Introduction
Styela clava (SC), of the class Ascidiacea
(ascidians or sea squirts) is a solitary, oviparous, hermaphrodite
tunicate characterized by a rough and wrinkled brown surface and a
club-shaped body (1). SC is found
in East Asia, Australia, New Zealand, North America and Europe
(2–4). The annual production of SC in
suspended culture increased to 2,759 MT in 2013 owing to demand for
use as a raw material in food production in Korea (5,6).
However, increasing SC consumption has resulted in rising levels of
SC tunic (SCT), which is regarded as waste, and may cause
environmental pollution (7,8).
Thus, many studies have been conducted to evaluate the possibility
of using novel bioactive materials derived from SCT to provide
solutions to this problem.
Natural polymers and bioactive compounds derived
from SCT have been extensively applied for the treatment of
inflammation, oxidative stress and surgical wounds. Cellulose
complex derived from SCT exerted significant therapeutic effects on
surgical wounds and bone defects in animals. Cellulose films (CF)
have been successfully prepared from solutions of SCT cellulose
powder completely dissolved in N-methylmorpholine-N-oxide
(NMMO)/H2O (87/13 wt %) (9). It has been demonstrated that Sprague
Dawley (SD) rats treated with SCT-CF showed no epidermal
hyperplasia, inflammatory cell infiltration, redness or edema on a
surgical wound during 2 weeks of observation (10). In addition, SD rats treated with
hydrocolloid membrane containing SCT (HCM-SCT) exhibited
significantly faster re-epithelization, decreased epidermal
thickness, decreased wound diameters and increased collagen levels
(11). Cellulose membrane,
obtained from the squirt skin of ascidians, was found to exert
bioinductive effects on bone and mesenchymal tissues in the
periosteum of cervical bone defects in SD rats (12). Furthermore, glycosaminoglycans,
which may serve as a lubricant or a shock absorber, were
successfully extracted from SCT using sodium phosphate at 105°C for
2 h, followed by deproteinization with trichloroacetic acid or
hydrochloride (7).
Bioactive compounds and extracts have also been
prepared from SCT by extraction with different solvents.
Chondroitin sulfate extracted from SCT was demonstrated to
effectively suppress tumor necrosis factor-α (TNF-α)-induced
nuclear factor-κB activation and the expression of two inflammatory
factors [vascular cell adhesion molecule-1 (VCAM-1) and inducible
nitric oxide synthase (iNOS)], through the blocking of Akt signals
in JB6 P+ cells derived from BALB/c mice (13). Moreover, carotenoids found in high
concentrations in SCT were found to exhibit strong hydroxyl radical
scavenging activities, reducing power as well as inhibitory effects
against linoleic acid peroxidation (14). Furthermore, nine extracts
collected from SCT using different solvents possessed high levels
of tyrosinase inhibition and antioxidant activity due to the high
total phenolic and flavonoid contents (8). However, to the best of our
knowledge, no other studies have examined whether these extracts
contribute to the homeostasis of the skin or the prevention of
photoaging induced by ultraviolet (UV) radiation, although
chondroitin sulfate and carotenoids have been reported to exert
potential therapeutic effects upon inflammation and oxidative
stress in vitro.
Thus, the protective effects of topically applied
ethanol extract of SCT (EtSCT) on skin morphology,
histopathological changes, endoplasmic reticulum (ER) stress,
inflammation and the antioxidant status of the skin of hairless
mice were examined in this study. The results presented herein
provide strong evidence for the potential use of EtSCT in the
prevention or alleviation of UV-induced skin aging, as well as the
underlying mechanism of action.
Materials and methods
Preparation of EtSCT
SCT powder was prepared as previously described
(15). Briefly, 330 g SCT in 10%
NaOH aqueous solution (9,900 ml) were boiled at 100°C for 2 h to
remove sediments and debris, after collecting SCT from the beach of
the South Sea in Goseong-gun, Korea. The samples were washed with
distilled water three times, boiled in 5% CH3COOH
solution at 100°C for 2 h to neutralize the NaOH solution, and then
washed with distilled water three times. The SCT samples were
subsequently bleached by separate boiling and washing in 10%
H2O2 solution. Following a final wash with
distilled water, the SCT samples were dried at 100–120°C for 2–3 h,
ground in a pin milling machine (DM-120, Youngin Scientific Co.,
Ltd., Seoul, Korea) using a proprietary commercial process in which
they were passed through a combination of 30-mesh sieves for 10 min
once, then 120-mesh sieves for 10 min twice.
EtSCT was prepared as previously described (8). Briefly, the ethanol extracts were
purified from 100 g of SCT powder for 3 h at 80°C using circulating
extraction equipment (IKA Labortechnik, Staufen, Germany) after
adding 1,000 ml of 100% ethanol. After repeating this process three
times, a solution of the extracts was concentrated to dry pellets
in a rotary evaporator (Eyela, Tokyo, Japan) after filtering
through Whatman No. 1 filter paper (Whatman International, Ltd.,
Maidstone, UK). The samples were then stored at −80°C until further
use.
Measurement of total phenolic and
flavonoid contents
Total phenolic contents were measured by the
Folin-Ciocalteu method, with slight modifications (16). Briefly, 1 ml EtSCT solution was
mixed with 5 ml Folin-Ciocalteu reagent (Sigma-Aldrich Co., St.
Louis, MO, USA) and then incubated at room temperature for 5 min.
The mixture was subsequently added to 15 ml 20%
Na2CO3 and vortexed for 30 sec, after which
the absorbance was repeatedly measured at 765 nm using a VersaMax
plate reader (Molecular Devices, Sunnyvale, CA, USA). A standard
calibration curve was prepared using different concentrations of
gallic acid (Sigma-Aldrich Co.) and the concentration of total
phenolic contents in EtSCT was presented as mg gallic acid
equivalents of extract.
The flavonoid contents were measured as previously
described (17). Briefly, 200
µl of several different concentrations of EtSCT were mixed
with 60 µl 5% NaNO2 and 60 µl 10%
AlCl3 (both from Sigma-Aldrich Co.). Following
incubation at 25°C for 5 min, the mixture was added to 400
µl 1 M NaOH and the absorbance was repeatedly measured at
510 nm using a VersaMax plate reader (Molecular Devices). A
standard calibration curve was then prepared using different
concentrations of catechin (Sigma-Aldrich Co.). The concentration
of flavonoid contents in Et-SCT was presented as mg catechin
equivalents of extract.
Analysis of antioxidant activity
The scavenging activity of
2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals was measured as
previously described (18).
Briefly, each sample (250 µl) of EtSCT was mixed with 500
µl 0.2 mM DPPH (Sigma-Aldrich Co.) in 95% ethanol solution
or 100 µl 95% ethanol solution, and then incubated for 30
min at room temperature. Subsequently, the absorbance of the
reaction mixture was measured at 517 nm using a VersaMax plate
reader (Molecular Devices). The DPPH radical scavenging activity of
the EtSCT was expressed as the percent decrease in absorbance
relative to the control.
The reducing power of EtSCT was determined as
previously described (19).
Briefly, an appropriate volume (250 µl) of EtSCT solution
was mixed with 250 µl 0.2 M sodium phosphate buffer (pH 6.6)
and 250 µl 1% potassium ferricyanide, and then incubated at
50°C for 20 min. Following centrifugation at 1,000 × g for 10 min,
the supernatant was collected (250 µl) and mixed with 50
µl distilled water and 50 µl 0.1% ferric chloride,
and then incubated at room temperature for 10 min. Finally, the
absorbance of the reaction mixture was measured at 700 nm using a
VersaMax plate reader (Molecular Devices). The reducing power was
expressed as the percentage increase in rate with absorbance of the
EtSCT-treated group relative to the absorbance level of a dimethyl
sulfoxide (DMSO; Duchefa Biochemie B.V., Haarlem,
Netherlands)-treated group.
The scavenging activity of nitric oxide (NO) was
measured as previously described (20). Briefly, each sample of EtSCT (500
µl) was mixed with 500 µl 10 mM sodium nitroprusside
(Sigma-Aldrich Co.) and then incubated at 25°C for 150 min. This
mixture was then added to 500 µl 1% sulfanilamide solution
and 500 µl 0.1% N-(1-naphthyl)ethylenediamine
dihydrochloride solution and incubated at room temperature for 10
min. The absorbance of the reaction mixture was subsequently
measured at 546 nm using a VersaMax plate reader (Molecular
Devices). The NO scavenging activity of the EtSCT was expressed as
the percentage absorbance relative to a control treated with
DMSO.
Animal experiments
The animal protocols used in this study were
reviewed and approved based on the ethical procedures and
scientific care of animals set by the Pusan National University
Institutional Animal Care and Use Committee (PNU-IACUC; approval
no. PNU-2015-0812). Six-week-old male SKH-1 hairless mice were
obtained from Orient Bio (Seongnam, Korea) and housed at the Pusan
National University Laboratory Animal Resources Center, which is
accredited by the Association for Assessment and Accreditation of
Laboratory Animal Care International (AAALAC International;
accredited unit no. 001525) in accordance with the United States
National Institutes of Health Guidelines and the Korea Food and
Drug Administration (KFDA; accredited unit no. 00231) and in
accordance with the Laboratory Animals Act. All mice were given a
standard irradiated chow diet (Purina Mills, Seoungnam, Korea)
ad libitum, and were maintained in a specific pathogen-free
state under a strict light cycle (lights on at 08:00 h and off at
18:00 h) at 23±2°C and 50±10% relative humidity.
The six-week-old hairless mice (n=24) were first
assigned to either a no radiation (n=6) or a UV radiation (n=18)
group. The UV radiation group was then further divided into a
vehicle-treated group, a low concentration EtSCT (LEtSCT)-treated
group and a high concentration EtSCT (HEtSCT)-treated group. The
two EtSCT-treated groups were treated with 5 or 10 mg/ml of EtSCT
solution in olive oil (1 ml) applied topically onto the dorsal skin
of hairless mice three times a week for 13 weeks, whereas the
vehicle-treated group was treated with a consistent volume of olive
oil. At 13 weeks after the application of the vehicle, LEtSCT and
HEtSCT treatments, the animals were sacrificed immediately using
CO2 gas, to acquire blood, skin tissue, liver tissue and
kidney tissue samples for further analysis.
UV radiation and topical
administration
The minimal erythemal dose (MED) from the UV
irradiation device was determined as suggested in previous studies
(21–23). Briefly, a UV irradiation device
was made from a TL20W/12RS UV lamp and a Kodacel filter in a
rectangular parallelepiped box. The UV lamp (Philips Lighting,
Eindhoven, The Netherlands) had an emission spectrum of 274 to 380
nm that was composed of the following types of UV radiation: 10.2%
UVC (275–290 nm), 53.5% UVB (290–320), 25.3% UVA1 (320–340 nm) and
11.2% UVA2 (340–380 nm). Kodacel Sheeting 6805 Product (Kodak,
Rochester, NY, USA) was used to remove UVC wavelengths <290 nm.
The irradiation intensity was measured at 30 cm from a light source
using a UVX radiometer (UVP, LLC, Upland, CA, USA). To determine
the 1 MED, the dorsal skin of the mice was exposed to different
doses of UV light and the formation of erythema was evaluated after
24 h. Skin aging was then induced by irradiation at 1 MED three
times/week (Monday, Wednesday and Friday) for 13 weeks.
During the first 4 weeks, the dose of UV radiation
was gradually increased by 1 MED per week from 1 to 4 MED, after
which the mice received a constant dose (4 MED) of UV radiation
from week 4 to 13. During each step of treatment, the mice in the
subset groups were treated with a topical application of 100
µl of either vehicle, LEtSCT or HEtSCT onto their back skin,
at 30 min after exposure to UV radiation.
Evaluation of wrinkle formation
Wrinkle formation was measured using a DETAX System
II (MIXPAC) and Double-Stick Disc (3M Health Care, Neuss, Germany),
according to a procedure established by our laboratory (23). After 12 weeks, skin surface
impressions (replica) were prepared by applying silicon rubber in
mixed liquid form secreted from a DETAX System II to the dorsal
skin of mice. Each replica was captured by a digital camera in
conjunction with the Leica Ez4HD (Leica Microsystems, Wetzlar,
Germany) at ×10 magnification. The depth and number of wrinkles on
each skin impression were analyzed, after which the samples were
classified into one of the four degrees suggested by Bissett et
al (24). Specifically, grade
0 indicated no wrinkle formation, grade 1 indicated some shallow
wrinkles, grade 2 indicated some wrinkles, and grade 3 indicated
several deep wrinkles.
Measurement of transepidermal water loss
(TEWL) and erythema index
Two factors related to skin homeostasis were
assessed on the dorsal skin of each mouse using the appropriate
devices (25). Specifically, TEWL
was detected using a Corneometer CM825, and the erythema index was
analyzed using a Mexameter MX18 (both from Courage and Khazaka
Electronics, Cologne, Germany) according to the manufacturer's
instructions. Each detection was performed three times on every
site on the dorsal skin of the hairless mice.
Western blot analysis
The proteins prepared from the skin tissues of the
vehicle-, LEtSCT- or HEtSCT-treated mice were separated by 4–20%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) for 3 h, after which the resolved proteins were
transferred to a nitrocellulose membrane for 2 h at 40 V. Each
membrane was then incubated separately with one of the following
primary antibodies overnight at 4°C: anti-matrix metalloproteinase
(MMP)-1/8 (sc-30069; 1:1,000) and anti-MMP-9 (sc-10737; 1:1,1000)
(both from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA);
anti-collagen I (ab34710; 1:1,000; Abcam, Cambridge, UK); anti-ERK
(sc-94; 1:1,000) and anti-phosphorylated (p-)ERK (sc-7383, 1:1,000)
(both from Santa Cruz Biotechnology, Inc.); anti-c-Jun N-terminal
kinase (JNK; 9252; 1:1,000), anti-p-JNK (9251; 1:1,000), anti-p38
(9212, 1:1,000) and anti-p-p38 (9211; 1:1,000) (all from Cell
Signaling Technology, Danvers, MA, USA); anti-inositol-requiring
enzyme (IRE)1β (sc-10511; 1:1,000; Santa Cruz Biotechnology, Inc.);
eukaryotic initiation factor (eIF)2α (9722; 1:1,000) and
anti-p-eIF2α (9721; 1:1,000) (both from Cell Signaling Technology);
anti-β-actin (A5316; 1:3,000; Sigma-Aldrich Co.) and anti-α-tubulin
(T6074; 1:1,000; Sigma-Aldrich Co.). The membranes were then washed
with washing buffer (137 mM NaCl, 2.7 mM KCl, 10 mM
Na2HPO4, 2 mM KH2PO4
and 0.05% Tween-20) and incubated with horseradish peroxidase
(HRP)-conjugated goat anti-rabbit IgG (81-6120; Zymed; Thermo
Fisher Scientific, Waltham, MA, USA) at a 1:1,000 dilution at room
temperature for 2 h. Finally, the membrane blots were developed
using a Chemiluminescence Reagent Plus kit (Pfizer, New York, NY,
USA).
Histological analysis and optical
microscopy
The dorsal skin in the region treated with the
EtSCTs was collected and fixed with 10% formalin for 48 h, embedded
in paraffin wax, and then sectioned into 4 µm thick slices.
The skin sections were subsequently stained with hematoxylin and
eosin (H&E) (Sigma-Aldrich Co.), after which they were examined
using a light microscope (DM500; Leica Microsystems) for evidence
of alterations of histological structures. Additionally, the
thickness of the epidermis and dermis as well as the number of
adipocytes were measured using the Leica Application Suite (Leica
Microsystems).
Toluidine blue staining
Mast cells were detected by staining with toluidine
blue as previously described (26). After deparaffinization and
dehydration, the skin sections were stained with 0.25% solution of
toluidine blue (Sigma-Aldrich Co.) and examined by light microscopy
for the presence of mast cells. The number of cells/specific area
was measured using the Leica Application Suite (Leica
Microsystems).
Immunohistochemical analysis
For immunohistochemical analysis, the dorsal skin in
the region treated with the EtSCTs was collected and fixed with 10%
formalin for 48 h, embedded in paraffin wax, and then sectioned
into 4 µm thick slices. These sections were deparaffinized
with xylene, rehydrated and pretreated for 30 min at room
temperature with phosphate-buffered saline (PBS) blocking buffer
containing 10% goat serum. The sections were then incubated with
mouse anti-CD31 antibody (ab24590; Abcam) at a dilution of 1:100 in
PBS blocking buffer. The antigen-antibody complexes were visualized
with biotinylated secondary antibody (goat anti-mouse)-conjugated
HRP (G21040; Invitrogen, Carlsbad, CA, USA) at a dilution of 1:100
in PBS blocking buffer. CD31 protein was detected using stable
3,3′-diaminobenzidine (DAB; ScyTek Laboratories Inc., Logan, UT,
USA) and observed using the Leica Application Suite (Leica
Microsystems).
Enzyme-linked immunosorbent assay (ELISA)
for interleukin (IL)-6
The concentration of IL-6 in the skin tissue was
measured using a Mouse IL-6 ELISA Max Deluxe kit (Biolegend, San
Diego, CA, USA) according to the manufacturer's instructions.
Briefly, the skin tissue (100 µg) was prepared for analysis
by gradually adding sucrose buffer (400 µl) solution to
tissue homogenate. The skin samples or standards and buffer C were
incubated in a 96-well plate at room temperature for 2 h while
shaking at 200 rpm, after which 100 µl IL-6 detection
antibody solution was added to each well and the samples were
incubated at room temperature for 1 h with shaking. After washing,
100 µl avidin-HRP D solution was added to each well and the
plate was incubated at room temperature for 30 min with shaking.
Next, 100 µl substrate solution was added to each well and
the plate was incubated for 10 min in the dark. The reaction was
then quenched by the addition of 100 µl Stop solution.
Subsequently, the plates were analyzed by evaluating absorbance at
450 nm using a VersaMax plate reader (Molecular Devices).
Measurement of body and organ
weights
Alterations in body weight were measured using an
electronic balance (Mettler Toledo, Greifensee, Switzerland) once a
week according to the KFDA guidelines. Finally, the weights of the
kidneys and livers collected from the sacrificed mice were
determined using the same method employed to detect the body
weight.
Serum biochemical analysis
Following the final treatment, all mice fasted for
24 h, after which blood was collected from the abdominal vein.
Serum was obtained by centrifuging the blood after incubation for
30 min at room temperature. The serum concentrations of alkaline
phosphatase (ALP), alanine transaminase (ALT), aspartate
transaminase (AST), lactate dehydrogenase (LDH), blood urea
nitrogen (BUN) and creatinine (CRE) were then assayed using a model
747 automated serum analyzer (Hitachi, Tokyo, Japan). All assays
were conducted with fresh serum using standard enzymatic methods
and all measurements were conducted in duplicate.
Analysis of malondialdehyde (MDA)
levels
The MDA level was assayed using a Lipid Peroxidation
(MDA) Assay kit (Sigma-Aldrich Co.) according to the manufacturer's
instructions. Briefly, the skin tissue of each mouse was
homogenized in MDA lysis buffer containing butylhydroxytoluene
(BHT), after which the homogenates were stored at −20°C until
analysis. Serum collected from each mouse was mixed with 42 mM
sulfuric acid (H2SO4) and 10% phosphotungstic
acid solution, after which the samples were centrifuged at 13,000 ×
g for 3 min. The pellet was resuspended with dH2O
containing BHT. The sample or standards and thiobarbituric acid
(TBA) solution (70 mM TBA, 5.0 M glacial acetic acid) were
incubated in a microcentrifuge tube at 95°C for 60 min, cooled to
room temperature in an ice bath for 10 min, and then absorbance at
450 nm was read using a VersaMax plate reader (Molecular
Devices).
Analysis of superoxide dismutase (SOD)
activity
The SOD activity in the skin tissue was detected
using a calorimetric assay and the reagents in the SOD assay kit
(Dojindo Molecular Technologies Inc., Kumamoto, Japan). Firstly,
the skin tissue (100 mg) was homogenized in 600 µl sucrose
buffer (0.25 mol/l sucrose, 10 mmol/l HEPES, 1 mmol/l EDTA, pH 7.4)
using a glass homogenizer. The lysate was then harvested from the
mixture by centrifugation at 10,000 × g for 60 min and stored at
−70°C until needed for the enzyme activity assay. To measure SOD
activity, the sample lysate was diluted with dilution buffer or
saline as follows: 1, 1/5, 1/52, 1/53,
1/54, 1/55 and 1/56. Next, 25
µl aliquots of each sample solution were placed in 96-well
plates, after which 200 µl of the WST working solution was
added. In addition, an enzyme working solution (20 µl) was
added to each well and the samples were then mixed thoroughly. The
enzyme reaction was induced by incubating the mixture plate at 37°C
for 20 min, and then measuring absorbance at 450 nm using a
spectrophotometer. SOD activity was calculated directly using the
following equation: SOD activity [inhibition rate (%)] =
[(Ablank 1-Ablank
3)-(Asample-Ablank 2)]/(Ablank
1-Ablank 3) ×100 (Ablank 1, absorbance
of blank 1; Ablank 2, absorbance of blank 2; Ablank
3, absorbance of blank 3; Asample, absorbance of
sample).
Statistical analysis
One-way ANOVA was used to determine the significant
differences between the no radiation group and the UV radiation
groups (SPSS for Windows, release 10.10, standard version; SPSS
Inc., Chicago, IL, USA). In addition, differences in the responses
of the vehicle-treated group and the EtSCT-treated groups within
the UV radiation group were evaluated using a post-hoc test (SPSS
for Windows, release 10.10, standard version; SPSS Inc.) of the
variance and significance levels. All values are reported as the
means ± SEM. A p-value of <0.05 was considered to indicate a
statistically significant difference.
Results
Antioxidant activity and total flavonoid
and phenolic levels in EtSCT
As shown in Table
I, EtSCT contained high concentrations of two important
antioxidants, flavonoids (15.3 mg/ml) and phenolics (36.8 mg/ml).
The free radical scavenging activity of DPPH and NO, as well as the
reducing power of EtSCT were analyzed following mix with single
doses of EtSCT. High DPPH scavenging activity (92.7%) was measured
in EtSCT. In addition, the reducing power and NO scavenging
activity of EtSCT were found to be 3.1 and 15.6%, respectively
(Table II). Taken together,
these results indicate that EtSCT has strong DPPH and NO scavenging
activity, as well as reducing power, and therefore has the
potential for use as an antioxidant.
 | Table IConcentration of total flavonoids and
phenolic contents in EtSCT. |
Table I
Concentration of total flavonoids and
phenolic contents in EtSCT.
| Categories | Concentration
(mg/ml) |
|---|
| Flavonoids | 15.3 |
| Phenolic
contents | 36.8 |
 | Table IIAntioxidant activity of EtSCT. |
Table II
Antioxidant activity of EtSCT.
| Categories | Level (%) |
|---|
| Reducing power | 3.1 |
| DPPH radical
scavenging activity | 92.7 |
| NO scavenging
activity | 15.6 |
Suppression of photodamage in response to
the topical application of EtSCT
Alterations in skin phenotypes were evaluated in the
vehicle- or EtSCT-treated mice after 13 weeks of treatment to
determine whether EtSCT application inhibits photoaging induced by
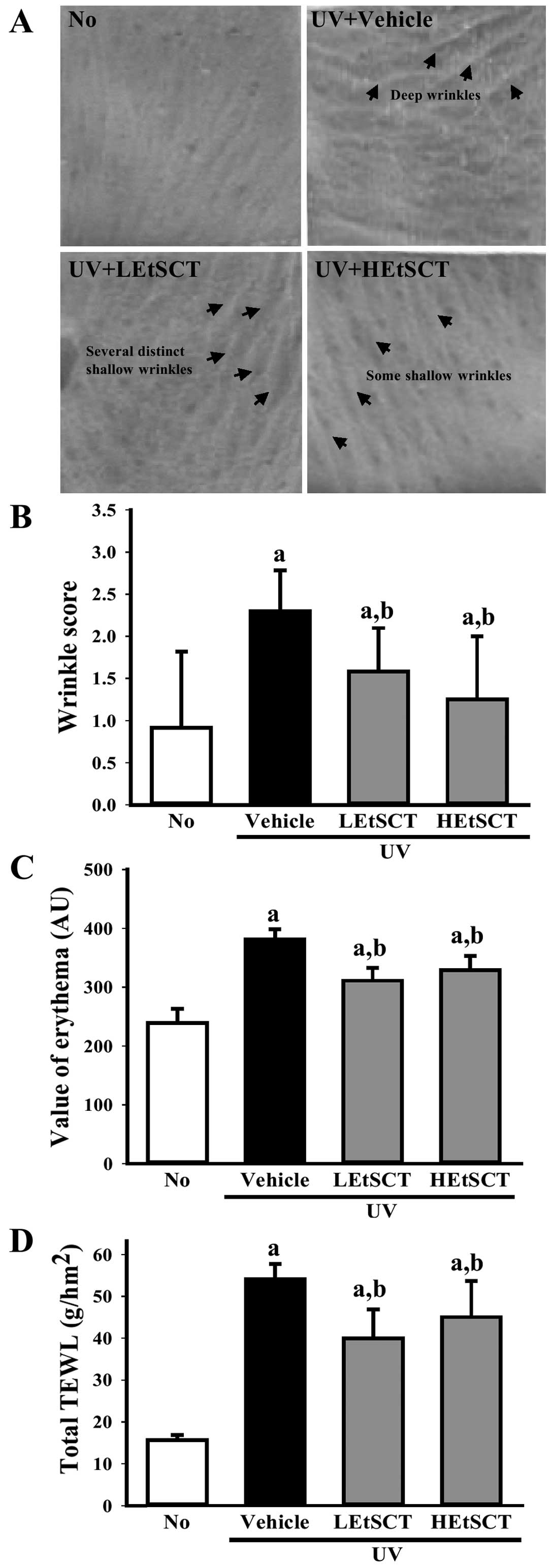
UV radiation. Following exposure to UV radiation, the wrinkle
score, including the depth and number of wrinkles, was
significantly higher in the UV + vehicle-treated group than in the
no radiation group. Conversely, there were significantly fewer
wrinkles induced by UV radiation in the LEtSCT- and HEtSCT-treated
mice (Fig. 1A and B). In
addition, the level of TEWL and the erythema index were
significantly higher in the mice exposed to UV light when this
level was compared with that of the no radiation group. However,
these levels were significantly lower in the UV + EtSCT-treated
groups than in the UV + vehicle-treated group, although this effect
was not dose-dependent (Fig. 1C and
D). Therefore, the topical application of EtSCT to the dorsal
skin of hairless mice effectively inhibited wrinkle formation while
preventing water loss and decreasing the erythema index.
Effects of EtSCT on the histological
structures of skin samples from hairless mice
It has been previously demonstrated that significant
histological changes in the dorsal skin of mice were induced by UV
radiation (27). Thus, in the
present study, the effects of the topical application of EtSCT on
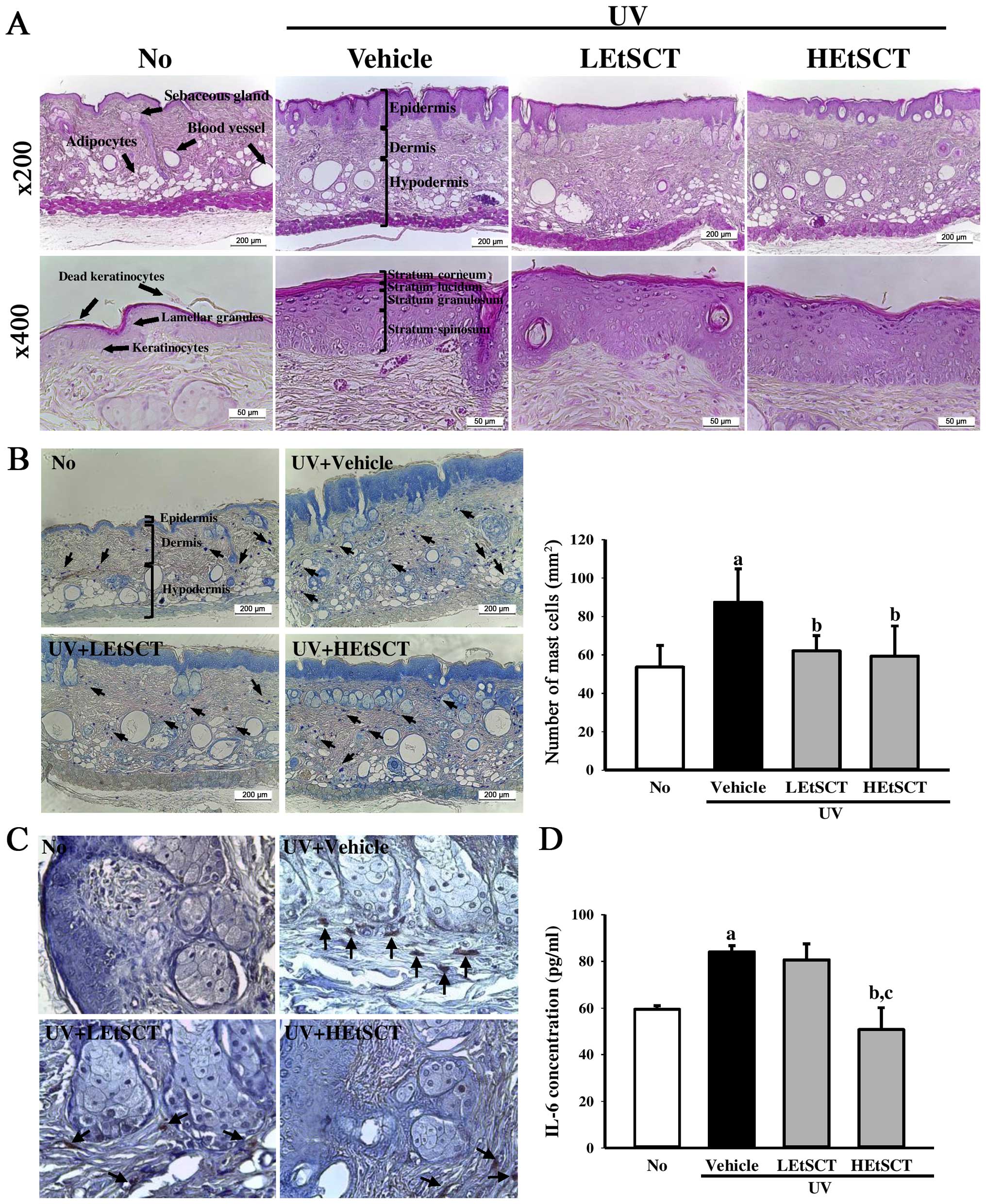
the histological structure of mouse skin were examined. Following
UV radiation exposure, the epidermis and dermis were significantly
thicker than in the no radiation group. However, the LEtSCT- and
HEtSCT-treated groups showed decreased epidermal and dermal
thickness, although their ratio was greater in the epidermis than
the dermis (Fig. 2A and Table III). This pattern of changes to
the epidermis was also detected in the subregions of the epidermis,
including the stratum corneum, stratum lucidum, stratum granulosum
and stratum spinosum (Fig. 2A).
Conversely, the number of adipocytes in the subcutaneous region was
also significantly lower in the UV + vehicle-treated group.
However, these levels were increased in the UV + LEtSCT- and UV +
HEtSCT-treated groups relative to that in the UV + vehicle-treated
group, although the average area of adipocytes was maintained at a
constant level (Fig. 2A and
Table III). Taken together,
these results suggest that the topical application of EtSCT for 13
weeks induced a decrease in the thickness of the epidermis and
dermis, as well as an increase in the number of adipocytes
following UV exposure.
 | Table IIIAlterations in the various
histological factors in skin tissue treated with UV + EtSCT. |
Table III
Alterations in the various
histological factors in skin tissue treated with UV + EtSCT.
| Categories | No radiation | UV
|
|---|
| Vehicle | LEtSCT | HEtSCT |
|---|
| Epidermis Th
(µm) | 43.2±6.88 | 165.7±24.18a | 97.9±8.50a,b | 64.2±11.62b,c |
| Dermis Th
(µm) | 251.3±27.25 | 355.0±29.27a | 305.8±23.21 | 313.0±16.60 |
| SC Th
(µm) | 5.3±0.90 | 18.4±0.76a | 13.4±1.66a,b | 9.6±0.75a–c |
| SL Th
(µm) | 2.8±0.30 | 5.3±1.12a | 3.9±0.69 | 4.4±0.52 |
| SG Th
(µm) | 12.7±2.36 | 40.9±4.62a | 30.3±4.98a,b | 32.2±6.54a,b |
| SS Th
(µm) | 30.3±4.98 | 49.6±6.03a | 34.8±3.64 | 38.5±4.07 |
| No. of
adipocytes | 55.6±5.85 | 29.0±9.16a | 50.2±11.23b | 42.4±9.76b |
| Average area of
adipocytes (cm2) | 36.8±22.32 | 19.9±16.14a | 15.2±12.78a | 21.0±11.26a |
Effects of EtSCT on skin
inflammation
To examine the suppressive effect of EtSCT on skin
inflammation induced by UV radiation, alterations in the mast cell
number, CD31 expression and IL-6 concentration were measured in the
skin tissues of mice. Many mast cells in the dermis region were
stained blue in the UV + vehicle-treated group relative to the no
radiation group. However, the number of mast cells was
significantly lower in the UV + LEtSCT-treated (62.0%) and the UV +
HEtSCT-treated (59.3%) groups (Fig.
2B). Moreover, changes in the number of CD31-stained cells were
very similar to those of mast cells in the dermis region. The UV +
EtSCT-treated groups showed a decrease in the number of
CD31-stained cells relative to the UV + vehicle-treated group
(Fig. 2C). A similar pattern was
also observed in the expression of IL-6, although the degree of
decrease or increase varied in each group. The expression of IL-6
decreased markedly only in the UV + HEtSCT-treated group (Fig. 2D). Overall, the above results
indicate that skin inflammation induced by UV radiation may be
suppressed by the topical application of EtSCT for 13 weeks.
Effect of topical application of EtSCT on
the mechanism responsible for the regulation of collagen
contents
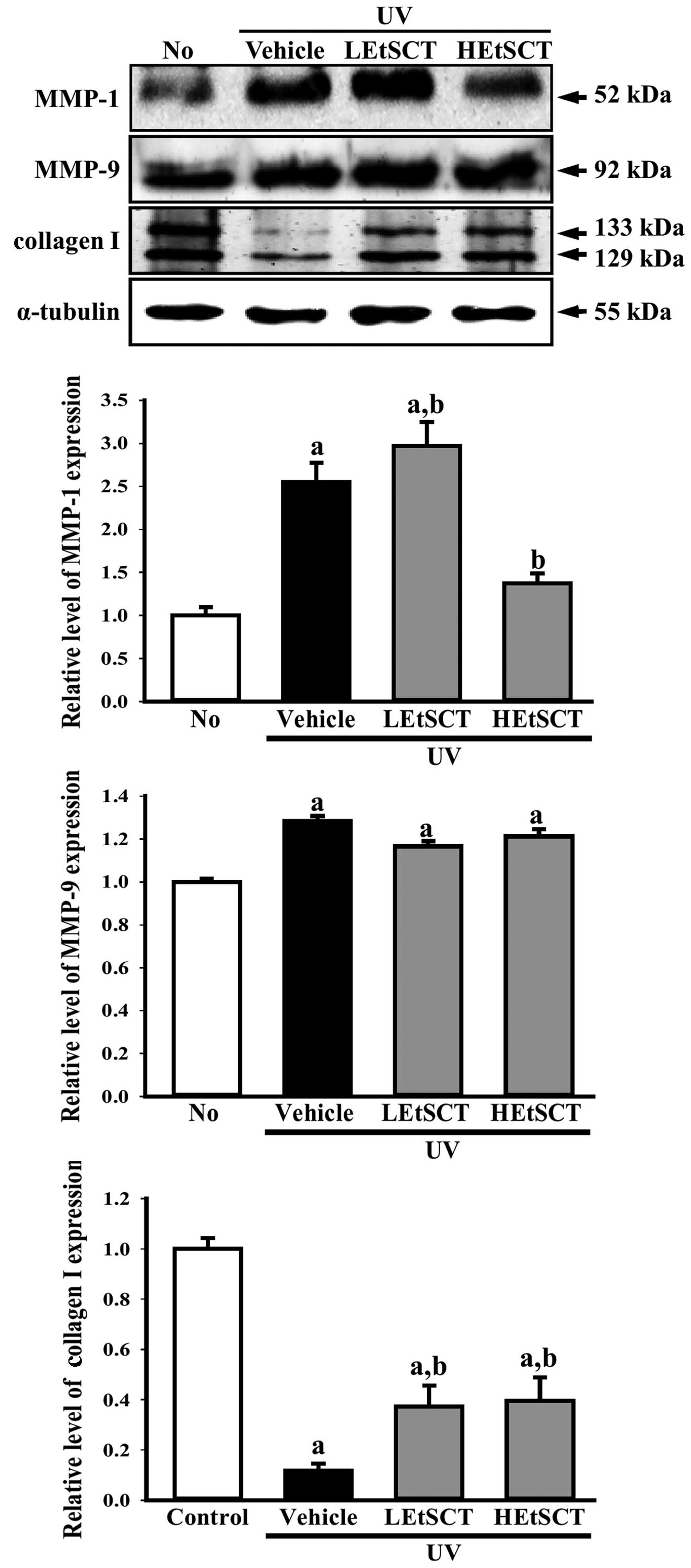
Alterations in the collagen content of the
extracellular matrix (ECM) are primarily responsible for the
clinical manifestations of skin aging such as wrinkles, sagging and
laxity (28,29). Therefore this study examined
whether the regulatory mechanism of collagen I contents in skin
tissue was recovered by the topical application of EtSCT for 13
weeks. The collagen I level in the UV + vehicle-treated group was
lower than that in the no radiation group. However, these levels
were significantly higher in the UV + LEtSCT- and the UV +
HEtSCT-treated groups (approximately 2.2 or 2.7-fold, respectively)
than in the UV + vehicle-treated group (Fig. 3).
To examine whether alterations in collagen
expression accompanied changes in the collagenase and elastinase in
skin tissue treated with EtSCT, the expression of MMP in the skin
of the UV + EtSCT-treated groups was measured. In the UV +
vehicle-treated group, the level of MMP-1 expression was
significantly higher than that in the no radiation group. However,
MMP-1 levels decreased significantly by 65% in the group treated
with HEtSCT alone, whereas this level was slightly increased in the
UV + LEtSCT-treated group (Fig.
3). Conversely, the expression pattern of elastinase (MMP-9)
was different from that of MMP-1 in the UV + HEtSCT-treated group.
The LEtSCT- and HEtSCT-treated groups did not show a significant
increase in MMP-9 expression in the UV + EtSCT-treated groups,
although the levels in these groups were higher than those in the
no radiation group (Fig. 3).
Taken together, these results suggest that the topical application
of EtSCT induces the recovery of collagen I content by suppressing
MMP-1 expression.
Effects of EtSCT on the mitogen-activated
protein (MAP) kinase signaling pathway activated by UV
radiation
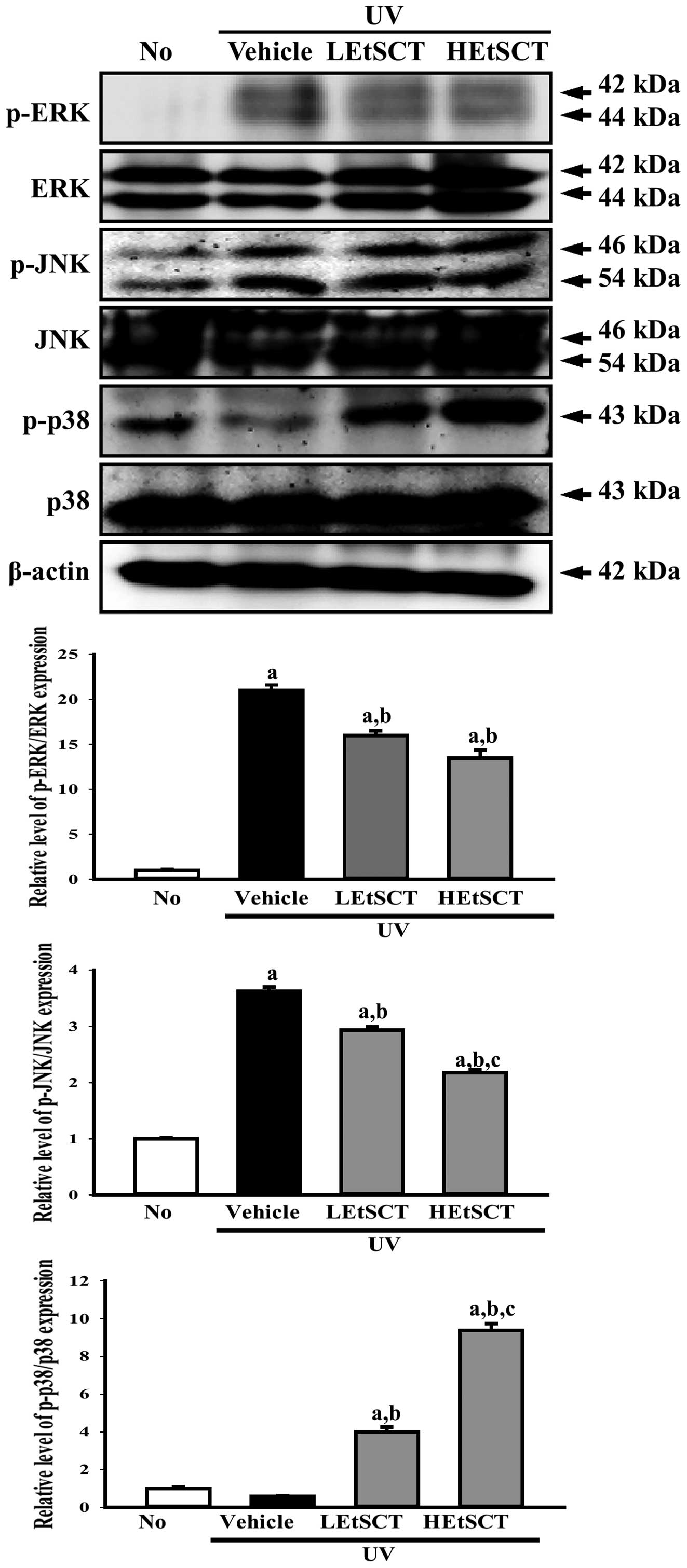
To determine whether activation of the MAP kinase
signaling pathway following exposure to UV radiation was inhibited
by the topical application of EtSCT for 13 weeks, the expression of
key proteins belonging to this pathway were measured in the dorsal
skin of EtSCT-treated mice. Western blot analysis using specific
antibodies revealed different phosphorylation patterns among the
three proteins (ERK, JNK and p38) in the subset groups. In the case
of ERK and JNK, the phosphorylation level was higher in the UV +
vehicle-treated group than in the no radiation group. Following
topical application of EtSCT, this level decreased significantly,
although the decrease did not differ among groups (Fig. 4). In the case of p38, the
phosphorylation level was slightly decreased in the UV +
vehicle-treated group when compared with that of the no radiation
group. However, EtSCT treatment induced a marked increase in the
phosphorylation level of p38, with the highest level of this
protein being observed in the HEtSCT-treated group (Fig. 4). The results of the present study
suggest that EtSCT application inhibits ERK and JNK phosphorylation
and activates p38 phosphorylation.
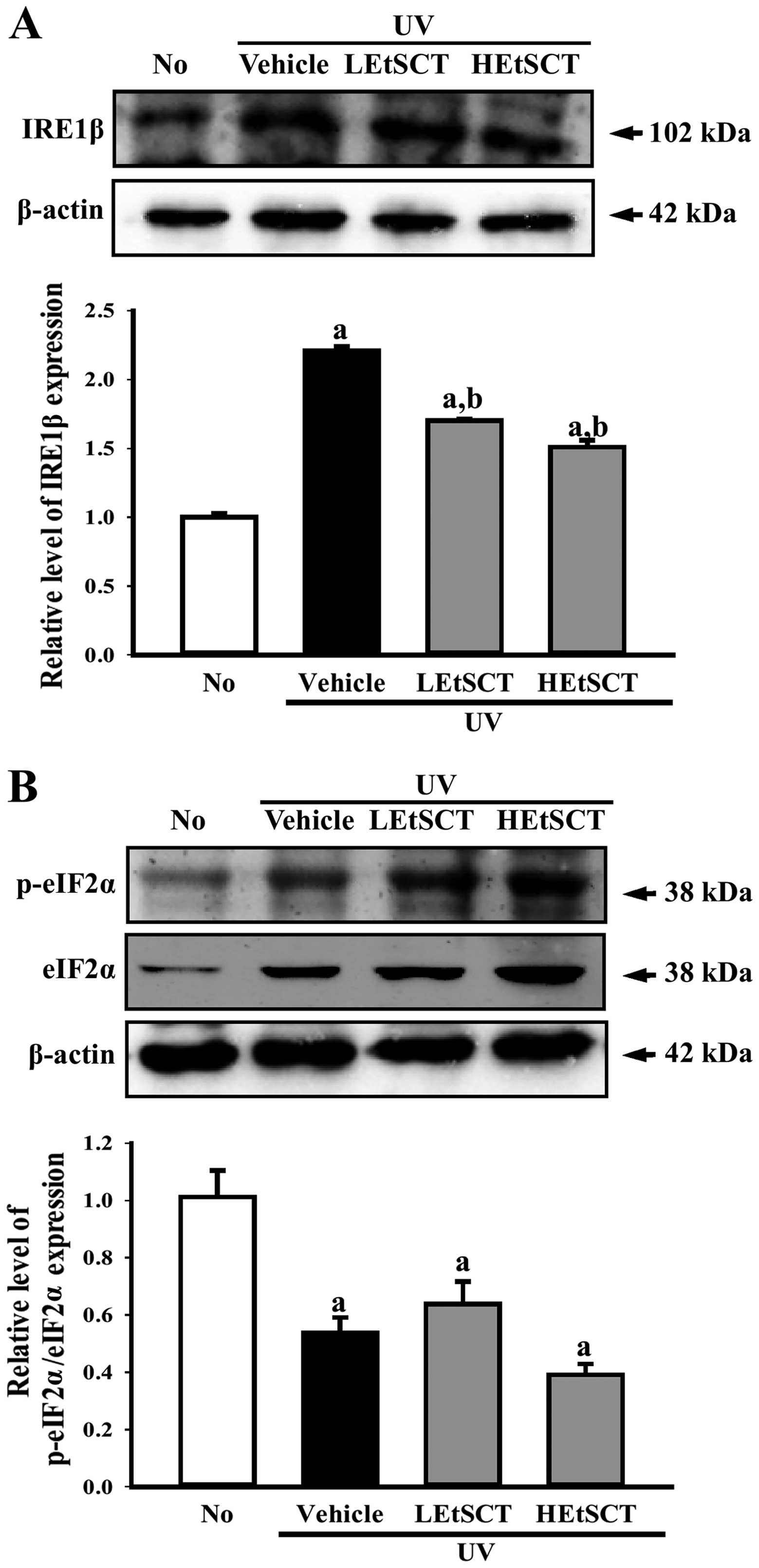
Effects of EtSCT on the ER stress
signaling pathway of following exposure to UV radiation
To determine whether the topical application of
EtSCT stimulates two different types of signaling in the ER stress
pathway, the levels of key proteins in the IRE1 and protein kinase
R-like endoplasmic reticulum kinase (PERK, also known as eukaryotic
translation initiation factor 2-α kinase 3) signaling pathway were
monitored in the skin tissues from all groups of mice by performing
western blot analysis with the corresponding antibodies. In the
case of the IRE1 signaling pathway, the UV + vehicle-treated group
showed higher protein expression of IRE1β than in the no radiation
group. The expression of this protein was significantly lower in UV
+ EtSCT-treated mice, and the greatest decrease was observed in the
HEtSCT-treated group. However, the phosphorylation level of eIF2α
was maintained in the LEtSCT- and HEtSCT-treated mice (Fig. 5A and B). Taken together, these
results suggest that the topical application of EtSCT effectively
inhibits the IRE1 signal within the ER stress signaling
pathway.
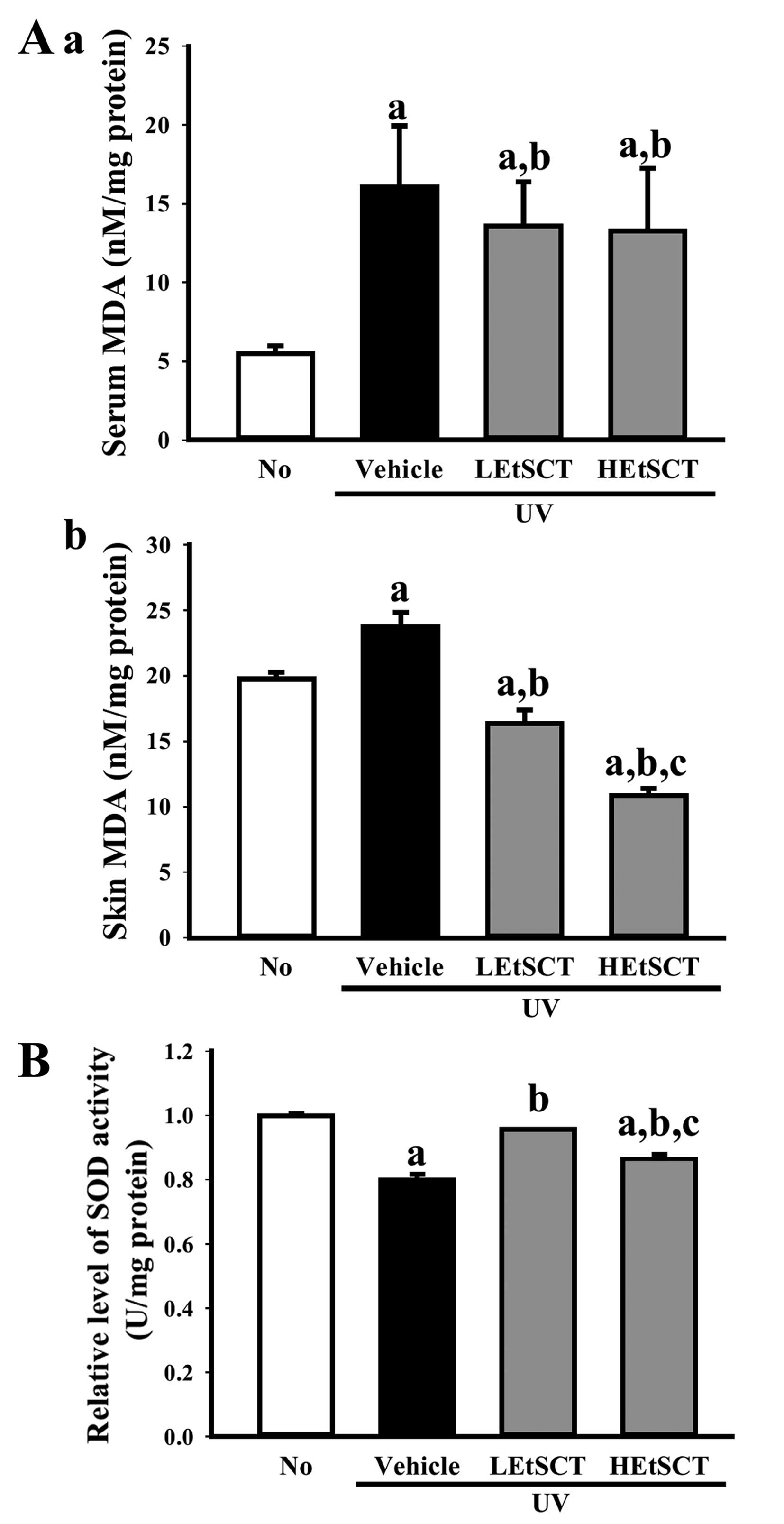
Antioxidant activity of skin tissue
Finally, we examined the inhibitory effects of EtSCT
on oxidative stress induced by UV radiation. To accomplish this,
the MDA concentration and SOD activity were measured in the skin
tissue of hairless mice. The concentration of MDA in the serum and
skin tissue was 190 and 26% higher, respectively, in the UV +
vehicle-treated group than in the no radiation group, whereas the
level decreased significantly in a dose-dependent manner in the
skin of EtSCT-treated groups. The highest level of decrease was
detected in the HEtSCT-treated group (Fig. 6A). The activity of SOD showed the
opposite pattern to the MDA concentration. Specifically, the UV +
vehicle-treated group showed lower (22%) SOD activity than the no
radiation group, although this level was significantly higher in
all EtSCT treated groups (Fig.
6B). Overall, these results suggest that EtSCT treatment
inhibits the oxidative stress and lipid peroxidation induced by UV
radiation.
Toxicity analysis of topically applied
EtSCT
Changes in body weight, serum biochemical indicators
and histological structures in the hairless mice treated with UV +
EtSCT were evaluated in order to determine the toxicity of EtSCT.
Although the body weights of the mice in the EtSCT-treated groups
were slightly higher than those of the mice in the control, there
were no significant differences in body weight between the treated
groups at 13 weeks (Table IV).
The hepatotoxicity of EtSCT was determined by measuring ALP, ALT
and AST concentrations, as well as by observing the
histopathological alterations in the mouse livers. No significant
alterations in the three liver enzymes were detected in the UV +
EtSCT-treated groups (Table IV).
Histological alterations in the liver sections stained with H&E
were observed upon microscopic evaluation. No significant
pathological changes were detected in the liver tissue of mice in
any groups (data not shown).
 | Table IVToxicity analysis of topically
applied EtSCT to hairless mice. |
Table IV
Toxicity analysis of topically
applied EtSCT to hairless mice.
| Categories | No radiation | UV
|
|---|
| Vehicle | LEtSCT | HEtSCT |
|---|
| Body weight
(g) | 34.9±0.64 | 34.2±1.89 | 36.2±0.70 | 35.1±3.30 |
| Liver weight
(g) | 192.31±2.43 | 188.13±3.26 | 192.91±2.98 | 192.58±2.32 |
| ALT (mg/dl) | 37.16±6.76 | 26.60±2.56 | 27.60±3.51 | 22.60±1.93 |
| AST (mg/dl) | 57.33±13.83 | 73.66±6.28 | 70.00±12.28 | 61.40±3.66 |
| ALP (mg/dl) | 148.20±7.12 | 153.50±7.30 | 156.50±1.04 | 149.40±6.72 |
| LDH (mg/dl) | 435.20±71.20 | 511.83±48.71 | 484.00±21.53 | 355.00±36.12 |
| Kidney weight
(g) | 0.28±0.00 | 0.26±0.01 | 0.27±0.01 | 0.27±0.00 |
| BUN (mg/dl) | 21.78±1.99 | 18.72±1.49 | 18.46±1.06 | 19.91±0.84 |
| CRE (mg/dl) | 0.37±0.01 | 0.32±0.01 | 0.32±0.04 | 0.33±0.05 |
The concentrations of BUN and CRE, which indicate
kidney toxicity, showed a similar pattern, with a constant level of
these factors maintained in the subset groups (Table IV). Furthermore, microscopic
observation revealed no specific pathological symptoms in any of
the EtSCT-treated groups (data not shown). These results suggest
that EtSCT treatment for 13 weeks does not induce specific toxic
effects in the livers or kidneys of hairless mice.
Discussion
Both the passage of time (intrinsic aging) and
cumulative exposure to external influences (extrinsic aging), such
as UV radiation and smoking, may induce skin aging (30,31). Among factors leading to extrinsic
aging, UV radiation is the most well-known cause of skin damage,
resulting in deep wrinkles, roughness, laxity and pigmentation
(31). However, skin aging
induced by UV radiation may be effectively prevented by
antioxidants and mixtures originating from natural products
(32). In this study, we
investigated the novel effects of EtSCT on UV-induced skin aging.
Although the antioxidant activity of various compounds and extracts
derived from SC have been investigated in several studies (9,33–36), there have been no investigations
of the therapeutic effects of SCT on photoaging induced by UV
radiation, to the best of our knowledge. In this study, EtSCT was
shown to have the ability to prevent skin aging, including wrinkle
formation, increasing epidermal thickness, skin inflammation,
activation of the MAP kinase pathway and enhanced lipid oxidation,
through the upregulation of antioxidant activity in the skin.
Many phytochemicals that are sources of natural
antioxidants, such as phenolic diterpenes, flavonoids, tannins and
polyphenolic acids, have been reported to be potential therapeutic
agents for the treatment of cancer, pathological angiogenesis and
cardiovascular disease (37,38). In a previous study, the
concentrations of flavonoids and phenolic contents were measured in
SCT extracts collected using several different solvents. The
highest flavonoid contents were detected in chloroform extract
(23.0 mg/g), followed by n-butanol extract (19.1 mg/g), acetone
extract (17.2 mg/g), ethanol extract (15.6 mg/g) and n-hexane
extract (8.9 mg/g) (8). In
addition, the total phenolic content in hot water extract of SCT
was 46.6 mg/g, whereas it was 37.5 mg/g in ethanol extract
(8). However, carotenoid extract
purified with acetone solvent contained 11.1–13.8 µg/g of
total phenolic content (14). In
the present study, we used ethanol extract to examine the
therapeutic effects against skin aging. These extracts showed
similar flavonoid and total phenolic contents to previous findings
(8).
Measurements of DPPH and NO scavenging activity as
well as reducing power are widely used to evaluate antioxidant
activity (39). Extracts
collected from SCT using different solvents showed various levels
of antioxidant activity in previous reports. The highest levels of
DPPH radical scavenging activity were detected in acetone,
ethylacetate, chloroform and n-butanol extract, and ethanol extract
showed 93.3% activity. However, the greatest NO radical scavenging
activity was measured in n-hexane extract, while that of ethanol
extract and methanol extract was 15.0 and 9.7%, respectively. The
reducing power of autoclave extract was highest (100%), followed by
that of hot water extract (97.8%), n-butanol extract (4.6%),
chloroform extract (3.1%) and ethanol extract (2.9%) (8). Moreover, the carotenoid-enriched
sample showed a scavenging capacity of 29.9% when tested against 5
mM DPPH radical for 30 min as well as strong reducing power with an
OD of 1.025 against a concentration of 120 µg/ml (14). The results of this study are in
agreement with those reported by Lee et al (8), whereas they differ from those
observed by Nacional et al (14). The differences among samples may
have been due to differences in the extraction solvents and test
systems employed.
Although many antioxidants and anti-photoaging
compounds that effectively prevent photodamage of the skin are
available, few studies have demonstrated correlations between
natural extracts with antioxidant activity and protection of the
skin against wrinkle formation, TEWL and erythema. Topical
treatment with Machilus thunbergii Siebold & Zucc.
effectively reduced wrinkle formation and skin thickness on
hairless mice subjected to UV radiation (40). The skin erythema index in a jujube
water extract (JWE)-treated group was significantly lower than that
of a control group (41), and the
oral intake of sea buckthorn (Hippophae rhamnoides L.) fruit
blend (SFB) induced decreased wrinkle formation and TEWL during
UV-induced skin aging (23).
However, no studies have shown that the administration of SCT
extracts closely correlate with the decreased formation of
wrinkles, TEWL and erythema in skin, to the best of our knowledge.
One possible mechanism of protection is the inhibition of
tyrosinase activity, which is the rate limiting enzyme that
regulates the production of melanin in skin melanocytes (42). Thus, this study is the first to
suggest that the topical application of EtSCT may prevent or
alleviate the formation of wrinkles, TEWL and erythema during
UV-induced skin aging, to the best of our knowledge.
The epidermis contains different levels of melanin
in the skin, which may play a significant role in determining
photoprotective effects against underlying cells (43). Following UV radiation, the
epidermal thickness and the number of adipocytes were recovered to
a similar level as that in the no radiation group. However, it has
been previously demonstrated that treatment with several extracts,
including M. thunbergii, Artocarpus communis and SFB,
resulted in a significant decrease in epidermal thickness and the
number of adipocytes relative to the control (23,40,44). In the present study, only
epidermal thickness was rapidly decreased in all EtSCT-treated
groups (Fig. 2 and Table III). These findings are similar
to those of a previous study in which a decrease in epidermal
thickness was shown to be related to treatment with antioxidant
extracts (45).
Collagen I and III, which are abundant in the
dermis, polymerize to produce extended mechanically stiff fibrils,
which impart tensile strength to the tissue (46,47). Thus, wrinkle formation is closely
correlated with the regulation of collagen synthesis and
degradation (48). The majority
of reports have shown that the level and distribution of collagen
in the skin increases significantly following treatment with
antioxidants, although this level is decreased after exposure to UV
radiation (23,49,50). Conversely, an opposite tendency in
collagen I expression was detected in the levels of MMP-1.
Specifically, MMP-1 plays important roles in the degradation of
dermal collagens in the ECM composed mainly of type 1 collagen
during the aging of human skin (45,51,52). In this study, the collagen levels
were significantly lower in the UV radiation group than in the no
radiation group, but this level was markedly higher in the LEtSCT-
or HEtSCT-treated groups than in the UV + vehicle-treated group.
Moreover, the increase in MMP-1 expression induced by UV radiation
was recovered in the HEtSCT-treated group. These changes were
similar to those reported in previous studies examining the effects
of tempol, retinoic acid and SFB on the collagen regulation of
skin, although the variations in the increase or decrease rate
differed (23,48,49,52). This is the first study, to the
best of our knowledge, to suggest that collagen expression may be
affected by the topical application of EtSCT.
The inflammation and oxidative stress induced by ROS
overproduction following exposure to UV radiation may be prevented
or decreased by treatment with traditional medicines (53), natural products (45) and some powerful antioxidants such
as anthocyanins and quercetin (54,55). Previous studies have shown that
the levels of pro-inflammatory cytokines, including IL-1β, IL-6,
IL-8 and TNF-α, were reduced by treatment with A. communis
and Butea monosperma flower extract (55,56). In addition, it has been
demonstrated that groups treated with JWE or
(-)-epigallocatechin-3-gallate (EGCG) exhibited a relatively lower
infiltration of mast cells and leukocytes in the dermis or
hyperdermis relative to the vehicle-treated group (41,57). The present study revealed that the
increase in mast cell numbers and cytokine expression induced by UV
radiation was attenuated by treatment with EtSCT, with marked
decreases in the infiltration of mast cells and the expression of
CD31 and IL-6 being observed. These findings are similar to the
results of previous studies (41,44,56). Significant changes were observed
in response to the LEtSCT and HEtSCT treatments. Specifically,
HEtSCT almost completely inhibited the infiltration of mast cells
and cytokine expression in the dorsal skin of hairless mice. Taken
together, these findings indicate that EtSCT is a strong candidate
for use as an inhibitor of inflammation.
The excessive production of ROS during chronic UV
radiation may induce an imbalance between pro-oxidant production
and antioxidant defense, resulting in the stimulation of oxidative
DNA damage and the peroxidation of lipids and proteins in the skin
(58). Treatment with M.
thunbergii and A. communis extracts as well as
α-tocopherol have been shown to reduce the formation of lipid
peroxides relative to the vehicle-treated group, although the
extent of the decrease varied (40,44,59). In the present study, lipid
peroxide levels were measured in the serum and skin tissue of
hairless mice following UV + EtSCT co-treatment for 13 weeks. The
MDA levels in both the serum and skin of the EtSCT-treated groups
were lower than those in the UV + vehicle-treated group, which is
in agreement with the results of previous studies (Fig. 6).
Taken together, these results provide the first
evidence to the best of our knowledge, that EtSCT contains high
levels of flavonoids and phenolic compounds that may contribute to
the protection and treatment of skin damaged by UV exposure. EtSCT
was particularly effective at preventing alterations in skin
phenotypes and histological structures as well as regulating
inflammation and oxidative conditions without exerting toxicity
against the liver and kidneys.
Acknowledgments
The present study was supported by a grant to
Professor Dae Youn Hwang from the Korea Institute of Marine Science
and Technology Promotion (no. 112088-3).
References
|
1
|
Millar RH: The identity of the ascidians
Styela mammiculata Carlisle and Styela clava Herdman. J Mar Biol
Assoc UK. 39:509–511. 1960. View Article : Google Scholar
|
|
2
|
Carlisle DB: Styela mammiculata n.sp, a
new species of ascidian from the Plymouth area. J Mar Biol Assoc
UK. 33:329–334. 1954. View Article : Google Scholar
|
|
3
|
Kang PA, Kim Y and Yoon DS: Studies on the
hanging culture of oyster, Crassostrea gigas, in the Korean coastal
waters (IV). On the fouling organisms associated with culturing
oysters at the oyster culture farms in Chungmu. Bull Natl Fish Res
Dev Agency. 25:29–34. 1980.
|
|
4
|
Davis MH and Davis ME: First record of
Styela clava (Tunicata, Ascidiacea) from the Mediterranean Sea.
Aquatic Invasions. 3:125–132. 2008. View Article : Google Scholar
|
|
5
|
Park JH and Suh YC: GIS-Based Suitable
Site Selection for aquaculture using scope for growth of Styela
clava. J Korean Assoc Geogr Inf Stud. 16:81–90. 2013. View Article : Google Scholar
|
|
6
|
Shin YK, Park JJ, Park MS, Myeong JI and
Hur YB: Effect of temperature and dissolved oxygen on the survival
rate and physiological response of the warty sea squirt Styela
clava. Korean J Environ Biol. 32:216–224. 2014. View Article : Google Scholar
|
|
7
|
Ahn SH, Jung SH, Kang SJ, Jeong TS and
Choi BD: Extraction of glycosaminoglycans from Styela clava tunic.
Korean J Biotechnol Bioeng. 18:180–185. 2003.
|
|
8
|
Lee SM, Kang EJ, Go TH, Jeong SY, Park GT,
Lee HS, Hwang DY, Jung YJ and Son HJ: Screening of biological
activity of solvent extract from Styela clava tunic for fishery
waste recycling. J Environ Sci Intern. 23:89–96. 2014. View Article : Google Scholar
|
|
9
|
Jung YJ: Properties of regenerated
cellulose films prepared from the tunicate Styela clava. J Kor Fish
Soc. 41:237–242. 2008.
|
|
10
|
Jung YJ, An BJ, Hwang DY, Kim HD, Park SM,
Cho H and Kim HS: Preparation and properties of regenerated
cellulosic biomaterial made from Styela clava tunics. Biomater Res.
12:71–76. 2008.
|
|
11
|
Kwak MH, Go J, Kim JE, Lee YJ, Lee SH, Lee
HS, Son HJ, Jung YJ and Hwang DY: Property and efficacy analysis of
hydrocolloid membrane containing Styela clava Tunic on the wound
repair of skin in SD rats. Biomater Res. 17:91–101. 2013.
|
|
12
|
Kim SM, Lee JH, Cho JA, Lee SC and Lee SK:
Development of a bioactive cellulose membrane from sea squirt skin
for bone regeneration - A preliminary research. J Korean Assoc Oral
Maxillofac Surg. 31:440–453. 2005.
|
|
13
|
Xu CX, Jin H, Chung YS, Shin JY, Woo MA,
Lee KH, Palmos GN, Choi BD and Cho MH: Chondroitin sulfate
extracted from the Styela clava tunic suppresses TNF-α-induced
expression of inflammatory factors, VCAM-1 and iNOS by blocking
Akt/NF-kappaB signal in JB6 cells. Cancer Lett. 264:93–100. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nacional LM, Kang SJ and Choi BD:
Antioxidative activity of carotenoids in mideodeok Styela clava.
Fish Aquat Sci. 14:243–249. 2011.
|
|
15
|
Song SH, Kim JE, Lee YJ, Kwak MH, Sung GY,
Kwon SH, Son HJ, Lee HS, Jung YJ and Hwang DY: Cellulose film
regenerated from Styela clava tunics have biodegradability,
toxicity and biocompatibility in the skin of SD rats. J Mater Sci
Mater Med. 25:1519–1530. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Singleton VL and Rossi JA: Colorimetry of
total phenolics with phosphomolybdic-phosphotungstic acid reagents.
Am J Enol Vitic. 16:144–158. 1965.
|
|
17
|
Zhishen J, Mengcheng T and Jianming W: The
determination of flavonoid contents in mulberry and their
scavenging effects on superoxide radicals. Food Chem. 64:555–559.
1999. View Article : Google Scholar
|
|
18
|
Oh H, Ko EK, Kim DH, Jang KK, Park SE, Lee
HS and Kim YC: Secoiridoid glucosides with free radical scavenging
activity from the leaves of Syringa dilatata. Phytother Res.
17:417–419. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oyaizu M: Studies on products of the
browning reaction. Antioxidative activities of browning reaction
products prepared from glucosamine. Jpn J Nutr. 44:307–315. 1986.
View Article : Google Scholar
|
|
20
|
Marcocci L, Maguire JJ, Droy-Lefaix MT and
Packer L: The nitric oxide-scavenging properties of Ginkgo biloba
extract EGb 761. Biochem Biophys Res Commun. 201:748–755. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park CH, Lee MJ, Kim JP, Yoo ID and Chung
JH: Prevention of UV radiation-induced premature skin aging in
hairless mice by the novel compound Melanocin A. Photochem
Photobiol. 82:574–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nam SH, Jung SE, Lee YK, Kim JE, Lee EP,
Choi HW, Kim HS, Lee JH, Jung YJ, Lee CY, et al: Topical
application of selenium can significantly relieve UV-induced skin
aging in hairless mice. Lab Anim Res. 26:37–45. 2010. View Article : Google Scholar
|
|
23
|
Hwang IS, Kim JE, Choi SI, Lee HR, Lee YJ,
Jang MJ, Son HJ, Lee HS, Oh CH, Kim BH, et al: UV radiation-induced
skin aging in hairless mice is effectively prevented by oral intake
of sea buckthorn (Hippophae rhamnoides L.) fruit blend for 6 weeks
through MMP suppression and increase of SOD activity. Int J Mol
Med. 30:392–400. 2012.PubMed/NCBI
|
|
24
|
Bissett DL, Hannon DP and Orr TV: An
animal model of solar-aged skin: histological, physical, and
visible changes in UV-irradiated hairless mouse skin. Photochem
Photobiol. 46:367–78. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Baba H, Masuyama A, Yoshimura C, Aoyama Y,
Takano T and Ohki K: Oral intake of Lactobacillus
helveticus-fermented milk whey decreased transepidermal water loss
and prevented the onset of sodium dodecylsulfate-induced dermatitis
in mice. Biosci Biotechnol Biochem. 74:18–23. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim HJ, Kim J, Kim SJ, Lee SH, Park YS,
Park BK, Kim BS, Kim SK, Cho SD, Jung JW, et al: Anti-inflammatory
effect of quercetin on picryl chloride-induced contact dermatitis
in BALB/c mice. Lab Anim Res. 26:7–13. 2010. View Article : Google Scholar
|
|
27
|
Koshiishi I, Horikoshi E, Mitani H and
Imanari T: Quantitative alterations of hyaluronan and dermatan
sulfate in the hairless mouse dorsal skin exposed to chronic UV
irradiation. Biochim Biophys Acta. 1428:327–333. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Philips N, Burchill D, O'Donoghue D,
Keller T and Gonzalez S: Identification of benzene metabolites in
dermal fibroblasts as nonphenolic: regulation of cell viability,
apoptosis, lipid peroxidation and expression of matrix
metalloproteinase 1 and elastin by benzene metabolites. Skin
Pharmacol Physiol. 17:147–152. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Philips N, Conte J, Chen YJ, Natrajan P,
Taw M, Keller T, Givant J, Tuason M, Dulaj L, Leonardi D and
Gonzalez S: Beneficial regulation of matrixmetalloproteinases and
their inhibitors, fibrillar collagens and transforming growth
factor-β by Polypodium leucotomos, directly or in dermal
fibroblasts, ultraviolet radiated fibroblasts, and melanoma cells.
Arch Dermatol Res. 301:487–495. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yaar M and Gilchrest BA: Photoageing:
mechanism, prevention and therapy. Br J Dermatol. 157:874–887.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Langton AK, Sherratt MJ, Griffiths CEM and
Watson REB: A new wrinkle on old skin: the role of elastic fibres
in skin ageing. Int J Cosmet Sci. 32:330–339. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sumiyoshi M and Kimura Y: Effects of a
turmeric extract (Curcuma longa) on chronic ultraviolet B
irradiation-induced skin damage in melanin-possessing hairless
mice. Phytomedicine. 16:1137–1143. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jung ES, Park E and Lee SC: Antioxidant
activities of extracts from parts of styela clava. J Kor Soc Food
Sci Nutr. 37:1674–1678. 2008. View Article : Google Scholar
|
|
34
|
Kim JJ, Kim SJ, Kim SH, Park HR and Lee
SC: Antioxidant and anticancer activities of extracts from Styela
plicata. J Korean Soc Food Sci Nutr. 34:937–941. 2005. View Article : Google Scholar
|
|
35
|
Kim JJ, Kim SJ, Kim SH, Park HR and Lee
SC: Antioxidant and anticancer activities of extracts from Styela
clava according to the processing methods and solvents. J Kor Soc
Food Nutr. 35:278–283. 2006. View Article : Google Scholar
|
|
36
|
Park JW, You DH, Bae MS, Kim JM, Lee JH,
Kim SJ, Jeon YJ, Park EJ and Lee SC: Antioxidant and
antihypertensive activities of Styela plicata according to
harvesting time and size. Korean Soc Food Sci Nutr. 40:350–356.
2010. View Article : Google Scholar
|
|
37
|
Yoysungnoen P, Wirachwong P, Changtam C,
Suksamrarn A and Patumraj S: Anti-cancer and anti-angiogenic
effects of curcumin and tetrahydrocurcumin on implanted
hepatocellular carcinoma in nude mice. World J Gastroenterol.
14:2003–2009. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Münzel T, Gori T, Bruno RM and Taddei S:
Is oxidative stress a therapeutic target in cardiovascular disease?
Eur Heart J. 31:2741–2748. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Roginsky V and Alegria AE: Oxidation of
tea extracts and tea catechins by molecular oxygen. J Agric Food
Chem. 53:4529–4535. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Uhm YK, Jung KH, Bu HJ, Jung MY, Lee MH,
Lee S, Lee S, Kim HK and Yim SV: Effects of Machilus thunbergii
Sieb et Zucc on UV-induced photoaging in hairless mice. Phytother
Res. 24:1339–1346. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Choi SY and Kim YC: Alleviative effects of
jujube water extract on the inflammation and barrier damage in
hairless mice skin. Environ Health Toxicol. 24:351–357. 2009.
|
|
42
|
Iozumi K, Hoganson GE, Pennella R, Everett
MA and Fuller BB: Role of tyrosinase as the determinant of
pigmentation in cultured human melanocytes. J Invest Dermatol.
100:806–11. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yamaguchi Y, Takahashi K, Zmudzka BZ,
Kornhauser A, Miller SA, Tadokoro T, Berens W, Beer JZ and Hearing
VJ: Human skin responses to UV radiation: pigment in the upper
epidermis protects against DNA damage in the lower epidermis and
facilitates apoptosis. FASEB J. 20:1486–1488. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee CW, Ko HH, Chai CY, Chen WT, Lin CC
and Yen FL: Effect of Artocarpus communis extract on UVB
irradiation-induced oxidative stress and inflammation in hairless
mice. Int J Mol Sci. 14:3860–3873. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pinnell SR: Cutaneous photodamage,
oxidative stress, and topical antioxidant protection. J Am Acad
Dermatol. 48:1–19. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gosline J, Lillie M, Carrington E,
Guerette P, Ortlepp C and Savage K: Elastic proteins: biological
roles and mechanical properties. Philos Trans R Soc Lond B Biol
Sci. 357:121–132. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Heim AJ, Matthews WG and Koob TJ:
Determination of the elastic modulus of native collagen fibrils via
radial indentation. Appl Phys Lett. 89:181902–181903. 2006.
View Article : Google Scholar
|
|
48
|
Chen S, Kiss I and Tramposch KM: Effects
of all-trans retinoic acid on UVB-irradiated and non-irradiated
hairless mouse skin. J Invest Dermatol. 98:248–254. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yan SX, Hong XY, Hu Y and Liao KH: Tempol,
one of nitroxides, is a novel ultraviolet-A1 radiation protector
for human dermal fibroblasts. J Dermatol Sci. 37:137–143. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Vicentini FT, Fonseca YM, Pitol DL,
Iyomasa MM, Bentley MV and Fonseca MJ: Evaluation of protective
effect of a water-in-oil microemulsion incorporating quercetin
against UVB-induced damage in hairless mice skin. J Pharm Pharm
Sci. 13:274–285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Brennan M, Bhatti H, Nerusu KC,
Bhagavathula N, Kang S, Fisher GJ, Varani J and Voorhees JJ: Matrix
metalloproteinase-1 is the major collagenolytic enzyme responsible
for collagen damage in UV-irradiated human skin. Photochem
Photobiol. 78:43–48. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Fisher GJ, Datta S, Wang Z, Li XY, Quan T,
Chung JH, Kang S and Voorhees JJ: c-Jun-dependent inhibition of
cutaneous procollagen transcription following ultraviolet
irradiation is reversed by all-trans retinoic acid. J Clin Invest.
106:663–670. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kang TH, Park HM, Kim YB, Kim H, Kim N, Do
JH, Kang C, Cho Y and Kim SY: Effects of red ginseng extract on UVB
irradiation-induced skin aging in hairless mice. J Ethnopharmacol.
123:446–451. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tsoyi K, Park HB, Kim YM, Chung JI, Shin
SC, Lee WS, Seo HG, Lee JH, Chang KC and Kim HJ: Anthocyanins from
black soybean seed coats inhibit UVB-induced inflammatory
cylooxygenase-2 gene expression and PGE2 production through
regulation of the nuclear factor-kappaB and phosphatidylinositol
3-kinase/Akt pathway. J Agric Food Chem. 56:8969–8974. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Casagrande R, Georgetti SR, Verri WA Jr,
Dorta DJ, dos Santos AC and Fonseca MJ: Protective effect of
topical formulations containing quercetin against UVB-induced
oxidative stress in hairless mice. J Photochem Photobiol B.
84:21–27. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Krolikiewicz-Renimel I, Michel T,
Destandau E, Reddy M, André P, Elfakir C and Pichon C: Protective
effect of a Butea monosperma (Lam) Taub flowers extract against
skin inflammation: antioxidant, anti-inflammatory and matrix
metalloproteinases inhibitory activities. J Ethnopharmacol.
148:537–543. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Katiyar SK and Mukhtar H: Green tea
polyphenol (-)-epigallo-catechin-3-gallate treatment to mouse skin
prevents UVB-induced infiltration of leukocytes, depletion of
antigen-presenting cells, and oxidative stress. J Leukoc Biol.
69:719–726. 2001.PubMed/NCBI
|
|
58
|
Hanson KM and Simon JD: Epidermal
trans-urocanic acid and the UV-A-induced photoaging of the skin.
Proc Natl Acad Sci USA. 95:10576–10578. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lopez-Torres M, Thiele JJ, Shindo Y, Han D
and Packer L: Topical application of alpha-tocopherol modulates the
antioxidant network and diminishes ultraviolet-induced oxidative
damage in murine skin. Br J Dermatol. 138:207–215. 1998. View Article : Google Scholar : PubMed/NCBI
|