Introduction
Chronic infection with hepatitis B virus (HBV) not
only greatly increases the risk of developing hepatic fibrosis,
hepatic sclerosis and hepatocellular carcinoma, but is also
associated with damage to several other extra-hepatic organs
(1). Many types of extra-hepatic
manifestations have been observed in patients with both acute and
chronic hepatitis with HBV infection (1). Renal involvement is among the most
common extra-hepatic manifestation and usually manifests in the
form of immune complex-mediated nephropathy, such as membranous
glomerulonephritis, membranoproliferative glomerulonephritis and
immunoglobulin A nephropathy (2–4).
To date, tubular and interstitial damage has
attracted much attention, although glomerular damage has been
proven to be the main pathological characteristic of HBV-associated
glomerulonephritis (HBV-GN). It has been reported that inflammatory
cell infiltration and tubulointerstitial fibrosis are observed in
patients with HBV-GN, and HBV DNA and RNA have been identified in
renal tubular cells. Thus, tubulointerstitial damage may play an
important role in HBV-GN (5,6).
The HBV X protein (HBx), a 17-kDa protein, is the
smallest open reading frame in the HBV genome, and it is located at
1374–1838 bp of the HBV genome. The overall length is 435 to 462
bp, and the code length is of a protein containing 154 amino acids.
HBx is a multifunctional protein and it activates multiple cellular
signal transduction pathways and regulates apoptosis. A number of
studies have suggested that HBx activates the nuclear factor-κB
(NF-κB), Janus kinase/signal transducers and activators of
transcription (JAK/STAT), Ras-Raf-mitogen-activated protein kinase
(MAPK), p38 MAPK, c-Jun N-terminal kinase (JNK), phosphoinositide
3-kinase (PI3K) and the Src tyrosine kinase signaling pathways
(7–11) to induce host cell apoptosis.
Recently, HBx was detected in renal tissues, mainly
in tubular epithelial cells (7).
Wang et al reported that HBx induced epithelial-mesenchymal
transition (EMT) and immunity disorder in renal tubule epithelial
cells through the Notch1 pathway (12). However, the transforming growth
factor-β (TGF-β) (13,14) pathway plays an important role in
renal EMT, and its downstream factors, such as Smads, p38,
extracellular signal-regulated kinase (ERK), PI3K and NF-κB also
play an important role in EMT. Thus, in the present study, we aimed
to determine the role of HBx in HBV-GN-induced renal EMT and to
elucidate the potential underlying mechanisms.
For this purpose, we successfully transfected HBx
plasmid into human renal proximal tubule epithelial cells (HK-2
cells), and determined that HBx promotes renal EMT through the
activation of NF-κB phosphorylation, but not through that of other
TGF-β downstream factors.
Materials and methods
HBx plasmid construction
HBx plasmid was provided by GeneCopoeia (Guangzhou,
China). Full-length HBx was PCR-amplified from the p1.2II plasmid
(HBV adr genome). The forward primer was 5′-GCGGTAGGCGTGTACGGT-3
and the reverse primer was 5′-GTGGCACCTTCCAGGGTC-3′. These were
synthesized and inserted into the pEZ-M09 vector GeneCopoeia). An
empty pEZ-M09 was used as a control. The PCR amplification protocol
consisted of an initial 4-min denaturation at 94°C, 25 cycles of
denaturation at 94°C (1 min each), annealing at 60°C for 1 min,
extension at 72°C for 1 min and a final extension at 72°C for 10
min. All ligated vectors were confirmed by sequence analysis. The
stable selection marker was neomycin.
Cell culture and treatment
HK-2 cells (a kind gift from Dr Huiyao Lan, Li Ka
Shing Institute of Health Sciences, Department of Chemical
Pathology, The Chinese University of Hong Kong, Hong Kong, China)
were cultured in DMEM-Ham's medium supplemented with 10% fetal
bovine serum (FBS) (both from Gibco, Life Techologies, Grand
Island, NY, USA). Cells at approximately 60% confluence were used
for the in vitro experiments.
The HBx plasmid and empty plasmid (pEZ-M09) were
mixed with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA)
separately and transfected into the cells following serum
starvation for 12 h at various concentrations (0, 0.5, 1.0, 1.5 and
2.0 µg/ml). The cells were cultured in medium without FBS
and harvested at different time points following transfection.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was prepared from the HK-2 cells using
TRIzol reagent, according to the manufacturer's instructions
(Invitrogen). The RNA concentration was calculated using a Nanodrop
ND1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE,
USA). Aliquots of each RNA extraction were reverse-transcribed
simultaneously into cDNA using the One-Step RT-PCR kit (Takara,
Tokyo, Japan) according to the manufacturer's instructions. Each
qPCR reaction was performed in a total volume of 25 µl in
duplicate using the SYBR® Premix Ex Taq™ kit (Takara)
and the Fast Real-Time PCR system 7500 (Applied Biosystems Inc.,
Foster City, CA, USA). The sequences of the primer pairs are listed
in Table I. The thermal cycling
conditions comprised 30 sec at 95°C, followed by 95°C for 5 sec and
60°C for 34 sec for 40 cycles with melting curve analysis. The
relative quantification of each gene was calculated following
normalization to GAPDH mRNA using the 2−ΔΔCT method.
 | Table IThe sequences of the primer pairs used
for RT-PCR. |
Table I
The sequences of the primer pairs used
for RT-PCR.
| Primer | Sequence |
|---|
| Human E-cadherin |
| Forward |
5′-CTCAGTGTTTGCTCGGCGTTTGC-3′ |
| Reverse |
5′-GCTCTGGGTTGGATTCAGAG-3′ |
| Human collagen I |
| Forward |
5′-ACGTCCTGGTGAAGTTGGTC-3′ |
| Reverse |
5′-ACCAGGGAAGCCTCTCTCTC-3′ |
| Human α-SMA |
| Forward |
5′-CATCACCAACTGGGACGACATGGAA-3′ |
| Reverse |
5′-GCATAGCCCTCATAGATGGGGACATTG-3′ |
| Human
fibronectin |
| Forward |
5′-TCCTTGCTGGTATCATGGCAG-3′ |
| Reverse |
5′-AGACCCAGGCTTCTCATACTTGA-3′ |
| Human GAPDH |
| Forward |
5′-GCTGGCGCTGAGTACGTCGTGGAGT-3 |
| Reverse |
5′-CACAGTCTTCTGGGTGGCAGTGATGG-3′ |
Western blot analysis
Western blot analysis was performed as previously
described (15). Briefly, total
protein was extracted from the cells by lysis in 500 µl of
buffer containing Nonidet P-40 (10%), Tris-HCl (25 mM), NaCl (150
mM), ethylenediaminetetraacetic acid (EDTA) (10 mM) and a 1:50
dilution of a protease inhibitor cocktail (Sigma, St. Louis, MO,
USA) for 30 min on ice. The cell lysates were centrifuged at 12,000
× g for 15 min (4°C). Cell lysates were heated at 95°C and
separated on sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) gels. Transferred membranes were
immunoblotted with the following primary antibodies, respectively:
anti-E-cadherin (610181; BD Biosciences, Franklin Lakes, NJ, USA),
anti-α-smooth muscle actin (α-SMA; A5228-200), anti-fibronectin
(F364B, Sigma), anti-HBx (ab235, Abcam, Cambridge, NK),
anti-collagen I (234167; Calbiochem, San Diego, CA, USA);
anti-neomycin (myc; #2276), anti-phosphorylated NF-κB ((#3033), and
anti-NF-κB (#3032); anti-p-Smad2 (#9510), anti-p-Smad3 (#9520),
anti-Smad2 (#5339), anti-Smad3 (#9523), anti-p-p38 (#4511),
anti-p38 (#8690), anti-p-PI3K (#4228), anti-PI3K (#4249),
anti-p-ERK (#4370) and anti-ERK (#9102) (all from Cell Signaling
Technology Inc., Beverly, MA, USA).
Following extensive washing, the membranes were
incubated with the secondary antibodies (anti-mouse IgG, #7076 and
anti-rabbit IgG, #4414; Cell Signaling Technology Inc.).
Immobilized antibodies were then detected using an Odyssey detector
(LI-COR Biosciences, Lincoln, NE, USA).
Immunofluorescence staining
Immunofluorescence staining was performed as
previously described (16).
Briefly, the cells cultured on cover slips were fixed,
permeabilized with 0.5% Triton X-100, and incubated with the
primary antibodies overnight at 4°C, followed by incubation with
secondary antibodies (anti-mouse IgG, #7076; anti-rabbit IgG #4414;
Cell Signaling Technology Inc.) conjugated to Alexa Fluor 488 or
588 (Invitrogen). The cells were then counterstained with
4′,6-diamidino-2-phenylindole (DAPI) to visualize the nuclei.
Images were acquired using a confocal microscope (Olympus Corp.,
Tokyo, Japan).
Statistical analysis
Data are expressed as the means ± SD. Comparisons
between 2 groups were conducted using a two-tailed t-test.
Comparisons between multiple groups was made using one-way ANOVA
followed by the Student-Newman-Keuls test. A value of p<0.05 was
considered to indicate a statistically significant difference.
Results
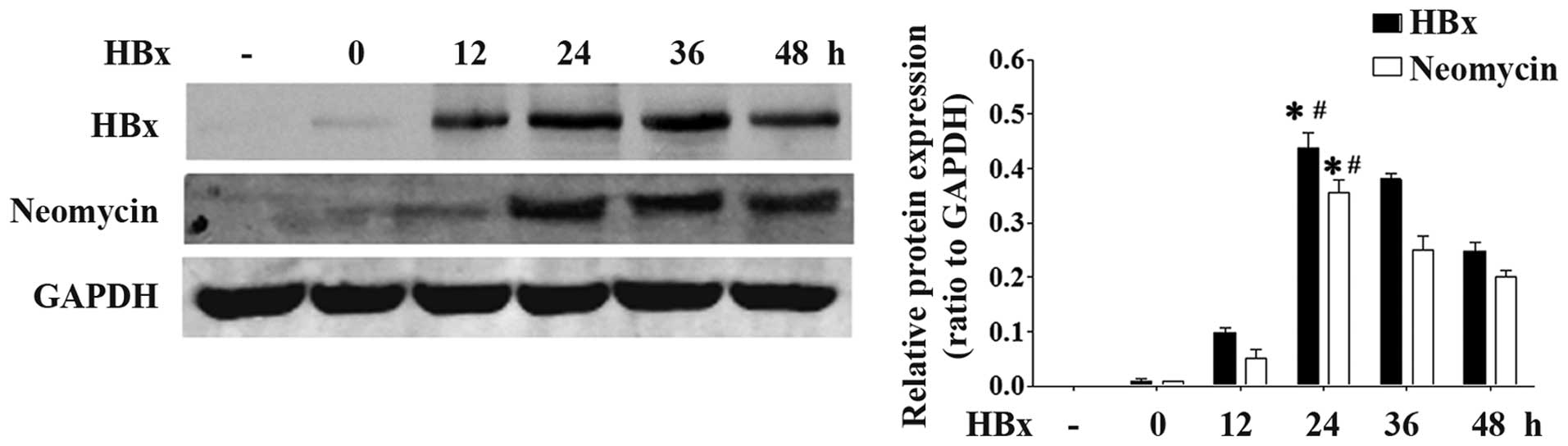
HBx gene is successfully expressed in
HK-2 cells following transfection
We first examined whether the HBx plasmid was
successfully transfected into the HK-2 cells. The levels of both
HBx (17 kDa) and neomycin (17 kDa) were markedly increased at 24 h
following co-transfection with the HBx plasmid (Fig. 1). These data suggested that the
HBx plasmid was successfully transfected into the HK-2 cells.
HBx induces renal EMT
We then examined whether HBx affects EMT-related
gene expression. As shown in Figs.
2 and 3, transfection with
the lower concentrations (0.5–1.0 µg/ml; Fig. 2) of the HBx plasmid and at the
earlier time points (12 and 24 h; Fig. 3) downregulated E-cadherin mRNA and
protein expression in the HK-2 cells in a concentration- and
time-dependent manner. Statistical significance was reached at the
concentration of 1.0 µg/ml and at the 24-h time point.
Transfection with the higher concentrations (1.5 and 2.0
µg/ml) of HBx and at the later time points (36 and 48 h)
slightly increased E-cadherin expression. At the same time,
transfection with the HBx plasmid upregulated the mRNA and protein
expression of α-SMA, collagen I and fibronectin in a concentration-
and time-dependent manner, again at the lower concentrations
(Fig. 2) and earlier time points
(Fig. 3). Statistical
significance was reached at the concentration of 1.0 µg/ml
and at the 24-h time point. At the higher concentrations and later
time points, there was a slight decrease in the levels of α-SMA,
collagen I and fibronectin (Figs.
2 and 3).
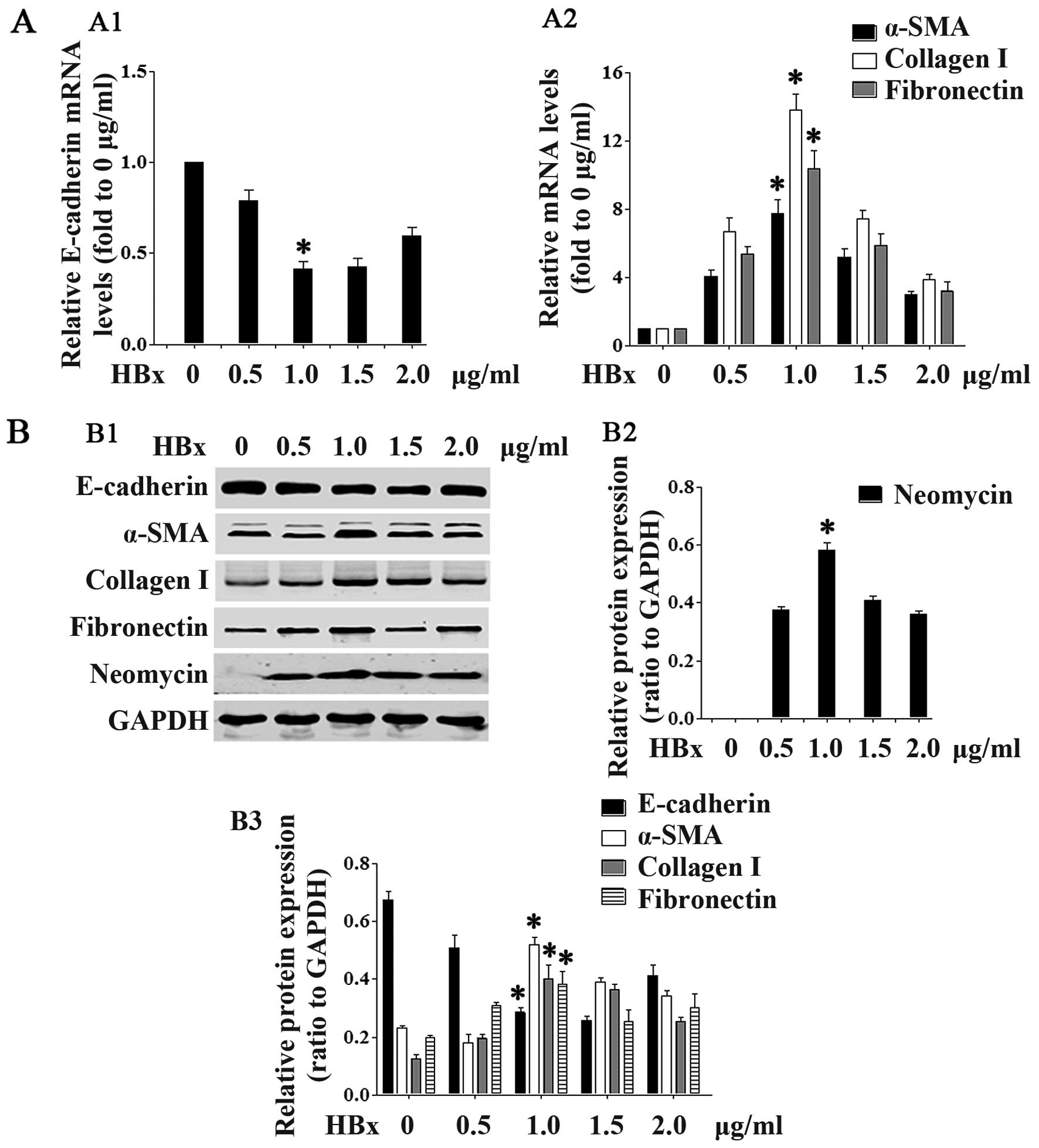 | Figure 2HBV X protein (HBx) protein promotes
renal epithelial-mesenchymal transition (EMT). In
concentration-dependent experiments, HK2 cells were transfected
with the pEZ-M09-HBx plasmid at the concentrations of 0.5, 1.0, 1.5
or 2.0 µg/ml. (A) Cells were collected 24 h after
transfection and RT-PCR was performed to detect the mRNA expression
of (A1) E-cadherin, and (A2) α-smooth muscle actin (α-SMA),
collagen I and fibronectin. (B) Cells were collected 36 h after
transfection, and western blot analyses were performed to detect
the protein expression of neomycin, E-cadherin, α-SMA, collagen I
and fibronectin. (B1) Representative blots showing the expression
of neomycin, E-cadherin, α-SMA, collagen I and fibronectin. (B2)
Quantification of neomycin. (B3) Quantification of E-cadherin,
α-SMA, collagen I and fibronectin. Data are expressed as the means
± SD of 3 independent experiments. *p<0.05 vs. 0
µg/ml groups in (A1, A2, B2 and B3), as shown by ANOVA. |
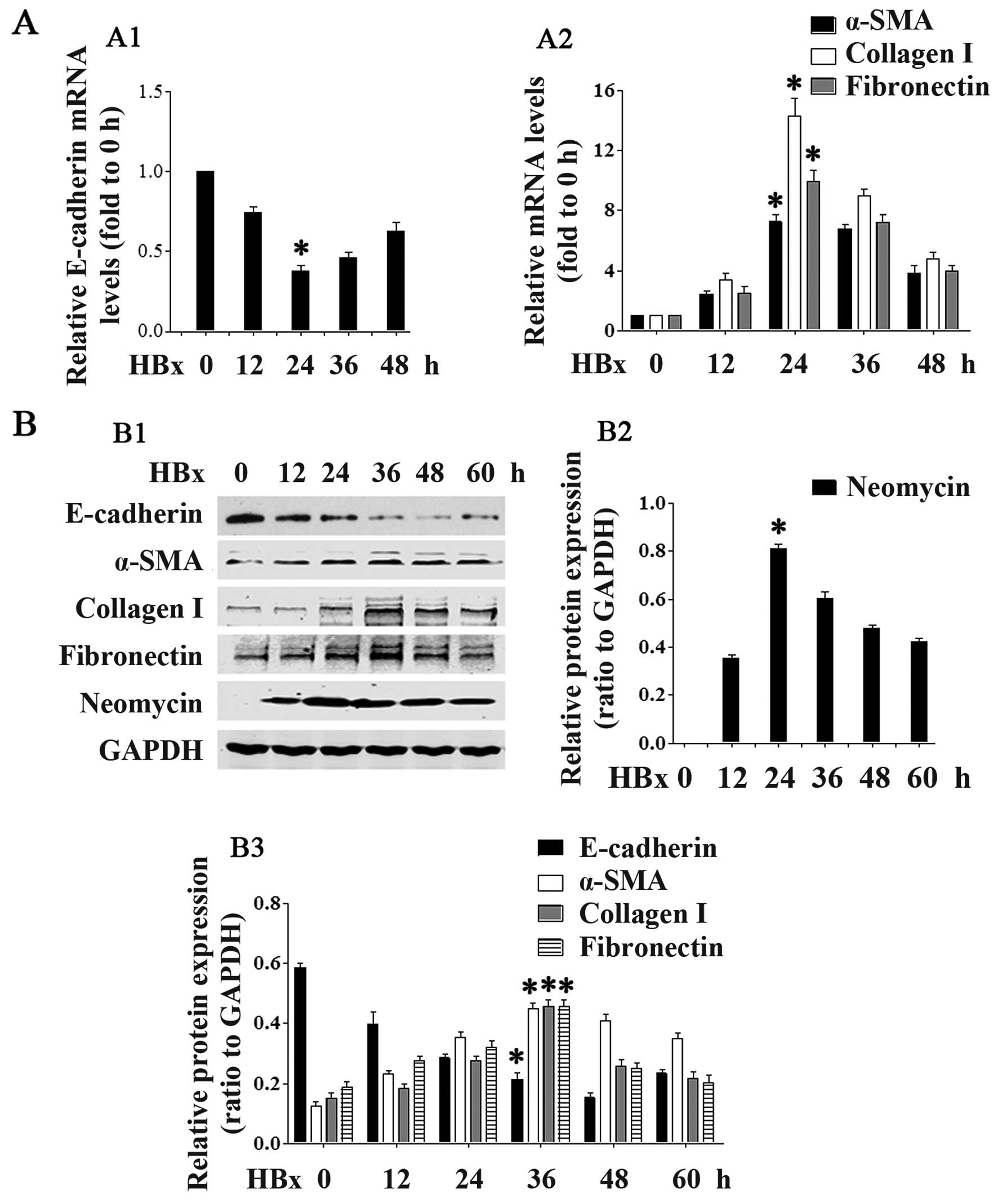 | Figure 3HBV X protein (HBx) promotes renal
epithelial-mesenchymal transition (EMT). In time-dependent
experiments, HK2 cells were transfected with the pEZ-M09-HBx
plasmid (1.0 µg/ml) for the indicated periods of time. (A)
Cells were collected and RT-PCR was performed to detect the mRNA
expression of (A1) E-cadherin, and (A2) α-smooth muscle actin
(α-SMA), collagen I and fibronectin. (B) Cells were collected and
western blot analyses were performed to detect the protein
expression of neomycin, E-cadherin, α-SMA, collagen I and
fibronectin. (B1) Representative blots showing the expression of
neomycin, E-cadherin, α-SMA, collagen I and fibronectin. (B2)
Quantification of neomycin. (B3) Quantification of E-cadherin,
α-SMA, collagen I and fibronectin. Data are expressed as the means
± SD of 3 independent experiments. *p<0.05 vs. 0 h
groups in (A1, A2, B2 and B3), as shown by ANOVA. |
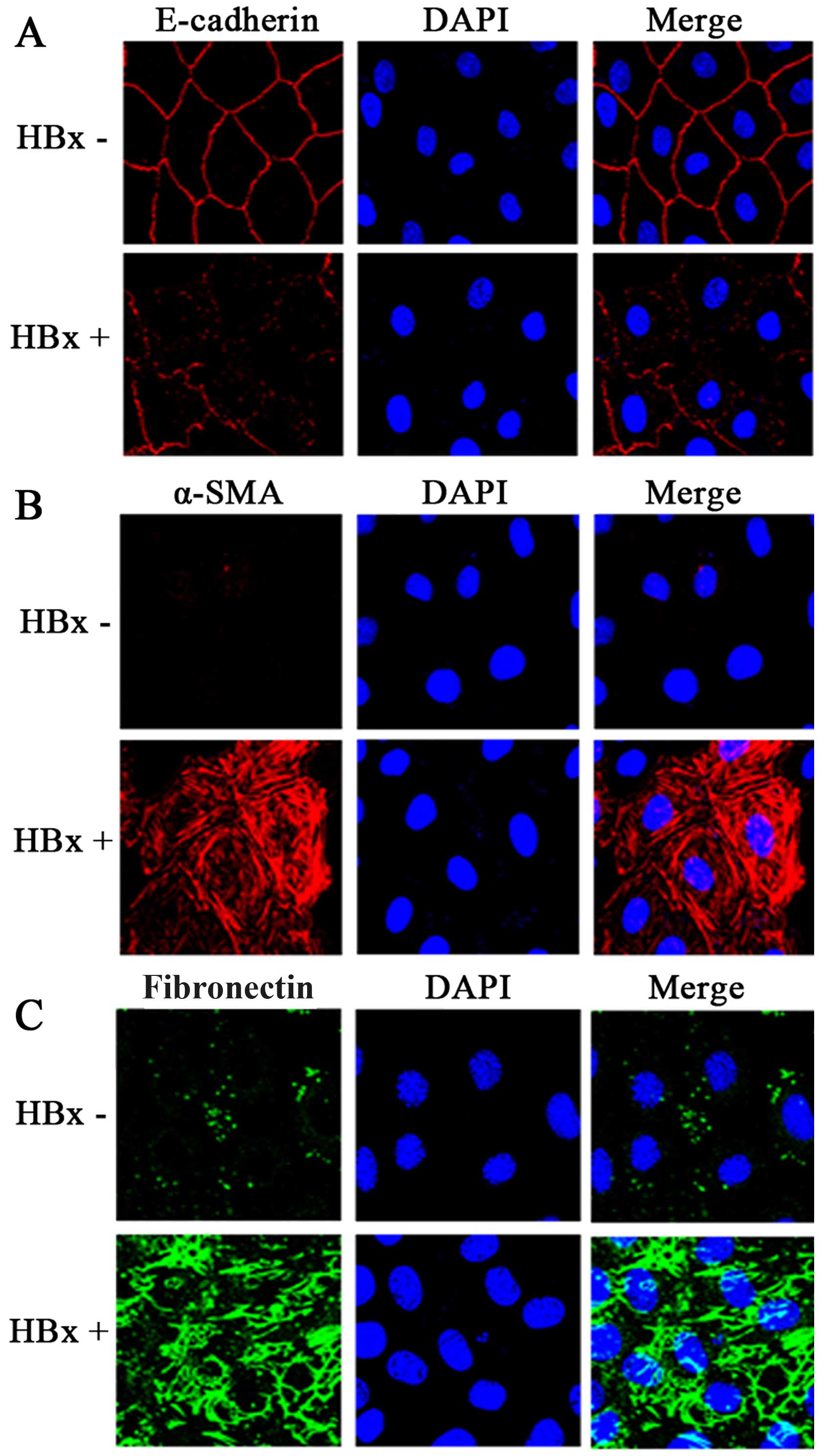
As shown by confocal immunofluorescence microscopy,
transfection with the HBx plasmid induced complete EMT in the HK-2
cells (as evidenced by the loss of E-cadherin expression, and the
strong expression of α-SMA and fibronectin) (Fig. 4).
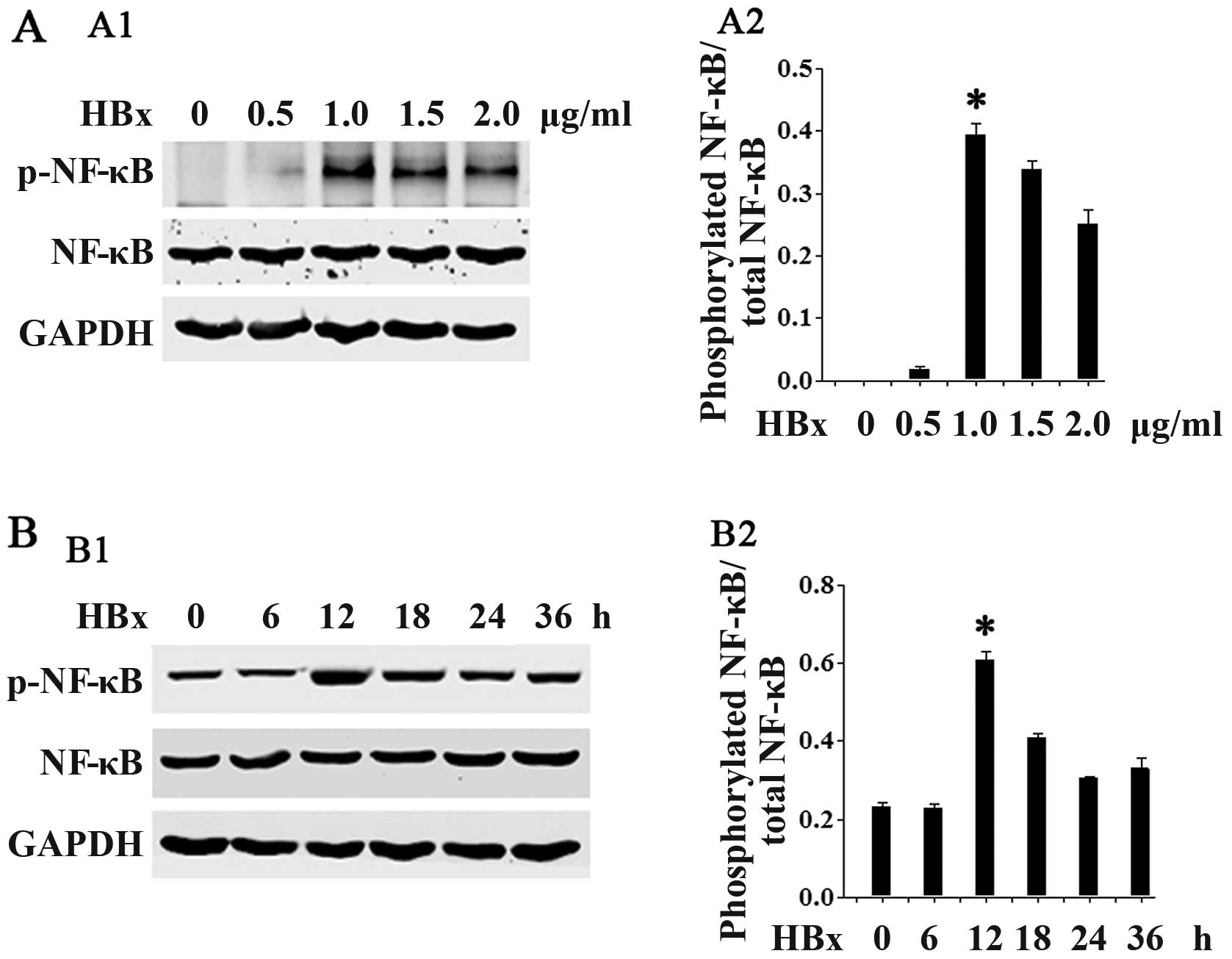
HBx increases NF-κB phosphorylation
We then examined the potential molecular mechanisms
responsible for the effects of HBx in renal fibrosis. Given the
critical role of NF-κB activation in renal fibrosis, we
hypothesized that HBx may be able to affect the phosphorylation of
NF-κB. As shown in Fig. 5,
transfection with HBx at the lower concentrations (0.5–1.0
µg/ml; Fig. 5A) and at the
earlier time points (6–12 h; Fig.
5B) increased NF-κB phosphorylation in a concentration- and
time-dependent manner. Statistical significance was reached at the
concentration of 1.0 µg/ml and at the 12-h time point. At
the higher concentrations (1.5–2.0 µg/ml) and later time
points (18–36 h), there was a slight decrease in NF-κB
phosphorylation.
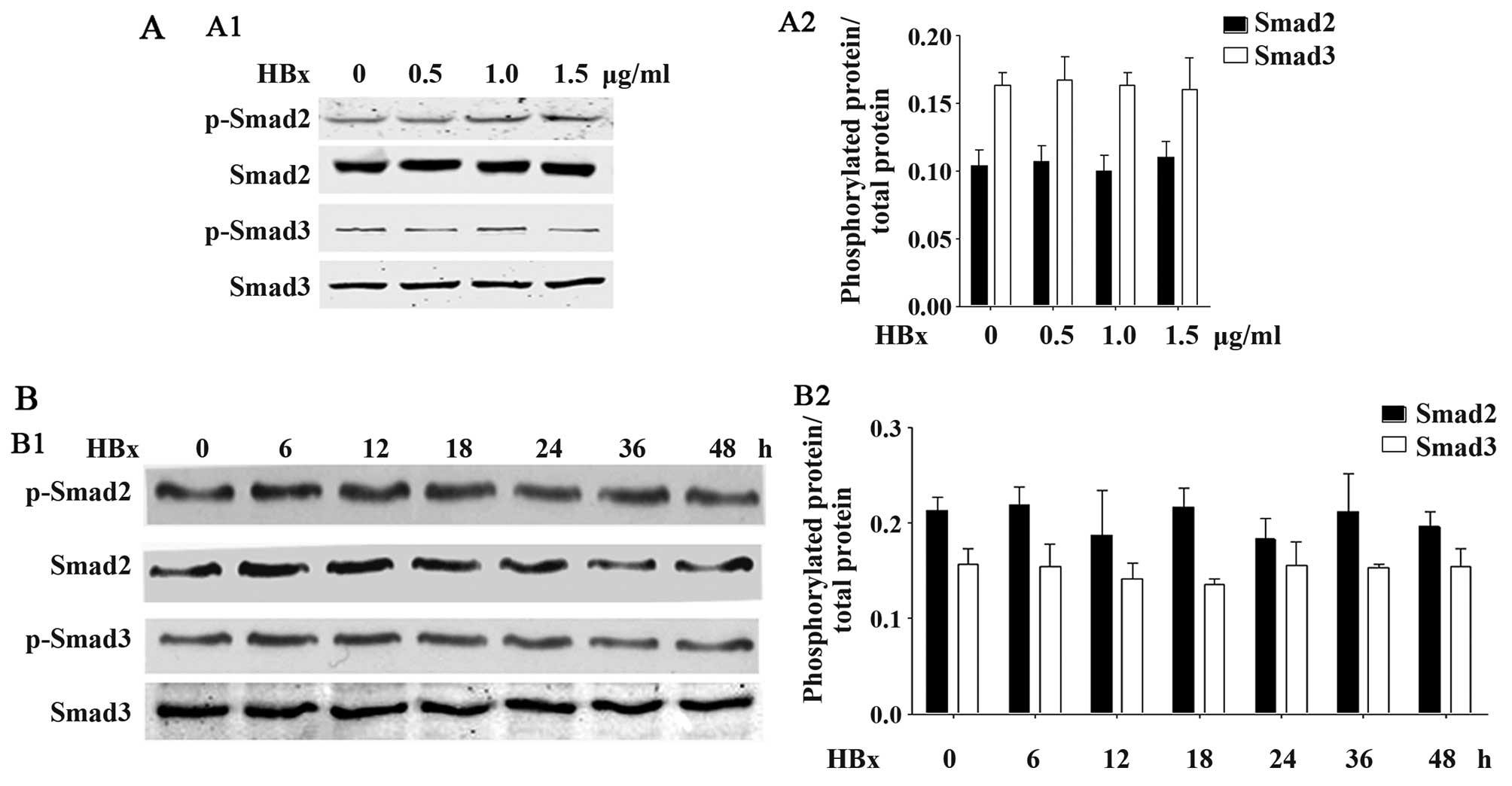
HBx does not affect TGF-β1-associated
Smad2, Smad3, p38, PI3K or ERK phosphorylation
We further examined whether HBx affects the other
typical phosphorylation-associated signaling pathways, such as
Smad2, Smad3, p38, PI3K and ERK. As shown in Fig. 6, HBx did affect Smad2 or Smad3
phosphorylation in the HK-2 cells. No significant differences were
observed at any of the concentrations of HBx (Fig. 6A) or at any time point (Fig. 6B). Furthermore, as shown in
Fig. 7, HBx was also unable to
affect the phosphorylation of p38, PI3K or ERK, at any of the
concentrations of HBx (Fig. 7A)
or at any time point (Fig. 7B).
These data suggested that HBx did not affect the phosphorylation of
associated signaling pathways.
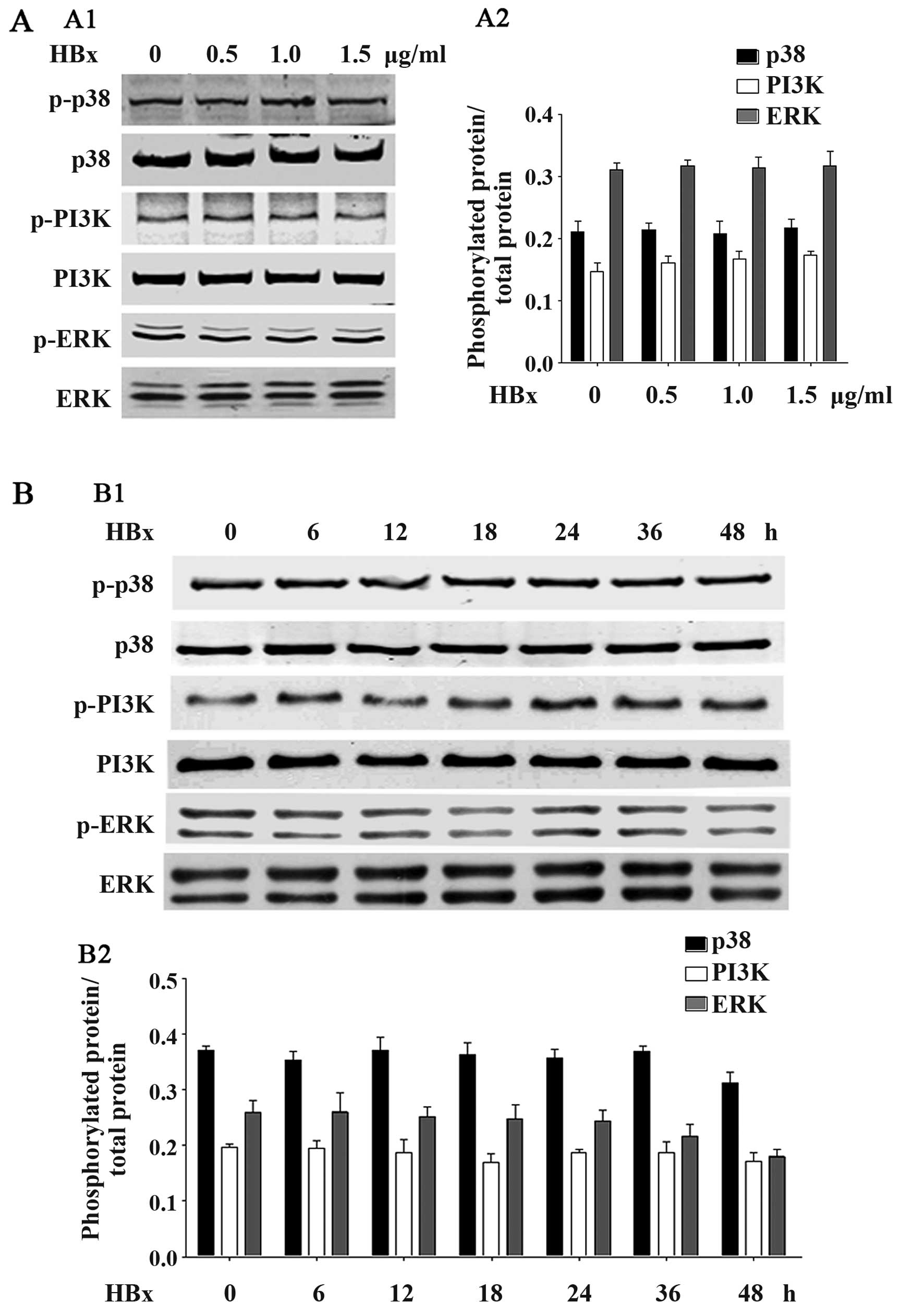 | Figure 7HBV X protein (HBx) protein does not
affect p38, PI3K or ERK phoshorylation. (A) HK2 cells were
transfected with the pEZ-M09-HBx plasmid at the dose of 0.5, 1.0 or
1.5 µg/ml, respectively. Cells were collected and western
blot analyses were performed to detect the levels of
phosphorylation of phosphorylated p38, total p38, phosphorylated
PI3K, total PI3K, phosphorylated ERK and total ERK. (B) HK2 cells
were transfected with the pEZ-M09-HBx plasmid (1.0 µg/ml)
for 6, 12, 18, 24, 36 or 48 h. Cells were collected and western
blot analyses were performed to detect the levels of phosphorylated
p38, total p38, phosphorylated PI3K, total PI3K, phosphorylated ERK
and total ERK. Data are expressed as the means ± SD of 3
independent experiments. |
Discussion
In the present study, we demonstrated that HBx, a
17-kDa protein, significantly promoted renal EMT in renal HK-2
cells. Furthermore, HBx may promote renal EMT by increasing the
phosphorylation of NF-κB.
HBV-GN has been recognized as the most prevalent
extra-hepatic manifestation caused by HBV infection (1–4).
Its pathogenesis has not yet been completely clarified. HBV-DNA,
covalently closed circular DNA (cccDNA) and even complete viral
particles (17,18) have been found in the kidneys of
patients with HBV-GN, supporting the view that HBV can directly
infect the kidneys and in situ reproduction to cause
diseases. Over the years, research has focused on the existence and
significance of HBV-related nucleic acid molecules in nephridial
tissue, such as HBeAg, HBsAg and HBcAg (19–23). Recently, HBx was detected in renal
tissues, mainly in tubular epithelial cells from patients with
HBV-GN (5). In this study, we
showed that HBx was highly expressed in HK-2 cells following
transfection with HBx plasmid.
In liver cells, HBx is the most important
determinant of viral pathogenesis, and it can modulate the
transcriptional activation of AP-1 and NF-κB (8,24),
activate the Ras/Raf/ERKs, PI3K-Akt and JAK/STAT signaling pathways
(7), promote cellular
proliferation (25), affect
apoptosis (26) and enhance the
invasive potential (27) of
infected cells. However, the influence of HBx in renal cells
remains far from being completely understood. Renal fibrosis,
characterized by massive interstitial myofibroblast activation and
excessive matrix protein accumulation, is the final common pathway
of virtually all types of progressive chronic kidney disease (CKD),
leading to end stage renal disease (ESRD) (28). HBV-GN plays a critical role in the
progression of CKD. Thus, we were wished to determine whether HBx
plays a role in renal fibrosis. The present study demonstrated that
co-transfection with HBx plasmid markedly downregulated E-cadherin
mRNA and protein expression, and upregulated α-SMA, collagen I, and
fibronectin expression at the same time, suggesting that HBx
promotes renal EMT. Although it has been demonstrated that the
major cell component that produces extracellular matrix in
unilateral ureteral obstruction is interstitial myofibroblasts
(29), the contribution of
tubular epithelial cell injury to ECM accumulation in fibrotic
kidneys cannot be excluded (30).
Clippinger et al (8) successively researched the location
of HBx protein in the mitochondrion and its influence on liver cell
apoptosis. As a result, it was found that in liver cells, the
function of HBx in apoptosis was mainly dependent on the NF-κB
signaling pathway. Considering the imperative role of NF-κB in
mediating renal EMT (31,32), in this study, we examined the
effects of HBx on NF-κB signaling. Indeed, our findings
demonstrated that HBx markedly increased NF-κB phosphorylation in
the HK-2 cells, in a concentration- and time-dependent manner (at
the lower concentrations and earlier time points). These data
suggest that HBx may promote renal EMT through the NF-κB pathway.
As the TGF-β1 signaling has been recognized as a typical pathway in
renal fibrosis and EMT, we also examined the effects of HBx on
TGF-β1-related signaling. Of note, co-transfection with the HBx
plasmid did not affect the phosphorylation of Smad2, Smad3, p38,
ERK or PI3K, suggesting that HBx may selectively promote NF-κB
phosphorylation.
Therefore, as there is still no well-used animal
model of HBV-GN, we could not confirm these results in animal
experiments. Further studies are warranted using other types of
renal cells, as well as animal models.
In conclusion, this study demonstrated that the
activation of the NF-κB signaling pathway and changes in the levels
of EMT-associated genes are involved in the process of EMT in HK-2
cells induced by HBx. These findings demonstrate that HBx may
promote renal EMT through the activation of NF-κB. Since an
immortalized cell strain was used as the object of investigation in
the present study, it is necessary to conduct further research in
order to fully confirm our findings.
Acknowledgments
This study was supported by a grant from the Nanfang
Hospital Foundation, Southern Medical University (no. 2012C08) to
J.A.
References
|
1
|
Yi Z, Jie YW and Nan Z: The efficacy of
anti-viral therapy on hepatitis B virus-associated
glomerulonephritis: A systematic review and meta-analysis. Ann
Hepatol. 10:165–173. 2011.PubMed/NCBI
|
|
2
|
Abbas NA, Pitt MA, Green AT and Solomon
LR: Successful treatment of hepatitis B virus (HBV)-associated
membranoproliferative glomerulonephritis (MPGN) with α interferon.
Nephrol Dial Transplant. 14:1272–1275. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Looi LM and Prathap K: Hepatitis B virus
surface antigen in glomerular immune complex deposits of patients
with systemic lupus erythematosus. Histopathology. 6:141–147. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khaira A, Upadhyay BK, Sharma A, Das P,
Mahajan S, Makhariya G, Dinda AK, Agarwal SK and Tiwari SC:
Hepatitis B virus associated focal and segmental glomerular
sclerosis: Report of two cases and review of literature. Clin Exp
Nephrol. 13:373–377. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou Y, Zhu N, Wang X, Wang L, Gu LJ and
Yuan WJ: The role of the toll-like receptor TLR4 in hepatitis B
virus-associated glomerulonephritis. Arch Virol. 158:425–433. 2013.
View Article : Google Scholar
|
|
6
|
Lee WM: Hepatitis B virus infection. N
Engl J Med. 337:1733–1745. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
He P, Zhang D, Li H, Yang X, Li D, Zhai Y,
Ma L and Feng G: Hepatitis B virus X protein modulates apoptosis in
human renal proximal tubular epithelial cells by activating the
JAK2/STAT3 signaling pathway. Int J Mol Med. 31:1017–1029.
2013.PubMed/NCBI
|
|
8
|
Clippinger AJ, Gearhart TL and Bouchard
MJ: Hepatitis B virus X protein modulates apoptosis in primary rat
hepatocytes by regulating both NF-kappaB and the mitochondrial
permeability transition pore. J Virol. 83:4718–4731. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arbuthnot P, Capovilla A and Kew M:
Putative role of hepatitis B virus X protein in
hepatocarcinogenesis: Effects on apoptosis, DNA repair,
mitogen-activated protein kinase and JAK/STAT pathways. J
Gastroenterol Hepatol. 15:357–368. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee YI, Kang-Park S, Do SI and Lee YI: The
hepatitis B virus-X protein activates a phosphatidylinositol
3-kinase-dependent survival signaling cascade. J Biol Chem.
276:16969–16977. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim H, Lee H and Yun Y: X-gene product of
hepatitis B virus induces apoptosis in liver cells. J Biol Chem.
273:381–385. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang X, Zhou Y, Zhu N, Wang L, Gu LJ and
Yuan WJ: The deposition of Notch1 in hepatitis B virus-associated
nephropathy and its role in hepatitis B virus X protein-induced
epithelial-mesenchymal transdifferentiation and immunity disorder
in renal tubular epithelial cells. J Viral Hepat. 21:734–743. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schnaper HW, Hayashida T and Poncelet AC:
It's a Smad world: Regulation of TGF-β signaling in the kidney. J
Am Soc Nephrol. 13:1126–1128. 2002.PubMed/NCBI
|
|
14
|
Böttinger EP and Bitzer M: TGF-β signaling
in renal disease. J Am Soc Nephrol. 13:2600–2610. 2002. View Article : Google Scholar
|
|
15
|
Hao S, Shen H, Hou Y, Mars WM and Liu Y:
tPA is a potent mitogen for renal interstitial fibroblasts: Role of
beta1 integrin/focal adhesion kinase signaling. Am J Pathol.
177:1164–1175. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fritschy JM, Benke D, Mertens S, Oertel
WH, Bachi T and Möhler H: Five subtypes of type A
gamma-aminobutyric acid receptors identified in neurons by double
and triple immunofluorescence staining with subunit-specific
antibodies. Proc Natl Acad Sci USA. 89:6726–6730. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He XY, Fang LJ, Zhang YE, Sheng FY, Zhang
XR and Guo MY: In situ hybridization of hepatitis B DNA in
hepatitis B-associated glomerulonephritis. Pediatr Nephrol.
12:117–120. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou SD, Zhang YE, Guo MY, Fang LJ, Zhang
XR, Zhang M, Wu Z, Lin SY and Liao LT: The study of the
significance of the appearance of HbcAg in glomerulonephritis. Chin
J Nephrol. 11:104–106. 1995.
|
|
19
|
Lin CY: Hepatitis B virus-associated
membraneous nephropathy: Clinical features, immunological profiles
and outcome. Nephron. 55:37–44. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hirose H, Udo K, Kojima M, Takahashi Y,
Miyakawa Y, Miyamoto K, Yoshizawa H and Mayumi M: Deposition of
hepatitis B e antigen in membranous glomerulonephritis:
Identification by F(ab′)2 fragments of monoclonal antibody. Kidney
Int. 26:338–341. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hsu HC, Lin GH, Chang MH and Chen CH:
Association of hepatitis B surface (HBs) antigenemia and membranous
nephropathy in children in Taiwan. Clin Nephrol. 20:121–129.
1983.PubMed/NCBI
|
|
22
|
Lai KN, Lai FM, Chan KW, Chow CB, Tong KL
and Vallance-Owen J: The clinico-pathologic features of hepatitis B
virus-associated glomerulonephritis. Q J Med. 63:323–333.
1987.PubMed/NCBI
|
|
23
|
Knecht GL and Chisari FV: Reversibility of
hepatitis B virus-induced glomerulonephritis and chronic active
hepatitis after spontaneous clearance of serum hepatitis B surface
antigen. Gastroenterology. 75:1152–1156. 1978.PubMed/NCBI
|
|
24
|
Lee DH, Choi BH and Rho HM: The
synergistic transactivation of the hepatitis B viral (HBV)
pregenomic promoter by the E6 protein of human papillomavirus type
16 (HPV-16 E6) with HBV X protein was mediated through the AP1 site
of E element in the enhancer I (EnI) in human liver cell. Biochem
Biophys Res Commun. 265:62–66. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ahuja R, Kapoor NR and Kumar V: The HBx
oncoprotein of hepatitis B virus engages nucleophosmin to promote
rDNA transcription and cellular proliferation. Biochim Biophys
Acta. 1853:1783–1795. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miao J, Chen GG, Chun SY and Lai PP:
Hepatitis B virus X protein induces apoptosis in hepatoma cells
through inhibiting Bcl-xL expression. Cancer Lett. 236:115–124.
2006. View Article : Google Scholar
|
|
27
|
Liu Y, Tong Z, Li T, Chen Q, Zhuo L, Li W,
Wu RC and Yu C: Hepatitis B virus X protein stabilizes amplified in
breast cancer 1 protein and cooperates with it to promote human
hepatocellular carcinoma cell invasiveness. Hepatology.
56:1015–1024. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zeisberg M and Neilson EG: Mechanisms of
tubulointerstitial fibrosis. J Am Soc Nephrol. 21:1819–1834. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Grande MT and López-Novoa JM: Fibroblast
activation and myofibroblast generation in obstructive nephropathy.
Nat Rev Nephrol. 5:319–328. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sutaria PM, Ohebshalom M, McCaffrey TA,
Vaughan ED Jr and Felsen D: Transforming growth factor-β receptor
types I and II are expressed in renal tubules and are increased
after chronic unilateral ureteral obstruction. Life Sci.
62:1965–1972. 1998. View Article : Google Scholar
|
|
31
|
Huber MA, Azoitei N, Baumann B, Grünert S,
Sommer A, Pehamberger H, Kraut N, Beug H and Wirth T: NF-kappaB is
essential for epithelial-mesenchymal transition and metastasis in a
model of breast cancer progression. J Clin Invest. 114:569–581.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Maier HJ, Schmidt-Strassburger U, Huber
MA, Wiedemann EM, Beug H and Wirth T: NF-kappaB promotes
epithelial-mesenchymal transition, migration and invasion of
pancreatic carcinoma cells. Cancer Lett. 295:214–228. 2010.
View Article : Google Scholar : PubMed/NCBI
|