Introduction
Psoriasis is a common skin disorder that affects 2%
of the worldwide population (1).
Typical lesions are sharply demarcated, thick, erythematous, scaly
plaques. Histologically, it is characterized by epidermal
acanthosis, papillomatosis and parakeratosis, infiltrating
leukocytes and neutrophils in the epidermis and dermis, as well as
neoangiogenesis (2).
Psoriasis is recognized as an organ-specific
autoimmune disease that is triggered by an activated cellular
immune system (3,4). Although psoriasis has traditionally
been classified as a T helper (Th)1-mediated disease due to the
abundance of interferon (IFN)-γ-producing Th1-polarized T cells
within psoriatic skin (5),
several studies have highlighted the significance of Th17 cells.
Th17 cells are a newly identified population of interleukin
(IL)-17-producing Th cells shown to be involved in the pathogenesis
of psoriasis and other autoimmune inflammatory disorders (6–8).
The development and maintenance of Th17 cells is driven by IL-23, a
key cytokine which initiates the development of autoimmunity
(9,10). IL-23, secreted by skin dendritic
cells (DCs), induces Th17 cell production of proinflammatory
cytokines, such as IL-17A, IL-17F, tumor necrosis factor-α (TNF-α)
and IL-22. The cytokine components of the Th17 cells play vital
roles in human psoriasis (11).
These mediators act on keratinocytes leading to their activation
and hyperproliferation. In the cross-talk between keratinocytes and
Th17 cells, activated keratinocytes produce key proinflammatory
cytokines, chemokines and antimicrobial peptides, which recruit and
activate immune cells in the inflamed skin (12,13). These events result in
amplification of the immune response leading to the clinical
features of the disease.
Tripterygium wilfordii Hook. f. is a
traditional medicinal herb which has been used for several
centuries in China. One component found in extracts of this plant
is a stable glycoside, known as multi-glycoside of T.
wilfordii Hook. f. (GTW). It is comprised of trace diterpenes,
a small quantity of alkaloids and some pentacyclic triterpenes. It
is generally considered that the most toxic components of T.
wilfordii Hook.f. are diterpenoids followed by alkaloids, and
GTW is the least toxic compound (14). GTW has been shown to exert
anti-inflammatory and immunosuppressive effects and has been used
for the clinical treatment of psoriasis for many years (15). However, the precise immunological
mechanisms underlying the therapeutic effects of GTW on psoriasis
remain poorly understood.
Recently, the topical administration of imiquimod
(IMQ) to mice, a ligand for Toll-like receptor (TLR)7 and TLR8, was
shown to induce psoriasis-like skin inflammation, including
characteristics such as acanthosis, parakeratosis and inflammatory
cell infiltration (16). In the
present study, we examined the effects of GTW on psoriasis-like
lesions induced by topical IMQ administration in a mouse model, as
well as the underlying mechanisms. Our results provide evidence
that GTW protected the mice against IMQ-induced psoriasis-like
inflammation through a mechanism involving the inhibition of STAT3
phosphorylation in Th17-mediated inflammatory responses.
Materials and methods
Mice and treatments
Fifty mice (BALB/c, aged 8 weeks) were purchased
from the Experimental Animal Center of the Chinese Academy of
Medical Sciences (Beijing, China), the dorsal area was shaved and
the mice were randomly divided into the following five groups (10
mice/group): i) the normal control group (normal) which received
applications of appropriate Vaseline spread on the exposed back
regularly each day and intragastric administration of saline (0.4
ml/day) for 8 continuous days; ii) the model group which received
regular applications of 42 mg of 5% IMQ cream on the exposed back
each day and intragastric administration of saline (0.4 ml/day) for
8 continuous days; and iii-v) the treatment groups which received
low-, medium- and high-doses of GTW (10, 20 and 40 mg/kg,
respectively) by intragastric administration of GWT solution (0.4
ml/day) and regular applications of 42 mg of 5% IMQ cream on the
exposed back each day and intragastric administration of GWT
solution (0.4 ml/day) for 8 continuous days. The GTW used in this
study was provided by DND Pharmaceutical Co., Ltd. (Zhejiang,
China). It was solubilized in sterile distilled water and
administered to the GTW group intragastrically via gavage (10, 20
and 40 mg/kg) once per day in parallel with the IMQ administration
from day 1 to 8. All mice were kept under pathogen-free conditions
and provided with food and water ad libitum. Six mice in
each group at day 4 or 8 were sacrificed. The mice were sacrificed
by cervical dislocation and the spleens were removed and weighed.
All animal experiments were performed in accordance with the
National Institutes of Health Guidelines on Laboratory Research and
approved by the Animal Ethics Committee of Capital Medical
University (Beijing, China).
Severity scoring of skin
inflammation
To score the severity of the inflammation induced in
the skin, an objective scoring system was developed based on the
clinical Psoriasis Area and Severity Index (PASI). However, the
scoring system for the mouse model did not take into account the
affected skin area in the overall score. Erythema, scaling, and
thickening were scored independently on a scale from 0 to 4: 0,
none; 1, slight; 2, moderate; 3, marked; 4, very marked. The
cumulative score (erythema plus scaling plus thickening) served as
a measure of the severity of inflammation (scale 0–12). For score
evaluation, 6 mice were taken from each group per day.
Skin histology and immunohistochemical
(IHC) staining
Tissue samples were taken from 6 mice in each group
on days 4 or 8 in accordance with the following procedure: i) skin
tissue samples were incised from the corresponding lesions using a
nine-grid method; part of the tissue sample was embedded in optimal
cutting temperature (OCT) compound for the preparation of frozen
sections; ii) part of the tissue sample was stored in liquid
nitrogen for mRNA and protein detection; iii) the remaining part of
the tissue sample was fixed in formalin for the preparation of
paraffin sections.
IMQ-exposed, psoriasis-like inflammatory skin was
paraffin-embedded, sectioned and stained with hematoxylin and eosin
(H&E) and the epidermal thickness was determined using Image
Pro Plus 6.0 software under a microscope (BX51; Olympus, Tokyo,
Japan) in a blinded manner.
For IHC staining, the paraffin sections were
deparaffinized and hydrated by washing the sections in xylene for
20 min, followed by a graded series of alcohol at concentrations
from 70 to 100%. To unmask antigens, the sections were incubated in
10 mM citric acid (pH 6.0) at 95°C for 20 min. Endogenous
peroxidase activity was quenched by treating the sections with 3%
hydrogen peroxide for 10 min at room temperature. The sections were
then incubated with primary antibodies including anti-CD3 (Cat. no.
ab16669) and anti-proliferating cell nuclear antigen (PCNA; Cat.
no. ab29) (both from Abcam, Cambridge, UK); as well as anti-F4/80
(Cat. no. 123106; BioLegend, Inc., San Diego, CA, USA) overnight at
4°C in buffer. This was followed by incubation with biotin-linked
secondary antibody (Abcam) for 30 min. Finally,
3,3′-diaminobenzidine (DAB) or NovaRED substrate (Vector
Laboratories, Inc., Burlingame, CA, USA) was used for chromogenic
detection. The cryosections were fixed, blocked and then stained
with rat-anti-mouse Gr-1 (Cat. no. 550291; BD Pharmingen, San Jose,
CA, USA) followed by secondary antibody (Vectastain Elite ABC kit;
Vector Laboratories, Inc.). The slides were developed with NovaRED
substrate solution (Vector Laboratories, Inc.). Additional slides
were stained with FITC-conjugated anti-mouse γδ TCR (Cat. no.
118105, BioLegend, Inc.) or involucrin antibody (Cat. no. ab28057;
Abcam) and DAPI to visualize the nuclei. All the stained sections
were counterstained with hematoxylin (BASO Diagnostics Inc.,
Zhuhai, China). The sections from at least six mice/group were
stained, and the images were captured at ×200 or ×400 magnification
using a microscope (BX51; Olympus) with a digital charge-coupled
device (CCD) camera (DP72; Olympus).
Flow cytometric analysis
Spleen samples from each group were minced through a
70 µm mesh to obtain single cell suspensions. For flow
cytometric analysis, 1×106 cells were fixed and
permeabilized with Cytofix/Cytoperm (BD Pharmingen) stained with
fluorescein isothiocyanate (FITC)-conjugated mouse monoclonal
anti-CD4 and allophycocyanin (APC)-conjugated anti-CD25, and
phycoerythrin (PE)-conjugated mouse anti-Foxp3 (all from
eBioscience, San Diego, CA, USA). For the detection of
intracellular cytokines, 1×106 spleen cells were
stimulated with phorbol myristate acetate (PMA) in 10 ng/ml
phosphate-buffered saline (PBS) and ionomycin 1 µg/ml in PBS
(Sigma, St. Louis, CA, USA) in the presence of GolgiStop
(eBioscience) for 5 h. The cells were harvested and stained with
FITC-conjugated anti-CD4 (eBioscience), followed by intracellular
staining using mouse monoclonal antibodies: PE-conjugated
anti-IL-17A, APC-conjugated anti-IFN-γ, and APC-conjugated
anti-IL-4 (all from BD Pharmingen) after fixation and
permeabilization with Cytofix/Cytoperm (BD Pharmingen). Inguinal
lymph nodes were separated and ground by the 50 µm nylon
membrane using sterile technique. The cells were stained with
APC-conjugated anti-CD3 (eBio-science) and PE-conjugated anti-γδ
TCR (BD Pharmingen). The samples were analyzed on a flow cytometer
(FACSCanto) using CellQuest software (both from BD Biosciences,
Franklin Lakes, NJ, USA). The expression levels of intracellular
IL-17, IFN-γ, IL-4 and Foxp3 were analyzed.
Isolation of naive CD4+ T
cells and in vitro induction and culture of Th17 cells
The CD4+CD62L+ T cell Iso kit
II (Miltenyi Biotec, Bergisch Gladbach, Germany) was adopted for
the direct sorting of the naive CD4+ T cells. A
single-cell suspension was prepared by the mild grinding of the
spleen samples and the CD4+CD62L+ T cells
were isolated according to the manufacturer's instructions.
The CD4+CD62L+ T cells were
seeded in 12-well plates (2×106/well) or 96-well plates
(5×104/well) which were coated with anti-CD3 (5
µg/ml) and anti-CD28 (2 µg/ml) and well mixed and
cultured with complete RPMI-1640 medium containing transforming
growth factor (TGF)-β1 (5 ng/ml), IL-6 (20 ng/ml), IL-1β (10
ng/ml), anti-IL-2 (10 µg/ml), anti-IL-4 (10 µg/ml),
anti-IFN-γ (10 µg/ml), IL-23 (15 ng/ml) and 10% fetal bovine
serum (FBS). On the 3rd day of culture, the cells were ready for
the flow cytometric detection of Th17 cell differentiation.
Different concentrations of GTW together with all
the inducible factors required for Th17 cell differentiation were
added to the culture medium. Detection was conducted after a 3-day
co-incubation. The experimental groups were as follows: i) neutral
condition group: CD4+CD62L+ T cells were
seeded onto the culture plates coated only with anti-CD3 (5
µg/ml) and anti-CD28 (2 µg/ml) that provided a normal
growth environment for the CD4+ CD62L+ T
cells. The cells were cultured for 3 days; ii) Th17 polarizing
condition group: CD4+CD62L+ T cells were
seeded onto the culture plates coated with anti-CD3 (5
µg/ml) and anti-CD28 (2 µg/ml), and inducible factors
(TGF-β1, IL-6, IL-1β, anti-IL-2, anti-IL-4, anti-IFN-γ and IL-23)
were also added during the 3-day culture; and iii) GTW (16
µg/ml) group: the conditions of the Th17 polarizing group
were used. CD4+CD62L+ T cells were seeded
onto the culture plates coated with anti-CD3 (5 µg/ml) and
anti-CD28 (2 µg/ml), and all the inducible factors and 16
µg/ml GTW were also added during the 3-day culture; iv) GTW
(1.6 µg/ml) group: the conditions of the Th17 polarizing
group were used. The specific steps were the same as the GTW (16
µg/ml) group; however, the dose of GTW was 1.6 µg/ml;
v) GTW (0.16 µg/ml) group: the specific steps were the same
as the GTW (1.6 µg/ml) group; however, the dose of GTW was
0.16 µg/ml.
Impact of GTW upon Th17 cell
differentiation detected by flow cytometry
After 3 days of culture, the cells were stimulated
and induced to secrete intracellular cytokines by PMA (10 ng/ml),
ionomycin (1 µg/ml) and brefeldin A (BFA; 10 µg/ml).
After being cultured for 6 h, the cells were first incubated with
anti-mouse CD4-FITC antibody, and then permeabilized and stained
with APC-labeled anti-mouse IL-17 antibody. The percentage of
CD4+ IL-17+ T cells was then analyzed on a
flow cytometer in order to establish the differentiation rate of
the Th17 cells.
Impact of GTW upon IL-17 secretion
detected by enzyme-linked immunosorbent assay (ELISA)
The levels of IL-17 secreted from Th17 cells were
measured using a commercial IL-17 ELISA kit according to the
manufacturer's instructions.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA extraction from mouse skin was performed
using TRIzol reagent according to the manufacturer's instructions
(Invitrogen, Carlsbad, CA, USA). Following the generation of cDNA
using a Reverse Transcription kit (CWbio Co., Ltd., Beijing,
China), RT-qPCR was performed on a Roche 480 II detection system
with SYBR-Green Supermix (Bio-Rad Laboratories, Hercules, CA, USA).
The gene-specific primers are summarized in Table I. The gene expression levels were
normalized to the housekeeping gene, β-actin, and data are
represented as fold-changes according to the 2−ΔΔCt
method, where ΔCt=Cttarget gene−Ctβ-actin and
ΔΔCt=ΔCtinduced−ΔCtreference.
ΔCtreference was chosen as the normal mice skin sample.
PCR was performed as follows: 95°C for 10 min; 50 cycles of 95°C
for 15 sec, and 60°C for 60 sec. Relative quantitative analysis was
carried out using the 2−ΔΔCt method.
 | Table IPrimers used for RT-qPCR. |
Table I
Primers used for RT-qPCR.
| Primer
sequences |
|---|
| IL-23 | F:
5′-GACTCAGCCAACTCCTCCAGCCAG-3′ |
| R:
5′-TTGGCACTAAGGGCTCAGTCAGA-3′ |
| IL-17A | F:
5′-CAGACTACCTCAACCGTTCCA-3′ |
| R:
5′-ACAATCGAGGCCACGCAGGTGCAGC-3′ |
| IL-17F | F:
5′-TGCTACTGTTGATGTTGGGAC-3′ |
| R:
5′-AATGCCCTGGTTTTGGTTGAA-3′ |
| IL-22 | F:
5′-CGTCAACCGCACCTTTAT-3′ |
| R:
5′-AGGGCTGGAACCTGTCTG-3′ |
| TNF-α | F:
5′-GAGAAGTTCCCAAATGGC-3′ |
| R:
5′-ACTTGGTGGTTTGCTACG-3′ |
| RORγt | F:
5′-AGTATGTGGTGGAGTTTGC-3′ |
| R:
5′-TAGGACGACTTCCATTGCT-3′ |
| IFN-γ | F:
5′-TAACTCAAGTGGCATAGATGTGGAAG-3′ |
| R:
5′-GACGCTTATGTTGTTGCTGATGG-3′ |
| IL-10 | F:
5′-CTGGACAACATACTGCTAACCGACTC-3′ |
| R:
5′-AACTGGATCATTTCCGATAAGGC-3′ |
| K14 | F:
5′-ACGCCCACCTTTCATCTTCCCAAT-3′ |
| R:
5′-ATCTGGCGGTTGGTGGAGGTCA-3′ |
| Foxp3 | F:
5′-CCCATCCCCAGGAGTCTTG-3′ |
| R:
5′-ACCATGACTAGGGGCACTGTA-3′ |
| IL-4 | F:
5′-TCGTCTGTAGGGCTTCCAAGGTGCT-3′ |
| R:
5′-GTGGACTTGGACTCATTCATGGTGC-3′ |
| β-actin | F:
5′-GCCTTCCTTCTTGGGTAT-3′ |
| R:
5′-GGCATAGAGGTCTTTACGG-3′ |
Western blot analysis
Whole cell lysates were prepared from each skin
tissue sample and protein concentrations were estimated using a BCA
protein assay kit (23235; Thermo Fisher Scientific, Rockford, IL,
USA). Protein samples were separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; 10% gel) and
transferred to a polyvinylidene difluoride (PVDF) membrane by
electroblotting at 4°C. The membranes were then blocked for 2 h at
room temperature with 5% non-fat dried milk powder in TBS, 0.1%
Tween-20 (TBST) before being probed with anti-phosphorylated
(p-)STAT3 (Cat. no. 9134) in 1/2,000 dilution, or anti-STAT3 (Cat.
no. 9132) at a 1/2,000 dilution (both from Cell Signaling
Technology, Inc., Beverly, MA, USA) overnight at 4°C. Subsequently,
the membrane was exposed to horseradish peroxidase-conjugated
secondary antibodies (Cell Signaling Technology, Inc.). The results
were visualized using a chemiluminescence detection system (Pierce
ECL Western Blotting Substrate Detection system; Thermo Fisher
Scientific) and exposed to autoradiography film. GAPDH was used as
the control. Target protein expression levels were analyzed using
Quantity One v4.4.0 software (Bio-Rad Laboratories).
Statistical analysis
All quantitative data are shown as the means ± SD.
Comparisons between two groups were assessed using the Student's
t-test, and comparisons among three or more group were evaluated
using one-way analysis of variance (ANOVA) followed by post-hoc
testing with SPSS 15.0 software. A P-value <0.05 was considered
to indicate a statistically significant difference.
Results
GTW protects mice against the development
of psoriasis-like lesions induced by IMQ
The anti-inflammatory effects of GTW were examined
in a mouse model of IMQ-induced psoriasis-like lesions. Consistent
with previous findings (16),
psoriasis-like lesions exhibiting signs of erythema, increased
thickness and scaling, were observed two or three days after
initiating the topical application of IMQ to shaved skin on the
dorsal area of the mice. The severity of these lesions increased
with the duration of IMQ application (Fig. 1B). Similar lesions were not
observed in the normal (vehicle-treated) mice (Fig. 1A, first panel on left).
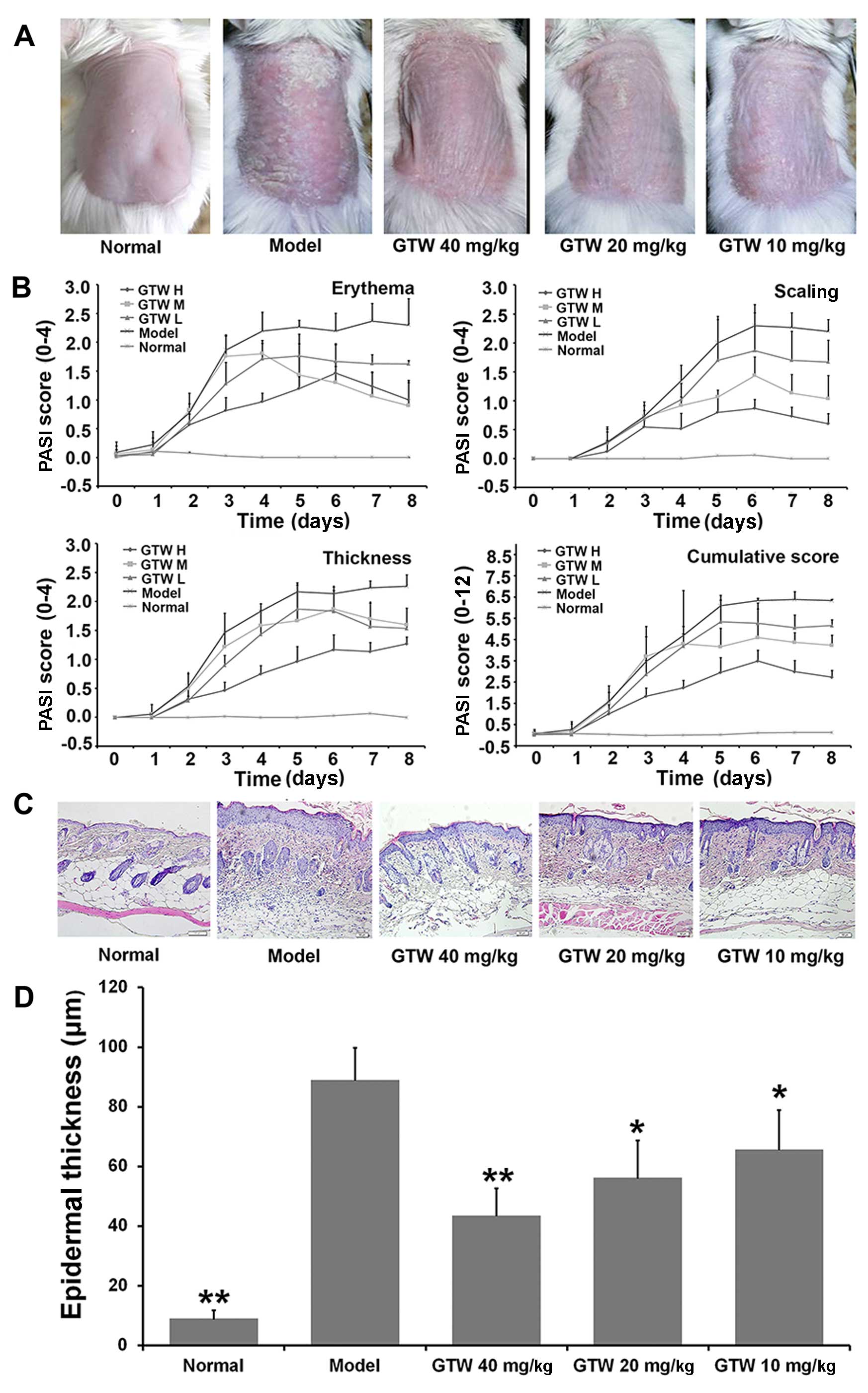 | Figure 1Therapeutic effect of multi-glycoside
of T. wilfordii Hook. f. (GTW) on psoriasis-like lesions
induced by imiquimod (IMQ) in mice. Vaseline was applied daily to
BALB/c mice in the normal group and IMQ was applied daily to mice
in the model and GTW-treated groups. The GTW groups were treated
simultaneously with GTW (10, 20 and 40 mg/kg) and IMQ. After 8
days, the mice were sacrificed for skin lesion analysis. (A)
Phenotypical presentation of back skin of mice after 8 days of
imiquimod treatment with the indicated compounds. Comparison of
skin changes in each group. (B) Erythema, scaling, and thickness of
the skin lesions were scored daily on a scale from 0 to 4 (0, none;
1, slight; 2, moderate; 3, marked; 4, very marked). In addition,
the cumulative score (erythema plus scaling plus thickness, scale
0–12) is depicted. Symbols indicate the means ± SD (n=6/group). (C)
Representative microscopic images of H&E-stained skin sections
from each group following 8 days of treatment. Acanthosis,
parakeratosis, pustules and desquamation were observed in the model
mice. Scale bar =50 µm. (D) Epidermal thickness was
evaluated using ImagePro Plus software under a microscope. Bars
represent the means ± SD (n=6/group). GTW H, 40 mg/kg; GTW M,
20mg/kg; GTW L, 10mg/kg. *P<0.05 and
**P<0.01 vs. the model group. |
By contrast, the severity of the skin lesions
observed in the IMQ-exposed mice was reduced by the
co-administration of GTW. In these mice, reduced redness,
desquamation and thickness was observed from day 2 compared with
the mice in the model group. The modified PASI scores showed that
GTW treatment resulted in a concentration- and time-dependent
inhibition of erythema, scaling, thickness and cumulative scores,
with an almost 40% reduction in all the measured parameters at a
dose of 40 mg/kg GTW (Fig.
1B).
Microscopic evaluation of H&E-stained mouse skin
sections obtained from the model group treated for 8 days with IMQ
showed characteristic changes associated with psoriasis lesions,
such as acanthosis (thickening of the epidermis), parakeratosis
(presence of nuclei in the stratum corneum), desquamation and
inflammatory infiltration. These characteristics were not observed
in the skin sections obtained from the mice in the normal group
(Fig. 1C). However, GTW treatment
partially inhibited these characteristic changes associated with
lesion development in the IMQ-exposed mice (Fig. 1C). Statistical analysis of
epidermal thickness using ImagePro Plus software showed significant
decreases in the epidermal thickness following GTW administration
in the IMQ-exposed mice (Fig.
1D).
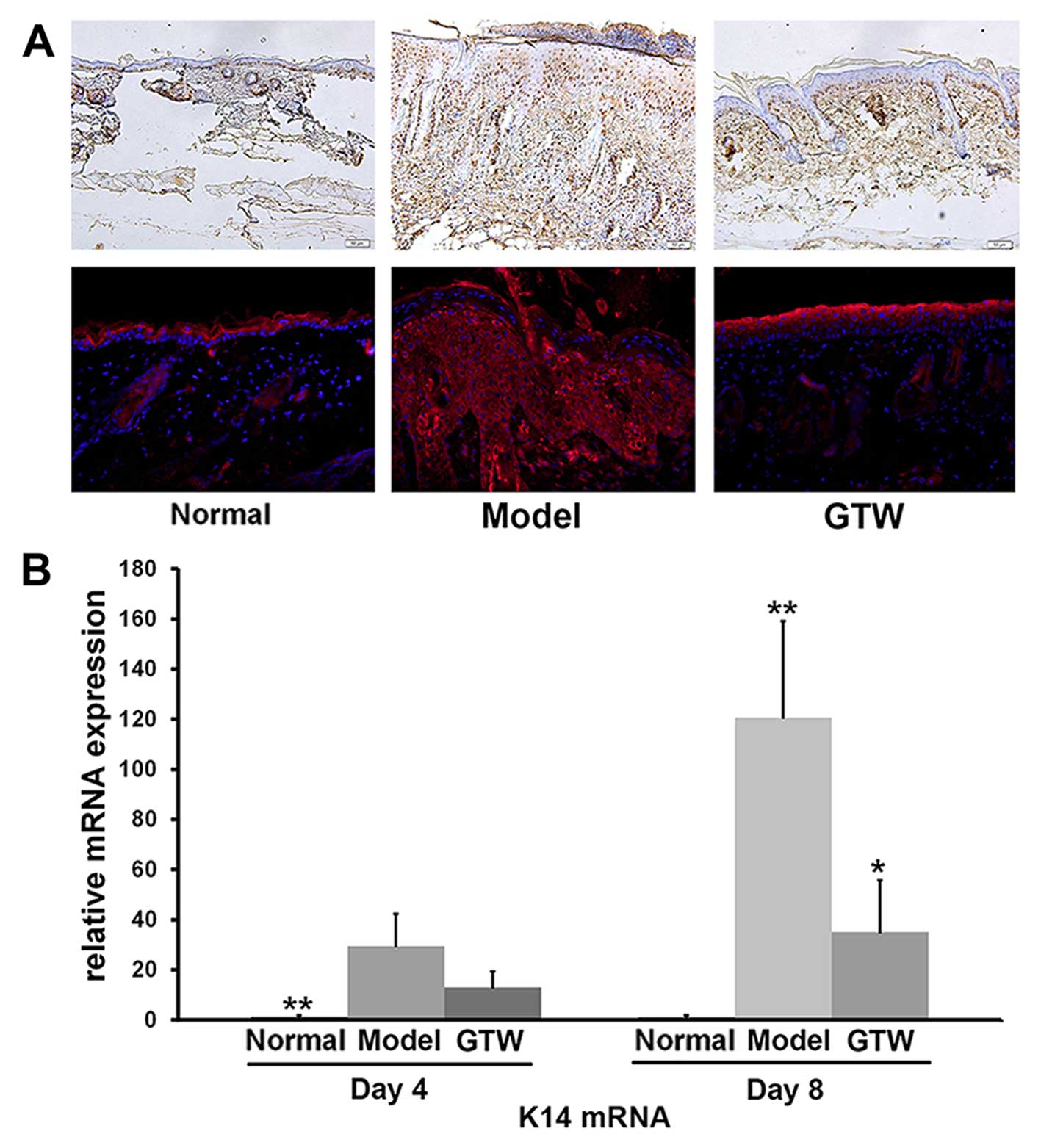
Hyperproliferation of keratinocytes was also
monitored by IHC staining of PCNA. In the IMQ-exposed mice, PCNA
was detected within keratinocytes throughout the different layers
of the epidermis, which was indicative of active proliferation.
However, similar to the normal group of mice (Fig. 2A, upper panels), PCNA-positive
cells were mainly restricted to the basal layer of the epidermis in
the IMQ plus GTW-treated mice although staining of the horny cell
layer was seen in all samples (Fig.
2A, upper panels).
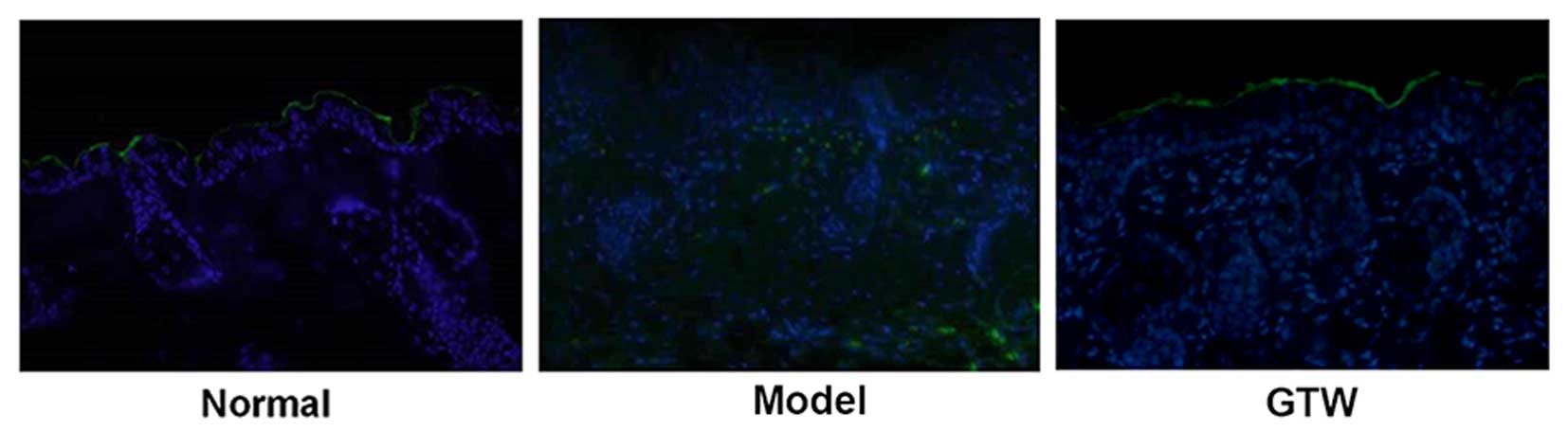
IHC staining of involucrin, a biological marker of
terminally differentiated keratinocytes in the epidermis, showed
typical expression in the stratum spinosum of the epidermis in
normal mice (Fig. 2A, lower
panels). By contrast, topical IMQ administration resulted in
widespread involucrin expression throughout the epidermis. However,
the expression of involucrin in the IMQ plus GTW-treated mice
showed a similar pattern to that observed in the normal
(vehicle-treated) mice. Apart from morphological and cellular
observations, we also evaluated the mRNA expression of keratin 14
(K14) as a marker of hyperproliferative keratinocytes, using
RT-qPCR. A significant increase in K14 mRNA expression was observed
in the IMQ-exposed mice compared with that in the normal control
mice. By contrast, a significant reduction (>60%) in K14 mRNA
expression was observed in the IMQ plus GTW-treated mice (Fig. 2B).
These results indicated that GTW significantly
inhibited IMQ-induced abnormalities in keratinocytes.
GTW attenuates IMQ-induced inflammatory
infiltration of T cells, neutrophils and macrophages
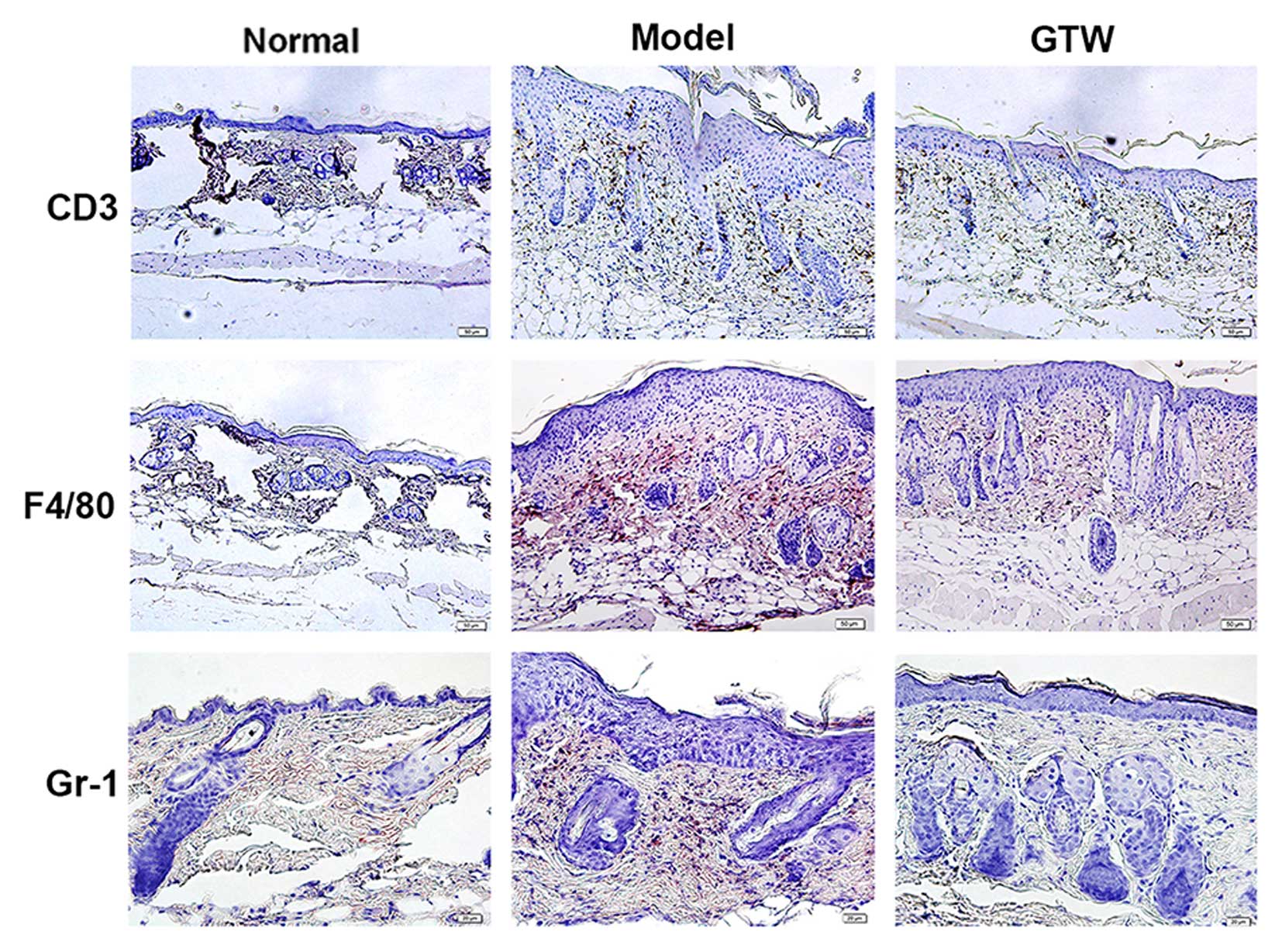
Psoriasis-like skin inflammation is the result of
abnormal immune cell activity. The induction and distribution of T
cells, neutrophils and macrophages infiltration is a hallmark of
the development of psoriasis-like skin inflammation (16). As expected, a marked infiltration
of both mononuclear and multinuclear cells was observed in the
H&E-stained skin sections from the IMQ-exposed mice (Fig. 3). To localize and quantify
leukocyte populations in the skin, we performed IHC staining for
CD3 (T cells), F4/80 (macrophages), and Gr-1 (neutrophils)
(Fig. 3). The numbers of cells of
each type for each high power field in normal skin (data not shown)
were 1.23±0.32 for F4/80, 3.37±0.68 for CD3+ and
2.84±0.55 for Gr-1. There were significantly fewer
F4/80+, CD3+ and Gr-1+ cells in
samples from the IMQ plus GTW group (11.85±3.79, 15.88±0.99 and
4.26±1.32, respectively) compared with the number observed in the
mice treated with IMQ alone (52.00±5.86, 43.49±2.14 and 23.28±4.
56, respectively, all P<0.01).
These data indicate that GTW suppressed the
differentiation of immune cells induced by IMQ.
GTW suppresses the expression of
Th17-type proinflammatory cytokines in IMQ-exposed skin
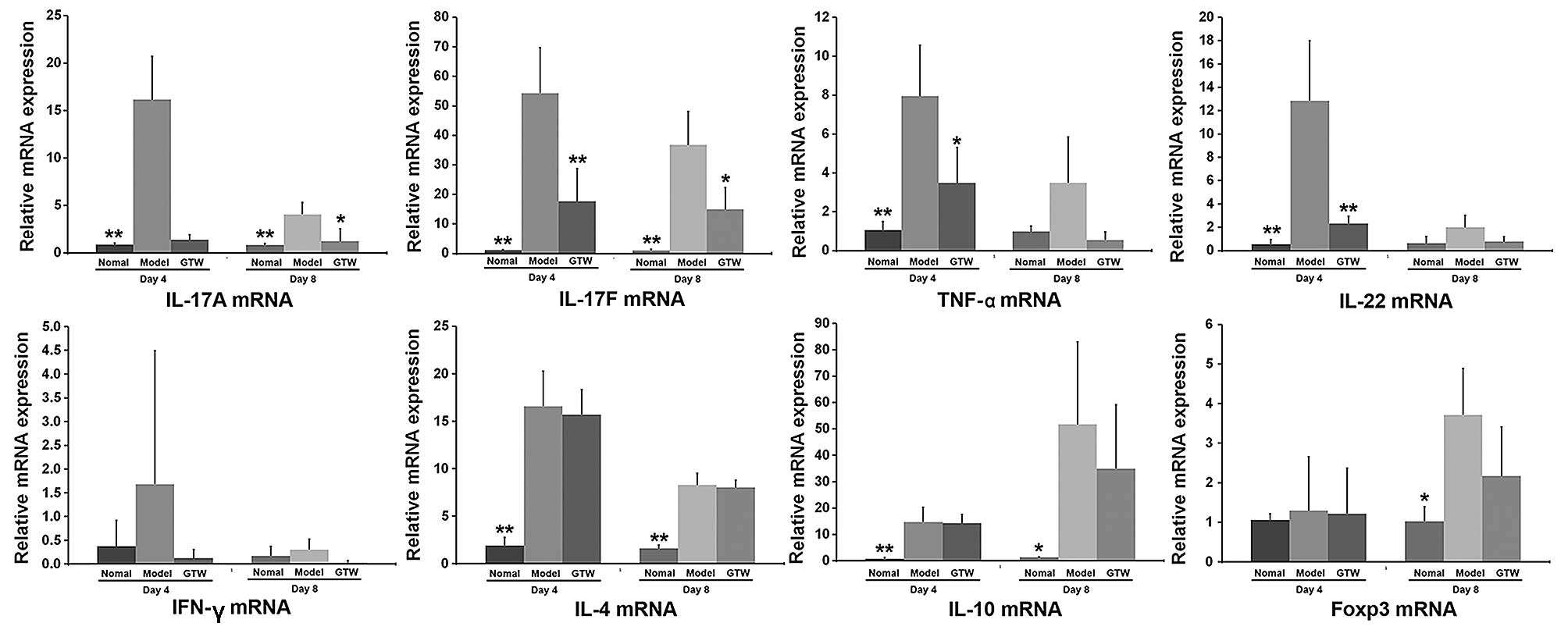
To characterize the immunosuppressive effect of GTW
on IMQ-induced skin lesions, the mRNA expression levels of a number
of cytokines were determined by RT-qPCR (Fig. 4).
Compared with the normal control mice, the mRNA
expression levels of Th17-type cytokines, including IL-17A, IL-17F,
IL-22 and TNF-α, were markedly increased in the skin of the
IMQ-exposed mice after 4 days (Fig.
4), although the mRNA levels of all these cytokines decreased
after 8 days. However, a clear suppressive effect on the mRNA
expression levels of these cytokines in the psoriasis-like lesions
was observed in the IMQ plus GTW-treated mice. GTW almost abolished
the transcription levels of IL-17A, TNF-α and IL-22, which were
similar to the levels observed in the vehicle-treated control mice.
However, the mRNA level of IL-17F was reduced by only 50%
(approximately) in the IMQ plus GTW group compared with the
IMQ-exposed group.
By contrast, the mRNA expression of the Th1 type
cytokine, IFN-γ, was not significantly induced by IMQ at any of the
time points (days 1, 2, 4, 6 and 8), with expression levels
remaining similar to those observed in control mice (data not
shown). GTW had no effect on the mRNA expression of IFN-γ.
The mRNA expression of IL-4, IL-10 and Foxp3 (a Treg
cell transcription factor) was significantly induced by IMQ,
although GTW did not inhibit their expression after treatment for 4
or 8 days (Fig. 4).
Unexpectedly, an increase in the mRNA expression of
IL-10 and Foxp3 mRNA expression was observed at day 8 in the
IMQ-exposed mice compared with that in the normal mice. However,
there was no significant difference between the IMQ-exposed and the
IMQ plus GTW-treated mice.
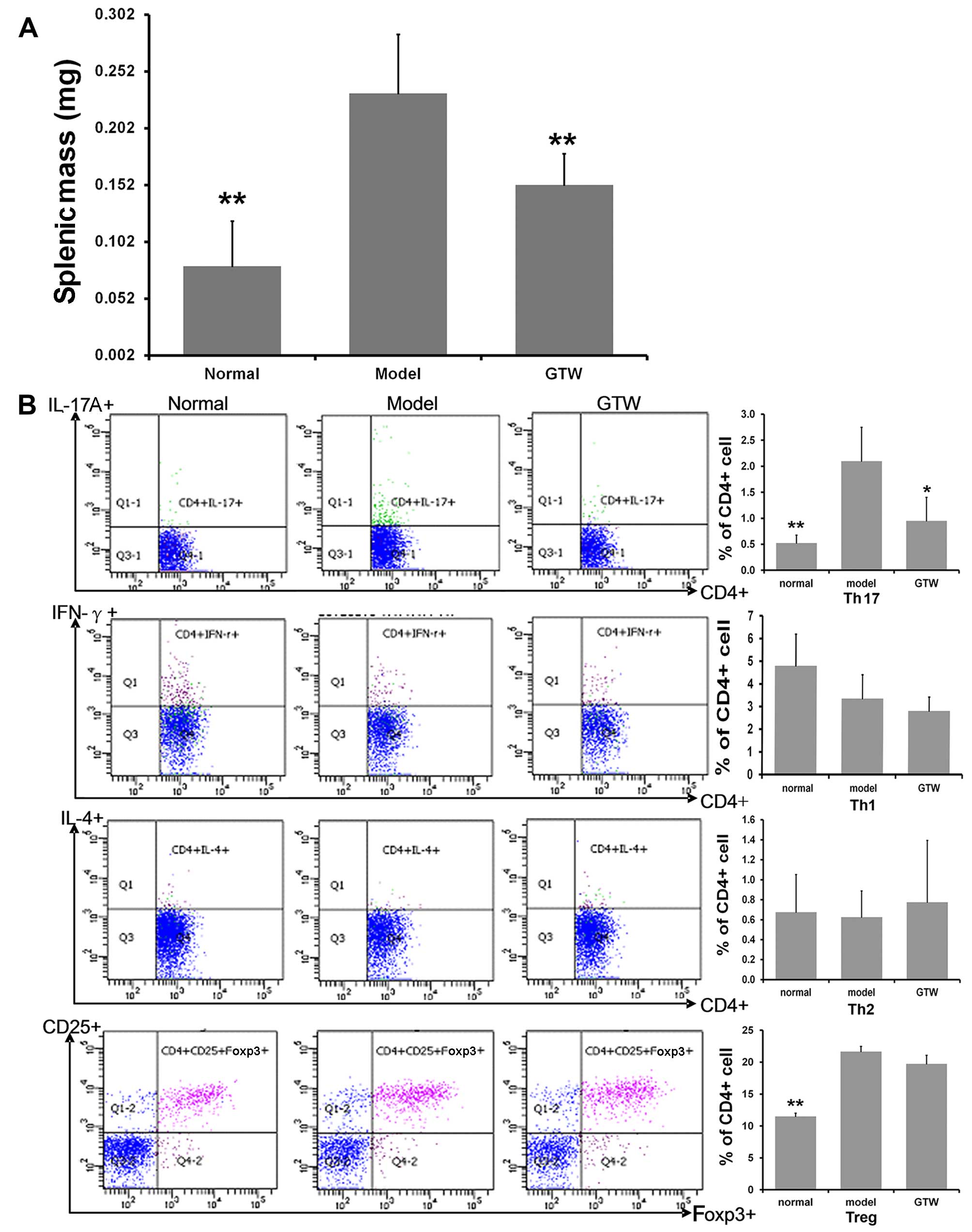
GTW decreases Th17 cell numbers in the
spleens of IMQ-exposed mice
Significant spleen enlargement, with a near
three-fold increase in splenic mass, was observed in the
IMQ-exposed mice after 8 days (Fig.
5A). A significantly lower splenic mass was recorded in the
mice treated with IMQ plus GTW, which was similar to that observed
in the normal group.
To determine the percentages of Th1, Th2, Th17 and
Treg cells in the spleen, the splenic cells were activated ex
vivo by PMA and ionomycin for 5 h, stained intracellularly for
IFN-γ, IL-4, IL-17A and Foxp3, and analyzed using flow cytometry.
Topical IMQ administration induced an increased percentage of
splenic CD4+ and IL-17A+ T cells compared
with that in the normal mice at day 8 (Fig. 5B). By contrast, the percentage of
CD4+ and IFN-γ+ T cells, as well as
CD4+ and IL-4+ T cells, was only marginally
decreased. Notably, increased percentages of splenic
CD4+ CD25+ Foxp3+ Treg cells were
found in the IMQ-exposed mice compared with that in the normal
group (Fig. 5B). GTW
administration resulted in the marked suppression of the percentage
of IMQ-induced CD4+ IL-17A+ T cells. However,
GTW was not found to affect the proportion of CD4+
IFN-γ+ T cells, CD4+ IL-4+ T cells
and CD4+ CD25+ Foxp3+ Treg
cells.
As the source of IL-17 may not be Th17 cells but γδT
cells, we also evaluated the numbers of γδT cells. Unfortunately,
there were too few to detect in the spleen samples so we detected
their levels in T cells isolated from lymph nodes. The results
showed 3.03±1.00% in the normal mice and 7.58±2.49% in the
IMQ-exposed mice, which was a significant increase (P<0.01).
However, the numbers following GTW treatment were not significantly
decreased (7.09±3.21%) (data not shown).
These data indicated that the effect of GTW was
mediated through Th17 cells rather than Th1, Th2 or Treg cells.
GTW decreases γδT cells in the dermis of
IMQ-exposed mice
We assessed the number of γδT cells in mouse skin by
performing IHC staining. The results showed that the number of γδT
cells in the dermis of mice with IMQ-induced psoriasis was
significantly decreased following GTW treatment (Fig. 6).
In vitro observation and identification
of Th17 cells
After a 3-day culture with naive
(CD4+CD62L+) T cells sorted by the magnetic
beams under a neutral condition and Th17 polarizing condition, the
cells were observed using an inverted microscope. Under the neutral
condition, the cells were growing in a good state, with visible
cell clusters that met with the growth characteristics of T cells.
Under the Th17 polarizing condition, the T cells were slightly
enlarged with abundant and large cell clusters and increased cell
numbers. The results of flow cytometric analysis indicated a
1.45±0.73 and 21.45±1.92% (data not shown) fraction of the Th17
(CD4+IL-17+) in the naïve T cells under the
neutral condition and Th17 polarizing condition, respectively;
thus, a significant difference was observed between the two groups
(P<0.01).
The results of the ELISA indicated a significantly
higher IL-17 secretion level in the Th17 polarizing condition group
than in the neutral condition group (P<0.05) and significantly
lower IL-17 secretion levels in all the GTW groups than in the Th17
polarizing condition group (data not shown; all P<0.05).
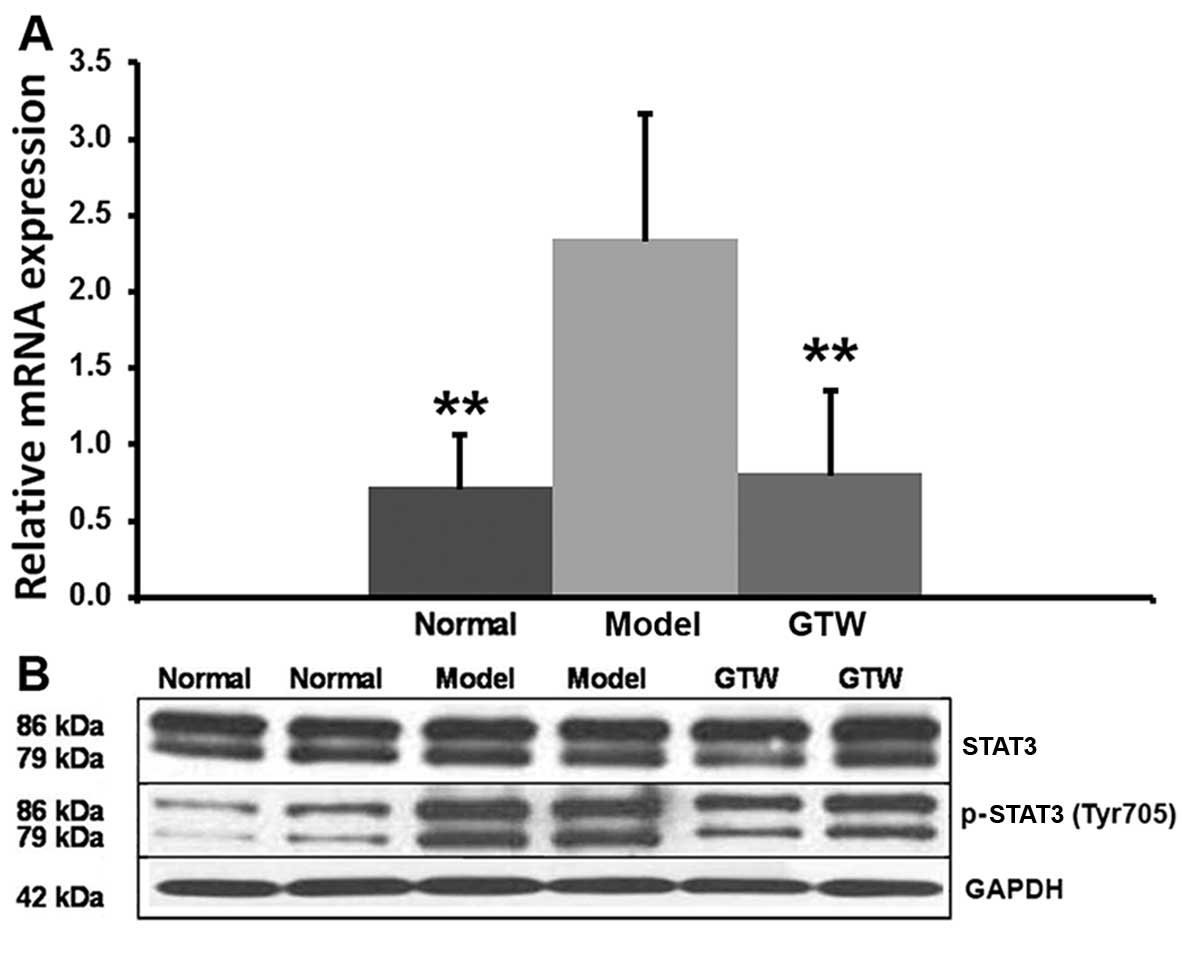
GTW inhibits the expression of retinoic
acid-related orphan receptor (ROR)γt and STAT3 phosphorylation in
IMQ-exposed mice
The transcription factor RORγt critically regulates
IL-17 production in Th17 cells. High mRNA levels of RORγt were
observed in the IMQ-induced mice skin lesions and were decreased by
the co-administration of GTW (Fig.
7A). STAT3 is a critical factor which is involved in Th17
differentiation through the regulation of IL-17 and RORγt. The
semi-quantitative analysis (data not shown) of the blots suggested
that the relative expression levels of STAT3 were 0.983±0.107 for
normal mice, 0.871±0.257 for IMQ-exposed mice and 0.784±0.304 for
GTW-treated mice, and the differences were not significant between
the groups. However, STAT3 phosphorylation was significantly
increased (P<0.01) in the IMQ-exposed mice, to a level of
1.348±0.371 compared with 0.235±0.137 in the normal mice. Analysis
of the effects of GTW on STAT3 activation indicated that GTW
treatment resulted in 0.673±0.126 relative levels showing
significantly reduced (P<0.01) STAT3 phosphorylation compared
with that in the IMQ-exposed mice (Fig. 7B).
Discussion
The Th17 cell response is considered to play a major
role in the pathogenesis of psoriasis. Th17 cells have been
implicated in the formation of IMQ-induced psoriatic lesions in a
mouse model (16). Thus, in this
study, we aimed to examine the known anti-inflammatory effects of
GTW in a mouse model, in order to further elucidate the mechanism
which is responsible for the anti-inflammatory effects of GTW and
protects against the formation of IMQ-induced psoriasis-like
lesions. Our results showed that GTW protected mice from developing
psoriasis-like lesions induced by topical IMQ administration.
Furthermore, our data indicated that this protection was achieved
by inhibiting Th17 cells rather than Th1, Th2 or Treg cells, and
GTW suppressed Th17 function through the inhibition of STAT3
phosphorylation.
The Th17 response is considered to play a major role
in psoriasis due to the increased activity of Th17 cells and
Th17-related cytokines in cutaneous lesions and in the serum of
patients with psoriasis (17–20).
In the present study, no apparent toxicity was
observed in the mice administered GTW intragastrically for 8 days
at doses of 10, 20 and 40 mg/kg (data not shown).
IMQ-induced psoriasis-like lesions in mice share a
substantial number of major hallmarks with human psoriatic plaques
in terms of histopathological and molecular features. In the
present study, inflammation was observed in the IMQ-exposed mouse
skin from days 2–3 onward, with significant thickening, erythema
and scaling of the skin, reaching peak severity at days 7–8. These
symptoms diminished gradually after 10–12 days (data not shown).
GTW exhibited protective activity against the formation of
IMQ-induced psoriasiform lesions at all time-points. Furthermore,
histopathological analysis confirmed that GTW prevented the
abnormal differentiation and proliferation of keratinocytes induced
by IMQ compared with the control mice, and flow cytometric analysis
revealed reduced inflammatory cell infiltration. However, a major
difference in K14 expression was observed in the present study
compared with that which has been observed in human psoriasis
lesions; it is generally agreed that K14 expression is
downregulated in psoriasis lesions, and this may be one of the
mechanisms through which the lesions develop (21). The results in this study
demonstrated that K14 expression was upregulated in IMQ-induced
psoriasiform lesions and then downregulated by GTW treatment. This
suggests that alternative mechanisms may be responsible for these
effects which occurred in our mouse model compared with those which
occur in human psoriatic plaques.
In the present study, comparisons with normal mice
revealed significantly increased levels of Th17 cytokines, such as
IL-17A, IL-17F and IL-22 mRNAs in the skin samples, and
IL-17-secreting splenic CD4+ lymphocytes in the
IMQ-exposed mice. Taken together, these findings confirmed that the
Th17 cell immune response was activated in a mouse model of
IMQ-induced psoriasis-like lesions. GTW exerted suppressive effects
on the Th17 response in this model. Notably, the mRNA levels of
IL-10 and Foxp3 were increased in the skin of the IMQ-exposed mice
in combination with a greater number of
CD4+CD25+ Foxp3+ T cells in the
spleen, which is in accordance with the results of a previous study
(22).
The balance between an efficient immune response and
pathological damage is maintained by the interplay between Treg and
effector T cells. We hypothesized that this balance is upset in
psoriasis by increased Th17 cell infiltration, leading to
concomitant Treg recruitment that acts as a feedback mechanism to
inhibit the hyper-immune response in IL-17-induced inflammation at
the lesion sites (22). This may
account for the increase in the number of Treg cells in IMQ-induced
psoriasis-like lesions in mice skin. By contrast, van der Fits
et al demonstrated only a marginal increase in the
percentage of splenic CD4+IFNγ+ T cells in
IMQ-exposed mice compared with normal mice, although in contrast to
our data, they reported increased percentages of splenic
CD4+IL-4+ T cells (16). These discrepancies may be due to
technical differences between these studies.
Emerging evidence has shown that high levels of
IL-17 are produced by dermal γδ T cells following IL-23 stimulation
and that these cells play a major role in the pathology of
psoriasis (23,24). Thus, we also performed a
preliminary investigation into γδ T cells. The results showed that
although the levels of γδ T cells were too low to detect with our
flow cytometry method in the spleen, levels in the lymph nodes were
clearly increased with IMQ induction; however, in this study, GTW
had no significant effect on these cell numbers. IHC staining
showed that the increased numbers of the γδ T cells in the dermis
of mice with IMQ-induced psoriasis were then decreased with GTW
treatment. Thus, these results suggest an important localized role
for γδ T cells and that further investigation is warranted, to
fully understand the interaction between γδ T cells and GTW
treatment in psoriasis.
In our experiments, GTW did not exert a
statistically significant effect on the proportion of
CD4+IFN-γ+ T cells,
CD4+IL-4+ T cells and
CD4+CD25+ Foxp3+ Treg cells in the
spleen cells. Taken together with the unchanged mRNA levels of Th1
cytokine IFNγ, Th2 cytokine IL-4 and Treg cytokine IL-10 in
IMQ-exposed mouse skin after GTW administration, these findings
suggest that the immunosuppressive effect of GTW in psoriasis is
exerted mainly on Th17 cells, rather than on Th1, Th2 or Treg
cells.
It has been proposed that RORγt is a 'master
regulator' of Th17 cell differentiation (25). RORγt deficiency has been reported
to result in a profound loss of function in Th17 cells and to
attenuate autoimmune development in an animal model of experimental
autoimmune encephalomyelitis (26). STAT3 is required for multiple
functions in Th17 cells such as gene expression of IL-17 and RORγt
(27,28). It has been reported that
homodimerization and nuclear translocation of p-STAT3 protein
induced the transcription of RORγt and Th17 cytokines, such as
IL-17A, IL-17F and IL-22 (6). We
demonstrated that GTW inhibited STAT3 phosphorylation and markedly
reduced the mRNA levels of RORγt, IL-17A, IL-17F and IL-22 in a
mouse model of IMQ-induced psoriasis-like lesions. As Th17
cytokines recruit neutrophils, the level of Gr-1+ cells
in IMQ-exposed mouse skin may be affected by the repressed
expression of IL-17, which is consistent with the results shown in
Fig. 3.
In conclusion, our data indicate that GTW inhibits
the formation of IMQ-induced skin lesions by downregulating the
function of Th17 cells rather than Th1, Th2 or Treg cells, and that
GTW suppresses Th17 function through the inhibition of STAT3
phosphorylation.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (nos. 81072810 and 81302985),
and the Beijing Science and Technology Projects (no.
D08050703550901).
References
|
1
|
Chan JR, Blumenschein W, Murphy E, Diveu
C, Wiekowski M, Abbondanzo S, Lucian L, Geissler R, Brodie S,
Kimball AB, et al: IL-23 stimulates epidermal hyperplasia via TNF
and IL-20R2-dependent mechanisms with implications for psoriasis
pathogenesis. J Exp Med. 203:2577–2587. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rácz E, Kurek D, Kant M, Baerveldt EM,
Florencia E, Mourits S, de Ridder D, Laman JD, van der Fits L and
Prens EP: GATA3 expression is decreased in psoriasis and during
epidermal regeneration; induction by narrow-band UVB and IL-4. PLoS
One. 6:e198062011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lowes MA, Bowcock AM and Krueger JG:
Pathogenesis and therapy of psoriasis. Nature. 445:866–873. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gaspari AA: Innate and adaptive immunity
and the pathophysiology of psoriasis. J Am Acad Dermatol. 54(Suppl
2): S67–S80. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lew W, Bowcock AM and Krueger JG:
Psoriasis vulgaris: cutaneous lymphoid tissue supports T-cell
activation and 'Type 1' inflammatory gene expression. Trends
Immunol. 25:295–305. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Di Cesare A, Di Meglio P and Nestle FO:
The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J
Invest Dermatol. 129:1339–1350. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Blauvelt A: T-helper 17 cells in psoriatic
plaques and additional genetic links between IL-23 and psoriasis. J
Invest Dermatol. 128:1064–1067. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pène J, Chevalier S, Preisser L, Vénéreau
E, Guilleux MH, Ghannam S, Molès JP, Danger Y, Ravon E, Lesaux S,
et al: Chronically inflamed human tissues are infiltrated by highly
differentiated Th17 lymphocytes. J Immunol. 180:7423–7430. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McGeachy MJ, Chen Y, Tato CM, Laurence A,
Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O'Shea JJ and Cua
DJ: The interleukin 23 receptor is essential for the terminal
differentiation of interleukin 17-producing effector T helper cells
in vivo. Nat Immunol. 10:314–324. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tonel G, Conrad C, Laggner U, Di Meglio P,
Grys K, McClanahan TK, Blumenschein WM, Qin JZ, Xin H, Oldham E, et
al: Cutting edge: a critical functional role for IL-23 in
psoriasis. J Immunol. 185:5688–5691. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mabuchi T, Takekoshi T and Hwang ST:
Epidermal CCR6+ γδ T cells are major producers of IL-22
and IL-17 in a murine model of psoriasiform dermatitis. J Immunol.
187:5026–5031. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Johnson-Huang LM, Suárez-Fariñas M,
Sullivan-Whalen M, Gilleaudeau P, Krueger JG and Lowes MA:
Effective narrow-band UVB radiation therapy suppresses the
IL-23/IL-17 axis in normalized psoriasis plaques. J Invest
Dermatol. 130:2654–2663. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nograles KE, Davidovici B and Krueger JG:
New insights in the immunologic basis of psoriasis. Semin Cutan Med
Surg. 29:3–9. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wan Y, Gu L, Suzuki K, Karasawa T, Fujioka
Y, Han GD, Koike H, Kawachi H and Shimizu F: Multi-glycoside of
Tripterygium wilfordii Hook.f. ameliorates proteinuria and acute
mesangial injury induced by anti-Thy1.1 monoclonal antibody.
Nephron, Exp Nephrol. 99:e121–e129. 2005. View Article : Google Scholar
|
|
15
|
Zhan QX and Xu LM: Effiency of
Tripterygium Wilfordii on treating psoriasis:A systematic review.
Chin J Dermatovenerol Integr Trad West Med. 6:192–196. 2007.
|
|
16
|
van der Fits L, Mourits S, Voerman JS,
Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens
EP and Lubberts E: Imiquimod-induced psoriasis-like skin
inflammation in mice is mediated via the IL-23/IL-17 axis. J
Immunol. 182:5836–5845. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kagami S, Rizzo HL, Lee JJ, Koguchi Y and
Blauvelt A: Circulating Th17, Th22, and Th1 cells are increased in
psoriasis. J Invest Dermatol. 130:1373–1383. 2010. View Article : Google Scholar :
|
|
18
|
Lowes MA, Kikuchi T, Fuentes-Duculan J,
Cardinale I, Zaba LC, Haider AS, Bowman EP and Krueger JG:
Psoriasis vulgaris lesions contain discrete populations of Th1 and
Th17 T cells. J Invest Dermatol. 128:1207–1211. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Harper EG, Guo C, Rizzo H, Lillis JV,
Kurtz SE, Skorcheva I, Purdy D, Fitch E, Iordanov M and Blauvelt A:
Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro
and in vivo: implications for psoriasis pathogenesis. J Invest
Dermatol. 129:2175–2183. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yilmaz SB, Cicek N, Coskun M, Yegin O and
Alpsoy E: Serum and tissue levels of IL-17 in different clinical
subtypes of psoriasis. Arch Dermatol Res. 304:465–469. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ota T, Takekoshi S, Takagi T, Kitatani K,
Toriumi K, Kojima T, Kato M, Ikoma N, Mabuchi T and Ozawa A: Notch
signaling may be involved in the abnormal differentiation of
epidermal keratinocytes in psoriasis. Acta Histochem Cytochem.
47:175–183. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang L, Yang XQ, Cheng J, Hui RS and Gao
TW: Increased Th17 cells are accompanied by FoxP3(+) Treg cell
accumulation and correlated with psoriasis disease severity. Clin
Immunol. 135:108–117. 2010. View Article : Google Scholar
|
|
23
|
Cai Y, Shen X, Ding C, Qi C, Li K, Li X,
Jala VR, Zhang HG, Wang T, Zheng J and Yan J: Pivotal role of
dermal IL-17-producing γδ T cells in skin inflammation. Immunity.
35:596–610. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pantelyushin S, Haak S, Ingold B, Kulig P,
Heppner FL, Navarini AA and Becher B: Rorγt+ innate
lymphocytes and γδ T cells initiate psoriasiform plaque formation
in mice. J Clin Invest. 122:2252–2256. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
O'Shea JJ, Steward-Tharp SM, Laurence A,
Watford WT, Wei L, Adamson AS and Fan S: Signal transduction and
Th17 cell differentiation. Microbes Infect. 11:599–611. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dong C: TH17 cells in development: an
updated view of their molecular identity and genetic programming.
Nat Rev Immunol. 8:337–348. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Y, Yu C, Zhu WM, Xie Y, Qi X, Li N and
Li JS: Triptolide ameliorates IL-10-deficient mice colitis by
mechanisms involving suppression of IL-6/STAT3 signaling pathway
and down-regulation of IL-17. Mol Immunol. 47:2467–2474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gomez-Rodriguez J, Sahu N, Handon R,
Davidson TS, Anderson SM, Kirby MR, August A and Schwartzberg PL:
Differential expression of interleukin-17A and -17F is coupled to T
cell receptor signaling via inducible T cell kinase. Immunity.
31:587–597. 2009. View Article : Google Scholar : PubMed/NCBI
|