Introduction
Myocardial infarction is a leading cause of
mortality and morbidity in developed countries. Although prompt
reperfusion therapy has been shown to reduce the infarct size and
improve left ventricular function in ST-elevation myocardial
infarction, reperfusion itself may cause lethal tissue injury and a
series of cellular events termed myocardial ischemia-reperfu-sion
(MI/R) injury (1–4).
The molecular mechanisms of reperfusion injury
appear to be multifactorial with various consequences on cellular
function (1). It has been
previously noted that apoptosis occurs shortly after myocardial
infarction and it is markedly enhanced during reperfusion (5,6).
The production of reactive oxygen species (ROS) in the ischemic
region and the surrounding myocardium is also generated, which
directly triggers cell death, including apoptosis. Inflammatory
responses, disruptions of energy metabolism and calcium overload
have also been proposed to underlie the pathology of MI/R injury
(7). Thus, a single therapeutic
agent may be incapable of hitting multiple targets to achieve
therapeutic efficacy.
Comprehensive investigations have been focusing on
combination drugs in order to optimize or amplify the therapeutic
effects (8,9). In traditional Chinese medicine
(TCM), a number of herbs are paired together in order to attenuate
toxicity, as well as to enhance the therapeutic effects (10). Radix Salvia miltiorrhiza
(S. miltiorrhiza) and Carthamus tinctorius L. (C.
tinctorius; also known as Flos Carthami) are usually used as a
combination herbal formulation, known as Danhong injection, which
can relieve the symptoms of angina pectoris, attenuate myocardial
ischemia and promotes atherosclerotic plaque regression (11). Due to their complex constituents,
studies have mainly focused on their combination effects. However,
previous studies have confirmed that Danshensu (DSS) and
hydroxysafflor yellow A (HSYA) are the main active ingredients of
Radix Salvia miltiorrhiza and Flos Carthami, respectively
(12,13). Hence, evaluating the combination
effects of these two active compounds may be of importance in
understanding the rationale for the combined use of the two herbs
in TCM.
There is evidence to indicate that S.
miltiorrhiza and C. tinctorius inhibit cellular
apoptosis and oxidative stress induced by MI/R (14,15). It would be of interest to
investigate the mechanisms reponsible for the combined effects of
DSS and HSYA. Thus, the present study aimed to evaluate the
cardioprotective effects of combination therapy with DSS and HSYA
in order to elucidate the mechanisms responsible for their combined
antioxidant and anti-apoptotic effects in vivo and in
vitro.
Materials and methods
Chemicals and drugs
DSS was purchased from the National Institute for
the Control of Pharmaceutical and Biological Products (Beijing,
China) as an amorphous powder. Its purity (>98%) was determined
using high-performance liquid chromatography (HPLC). The molecular
formula of DSS is C9H10O5 and its
molecular weight is 162.14. HSYA was obtained from C.
tinctorius as a yellow amorphous powder. Its purity (>99%)
was determined using HPLC. Being soluble in water, it has a
molecular formula of C27H32O16 and
a molecular weight of 611.1614.
The creatine kinase-MB (CK-MB) and cardiac troponin
I (cTnI) kits were purchased from Roche Molecular Biochemicals
(Mannheim, Germany). The lactate dehydrogenase (LDH), superoxide
dismutase (SOD) and malondialdehyde (MDA) kits were obtained from
Jiancheng Bioengineering Institute (Nanjing, China).
Dulbecco's modified Eagle's medium (DMEM) and other
cell culture supplies were purchased from Gibco-BRL (Grand Island,
NY, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT), zinc protoporphyrin IX [ZnPP-IX; a heme oxygenase-1
(HO-1) inhibitor], 2,3,5-triphe-nyltetrazolium chloride (TTC),
Evans blue, and hematoxylin and eosin were the products of Sigma
Chemical Co. (St. Louis, MO, USA). The phosphoinositide 3-kinase
(PI3K) inhibitor, LY294002 (#9901), and rabbit polyclonal
antibodies specific for Bcl-2 (#2870), Bax (#2772), β-actin
(#4970), total protein kinase B (Akt; t-Akt, serine 473; #9272),
phosphorylated Akt (p-Akt; #9271), cleaved caspase-3 (#9661) and
caspase-3 (#9662) were obtained from Cell Signaling Technology
(Beverly, MA, USA). Rabbit polyclonal antibodies specific for HO-1
(# sc-10789), 8-hydroxydeoxyguanosine (8-OHdG; #sc-66036) and
nuclear factor erythroid 2-related factor 2 (Nrf2; #sc-722) were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Rabbit polyclonal antibody specific for histone 3 (H3;
#ab4729) was purchased from Abcam (Cambridge, UK). All materials
for sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) were obtained from Bio-Rad Laboratories (Hercules, CA,
USA).
Experimental protocols
In vivo experiments
In consideration of the failures which may occur
when performing coronary ligation, such as no infarcts or death, 10
rats were randomly selected in each group so that a sufficient
number of animals (at least 6 rats) was maintained. The
concentrations of DSS and HSYA were 10, 20, 30, 45 and 60 mg/kg,
and 10, 20, 30, 50 and 70 mg/kg, respectively. They were combined
at concentrations of 10, 20, 30, 50 and 70 mg/kg (at a ratio of 1:1
according to each IC50 value) to analyze the synergistic
effects on myocardial infarct size. In the subsequent experiments,
the rats were randomly divided into 5 groups as follows: i) the
sham-operated group, ii) the MI/R group, iii) the MI/R + DSS (60
mg/kg) group, iv) the MI/R + HSYA (70 mg/kg) group, and v) the MI/R
+ DSS + HSYA (DH; 35 mg/kg DSS + 35 mg/kg HSYA) group. All drugs
were administered via tail vein injection at the time of
reperfusion. The concentrations of DSS and HSYA were selected on
the basis of the reported literature and our preliminary dose
selection experiments (15,16).
In vitro experiments
To further explore the mechanisms responsible for
the combined effects of DSS and HSYA, H9c2 cardiomyocytes were
used. The concentrations of DSS and HSYA were 1, 10, 35, 60 and 80
µM. DSS and HSYA at concentrations of 1, 10, 35, 60 and 80
µM were used in combination (at a ratio of 1:1 according to
each IC50 value) to analyze the synergistic effects on
cell viability. In the subsequent experiments, the H9c2 cells were
randomly divided into the following groups: i) the control (Con)
group, ii) the hypoxia/reoxygenation (H/R) group, iii) the H/R +
DSS (80 µM) group, iv) the H/R + HSYA (80 µM) group,
v) the H/R + DH (40 µM DSS + 40 µM HSYA) group, vi)
the H/R + DH + ZnPP-IX (10 µM) group, and vii) the H/R + DH
+ LY294002 (50 µM) group. The doses of each agent were
selected according to the published literature and our preliminary
experiments (17,18).
Animals and the establishment of an
animal model of MI/R injury
Adult male Sprague-Dawley rats (n=220, approximately
2 months old, weighing 250±20 g) were purchased from the
Experimental Animal Research Center at the Fourth Military Medical
University (Xi'an, China). The experimental protocols involving
animals were performed in adherence with Institutional Animal Care
and were approved by the Animal Care and Use Committee of the
Fourth Military Medical University.
The rats were anesthetized with sodium pentobarbital
(40 mg/kg intraperitoneally) and the tracheas were cannulated with
a polyethylene-90 (PE-90) tube connected to a rodent ventilator
with a tidal volume of 1.2 l/kg (75 breaths/min). A left
thoracotomy was performed between the fourth and fifth ribs. The
pericardium was removed and the left anterior descending (LAD)
artery was visualized and ligated with a 6–0 Prolene suture, as
previously described (19). The
appearance of myocardial pallor was confirmed as ischemia. After 30
min of LAD ligation, the ligature was removed to allow for 180 min
of reperfusion. In the sham-operated group, Prolene was drilled
underneath the LAD, but not ligated.
Assessment of myocardial infarct
size
After the completion of 180 min of reperfusion, the
myocardial infarct size was assessed by a double-staining technique
using 2% TTC and 3% Evans blue as previously described (20). Briefly, the LAD was re-ligated and
2 ml of 3% Evans blue dye was retrogradely infused into the carotid
artery to demarcate the area at risk (AAR; area not perfused with
blue dye) from the area not at risk (stained with blue dye). After
the dye was uniformly distributed, the hearts were rapidly excised
and frozen at −20°C, and subsequently the heart tissue was cut into
5 transverse slices. The sections were incubated in 2% TTC solution
in phosphate buffer (pH 7.4) at 37°C in the dark for 15 min and
then stored in 4% paraformaldehyde overnight to delineate the
infarct size (IS; pale area). The IS and AAR were measured using
Image-Pro Plus software (Media Cybernetics, Inc., Silver Spring,
MD, USA) after capturing images. The myocardial infarct size was
calculated as a percentage of the infarct size over the total AAR.
The dose-effect curve and fraction versus combination index (Fa-CI)
curve were analyzed using CompuSyn software (MIT, Cambridge, MA,
USA).
Determination of CK-MB and cTnI
release in serum
After being anesthetized, the rats were subjected to
MI/R surgery. Following 3 h of reperfusion, blood samples were
collected from the abdominal aorta of the rats using a 10 ml
syringe. The blood of the experimental rats was collected and serum
was separated by centrifugation and then kept at −20°C. The levels
of CK-MB, and cTnI were estimated using a commercially available
enzyme-linked immunosorbent assay kit with a microplate reader
(Thermo Fisher Scientific, Waltham, MA, USA) according to the
manufacturer's instructions.
Assessment of markers for oxidative
stress
The serum and the cell culture supernatant were used
to assay the MDA content and SOD activity using a microplate reader
(Multiskan GO; Thermo Fisher Scientific) according to the
instructions provided by the manufacturer.
Immunohistochemical assay for the
evaluation of 8-OHdG expression
The paraffin-embedded tissue samples were
deparaffinaged in xylene and then dehydrated with ethanol.
Subsequently, the samples were subjected to antigen retrieval and
then incubated in 3% H2O2 in 0.01 M
phosphate-buffered saline (PBS) and in 5% bovine serum albumin
(BSA) successively. The sections were incubated overnight at 4°C
with a primary antibody anti-8-OHdG (1:100) and then incubated for
1 h with a secondary antibody (Boster Biological Technology, Wuhan,
China). The reaction was visualized with a solution of
diaminobenzidine (DAB) and counterstained with hematoxylin. For
quantification, the number of 8-OHdG-stained positive cells was
calculated using Image-Pro Plus 6.0 software (Media Cybernetics,
Inc.).
Cell culture and H/R injury
The H9c2 cardiomyocyte cell line was purchased from
the Chinese Academy of Sciences Cell Bank (Shanghai, China) and
maintained in DMEM supplemented with 10% (v/v) fetal bovine serum
at 37°C in a CO2 incubator. The medium was replaced
every 2–3 days, and the cells were subcultured or subjected to
experimental procedures at 80–90% confluence.
To mimic ischemic injury in vivo, the
procedures for inducing H/R injury were modified from a previously
described method (21). Briefly,
the cells were maintained in serum-free DMEM (glucose-free) instead
of routine culture medium. Hypoxic conditions were established by
equilibrating a humidified chamber containing the cells with 95%
N2 and 5% CO2 via a gas transfusion apparatus
(Billups-Rothenberg, Del Mar, CA, USA). Following 4 h of
incubation, the cells were transferred to normal conditions in a
CO2 incubator and the medium was replaced with routine
culture medium to achieve re-oxygenation. The drugs were
administered at the beginning of re-oxygenation. Following 20 h of
re-oxygenation, the culture medium was collected and stored at
−80°C until analysis. In the control group, the H9c2 cells were
cultured under normal conditions for 24 h.
Analysis of cell viability and LDH
activity
The cells in the exponential phase were seeded at
1×104 cells/well in 96-well plates. After being
subjected to the different treatments, 20 µl of MTT solution
were added to the medium (0.5 mg/ml final concentration in medium)
and the cells were incubated for an additional 4 h at 37°C. The
supernatants were removed and the formazan crystals were dissolved
in 150 µl DMSO. The absorbance was read at 490 nm using a
microplate reader (Multiskan GO; Thermo Fisher Scientific). A
reduction in optical density reduction was considered to indicate a
decrease in cell viability. The cells in the control group were
considered 100% viable. The results were also assessed using
Compusyn software to calculate the CI value.
In order to confirm the degree of cardiomyocyte
injury in H/R, the release of LDH was measured. After being
subjected to the different treatments, the cell culture
supernatants were collected to assay LDH activity immediately
according to the manufacturer's instructions using a microplate
reader (Multiskan GO; Thermo Fisher Scientific) at 450 nm.
Protein extraction and western blot
analysis
The cardiomyocytes were resuspended in
radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime
Institute of Biotechnology, Jiangsu, China) on ice for 30 min. They
were then centifuged at 4°C for 20 min at 10,000 rpm to separate
the supernatant before being stored at −80°C. The nuclear proteins
were extracted separately from cultured myocytes using NE-PER
nuclear and cytoplasmic extraction reagents according to the
manufacturer's instructions (Thermo Fisher Scientific). A
Mitochondria/Cytosol Fractionation kit (BioVision, San Francisco,
CA, USA) was used to prepare mitochondrial protein. Protein
concentrations were measured using the Bradford method with the
Bio-Rad protein assay kit (Bio-Rad Laboratories). Denatured protein
was separated by SDS-PAGE and then electrotransferred onto
polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica,
MA, USA). After being blocked with 5% (w/v) non-fat milk at 37°C
for 30 min, the membranes were incubated overnight at 4°C with
primary antibodies including p-Akt, t-Akt, Bcl-2, Bax, cleaved
caspase-3 and caspase-3, HO-1, Nrf2, β-actin and H3 (1:1,000),
respectively. After washing in TBST 3 times, the membranes were
incubated at room temperature for 1 h with secondary antibody
diluted in TBST. The labeled protein bands were detected using
chemiluminescent reagents and were exposed to film. The band
intensity was determined using an image analyzer (Quantity One
System; Bio-Rad, Richmond, CA, USA).
Detection of apoptotic cell death
Cell apoptosis was determined by terminal
deoxy-nucleotidyl transferase-mediated dUTP nick-end labeling
(TUNEL) assay, as previously described (22). The H9c2 cells grown on a 6-mm
plate were fixed with 4% paraformaldehyde solution for 30 min at
room temperature. The cells were then treated with permeation
solution. Subsequently, the samples were incubated with TUNEL
reagent. The cells were also stained with 1 µg/ml
4′,6-diamidino-2-phenylindole (DAPI) for 30 min to detect cell
nuclei (blue). The number of TUNEL-positive cells was presented as
a percentage of the total cardiomyocytes and was evaluated at ×400
magnification.
Determination of combined effects
The manner in which DSS and HSYA act with regard to
myocardial infarct size and cell viability was determined by a
median-effect method proposed by Chou (23). Synergism or antagonism was
determined with CI values. CompuSyn software (MIT) was used to
determine the CI value. The CI was plotted as the fractional
inhibition (Fa) by computer simulation from 0.10 to 0.95. In this
analysis, the combined effect at the 50% fractional inhibition
(CI50) was reported as synergistic, antagonistic or
additive when the CI50 value was <1, >1 and equal
to 1, respectively.
Statistical analysis
All values are expressed as the means ± standard
deviation (SD). Statistical analysis was performed using one-way
analysis of variance (ANOVA) followed by a least significant
difference (LSD) test for multiple comparisons, using SPSS 19.0
software for Windows (SPSS Inc., Chicago, IL, USA). A value of
P<0.05 was considered to indicate a statistically significant
difference.
Results
DSS and HSYA synergistically alleviate
myocardial injury in rats with MI/R injury
Infarct size and the release of cTnI, and CK-MB in
serum were measured to determine the mechanisms through which DSS
and HSYA influence MI/R injury. The myocardial infarct size was
significantly reduced in the groups treated with the compounds
compared with that in the MI/R group (Fig. 1A and B). Combined treatment (DH)
enhanced this effect to a greater extent than treatment with either
DSS or HSYA alone. In order to determine whether DSS and HSYA have
a synergistic effect on reducing the infarct size, the dose-effect
curves of the single or combined treatment were analyzed by the
median-effect method. We found that DSS, HSYA and combination
therapy (Fig. 1C-1) at
concentrations of 10–60, 10–70 and 10–70 mg/kg yielded
CI50 values <1 (Fig.
1C-2), indicating synergistic effects between the agents. As
these effects occurred in a dose-dependent manner, the highest
doses were selected for use in subsequent experiments. The numbers
of rats that failed the coronary ligation procedure in each group
shown in Fig. 1 are listed in
Table I.
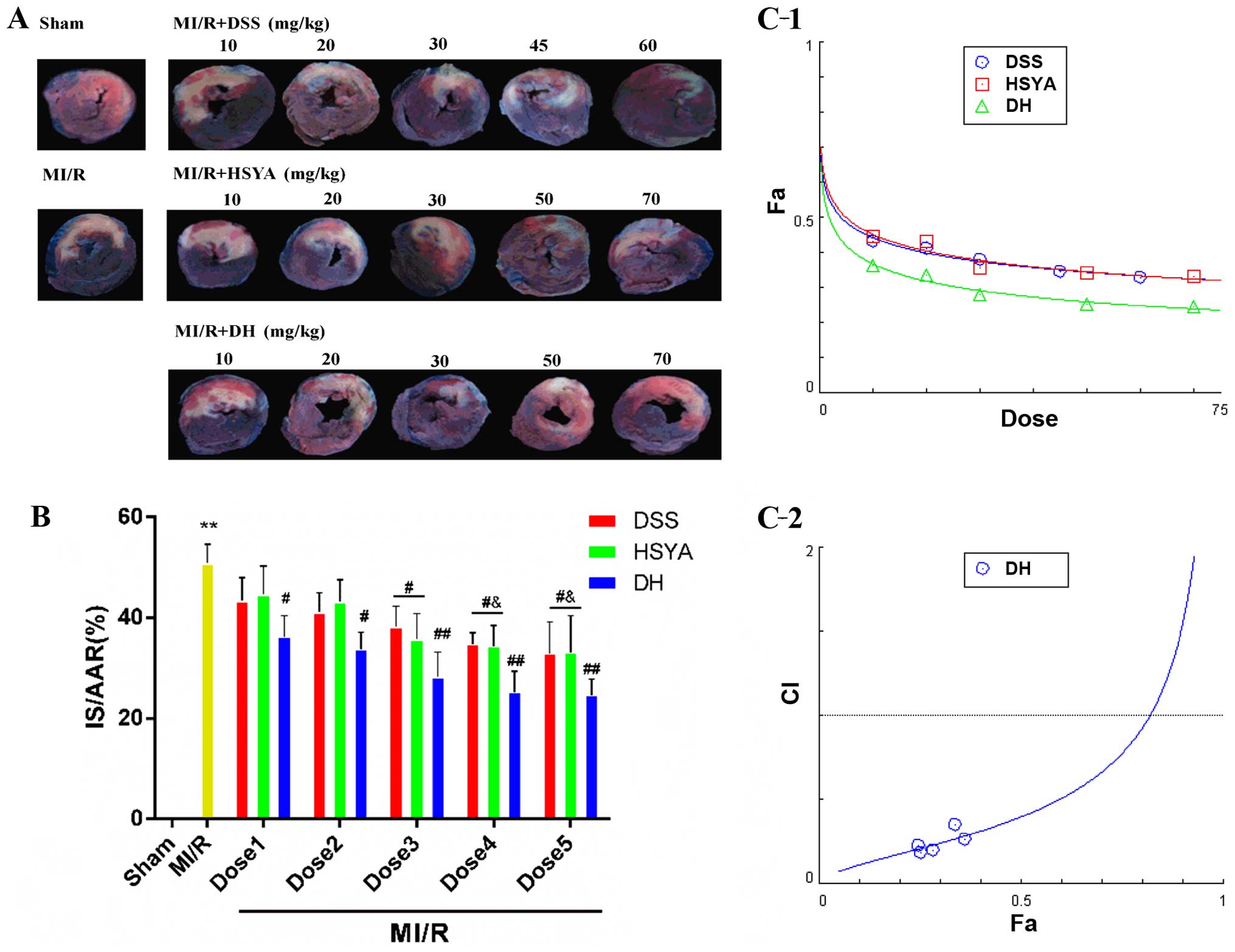 | Figure 1Danshensu (DSS) and hydroxysafflor
yellow A (HSYA) administered individually and in combination (DH)
reduce myocardial infarct size in rats subjected to myocardial
ischemia-reperfusion (MI/R) injury, and in combination exert a
synergistic effect. (A) A double-staining technique using TTC and
Evans blue assessed the effect of DSS and HSYA, individually and in
combination at different doses on infarct size and myocardial risk
area. (B) Myocardial infarct size expressed as a percentage of the
area at risk (IS/AAR%). Dose 1 indicates DSS (10 mg/kg), HSYA (10
mg/kg), DH (10 mg/kg); Dose 2 indicates DSS (20 mg/kg), HSYA (20
mg/kg), DH (20 mg/kg); Dose 3 indicates DSS (30 mg/kg), HSYA (30
mg/kg), DH (30 mg/kg); Dose 4 indicates DSS (45 mg/kg), HSYA (50
mg/kg), DH (50 mg/kg); Dose 5 indicates DSS (60 mg/kg), HSYA (70
mg/kg), DH (70 mg/kg). (C-1) The dose-effect curves of the single
or combined drug treatment. (C-2) The fraction versus combination
index (Fa-CI) curve of DSS and HSYA in combination reveals that
they exert a synergistic effect (CI50 <1) as
reflected by the median-effect method. The dashed line at CI=1
represents the additive effect. Values are expressed as the means ±
SD (n=7–9 rats in each group). **P<0.01 vs.
sham-operated (sham) group; ##P<0.01 and
#P<0.05 vs. MI/R group; &P<0.05 vs.
MI/R + DSS or MI/R + HSYA group. |
 | Table INumber of rats that failed the
coronary ligation procedure in each group shown in Fig. 1. |
Table I
Number of rats that failed the
coronary ligation procedure in each group shown in Fig. 1.
| Group | No. | Group | No. |
|---|
| Sham | 1 | MI/R + HSYA30 | 1 |
| MI/R | 3 | MI/R + HSYA50 | 1 |
| MI/R + DSS10 | 2 | MI/R + HSYA70 | 3 |
| MI/R + DSS20 | 3 | MI/R + DH10 | 1 |
| MI/R + DSS30 | 2 | MI/R + DH20 | 2 |
| MI/R + DSS45 | 3 | MI/R + DH30 | 6 |
| MI/R + DSS60 | 2 | MI/R + DH50 | 2 |
| MI/R + HSYA10 | 1 | MI/R + DH70 | 1 |
| MI/R + HSYA20 | 2 | | |
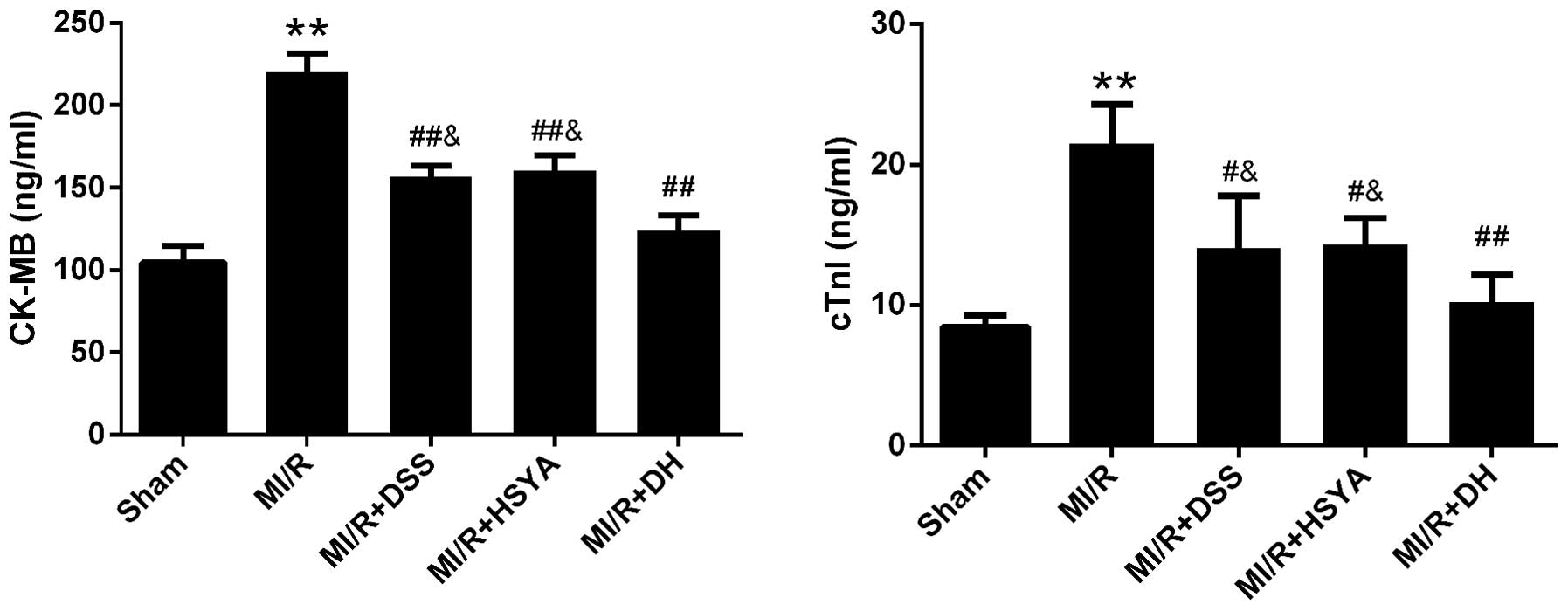
As markers of cardiomyocyte injury, the serum levels
of CK-MB and cTnI were 104.68±9.93 and 8.47±0.80 ng/ml in the
sham-operated group and were significantly increased in the MI/R
group to 220.78±10.63 and 21.52±2.79 ng/ml, respectively. Following
treatment with 60 mg/kg DSS or 70 mg/kg HSYA, the CK-MB and cTnI
levels were significantly decreased (P<0.01 and P<0.05 vs.
MI/R). The DH regimen (35+35 mg/kg) further enhanced these effects
compared to treatment with each agent alone (Fig. 2). The numbers of rats that failed
the coronary ligation procedure in each group shown in Fig. 2 are listed in Table II.
 | Table IINumber of rats that failed the
coronary ligation procedure in each group shown in Fig. 2. |
Table II
Number of rats that failed the
coronary ligation procedure in each group shown in Fig. 2.
| Group | Sham | MI/R | MI/R + DSS | MI/R + HSYA | MI/R + DH |
|---|
| No. | 1 | 3 | 3 | 1 | 2 |
DSS and HSYA exert antioxidant effect on
rats with MI/R injury
The rats in the MI/R group exhibited a significant
increase in the level of MDA to 17.01±3.58 nmol/ml (P<0.01 vs.
sham-operated group) and a significant decrease in SOD activity to
69.66±16.32 U/ml (P<0.01 vs. sham-operated group). The
administration of DSS and HSYA in combination to the rats with MI/R
injury exerted more significant cardioprotective effects (P<0.01
vs. MI/R) than the administration of each agent alone (P<0.05
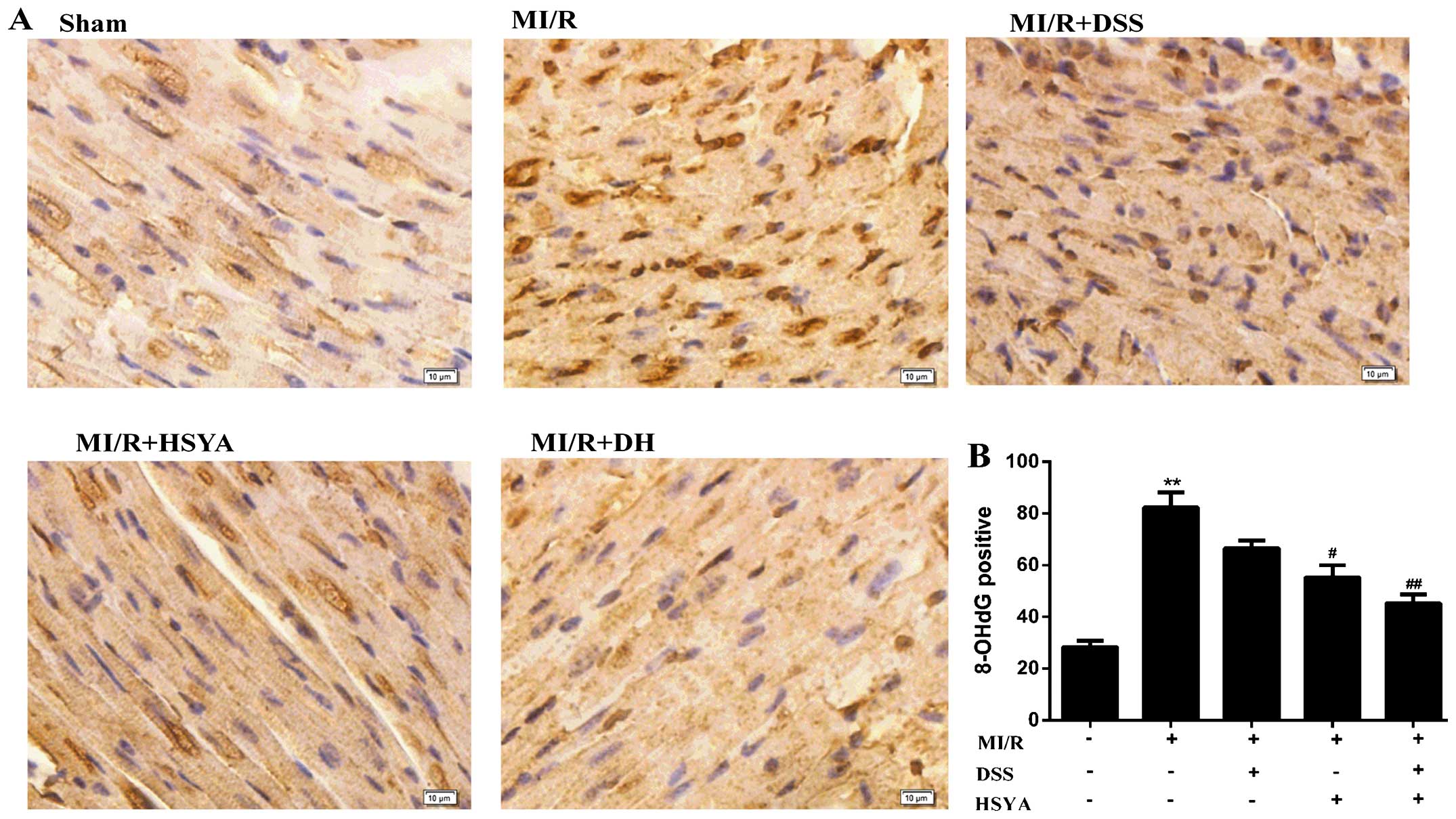
vs. MI/R), alleviating the parameters of oxidative stress (Table III). 8-OHdG is regarded as a
hallmark of oxidative DNA damage (24); thus, nuclear oxidative stress was
assessed using 8-OHdG immunohistochemical staining, which was
significantly increased (P<0.01 vs. sham-operated group) in the
MI/R group, while it was significantly reduced (P<0.01 vs. MI/R)
in the combined treatment group. The protocol used in the DH group
further potentiated the cardioprotective effects compared with
those observed in the individual treatment groups (Fig. 3). Additionally, HSYA exerted a
more potent antioxidant effect than DSS. The number of rats that
failed the coronary ligation procedure in each group shown Fig. 3 are listed in Table IV.
 | Table IIILevels of malondialdehyde (MDA) and
superoxide dismutase (SOD) activity in the serum of rats. |
Table III
Levels of malondialdehyde (MDA) and
superoxide dismutase (SOD) activity in the serum of rats.
| Treatment | MDA (nmol/ml) | SOD (U/ml) |
|---|
| Sham | 7.17±2.61 | 186.10±16.23 |
| MI/R | 17.01±3.58a | 69.66±16.32a |
| MI/R + DSS | 11.26±2.03b |
110.60±14.75b |
| MI/R + HSYA | 9.05±2.48b |
121.80±21.44b |
| MI/R + DH | 7.73±1.62c | 152.5±12.73c |
 | Table IVNumber of rats that failed the
coronary ligation procedure in each group shown in Fig. 3. |
Table IV
Number of rats that failed the
coronary ligation procedure in each group shown in Fig. 3.
| Group | Sham | MI/R | MI/R + DSS | MI/R + HSYA | MI/R + DH |
|---|
| No. | 2 | 2 | 3 | 3 | 1 |
DSS and HSYA synergistically protect H9c2
cardiomyocytes against injury induced by H/R
The results of MTT assay demonstrated that H/R
significantly reduced (P<0.05 vs. Con) cell viability to
35.26±6.10%, while DSS and HSYA used alone or in combination
protected the H9c2 cardiomyocytes against H/R injury, and the DH
group exhibited a significant increase in viability at dose 3 to
68.38±3.35% (P<0.05 vs. H/R + DSS or H/R + HSYA) (Fig. 4A). The dose-effect curves of DSS
and HSYA used alone or in combination were analyzed using the
median-effect method to determine whether they acted
synergistically to protect the H9c2 cardiomyocytes against H/R
injury (Fig. 4B-1). We found that
treatment with DSS and HSYA or in combination at concentrations of
1–80 µM yielded CI50 values <1 (Fig. 4B-2), indicating synergistic
effects between the agents. Since the agents exerted
cardioprotective effects in a dose-dependent manner, the highest
doses were selected for use in subsequent studies. As an
acknowledged marker of cell damage, the LDH levels in the cell
supernatant significantly increased (P<0.01 vs. Con) to
91.14±9.71 U/l in the H/R group. The DSS or HSYA groups exhibited
levels of 53.50±12.54 U/l and 65.11±5.51 U/l, respectively
(P<0.05 vs. H/R). These effects were markedly enhanced in the DH
group compared with the H/R group (P<0.01; Fig. 4C).
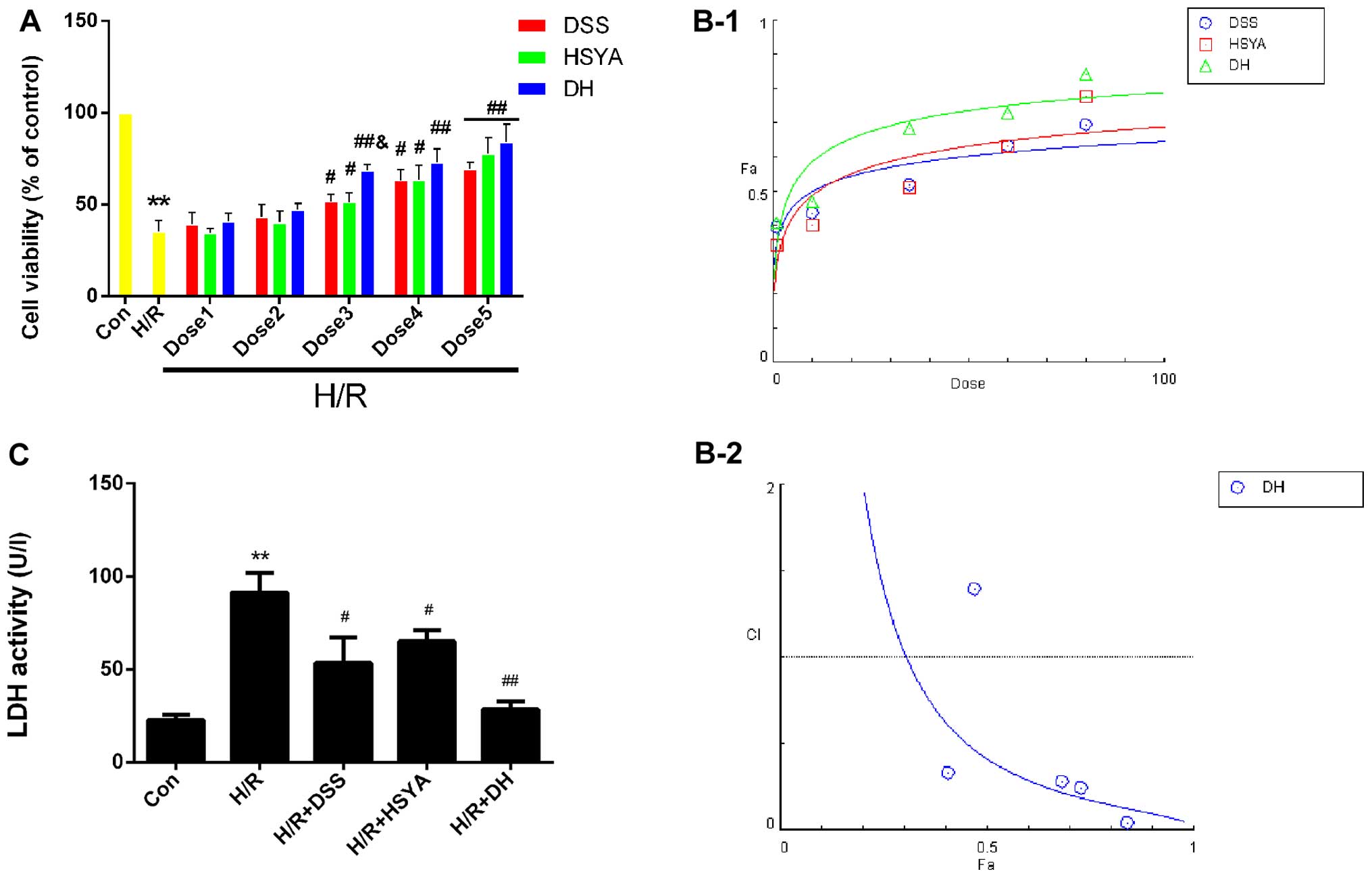 | Figure 4Danshensu (DSS), hydroxysafflor
yellow A (HSYA) administered individually or in combination protect
the H9c2 cardiomyocytes from hypoxia/reox-ygentaion (H/R) injury,
and in combitation, exert a synergistic effect. (A) Cell viability
was detected by an MTT reduction assay. H9c2 cardiomyocytes in the
control (Con) group were considered 100% viable. Dose 1 indicates
DSS (1 µM), HSYA (1 µM), DH (1 µM); Dose 2
indicates DSS (10 µM), HSYA (10 µM), DH (10
µM; Dose 3 indicates DSS (35 µM), HSYA (35
µM), DH (35 µM); Dose 4 indicates DSS (60 µM),
HSYA (60 µM), DH (60 µM); Dose 5 indicates DSS (80
µM), HSYA (80 µM), DH (80 µM). (B-1) The
dose-effect curves of the single or combined drug treatment. (B-2)
The fraction versus combination index (Fa-CI) curve of DSS and HSYA
in combination reveals that they exert a synergistic effect
(CI50 <1) as reflected by the median-effect method.
The dashed line at the combination index (CI=1) represents the
additive effect. (C) The effect of DSS and HSYA on LDH activity in
the Con, H/R, H/R + DSS (80 µM), H/R + HSYA (80 µM)
and H/R + DH (40+40 µM) groups. Values are expressed as the
means ± SD (n=6). **P<0.01 vs. Con group;
#P<0.05 and ##P<0.01 vs. H/R group;
&P<0.05 vs. H/R + DSS or H/R + HSYA group. |
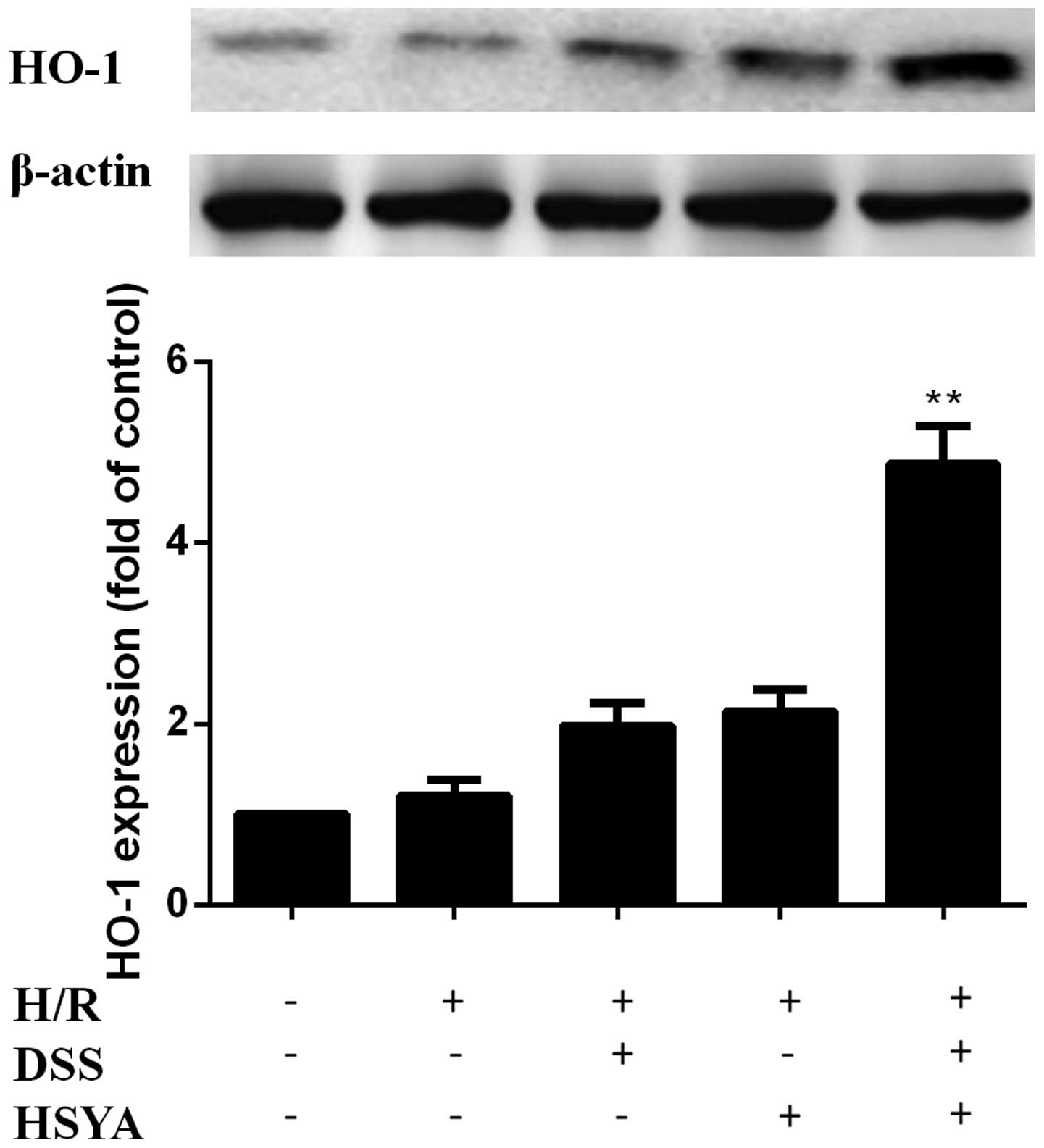
DSS and HSYA increase HO-1 expression in
H9c2 cardiomyocytes
HO-1 expression was increased in the H/R + DSS group
(80 µM) and the H/R + HSYA group (80 µM). HO-1
expression was further increased in the H/R + DH group (40+40
µM) to a level higher than that in the single treatment
groups (P<0.01). There were no significant differences between
the H/R group and the groups treated with each agent alone
(Fig. 5).
The antioxidant effects of DSS and HSYA
in H9c2 cardiomyocytes are negated by ZnPP-IX
The analysis of the MDA content and SOD activity
revealed that H/R deteriorated the parameters of oxidative stress
significantly compared with the control group (P<0.01). The MDA
content markedly decreased to 2.47±0.44 nmol/ml in the DSS group
and to 2.05±0.53 nmol/ml in the HSYA group (P<0.05 vs. H/R), and
the activity of SOD increased to 11.06±1.64 and 13.53±2.30 nmol/ml
in the DSS and the HSYA group, respectively. These protective
effects were more significantly enhanced in the DH group compared
with the H/R group (MDA, P<0.01; SOD, P<0.05). HSYA also
exerted a more potent antioxidant effect than DSS. The antioxidant
effects were markedly abrogated by ZnPP-IX (P<0.05 vs. H/R + DH)
(Table V).
 | Table VMDA content and SOD activity in
culture supernatants of cardiomyocytes. |
Table V
MDA content and SOD activity in
culture supernatants of cardiomyocytes.
| Treatment | MDA (nmol/ml) | SOD (U/ml) |
|---|
| Con | 1.72±0.70 | 17.98±2.84 |
| H/R | 4.01±0.85a | 7.40±2.90a |
| H/R + DSS | 2.47±0.44 | 11.06±1.64 |
| H/R + HSYA | 2.05±0.53b | 13.53±2.30 |
| H/R + DH | 1.74±0.63c | 15.58±3.04b |
| H/R + DH + Z | 3.62±0.42d | 7.11±2.64d |
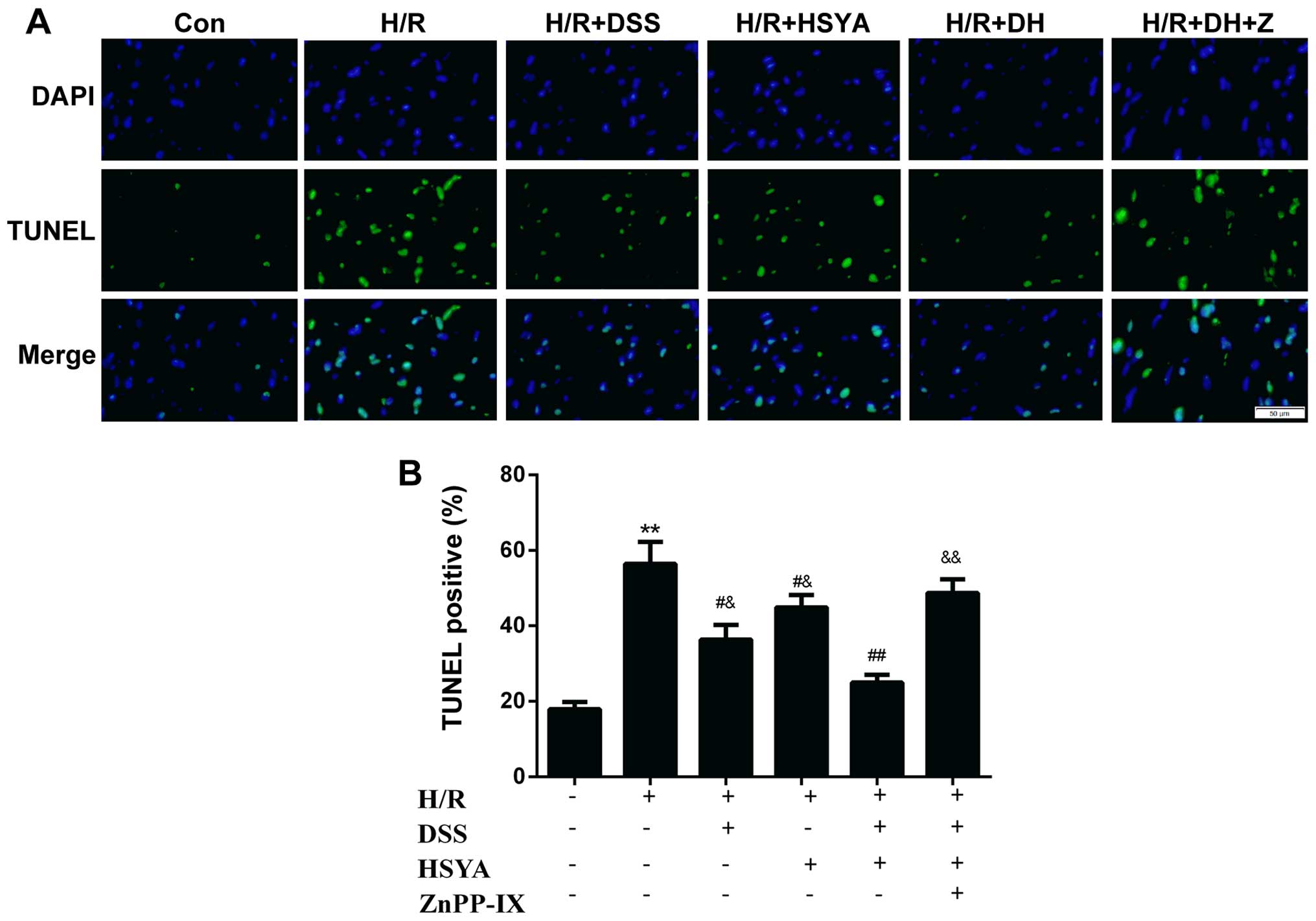
ZnPP-IX inhibits the anti-apoptotic
effect of DSS and HSYA in H9c2 cardiomyocytes
TUNEL staining of apoptotic cells demonstrated that
H/R led to a significant augmentation in cell apoptosis to
56.41±14.23% (P<0.01 vs. Con). Apoptosis was alleviated in the
group treated with DSS or HSYA to 36.38±9.46 and 44.88±8.10%,
respectively (P<0.05 vs. H/R). Apoptosis was decreased in the DH
group to 24.95±5.02% (P<0.05 vs. H/R + DSS or H/R + HSYA).
ZnPP-IX abrogated the anti-apoptotic effect of DSS and HSYA
(P<0.01 vs. H/R + DH) (Fig.
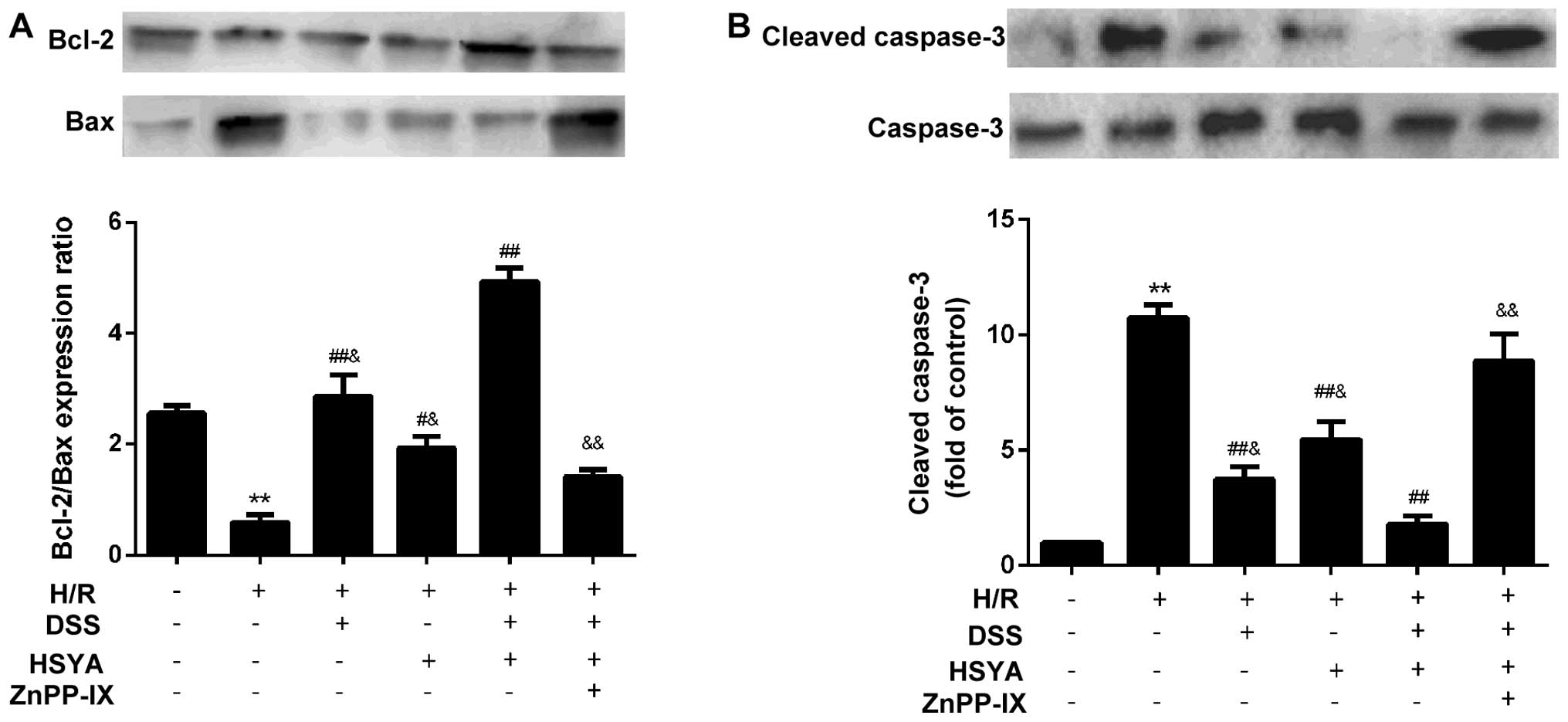
6). Following 20 h of reoxygenation, the Bcl-2/Bax ratio
decreased to 0.58±0.34 in the H/R-exposed cardiomyocytes. DSS or
HSYA reversed the ratio to 2.86±0.95 and 1.93±0.49, respectively
(P<0.01 and P<0.05 vs. H/R). In addition, treatment with DH
increased the Bcl-2/Bax ratio to 4.93±0.60 (P<0.01 vs. H/R + DSS
or H/R + HSYA). The Bcl-2/Bax ratio was downregulated in the group
treated with ZnPP-IX to 1.41±0.32 (P<0.01 vs. H/R + DH)
(Fig. 7A). H/R evoked a marked
increase in the cleaved caspase-3 levels to 10.74±1.39 (P<0.01
vs. Con), while DSS or HSYA decreased cleaved caspase-3 expression
to 3.74±1.34 and 5.46±1.90, respectively (P<0.01 vs. H/R).
Treatment with DH decreased the cleaved caspase-3 level to
1.80±0.85 (P<0.01 vs. H/R + DSS or H/R + HSYA), and in the group
co-incubated with ZnPP-IX, this effect was abolished (8.87±2.82;
P<0.01 vs. H/R + DH) (Fig.
7B). Furthermore, we found that DSS exerted a more potent
anti-apoptotic effect than HSYA.
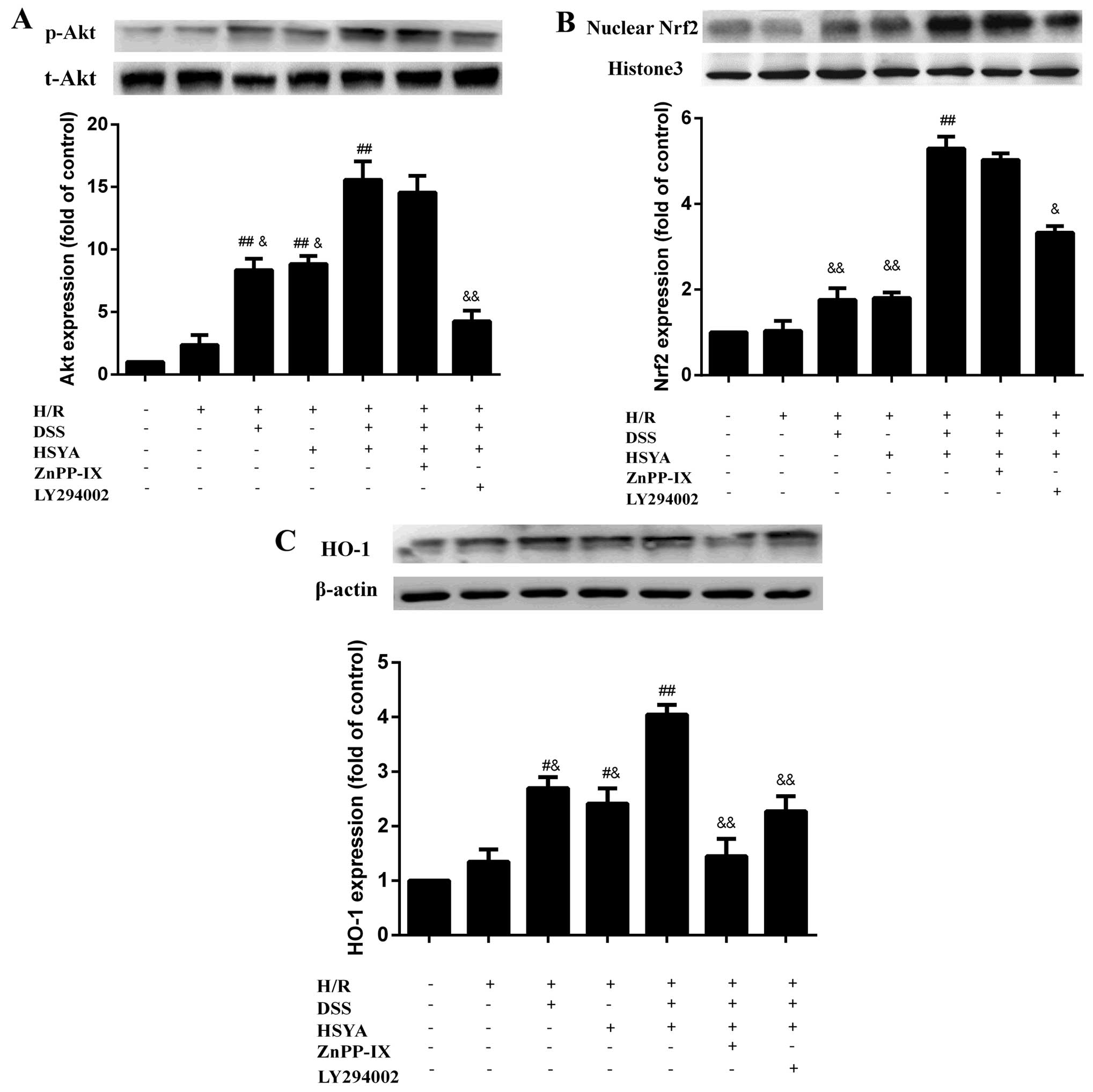
DSS and HSYA modulate the acvitation of
the Akt/Nrf2/HO-1 signaling pathway in H9c2 cardiomyocytes
Following 20 h of reoxygenation, treatment with DSS
or HSYA increased the expression of p-Akt, HO-1 and nuclear Nrf2,
and combined treatment exerted even more significant effects than
treatment with each agent alone (P<0.01). LY294002 markedly
abolished the effects of DSS and HSYA on p-Akt expression
(P<0.01 vs. H/R + DH), and partly blocked the expression of
nuclear Nrf2 and HO-1 (P<0.05 and P<0.01 vs. H/R + DH).
ZnPP-IX had no significant effect on p-Akt and nuclear Nrf2
expression compared with the H/R + DH group; however, it markedly
negated HO-1 expression (P<0.01 vs. H/R + DH) (Fig. 8).
Discussion
A number of studies have illustrated the protective
effects of DSS or HSYA in cardiovascular diseases (15,16,18); however, limited attention has been
paied to their use as a combination therapy and the mechanisms
responsible for their combined effects have yet to be elucidated.
In the present study, we investigated the synergistic protective
effects of DSS and HSYA on myocardial injury through in vivo
experiments using rats and in vitro experiments using H9c2
cardiomyocytes. To the best of our knowledge, this is the first
study to demonstrate the role of DSS and HSYA in combination to
protect against cellular injury through antioxidant and
anti-apoptotic effects, which are associated with the activation of
the Akt/Nrf2/HO-1 signaling pathway.
Infarct size is regarded as the gold standard in
assessing the severity of MI/R injury (25). Thus, the first investigations of
the combination effects examined infarct size. Following the
administration of a series of doses of DSS and/or HSYA, the infarct
size was reduced in a dose-dependent manner. The CI50
value of the infarct size (<1) determined by the median-effect
method verified the combination effects to be synergistic between
the agents. Additionally, the sensitive cardiac injury markers
(26–28) CK-MB and cTnI, were measured to
determine whether DSS and HSYA are capable of alleviating the
degree of myocardial injury caused by MI/R. The decreased release
of CK-MB and cTnI in the treatment groups demonstrated the
protective effects of DSS and HSYA. Furthermore, combined treatment
exerted a more potent protective effect compared to treatment with
either agent alone. To further confirm the combination effects, an
MTT assay was performed. The CI50 value of cell
viability was revealed to be <1, indicating a synergistic effect
between DSS and HSYA. LDH is one of the specific enzymes (29) present in the myocardial cytoplasm,
and its values may indirectly reflect the degree of damage of the
myocardium exposed to H/R. In the present study, the LDH levels in
the cell supernatant were significantly decreased following
treatment with DH. These results indicated that DSS and HSYA used
in combination exerted a synergistic cardio-protective effect in
vitro and in vivo.
Having determined that DSS and HSYA exerted a
synergistic cardioprotective effect, we then proceeded to elucidate
the possible mechanisms responsible for these effects. The
increased production of oxygen-free radicals in conjunction with
the decreased activity of antioxidant defenses are considered to be
an significant factor for myocardial reperfusion injury (30). SOD is the first line of cellular
defense against oxidative injury, which decomposes O2
and H2O2 before they interact to form the
more reactive hydroxyl radical (31,32). MDA is a product of lipid
peroxidation that may cause the crosslinking polymerization of
proteins, nucleic acids and some macromolecules, and thus, it has
been found that the amount of MDA often reflects the degree of
lipid peroxidation (33). 8-OHdG
appears to be a sensitive and integral marker of oxidative damage
to DNA due to any imbalance between OH• generation,
antioxidant defenses and the repair of damaged DNA sequences
(34). Using these three
important markers of oxidative stress, we confirmed that DSS and
HSYA significantly alleviated the parameters of oxidative stress
and HSYA exerted a profound protective effect in vitro and
in vivo. This finding indicated that DSS and HSYA played a
pivotal role in ameliorating the oxidative stress injury caused by
MI/R or H/R.
HO-1 is an inducible enzyme with potent antioxidant
activities due to its ability to degrade heme into biliverdin or
bilirubin, carbon monoxide and free iron (35,36). Furthermore, it has been
demonstrated that plant-derived chemical substances may act as
inducers of the HO-1, and these substances have been found to exert
cardioprotective effects against oxidative injury (37). Thus, we hypothesized that DSS and
HSYA may protect myocardial tissue and H9c2 cardiomyocytes from
oxidative stress-induced injury in association with the
upregulation of HO-1. Western blot analysis revealed that HO-1
expression was profoundly enhanced in the combined treatment group
compared with the groups treated with either agent alone. This
result not only verified the above-mentioned hypothesis, but also
indicated that combination therapy may be more effective in
increasing HO-1 expression than monotherapy. It has been noted that
ZnPP-IX, which is a competitive inhibitor of the HO-1 enzyme,
conjugates to HO-1 and reduces the conjugation between HO-1 and
heme (38,39). Following treatment with ZnPP-IX
(10 µM), the decreased MDA content and the increased
activity of SOD were both revoked. These results illustrated that
DSS and HSYA upregulated HO-1 expression and that the antioxidant
effects were partly dependent on HO-1 expression.
Apoptosis plays an important role in the loss of
cardiomyocytes in a variety of pathologies, including H/R injury
(18,40). It has been demonstrated that the
mitochondrial apoptotic pathway may be activated when a number of
pro-apoptotic factors are released (41). Bax is a pro-apoptotic protein,
whereas Bcl-2 is an anti-apoptotic protein. It has been noted that
the ratio of Bcl-2 to Bax acts as an essential element in
determining the threshold of apoptosis (18,42). Caspases transduce and execute
apoptotic signaling. Caspase-3 has been found to be activated by
the apoptotic pathway and then autocatalyzed, and processed into
activated fragments such as cleaved caspase-3, which are considered
as an index of apoptosis (43).
In the present study, DSS and HSYA increased the Bcl-2/Bax ratio in
the mitochondria, downregulated cleaved caspase-3 expression and
reduced TUNEL staining compared with the H/R group. DH therapy
enhanced the effect to a greater extent compared with DSS or HSYA
alone. The anti-apoptotic effects were markedly negated by ZnPP-IX.
Additionally, DSS exerted a more potent anti-apoptotic effect than
HSYA. These results confirmed that the overexpression of HO-1
induced by the agents and the combined regimen led to an enhanced
anti-apoptotic effect.
It has been proven that a specific molecular pathway
is important for compound-induced HO-1 expression. The PI3K/Akt
pathway has been demonstrated to be associated with increased HO-1
expression (44). In this study,
Akt phosphorylation was significantly enhanced in the H9c2
cardiomyocytes treated with DSS and HSYA compared with that
observed in the H/R group. The Akt inhibitor, LY294002,
significantly blocked this effect, while ZnPP-IX had no obvious
impact on Akt phosphorylation induced by the two agents. Nrf2 is a
key regulator of HO-1 expression in cells (45). It has been demonstrated that the
nuclear factor Nrf2 binds to Kelch-like ECH-associated protein-1
(Keap1) to form the Keap1-Nrf2 complex and limit Nrf2-mediated gene
expression in the cytoplasm under normal physiological
circumstances. Nrf2 is released from Keap1 under conditions of
oxidative stress or other potential damage and then translocates to
the nucleus, where it binds to antioxidant response element (ARE)
sequences, leading to the transcriptional activation of
anti-apoptotic genes, including HO-1 (18,46–49). In the present study, the two
agents enhanced the expression of nuclear Nrf2. LY294002 partly
blocked the increase in Nrf2 translocation to the nucleus and the
upregulation of HO-1 expression, while ZnPP-IX had no notable
effect on nuclear Nrf2 expression induced by DSS and HSYA. These
results indicated that the DSS- and HSYA-mediated upregulation of
Akt phosphorylation and nuclear Nrf2 did not rely on the
upregulation of HO-1 expression. On the contrary, the upregulation
of HO-1 expression was dependent on Akt phosphorylation. Akt
phosphorylation, as well as nuclear Nrf2 and HO-1 expression were
enhanced in the DH-treated group compared with that observed in the
groups treated with either agent alone. As we confirmed above,
treatment with DH exerted a synergistic effect. However, it would
be interesting to determine the possible mechanisms underlying
these synergistic effects. It has been pointed out that each single
constituent of a combination affects several targets, such as
enzymes, substrates, receptors and transport proteins (8,50).
Most notably, DSS mainly exerted anti-apoptotic effects, while HSYA
exerted significant antioxidant effects. They acted on
apoptosis-related proteins and oxidative-related enzymes,
respectively; thus, in combination they exerted complementary and
synergistic effects. Our results revealed that the combination of
DSS and HSYA conferred a synergistic effect on the activation of
the Akt/Nrf2/HO-1 pathway, which is a potential mechanism for
enhancing the anti-apoptotic and antioxidant effects.
In the present study, we only investigated the
antioxidant and anti-apoptotic effects involving the Akt/Nrf2/HO-1
pathway. It would be of interest to determine whether other aspects
contribute to these synergistic effects. Further studies are
warranted to clarify the synergyistic effects of DSS and HSYA on
the regulation of other pathways.
In conclusion, we found that DSS and HSYA acted
synergistically to significantly attenuate myocardial injury in
vitro and in vivo by exerting antioxidant and
anti-apoptotic effects through the Akt/Nrf2/HO-1 signaling pathway.
The results from the present study provide insight into the effects
and the mechanisms responsible for these synergistic effects. This
may lead to the development of effective combined therapeutic TCM
regimens so as to combat myocardial complications in clinical
practice.
Abbreviations:
|
MI/R
|
myocardial ischemia-reperfusion
|
|
Nrf2
|
nuclear factor erythroid 2-related
factor 2
|
|
CI
|
combination index
|
|
DSS
|
Danshensu
|
|
SOD
|
superoxide dismutase
|
|
HSYA
|
hydroxysafflor yellow A
|
|
ROS
|
reactive oxygen species
|
|
Akt
|
protein kinase B
|
|
HO-1
|
heme oxygenase-1
|
|
H/R
|
hypoxia/reoxygenation
|
|
CK-MB
|
creatine kinase-MB
|
|
cTnI
|
cardiac troponin I
|
|
MDA
|
malondialdehyde
|
|
LDH
|
lactate dehydrogenase
|
|
TUNEL
|
terminal deoxy-nucleotidyl
transferase-mediated dUTP nick-end labeling
|
|
8-OHdG
|
8-hydroxyde oxyguanosine
|
Acknowledgments
This study was financially supported by the National
Natural Science Foundation of China (nos. 81173514, 81403135,
81403182 and 81403134). The authors would like to thank their
colleagues at the New Drug Research and Development Center of
Xijing Hospital and the staff of China National Corp. of
Traditional and Herbal Medicine for their technical assistance.
References
|
1
|
Bainey KR and Armstrong PW: Clinical
perspectives on reperfusion injury in acute myocardial infarction.
Am Heart J. 167:637–645. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maxwell SR and Lip GY: Reperfusion injury:
a review of the pathophysiology, clinical manifestations and
therapeutic options. Int J Cardiol. 58:95–117. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yellon DM and Hausenloy DJ: Myocardial
reperfusion injury. N Engl J Med. 357:1121–1135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Buja LM: Myocardial ischemia and
reperfusion injury. Cardiovasc Pathol. 14:170–175. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Olivetti G, Quaini F, Sala R, Lagrasta C,
Corradi D, Bonacina E, Gambert SR, Cigola E and Anversa P: Acute
myocardial infarction in humans is associated with activation of
programmed myocyte cell death in the surviving portion of the
heart. J Mol Cell Cardiol. 28:2005–2016. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao ZQ, Nakamura M, Wang NP, Wilcox JN,
Shearer S, Ronson RS, Guyton RA and Vinten-Johansen J: Reperfusion
induces myocardial apoptotic cell death. Cardiovasc Res.
45:651–660. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hori M and Nishida K: Oxidative stress and
left ventricular remodelling after myocardial infarction.
Cardiovasc Res. 81:457–464. 2009. View Article : Google Scholar
|
|
8
|
Wagner H: Synergy research: approaching a
new generation of phytopharmaceuticals. Fitoterapia. 82:34–37.
2011. View Article : Google Scholar
|
|
9
|
Combination Pharmacotherapy and Public
Health Research Working Group: Combination pharmacotherapy for
cardiovascular disease. Ann Intern Med. 143:593–599. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang L, Zhou GB, Liu P, Song JH, Liang Y,
Yan XJ, Xu F, Wang BS, Mao JH, Shen ZX, et al: Dissection of
mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as
an effective treatment for promyelocytic leukemia. Proc Natl Acad
Sci USA. 105:4826–4831. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li SJ, Tang YP, Shen J, Li JP, Guo JM and
Duan JA: Research of Chinese medicine pairs (VIII) - Salviae
Miltiorrhizae Radix et Rhizoma-Carthami flos. Zhongguo Zhong Yao Za
Zhi. 38:4227–4231. 2013.In Chinese.
|
|
12
|
Guan Y, Yin Y, Zhu YR, Guo C, Wei G, Duan
JL, Wang YH, Zhou D, Quan W, Weng Y, et al: Dissection of
mechanisms of a chinese medicinal formula: Danhong injection
therapy for myocardial ischemia/reperfusion injury in vivo and in
vitro. Evid Based Complement Alternat Med. 2013:9723702013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou D, Zhu YR, Guan Y, Quan W, Guo C,
Weng Y, Cui J, Chen L, Ding Y, Xi M and Wen A: Development of
chemical fingerprint and metabolic fingerprint of Danhong injection
by HPLC-UV and HPLC-MS. Asian J Chem. 25:6285–6292. 2013.
|
|
14
|
Duan JL, Wang JW, Guan Y, Yin Y, Wei G,
Cui J, Zhou D, Zhu YR, Quan W, Xi MM and Wen AD: Safflor yellow A
protects neonatal rat cardiomyocytes against anoxia/reoxygenation
injury in vitro. Acta Pharmacol Sin. 34:487–495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yin Y, Guan Y, Duan J, Wei G, Zhu Y, Quan
W, Guo C, Zhou D, Wang Y, Xi M and Wen A: Cardioprotective effect
of Danshensu against myocardial ischemia/reperfusion injury and
inhibits apoptosis of H9c2 cardiomyocytes via Akt and ERK1/2
phosphorylation. Eur J Pharmacol. 699:219–226. 2013. View Article : Google Scholar
|
|
16
|
Zhou MX, Fu JH, Zhang Q and Wang JQ: The
effect of hydroxy safflower yellow A on inflammatory reaction in
myocardium of the rats after acute myocardial infarction. Afr J
Pharm Pharmacol. 7:643–649. 2013. View Article : Google Scholar
|
|
17
|
Cui G, Shan L, Hung M, Lei S, Choi I,
Zhang Z, Yu P, Hoi P, Wang Y and Lee SM: A novel Danshensu
derivative confers cardioprotection via PI3K/Akt and Nrf2 pathways.
Int J Cardiol. 168:1349–1359. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu SX, Zhang Y, Wang YF, Li XC, Xiang MX,
Bian C and Chen P: Upregulation of heme oxygenase-1 expression by
hydroxysafflor yellow A conferring protection from
anoxia/reoxygenation-induced apoptosis in H9c2 cardiomyocytes. Int
J Cardiol. 160:95–101. 2012. View Article : Google Scholar
|
|
19
|
Kin H, Zhao ZQ, Sun HY, Wang NP, Corvera
JS, Halkos ME, Kerendi F, Guyton RA and Vinten-Johansen J:
Postconditioning attenuates myocardial ischemia-reperfusion injury
by inhibiting events in the early minutes of reperfusion.
Cardiovasc Res. 62:74–85. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sachdeva J, Dai W, Gerczuk PZ and Kloner
RA: Combined remote perconditioning and postconditioning failed to
attenuate infarct size and contractile dysfunction in a rat model
of coronary artery occlusion. J Cardiovasc Pharmacol Ther.
19:567–573. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mao X, Wang T, Liu Y, Irwin MG, Ou JS,
Liao XL, Gao X, Xu Y, Ng KF, Vanhoutte PM and Xia Z:
N-acetylcysteine and allopurinol confer synergy in attenuating
myocardial ischemia injury via restoring HIF-1α/HO-1 signaling in
diabetic rats. PLoS One. 8:e689492013. View Article : Google Scholar
|
|
22
|
Tsai CY, Wang CC, Lai TY, Tsu HN, Wang CH,
Liang HY and Kuo WW: Antioxidant effects of diallyl trisulfide on
high glucose-induced apoptosis are mediated by the
PI3K/Akt-dependent activation of Nrf2 in cardiomyocytes. Int J
Cardiol. 168:1286–1297. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chou TC: Drug combination studies and
their synergy quantification using the Chou-Talalay method. Cancer
Res. 70:440–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Won MH, Kang TC, Jeon GS, Lee JC, Kim DY,
Choi EM, Lee KH, Choi CD, Chung MH and Cho SS: Immunohistochemical
detection of oxidative DNA damage induced by ischemia-reperfusion
insults in gerbil hippocampus in vivo. Brain Res. 836:70–78. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Panteghini M, Cuccia C, Bonetti G,
Giubbini R, Pagani F and Bonini E: Single-point cardiac troponin T
at coronary care unit discharge after myocardial infarction
correlates with infarct size and ejection fraction. Clin Chem.
48:1432–1436. 2002.PubMed/NCBI
|
|
26
|
Turer AT, Mahaffey KW, Gallup D, Weaver
WD, Christenson RH, Every NR and Ohman EM: Enzyme estimates of
infarct size correlate with functional and clinical outcomes in the
setting of ST-segment elevation myocardial infarction. Curr Control
Trials Cardiovasc Med. 6:122005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Christenson RH, Vollmer RT, Ohman EM, Peck
S, Thompson TD, Duh SH, Ellis SG, Newby LK and Topol EJ: Relation
of temporal creatine kinase-MB release and outcome after
thrombolytic therapy for acute myocardial infarction. Am J Cardiol.
85:543–547. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thygesen K, Alpert JS, White HD, Jaffe AS,
Apple FS, Galvani M, Katus HA, Newby LK, Ravkilde J, Chaitman B, et
al: Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of
Myocardial Infarction: Universal definition of myocardial
infarction. Eur Heart J. 28:2525–2538. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Quan W, Yin Y, Xi M, Zhou D, Zhu Y, Guan
Y, Guo C, Wang Y, Duan J and Wen A: Antioxidant properties of
magnesium lithospermate B contribute to the cardioprotection
against myocardial ischemia/reperfusion injury in vivo and in
vitro. J Tradit Chin Med. 33:85–91. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Das DK, Engelman RM, Rousou JA, Breyer RH,
Otani H and Lemeshow S: Pathophysiology of superoxide radical as
potential mediator of reperfusion injury in pig heart. Basic Res
Cardiol. 81:155–166. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu J, Hecker JG and Chiamvimonvat N:
Antioxidant enzyme gene transfer for ischemic diseases. Adv Drug
Deliv Rev. 61:351–363. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wattanapitayakul SK and Bauer JA:
Oxidative pathways in cardiovascular disease: Roles, mechanisms,
and therapeutic implications. Pharmacol Ther. 89:187–206. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Günfer T, Yaşar E and Bünyamin K: Changes
in the levels of MDA and GSH in mice serum, liver and spleen after
aluminum administration. East J Med. 11:7–12. 2006.
|
|
34
|
Cordis GA, Maulik G, Bagchi D, Riedel W
and Das DK: Detection of oxidative DNA damage to ischemic
reperfused rat hearts by 8-hydroxydeoxyguanosine formation. J Mol
Cell Cardiol. 30:1939–1944. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zeng B, Chen H, Zhu C, Ren X, Lin G and
Cao F: Effects of combined mesenchymal stem cells and heme
oxygenase-1 therapy on cardiac performance. Eur J Cardiothorac
Surg. 34:850–856. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tang YL, Tang Y, Zhang YC, Qian K, Shen L
and Phillips MI: Improved graft mesenchymal stem cell survival in
ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J
Am Coll Cardiol. 46:1339–1350. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu CC, Hsu MC, Hsieh CW, Lin JB, Lai PH
and Wung BS: Upregulation of heme oxygenase-1 by
epigallocatechin-3-gallate via the phosphatidylinositol
3-kinase/Akt and ERK pathways. Life Sci. 78:2889–2897. 2006.
View Article : Google Scholar
|
|
38
|
Maines MD: The heme oxygenase system: a
regulator of second messenger gases. Annu Rev Pharmacol Toxicol.
37:517–554. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kutty RK, Kutty G, Rodriguez IR, Chader GJ
and Wiggert B: Chromosomal localization of the human heme oxygenase
genes: Heme oxygenase-1 (HMOX1) maps to chromosome 22q12 and heme
oxygenase-2 (HMOX2) maps to chromosome 16p13.3. Genomics.
20:513–516. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Scarabelli TM, Knight R, Stephanou A,
Townsend P, Chen-Scarabelli C, Lawrence K, Gottlieb R, Latchman D
and Narula J: Clinical implications of apoptosis in ischemic
myocardium. Curr Probl Cardiol. 31:181–264. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Saelens X, Festjens N, Vande Walle L, van
Gurp M, van Loo G and Vandenabeele P: Toxic proteins released from
mitochondria in cell death. Oncogene. 23:2861–2874. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Murphy KM, Streips UN and Lock RB: Bax
membrane insertion during Fas(CD95)-induced apoptosis precedes
cytochrome c release and is inhibited by Bcl-2. Oncogene.
18:5991–5999. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Salvesen GS: Caspases and apoptosis.
Essays Biochem. 38:9–19. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kim KC, Kang KA, Zhang R, Piao MJ, Kim GY,
Kang MY, Lee SJ, Lee NH, Surh YJ and Hyun JW: Upregulation of
Nrf2-mediated heme oxygenase-1 expression by eckol, a phlorotannin
compound, through activation of Erk and PI3K/Akt. Int J Biochem
Cell Biol. 42:297–305. 2010. View Article : Google Scholar
|
|
45
|
Minelli A, Conte C, Grottelli S, Bellezza
I, Emiliani C and Bolaños JP: Cyclo(His-Pro) upregulates heme
oxygenase 1 via activation of Nrf2-ARE signalling. J Neurochem.
111:956–966. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lee IS, Lim J, Gal J, Kang JC, Kim HJ,
Kang BY and Choi HJ: Anti-inflammatory activity of xanthohumol
involves heme oxygenase-1 induction via NRF2-ARE signaling in
microglial BV2 cells. Neurochem Int. 58:153–160. 2011. View Article : Google Scholar
|
|
47
|
Ungvari Z, Bagi Z, Feher A, Recchia FA,
Sonntag WE, Pearson K, de Cabo R and Csiszar A: Resveratrol confers
endothelial protection via activation of the antioxidant
transcription factor Nrf2. Am J Physiol Heart Circ Physiol.
299:H18–H24. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mann GE, Niehueser-Saran J, Watson A, Gao
L, Ishii T, de Winter P and Siow RC: Nrf2/ARE regulated antioxidant
gene expression in endothelial and smooth muscle cells in oxidative
stress: implications for atherosclerosis and preeclampsia. Sheng Li
Xue Bao. 59:117–127. 2007.PubMed/NCBI
|
|
49
|
Lee S, Park Y, Zuidema MY, Hannink M and
Zhang C: Effects of interventions on oxidative stress and
inflammation of cardiovascular diseases. World J Cardiol. 3:18–24.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Imming P, Sinning C and Meyer A: Drugs,
their targets and the nature and number of drug targets. Nat Rev
Drug Discov. 5:821–834. 2006. View Article : Google Scholar : PubMed/NCBI
|