Introduction
Acute respiratory distress syndrome (ARDS) is
characterized by overwhelming lung inflammation and increased
microvascular permeability, causing the diffuse infiltration of
neutrophils and lung edema (1).
Neutrophils, rapidly recruited from the circulation to sites of
infection or tissue injury, play a prominent role in host defense
against invading pathogens (2).
The effective resolution of inflammation and the restoration of
tissue homeostasis critically depends on the neutrophil influx and
the timely removal of infiltrating neutrophils (3). However, as these neutrophil
functions lack specificity and can be injurious to host tissues, it
is important that neutrophil activity be tightly regulated to
prevent the perpetuation of inflammation. Actually, neutrophil
apoptosis can be delayed at infection sites by a range of factors,
including bacterial products such as lipopolysaccharide (LPS)
(4), and pro-inflammatory
cytokines (5), as well as hypoxia
(6), ostensibly to extend their
functional lifespan (7). Once an
episode of acute neutrophilic inflammation is complete, it is
essential that neutrophil recruitment is halted and that recruited
neutrophils undergo apoptosis, before disposal of the apoptotic
cells by surrounding phagocytes, such as alveolar macrophages
(3,8), to ensure the efficient resolution of
inflammation (9). However, in
reality, the natural resolution process of neutrophil apoptosis
followed by phagocytosis is impaired in numerous human inflammatory
disease states, with delayed neutrophil apoptosis observed in
conditions such as ARDS (10).
Observations in medical patients showed that neutrophil apoptosis
is inversely proportional to the severity of sepsis and
sepsis-induced ARDS, suggesting that neutrophil apoptosis may be a
marker of the severity of sepsis (10). Furthermore, accumulating evidence
has indicated a consistent association between neutrophils and ARDS
in human and animal models and the propensity of neutrophils and
their products to cause lung injury in experimental systems,
leading to the conclusion that neutrophils have an important
causative role in ARDS, and neutrophil apoptosis could emerge as a
critical control point in resolving inflammation. Optimistically,
recent studies using a variety of gene knockout, transgenic and
pharmacological strategies in diverse models of inflammation, have
demonstrated that modulating neutrophil apoptosis can profoundly
affect the outcome of inflammation (11–15).
Ghrelin is a 28-amino-acid peptide esterified with
octanoic acid on Ser 3 that is principally released from the
stomach mucosa (16). Apart from
its regulatory roles in growth hormone release, appetite and
gastric motility, ghrelin also has numerous peripheral actions,
including anti-inflammatory, anti-fibrotic and antioxidant
properties (16,17). The administration of ghrelin has
been reported to attenuate neutrophil migration, reduce
inflammatory cytokine production, promote the generation of
anti-inflammatory cytokines, and promote the phagocytosis of
apoptotic neutrophils by macrophages (16,17), thereby attenuating inflammation in
several disease models, including respiratory infection (18), polymicrobial sepsis (19,20), arthritis (21) and ARDS (20,22). However, little is known about
whether ghrelin is able to regulate neutrophil apoptosis, a
critical control point in the resolution of inflammation.
Based on previous observations, we hypothesized that
ghrelin may ameliorate lung impairment in ARDS by promoting
neutrophil apoptosis. Therefore, in this study, we examined this
hypothesis by investigating the role of ghrelin in ARDS using
primary cultured human peripheral blood neutrophils. We examined
the effects of ghrelin on neutrophil apoptosis using flow
cytometry, as well as by performing mitochondrial membrane
potential (ΔΨm) and terminal deoxynucleotidyl transferase
(TdT)-mediated dUTP nick-end labeling (TUNEL) assays. We also
performed western blot analysis and immunochemistry to detect the
expression of B-cell lymphoma 2 (Bcl-2) family proteins and cleaved
caspase-3. Our study aimed at providing laboratory evidence and a
potential theoretical basis for the effects of ghrelin on
neutrophil apoptosis in order to further elucidate the molecular
mechanisms responsible for the anti-inflammatory function of
ghrelin in ARDS.
Materials and methods
Reagents and antibodies
Human recombinant ghrelin was purchased from Enzo
Life Sciences (Farmingdale, NY, USA). Percoll was purchased from
Axis-Shield (Oslo, Norway). RPMI-1640 medium, fetal bovine serum
(FBS), and penicillin-streptomycin were obtained from Life
Technologies (Grand Island, NY, USA). Dimethyl sulfoxide (DMSO),
4′,6-diamidino-2-phenylindole (DAPI), Hoechst 33342, Tween-20, and
all buffers and salt solutions were purchased from Sigma-Aldrich
(St. Louis, MO, USA). The TUNEL assay kit, protease inhibitor
cocktail, and phosphatase inhibitor cocktails were from Roche
(Basel, Switzerland). The cleaved caspase-3 antibody (#AB3623) was
from Millipore (Billerica, MA, USA), and the Annexin V-fluorescein
isothiocyanate (FITC) cell apoptosis kit was obtained from BD
Biosciences (San Jose, CA, USA). Antibodies against Bax (#2772),
Bcl-2 (#2876), GAPDH (#2118) and peroxidase-conjugated secondary
antibody (#7074) were all purchased from Cell Signaling Technology,
Inc. (Danvers, MA, USA). Alexa Fluor-labeled secondary antibody
(#R37167) was purchased from Invitrogen (Carlsbad, CA, USA).
Neutrophil isolation and treatment
Venous blood collected from 20 healthy subjects was
fractionated on Ficoll-Hypaque and dextran gradients to separate
neutrophils. All blood samples were collected after obtaining
written informed consent from each subject and following the
approval of the Medical Ethics Committe of the First Affiliated
Hospital, Sun Yat-Sen University, Guangzhou, China. The neutrophil
layer was then transferred to a fresh tube and centrifuged for 10
min at 4°C. Hypotonic lysis with 0.2% NaCl was performed to remove
the remaining erythrocytes. The morphology and purity of the
neutrophils were assessed by Giemsa (Beijing Leagene Biotech Co.,
Ltd., Beijing, China) and Hoechst staining. For all experiments,
isolated neutrophils were resuspended in RPMI-1640 medium,
containing 100 U/ml penicillin and 100 µg/ml streptomycin
supplemented with 10% FBS. The prepared neutrophils were divided
into the following groups: i) the control group (neutrophils +
medium); ii) the LPS group (neutrophils + medium + 100 ng/ml LPS);
iii) the LPS + ghrelin group (neutrophils + medium + 100 ng/ml LPS
+ 100 nM ghrelin); and iv) the ghrelin group (neutrophils + medium
+ 100 nM ghrelin). The isolated human neutrophils were incubated
with or without ghrelin (100 nM) for 1.5 h prior to the addition of
LPS (100 ng/ml) for 8 h. The concentrations of the drugs were
selected based on previous studies (23,37) and on preliminary experiments (data
not shown).
Assessment of neutrophil apoptosis
Apoptosis was determined by 3 methods. Apoptosis was
first evaluated by cytofluorometry using the Annexin V-FITC kit.
Briefly, the cells in the different treatment groups were
centrifuged at 100 × g for 5 min, washed twice with cold
phosphate-buffered saline (PBS), and then resuspended in 500
µl binding buffer. The cells were then labeled with Annexin
V-FITC (5 µl) and propidium iodide (PI, 5 µl) for 15
min in the dark at room temperature before being analyzed using a
FACScan flow cytometer (BD Biosciences).
The collapse of the electrochemical gradient across
the mitochondrial membrane during apoptosis was measured using the
JC-1 mitochondrial membrane potential detection kit (Beyotime,
Beijing, China) using flow cytometry as described by the
manufacturer. This kit uses a unique cationic dye, JC-1, to signal
the loss of the ΔΨm. Briefly, following treatment with the
indicated agents and concentrations for 8 h, the cells were
collected, resuspended in JC-1 reagent solution, and incubated for
15 min at 37°C in the dark. The cells were then washed with a
buffer solution and resuspended in assay buffer, and analyzed by a
FACScan flow cytometer.
To measure the double-stranded cleavage of DNA,
TUNEL assay was performed with an in situ cell death
detection kit following the manufacturer's instructions. Briefly,
the cells grown on coverslips were washed with PBS and fixed with
4% paraformaldehyde for 15 min at room temperature. After washing,
the cells were incubated in permeabilization solution (0.1% Triton
X-100 in 0.1% sodium citrate, freshly prepared) for 2 min on ice.
The cells were then incubated with the TUNEL reaction mixture in a
humidified chamber at 37°C for 1 h. Subsequently, the cells were
briefly rinsed with PBS and counterstained with DAPI for 5 min in
order to visualize the nuclei. Stained sections were examined by a
fluorescence microscope (Carl Zeiss, Tokyo, Japan), and images of 5
random and non-overlapping fields were examined at an objective
magnification of ×200 for analysis.
Western blot analysis
Following BCA protein assaying (Thermo Fisher
Scientific, Cramlington, UK), equal amounts of proteins were
separated by 10% sodium dodecyl sulfate-polyacrymide gel
electrophoresis and transferred onto nitrocellulose membranes,
which were blocked using 5% non-fat milk for 1 h at room
temperature. The membranes were then incubated overnight at 4°C
with primary antibodies specific for Bax (1:1,000 dilution), Bcl-2
(1:1,000 dilution), or cleaved caspase-3 (1:200 dilution). After
washing, the membranes were incubated with horseradish
peroxidase-conjugated anti-mouse or anti-rabbit secondary
antibodies for 1 h at room temperature. Proteins were visualized
using an enhanced chemiluminescence kit (Millipore). The relative
protein expression levels were determined by densitometry and
normalized to the GAPDH levels using Image Pro Plus 6.0 software
(Media Cybernetics, Rockville, MD, USA).
Detection of cleaved caspase-3 by
immunofluorescence
The cells grown on coverslips were fixed with
paraformaldehyde for 15 min at room temperature. After washing with
PBS 3 times for 5 min, the cells were incubated for 30 min with 1%
bovine serum albumin (BSA) to block the non-specific binding of the
antibodies. The cells were then incubated with primary antibody
(1:200 dilution) in 1% BSA in a humidified chamber overnight at
4°C. After washing 3 times in PBS, the cell samples were incubated
with Alexa Fluor 488-conjugated goat antirabbit IgG secondary
antibody (1:200) in 1% BSA for 1 h in the dark at room temperature,
and then washed and counterstained with DAPI for 5 min. The
coverslips were then visualized under a fluorescence microscope at
×200 magnification. Quantification was performed using Image Pro
Plus 6.0 software.
Statistical analyses
Statistical significance of the differences between
groups among repeated experiments was calculated by one-way ANOVA
and Fisher's LSD-test using GraphPad Prism 4 software (GraphPad
Software Ltd, La Jolla, CA, USA). The results are expressed as the
mean values ± standard devitation. In all statistical tests, a
P-value <0.05 was considered to indicate a statistically
significant difference.
Results
Determination of the viability and purity
of the isolated neutrophils by Giemsa and Hoechst staining
The use of a percoll gradient to purify peripheral
blood neutrophils has been previously described (24). This approach results in a highly
purified population of neutrophils, with a purity >95% (as shown
by Giemsa staining) and a viability >98% (as shown by trypan
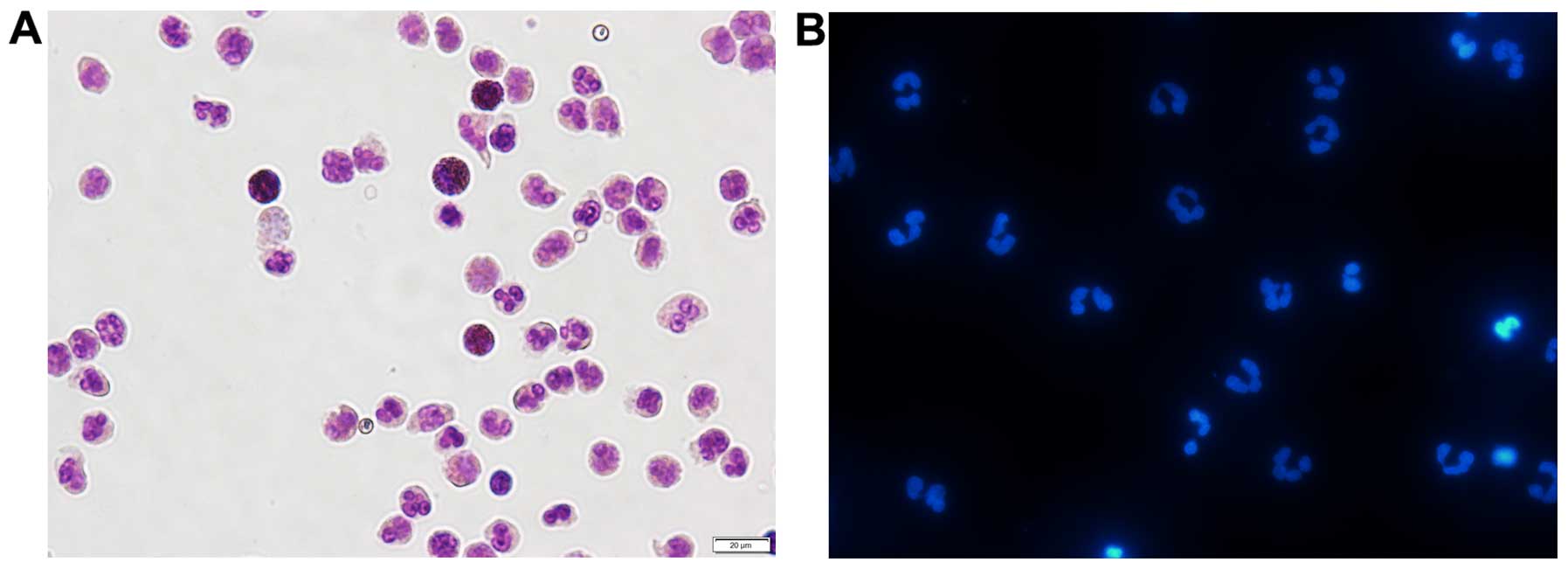
blue staining) (data not shown). As shown in Fig. 1A, after Giemsa staining, normal
cell nuclei were dyed blue or hyacinthine, with homogeneous color
and luster. Hoechst 33342 staining revealed a typical nuclear
morphology in the freshly isolated neutrophils (Fig. 1B). The examination of cell purity
proved that the isolated neutrophils perfectly met the requirements
for all the subsequent experiments.
Detection of neutrophil apoptosis by flow
cytometry
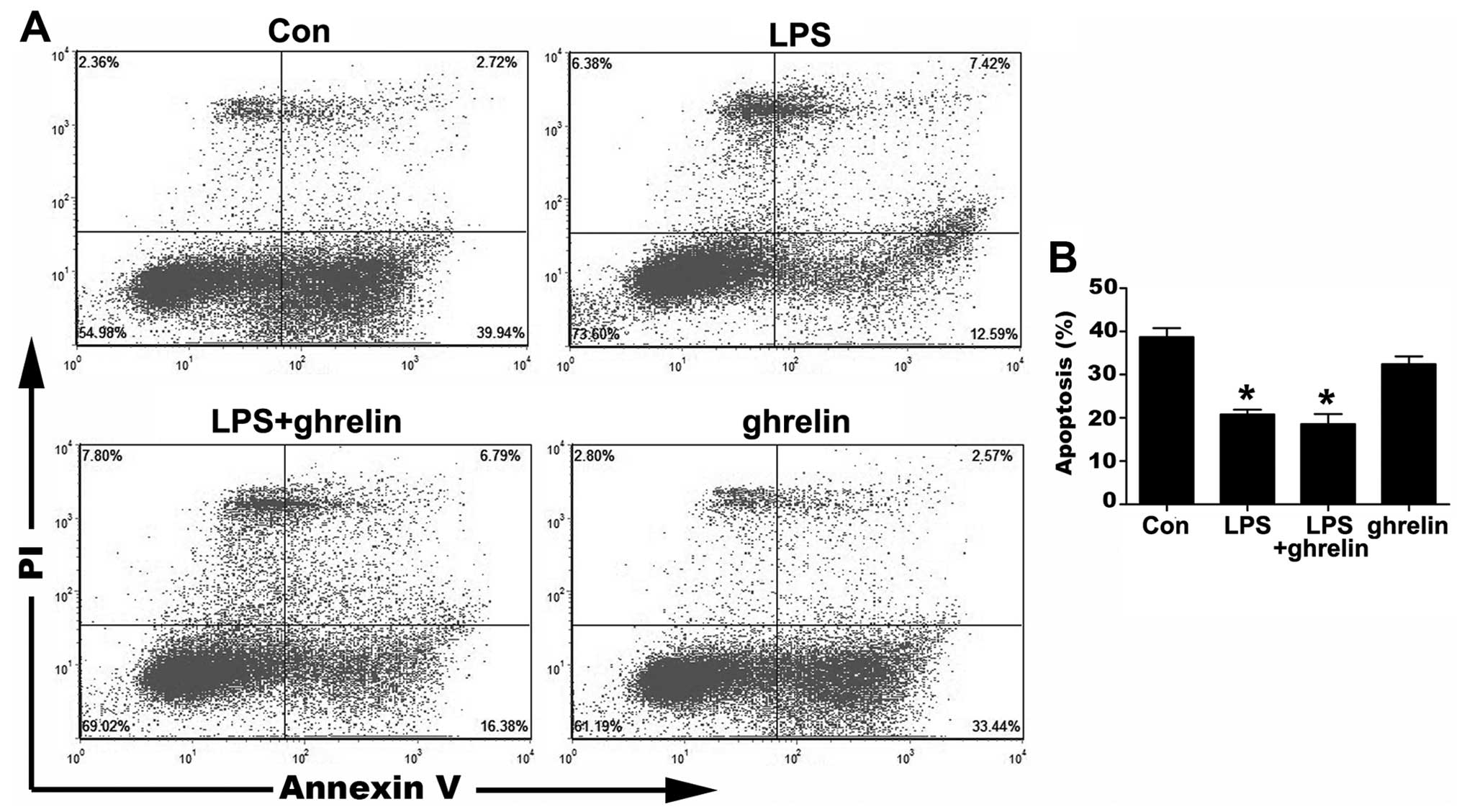
The occurrence of neutrophil apoptosis was
demonstrated using the Annexin V-FITC staining methods (Fig. 2). The results of flow cytometry
assay revealed that the ratio of apoptotic cells significantly
decreased from 38.67±2.09 in the untreated cells to 20.80±1.09% and
18.57±2.32% in the LPS-stimulated cells and LPS + ghrelin-treated
cells, respectively, indicating that LPS significantly prolonged
the neutrophil lifespan. However, treatment with ghrelin neither
induced nor inhibited neutrophil apoptosis, since there were no
apparent differences observed in the percentages of apoptotic
neutrophils between the LPS group and the LPS + ghrelin group, and
between the control and ghrelin groups (32.39±1.84%) (Fig. 2).
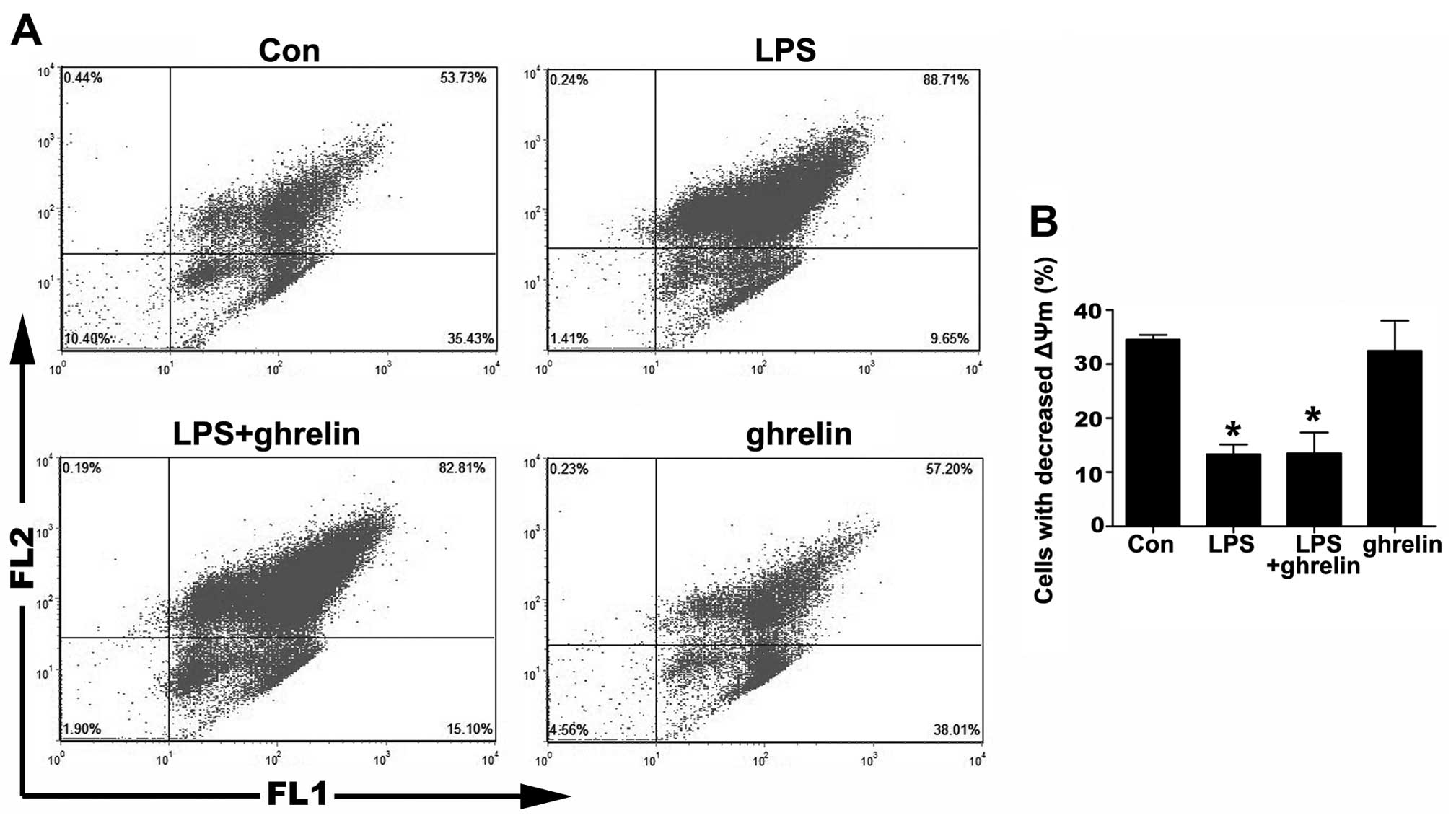
Detection of neutrophil apoptosis by ΔΨm
assay
The depolarization of ΔΨm is implicated in apoptosis
(25). Therefore, we then
measured ΔΨm using JC-1 staining. As shown in Fig. 3, the percentage of cells that lost
their ΔΨm in the LPS group was markedly decreased compared to the
control group, indicating that LPS was helpful in sustaining a
stable ΔΨm. On the other hand, there was no notable change in the
percentage of cells that lost their ΔΨm between the LPS + ghrelin
group and the LPS group, as well as between the ghrelin group and
the control group, indicating that ΔΨm in neutrophils was not
affected by ghrelin whatsoever.
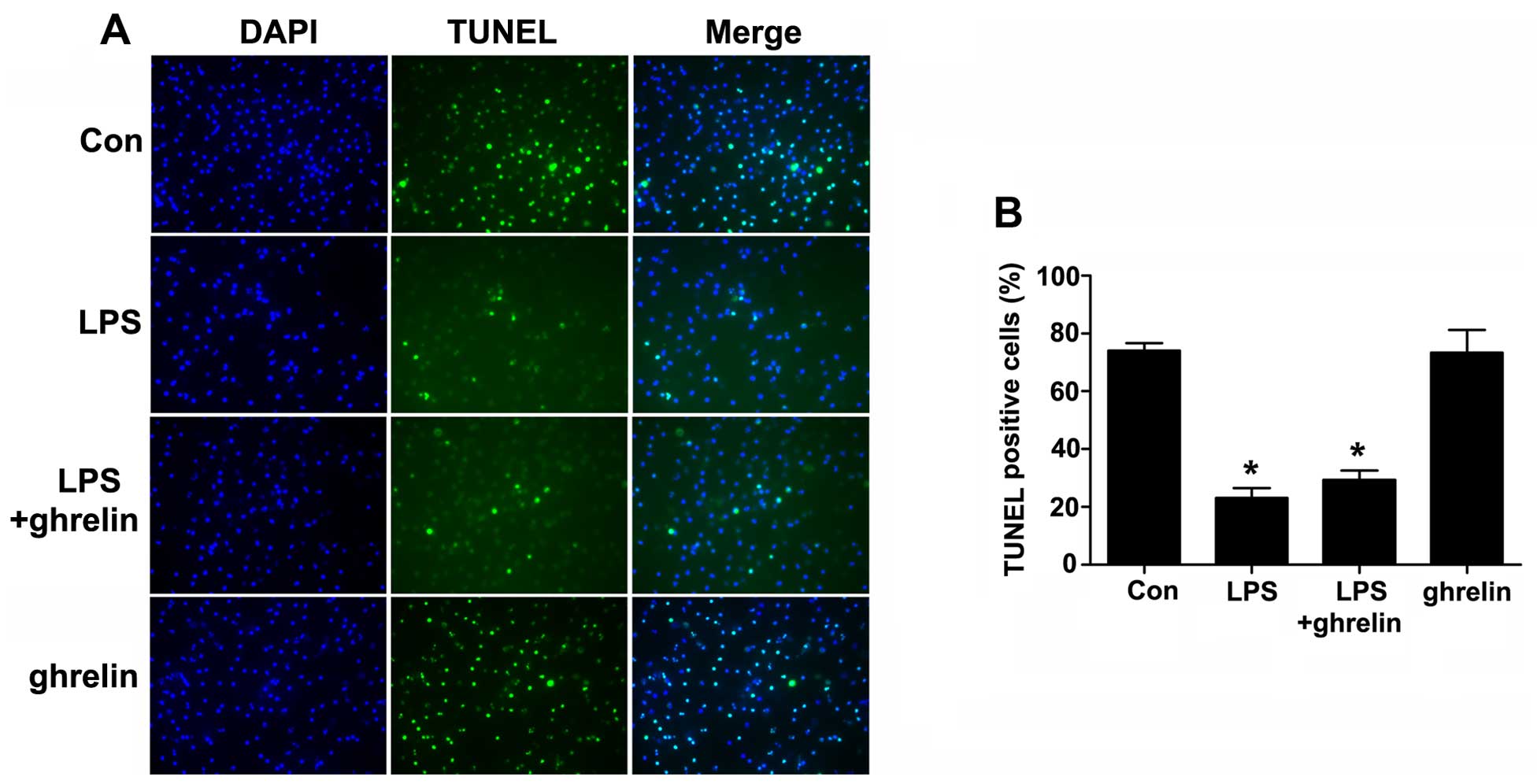
Detection of neutrophil by apoptosis
TUNEL assay
Cell apoptosis was then examined using a TUNEL assay
(Fig. 4). The TUNEL-positive
rates were 74.00±2.65, 23.00±3.46, 29.33±3.284 and 73.33±7.86% for
the control, LPS, LPS + ghrelin and ghrelin groups, respectively.
Our results revealed that LPS markedly decreased the number of
TUNEL-positive cells compared with the controls. However, there
were no apparent differences in the TUNEL positivity rates between
the LPS group and the LPS + ghrelin group, and between the control
and ghrelin groups, indicating that treatment with ghrelin may not
affect the incidence of neutrophil apoptosis.
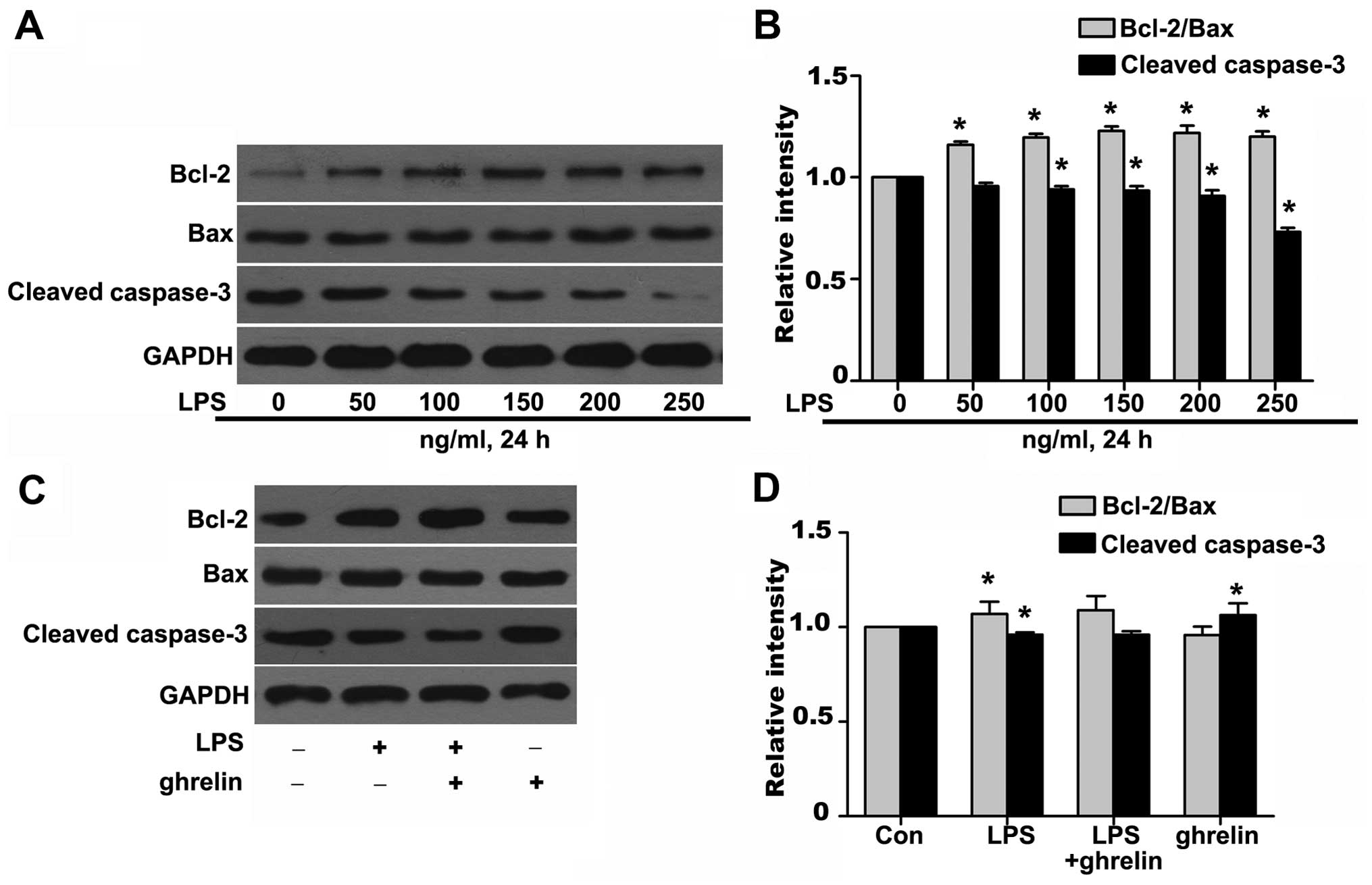
Effects of ghrelin on the Bcl-2/Bax ratio
and cleaved caspase-3 expression
To further investigate the molecular mechanisms
responsible for the ability of ghrelin to induce neutrophil
apoptosis, western blot analysis was carried out to determine the
levels of key neutrophil intracellular Bcl-2 family proteins and
caspase activation following drug stimulation. It has been shown
that Bcl-2 forms a heterodimeric complex with the apoptotic protein
Bax, thereby neutralizing its apoptotic effects (26). Therefore, the ratio of Bcl-2/Bax
is often considered as a decisive factor in determining whether
cells will undergo death or survive. The values of the Bcl-2/Bax
ratio were obtained by dividing the OD for Bcl-2 with the OD for
Bax in a different group. We observed that stimulation of the
neutrophils with LPS resulted in a noted dose-dependent rise in the
Bcl-2/Bax ratio and in the downregulation of cleaved caspase-3 at
the protein level (Fig. 5A and
B), which favors anti-apoptosis. It should be noted that
treatment with ghrelin at the concentration of 100 nM appeared to
hardly have any effect on the Bcl-2, Bax and cleaved caspase-3
expression levels after 8 h, as the Bcl-2/Bax ratio and cleaved
caspase-3 expression in the LPS + ghrelin group did not show
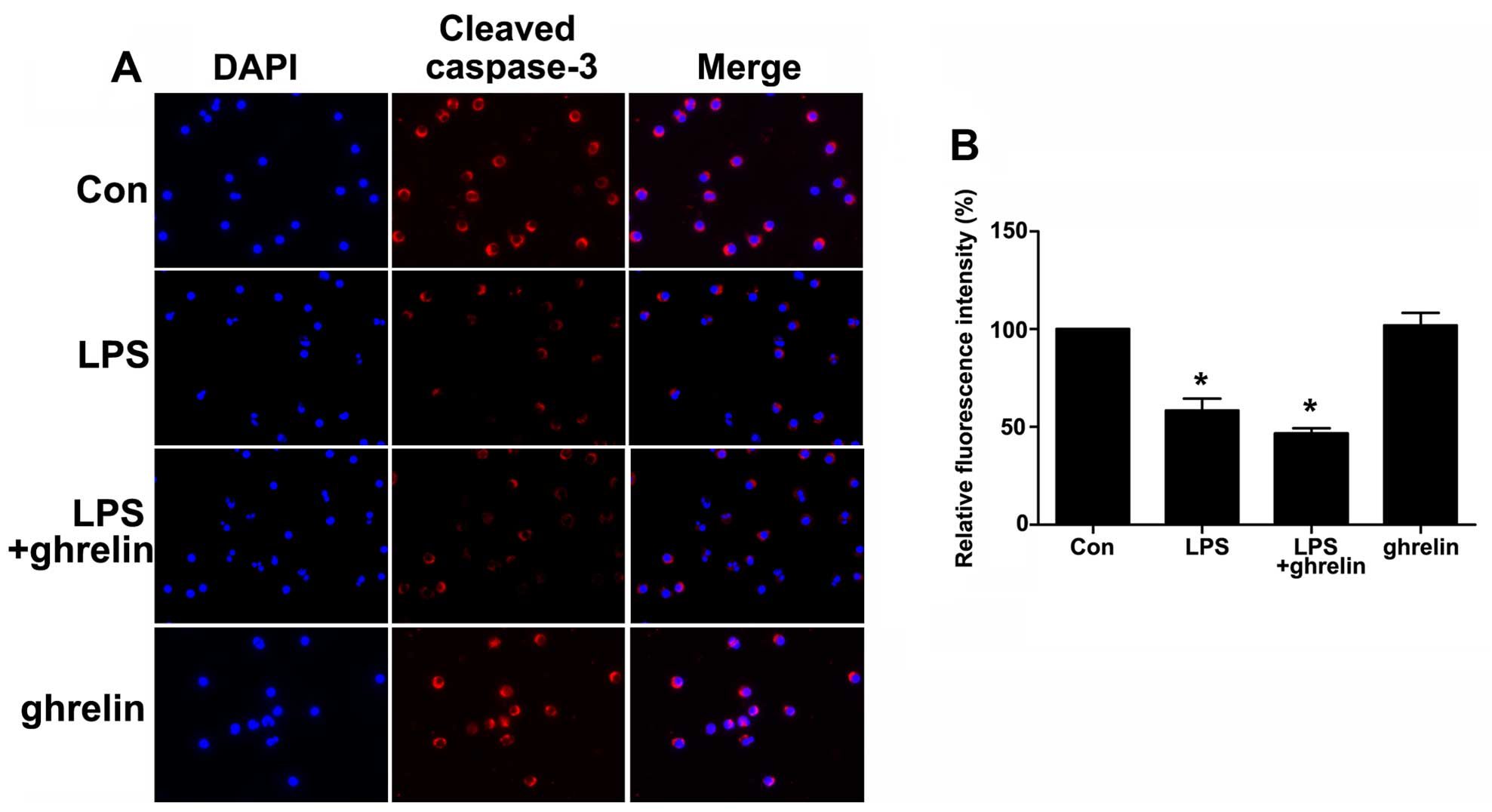
significant changes when compared with the LPS group (Fig. 5C and D). To determine whether
caspases (cysteine aspartic specific proteases) are involved in
neutrophil apoptosis in the cells exposed to LPS or ghrelin, we
also used immunofluorescence staining to evaluate active cleaved
caspases-3 levels in the cells. The data presented in Fig. 6 show a significant decrease in the
levels of cleaved caspase-3 in the neutrophils stimulated with LPS.
Notably, pre-treatment with ghrelin did not increase the cleavage
of caspase-3 as previously thought.
Discussion
The precise mechanisms repsonbilse for the
development of ARDS are complex and are not yet clear; however,
neutrophil apoptosis is one of the mechanisms involved (1,10).
Neutrophils, the most numerous immune cells in the first line of
protection against invading microbes, are characterized by a very
short lifespan (27); their
normally short lifespan in the circulation can be manipulated and
extended at sites of inflammation, to ensure that an appropriate
host response is mounted in response to infection or tissue injury.
However, uncontrolled neutrophil recruitment or inappropriate
neutrophil longevity is pathophysiologically involved in the
development of inflammatory diseases with unresolved neutrophilic
inflammation (28). For instance,
a delay in apoptosis may lead to the prolonged release of
neutrophil products and direct tissue injury, thus participating in
the development of ARDS (10).
The pharmacological manipulation of such a defective resolution
step is therefore an attractive avenue for the development of novel
pro-resolution and anti-inflammatory treatments (29).
Ghrelin has gained attention for its broad range of
roles in various biological systems (30). One of the most extensively studied
characteristics is that ghrelin plays a positive role during the
resolution phase of acute inflammation through the attenuation of
the pro-inflammatory mediator response (31,32), neutrophil infiltration (18,22,32), and the enhancement of the
phagocytosis of apoptotic cells (33), resulting in therapeutic benefits
in pathological conditions associated with inflammation (16). Previous studies have suggested
that ghrelin and ghrelin receptor are expressed in immune cells,
including neutrophils (34,35), alveolar macrophages and human lung
tissue (36), indicating that
ghrelin has direct modulatory effects on pulmonary function.
Further studies have indicated that ghrelin displays
anti-inflammatory properties in ARDS, leading to ameliorated
pulmonary injury in rats with ARDS (20). In our previous study, we
demonstrated that ghrelin inhibited apoptosis and enhanced the
phagocytosis of alveolar macrophages, thus mitigating lung injury
in septic ARDS (37). Therefore,
we hypothesized that ghrelin has a potential modulatory role in
neutrophil apoptosis for two reasons: first, ghrelin has been
extensively reported to play a positive role in various
inflammatory diseases; second, the ability of ghrelin to modulate
apoptosis has been proven in several cell types previously
(22,38,39). In contrast to the induction of the
apoptosis of endothelial and epithelial cells, the apoptosis of
neutrophils is decreased during sepsis-induced ARDS (40). Thus, we originally predicted that
ghrelin may act as a pro-apoptotic modulator of neutrophil
apoptosis, subsequently relieving lung injury in the pathological
process of ARDS. We believe that the careful analysis of neutrophil
apoptosis in response to ghrelin treatment is warranted in order to
better understand the mechanisms responsible for the resolution of
inflammation after the administration of ghrelin. Our findings
using flow cytometry demonstrated that stimulation of the cells
with 100 ng/ml of LPS decreased the apoptotic ratio of the
neutrophils, which was consistent with previous reports (23,41). However, pre-treatment with 100 nM
ghrelin did not increase the apoptotic ratio (Fig. 2), indicating that ghrelin may not
enhance the resolution of inflammation by promoting neutrophil
apoptosis.
The apoptotic pathway is controlled by the balance
of pro- vs. anti-apoptotic proteins from the Bcl-2 family, which
together regulate mitochondrial outer membrane permeabilization and
the release of pro-apoptotic substances from the mitochondria
(42). Neutrophils also express
Bcl-2 family members, the most relevant in regulating their
survival (42,43), which positively correlate with the
neutrophil lifespan (27,44). Our data clearly demonstrated that
the ratio of Bcl-2/Bax markedly rose in the LPS-stimulated
neutrophils, whereas we did not observe any notable changes in the
ratio of Bcl-2/Bax following pre-treatment with ghrelin (Fig. 5). The stabilization of ΔΨm plays
an essential role in the mitochondrial apoptotic pathway (45). Our study demonstrated that LPS can
help sustain the stabilization of ΔΨm to prolong the lifespan of
neutrophils. However, treatment with ghrelin did not alter the ΔΨm,
revealing for the first time that the anti-apoptotic effects of
ghrelin through mitochondrial signaling do not occur in human
neutrophils (Fig. 3). Using TUNEL
assay, we also investigated nuclear morphological changes following
drug treatment. Consistent with the results of flow cytometry and
ΔΨm assay, the results of TUNEL assay revealed that LPS markedly
decreased the double-stranded cleavage of DNA, while treatment with
ghrelin hardly had any influence on DNA double-stranded cleavage in
neutrophils (Fig. 4).
Apoptosis can proceed via either an extrinsic
pathway upon the ligation of a death receptor or an intrinsic
pathway in response to cellular stress, chemotherapeutic agents, or
ultraviolet damage (46). Both
apoptotic pathways eventually act through the activation of enzymes
known as caspases, which are responsible for the numerous
morphological and biochemical changes in apoptosis (46). Cleaved caspase-3 has been proposed
as a regulator of the neutrophil lifespan, as it is present in
mature neutrophils and its expression correlates with increased
apoptosis (43,46). Consistent with the dependence on
activated caspases for apoptosis, we observed that LPS caused a
decrease in caspase-3 activation. We also observed that ghrelin had
no influence on the cleavage of caspase-3, as pre-treatment with
ghrelin did not alter the cleaved caspase-3 expression level, as
shown by western blot analysis or immunofluorescence staining
(Figs. 5 and 6).
The important role of neutrophils in ARDS has been
demonstrated in previous studies, in which the severity of lung
injury was shown to decrease when neutrophils were eliminated
(10,40). Therefore, we originally predicted
that ghrelin may be identified as a modulator of neutrophil
apoptosis and sought insight into the underlying molecular
mechanisms. Interestingly, we found that ghrelin neither abrogated
the pro-survival influence of LPS that prolongs the neutrophil
lifespan nor induced neutrophil apoptosis. It is not entirely clear
why the effect of ghrelin on apoptosis varies between cell types;
however, there is evidence to suggest that ghrelin indeed possesses
anti- and pro-apoptotic functions towards different cells (47–50). For example, ghrelin has been
previously shown to inhibit or induce the apoptosis of lung
epithelial cells (22) and human
colorectal adenocarcinoma cells (51). However, to the best of our
knowledge, there is no previous available study characterizing the
direct effects of ghrelin on the regulation of the neutrophil
lifespan. In this study, we firstly reported that ghrelin neither
inhibited nor induced neutrophil apoptosis, which was not
consistent with our initial hypothesis.
We consider that there are several possible reasons
to explain this result. To begin with, past studies have suggested
that aside from the regulation of apoptosis, many other modulatory
mechanisms also contribute to the pharmacological anti-inflammatory
functions of ghrelin, varying from the inhibition of intracellular
signaling cascades (52), to
reduced cytokine production (52–54), an enhanced ability to eliminate
invading microorganisms (37),
and the inhibition of immunocyte apoptosis (55). Ghrelin may inhibit the production
and release of cytokines, reactive oxygen species, antimicrobial
and proteolytic granule proteins in neutrophils or other cells, and
enhance the ability of neutrophils to engulf pathogens, rather than
boosting neutrophil apoptosis. As is known to us, activated
neutrophils synthesize chemokines and cytokines, which recruit and
regulate the inflammatory response of other effector cells,
including macrophages, T cells and neutrophils themselves (3,56).
For example, pro-inflammatory cytokines, such as interleukin
(IL)-1β, tumor necrosis factor (TNF)-α, interferon (IFN)-μ, and
granulocyte/macrophage colony stimulating factor, can inhibit
neutrophil apoptosis, whereas IL-10 counteracts this inhibition
(27,57). Taken together, the elimination of
microorganisms by neutrophils can be regarded as a dynamic process,
integrating the synthesis of granule proteins during
differentiation, migration to sites of infection, phagocytosis and
the killing of microorganisms, the modulation of other effector
cells and, finally, apoptosis. We speculate that although ghrelin
did not influence neutrophil apoptosis in vitro, it may
indirectly affect neutrophil apoptosis by modulating the secretion
of various chemokines and cytokines, phagocytosis, and other steps
involved in the cascade of defense mechanisms in response to
various physiological and pathological conditions in vivo,
thus enhancing the resolution of inflammation and exerting
therapeutic benefits. Second, this result may in part be explained
by stimulated, inflammatory neutrophils having an altered
sensitivity towards apoptosis-inducing stimuli. As an essential
role to launch a first line of defense, substantial evidence has
suggested that neutrophils experience an extensive change in gene
expression and function, participating in previously unanticipated
ways in a whole host of immune, inflammatory, and tissue remodeling
responses that sustain life during stress (58). For example, TNF-related
apoptosis-inducing ligand (TRAIL)-deficient mice show normal
circulating neutrophil numbers and normal constitutive neutrophil
apoptosis, but demonstrate impaired apoptosis when exposed to an
inflammatory environment (59).
Thus, we hypothesized that the in vitro freshly isolated and
LPS-stimulated neutrophils may also alter their sensitivity toward
ghrelin. Third, we adopted the concentration of 100 nM ghrelin in
this study as it was extensively accepted in previous literature
(60,61). As we mentioned above, the
sensitivity toward apoptosis-inducing stimuli in neutrophils may
vary from normal physiological conditions, and thus the apoptotic
response to ghrelin may also be altered and the physiological
concentration of 100 nM ghrelin was possibly not intense enough to
cause neutrophil apoptosis in the freshly isolated and
LPS-stimulated neutrophils. Thus, dose-dependent effects need to be
investigated to better explain the observed anti-inflammatory
effects and beneficial consequences of ghrelin administration in
ARDS.
In conclusion, to the best of our knowledge, our
findings demonstrate for the first time that ghrelin does not drive
neutrophil apoptosis in a mitochondrial-dependent manner to
override the LPS-induced delay of apoptosis. Thus, the
identification of specific regulatory mechanisms responsible for
the ability of ghrelin to ameliorate inflammation in ARDS warrants
further investigation in future studies, helping to explain the
beneficial effects of ghrelin administration in various
pathological states associated with inflammation.
Acknowledgments
This study was supported by Grants from the Science
and Technology Program of Guangzhou City of China (2014Y2-00136),
the Science and Technology Program of Guangdong Province of China
(2014A020212151) and the Science and Technology Program of
Guangdong Province of China (2016A020216009).
References
|
1
|
Ranieri VM, Rubenfeld GD, Thompson BT,
Ferguson ND, Caldwell E, Fan E, Camporota L and Slutsky AS; ARDS
Definition Task Force: Acute respiratory distress syndrome: the
berlin definition. JAMA. 307:2526–2533. 2012.PubMed/NCBI
|
|
2
|
Nathan C: Neutrophils and immunity:
challenges and opportunities. Nat Rev Immunol. 6:173–182. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Martin KR, Ohayon D and Witko-Sarsat V:
Promoting apoptosis of neutrophils and phagocytosis by macrophages:
novel strategies in the resolution of inflammation. Swiss Med Wkly.
145:w140562015.PubMed/NCBI
|
|
4
|
Ma HJ, Huang XL, Liu Y and Fan YM: Sulfur
dioxide attenuates LPS-induced acute lung injury via enhancing
polymorphonuclear neutrophil apoptosis. Acta Pharmacol Sin.
33:983–990. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Recher M, Malipiero U, Schaer DJ, Koedel
U, Pfister HW, Birchler T, Petrausch U, Claus H, Gast H and Fontana
A: Inhibition of meningitis-associated neutrophil apoptosis by
TNF-α depends on functional PI3-kinase in monocytes. J Leukoc Biol.
93:259–266. 2013. View Article : Google Scholar
|
|
6
|
Walmsley SR, Print C, Farahi N,
Peyssonnaux C, Johnson RS, Cramer T, Sobolewski A, Condliffe AM,
Cowburn AS, Johnson N and Chilvers ER: Hypoxia-induced neutrophil
survival is mediated by HIF-1alpha-dependent NF-kappaB activity. J
Exp Med. 201:105–115. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pryde JG, Walker A, Rossi AG, Hannah S and
Haslett C: Temperature-dependent arrest of neutrophil apoptosis.
Failure of Bax insertion into mitochondria at 15 degrees C prevents
the release of cytochrome c. J Biol Chem. 275:33574–33584. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Savill JS, Wyllie AH, Henson JE, Walport
MJ, Henson PM and Haslett C: Macrophage phagocytosis of aging
neutrophils in inflammation. Programmed cell death in the
neutrophil leads to its recognition by macrophages. J Clin Invest.
83:865–875. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Simon HU: Targeting apoptosis in the
control of inflammation. Eur Respir J. Suppl 44(Suppl 44): 20s–21s.
2003. View Article : Google Scholar
|
|
10
|
Fialkow L, Fochesatto Filho L, Bozzetti
MC, Milani AR, Rodrigues Filho EM, Ladniuk RM, Pierozan P, de Moura
RM, Prolla JC, Vachon E and Downey GP: Neutrophil apoptosis: a
marker of disease severity in sepsis and sepsis-induced acute
respiratory distress syndrome. Crit Care. 10:R1552006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Persson CG and Uller L: Increased lung
neutrophil apoptosis and inflammation resolution. Eur Respir J.
39:789–790; author reply 790–791. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hutcheson R, Terry R, Hutcheson B, Jadhav
R, Chaplin J, Smith E, Barrington R, Proctor SD and Rocic P:
miR-21-mediated decreased neutrophil apoptosis is a determinant of
impaired coronary collateral growth in metabolic syndrome. Am J
Physiol Heart Circ Physiol. 308:H1323–H1335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Elks PM, van Eeden FJ, Dixon G, Wang X,
Reyes-Aldasoro CC, Ingham PW, Whyte MK, Walmsley SR and Renshaw SA:
Activation of hypoxia-inducible factor-1α (Hif-1α) delays
inflammation resolution by reducing neutrophil apoptosis and
reverse migration in a zebrafish inflammation model. Blood.
118:712–722. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kolaczkowska E, Plytycz B, Arnold B,
Piccard H and Opdenakker G: Increased cyclooxygenase activity
impairs apoptosis of inflammatory neutrophils in mice lacking
gelatinase B/matrix metalloproteinase-9. Immunology. 128(Suppl 1):
e262–e274. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koedel U, Frankenberg T, Kirschnek S,
Obermaier B, Häcker H, Paul R and Häcker G: Apoptosis is essential
for neutrophil functional shutdown and determines tissue damage in
experimental pneumococcal meningitis. Plos Pathog. 5:e10004612009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baatar D, Patel K and Taub DD: The effects
of ghrelin on inflammation and the immune system. Mol Cell
Endocrinol. 340:44–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dixit VD and Taub DD: Ghrelin and
immunity: a young player in an old field. Exp Gerontol. 40:900–910.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kodama T, Ashitani J, Matsumoto N, Kangawa
K and Nakazato M: Ghrelin treatment suppresses neutrophil-dominant
inflammation in airways of patients with chronic respiratory
infection. Pulm Pharmacol Ther. 21:774–779. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang L, Zhao J, Yang J, Zhang Z, Du J and
Tang C: Therapeutic effects of ghrelin on endotoxic shock in rats.
Eur J Pharmacol. 473:171–176. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu R, Dong W, Zhou M, Zhang F, Marini CP,
Ravikumar TS and Wang P: Ghrelin attenuates sepsis-induced acute
lung injury and mortality in rats. Am J Respir Crit Care Med.
176:805–813. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Granado M, Priego T, Martín AI, Villanúa
MA and López-Calderón A: Anti-inflammatory effect of the ghrelin
agonist growth hormone-releasing peptide-2 (GHRP-2) in arthritic
rats. Am J Physiol Endocrinol Metab. 288:E486–E492. 2005.
View Article : Google Scholar
|
|
22
|
Imazu Y, Yanagi S, Miyoshi K, Tsubouchi H,
Yamashita S, Matsumoto N, Ashitani J, Kangawa K and Nakazato M:
Ghrelin ameliorates bleomycin-induced acute lung injury by
protecting alveolar epithelial cells and suppressing lung
inflammation. Eur J Pharmacol. 672:153–158. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jia SH, Parodo J, Kapus A, Rotstein OD and
Marshall JC: Dynamic regulation of neutrophil survival through
tyrosine phosphorylation or dephosphorylation of caspase-8. J Biol
Chem. 283:5402–5413. 2008. View Article : Google Scholar
|
|
24
|
Pouliot M, Fiset ME, Massé M, Naccache PH
and Borgeat P: Adenosine upregulates cyclooxygenase-2 in human
granulocytes: impact on the balance of eicosanoid generation. J
Immunol. 169:5279–5286. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hirsch T, Marzo I and Kroemer G: Role of
the mitochondrial permeability transition pore in apoptosis. Biosci
Rep. 17:67–76. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang K, Gross A, Waksman G and Korsmeyer
SJ: Mutagenesis of the BH3 domain of BAX identifies residues
critical for dimerization and killing. Mol Cell Biol. 18:6083–6089.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Akgul C, Moulding DA and Edwards SW:
Molecular control of neutrophil apoptosis. FEBS Lett. 487:318–322.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nishiura H, Zhao R, Chen J, Taniguchi K
and Yamamoto T: Involvement of regional neutrophil apoptosis
promotion by ribosomal protein S19 oligomers in resolution of
experimental acute inflammation. Pathol Int. 63:581–590. 2013.
View Article : Google Scholar
|
|
29
|
Jančinová V, Perečko T, Nosáĺ R, Mihalová
D, Bauerová K and Drábiková K: Pharmacological regulation of
neutrophil activity and apoptosis: contribution to new strategy for
modulation of inflammatory processes. Interdiscip Toxicol. 4:11–14.
2011. View Article : Google Scholar
|
|
30
|
Khatib MN, Shankar A, Kirubakaran R, Agho
K, Simkhada P, Gaidhane S, Saxena D, B U, Gode D, Gaidhane A and
Zahiruddin SQ: Effect of ghrelin on mortality and cardiovascular
outcomes in experimental rat and mice models of heart failure: a
systematic review and meta-analysis. PLoS One. 10:e01266972015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang D, Wang H, Luo P, Hwang A, Sun D,
Wang Y, Zhang Z, Liu N, Wang S, Li C and Cao F: Effects of ghrelin
on homocysteine-induced dysfunction and inflammatory response in
rat cardiac microvascular endothelial cells. Cell Biol Int.
36:511–517. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sehirli O, Sener E, Sener G, Cetinel S,
Erzik C and Yeğen BC: Ghrelin improves burn-induced multiple organ
injury by depressing neutrophil infiltration and the release of
pro-inflammatory cytokines. Peptides. 29:1231–1240. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yada T, Kaiya H, Mutoh K, Azuma T, Hyodo S
and Kangawa K: Ghrelin stimulates phagocytosis and superoxide
production in fish leukocytes. J Endocrinol. 189:57–65. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hattori N, Saito T, Yagyu T, Jiang BH,
Kitagawa K and Inagaki C: GH, GH receptor, GH secretagogue
receptor, and ghrelin expression in human T cells, B cells, and
neutrophils. J Clin Endocrinol Metab. 86:4284–4291. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hattori N: Expression, regulation and
biological actions of growth hormone (GH) and ghrelin in the immune
system. Growth Horm IGF Res. 19:187–197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Volante M, Fulcheri E, Allìa E, Cerrato M,
Pucci A and Papotti M: Ghrelin expression in fetal, infant, and
adult human lung. J Histochem Cytochem. 50:1013–21. 2012.
View Article : Google Scholar
|
|
37
|
Li B, Zeng M, He W, Huang X, Luo L, Zhang
H and Deng DY: Ghrelin protects alveolar macrophages against
lipopolysaccharide-induced apoptosis through growth hormone
secretagogue receptor 1a-dependent c-Jun N-terminal kinase and
Wnt/β-catenin signaling and suppresses lung inflammation.
Endocrinology. 156:203–217. 2015. View Article : Google Scholar
|
|
38
|
Lee JY, Chung H, Yoo YS, Oh YJ, Oh TH,
Park S and Yune TY: Inhibition of apoptotic cell death by ghrelin
improves functional recovery after spinal cord injury.
Endocrinology. 151:3815–3826. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Baldanzi G, Filigheddu N, Cutrupi S,
Catapano F, Bonissoni S, Fubini A, Malan D, Baj G, Granata R,
Broglio F, et al: Ghrelin and des-acyl ghrelin inhibit cell death
in cardiomyocytes and endothelial cells through ERK1/2 and PI
3-kinase/AKT. J Cell Biol. 159:1029–1037. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang H, Xu L, Zhao J, Wang D, Guo R, Wang
J, Gong W, Liu T, Zhang Y and Dong L: Regulatory mechanism of
pyrrolidine dithiocarbamate is mediated by nuclear factor-κB and
inhibits neutrophil accumulation in ARDS mice. Exp Ther Med.
8:614–622. 2014.PubMed/NCBI
|
|
41
|
Turina M, Miller FN, Tucker C and Polk HC:
Effects of hyperglycemia, hyperinsulinemia, and hyperosmolarity on
neutrophil apoptosis. Surg Infect (Larchmt). 7:111–121. 2006.
View Article : Google Scholar
|
|
42
|
Croker BA, O'Donnell JA, Nowell CJ,
Metcalf D, Dewson G, Campbell KJ, Rogers KL, Hu Y, Smyth GK, Zhang
JG, et al: Fas-mediated neutrophil apoptosis is accelerated by Bid,
Bak, and Bax and inhibited by Bcl-2 and Mcl-1. Proc Natl Acad Sci
USA. 108:13135–13140. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Maianski NA, Roos D and Kuijpers TW: Bid
truncation, bid/bax targeting to the mitochondria, and caspase
activation associated with neutrophil apoptosis are inhibited by
granulocyte colony-stimulating factor. J Immunol. 172:7024–7030.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Edwards SW, Moulding DA, Derouet M and
Moots RJ: Regulation of neutrophil apoptosis. Chem Immunol Allergy.
83:204–224. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Maianski NA, Geissler J, Srinivasula SM,
Alnemri ES, Roos D and Kuijpers TW: Functional characterization of
mitochondria in neutrophils: a role restricted to apoptosis. Cell
Death Differ. 11:143–153. 2004. View Article : Google Scholar
|
|
46
|
Espino J, Bejarano I, Redondo PC, Rosado
JA, Barriga C, Reiter RJ, Pariente JA and Rodríguez AB: Melatonin
reduces apoptosis induced by calcium signaling in human leukocytes:
evidence for the involvement of mitochondria and Bax activation. J
Membr Biol. 233:105–118. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cassoni P, Papotti M, Ghè C, Catapano F,
Sapino A, Graziani A, Deghenghi R, Reissmann T, Ghigo E and
Muccioli G: Identification, characterization, and biological
activity of specific receptors for natural (ghrelin) and synthetic
growth hormone secretagogues and analogs in human breast carcinomas
and cell lines. J Clin Endocrinol Metab. 86:1738–1745.
2001.PubMed/NCBI
|
|
48
|
Ghè C, Cassoni P, Catapano F, Marrocco T,
Deghenghi R, Ghigo E, Muccioli G and Papotti M: The
antiproliferative effect of synthetic peptidyl GH secretagogues in
human CALU-1 lung carcinoma cells. Endocrinology. 143:484–491.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cassoni P, Ghé C, Marrocco T, Tarabra E,
Allia E, Catapano F, Deghenghi R, Ghigo E, Papotti M and Muccioli
G: Expression of ghrelin and biological activity of specific
receptors for ghrelin and des-acyl ghrelin in human prostate
neoplasms and related cell lines. Eur J Endocrinol. 150:173–84.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang GG, Cai HQ, Li YH, Sui YB, Zhang JS,
Chang JR, Ning M, Wu Y, Tang CS, Qi YF and Yin XH: Ghrelin protects
heart against ERS-induced injury and apoptosis by activating
AMP-activated protein kinase. Peptides. 48:156–165. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bonfili L, Cuccioloni M, Cecarini V,
Mozzicafreddo M, Palermo FA, Cocci P, Angeletti M and Eleuteri AM:
Ghrelin induces apoptosis in colon adenocarcinoma cells via
proteasome inhibition and autophagy induction. Apoptosis.
20:1188–200. 2013. View Article : Google Scholar
|
|
52
|
Li WG, Gavrila D, Liu X, Wang L,
Gunnlaugsson S, Stoll LL, McCormick ML, Sigmund CD, Tang C and
Weintraub NL: Ghrelin inhibits proinflammatory responses and
nuclear factor-kappaB activation in human endothelial cells.
Circulation. 109:2221–2226. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dixit VD, Schaffer EM, Pyle RS, Collins
GD, Sakthivel SK, Palaniappan R, Lillard JW Jr and Taub DD: Ghrelin
inhibits leptin- and activation-induced proinflammatory cytokine
expression by human monocytes and T cells. J Clin Invest.
114:57–66. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Xia Q, Pang W, Pan H, Zheng Y, Kang JS and
Zhu SG: Effects of ghrelin on the proliferation and secretion of
splenic T lymphocytes in mice. Regul Pept. 122:173–178. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhu J, Zheng C, Chen J, Luo J, Su B, Huang
Y, Su W, Li Z and Cui T: Ghrelin protects human umbilical vein
endothelial cells against high glucose-induced apoptosis via
mTOR/P70S6K signaling pathway. Peptides. 52:23–28. 2014. View Article : Google Scholar
|
|
56
|
Scapini P and Cassatella MA: Social
networking of human neutrophils within the immune system. Blood.
124:710–719. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Jia SH, Li Y, Parodo J, Kapus A, Fan L,
Rotstein OD and Marshall JC: Pre-B cell colony-enhancing factor
inhibits neutrophil apoptosis in experimental inflammation and
clinical sepsis. J Clin Invest. 113:1318–1327. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Burton JL, Madsen SA, Chang LC, Weber PS,
Buckham KR, van Dorp R, Hickey MC and Earley B: Gene expression
signatures in neutrophils exposed to glucocorticoids: a new
paradigm to help explain 'neutrophil dysfunction' in parturient
dairy cows. Vet Immunol Immunopathol. 105:197–219. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
McGrath EE, Marriott HM, Lawrie A, Francis
SE, Sabroe I, Renshaw SA, Dockrell DH and Whyte MK: TNF-related
apoptosis-inducing ligand (TRAIL) regulates inflammatory neutrophil
apoptosis and enhances resolution of inflammation. J Leukoc Biol.
90:855–865. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chung H, Kim E, Lee DH, Seo S, Ju S, Lee
D, Kim H and Park S: Ghrelin inhibits apoptosis in hypothalamic
neuronal cells during oxygen-glucose deprivation. Endocrinology.
148:148–159. 2007. View Article : Google Scholar
|
|
61
|
Kim SW, Her SJ, Park SJ, Kim D, Park KS,
Lee HK, Han BH, Kim MS, Shin CS and Kim SY: Ghrelin stimulates
proliferation and differentiation and inhibits apoptosis in
osteoblastic MC3T3-E1 cells. Bone. 37:359–369. 2005. View Article : Google Scholar : PubMed/NCBI
|