1. Introduction
Pesticides are widely utilized chemicals in
agriculture, intended to preserve the productivity of crops and the
quality of harvests. The term pesticide includes compounds of
different chemical structures and specific mechanisms of action,
which allow them to prevent, destroy, repel, or mitigate target
pests (1). Based on their
specific chemical structure, pesticides can be classified into
various classes, e.g., carbamates, coumarin derivatives,
organochlorine compounds, organophosphorus compounds and
pyrethroids.
The universal use of assorted groups of pesticides
causes global environmental pollution, as well as the accidental
exposure of humans to these pesticides (2). Pesticides settle into the soil, are
discharged into the groundwater and consecutively to rivers and
seas, entering the food chain and thus, indirectly, human bodies.
Environmental contamination is mostly significant in developing
countries, where the use of pesticides is extensive and
indiscriminate, while the inadequacy of preparation and equipment
to carefully manage pesticides and the absence of strict control
increase health risks (3). Of
note, among the well-developed countries of the European Union
(EU), Italy ranks first in the consumption of pesticides per
agricultural area unit (4).
With regard to the general population, exposure to
pesticides occurs at relatively low levels. Usually, at these doses
of exposure, pesticides do not produce any permanent harmful
effects to adult humans. However, several groups of individuals run
a considerable risk either due to increased exposure (e.g.,
agricultural workers and their families; individuals who reside
close to fields where pesticides are applied) or due to increased
susceptibility to pesticide toxicity (e.g., children) (5–10).
Health effects resulting from pesticide exposure via the dermal,
oral, or inhalatory routes vary according to the specific compound
involved and may be acute or chronic.
Experimental studies have reported that exposure to
pesticides can exert damaging effects on the immune system
(11–16). The immune system is a composite
network of anatomical sites and various specialized cell types
which are involved in the defence of an organism against potential
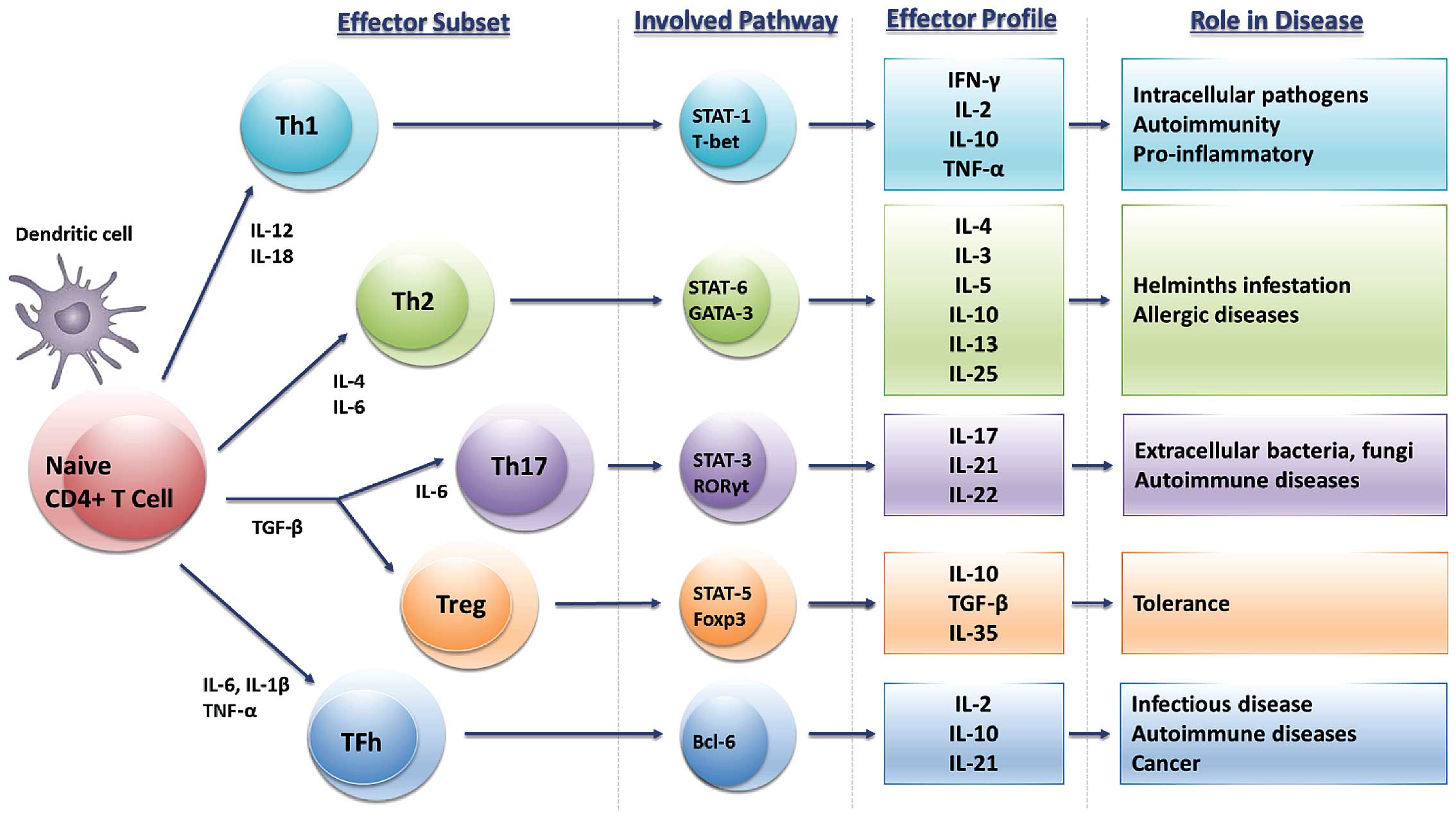
pathogens and neoplastic cells (Fig.
1). The immune response consists of the antigen-non-specific
response (innate) andthe antigen-specific one (adaptive).
Immunocompetent cells secrete inflammatory mediators, such as
cytokines, chemokines, and reactive oxygen species (ROS) and
reactive nitrogen species (RNS). In particular, cytokines can
regulate innate or adaptive immunity, hematopoiesis, inflammatory
processes and many other cellular activities through specific
binding to their respective receptors. It has been demonstrated
that while in vitro cytokines can act alone, their in
vivo actions are synergistic or antagonistic; thus they form an
intercommunicative network that results from a delicate balance
supporting homeostasis (17).
This balance shows vulnerability to the actions of a
number of chemicals, including pesticides, leading to structural
and functional alterations to the system, which ultimately result
in a detrimental outcome.
The involvement of the immune system, contributing
to the development of pathological conditions, has been previously
demonstrated also in the context of different occupational risks
(18,19). Furthermore, work exposure to
biological risks may cause infection from different agents that
activate the immune response (20–22). Such an activation may induce
specific B-cell clones to proliferate as a consequence of the
chronic antigenic stimulation sustained by different infectious
agents (23,24). Moreover, the involvement of the
tumor microenvironment along with cytokine release may be
associated with tumor development in workers exposed to asbestos or
to other fibers (25–27).
Although several studies have investigated the
effects of exposure to pesticides on the cytokine network, the
majority of these studies have analyzed the in vitro
effects, whereas in vivo studies have mostly focused on
animal models. Therefore, there is a discrepancy in the knowledge
of the real impact of these alterations on humoral cytokine levels
in humans and, in particular, on the minimum doses that can promote
the development of chronic diseases. Herein, we reviewed the
literature, focusing on the effects of occupational and
environmental exposure to pesticides on cytokine pathways and on
the impact of alterations in cytokine levels on health, with
particular emphasis on the development of several chronic
diseases.
2. Data collection
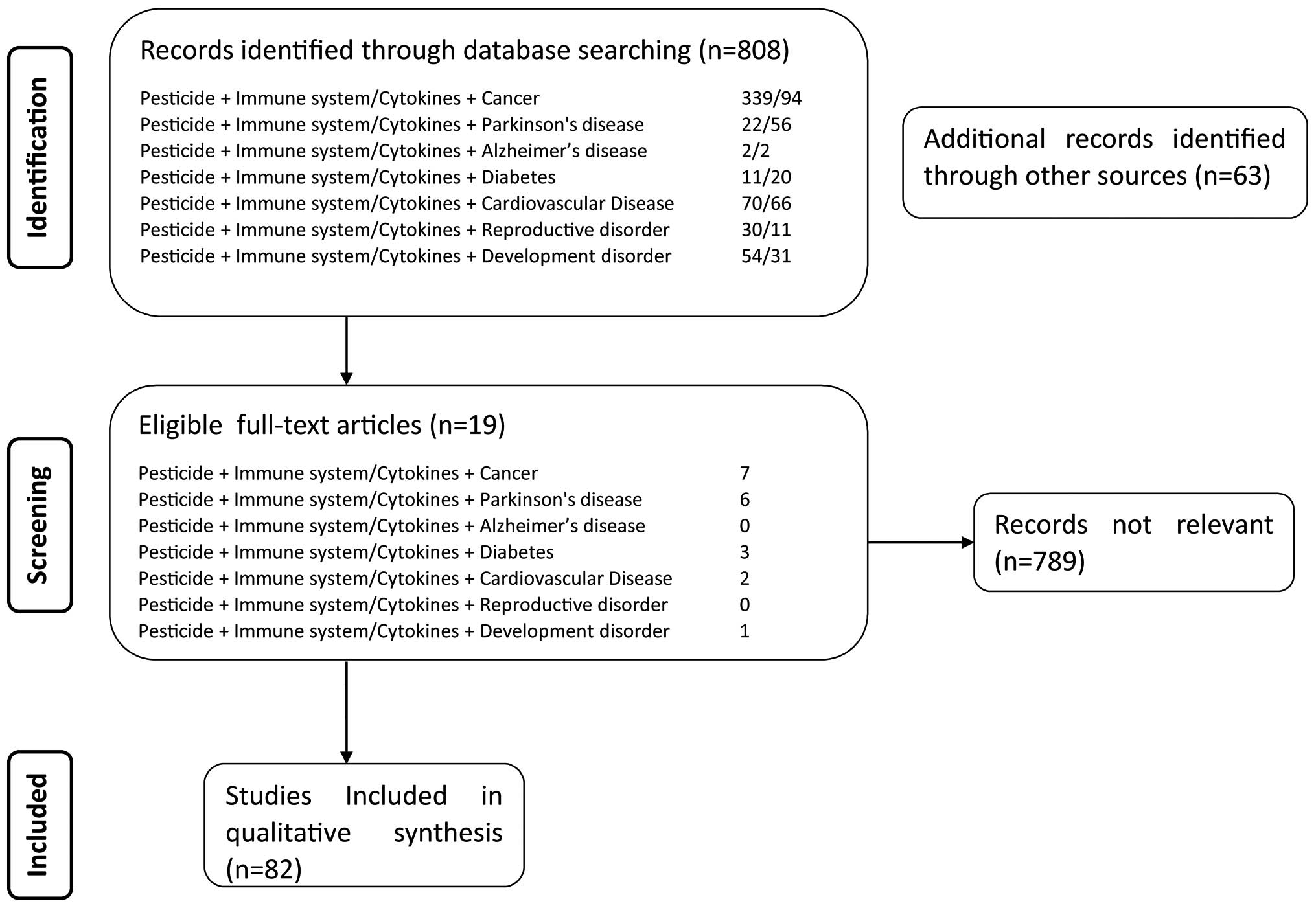
In the present review, data were obtained from a
focused search of PubMed scientific databases in order to identify
articles published in the English language correlating the exposure
to pesticides with several chronic immune-related diseases. The
majority of studies were identified through a PubMed search using
the following terms: 'cancer', 'Parkinson's disease', 'Alzheimer's
disease', 'diabetes', 'cardiovascular disease', 'reproductive
disorder', 'development disorder' each in combination with
'pesticide' and 'immune system' or 'cytokine'.
Moreover, further publications were identified among
the reference list of the screened articles. No restrictions were
placed with regard to the country of origin, ethnicity, gender,
environmental and occupational settings, or the type or date of
publication (Fig. 2).
3. Cancer
The hypothesis of the possible association between
exposure to pesticides and cancer has been widely investigated; the
literature provides substantial evidence that chronic exposure to
pesticides, even at low doses, in agricultural, commercial,
domestic and garden administrations is associated with an increased
risk of cancer, including prostate, lung, liver, breast and colon
cancer, as well as non-Hodgkin lymphoma, leukemia and multiple
myeloma (28–41).
A wide body of data have reported the ability of the
immune system to recognize and control tumor growth. There seems to
be a tight link between the risk of cancer and the immunotoxic
effect of chemical compounds influencing the activity of natural
killer (NK) and natural killer T (NKT) cells, macrophages,
cytotoxic T cells and cytokine secretion: these alterations have
been strongly suggested to affect cancer immunosurveillance, and
several pesticides (organophosphates, organochlorines,
dithiocarbamates and some fungicides) have been reported to induce
the activation of these cells both in vitro, in vivo
and in exposed human populations (42). Data from in vitro and in
vivo studies have suggested that the immune system is able to
recognize transformed cells, and now it is generally established
that avoiding immune detection and elimination is a hallmark of
cancer; on the contrary, it has become clear that the immune system
can also promote tumor progression by promoting chronic
inflammation, influencing tumor immunogenicity and suppressing
antitumor immunity (43). This
dual role of the immune system in inhibiting or facilitating tumor
growth is known as cancer immunoediting and consists of three
phases: elimination (immune surveillance of cancer), equilibrium
(with immunity controlling tumor expansion) and escape (tumor cells
differentiate reducing their immunogenicity or inducing
immunosuppressive mechanisms, leading to tumor growth).
Chemical-induced immunotoxicity, due to pesticide exposure, can
compromise any of the phases of immunoediting (42,44).
Another possible mechanism of tumorigenicity is
suggested by the evidence of neoplastic transformation of chronic
inflammatory sites where pesticides can activate innate immune
dysfunction, leading to chronic inflammation. Indeed, the increased
production of growth factors required to repair tissues damaged by
the increased secretion of inflammatory chemokines and cytokines
[e.g., tumor necrosis factor (TNF), interleukin (IL)-1, -6 and -8]
facilitates tumor cell growth (16,45).
If note, our database search did not apprehend any
study clearly including human pesticide exposure, cancer and
cytokine expression. However, the putative association is strongly
prompted by other publications suggesting that modifications in
cytokine profiles and other immunological targets can increase
cancer risk.
Cassidy et al investigated the correlation
between exposure to some insecticides and breast cancer. In
biopsies of women evaluated for breast lesions, they found a
positive association between the presence of heptachlor epoxide and
the prevalence of cancer (46).
Moreover, when these authors exposed isolated human lymphocytes to
heptachlor epoxide at concentrations similar to those found in
breast biopsies, it was demonstrated that TNF-α, in synergy with
estrogens, induced DNA damage via nitric oxide (NO) signaling and
initiated neoplastic transformation. In another study, the
immunotoxicity of a synthetic pyrethroid was evaluated in 30
greenhouse workers occupationally exposed to α-cypermethrin, by
comparing the plasma levels of IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8,
IL-10, IL-12p70, TNF-α, TNF-β and interferon (IFN)-γ. Exposed
workers showed neither clinical signs of immunosuppression nor
alterations in total leukocyte or leukocyte subpopulations, while
significant differences (p<0.05) were demonstrated for IL-12p70
and highly significant differences (p<0.001) for IFN-γ, IL-2 and
IL-8 levels. These findings are highly relevant since the
respective mediators are implicated in antitumor immunity and
response to infection. These results therefore, support the
hypothesis that pyrethroid exposure may reduce host defenses
against infection and cancer, mostly in subjects with decreased
immune capacity (11).
We have previously investigated whether exposure to
multiple pesticides (including neonicotinoids, pyrethroids and
organophosphates), all suspected to be carcinogenic, can influence
the Th17/Th22 network. IL-17 and IL-22 serum levels were analyzed
in occupationally-exposed greenhouse workers, indicating a
significant increase in the levels of IL-22, which has been
established to be involved in cancer initiation from inflammation
through the activation of the signal transducer and activator of
transcription 3 (STAT-3) signaling pathway, while it promotes
tumorigenesis via mitogen-activated protein kinase kinase kinase 8
(MAP3K8) signaling. Our results support the hypothesis that
oxidative stress and impairment of Th1 lymphocyte function may
affect host defenses against cancer in workers exposed to
pesticides (12).
Recently, Dhouib et al reviewed the
association between carbamate pesticide exposure, alterations in
the immune system and susceptibility to different types of cancer.
The authors concluded that carbamates can cause cancer through
several non-immunological and immunological mechanisms, including
oxidative stress, the suppression of cytokine production in T
helper 1 (Th1) cells and the IFN-γ-induced production of NO in
macrophages (47).
The study by Jorsaraei et al showed that
carbaryl, another carbamate, reduced both the thymus and spleen
weight in rats; in addition, carbaryl significantly decreased
spleen lymphocyte proliferation and IL-2 production, altered the
Th1/Th2 balance towards the Th2 response through the inhibition of
IFN-γ and the increase of IL-4 and IL-10 production, and also
reduced pro-inflammatory cytokine (IL-1β and TNF-α) production by
macrophages (48). Thus,
according to the same authors, carbaryl may promote cancer
development through the utilization of immune response
mechanisms.
Chlorpyrifos and its derivatives have also been
implicated in carcinogenesis. In a recent study, an in vivo
administration of chlorpyrifos to rats demonstrated a modest
negative correlation between exposure levels and the number of NK
cells, which are known to play an important role in cancer
immunosurveillance (49).
Moreover, it has been recently demonstrated that methyl parathion
and chlorpyrifos promote the production of inflammatory citokines,
such as TNF-α, IL-6 and IL-1β in human hepatocellular carcinoma
(HepG2) cells, thus negatively influencing the expression of the
paraoxonase 1 (PON1) gene. Importantly, a decreased expression of
the PON1 gene may increase susceptibility to organophosphate
toxicity and the incidence of diseases related to chronic
inflammation, such as hepatoceullular carcinogenesis (1,50,51).
4. Parkinson's disease
Parkinson's disease (PD) is a neurodegenerative
disease mostly affecting the motor system and is characterized by
tremor, rigidity, postural reflex impairment and slowness. This
debilitating condition is caused by the selective depletion of
dopamine (DA) neurons in the substantia nigra pars compacta (SNpc)
region and DA levels in the corpus striatum of the nigrostriatal DA
pathway (52). DA neurons are
particularly susceptible to oxidative stress owing to their low
antioxidant capacity and elevated content of oxidation-prone
molecules (DA, lipids and iron). The exact etiology and mechanisms
of action of PD are still unclear.; however, there is strong
evidence to suggest that glial reaction and neuroinflammation play
a role in its pathogenesis (53).
Glial cell activation is a frequent characteristic
both in patients and in animal models of PD, and microglia and
astrocytes are considered to play pivotal roles in the
neuroinflammatory processes related to PD development. Moreover, it
has been hypothesized that the excessive activation of microglia
leads to the increased induction of pro-inflammatory cytokines,
such as TNF-α, IL-1β, IL-6 and IFN-γ, inducing the degeneration of
SNpc dopaminergic neurons. Furthermore, microglial activation
promotes the release of pro-inflammatory mediators which are
responsible for astrocyte activation, which in turn contributes to
neuroinflammatory processes in PD (54).
Several studies have suggested that environmental
factors, such as neurotoxins, pesticides, insecticides and DA
itself may play a role in the pathophysiology of PD through the
neurodegeneration of SNpc dopaminergic neurons, impairing
mitochondrial function and activating microglia, thus finally
inducing the accumulation of ROS and inflammatory factors (55–58).
PD is probably the neurological disease most
frequently associated, by epidemiological studies, with exposure to
pesticides in occupational or environmental settings. Overall,
available data have provided sufficient evidence to establish an
association between exposure to pesticides in general and an
increased risk of developing PD; however, the evidence does not
appear so convincing in order to confirm a causal association
(59). Thus, experimental data on
rodents have suggested that pesticide exposure represents a risk
factor for PD. Importantly, paraquat and rotenone, known to cause
neuroinflammation and dopaminergic cell loss, are the most common
pesticides used to establish toxin-based animal models of PD
(60–63). Moreover, Mitra et al
demonstrated that treatment with paraquat caused behavioral
impairment, as well as an increase in ROS and TNF-α levels in the
substantia nigra in rats (64).
In another study, Mangano et al demonstrated that a genetic
deletion of IFN-γ protected SNpc DA neurons from the harmful
effects of paraquat, and normalized modifications in inflammatory
and oxidative factors (IL-1β, TNF-α) within this brain region
(65). Therefore, the authors
concluded that IFN-γ influences paraquat-induced neurodegeneration,
implicating oxidative and pro-inflammatory pathways. Moreover, the
release of TNF-α and IL-1β from microglial BV-2 cells is also
increased following treatment with rotenone as reported by Yuan
et al (66). Exposure to
some organochlorine pesticides, particularly dieldrin, has been
shown to result in selective dopaminergic neurotoxicity that may be
mediated by intracellular ROS production, regulating NOX2 and
microglial activation (67–70). In another example of the
deleterious effects of pesticides, the chronic subjection of rats
to dichlorvos, an organophosphate insecticide, causes microglial
activation with the induction of NADPH oxidase and pro-inflammatory
cytokines (TNF-α, IL-1β and IL-6) (71). It is important to note that
microglial activation itself represents a risk factor for PD by
enhancing the susceptibility of DA cells to dichlorvos toxicity
(71).
5. Diabetes
Diabetes mellitus (DM) is a chronic progressive
disease characterized by systemic hyperglycemia which results from
alterations in insulin action, insulin secretion or both. DM,
particularly type 2, is considered a multifactorial disease in
which genetics and lifestyle play a significant role, as well as
environmental and occupational factors. Indeed, Raafat et al
demonstrated a strong correlation between the blood concentration
of malathion, an organophosphate insecticide, and insulin
resistance among farmers (72).
Type 1 DM is an autoimmune disease, in which the
immune system incorrectly targets insulin-producing β-cells in the
pancreas. Therefore, this type of diabetes can be considered an
inflammatory disease (73). On
the other hand, type 2 DM is a metabolic disorder associated with
hyperglycemia, insulin resistance and a relative lack of insulin.
This pathology seems to originate from alterations of the immune
system, particularly as regards patterns of cytokine expression,
resulting in a condition known as 'systemic low grade inflammation'
(74). Furthermore, cytokines
produced by adipose tissue, such as TNF-α and IL-6, control the
secretion of the C-reactive protein from the liver (75,76). The stimulation of this
inflammatory mechanism seems to be the trigger of insulin
resistance in the peripheral tissues (77).
Recent epidemiological data have demonstrated a
marked positive association between human exposure to
organochlorines and organophosphate pesticides and DM (78). On the whole, a positive
association of exposure with some organochlorine pesticides
[trans-nonachlor, hexachlorobenzene,
dichlorodiphenyltrichloroethane (DDT) and
dichlorodiphenyldichloroethylene (DDE)] with type 2 diabetes has
been evidenced. However, the available bibliographic data are not
adequately numerous in order to confirm causality (79–81).
However, no human study, to date, to the best of our
knowledge, has been performed focusing on the relation between DM
or insulin resistance with mechanistic hypotheses which involve the
cytokine network and pesticide exposure. Conversely, this putative
association has been examined only in a few experimental studies in
rodent models of insulin resistance. Thus, a recent study revealed
that the increment of immune cells may be a consequence of the
increased expression of cytokines, including IL-1, IL-6 and IFN-γ
in the liver of rats exposed to malathion (82). Moreover, Zhang et al
demonstrated enhanced levels of malondialdehyde, TNF-α and IL-6 in
the muscles of rats exposed to omethoate, a commonly used
insecticide in developing countries (83). The authors concluded with the
postulation that omethoate has a potential to cause insulin
resistance. Furthermore, a cross-sectional study suggested that
farmers occupationally exposed to organophosphosphate pesticides
are susceptible to developing neuropsychological disorders, as well
as diabetes (84).
In addition, certain pesticides, belonging to the
organochlorine family, seem to be linked with an enhanced risk of
diabetes (85). Duramad et
al investigated the effects of environmental exposure to
pesticides in children during prenatal and early postnatal
development; these authors hypothesized that changes in Th1/Th2
cytokine profiles are involved in the development of several
disorders, such as respiratory diseases, allergies, cancer and
diabetes (86).
Other studies have demonstrated increased levels of
pro-inflammatory cytokines in rats exposed to organophosphates
(87–89). Additionally, it has been
demonstrated that the peripheral administration of IL-6 induces
hyperglycemia and insulin resistance in humans and rodents
(90). A recent study
hypothesized that organophosphate pesticides can attenuate the
incretin effect and produce insulin resistance via lipotoxic
effects, inflammatory stimulation and the induction of oxidative
stress. Furthermore, organophosphates were found to cause β-cell
dysfunction by overstimulation and the consequent downregulation of
muscarinic receptors owing to the accumulation of acetylcholine
(ACh), which plays a role in glucose-dependent insulin production
(91).
6. Cardiovascular disease
Cardiovascular disease (CVD) represents the most
important cause of death worldwide and the second most common cause
of death in high-income countries. A great number of
epidemiological studies have supported the correlation between
exposure to pesticides and the development of CVD. A survey
performed in Kenyan rural regions revealed that most of the active
farm workers suffered from health problems related to the use of
different agrochemical compounds. The study analyzed the
association of various work tasks, such as horticulture,
floriculture and greenhouse (those with a heavy application of
agrochemicals) in order to detect cardiovascular symptoms (chest
pain, palpitation and leg swelling) in respective workers. Indeed,
a significant correlation between agrochemical exposure and CVD was
detected (92). Moreover, a
possible association has been suggested between the application of
agrochemicals (including herbicides, phenoxy compounds,
insecticides, fungicides, carbamates, organophosphates and
organochlorines) with mortality due to CVD, such as ischemic heart
disease and cerebrovascular accidents among farm workers (93).
According to the available literature, there is no
study to date clearly including human pesticide exposure, CVD and
cytokine expression, although a limited number of animal studies
describing this complex association is available. Thus, Vadhana
et al evaluated the effect of early life permethrin
treatment on the hearts of adult rats (94). They reported higher levels of
cholesterol, IL-1β, IL-2, IL-10, IFN-γ and Rantes in plasma,
supporting the evidence that the modifications detected in adult
rats may be the result of epigenetic alterations occurring during
the neonatal age, leading to alterations in chemokines related to
cardiac function. Zafiropoulos et al evaluated the effect of
repeated low-level exposure to diazinon, propoxur and chlorpyrifos
on the cardiac function of rabits and showed that these produce
modifications in genomic DNA from cardiac tissues and increase the
oxidative stress (95). In
another study, following the administration of omethoate to rats,
it was shown that the TNF-α level in cerebral tissue was
significantly higher than that observed in the control group
(96).
7. Other chronic diseases
There are a limited number of studies evaluating the
correlation between exposure to pesticides and other respective
chronic diseases in humans caused by cytokine dysregulation. This
association however, still lacks robust support from bibliography.
These conditions include: Alzheimer's disease (97–101), reproductive (8,102–105) and neurodevelopmental disorders
(106–109) which require further
investigation for more proof.
8. Limitations of the database
Pesticides can exert various effects on human health
by influencing the immune system in the case of both environmental
and occupational exposure. Exposure to pesticides induces
modifications in the immune system according to the specific
pesticide and includes the alteration of well-regulated immune
responses to tumor antigens, allergens, self-antigens and microbial
antigens that can increase the susceptibility of the organism to
develop various types of cancers, allergies, autoimmune and
infectious diseases. However, the exact immunological mechanisms
which underlie pesticide toxicity remain unclear.
Animal studies have demonstrated that specific
pesticides can modify the immune system either morphologically or
functionally. Nevertheless, in some cases, the concentrations or
doses tested did not indicate relevant exposure concentrations for
humans, thus the identification of a link between immunotoxic
effects in animal models and human populations remains complex.
The exposure assessments in these studies were
generally limited to evaluating broad categories of pesticides or
target populations, which may conceal the involvement of specific
pesticide or other agricultural exposure (110). Other limiting factors are
exposure to a single pesticide in most immunotoxicity studies,
while in real-life situations, individuals are exposed to a wide
range of various chemicals and/or pesticides. Owing to the
potential antagonism or synergistic effects of chemicals when they
are mixed together, the toxicity and the resulting risks may differ
as compared to their own effects. This is important in the case of
individuals who are occupationally exposed, as the levels of
combined pesticides to which humans are exposed to can achieve
extremely high concentrations, particularly in manufacturing
industries and circumscribed farming areas (111).
The studies which report immune system disorders in
human exposed to pesticides have many limitations, such as a lack
of information on exposure levels, differences in pesticide
administration procedures, difficulty in characterizing a
prognostic significance to the weak modifications often observed
and the interpretation of obtained results. It seems that although
the exposure to pesticides can affect immune function, the
occurrence of immune disorders depends on the levels and duration
of exposure to pesticides (112).
9. Conclusions
There is evidence from experimental and
epidemiological studies to indicate that pesticide exposure may
affect the immune system through disturbances of the cytokine
balance. Equally, it is now well-established that the immune system
plays an important role in the development of several chronic
diseases. However, it is as yet not clear whether pesticide
exposure may lead to chronic illness through direct or indirect
effects on cytokines. The main challenge is not just to identify
the effects of individual pesticides and their combinations on the
immune system, but also to determine the way and the duration of
exposure, since the toxic effects on the immune system cannot be
separated from these considerations. Therefore, there is a clear
need of more well-designed and standardized epidemiological and
experimental studies to identify the exact association between
exposure levels and toxic effects in order to establish novel
biomarkers of exposure.
References
|
1
|
Costa C, Gangemi S, Giambò F, Rapisarda V,
Caccamo D and Fenga C: Oxidative stress biomarkers and paraoxonase
1 polymorphism frequency in farmers occupationally exposed to
pesticides. Mol Med Rep. 12:6353–6357. 2015.PubMed/NCBI
|
|
2
|
Kapka-Skrzypczak L, Cyranka M, Skrzypczak
M and Kruszewski M: Biomonitoring and biomarkers of organophosphate
pesticides exposure - state of the art. Ann Agric Environ Med.
18:294–303. 2011.
|
|
3
|
farming on public and environmental health
in The Netherlands, India, Ethiopia, and Uganda, considering the
use of antibiotics and other agro-chemicals. Front Public Health.
4:122016.
|
|
4
|
No authors listed. Infographic: pesticide
planet. Science. 341:730–731. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coronado GD, Holte S, Vigoren E, Griffith
WC, Barr DB, Faustman E and Thompson B: Organophosphate pesticide
exposure and residential proximity to nearby fields: evidence for
the drift pathway. J Occup Environ Med. 53:884–891. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Van Maele-Fabry G, Hoet P and Lison D:
Parental occupational exposure to pesticides as risk factor for
brain tumors in children and young adults: A systematic review and
meta-analysis. Environ Int. 56:19–31. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dolapsakis G, Vlachonikolis IG, Varveris C
and Tsatsakis AM: Mammographic findings and occupational exposure
to pesticides currently in use on Crete. Eur J Cancer.
37:1531–1536. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mehrpour O, Karrari P, Zamani N, Tsatsakis
AM and Abdollahi M: Occupational exposure to pesticides and
consequences on male semen and fertility: A review. Toxicol Lett.
230:146–156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsakirakis A, Kasiotis KM, Arapaki N,
Charistou A, Tsatsakis A, Glass CR and Machera K: Determination of
operator exposure levels to insecticide during bait applications in
olive trees: Study of coverall performance and duration of
application. Int J Hyg Environ Health. 214:71–78. 2011. View Article : Google Scholar
|
|
10
|
García-García CR, Parrón T, Requena M,
Alarcón R, Tsatsakis AM and Hernández AF: Occupational pesticide
exposure and adverse health effects at the clinical, hematological
and biochemical level. Life Sci. 145:274–283. 2016. View Article : Google Scholar
|
|
11
|
Costa C, Rapisarda V, Catania S, Di Nola
C, Ledda C and Fenga C: Cytokine patterns in greenhouse workers
occupationally exposed to α-cypermethrin: An observational study.
Environ Toxicol Pharmacol. 36:796–800. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fenga C, Gangemi S, Catania S, De Luca A
and Costa C: IL-17 and IL-22 serum levels in greenhouse workers
exposed to pesticides. Inflamm Res. 63:895–897. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen D, Zhang Z, Yao H, Cao Y, Xing H and
Xu S: Pro- and anti-inflammatory cytokine expression in immune
organs of the common carp exposed to atrazine and chlorpyrifos.
Pestic Biochem Physiol. 114:8–15. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Díaz-Resendiz KJ, Toledo-Ibarra GA and
Girón-Pérez MI: Modulation of immune response by organophosphorus
pesticides: fishes as a potential model in immunotoxicology. J
Immunol Res. 2015:2138362015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma J and Li X: Alteration in the cytokine
levels and histopathological damage in common carp induced by
glyphosate. Chemosphere. 128:293–298. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mokarizadeh A, Faryabi MR, Rezvanfar MA
and Abdollahi M: A comprehensive review of pesticides and the
immune dysregulation: Mechanisms, evidence and consequences.
Toxicol Mech Methods. 25:258–278. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brundage KM and Barnett JB: Immunotoxicity
of pesticides. Hayes handbook of pesticide toxicology. Academic
Press/Elsevier; New York, NY: pp. 483–497. 2010, View Article : Google Scholar
|
|
18
|
Gangemi S, Merendino RA, Minciullo PL,
Ferlazzo B, Germanò D and Fenga C: Serum levels of interleukin-18
in subjects affected by occupational allergic contact dermatitis. J
Dermatol Sci. 33:187–188. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gangemi S, Rapisarda V, Minciullo PL, Di
Pasquale G, Lombardo G, Valentino M and Fenga C: Circulating levels
of interleukin-18 in asbestos-exposed workers. Toxicol Ind Health.
21:125–129. 2005. View Article : Google Scholar
|
|
20
|
Fenga C, Cacciola A, Di Nola C, Calimeri
S, Lo Giudice D, Pugliese M, Niutta PP and Martino LB: Serologic
investigation of the prevalence of Chlamydophila psittaci in
occupationally-exposed subjects in eastern Sicily. Ann Agric
Environ Med. 14:93–96. 2007.PubMed/NCBI
|
|
21
|
Rapisarda V, Marconi A, Candido S,
Nicolosi D, Salmeri M, Gangemi P, Proietti L, Spandidos DA, Bracci
M, Fenga C and Libra M: A tailored health surveillance program
unveils a case of MALT lymphoma in an HCV-positive health-care
worker. Oncol Lett. 5:651–654. 2013.PubMed/NCBI
|
|
22
|
Libra M, Mangano K, Anzaldi M, Quattrocchi
C, Donia M, di Marco R, Signorelli S, Scalia G, Zignego AL, de Re
V, et al: Analysis of interleukin (IL)-1β IL-1 receptor antagonist,
soluble IL-1 receptor type II and IL-1 accessory protein in
HCV-associated lymphoproliferative disorders. Oncol Rep.
15:1305–1308. 2006.PubMed/NCBI
|
|
23
|
Libra M, Polesel J, Russo AE, De Re V,
Cinà D, Serraino D, Nicoletti F, Spandidos DA, Stivala F and
Talamini R: Extrahepatic disorders of HCV infection: A distinct
entity of B-cell neoplasia? Int J Oncol. 36:1331–1340. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ferreri AJ, Dolcetti R, Magnino S,
Doglioni C and Ponzoni M: Chlamydial infection: The link with
ocular adnexal lymphomas. Nat Rev Clin Oncol. 6:658–669. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Loreto C, Rapisarda V, Carnazza ML,
Musumeci G, Valentino M, Fenga C and Martinez G: Fluoro-edenite
fibres induce lung cell apoptosis: An in vivo study. Histol
Histopathol. 23:319–326. 2008.
|
|
26
|
Malaponte G, Hafsi S, Polesel J,
Castellano G, Spessotto P, Guarneri C, Canevari S, Signorelli SS,
McCubrey JA and Libra M: Tumor microenvironment in diffuse large
B-cell lymphoma: Matrixmetalloproteinases activation is mediated by
osteopontin overexpression. Biochim Biophys Acta. 1863:483–489.
2016. View Article : Google Scholar
|
|
27
|
Musumeci G, Cardile V, Fenga C, Caggia S
and Loreto C: Mineral fibre toxicity: Expression of retinoblastoma
(Rb) and phospho-retinoblastoma (pRb) protein in alveolar
epithelial and mesothelial cell lines exposed to fluoro-edenite
fibres. Cell Biol Toxicol. 27:217–225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Alavanja MC, Samanic C, Dosemeci M, Lubin
J, Tarone R, Lynch CF, Knott C, Thomas K, Hoppin JA, Barker J, et
al: Use of agricultural pesticides and prostate cancer risk in the
Agricultural Health Study cohort. Am J Epidemiol. 157:800–814.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Alavanja MC, Ross MK and Bonner MR:
Increased cancer burden among pesticide applicators and others due
to pesticide exposure. CA Cancer J Clin. 63:120–142. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Beane Freeman LE, Bonner MR, Blair A,
Hoppin JA, Sandler DP, Lubin JH, Dosemeci M, Lynch CF, Knott C and
Alavanja MC: Cancer incidence among male pesticide applicators in
the Agricultural Health Study cohort exposed to diazinon. Am J
Epidemiol. 162:1070–1079. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fenga C: Occupational exposure and risk of
breast cancer. Biomed Rep. 4:282–292. 2016.PubMed/NCBI
|
|
32
|
Gomaa AI, Khan SA, Toledano MB, Waked I
and Taylor-Robinson SD: Hepatocellular carcinoma: Epidemiology,
risk factors and pathogenesis. World J Gastroenterol. 14:4300–4308.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Khuder SA and Mutgi AB: Meta-analyses of
multiple myeloma and farming. Am J Ind Med. 32:510–516. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee WJ, Blair A, Hoppin JA, Lubin JH,
Rusiecki JA, Sandler DP, Dosemeci M and Alavanja MC: Cancer
incidence among pesticide applicators exposed to chlorpyrifos in
the Agricultural Health Study. J Natl Cancer Inst. 96:1781–1789.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee WJ, Sandler DP, Blair A, Samanic C,
Cross AJ and Alavanja MC: Pesticide use and colorectal cancer risk
in the Agricultural Health Study. Int J Cancer. 121:339–346. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Merhi M, Raynal H, Cahuzac E, Vinson F,
Cravedi JP and Gamet-Payrastre L: Occupational exposure to
pesticides and risk of hematopoietic cancers: Meta-analysis of
case-control studies. Cancer Causes Control. 18:1209–1226. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Purdue MP, Hoppin JA, Blair A, Dosemeci M
and Alavanja MC: Occupational exposure to organochlorine
insecticides and cancer incidence in the Agricultural Health Study.
Int J Cancer. 120:642–649. 2007. View Article : Google Scholar :
|
|
38
|
Vakonaki E, Androutsopoulos VP, Liesivuori
J, Tsatsakis AM and Spandidos DA: Pesticides and oncogenic
modulation. Toxicology. 307:42–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Duke TJ, Jahed NC, Veneroso CC, Da Roza R,
Johnson O, Hoffman D, Barsky SH and Levine PH: A cluster of
inflammatory breast cancer (IBC) in an office setting: Additional
evidence of the importance of environmental factors in IBC
etiology. Oncol Rep. 24:1277–1284. 2010.PubMed/NCBI
|
|
40
|
Belpomme D, Irigaray P, Ossondo M, Vacque
D and Martin M: Prostate cancer as an environmental disease: An
ecological study in the French Caribbean islands, Martinique and
Guadeloupe. Int J Oncol. 34:1037–1044. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hardell L, Eriksson M and Degerman A:
Metaanalysis of 4 Swedish case-control studies on exposure to
pesticides as risk-factor for soft-tissue sarcoma including the
relation to tumor-localization and histopathological type. Int J
Oncol. 6:847–851. 1995.PubMed/NCBI
|
|
42
|
Corsini E, Sokooti M, Galli CL, Moretto A
and Colosio C: Pesticide induced immunotoxicity in humans: A
comprehensive review of the existing evidence. Toxicology.
307:123–135. 2013. View Article : Google Scholar
|
|
43
|
Teng MW, Galon J, Fridman WH and Smyth MJ:
From mice to humans: Developments in cancer immunoediting. J Clin
Invest. 125:3338–3346. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dunn GP, Old LJ and Schreiber RD: The
three Es of cancer immunoediting. Annu Rev Immunol. 22:329–360.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Grivennikov SI and Karin M: Inflammatory
cytokines in cancer: Tumour necrosis factor and interleukin 6 take
the stage. Ann Rheum Dis. 70(Suppl 1): i104–i108. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cassidy RA, Natarajan S and Vaughan GM:
The link between the insecticide heptachlor epoxide, estradiol, and
breast cancer. Breast Cancer Res Treat. 90:55–64. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dhouib I, Jallouli M, Annabi A, Marzouki
S, Gharbi N, Elfazaa S and Lasram MM: From immunotoxicity to
carcinogenicity: The effects of carbamate pesticides on the immune
system. Environ Sci Pollut Res Int. 23:9448–9458. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jorsaraei SG, Maliji G, Azadmehr A,
Moghadamnia AA and Faraji AA: Immunotoxicity effects of carbaryl in
vivo and in vitro. Environ Toxicol Pharmacol. 38:838–844. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Noworyta-Głowacka J, Beresińska M,
Bańkowski R, Wiadrowska B, Siennicka J and Ludwicki JK: Effect of
chlorpyrifos on the profile of subpopulations immunocompetent cells
B, T and NK in in vivo model. Rocz Panstw Zakl Hig. 65:311–316.
2014.
|
|
50
|
Jung IH, Choi JH, Chung YY, Lim GL, Park
YN and Park SW: Predominant Activation of JAK/STAT3 Pathway by
Interleukin-6 Is Implicated in Hepatocarcinogenesis. Neoplasia.
17:586–597. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Medina-Díaz IM, Ponce-Ruiz N,
Ramírez-Chávez B, Rojas-García AE, Barrón-Vivanco BS, Elizondo G
and Bernal-Hernández YY: Downregulation of human paraoxonase 1
(PON1) by organophosphate pesticides in HepG2 cells. Environ
Toxicol. Mar 7–2016.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kish SJ, Shannak K, Rajput A, Deck JH and
Hornykiewicz O: Aging produces a specific pattern of striatal
dopamine loss: Implications for the etiology of idiopathic
Parkinson's disease. J Neurochem. 58:642–648. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Blesa J, Trigo-Damas I, Quiroga-Varela A
and Jackson-Lewis VR: Oxidative stress and Parkinson's disease.
Front Neuroanat. 9:912015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hong H, Kim BS and Im HI:
Pathophysiological role of neuroinflammation in neurodegenerative
diseases and psychiatric disorders. Int Neurourol J. 20(Suppl 1):
S2–S7. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Baltazar MT, Dinis-Oliveira RJ, de Lourdes
Bastos M, Tsatsakis AM, Duarte JA and Carvalho F: Pesticides
exposure as etiological factors of Parkinson's disease and other
neurodegenerative diseases - a mechanistic approach. Toxicol Lett.
230:85–103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Freire C and Koifman S: Pesticide exposure
and Parkinson's disease: Epidemiological evidence of association.
Neurotoxicology. 33:947–971. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Van Maele-Fabry G, Hoet P, Vilain F and
Lison D: Occupational exposure to pesticides and Parkinson's
disease: A systematic review and meta-analysis of cohort studies.
Environ Int. 46:30–43. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Dardiotis E, Xiromerisiou G,
Hadjichristodoulou C, Tsatsakis AM, Wilks MF and Hadjigeorgiou GM:
The interplay between environmental and genetic factors in
Parkinson's disease susceptibility: The evidence for pesticides.
Toxicology. 307:17–23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Moretto A and Colosio C: The role of
pesticide exposure in the genesis of Parkinson's disease:
Epidemiological studies and experimental data. Toxicology.
307:24–34. 2013. View Article : Google Scholar
|
|
60
|
Litteljohn D, Mangano E, Clarke M, Bobyn
J, Moloney K and Hayley S: Inflammatory mechanisms of
neurodegeneration in toxin-based models of Parkinson's disease.
Parkinsons Dis. 2011:7135172010.
|
|
61
|
Mitra S, Chakrabarti N, Dutta SS, Ray S,
Bhattacharya P, Sinha P and Bhattacharyya A: Gender-specific brain
regional variation of neurons, endogenous estrogen,
neuroinflammation and glial cells during rotenone-induced mouse
model of Parkinson's disease. Neuroscience. 292:46–70. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Panaro MA and Cianciulli A: Current
opinions and perspectives on the role of immune system in the
pathogenesis of Parkinson's disease. Curr Pharm Des. 18:200–208.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Taetzsch T and Block ML: Pesticides,
microglial NOX2, and Parkinson's disease. J Biochem Mol Toxicol.
27:137–149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Mitra S, Chakrabarti N and Bhattacharyya
A: Differential regional expression patterns of α-synuclein, TNF-α,
and IL-1β; and variable status of dopaminergic neurotoxicity in
mouse brain after Paraquat treatment. J Neuroinflammation.
8:1632011. View Article : Google Scholar
|
|
65
|
Mangano EN, Litteljohn D, So R, Nelson E,
Peters S, Bethune C, Bobyn J and Hayley S: Interferon-γ plays a
role in paraquat-induced neurodegeneration involving oxidative and
proinflammatory pathways. Neurobiol Aging. 33:1411–1426. 2012.
View Article : Google Scholar
|
|
66
|
Yuan YH, Sun JD, Wu MM, Hu JF, Peng SY and
Chen NH: Rotenone could activate microglia through NFκB associated
pathway. Neurochem Res. 38:1553–1560. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Liu B, Gao HM and Hong JS: Parkinson's
disease and exposure to infectious agents and pesticides and the
occurrence of brain injuries: Role of neuroinflammation. Environ
Health Perspect. 111:1065–1073. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Gao HM, Liu B, Zhang W and Hong JS:
Synergistic dopaminergic neurotoxicity of MPTP and inflammogen
lipopolysaccharide: Relevance to the etiology of Parkinson's
disease. FASEB J. 17:1957–1959. 2003.PubMed/NCBI
|
|
69
|
Hatcher JM, Richardson JR, Guillot TS,
McCormack AL, Di Monte DA, Jones DP, Pennell KD and Miller GW:
Dieldrin exposure induces oxidative damage in the mouse
nigrostriatal dopamine system. Exp Neurol. 204:619–630. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Weisskopf MG, Knekt P, O'Reilly EJ,
Lyytinen J, Reunanen A, Laden F, Altshul L and Ascherio A:
Persistent organochlorine pesticides in serum and risk of Parkinson
disease. Neurology. 74:1055–1061. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Binukumar BK, Bal A and Gill KD: Chronic
dichlorvos exposure: microglial activation, proinflammatory
cytokines and damage to nigrostriatal dopaminergic system.
Neuromolecular Med. 13:251–265. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Raafat N, Abass MA and Salem HM: Malathion
exposure and insulin resistance among a group of farmers in
Al-Sharkia governorate. Clin Biochem. 45:1591–1595. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Donath MY and Shoelson SE: Type 2 diabetes
as an inflammatory disease. Nat Rev Immunol. 11:98–107. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Duncan BB, Schmidt MI, Pankow JS,
Ballantyne CM, Couper D, Vigo A, Hoogeveen R, Folsom AR and Heiss
G; Atherosclerosis Risk in Communities Study: Low-grade systemic
inflammation and the development of type 2 diabetes: The
atherosclerosis risk in communities study. Diabetes. 52:1799–1805.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wellen KE and Hotamisligil GS:
Inflammation, stress, and diabetes. J Clin Invest. 115:1111–1119.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhang K, Shen X, Wu J, Sakaki K, Saunders
T, Rutkowski DT, Back SH and Kaufman RJ: Endoplasmic reticulum
stress activates cleavage of CREBH to induce a systemic
inflammatory response. Cell. 124:587–599. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Yudkin JS, Stehouwer CD, Emeis JJ and
Coppack SW: C-reactive protein in healthy subjects: associations
with obesity, insulin resistance, and endothelial dysfunction: a
potential role for cytokines originating from adipose tissue?
Arterioscler Thromb Vasc Biol. 19:972–978. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Swaminathan K: Pesticides and human
diabetes: A link worth exploring? Diabet Med. 30:1268–1271. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Taylor KW, Novak RF, Anderson HA, Birnbaum
LS, Blystone C, Devito M, Jacobs D, Köhrle J, Lee DH, Rylander L,
et al: Evaluation of the association between persistent organic
pollutants (POPs) and diabetes in epidemiological studies: A
national toxicology program workshop review. Environ Health
Perspect. 121:774–783. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Jaacks LM and Staimez LR: Association of
persistent organic pollutants and non-persistent pesticides with
diabetes and diabetes-related health outcomes in Asia: A systematic
review. Environ Int. 76:57–70. 2015. View Article : Google Scholar
|
|
81
|
Magliano DJ, Loh VH, Harding JL, Botton J
and Shaw JE: Persistent organic pollutants and diabetes: A review
of the epidemiological evidence. Diabetes Metab. 40:1–14. 2014.
View Article : Google Scholar
|
|
82
|
Lasram MM, Dhouib IB, Bouzid K, Lamine AJ,
Annabi A, Belhadjhmida N, Ahmed MB, Fazaa SE, Abdelmoula J and
Gharbi N: Association of inflammatory response and oxidative injury
in the pathogenesis of liver steatosis and insulin resistance
following subchronic exposure to malathion in rats. Environ Toxicol
Pharmacol. 38:542–553. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Zhang Y, Ren M, Li J, Wei Q, Ren Z, Lv J,
Niu F and Ren S: Does omethoate have the potential to cause insulin
resistance? Environ Toxicol Pharmacol. 37:284–290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Malekirad AA, Faghih M, Mirabdollahi M,
Kiani M, Fathi A and Abdollahi M: Neurocognitive, mental health,
and glucose disorders in farmers exposed to organophosphorus
pesticides. Arh Hig Rada Toksikol. 64:1–8. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Starling AP, Umbach DM, Kamel F, Long S,
Sandler DP and Hoppin JA: Pesticide use and incident diabetes among
wives of farmers in the Agricultural Health Study. Occup Environ
Med. 71:629–635. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Duramad P, Tager IB and Holland NT:
Cytokines and other immunological biomarkers in children's
environmental health studies. Toxicol Lett. 172:48–59. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ayub S, Verma J and Das N: Effect of
endosulfan and malathion on lipid peroxidation, nitrite and
TNF-alpha release by rat peritoneal macrophages. Int
Immunopharmacol. 3:1819–1828. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Hariri AT, Moallem SA, Mahmoudi M, Memar B
and Hosseinzadeh H: Sub-acute effects of diazinon on biochemical
indices and specific biomarkers in rats: Protective effects of
crocin and safranal. Food Chem Toxicol. 48:2803–2808. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Yurumez Y, Cemek M, Yavuz Y, Birdane YO
and Buyukokuroglu ME: Beneficial effect of N-acetylcysteine against
organophosphate toxicity in mice. Biol Pharm Bull. 30:490–494.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zou C and Shao J: Role of adipocytokines
in obesity-associated insulin resistance. J Nutr Biochem.
19:277–286. 2008. View Article : Google Scholar
|
|
91
|
Rathish D, Agampodi SB, Jayasumana MA and
Siribaddana SH: From organophosphate poisoning to diabetes
mellitus: The incretin effect. Med Hypotheses. 91:53–55. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Tsimbiri PF, Moturi WN, Sawe J, Henley P
and Bend JR: Health impact of pesticides on residents and
horticultural workers in the lake Naivash region, Kenya. Occup Dis
Environ Med. 3:24–34. 2015. View Article : Google Scholar
|
|
93
|
Weichenthal S, Villeneuve PJ, Burnett RT,
van Donkelaar A, Martin RV, Jones RR, DellaValle CT, Sandler DP,
Ward MH and Hoppin JA: Long-term exposure to fine particulate
matter: Association with nonaccidental and cardiovascular mortality
in the agricultural health study cohort. Environ Health Perspect.
122:609–615. 2014.PubMed/NCBI
|
|
94
|
Vadhana MS, Carloni M, Nasuti C, Fedeli D
and Gabbianelli R: Early life permethrin insecticide treatment
leads to heart damage in adult rats. Exp Gerontol. 46:731–738.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zafiropoulos A, Tsarouhas K, Tsitsimpikou
C, Fragkiadaki P, Germanakis I, Tsardi M, Maravgakis G,
Goutzourelas N, Vasilaki F, Kouretas D, et al: Cardiotoxicity in
rabbits after a low-level exposure to diazinon, propoxur, and
chlorpyrifos. Hum Exp Toxicol. 33:1241–1252. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Wang T, Jiang N, Han B, Liu W, Liu T, Fu F
and Zhao D: Escin attenuates cerebral edema induced by acute
omethoate poisoning. Toxicol Mech Methods. 21:400–405. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Campdelacreu J: Parkinson disease and
Alzheimer disease: Environmental risk factors. Neurologia.
29:541–549. 2014. View Article : Google Scholar
|
|
98
|
Chin-Chan M, Navarro-Yepes J and
Quintanilla-Vega B: Environmental pollutants as risk factors for
neurodegenerative disorders: Alzheimer and Parkinson diseases.
Front Cell Neurosci. 9:1242015. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Hayden KM, Norton MC, Darcey D, Ostbye T,
Zandi PP, Breitner JC and Welsh-Bohmer KA; Cache County Study
Investigators: Occupational exposure to pesticides increases the
risk of incident AD: The Cache County study. Neurology.
74:1524–1530. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Richardson JR, Roy A, Shalat SL, von Stein
RT, Hossain MM, Buckley B, Gearing M, Levey AI and German DC:
Elevated serum pesticide levels and risk for Alzheimer disease.
JAMA Neurol. 71:284–290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Zaganas I, Kapetanaki S, Mastorodemos V,
Kanavouras K, Colosio C, Wilks MF and Tsatsakis AM: Linking
pesticide exposure and dementia: What is the evidence? Toxicology.
307:3–11. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Den Hond E, Tournaye H, De Sutter P,
Ombelet W, Baeyens W, Covaci A, Cox B, Nawrot TS, Van Larebeke N
and D'Hooghe T: Human exposure to endocrine disrupting chemicals
and fertility: A case-control study in male subfertility patients.
Environ Int. 84:154–160. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Jamal F, Haque QS, Singh S and Rastogi S:
The influence of organophosphate and carbamate on sperm chromatin
and reproductive hormones among pesticide sprayers. Toxicol Ind
Health. Jan 29–2015.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Miranda-Contreras L, Cruz I, Osuna JA,
Gómez-Pérez R, Berrueta L, Salmen S, Colmenares M, Barreto S, Balza
A, Morales Y, et al: Effects of occupational exposure to pesticides
on semen quality of workers in an agricultural community of Merida
state, Venezuela. Invest Clin. 56:123–136. 2015.In Spanish.
PubMed/NCBI
|
|
105
|
Radwan M, Jurewicz J, Wielgomas B,
Piskunowicz M, Sobala W, Radwan P, Jakubowski L, Hawuła W and Hanke
W: The association between environmental exposure to pyrethroids
and sperm aneuploidy. Chemosphere. 128:42–48. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Furlong MA, Engel SM, Barr DB and Wolff
MS: Prenatal exposure to organophosphate pesticides and reciprocal
social behavior in childhood. Environ Int. 70:125–131. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Ornoy A, Weinstein-Fudim L and Ergaz Z:
Prenatal factors associated with autism spectrum disorder (ASD).
Reprod Toxicol. 56:155–169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Shelton JF, Geraghty EM, Tancredi DJ,
Delwiche LD, Schmidt RJ, Ritz B, Hansen RL and Hertz-Picciotto I:
Neurodevelopmental disorders and prenatal residential proximity to
agricultural pesticides: The CHARGE study. Environ Health Perspect.
122:1103–1109. 2014.PubMed/NCBI
|
|
109
|
Vrijheid M, Casas M, Gascon M, Valvi D and
Nieuwenhuijsen M: Environmental pollutants and child health-A
review of recent concerns. Int J Hyg Environ Health. 219:331–342.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Aschebrook-Kilfoy B, Ward MH, Della Valle
CT and Friesen MC: Occupation and thyroid cancer. Occup Environ
Med. 71:366–380. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Voccia I, Blakley B, Brousseau P and
Fournier M: Immunotoxicity of pesticides: A review. Toxicol Ind
Health. 15:119–132. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Taghavian F, Vaezi G, Abdollahi M and
Malekirad AA: Comparative Toxicological Study between Exposed and
Non-Exposed Farmers to Organophosphorus Pesticides. Cell J.
18:89–96. 2016.PubMed/NCBI
|