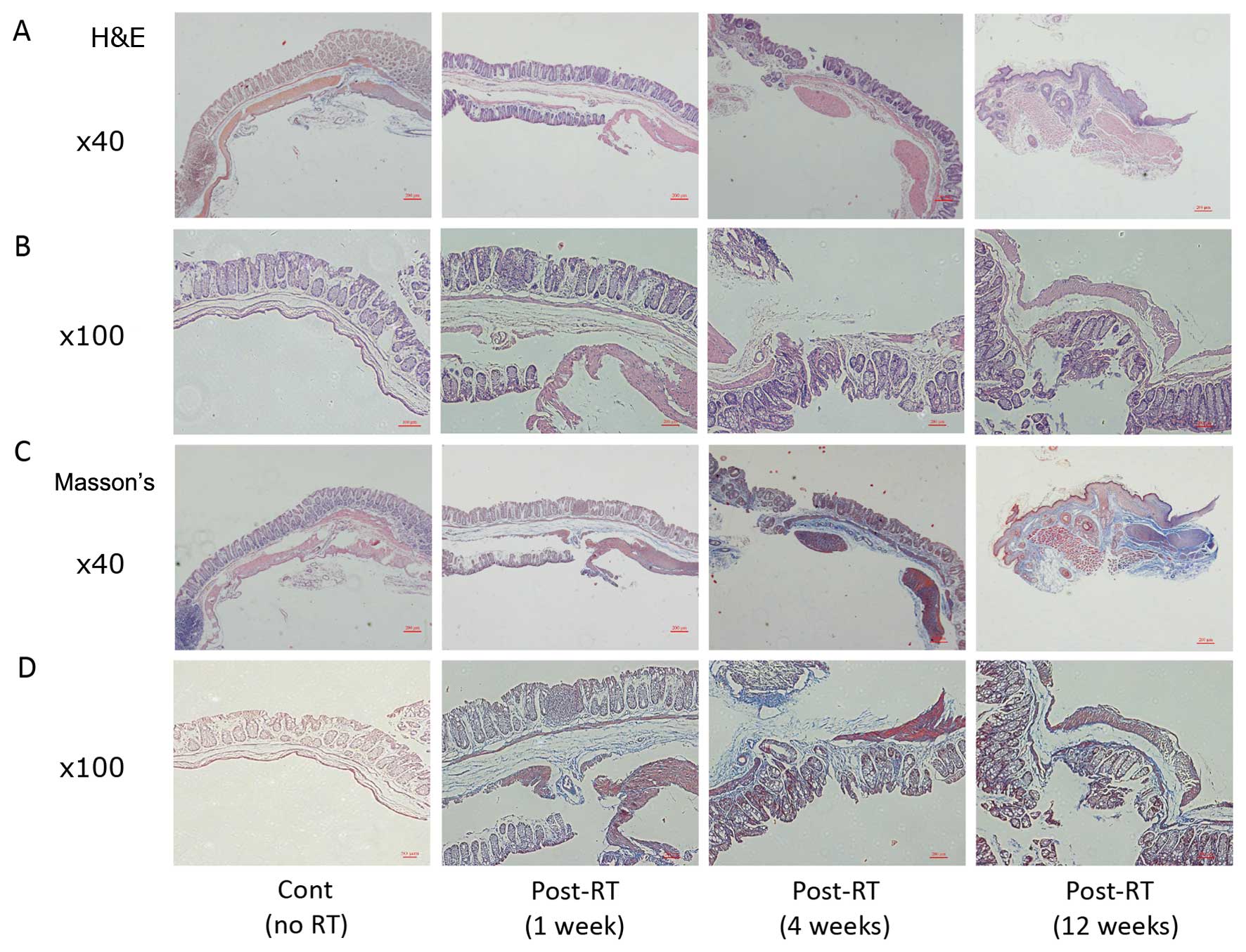
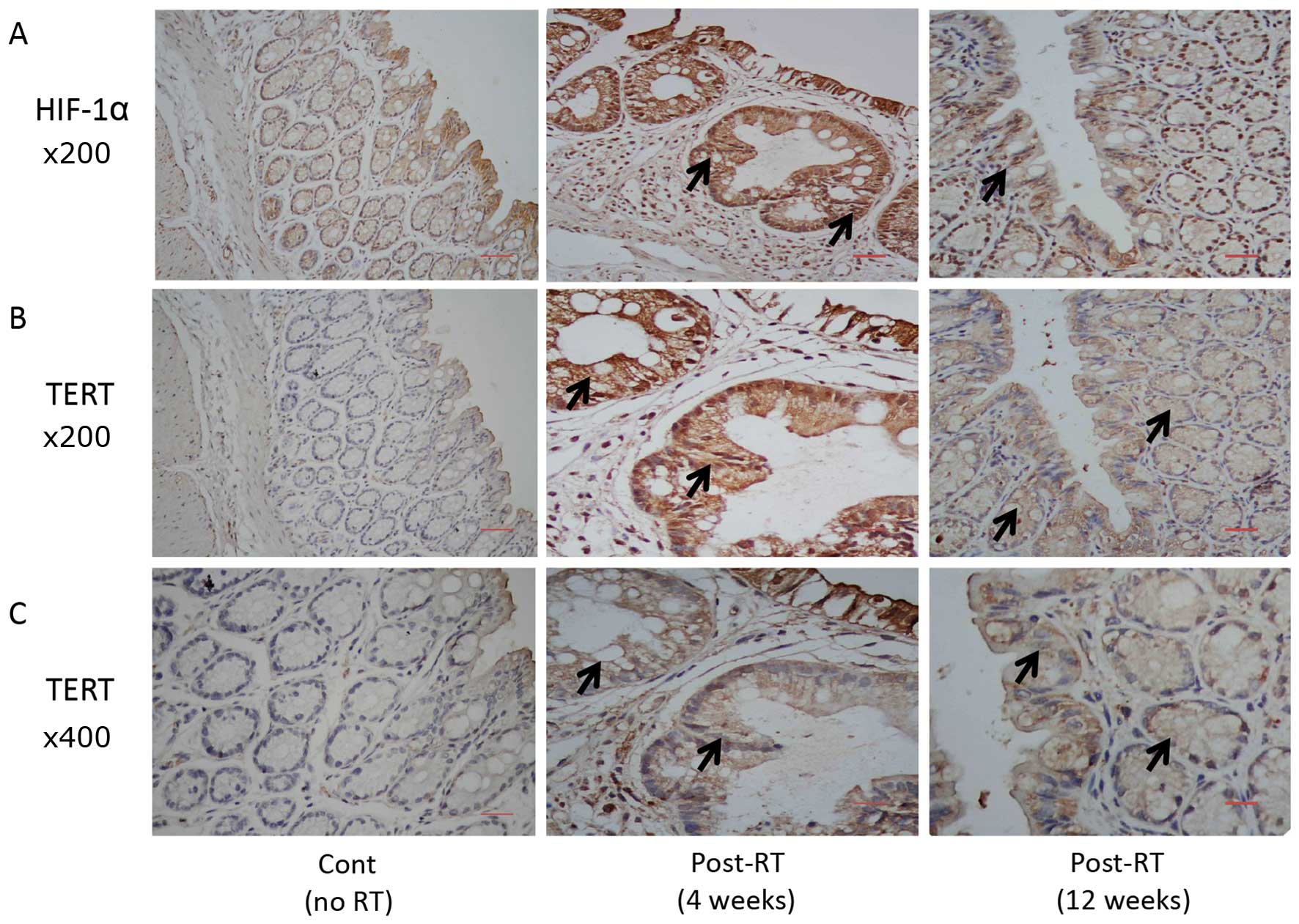
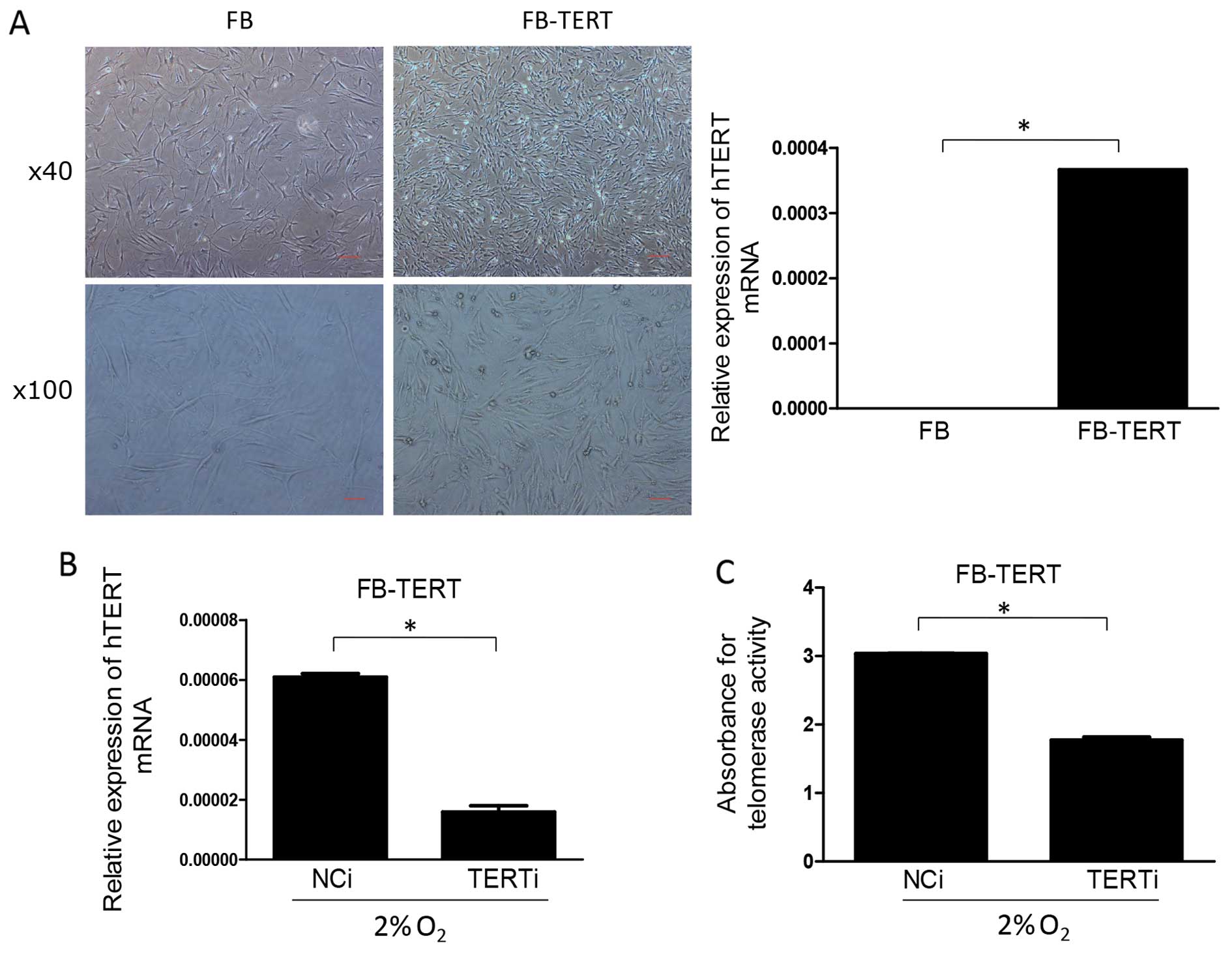
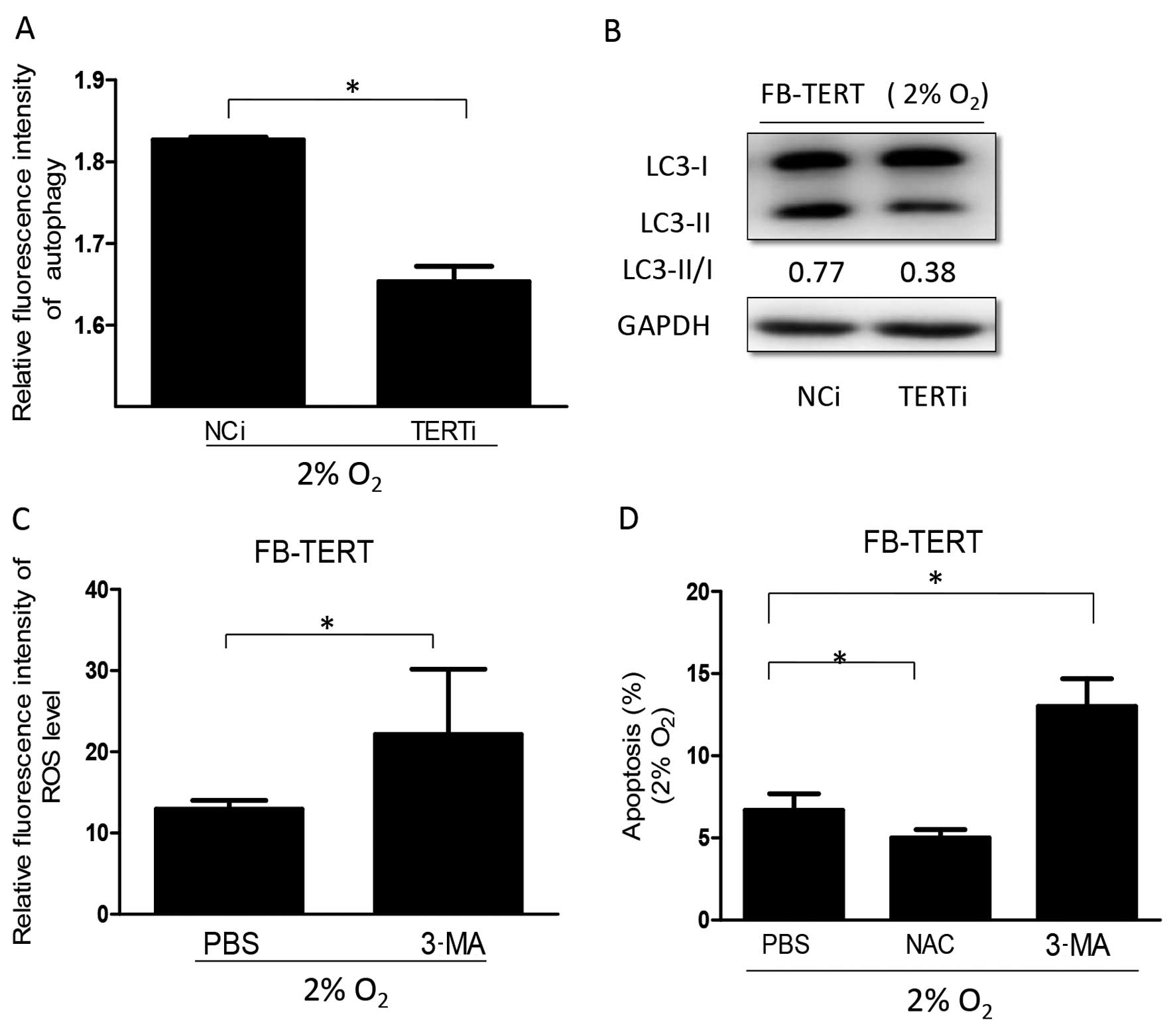
|
1
|
Grodsky MB and Sidani SM: Radiation
proctopathy. Clin Colon Rectal Surg. 28:103–111. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Christofidou-Solomidou M, Pietrofesa RA,
Arguiri E, Schweitzer KS, Berdyshev EV, McCarthy M, Corbitt A,
Alwood JS, Yu Y and Globus RK: Space radiation-associated lung
injury in a murine model. Am J Physiol Lung Cell Mol Physiol.
308:L416–L428. 2015. View Article : Google Scholar :
|
|
3
|
Nordal RA, Nagy A, Pintilie M and Wong CS:
Hypoxia and hypoxia-inducible factor-1 target genes in central
nervous system radiation injury: a role for vascular endothelial
growth factor. Clin Cancer Res. 10:3342–3353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu Y, Kudo K, Abe Y, Aoki M, Hu DL,
Kijima H and Nakane A: Hypoxia expression in radiation-induced late
rectal injury. J Radiat Res (Tokyo). 49:261–268. 2008. View Article : Google Scholar
|
|
5
|
Liu Y, Kudo K, Abe Y, Hu DL, Kijima H,
Nakane A and Ono K: Inhibition of transforming growth factor-beta,
hypoxia-inducible factor-1alpha and vascular endothelial growth
factor reduced late rectal injury induced by irradiation. J Radiat
Res (Tokyo). 50:233–239. 2009. View Article : Google Scholar
|
|
6
|
Distler JH, Jüngel A, Pileckyte M, Zwerina
J, Michel BA, Gay RE, Kowal-Bielecka O, Matucci-Cerinic M, Schett
G, Marti HH, et al: Hypoxia-induced increase in the production of
extracellular matrix proteins in systemic sclerosis. Arthritis
Rheum. 56:4203–4215. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Haroon ZA, Raleigh JA, Greenberg CS and
Dewhirst MW: Early wound healing exhibits cytokine surge without
evidence of hypoxia. Ann Surg. 231:137–147. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schmidt-Ullrich RK, Dent P, Grant S,
Mikkelsen RB and Valerie K: Signal transduction and cellular
radiation responses. Radiat Res. 153:245–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Spilsbury A, Miwa S, Attems J and Saretzki
G: The role of telomerase protein TERT in Alzheimer's disease and
in tau-related pathology in vitro. J Neurosci. 35:1659–1674. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Greider CW: Chromosome first aid. Cell.
67:645–647. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
De Boeck G, Forsyth RG, Praet M and
Hogendoorn PC: Telomere-associated proteins: cross-talk between
telomere maintenance and telomere-lengthening mechanisms. J Pathol.
217:327–344. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ahmed S, Passos JF, Birket MJ, Beckmann T,
Brings S, Peters H, Birch-Machin MA, von Zglinicki T and Saretzki
G: Telomerase does not counteract telomere shortening but protects
mitochondrial function under oxidative stress. J Cell Sci.
121:1046–1053. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Masutomi K, Possemato R, Wong JM, Currier
JL, Tothova Z, Manola JB, Ganesan S, Lansdorp PM, Collins K and
Hahn WC: The telomerase reverse transcriptase regulates chromatin
state and DNA damage responses. Proc Natl Acad Sci USA.
102:8222–8227. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jan HM, Wei MF, Peng CL, Lin SJ, Lai PS
and Shieh MJ: The use of polyethylenimine-DNA to topically deliver
hTERT to promote hair growth. Gene Ther. 19:86–93. 2012. View Article : Google Scholar
|
|
15
|
Qu Y, Duan Z, Zhao F, Wei D, Zhang J, Tang
B, Li J, Yang C and Mu D: Telomerase reverse transcriptase
upregulation attenuates astrocyte proliferation and promotes
neuronal survival in the hypoxic-ischemic rat brain. Stroke.
42:3542–3550. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tao X, Ming-Kun Y, Wei-Bin S, Hai-Long G,
Rui K and Lai-Yong T: Role of telomerase reverse transcriptase in
glial scar formation after spinal cord injury in rats. Neurochem
Res. 38:1914–1920. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Osanai M, Tamaki T, Yonekawa M, Kawamura A
and Sawada N: Transient increase in telomerase activity of
proliferating fibroblasts and endothelial cells in granulation
tissue of the human skin. Wound Repair Regen. 10:59–66. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nishi H, Nakada T, Kyo S, Inoue M, Shay JW
and Isaka K: Hypoxia-inducible factor 1 mediates upregulation of
telomerase (hTERT). Mol Cell Biol. 24:6076–6083. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Indran IR, Hande MP and Pervaiz S: hTERT
overexpression alleviates intracellular ROS production, improves
mitochondrial function, and inhibits ROS-mediated apoptosis in
cancer cells. Cancer Res. 71:266–276. 2011. View Article : Google Scholar
|
|
20
|
Okunieff P, Cornelison T, Mester M, Liu W,
Ding I, Chen Y, Zhang H, Williams JP and Finkelstein J: Mechanism
and modification of gastrointestinal soft tissue response to
radiation: role of growth factors. Int J Radiat Oncol Biol Phys.
62:273–278. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang S, Zhang M, Chen C, Cao Y, Tian Y,
Guo Y, Zhang B, Wang X, Zhang L, et al: Triptolide mitigates
radiation-induced pulmonary fibrosis. Radiat Res. 184:509–517.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Martin M, Lefaix J and Delanian S:
TGF-beta1 and radiation fibrosis: a master switch and a specific
therapeutic target? Int J Radiat Oncol Biol Phys. 47:277–290. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Murphy MP: How mitochondria produce
reactive oxygen species. Biochem J. 417:1–13. 2009. View Article : Google Scholar
|
|
24
|
Lee J, Giordano S and Zhang J: Autophagy,
mitochondria and oxidative stress: cross-talk and redox signalling.
Biochem J. 441:523–540. 2012. View Article : Google Scholar :
|
|
25
|
Meister A and Anderson ME: Glutathione.
Annu Rev Biochem. 52:711–760. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun Y, Xing X, Liu Q, Wang Z, Xin Y, Zhang
P, Hu C and Liu Y: Hypoxia-induced autophagy reduces
radiosensitivity by the HIF-1α/miR-210/Bcl-2 pathway in colon
cancer cells. Int J Oncol. 46:750–756. 2015.
|
|
27
|
Kroemer G and Jäättelä M: Lysosomes and
autophagy in cell death control. Nat Rev Cancer. 5:886–897. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Baehrecke EH: Autophagy: dual roles in
life and death? Nat Rev Mol Cell Biol. 6:505–510. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
He WS, Dai XF, Jin M, Liu CW and Rent JH:
Hypoxia-induced autophagy confers resistance of breast cancer cells
to ionizing radiation. Oncol Res. 20:251–258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Apel A, Herr I, Schwarz H, Rodemann HP and
Mayer A: Blocked autophagy sensitizes resistant carcinoma cells to
radiation therapy. Cancer Res. 68:1485–1494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sannigrahi MK, Singh V, Sharma R, Panda NK
and Khullar M: Role of autophagy in head and neck cancer and
therapeutic resistance. Oral Dis. 21:283–291. 2015. View Article : Google Scholar
|
|
32
|
Rouschop KM, Ramaekers CH, Schaaf MB,
Keulers TG, Savelkouls KG, Lambin P, Koritzinsky M and Wouters BG:
Autophagy is required during cycling hypoxia to lower production of
reactive oxygen species. Radiother Oncol. 92:411–416. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zeng M, Wei X, Wu Z, Li W, Li B, Zhen Y,
Chen J, Wang P and Fei Y: NF-κB-mediated induction of autophagy in
cardiac ischemia/reperfusion injury. Biochem Biophys Res Commun.
436:180–185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang Y, Wu Y, Wu D, Tashiro S, Onodera S
and Ikejima T: NF-kappab facilitates oridonin-induced apoptosis and
autophagy in HT1080 cells through a p53-mediated pathway. Arch
Biochem Biophys. 489:25–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bubici C, Papa S, Dean K and Franzoso G:
Mutual cross-talk between reactive oxygen species and nuclear
factor-kappa B: molecular basis and biological significance.
Oncogene. 25:6731–6748. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun Z, Yin Z, Liu C and Tian J: The
changes in the expression of NF-KB in a degenerative human
intervertebral disc model. Cell Biochem Biophys. 72:115–122. 2015.
View Article : Google Scholar
|