Introduction
Breast cancer is the most common type of cancer
affecting females worldwide, and ranks as the second most common
type of cancer with 1.38 million new cases diagnosed and 458,100
associated deaths in 2008 (1).
Despite advances in the diagnosis and the implementation of novel
strategies for the treatment of breast cancer, interventions are
often ineffective due to the high proliferative ability of cancer
cells and intrinsic resistance to clinical therapies, including
chemotherapy (2). Therefore,
there is an urgent need to develop novel therapeutic strategies by
elucidating the molecular mechanisms responsible for the
development and progression of breast cancer.
MicroRNAs (miRNAs or miRs) are a class of small
(21–24 nucleotides), non-coding RNA molecules which function as
pivotal regulators of gene expression by interacting with the
3′-untranslated region (3′UTR) of their target mRNA. miRNAs have
been demonstrated to regulate thousands of human genes and are of
fundamental importance in various human diseases, such as
cardiovascular disease, Alzheimer's disease and autoimmune diseases
(3,4). Several studies have confirmed the
abnormal expression of miRNAs in several types of cancer, including
colon cancer, hepatocellular carcinoma, lung cancer and breast
cancer (5–7). Emerging evidence has indicated that
miRNAs are capable of modulating various biological processes which
occur in cancer cells, such as cell proliferation, invasion, cell
cycle arrest and survival (8,9).
Of note, many miRNAs are located at fragile sites or
cancer-associated regions, which may explain the reason for the
correlation between tumorigenesis and the aberrant expression of
certain miRNAs. Among these, miR-214 has been demonstrated to be
dysregulated in several types of human malignancies (8,10,11). However, miR-214 plays various
roles, which may even be opposing roles, in different types of
cancer. In hepatoma, miR-214 is downregulated and acts as an
anti-oncogene, affecting hepatoma cancer cell growth, metastasis
and tumor angiogenesis (10,12). By contrast, miR-214 is
significantly upregulated in osteosarcoma tissues with a large
tumor size and positive metastasis, and it is recognized as an
independent prognostic factor of unfavorable survival in pediatric
osteosarcoma (11). The serum
concentrations of miR-214 have been proven to be significantly
higher in patients with breast cancer in contrast to those in
healthy women (13). However, the
role of miR-124 in breast cancer is unclear as its underlying
mechanisms of action remain poorly understood.
In the present study, the expression level of
miR-214 in breast cancer cell lines was determined. Importantly, we
examine the effects of miR-214 on cell proliferation and resistance
to apoptosis. Furthermore, the underlying mechanisms of action of
miR-214, as well as its potential targets were also
investigated.
Materials and methods
Reagents and antibodies
The phosphoinositide 3-kinase (PI3K) inhibitor,
LY294002 (#440202), was purchased from Calbiochem (San Diego, CA,
USA). The polyclonal antibodies against human Akt (#9272) and
phosphorylated (p-)Akt (#9271) were obtained from Cell Signaling
Technology (Beverly, MA, USA). The antibodies against Bcl-2
(#sc-509) were purchased from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). Rabbit polyclonal antibodies against cyclin D1
(ab15196) and p27 (ab7961) was purchased from Abcam (Cambridge,
UK). The antibodies against phosphatase and tensin homolog (PTEN;
#9559) were purchased from Cell Signaling Technology (Danvers, MA,
USA).
Human cancer cell lines and cell
culture
The human breast cancer cell lines, MCF-7,
MDA-MB-231, MDA-MB-453 and T47D, were obtained from the American
Type Culture Collection (ATCC; Manassas, VA, USA). The
non-malignant breast epithelial cell line, MCF-10A, was also from
ATCC and was used as the ‛normal' control for human breast cancer
cell analysis. The above-mentioned 4 breast cancer cell lines were
grown in RPMI-1640 medium supplemented with 10% fetal calf serum.
The MCF-10A cells were cultured in Mammary Epithelial Cell Basal
Medium (Cambrex, Walkersville, MD, USA). All media were
supplemented with 2 mM glutamine, 100 µg/ml streptomycin and
penicillin. The cells were all incubated in a humidified atmosphere
at 37°C with 5% CO2.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
The expression of miR-214 was analyzed using the
TaqMan® microRNA reverse transcription kit (Applied
Biosystems, Foster City, CA, USA). Briefly, total RNA was extracted
from the cultured cells using TRIzol reagent (Invitrogen, Carlsbad,
CA, USA) according to the manufacturer's instructions. The
resulting total RNA was then used for the primer-specific reverse
transcription of miR-214 and U6. To analyze the expression of
miR-214 in breast cancer cells, approximately 2 µl cDNA was
subjected to qPCR using a 7900HT Fast Real-Time PCR System (Applied
Biosystems). The reaction conditions and procedures were performed
according to the instructions provided with the TaqMan microRNA
reverse transcription kit (Applied Biosystems). U6 was used to
normalize the miRNA. All results were calculated using the
2−ΔΔCt method.
Oligonucleotide transfection
The miR-214 mimics and scramble control miRNA
(miR-con) sequences were used as previously described (14). All the oligonucleotides were
obtained from and purified by RiboBio (Guangzhou, Guangdong,
China). For transfection, approximately 1×105 cells were
seeded into 12-well plates. When the cells reached 50–80%
confluence, 0.4 nmol miRNA mimics or miR-con were mixed with 15
µl GenePORTER 2 Transfection Reagent (GTS, San Diego, CA,
USA). The above mixture was then separately transfected into the
cells. Following incubation for 6 h, fresh medium was added for a
further 48 h. The transfection efficiency of the miR-214 mimics was
evaluated using RT-qPCR.
Construction and transfection of PTEN
expression vectors
To obtain the PTEN expression vectors, wild-type
PTEN lacking the 3′UTR region was cloned into the pcDNA3.1 vector
by Genesil Biotechnology Co. Ltd. (Wuhan, China). When the cells
grew to 70–80% confluency, 15 µg of pcDNA-PTEN or pcDNA3.1
empty vector were transfected into the MCF-7 cells using
Lipofectamine 2000 (Invitrogen) in accordance with the
manufacturer's instructions. Following incubation for 6 h, the
medium was replaced with the fresh RPMI-1640 medium containing 10%
fetal bovine serum (FBS). The transfected cells were evaluated by
western blot analysis.
Dual-luciferase reporter assay in
vitro
For luciferase reporter experiments, the 3′UTR
sequence of PTEN predicted to interact with miR-214 or a mutated
sequence within the predicted target sites was synthesized and
inserted into the MluI and HindIII sites of a pGL3
vector (Promega, Madison, WI, USA). These constructs were known as
pGL3-PTEN-3′UTR-wt or pGL3-PTEN-3′UTR-mut, respectively. For the
reporter assay, the MCF-7 cells were plated onto 12-well plates,
and then co-transfected with the above-mentioned constructs and 5
ng of pRL-TK (Promega), with or without miR-214 or miR-con using
Lipofectamine 2000 reagent (Invitrogen). Approximately 48 h later,
the cells were harvested. The luciferase and Renilla signals
were determined using the Dual-Luciferase Reporter Assay system
(Promega) according to the manufacturer's instructions.
Cell viability assay
The cells were seeded in a 96-well culture plate
(1×105 cells/well) and allowed to attach for 24 h.
Following pre-treatment with the PI3K/Akt inhibitor, LY294002, the
cells were transfected as described above. The culture medium was
removed from each well and replaced with fresh medium containing 5
mg/ml MTT solution (Sigma-Aldrich, St. Louis, MO, USA). The plate
was then incubated at 37°C for an additional 5 h. After removing
the remaining supernatant, 100 µl of DMSO were added and
mixed thoroughly to dissolve the formed formazan crystals. Cell
viability was then analyzed by measuring the absorbance of each
well at 570 nm. Relative cell viability was calculated as the
absorbance percentage of the treatment group relative to the
control group.
Analysis of cell apoptosis using flow
cytometry (FCM)
Cell apoptosis was detected by FCM. Briefly,
following incubation with RPMI-1640 medium without serum for 24 h,
the trans-fected cells were pre-treated with 20 µM LY294002.
The cells were trypsinized and washed with PBS. The cells were then
centrifuged at 1,000 × g for 10 min at room temperature and
resuspended with 500 µl of binding buffer, followed by
incubation with 5 µl Annexin V-FITC and 5 µl PI
(Sigma-Aldrich, Carlsbad, CA, USA) for 15 min at room temperature.
All specimens were analyzed on a FACScan flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA) for the relative
quantification of apoptosis.
Western blot analysis
Total protein from the treatment groups was
extracted using RIPA lysis buffer (Beyotime, Nantong, China) and
quantified using a BCA assay kit (Pierce, Rockford, IL, USA). A
total of 200 µg of protein was separated by SDS-PAGE and
transferred onto PVDF membranes (Schleicher & Schuell GmbH,
Dassel, Germany). After blocking with 5% non-fat milk, the
membranes were incubated with antibodies against p-Akt, Akt, cyclin
D1, p27, Bcl-2 and PTEN. Incubation with the primary antibody was
carried out overnight at 4°C. The membranes were then incubated
with a secondary antibody conjugated to horseradish peroxidase
(HRP; Jackson ImmunoResearch, West Grove, MA, USA). The LumiGLo
reagent (KPL Inc., Gaithersburg, MD, USA) was introduced to
visualize the bound antibodies. The protein expression levels were
normalized by β-actin. The intensity of protein expression was
quantified with ImageJ software (National Institutes of Health,
Bethesda, MD, USA).
Statistical analysis
All results are presented as the means ± SEM. The
statistical significance of the differences between groups was
analyzed using a Student's t-test. A P-value <0.05 was
considered to indicate a statistically significant difference.
Results
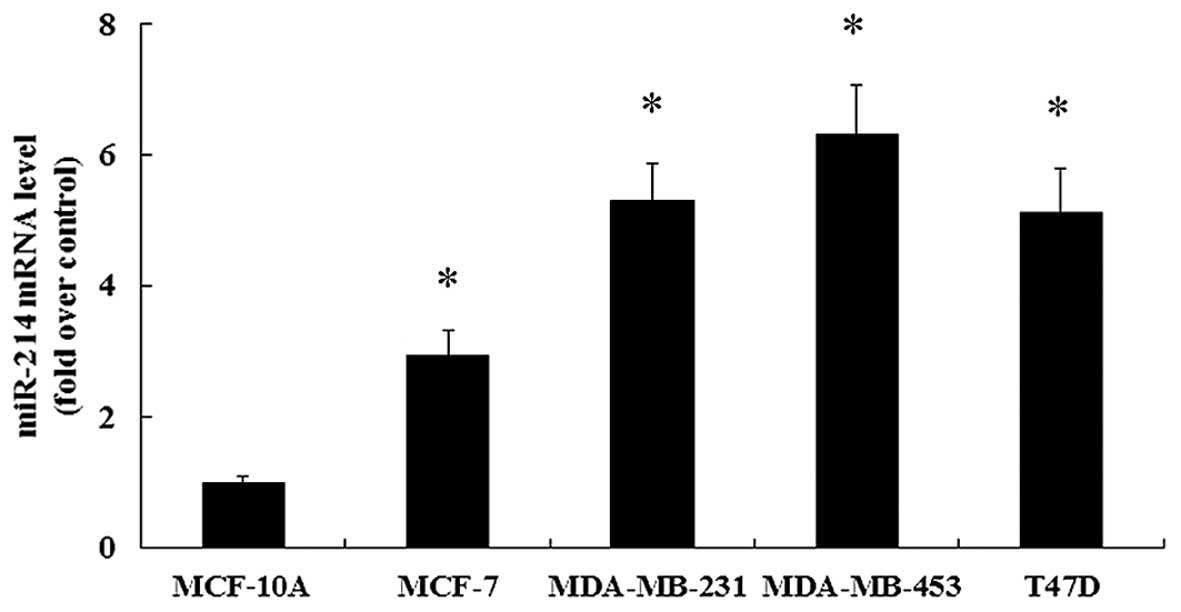
Expression of miR-214 is increased in
breast cancer cell lines
It has been demonstrated that miR-214 plays an
important role in the progression of different types of cancer
(8,14). However, research into the role of
miR-214 in breast cancer remains limited. To clarify this issue, in
this study, miR-214 expression in 4 human breast cancer cell lines
(MCF-7, MDA-MB-231, MDA-MB-453 and T47D) was assessed. Compared
with the non-malignant breast epithelial cell line, MCF-10A, the
marked upregulation of miR-214 expression was found in the 4 breast
cancer cell lines (Fig. 1).
Consequently, these results confirmed the notable upregulation of
miR-214 in breast cancer cells, which may be important in the
development and progression of human breast cancer. Furthermore,
the relative lower expression of miR-214 was observed in the breast
cancer MCF-7 cells in contrast to the other 3 breast cancer cell
lines. Thus, to better investigate the effects of miR-214
upregulation in breast cancer cells, the MCF-7 cells were selected
for use in the subsequent experiments.
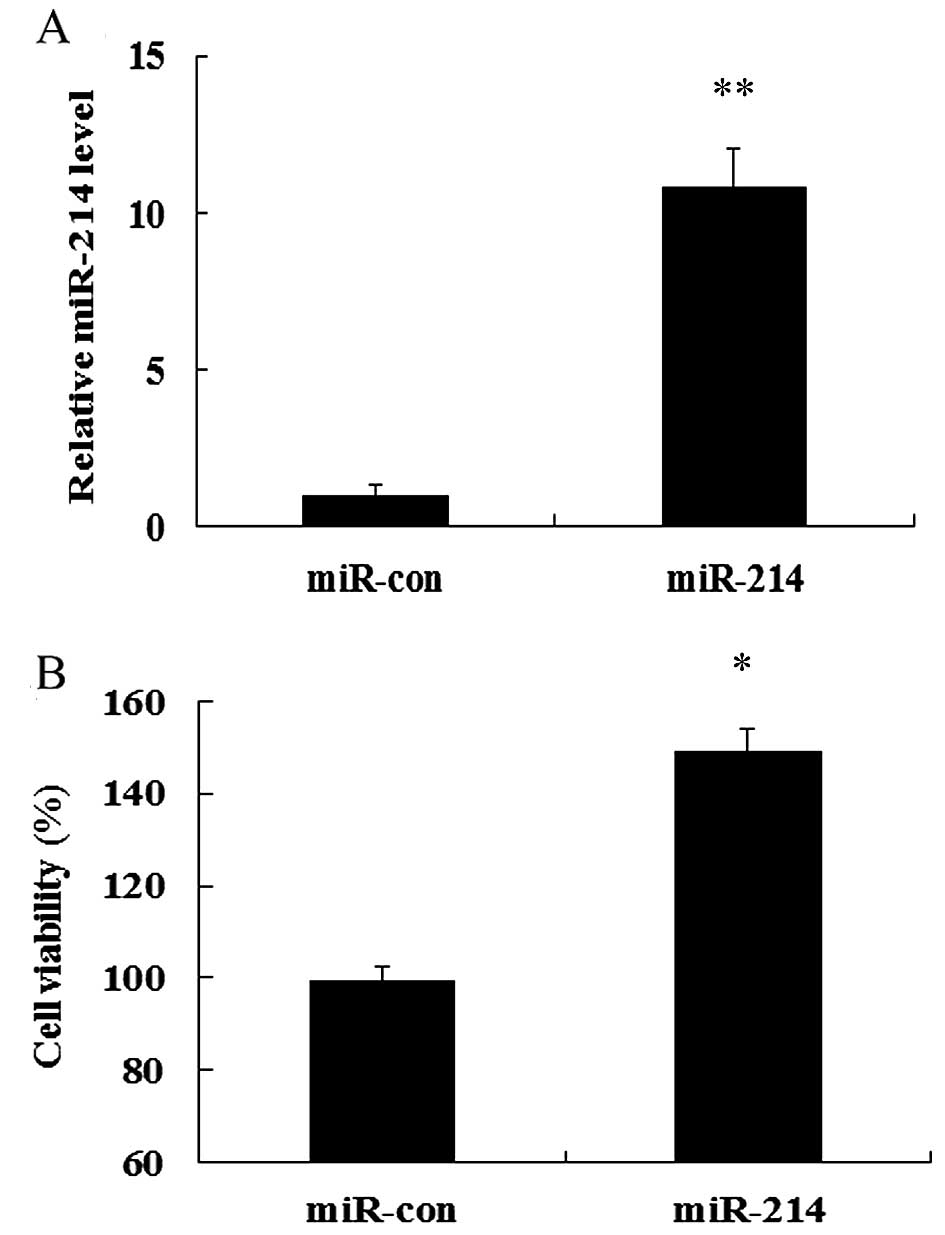
miR-214 overexpression enhances cell
viability
To explore the role of miR-214 in the development
and progression of breast cancer, we evaluated the effects of the
overexpression of miR-214 on MCF-7 cell growth. To determine the
direct contribution of miR-214 in breast cell growth, we
successfully induced the expression of miR-214 in MCF-7 cells by
transfecting the cells with miR-214 mimics which were detected
using RT-qPCR (Fig. 2A).
Importantly, MTT assay confirmed that the overexpression of miR-214
markedly induced a 1.49-fold increase in cell viability, compared
with that in the control group (transfected with miR-con; Fig. 2B). Therefore, these results
indicate that the overexpressoin of miR-214 markedly enhances cell
viability.
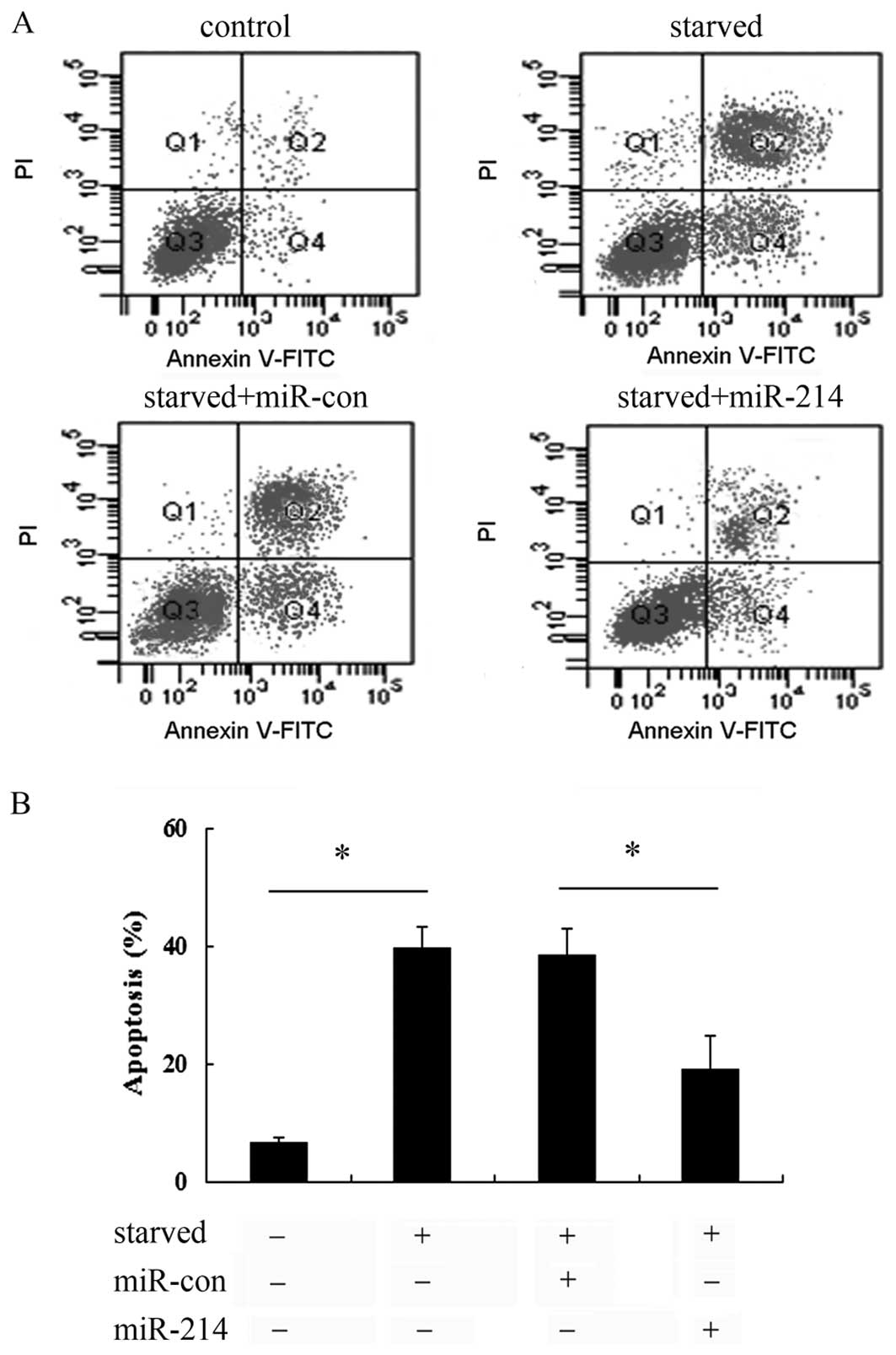
Overexpression of miR-214 abrogated cell
apoptosis induced by serum starvation
To further examine the effect of miR-214 on cell
apoptosis induced by serum starvation, Annexin V-FITC and PI
staining was used. As shown in Fig.
3A, a notable increase in the cell apoptotic rate was observed
when the cells were exposed to serum starvation conditions.
However, transfection with miR-214 mimics markedly abrogated the
increase in cell apoptosis, compared with that in the control
groups (untreated control and miR-con-transfected cells). Further
quantitative analysis revealed that serum starvation induced a
0.39-fold increase in the cell apoptotic rate, which was markedly
abrogated when the cells were transfected with miR-214 mimics
(Fig. 3B), implying that miR-214
exerts protective effects against the apoptosis of breast cancer
cells, thus playing a critical role in the development of breast
cancer cells.
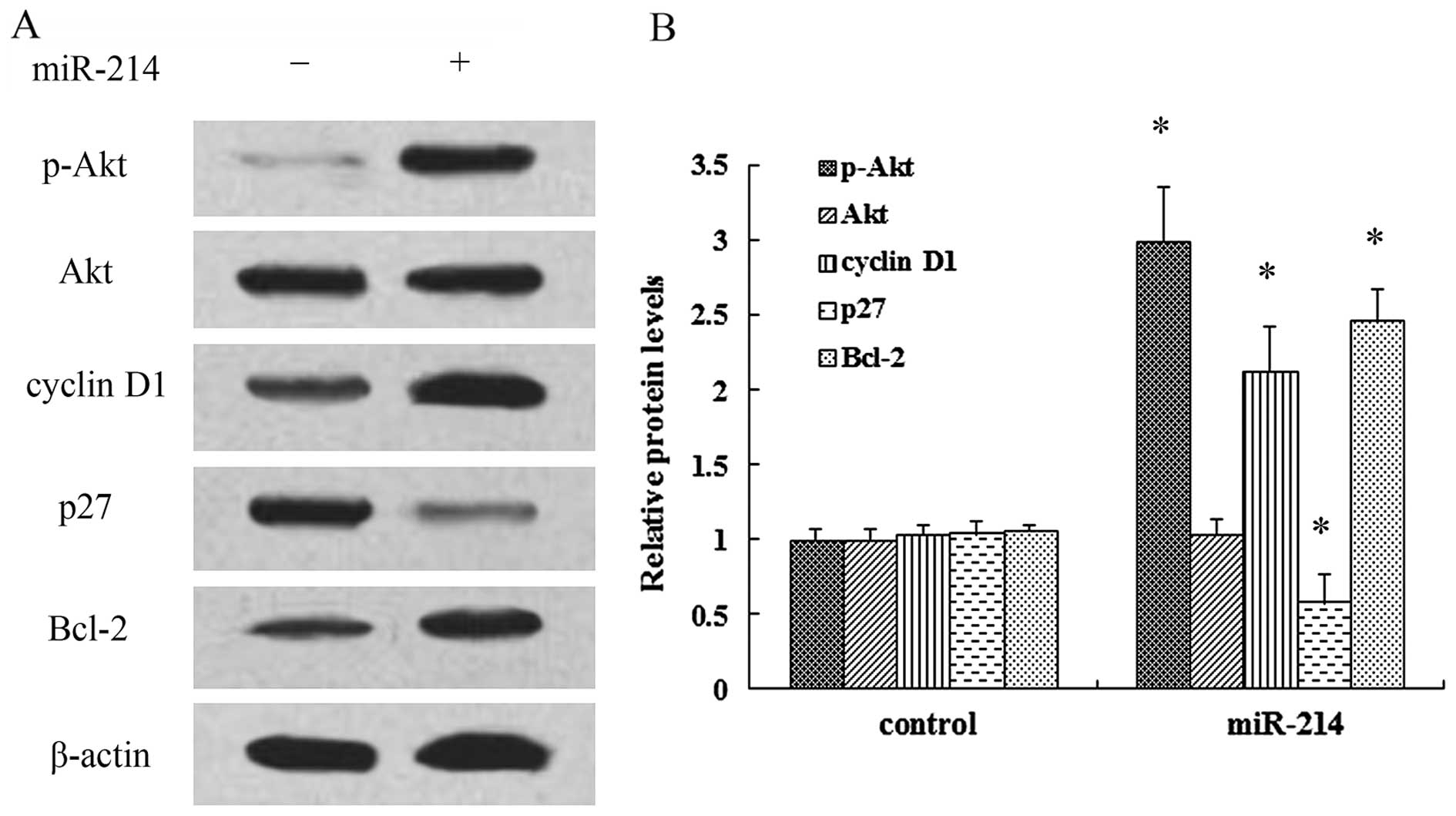
miR-214 exerts a positive effect on cell
growth by regulating the activation of the PI3K/Akt pathway
It has been demonstrated that PI3K/Akt signaling
plays a critical role in the development of cancer and multiple
physiological processes (15).
Thus, in order to elucidate the mechanisms responsible for the
promoting effects of miR-214 on cell growth, the activation of the
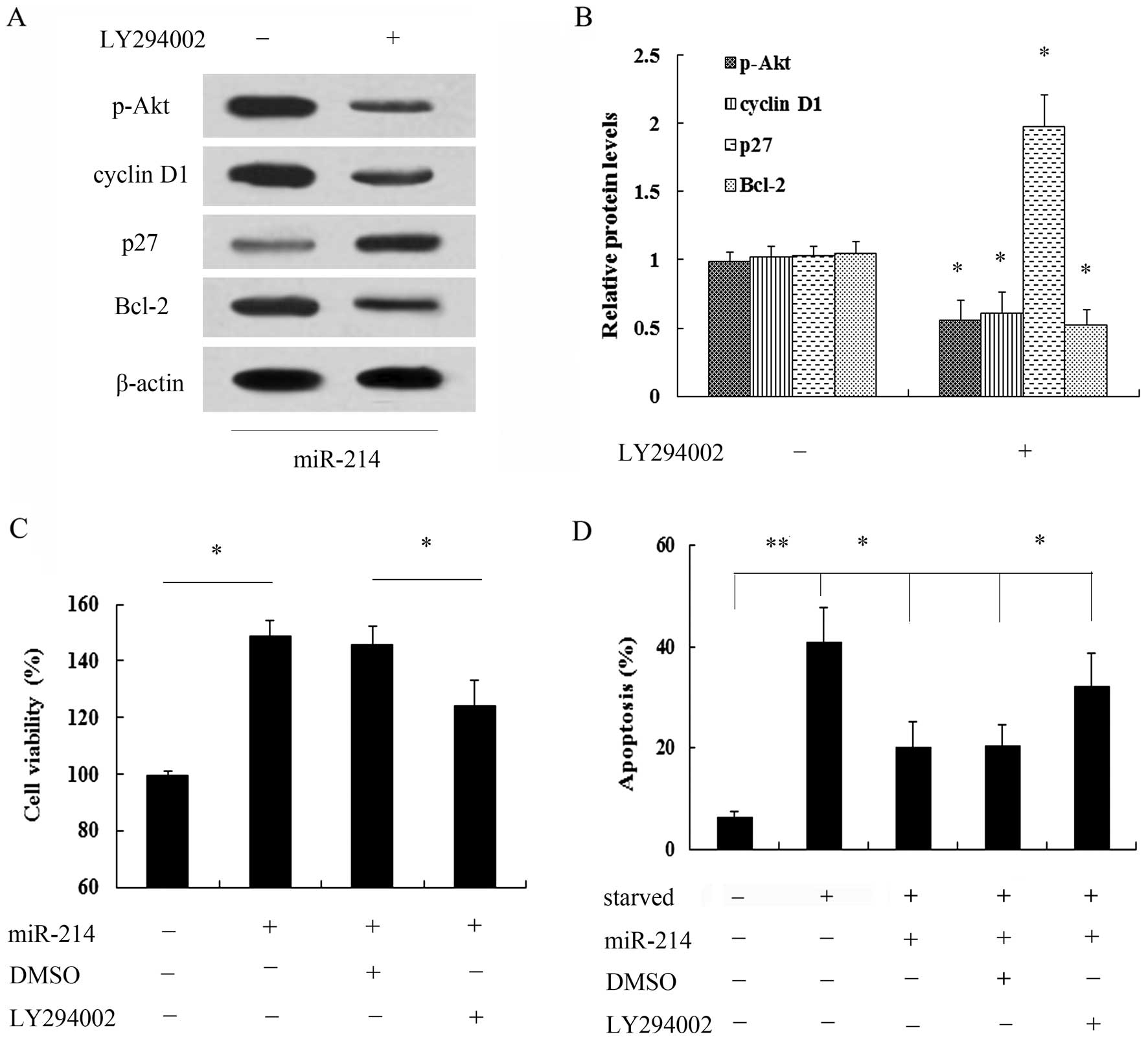
PI3K/Akt pathway was examined. As shown in Fig. 4A, transfection with miR-214 mimics
markedly induced the expression of p-Akt, but not that of Akt.
Moreover, the expression of the cell cycle inhibitory protein, p27,
was notably decreased when the cells were transfected with miR-214
mimics, whereas the expression of cyclin D1 was increased.
Furthermore, the overexpression of miR-214 induced a 2.46-fold
increase in the expression of the anti-apoptotic protein, Bcl-2
(Fig. 4B). Thus, these findings
prompted us to hypothesize that the PI3K/Akt signaling pathway is
associated with the promoting effects of miR-214 on the growth of
breast cancer cells. Following pre-treatment with the PI3K
inhibitor, LY294002, the miR-214-induced activation of the PI3K/Akt
signaling pathway was markedly abrogated (Fig. 5A and B). Of note, pre-treatment
with LY294002 markedly attenuated the miR-214-induced increase in
cell viability (Fig. 5C).
Importantly, miR-214 exerted a protective effect agasint cell
apoptosis induced by serum starvation; this effect was also
abrogated when the cells were pre-treated with LY294002 (Fig. 5D). Taken together, these data
suggest that miR-214 exerts a protective effect against the
apoptosis of breast cancer cells and promotes their growth, mainly
by regulating the activation of the PI3K/Akt signaling pathway.
PTEN is a target of miR-214
It has been confirmed that PTEN negatively regulates
PI3K/Akt signaling (16,17). Thus, to further elucidate the
mechanisms involved in the miR-214-mediated activation of the
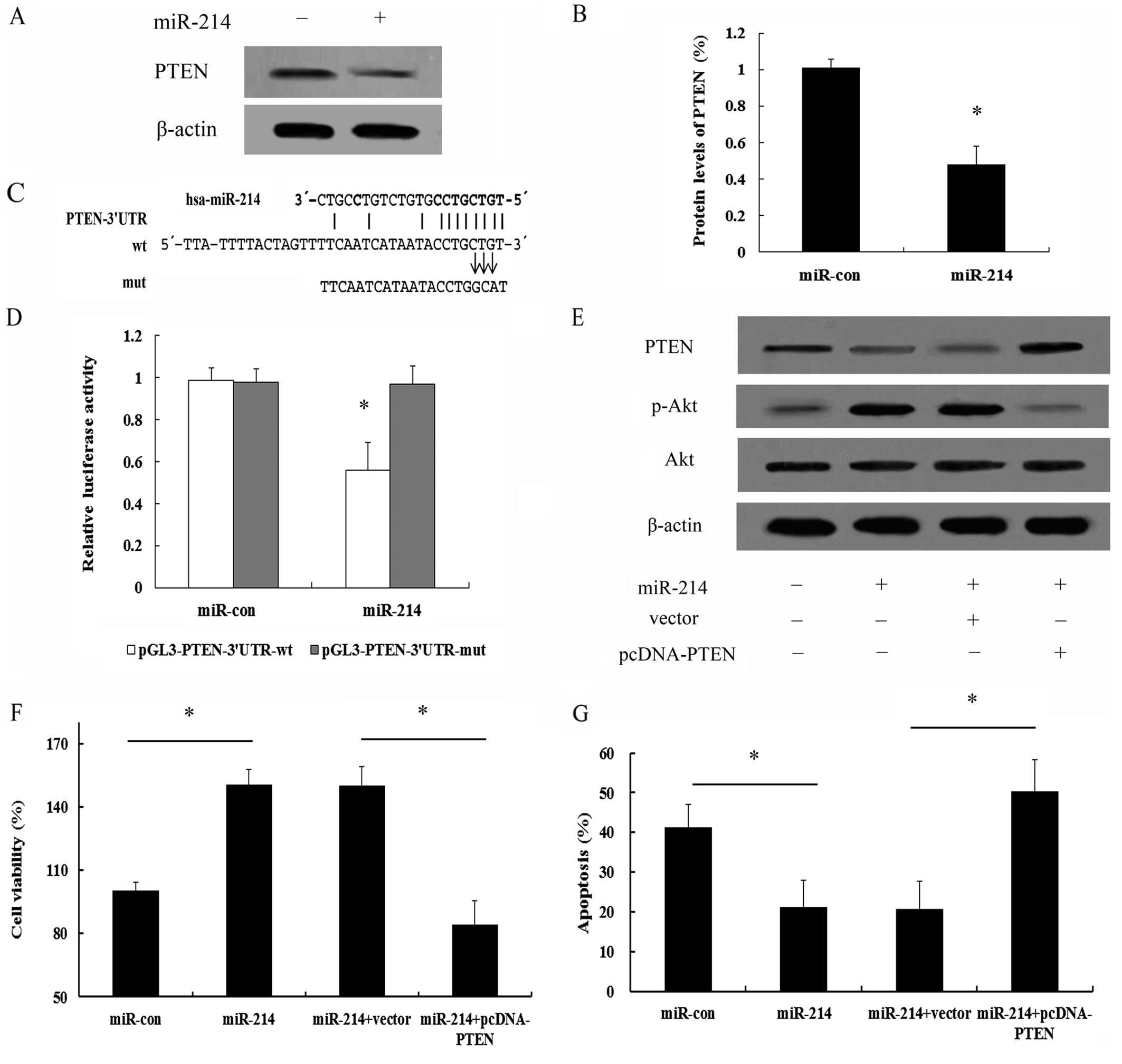
PI3K/Akt signaling pathway, we analyzed the expression of PTEN. As
shown in Fig. 6A, a high PTEN
expression was detected in the MCF-7 cells. However, transfection
with miR-214 induced a marked downregulation (0.48-fold) in PTEN
expression (Fig. 6B). It has been
documented that miRNAs negatively regulate the expression of their
targets, primarily by interacting with the 3′UTR of their mRNA,
which ultimately leads to mRNA degradation or translational
inhibition (8,9). Further bioinformatics analysis
indicated that there was a conservative binding site for miR-214 in
PTEN (Fig. 6C). The 3′UTR
sequence of PTEN which was predicted to interact with miR-214 was
further synthesized and inserted into a pGL3 vector and separately
termed pGL3-PTEN-3′UTR-wt and pGL3-PTEN-3′UTR-mut. Co-transfection
of the MCF-7 cells with miR-214 and pGL3-PTEN-3′UTR-wt
significantly suppressed the luciferase activity; however, this
effect was not observed in the pGL3-PTEN-3′UTR-mut-trasnfected
group (Fig. 6D). Taken together,
these results confirmed that miR-214 downregulated PTEN expression
by directly binding to the 3′UTR of PTEN mRNA in breast cancer
cells.
PTEN is responsible for the promoting
effects of miR-214 on the survival and resistance to apoptosis of
breast cancer cdells through PI3K/Akt signaling
To determine whether PTEN is repsonsible for the
promoting effects of miR-214 on cell survival and the protective
effects of this miRNA on the apoptosis of breast cancer cells, as
well as the involvement of the PI3K/Akt signaling pathway, the
expression of PTEN was ectopically induced by transfecting the
cells with cDNA that contained only the coding region of PTEN,
which should escape regulation by miR-214 and thus, inhibit the
function of miR-214. Following transfection of pcDNA-PTEN lacking
3′UTR into the MCF-7 cells, the expression of PTEN which had been
inhibited by miR-214, was markedly increased; however, the increase
in the levels of p-Akt induced by miR-214 was clearly abrogated
(Fig. 6E), implying that miR-214
regulates the activation of the PI3K/Akt signaling pathway by
targeting PTEN. Importantly, the overexpression of PTEN
significantly abrogated the protective effects of miR-214 on cell
survival (Fig. 6F).
Simultaneously, PTEN expression also sensitized the
miR-214-expressing MCF-7 cells to apoptosis induced by serum
starvation (Fig. 6G). Therefore,
these data further validate that the PTEN/Akt pathway is a major
target of miR-214 and that it is largely responsible for
miR-214-mediated cell survival and anti-apoptotic effects.
Discussion
Breast cancer ranks as the leading cause of
cancer-related mortality in women worldwide (1,2). A
substantial increase in the global incidence rates of breast cancer
has been documented over the past few years. Therefore, elucidating
the molecular mechanisms involved in the progression of breast
cancer is pivotal for the development of therapies for breast
cancer. This study presents the important finding that the
expression of miR-214 was upregulated in several breast cancer cell
lines compared with that in the non-malignant breast epithelial
cell line, MCF-10A. Additionally, the overexpression of miR-214
markedly enhanced cell viability and promoted resistance to
apoptosis. Furthermore, miR-214 was shown to be involved in the
modulation of PI3K/Akt signaling by directly targeting PTEN. Taken
together, these results suggest that miR-214 plays a critical role
in the development and progression of breast cancer.
miRNAs are endogenous, single-stranded, non-coding
RNAs which are approximately 22 nucleotides in length and may
suppress the post-transcriptional expression of target genes and
are consequently involved in the regulation of various cellular
processes, e.g., cell apoptosis, differentiation, development and
metastasis (9,18,19). Growing evidence has reinforced the
fact that miRNAs are frequently deregulated and aberrantly
expressed in certain types of cancer, by acting as either tumor
suppressors (9,20) or oncogenes (21,22), and that they play a central role
in carcinogenesis. Among these miRNAs, miR-214 has attracted
increasing attention due to its critical role in the development of
different types of cancer (8,10);
however, accumulating evidence has confirmed that miR-214 exerts
various, even inverse, effects in different types of cancer. For
example, miR-214 has been shown to function as an oncogene in
melanoma to mediate tumorigenesis (8), whereas it acts as an anti-oncogene
in hepatoma (10). Nevertheless,
the roles of miR-214 in breast cancer remain unclear, as well as
the underlying mechanisms. In this study, miR-214 expression was
significantly elevated in four human breast cancer cell lines in
contrast to its expression level in the non-malignant breast
epithelial cell line, MCF-10A, which is consistent with the finding
that miR-214 is expressed at higher levels in the serum of breast
cancer patients compared with that in healthy patients or those
with benign breast disease (13).
Moreover, the overexpression of miR-214 markedly enhanced cell
viability and abrogated cell apoptosis triggered by serum
starvation, indicating a pivotal role of this miRNA in breast
cancer cell growth. Consequently, these data suggest that miR-214
acts as a novel oncogene which regulates the progression of breast
cancer.
It is widely accepted that carcinogenesis often
results from the dirsupted balance between cell growth and
programmed cell death (i.e., apoptosis). The PI3K/Akt signaling
pathway has been proven to be involved in the above-mentioned
processes and even other processes, and its expression is
frequently disrupted in human cancer (15,23). The abnormal activation of this
pathway has been corroborated by epidemiological and experimental
studies as a critical step toward the initiation and maintenance of
human tumors. Previous research has demonstrated that PI3K/Akt
triggers a cascade of multiple signals that regulate cancer cell
proliferation, invasion, metastasis, survival as well as affecting
prognosis (24,25). Thus, in this study, to explore the
mechanisms involved in the miR-214-mediated increase in cell
viability and resistance to apoptosis, we examined the involvement
of PI3K/Akt signaling in these processes. In accordance with our
hypothesis, the overexpression of miR-214 evidently induced the
activation of p-Akt. It is generally believed that cyclin D1, p27
and Bcl-2 are common downstream molecules of the PI3K/Akt pathway,
which are all associated with cell proliferation and apoptosis
(26,27). The consistently higher expression
of cyclin D1 and the anti-apoptotic protein, Bcl-2, was observed in
the cells transfected with miR-214 mimics; however, the expression
of the cell cycle inhibitory protein, p27, was downregulated. The
above-mentioned results confirmed that the upregulation of miR-214
induced the activation of the PI3K/Akt pathway. Further mechanistic
analysis validated that pre-treatment with the inhibitor, LY294002,
notably blocked the activation of the PI3K/Akt pathway induced by
miR-214. Simultaneously, the promoting effects of miR-214 on cell
viability and its protective effects against apoptosis were
markedly abrogated by pre-treatment with LY294002. Taken together,
these results suggest that miR-214 positively promotes breast
cancer cell growth by regulating PI3K/Akt signaling.
A number of studies have confirmed that the
dysregulation of PTEN occurs in various types of cancer and that it
plays a role as a tumor suppressor in the progression of cancer
(28,29). Recently, PTEN has been recognized
as a negative regulator of PI3K/Akt signaling (16,17). In ovarian cancer, the decrease in
PTEN expression has been shown to induce the activation of the
PI3K/Akt pathway (30). In the
present study, the overexpression of miR-214 markedly decreased
PTEN expression. Further bioinformatics analysis indicated that
there was a conservative binding site of miR-214 in the 3′UTR of
PTEN. Importantly, luciferase activity was markedly reduced when
the cells were transfected with the PTEN-3′UTR-containing
luciferase reporter system, but not in the PTEN-3′UTR-mutation
groups, indicating that PTEN is a direct target of miR-214. Further
mechanistic analysis validated that the miR-214-mediated activation
of the PI3K/Akt pathway was markedly abrogated following
transfection with pcDNA-PTEN lacking 3′UTR, implying that miR-214
induced the activation of the PI3K/Akt signaling pathway by
directly targeting PTEN. Of note, the expression of PTEN
significantly abrogated the protective effects of miR-214 on cell
survival and resistance to apoptosis. Therefore, these data further
confirm that miR-214 regulates cell survival and exerts
anti-apoptotic effects mainly by targeting the PTEN-PI3K/Akt
pathway.
In conclusion, the present study observed an
upregulation of miR-214 in breast cancer cell lines. Importantly,
miR-214 enhanced cell viability and promoted resistance to
apoptosis by directly targeting PTEN-PI3K/Akt signaling, which
ultimately facilitates the development of breast cancer.
Consequently, pharmaceutical interventions targeting miR-214 may
provide a promising therapeutic strategy for the treatment of
breast cancer.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Benson JR and Jatoi I: The global breast
cancer burden. Future Oncol. 8:697–702. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van Rooij E and Olson EN: MicroRNA
therapeutics for cardiovascular disease: opportunities and
obstacles. Nat Rev Drug Discov. 11:860–872. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dai R and Ahmed SA: MicroRNA, a new
paradigm for understanding immunoregulation, inflammation, and
autoimmune diseases. Transl Res. 157:163–179. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang J, Lyu H, Wang J and Liu B: MicroRNA
regulation and therapeutic targeting of survivin in cancer. Am J
Cancer Res. 5:20–31. 2015.PubMed/NCBI
|
|
6
|
Cheng H, Zhang L, Cogdell DE, Zheng H,
Schetter AJ, Nykter M, Harris CC, Chen K, Hamilton SR and Zhang W:
Circulating plasma miR-141 is a novel biomarker for metastatic
colon cancer and predicts poor prognosis. PLoS One. 6:e177452011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fornari F, Milazzo M, Chieco P, Negrini M,
Marasco E, Capranico G, Mantovani V, Marinello J, Sabbioni S,
Callegari E, et al: In hepatocellular carcinoma miR-519d is
up-regulated by p53 and DNA hypomethylation and targets CDKN1A/p21,
PTEN, AKT3 and TIMP2. J Pathol. 227:275–285. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Penna E, Orso F, Cimino D, Tenaglia E,
Lembo A, Quaglino E, Poliseno L, Haimovic A, Osella-Abate S, De
Pittà C, et al: microRNA-214 contributes to melanoma tumour
progression through suppression of TFAP2C. EMBO J. 30:1990–2007.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yamada Y, Hidaka H, Seki N, Yoshino H,
Yamasaki T, Itesako T, Nakagawa M and Enokida H: Tumor-suppressive
microRNA-135a inhibits cancer cell proliferation by targeting the
c-MYC oncogene in renal cell carcinoma. Cancer Sci. 104:304–312.
2013. View Article : Google Scholar
|
|
10
|
Shih TC, Tien YJ, Wen CJ, Yeh TS, Yu MC,
Huang CH, Lee YS, Yen TC and Hsieh SY: MicroRNA-214 downregulation
contributes to tumor angiogenesis by inducing secretion of the
hepatoma-derived growth factor in human hepatoma. J Hepatol.
57:584–591. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Z and Cai H, Lin L, Tang M and Cai H:
Upregulated expression of microRNA-214 is linked to tumor
progression and adverse prognosis in pediatric osteosarcoma.
Pediatr Blood Cancer. 61:206–210. 2014. View Article : Google Scholar
|
|
12
|
Wang J, Li J, Wang X, Zheng C and Ma W:
Downregulation of microRNA-214 and overexpression of FGFR-1
contribute to hepatocellular carcinoma metastasis. Biochem Biophys
Res Commun. 439:47–53. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schwarzenbach H, Milde-Langosch K,
Steinbach B, Müller V and Pantel K: Diagnostic potential of
PTEN-targeting miR-214 in the blood of breast cancer patients.
Breast Cancer Res Treat. 134:933–941. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li B, Han Q, Zhu Y, Yu Y, Wang J and Jiang
X: Down-regulation of miR-214 contributes to intrahepatic
cholangiocarcinoma metastasis by targeting Twist. FEBS J.
279:2393–2398. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Osaki M, Oshimura M and Ito H: PI3K-Akt
pathway: its functions and alterations in human cancer. Apoptosis.
9:667–676. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moon S-H, Kim D-K, Cha Y, Jeon I, Song J
and Park K-S: PI3K/Akt and Stat3 signaling regulated by PTEN
control of the cancer stem cell population, proliferation and
senescence in a glioblastoma cell line. Int J Oncol. 42:921–928.
2013.PubMed/NCBI
|
|
17
|
Abdulaziz S, Al-Shahid M and Al-Thenayan
E: A 49-year-old man with acute pulmonary hypertension post lung
transplantation. Chest. 144:704–707. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Baumjohann D, Kageyama R, Clingan JM,
Morar MM, Patel S, de Kouchkovsky D, Bannard O, Bluestone JA,
Matloubian M, Ansel KM and Jeker LT: The microRNA cluster MIR-17~92
promotes TFH cell differentiation and represses
subset-inappropriate gene expression. Nat Immunol. 14:840–848.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou Y, Xiong M, Niu J, Sun Q, Su W, Zen
K, Dai C and Yang J: Secreted fibroblast-derived miR-34a induces
tubular cell apoptosis in fibrotic kidney. J Cell Sci.
127:4494–4506. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zha R, Guo W, Zhang Z, Qiu Z, Wang Q, Ding
J, Huang S, Chen T, Gu J, Yao M and He X: Genome-wide screening
identified that miR-134 acts as a metastasis suppressor by
targeting integrin β1 in hepatocellular carcinoma. PLoS One.
9:e876652014. View Article : Google Scholar
|
|
21
|
Yau WL, Lam CSC, Ng L, Chow AK, Chan ST,
Chan JY, Wo JY, Ng KT, Man K, Poon RT and Pang RW: Over-expression
of miR-106b promotes cell migration and metastasis in
hepatocellular carcinoma by activating epithelial-mesenchymal
transition process. PLoS One. 8:e578822013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Josson S, Gururajan M, Hu P, Shao C, Chu
GY, Zhau HE, Liu C, Lao K, Lu CL, Lu YT, et al: miR-409-3p/-5p
promotes tumorigenesis, epithelial-to-mesenchymal transition, and
bone metastasis of human prostate cancer. Clin Cancer Res.
20:4636–4646. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Slomovitz BM and Coleman RL: The
PI3K/AKT/mTOR pathway as a therapeutic target in endometrial
cancer. Clin Cancer Res. 18:5856–5864. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yulyana Y, Ho IA, Sia KC, Newman JP, Toh
XY, Endaya BB, Chan JK, Gnecchi M, Huynh H, Chung AY, et al:
Paracrine factors of human fetal MSCs inhibit liver cancer growth
through reduced activation of IGF-1R/PI3K/Akt signaling. Mol Ther.
23:746–756. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vo BT, Morton D Jr, Komaragiri S, Millena
AC, Leath C and Khan SA: TGF-β effects on prostate cancer cell
migration and invasion are mediated by PGE2 through activation of
PI3K/AKT/mTOR pathway. Endocrinology. 154:1768–1779. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yakes FM, Chinratanalab W, Ritter CA, King
W, Seelig S and Arteaga CL: Herceptin-induced inhibition of
phosphati-dylinositol-3 kinase and Akt is required for
antibody-mediated effects on p27, cyclin D1, and antitumor action.
Cancer Res. 62:4132–4141. 2002.PubMed/NCBI
|
|
27
|
Kumar P, Miller AI and Polverini PJ: p38
MAPK mediates γ-irradiation-induced endothelial cell apoptosis, and
vascular endothelial growth factor protects endothelial cells
through the phosphoinositide 3-kinase-Akt-Bcl-2 pathway. J Biol
Chem. 279:43352–43360. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Song MS, Salmena L and Pandolfi PP: The
functions and regulation of the PTEN tumour suppressor. Nat Rev Mol
Cell Biol. 13:283–296. 2012.PubMed/NCBI
|
|
29
|
Mulholland DJ, Tran LM, Li Y, Cai H, Morim
A, Wang S, Plaisier S, Garraway IP, Huang J, Graeber TG and Wu H:
Cell autonomous role of PTEN in regulating castration-resistant
prostate cancer growth. Cancer Cell. 19:792–804. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee S, Choi EJ, Jin C and Kim DH:
Activation of PI3K/Akt pathway by PTEN reduction and PIK3CA mRNA
amplification contributes to cisplatin resistance in an ovarian
cancer cell line. Gynecol Oncol. 97:26–34. 2005. View Article : Google Scholar : PubMed/NCBI
|