Introduction
High mobility group nucleosomal binding domain 2
(HMGN2) is a member of the HMG superfamily of non-histone,
chromatin-binding proteins, which may be released into the
extracellular fluid under pathological conditions of inflammation
or cell damage (1–3). The expression of HMG proteins is
developmentally regulated. It has been well documented that
intranuclear HMG proteins are major regulators of chromosome
architecture and gene transcription. Therefore, HMGN2 has been
initially regarded as a general transcriptional activator. However,
there is increasing evidence to suggest that HMGN2 has multiple
intercellular and extracellular functions. It has been well
documented that HMGNs may specifically regulate the expression of
glycine transporter 1 (GLY1) (4),
the transcription factor heat shock protein (HSP)70 (5) and the estrogen-regulated genes TEF1
and FOS (6). β-defensin is an
important innate immune mediator and we have found previously that
endogenous HMGN2 may regulate the lipopolysaccharide (LPS)-induced
expression of β-defensin in respiratory epithelial cells (7,8).
Our previous research has shown that HMGN2 may be released by
interleukin (IL)-2- and phytohemagglutinin (PHA)-stimulated
peripheral blood mononuclear cells (PBMCs) (9). Furthermore, exogenous HMGN2 has also
been found to exert antimicrobial effects against bacteria, viruses
and fungi. In addition, our previous studies showed that exogenous
HMGN2 inhibited the invasion of Klebsiella pneumoniae into
bladder epithelial cells (10,11) and respiratory epithelial cells
(data unpublished). Therefore, HMGN2 as an HMG protein may play a
critical role in the innate immune responses induced by mucosal
pathogens.
K. pneumoniae infection is one of the most
frequent hospital-acquired infections, particularly in elderly and
immunocompromised individuals. The respiratory tract is the portal
of entry and target organ of K. pneumoniae; the persistent
localization of K. pneumoniae subsequently leads to severe
pulmonary infections second only to Pseudomonas aeruginosa
in China (12). The integrins are
a large family of αβ heterodimeric transmembrane adhesion receptors
that mediate cellular interactions with microbes. It has been
demonstrated that integrin receptors served as the most important
intermediary for the internalization of a series of bacteria by
respiratory epithelial cells, including Streptococcus
pneumoniae, Staphylococcus aureus, Streptococcus
pyogenes and P. aeruginosa (13). Therefore, modulating the
expression and activity of integrin may interfere with the ability
of bacteria to invade host cells. Moreover, our cDNA microarray
analysis showed that gene silencing of HMGN2 induced the
upregulation of α5β1 integrin in A549 cells (7). With regard to the multifunctional
role of HMGN2 in regulating the expression of genes involved in the
specific innate immune response, we aimed to determine whether the
silencing of HMGN2 promotes the internalization of K.
pneumoniae by increasing the expression of α5β1 integrin in
respiratory epithelial cells.
Materials and methods
Reagents and antibodies
Rabbit anti-human α5 integrin (ab25251) and β1
integrin (ab52971) monoclonal antibodies were purchased from Abcam
(Cambridge, UK). Talin (T3287) was purchased from Sigma-Aldrich
(Shanghai, China). HMGN2 (9437P), phospho-FAK (3284), FAK (3285);
phospho-Src (6943) and Src (2109) were purchased from Cell
Signaling Technology (Danvers, MA, USA). Rhodamine-conjugated
phalloidin, DAPI and FITC were purchased from Sigma-Aldrich.
RBITC-conjugated secondary antibody was purchased from Beyotime
(Shanghai, China). Cytochalasin B and fibronectin peptide were
acquired from Sigma-Aldrich. TRIzol reagent was obtained from
Invitrogen (Carlsbad, CA, USA). RevertAid First Strand cDNA
Synthesis kit and Maxima® SYBR-Green were obtained from
Thermo Fisher Scientific (Vilnius, Lithuania). The PCR primers were
obtained from Sangon Biotech Co., Ltd. (Shanghai, China). RPMI-1640
medium was purchased from HyClone, Thermo Scientific (Beijing,
China). Fetal bovine serum (FBS) was obtained from FuMeng Gene Co.,
Ltd. (Shanghai, China). Penicillin-streptomycin was purchased from
Beijing Solarbio Science and Technology Co., Ltd. (Beijing, China).
Other chemical reagents were all analytical grade.
Strain and cell culture
K. pneumoniae strain 33 was isolated from a
sputum sample obtained from a patient with a respiratory infection,
which was identified as K. pneumoniae by API 20E
(bioMérieux, Marcy-l'Étoile, France), at the Medical Department,
West China Hospital of Sichuan University (Chengdu, China).
Single-colony isolates of K. pneumoniae were maintained at
37°C on Luria Broth (LB) agar. To infect the epithelial cells, a
single colony was grown overnight at 37°C in LB medium, and then 50
µl of this culture was grown in LB medium for 3 h until
mid-log-phase, washed, and resuspended in phosphate-buffered saline
(PBS). The human pulmonary epithelial cell lines A549 and SPC-A-1
as well as the human bronchial epithelial cell line HBE16 from our
laboratory were cultured in RPMI-1640 containing 10% FBS and 1%
penicillin-streptomycin at 37°C, in 5% CO2.
Bacterial adhesion and invasion
assays
As described in our previous study (10), the A549, SPC-A-1 and HBE16 cells
(1×105 cells/well) were seeded in a 24-well plate and
allowed to adhere overnight. After being washed with PBS, the cells
were infected with K. pneumoniae strain 33 at a multiplicity
of infection (MOI) of 200:1 for 2 h. Non-adherent bacterial cells
were removed by washing the cells with PBS. In order to lyse the
cells, 200 µl 0.25% Triton X-100 was added to each well and
incubated at 37°C for 10 min. The cells were removed by scraping
and plated onto LB agar plates. The colonies were counted to
quantify the number of adherent bacteria. For the invasion assay,
100 µg/ml gentamicin (Sichuan Long March Pharmaceutical Co.,
Ltd., Sichuan, China) was added to kill extracellular bacteria and
the cells were incubated at 37°C for an additional 2 h. Duplicate
samples were included and the experiment was repeated 3 times. We
examined the bacterial invasion rate in all three cell lines as
well as the time course of invasion by K. pneumoniae strain
33 using HMGN2-deficient A549 cells.
RNA interference (RNAi) using small
interfering RNA (siRNA) and short hairpin RNA (shRNA) plasmid
constructs
The cells were seeded at a density of
5×105 cells/well in 6-well plates and allowed to reach
60% confluence on the day of transfection. The small interfering
RNA (siRNA) and shRNA for HMGN2 were synthesized at our laboratory
as previously described as well as shRNA control (shControl) and
siRNA control (siControl) (7).
HMGN2-overexpressing (pexHMGN2) and control (pexControl) vectors
were constructed using a pEX-1-HMGN2 vector (GenePharma. Inc,
Shanghai, China). shRNA HMGN2, 5′-GCAAAGGTGAAGGACGAACCA-3′ or shRNA
control, 5′-GCTTCGCGCCGTAGTCTTA-3′ were cloned into a psi-LVRH1GP
vector (Fulengen. Inc, Guangzhou, China). The cells were
transfected for 24 h with siRNA or shRNA plasmids using
Lipofectamine 2000 reagent in Opti-MEM medium.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The effects of siHMGN2 on the expression of the cell
integrin gene encoding α5/β1 integrin, which is critical for the
invasion of K. pne umoniae into human lung epithelial cells,
were investigated in A549 cells by RT-qPCR. Total RNA was extracted
using TRIzol reagent. cDNA synthesis was achieved using the
RevertAid First Strand cDNA Synthesis kit. The cDNA synthesized
served as the template for RT-qPCR amplification of α5/β1 integrin.
The PCR products were detected using Thermo Fisher Scientific
Maxima SYBR-Green. The primer sequences were as follows: α5
integrin forward, 5′-TGCAGTGTGAGGCTGTGTACA-3′ and reverse,
5′-GTGGCCACCTGACGCTCT-3′; and β1 integrin forward,
5′-CTCAAGCCAGAGGATATTAC-3′ and reverse, 5′-TCATTGAGTAAGACAGGTCC-3′
(14,15). The relative levels of α5/β1
integrin mRNA transcripts in different groups were evaluated using
the 2−ΔΔCt method.
Western blot analysis
Western blot analysis was performed as previously
described (10). The A549 cells
were collected and lysed in lysis buffer (1 ml containing 5
µl PMSF, 10 µl phosphatase inhibitors and 1 µl
protease inhibitors). The cell lysates were centrifuged at 13,400 ×
g for 20 min at 4°C. The total protein concentration was determined
using the Bradford assay. Equal amounts of the lysate were added
into the wells of the SDS-PAGE gel. The proteins were then
transferred onto a PVDF membrane and blocked with 5% non-fat milk
in Tris-buffered saline solution for 1 h, and incubated with the
primary antibodies overnight at 4°C. The membrane was incubated
with the secondary antibody (1:1,000) for 2 h at room temperature.
The intensity of the bands was quantified relative to corresponding
GAPDH bands with the Bio-Rad VersaDoc™ Imaging system (Berkeley,
CA, USA).
Flow cytometric analysis
The A549 or HBE16 cells were infected with K.
pneumoniae pre-treated with siRNA HMGN2 or siRNA control at an
MOI of 200:1 for 1 h at 37°C. The cells were then washed
vigorously, detected, fixed with 4% paraformaldehyde and
permeabilized with 0.1% Triton X-100. The cells were stained with
rhodamine-conjugated phalloidin for 60 min. For the α5/β1 integrin
assay, the cells were incubated with the rabbit monoclonal
antibodies at 4°C overnight, and then incubated with anti-rabbit
secondary antibody for 1 h. Flow cytometric analysis was performed
using a BD Accuri™ C6 flow cytometer (BD Biosciences, Franklin
Lakes, NJ, USA) and repeated thrice.
Immunofluorescence microscopy
The A549 cells were washed thrice in PBS, and
subsequently fixed with 4% paraformaldehyde for 30 min. The cells
were then permeabilized for 10 min in PBS containing 0.1% Triton
X-100, and washed 3 times in PBS. The cells were stained with
rhodamine-conjugated phalloidin for 1 h to visualize filamentous
actin (F-actin), and the cell nuclei were stained with
4′,6-diamidino-2-phenylindole (DAPI) for 5 min. The bacteria were
stained with FITC for 1 h. For the α5/β1 integrin stain, the cells
were incubated with the rabbit monoclonal antibodies at 4°C
overnight, then recovered at 37°C for 30 min and incubated with
anti-rabbit secondary antibody at 37°C for 30 min. The stained
cells were observed under an immunofluorescence microscope (VMF30A;
Carl Zeiss, Oberkochen, Germany).
Cell adhesion assay
A cell adhesion assay was performed as previously
described by Docheva et al (16). Briefly, a 96-well plate was coated
with 50 µg/ml fibronectin overnight at 4°C and then blocked
with 1% bovine serum albumin (BSA) powder in PBS for 1 h at 37°C.
The A549 cells (3×104 cells/well) were plated and
incubated for 2 h at 37°C. Non-adherent cells were removed by
washing with PBS. The number of adherent cells was estimated using
a cell counting kit-8 (CCK-8; purchased from Vazyme Biotech,
Shanghai, China) and the absorbance at 405 nm was measured by a
Bio-Rad iMark™ microplate reader. The percentage of adherent cells
on fibronectin was calculated to a maximal reference. Three
independent experiments were performed in triplicate.
Statistical analysis
The two-tailed Student's t-test was used to assess
the differences in values between the experimental groups. A
P-value <0.05 was considered to indicate a statistically
significant difference.
Results
Gene expression profiles associated with
integrin in HMGN2-silenced A549 cells
To explore the role of HMGN2 in the regulation of
gene transcription in respiratory epithelial cells, we employed
siRNA targeted against HMGN2 in A549 cells. In our previous study,
we used a cDNA microarray to test approximately 31,000 genes; the
expression of >4% of the genes was changed by ≥2-fold in the
HMGN2-silenced A549 cells (7).
The results of cDNA microarray analysis revealed that disruption of
HMGN2 induced the expression of 10 integrin subunits and a series
of related genes changed. Specifically, integrin alpha 5 gene
(ITGA5) was increased 1.64-fold and integrin beta 1 gene
(ITGB1) was upregulated 2.06-fold in the A549 cells depleted
of HMGN2. Furthermore, we found that the integrin-activated protein
gene integrin-linked kinase (ILK) was upregulated and the
integrin activity inhibiting proteins PTENP1 and TENS1 were
downregulated. In addition, Src kinase-associated phosphoprotein 2
(SCAP2) gene was increased 1.61-fold compared with the siRNA
control A549 cells (Table I).
These changes in gene expression profiles are associated with
cytoskeletal rearrangement, cell adhesion, migration, proliferation
and mechanical sensing.
 | Table IList of integrin and associated
partial genes with changed expression identified by microarray
analysis. |
Table I
List of integrin and associated
partial genes with changed expression identified by microarray
analysis.
| GenBank | CGAP gene
symbol | Log2 ratio CVA | Entrez gene
description |
|---|
| NM_014288.3 | ITGB3BP | 0.916241 | Integrin beta 3
binding protein (beta3-endonexin) |
| NM_002214.1 | ITGB8 | 0.9928003 | Integrin beta
8 |
| NM_002211.2 | ITGB1 | 1.042064 | Integrin beta 1
(fibronectin receptor, beta polypeptide, antigen CD29 includes
MDF2) |
| NM_002203.2 | ITGA2 | 1.3149265 | Integrin alpha 2
(CD49B, alpha 2 subunit of VLA-2 receptor) |
| NM_000211.1 | ITGB2 | 1.5798765 | Integrin beta 2
(antigen CD18 (p95), lymphocyte function-associated antigen 1) |
| NM_000889.1 | ITGB7 | 0.437597 | Integrin beta
7 |
| NM_002210.2 | ITGAV | 0.712578 | Integrin alpha V
(vitronectin receptor, alpha polypeptide, antigen CD51) |
| AH008066.2 | ITGA6 | 4.42582719 | Integrin alpha
6 |
| NM_002207.2 | ITGA9 | 0.7271413 | Integrin alpha
9 |
| NM_004791.2 | ITGBL1 | 0.7609444 | Homo sapiens
integrin subunit beta like 1 |
| BU738798 | ILK | 0.2331936 | Integrin-linked
kinase |
| NM_003930.3 | SCAP2 | 0.683518 | Src
kinase-associated phosphoprotein 2 |
| NM_032214.2 | SLA2 | 0.1621274 | Src-like-adaptor
2 |
| NM_020209.2 | SHD | −1.2045916 | Src homology 2
domain containing transforming protein D |
| NM_000261.1 | MYOC | 2.2054772 | Myocilin,
trabecular meshwork inducible glucocorticoid response |
| NM_023940.2 | RASL11B | 2.3479829 | RAS-like, family
11, member B |
| NM_005406.1 | ROCK1 | −2.461461 | Rho-associated,
coiled-coil containing protein kinase 1 |
| NM_004333.2 | BRAF | −2.31846 | v-raf murine
sarcoma viral oncogene homolog B1 |
| BC038293 | PTENP1 | −1.166008 | Phosphatase and
tensin homolog (mutated in multiple advanced cancers 1), pseudogene
1 |
| NM_022748.6 | TENS1 | −1.0666966 | Tensin-like SH2
domain containing 1 |
| NM_006289.2 | TLN1 | −0.527023 | Talin 1 |
| NM_015059.1 | TLN2 | −0.1582942 | Talin 2 |
HMGN2 gene knockdown increases the
internalization of K. pneumoniae strain 33 by pulmonary and
bronchial epithelial cells
HMGN2 has been suggested to significantly inhibit
the internalization of K. pneumoniae by bladder epithelial
cells (10). In addition, HMGN2
may generally or specifically modulate gene expression. Herein, we
examined whether disrupting the expression of HMGN2 affected the
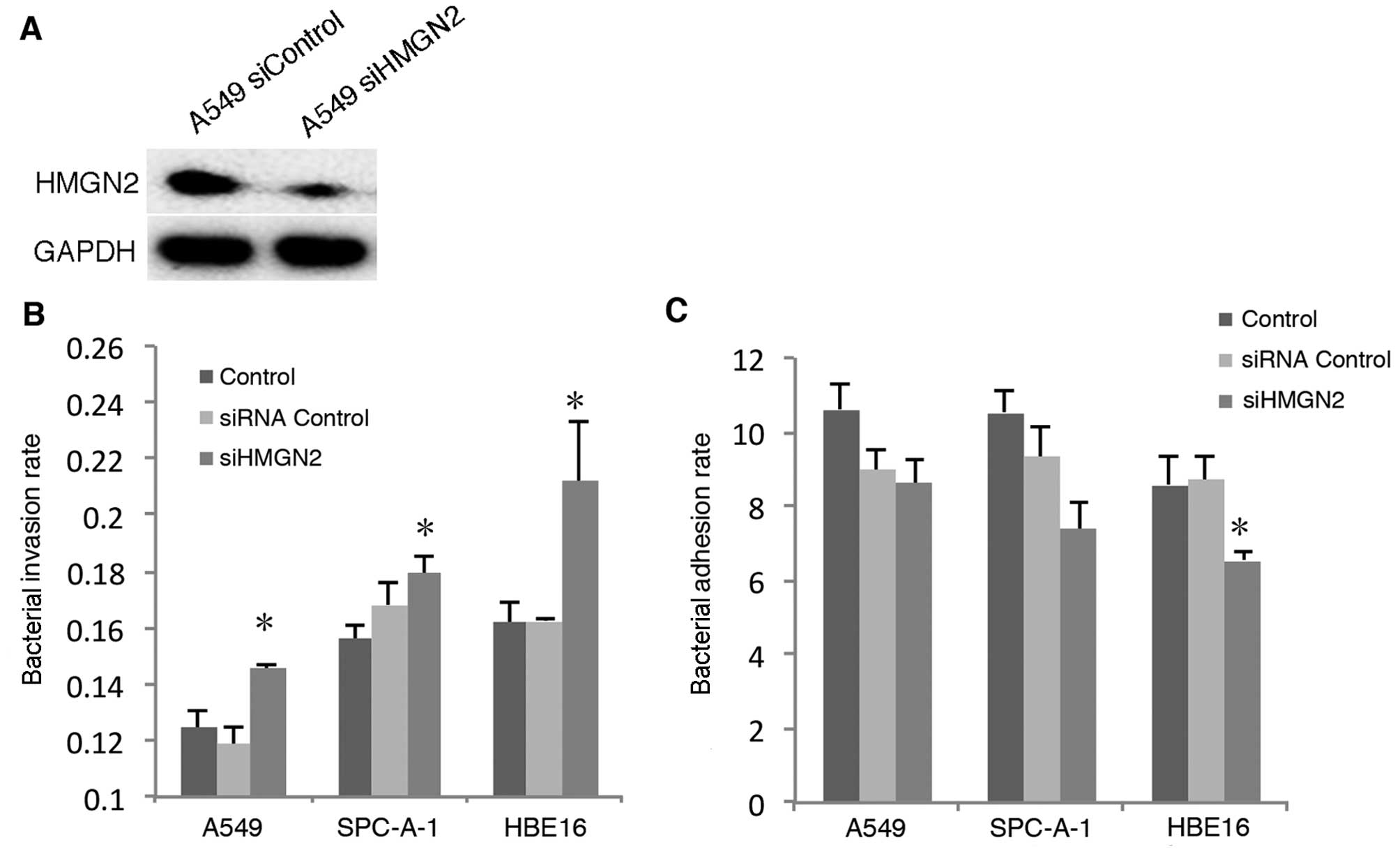
ability of the host cell to interact with bacteria. We used siRNA
to silence the expression of HMGN2 in the following cell lines:
A549 (Fig. 1A); SPC-A-1 and HBE16
(data not shown).
K. pneumoniae internalization by the
epithelial cells was examined using a bacterial invasion assay. The
cells were co-incubated with K. pneumoniae strain 33 at an
MOI of 200:1 for 2 h, and the bacterial adhesion and invasion rates
were detected, respectively. The results showed that the pulmonary
epithelial cells (A549 and SPC-A-1) and the bronchial epithelial
cells (HBE16) depleted of HMGN2 demonstrated enhanced
internalization of K. pneumoniae strain 33 when compared
with the controls (Fig. 1B). The
results indicate that depleted HMGN2 induced the internalization of
K. pneumoniae strain 33 by respiratory epithelial cells.
However, the adhesion of K. pneumoniae strain 33 to HBE16
cells was decreased (Fig.
1C).
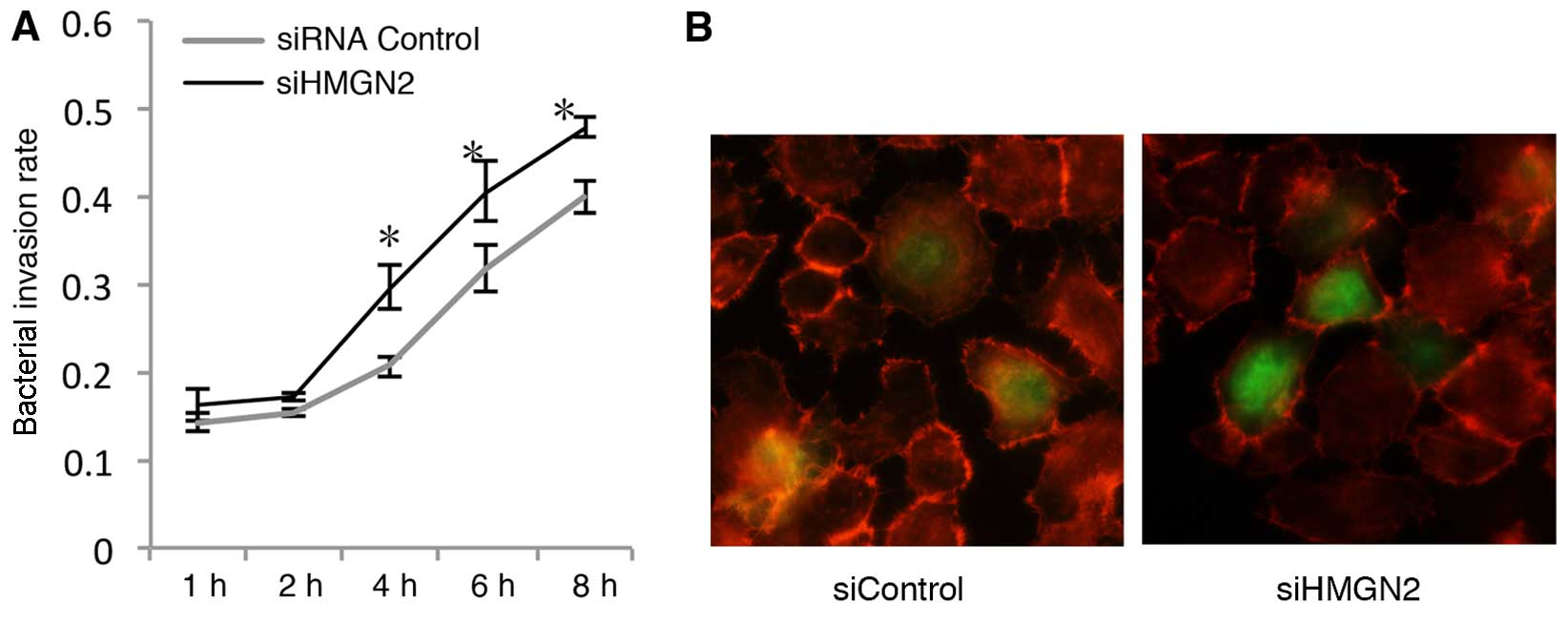
Invasion kinetics of K. pneumoniae strain
33 in A549 cells transfected with HMGN2 RNAi
In order to further explore bacterial
internalization by respiratory epithelial cells, we examined the
time course of invasion by K. pneumoniae strain 33 using
HMGN2 RNAi in A549 cells. The A549 cells were infected with K.
pneumoniae strain 33 at an MOI of 200:1, and the numbers of
intracellular K. pneumoniae bacteria were quantified at
various time intervals (Fig. 2A).
Bacterial internalization by HMGN2-deficient A549 epithelial cells
was completed after a 1-h incubation. The bacterial invasion rate
increased in a time-dependent manner, and the rate in the HMGN2
siRNA group was always higher in comparison with that in the
control group at each time point. In this assay, at 6 h a bacterial
invasion rate of 0.405±0.035 bacteria per cell was detected in the
HMGN2 siRNA group, which was 2.5-fold higher than the bacterial
invasion rate at 1 h.
To further confirm the increased intracellular
presence of bacteria by silencing HMGN2 in the cells,
HMGN2-silenced A549 monolayers were infected with FITC-labeled
K. pneu-moniae strain 33 at an MOI of 200:1 at 37°C for 2 h.
We showed that the bacteria interacted with the cells using
immunofluorescence microscopy (Fig.
2B).
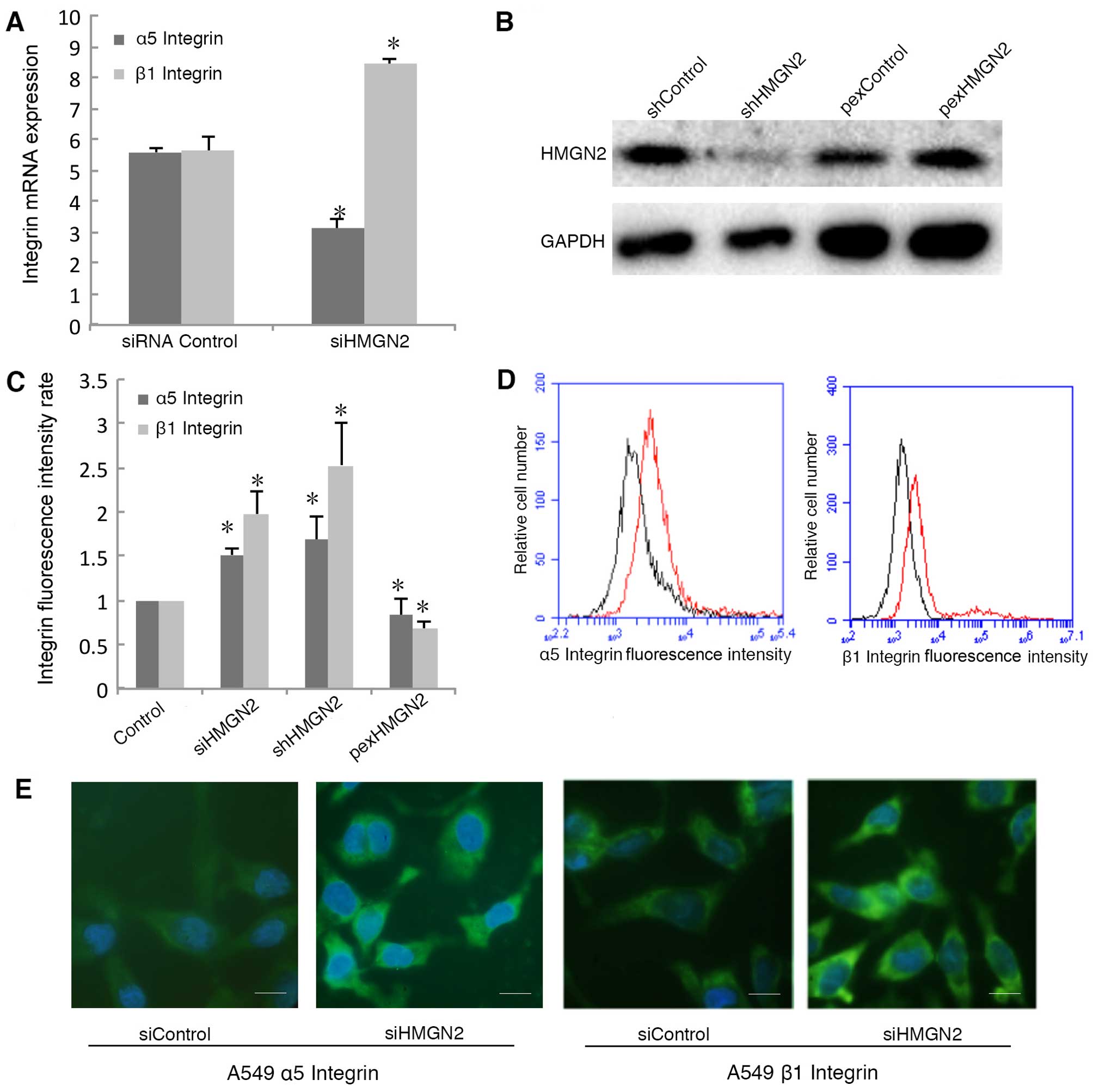
Silencing HMGN2 mediates the upregulation
of α5β1 integrin on cell membranes
To elucidate the mechanism responsible for the
induction of bacterial internalization by HMGN2-silenced A549
cells, we measured α5β1 integrin mRNA levels in the HMGN2-silenced
A549 cells. Silencing HMGN2 in the A549 cells increased the mRNA
expression of β1 integrin, whereas it decreased the mRNA expression
of α5 integrin (Fig. 3A). We used
shRNA to silence the expression of HMGN2 and an overexpression
plasmid to overexpress HMGN2 in A549 cells (Fig. 3B). To explore the functions of
integrins on the cell membrane, we examined the expression of α5β1
integrin on the surface of A549 cells using flow cytometry. α5 and
β1 integrin proteins located on the cell surface were also studied
by employing an RBITC-conjugated secondary antibody, which emits
green fluorescence (FL1-positive; green). The expression of α5
integrin was upregulated in the HMGN2-deficient A549 cells both by
siRNA and shRNA silencing, and the expression of β1 integrin was
also higher when compared with the siRNA control A549 cells
(Fig. 3C and D). Overexpression
of HMGN2 downregulated α5 integrin and β1 integrin protein
expression on the cell membrane. In addition, α5/β1 integrin was
detected on the cell membrane by fluorescence microscopy (Fig. 3E).
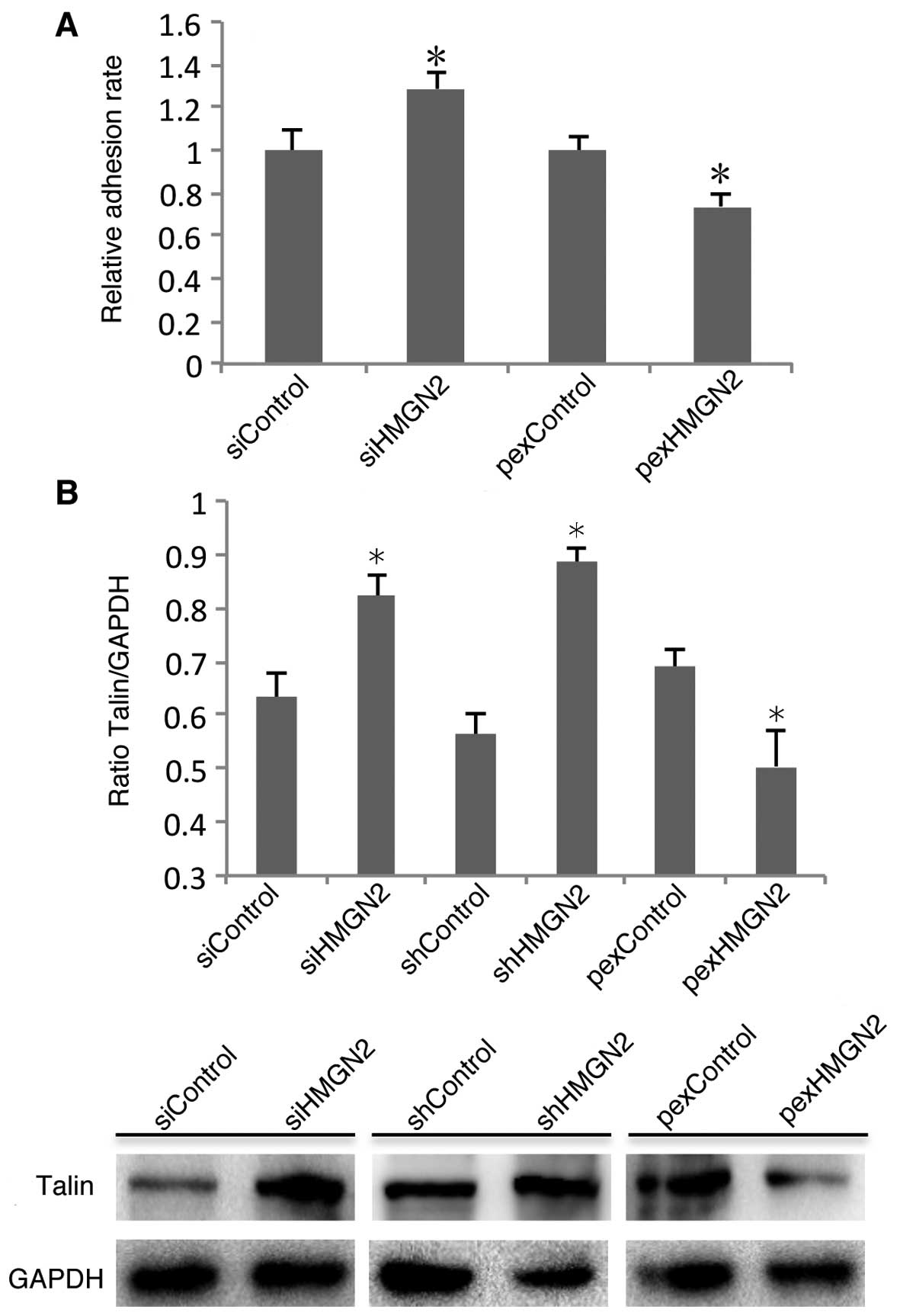
Talin is involved in silencing
HMGN2-induced α5β1 integrin activity
Integrin activity is a key factor which determines
the host cell interaction with pathogens. Integrin activation is
determined by the conformation of α and β subunits on the cell
membrane, which are regulated by inside-out activation (17). Having established that silencing
HMGN2 induces the internalization of K. pneumoniae by
respiratory epithelial cells, we next examined whether it is due to
effects on α5β1 integrin activity. Fibronectin is known to be an
important integrin ligand existing in the extracellular matrix
(ECM). Therefore, we examined the ability of A549 cells to adhere
to fibronectin. The cell adhesion assay indicated that HMGN2
knockdown in the A549 cells induced the adhesion of α5β1 integrin
to fibronectin (Fig. 4A). It has
been reported that talin plays an important role in α5β1 integrin
activation (18). To examine the
role of talin in the HMGN2-mediated regulation of α5β1 integrin
expression and activity, western blot analysis was used to examine
the protein levels of talin. The results showed that the
transfection of cells with HMGN2 siRNA or shRNA increased talin
expression, and the overexpression of HMGN2 in A549 cells
suppressed talin expression (Fig.
4B).
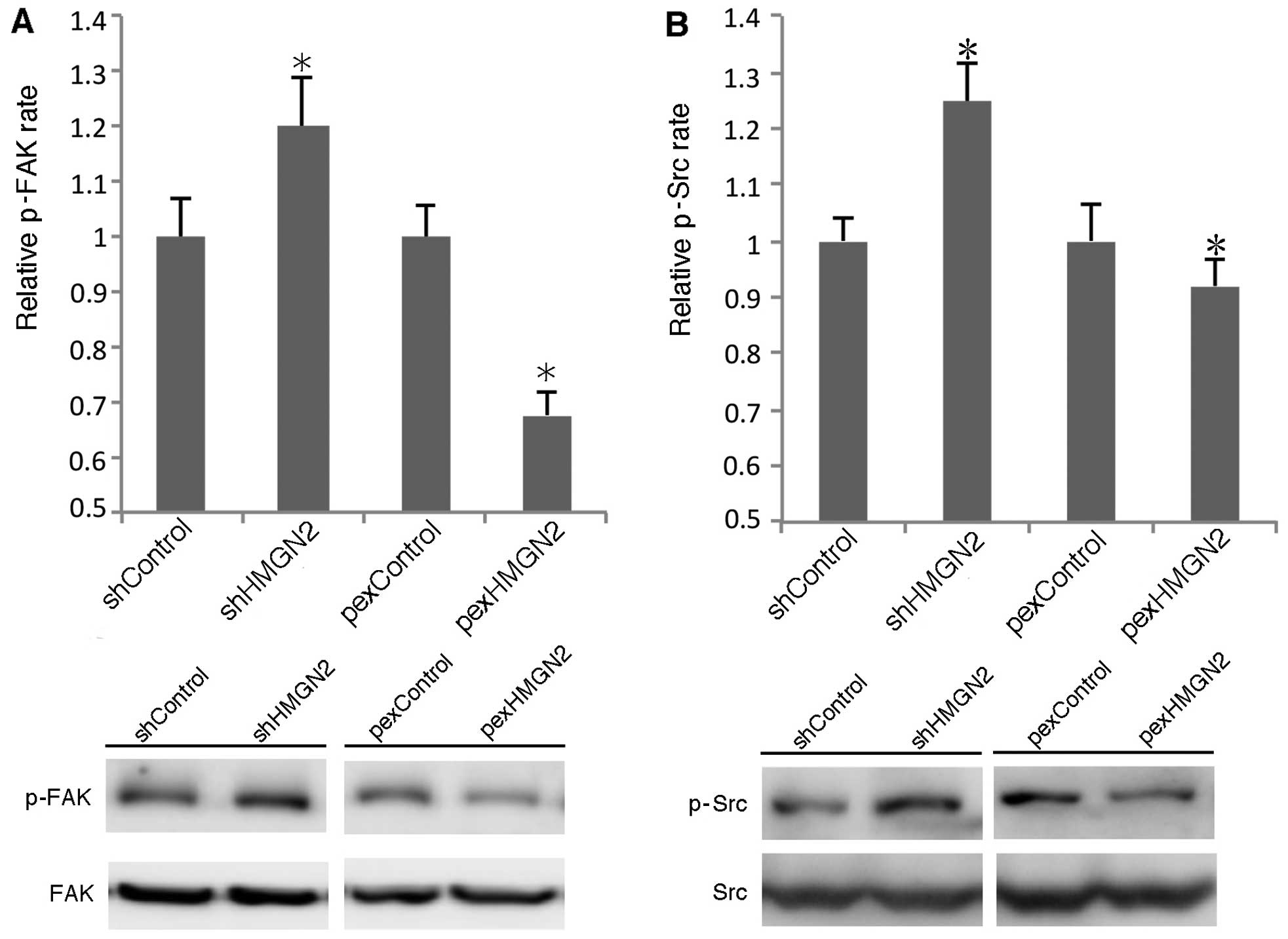
FAK/Src signaling pathway is involved in
silencing HMGN2 in A549 cells
FAK/Src may be recruited to cell membranes and
phosphorylated by activated α5β1 integrin. We examined whether
silencing HMGN2 enhanced FAK/Src activation. As shown in Fig. 5, knockdown of HMGN2 using shRNA
induced the phosphorylation of FAK and Src by >20 and 25%,
respectively. The overexpression of HMGN2 in the A549 cells
decreased the phosphorylation levels of FAK and Src. These data
indicate that silencing HMGN2 in A549 cells induces the activation
of FAK and Src.
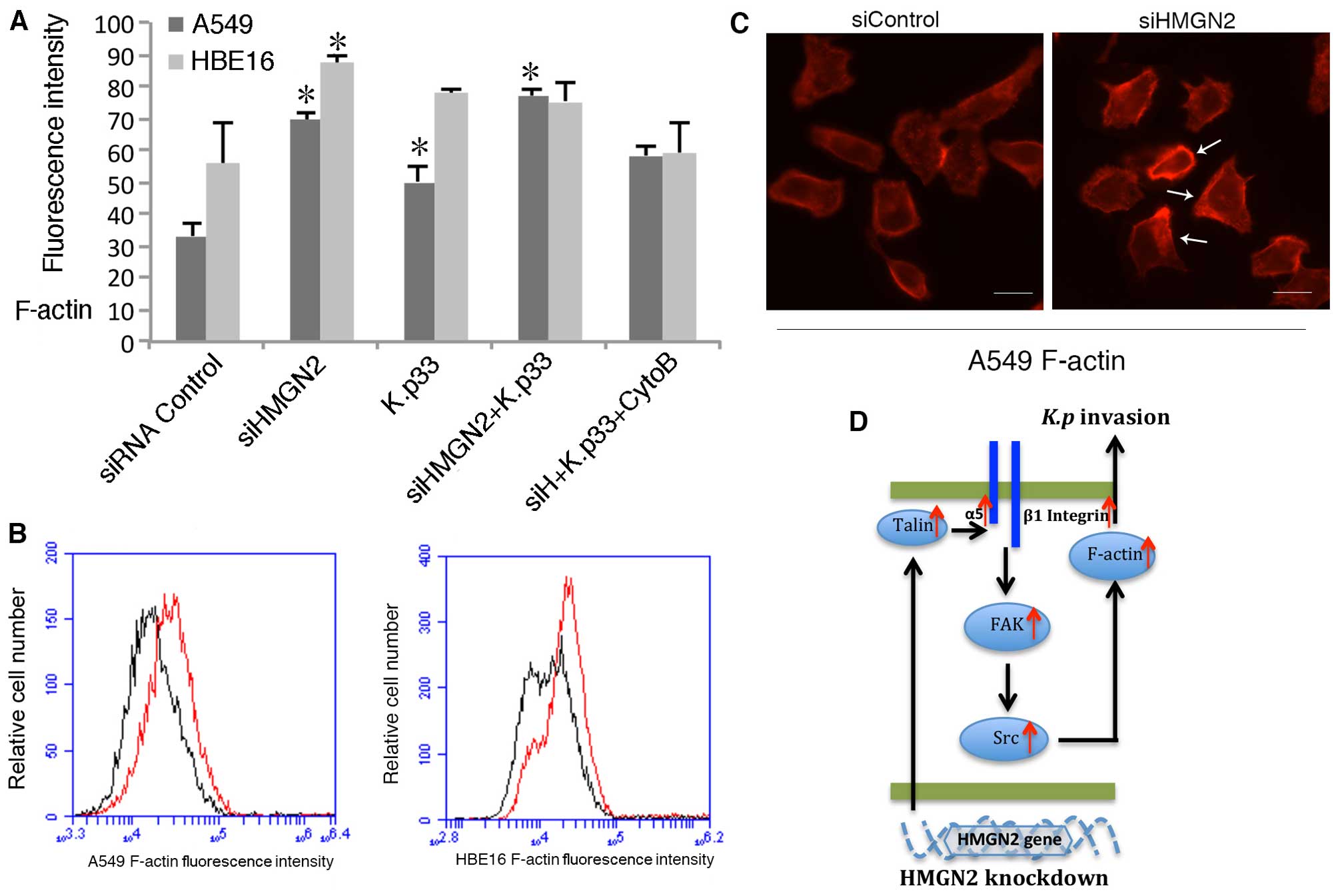
Knockdown of endogenous HMGN2 induces
actin cytoskeleton reorganization
Microfilament polymerization is an important process
for most bacteria entering eukaryotic cells. Our previous studies
(10,11) revealed that K. pneumoniae
infection induces actin polymerization. To examine the
polymerization of actin, respiratory epithelial cells were
transfected with HMGN2 siRNA for 24 h, and as a positive control,
the cells were pre-treated with 10 µg/ml cytochalasin B, an
inhibitor of actin rearrangement. The cells were then incubated
with K. pneumoniae stain 33 for 2 h at 37°C, and analyzed
using flow cytometry. As shown in Fig. 6A and B, K. pneumoniae 33
infection induced actin polymerization and HMGN2-silenced A549 and
HBE16 cells showed significantly higher amounts of F-actin. The
data indicates that the depletion of HMGN2 promoted the assembly of
actin. Morphological evidence was obtained by fluorescence
microscopy. Staining of the cells with rhodamin-conjugated
phalloidin revealed that well-organized actin filaments were more
clear in the HMGN2-silenced A549 cells (Fig. 6C).
Discussion
The airway epithelium represents a primary site for
contact between microbes and their hosts. Entry into epithelial
cells is considered to be an important strategy for microorganisms
to escape immune clearance (19).
Our previous research has suggested that exogenous HMGN2
significantly inhibits the internalization of K. pneumoniae
by bladder epithelial cells (10). However, the role of endogenous
HMGN2 in the regulation of K. pneumoniae internalization
remains unclear. In the present study, we firstly investigated
whether endogenous HMGN2 was involved in the internalization of
K. pneumoniae by respiratory epithelial cells. We found that
the transfection of pulmonary and bronchial cells with HMGN2 siRNA
induced the increased internalization of K. pneumoniae
strain 33; however, the decreased adhesion of K. pneumoniae
strain 33 to the bronchial epithelial HBE16 cells was observed. The
results suggested that endogenous HMGN2 may also be engaged in the
epithelial cells to anti-microbial invasion process of the
respiratory tract, which is conducive to the innate immune defenses
as well as beneficial for the elimination of bacteria.
The adhesion of bacteria to mucosal and epithelial
surfaces is often the first stage in host cell invasion. In
general, it has been revealed that the adhesion of bacteria to
respiratory epithelial cells may promote bacterial cell invasion
(20,21). However, we observed that the K.
pneumoniae adhesion rate was not significantly altered or even
reduced following HMGN2 knockdown in respiratory epithelial cells,
which indicated that the increased K. pneumoniae invasion
rate induced by HMGN2 knockdown in respiratory epithelial cells is
not affected by the adhesion rate.
Adhesion and invasion are both mutually interrelated
and independent complex processes. The internalization of bacteria
by epithelial cells is not only associated with the adhesion of
bacteria to the cell membrane receptors, but it is also associated
with the intracellular signals that regulate the cell surface
receptors and cytoskeleton polymerization state (22,23). Cue et al (24) have reported that a nonpeptide
integrin antagonist may inhibit S. pyogenes invasion whereas
it increases bacterial adhesion to epithelial cells through
blocking α5β1 integrin. Takagi et al (17) and Vinogradova et al
(25) developed the 'inside-out'
integrin signaling model, which stated that the intracellular
signal may influence the conformation and ligand-binding affinity
of the extracellular domain of integrins. This 'inside-out' signal
transduction appears to be mediated through the integrin
cytoplasmic domains. Our cDNA microarray results showed that α5 and
β1 integrin were upregulated in the HMGN2-silenced A549 cells
(7). Therefore, we hypothesized
that the upregulated expression of α5β1 integrin, induced by HMGN2
knockdown, mediated the increased internalization of K.
pneumoniae by A549 cells.
Integrins are cell transmembrane adhesion receptors,
which contain at least 18α and 8β subunits that form 24 different
αβ integrins in distinct cells (26). These heterodimers on the cell
surface have been demonstrated to play a pivotal role in regulating
cell-cell, cell-ECM and cell-bacteria interactions to control cell
fate in cancer biology and inflammation. α5β1 integrin, is composed
of α5 and β1 subunits and it has been described as a receptor which
binds to fibronectin as well as having a well-defined role in cell
and bacterial adhesion (27). It
has been reported that HMGB1, another type of HMG protein, may
induce motility in chondrosarcoma cells through upregulating α5β1
integrin expression (28).
Moreover, previous findings have indicated that α5β1 integrin acts
as a membrane receptor to recognize a series of Gram negative and
positive pathogens (13).
Therefore, we postulated that HMGN2 has a similar function and it
regulates α5β1 integrin expression and subsequently affects
cellular behavior.
To determine whether HMGN2 regulates the expression
of α5β1 integrin in respiratory epithelial cells, the RNAi
technique was used to disrupt HMGN2 expression in A549 cells.
Subsequently, both the mRNA and protein levels of α5β1 integrin
were analyzed. Flow cytometric analysis and immunofluorescence
microscopy revealed that the expression of α5 and β1 integrin on
cell membranes was increased by HMGN2 knockdown in A549 cells.
However, RT-qPCR revealed only the increased mRNA expression of the
β1 subunit, whereas the mRNA level of α5 integrin was decreased,
which differs from the cDNA microarray data (7). For this paradox, there are at least
two main reasons. One possibility is that false positive results
existed in the micro-array analysis; another possible reason is
that HMGN2 may regulate the transportation of α5β1 integrin from
the cytoplasm to the cell membrane leading to the α5β1 integrin
distribution change, which possibly explains the decreased presence
of α5 integrin on the cell membranes. Johnson et al have
demonstrated that integrin α and β subunits are combined in the
endoplasmic reticulum and transported to the cell surface before
functioning as a molecular bridge between cellular 'inside and
outside' signaling (29).
The activation of cell surface integrin is
determined by integrin α and β subunit conformation. The integrin
heterodimers have three different affinity phases: inactive; active
and ligand occupied states; a dynamic equilibrium exists among them
(30). External signal
stimulation may break up the salt bridge between the α and β
subunit and therefore inhibit the activation of integrin.
Fibronectin, a α5β1 integrin ligand, exists in the ECM. An
established cell adhesion assay testing the ability of α5β1
integrin to bind to fibronectin was used to determine the integrin
binding activity. Our results showed that silencing HMGN2 in the
A549 cells increased the adhesion rate of epithelial cells to
fibronectin, which indicates that HMGN2 may play a role in
switching integrin active states (Fig. 4A).
Integrin activation is a dynamic, tightly-regulated
process, which is regulated by both positive and negative
regulators (30). Talins and
kindlins, two families of FERM-domain proteins, bind to the
cytoplasmic tail of integrins and recruit cytoskeletal and
signaling proteins, which have been well documented to positively
regulate the activation of integrin (26). It has been demonstrated that talin
may loosen the integrin transmembrane coiled-coil structure, which
further affects the affinity of integrin for extracellular ligands
as well as cytoskeleton polymerization (18,31). We found that silencing HMGN2
upregulated the protein expression of talin, which was decreased by
the overexpression of HMGN2 in A549 cells. This result further
confirmed the cell adhesion assay data that HMGN2 knockdown
increased α5β1 integrin activity by increasing talin expression.
The activation of integrin leads to the connection of the α subunit
to extracellular ligands and the β-subunit mediates the transfer
signal from the outside to the inside of the cell, which affects
the biological behavior of cell cytoskeletal rearrangement,
adhesion, migration, proliferation and differentiation (32).
The conformation of the host cell cytoskeleton plays
a crucial role in the process of bacterial internalization
(33). Actin filaments are the
basic component of the cytoskeleton that exist in two essential
forms: monomeric (G-actin) and filamentous (F-actin). The F-actin
filaments are highly dynamic structures, and their supermolecular
organization is constantly modified according to cellular needs
(34). Previous studies have
suggested that the reorganization of the cytoskeleton is beneficial
to bacterial internalization. Activated integrin transfers signal
to downstream molecules and changes the actin polymerization state
through integrin adaptor proteins (27,35). FAK/Src as the integrin adaptor
protein may bridge a number of downstream signaling pathways. Some
previous studies have indicated that the FAK phosphorylation
induces actin rearrangement in intestinal and reproductive tract
epithelial cells (36,37). Another study has reported that
actin polymerization is induced by Src signaling in kidney
epithelial cells (38).
Therefore, we examined FAK/Src signaling in A549 cells following
HMGN2 silencing. The results of western blot analysis revealed that
knockdown of HMGN2 activated FAK/Src signaling. In addition, our
microarray analysis showed that the expression of SCAP2 that is
downstream of Src signaling, was also induced by HMGN2 knockdown in
A549 cells (7). In this context,
we suggest that FAK/Src signaling downstream of integrin activation
was involved in the HMGN2-regulated internalization of
bacteria.
Furthermore, F-actin fluorescence data showed that
F-actin was more polymerized in the pulmonary epithelial cells than
in the bronchial epithelial cells following HMGN2 depletion and
subsequent bacterial infection. Collectively, we believe that the
role of HMGN2 in the regulation of bacterial internalization by
respiratory epithelial cells is physiologically and pathologically
meaningful. As we have indicated, HMGN2 knockdown increased the
rearrangement of actin filaments and bacterial internalization, and
thus, the sensitivity to respiratory infections would also be
increased in HMGN2-depleted organisms. Furthermore, it has been
reported that HMGN2 expression is absent in adult animals (39). Moreover, it is well known that
K. pneumoniae often occurs in the elderly (40); thus, determining whether HMGN2
plays a unique role in aging-related infectious diseases warrants
further investigation.
In conclusion, our results indicate that the
inhibition of HMGN2 in respiratory epithelial cells with
HMGN2-specific siRNA increased the internalization of K. pneumoniae
through the enhanced expression and activity of α5β1 integrin. The
precise signaling pathway involved in this process is that of
talin-mediated integrin activation, which led to the
phosphorylation of FAK and Src, and further induced F-actin
polymerization (Fig. 6D). From a
therapeutic point of view, targeting HMGN2 may prove to be a
valuable defense tool against pulmonary infection caused by K.
pneumoniae, through the inhibition of the interactions and
crosstalk between respiratory epithelial cells and K.
pneumoniae.
Acknowledgments
The present study was supported, in whole or in
part, by the National Natural Science Foundation of China (nos.
30470763, 81470931 and 31401188), and the Youth Foundation of
Sichuan University (no. 2014SCU11042).
References
|
1
|
Körner U, Bustin M, Scheer U and Hock R:
Developmental role of HMGN proteins in Xenopus laevis. Mech Dev.
120:1177–1192. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mosevitsky MI, Novitskaya VA, Iogannsen MG
and Zabezhinsky MA: Tissue specificity of nucleocytoplasmic
distribution of HMG1 and HMG2 proteins and their probable
functions. Eur J Biochem. 185:303–310. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reeves R and Adair JE: Role of high
mobility group (HMG) chromatin proteins in DNA repair. DNA Repair
(Amst). 4:926–938. 2005. View Article : Google Scholar
|
|
4
|
West KL, Castellini MA, Duncan MK and
Bustin M: Chromosomal proteins HMGN3a and HMGN3b regulate the
expression of glycine transporter 1. Mol Cell Biol. 24:3747–3756.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Belova GI, Postnikov YV, Furusawa T,
Birger Y and Bustin M: Chromosomal protein HMGN1 enhances the heat
shock-induced remodeling of Hsp70 chromatin. J Biol Chem.
283:8080–8088. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu N and Hansen U: HMGN1 modulates
estrogen-mediated transcriptional activation through interactions
with specific DNA-binding transcription factors. Mol Cell Biol.
27:8859–8873. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Deng LX, Wu GX, Cao Y, Fan B, Gao X, Luo L
and Huang N: The chromosomal protein HMGN2 mediates
lipopolysaccharide-induced expression of β-defensins in A549 cells.
FEBS J. 278:2152–2166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Deng LX, Wu GX, Cao Y, Fan B, Gao X, Tang
XH and Huang N: The chromosomal protein HMGN2 mediates the
LPS-induced expression of β-defensins in mice. Inflammation.
35:456–473. 2012. View Article : Google Scholar
|
|
9
|
Feng Y, Huang N, Wu Q and Wang B: HMGN2: a
novel antimicrobial effector molecule of human mononuclear
leukocytes? J Leukoc Biol. 78:1136–1141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cao Y, Wu G, Fan B, Zheng F, Gao X, Liu N,
Liu X and Huang N: High mobility group nucleosomal binding domain 2
protein protects bladder epithelial cells from Klebsiella
pneumoniae invasion. Biol Pharm Bull. 34:1065–1071. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu G, Cao Y, Fan B, Zheng F, Gao X, Liu N,
Liu X and Huang N: High-mobility group protein N2 (HMGN2) inhibited
the internalization of Klebsiella pneumoniae into cultured bladder
epithelial cells. Acta Biochim Biophys Sin (Shanghai). 43:680–687.
2011. View Article : Google Scholar
|
|
12
|
Cossart P and Sansonetti PJ: Bacterial
invasion: the paradigms of enteroinvasive pathogens. Science.
304:242–248. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ulanova M, Gravelle S and Barnes R: The
role of epithelial integrin receptors in recognition of pulmonary
pathogens. J Innate Immun. 1:4–17. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dingemans AM, van den Boogaart V, Vosse
BA, van Suylen RJ, Griffioen AW and Thijssen VL: Integrin
expression profiling identifies integrin alpha5 and beta1 as
prognostic factors in early stage non-small cell lung cancer. Mol
Cancer. 9:1522010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sayeed A, Fedele C, Trerotola M, Ganguly
KK and Languino LR: IGF-IR promotes prostate cancer growth by
stabilizing α5β1 integrin protein levels. PLoS One. 8:e765132013.
View Article : Google Scholar
|
|
16
|
Docheva D, Padula D, Schieker M and
Clausen-Schaumann H: Effect of collagen I and fibronectin on the
adhesion, elasticity and cytoskeletal organization of prostate
cancer cells. Biochem Biophys Res Commun. 402:361–366. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takagi J, Erickson HP and Springer TA:
C-terminal opening mimics 'inside-out' activation of integrin α5β1.
Nat Struct Mol Biol. 8:412–416. 2001. View
Article : Google Scholar
|
|
18
|
Calderwood DA, Zent R, Grant R, Rees DJG,
Hynes RO and Ginsberg MH: The Talin head domain binds to integrin β
subunit cytoplasmic tails and regulates integrin activation. J Biol
Chem. 274:28071–28074. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lafont F and van der Goot FG: Bacterial
invasion via lipid rafts. Cell Microbiol. 7:613–620. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Agarwal V, Ahl J, Riesbeck K and Blom AM:
An alternative role of C1q in bacterial infections: facilitating
Streptococcus pneumoniae adherence and invasion of host cells. J
Immunol. 191:4235–4245. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
March C, Moranta D, Regueiro V, Llobet E,
Tomás A, Garmendia J and Bengoechea JA: Klebsiella pneumoniae outer
membrane protein A is required to prevent the activation of airway
epithelial cells. J Biol Chem. 286:9956–9967. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mohan Nair MK and Venkitanarayanan K: Role
of bacterial OmpA and host cytoskeleton in the invasion of human
intestinal epithelial cells by Enterobacter sakazakii. Pediatr Res.
62:664–669. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alexander EH and Hudson MC: Factors
influencing the internalization of Staphylococcus aureus and
impacts on the course of infections in humans. Appl Microbiol
Biotechnol. 56:361–366. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cue D, Southern SO, Southern PJ, Prabhakar
J, Lorelli W, Smallheer JM, Mousa SA and Cleary PP: A nonpeptide
integrin antagonist can inhibit epithelial cell ingestion of
Streptococcus pyogenes by blocking formation of integrin alpha 5
beta 1-fibronectin-M1 protein complexes. Proc Natl Acad Sci USA.
97:2858–2863. 2000. View Article : Google Scholar
|
|
25
|
Vinogradova O, Velyvis A, Velyviene A, Hu
B, Haas T, Plow E and Qin J: A structural mechanism of integrin
alpha(IIb)beta(3) 'inside-out' activation as regulated by its
cytoplasmic face. Cell. 110:587–597. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Luo B-H, Carman CV and Springer TA:
Structural basis of integrin regulation and signaling. Annu Rev
Immunol. 25:619–647. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiao T, Takagi J, Coller BS, Wang JH and
Springer TA: Structural basis for allostery in integrins and
binding to fibrinogen-mimetic therapeutics. Nature. 432:59–67.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tang CH, Keng YT and Liu JF: HMGB-1
induces cell motility and α5β1 integrin expression in human
chondrosarcoma cells. Cancer Lett. 322:98–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Johnson MS, Lu N, Denessiouk K, Heino J
and Gullberg D: Integrins during evolution: evolutionary trees and
model organisms. Biochim Biophys Acta. 1788:779–789. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Anderson LR, Owens TW and Naylor MJ:
Structural and mechanical functions of integrins. Biophys Rev.
6:203–213. 2014. View Article : Google Scholar
|
|
31
|
Anthis NJ, Wegener KL, Ye F, Kim C, Goult
BT, Lowe ED, Vakonakis I, Bate N, Critchley DR, Ginsberg MH and
Campbell ID: The structure of an integrin/talin complex reveals the
basis of inside-out signal transduction. EMBO J. 28:3623–3632.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Margadant C, Monsuur HN, Norman JC and
Sonnenberg A: Mechanisms of integrin activation and trafficking.
Curr Opin Cell Biol. 23:607–614. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Campellone KG: Cytoskeleton-modulating
effectors of enteropathogenic and enterohaemorrhagic Escherichia
coli: Tir, EspFU and actin pedestal assembly. FEBS J.
277:2390–2402. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Navarro-Garcia F, Serapio-Palacios A,
Ugalde-Silva P, Tapia-Pastrana G and Chavez-Dueñas L: Actin
cytoskeleton manipulation by effector proteins secreted by
diarrheagenic Escherichia coli pathotypes. BioMed Res Int.
2013:3743952013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhu J, Carman CV, Kim M, Shimaoka M,
Springer TA and Luo BH: Requirement of α and β subunit
transmembrane helix separation for integrin outside-in signaling.
Blood. 110:2475–2483. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dia VP and Gonzalez de Mejia E: Lunasin
potentiates the effect of oxaliplatin preventing outgrowth of colon
cancer metastasis, binds to α5β1 integrin and suppresses
FAK/ERK/NF-κB signaling. Cancer Lett. 313:167–180. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Siu MK, Wong CH, Xia W, Mruk DD, Lee WM
and Cheng CY: The β1-integrin-p-FAK-p130Cas-DOCK180-RhoA-vinculin
is a novel regulatory protein complex at the apical ectoplasmic
specialization in adult rat testes. Spermatogenesis. 1:73–86. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pawlak G and Helfman DM: MEK mediates
v-Src-induced disruption of the actin cytoskeleton via inactivation
of the Rho-ROCK-LIM kinase pathway. J Biol Chem. 277:26927–26933.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Crippa MP, Nickol JM and Bustin M:
Developmental changes in the expression of high mobility group
chromosomal proteins. J Biol Chem. 266:2712–2714. 1991.PubMed/NCBI
|
|
40
|
Fein AM: Pneumonia in the elderly:
overview of diagnostic and therapeutic approaches. Clin Infect Dis.
28:726–729. 1999. View
Article : Google Scholar
|