Introduction
Thyroid cancer is the most common endocrine
malignancy, and incidences are on the rise worldwide. Thyroid
tumours frequently possess genetic alterations which lead to the
activation of the mitogen-activated protein kinase (MAPK)
signalling pathway (1). The
expression of microRNAs (miRNAs or miRs) has been studied in
thyroid cancers, and as with other types of cancer, miRNA profiles
have been found to be significantly different between tumours of
the thyroid compared to normal thyroid tissue. For instance
miR-146, miR-221, miR-222, miR-155, miR-181a and miR-181b have been
shown to differentiate papillary thyroid cancer from normal thyroid
tissue (2,3).
miRNAs have also shown differential expression
between different types of thyroid cancer (4) and within a multifocal pluriform
tumour from an individual thyroid gland (5), thus illustrating that miRNA profiles
have demonstrated both intra- and inter-tumour variability. The
functions of some of these differentially expressed miRNAs have
also been elucidated: for instance, miR-221 has been shown to
regulate HOXB5 (6) and the
human telomerase reverse transcriptase (hTERT) gene is
regulated by miR-138 (7).
Furthermore, the potential utility of these small molecules to aid
thyroid cancer diagnosis (8) and
prognosis (9) has also been
investigated.
The aim of the present study was to elucidate the
target mRNAs of two miRNAs that we had previously found to be
differentially expressed in thyroid cancer (5): miR-222 and miR-25. miR-222 is
commonly found to be upregulated in thyroid cancer (2–4)
and is part of an intergenic miRNA cluster (that also contains
miR-221) on the p11.3 region of the X chromosome. It is one of the
most well-known miRNAs linked to thyroid cancer, and some of its
gene targets have been elucidated, including the KIT gene
(2) and the p27Kip1
protein (10).
miR-25 is also located in a miRNA cluster, termed
the mi-106b-25 cluster. miR-106b and miR-93 are the two other
miRNAs in this highly conserved cluster which is located in a
515-bp region at chromosome 7q22, in intron 13 of the host gene
MCM7. The miRNAs are co-transcribed in the context of the
MCM7 primary transcript and have been found to accumulate in
different types of cancer, including gastric, prostate, pancreatic
neuroendocrine tumours, neuroblastoma and multiple myeloma
(11). miR-25 has been shown to
regulate p57 (12) and E2F1 as
part of a negative feedback loop in gastric cancer (11). It has also been shown to promote
cell invasion and migration in esophageal squamous cell carcinoma
(13) and regulate apoptosis by
targeting the Bim protein in ovarian (14) and eosophangeal cells (15). miR-25 has been found to be
downregulated in anaplastic thyroid carcinoma (16) and, along with miR-30d, to target
the polycomb protein enhancer of zeste 2 (EZH2) in this disease
context (17).
In this study, we describe work in which we examined
the impact of upregulating the thyroid cancer-associated miRNA
miR-222 in benign Nthy-ori cells, and miR-25, a miRNA downregulated
in anaplastic thyroid cancer, in the anaplastic cancer-derived
8505C cell line. Microarray technologies were utilised to monitor
global gene expression changes in response to altered expression of
miR-222 and miR-25. This unbiased genome-wide approach provided by
the microarrays yielded the discovery of almost 100 mRNAs that are
either directly or indirectly targeted by each miRNA in thyroid
cells and have not been previously described to the best of our
knowledge. These gene lists provide insights as to the functions of
these miRNAs within thyroid cells; they contain both predicted and
novel targets of the miRNAs, a subset of which were validated at
the mRNA and protein level.
Materials and methods
Cell culture
The human thyroid follicular epithelial cell line
Nthy-ori 3-1 (cat no. 90011609; ECACC, Salisbury, UK) was grown in
RPMI media containing 10% foetal bovine serum (FBS), 2%
penicillin/streptomycin (5,000 U/ml). An undifferentiated human
thyroid carcinoma cell line, 8505C, (cat no. 94090184; ECACC) was
grown in Eagle's minimum essential medium (EMEM) with Hank's
buffered salt solution (HBSS) containing 2 mM glutamine and 1%
non-essential amino acids (NEAA), 10% FBS, and 2%
penicillin/streptomycin (5,000 U/ml). All cell culture reagents
were purchased from Lonza (Basel, Switzerland) and cells were
incubated at 37°C in a 5% CO2 humidified chamber (series
II water jacketed CO2 incubator; Thermo Fisher
Scientific, Waltham, MA, USA).
Transfections
For transfections, Nthy-ori 3-1 and 8505C cells were
plated at a density of 1.5×105 cells/ml in 12-well
plates (Nalge Nunc, Penfield, NY, USA) with three replicate wells
for each condition. Cells were reverse transfected using
Lipofectamine 2000 (Invitrogen, Grand Island, NY, USA) according to
the manufacturer's instructions with 50 nM pre-miR positive control
(cat. # AM17150), pre-miR negative control #1 (cat. # AM17110),
pre-miR-222 (cat. # PM11376) or pre-miR-25 (cat. # PM12401)
(Ambion, Austin, TX, USA).
Transfection efficiency was evaluated using TaqMan
real-time polymerase chain reaction (PCR) as follows. Pre-miR
hsa-miR-1 miRNA precursor was used as a positive control in
transfection experiments as, upon delivery into cells, it
effectively downregulates the expression of PTK9 at the mRNA
level. Effective delivery and activity of the pre-miR hsa-miR-1
miRNA precursor was detected by real-time PCR using a TaqMan Gene
Expression assay to PTK9 (assay ID: Hs00702289_s1), and GAPDH mRNA
was measured as an endogenous control (assay ID: Hs02758991_g1)
(both from Applied Biosystems, Foster City, CA, USA). A high
capacity cDNA reverse transcription kit (cat. # 4374966; Applied
Biosystems) was used to convert total RNA to single-stranded cDNA
for positive control analysis. Reactions contained 2 μl of
RT buffer (10X), 0.8 μl of deoxynucleotide triphosphate
(25X), 2 μl of random primers (10X), 1 μl of
multiscribe RT enzyme (500 U/μl), 1 μl of RNase
inhibitor, 3.2 μl of nuclease-free water and 10 μl of
extracted total RNA. The reactions were incubated at 25°C for 10
min, 37°C for 2 h, and 85°C for 5 sec (Perkin Elmer 9600 GeneAmp
PCR system; Applied Biosystems). Real-time PCR reactions were
performed with TaqMan 2X Universal master mix (no AmpErase UNG)
(cat. # 4304437; Applied Biosystems) and TaqMan gene expression
assays (listed previously). Reactions contained 10 μl of
TaqMan Universal PCR master mix (no AmpErase UNG), 1 μl of
TaqMan gene expression assay (20X), 2 μl of cDNA template
and 7 μl of nuclease-free water.
For miRNA analysis, total RNA from transfections was
used to synthesise cDNA using the high-capacity cDNA reverse
transcription kit and miRNA-specific RT primers for miR-222 (cat. #
4427975), miR-25 (cat. # 4427975), and endogenous control RNU6B
(cat. # 4427975) (Applied Biosystems) in 15 μl reactions.
Reactions contained 0.15 μl of 25X dNTP mixture (100 mM), 1
μl of multiScribe reverse transcriptase (50 U/μl),
1.5 μl of reverse transcription buffer (10X), 0.19 μl
of RNase inhibitor, 4.16 μl of nuclease-free water, 3
μl of RT primer and 5 μl of extracted total RNA. The
reactions were incubated at 16°C for 30 min, 42°C for 30 min, 85°C
for 5 min and then an indefinite hold at 4°C (Perkin Elmer 9600
GeneAmp PCR system; Applied Biosystems). miRNA expression was then
assessed using sequence specific primers from the TaqMan microRNA
assays and TaqMan 2X Universal master mix (no AmpErase UNG) (cat. #
4304437; Applied Biosystems) in 20 μl reactions according to
the manufacturer's instructions. Reactions contained 1 μl of
TaqMan miRNA assay mix (20X), 1.33 μl of product from RT
reaction (1:15 dilution), 10 μl of TaqMan 2X Universal
master mix (no AmpErase UNG) and 7.67 μl of nuclease free
water. Each assay was performed in triplicate for each sample.
All real-time PCR reactions were incubated in a
96-well optical plate (cat. # N8010560; Applied Biosystems) at 95°C
for 10 min, following by 40 cycles of 95°C for 15 sec and 60°C for
1 min on the Applied Biosystems 7900HT Real-Time PCR system
(Applied Biosystems). Study files were generated using a fixed
threshold of 0.1 on the SDS2.2.2 software (Applied Biosystems), and
Microsoft Excel (Microsoft, Redmond, WA, USA) was used to perform
ΔΔCt analysis on the real-time PCR output (18).
RNA isolation and mRNA microarray
analysis
Total RNA was isolated from transfected cell lines
using PARIS™ Protein and RNA isolation kit (cat. # AM1556; Ambion)
and RNeasy mini kit (cat. # 74104; Qiagen, Venlo, The Netherlands).
Briefly, the cells were lysed with PARIS Cell Disruption Buffer and
2X Lysis/Binding Solution. Cell lysates were then transferred to
Qiagen QIAshredder columns followed by RNA extraction and on column
DNase treatment (Cat. # 79254; Qiagen, Venlo, The Netherlands)
according to the manufacturer's instructions. RNA nanogram
concentration per microlitre was verified using a NanoDrop
spectrophotometer (ND-1000; Labtech International, Lewes, UK) and
RNA integrity was verified using a 2100 Bioanalyser (Agilent, Santa
Clara, CA, USA).
Affymetrix GeneChip Human Gene 1.0 ST arrays were
used for gene expression analysis according to the manufacturer's
instructions (cat. # 902461; Affymetrix, Santa Clara, CA, USA).
Three biological repeats were used for each treatment. Microarray
statistical analysis was performed on. CEL files using the Partek
Genomics Suite (Partek Inc., St. Louis, MO, USA; www.partek.com). Data were normalised and summarised
using the robust multi-average method, as previously described
(19). Paired t-tests were
performed to compare the data from the pre-miR transfections to the
negative control transfections. Comparisons were corrected for
multiple testing using the false discovery rate (FDR). Genes were
deemed to be differentially regulated in the pre-miR™ transfected
cells if they possessed a FDR ≤0.05 and a fold change ≥2. Gene
functional and pathway enrichment analysis was assessed by the
PANTHER database (http://www.pantherdb.org/).
Fluidigm reverse transcription-PCR
analysis of target genes downregulated by miR-222 and miR-25
Total RNA from transfections was converted to
single-stranded cDNA using the High Capacity cDNA Reverse
Transcription kit (cat. # 4374966; Applied Biosystems) in 20
μl reactions, as described in the transfection section of
this manuscript.
Pre-amplification of cDNA was performed using the
TaqMan PreAmp Master Mix kit (cat. # 4384267; Applied Biosystems).
The assays used were as follows: MAL2; Hs00294541_m1, MAL;
Hs00242748_m1, TLR3; Hs01551078_m1, ADM; Hs00181605_m1, RAB19;
Hs01397748_m1, PDCD1LG2; Hs00228839_m1, RAD51; Hs00947968_m1,
ADAM21; Hs01652548_s1, HYOU1; Hs01026180_m1, THBS1; Hs00962908_m1,
ETS1; Hs00428287_m1, FGFR2; Hs03466165_gH, CYR61; Hs00609994_m1,
PHLDB2; Hs01083801_m1, BIRC3; Hs00154109_m1, PRKAA2; Hs00178903_m1,
CYP24A1; Hs00989011_g1, AOX1; Hs00154079_m1, MYO5C; Hs00218921_m1,
GPR126; Hs01097890_m1, CDK6; Hs00608037_m1, PPME1; Hs00211693_m1,
RBM24; Hs00290607_m1, TNFSF10/TRAIL; Hs00921974_m1, ITGA6;
Hs01041012_m1, MPP1; Hs00609971_m1, TIMP2; Hs00234278_m1, ITGA3;
Hs01076876_m1, ADAMTS1; Hs00199608_m1, ITGA5; Hs00233732_m1,
TRIP13; Hs01020073_m1, MAP2K4; Hs00387426_m1, RAB23; Hs00212407_m1,
ADAM10; Hs00153853_m1, TRHDE; Hs00183821_m1, RAB14; Hs00249440_m1,
RARB; Hs00233405_m1, MAN2A1; Hs00159007_m1, RGS4; Hs00194501_m1,
PTGS2; Hs00153133_m1, EPGN; Hs02385425_m1, SNAP23; Hs01047498_m1,
LHFPL2; Hs00299613_m1, MYO1B; Hs01031676_m1, NFIA; Hs00906448_m1,
RNF38; Hs01014398_m1 (all from Applied Biosystems). Before running
the pre-amplification reaction, the intended assays were pooled
together, as per the manufacturer's instructions, with 1X Tris-EDTA
(TE) buffer in a 0.2X pooled assay mix. The pre-amplification
reaction was performed in 5 μl reactions containing 2.5
μl of TaqMan PreAmp master mix (2X), 1.25 μl of the
pooled assay mix (0.2X), and 1.25 μl of cDNA sample (100
ng). The pre-amplification reaction was performed for 10 min at
95°C, and 14 cycles of 15 sec at 95°C and 4 min at 60°C (PE 9600
GeneAmp PCR system; Applied Biosystems).
The Fluidigm Dynamic Arrays facilitate the testing
of the expression of 48 genes in 48 samples by performing 2,304 PCR
assays per chip. The TaqMan gene expression assays listed in the
previous paragraph were diluted to a final concentration of 10X
using Fluidigm DA Assay Loading Reagent (cat. # 100-7611; Fluidigm,
South San Francisco, CA, USA), and the pre-amplified products,
diluted to 1:5 using 1X TE buffer. The cDNA product was combined
with real-time PCR reagents in 5 μl reactions as follows:
2.25 μl of diluted pre-amplified cDNA, 2.25 μl
Universal PCR master mix (2X) (cat. # 4304437; Applied Biosystems),
and 0.25 μl Fluidigm sample loading agent (cat. # 100-7610;
Fluidigm). Samples, primers and probes were then loaded onto the
Fluidigm 48.48 Dynamic Arrays and placed on the IFC controller
(both from Fluidigm) which pressure-loads the assay components into
the reaction chambers. Assay components are automatically combined
on-chip. The Biomark Real-Time PCR system (Fluidigm) was then used
for thermal cycling and fluorescence detection. cDNA from
transfections was loaded in duplicate on three replicate dynamic
arrays. Microsoft Excel (Microsoft) was used to perform ΔΔCt
analysis on the real-time PCR output, as previously described
(18).
Protein extraction and western
blotting
Protein was isolated from transfections using
ice-cold radioimmunoprecipitation assay (RIPA) buffer (cat. #
R0278; Medical Supply Co., Dublin, Ireland) with added protease and
phosphatase inhibitor cocktails (cat. # 04693116001 and 04693116001
respectively; Roche, Indianapolis, IN, USA). Samples were suspended
in SDS-PAGE sample buffer (cat. # S3401; Sigma-Aldrich, St. Louis,
MO, USA), boiled for 4 min, and resolved on 10% SDS-PAGE gels. The
separated proteins were electrophoretically transferred to PVDF
membranes by the semi-dry transfer technique for 1 h. PVDF
membranes were subsequently blocked in 5% non-fat dried milk in
TBS-0.1% Tween-2 (TBS-T) for 1 h at room temperature, washed three
times in TBS-T, and incubated with primary antibodies (polyclonal
rabbit anti-human mitogen-activated protein kinase kinase 4 (MEK4)
(sc-964; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA)
diluted to 1:50, or monoclonal mouse anti-human TNF-related
apoptosis-inducing ligand (TRAIL) (ab12124) at 2 μg/ml, or
monoclonal mouse anti-human GAPDH (ab8245) (diluted to 1:25,000)
(both from Abcam, Cambridge, UK) overnight at 4°C with gentle
rocking. After three washes in TBS-T, the membranes were incubated
with the appropriate HRP-labelled secondary antibodies [goat
anti-mouse IgG-HRP, goat anti-rabbit IgG-HRP (cat. # 7076 and 7074
respectively; Cell Signaling Technology, Danvers, MA, USA)] for 1 h
at room temperature. The membranes were washed three times in TBS-T
and immunoreactive bands were visualised using a HRP development
solution containing 100 mM Tris-HCl pH 8.8, 30%
H2O2, 250 mM luminol, and 90 mM
4-iodophenylboronic acid (4-IPBA), and subsequent exposure to Kodak
light-sensitive film (cat. # F5763-50EA; Sigma-Aldrich). Jurkat T
cell lysates were a gift from Dr Michael Freeley and were used as
postive control lysates in western blot experiments
Results
As has been previously noted, expression of miR-222
is upregulated in thyroid cancers compared to their normal
counterparts (2–5), while expression of miR-25 is
downregulated in anaplastic thyroid carcinoma (5,16).
We therefore investigated the impact of overexpression of miR-222
in normal Nthy-ori cells, and of miR-25 in the 8505C cells. An
average 2.9-fold upregulation of miR-222 and 10,939-fold
upregulation of miR-25 was observed with the pre-miR transfections
(n=3). The very large miR-25 fold change increase resulted from an
average Ct change of 20.95 in the pre-miR-25 treated cells compared
to 33.53 in the cells treated with the negative control pre-miR.
Reduced transfection efficiency in the normal Nthy-ori cells may
explain the more modest increase in miR-222 post-transfection;
however, the positive control gene, PTK9, was downregulated
to a similar extent in both cell lines; 82.9 and 76.9% in the 8505C
and Nthy-ori cells respectively. Microarray analysis elucidated the
genes significantly downregulated (FDR value ≤0.05 and fold-change
≥2) by pre-miR-222 in Nthy-ori cells and pre-miR-25 in 8505C cells.
We found that overexpression of miR-222 in normal Nthy-ori cells
resulted in the downregulation of 82 target genes, while
overexpression of miR-25 resulted in the downregulation of 98
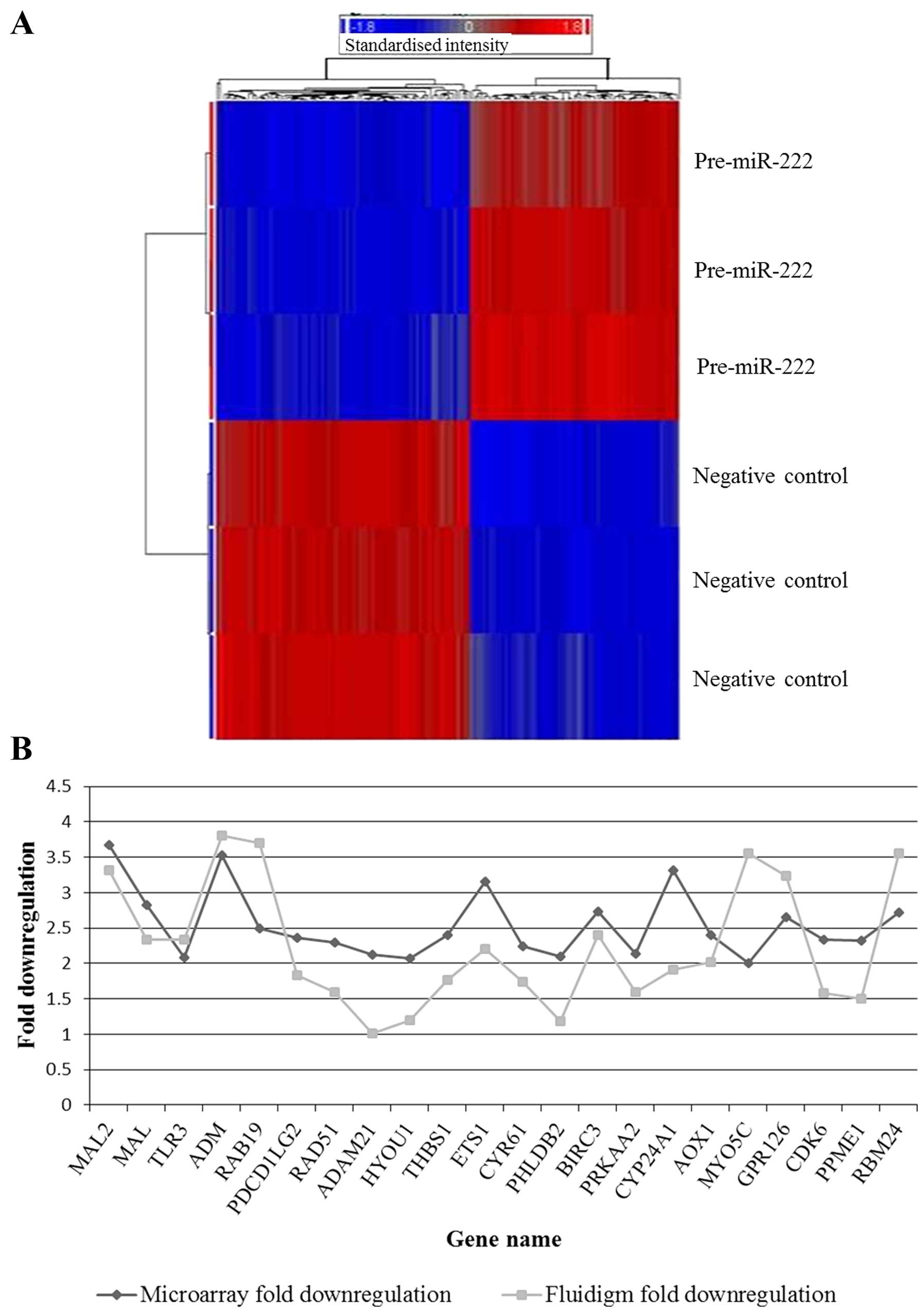
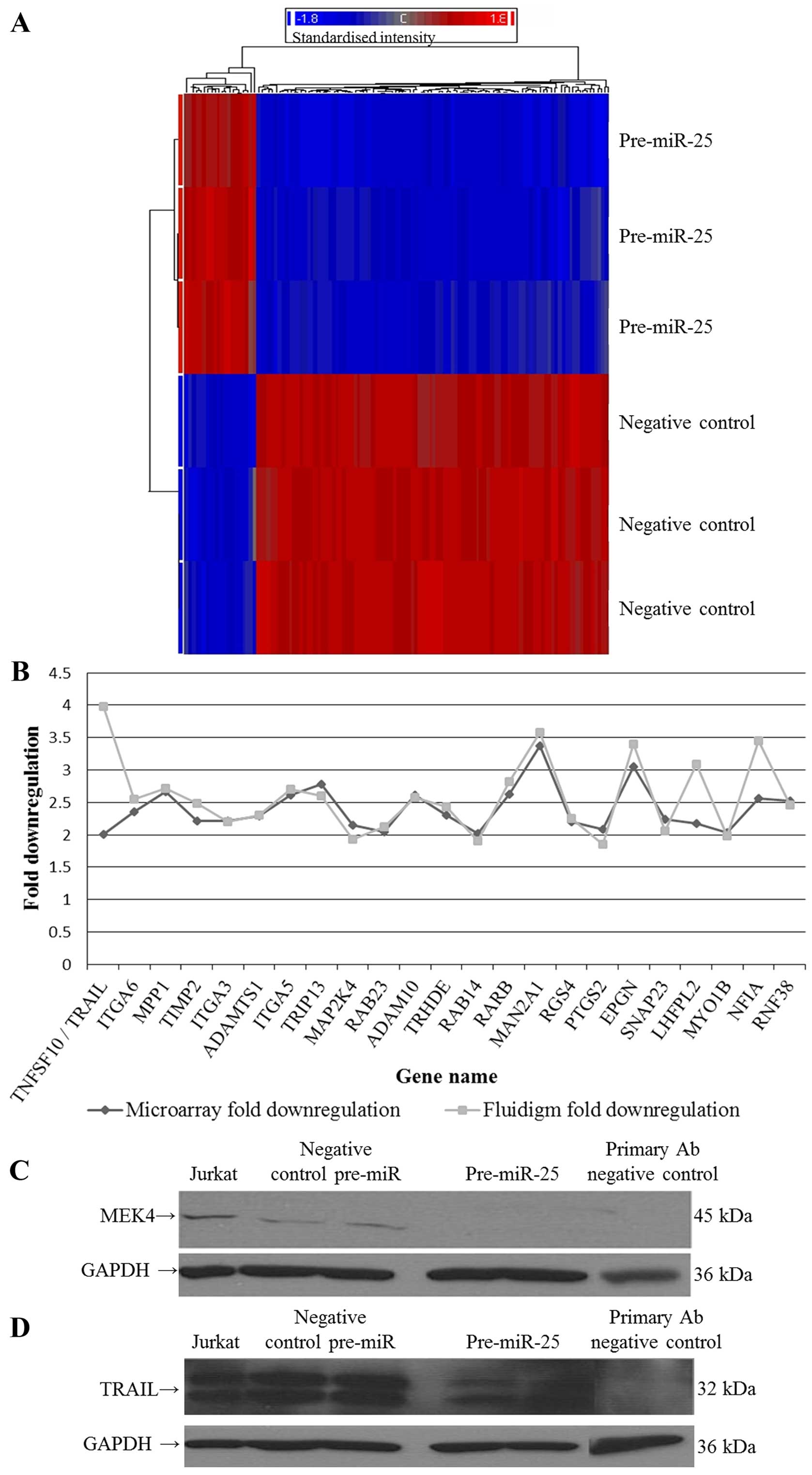
target genes in 8505C cells. Figs.
1A and 2A illustrate the
genes that were significantly downregulated by pre-miR-222 and
pre-miR-25, respectively (see Tables
I and II for full gene
lists). The PANTHER database was used to perform gene ontology
analysis on these pre-miR target lists. This analysis illustrated
that the two miRNAs target genes of diverse functions in the
thyroid cells, as no pathway was significantly over- or
under-represented in either list. This observation concurs with
other studies on miRNA target prediction and elucidation (20–22). miR-222 and miR-25 target lists
were also cross-referenced with in silico prediction results
from three prediction algorithms: miRanda, PicTar and
TargetScan.
 | Table IAll significantly downregulated genes
in pre-miR-222 treated cells compared to cells treated with
negative control pre-miRs. |
Table I
All significantly downregulated genes
in pre-miR-222 treated cells compared to cells treated with
negative control pre-miRs.
| RefSeq | Gene symbol | Gene
assignment | Fold change | P-value (FDR) |
|---|
| NM_015913 | TXNDC12 | Thioredoxin domain
containing 12 (endoplasmic reticulum) | −4.807 | 1.02E-07 |
| NM_000700 | ANXA1 | Annexin A1 | −3.881 | 2.24E-08 |
| NM_052886 | MAL2 | Mal, T cell
differentiation protein 2 | −3.681 | 1.16E-07 |
| NM_005139 | ANXA3 | Annexin A3 | −3.595 | 1.48E-09 |
| NM_004493 | HSD17B10 | Hydroxysteroid
(17-beta) dehydrogenase 10 | −3.548 | 3.73E-10 |
| NM_001124 | ADMb | Adrenomedullin | −3.526 | 1.08E-09 |
| NM_020373 | ANO2 | Anoctamin 2 | −3.475 | 1.68E-07 |
| NM_000782 | CYP24A1 | Cytochrome P450,
family 24, subfamily A, polypeptide 1 | −3.321 | 1.71E-06 |
| NM_005238 | ETS1b | V-ets
erythroblastosis virus E26 oncogene homolog 1 (avian) | −3.159 | 2.16E-08 |
| NM_003494 | DYSF | Dysferlin, limb
girdle muscular dystrophy 2B (autosomal recessive) | −3.057 | 8.62E-09 |
| NM_019026 | TMCO1 | Transmembrane and
coiled-coil domains 1 | −2.935 | 4.46E-07 |
| NM_007257 | PNMA2 | Paraneoplastic
antigen MA2 | −2.916 | 4.12E-08 |
| NM_031302 | GLT8D2 | Glycosyltransferase
8 domain containing 2 | −2.853 | 1.19E-07 |
| NM_002371 | MAL | Mal, T cell
differentiation protein | −2.830 | 4.19E-08 |
| NM_001165 | BIRC3 | Baculoviral IAP
repeat-containing 3 | −2.734 | 6.86E-08 |
| NM_153020 | RBM24b | RNA binding motif
protein 24 | −2.717 | 1.34E-05 |
| NM_020651 | PELI1 | Pellino homolog 1
(Drosophila) | −2.668 | 4.42E-06 |
| NM_020455 | GPR126 | G protein-coupled
receptor 126 | −2.659 | 9.57E-08 |
| NM_004572 | PKP2 | Plakophilin 2 | −2.630 | 7.81E-10 |
| NM_012431 | SEMA3E | Sema domain,
immunoglobulin domain (Ig), short basic doma in, secreted,
(semaphorin) 3E | −2.625 | 1.83E-05 |
| NM_002780 | PSG4 | Pregnancy specific
beta-1-glycoprotein 4 | −2.606 | 8.80E-08 |
| NM_002764 | PRPS1 | Phosphoribosyl
pyrophosphate synthetase 1 | −2.599 | 1.99E-08 |
| NM_014322 | OPN3 | Opsin 3 | −2.581 | 9.04E-07 |
| NM_013322 | SNX10 | Sorting nexin
10 | −2.569 | 2.18E-07 |
| NM_182757 | RNF144B | Ring finger
144B | −2.519 | 1.61E-09 |
| NM_000716 | C4BPB | Complement
component 4 binding protein beta | −2.519 | 6.66E-05 |
| NM_020127 | TUFT1 | Tuftelin 1 | −2.519 | 3.51E-08 |
| NM_001008749 | RAB19 | RAB19, member RAS
oncogene family | −2.501 | 8.91E-07 |
| NM_017439 | PION | Pigeon homolog
(Drosophila) | −2.499 | 3.05E-05 |
| NM_018639 | WSB2 | WD repeat and SOCS
box-containing 2 | −2.482 | 4.86E-08 |
| NM_014143 | CD274 | CD274 molecule | −2.420 | 5.96E-07 |
| NM_003246 | THBS1 | Thrombospondin
1 | −2.403 | 1.78E-08 |
| NM_001159 | AOX1 | Aldehyde oxidase
1 | −2.403 | 2.50E-06 |
| NM_000141 | FGFR2 | Fibroblast growth
factor receptor 2 | −2.394 | 2.56E-06 |
| NM_003901 | SGPL1 |
Sphingosine-1-phosphate lyase 1 | −2.380 | 1.82E-06 |
| NM_002296 | LBR | Lamin B
receptor | −2.368 | 2.36E-08 |
| NM_025239 | PDCD1LG2 | Programmed cell
death 1 ligand 2 | −2.360 | 7.42E-06 |
| NM_004741 | NOLC1 | Nucleolar and
coiled-body phosphoprotein 1 | −2.351 | 6.16E-07 |
| NM_002998 | SDC2a | Syndecan 2 | −2.333 | 2.69E-07 |
| NM_001259 | CDK6c | Cyclin-dependent
kinase 6 | −2.332 | 2.46E-07 |
| NM_016147 | PPME1 | Protein phosphatase
methylesterase 1 | −2.326 | 1.44E-08 |
| NM_004482 | GALNT3 |
UDP-N-acetyl-alpha-D-galactosamine:
polypeptide N-acetylgalactosaminyltransferase 3 (GalNAc-T3) | −2.314 | 4.48E-06 |
| NM_021069 | SORBS2 | Sorbin and SH3
domain containing 2 | −2.314 | 2.89E-07 |
| NM_005264 | GFRA1 | GDNF family
receptor alpha 1 | −2.304 | 5.49E-07 |
| NM_022131 | CLSTN2 | Calsyntenin 2 | −2.298 | 7.38E-08 |
| NM_002875 | RAD51 | RAD51 homolog (RecA
homolog, E. coli) (S. cerevisiae) | −2.295 | 2.71E-06 |
| BC066649 | C1orf198 | Chromosome 1 open
reading frame 198 | −2.275 | 2.03E-07 |
| AK131040 | LOC388022 | Hypothetical gene
supported by AK131040 | −2.259 | 8.18E-05 |
| BC016278 | LOH3CR2A | Loss of
heterozygosity, 3, chromosomal region 2, gene A | −2.243 | 0.0004969 |
| NM_001554 | CYR61 | Cysteine-rich,
angiogenic inducer, 61 | −2.241 | 6.15E-07 |
| NM_033393 | FHDC1 | FH2 domain
containing 1 | −2.237 | 3.51E-07 |
| NM_002492 | NDUFB5 | NADH dehydrogenase
(ubiquinone) 1 beta subcomplex, 5 | −2.225 | 1.36E-07 |
| NM_018327 | SPTLC3 | Serine
palmitoyltransferase, long chain base subunit 3 | −2.225 | 6.97E-07 |
| NM_080670 | SLC35A4 | Solute carrier
family 35, member A4 | −2.211 | 6.39E-07 |
| NM_022772 | EPS8L2 | EPS8-like 2 | −2.202 | 3.77E-08 |
| NM_014391 | ANKRD1 | Ankyrin repeat
domain 1 (cardiac muscle) | −2.191 | 7.78E-08 |
| NM_016206 | VGLL3 | Vestigial like 3
(Drosophila) | −2.191 | 8.67E-07 |
| Ensembl no:
ENST00000319426 | - | Partially
transcribed sequence | −2.188 | 4.57E-06 |
| NM_004694 | SLC16A6 | Solute carrier
family 16, member 6 (monocarboxylic acid transporter 7) | −2.184 | 0.0003082 |
| NM_021623 | PLEKHA2 | Pleckstrin homology
domain containing, family A (phosphoinositide binding specific)
member 2 | −2.160 | 1.74E-06 |
| NR_002836 | PGM5P2 | Phosphoglucomutase
5 pseudogene 2 | −2.159 | 2.55E-07 |
| NM_001145204 | LOC729993 | Hypothetical
protein LOC729993, transcript variant 1, mRNA | −2.148 | 2.73E-07 |
| NM_153345 | TMEM139 | Transmembrane
protein 139 | −2.143 | 3.58E-06 |
| NR_002836 | PGM5P2 | Phosphoglucomutase
5 pseudogene 2 | −2.141 | 4.39E-08 |
| NM_006252 | PRKAA2 | Protein kinase,
AMP-activated, alpha 2 catalytic subunit | −2.137 | 7.04E-07 |
| NM_002547 | OPHN1 | Oligophrenin 1 | −2.137 | 2.60E-07 |
| NM_016132 | MYEF2 | Myelin expression
factor 2 | −2.136 | 4.39E-05 |
| NM_003813 | ADAM21 | ADAM
metallopeptidase domain 21 | −2.121 | 0.0005355 |
| Ensembl no:
ENST00000384701 | – | Expressed sequence
tag (EST) | −2.116 | 0.0002848 |
| NM_145753 | PHLDB2 | Pleckstrin
homology-like domain, family B, member 2 | −2.099 | 4.55E-05 |
| NM_001415 | EIF2S3 | Eukaryotic
translation initiation factor 2, subunit 3 gamma, 52 kDa | −2.096 | 2.72E-07 |
| NM_000331 | SAA1 | Serum amyloid
A1 | −2.086 | 0.0041182 |
| NM_003265 | TLR3 | Toll-like receptor
3 | −2.082 | 5.79E-05 |
| NM_006389 | HYOU1 | Hypoxia
up-regulated 1 | −2.077 | 1.02E-07 |
| NM_015238 | WWC1 | WW and C2 domain
containing 1 | −2.069 | 1.56E-07 |
| NM_001001522 | TAGLN | Transgelin | −2.056 | 9.60E-07 |
| Ensembl no:
ENST00000322446 | – | Partially
transcribed sequence | −2.042 | 0.0007311 |
| NM_006832 | FERMT2 | Fermitin family
homolog 2 (Drosophila) | −2.005 | 3.07E-05 |
| NM_018728 | MYO5C | Myosin VC | −2.003 | 9.51E-07 |
| NM_002354 | EPCAM | Epithelial cell
adhesion molecule | −2.018 | 0.0224988 |
 | Table IIAll significantly downregulated genes
in pre-miR-25 treated cells compared to cells treated with negative
control pre-miRs. |
Table II
All significantly downregulated genes
in pre-miR-25 treated cells compared to cells treated with negative
control pre-miRs.
| RefSeq | Gene symbol | Gene
assignment | Fold change | P-value (FDR) |
|---|
| NM_000210 | ITGA6 | Integrin alpha
6 | −2.352 | 6.17E-09 |
| NM_002372 | MAN2A1b | Mannosidase, alpha,
class 2A, member 1 | −3.370 | 4.98E-10 |
| NM_002436 | MPP1 | Membrane protein,
palmitoylated 1 | −2.669 | 1.08E-09 |
| NM_003255 | TIMP2 | TIMP
metallopeptidase inhibitor 2 | −2.218 | 1.75E-09 |
| NM_021913 | AXLd | AXL receptor
tyrosine kinase | −2.594 | 2.49E-09 |
| NM_015881 | DKK3 | Dickkopf homolog 3
(Xenopus laevis) | −3.428 | 2.89E-09 |
| NM_018442 | IQWD1c | IQ motif and WD
repeats 1 | −2.134 | 2.97E-09 |
| NM_002204 | ITGA3 | Integrin alpha 3
(antigen CD49C, alpha 3 subunit of VLA-3 receptor) | −2.209 | 7.00E-09 |
| NM_004035 | ACOX1 | Acyl-Coenzyme A
oxidase 1, palmitoyl | −2.206 | 1.42E-08 |
| NM_005562 | LAMC2 | Laminin gamma
2 | −2.803 | 1.12E-08 |
| NM_006520 | DYNLT3d | Dynein, light
chain, Tctex-type 3 | −2.694 | 1.07E-08 |
| NM_006988 | ADAMTS1 | ADAM
metallopeptidase with thrombospondin type 1 motif 1 | −2.285 | 1.34E-08 |
| NM_006080 | SEMA3Ae | Sema domain,
immunoglobulin domain (Ig), short basic domain, secreted,
(semaphorin) 3A | −2.450 | 2.61E-08 |
| NM_000351 | STS | Steroid sulfatase
(microsomal), isozyme S | −2.410 | 2.36E-08 |
| NM_019004 | ANKIB1 | Ankyrin repeat and
IBR domain containing 1 | −2.304 | 1.15E-08 |
| NM_181659 | NCOA3 | Nuclear receptor
coactivator 3 | −2.006 | 1.41E-08 |
| NM_002844 | PTPRK | Protein tyrosine
phosphatase receptor type K | −2.152 | 1.97E-08 |
| NM_002205 | ITGA5a | Integrin alpha 5
(fibronectin receptor, alpha polypeptide) | −2.614 | 9.07E-09 |
| NM_000916 | OXTR | Oxytocin
receptor | −2.473 | 4.16E-08 |
| NM_005349 | RBPJ | Recombination
signal binding protein for immunoglobulin kappa J region | −2.033 | 4.83E-08 |
| NM_005471 | GNPDA1 |
Glucosamine-6-phosphate deaminase 1 | −2.236 | 1.42E-08 |
| NM_004237 | TRIP13 | Thyroid hormone
receptor interactor 13 | −2.781 | 1.02E-08 |
| NM_001418 | EIF4G2e | Eukaryotic
translation initiation factor 4 gamma, 2 | −2.494 | 1.95E-08 |
| NM_021999 | ITM2B | Integral membrane
protein 2B | −2.070 | 3.95E-08 |
| NM_000138 | FBN1b | Fibrillin 1 | −2.355 | 2.18E-07 |
| NM_003010 | MAP2K4b | Mitogen-activated
protein kinase kinase 4 | −2.147 | 1.34E-08 |
| NM_016275 | SELT | Selenoprotein
T | −3.175 | 2.35E-08 |
| NM_001102445 | RGS4 | Regulator of
G-protein signaling 4 | −2.194 | 3.62E-08 |
| NM_001001521 | UGP2b | UDP-glucose
pyrophosphorylase 2 | −3.032 | 6.61E-08 |
| NM_002948 | RPL15 | Ribosomal protein
L15 | −2.659 | 4.97E-08 |
| NM_003139 | SRPRb | Signal recognition
particle receptor ('docking protein') | −2.195 | 1.33E-07 |
| NM_001190 | BCAT2b | Branched chain
aminotransferase 2, mitochondrial | −3.040 | 7.52E-08 |
| NM_000693 | ALDH1A3 | Aldehyde
dehydrogenase 1 family, member A3 | −2.168 | 2.58E-06 |
| NM_012242 | DKK1 | Dickkopf homolog 1
(Xenopus laevis) | −2.060 | 3.20E-07 |
| NM_006178 | NSF |
N-ethylmaleimide-sensitive factor | −2.202 | 4.09E-07 |
| NM_199511 | CCDC80 | Coiled-coil domain
containing 80 | −2.016 | 1.25E-07 |
| NM_014674 | EDEM1b | ER degradation
enhancer, mannosidase alpha-like 1 | −2.391 | 8.84E-08 |
| NM_213611 | SLC25A3 | Solute carrier
family 25 (mitochondrial carrier; phosphate carrier) | −2.086 | 1.17E-07 |
| NM_133494 | NEK7 | NIMA (never in
mitosis gene a)-related kinase 7 | −2.424 | 3.00E-07 |
| NM_017958 | PLEKHB2 | Pleckstrin homology
domain containing, family B2 (evectins) member | −3.420 | 8.84E-08 |
| NM_000259 | MYO5A | Myosin VA (heavy
chain 12, myoxin) | −2.426 | 2.11E-07 |
| NM_016277 | RAB23b | RAB23, member RAS
oncogene family | −2.051 | 1.26E-07 |
| NM_001110 | ADAM10b | ADAM
metallopeptidase domain 10 | −2.616 | 3.21E-07 |
| NM_001693 | ATP6V1B2 | ATPase,
H+ transporting, lysosomal 56/58 kDa, V1 subunit B2 | −2.064 | 1.30E-07 |
| NM_005779 | LHFPL2a | Lipoma HMGIC fusion
partner-like 2 | −2.173 | 1.72E-07 |
| NM_016422 | RNF141e | Ring finger protein
141 | −2.260 | 8.57E-08 |
| NM_002095 | GTF2E2 | General
transcription factor IIE, polypeptide 2, beta 34k | −2.570 | 7.22E-07 |
| NM_003483 | HMGA2e | High mobility group
AT-hook 2 | −2.654 | 1.24E-07 |
| NM_014223 | NFYCc | Nuclear
transcription factor Y, gamma | −2.112 | 1.57E-07 |
| NM_013381 | TRHDE |
Thyrotropin-releasing hormone degrading
enzyme | −2.306 | 2.96E-07 |
| NM_006287 | TFPI | Tissue factor
pathway inhibitor (lipoprotein-associated coagulation
inhibitor) | −2.145 | 5.36E-07 |
| NM_033540 | MFN1 | Mitofusin 1 | −2.014 | 3.46E-07 |
| NM_024664 | PPCSb |
Phosphopantothenoylcysteine
synthetase | −2.386 | 3.87E-07 |
| NM_023080 | C8orf33 | Chromosome 8 open
reading frame 33 | −2.006 | 1.98E-07 |
| NM_016352 | CPA4 | Carboxypeptidase
A4 | −2.060 | 1.26E-06 |
| NM_012223 | MYO1Bb | Myosin IB | −2.030 | 1.35E-06 |
| NM_020223 | FAM20C | Family with
sequence similarity 20 member C | −2.387 | 1.16E-06 |
| NM_004670 | PAPSS2 | 3′-phosphoadenosine
5′-phosphosulfate synthase 2 | −2.347 | 9.66E-07 |
| NM_005896 | IDH1 | Isocitrate
dehydrogenase 1 (NADP+), soluble | −2.599 | 3.89E-06 |
| NM_016322 | RAB14b | RAB14, member RAS
oncogene family | −2.021 | 4.30E-07 |
| NM_002971 | SATB1 | SATB homeobox
1 | −2.080 | 1.43E-06 |
| NM_000405 | GM2A | GM2 ganglioside
activator | −2.249 | 4.07E-07 |
| NM_001547 | IFIT2 | Interferon-induced
protein with tetratricopeptide repeats | −4.287 | 8.87E-07 |
| NM_016570 | ERGIC2 | ERGIC and golgi
2 | −2.858 | 9.73E-07 |
| NM_005595 | NFIAb | Nuclear factor
I/A | −2.567 | 6.45E-07 |
| NM_005981 | TSPAN31 | Tetraspanin 31 | −2.127 | 6.55E-07 |
| NM_001085471 | FOXN3 | Forkhead box
N3 | −2.314 | 1.11E-06 |
| BC016048 | LRRC38 | Leucine rich repeat
containing 38 | −2.394 | 5.78E-07 |
| NM_005314 | GRPR | Gastrin-releasing
peptide receptor | −2.545 | 6.82E-06 |
| NM_002783 | PSG7 | Pregnancy specific
beta-1-glycoprotein 7 | −2.015 | 3.89E-06 |
| NM_002781 | PSG5 | Pregnancy specific
beta-1-glycoprotein 5 | −2.222 | 4.73E-06 |
| NM_003884 | KAT2B | K(lysine)
acetyltransferase 2B | −2.108 | 4.43E-06 |
| NM_001040409 | MTHFD2 |
Methylenetetrahydrofolate dehydrogenase
(NADP+ dependent) 2, methenyltetrahydrofolate
cyclohydrolase | −2.312 | 1.92E-05 |
| NM_003580 | NSMAFb | Neutral
sphingomyelinase (N-SMase) activation associated factor | −2.072 | 2.33E-06 |
| NM_001013442 | EPGN | Epithelial mitogen
homolog (mouse) | −3.057 | 1.89E-06 |
| NM_012319 | SLC39A6 | Solute carrier
family 39 (zinc transporter), member 6 | −2.026 | 2.95E-06 |
| NM_000965 | RARB | Retinoic acid
receptor, beta | −2.624 | 5.42E-06 |
| NM_152772 | TCP11L2 | T-complex 11
(mouse)-like 2 | −2.105 | 4.63E-05 |
| BC017297 | FAM49B | Family with
sequence similarity 49 member B | −2.051 | 1.87E-06 |
| NM_001002265 | MARCH8 | Membrane-associated
ring finger (C3HC4) 8 | −2.232 | 7.22E-06 |
| NM_015509 | NECAP1b | NECAP endocytosis
associated 1 | −2.013 | 1.85E-06 |
| NM_001080512 | BICC1 | Bicaudal C homolog
1 (Drosophila) | −2.193 | 9.59E-06 |
| NM_001031850 | PSG6 | Pregnancy specific
beta-1-glycoprotein 6 | −2.806 | 7.76E-06 |
| NM_020841 | OSBPL8 | Oxysterol binding
protein-like 8 | −2.135 | 8.37E-06 |
| NM_007036 | ESM1 | Endothelial
cell-specific molecule 1 | −2.059 | 5.81E-06 |
| NM_016075 | VPS36 | Vacuolar protein
sorting 36 homolog (S. cerevisiae) | −2.021 | 3.55E-06 |
| NM_000963 | PTGS2 |
Prostaglandin-endoperoxide synthase 2
(prostaglandin G/H synthase and cyclooxygenase) | −2.084 | 9.13E-06 |
| NM_017946 | FKBP14 | FK506 binding
protein 14, 22 kDa | −2.240 | 1.13E-05 |
| NM_003825 | SNAP23 |
Synaptosomal-associated protein, 23
kDa | −2.233 | 6.08E-06 |
| NM_194328 | RNF38a | Ring finger protein
38 | −2.526 | 7.91E-06 |
| NM_006636 | MTHFD2 |
Methylenetetrahydrofolate dehydrogenase
(NADP+ dependent) 2, methenyltetrahydrofolate
cyclohydrolase | −2.362 | 0.0001745 |
| NM_006905 | PSG1 | Pregnancy specific
β-1-glycoprotein 1 | −2.016 | 0.0001079 |
| Ensembl no:
ENST00000390952 | – | Expressed Sequence
Tag (EST) | −2.078 | 0.0001661 |
| NM_030920 | ANP32Ee | Acidic
(leucine-rich) nuclear phosphoprotein 32 family, member E | −2.663 | 6.01E-05 |
| NM_000262 | NAGA |
N-acetylgalactosaminidase, alpha | −2.055 | 4.77E-05 |
| NM_001548 | IFIT1 | Interferon-induced
protein with tetratricopeptide repeats | −2.439 | 3.00E-05 |
| NM_001039569 | AP1S3 | Adaptor-related
protein complex 1, sigma 3 subunit | −2.193 | 0.0003395 |
| NM_003810 | TNFSF10 | Tumour necrosis
factor (ligand) superfamily, member 10 | −2.003 | 0.0011012 |
Overexpression of miR-222 in Nthy-ori
cells
Five of the 82 genes (6.1%) significantly
downregulated by miR-222 overexpression in the Nthy-ori 3-1 cells
were predicted gene targets of the miRNA in miRanda, PicTar or
TargetScan, or a combination of the databases. Gene ontology
analysis revealed that no molecular function or biological process
was significantly over- or underrepresented in the miR-222 target
list. Notwithstanding this observation, several genes that were
found to be targets of this miRNA have interesting molecular
functions. For instance, seven receptors are in the lists
(MAL, OPN3, PSG4, FGFR2, GPR126,
TLR3 and LBR), four transcription factors
(PSG4, ETS1, MAL and RBM24), five
signalling molecules (THBS1, ETS1, CYR61,
SEMA3E and ADM), and five genes involved in defence
and immunity (PNMA2, C4BPB, SDC2, MAL,
TLR3 and SAA1).
The downregulation of 22 genes in response to
miR-222 overexpression identified by the microarray analysis (both
predicted and novel targets) was successfully validated using
Fluidigm real-time PCR technologies (Table III and Fig. 1B) (Pearson's correlation,
0.506).
 | Table IIIDownregulated genes following
expression of pre-miR-222 in Nthy-ori 3-1. |
Table III
Downregulated genes following
expression of pre-miR-222 in Nthy-ori 3-1.
| Gene | Microarray fold
downregulation | Microarray P-value
(FDR) | Fluidigm qPCR fold
downregulation | Fluidigm P-value
(t-test) |
|---|
| MAL2 | 3.681 | 1.16E-07 | 3.314 | 3.55E-05 |
| MAL | 2.83 | 4.19E-08 | 2.332 | 1.54E-05 |
| TLR3 | 2.082 | 5.79E-05 | 2.341 | 0.0001 |
| ADMa | 3.526 | 1.08E-09 | 3.81 | 2.93E-05 |
| RAB19 | 2.501 | 8.91E-07 | 3.7 | 4.48E-06 |
|
PDCD1LG2 | 2.36 | 7.42E-06 | 1.827 | 0.004 |
| RAD51 | 2.295 | 2.71E-06 | 1.597 | 9.59E-05 |
| ADAM21 | 2.121 | 0.0005 | 1.008 | 0.9517 |
| HYOU1 | 2.077 | 1.02E-07 | 1.198 | 0.0001 |
| THBS1 | 2.403 | 1.78E-08 | 1.769 | 0.0002 |
| ETS1a | 3.159 | 2.16E-08 | 2.208 | 0.0003 |
| CYR61 | 2.241 | 6.15E-07 | 1.745 | 0.0006 |
| PHLDB2 | 2.099 | 4.55E-05 | 1.178 | 0.003 |
| BIRC3 | 2.734 | 6.86E-08 | 2.406 | 0.0005 |
| PRKAA2 | 2.137 | 7.04E-07 | 1.595 | 0.001 |
| CYP24A1 | 3.321 | 1.71E-06 | 1.911 | 0.0006 |
| AOX1 | 2.403 | 2.50E-06 | 2.024 | 0.0006 |
| MYO5C | 2.003 | 9.51E-07 | 3.549 | 0.0002 |
| GPR126 | 2.659 | 9.57E-08 | 3.24 | 6.8E-05 |
| CDK6b | 2.332 | 2.46E-07 | 1.588 | 0.0004 |
| PPME1 | 2.326 | 1.44E-08 | 1.5 | 0.0006 |
|
RBM24a | 2.717 | 1.34E-05 | 3.55 | 3.11E-06 |
Overexpression of miR-25 in 8505C
cells
The list of 98 miR-25 target genes was also
cross-referenced with in silico prediction results. This
comparison revealed that the list of genes that were significantly
downregulated by pre-miR-25 in the 8505C cells is enriched with
27/98 or 27.55% predicted gene targets for miR-25. Gene ontology
analysis of the miR-25 gene target list suggests that cell adhesion
is an important aspect of miR-25 functioning in the thyroid cells
as biological processes including cell adhesion, cell
communication, signal transduction, and cell adhesion-mediated
signalling were significantly enriched for in this list (P-value
1.47E-02, 1.18E-03, 3.13E-02 and 1.59E-02, respectively). The list
is also significantly enriched for cell adhesion molecules:
ITGA3, ITGA5 (which is also a predicted target of the
miRNA in all three databases used); and ITGA6 (P-value
3.10E-03) and CAM family adhesion molecules: PSG1,
PSG5, PSG6 and PSG7 (P-value 2.47E-02). Seven
receptors are also in the miR-25 list: TCP11L2, RARB,
GRPR, PTPRK, AXL, LRRC38 and
OXTR; six transcription factors: RARB, FOXN3,
SATB1, NCOA3, GTF2E2 and NFIA; and five
kinases: MPP1, AXL, MAP2K4, PAPSS2 and
NEK7, and a member of the tumour necrosis family,
TNFSF10/TRAIL.
Fluidigm real-time PCR technologies were used to
confirm the downregulation of 23 predicted and novel gene targets
in response to miR-25 expression in the 8505C cells (Table IV and Fig. 2B). All genes tested were
downregulated in both the microarray and Fluidigm analyses
(Pearson's correlation, 0.538; TRAIL removed, 0.810). In
addition, two novel targets of miR-25 were successfully validated
at the protein level by western blotting; predicted target MEK4 and
novel target TRAIL. Fig. 2C and D
illustrate the reduction in MEK4 and TRAIL protein expression
following treatment of anaplastic thyroid cells with pre-miR-25,
demonstrating that they are direct or indirect targets of this
miRNA.
 | Table IVDownregulated genes following
expression of pre-miR-25 in 8505C cells. |
Table IV
Downregulated genes following
expression of pre-miR-25 in 8505C cells.
| Gene | Microarray fold
downregulation | Microarray P-value
(FDR) | Fluidigm qPCR fold
downregulation | Fluidigm P-value
(t-test) |
|---|
| TNFSF10/TRAIL | 2.003 | 0.001 | 3.982 | 0.003 |
| ITGA6 | 2.352 | 6.17E-09 | 2.552 | 0.0002 |
| MPP1 | 2.669 | 1.08E-09 | 2.718 | 7.3E-06 |
| TIMP2 | 2.218 | 1.75E-09 | 2.483 | 2.19E-05 |
| ITGA3 | 2.209 | 7.00E-09 | 2.194 | 2.40E-07 |
| ADAMTS1 | 2.285 | 1.34E-08 | 2.304 | 0.002 |
| ITGA5b | 2.614 | 9.07E-09 | 2.699 | 4.68E-07 |
| TRIP13 | 2.781 | 1.02E-08 | 2.606 | 4.92E-06 |
| MAP2K4a | 2.147 | 1.34E-08 | 1.925 | 7.70E-05 |
| RAB23a | 2.051 | 1.26E-07 | 2.122 | 8.45E-05 |
| ADAM10a | 2.616 | 3.21E-07 | 2.571 | 1.57E-05 |
| TRHDE | 2.306 | 2.96E-07 | 2.43 | 2.09E-06 |
| RAB14a | 2.021 | 4.30E-07 | 1.907 | 2.91E-05 |
| RARB | 2.624 | 5.42E-06 | 2.816 | 7.68E-06 |
| MAN2A1a | 3.37 | 4.98E-10 | 3.576 | 2.19E-07 |
| RGS4 | 2.194 | 3.62E-08 | 2.255 | 0.0009 |
| PTGS2 | 2.084 | 9.13E-06 | 1.853 | 0.0098 |
| EPGN | 3.057 | 1.89E-06 | 3.394 | 0.0003 |
| SNAP23 | 2.233 | 6.08E-06 | 2.057 | 8.58E-05 |
| LHFPL2b | 2.173 | 1.72E-07 | 3.092 | 0.0002 |
| MYO1Ba | 2.03 | 1.35E-06 | 1.986 | 1.997E-06 |
| NFIAa | 2.567 | 6.45E-07 | 3.445 | 0.015 |
Discussion
The aim of this study was to elucidate the mRNA
targets of two miRNAs that we had previously found to be
differentially expressed in thyroid cancer (5): miR-222 and miR-25. This was achieved
by overexpressing the thyroid cancer-associated miRNA miR-222 in
the transformed normal Nthy-ori cells, and miR-25 in the anaplastic
cancer-derived 8505C cells. The use of microarrays to analyse the
RNA from these cells exploited an unbiased genome-wide approach and
yielded the discovery of a set of mRNAs that are either directly or
indirectly targeted by each miRNA in thyroid cells, and have not
been previously described.
The gene target lists produced by this genome-wide
investigation compare favourably with previously published studies
on miRNA target elucidation and prediction. Similar to prior
studies (21,23), the two pre-miRs used in this body
of study each downregulated almost 100 target transcripts: 98 by
pre-miR-25 in the 8505C cells and 82 by pre-miR-222 in the Nthy-ori
3-1 cells. The subtle change in gene expression observed in the
genes targeted by pre-miR-222 and pre-miR-25 (between 2- and
4-fold) is also similar to other studies on the subject (6,21).
Finally, the relatively low number of predicted miRNA targets
significantly differentially expressed has been noted previously
(6), and the divergent assortment
of molecular functions and biological processes encompassed within
the gene target lists is also reflective of published accounts of
target prediction and elucidation (20,21). These diverse lists allow us
insight into the functions of these two miRNAs within the
respective thyroid cell lines. For instance, upregulation of
miR-222 in Nthy-ori cells impacts on several transcription factors,
cell signalling molecules, and genes involved in defence and
immunity (PNMA2, C4BPB, SDC2, MAL,
TLR3 and SAA1).
Expression of miR-25 is downregulated in anaplastic
thyroid carcinoma, so we upregulated the expression of miR-25 in
the anaplastic thyroid carcinoma 8505C cell line. Although miR-25
was found to target genes with a wide variety of functions, the
gene ontology analysis of this list highlights the predilection of
this miRNA for regulating genes involved in cell adhesion, with
both this cellular process and molecules of this molecular function
being significantly over-represented in this list. Two main groups
of cell adhesion molecules are over-represented in the miR-25
target list; inte-grin α genes ITGA3, ITGA5 (which is
a predicted target of the miR-25 in miRanda, PicTar and TargetScan)
and ITGA6, and the pregnancy-specific glycoproteins (PSGs);
PSG1, PSG5, PSG6 and PSG7. Loss of
miR-25 expression in anaplastic thyroid carcinoma is therefore
associated with upregulation of genes encoding integrins and
glycoproteins. The ITGA genes appear to be markers of
aggression in the cancers in which they have been explored. For
instance, ITGA3 and ITGA5 have been shown to be
markers of invasiveness in head and neck squamous cell carcinoma
(24), ITGA3, along with ITGB4
and 5 were found to be candidate biomarkers for cervical lymph node
metastasis or death in tongue squamous cell carcinoma (25) and ITGA6 was found to be necessary
for the tumourigenicity of a stem cell-like subpopulation within
the MCF7 breast cancer cell line (26).
This is not the first occasion miR-25 has been
linked to a role in cell adhesion, as Xu et al have
previously highlighted the role of miR-25 in oesophageal squamous
cell carcinoma (ESCC). They found upregulation of this miRNA in
ESCC cells promoted migration and invasion, and also found that
miR-25 directly targets E-Cadherin, an important cellular adhesion
protein in the ESCC cells (13).
Gerson et al have recently identified miR-25, and indeed
miR-222, to be responsive to β4 integrin expression in breast
carcinoma cell lines (27). Our
observation, along with these previous studies in breast cancer and
ESCC, indicates a role for miR-25 in tumour progression through the
disruption of cell adhesion.
Additional functions of miR-25 are highlighted with
the two proteins (MEK4 and TRAIL) that were shown to be
down-regulated in response to the expression of miR-25. MEK4 is a
member of the MAP kinase kinase family that directly phosphorylates
and activates c-Jun NH2-terminal kinase (JNK) in response to
cellular stresses and pro-inflammatory cytokines, and can also
activate p38 (28). MAP kinase
signalling is often disrupted in thyroid cancer through RAS,
BRAF or RET/PTC mutations (29). Both tumour suppressor and
oncogenic functions have been attributed to MEK4 in cancer
(30,31). Little is known of the role of MEK4
in thyroid cancer; however, Chiariello et al demonstrated
that Ret signalling involves MEK4 (32). It remains unclear as to whether
MEK4 expression influences anaplastic thyroid carcinoma in a tumour
suppressive or oncogenic manner. However, as pre-miR-25 was shown
to downregulate MEK4 mRNA and protein in ATC 8505C cells in this
study, it is interesting to speculate that the endogenous
downregulation of miR-25 may lead to the upregulation of MEK4
expression and its pro-oncogenic characteristics in ATC cells.
TRAIL expression was also decreased in response to
miR-25 expression in 8505C cells. TRAIL has five cellular receptors
and can activate the extrinsic and intrinsic pathways to regulate
intercellular apoptotic responses in the immune system (33). Approximately a decade and a half
ago it was noted that TRAIL could induce apoptosis in transformed
and malignant cells but not in normal cells (34). TRAIL was subsequently found to be
capable of triggering apoptosis in eight PTC and two ATC derived
thyroid carcinoma cell lines but not in normal thyrocytes (35). As a result of this tumour-specific
effect, a great deal of work was done to develop anticancer
therapies to mimic the effect of TRAIL. The TRAIL and MAP kinase
pathways appear to overlap in TRAIL therapy signalling. Ohtsuka
et al reported that the combination of anti-death receptor
antibodies and chemotherapy agents led to a synergistic activation
of the JNK/p38 MAP kinase which was mediated by MEK4 in breast,
prostate and colon cancer cells (36). Moreover, Söderström et al
showed that MAP/extracellular regulated kinase (ERK) signalling
(which is frequently over-activated in thyroid cancer) protected
activated T cells from TRAIL-induced apoptosis (37).
miR-25 involvement in the TRAIL-mediated apoptosis
pathway was confirmed further with the observation by Razumilava
et al that this miRNA targets TRAIL death receptor-4 (DR4)
and promotes apoptosis resistance in cholangiocarcinoma (38). If one considers the pro-apoptotic
role of TRAIL, it is difficult to elucidate how an upregulation of
this gene through the endogenous downregulation of miR-25 would be
beneficial to the progress of anaplastic thyroid carcinoma.
However, a review by Newsom-Davis et al outlines in
vitro and in vivo experiments in which TRAIL expression
induced proliferation, migration and invasion of tumour cells which
were resistant to TRAIL-mediated apoptosis. They describe how
secondary intracellular signalling complexes, following TRAIL DISC
formation, can activate NF-κB via the inhibitor of κB kinase
complex (IKK complex) which signals through MAPK, JNK and p38
(39). Other groups have shown
that TRAIL-induced survival and proliferation does not involve the
p38 kinase pathway but is dependent on the MAP kinase ERKs.
Therefore, in the anaplastic thyroid cancer cells, the endogenous
downregulation of miR-25 may enable the upregulation of TRAIL to
activate its pro-survival MAP kinase responses (perhaps involving
MEK4). Future studies may also benefit from investigating whether
miR-25 is involved in regulating MEK4 and TRAIL in response to
TRAIL cancer therapies.
In conclusion, this study used an unbiased approach
to elucidate almost 100 genes that are either directly or
indirectly targeted by miR-25 and miR-222 in thyroid cells. The
number of genes in these target lists, the extent to which the
genes are regulated and the diversity of their functions is
reflective of other published accounts of miRNA target prediction
and elucidation. The gene targets of these two miRNAs confirm the
diverse nature of miRNA-target interactions within cells. A
considerable proportion of these targets have been validated using
RT-PCR with a further two, MEK4 and TRAIL, being confirmed at the
protein level. We provide an interesting insight into the functions
of these two miRNAs in thyroid cells, in particular that cell
adhesion and apoptosis are important aspects of miR-25 functioning
in thyroid cells. In addition to this, there is broad scope for
further investigation of the many results produced by this
study.
Abbreviations:
|
MAPK
|
mitogen-activated protein kinase
|
|
hTERT
|
human telomerase reverse
transcriptase
|
|
FDR
|
false discovery rate
|
|
RIPA
|
radioimmunoprecipitation assay
|
|
DR4
|
TRAIL death receptor-4
|
|
PSG
|
pregnancy-specific glycoprotein
|
|
TRAIL
|
TNF-related apoptosis-inducing
ligand
|
|
IKK complex
|
inhibitor of κB kinase complex
|
|
ERK
|
extracellular regulated kinases
|
|
JNK
|
c-Jun NH2-terminal kinase
|
Acknowledgments
The authors would like to acknowledge grants from
the Health Research Board Molecular Medicine PhD training programme
and the Science Foundation Ireland funded Molecular Therapeutics
for Cancer Ireland.
References
|
1
|
Smallridge RC, Marlow LA and Copland JA:
Anaplastic thyroid cancer: molecular pathogenesis and emerging
therapies. Endocr Relat Cancer. 16:17–44. 2009. View Article : Google Scholar
|
|
2
|
He H, Jazdzewski K, Li W, Liyanarachchi S,
Nagy R, Volinia S, Calin GA, Liu CG, Franssila K, Suster S, et al:
The role of microRNA genes in papillary thyroid carcinoma. Proc
Natl Acad Sci USA. 102:19075–19080. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pallante P, Visone R, Ferracin M, Ferraro
A, Berlingieri MT, Troncone G, Chiappetta G, Liu CG, Santoro M,
Negrini M, et al: MicroRNA deregulation in human thyroid papillary
carcinomas. Endocr Relat Cancer. 13:497–508. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nikiforova MN, Tseng GC, Steward D, Diorio
D and Nikiforov YE: MicroRNA expression profiling of thyroid
tumors: biological significance and diagnostic utility. J Clin
Endocrinol Metab. 93:1600–1608. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aherne ST, Smyth PC, Flavin RJ, Russell
SM, Denning KM, Li JH, Guenther SM, O'Leary JJ and Sheils OM:
Geographical mapping of a multifocal thyroid tumour using genetic
alteration analysis and miRNA profiling. Mol Cancer. 7:892008.
View Article : Google Scholar
|
|
6
|
Kim HJ, Kim YH, Lee DS, Chung J-K and Kim
S: In vivo imaging of functional targeting of miR-221 in papillary
thyroid carcinoma. J Nucl Med. 49:1686–1693. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mitomo S, Maesawa C, Ogasawara S, Iwaya T,
Shibazaki M, Yashima-Abo A, Kotani K, Oikawa H, Sakurai E, Izutsu
N, et al: Downregulation of miR-138 is associated with
overexpression of human telomerase reverse transcriptase protein in
human anaplastic thyroid carcinoma cell lines. Cancer Sci.
99:280–286. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Keutgen XM, Filicori F, Crowley MJ, Wang
Y, Scognamiglio T, Hoda R, Buitrago D, Cooper D, Zeiger MA,
Zarnegar R, et al: A panel of four miRNAs accurately differentiates
malignant from benign indeterminate thyroid lesions on fine needle
aspiration. Clin Cancer Res. 18:2032–2038. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chou CK, Yang KD, Chou FF, Huang CC, Lan
YW, Lee YF, Kang HY and Liu RT: Prognostic implications of miR-146b
expression and its functional role in papillary thyroid carcinoma.
J Clin Endocrinol Metab. 98:E196–E205. 2013. View Article : Google Scholar
|
|
10
|
Visone R, Russo L, Pallante P, De Martino
I, Ferraro A, Leone V, Borbone E, Petrocca F, Alder H, Croce CM and
Fusco A: MicroRNAs (miR)-221 and miR-222, both overexpressed in
human thyroid papillary carcinomas, regulate p27Kip1
protein levels and cell cycle. Endocr Relat Cancer. 14:791–798.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Petrocca F, Vecchione A and Croce CM:
Emerging role of miR-106b-25/miR-17-92 clusters in the control of
transforming growth factor beta signaling. Cancer Res.
68:8191–8194. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim Y-K, Yu J, Han TS, Park SY, Namkoong
B, Kim DH, Hur K, Yoo MW, Lee HJ, Yang HK and Kim VN: Functional
links between clustered microRNAs: Suppression of cell-cycle
inhibitors by microRNA clusters in gastric cancer. Nucleic Acids
Res. 37:1672–1681. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu X, Chen Z, Zhao X, Wang J, Ding D, Wang
Z, Tan F, Tan X, Zhou F, Sun J, et al: MicroRNA-25 promotes cell
migration and invasion in esophageal squamous cell carcinoma.
Biochem Biophys Res Commun. 421:640–645. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang H, Zuo Z, Lu X, Wang L, Wang H and
Zhu Z: MiR-25 regulates apoptosis by targeting Bim in human ovarian
cancer. Oncol Rep. 27:594–598. 2012.
|
|
15
|
Kan T, Sato F, Ito T, Matsumura N, David
S, Cheng Y, Agarwal R, Paun BC, Jin Z, Olaru AV, et al: The
miR-106b-25 polycistron, activated by genomic amplification,
functions as an oncogene by suppressing p21 and Bim.
Gastroenterology. 136:1689–1700. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Visone R, Pallante P, Vecchione A,
Cirombella R, Ferracin M, Ferraro A, Volinia S, Coluzzi S, Leone V,
Borbone E, et al: Specific microRNAs are downregulated in human
thyroid anaplastic carcinomas. Oncogene. 26:7590–7595. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Esposito F, Tornincasa M, Pallante P,
Federico A, Borbone E, Pierantoni GM and Fusco A: Down-regulation
of the miR-25 and miR-30d contributes to the development of
anaplastic thyroid carcinoma targeting the polycomb protein EZH2. J
Clin Endocrinol Metab. 97:E710–E718. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
19
|
Irizarry RA, Hobbs B, Collin F,
Beazer-Barclay YD, Antonellis KJ, Scherf U and Speed TP:
Exploration, normalization, and summaries of high density
oligonucleotide array probe level data. Biostatistics. 4:249–264.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lewis BP, Shih IH, Jones-Rhoades MW,
Bartel DP and Burge CB: Prediction of mammalian microRNA targets.
Cell. 115:787–798. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lim LP, Lau NC, Garrett-Engele P, Grimson
A, Schelter JM, Castle J, Bartel DP, Linsley PS and Johnson JM:
Microarray analysis shows that some microRNAs downregulate large
numbers of target mRNAs. Nature. 433:769–773. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bartel DP: MicroRNAs: target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brennecke J, Stark A, Russell RB and Cohen
SM: Principles of microRNA-target recognition. PLoS Biol.
3:e852005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu YH, Kuo HK and Chang KW: The evolving
transcriptome of head and neck squamous cell carcinoma: a
systematic review. PLoS One. 3:e32152008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kurokawa A, Nagata M, Kitamura N, Noman
AA, Ohnishi M, Ohyama T, Kobayashi T, Shingaki S and Takagi R;
Oral, Maxillofacial Pathology, and Surgery Group: Diagnostic value
of integrin alpha3, beta4, and beta5 gene expression levels for the
clinical outcome of tongue squamous cell carcinoma. Cancer.
112:1272–1281. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cariati M, Naderi A, Brown JP, Smalley MJ,
Pinder SE, Caldas C and Purushotham AD: Alpha-6 integrin is
necessary for the tumourigenicity of a stem cell-like subpopulation
within the MCF7 breast cancer cell line. Int J Cancer. 122:298–304.
2008. View Article : Google Scholar
|
|
27
|
Gerson KD, Maddula VSRK, Seligmann BE,
Shearstone JR, Khan A and Mercurio AM: Effects of β4 integrin
expression on microRNA patterns in breast cancer. Biol Open.
1:658–666. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cuenda A: Mitogen-activated protein kinase
kinase 4 (MKK4). Int J Biochem Cell Biol. 32:581–587. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fagin JA and Mitsiades N: Molecular
pathology of thyroid cancer: diagnostic and clinical implications.
Best Pract Res Clin Endocrinol Metab. 22:955–969. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang L, Pan Y and Dai JL: Evidence of MKK4
pro-oncogenic activity in breast and pancreatic tumors. Oncogene.
23:5978–5985. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xin W, Yun KJ, Ricci F, Zahurak M, Qiu W,
Su GH, Yeo CJ, Hruban RH, Kern SE and Iacobuzio-Donahue CA:
MAP2K4/MKK4 expression in pancreatic cancer: genetic validation of
immunohistochemistry and relationship to disease course. Clin
Cancer Res. 10:8516–8520. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chiariello M, Visconti R, Carlomagno F,
Melillo RM, Bucci C, de Franciscis V, Fox GM, Jing S, Coso OA,
Gutkind JS, et al: Signalling of the Ret receptor tyrosine kinase
through the c-Jun NH2-terminal protein kinases (JNKS): evidence for
a divergence of the ERKs and JNKs pathways induced by Ret.
Oncogene. 16:2435–2445. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mahalingam D, Szegezdi E, Keane M, de Jong
S and Samali A: TRAIL receptor signalling and modulation: are we on
the right TRAIL? Cancer Treat Rev. 35:280–288. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ashkenazi A and Dixit VM: Apoptosis
control by death and decoy receptors. Curr Opin Cell Biol.
11:255–260. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mitsiades N, Poulaki V, Tseleni-Balafouta
S, Koutras DA and Stamenkovic I: Thyroid carcinoma cells are
resistant to FAS-mediated apoptosis but sensitive to tumor necrosis
factor-related apoptosis-inducing ligand. Cancer Res. 60:4122–4129.
2000.PubMed/NCBI
|
|
36
|
Ohtsuka T, Buchsbaum D, Oliver P, Makhija
S, Kimberly R and Zhou T: Synergistic induction of tumor cell
apoptosis by death receptor antibody and chemotherapy agent through
JNK/p38 and mitochondrial death pathway. Oncogene. 22:2034–2044.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Söderström TS, Poukkula M, Holmström TH,
Heiskanen KM and Eriksson JE: Mitogen-activated protein
kinase/extracellular signal-regulated kinase signaling in activated
T cells abrogates TRAIL-induced apoptosis upstream of the
mitochondrial amplification loop and caspase-8. J Immunol.
169:2851–2860. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Razumilava N, Bronk SF, Smoot RL, Fingas
CD, Werneburg NW, Roberts LR and Mott JL: miR-25 targets
TNF-related apoptosis inducing ligand (TRAIL) death receptor-4 and
promotes apoptosis resistance in cholangiocarcinoma. Hepatology.
55:465–475. 2012. View Article : Google Scholar
|
|
39
|
Newsom-Davis T, Prieske S and Walczak H:
Is TRAIL the holy grail of cancer therapy? Apoptosis. 14:607–623.
2009. View Article : Google Scholar : PubMed/NCBI
|