurate and robust than previous
thyroid biomarkers in tumors and indeterminate FNA samples.
Measuring the biomarker may be made relatively easy by RT-PCR
facilitating implementation. Changes in the gene expression
correlations between benign and malignant samples may be associated
with tumor progression and may explain the presence and robustness
of the gene differences that compose the proposed biomarker.
Abbreviations:
|
FNA
|
fine-needle aspiration
|
|
RT-PCR
|
real-time-polymerase chain
reaction
|
|
NC
|
nearest centroid
|
Acknowledgments
The present study was supported by Grupo de
Investigación con Enfoque Estratégico en Bioinformática of the
Instituto Tecnológico y de Estudios Superiores of Monterrey,
CONACyT (Posgrado Nacional 002087 and grant scholarship 339770). We
thank the Instituto Tecnológico y de Estudios Superiores of
Monterrey, Hospital San José de Monterrey, and the Instituto
Mexicano del Seguro Social for supporting this study.
References
|
1
|
Aschebrook-Kilfoy B, Ward MH, Sabra MM and
Devesa SS: Thyroid cancer incidence patterns in the United States
by histologic type, 1992–2006. Thyroid. 21:125–134. 2011.
View Article : Google Scholar :
|
|
2
|
Sadowski SM, Köhler BB, Meyer P,
Pusztaszeri M, Robert JH and Triponez F: Treatment of
differentiated thyroid cancer. Rev Med Suisse. 8:1321–1325. 2012.In
French. PubMed/NCBI
|
|
3
|
Kazaure HS, Roman SA and Sosa JA:
Aggressive variants of papillary thyroid cancer: Incidence,
characteristics and predictors of survival among 43,738 patients.
Ann Surg Oncol. 19:1874–1880. 2012. View Article : Google Scholar
|
|
4
|
Liénart F: Thyroid nodule: Benign or
malignant? Rev Med Brux. 33:254–262. 2012.In French.
|
|
5
|
Lew JI, Snyder RA, Sanchez YM and
Solorzano CC: Fine needle aspiration of the thyroid: correlation
with final histopathology in a surgical series of 797 patients. J
Am Coll Surg. 213:188–195. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Knezević-Usaj S, Eri Z, Panjković M, Klem
I, Petrović T, Ivković-Kapicl T, Karapandzić A and Jelić J:
Diagnostic relevance of fine needle aspiration cytology in nodular
thyroid lesions. Vojnosanit Pregl. 69:555–561. 2012.In Serbian.
View Article : Google Scholar
|
|
7
|
American Thyroid Association (ATA)
Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid
Cancer; Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel
SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, et al
Revised American Thyroid Association management guidelines for
patients with thyroid nodules and differentiated thyroid cancer.
Thyroid. 19:1167–1214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mehanna R, Murphy M, McCarthy J, O'Leary
G, Tuthill A, Murphy MS and Sheahan P: False negatives in thyroid
cytology: impact of large nodule size and follicular variant of
papillary carcinoma. Laryngoscope. 123:1305–1309. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Duick DS: Overview of molecular biomarkers
for enhancing the management of cytologically indeterminate thyroid
nodules and thyroid cancer. Endocr Pract. 18:611–615. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Prasad NB, Kowalski J, Tsai HL, Talbot K,
Somervell H, Kouniavsky G, Wang Y, Dackiw AP, Westra WH, Clark DP,
et al: Three-gene molecular diagnostic model for thyroid cancer.
Thyroid. 22:275–284. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alexander EK, Kennedy GC, Baloch ZW, Cibas
ES, Chudova D, Diggans J, Friedman L, Kloos RT, LiVolsi VA, Mandel
SJ, et al: Preoperative diagnosis of benign thyroid nodules with
indeterminate cytology. N Engl J Med. 367:705–715. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tamez-Pérez HE, Gutiérrez-Hermosillo H,
Forsbach-Sánchez G, Gómez-de Ossio MD, González-González G,
Guzmán-López S, Tamez-Peña AL, Mora-Torres NE and González-Murillo
EA: Nondiagnostic thyroid fine needle aspiration cytology: outcome
in surgical treatment. Rev Invest Clin. 59:180–183. 2007.PubMed/NCBI
|
|
13
|
Giordano TJ, Kuick R, Thomas DG, Misek DE,
Vinco M, Sanders D, Zhu Z, Ciampi R, Roh M, Shedden K, et al:
Molecular classification of papillary thyroid carcinoma: Distinct
BRAF, RAS, and RET/PTC mutation-specific gene expression profiles
discovered by DNA microarray analysis. Oncogene. 24:6646–6656.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Borup R, Rossing M, Henao R, Yamamoto Y,
Krogdahl A, Godballe C, Winther O, Kiss K, Christensen L, Høgdall E
and Nielsen FC: Molecular signatures of thyroid follicular
neoplasia. Endocr Relat Cancer. 17:691–708. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Issaq HJ, Waybright TJ and Veenstra TD:
Cancer biomarker discovery: opportunities and pitfalls in
analytical methods. Electrophoresis. 32:967–975. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Drucker E and Krapfenbauer K: Pitfalls and
limitations in translation from biomarker discovery to clinical
utility in predictive and personalised medicine. EPMA J. 4:72013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Walsh PS, Wilde JI, Tom EY, Reynolds JD,
Chen DC, Chudova DI, Pagan M, Pankratz DG, Wong M, Veitch J, et al:
Analytical performance verification of a molecular diagnostic for
cytology-indeterminate thyroid nodules. J Clin Endocrinol Metab.
97:E2297–E2306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Duick DS, Klopper JP, Diggans JC, Friedman
L, Kennedy GC, Lanman RB and McIver B: The impact of benign gene
expression classifier test results on the endocrinologist-patient
decision to operate on patients with thyroid nodules with
indeterminate fine-needle aspiration cytopathology. Thyroid.
22:996–1001. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ali SZ, Fish SA, Lanman R, Randolph GW and
Sosa JA: Use of the Afirma® gene expression classifier
for preoperative identification of benign thyroid nodules with
indeterminate fine needle aspiration cytopathology. PLoS Curr.
5:52013.
|
|
20
|
Alexander EK, Schorr M, Klopper J, Kim C,
Sipos J, Nabhan F, Parker C, Steward DL, Mandel SJ and Haugen BR:
Multicenter clinical experience with the Afirma gene expression
classifier. J Clin Endocrinol Metab. 99:119–125. 2014. View Article : Google Scholar
|
|
21
|
Ward LS and Kloos RT: Molecular markers in
the diagnosis of thyroid nodules. Arq Bras Endocrinol Metabol.
57:89–97. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Harrell RM and Bimston DN: Surgical
utility of Afirma: effects of high cancer prevalence and oncocytic
cell types in patients with indeterminate thyroid cytology. Endocr
Pract. 20:364–369. 2014. View Article : Google Scholar
|
|
23
|
Vriens D, Adang EM, Netea-Maier RT, Smit
JW, de Wilt JH, Oyen WJ and de Geus-Oei LF: Cost-effectiveness of
FDG-PET/CT for cytologically indeterminate thyroid nodules: a
decision analytic approach. J Clin Endocrinol Metab. 99:3263–3274.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McIver B, Castro MR, Morris JC, Bernet V,
Smallridge R, Henry M, Kosok L and Reddi H: An independent study of
a gene expression classifier (Afirma) in the evaluation of
cytologically indeterminate thyroid nodules. J Clin Endocrinol
Metab. 99:4069–4077. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bustin SA: Absolute quantification of mRNA
using real-time reverse transcription polymerase chain reaction
assays. J Mol Endocrinol. 25:169–193. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Grate LR: Many accurate
small-discriminatory feature subsets exist in microarray transcript
data: biomarker discovery. BMC Bioinformatics. 6:972005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Trevino V and Falciani F: GALGO: an R
package for multivariate variable selection using genetic
algorithms. Bioinformatics. 22:1154–1156. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dabney AR: Classification of microarrays
to nearest centroids. Bioinformatics. 21:4148–4154. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tomei S, Marchetti I, Zavaglia K, Lessi F,
Apollo A, Aretini P, Di Coscio G, Bevilacqua G and Mazzanti C: A
molecular computational model improves the preoperative diagnosis
of thyroid nodules. BMC Cancer. 12:3962012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Meng H, Murrelle EL and Li G:
Identification of a small optimal subset of CpG sites as biomarkers
from high-throughput DNA methylation profiles. BMC Bioinformatics.
9:4572008. View Article : Google Scholar
|
|
31
|
Price ND, Trent J, El-Naggar AK, Cogdell
D, Taylor E, Hunt KK, Pollock RE, Hood L, Shmulevich I and Zhang W:
Highly accurate two-gene classifier for differentiating
gastrointestinal stromal tumors and leiomyosarcomas. Proc Natl Acad
Sci USA. 104:3414–3419. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang X and Gotoh O: Accurate molecular
classification of cancer using simple rules. BMC Med Genomics.
2:642009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fluge Ø, Bruland O, Akslen LA, Lillehaug
JR and Varhaug JE: Gene expression in poorly differentiated
papillary thyroid carcinomas. Thyroid. 16:161–175. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Prazeres H, Torres J, Rodrigues F, Pinto
M, Pastoriza MC, Gomes D, Cameselle-Teijeiro J, Vidal A, Martins
TC, Sobrinho-Simões M and Soares P: Chromosomal, epigenetic and
microRNA-mediated inactivation of LRP1B, a modulator of the
extracellular environment of thyroid cancer cells. Oncogene.
30:1302–1317. 2011. View Article : Google Scholar
|
|
35
|
Kim HS, Kim H, Kim JY, Jeoung NH, Lee IK,
Bong JG and Jung ED: Microarray analysis of papillary thyroid
cancers in Korean. Korean J Intern Med. 25:399–407. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kenney MC, Chwa M, Atilano SR, Tran A,
Carballo M, Saghizadeh M, Vasiliou V, Adachi W and Brown DJ:
Increased levels of catalase and cathepsin V/L2 but decreased
TIMP-1 in keratoconus corneas: evidence that oxidative stress plays
a role in this disorder. Invest Ophthalmol Vis Sci. 46:823–832.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hawthorn L, Stein L, Varma R, Wiseman S,
Loree T and Tan D: TIMP1 and SERPIN-A overexpression and TFF3 and
CRABP1 underexpression as biomarkers for papillary thyroid
carcinoma. Head Neck. 26:1069–1083. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kebebew E, Peng M, Reiff E, Duh QY, Clark
OH and McMillan A: Diagnostic and prognostic value of
angiogenesis-modulating genes in malignant thyroid neoplasms.
Surgery. 138:1102–1110. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fu M, Wang C, Li Z, Sakamaki T and Pestell
RG: Minireview: Cyclin D1: normal and abnormal functions.
Endocrinology. 145:5439–5447. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Seybt TP, Ramalingam P, Huang J, Looney SW
and Reid MD: Cyclin D1 expression in benign and differentiated
malignant tumors of the thyroid gland: diagnostic and biologic
implications. Appl Immunohistochem Mol Morphol. 20:124–130. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cagnoni G and Tamagnone L: Semaphorin
receptors meet receptor tyrosine kinases on the way of tumor
progression. Oncogene. 33:4795–4802. 2014. View Article : Google Scholar
|
|
42
|
Kigel B, Varshavsky A, Kessler O and
Neufeld G: Successful inhibition of tumor development by specific
class-3 semaphorins is associated with expression of appropriate
semaphorin receptors by tumor cells. PLoS One. 3:e32872008.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Vriens MR, Moses W, Weng J, Peng M,
Griffin A, Bleyer A, Pollock BH, Indelicato DJ, Hwang J and Kebebew
E: Clinical and molecular features of papillary thyroid cancer in
adolescents and young adults. Cancer. 117:259–267. 2011. View Article : Google Scholar
|
|
44
|
Nillni EA, Xie W, Mulcahy L, Sanchez VC
and Wetsel WC: Deficiencies in prothyrotropin-releasing hormone
processing and abnormalities in thermoregulation in Cpefat/fat
mice. J Biol Chem. 277:48587–48595. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Murthy SR, Pacak K and Loh YP:
Carboxypeptidase E: elevated expression correlated with tumor
growth and metastasis in pheochromocytomas and other cancers. Cell
Mol Neurobiol. 30:1377–1381. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liguori L, Andolfo I, de Antonellis P,
Aglio V, di Dato V, Marino N, Orlotti NI, De Martino D, Capasso M,
Petrosino G, et al: The metallophosphodiesterase Mpped2 impairs
tumorigenesis in neuroblastoma. Cell Cycle. 11:569–581. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sharma MK, Watson MA, Lyman M, Perry A,
Aldape KD, Deák F and Gutmann DH: Matrilin-2 expression
distinguishes clinically relevant subsets of pilocytic astrocytoma.
Neurology. 66:127–130. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Long W, Foulds CE, Qin J, Liu J, Ding C,
Lonard DM, Solis LM, Wistuba II, Qin J, Tsai SY, et al: ERK3
signals through SRC-3 coactivator to promote human lung cancer cell
invasion. J Clin Invest. 122:1869–1880. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Medici M, Porcu E, Pistis G, Teumer A,
Brown SJ, Jensen RA, Rawal R, Roef GL, Plantinga TS, Vermeulen SH,
et al: Identification of novel genetic Loci associated with thyroid
peroxidase antibodies and clinical thyroid disease. PLoS Genet.
10:e10041232014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yang JJ, Cheng C, Devidas M, Cao X,
Campana D, Yang W, Fan Y, Neale G, Cox N, Scheet P, et al:
Genome-wide association study identifies germline polymorphisms
associated with relapse of childhood acute lymphoblastic leukemia.
Blood. 120:4197–4204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tomás G, Tarabichi M, Gacquer D, Hébrant
A, Dom G, Dumont JE, Keutgen X, Fahey TJ III, Maenhaut C and
Detours V: A general method to derive robust organ-specific gene
expression-based differentiation indices: application to thyroid
cancer diagnostic. Oncogene. 31:4490–4498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Dom G, Tarabichi M, Unger K, Thomas G,
Oczko-Wojciechowska M, Bogdanova T, Jarzab B, Dumont JE, Detours V
and Maenhaut C: A gene expression signature distinguishes normal
tissues of sporadic and radiation-induced papillary thyroid
carcinomas. Br J Cancer. 107:994–1000. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Treviño Alvarado VM and Falciani F:
Edinburgh DTo and Mexico CNdCyTC: Identifying the molecular
components that matter: a statistical modelling approach to linking
functional genomics data to cell physiology. PhD thesis. School of
Biosciences, University of Birmingham; England: 2007
|
|
54
|
Anglani R, Creanza TM, Liuzzi VC, Piepoli
A, Panza A, Andriulli A and Ancona N: Loss of connectivity in
cancer co-expression networks. PLoS One. 9:e870752014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Prasad NB, Somervell H, Tufano RP, Dackiw
AP, Marohn MR, Califano JA, Wang Y, Westra WH, Clark DP, Umbricht
CB, et al: Identification of genes differentially expressed in
benign versus malignant thyroid tumors. Clin Cancer Res.
14:3327–3337. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tibshirani R, Hastie T, Narasimhan B and
Chu G: Diagnosis of multiple cancer types by shrunken centroids of
gene expression. Proc Natl Acad Sci USA. 99:6567–6572. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Guyon I, Weston J, Barnhill S and Vapnik
V: Gene selection for cancer classification using support vector
machines. Mach Learn. 46:389–422. 2002. View Article : Google Scholar
|