Introduction
Excessive alcohol consumption increases the risk of
heart disease, which continues to be one of the major causes of
mortality and morbidity in many countries. High doses of ethanol
can induce or exacerbate a series of pathophysiological disorders,
leading to cardiomyocyte apoptosis, myocardial fibrosis,
cardiomyopathy and congestive heart failure, all associated with
alcoholic cardiomyopathy (ACM) (1).
Several mechanisms are involved in mediating the
adverse effects of ethanol, including the induction of oxidative
stress and apoptotic cell death (1). There is abundant evidence indicating
that ethanol promotes cellular apoptosis and this in turn causes
the loss of cardiomyocytes (2–5).
Myocyte loss or cell death may be an important mechanism of organ
dysfunction and pathology. Several early studies on animal models
of ACM and patients with ACM support a role for myocyte loss as an
underlying mechanism of ethanol-induced cardiac dysfunction
(2,6,7).
These observations collectively imply that high-dose ethanol can
diminish the cardiomyocyte population by the induction of
apoptosis, which appears to result in subsequent abnormalities.
Compelling evidence indicates that oxidative stress,
excessive intracellular reactive oxygen species (ROS) production,
exceeding the antioxidant capacity of cells, plays a critical role
in ethanol-induced apoptosis (8–11).
ROS generation has been observed in ethanol-exposed cultured cells,
including cardiac cells (8–10),
as well as in the pathologies of several other types of
cardiovascular insults, such as ischemia-reperfusion injury and
cardiomyopathies (12). Others
have suggested that ethanol-induced excessive ROS generation and
oxidative stress may result from several processes or mechanisms
involving mitochondrial cytochrome p450, xanthine oxidase, and
NADPH oxidase (13,14). Once produced, ROS may not only
cause oxidative damage to biomolecules, such as DNA, protein and
lipids, but can also regulate the expression of genes related to
growth and cell death (15,16). Of note, a number of events typical
of oxidative stress are observed in cardiomyocytes following
exposure to ethanol, which include myocyte loss and disarray
(2), as well as changes in
intracellular organelles. In addition, studies have also revealed
that not only antioxidants, such as vitamin E and vitamin C
(9), but also antioxidant
enzymes, including superoxide dismutase (SOD), catalase (CAT) and
heme oxygenase-1 (HO-1), all can inhibit ethanol-induced oxidative
stress and apoptosis (5,14,17). Therefore, it is important to find
effective antioxidants or means with which to improve myocardial
cell oxidative stress states caused by ethanol.
Nuclear factor erythroid 2-related factor 2 (Nrf2),
a member of the cap 'n' collar family of basic region-leucine
zipper (bZIP) transcription factors, which is expressed in a
variety of tissues, is considered as one of the major intracellular
defense systems with which to combat oxidative stress (18,19). Under basal conditions, Nrf2 is
located mainly in the cytoplasm bound to the Kelch-like
ECH-associated protein 1 (Keap1), an adaptor protein for the
Cul3-dependent ubiquitination and degradation of Nrf2. When
activated, Nrf2 stabilizes and translocates to the nucleus, leading
to the induction of the expression of phase II detoxifying and
antioxidant genes, such as SOD, HO-1 and CAT (18,19). Nrf2 has been shown to provide
protection against a number of oxidative stress-related
cardiovascular diseases (19–22), and may be a target for the
treatment of cardiomyocyte injury (23). Preliminary data from a recent
review revealed that low doses (5 mM ethanol) of alcohol increased
the expression of Nrf2 and its homolog Nrf1 to provide
cardioprotection (24). Recently,
studies have also evidenced that the upregulation of Nrf2 by
inducers, such as 3H-1,2-dithiole-3-thione (D3T) or sulforaphane
(SFN), significantly decrease the ethanol-induced ROS generation in
and the apoptosis of cells i the nervous system (25,26). However, an inducer of Nrf2
successfully employed to prevent ACM has not yet been
identified.
Tert-butylhydroquinone (tBHQ), a potent inducer of
Nrf2, has received increasing attention due to its ability to
activate many cytoprotective and detoxifying enzymes (27–31). Of note, tBHQ has been approved for
human use as a synthetic food antioxidant to protect oils and fats
from oxidative deterioration and rancidity (32). tBHQ can enhance Nrf2-mediated
transcriptional activation depending on the increase of Nrf2
protein stability through the inhibition of the Keap1-mediated
ubiquitination (27,28). There is substantial evidence to
support the finding that tBHQ can aid in protecting various cells
and organs against oxidative insults through the activation of Nrf2
signaling (29–31). A recent study revealed that tBHQ
prevented ethanol-induced apoptosis by the induction of the
Nrf2-driven antioxidant response in cranial neural crest cells
(33). Nevertheless, to date, in
spite of being a well-recognized and effective strategy for the
protection of a variety of different cell types and organs under
oxidative stress conditions, the cardioprotective effects of tBHQ
have not been investigated, at least to the best of our
knowledge.
This study aimed to examine the effects of tBHQ on
ethanol-induced oxidative stress and the apoptosis of a cultured
H9c2 cell line, a cloned heart muscle cell line originating from
embryonic rat hearts that presents many cardiomyocyte
phenotypes.
Materials and methods
Reagents
Cell culture and treatment
The H9c2 cells (from the Shanghai Institutes for
Biological Sciences, Shanghai, China) were cultured in Dulbecco's
modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS),
penicillin G (100 U/ml) and streptomycin (100 mg/ml) (Gibco-BRL,
Grand Island, NY, USA). The cells were maintained in a tissue
culture incubator at 37°C in a 5% CO2 atmosphere. The
medium was changed every 2–3 days, and the cells were subcultured
when the cell population density reached 70–80% confluence. In
order to determine the optimal concentrations of ethanol (EtOH;
Beijing Chemical Reagent Co., Ltd., Beijing, China) and tBHQ, the
cells were exposed to various concentrations of both agents [EtOH:
0, 25, 50, 100, 200, 400 and 800 mM for 24 h; and tBHQ (Sigma
Chemical Co., St. Louis, MO, USA): 0, 0.625, 1.25, 2.5, 5, 10, 20,
50 and 100 µM for 48 h]. Four sets of experiments were then
performed under standard culture conditions: i) untreated control
cells; ii) cell treatment with 200 mM ethanol; iii) cell treatment
with 5 µM tBHQ; and iv) cell pre-treatment with 5 µM
tBHQ for 24 h, followed by medium change and co-culture with 200 mM
ethanol containing 5 µM tBHQ for a further 24 h.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
Cell viability was examined by MTT assay. The H9c2
cells were seeded on 96-well plates at a density of
1.5×104 cells/well and were maintained in regular growth
medium for 24 h. The cells were then subjected to the ethanol and
tBHQ treatments, as described above. After the treatments, the H9c2
cells were incubated with 20 µl MTT solution (5 mg/ml
phosphate buffer; obtained from Sigma Chemical Co.) at 37°C for 4
h, and the purple formazan crystals were then solubilized with 150
µl DMSO (Sigma Chemical Co.) at room temperature for 10 min.
The absorbance at 490 nm was measured using a microplate reader
(Sunrise RC; Tecan Group, Ltd., Mannedorf, Switzerland), and cell
viability was expressed as a percentage of the control culture
value.
Detection of apoptosis using Annexin
V-FITC/propidium iodide (PI)
The apoptotic cells were detected by using Annexin
V-FITC apoptosis detection kits (Nanjing KeyGen Biotech Co., Ltd.,
Nanjing, China). The cells were collected, washed twice with PBS,
resuspended in binding buffer (10 mM HEPES/NaOH pH 7.4, 140 mM NaCl
and 2.5 mM CaCl2) and incubated with Annexin V at room
temperature in the dark for 15 min. The cells were then
centrifuged, resuspended in binding buffer, and incubated with PI.
Subsequently, binding buffer (500 µl) was added, and the
apoptotic cells were assessed using a flow cytometer (FACSCalibur;
BD Biosciences, Franklin Lakes, NJ, USA).
Measurement of ROS production
ROS generation was measured using a 5(6)-carboxy-2′,7′-dichlorofluorescein
diacetate (cDCFH-DA) detection kit (Beyotime Biotechnology,
Jiangsu, China). After harvest, the cells were washed with PBS and
then incubated with 10 µM of cDCFH-DA diluted in serum-free
medium at 37°C for 30 min. The fluorescence intensity was monitored
for 30 min after excitation at 488 nm and emission at 525 nm using
a FACSort cell sorter (Becton-Dickinson, San Diego, CA, USA).
Western blot analysis
The cells were lysed in ice-cold RIPA buffer [50 mM
Tris (pH 7.4), 150 mM NaCl, 1% Triton, 0.5% deoxycholate, 0.1% SDS,
1 mM EDTA, 10 mM NaF, and 0.1 mM phenylmethylsulfonyl fluoride
(PMSF)] to obtain total proteins. Nuclear protein was extracted
using a nuclear and cytoplasmic protein extraction kit following
the manufacturer's instructions. The protein concentration was
detected via the BCA protein assay (Beyotime Biotechnology). Equal
amounts of protein samples (50 µg protein/lane) were
separated by 10–12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred onto polyvinylidene
difluoride (PVDF) membranes. The non-specific antibodies were
blocked with 5% non-fat dried milk in PBS for 2 h at room
temperature. Membranes were then incubated overnight at 4°C with
primary antibodies directed against the Nrf2 (ab31163) at 1:200
dilution, and HO-1 (ab13248) (both from Abcam, Cambridge, UK), SOD
(sc-101523) and CAT (A-4; sc-271358) (both from Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), caspase-3 (3CSP03;
ab2171; Abcam), Bcl-2-associated X protein (Bax; P-19; sc-526) and
B-cell lymphoma-2 (Bcl-2; N-19; sc-492) (Santa Cruz Biotechnology,
Inc.) at 1:500 dilution. The membranes were then washed with TBST 3
times and further incubated with horseradish peroxidase-conjugated
secondary antibody (1:2,000) for 1 h at room temperature. After
washing, the membranes were processed using an
electroche-miluminescence (ECL) reagent (Pierce Biotechnology,
Inc., Rockford, IL, USA) and the light emission was captured on
X-ray film. The signals were visualized by chemiluminescent
horseradish peroxidase substrate and then subjected to a
densi-tometric analysis and normalized to tubulin or lamin-B1.
Immunofluorescence staining
The cellular localization of Nrf2 was determined by
immunofluorescence staining. The H9c2 cells were seeded at
1.5×104 cells/well in glass chamber slides (glass
coverslips) and cultured overnight at 37°C. The cells were then
exposed to ethanol or tBHQ alone, or in combination, as described
above. At the end of incubation, the cells were washed twice in PBS
and fixed in 4% paraformaldehyde for 10 min at room temperature.
The cells were then permeabilized with 0.1% Triton X-100, washed
and incubated with blocking buffer (10% NGS) for 1 h at room
temperature. The cells were then incubated overnight with primary
antibodies against AC-tubulin (6-11B-1; ab24610) at 1:200 dilution
and Nrf2 (both from Abcam) at a 1:200 dilution in a humidified
chamber at 4°C. Subsequently, the cells were washed with PBS and
incubated with secondary antibodies conjugated to either FITC or
TRITC at a dilution of 1:500 (Jackson ImmunoResearch Laboratories,
Inc., West Grove, PA, USA) for 1 h at room temperature in the dark,
and were then counterstained with DAPI dye to show the nuclear
morphology. After the slides were rinsed with PBS, coverslips were
mounted on slides, and images of the labeled cells were visualized
and photographed using a confocal fluorescence microscope (TCS SP2;
Leica, Wetzlar, Germany).
Statistical analysis
The data are presented as the means ± SD. One-way
analysis of variance (ANOVA) followed by the Student-Newman-Keuls
test was applied to calculate the statistical significance between
various groups. The differences were considered to be statistically
significant at p<0.05.
Results
Treatment with tBHQ markedly enhances the
viability of H9c2 cardiomyocytes exposed to ethanol
As shown in Fig.
1A, the exposure to ethanol at 200 mM for 24 h significantly
decreased the percentage of surviving cells. Thus, the
concentration of 200 mM ethanol was used as a standard
concentration with which to induce apoptosis in the subsequent
experiments. The results of MTT assay demonstrated no decrease in
the viability of cells exposed to various concentrations of tBHQ
(0, 0.625, 1.25, 2.5, 5, 10, 20, 50 and 100 µM) for 48 h
(Fig. 1B). Furthermore, the
results revealed that pre-incubation of the H9c2 cardiomyocytes
with various concentrations of tBHQ (0, 0.625, 1.25, 2.5, 5, 10,
20, 50 and 100 µM) enhanced cell viability which was
decreased due to exposure to ethanol in a dose-dependent manner
(Fig. 1C). Maximum viability was
apparent at a concentra tion of 5 µM. However, higher
concentrations (50 and 100 µM) of tBHQ did not cause any
attenuation of the inhibitory effects of ethanol on cell viability.
Therefore, we employed 5 µM of tBHQ as a concentration for
use in our subsequent experiments.
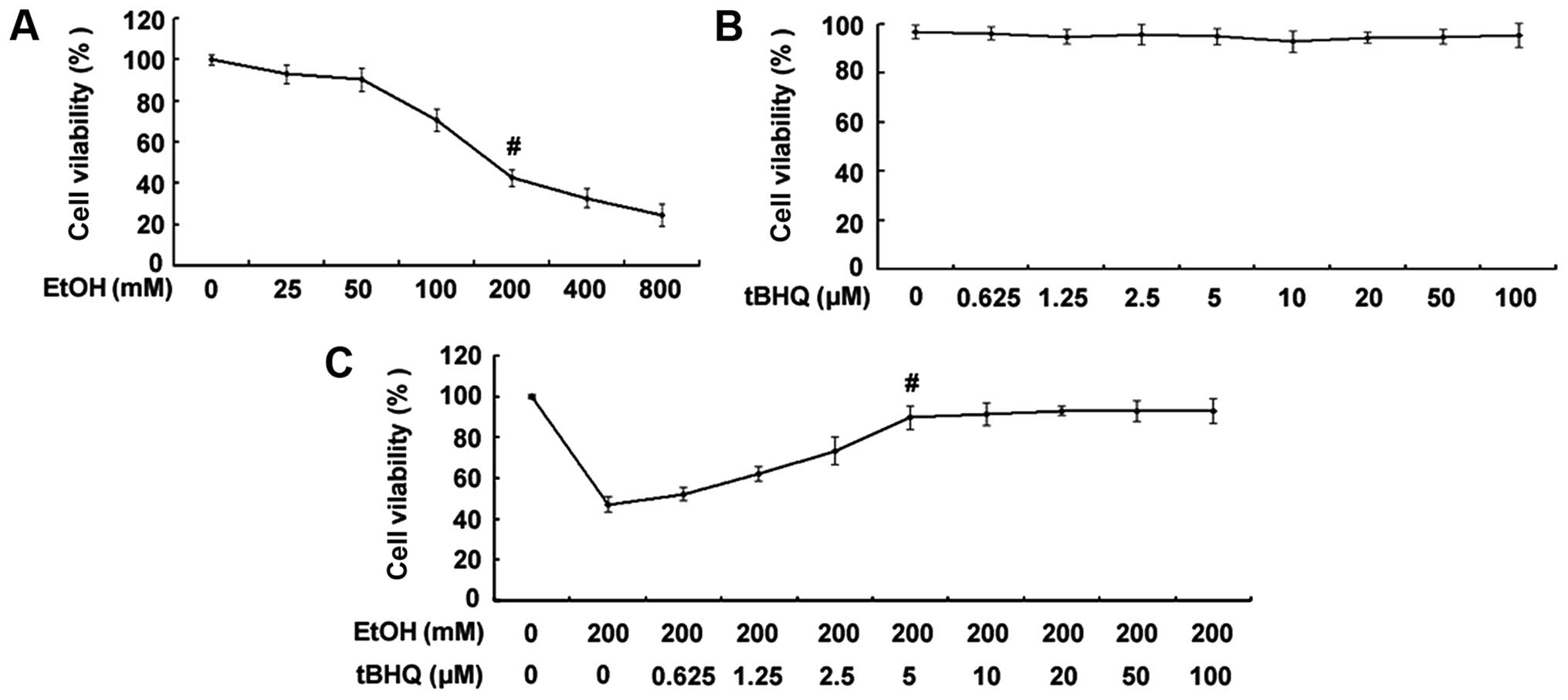 | Figure 1(A) Effect of various concentrations
of ethanol (EtOH) on cell viability. H9c2 cardiomyocytes were
exposed to various concentrations of ethanol (0, 25, 50, 100, 200,
400 and 800 mM) for 24 h. Viable cells were identified by MTT
assay. The data are shown as the means ± SD; n=5,
#p<0.01 vs. control group. (B) Effect of various
concentrations of tert-butylhydroquinone (tBHQ) on cell viability.
The H9c2 cardiomyocytes were incubated with various concentrations
of tBHQ (0, 0.625, 1.25, 2.5, 5, 10, 20, 50 and 100 µM) for
48 h. Cell viability was measured by MTT assay. No decrease in the
viability of cells exposed to various concentrations of tBHQ for 48
h was established. (C) tBHQ protects against EtOH-induced cell
death of cultured H9c2 cardiomyocytes in a dose-dependent manner.
The cells were treated with various concentrations of tBHQ (0,
0.625, 1.25, 2.5, 5, 10, 20, 50 and 100 µM) for 24 h,
followed by incubation for 24 h with 200 mM of ethanol. Cell
viability was analyzed by MTT assay. The values are expressed as
the means ± SD; n=5 experiments, #p<0.05 vs. EtOH
alone. |
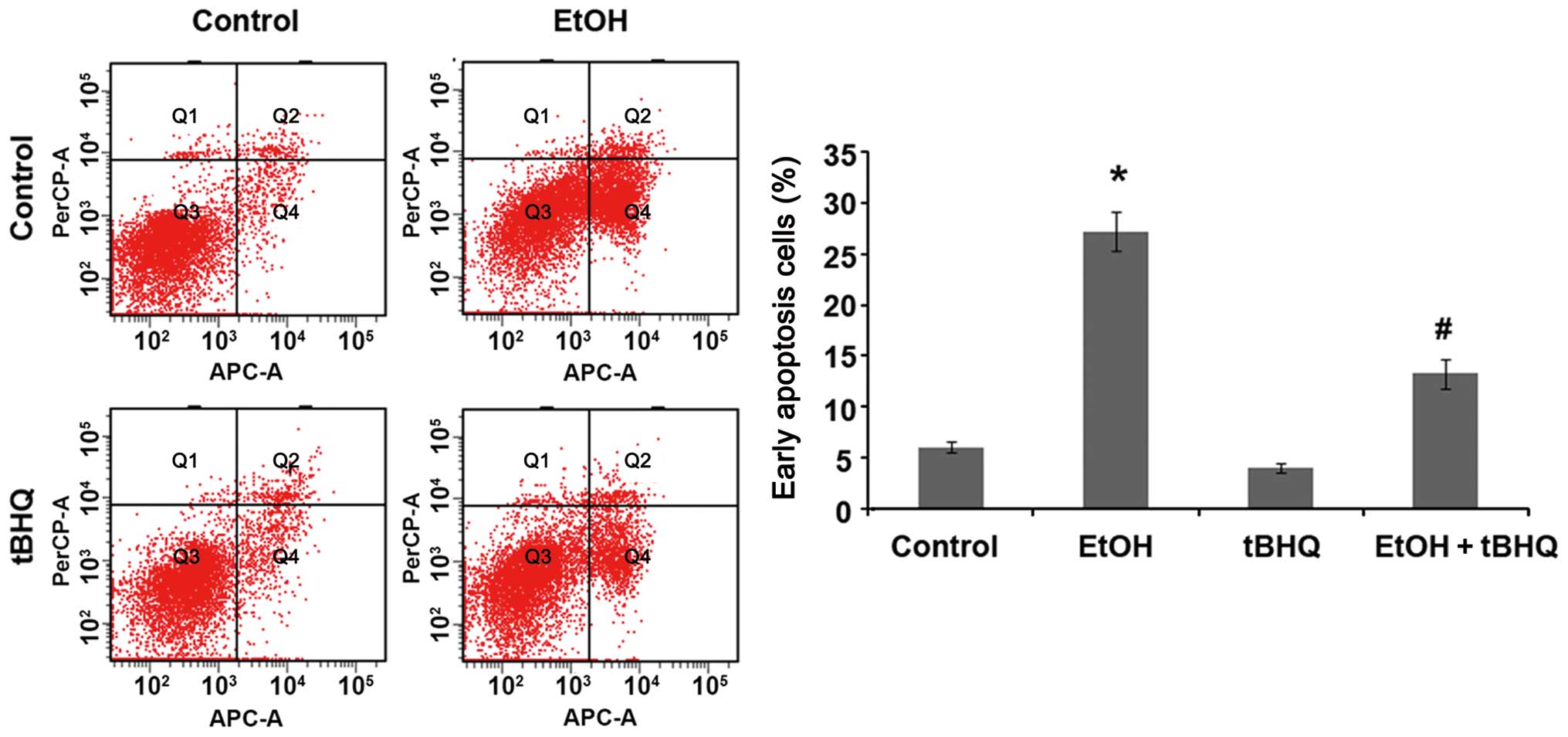
Treatment with tBHQ prevents the
ethanol-induced apoptosis of H9c2 cells
We performed a flow cytometric analysis and found
that ethanol caused a substantial increase in the number of
apoptotic cells, whereas pre-treatment with tBHQ significantly
lowered the amount of apoptotic cells compared with the cells
exposed to ethanol alone (Fig.
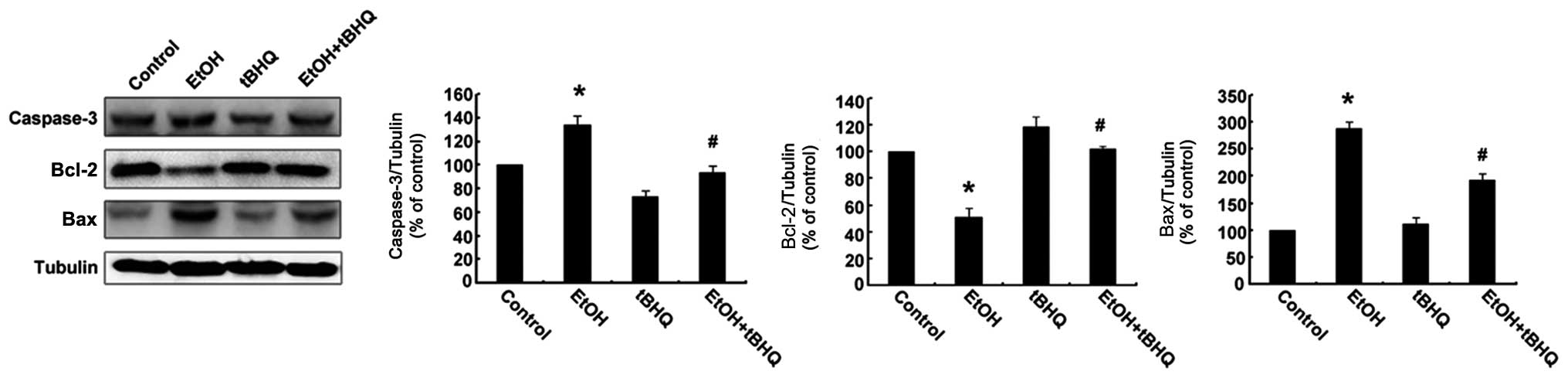
2). In addition, the results of western blot analysis revealed
that the H9c2 cells exposed to ethanol manifested a marked increase
in the expression of the apoptotic protein, caspase-3, and the
pro-apoptotic protein, Bax, while a significant decrease in the
expression of the anti-apoptotic protein, Bcl-2, was observed.
However, the pre-treatment with tBHQ markedly inhibited the
ethanol-induced increase in caspase-3 and Bax expression, and
enhanced Bcl-2 expression (Fig.
3).
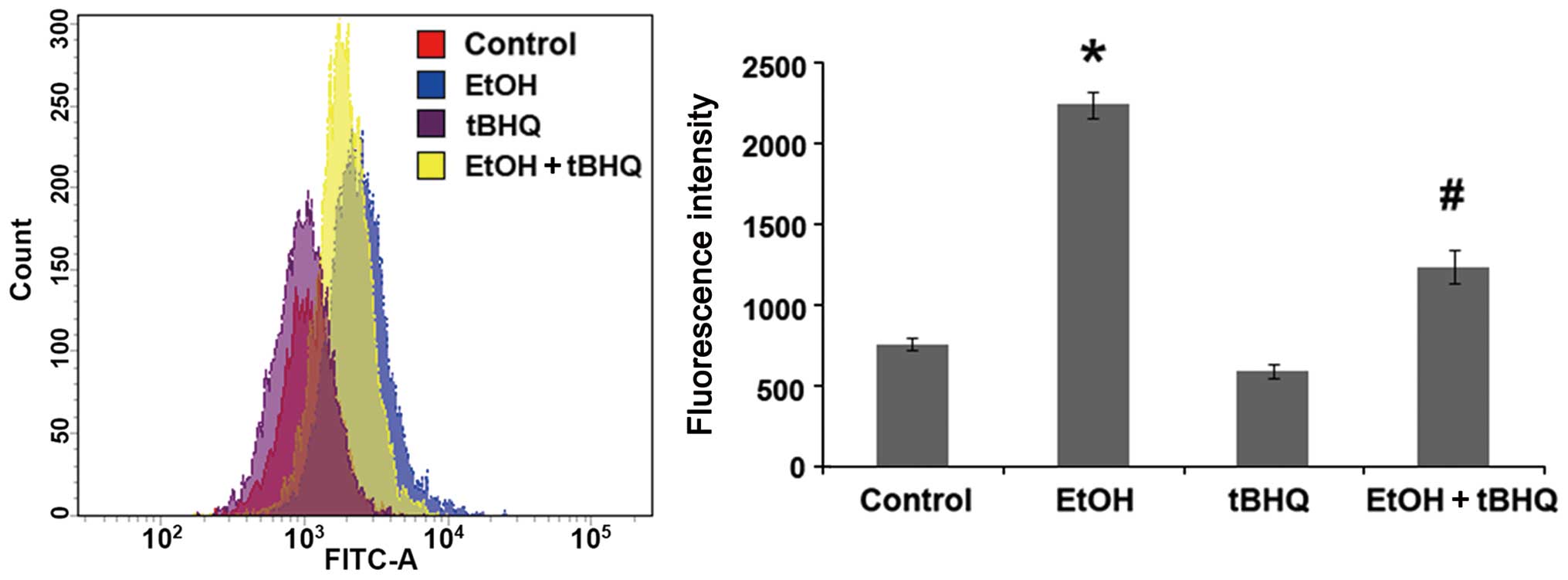
Treatment with tBHQ significantly
prevents the ethanol- induced excessive production of ROS in H9c2
cells
To determine whether tBHQ can prevent the oxidative
stress induced by ethanol in cardiomyocytes, a cDCFH-DA assay
(Beyotime Biotechnology) was performed to measure ROS production,
representing one of the key events in apoptotic cell death. We
found that the exposure to ethanol at 200 mM induced considerable
ROS production, which was attenuated significantly by pre-treatment
with BHQ (Fig. 4).
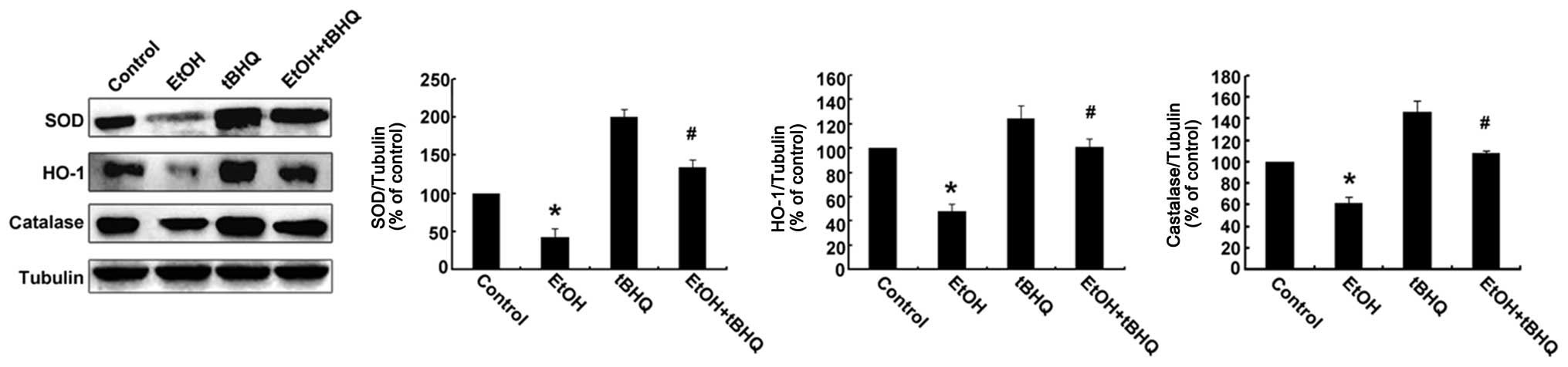
Treatment with tBHQ restores the
expression of antioxidant proteins, including SOD, CAT and HO-1 in
ethanol-exposed H9c2 cells
Western blot analysis was performed to determine
whether tBHQ affects the expression of Nrf2 downstream antioxidant
proteins (SOD, HO-1, and CAT) in H9c2 cells exposed to ethanol. As
illustrated in Fig. 5, the
protein expression of SOD, HO-1 and CAT was decreased in the
ethanol-exposed cells compared with that in the ethanol-untreated
cells, whereas it was restored by tBHQ in the ethanol-exposed H9c2
cells.
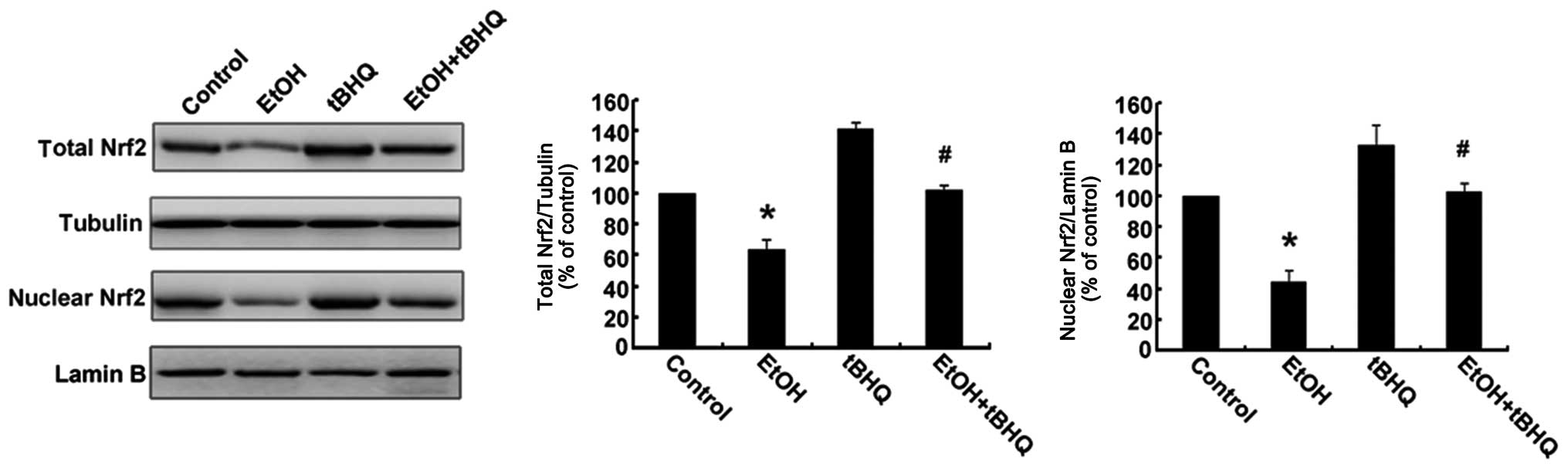
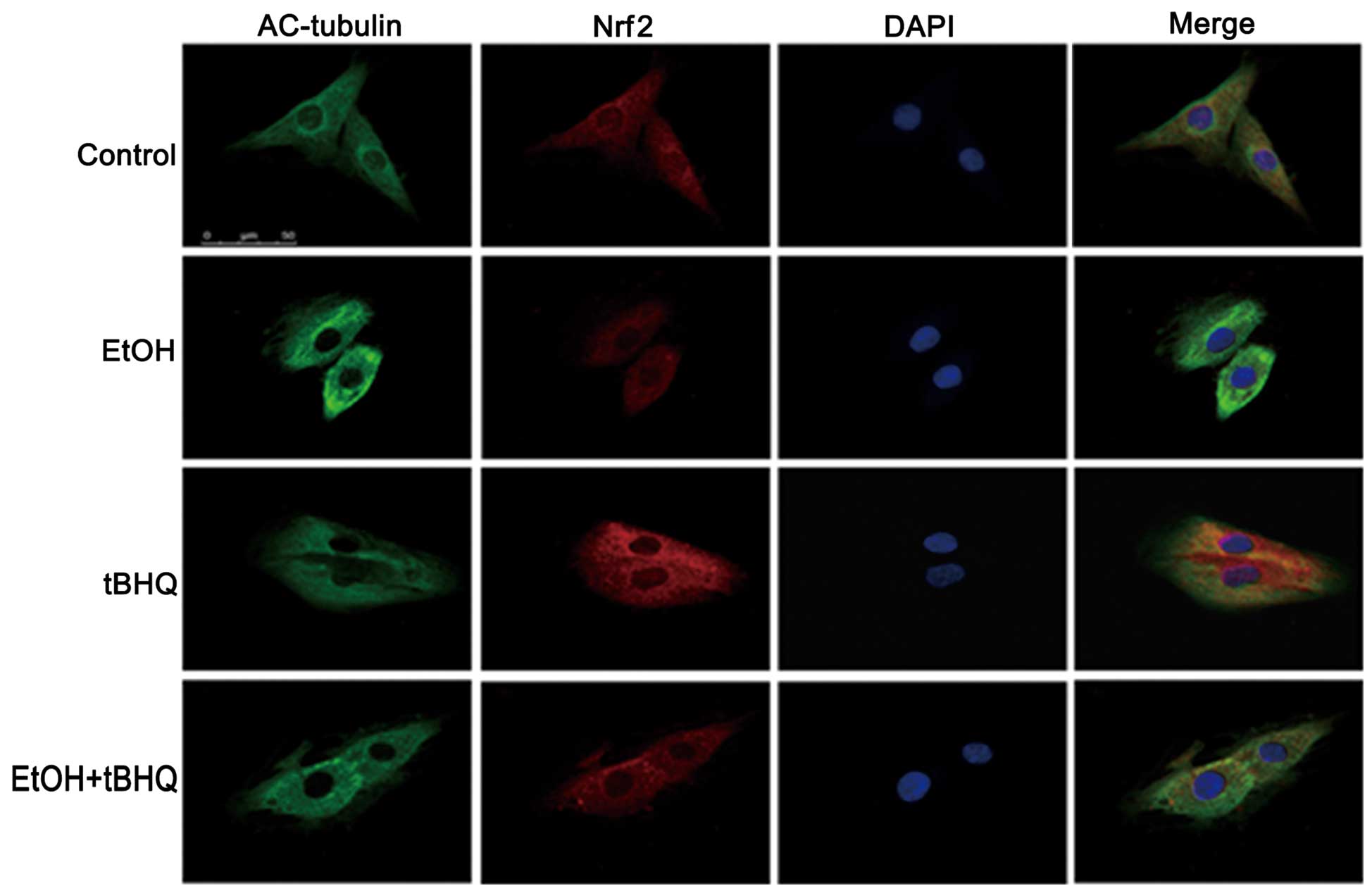
Treatment with tBHQ increases the protein
expression and promotes the nuclear translocation of Nrf2 which is
inhibited by exposure to ethanol in H9c2 cells
As depicted in Figs.
6 and 7, ethanol inhibited
not only Nrf2 protein expression, but also Nrf2 nuclear
translocation, which may have made the cells more sensitive to
ethanol-induced toxicity. tBHQ alone partially, but significantly
increased the Nrf2 protein level in the H9c2 cells. Pre-treatment
wit htBHQ restored the level of Nrf2 protein and promoted its
nuclear accumulation in the H9c2 cells, which were inhibited by
exposure to ethanol, which may have elicited the cytoprotective
response, resulting in the increased resistance of the cells to the
ethanol challenge. The above-mentioned results were confirmed by
western blot analysis and immunofluorescence staining.
Discussion
Previous studies have demonstrated that apoptosis is
the major factor responsible for ethanol-induced myocardial cell
death, which is closely associated with ACM (1). For this reason, for preventing ACM,
it is crucial to find an effective anti-apoptotic agent. In this
study, treatment with tBHQ significantly reduced the occurrence of
ethanol-induced cardiomyocyte apoptosis and promoted cell survival,
as confirmed by the results of MTT assay, flow cytometry and the
expression of caspase-3. Simultaneously, treatment withy tBHQ
significantly reduced the ethanol-induced generation of ROS (as
shown in Fig. 4). Furthermore,
treatment with tBHQ not only promoted the nuclear translocation of
Nrf2 (as depicted in Fig. 7), but
also increased Nrf2 expression which was decreased by the exposure
of the H9c2 cells to ethanol, which in turn led to the enhancement
of the levels of antioxidant proteins, including SOD, HO-1 and CAT.
In addition, the results also revealed that tBHQ protected the H9c2
cells from ethanol-induced apoptosis by participating in the
upregulation of Bcl-2 expression and the downregulation of Bax
expression. Taken together, these results provide substantial
evidence that tBHQ can protect H9c2 cardiomyocytes against
ethanol-induced oxidative stress and apoptosis, which may be
associated with the activation of the Nrf2 antioxidant response
pathway. To the best of our knowledge, this study is the first to
demonstrate the cardioprotec tive effects of tBHQ on the apoptosis
of H9c2 cardiomyocytes induced by ethanol.
Oxidative stress is well known as a major mechanism
leading to apoptosis induced by ethanol (8–10).
ROS are the major cause of cellular oxidative stress, which can
both oxidize biomolecules and regulate the expression of genes
related to apoptosis, and can serve as a signaling link between
ethanol-induced oxidative stress and apoptosis, leading to cell
apoptosis (16,34). In the present study, we confirmed
that the exposure of H9c2 cells to 200 mM ethanol increased ROS
generation excessively (Fig. 4).
In our experiments, 200 mM ethanol was used as an inducing
concentration according to the results of MTT assay and our pilot
trial. It is a high-dose concentration which has been confirmed to
effectively induce the ROS-mediated apoptosis of cardiomyocytes
(34). The results from this
study also demonstrated that ethanol markedly induced the apoptosis
of H9c2 cells, as indicated by the results of flow cytometric
analysis, which revealed a significant increase in the apoptotic
cell number in the ethanol-exposed H9c2 cells as compared to the
controls (Fig. 2). In parallel,
ethanol activated caspase-3 and Bax, but inhibited Bcl-2, as shown
in Fig. 3. These results further
confirmed that the oxidative stress induced by ethanol played a
crucial role in eliciting cardiomyocyte apoptosis. Thus,
anti-oxidative stress processes may indeed be critically beneficial
to prevent apoptosis and protect cardiac function.
tBHQ is a potent antioxidant compound which exerts
protective effects on multiple cells and organs, including
neuroprotective (35–37), hepa toprotective (38) and renal protective effects
(30,39). It has been shown that tBHQ
diminishes apoptosis by reducing oxidative stress (37). More importantly, tBHQ inhibits
ethanol-induced oxidative stress and the apoptosis of cranial
neural crest cells (33). Through
examination, we further evidenced that tBHQ markedly inhibited the
apoptosis of cardiomyocytes exposed to ethanol (as confirmed by MTT
assay, Annexin V-FITC staining and the protein expression of
caspase-3) accompanied by a decrease of ROS (as illustrated in
Fig. 4). As a central regulator
of oxidative stress, Nrf2 can effectively inhibit ROS generation
and prevent apoptosis (40,41). Following activation, Nrf2 can
translocate to the nucleus to promote the expression of antioxidant
enzymes to reduce ROS and prevent apoptosis (42). Moreover, other researchers have
found that Nrf2 increases the levels of the anti-apoptotic
proteins, Bcl-2 and Bcl-xL, which inhibits apoptosis (43). Nrf2 can be activated by a chemical
inducer. tBHQ has long been known as an effective inducer of Nrf2
(36). Substantial evidence has
indicated that activating the Nrf2 pathway by tBHQ can prevent
apoptosis (27,29). Independently of Nrf2 activation,
other protective mechanisms of tBHQ are also involved in the
induction of autophagy (44,45). In addition, a recent study
demonstrated that tBHQ activated Akt rather than Nrf2 to suppress
apoptosis (46). In our study,
pre-treatment with tBHQ significantly restored the level of Nrf2
protein (Fig. 6) and promoted
nuclear translocation (as shown in Fig. 7) previously inhibited by ethanol
exposure in H9c2 cells, with an increase in the levels of the
antioxidant proteins, SOD, HO-1 and CAT. All the findings mentioned
above provide substantial evidence that tBHQ enhances the
resistance against ethanol-induced cytotoxicity in H9c2 cells, and
activates the Nrf2 cytoprotective response pathway, thus reducing
apoptosis.
There are many studies demonstrating that the
anti-apoptotic Bcl-2 and proapoptotic Bax genes play a major role
in maintaining the balance of cell death and survival, and the
upregulation of the anti-apoptotic Bcl-2 protein can antagonize the
pro-apoptotic activities of Bax (47). Moreover, Niture and Jaiswal found
that tBHQ antagonized Bcl-2, INrf2 (inhibitor of Nrf2) interaction,
led to the release and stabilization of Bcl-2, increased Bcl-2, Bax
heterodimers, and reduced the apoptosis of mouse Hepa-1 cells
(48). Further examination
indicated that pre-treatment with tBHQ destabilized
Nrf2-Keapl/PGAM5 (phosphog1ycerate mutase 5)-Bcl-xL, increased the
release of Bcl-xL and inhibited apoptosis (49). In the present study, we found that
ethanol markedly downregulated Bcl-2 expression, while it
upregulated Bax expression. Conversely, pre-treatment with tBHQ
reversed these changes in Bax and Bcl-2 protein expression in the
H9c2 cardiomyocytes exposed to ethanol, as confirmed by the resutls
of western blot analysis. Consistently, pre-treatment with tBHQ
markedly inhibited the ethanol-induced increase in the expression
of caspase-3 in H9c2 cells.
In conclusion, these new experimental findings
indicate that pre-treatment with tBHQ protects H9c2 cardiomyocytes
against ethanol-induced oxidative stress and apoptosis. Our results
also suggest that the anti-apoptotic effects of tBHQ are at least
partly mediated by the inhibition of ROS generation, the activation
of Nrf2 signaling pathway, the reduction of caspase-3 expres sion
and the modulation of Bcl-2 and Bax expres sion. tBHQ may thus
provide a valuable therapeutic intervention for the treatment of
ACM.
References
|
1
|
Piano MR and Phillips SA: Alcoholic
cardiomyopathy: pathophysiologic insights. Cardiovasc Toxicol.
14:291–308. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Capasso JM, Li P, Guideri G, Malhotra A,
Cortese R and Anversa P: Myocardial mechanical, biochemical, and
structural alterations induced by chronic ethanol ingestion in
rats. Circ Res. 71:346–356. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li SY, Li Q, Shen JJ, Dong F, Sigmon VK,
Liu Y and Ren J: Attenuation of acetaldehyde-induced cell injury by
overexpression of aldehyde dehydrogenase-2 (ALDH2) transgene in
human cardiac myocytes: role of MAP kinase signaling. J Mol Cell
Cardiol. 40:283–294. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen DB, Wang L and Wang PH: Insulin-like
growth factor I retards apoptotic signaling induced by ethanol in
cardiomyocytes. Life Sci. 67:1683–1693. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tan Y, Li X, Prabhu SD, Brittian KR, Chen
Q, Yin X, McClain CJ, Zhou Z and Cai L: Angiotensin II plays a
critical role in alcohol- induced cardiac nitrative damage, cell
death, remodeling, and cardiomyopathy in a protein kinase
C/nicotinamide adenine dinucleotide phosphate oxidase-dependent
manner. J Am Coll Cardiol. 59:1477–1486. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fernández-Solà J, Fatjó F, Sacanella E,
Estruch R, Bosch X, Urbano-Márquez A and Nicolás JM: Evidence of
apoptosis in alcoholic cardiomyopathy. Hum Pathol. 37:1100–1110.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fernández-Solà J, Lluis M, Sacanella E,
Estruch R, Antúnez E and Urbano-Márquez A: Increased myostatin
activity and decreased myocyte proliferation in chronic alcoholic
cardiomyopathy. Alcohol Clin Exp Res. 35:1220–1229. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kannan M, Wang L and Kang YJ: Myocardial
oxidative stress and toxicity induced by acute ethanol exposure in
mice. Exp Biol Med (Maywood). 229:553–559. 2004.
|
|
9
|
Guan Z, Lui CY, Morkin E and Bahl JJ:
Oxidative stress and apoptosis in cardiomyocyte induced by
high-dose alcohol. J Cardiovasc Pharmacol. 44:696–702. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jing L, Jin CM, Li SS, Zhang FM, Yuan L,
Li WM, Sang Y, Li S and Zhou LJ: Chronic alcohol intake-induced
oxidative stress and apoptosis: role of CYP2E1 and calpain-1 in
alcoholic cardiomyopathy. Mol Cell Biochem. 359:283–292. 2012.
View Article : Google Scholar
|
|
11
|
Umoh NA, Walker RK, Al-Rubaiee M, Jeffress
MA and Haddad GE: Acute alcohol modulates cardiac function as
PI3K/Akt regulates oxidative stress. Alcohol Clin Exp Res.
38:1847–1864. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ho E, Karimi Galougahi K, Liu CC, Bhindi R
and Figtree GA: Biological markers of oxidative stress:
applications to cardiovascular research and practice. Redox Biol.
1:483–491. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lucas DL, Brown RA, Wassef M and Giles TD:
Alcohol and the cardiovascular system: research challenges and
opportunities. J Am Coll Cardiol. 45:1916–1924. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang RH, Gao JY, Guo HT, Scott GI, Eason
AR, Wang XM and Ren J: Inhibition of CYP2E1 attenuates chronic
alcohol intake-induced myocardial contractile dysfunction and
apoptosis. Biochim Biophys Acta. 1832:128–141. 2013. View Article : Google Scholar
|
|
15
|
Kannan K and Jain SK: Oxidative stress and
apoptosis. Pathophysiology. 7:153–163. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zu L, Zheng X, Wang B, Parajuli N,
Steenbergen C, Becker LC and Cai ZP: Ischemic preconditioning
attenuates mitochondrial localization of PTEN induced by
ischemia-reperfusion. Am J Physiol Heart Circ Physiol.
300:H2177–H2186. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kino M: Chronic effects of ethanol under
partial inhibition of catalase activity in the rat heart: light and
electron microscopic observations. J Mol Cell Cardiol. 13:5–21.
1981. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jaiswal AK: Nrf2 signaling in coordinated
activation of antioxidant gene expression. Free Radic Biol Med.
36:1199–1207. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li J, Ichikawa T, Villacorta L, Janicki
JS, Brower GL, Yamamoto M and Cui T: Nrf2 protects against
maladaptive cardiac responses to hemodynamic stress. Arterioscler
Thromb Vasc Biol. 29:1843–1850. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Calvert JW, Elston M, Nicholson CK,
Gundewar S, Jha S, Elrod JW, Ramachandran A and Lefer DJ: Genetic
and pharmacologic hydrogen sulfide therapy attenuates
ischemia-induced heart failure in mice. Circulation. 122:11–19.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li J, Zhang C, Xing Y, Janicki JS,
Yamamoto M, Wang XL, Tang DQ and Cui T: Up-regulation of p27(kip1)
contributes to Nrf2-mediated protection against angiotensin
II-induced cardiac hypertrophy. Cardiovasc Res. 90:315–324. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li S, Wang W, Niu T, Wang H, Li B, Shao L,
Lai Y, Li H, Janicki JS, Wang XL, et al: Nrf2 deficiency
exaggerates doxorubicin-induced cardiotoxicity and cardiac
dysfunction. Oxid Med Cell Longev. 2014:7485242014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li J, Ichikawa T, Janicki JS and Cui T:
Targeting the Nrf2 pathway against cardiovascular disease. Expert
Opin Ther Targets. 13:785–794. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Walker RK, Cousins VM, Umoh NA, Jeffress
MA, Taghipour D, Al-Rubaiee M and Haddad GE: The good, the bad, and
the ugly with alcohol use and abuse on the heart. Alcohol Clin Exp
Res. 37:1253–1260. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dong J, Yan D and Chen SY: Stabilization
of Nrf2 protein by D3T provides protection against ethanol-induced
apoptosis in PC12 cells. PLoS One. 6:e168452011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen X, Liu J and Chen SY: Sulforaphane
protects against ethanol-induced oxidative stress and apoptosis in
neural crest cells by the induction of Nrf2-mediated antioxidant
response. Br J Pharmacol. 169:437–448. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li J, Johnson D, Calkins M, Wright L,
Svendsen C and Johnson J: Stabilization of Nrf2 by tBHQ confers
protection against oxidative stress-induced cell death in human
neural stem cells. Toxicol Sci. 83:313–328. 2005. View Article : Google Scholar
|
|
28
|
Kaspar JW and Jaiswal AK: An
autoregulatory loop between Nrf2 and Cul3-Rbx1 controls their
cellular abundance. J Biol Chem. 285:21349–21358. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu J, Cheng M, Liu Q, Yang J, Wu S, Lu X,
Jin C, Ma H and Cai Y: Protective role of tert-butylhydroquinone
against sodium fluoride-induced oxidative stress and apoptosis in
PC12 cells. Cell Mol Neurobiol. 35:1017–1025. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li H, Zhang L, Wang F, Shi Y, Ren Y, Liu
Q, Cao Y and Duan H: Attenuation of glomerular injury in diabetic
mice with tert-butylhydroquinone through nuclear factor erythroid
2-related factor 2-dependent antioxidant gene activation. Am J
Nephrol. 33:289–297. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Z, Ji C, Wu L, Qiu J, Li Q, Shao Z
and Chen G: Tert-butylhydroquinone alleviates early brain injury
and cognitive dysfunction after experimental subarachnoid
hemorrhage: role of Keap1/Nrf2/ARE pathway. PLoS One. 9:e976852014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gharavi N, Haggarty S and El-Kadi AO:
Chemoprotective and carcinogenic effects of tert-butylhydroquinone
and its metabolites. Curr Drug Metab. 8:1–7. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yan D, Dong J, Sulik KK and Chen SY:
Induction of the Nrf2-driven antioxidant response by
tert-butylhydroquinone prevents ethanol-induced apoptosis in
cranial neural crest cells. Biochem Pharmacol. 80:144–149. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Y, Zhao J, Yang W, Bi Y, Chi J, Tian
J and Li W: High-dose alcohol induces reactive oxygen
species-mediated apoptosis via PKC-β/p66Shc in mouse primary
cardiomyocytes. Biochem Biophys Res Commun. 456:656–661. 2015.
View Article : Google Scholar
|
|
35
|
Jin W, Ni H, Dai Y, Wang H, Lu T, Wu J,
Jiang J and Liang W: Effects of tert-butylhydroquinone on
intestinal inflammatory response and apoptosis following traumatic
brain injury in mice. Mediators Inflamm. 2010:5025642010.
View Article : Google Scholar
|
|
36
|
Kraft AD, Johnson DA and Johnson JA:
Nuclear factor E2-related factor 2-dependent antioxidant response
element activation by tert-butylhydroquinone and sulforaphane
occurring preferentially in astrocytes conditions neurons against
oxidative insult. J Neurosci. 24:1101–1112. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun J, Ren X and Simpkins JW: Sequential
upregulation of superoxide dismutase 2 and heme oxygenase 1 by
tert-butylhy-droquinone protects mitochondria during oxidative
stress. Mol Pharmacol. 88:437–449. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Duan X, Liu D, Xing X, Li J, Zhao S, Nie
H, Zhang Y, Sun G and Li B: Tert-butylhydroquinone as a phenolic
activator of Nrf2 antagonizes arsenic-induced oxidative
cytotoxicity but promotes arsenic methylation and detoxication in
human hepatocyte cell line. Biol Trace Elem Res. 160:294–302. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang C, Li C, Peng H, Ye Z, Zhang J, Liu X
and Lou T: Activation of the Nrf2-ARE pathway attenuates
hyperglycemia-mediated injuries in mouse podocytes. Cell Physiol
Biochem. 34:891–902. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
He X, Kan H, Cai L and Ma Q: Nrf2 is
critical in defense against high glucose-induced oxidative damage
in cardiomyocytes. J Mol Cell Cardiol. 46:47–58. 2009. View Article : Google Scholar
|
|
41
|
Chen X, Liu J and Chen SY: Over-expression
of Nrf2 diminishes ethanol-induced oxidative stress and apoptosis
in neural crest cells by inducing an antioxidant response. Reprod
Toxicol. 42:102–109. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Huang XS, Chen HP, Yu HH, Yan YF, Liao ZP
and Huang QR: Nrf2-dependent upregulation of antioxidative enzymes:
a novel pathway for hypoxic preconditioning-mediated delayed
cardioprotection. Mol Cell Biochem. 385:33–41. 2014. View Article : Google Scholar
|
|
43
|
Calvert JW, Jha S, Gundewar S, Elrod JW,
Ramachandran A, Pattillo CB, Kevil CG and Lefer DJ: Hydrogen
sulfide mediates cardioprotection through Nrf2 signaling. Circ Res.
105:365–374. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li S, Li J, Shen C, Zhang X, Sun S, Cho M,
Sun C and Song Z: Tert-butylhydroquinone (tBHQ) protects
hepatocytes against lipotoxicity via inducing autophagy
independently of Nrf2 activation. Biochim Biophys Acta. 1841:22–33.
2014. View Article : Google Scholar
|
|
45
|
Li T, Sun KJ, Wang HD, Zhou ML, Ding K, Lu
XY, Wei WT, Wang CX and Zhou XM: Tert-butylhydroquinone ameliorates
early brain injury after experimental subarachnoid hemorrhage in
mice by enhancing Nrf2-independent autophagy. Neurochem Res.
40:1829–1838. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang Y, Liu FF, Bi X, Wang S, Wu X and
Jiang F: The antioxidant compound tert-butylhydroquinone activates
Akt in myocardium, suppresses apoptosis and ameliorates pressure
overload-induced cardiac dysfunction. Sci Rep. 5:130052015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Reed JC: Double identity for proteins of
the Bcl-2 family. Nature. 387:773–776. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Niture SK and Jaiswal AK: INrf2 (Keap1)
targets Bcl-2 degradation and controls cellular apoptosis. Cell
Death Differ. 18:439–451. 2011. View Article : Google Scholar
|
|
49
|
Niture SK and Jaiswal AK: Inhibitor of
Nrf2 (INrf2 or Keap1) protein degrades Bcl-xL via phosphoglycerate
mutase 5 and controls cellular apoptosis. J Biol Chem.
286:44542–44556. 2011. View Article : Google Scholar : PubMed/NCBI
|