Introduction
Bronchial asthma is a chronic inflammatory disease
of the respiratory tract which is characterized by airway
hyper-responsiveness and reversible airflow restriction. Currently,
standardized treatment and self-management by patients have
significantly reduced the incidence of acute asthmatic attacks.
However, asthma remains a disease which does not yet have a
complete cure (1). Psychological
problems are common in asthmatic patients, even in patients who
have well-controlled asthma. Valença et al (2) reported that 43.5% of patients with
asthma had at least one type of psychiatric disorder. Anxiety and
depression are the two most common disorders encountered in
asthmatic patients, and tend to correlate with asthma control
(3,4). A recent study reported that anxiety
and depression occurred in 33.3 and 47.7% asthmatic patients,
respectively (5).
Asthmatic patients are at a higher risk of
psychiatric morbidity, with anxiety and depression being the two
most common disorders in these patients (3,4,6–12).
Asthma significantly affects psychological and social wellbeing due
to the impact it has on routine activities, sleep and the daily
life of patients (3,4,6–12).
Conversely, psychological factors may be a risk factor for the
exacerbation of this disorder. Another theory regarding the link
between asthma and psychological factors describes asthma as a
classic psychosomatic disorder caused by specific psychological
conflicts (13).
The serotonin transporter (5-HTT) is known to be
associated with depression and emotional stress (14). The 5′-flanking promoter region of
the 5-HTT gene has a biallelic insertion/deletion (5-HTTLPR)
(15). Furthermore, it has been
reported that the 5-HTTLPR genotype is linked with severe
depression (15); the
polymorphisms within this region are associated with improved
responses to treatment with selective serotonin reuptake inhibitors
(16). Brain-derived neurotrophic
factor (BDNF) is a growth factor which belongs to the neurotrophin
family and is highly expressed in the hippocampus and amygdala of
the brain (17). It regulates the
pathophysiology of mood disorders, including major depressive
disorder (MDD) and bipolar disorder (BD), as well as learning and
memory (17,18). Patients with anxiety and BD are
known to have decreased levels of BDNF (17,18). Previous studies have also
implicated BDNF in the pathogenesis of asthma (19–21). Furthermore, the BDNF gene
polymorphism has been reported to be associated with allergic
asthma and allergic rhinitis (22).
Neuropeptide S receptor 1 (NPSR1) gene has been
suggested to be a candidate gene which is associated with increased
susceptibility to asthma and other atopic disorders in different
populations (23). The signaling
system of NPSR1 and its ligand neuropeptide S (NPS) have been
implicated in various pathophysiological pathways, including
regulation of the immunologic phenotype and various neurobiological
phenomena such as wakefulness, arousal, anxiety, learning and
memory (23).
To the best of our knowledge, the association of
NPSR1, BDNF and 5-HTT gene polymorphisms with anxiety or depression
in asthmatic patients has not been previously examined. Hence, this
study was undertaken in order to correlate anxiety and depression
with the clinical characteristics of asthmatic patients, and to
explore their association with 5-HTT, BDNF and NPSR1 gene
polymorphisms.
Subjects and methods
Subjects
This study was approved by the Medical Ethics
Committee for Clinical Research, Zhongda Hospital Affiliated to
Southeast University (Nanjing, China). Written informed consent was
obtained from all subjects. The inclusion criteria were as follows:
patients were of Han Chinese ethnicity aged >18 years, who had
been diagnosed with bronchial asthma as per the diagnostic criteria
established by the Respiratory Diseases Branch of the Chinese
Medical Association (24),
without mental disorders according to the diagnostic standard of
the Diagnostic and Statistical Manual of Mental Disorders, 4th
Edition (DSM2-IV), and had completed at least primary education.
Subjects with chronic diseases (diabetes, hypertension, coronary
heart disease, chronic obstructive pulmonary disease and genetic
diseases), pregnant or lactating women or individuals with a
history of drug abuse were excluded from the study. The healthy
controls were volunteers from Zhongda Hospital, which included
hospital staff, doctors and medical students.
Assessment of asthma control, anxiety and
depression
All patients completed questionnaires using the
Hamilton Anxiety Rating Scale (HAMA), the Hamilton Depression
Rating Scale (HAMD) and the Asthma Control Test (ACT) score
(11). Asthma without anxiety was
defined as a HAMA anxiety score ≤14 points; asthma with anxiety was
defined as a HAMA anxiety score >14 points; asthma without
depression was defined as a HAMD depression score ≤7 points; and
asthma with depression was defined as a HAMD depression score >7
points.
Genomic DNA preparation
Blood for genomic studies was collected from all
asthma patients and healthy controls in an
ethylenediaminetetraacetic acid (EDTA) vial. The genomic DNA was
extracted from 3 ml of EDTA-anticoagulated whole blood using a
Blood Genomic DNA Extraction kit (cat. no. DP318; Tiangen Biotech
(Beijing) Co., Ltd., Beijing, China) according to the
manufacturer's instructions. The quality and quantity of genomic
DNA extracted was examined with spectrophotometry (ND-1000;
NanoDrop products, Thermo Fisher Scientific, Wilmington, DE, USA).
All extracted DNA had an OD260/280 between 1.7 and 1.9. The
purified DNA samples were stored at −80°C until further
testing.
Polymerase chain reaction (PCR)
amplification and genotyping
Genotyping of BDNF, NPSR1 and 5-HTT was performed
using the PCR-restriction fragment length polymorphism (PCR-RFLP)
and direct sequencing (GenScript Corp., Nanjing, China). For
detecting single nucleotide polymorphisms (SNPs), 5-HTT (5-HTTLPR),
BDNF (rs6265) and NPSR1 (rs324981) genes were amplified using PCR.
The sequences of all primers and the conditions of PCR-RFLP
analysis for the three polymorphisms (5-HTTLPR for 5-HTT, rs324981
for NPSR1 and rs6265 for BDNF) are summarized in Table I. Each SNP site for DNA sequencing
was randomly selected from 10% of the samples. The sequencing was
performed using the dideoxynucleotide termination method.
 | Table ICharacterization of PCR reaction
conditions for the analyzed single nucleotide polymorphisms. |
Table I
Characterization of PCR reaction
conditions for the analyzed single nucleotide polymorphisms.
| Target | Primer sequence
(5′→3′) | Tm (°C) | Restriction
enzyme | Allele sizes
(bp) |
|---|
| 5-HTT | F:
GGCGTTGCCGCTCTGAATGC | 59 | | |
| R:
GAGGGACTGAGCTGGACAACC | | | |
| BDNF (rs6265) | F:
ACTCTGGAGAGCGTGAATGG | 60 | Eco72I | A: 171 |
| R:
ACTACTGAGCATCACCCTGGA | | | G: 99, 72 |
| NPSR1
(rs324981) | F:
GCTTTGCATTTCCTCAGTGG | 60 | AseI | T: 471 |
| R:
ATTTGTGGCTCGTTTGTGTTTTCT | | | A: 277, 194 |
Statistical analysis
All statistical analyses were conducted using SPSS
statistical analysis software (version 19.0; IBM SPSS, Chicago, IL,
USA). Hardy-Weinberg equilibrium (HWE) was evaluated for each SNP
in all groups. The Satterthwaite test was used to compare the
differences in age between groups with and without anxiety or
depression. The Chi-square test was used to compare the differences
in genotypic distribution among groups. The interactive effects of
different genotypes on the anxiety/depression scores were analyzed
using the Scheirer-Ray-Hare test. A P-value <0.05 was considered
to indicate a statistically significant difference.
Results
Baseline characteristics of subjects
The baseline characteristics of the subjects are
summarized in Table II. A total
of 143 (male 63, female 80) asthmatic patients were examined in
this study. There were 49 patients with anxiety and 94 without
anxiety. There were 12 patients with depression and 131 without
depression. There were 20 males and 29 females with anxiety, and 7
males and 5 females with depression. A total of 175 (male 51,
female 124) healthy controls, (mean age, 39.11±14.98 years) were
included in this study. The mean age of the asthmatic patients was
50.22±12.95 years. The mean age of the patients with and without
anxiety was 52.76±10.13 and 48.9±14.08 years, respectively, with no
significant difference in age between the two groups (t=−1.88,
P=0.063). Similarly, no significant difference was observed between
the mean ages of patients with and without depression [54.08±8.21
and 49.87±13.27 years, respectively (t=−1.08, P=0.283)]. These
results suggest that the occurrence of both anxiety and depression
in asthmatic patients is not associated with age. Both age and
gender were significantly different in the group with asthma and
the group with asthma and anxiety (age, F=25.74, P<0.0001;
gender, χ2=7.95, P=0.019), when compared with healthy
controls.
 | Table IIBaseline characteristics of study
subjects. |
Table II
Baseline characteristics of study
subjects.
| Variable | Healthy | Asthma | Asthma without
anxiety | Asthma with
anxiety | Statistics | Asthma without
depression | Asthma with
depression | Statistics |
|---|
| Total | 175 | 143 | 94 | 49 | | 131 | 12 | |
| Age (years) | 39.11±14.98 | 50.22±12.95 | 48.9±14.08 | 52.76±10.13 | t=−1.88,
P=0.063 | 49.87±13.27 | 54.08±8.21 | t=−1.08,
P=0.283
F=25.74,
P<0.0001 |
| Gender | | | | | | | | |
| Male | 51 | 63 | 43 | 20 | | 56 | 7 |
χ2=7.95, |
| Female | 124 | 80 | 51 | 29 | | 75 | 5 | P=0.019 |
| Education | | | | | | | | |
| 1 | | | 4 (4.35) | 2 (4.08) |
χ2=0.29, | 5 (3.88) | 1 (8.33) |
χ2=5.70, |
| 2 | | | 22 (23.91) | 14 (28.57) | P=0.59 | 29 (22.48) | 7 (58.33) | P=0.017 |
| 3 | | | 34 (36.96) | 18 (36.73) | | 50 (38.76) | 2 (16.67) | |
| 4 | | | 32 (4.78) | 15 (30.61) | | 45 (34.88) | 2 (16.67) | |
| ACT score | N/A | 18.49±4.51 | 19.77±4 | 16.04±4.47 | P<0.05 | 18.9±4.37 | 14±3.67 | t=0.76,
P=0.0002 |
In the present study, patients who were less
educated were more likely to suffer from depression (χ2
=5.70, P=0.017) rather than anxiety (χ2=0.29, P=0.59).
Our results suggest a negative correlation between depression and
education levels, possibly due to the educated patients having a
better understanding of asthma and other diseases. Upon evaluation
of the ACT score, we noted that asthma patients with anxiety and
depression had lower scores as compared to those without any
psychiatric illness (anxiety, 16.04±4.47; P<0.05 and depression,
14±3.67; P=0.0002). These findings suggest a negative correlation
of the ACT score with both anxiety and depression in asthmatic
patients.
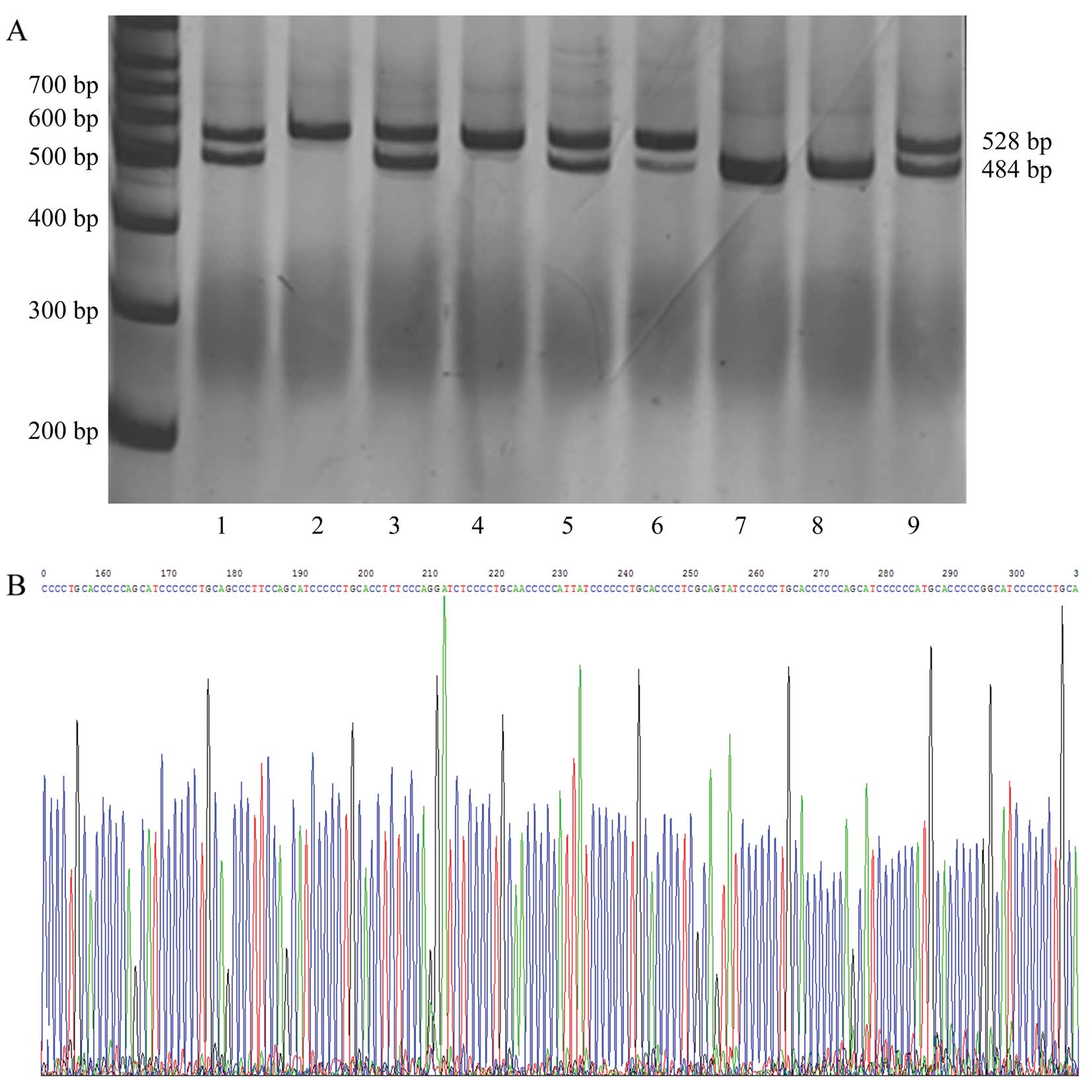
Association of 5-HTT gene polymorphisms
with anxiety and depression in asthmatic patients
To examine the association of 5-HTT gene
polymorphisms with anxiety and depression in asthmatic patients, we
analyzed the frequency of 5-HTT genotypes in the healthy control
group, the groups with asthma but without anxiety/depression, and
the groups with asthma and anxiety/depression (Table III and Fig. 1). In addition, 5-HTT gene
polymorphisms were not detected in 6 healthy subjects. Therefore,
there were data from 169 healthy subjects shown in Table III.
 | Table IIIGenotype distributions and allele
frequencies of 5-HTT polymorphisms for asthmatic patients vs.
control group. |
Table III
Genotype distributions and allele
frequencies of 5-HTT polymorphisms for asthmatic patients vs.
control group.
| Subject | Genotype
| Total | Statistics |
|---|
| LL | LS | SS |
|---|
| Healthy | 6 (3.55) | 63 (37.28) | 100 (59.17) | 169 | |
| Male | 3 (6.12) | 17 (34.69) | 29 (59.18) | 49 | |
| Female | 3 (2.50) | 46 (38.33) | 71 (59.17) | 120 | |
| Asthma without
anxiety | 10 (11.11) | 42 (56.67) | 38 (42.22) | 90 | |
| Male | 4 (9.76) | 20 (48.78) | 17 (41.46) | 41 | |
| Female | 6 (12.24) | 22 (44.90) | 21 (42.86) | 49 | |
| Asthma with
anxiety | 3 (6.38) | 21 (44.68) | 23 (48.94) | 47 |
χ2=10.18, P=0.038 |
| Male | 3 (15.79) | 7 (36.84) | 9 (47.37) | 19 | χ2=4.02,
P=0.43 |
| Female | 0 (0.00) | 14 (50.00) | 14 (50.00) | 28 |
χ2=10.97, P=0.027 |
| Asthma without
depression | 12 (9.52) | 59 (46.83) | 55 (43.65) | 126 | |
| Male | 6 (11.32) | 24 (45.28) | 23 (43.40) | 53 | |
| Female | 6 (8.22) | 35 (47.95) | 32 (43.84) | 73 | |
| Asthma with
depression | 1 (9.09) | 4 (36.36) | 6 (54.55) | 11 | χ2=9.34,
P=0.053 |
| Male | 1 (14.29) | 3 (42.86) | 3 (42.86) | 7 | χ2=3.04,
P=0.55 |
| Female | 0 (0.00) | 1 (25.00) | 3 (75.00) | 4 | χ2=7.09,
P=0.13 |
The majority of healthy individuals had the SS
(59.17%) genotype. However, in the group with asthma but without
anxiety, the majority had the LS (56.67%) genotype, followed by SS
(42.22%) and LL (11.11%) genotypes. In the group with asthma and
anxiety, the LS and SS genotypes accounted for 44.68 and 48.94%,
respectively, whereas LL accounted for only 6.38%. None of the
females had the LL genotype in this group. There were significant
differences in the distribution of 5-HTT genotypes in the group
with asthma but without anxiety, and the group with asthma and
anxiety, when compared with the healthy controls
(χ2=10.18, P=0.038). Furthermore, there were significant
differences in the distribution of 5-HTT genotypes among female
patients (χ2=10.97, P=0.027) with asthma and anxiety
when compared with healthy females. However, similar results were
not noted for males. These findings suggest an association between
the 5-HTT gene polymorphisms and female asthmatic patients with
anxiety.
In the group with asthma without depression, the LS
and SS genotypes accounted for 46.83 and 43.65%, respectively; LL
accounted for only 9.52%. In the group with asthma and depression,
LS and SS accounted for 36.36 and 54.55%, respectively, whereas LL
accounted for only 9.09%. It is important to note that none of the
females had the LL genotype. The Chi-square test showed that 5-HTT
gene distributions in the healthy group, and in asthma patients
with or without depression group were similar (χ2=9.34,
P=0.053). Also, there was no gender bias between the three groups.
These findings suggest that there is no association between 5-HTT
gene polymorphisms and asthmatic patients with depression.
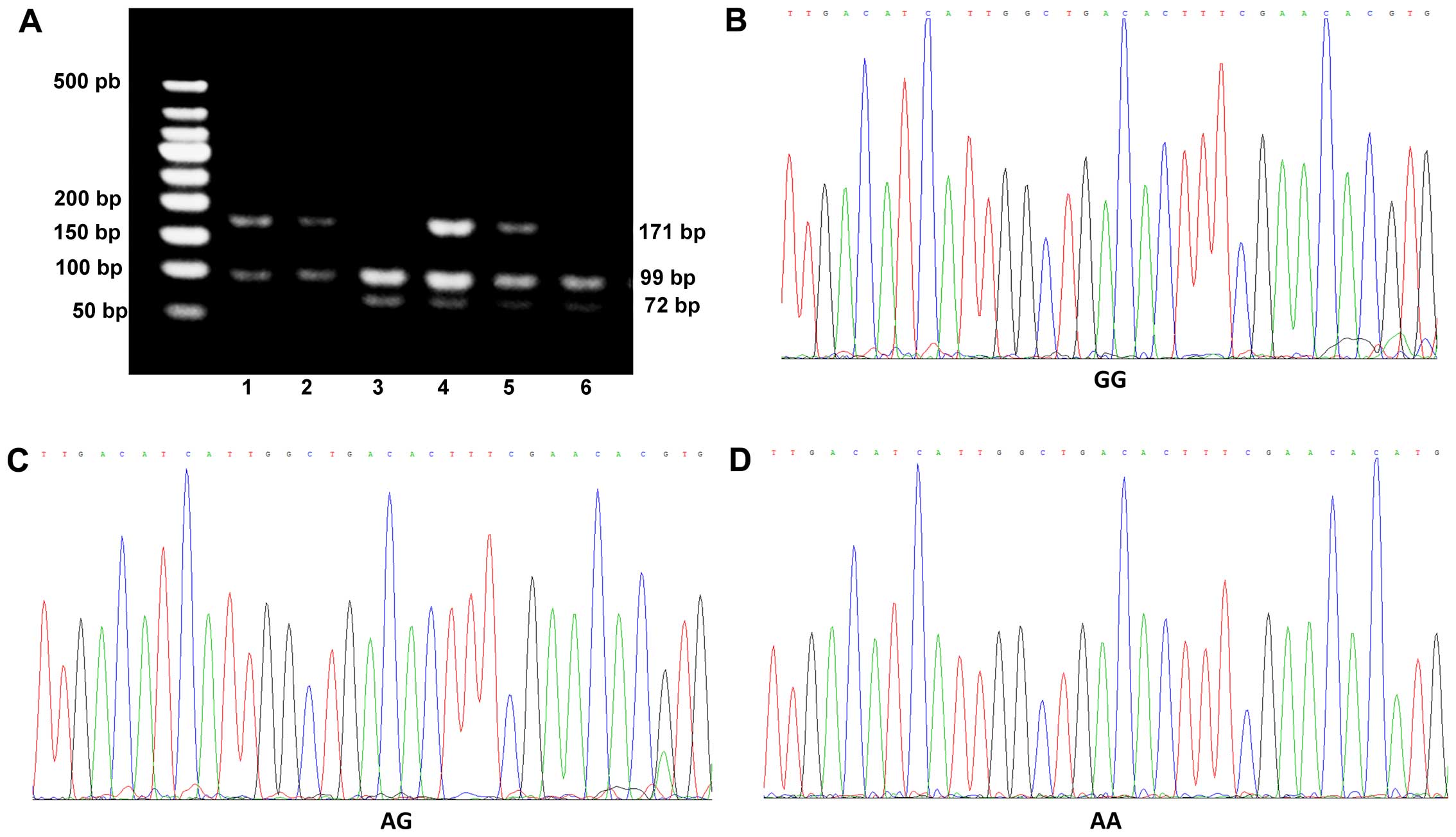
Association of BDNF gene polymorphisms
with anxiety and depression
The frequency of BDNF gene polymorphisms in the
healthy controls, asthmatic patients without anxiety/depression,
and groups with asthma and anxiety/depression was also examined
(Table IV and Fig. 2). In addition, BDNF gene
polymorphisms were not detected in 4 healthy subjects. Therefore,
there were data from 171 healthy subjects shown in Table IV. We noted that 52.63% of
healthy individuals had the AG genotype. In the group with asthma
but not anxiety, the major genotype of BNDF was AA (40.66%),
followed by AG (32.97%) and GG (26.37%). In the group with asthma
and anxiety, the major genotype of BNDF was AA (44.44%), followed
by AG (31.11%) and GG (24.44%). BDNF genotype distributions in the
group with asthma but not anxiety, and the group with asthma and
anxiety (χ2=24.72, P<0.0001) were significantly
different when compared with the control group. Furthermore, there
were significant differences in the distribution of the BNDF
genotype among the female asthmatic patients with anxiety when
compared with healthy females (χ2=19.35, P=0.0007). A
corresponding association was not observed in male patients. These
findings suggest an association between BDNF gene polymorphisms and
female asthmatic patients with anxiety.
 | Table IVGenotype distribution and allele
frequencies of BDNF polymorphisms in asthmatic patients vs.
controls. |
Table IV
Genotype distribution and allele
frequencies of BDNF polymorphisms in asthmatic patients vs.
controls.
| Subject | Genotype
| Total | Statistics |
|---|
| AA | AG | GG |
|---|
| Healthy | 69 (40.35) | 90 (52.63) | 12 (7.02) | 171 | |
| Male | 22 (43.14) | 27 (52.94) | 2 (3.92) | 51 | |
| Female | 47 (39.17) | 63 (52.50) | 10 (8.33) | 120 | |
| Asthma without
anxiety | 37 (40.66) | 30 (32.97) | 24 (26.37) | 91 | |
| Male | 17 (40.48) | 17 (40.48) | 8 (19.05) | 42 | |
| Female | 20 (40.82) | 13 (26.53) | 16 (32.65) | 49 | |
| Asthma with
anxiety | 20 (44.44) | 14 (31.11) | 11 (24.44) | 45 |
χ2=24.72, P<0.0001 |
| Male | 9 (47.37) | 5 (26.32) | 5 (26.32) | 19 | χ2=9.34,
P=0.053 |
| Female | 11 (42.31) | 9 (34.62) | 6 (23.08) | 26 |
χ2=19.35, P=0.0007 |
| Asthma without
depression | 52 (41.6) | 42 (33.6) | 31 (24.8) | 125 | |
| Male | 22 (40.74) | 21 (38.89) | 11 (20.37) | 54 | |
| Female | 30 (42.25) | 21 (29.58) | 20 (28.17) | 71 | |
| Asthma with
depression | 5 (45.45) | 2 (18.18) | 4 (36.36) | 11 |
χ2=26.40, P<0.0001 |
| Male | 4 (57.14) | 1 (14.29) | 2 (28.57) | 7 |
χ2=10.82, P=0.029 |
| Female | 1 (25.00) | 1 (25.00) | 2 (50.00) | 4 |
χ2=18.92, P=0.0008 |
In the group of asthmatic patients without
depression AA, AG and GG genotypes of BNDF accounted for 41.6, 33.6
and 24.8%, respectively. In the group with asthma and depression,
AA, AG and GG genotypes accounted for 45.45, 18.18 and 36.36%,
respectively. In this group, AA, AG and GG genotypes were present
in 57.14, 14.29 and 28.57% of males, respectively, and 25.00, 25.00
and 50.00% of females, respectively. There were significant
differences in the distribution of BDNF genotypes in the asthmatic
patients with and without depression (χ2=26.40,
P<0.0001), when compared with the healthy controls. Further
analysis revealed significant differences in the distribution of
BNDF genotypes among both male (χ2=10.82, P=0.029) and
female patients (χ2=18.92, P=0.0008) with asthma and
depression, when compared with healthy males and females,
respectively. These findings suggest an association between BDNF
gene polymorphisms and asthmatic patients with depression
regardless of gender.
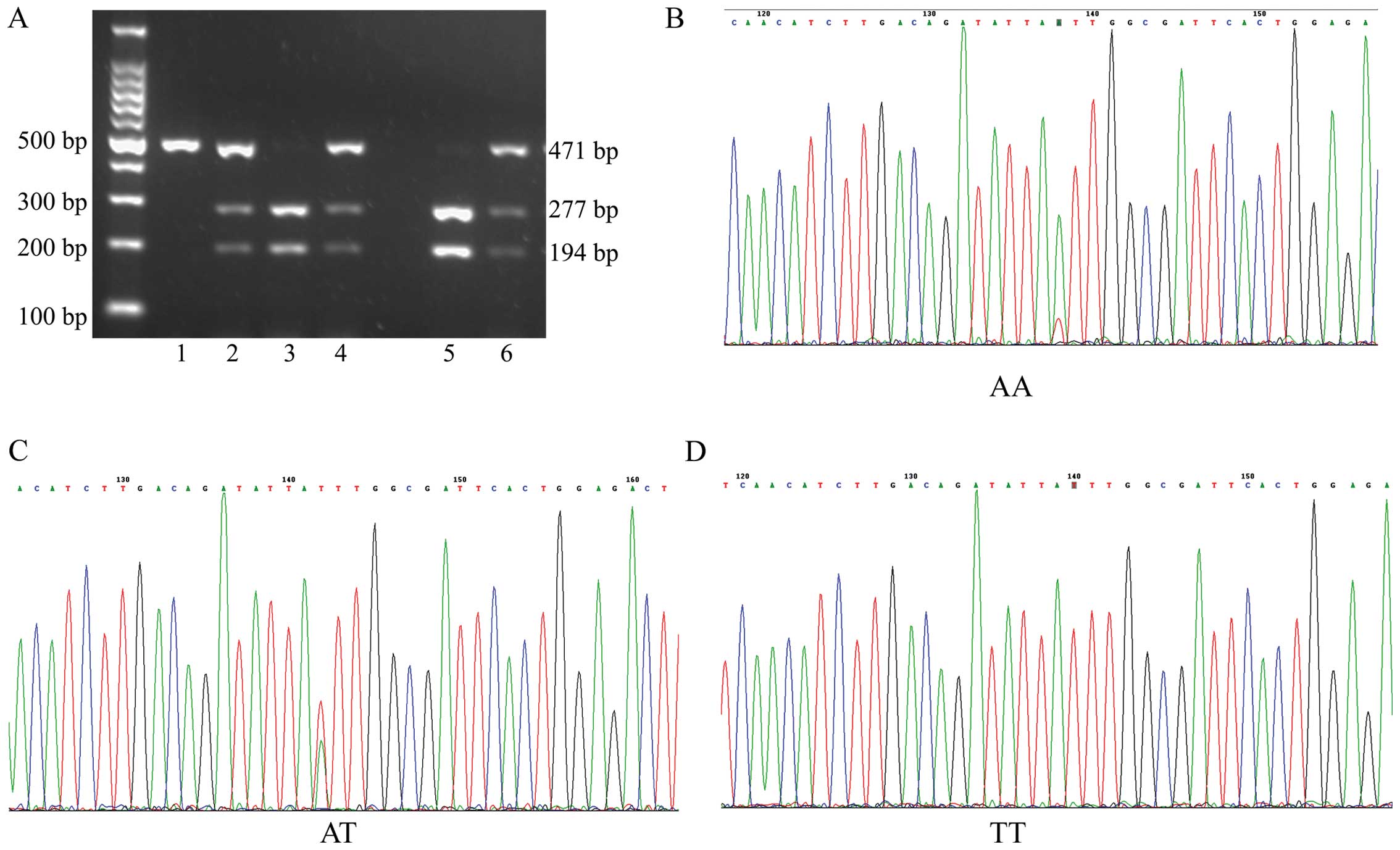
Association of NPSR1 gene polymorphisms
with anxiety and depression
To examine the association between NPSR1 gene
polymorphisms and both anxiety and depression in asthmatic
patients, we analyzed the frequencies of NPSR1 genotypes in the
healthy control group, the groups with asthma but without
anxiety/depression, and the groups with asthma and
anxiety/depression (Table V and
Fig. 3). In addition, NPSR1 gene
polymorphisms were not detected in 4 healthy subjects. Therefore,
there were data from 171 healthy subjects shown in Table V.
 | Table VGenotype distribution and allele
frequencies of NPSR1 polymorphisms in asthmatic patients vs.
controls. |
Table V
Genotype distribution and allele
frequencies of NPSR1 polymorphisms in asthmatic patients vs.
controls.
| Subject | Genotype
| Total | Statistics |
|---|
| TT | AT | AA |
|---|
| Healthy | 59 (34.50) | 79 (46.20) | 33 (19.30) | 171 | |
| Male | 19 (37.25) | 26 (50.98) | 6 (11.76) | 51 | |
| Female | 40 (33.33) | 53 (44.17) | 27 (22.50) | 120 | |
| Asthma without
anxiety | 26 (28.57) | 49 (53.85) | 16 (17.58) | 91 | |
| Male | 13 (30.95) | 23 (54.76) | 6 (14.29) | 42 | |
| Female | 13 (26.53) | 26 (53.06) | 10 (20.41) | 49 | |
| Asthma with
anxiety | 16 (34.78) | 20 (43.48) | 10 (21.74) | 46 | χ2=1.94,
P=0.75 |
| Male | 7 (36.84) | 8 (42.11) | 4 (21.05) | 19 | χ2=1.53,
P=0.82 |
| Female | 9 (33.33) | 12 (44.44) | 6 (22.22) | 27 | χ2=1.23,
P=0.87 |
| Asthma without
depression | 40 (31.75) | 62 (49.21) | 24 (19.05) | 126 | |
| Male | 19 (35.19) | 26 (48.15) | 9 (16.67) | 54 | |
| Female | 21 (29.17) | 36 (50.00) | 15 (20.83) | 72 | |
| Asthma with
depression | 2 (18.18) | 7 (63.64) | 2 (18.18) | 11 | χ2=1.72,
P=0.79 |
| Male | 1 (14.29) | 5 (71.43) | 1 (14.29) | 7 | χ2=2.22,
P=0.70 |
| Female | 1 (25.00) | 2 (50.00) | 1 (25.00) | 4 | χ2=0.72,
P=0.95 |
In the healthy controls, NPSR1 genotypes AT, TT and
AA accounted for 46.20, 34.50 and 19.30%, respectively. Among the
asthmatic patients, AT was the most common genotype that accounted
for 53.85, 43.48, 49.21 and 63.64%, in asthma without anxiety,
asthma with anxiety, asthma without depression, and asthma with
depression, respectively; followed by TT (28.57, 34.78, 31.75 and
18.18%, respectively); and AA (17.58, 21.74, 19.05 and 18.18%,
respectively). NPSR1 genotype distribution in the asthma without
anxiety group, and the asthma with anxiety group
(χ2=1.94, P=0.75) were not found to be significantly
different when compared with healthy controls. Furthermore, there
were no significant differences in genotype distribution among male
(χ2=1.53, P=0.82) and female patients
(χ2=1.23, P=0.87) in the asthma with anxiety group, when
compared with healthy males and females, respectively. These
findings suggest a lack of association between NPSR1 gene
polymorphisms (both overall and gender related) and asthmatic
patients with anxiety.
NPSR1 genotype distribution was not found to be
significantly different in the asthma without depression group, and
the asthma with depression group (χ2=1.72, P=0.79), when
compared with healthy controls. Furthermore, there were no
significant differences in genotype distribution among male
(χ2=2.22, P=0.70) and female asthmatic patients
(χ2=0.72, P=0.95) with depression, when compared with
healthy males and females, respectively. These findings also
indicate a lack of association between NPSR1 gene polymorphisms
(both overall and gender related) and depression in asthmatic
patients.
Allele frequencies of 5-HTT, BDNF and
NPSR1 in asthma patients with and without anxiety or
depression
We analyzed the allele frequencies of 5-HTT, BDNF
and NPSR1 in the asthmatic patients with and without anxiety or
depression (Table VI). The
majority of patients with asthma had the S+ allele in
the 5-HTT gene, A+ in the BDNF gene and T+ in
the NPSR1 gene. The frequency of the S+ allele in the
5-HTT gene was 89.36% (without anxiety), 93.88% (with anxiety),
90.84% (without depression) and 91.67% (with depression). LL only
accounted for 10.64, 6.12, 9.16 and 8.33%, respectively. The
frequency of the A+ allele in the BDNF gene was 74.47%
(without anxiety), 77.55% (with anxiety), 76.34% (without
depression) and 66.67% (with depression); GG accounted for 25.53,
22.45, 23.66 and 33.33%, respectively. The frequency of the
T+ allele in the NPSR1 gene was 82.98% (without
anxiety), 79.59% (with anxiety), 81.68% (without depression), and
83.33% (with depression); and AA accounted for 17.02, 20.41, 18.32
and 16.67%, respectively.
 | Table VIGenotype distributions of 5-HTT and
BDNF and NPSR1 polymorphisms in asthmatic patients with and without
anxiety or depression. |
Table VI
Genotype distributions of 5-HTT and
BDNF and NPSR1 polymorphisms in asthmatic patients with and without
anxiety or depression.
| 5-HTT
| BDNF
| NPSR1
|
|---|
| S+ | LL | A+ | GG | T+ | AA |
|---|
| Asthma without
anxiety | 84 (89.36) | 10 (10.64) | 70 (74.47) | 24 (25.53) | 78 (82.98) | 16 (17.02) |
| Asthma with
anxiety | 46 (93.88) | 3 (6.12) | 38 (77.55) | 11 (22.45) | 39 (79.59) | 10 (20.41) |
| Asthma without
depression | 119 (90.84) | 12 (9.16) | 100 (76.34) | 31 (23.66) | 107 (81.68) | 24 (18.32) |
| Asthma with
depression | 11 (91.67) | 1 (8.33) | 8 (66.67) | 4 (33.33) | 10 (83.33) | 2 (16.67) |
Correlation of 5-HTT, BDNF and NPSR1 gene
polymorphisms with anxiety/depression scores
When the alleles were LL (5-HTT) and A+
(BDNF), the anxiety score had the highest median value.
Scheirer-Ray-Hare analysis demonstrated that the anxiety score was
significantly affected by the interactions between 5-HTT (LL,
S+) and BDNF (A+, GG) (H=5.99, P=0.015), as
shown in Table VII. In
addition, BDNF gene polymorphisms were not detected in 1 patient.
Therefore, there were data from 142 patients shown in Table VII. This suggests a synergistic
effect of the interaction between 5-HTT and BDNF on the anxiety
score. When the alleles were GG (BDNF) and AA (NPSR1), the
depression score had the maximum median value. Scheirer-Ray-Hare
analysis showed that the depression score was significantly
affected by the interactions between BDNF (A+, GG) and
NPSR1 (AA, T+) (H=4.22, P=0.04), as shown in Table VIII. The results suggest that
the interaction between BDNF and NPSR1 had a synergistic effect on
the depression score.
 | Table VIIInteraction between 5-HTT and BDNF
polymorphisms and its affect on the anxiety score of asthmatic
patients. |
Table VII
Interaction between 5-HTT and BDNF
polymorphisms and its affect on the anxiety score of asthmatic
patients.
| BDNF | 5-HTT | Frequency | Anxiety score
| Statistics |
|---|
| Median | Quartile |
|---|
| A+ | S+ | 97 | 11 | 9 | H=5.99, |
| GG | S+ | 33 | 8 | 8 | P=0.015 |
| A+ | LL | 10 | 82 | 6 | |
| GG | LL | 2 | 20 | 12 | |
 | Table VIIIInteraction between BDNF and NPSR1
polymorphisms and its affect on the depression score of asthmatic
patients. |
Table VIII
Interaction between BDNF and NPSR1
polymorphisms and its affect on the depression score of asthmatic
patients.
| BDNF | NPSR1 | Frequency | Depression score
| Statistics |
|---|
| Median | Quartile |
|---|
| A+ | T+ | 87 | 6 | 6 | H=4.22, |
| A+ | AA | 21 | 3 | 6 | P=0.04 |
| GG | T+ | 30 | 4.5 | 7 | |
| GG | AA | 5 | 8 | 7 | |
Discussion
Asthmatic patients are known to have a higher risk
of developing mental disorders (25). The interactions between
behavioral, neural, endocrine and immune processes have been
studied in asthmatic patients and it has been noted that a major
role is played by psychological factors in the development of
asthma (26). The higher risk of
anxiety and depression in asthmatic patients is also well
documented (5,12,27–29). Furthermore, anxiety and depression
are closely associated with poor asthma control and aggravated
symptomatology (2,3,30).
Genetic polymorphisms may predispose asthmatic patients to anxiety
or depression. In this study, we sought to examine the correlation
between both anxiety and depression and the clinical
characteristics of asthmatic patients, and we explored the
association between NPSR1, BDNF and 5-HTT gene polymorphisms and
anxiety and depression in these patients. We found no evidence of
any association between age or education level and the occurrence
of anxiety. However, depression was associated with a lower level
of education, but not age. Both anxiety and depression negatively
correlated with the ACT score.
Genetic factors are thought to influence the
susceptibility of asthmatic patients to anxiety or depression. We
noted that the interaction between 5-HTT (LL) and BDNF
(A+) exerted a synergistic effect to increase the
anxiety score in the asthmatic patients whereas the interaction
between BDNF (A+, GG) and NPSR1 (AA, T+)
exerted a synergistic effect to increase the depression score in
asthmatic patients.
To explore a potential association between
anxiety/depression and the demographic and clinical characteristics
of the patients, we disaggregated the study subjects by age,
gender, educational level and ACT scores. Consistent with a
previous study (31), the
incidence of asthma correlated with age and gender. However,
significant differences in asthmatic patients with or without
anxiety and with or without depression were not noted with respect
to age or gender. The incidence of anxiety or depression was
similar between males and females, as well as with respect to age.
The results of our study differ from some earlier studies in this
respect (32,33). For example, in a study by
Fernandes et al (33), the
risk of anxiety was noted as being directly proportional to age,
and inversely proportional to the socioeconomic level, particularly
in women. Notably, we found that depression rather than anxiety was
associated with a lower level of education. Consistent with the
findings of previous studies (2,3,5,7,10,34), both anxiety and depression were
associated with poor asthma control, as evaluated by the ACT score
in our study. Amelink et al (7) reported that patients with severe
prednisone-dependent asthma were at a higher risk of emotional
distress than those with severe non-prednisone dependent asthma, or
those with mild to moderate asthma. Thus, co-existing anxiety
and/or depression may be a major determinant of treatment outcomes
in asthmatic patients. These findings underline the importance of
screening asthmatic patients for anxiety and depression, as part of
the routine management of these patients. Furthermore, adjunctive
behavioral therapy or psychiatric intervention should be considered
for these patients.
Our study also assessed the role of genetic factors
in contributing to the increased risk of anxiety or depression in
asthmatic patients. We explored the association between SNPs of
5-HTT, BDNF and NPSR1 genes and anxiety or depression in asthmatic
patients. Our results demonstrated an association between 5-HTT
gene polymorphisms and anxiety in female asthmatic patients. To the
best of our knowledge, this is the first report linking 5-HTT gene
polymorphisms with anxiety in asthmatic patients. Caspi et
al (35) reported an
association between polymorphisms in the promoter region of the
5-HTT gene and the influence of stressful life events on
depression. However, a meta-analysis of 14 independent studies
(14) did not support this
conclusion. This previous analysis showed that the 5-HTT
polymorphism was not associated with an increased risk of
depression in males alone, females alone or both males and females
(14). BDNF is a neurotrophic
factor which plays a critical role in maintaining the function of
the neurons (21). It has also
been reported to play a pivotal role in the pathogenesis of major
depression and other mood disorders (21,36). In addition to being highly
expressed in the brain, it is also expressed in the lungs (37). Braun et al (38) suggested a link between BDNF and
allergic bronchial asthma. Genetic variants of BDNF have been
implicated in the causation of allergic asthma in various
populations (19,22,39–41). However, whether polymorphisms of
the BDNF gene predispose asthmatic patients to be affected by mood
disorders has not yet been established. In the present study, gene
polymorphisms in BDNF were not only associated with anxiety in
female asthmatic patients, but were also linked with depression in
the asthmatic population regardless of gender. These results
suggest that BDNF genetic variants play an important role in the
pathogenesis of anxiety and depression in asthmatic patients.
The NPS receptor signaling pathway is involved in
the pathogenesis of asthma and allergies (42). It also regulates anxiety and
arousal in rodents (43). Donner
et al (22) suggested that
the NPS and NPSR1 signaling pathway is involved in regulating human
susceptibility to anxiety disorders. The NPSR1 gene has been linked
with asthma, high serum total IgE levels and other atopic diseases
(44). By contrast, the present
study showed that NPSR1 gene polymorphisms were not associated with
either anxiety or depression in asthmatic patients.
Furthermore, we found that the S+
(5-HTT), A+ (BDNF) and T+ (NPSR1) alleles had
the highest frequency in the asthmatic patients regardless of
anxiety or depression. More importantly, the risk and severity of
anxiety or depression appeared to be influenced by the interaction
between gene-gene polymorphisms. Asthmatic patients carrying both
risk alleles 5-HTT (LL) and BDNF (A+) had enhanced
anxiety scores, and those carrying risk alleles BDNF
(A+, GG) and NPSR1 (AA, T+) had increased
depression scores. A previous study demonstrated that the NPSR1
polymorphism in AA carriers was linked to increased anxiety scores.
However, the fear-potentiated startle is affected by the
interaction between 5-HTT and NPSR1 polymorphisms (45). In our study, there was no evidence
to suggest that the interaction between 5-HTT and NPSR1
polymorphisms plays a role in inducing anxiety or depression in
asthmatic patients. Our findings suggest that anxiety and
depression in asthmatic patients are influenced not only by
polymorphisms of individual genes, but also by interactions between
different gene polymorphisms.
It is necessary to acknowledge some limitations of
our study. In the present study, the assessment of anxiety and
depression was undertaken using a simple questionnaire, without
conducting a structured interview. Another limitation is the
cross-sectional study design, due to which the results may not be
consistent over time. The third possible limitation is the
existence of selection bias in the study, as all the patients were
enrolled from a single hospital.
In conclusion, the present study demonstrates
differences in the risk factors for anxiety and depression in
asthmatic patients. Depression was negatively associated with the
level of education. There was a correlation between both anxiety
and depression and poor asthma control. The interaction between
5-HTT (LL) and BDNF (A+) appeared to increase the risk
of anxiety, whereas the interaction between BDNF (A+,
GG) and NPSR1 (AA, T+) appeared to increase the risk of
depression in asthmatic patients.
Acknowledgments
We would like to thank Dr Xiaojin Yu and his
research team at the Southeast University School of Public Health
for assistance with statistical analysis. We would like to thank
Professor Ran Liu at the Southeast University School of Public
Health for assistance in genetic testing.
References
|
1
|
Grainge C, Thomas PS, Mak JC, Benton MJ,
Lim TK and Ko FW: Year in review 2015: Asthma and chronic
obstructive pulmonary disease. Respirology. 21:765–775. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Valença AM, Falcão R, Freire RC,
Nascimento I, Nascentes R, Zin WA and Nardi AE: The relationship
between the severity of asthma and comorbidities with anxiety and
depressive disorders. Rev Bras Psiquiatr. 28:206–208. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Di Marco F, Verga M, Santus P, Giovannelli
F, Busatto P, Neri M, Girbino G, Bonini S and Centanni S: Close
correlation between anxiety, depression, and asthma control. Respir
Med. 104:22–28. 2010. View Article : Google Scholar
|
|
4
|
Thoren C and Petermann F: Reviewing asthma
and anxiety. Respir Med. 94:409–415. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coban H and Aydemir Y: The relationship
between allergy and asthma control, quality of life, and emotional
status in patients with asthma: a cross-sectional study. Allergy
Asthma Clin Immunol. 10:672014. View Article : Google Scholar
|
|
6
|
Katon WJ, Richardson L, Lozano P and
McCauley E: The relationship of asthma and anxiety disorders.
Psychosom Med. 66:349–355. 2004.PubMed/NCBI
|
|
7
|
Amelink M, Hashimoto S, Spinhoven P, Pasma
HR, Sterk PJ, Bel EH and ten Brinke A: Anxiety, depression and
personality traits in severe, prednisone-dependent asthma. Respir
Med. 108:438–444. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng Z, Dai LL, Li F, Liu Y, Kang Y, Chen
HJ, Wang X, Zhang H and Ni R: Relationship between anxiety,
depression and asthma control. Zhonghua Yi Xue Za Zhi.
92:2128–2130. 2012.In Chinese. PubMed/NCBI
|
|
9
|
Deshmukh VM, Toelle BG, Usherwood T,
O'Grady B and Jenkins CR: The association of comorbid anxiety and
depression with asthma-related quality of life and symptom
perception in adults. Respirology. 13:695–702. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kullowatz A, Kanniess F, Dahme B,
Magnussen H and Ritz T: Association of depression and anxiety with
health care use and quality of life in asthma patients. Respir Med.
101:638–644. 2007. View Article : Google Scholar
|
|
11
|
Letitre SL, de Groot EP, Draaisma E and
Brand PL: Anxiety, depression and self-esteem in children with
well-controlled asthma: case-control study. Arch Dis Child.
99:744–748. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu S, Wu R, Li L, Liu L, Li G, Zhang X,
Guo Y, Wang Y, Zhang H, Li G and Li H: The prevalence of anxiety
and depression in Chinese asthma patients. PLoS One. 9:e1030142014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alvarez GG and Fitzgerald JM: A systematic
review of the psychological risk factors associated with near fatal
asthma or fatal asthma. Respiration. 74:228–236. 2007. View Article : Google Scholar
|
|
14
|
Risch N, Herrell R, Lehner T, Liang KY,
Eaves L, Hoh J, Griem A, Kovacs M, Ott J and Merikangas KR:
Interaction between the serotonin transporter gene (5-HTTLPR),
stressful life events, and risk of depression: a meta-analysis.
JAMA. 301:2462–2471. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mann JJ, Huang YY, Underwood MD, Kassir
SA, Oppenheim S, Kelly TM, Dwork AJ and Arango V: A serotonin
transporter gene promoter polymorphism (5-HTTLPR) and prefrontal
cortical binding in major depression and suicide. Arch Gen
Psychiatry. 57:729–738. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Smeraldi E, Zanardi R, Benedetti F, Di
Bella D, Perez J and Catalano M: Polymorphism within the promoter
of the serotonin transporter gene and antidepressant efficacy of
fluvoxamine. Mol Psychiatry. 3:508–511. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park MH, Chang KD, Hallmayer J, Howe ME,
Kim E, Hong SC and Singh MK: Preliminary study of anxiety symptoms,
family dysfunction, and the brain-derived neurotrophic factor
(BDNF) Val66Met genotype in offspring of parents with bipolar
disorder. J Psychiatr Res. 61:81–88. 2015. View Article : Google Scholar
|
|
18
|
Hashimoto K, Shimizu E and Iyo M: Critical
role of brain-derived neurotrophic factor in mood disorders. Brain
Res Brain Res Rev. 45:104–114. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Watanabe T, Fajt ML, Trudeau JB, Voraphani
N, Hu H, Zhou X, Holguin F and Wenzel SE: Brain Derived
Neurotrophic Factor (BDNF) Expression in Asthma. Association with
Severity and Type-2 Inflammatory Processes. Am J Respir Cell Mol
Biol. 844–852. 2015. View Article : Google Scholar
|
|
20
|
Ozek E and Ekici B: Asthma and suicide:
possible role of brain-derived neurotrophic factor. Med Hypotheses.
77:261–262. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsai SJ: Asthma, major depression and
brain-derived neurotrophic factor. Med Hypotheses. 65:417–418.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Andiappan AK, Parate PN, Anantharaman R,
Suri BK, Wang Y and Chew FT: Genetic variation in BDNF is
associated with allergic asthma and allergic rhinitis in an ethnic
Chinese population in Singapore. Cytokine. 56:218–223. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Donner J, Haapakoski R, Ezer S, Melén E,
Pirkola S, Gratacòs M, Zucchelli M, Anedda F, Johansson LE,
Söderhäll C, et al: Assessment of the neuropeptide S system in
anxiety disorders. Biol Psychiatry. 68:474–483. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Asthma Workgroup CTS; Chinese Medical
Association: Guideline Chinese expert consensus on bronchial asthma
control. Chin J Tuberc Respir Dis. 31:177–185. 2008.In Chinese.
|
|
25
|
Feldman JM, Siddique MI, Morales E,
Kaminski B, Lu SE and Lehrer PM: Psychiatric disorders and asthma
outcomes among high-risk inner-city patients. Psychosom Med.
67:989–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Di Marco F, Santus P and Centanni S:
Anxiety and depression in asthma. Curr Opin Pulm Med. 17:39–44.
2011. View Article : Google Scholar
|
|
27
|
Adams RJ, Wilson DH, Taylor AW, Daly A,
Tursan d'Espaignet E, Dal Grande E and Ruffin RE: Psychological
factors and asthma quality of life: a population based study.
Thorax. 59:930–935. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Alonso J, de Jonge P, Lim CC,
Aguilar-Gaxiola S, Bruffaerts R, Caldas-de-Almeida JM, Liu Z,
O'Neill S, Stein DJ, Viana MC, et al: Association between mental
disorders and subsequent adult onset asthma. J Psychiatr Res.
59:179–188. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Scott KM, Von Korff M, Ormel J, Zhang MY,
Bruffaerts R, Alonso J, Kessler RC, Tachimori H, Karam E, Levinson
D, et al: Mental disorders among adults with asthma: results from
the World Mental Health Survey. Gen Hosp Psychiatry. 29:123–133.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Leander M, Lampa E, Rask-Andersen A,
Franklin K, Gislason T, Oudin A, Svanes C, Torén K and Janson C:
Impact of anxiety and depression on respiratory symptoms. Respir
Med. 108:1594–1600. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
de Marco R, Locatelli F, Sunyer J and
Burney P: Differences in incidence of reported asthma related to
age in men and women. A retrospective analysis of the data of the
European Respiratory Health Survey. Am J Respir Crit Care Med.
162:68–74. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sundberg R, Torén K, Franklin KA, Gislason
T, Omenaas E, Svanes C and Janson C: Asthma in men and women:
treatment adherence, anxiety, and quality of sleep. Respir Med.
104:337–344. 2010. View Article : Google Scholar
|
|
33
|
Fernandes L, Fonseca J, Martins S, Delgado
L, Pereira AC, Vaz M and Branco G: Association of anxiety with
asthma: subjective and objective outcome measures. Psychosomatics.
51:39–46. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Trzcinska H, Zwierzchowska B, Kozlowski B,
Derdowski S and Przybylski G: Analysis of the role of selected
demographic and psychological variables (anxiety and depression) as
risk factors of inadequate control of bronchial asthma. Ann Agric
Environ Med. 20:504–508. 2013.PubMed/NCBI
|
|
35
|
Caspi A, Sugden K, Moffitt TE, Taylor A,
Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A
and Poulton R: Influence of life stress on depression: moderation
by a polymorphism in the 5-HTT gene. Science. 301:386–389. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Castrén E, Võikar V and Rantamäki T: Role
of neurotrophic factors in depression. Curr Opin Pharmacol.
7:18–21. 2007. View Article : Google Scholar
|
|
37
|
Lommatzsch M, Braun A, Mannsfeldt A,
Botchkarev VA, Botchkareva NV, Paus R, Fischer A, Lewin GR and Renz
H: Abundant production of brain-derived neurotrophic factor by
adult visceral epithelia. Implications for paracrine and
target-derived Neurotrophic functions. Am J Pathol. 155:1183–1193.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Braun A, Lommatzsch M, Mannsfeldt A,
Neuhaus-Steinmetz U, Fischer A, Schnoy N, Lewin GR and Renz H:
Cellular sources of enhanced brain-derived neurotrophic factor
production in a mouse model of allergic inflammation. Am J Respir
Cell Mol Biol. 21:537–546. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jesenak M, Babusikova E, Evinova A,
Banovcin P and Dobrota D: Val66Met polymorphism in the BDNF gene in
children with bronchial asthma. Pediatr Pulmonol. 50:631–637. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Szczepankiewicz A, Bręborowicz A,
Sobkowiak P and Popiel A: Association of BDNF gene polymorphism
with asthma in Polish children. World Allergy Organ J. 3:235–238.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zeilinger S, Pinto LA, Nockher WA, Depner
M, Klopp N, Illig T, von Mutius E, Renz H and Kabesch M: The effect
of BDNF gene variants on asthma in German children. Allergy.
64:1790–1794. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ilmarinen P, James A, Moilanen E,
Pulkkinen V, Daham K, Saarelainen S, Laitinen T, Dahlen SE, Kere J,
Dahlen B and Kankaanranta H: Enhanced expression of neuropeptide S
(NPS) receptor in eosinophils from severe asthmatics and subjects
with total IgE above 100IU/ml. Peptides. 51:100–109. 2014.
View Article : Google Scholar
|
|
43
|
Xu YL, Gall CM, Jackson VR, Civelli O and
Reinscheid RK: Distribution of neuropeptide S receptor mRNA and
neurochemical characteristics of neuropeptide S-expressing neurons
in the rat brain. J Comp Neurol. 500:84–102. 2007. View Article : Google Scholar
|
|
44
|
Bernier V, Stocco R, Bogusky MJ, Joyce JG,
Parachoniak C, Grenier K, Arget M, Mathieu MC, O'Neill GP, Slipetz
D, et al: Structure-function relationships in the neuropeptide S
receptor: molecular consequences of the asthma-associated mutation
N107I. J Biol Chem. 281:24704–24712. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Glotzbach-Schoon E, Andreatta M, Reif A,
Ewald H, Tröger C, Baumann C, Deckert J, Mühlberger A and Pauli P:
Contextual fear conditioning in virtual reality is affected by
5HTTLPR and NPSR1 polymorphisms: effects on fear-potentiated
startle. Front Behav Neurosci. 7:312013. View Article : Google Scholar : PubMed/NCBI
|