Introduction
Sepsis is one of the common causes of death in
intensive care unit (ICU) patients. As inflammation spreads into
the bloodstream, the deregulated production of cytokines, such as
tumor necrosis factor (TNF)-α and interleukin (IL)-1b, triggers
high mobility group box (HMGB)1 expression as a late mediator of
inflammation (1). Systemic and
persistent inflammation may lead to organ dysfunction and
ultimately, death (2,3). Despite evidence suggesting that
TNF-α, IL-1b and HMGB1 are associated with endotoxin lethality and
septic syndrome, unfortunately, monoclonal antibodies against TNF-α
and TNF-binding proteins fail to decrease sepsis (2).
Despite the availability of 170 different
biomarkers, the complex clinical syndrome of sepsis and related
mortality have increased in incidence (4). Microbial infection underlying sepsis
accounts for an almost 30% increase in mortality, despite advances
in antimicrobial research (5).
The majority of studies on sepsis have focused on biomarkers for
early diagnosis, treatment or prognosis. Treatment is very
unsatisfactory, as early inflammatory cytokines are restored to
their basic levels only after sepsis-related death (9).
A more appropriate cytokine to block sepsis includes
the late onset inflammatory mediator, HMGB1, a crucial late-phase
cytokine in the course of sepsis (6). A high level of HMGB1 is associated
with a poor outcome in sepsis (7). Superfluous HMGB1, secreted by
macrophages and monocytes, plays a key role in the pathogenesis of
sepsis due to bacterial infections (7), as well as in cancers (8). Future breakthroughs in sepsis
treatment may depend on addressing the issue of the production of
late inflammatory cytokines, such as HMGB1 (9).
As non-coding transcripts of 18–25 nucleotides,
microRNAs (miRNAs or miRs) usually target mRNAs to modulate gene
expression by 1.2- to 4.0-fold rather than acting as a genetic
switch (10). Several miRNAs have
been shown to regulate sepsis by targeting different mRNAs. The
suppression of the expression of miR-15a/16 in bone marrow-derived
macrophages caused by bacterial-derived lipopolysaccharide (LPS)
has been shown to increase phagocytosis and bacterial clearance
through Toll-like receptor (TLR) 4-associated pathways,
subsequently affecting the survival of septic mice (11). miR-147b represents an endothelial
barrier of protection against endotoxin-mediated inflammation by
decreasing ADAM metallopeptidase domain 15 (ADAM15) expression and
attenuating LPS-induced barrier dysfunction during septic challenge
by bacterial LPS in endothelial cells (12). Serum levels of miR-122 have been
shown to be increased in patietns with sepsis with clotting
abnormalities (13). miR-133a
levels have also been shown to correlate with the severity of
sepsis and, at significantly higher levels, predicted an
unfavorable prognosis of critically ill patients (14).
The cholinergic anti-inflammatory pathway (CAP) is a
mechanism associated with the vagus nerve. CAP plays a critical
role in controlling the inflammatory response by modulating
systemic and local inflammation (15). Its molecular mechanisms of action
are mediated by the major neurotransmitter, acetylcholine, which
activates the nicotinic acetylcholine receptor subunit α7
(α7nAchR). α7nAchR is expressed on macrophages and is an essential
component of CAP (3).
3-(2,4-Dimethoxybenzylidene)anabaseine (GTS-21) is an
α7nAchR-specific agonist (16).
miR-124 reduces early inflammatory cytokine levels in the
cholinergic anti-inflammatory response. miR-124 reduces IL-6
production by affecting the signal transducer and activator of
transcription 3 (STAT3), and attenuates the release of TNF-α via
TNF-α converting enzyme (TACE) (17).
The role of the late inflammatory cytokine HMGB1 as
regards its regulation by miRNAs have yet to be reported in sepsis.
In this study, using miRNA microarray analysis, we screened 3
target miRNAs: miR-205-5b, miR-196a and miR-193b. Of the 3 miRNAs,
miR-205-5b was the most highly expressed followed by decreased
HMGB1 levels. Given the important role of HMGB1 in sepsis (18), miR-205-5b is a potential biomarker
or therapeutic target in sepsis. In this study, we elucidated the
expression of miR-205-5b in different tissues using a mouse model
of LPS-induced sepsis and identified the mechanisms of action of
miR-205-5b action and its effects on HMGB1.
Materials and methods
Cell culture
The mouse macrophage cell line, RAW264.7, was
obtained from the Medical Experimental Center of Zhongnan Hospital
of Wuhan University. The RAW264.7 cells were cultured in DMEM with
heat-inactivated 10% fetal bovine serum (FBS) (HyClone, Logan, UT,
USA) and seeded into 48-well microtiter plates at a density of
2×104 cells/well.
Enzyme-linked immunosorbent assay
(ELISA)
A 200 µl supernatant was obtained at 1, 6,
12, 18, 24 and 30 h to confirm the optimal concentration. The
levels of TNF-α and HMGB1 in the supernatant were determined by
ELISA using a specific kit (eBioscience, Inc., San Diego, CA, USA)
according to the manufacturer's instructions with a microplate
reader (RT-2100C; Rayto, USA).
miRNA microarrays
Following culture of the RAW264.7 cells for 24 h,
cells in the LPS group were stimulated with 50 ng/ml LPS
(Sigma-Aldrich, St. Louis, MO, USA), those in the GTS-21 group with
50 ng/ml LPS and 8 µg/ml GTS-21 (Abcam, Cambridge, UK) at 24
h, along with the control group (untreated cells) on an Affymetrix
miRNA 3.0 technology platform, which contained 1,111 mature mouse
miRNAs. NanoDrop ND-2100 (Thermo Fisher Scientific, Waltham, MA,
USA) was used with Agilent 2100 (Agilent Technologies, Inc., Santa
Clara, CA, USA) to identify and quantify the total RNAs and ensure
their integrity.
Total RNA was tailed with with poly(A) polymerase
and labeled with biotin, followed by hybridization, washing,
staining and scanning with Affymetrix Scanner 3000 (Affymetrix,
Inc., Santa Clara, CA, USA) to obtain the raw image. Raw image
processing was performed using Affymetrix GeneChip Command Console
software (version 4.0), read by Expression Console (version 1.3.1)
(both from Affymetrix, Inc.) and RMA normalization. GeneSpring NGS
(version 12.5) (Agilent Technologies) was used for subsequent data
analysis. Probes meeting at least one out of two conditions and
containing flags in 'P' were selected for further analysis.
Differentially expressed miRNAs were then identified
through fold change. The threshold set for upregulated and
downregulated genes was a fold change ≥2.0 and p≤0.05. Target genes
of differentially expressed miRNAs that represented the
intersection of the 3 databases (TargeScan, PITA and microRNA.org) were selected as candidate target genes
for further analysis. GO analysis and KEGG analysis were used to
determine the roles of these target genes. Unsupervised
hierarchical clustering was performed to show the distinguishable
miRNA expression pattern among samples.
Experimental animals
We purchased 5-to 6-week-old BALB/c mice (weighing
20–22 g) from the Experimental Animal Center of Wuhan University,
an accredited SPF facility according to national and institutional
guidelines. The animals were bred under standard conditions with a
temperature of 22°C with a 12-h light/dark cycle and had free
access to water and a standard rodent diet. In addition, the
animals received humane care in compliance with the Principles of
Laboratory Animal Care of Wuhan University. All protocols were
approved by the Wuhan University of Science and Technology Animal
Care and Use Committee (certificate number: TY20120025).
Experimental protocols
The experimental animals were randomly divided into
3 groups as follows: the control, LPS, and GTS-21 treatment (LPS +
GTS-21) groups, with 6 mice in each group.
In the LPS group, a single intraperitoneal injection
of 15 mg/kg LPS was used to induce peripheral inflammation. The
mice in the control group were treated similar to those in the LPS
group, but received saline instead of LPS. In the GTS-21 treatment
group, 15 mg/kg LPS and 4 mg/kg GTS-21 were injected into the mouse
abdominal cavity.
Twenty-four hours after the intraperitoneal
injection, blood, lungs, livers, colons and spleens were harvested
immediately after the mice were euthanized. Blood was centrifuged
at 3,000 rpm for 10 min at 4°C, to obtain the supernatant for
further testing. Other tissues were stored in TRIzol for later
use.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) for the determination of miRNA
and mRNA expression
Total RNA extracted from the lung, heart, liver,
spleen, gut and renal tissues of the septic mice using TRIzol
reagent (Invitrogen, Carslbad, CA, USA), and was
reverse-transcribed into cDNA using the RevertAid First Strand cDNA
Synthesis kit (Thermo Fisher Scientific). qPCR for TNF-α and
β-actin was performed using the 7300 Real-Time PCR system (Applied
Biosystems Life Technologies, Foster City, CA, USA). The primer
sequences were synthesized for TNF-α (Invitrogen). RT-qPCR was
performed in triplicate and data were calculated using the
2−ΔΔCT method, where ΔΔ Ct = ΔCt - ΔCtNC
group, ΔCt = CtmiR-205 - CtU6. The
primers used for RT-qPCR are listed in Table I.
 | Table IPrimers used for RT-qPCR. |
Table I
Primers used for RT-qPCR.
| Primers | Sequences |
|---|
| U6-S |
5′-CTCGCTTCGGCAGCACA-3′ |
| U6-A |
5′-AACGCTTCACGAATTTGCGT-3′ |
| M-miR-205-5p-RT |
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCAGACTCC-3′ |
| M-miR-205-5p-S |
5′-ACACTCCAGCTGGGTCCTTCATTCCACCGG-3′ |
| Universal primer |
5′-TGGTGTCGTGGAGTCG-3′ |
| M-HMGB1-S |
5′-CGGATGCTTCTGTCAACTTCTC-3′ |
| M-HMGB1-A |
5′-GTTTCTTCGCAACATCACCAAT-3′ |
| M-β-actin-S |
5′-GTGACGTTGACATCCGTAAAGA-3′ |
| M-β-actin-A |
5′-GTAACAGTCCGCCTAGAAGCAC-3′ |
Transfection of miRNAs
The RAW264.7 cells were seeded into 6-well plates
(5×104 cells/well) and cultured in 2 ml Dulbecco's
modified Eagle's medium (DMEM) with 5% FBS, maintained at 37°C in
the presence of 5% CO2. miR-205 mimics, inhibitor and
negative control were synthesized by GenePharma (Shanghai, China).
When the cells reached 70–90% confluency, they were transfected
with miR-205 mimics or inhibitor, respectively according to the
manufacturer's instructions with adherent cell transfection
procedures. A negative control of Lipofectamine 2000 (Invitrogen)
was maintained as well.
Western blot analysis
In order to obtain the total cellular lysates, the
transfected cells and controls were lysed in ice-cold cell lysis
buffer (Wuhan Goodbio Technology Co., Ltd., Wuhan, China). The
protein concentration was determined using the Bradford protein
assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA) using a
c-globulin standard curve. Proteins were separated by standard
SDS-PAGE and transferred onto PVDF membranes (Millipore Corp.,
Billerica, MA, USA). Non-specific binding sites were blocked using
5% dry skimmed milk, 0.2% Tween-20 in PBS (pH 7.4) and then
incubated with HMGB1 antibodies (Boster Inc., Wuhan, China)
overnight at 4°C. The PVDF membranes were decolorized 3 times for 5
min each on a shaking bed with TBST at room temperature. The
secondary antibody (Wuhan Goodbio Technology Co., Ltd.) was diluted
(1:3,000) with TBST, and incubated for 30 min. The PVDF membranes
were washed with TBST 3 times for 5 min again. After full access to
substrate working solution, developments and fixing, the gray scale
was analyzed using the Alpha software processing system (Alpha
Innotech Corp., San Leandro, CA, USA).
Statistical analysis
All data are expressed as the means ± standard error
of the mean and analyzed using GraphPad Prism version 6.01
(GraphPad Software, Inc., San Diego, CA; USA). Statistical
signifiance was determined using a Student's t-test, and a value of
p<0.05 was considered to indicate a statistically significant
difference.
Results
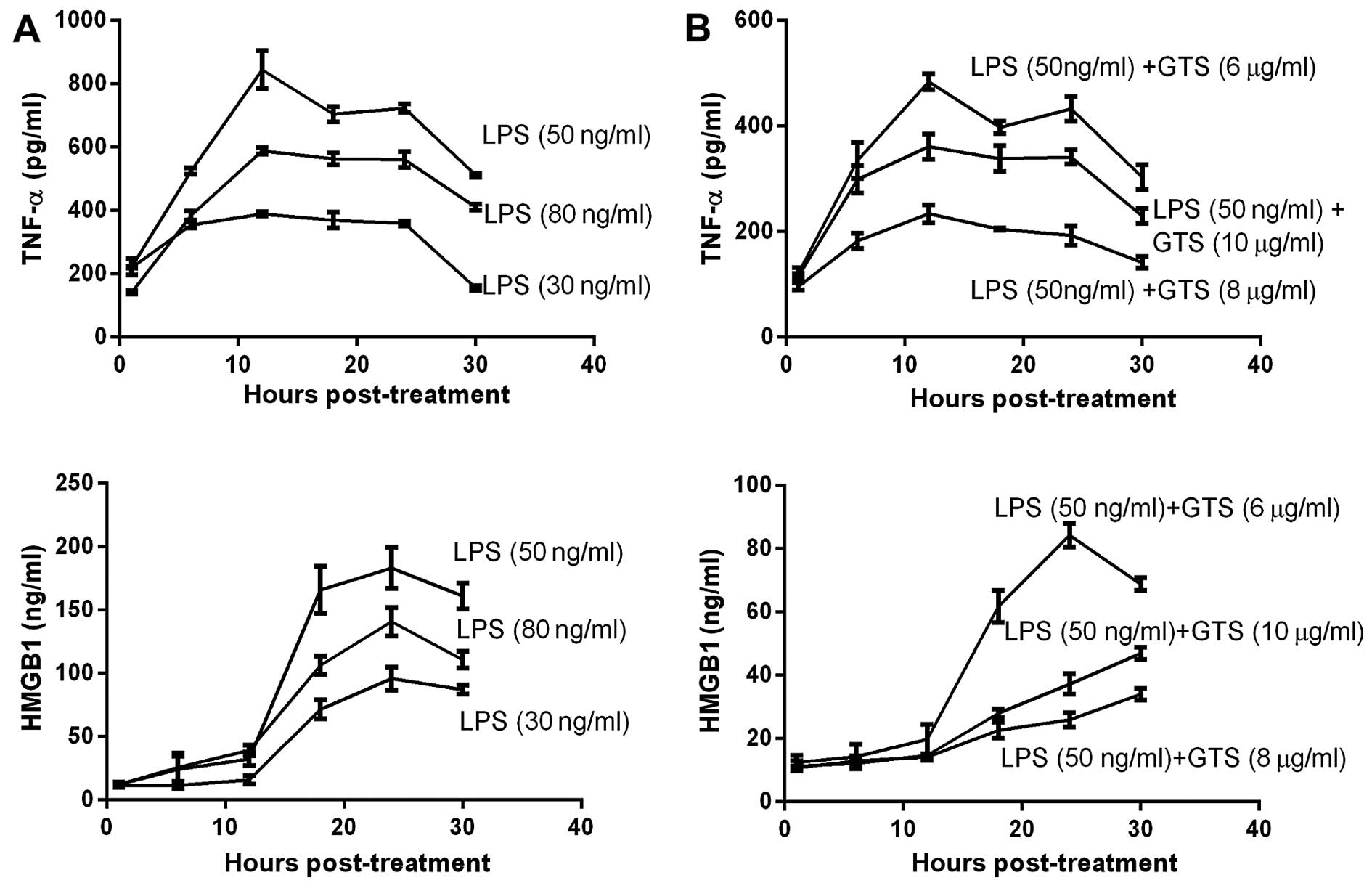
Optimal LPS and GTS-21 concentration
In order to select the optimal concentrations of LPS
and GTS-21, we designed the LPS and GTS concentration gradient. The
levels of TNF-α and HMGB1 in the cells were examined by ELISA in
order to determine the optimal concentration of LPS needed to
induce maximal levels of TNF-α and HMGB1 and the optimal
concentration of GTS-21 needed to reduce the levels of TNF-α and
HMGB1. Following incubation for 24 h at 37°C, we stimulated the
RAW264.7 cells with LPS using the 30, 50 and 80 ng/ml concentration
gradients following pre-treatment with GTS-21 using the 6, 8 and 10
µg/ml concentration gradients half an hour earlier. The
results suggested that macrophages stimulated with LPS 50 ng/ml
expressed more TNF-α and HMGB1 than the other groups; thus, this
concentration was considered ideal (Fig. 1A). When the macrophages were
stimulated with 50 ng/ml LPS and 3 different concentrations of
GTS-21, the decrease in the expression of TNF-α and HMGB1 was
greatest at 8 µg/ml of GTS-21 (Fig. 1B). The optimal GTS-21
concentration for treatment was 8 µg/ml, as this
concentration achieved optimal inhibitory effects.
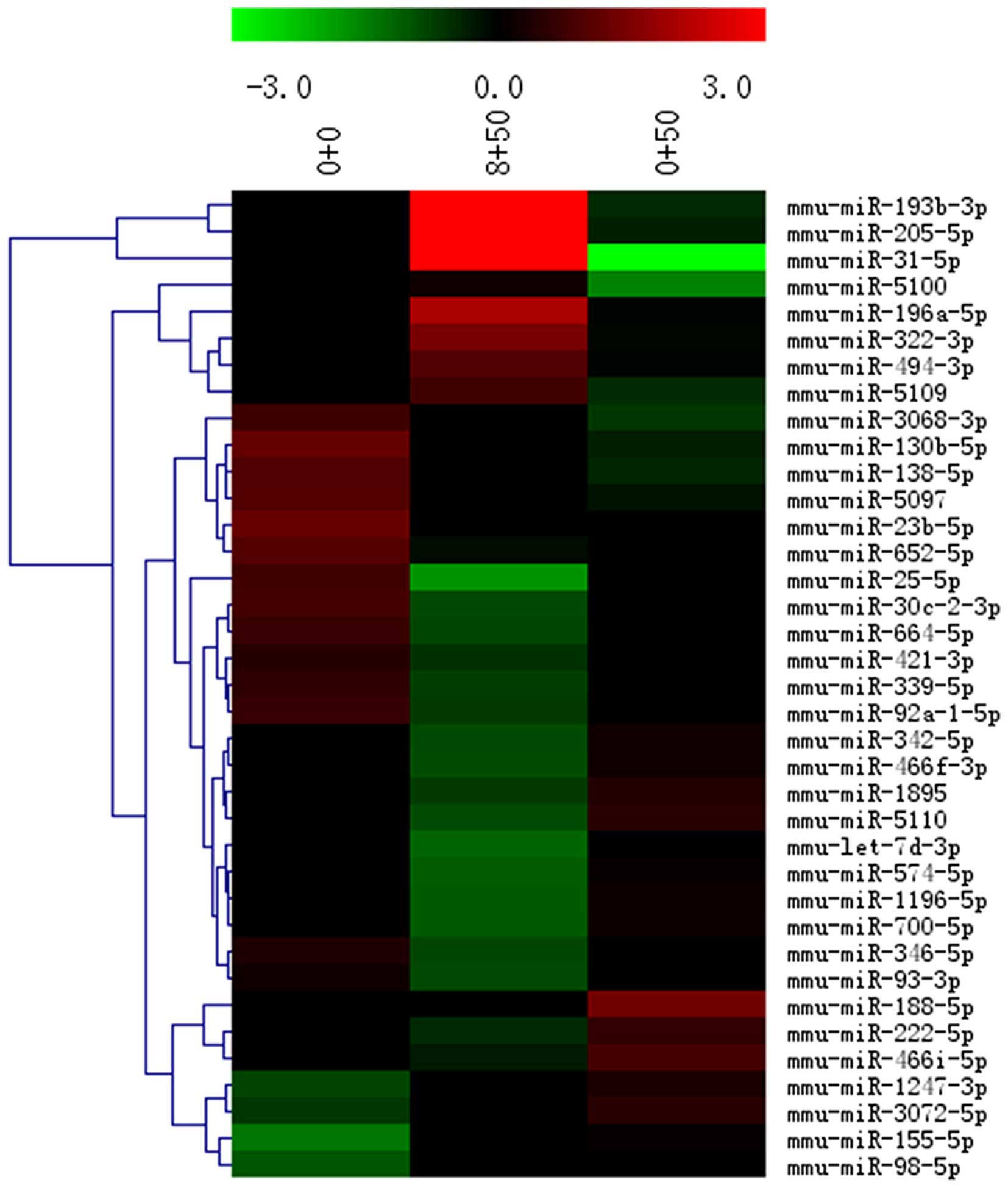
Upregulated miRNA targets in microarray
analysis
In the heatmap shown in Fig. 2, each row represents a single
miRNA, and each column represents a plasma sample. The legend on
the right indicates the miRNA representing the corresponding row.
The relative miRNA expression is displayed according to the color
scale: red indicates upregulation; green indicates downregulation,
as previously described (19).
Differentially expressed miRNAs were identified
through fold change (FC). Among the different miRs (miR-205-5b,
miR-196a and miR-193b), miR-205 was highly expressed in the GTS-21
group, and its levels were 18.478138-fold those of the LPS group,
and 14.27163-fold those of the control group.
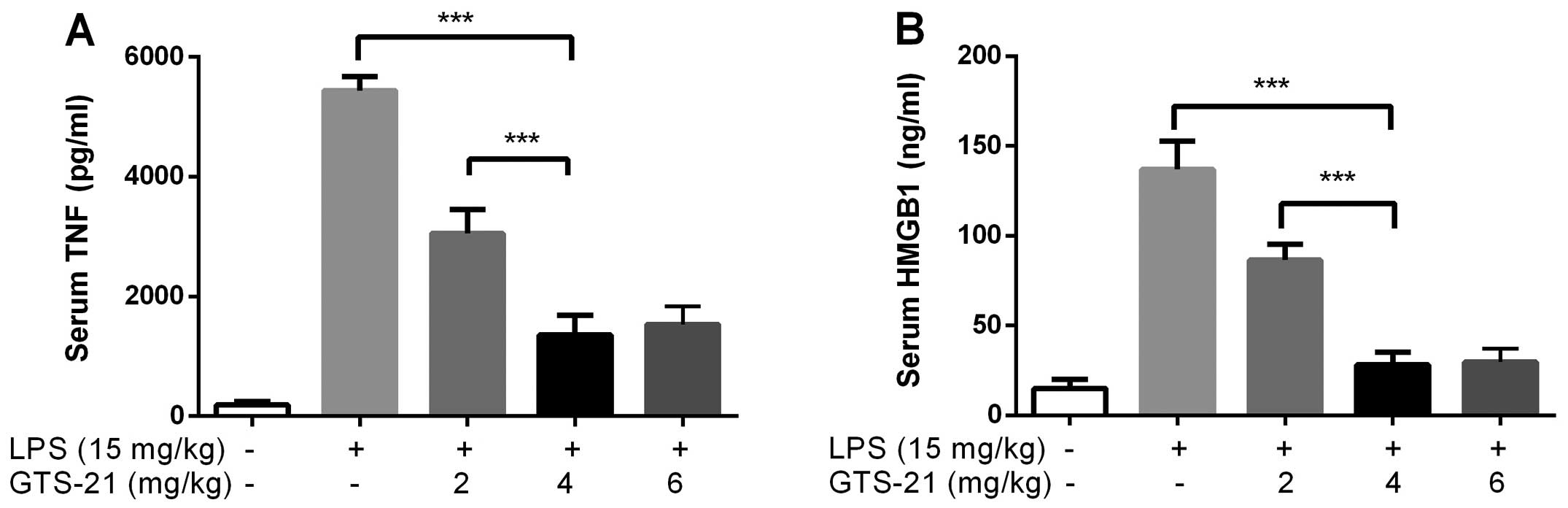
Optimal dosage of GTS-21 in mice
The mice (n=6) were intraperitoneally injected with
GTS-21 (2, 4 and 6 mg/kg) or saline 30 min prior to LPS
administration. The serum TNF-α and HMGB1 levels were analyzed 12
and 24 h later. The ideal dose of GTS-21 was 4 mg/kg as it induced
the most prominent inhibitory effects on TNF-α and HMGB1 expression
(Fig. 3).
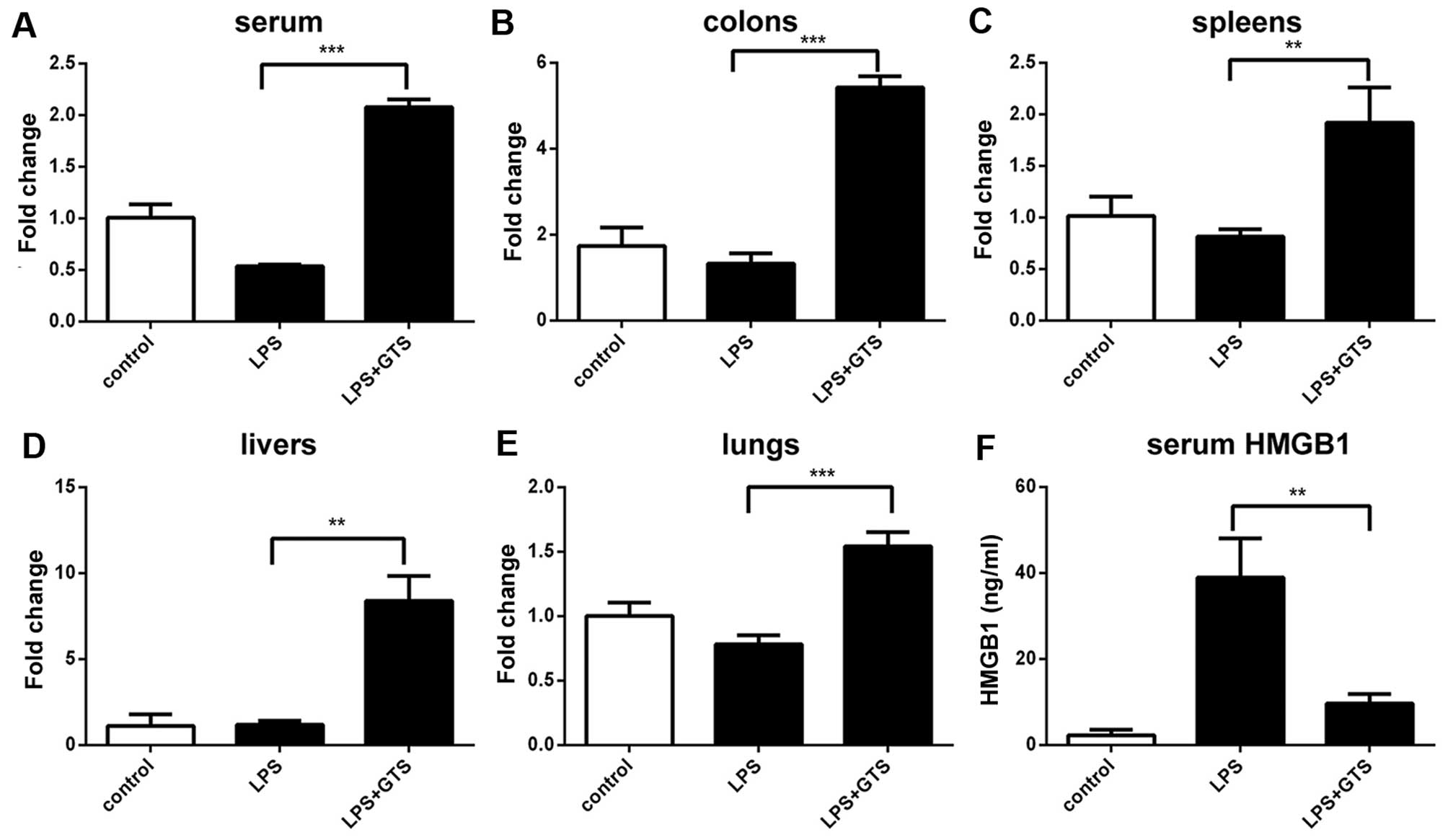
Expression profiles of miR-205-5p in mice
with sepsis
The expression levels of miR-205-5p targets in the
serum, livers, colons, lungs and spleen were quantified by RT-qPCR
to verify the upregulation detected in the samples of
GTS-21-treated septic mice. The miR-205-5p levels were not similar
in the different organs (Fig.
4A–E). The levels of miR-205-5p in the different mouse organs
were as follows: serum (LPS vs. control p=0.0033; GTS vs. control
p<0.0002; GTS vs. LPS p=0.0001), colon (LPS vs. control
p=0.2915; GTS vs. control p<0.0002; GTS vs. LPS p=0.0001);
spleen (LPS vs. control p=0.1640; GTS vs. control p<0.0157; GTS
vs. LPS p=0.0054); liver (LPS vs. control p=0.8472; GTS vs. control
p<0.0015; GTS vs. LPS p=0.0011); and lungs (LPS vs. control
p=0.0364; GTS vs. control p<0.0032; GTS vs. LPS p=0.0005)
(Fig. 4A–E). In general,
treatment with GTS-21 induced a significant increase in miR-205-5p
expression.
The serum concentration of HMGB1 (LPS vs. GTS
p=0.0054; LPS vs. control p=0.0022; LPS + GTS vs. control p=0.0063)
(Fig. 4F) was significantly
increased following the administration of LPS. HMGB1 expression was
markedly decreased in serum following treatment with GTS-21
(Fig. 4F).
miR-205-5b attenuates the LPS-induced
increase in HMGB1 expression
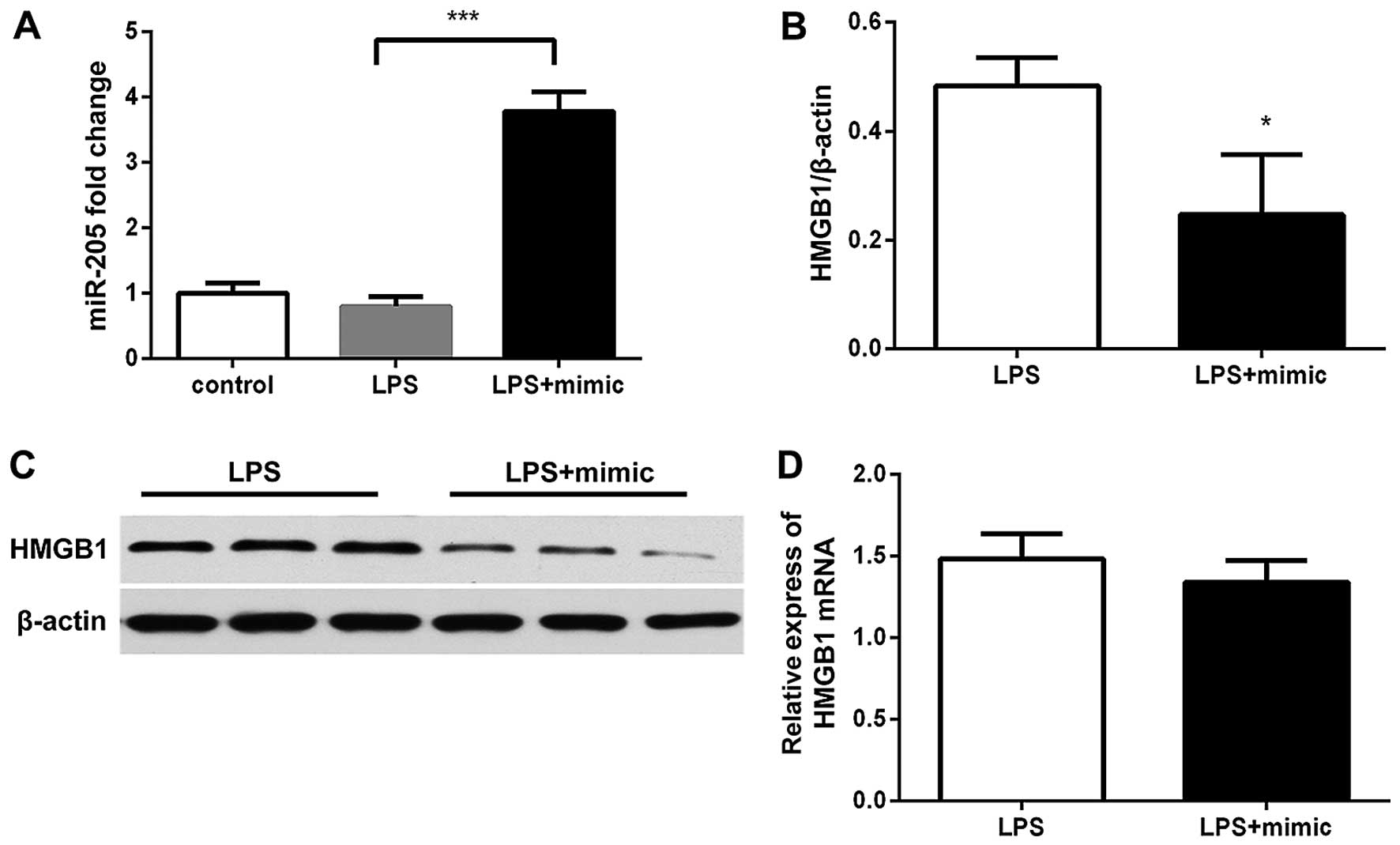
We first confirmed that transfection with miR-205-5b
mimics significantly increased miR-205-5b expression in the cells
(Fig. 5A). When the RAW264.7
cells were transfected with the miR-205 mimics, or negative control
for 24 h, as shown in Fig. 5,
HMGB1 protein expression was altered (p<0.05). Western blot
analysis of HMGB1 protein expression revealed that the HMGB1 levels
in the group transfected with miR-205-5b mimics were significantly
lower than those in the negative control-transfected group
(p=0.0280; Fig. 5B and C).
However, the miR-205-5b mimics did not affect the HMGB1 mRNA level
(p=0.2896; Fig. 5D).
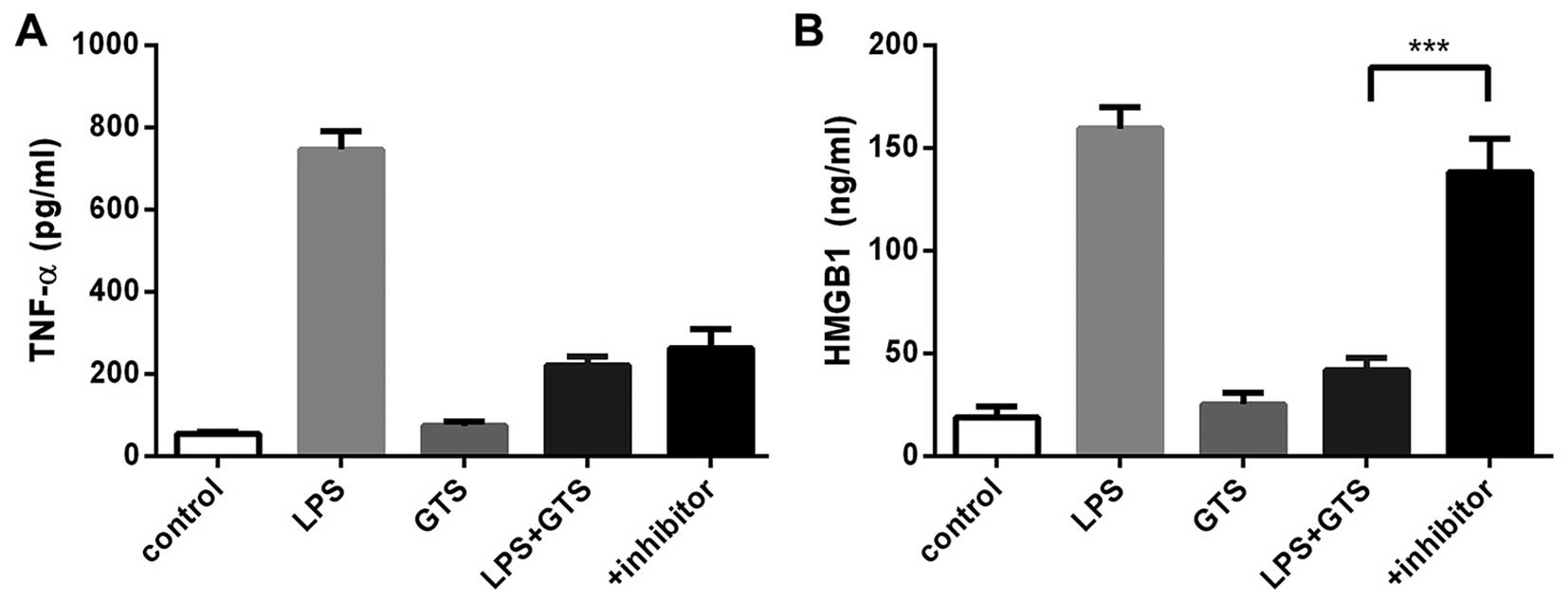
Transfection with the miR-205 inhibitor did not
affect the GTS-21-induced suppression of the expression of TNF-α
(Fig. 6A); however, the miR-205
inhibitor increased HMGB1 expression 24 h later (Fig. 6B).
Discussion
CAP mediates the inhibition of the production of
inflammatory cytokines, including TNF-α, nuclear factor (NF)-κB and
HMGB1 in sepsis (3). It acts
similarly in other inflammatory syndromes, such as arthritis and
viral myocarditis (20,21).
The mechanisms of action of CAP entail α7nAchR
activation by electrical vagal stimulation or pharmacological
agonists. Electrical vagal stimulation is impracticable in sepsis.
GTS-21, a selective partial α7nAchR agonist (22), has been proven to effectively
reduce the inflammatory reaction and improve prognosis in animals
(23–25). Similar to previous studies
(22), our findings suggest that
GTS-21 suppresses TNF-α and HMGB1 expression in a dose-dependent
manner in experiments using mouse macrophages. The curves indicated
the optimal concentration of GTS-21 as 8 µg/ml for
marophages.
Mammalian miRNAs have emerged as meaningful
modulators of cellular function in different organ systems since
their discovery (10). Previous
studies have shown that miR-124 mediates the cholinergic
anti-inflammatory action against early inflammatory factors,
including TNF-α maturation by TACE and IL-6 transcription by
targeting STAT3 (17); however,
it is unknown whether miRNAs affect HMGB1. To elucidate the
mechanisms underlying the cholinergic anti-inflammatory activity
against one of the most important late inflammatory factors, which
correlates with prognosis (7), we
performed miRNA microarray analysis to identify potential miRNAs
involved. To the best of our knowledge, our study has confirmed for
the first time that miR-205-5b expression significantly increased
following the activation of α7nAchR in macrophages.
In vitro experiments were more credible as
they ruled out the possibility of HMGB1 release from the necrotic
tissue. We reported that LPS-stimulated RAW264.7 cells transfected
with miR-205-5b mimics displayed a low HMGB1 expression, whereas
the expression of TNF-α was not altered. However, transfection with
miR-205-5b inhibitor neutralized the effects of GTS-21 on HMGB1
expression. Therefore, our results indicate that miR-205-5b targets
HMGB1 expression in the CAP. The results of RT-qPCR demonstrated
that miR-205-5b mimics did not affect the HMGB1 mRNA levels, with
no significant differences observed between the mimics- and
negative control-transfected groups, suggesting a
post-transcriptional regulation.
In animal experiments, miR-205-5b expression in the
LPS group exhibited a decrease, although this was not significant
in the different tissues. This indicates that LPS cannot suppress
the expression of miR-205-5b. miR-205-5b targets different proteins
in different pathophysiologies (26–29). In this study, we first found that
following the activation of CAP, superfluous miR-205-5b suppressed
HMGB1 via the transcriptional inhibition of HMGB1 in serum.
Decreased HMGB1 expression leads to sharply lowered blood HMGB1
levels.
In conclusion, miR205-5b upregulation suppresses
HMGB1 expression in cells and tissues in sepsis. The regulatory
mechanisms involved include the suppression of HMGB1 at the
post-transcriptional level. miR-205-5b may thus be a potential
therapeutic target for the treatment of inflammatory diseases and a
possible novel therapeutic strategy against late sepsis.
Acknowledgments
The present study was financially supported by the
National Natural Science Foundation of China (no. 30972852).
References
|
1
|
Kang R, Chen R, Zhang Q, Hou W, Wu S, Cao
L, Huang J, Yu Y, Fan XG, Yan Z, et al: HMGB1 in health and
disease. Mol Aspects Med. 40:1–116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tracey KJ: The inflammatory reflex.
Nature. 420:853–859. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pavlov VA, Wang H, Czura CJ, Friedman SG
and Tracey KJ: The cholinergic anti-inflammatory pathway: a missing
link in neuroimmunomodulation. Mol Med. 9:125–134. 2003.PubMed/NCBI
|
|
4
|
Silman NJ: Rapid diagnosis of sepsis using
biomarker signatures. Crit Care. 17:10202013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lyle NH, Pena OM, Boyd JH and Hancock R:
Barriers to the effective treatment of sepsis: antimicrobial
agents, sepsis definitions, and host-directed therapies. Ann NY
Acad Sci. 1323:101–114. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu L, Bao H, Si Y and Wang X: Effects of
dexmedetomidine on early and late cytokines during polymicrobial
sepsis in mice. Inflamm Res. 62:507–514. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Charoensup J, Sermswan RW, Paeyao A,
Promakhejohn S, Punasee S, Chularari C, Krabkraikaew S,
Lertanekawattana S and Wongratanacheewin S: High HMGB1 level is
associated with poor outcome of septicemic melioidosis. Int J
Infect Dis. 28:111–116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo ZS, Liu ZQ, Bartlett DL, Tang DL and
Lotze MT: Life after death: targeting high mobility group box 1 in
emergent cancer therapies. Am J Cancer Res. 3:1–20. 2013.PubMed/NCBI
|
|
9
|
Wang H, Liao H, Ochani M, Justiniani M,
Lin X, Yang L, Al-Abed Y, Wang H, Metz C, Miller EJ, et al:
Cholinergic agonists inhibit HMGB1 release and improve survival in
experimental sepsis. Nat Med. 10:1216–1221. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
O'Connell RM, Rao DS and Baltimore D:
microRNA regulation of inflammatory responses. Annu Rev Immunol.
30:295–312. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moon HG, Yang J, Zheng Y and Jin Y:
miR-15a/16 regulates macrophage phagocytosis after bacterial
infection. J Immunol. 193:4558–4567. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chatterjee V, Beard RS Jr, Reynolds JJ,
Haines R, Guo M, Rubin M, Guido J, Wu MH and Yuan SY: MicroRNA-147b
regulates vascular endothelial barrier function by targeting ADAM15
expression. PLoS One. 9:e1102862014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang HJ, Deng J, Wang JY, Zhang PJ, Xin Z,
Xiao K, Feng D, Jia YH, Liu YN and Xie LX: Serum miR-122 levels are
related to coagulation disorders in sepsis patients. Clin Chem Lab
Med. 52:927–933. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tacke F, Roderburg C, Benz F, Cardenas DV,
Luedde M, Hippe HJ, Frey N, Vucur M, Gautheron J, Koch A, et al:
Levels of circulating miR-133a are elevated in sepsis and predict
mortality in critically ill patients. Crit Care Med. 42:1096–1104.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pavlov VA and Tracey KJ: Controlling
inflammation: the cholinergic anti-inflammatory pathway. Biochem
Soc Trans. 34:1037–1040. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang H, Yu M, Ochani M, Amella CA, Tanovic
M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, et al: Nicotinic
acetylcholine receptor alpha7 subunit is an essential regulator of
inflammation. Nature. 421:384–388. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun Y, Li Q, Gui H, Xu DP, Yang YL, Su DF
and Liu X: MicroRNA-124 mediates the cholinergic anti-inflammatory
action through inhibiting the production of pro-inflammatory
cytokines. Cell Res. 23:1270–1283. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Musumeci D, Roviello GN and Montesarchio
D: An overview on HMGB1 inhibitors as potential therapeutic agents
in HMGB1-related pathologies. Pharmacol Ther. 141:347–357. 2014.
View Article : Google Scholar
|
|
19
|
Jia SZ, Yang Y, Lang J, Sun P and Leng J:
Plasma miR-17-5p, miR-20a and miR-22 are down-regulated in women
with endometriosis. Hum Reprod. 28:322–330. 2013. View Article : Google Scholar :
|
|
20
|
Cheng Z, Li-Sha G, Jing-Lin Z, Wen-Wu Z,
Xue-Si C, Xing-Xing C and Yue-Chun L: Protective role of the
cholinergic anti-inflammatory pathway in a mouse model of viral
myocarditis. PLoS One. 9:e1127192014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Koopman FA, Schuurman PR, Vervoordeldonk
MJ and Tak PP: Vagus nerve stimulation: a new bioelectronics
approach to treat rheumatoid arthritis? Best Pract Res Clin
Rheumatol. 28:625–635. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sitapara RA, Antoine DJ, Sharma L, Patel
VS, Ashby CR Jr, Gorasiya S, Yang H, Zur M and Mantell LL: The α7
nicotinic acetylcholine receptor agonist GTS-21 improves bacterial
clearance in mice by restoring hyperoxia-compromised macrophage
function. Mol Med. 20:238–247. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cai B, Chen F, Ji Y, Kiss L, de Jonge WJ,
Conejero-Goldberg C, Szabo C, Deitch EA and Ulloa L: Alpha7
cholinergic-agonist prevents systemic inflammation and improves
survival during resuscitation. J Cell Mol Med. 13(9B): 3774–3785.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kox M, Pompe JC, Peters E, Vaneker M, van
der Laak JW, van der Hoeven JG, Scheffer GJ, Hoedemaekers CW and
Pickkers P: α7 nicotinic acetylcholine receptor agonist GTS-21
attenuates ventilator-induced tumour necrosis factor-α production
and lung injury. Br J Anaesth. 107:559–566. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pavlov VA, Ochani M, Yang LH,
Gallowitsch-Puerta M, Ochani K, Lin X, Levi J, Parrish WR,
Rosas-Ballina M, Czura CJ, et al: Selective alpha7-nicotinic
acetylcholine receptor agonist GTS-21 improves survival in murine
endotoxemia and severe sepsis. Crit Care Med. 35:1139–1144. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang P, Wang L, Rodriguez-Aguayo C, Yuan
Y, Debeb BG, Chen D, Sun Y, You MJ, Liu Y, Dean DC, et al: miR-205
acts as a tumour radiosensitizer by targeting ZEB1 and Ubc13. Nat
Commun. 5:56712014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin D, Halilovic A, Yue P, Bellner L, Wang
K, Wang L and Zhang C: Inhibition of miR-205 impairs the
wound-healing process in human corneal epithelial cells by
targeting KIR4.1 (KCNJ10). Invest Ophthalmol Vis Sci. 54:6167–6178.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Orang AV, Safaralizadeh R, Hosseinpour
Feizi MA and Somi MH: Diagnostic and prognostic value of miR-205 in
colorectal cancer. Asian Pac J Cancer Prev. 15:4033–4037. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qiao W, Chen L and Zhang M: MicroRNA-205
regulates the calcification and osteoblastic differentiation of
vascular smooth muscle cells. Cell Physiol Biochem. 33:1945–1953.
2014. View Article : Google Scholar : PubMed/NCBI
|