Introduction
Bronchial asthma is a complex inflammatory airway
disease mainly characterized by the presence of inflammation,
airway hyperresponsiveness (AHR) and airway remodeling. While these
are disease-defining characteristics, asthma has recently been
recognized as a widely heterogeneous disease [Global Initiative for
Asthma (GINA), 2014 update]. To date, it is estimated that 300
million individuals are affected by the disease worldwide and an
effective treatment has not yet been found (1). Common allergens such as house dust,
animal dander, inhalants, drugs, pollens, and air pollutants
contribute to the induction of asthma. Cumulative evidence has
indicated that CC chemokine receptor 7 (CCR7) and its ligands,
CCL19/CCL21, are involved in these inflammatory responses (2–4);
they are also associated with the recruitment and the activation of
multiple cell types, such as dendritic cells (DCs), eosinophils
(EOS), T cells and B cells (5–8),
and also interact with inflammatory cytokines, such as tumor
necrosis factor (TNF)-α and interleukin (IL)-6 (9,10).
Chemokines are small (8–14 kDa) chemotactic
cytokines that bind to seven transmembrane domain G protein-coupled
receptors (GPCR) and are secreted by a great variety of cell types.
Chemokines are important mediators involved in the migration and
activation of various subsets of leukocytes (11), as well as lymphoid development and
lymphocyte homing into and out of secondary and tertiary lymphoid
organs.
CCR7 is a member of the GPCR family and is commonly
expressed in memory T cells, B cells and mature DCs (12). CCL19 and CCL21 are the only
ligands for CCR7. T cells and antigen-presenting DCs establish
close physical contacts within the lymph nodes, which jointly
contribute to a myriad of adaptive immune functions, including
secondary lymphoid organogenesis, and regulatory and memory T cell
function (13).
It has been previously described that the CCR7
promoter includes potential binding sites for nuclear factor-κB
(NF-κB) (14), which is involved
in many inflammatory diseases. CCR7 is a direct target of NF-κB and
the induction of CCR7 expression has been shown to be regulated by
NF-κB (14), indicating that the
modulation of inflammation may be beneficial to the control and
treatment of asthma.
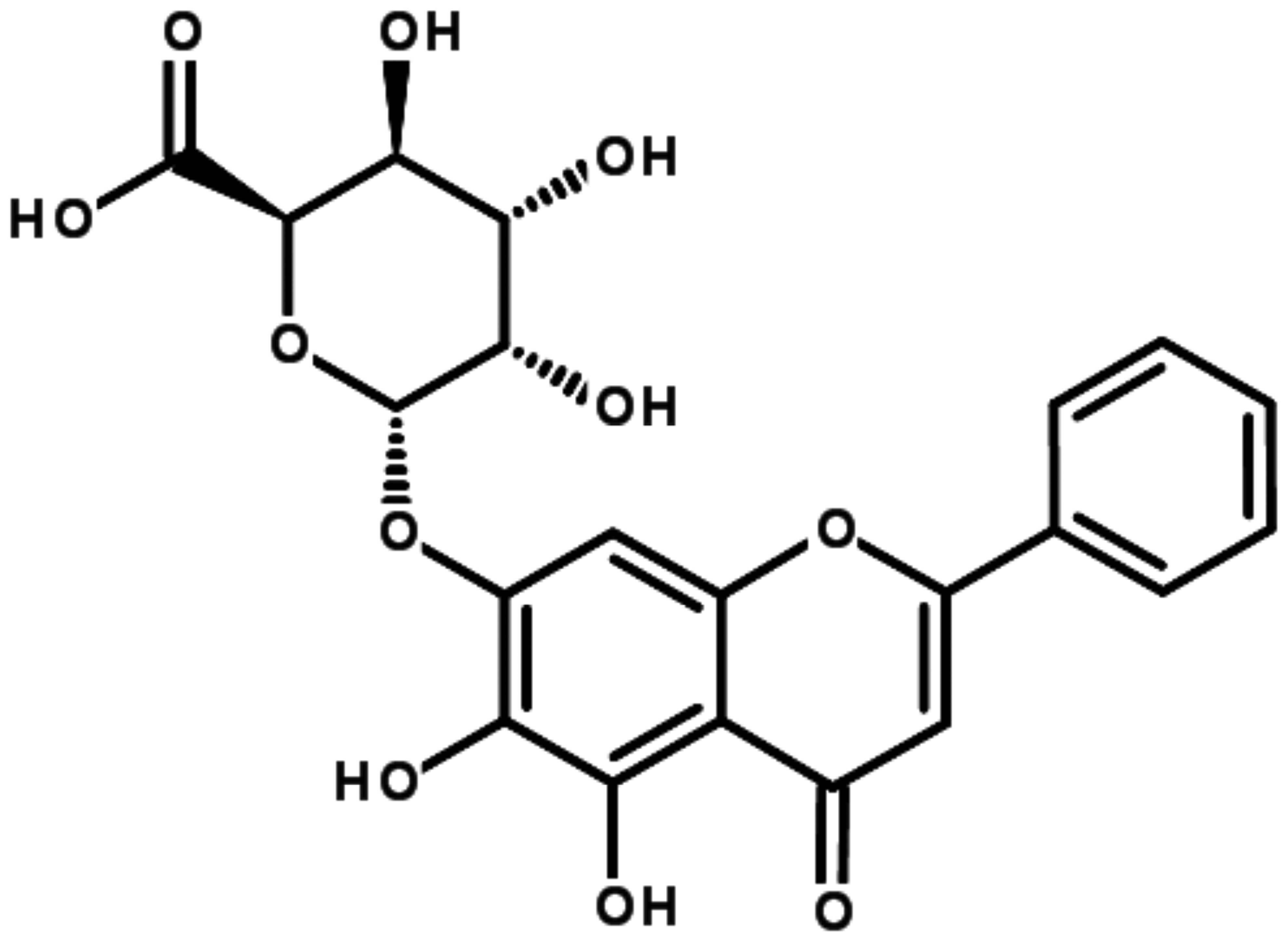
Baicalin, as a small molecule flavonoid compound
(Fig. 1), which is the major
active constituent of Scutellariae radix, has been extensively
employed in traditional Chinese medicine (TCM) for the treatment of
asthma. It has been demonstrated that baicalin exerts
anti-inflammatory effects on experimental colitis (15). In a previous study of ours, we
demonstrated the anti-airway remodeling effect of baicalin
(16). Moreover, baicalin has
been shown to rectify the Th1/Th2 and Treg/Th17 imbalance in
asthmatic mice (17). However,
the effects and related molecular mechanisms of action of baicalin
on CCR7 and its ligands associated with airway inflammation have
not been fully elucidated. The present study was designed to
examine the effects and the underlying molecular mechanisms of
action of baicalin on airway inflammation in a mouse model of
ovalbumin (OVA)-induced asthma.
Materials and methods
Animals and reagents
Seven-week-old female BALB/c mice were purchased
from SIPPR/BK Laboratory Animal Co., Ltd., (Shanghai, China), and
reared under specific pathogen-free conditions following the
guidelines and approval of the Animal Care and Use Committee on the
Ethics of Animal Experiments of Fudan University, Shanghai,
China.
Baicalin (purity ≥98%; chemical structure shown in
Fig. 1) was provided by Must
Biotechnology Co., Ltd. (Chengdu, China). OVA (grade V), aluminum
hydroxide and methacholine (Mch) were purchased from Sigma-Aldrich
(St. Louis, MO, USA). The OVA-specific IgE ELISA kit was obtained
from Shibayagi (Gunma, Japan). The CCL19 and CCL21, and the TNF-α
and IL-6 ELISA kits were purchased from RayBiotech (Norcross, GA,
USA). Anti-mouse CCR7 antibody was purchased from Abcam (Cambridge,
MA, USA).
Mouse model of OVA-induced asthma and
treatment
Asthma was initiated by an intraperitoneal injection
of 0.2 ml saline containing 20 µg OVA and 2 mg aluminum
hydroxide on days 0, 7, 14 and 21; from day 25 to 31, each mouse
was placed into an individual chamber and challenged with 3% OVA
nebulization (30 min/day) utilizing an ultrasonic nebulizer model
402AI; Yuyue, Jiangsu, China).
The mice were divided into 6 experimental groups as
follows (n=12/group): i) the normal control (NC) group; ii) the
asthma (A) group; iii) asthmatic mice treated with 10 mg/kg
baicalin (B10) group; iv) asthmatic mice treated with 25 mg/kg
baicalin (B25) group; v) asthmatic mice treated with 50 mg/kg
baicalin (B50) group; and vi) asthmatic mice treated with
dexamethasone (DXM) (0.085 mg/kg). The mice in the NC group were
sensitized and challenged with an equivalent volume of normal
saline. Baicalin and DXM were intragastrically administered from
day 24 to 31 prior to the OVA challenge. The following analyses
were implemented within 24 h of the final OVA challenge:
Determination of lung function
AHR was detected by an invasive pulmonary facility
for small animals (Buxco Electronics, Troy, NY, USA) after the
final OVA challenge, as previously described by Pichavant et
al (18). Briefly, each mouse
was tracheostomized and intubated under anesthesia and the
administration by nebulization to gradients of Mch (3.125, 6.25 and
12.5 mg/ml) was carried out to evaluate pulmonary resistance
(RL) and pulmonary dynamic compliance (Cdyn). Data were
expressed as a percentage change from the baseline value.
Inflammatory cell classification in
bronchoalveolar lavage fluid (BALF) and collection of serum
Immediately following the assessment of AHR, and
while the mice were still under anesthesia, blood was collected
from the left eyeball and stored at 4°C for 2 h and then
centrifuged at 5,000 × g, 4°C for 30 min. The serum was collected,
repackaged and stored at −80°C for use in ELISA. The lung tissues
of the mice were then exposed, the right lung was tied and BALF was
collected by inserting a tracheal tube and lavaging the left lung 3
times with 0.3 ml aliquots of phosphate-buffered saline (PBS) and
centrifuged at 800 × g, 4°C for 10 min. The supernatants were
gathered and stored at −80°C for ELISA and the sediments were
resuspended in 0.1 ml PBS for determining the cell count using a
hemacytometer (Hemavet 950FS; Drew Scientific, Inc., Waterbury, CT,
USA).
Lung histopathological assessment
Following the collection of BALF, the mice were
euthanized and the lung tissue from the middle lobe of the right
lung was detached and fixed in 4% paraformaldehyde, embedded in
paraffin and thin slices (4-µm-thick) were cut from blocks
and stained with hematoxylin and eosin (H&E). The
histopathological changes were determined under an optical
microscope (Eclipse 80i; Nikon Corporation, Tokyo, Japan) to assess
the inflammatory changes in the lung tissues of the mice.
Histopathological evaluation was performed in a
blinded manner on randomized sections. The severity of inflammatory
cell infiltration into the lungs was assessed by a 5 point scoring
system as previously described by Underwood et al (19).
Detection of IgE, chemokine and cytokine
levels in BALF or serum by ELISA
The levels of CCL19, CCL21 and OVA-specific IgE in
BALF, as well as the levels of IL-6 and TNF-α in serum were
detected using respective ELISA kits, following the manufacturer's
instructions. The assays employed antibodies specific for each
cytokine coated on 96-well plates. All reagents, samples and
standards were prepared as instructed. Subsequently, 100 µl
standard or sample were added to each well followed by incubation
for 2.5 h at room temperature. The solution was then discarded and
the sampels were washed 3–4 times with 1X Wash Solution. This was
followed by the addition of 100 µl prepared biotin antibody
to each well and incubation for 1 h at room temperature, and
further washing with 1X Wash Solution for 3 times. Subsequently,
100 µl prepared streptavidin solution was added followed by
incubation for 45 minutes at room temperature. TMB One-Step
Substrate Reagent (100 µl) was then added to each well,
followed by incubation for 30 min at room temperature. Finally, 50
µl Stop Solution were added to each well and the absorbance
was immediately read at 450 nm.
Reverse transcription-quantitative PCR
(RT-qPCR)
The expression of CCR7 in the lung tissue was
detected by RT-qPCR. Briefly, total RNA was extracted and reverse
transcripted into first-strand cDNA. qPCR amplification was then
performed according to the manufacturer's instructions using a PCR
Amplifier (FTC-3000 qPCR system; Funglyn Biotech, Inc., Toronto,
ON, Canada). The cycling conditions were as follows: 1 cycle at
95°C for 3 min, 40 cycles at 95°C for 5 sec and 60°C for 30 sec;
and 1 cycle for melting at 94°C for 90 sec, 60°C for 3 min and 94°C
for 10 sec. The fold change in the expression of each gene was
calculated using the 2−ΔΔCt method, with the
housekeeping gene, GAPDH, as an internal control. The final data
were presented as the fold change compared to the expression level
in the mice in the NC group. The sequences of the primers used are
listed in Table I.
 | Table IPrimers used for PCR
amplification. |
Table I
Primers used for PCR
amplification.
| Gene | Primer
sequences |
|---|
| CCR7 | F:
5′-CGCAACTTTGAGCGGAACAA-3′ |
| (NM_001301713) | R:
5′-TTCGCAGCTGCTATTGGTGA-3′ |
| GAPDH | F:
5′-TGGCCTTCCGTGTTCCTAC-3′ |
Western blot analysis
Lung tissues were collected for the examination of
the protein expression of CCR7, as well as that of phosphorylated
(p-)IκBα and p-p65 by western blot analysis. Sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (10%) was
used to isolate the total protein following extraction. The lung
protein samples were electrophoretically transferred onto PVDF
membranes. Subsequently, the PVDF membranes were blocked with 5%
BSA for 2 h at room temperature followed by incubation with primary
and secondary antibodies. The immunoblots were incubated with the
following antibodies: primary antibodies overnight at 4°C with CCR7
(1:2,000 dilution; Abcam), p-IκBα (1:1,000 dilution) and p-p65
(1:1,000 dilution) (both from Cell Signaling Technology, Inc.,
Danvers, MA, USA); and secondary antibodies at 37°C for 2 h with
1:2,000 dilution. Finally, the immunoblots were visualized and
analyzed using Image Lab software (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Statistical analysis
All analyses were undertaken using SPSS 19.0
statistical software. Data are expressed as the means ± SEM. For
comparisons of multiple parameters, ANOVA with the LSD test or
Dunnett's test were used to evaluate the statistical significance
of the differences between groups. A P-value <0.05 was
considered to indicate a statistically significant difference.
Results
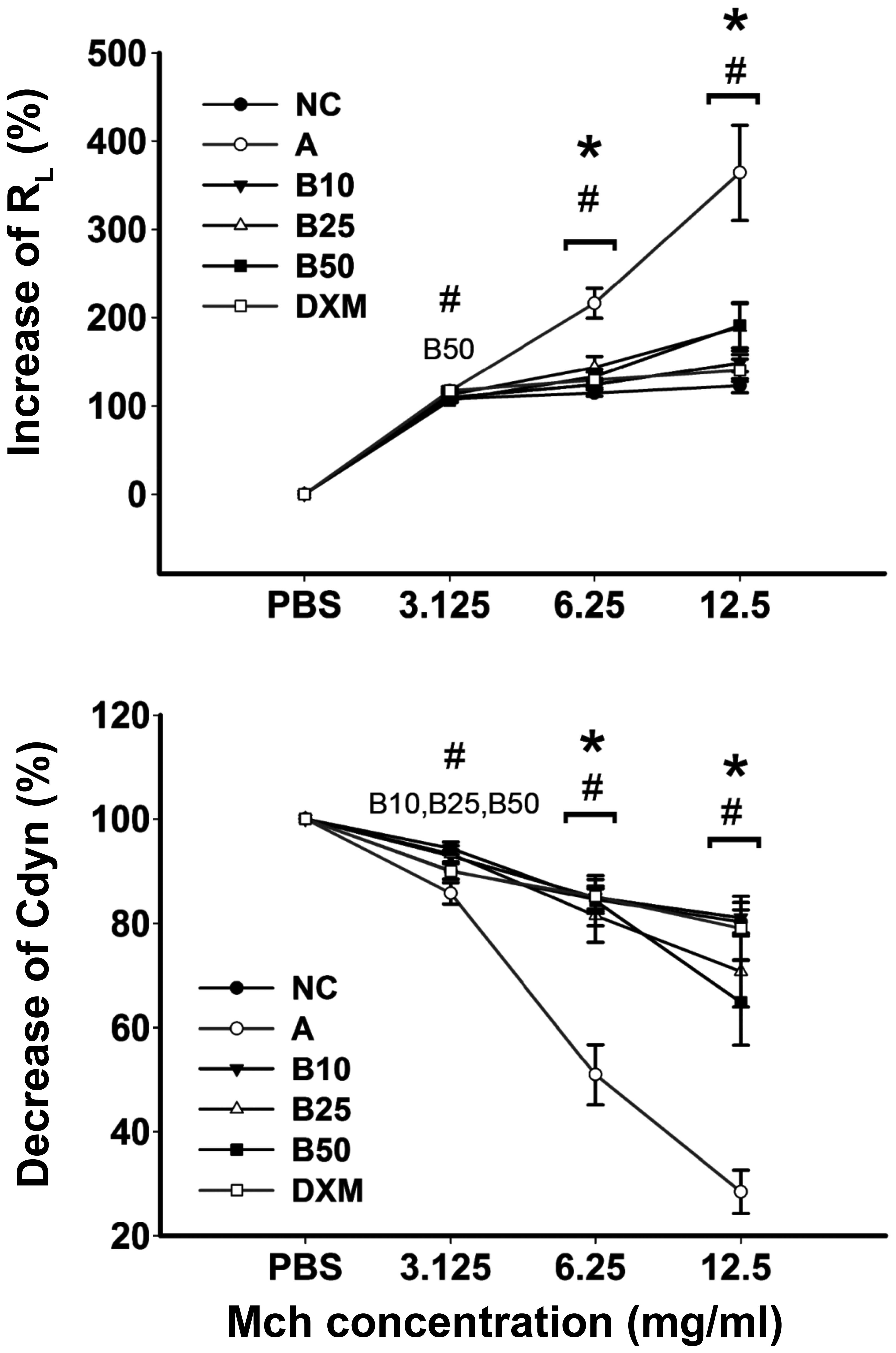
Baicalin mitigates AHR in asthmatic
mice
The efficacy of baicalin against OVA-induced AHR was
examined by means of the airway responsiveness to aerosolized PBS
or Mch. As shown in Fig. 2, only
inconspicuous alterations in RL were observed in the
normal mice, while the mice with OVA-induced asthma exhibited an
obvious increase in RL and a decrease in Cdyn (Mch of
6.25 and 12.5 mg/ml) compared with the mice in the NC group
(P<0.05). Treatment with all concentrations of baicalin led to
an obvious increase in Cdyn and a reduction in RL in the
asthmatic mice (P<0.05) and these effects occurred in a
dose-dependent manner.
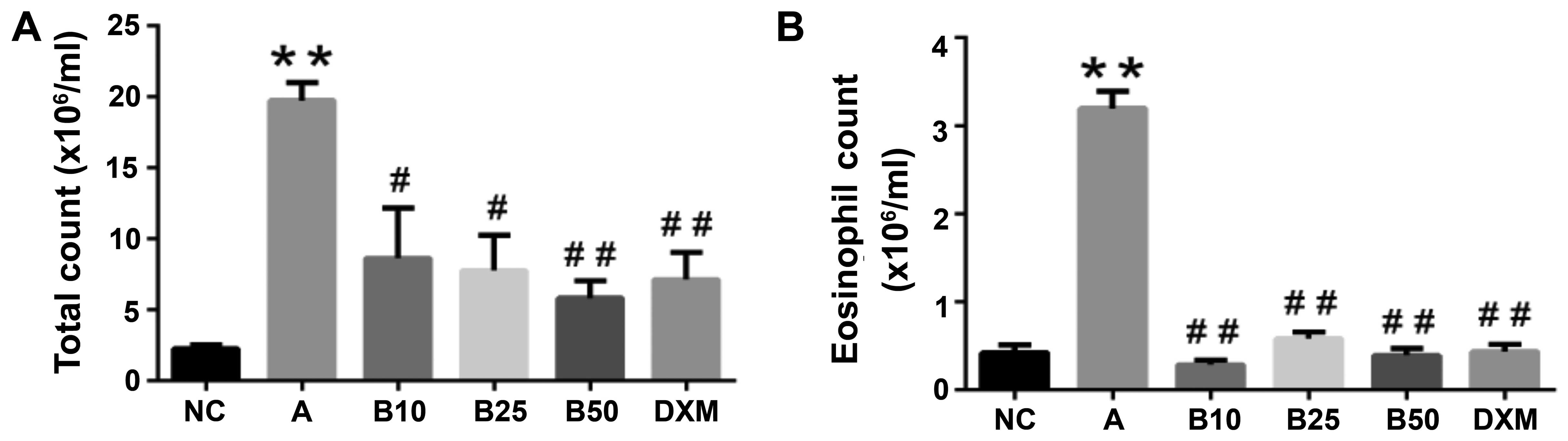
Inflammatory cell recruitment in BALF is
suppressed by baicalin
As shown in by our results, the OVA-sensitized and
challenged mice exhibited a large quantity of inflammatory cells
infiltrating the lungs compared to the mice in the NC group
(P<0.01; Fig. 3). In order to
ascertain the effects of baicalin on inflammatory cell counts, we
detected general white blood cells (WBCs) and EOS. The WBC and EOS
counts in BALF were significantly decreased in the baicalin-treated
mice. Moreover, the mice treated with 50 mg/kg baicalin (B50)
exhibited a marked reduction in the WBC and EOS counts in BALF
(P<0.01), and this reduction in inflammatory cell count in the
B50 group was greater than that observed in the DXM-treated
mice.
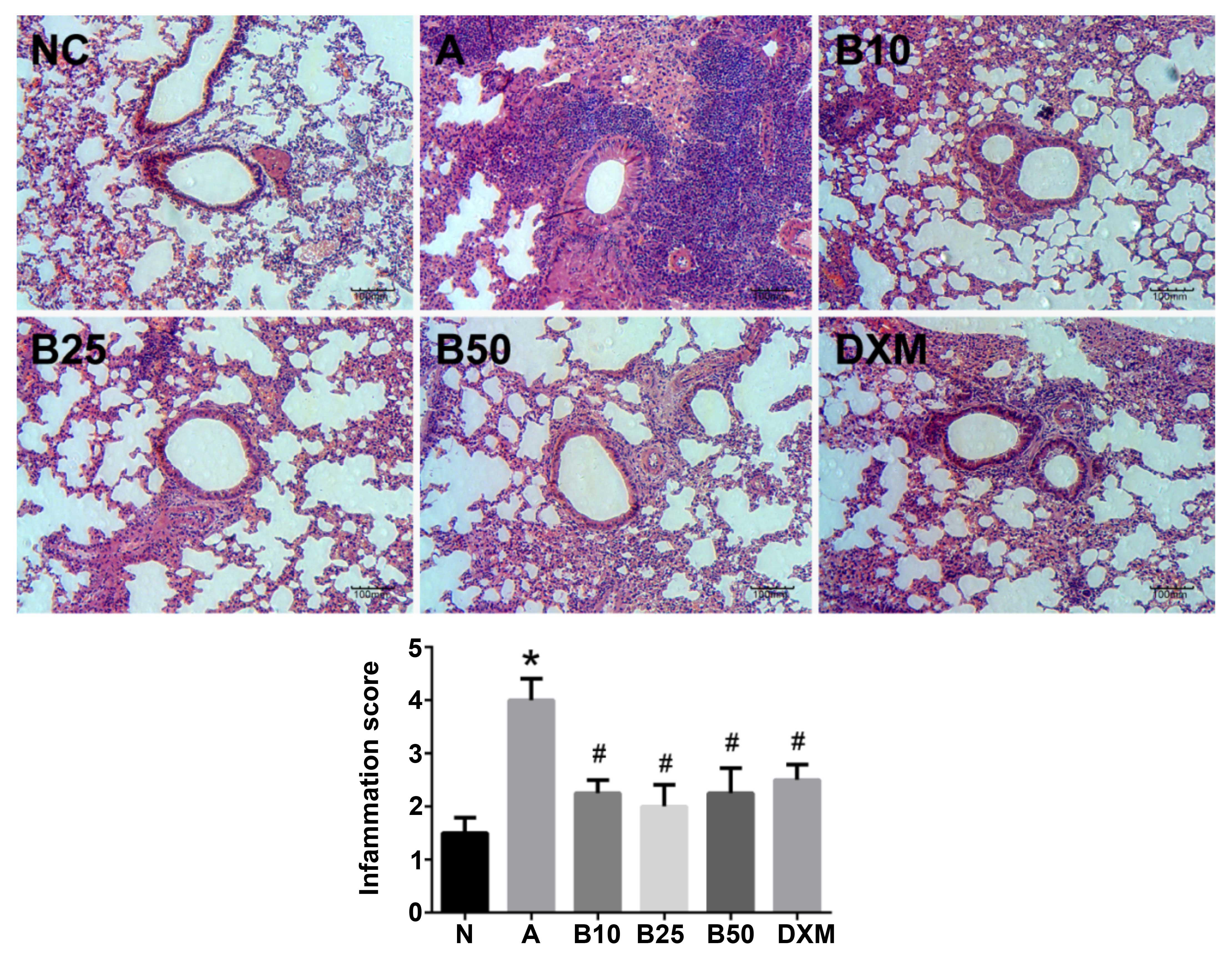
Histological lung inflammation caused by
OVA inhalation is reduced in baicalin-treated mice
In the lung tissues from the mice exposed to OVA, a
distinct infiltration of EOS and mucus secretion into the
tracheobronchial mucosa and airway lumen was observed as compared
with the mice in the NC group (P<0.05; Fig. 4). Conversely, the baicalin and
DXM-treated mice exhibited less infiltration of EOS into the
tracheobronchial mucosa and airway lumen (P<0.05).
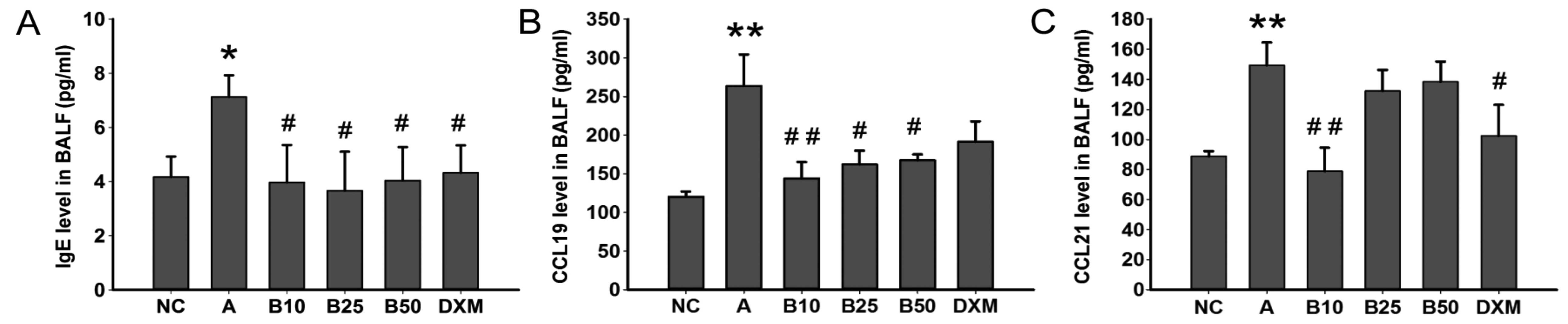
Effect of baicalin treatment on IgE,
CCL19 and CCL21 levels in OVA-challenged mice
The results of ELISA indicated that the OVA-specific
IgE level in BALF was significantly increased by the OVA challenge
when compared to the mice in the NC group (P<0.05; Fig. 5A). Gavage of baicalin in each dose
group showed an obvious reduction in level of OVA-specific IgE in
BALF (P<0.05). Simultaneously, the mice with asthma exhibited a
marked increase in the levels of CCL19 and CCL21 (P<0.01;
Fig. 5B and C) in BALF. Treatment
with 10 mg/kg baicalin (B10) markedly decreased the level of CCL19
and CCL21 (P<0.01), and treatment with baicalin at 25 and 50
mg/kg (B25 and B50) resulted in a marked reduction in the CCL19
level (P<0.05; Fig. 5B).
However, treatment with baicalin at 25 and 50 mg/kg did not
significantly inhibit the CCL21 level in BALF, whereas DXM did
(Fig. 5C).
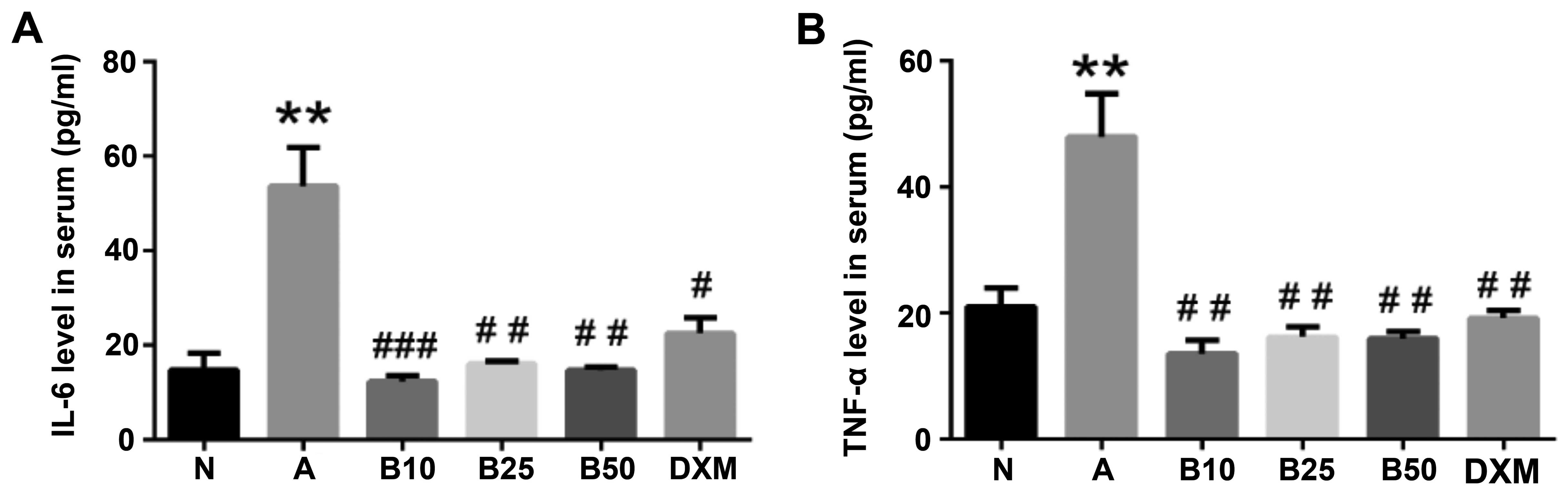
Effect of baicalin treatment on IL-6 and
TNF-α levels in serum of mice with OVA-induced asthma
IL-6 and TNF-α have been shown to be implicated in
many aspects of airway pathology and in evoking allergic
inflammatory responses (20–22). In this study, to examine the
effect of baicalin on these cytokines, the secretion of these
cytokines in the serum of mice in each group were also evaluated by
ELISA. As shown in Fig. 6, there
were significant elevations in the expression levels of IL-6 and
TNF-α in the mice with OVA-induced asthma compared with the mice in
the NC group (P<0.01). The increased expression levels of these
cytokines in serum were evidently suppressed by baicalin treatment
(P<0.01). Enzyme immunoassays revealed that compared with the
asthmatic mice, the mice treated with baicalin exhibited a marked
decrease in the levels of IL-6 and TNF-α, and this effect was more
prominent than that observed with DXM treatment.
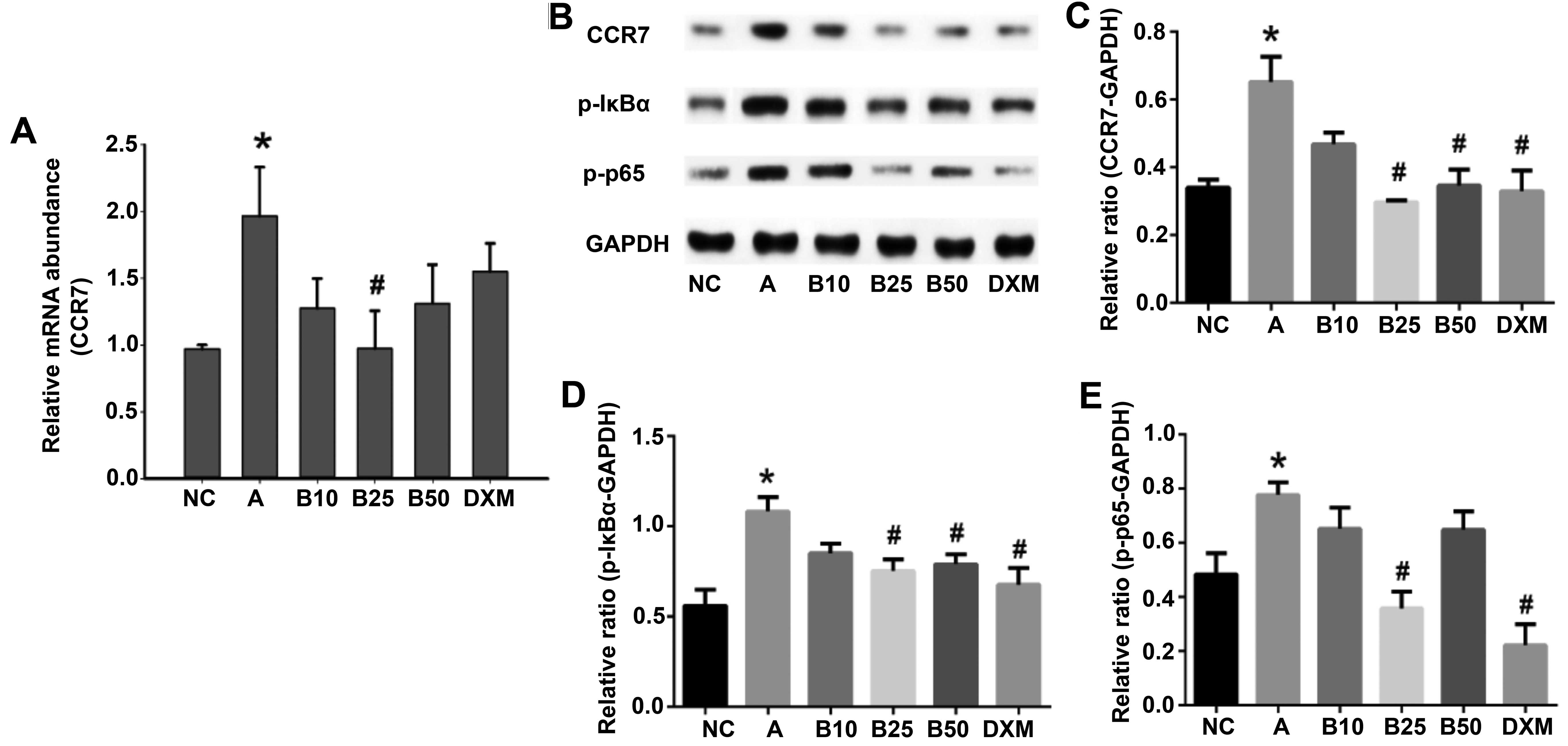
Baicalin inhibits CCR7 mRNA and CCR7
protein, as well as p-IκBα and p-p65 protein expression in lung
tissue
To determine whether the effects of baicalin on CCR7
and NF-κB were transcriptionally regulated, CCR7 mRNA expression
was determined by RT-qPCR, and the protein expressio levels of CCR7
and p-IκBα and p-p65 were detected by western blot analysis. As
shown in Fig. 7, CCR7 mRNA
expression and CCR7, p-IκBα (P<0.05) and p-p65 (P<0.01)
protein expression were significantly increased in the
OVA-challenged mice. Notably, treatment with baicalin at 25 mg/kg
(B25) had a marked inhibitory effect on the CCR7 mRNA and protein
levels, as well as on the p-IκBα and p-p65 protein levels in the
lung tissue of the OVA sensitized and challenged mice.
Discussion
Asthma is a highly complex airway inflammatory
disorder, the incidence of which is continually increasing. Airway
inflammation, AHR and mucus overproduction are the major
pathological characteristics of the disease (1). Chemokine receptors with their
ligands and NF-κB together with related cytokines are considered to
be involved in the pathogenesis of asthma (4,23–25). In this study, the effects of
baicalin on inflammation in a mouse model of OVA-induced asthmat
and on the associated chemokine receptors and their ligands were
investigated. Our results revealed that the OVA-exposed mice
exhibited substantial airway inflammatory changes compared to the
NC mice. Consistent with previous discoveries (26,27), in this study, AHR was markedly
increased in the OVA-exposed asthmatic mice; however, treatment
with baicalin led to a significant increase in Cdyn and a reduction
in RL in the asthmatic mice and these effects occurred
in a dose-dependent manner, which indicated that baicalin treatment
had a protective effect on pulmonary function in the asthmatic
mice. Furthermore, the increased amount of inflammatory cells, as
well as the OVA-specific IgE level in BALF and the secretion of
inflammatory cytokines into the blood of asthmatic mice were
markedly reduced by baicalin.
The homeostatic chemokines, CCL19 and CCL21, are the
sole ligands for CCR7, and they have a potent chemotactic activity
for antigen-presenting mDCs (28)
and have been shown to be important in the homing and traffic of
naive lymphocytes within lymphoid tissues (29). Studies have demonstrated that the
interaction of recruited DCs and T cells at the site of regional
lymph nodes is promoted by CCL19 and CCL21 (30,31). Mice lacking both CCL21 and CCL19
gene expression exhibit a significantly reduced airway inflammatory
response (4). In this study,
treatment with various concentrations of baicalin exerted a marked
inhibitory effect on the CCL19 level, and treatment with baicalin
at 10 mg/kg significantly reduced the CCL21 level in BALF, which
may support the anti-inflammatory effect of baicalin in asthmatic
mice.
CCR7 is considered to be involved in the lymph node
homing of naive T cells and DCs, and also plays a crucial role in
regulating immune cell migration for the balance of immunity
against pathogens and the maintenance of central and peripheral
immunological tolerance (32,33). It has been shown that
CCR7−/− mice exhibit a strongly impaired migration of
lung-derived DCs to the bronchial lymph nodes (34). In addition, defective CCR7
expression has been shown to contribute to the reduced efficiency
of the interaction between DCs and T cells, as well as to the
decreased responsiveness to CCR7 ligands (35). Based on such discoveries, CCR7 has
received considerable attention as a potential therapeutic target
in allergies (36). In this
study, the protein and mRNA expression level of CCR7 were
remarkably elevated in asthmatic mice; however, CCR7 protein
expression was significantly reduced by treatment with baicalin at
25 and 50 mg/kg and by DXM, and CCR7 mRNA expression was markedly
decreased by treatment with baicalin at 25 mg/kg, suggesting that
baicalin may be an effective medication for allergic
inflammation.
Crucial to the pathogenesis of asthma, the
inflammatory cytokines, IL-6 and TNF-α, which have been reported to
be implicated in the airway allergic inflammatory responses,
regulate many aspects in adaptive immune disorders. IL-6 and TNF-α,
which can be expressed by several cell types (24,37), aggregate into the airways and lung
tissues when allergy occurs. A previous study demonstrated that
IL-6 levels were markedly elevated both in symptomatic and
asymptomatic asthma patients (38). A recent study also found that IL-6
trans-signaling increased the expression of airway disease-related
genes in airway smooth muscle (25). In addition, the anti-inflammatory
effect exerted by targeting TNF-α is currently being widely
investigated and studies have shown that the blockade of TNF-α
contributes to the suppression of murine allergic airway
inflammation (22,39). Various studies have demonstrated
that the inhibition of the critical inflammatory cytokines, IL-6
and TNF-α, represent a prominent new approach for the treatment of
IL-6- and TNF-α-associated inflammatory diseases (40–43). The findings of the present study
demonstrated that compared with the mice in the NC group, the IL-6
and TNF-α levels in serum were significantly decreased by treatment
with all concentrations of baicalin, and these effects were more
prominent than those observed with DXM treatment. Our data may
confirm the hypothesis that baicalin exerts an inhibitory effect on
regulatory Th17 cells in asthmatic mice.
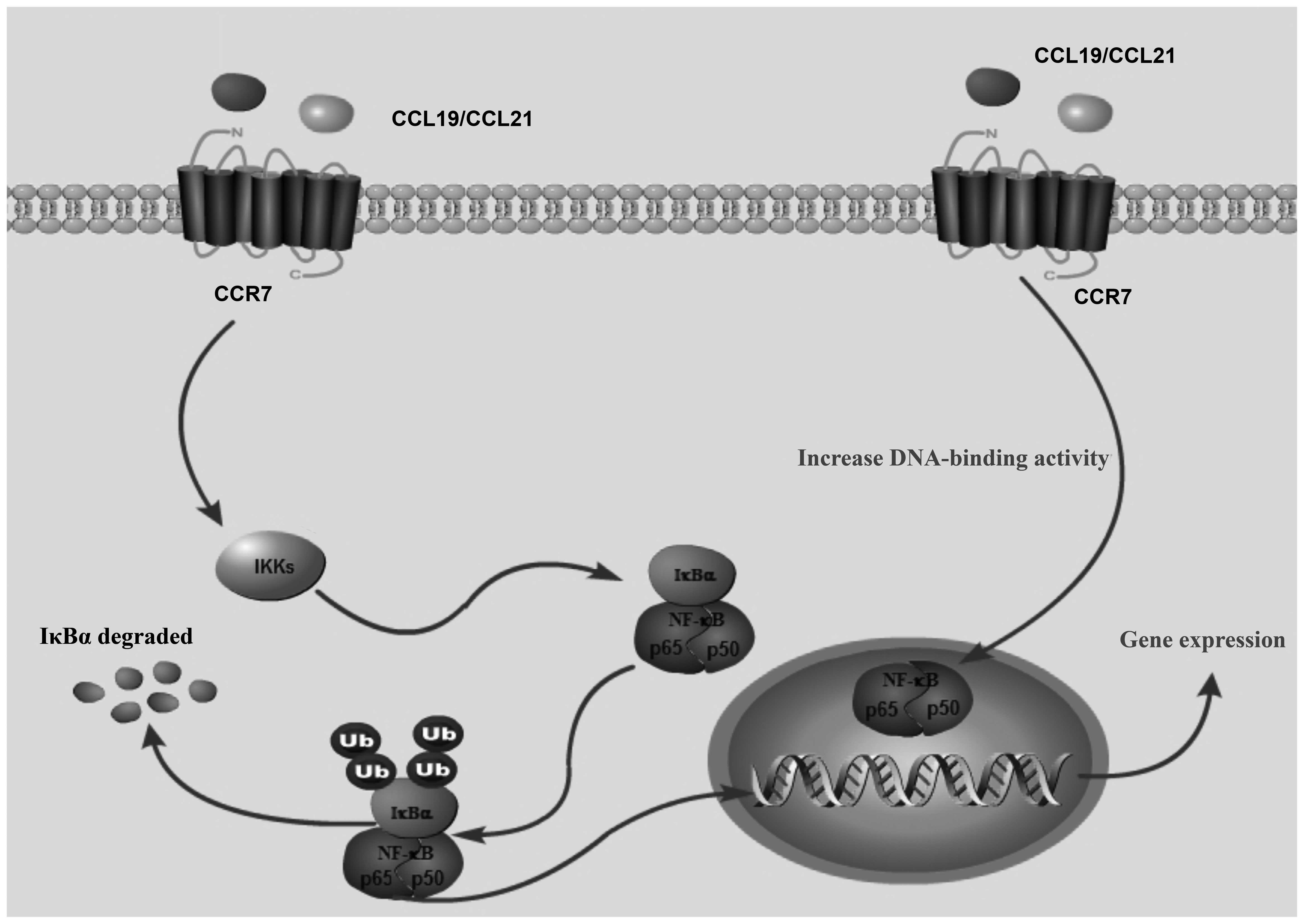
NF-κB is a master regulatory transcription factor
that is crucial to the expression of inflammatory cytokines,
chemokine receptors and the counterpart ligands in
inflammation-related diseases (44). The inhibitor of IκB kinase (IKKs),
p65/p50 complex and IκB are key cytokines in the NF-κB pathway;
however, among the p65/p50 complex, only p65 has transcriptional
activity (45). It has been
demonstrated that the interaction between CCR7 and its ligand,
CCL19, activate p-IκB and cause NF-κB to translocate to the
nucleus, increasing the DNA-binding capacity of NF-κB (46). In addition, it has been
demonstrated that the stimulation of DCs with CCL19 or CCL21
induces the activation of NF-κB (47). Therefore, CCR7-mediated NF-κB
activation may play a key role in the pathophysiological process of
asthma (Fig. 8), and the
suppression of NF-κB activation and the interaction with CCR7 and
its ligands are associated with reducing airway inflammation.
Accordingly, we investigated whether baicalin treatment could
interfere with CCR7 and the NF-κB pathway. The data from western
blot analysis revealed that the expression of p-IκBα and p-p65
protein were markedly elevated in the lung tissue of OVA-exposed
mice as compared to the NC mice and were markedly inhibited by
treatment with baicalin at the dose of 25 mg/kg. Collectively,
these data suggest the potential of baicalin to inhibit the
functions of CCR7 and its ligands via NF-κB, thus attenuating
inflammation, and the development and progression of asthma.
In conclusion, in the present study, we demonstrated
that baicalin improves pulmonary function and attenuates airway
inflammation. Our data provide novel mechanistic insight into the
anti-inflammatory effects of baicalin. Importantly, the levels of
IgE and CCR7, and those of its ligand, CCL19, were prominently
suppressed by treatment with baicalin. Furthermore, baicalin exerts
an inhibitory effect on the NF-κB pathway. These discoveries may
demonstrate the potential of baicalin as an agent for the
management of allergic asthma.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81173390, 81403476 and
81573758) and the Development Project of Shanghai Peak
Disciplines-Integrated Chinese and Western Medicine.
References
|
1
|
Olin JT and Wechsler ME: Asthma:
pathogenesis and novel drugs for treatment. BMJ. 349:g55172014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kaur D, Saunders R, Berger P, Siddiqui S,
Woodman L, Wardlaw A, Bradding P and Brightling CE: Airway smooth
muscle and mast cell-derived CC chemokine ligand 19 mediate airway
smooth muscle migration in asthma. Am J Respir Crit Care Med.
174:1179–1188. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saunders R, Sutcliffe A, Kaur D, Siddiqui
S, Hollins F, Wardlaw A, Bradding P and Brightling C: Airway smooth
muscle chemokine receptor expression and function in asthma. Clin
Exp Allergy. 39:1684–1692. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamashita N, Tashimo H, Matsuo Y, Ishida
H, Yoshiura K, Sato K, Yamashita N, Kakiuchi T and Ohta K: Role of
CCL21 and CCL19 in allergic inflammation in the ovalbumin-specific
murine asthmatic model. J Allergy Clin Immunol. 117:1040–1046.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sennikov SV, Falaleeva SA, Shkaruba NS,
Chumasova OA, Obleukhova IA, Sizikov AE and Kurilin VV: Maturation
and cytokine production potential of dendritic cells isolated from
rheumatoid arthritis patients peripheral blood and induced in
vitro. Hum Immunol. pii: S0198–8859(16)30158-6. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Akuthota P, Ueki S, Estanislau J and
Weller PF: Human eosinophils express functional CCR7. Am J Respir
Cell Mol Biol. 48:758–764. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jung YW, Kim HG, Perry CJ and Kaech SM:
CCR7 expression alters memory CD8 T-cell homeostasis by regulating
occupancy in IL-7- and IL-15-dependent niches. Proc Natl Acad Sci
USA. 113:8278–8283. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moschovakis GL, Bubke A, Dittrich-Breiholz
O, Braun A, Prinz I, Kremmer E and Förster R: Deficient CCR7
signaling promotes TH2 polarization and B-cell activation in vivo.
Eur J Immunol. 42:48–57. 2012. View Article : Google Scholar
|
|
9
|
Boislève F, Kerdine-Romer S,
Rougier-Larzat N and Pallardy M: Nickel and DNCB induce CCR7
expression on human dendritic cells through different signalling
pathways: role of TNF-alpha and MAPK. J Invest Dermatol.
123:494–502. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pahne-Zeppenfeld J, Schröer N,
Walch-Rückheim B, Oldak M, Gorter A, Hegde S and Smola S: Cervical
cancer cell-derived interleukin-6 impairs CCR7-dependent migration
of MMP-9-expressing dendritic cells. Int J Cancer. 134:2061–2073.
2014. View Article : Google Scholar
|
|
11
|
Johnston B and Butcher EC: Chemokines in
rapid leukocyte adhesion triggering and migration. Semin Immunol.
14:83–92. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ben-Baruch A: Organ selectivity in
metastasis: regulation by chemokines and their receptors. Clin Exp
Metastasis. 25:345–356. 2008. View Article : Google Scholar
|
|
13
|
Comerford I, Harata-Lee Y, Bunting MD,
Gregor C, Kara EE and McColl SR: A myriad of functions and complex
regulation of the CCR7/CCL19/CCL21 chemokine axis in the adaptive
immune system. Cytokine Growth Factor Rev. 24:269–283. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Höpken UE, Foss HD, Meyer D, Hinz M, Leder
K, Stein H and Lipp M: Up-regulation of the chemokine receptor CCR7
in classical but not in lymphocyte-predominant Hodgkin disease
correlates with distinct dissemination of neoplastic cells in
lymphoid organs. Blood. 99:1109–1116. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cui L, Feng L, Zhang ZH and Jia XB: The
anti-inflammation effect of baicalin on experimental colitis
through inhibiting TLR4/NF-κB pathway activation. Int
Immunopharmacol. 23:294–303. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun J, Li L, Wu J, Liu B, Gong W, Lv Y,
Luo Q, Duan X and Dong J: Effects of baicalin on airway remodeling
in asthmatic mice. Planta Med. 79:199–206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma C, Ma Z, Fu Q and Ma S: Anti-asthmatic
effects of baicalin in a mouse model of allergic asthma. Phytother
Res. 28:231–237. 2014. View
Article : Google Scholar
|
|
18
|
Pichavant M, Goya S, Hamelmann E, Gelfand
EW and Umetsu DT: Animal models of airway sensitization. Curr
Protoc Immunol. Chapter 15: Unit15.18. 2007. View Article : Google Scholar
|
|
19
|
Underwood S, Foster M, Raeburn D, Bottoms
S and Karlsson JA: Time-course of antigen-induced airway
inflammation in the guinea-pig and its relationship to airway
hyperresponsiveness. Eur Respir J. 8:2104–13. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chu DK, Al-Garawi A, Llop-Guevara A,
Pillai RA, Radford K, Shen P, Walker TD, Goncharova S, Calhoun WJ,
Nair P and Jordana M: Therapeutic potential of anti-IL-6 therapies
for granulocytic airway inflammation in asthma. Allergy Asthma Clin
Immunol. 11:142015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rose-John S, Scheller J, Elson G and Jones
SA: Interleukin-6 biology is coordinated by membrane-bound and
soluble receptors: role in inflammation and cancer. J Leukoc Biol.
80:227–236. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brightling C, Berry M and Amrani Y:
Targeting TNF-α: a novel therapeutic approach for asthma. J Allergy
Clin Immunol. 121:5–10; quiz 11–12. 2008. View Article : Google Scholar
|
|
23
|
Gagliardo R, Chanez P, Profita M, Bonanno
A, Albano GD, Montalbano AM, Pompeo F, Gagliardo C, Merendino AM
and Gjomarkaj M: IkappaB kinase-driven nuclear factor-kappaB
activation in patients with asthma and chronic obstructive
pulmonary disease. J Allergy Clin Immunol. 128:635–645. 2011.
View Article : Google Scholar
|
|
24
|
Berry M, Brightling C, Pavord I and
Wardlaw A: TNF-α in asthma. Curr Opin Pharmacol. 7:279–282. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Robinson MB, Deshpande DA, Chou J, Cui W,
Smith S, Langefeld C, Hastie AT, Bleecker ER and Hawkins GA: IL-6
trans-signaling increases expression of airways disease genes in
airway smooth muscle. Am J Physiol Lung Cell Mol Physiol.
309:L129–L138. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun J, Wu J, Xu C, Luo Q, Li B and Dong J:
Paeoniflorin attenuates allergic inflammation in asthmatic mice.
Int Immunopharmacol. 24:88–94. 2015. View Article : Google Scholar
|
|
27
|
Wei Y, Luo QL, Sun J, Chen MX, Liu F and
Dong JC: Bu-Shen-Yi-Qi formulae suppress chronic airway
inflammation and regulate Th17/Treg imbalance in the murine
ovalbumin asthma model. J Ethnopharmacol. 164:368–377. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Le Y, Zhou Y, Iribarren P and Wang J:
Chemokines and chemokine receptors: their manifold roles in
homeostasis and disease. Cell Mol Immunol. 1:95–104. 2004.
|
|
29
|
Ebert LM, Schaerli P and Moser B:
Chemokine-mediated control of T cell traffic in lymphoid and
peripheral tissues. Mol Immunol. 42:799–809. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lambrecht BN: Allergen uptake and
presentation by dendritic cells. Curr Opin Allergy Clin Immunol.
1:51–59. 2001. View Article : Google Scholar
|
|
31
|
Choi B, Lim HC, Lee ES, Anower AKMM and
Sohn S: CCL21 attenuates HSV-induced inflammation through
up-regulation of CD8+ memory cells. Immunobiology.
218:579–590. 2013. View Article : Google Scholar
|
|
32
|
Förster R, Davalos-Misslitz AC and Rot A:
CCR7 and its ligands: balancing immunity and tolerance. Nat Rev
Immunol. 8:362–371. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Worbs T and Förster R: A key role for CCR7
in establishing central and peripheral tolerance. Trends Immunol.
28:274–280. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hintzen G, Ohl L, del Rio ML,
Rodriguez-Barbosa JI, Pabst O, Kocks JR, Krege J, Hardtke S and
Förster R: Induction of tolerance to innocuous inhaled antigen
relies on a CCR7-dependent dendritic cell-mediated antigen
transport to the bronchial lymph node. J Immunol. 177:7346–7354.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ato M, Stäger S, Engwerda CR and Kaye PM:
Defective CCR7 expression on dendritic cells contributes to the
development of visceral leishmaniasis. Nat Immunol. 3:1185–1191.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Saban DR: The chemokine receptor CCR7
expressed by dendritic cells: a key player in corneal and ocular
surface inflammation. Ocul Surf. 12:87–99. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schmidt-Weber CB, Akdis M and Akdis CA:
TH17 cells in the big picture of immunology. J Allergy Clin
Immunol. 120:247–54. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yokoyama A, Kohno N, Fujino S, Hamada H,
Inoue Y, Fujioka S, Ishida S and Hiwada K: Circulating
interleukin-6 levels in patients with bronchial asthma. Am J Respir
Crit Care Med. 151:1354–1358. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hutchison S, Choo-Kang BSW, Bundick RV,
Leishman AJ, Brewer JM, McInnes IB and Garside P: Tumour necrosis
factor-α blockade suppresses murine allergic airways inflammation.
Clin Exp Immunol. 151:114–122. 2008. View Article : Google Scholar
|
|
40
|
Hunter CA and Jones SA: IL-6 as a keystone
cytokine in health and disease. Nat Immunol. 16:448–457. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nishimoto N and Kishimoto T: Inhibition of
IL-6 for the treatment of inflammatory diseases. Curr Opin
Pharmacol. 4:386–391. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Berry MA, Hargadon B, Shelley M, Parker D,
Shaw DE, Green RH, Bradding P, Brightling CE, Wardlaw AJ and Pavord
ID: Evidence of a role of tumor necrosis factor alpha in refractory
asthma. N Engl J Med. 354:697–708. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Howarth PH, Babu KS, Arshad HS, Lau L,
Buckley M, McConnell W, Beckett P, Al Ali M, Chauhan A, Wilson SJ,
et al: Tumour necrosis factor (TNF alpha) as a novel therapeutic
target in symptomatic corticosteroid dependent asthma. Thorax.
60:1012–1018. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kuwabara T, Tanaka Y, Ishikawa F, Kondo M,
Sekiya H and Kakiuchi T: CCR7 ligands up-regulate IL-23 through
PI3-kinase and NF-kappa B pathway in dendritic cells. J Leukoc
Biol. 92:309–318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang H and Sun SC: NF-κB in inflammation
and renal diseases. Cell Biosci. 5:632015. View Article : Google Scholar
|
|
46
|
Liu FY, Zhao ZJ, Li P, Ding X, Guo N, Yang
LL, Zong ZH and Sun CF: NF-κB participates in chemokine receptor
7-mediated cell survival in metastatic squamous cell carcinoma of
the head and neck. Oncol Rep. 25:383–391. 2011.
|
|
47
|
Sánchez-Sánchez N, Riol-Blanco L, de la
Rosa G, Puig-Kröger A, García-Bordas J, Martín D, Longo N, Cuadrado
A, Cabañas C, Corbí AL, et al: Chemokine receptor CCR7 induces
intracellular signaling that inhibits apoptosis of mature dendritic
cells. Blood. 104:619–625. 2004. View Article : Google Scholar : PubMed/NCBI
|