1. Introduction
Glaucoma is the second leading cause of blindness
worldwide (1). It is
characterized by optic disk changes and progressive visual field
loss, and eventually leads to the apoptosis of retinal ganglion
cells and axon loss (2). Elevated
intraocular pressure (IOP) is the most important risk factor for
glaucoma (3,4). High IOP usually occurs as a result
of an increase in the aqueous humor outflow resistance of the
trabecular meshwork (TM). The TM is composed of trabecular beams
made of extracellular matrix (ECM) elements, including fibronectin,
laminin and collagen (5). Cells
that line the trabecular beams are believed to be essential for
regulating the aqueous humor outflow that controls IOP. TM
abnormalities are the most common pathogenesis of glaucoma
(6–9). Still, the pathogenesis of glaucoma
is unclear as is the reason why the TM fails to maintain normal
levels of aqueous humor outflow resistance. Oxidative stress and
vascular damage (6) are
considered two major alterations in the TM related to glaucoma
(10). In this review, we discuss
findings related to oxidative damage to the TM.
2. Oxidative stress
Free radicals are moieties with an unpaired electron
and occur as normal metabolites. Under physiological conditions,
cells produce up to 1011 free radicals per day. Free
radicals may be classified as oxygen and non-oxygen moieties. Among
these, oxygen radicals account for 95%. Oxygen radicals include
oxygen and highly reactive oxygenated molecules, such as hydrogen
peroxide (H2O2), hydroxyl radical
(OH•), peroxide hydroxyl radicals, alkoxy radicals,
superoxide and the anion radical (O2−), which
are collectively known as reactive oxygen species (ROS) (11). Reactive nitrogen species also play
an important role in oxidative stress (12); however, they will not be discussed
in this review.
Under physiological conditions, the production and
elimination of ROS are in equilibrium; however, some xenobiotics,
ionizing radiation, illnesses, or aging may cause the production of
ROS to levels that exceed the neutralizing capacity of an organism,
thus leading to a series of pathological changes, which in turn
leads to oxidative stress. This process is related to defense
mechanisms, such as neutrophil inflammatory infiltrates, an
increase in protease secretion and the generation of oxide
intermediates. This process is similar to the normal aging process,
although more severe (11). It is
a prominent feature of many acute and chronic diseases (13). Oidative stress plays an important
role in pulmonary fibrosis, epilepsy, hypertension,
atherosclerosis, Parkinson's disease and sudden death, and it is
also known to be associated with a number of ophthalmic diseases,
such as age-related macular degeneration, cataract and glaucoma
(14,15).
3. Sources of ROS in the TM
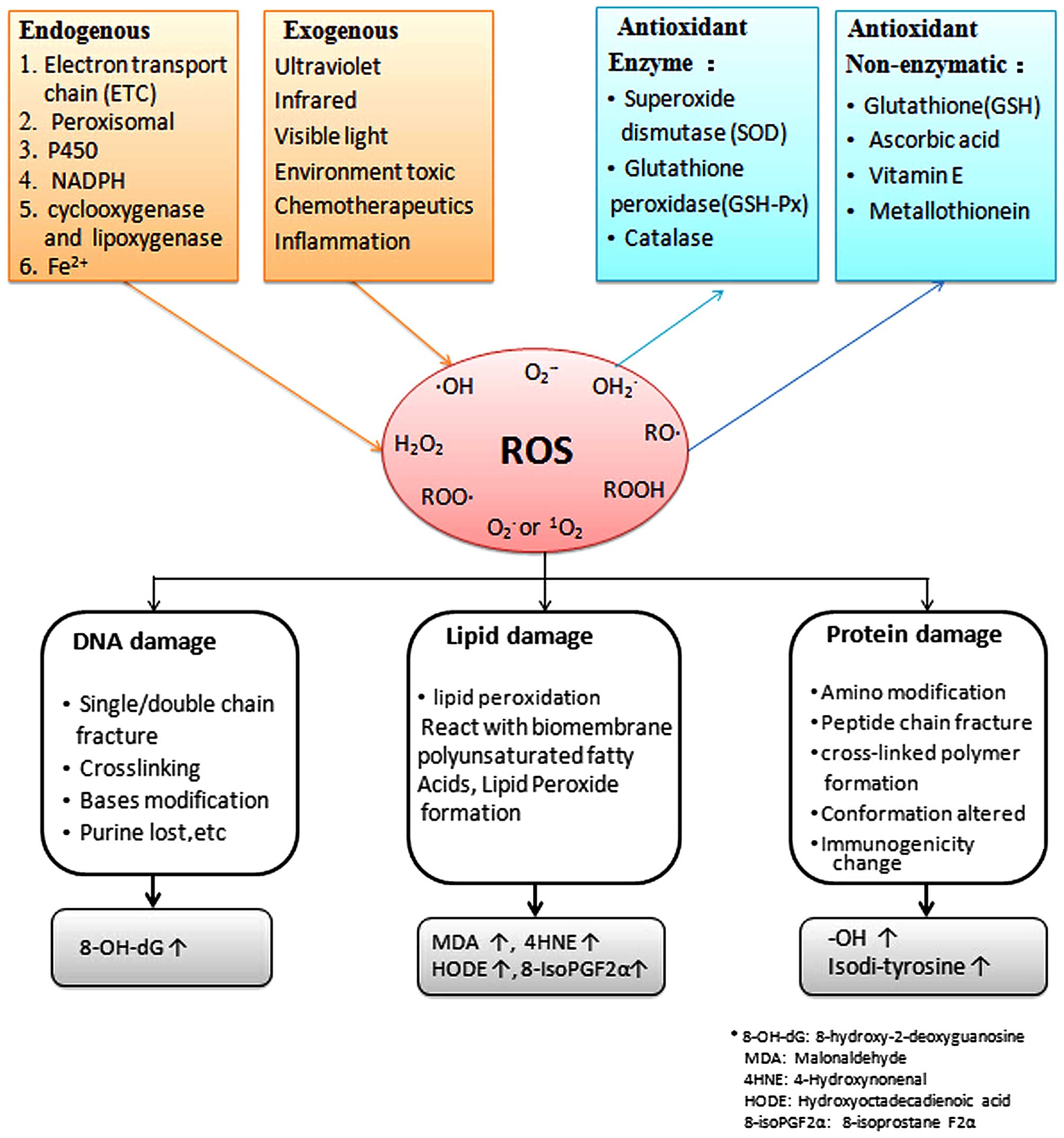
ROS in cells are derived from both endogenous and
exogenous sources. In the endogenous process, the majority of ROS
are generated as a by product of normal metabolism (16). The sources of ROS are summarized
in Fig. 1.
Mitochondrial ROS production
The mitochondria consume >90% of cellular oxygen
in aerobic organisms under physiological conditions. Of this,
approximately 1–5% of the oxygen is converted to ROS (17,18). In the mitochondria, the electron
transport chain resides in the inner membrane where electrons are
transmitted from NADH/FADH2 to oxygen to produce
H2O. However, some electrons leak before they reach the
final step, prematurely reacting with O2 to form
superoxide instead of H2O (19–21).
Peroxisomal ROS production
Peroxisomes are monolayer vesicles, 0.5–1.0
μm in diameter, that generally exist in eukaryotic cells.
Peroxisomes contain a variety of enzymes, such as flavoenzymes and
oxidoreductases. All these enzymes are either involved in the
oxidation of fatty acids, D-amino acid catabolism and anabolism,
glyoxylate/dicarboxylate metabolism, or in the production of
spermidine, an autophagy-stimulating, life-prolonging substance.
Peroxisomes produce H2O2 as a byproduct
(18,22) and also produce
O2−, which mainly originates from the enzyme,
xanthine oxidase, that is also found in the cytosol and is
essential for purine degradation (23).
Endoplasmic reticulum ROS production
The mitochondria were believed to be the main
producer of ROS in the cell; however, an increasing number of
studies over the past decade have indicate dthat the endoplasmic
reticulum, as well as peroxisomes produce as much or even more ROS
than the mitochondria (18,22). In the endoplasmic reticulum, ROS
are mainly produced by cytochrome P450 mono-oxygenases (P450), a
superfamily of heme thiol proteins that are also distributed in the
mitochondrial inner membrane. P450 is responsible for the synthesis
and degradation of endogenous substances (i.e., fatty acids and
hormones) and the detoxification of xenobiotics and lipophilic
compounds. In this process, electrons are transferred from NADPH to
cytochrome P450 via cytochrome P450 reductase, leading to the
hydroxylation of xenobiotics. The leakage of electrons from this
system can result in the formation of oxygen radicals, particularly
O2− (24,25).
The endoplasmic reticulum is the main organelle
responsible for protein processing. At the early stage of the
protein unfolding process, the level of protein disulfide isomerase
increases to correct misfolded proteins by forming correct
disulfide bonds. Via the folding protein process, protein disulfide
isomerase is reduced and an electron is transferred to molecular
oxygen and glutathione (GSH). Incomplete transfer leads to the
production of superoxide (26,27).
ROS produced in membranes and in the
cytosol
Superoxide produced in mitochondrial and plasma
membranes (29–31) is due to the activity of NADPH
oxidases which is different from all other byproduct process. The
superoxide produced in these membranes acts as a signaling molecule
to protect against invading microorganisms (28). Electrons are passed on from NADPH
to FAD to two b-type hemes and finally to O2, resulting
in the formation of superoxide.
In the cytosol, ROS are produced as a byproduct of
arachidonic acid metabolism. With NADH or NADPH, superoxide is
generated by cyclooxygenase and lipoxygenase enzymes that use
arachidonic acid to synthesize prostaglandin H2 or
leukotrienes (32). Additionally,
in the cytosol, ferrous iron reacts with H2O2
and, via the Fenton reaction, generates ferric iron, the very
reactive hydroxyl radical (OH•), and hydroxide
(OH−).
Exogenous factors
Infrared, ultraviolet (UV) and visible light can
cause oxidative damage to the eye. UV light induces mutations that
have been linked to a variety of ophthalmic pathological changes.
UV light can be categorized by its wavelength as: UVA (315–400 nm),
UVB (280–315 nm) and UVC (100–280 nm). All UVC and the majority of
UVB light are absorbed by the cornea. Only UV wavelengths longer
than 295 nm can be transmitted through the cornea to the anterior
chamber; thus the aqueous humor and TM are only exposed to a small
amount of UVA. However, even this small amount of UVA leads to the
generation of ROS that are one of the main causes of oxidative
stress in the TM (33,34). The UV-irradiation process can
affect DNA, particularly mitochondrial DNA (mtDNA). It often leads
to a 'common' 4977-bp long deletion of mtDNA that can increase
mitochondrial ROS production. Increased levels of ROS can also lead
to increased levels of mtDNA damage. Infrared light is absorbed by
the mitochondrial electron transport chain, particularly at complex
IV, leading to an increased leakage of ROS into the mitochondrial
matrix. Besides, X-rays, some environmentally toxic moieties and
some chemotherapies, as well as inflammation can could cause
oxidative damage (18,35,36).
Endogenous and exogenous factors together contribute
to pathogenic events that set forth the process of oxidative
stress-induced damage. Intense exposure to light, robust metabolic
activity, and high oxygen tension are considered the major causes
of pathological ROS (37).
The TM is surrounded by aqueous humour and thus the
maintenance of the redox state of the aqueous humor is of vital
importance to the TM. Inner TM cells resident near the anterior
chamber are more severely exposed to oxidative damage (38,39). In the anterior chamber,
H2O2 and other ROS are mainly generated by a
light-dependent reaction with iris melanin (40). Metabolic pathways, inflammatory
processes and phagocytosis are also important generating pathways.
The concentration of H2O2 in the aqueous
humor is believed to be 25 μM (41).
4. Oxidative damage in the TM
Free radicals take part in many important life
processes. They are closely related to cell proliferation,
differentiation, apoptosis, muscle contraction, nerve conduction
and gene expression, and act as second messengers in cell signal
transduction (42,43). Due to or under some external
causes, such as illness or aging, the oxidative balance of the body
is compromised, leading to pathological changes. ROS damage
proteins, lipids, and in particular, DNA molecules (44); these process are associated with
the development of cell aging, chronic inflammation, cancer and
apoptosis.
OH• is the most reactive ROS. It can
react with different DNA moieties, including purine, pyrimidine and
the deoxyribose backbone, resulting in irreversible mutations, such
as single and double chain fracture, crosslinking between or within
the chain, base modification and purine loss (16). mtDNA is less protected than
nuclear DNA (45) and is more
sensitive to oxidative stress (46). Superoxide anions mainly damage
biological membranes, causing lipid peroxidation that generates
cytotoxic secondary products of lipid oxidation. ROS also damage
amino acid residues, particularly cysteine and methionine residues,
and damage the structure of critical areas, which leads to
misfolding or dysfunction (47).
The TM is the most sensitive tissue to oxidative
damage in the anterior chamber (48). Oxidative stress to the TM can
cause much damage, such as reduce TM mitochondrial respiratory
activity, leading to growth arrest (49), affect ECM structure (50) and lead to ECM accumulation
(51), damage TM cellular DNA
(52), alter membrane
permeability (53), cause the
rearrangement of TM cell cytoskeletal structures, cause the loss of
cell-matrix adhesion (54),
affect cell cycle progression (55), cause inflammatory cytokine release
(56,57), and trigger apoptosis (58,59), as well as many forms of cell death
(60). Cell death may cause a
free radical attack (61,62) and the loss or altered
functionality of TM cells, leading to even more oxidative stress,
thus beginning a vicious cycle (63). At least, ROS alter the morphology,
function and drainage of the anterior chamber filter channel that
eventually leads to an increase in IOP (40,39,54,63–70). In patients with glaucoma, the
levels of mtDNA damage and lipid peroxidation products in the TM
are significantly higher compared with the controls (14,69,71) and their visual field defects, due
to retinal ganglion cell degeneration, are directly proportional to
oxidative damage to the TM (69,70).
5. Antioxidants in the TM
The ability of antioxidants in TM cells to counter
oxidative damage is critical to their survival.
In a biological system, antioxidants can be
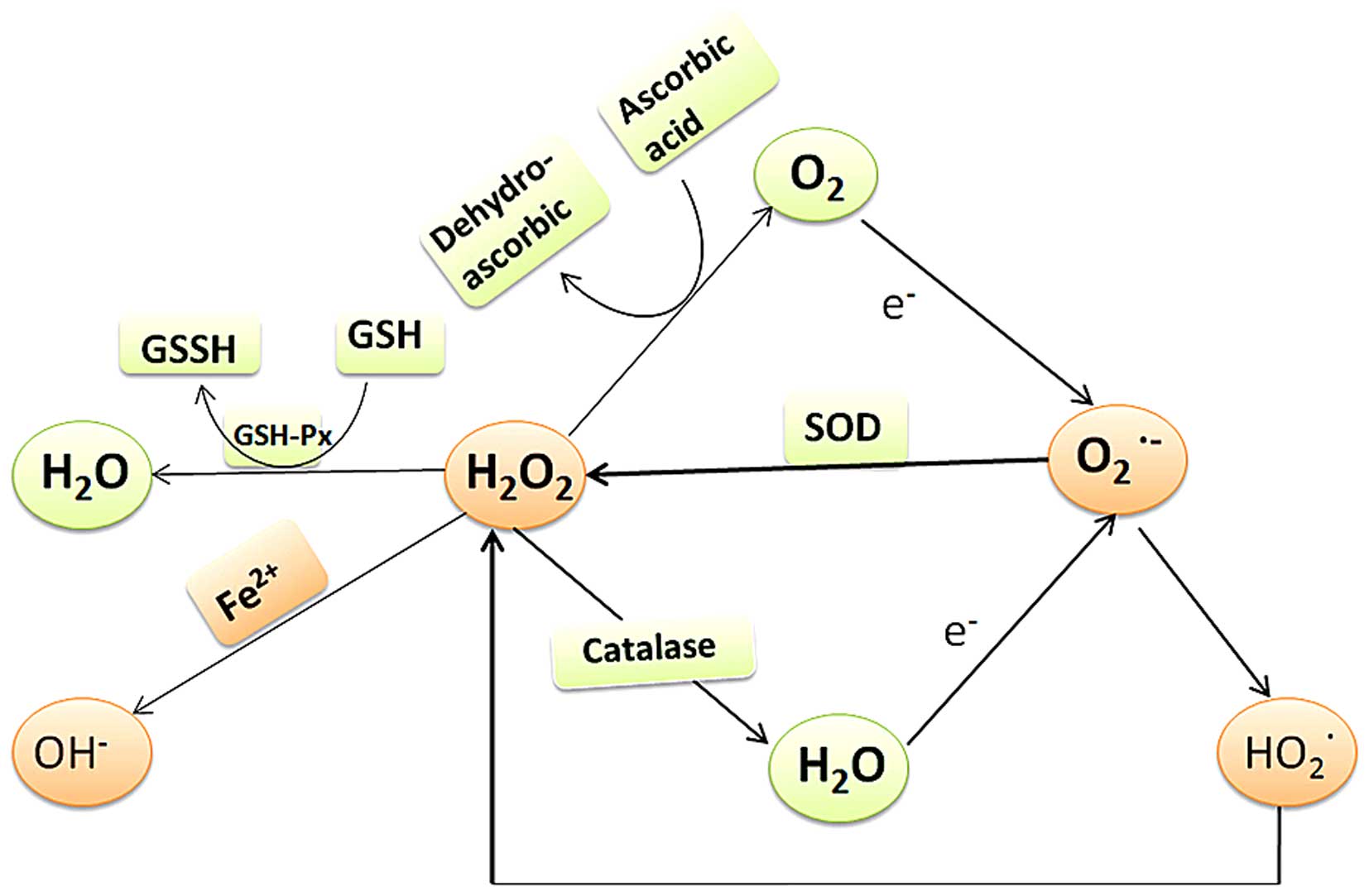
categorized as enzymatic or non-enzymatic (Figs. 1 and 2). Antioxidant enzymes in the TM include
superoxide dismutase (SOD), glutathione peroxidase (GSH-Px),
catalase and glutathione reductase (GSH-Re) (63,72). Non-enzymatic antioxidants include
endogenously produced GSH or dietary compounds, such as vitamins C
and E, and certain metal reduction proteins. The function of these
molecules is to capture free radicals by accepting the unpaired
electron and passing it on. In nocturnal animals, the levels of
antioxidants in aqueous humor are much lower than in diurnal
animals, suggesting that non-enzymatic antioxidants are consumed to
protect the eyes from exogenous light damage (73–75).
In addition to the antioxidants mentioned above, TM
cells have been shown to be able to synthesize a specific set of
proteins, such as β-crystalline, that may act as molecular
chaperones to prevent oxidative damage (76). Compared with plasma, the
concentrations of ascorbic acid (530 μM) and GSH (5.5
μM) in aqueous humor are higher, which is important for
maintaining the anterior chamber and TM oxidation balance (64). Ascorbic acid is considered to be
the main antioxidant in the eye due to its high concentration in
many ocular tissues (77–79). In the aqueous humor, the
concentration of ascorbic acid is 15-fold higher than that in
plasma (80). The mechanisms
responsible for the antioxidant activity of ascorbic acid include
the direct absorption of UV light (81), quenching the fluorescence of
biomolecules, and controlling fluorescence-mediated
bio-transformations (82). A
number of studies have demonstrated that the antioxidant activity
of ascorbic acid depends on its concentration; ascorbic acid can
also promote oxidation (83–85). Ascorbic acid can cause the
decomposition of lipid peroxide and the generation of endogenous
genotoxic substances; these substances can damage DNA and the level
of these substance increases with the ascorbic acid concentration
(86).
Oxidation and antioxidant systems in the eye
crossover to maintain balance. Classical examples include the
GPX-GSH-GR-NADPH and GSH-vitamin C and E systems. These systems
work together so a deficiency in one antioxidant is not always
associated with eye pathologies (73). ROS production essentially depends
on mitochondrial function and on the levels of antioxidant defenses
(87). Age (88–90), diet and gene polymorphisms
(91) also affect the ability of
the body to resist and protect itself against oxidative damage.
In patients with primary open-angle glaucoma, the
levels of circulating GSH are decreased, which indicates that the
antioxidant defense system has been compromised (92). The levels of total reactive
antioxidant potential and water soluble antioxidants, such as
ascorbate and tyrosine in aqueous humor also decrease (93). The level of antioxidant enzymes in
the aqueous humor of patients with primary open-angle glaucoma is
controversial. Some articles have reported an increase in
antioxidant enzymes (94,95), whereas others have reported a
decrease (96,97). Whether the content of antioxidant
enzymes correlates with the clinical course of primary open-angle
glaucoma remains to be elucidated.
6. TM and oxidative stress in
vitro/in vivo
Establishing a reliable oxidative stress model is
essential to elucidating the mechanisms of oxidative stress and the
efficacy of antioxidant drugs. In in vitro experiments,
H2O2 is the most widely used agent in
oxidative stress models. H2O2 can easily pass
through cell membranes and into cells, where it may react with iron
ions to produce very active free radicals. In TM cells, the
concentration of H2O2 usually ranges from 100
μM to 1 mM (54,57,98–100). Treatment concentrations and
times vary significantly among different studies. In some studies,
TM cells were exposed to 200 μM H2O2
for 30 min (57) or 300 μM
for 1 h (103) which caused a
60% reduction in mitochondrial activity. In other studies, TM cells
were exposed to 1 mM H2O2 for 24 h, resulting
in a rate of cell death of apprximately 50% (98,99). The difference here may be due to
the instability of H2O2.
H2O2 is usually stable in solutions with a pH
between 3.5–4.5; however, it easily decomposes in alkaline
solutions or when exposed to bright light, particularly shortwave
radiation. Tert-butyl hydroperoxide (tBHP) is a common lipid
hydroperoxide. Unlike H2O2, tBHP is not
degraded by catalase, which allows it to cause oxidative stress for
a longer period of time compared with H2O2
(101).
In the past, the degree of oxidative stress was
usually measured by quantifying the activity of SOD, catalase and
GSH-Px (93); however, currently
the levels of products of oxidation such as oxidized lipids,
proteins, amino acids and DNA are measured as they are more stable.
Measured products include the lipid peroxidation products,
hydroxyoctadecadienoic acid and malondialdehyde, and the DNA
oxidative modification marker, 8-OH deoxyguanosine (Fig. 1) (11).
In TM cells, after exogenous oxidative treatment,
the following damage has been observed: a decrease in cellular
activity (102), a change in
cell cycle progression, the inhibition of cell proliferation
(103), and the promotion of
cellular senescence (100).
Oxidation treatment can rearrange the cell cytoskeleton structure
(actin and vimentin) (54,102),
increase the synthesis of ECM (fibronectin, plasminogen activator
inhibitor 1, connective tissue growth factor) (103), decrease the adhesion to ECM
(fibronectin, laminin, collagen types I and IV) (54), and increase the expression of some
inflammatory mediators [interleukin (IL)-1α, IL-6, IL-8 and
endothelial-leukocyte adhesion molecule 1 (ELAM-1)] (57,103), leading to cell apoptosis and
death (100). Nuclear factor
(NF)-κB is the most relevant pathway associated with
H2O2-induced changes (54,103). NF-kB expression increases, and
activate downstream target genes, including mitogen-activated
protein kinase (MAPK) signaling path ways; phosphoinositide
3-kinase (PI3K)-Akt, extracellular signal-regulated kinase (ERK)
and p38 have all been reported to contribute to cellular damage
(101,102).
Oxidative in vivo models are diverse;
however, few studies have examined oxidative stress in the TM.
Non-specific methods include the use of irradiation, inhaled ozone
and hypoxia-reperfusion, which may hardly reach effective
concentrations in the TM during a short period of time. For the
research of oxidative stress in the TM, methods involving the
injection of drugs near targeted tissues, which have been widely
reported in many other ocular tissues, such as the retina and lens
(104,105) may be considered for future
studies (106).
7. Clinical studies on protection against
oxidative stress for the treatment of glaucoma
A series of substances have been reported to have
potential antioxidant effects, such as creatine, α-lipoic acid,
nicotinamide and catechins. These substances mainly include some
antioxidant enzymes, oxidase inhibitors, vitamins C and E, some
metal ions such as Se and Zn, and some hormones. Some foods which
have ingredients such as as polyphenolic flavonoids (107) such as tea, coffee, dark
chocolate (108), red wine
(109), anthocyanosides
(110) found in blueberries and
Ginkgo biloba (111) also
have antioxidant effects. However, all of these antioxidant
substances lack targeting and specificity. There are some compounds
that have been shown to protect TM cells from oxidative stress
in vitro (103,112); however, their effects are
limited in vitro. In vivo, dorzolamide, a carbonic
anhydrase inhibitor, has been reported to reduce oxidative products
and increase antioxidant enzyme activity in the aqueous humor of
patients with primary open-angle glaucoma (113).
It is worth emphasizing that although oxidative
stress has been confirmed to play a role in many diseases,
antioxidant supplements are not always good for the health
(114) and sometimes may even
cause harm (115–117). In a 2-year randomized controlled
trial, oral antioxidant supplementation in 117 patients with mild
or moderate glaucoma had no effect (118), and some researchers have
indicated that antioxidants promote cancer cell metastasis
(119). As in studies of other
systems, the research of antioxidant treatments for TM protection
or glaucoma needs to be designed to elucidate how to use
antioxidant compounds, determine when is the best intervention time
(to prevent or to treat), and who (healthy or unhealthy
individuals) can benefit from these compounds.
8. Conclusion and future perspectives
Although many questions remain unanswered, it is
becoming increasingly clear that oxidative stress-induced damage to
the TM is related to glaucoma, which may inspire futher studies to
find better and more stable antioxidants and better models with
which to elucidate the mechanisms involved, and to determine
whether in vitro findings can translate into in vivo
observations. The regulation of the oxidative/redox balance may be
the ultimate target for protecting the TM from oxidative stress and
preventing glaucoma.
Acknowledgments
This study was partially supported by the National
Natural Science Foundation of China (no. 81271002). We would like
to thank Dr He Y.X. and Dr Zhang G.X. for providing inspiration and
encouragement.
References
|
1
|
Kingman S: Glaucoma is second leading
cause of blindness globally. Bull World Health Organ. 82:887–888.
2004.
|
|
2
|
Bojikian KD, Moore DB, Chen PP and
Slabaugh MA: Optic disc hemorrhage after phacoemulsification in
patients with glaucoma. ISRN Ophthalmol. 2014:5740542014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kass MA, Heuer DK, Higginbotham EJ,
Johnson CA, Keltner JL, Miller JP, Parrish RK II, Wilson MR and
Gordon MO: The Ocular Hypertension Treatment Study: A randomized
trial determines that topical ocular hypotensive medication delays
or prevents the onset of primary open-angle glaucoma. Arch
Ophthalmol. 120:701–830. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Heijl A, Leske MC, Bengtsson B, Hyman L,
Bengtsson B and Hussein M; Early Manifest Glaucoma Trial Group:
Reduction of intraocular pressure and glaucoma progression: Results
from the Early Manifest Glaucoma Trial. Arch Ophthalmol.
120:1268–1279. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yue BY: The extracellular matrix and its
modulation in the trabecular meshwork. Surv Ophthalmol. 40:379–390.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Flammer J: The vascular concept of
glaucoma. Surv Ophthalmol. 38(Suppl): S3–S6. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shen F, Chen B, Danias J, Lee KC, Lee H,
Su Y, Podos SM and Mittag TW: Glutamate-induced glutamine
synthetase expression in retinal Muller cells after short-term
ocular hypertension in the rat. Invest Ophthalmol Vis Sci.
45:3107–3112. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Galassi F, Renieri G, Sodi A, Ucci F,
Vannozzi L and Masini E: Nitric oxide proxies and ocular perfusion
pressure in primary open angle glaucoma. Br J Ophthalmol.
88:757–760. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chung HS, Harris A, Evans DW, Kagemann L,
Garzozi HJ and Martin B: Vascular aspects in the pathophysiology of
glaucomatous optic neuropathy. Surv Ophthalmol. 43(Suppl 1):
S43–S50. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lütjen-Drecoll E: Morphological changes in
glaucomatous eyes and the role of TGFbeta2 for the pathogenesis of
the disease. Exp Eye Res. 81:1–4. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dalle-Donne I, Rossi R, Colombo R,
Giustarini D and Milzani A: Biomarkers of oxidative damage in human
disease. Clin Chem. 52:601–623. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weidinger A and Kozlov AV: Biological
activities of reactive oxygen and nitrogen species: Oxidative
stress versus signal transduction. Biomolecules. 5:472–484. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Giustarini D, Dalle-Donne I, Tsikas D and
Rossi R: Oxidative stress and human diseases: Origin, link,
measurement, mechanisms, and biomarkers. Crit Rev Clin Lab Sci.
46:241–281. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Babizhayev MA: Lipid fluorophores of the
human crystalline lens with cataract. Graefes Arch Clin Exp
Ophthalmol. 227:384–391. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Green K: Free radicals and aging of
anterior segment tissues of the eye: A hypothesis. Ophthalmic Res.
27(Suppl 1): 143–149. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jezek P and Hlavatá L: Mitochondria in
homeostasis of reactive oxygen species in cell, tissues, and
organism. Int J Biochem Cell Biol. 37:2478–2503. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Turrens JF: Mitochondrial formation of
reactive oxygen species. J Physiol. 552:335–344. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rinnerthaler M, Bischof J, Streubel MK,
Trost A and Richter K: Oxidative stress in aging human skin.
Biomolecules. 5:545–589. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Harman D: The biologic clock: The
mitochondria? J Am Geriatr Soc. 20:145–147. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chance B, Sies H and Boveris A:
Hydroperoxide metabolism in mammalian organs. Physiol Rev.
59:527–605. 1979.PubMed/NCBI
|
|
21
|
Muller FL, Lustgarten MS, Jang Y,
Richardson A and Van Remmen H: Trends in oxidative aging theories.
Free Radic Biol Med. 43:477–503. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fransen M, Nordgren M, Wang B and
Apanasets O: Role of peroxisomes in ROS/RNS-metabolism:
Implications for human disease. Biochim Biophys Acta.
1822:1363–1373. 2012. View Article : Google Scholar
|
|
23
|
Harrison R: Structure and function of
xanthine oxidoreductase: Where are we now? Free Radic Biol Med.
33:774–797. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bae YS, Oh H, Rhee SG and Yoo YD:
Regulation of reactive oxygen species generation in cell signaling.
Mol Cells. 32:491–509. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gorsky LD, Koop DR and Coon MJ: On the
stoichiometry of the oxidase and monooxygenase reactions catalyzed
by liver microsomal cytochrome P-450. Products of oxygen reduction.
J Biol Chem. 259:6812–6817. 1984.PubMed/NCBI
|
|
26
|
Benham AM, van Lith M, Sitia R and
Braakman I: Ero1-PDI interactions, the response to redox flux and
the implications for disulfide bond formation in the mammalian
endoplasmic reticulum. Philos Trans R Soc Lond B Biol Sci.
368:201104032013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bhandary B, Marahatta A, Kim HR and Chae
HJ: An involvement of oxidative stress in endoplasmic reticulum
stress and its associated diseases. Int J Mol Sci. 14:434–456.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rinnerthaler M, Büttner S, Laun P, Heeren
G, Felder TK, Klinger H, Weinberger M, Stolze K, Grousl T, Hasek J,
et al: Yno1p/Aim14p, a NADPH-oxidase ortholog, controls
extramitochondrial reactive oxygen species generation, apoptosis,
and actin cable formation in yeast. Proc Natl Acad Sci USA.
109:8658–8663. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Block K, Gorin Y and Abboud HE:
Subcellular localization of Nox4 and regulation in diabetes. Proc
Natl Acad Sci USA. 106:14385–14390. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Krause KH: Tissue distribution and
putative physiological function of NOX family NADPH oxidases. Jpn J
Infect Dis. 57:S28–S29. 2004.PubMed/NCBI
|
|
31
|
Nauseef WM: Biological roles for the NOX
family NADPH oxidases. J Biol Chem. 283:16961–16965. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kukreja RC, Kontos HA, Hess ML and Ellis
EF: PGH synthase and lipoxygenase generate superoxide in the
presence of NADH or NADPH. Circ Res. 59:612–619. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Norren DV and Vos JJ: Spectral
transmission of the human ocular media. Vision Res. 14:1237–1244.
1974. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Spector A: Oxidative stress-induced
cataract: Mechanism of action. FASEB J. 9:1173–1182.
1995.PubMed/NCBI
|
|
35
|
Bryszewska M, Piasecka A, Zavodnik LB,
Distel L and Schüssler H: Oxidative damage of Chinese hamster
fibroblasts induced by t-butyl hydroperoxide and by X-rays. Biochim
Biophys Acta. 1621:285–291. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Saccà SC, Pulliero A and Izzotti A: The
dysfunction of the trabecular meshwork during glaucoma course. J
Cell Physiol. 230:510–525. 2015. View Article : Google Scholar
|
|
37
|
Shoham A, Hadziahmetovic M, Dunaief JL,
Mydlarski MB and Schipper HM: Oxidative stress in diseases of the
human cornea. Free Radic Biol Med. 45:1047–1055. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Alvarado J, Murphy C and Juster R:
Trabecular meshwork cellularity in primary open-angle glaucoma and
nonglaucomatous normals. Ophthalmology. 91:564–579. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kahn MG, Giblin FJ and Epstein DL:
Glutathione in calf trabecular meshwork and its relation to aqueous
humor outflow facility. Invest Ophthalmol Vis Sci. 24:1283–1287.
1983.PubMed/NCBI
|
|
40
|
Wielgus AR and Sarna T: Ascorbate enhances
photogeneration of hydrogen peroxide mediated by the iris melanin.
Photochem Photobiol. 84:683–691. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Spector A and Garner WH: Hydrogen peroxide
and human cataract. Exp Eye Res. 33:673–681. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sundaresan M, Yu ZX, Ferrans VJ, Irani K
and Finkel T: Requirement for generation of
H2O2 for platelet-derived growth factor
signal transduction. Science. 270:296–299. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gulati P, Klöhn PC, Krug H, Göttlicher M,
Markova B, Böhmer FD and Herrlich P: Redox regulation in mammalian
signal transduction. IUBMB Life. 52:25–28. 2001. View Article : Google Scholar
|
|
44
|
Beckman KB and Ames BN: Mitochondrial
aging: Open questions. Ann NY Acad Sci. 854:118–127. 1998.
View Article : Google Scholar
|
|
45
|
Balansky R, Izzotti A, Scatolini L,
D'Agostini F and De Flora S: Induction by carcinogens and
chemoprevention by N-acetylcysteine of adducts to mitochondrial DNA
in rat organs. Cancer Res. 56:1642–1647. 1996.PubMed/NCBI
|
|
46
|
de Grey AD: A proposed refinement of the
mitochondrial free radical theory of aging. Bioessays. 19:161–166.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dean RT, Fu S, Stocker R and Davies MJ:
Biochemistry and pathology of radical-mediated protein oxidation.
Biochem J. 324:1–18. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Izzotti A, Saccà SC, Longobardi M and
Cartiglia C: Sensitivity of ocular anterior chamber tissues to
oxidative damage and its relevance to the pathogenesis of glaucoma.
Invest Ophthalmol Vis Sci. 50:5251–5258. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Clopton DA and Saltman P: Low-level
oxidative stress causes cell-cycle specific arrest in cultured
cells. Biochem Biophys Res Commun. 210:189–196. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Knepper PA, Goossens W and Palmberg PF:
Glycosaminoglycan stratification of the juxtacanalicular tissue in
normal and primary open-angle glaucoma. Invest Ophthalmol Vis Sci.
37:2414–2425. 1996.PubMed/NCBI
|
|
51
|
Knepper PA, Goossens W, Hvizd M and
Palmberg PF: Glycosaminoglycans of the human trabecular meshwork in
primary open-angle glaucoma. Invest Ophthalmol Vis Sci.
37:1360–1367. 1996.PubMed/NCBI
|
|
52
|
Izzotti A, Longobardi M, Cartiglia C and
Saccà SC: Mitochondrial damage in the trabecular meshwork occurs
only in primary open-angle glaucoma and in pseudoexfoliative
glaucoma. PLoS One. 6:e145672011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Alvarado JA, Alvarado RG, Yeh RF,
Franse-Carman L, Marcellino GR and Brownstein MJ: A new insight
into the cellular regulation of aqueous outflow: How trabecular
meshwork endothelial cells drive a mechanism that regulates the
permeability of Schlemm's canal endothelial cells. Br J Ophthalmol.
89:1500–1505. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhou L, Li Y and Yue BY: Oxidative stress
affects cytoskeletal structure and cell-matrix interactions in
cells from an ocular tissue: The trabecular meshwork. J Cell
Physiol. 180:182–189. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Giancotti FG: Integrin signaling:
Specificity and control of cell survival and cell cycle
progression. Curr Opin Cell Biol. 9:691–700. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Welge-Lüssen U and Birke K: Oxidative
stress in the trabecular meshwork of POAG. Klin Monbl Augenheilkd.
227:99–107. 2010.In German.
|
|
57
|
Li G, Luna C, Liton PB, Navarro I, Epstein
DL and Gonzalez P: Sustained stress response after oxidative stress
in trabecular meshwork cells. Mol Vis. 13:2282–2288. 2007.
|
|
58
|
Dröge W: Free radicals in the
physiological control of cell function. Physiol Rev. 82:47–95.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Frisch SM and Ruoslahti E: Integrins and
anoikis. Curr Opin Cell Biol. 9:701–706. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Martindale JL and Holbrook NJ: Cellular
response to oxidative stress: Signaling for suicide and survival. J
Cell Physiol. 192:1–15. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yan DB, Trope GE, Ethier CR, Menon IA and
Wakeham A: Effects of hydrogen peroxide-induced oxidative damage on
outflow facility and washout in pig eyes. Invest Ophthalmol Vis
Sci. 32:2515–2520. 1991.PubMed/NCBI
|
|
62
|
Padgaonkar V, Giblin FJ, Leverenz V, Lin
LR and Reddy VN: Studies of H2O2-induced
effects on cultured bovine trabecular meshwork cells. J Glaucoma.
3:123–131. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
De La Paz MA and Epstein DL: Effect of age
on superoxide dismutase activity of human trabecular meshwork.
Invest Ophthalmol Vis Sci. 37:1849–1853. 1996.PubMed/NCBI
|
|
64
|
Alvarado J, Murphy C, Polansky J and
Juster R: Age-related changes in trabecular meshwork cellularity.
Invest Ophthalmol Vis Sci. 21:714–727. 1981.PubMed/NCBI
|
|
65
|
Freedman SF, Anderson PJ and Epstein DL:
Superoxide dismutase and catalase of calf trabecular meshwork.
Invest Ophthalmol Vis Sci. 26:1330–1335. 1985.PubMed/NCBI
|
|
66
|
Tan JC, Peters DM and Kaufman PL: Recent
developments in understanding the pathophysiology of elevated
intraocular pressure. Curr Opin Ophthalmol. 17:168–174.
2006.PubMed/NCBI
|
|
67
|
Gabelt BT and Kaufman PL: Changes in
aqueous humor dynamics with age and glaucoma. Prog Retin Eye Res.
24:612–637. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kumar DM and Agarwal N: Oxidative stress
in glaucoma: A burden of evidence. J Glaucoma. 16:334–343. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Izzotti A, Saccà SC, Cartiglia C and De
Flora S: Oxidative deoxyribonucleic acid damage in the eyes of
glaucoma patients. Am J Med. 114:638–646. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Saccà SC, Pascotto A, Camicione P, Capris
P and Izzotti A: Oxidative DNA damage in the human trabecular
meshwork: Clinical correlation in patients with primary open-angle
glaucoma. Arch Ophthalmol. 123:458–463. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zanon-Moreno V, Marco-Ventura P,
Lleo-Perez A, Pons-Vazquez S, Garcia-Medina JJ, Vinuesa-Silva I,
Moreno-Nadal MA and Pinazo-Duran MD: Oxidative stress in primary
open-angle glaucoma. J Glaucoma. 17:263–268. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Nguyen KP, Chung ML, Anderson PJ, Johnson
M and Epstein DL: Hydrogen peroxide removal by the calf aqueous
outflow pathway. Invest Ophthalmol Vis Sci. 29:976–981.
1988.PubMed/NCBI
|
|
73
|
Chen Y, Mehta G and Vasiliou V:
Antioxidant defenses in the ocular surface. Ocul Surf. 7:176–185.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Behndig A, Svensson B, Marklund SL and
Karlsson K: Superoxide dismutase isoenzymes in the human eye.
Invest Ophthalmol Vis Sci. 39:471–475. 1998.PubMed/NCBI
|
|
75
|
Reiss GR, Werness PG, Zollman PE and
Brubaker RF: Ascorbic acid levels in the aqueous humor of nocturnal
and diurnal mammals. Arch Ophthalmol. 104:753–755. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Tamm ER, Russell P, Johnson DH and
Piatigorsky J: Human and monkey trabecular meshwork accumulate
alpha B-crystallin in response to heat shock and oxidative stress.
Invest Ophthalmol Vis Sci. 37:2402–2413. 1996.PubMed/NCBI
|
|
77
|
Hanashima C and Namiki H: Reduced
viability of vascular endothelial cells by high concentration of
ascorbic acid in vitreous humor. Cell Biol Int. 23:287–298. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Brubaker RF, Bourne WM, Bachman LA and
McLaren JW: Ascorbic acid content of human corneal epithelium.
Invest Ophthalmol Vis Sci. 41:1681–1683. 2000.PubMed/NCBI
|
|
79
|
Dreyer R and Rose RC: Lacrimal gland
uptake and metabolism of ascorbic acid. Proc Soc Exp Biol Med.
202:212–216. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Becker B: Chemical composition of human
aqueous humor; effects of acetazoleamide. AMA Arch Opthalmol.
57:793–800. 1957. View Article : Google Scholar
|
|
81
|
Giblin FJ, McCready JP, Kodama T and Reddy
VN: A direct correlation between the levels of ascorbic acid and
H2O2 in aqueous humor. Exp Eye Res. 38:87–93.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Ringvold A: The significance of ascorbate
in the aqueous humour protection against UV-A and UV-B. Exp Eye
Res. 62:261–264. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Griffiths HR and Lunec J: Ascorbic acid in
the 21st century - more than a simple antioxidant. Environ Toxicol
Pharmacol. 10:173–182. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Andorn AC, Britton RS and Bacon BR:
Ascorbate-stimulated lipid peroxidation in human brain is dependent
on iron but not on hydroxyl radical. J Neurochem. 67:717–722. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Song JH, Shin SH and Ross GM: Oxidative
stress induced by ascorbate causes neuronal damage in an in vitro
system. Brain Res. 895:66–72. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Lee SH, Oe T and Blair IA: Vitamin
C-induced decomposition of lipid hydroperoxides to endogenous
genotoxins. Science. 292:2083–2086. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Camougrand N and Rigoulet M: Aging and
oxidative stress: Studies of some genes involved both in aging and
in response to oxidative stress. Respir Physiol. 128:393–401. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Cejková J, Vejrazka M, Pláteník J and
Stípek S: Age-related changes in superoxide dismutase, glutathione
peroxidase, catalase and xanthine oxidoreductase/xanthine oxidase
activities in the rabbit cornea. Exp Gerontol. 39:1537–1543. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Samiec PS, Drews-Botsch C, Flagg EW, Kurtz
JC, Sternberg P Jr, Reed RL and Jones DP: Glutathione in human
plasma: Decline in association with aging, age-related macular
degeneration, and diabetes. Free Radic Biol Med. 24:699–704. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Serbecic N and Beutelspacher SC: Vitamins
inhibit oxidant-induced apoptosis of corneal endothelial cells. Jpn
J Ophthalmol. 49:355–362. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Wallace DC: A mitochondrial paradigm of
metabolic and degenerative diseases, aging, and cancer: A dawn for
evolutionary medicine. Annu Rev Genet. 39:359–407. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Gherghel D, Griffiths HR, Hilton EJ,
Cunliffe IA and Hosking SL: Systemic reduction in glutathione
levels occurs in patients with primary open-angle glaucoma. Invest
Ophthalmol Vis Sci. 46:877–883. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Ferreira SM, Lerner SF, Brunzini R,
Evelson PA and Llesuy SF: Oxidative stress markers in aqueous humor
of glaucoma patients. Am J Ophthalmol. 137:62–69. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Ghanem AA, Arafa LF and El-Baz A:
Oxidative stress markers in patients with primary open-angle
glaucoma. Curr Eye Res. 35:295–301. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Yang J, Tezel G, Patil RV, Romano C and
Wax MB: Serum autoantibody against glutathione S-transferase in
patients with glaucoma. Invest Ophthalmol Vis Sci. 42:1273–1276.
2001.PubMed/NCBI
|
|
96
|
Bagnis A, Izzotti A, Centofanti M and
Saccà SC: Aqueous humor oxidative stress proteomic levels in
primary open angle glaucoma. Exp Eye Res. 103:55–62. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Majsterek I, Malinowska K, Stanczyk M,
Kowalski M, Blaszczyk J, Kurowska AK, Kaminska A, Szaflik J and
Szaflik JP: Evaluation of oxidative stress markers in pathogenesis
of primary open-angle glaucoma. Exp Mol Pathol. 90:231–237. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Famili A, Ammar DA and Kahook MY: Ethyl
pyruvate treatment mitigates oxidative stress damage in cultured
trabecular meshwork cells. Mol Vis. 19:1304–1309. 2013.PubMed/NCBI
|
|
99
|
Ammar DA, Hamweyah KM and Kahook MY:
Antioxidants protect trabecular meshwork cells from hydrogen
peroxide-induced cell death. Transl Vis Sci Technol. 1:42012.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Yu AL, Fuchshofer R, Kampik A and
Welge-Lüssen U: Effects of oxidative stress in trabecular meshwork
cells are reduced by prostaglandin analogues. Invest Ophthalmol Vis
Sci. 49:4872–4880. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Yang Y, Liu X, Huang J, Zhong Y, Mao Z,
Xiao H, Li M and Zhuo Y: Inhibition of p38 mitogen-activated
protein kinase phosphorylation decrease tert-butyl
hydroperoxide-induced apoptosis in human trabecular meshwork cells.
Mol Vis. 18:2127–2136. 2012.PubMed/NCBI
|
|
102
|
Awai-Kasaoka N, Inoue T, Kameda T,
Fujimoto T, Inoue-Mochita M and Tanihara H: Oxidative stress
response signaling pathways in trabecular meshwork cells and their
effects on cell viability. Mol Vis. 19:1332–1340. 2013.PubMed/NCBI
|
|
103
|
Tourtas T, Birke MT, Kruse FE,
Welge-Lüssen UC and Birke K: Preventive effects of omega-3 and
omega-6 fatty acids on peroxide mediated oxidative stress responses
in primary human trabecular meshwork cells. PLoS One. 7:e313402012.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Ham WT Jr, Mueller HA, Ruffolo JJ Jr,
Millen JE, Cleary SF, Guerry RK and Guerry D III: Basic mechanisms
underlying the production of photochemical lesions in the mammalian
retina. Curr Eye Res. 3:165–174. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Yokoyama Y, Maruyama K, Yamamoto K,
Omodaka K, Yasuda M, Himori N, Ryu M, Nishiguchi KM and Nakazawa T:
The role of calpain in an in vivo model of oxidative stress-induced
retinal ganglion cell damage. Biochem Biophys Res Commun.
451:510–515. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Liu Q, Wu K, Qiu X, Yang Y, Lin X and Yu
M: siRNA silencing of gene expression in trabecular meshwork: RhoA
siRNA reduces IOP in mice. Curr Mol Med. 12:1015–1027. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Romagnolo DF and Selmin OI: Flavonoids and
cancer prevention: A review of the evidence. J Nutr Gerontol
Geriatr. 31:206–238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Miller KB, Stuart DA, Smith NL, Lee CY,
McHale NL, Flanagan JA, Ou B and Hurst WJ: Antioxidant activity and
polyphenol and procyanidin contents of selected commercially
available cocoa-containing and chocolate products in the United
States. J Agric Food Chem. 54:4062–4068. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Haufschild T, Kaiser HJ, Preisig T,
Pruente C and Flammer J: Influence of red wine on visual function
and endothelin-1 plasma level in a patient with optic neuritis. Ann
Neurol. 53:825–826. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Määttä-Riihinen KR, Kähkönen MP, Törrönen
AR and Heinonen IM: Catechins and procyanidins in berries of
vaccinium species and their antioxidant activity. J Agric Food
Chem. 53:8485–8491. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Cybulska-Heinrich A, Mozaffarieh M and
Flammer J: Value of non-IOP lowering therapy for glaucoma. Klin
Monbl Augenheilkd. 230:114–119. 2013.In German. PubMed/NCBI
|
|
112
|
Luna C, Li G, Liton PB, Qiu J, Epstein DL,
Challa P and Gonzalez P: Resveratrol prevents the expression of
glaucoma markers induced by chronic oxidative stress in trabecular
meshwork cells. Food Chem Toxicol. 47:198–204. 2009. View Article : Google Scholar :
|
|
113
|
Zanon-Moreno V, Garcia-Medina JJ,
Gallego-Pinazo R, Vinuesa-Silva I, Moreno-Nadal MA and Pinazo-Duran
MD: Antioxidant status modifications by topical administration of
dorzolamide in primary open-angle glaucoma. Eur J Ophthalmol.
19:565–571. 2009.PubMed/NCBI
|
|
114
|
Gutteridge JM and Halliwell B:
Antioxidants: Molecules, medicines, and myths. Biochem Biophys Res
Commun. 393:561–564. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Bjelakovic G, Nikolova D, Simonetti RG and
Gluud C: Antioxidant supplements for prevention of gastrointestinal
cancers: A systematic review and meta-analysis. Lancet.
364:1219–1228. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Talaulikar VS and Manyonda IT: Vitamin C
as an antioxidant supplement in women's health: A myth in need of
urgent burial. Eur J Obstet Gynecol Reprod Biol. 157:10–13. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Kaisar MA and Cucullo L: OTC antioxidant
products for the treatment of cardiovascular and other disorders:
Popular myth or fact? J Pharmacovigil. 3:e1362015. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Garcia-Medina JJ, Garcia-Medina M,
Garrido-Fernandez P, Galvan-Espinosa J, Garcia-Maturana C,
Zanon-Moreno V and Pinazo-Duran MD: A two-year follow-up of oral
antioxidant supplementation in primary open-angle glaucoma: An
open-label, randomized, controlled trial. Acta Ophthalmol.
93:546–554. 2015. View Article : Google Scholar
|
|
119
|
Piskounova E, Agathocleous M, Murphy MM,
Hu Z, Huddlestun SE, Zhao Z, Leitch AM, Johnson TM, DeBerardinis RJ
and Morrison SJ: Oxidative stress inhibits distant metastasis by
human melanoma cells. Nature. 527:186–191. 2015. View Article : Google Scholar : PubMed/NCBI
|