Introduction
Caused by impaired bile formation or bile acid
excretion from hepatocytes into the bile canaliculi, cholestasis
occurs in a wide variety of human diseases, including biliary
atresia, Alagille syndrome, primary biliary cirrhosis, and primary
sclerosing cholangitis (1–3).
The accumulation of toxic bile salt damages hepatocytes and
cholangiocytes, resulting in apoptosis and oncosis (characteristic
hepatocyte necrosis in response to obstructive cholestasis);
together with the inflammatory reactions, the resultant cell death
exacerbates liver injuries, ultimately leading to fibrosis,
cirrhosis and liver failure (4–9).
There is no effective pharmacologic treatment for cholestatic liver
disease, and, with the exception of liver transplantation, surgical
treatments are often ineffective, particularly in the advanced
stages of these disorders (1,2,10).
Indeed, cholestatic liver disease is best treated via liver
transplantation, accounting for 24 % of all liver transplantation
cases (10). Yet liver
transplantation is associated with several limitations, including
shortages of available donors, postoperative complications and
immunologic rejection (10–12). In this regard, current efforts are
focusing on innovative pharmacotherapy to attenuate cholestatic
liver injuries and/or inhibit the progression of these
disorders.
Certain growth factors are hepatogenic and
hepatotrophic, playing essential roles in liver development and
homeostasis. One well-studied hepatotrophic factor is hepatocyte
growth factor (HGF), a heterodimer composed of a 69-kDa α chain and
34-kDa β chain that was originally identified and cloned as a
potent mitogen for hepatocytes (13,14). Preclinical studies in animal
disease models suggest that HGF can be therapeutic for several
liver disorders, including acute hepatitis, fulminant hepatic
failure, chronic hepatitis, liver fibrosis and cirrhosis, and liver
cancer (15–22). Moreover, three independent studies
using recombinant HGF protein, naked HGF expression plasmid, or
HGF-expressing adeno-associated viral vector demonstrated that HGF
prevented and/or treated bile duct ligation (BDL)-induced
cholestatic liver injury (23–25).
On the other hand, heparin-binding epidermal growth
factor-like growth factor (HB-EGF), a new member of the epidermal
growth factor (EGF) family, is expressed in normal liver tissues,
and HB-EGF mRNA levels increase more rapidly than those of HGF
after liver injury (26,27). The unique feature of HB-EGF is
that its membrane-anchored precursor form (proHB-EGF) is initially
synthesized and subsequently cleaved at the juxtamembrane domain by
a specific metalloproteinase; the resultant soluble form (sHB-EGF)
acts on certain cell types, including hepatocytes, to induce
mitogenic and regenerative activities (28–31). In contrast to HGF, which has been
extensively studied, the potential therapeutic effects of HB-EGF
remain controversial. For instance, a study using HB-EGF-null mice
demonstrated a suppressive effect of HB-EGF in experimental liver
fibrosis (32), whereas other
studies have suggested that HB-EGF induces fibrosis (33–35). This fact, together with historical
lessons from the opposing results of HGF on hepatocarcinogenesis
using different transgenic mouse models (36,37), suggests that a direct assessment
of therapeutic potential by the administration of the HB-EGF agent
is crucial in order to come to a definitive conclusion. In
addition, simultaneous comparison of the therapeutic effects of the
HB-EGF agent to those of the well-characterized HGF agent in the
same disease model should provide a great deal of information about
the differences between these growth factors with regard to their
biologic roles and therapeutic effects. Our previous studies have
revealed that treatment of mice with HB-EGF was effective against
acute liver injury and lethal fulminant hepatic failure caused by
Fas-mediated hepatocyte apoptosis (29,30); in these models, HB-EGF produced
greater protective and mitogenic effects in hepatocytes than HGF
(29). However, the therapeutic
effects of HB-EGF in other liver disorders - particularly, its
potential inhibition of hepatocyte necrosis, liver fibrosis and
cholestasis - have not yet been elucidated.
In this study, the HB-EGF and/or HGF genes were
adenovirally transduced into the livers of mice subjected to BDL.
We assessed liver regeneration and inhibition of liver injuries,
including the apoptosis and oncosis (necrosis) of hepatocytes,
cholestasis and liver fibrosis. This study elucidates not only that
HB-EGF is therapeutic for cholestatic liver injury but also that
HB-EGF exerts potent antinecrotic (antioncotic) and moderate
antifibrotic effects in preclinical animal experiments for liver
diseases. Moreover, the effects of HB-EGF, HGF, and the combination
of the two were examined to clarify the biologic roles and
therapeutic potential of these hepatotrophic growth factors.
Materials and methods
Recombinant adenoviral vectors
Replication-defective recombinant adenoviral vectors
(Ads) under the transcriptional control of cytomegalovirus
immediate-early gene enhancer and the chicken β-actin promoter were
generated and prepared as described previously (20,29,35,38–40). Vectors included Ad.LacZ, Ad.HB-EGF
and Ad.HGF, which express β-galactosidase, human HB-EGF and human
HGF, respectively.
Animal experiments and ethics
statement
The protocol for mouse experiments is shown in
Fig. 1A. On day 0, male
5–6-week-old C57BL/6J mice (Japan Charles River Co., Yokohama,
Japan) were injected in the tail vein with 1×1011
particles of Ad.LacZ (day 3, n=9; day 14, n=15), Ad.HB-EGF (day 3,
n=11; day 14, n=13), or Ad.HGF (n=10), or a combination of
Ad.HB-EGF and Ad.HGF (2×1011 total particles; n=10). The
mice were then subjected to double ligation of the common bile duct
essentially as described previously with minor modifications
(5,41). Some animals were sacrificed by an
overdose of diethyl ether anesthesia on day 3, whereas blood was
collected from the tail veins of other animals on day 5 prior to
sacrifice on day 14 (Fig. 1A).
Liver and blood samples were collected at the time of
sacrifice.
 | Figure 1Adenoviral gene transduction and
expression in mice subjected to BDL. (A) Experimental protocol for
the following experiments. On day 0, mice received an intravenous
injection of 1 × 1011 particles of either Ad.LacZ,
Ad.HB-EGF, or Ad.HGF, or a combination of Ad.HB-EGF and Ad.HGF (2 ×
1011 particles in total). Some animals were sacrificed
(S) on day 3, whereas blood was collected (C) from other animals
under anesthesia on day 5 prior to sacrifice on day 14. Liver and
blood samples were collected at the time of sacrifice. (B) X-gal
staining of liver samples, 3 and 14 days after Ad.LacZ injection
and BDL (×200 magnification). (C) Immunohistochemical staining for
human HB-EGF in liver samples 3 and 14 days after BDL and
intravenous injection of control Ad.LacZ, Ad.HB-EGF, or a
combination of Ad.HB-EGF and Ad.HGF (×200 magnification). (D)
Western blot analysis for human HB-EGF and human HGF on days 3 and
14 after BDL and injection of control Ad.LacZ, Ad.HB-EGF, Ad.HGF,
or a combination of Ad.HB-EGF and Ad.HGF. BDL, bile duct ligation.
Ad, adenoviral vector; HB-EGF, heparin-binding epidermal growth
factor-like growth factor; HGF, hepatocyte growth factor. |
All animal studies were performed in accordance with
the National Institutes of Health guidelines and with the approval
of the Frontier Science Research Centre, Kagoshima University
(permit number: MD09013). All efforts were made to minimize
suffering.
Biochemical analysis
Serum levels of aspartate aminotransferase (AST),
alanine aminotransferase (ALT), and total bilirubin (T-Bil) were
measured using an automated analyzer (SPOTCHEM EZ SP-4430; Arkray,
Kyoto, Japan).
Histopathologic analysis
Formalin-fixed, paraffin-embedded liver tissue
samples were stained with hematoxylin (Merck KGaA, Darmstadt,
Germany) and eosin (Wako, Tokyo, Japan), or Masson's trichrome
(Trichrome Stain (Masson) kit; Sigma-Aldrich, St Louis, MO, USA).
For morphometric analysis of hepatocyte death, the areas containing
biliary infarcts (clusters of characteristic oncotic hepatocytes)
were assessed by counting 13,280 points on an image (×40,
magnification; Powered BX-41; Olympus, Tokyo, Japan) as previously
described with some modifications (42–45). To assess liver fibrosis
quantitatively, areas stained blue with Masson's trichrome were
measured using Image J software (National Institutes of Health). To
assess in vivo adenoviral gene transduction efficiency,
staining was performed using O-nitrophenyl-β-D-galactopyranoside
(X-gal) and frozen liver tissues from the mice that received
Ad.LacZ injections as described previously (20,35,44).
Immunohistochemistry was performed using antibodies
specific for goat polyclonal anti-human HB-EGF (AF-259-NA; R&D
systems, Minneapolis, MN, USA), rat monoclonal anti-mouse Ki-67
(M7249; Dako, Glostrup, Denmark) and rabbit polyclonal anti-mouse
collagen IV (ab6586; Abcam, Cambridge, UK) as described previously
(29,30,35). The immunofluorescent images were
captured using a fluorescent microscope (AxioObserver.A1; Carl
Zeiss, Oberkochen, Germany.
To detect apoptotic cells, a terminal
deoxynucleotidyl transferase mediated deoxyuridine triphosphate
biotin nick-end labeling (TUNEL) assay (ApopTag Kit; Chemicon
International, Billerica, MA, USA) was performed according to the
manufacturer's instructions.
TUNEL-positive or Ki-67-positive hepatocytes were
counted and averaged in 30 randomly selected fields at ×200,
magnification (17,29). TUNEL-positive, Ki-67-positive, and
all epithelial cells in the interlobular bile ducts were counted
and the percentages of positive cells were calculated.
Western blot analysis
Liver tissue was homogenized in lysis buffer
containing 15 mM 3-[(3-cholamidopropyl)
dimethyl-ammonio]-1-propanesulfonate, 0.15 M NaCl, 2 mM
ethylenediaminetetraacetic acid (pH 8), 1 mM phenylmethanesulfonyl
fluoride, 1 mM Na3VO4, and Tris-Cl (pH 7.5).
Western blot analysis was performed as described previously
(17,30) using primary antibodies specific
for goat polyclonal anti-human HB-EGF, goat polyclonal anti-human
HGF (AB-294-NA; R&D Systems), mouse monoclonal anti-mouse
α-smooth muscle actin (α-SMA; A2547; Sigma-Aldrich), rabbit
polyclonal anti-mouse Bcl-2 (sc-492; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA), rabbit polyclonal anti-mouse Bcl-xL
(sc-634; Santa Cruz Biotechnology, Inc.), mouse monoclonal
anti-mouse Bax (sc-7480; Santa Cruz Biotechnology, Inc.), mouse
monoclonal anti-mouse collagen I (ab88147; Abcam) or mouse
monoclonal anti-mouse α-tubulin (T6074; Sigma-Aldrich). The
densities of the bands were measured, and the signal ratios of
α-SMA/α-tubulin and collagen I/α-tubulin were calculated. For
detection of each molecule, 25 µg of protein was loaded (40
µg in the case of α-SMA). Band densities were measured using
ImageJ software.
Statistical analysis
Values are expressed as the means ± standard errors
(SE). Differences between/among groups were evaluated using the
unpaired Student's t-test for comparisons between two groups or
one-way ANOVA for multiple-group comparisons (Statmate III
software, ATMS Co., Ltd., Tokyo, Japan). P <0.05 was considered
to indicate a statistically significant difference.
Results
Adenoviral gene transduction efficiency
and expression in mice subjected to BDL
We first examined adenoviral gene transduction
efficiency and the persistence of transgene expression in mouse
livers after BDL (Fig. 1B). The
intravenous injection of 1 × 1011 particles of Ad.LacZ
resulted in close to 100% gene transduction among hepatocytes 3
days after Ad injection and BDL, whereas approximately half of the
hepatocytes showed transgene expression on day 14 (Fig. 1B). On day 3, hepatocytes from mice
treated with Ad.HB-EGF or the combination of Ad.HB-EGF and Ad.HGF
immunohistochemically showed intense membrane staining of human
HB-EGF (membrane-anchored proHB-EGF) and moderate staining
intensity in the cytoplasm (likely endocytosed sHB-EGF) (Fig. 1C). The percentages of hepatocytes
showing strong human HB-EGF expression and human HB-EGF-specific
staining in individual hepatocytes were markedly lower on day 14.
The high and low expression of human HB-EGF on days 3 and 14,
respectively, was confirmed by western blot analysis (Fig. 1D). Similarly, western blot
analysis demonstrated that the expression of human HGF was high on
day 3 and low on day 14 after BDL and injection of Ad.HGF or the
combination of Ad.HB-EGF and Ad.HGF (Fig. 1D); immunohistochemical
confirmation of this result was hampered owing to the lack of a
reliable anti-HGF antibody for immunohistochemistry. Taken
together, the results showed efficient adenoviral gene
transduction, robust expression of the transgenes (human HB-EGF and
HGF), and limited periods of (i.e., several days) transgene
expression in the BDL livers following intravenous Ad injection.
These findings are consistent with those from previous studies,
although the percentages of transduced hepatocytes and transgene
expression levels vary based on the transgenes and transcriptional
regulatory elements (i.e., promoters) in the Ads (29,44,46).
Liver enzyme levels after HB-EGF and/or
HGF gene therapy in mice subjected to BDL
By carefully examining the time course of pathologic
changes after BDL, previous studies demonstrated that hepatocyte
injury and proliferation peaked at 3 and 5 days after BDL,
respectively, and that fibrosis was fully established 14 days after
BDL (5,41). Based on these results and the
limited periods during which transgenes were expressed (Fig. 1), we selected 3, 5, and 14 days
after BDL to experimentally represent the acute, subacute, and
chronic phases, respectively.
In accordance with previous findings, AST and ALT
levels peaked 3 days after BDL and the injection of control Ad.LacZ
(Fig. 2); AST levels were 60
times higher than normal, whereas ALT levels increased 42 fold
(1260±312 IU/l). The increases in AST and ALT levels were
significantly attenuated in mice injected with Ad.HB-EGF (AST,
1586±309 IU/l and ALT, 517±93 IU/l; 2.6 and 2.4 times as low as
those in the control Ad.LacZ-treated mice, respectively). By
contrast, Ad.HGF injection mildly, but not to a statistically
significantly degree, inhibited the elevations in AST and ALT
levels on day 3. On days 5 and 14 after BDL in all treatment
groups, AST and ALT levels returned to approximately 2000 IU/l and
1000 IU/l, respectively. On day 5, no significant differences in
AST and ALT levels were observed among mice injected with control
Ad.LacZ, Ad.HB-EGF, Ad.HGF or the combination of Ad.HB-EGF and
Ad.HGF. On day 14, significant differences were observed between
either Ad.HGF or the combination versus the control Ad.LacZ in AST
levels and between Ad.HGF and the combination in ALT levels.
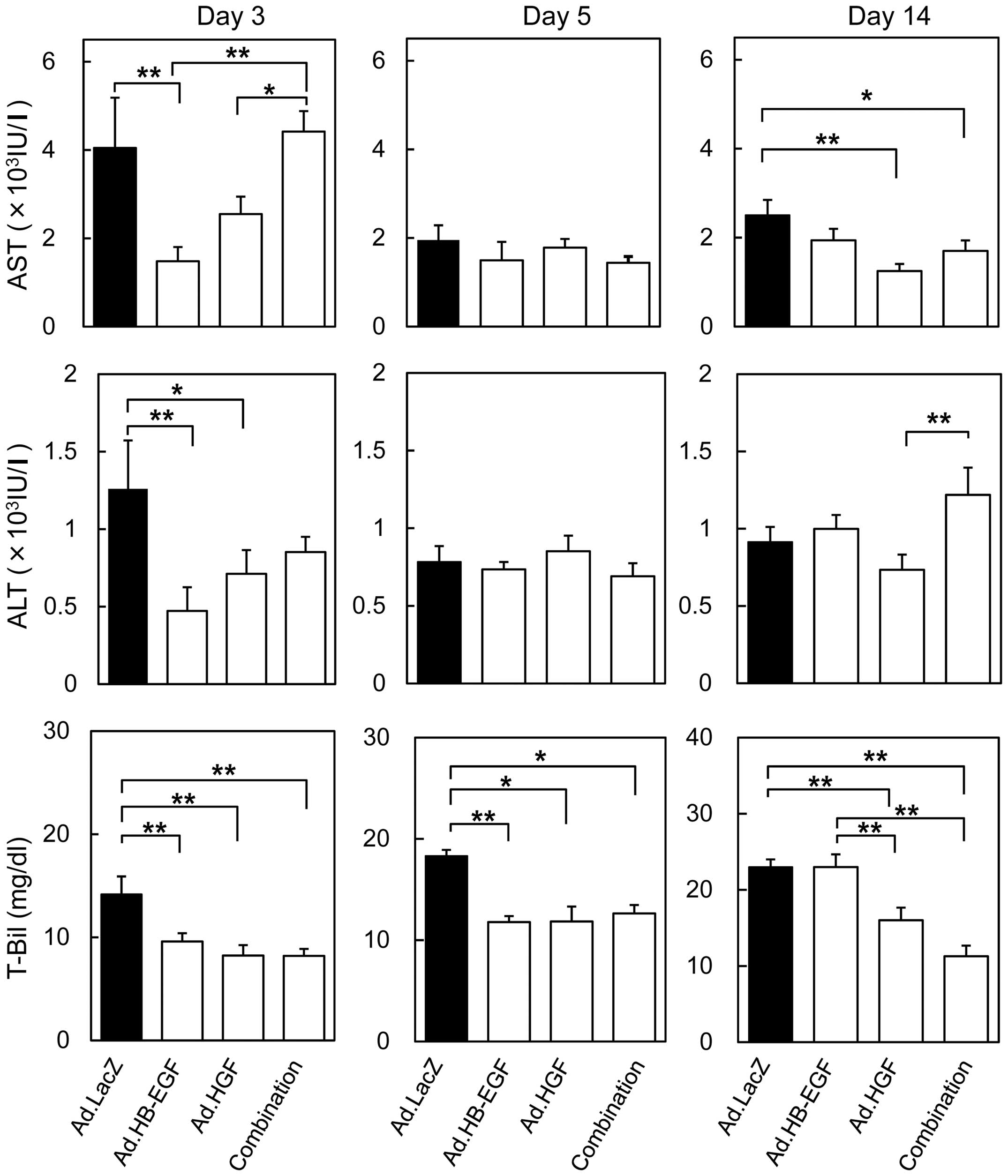 | Figure 2Serum biochemical analysis following
Ad injection and BDL. Blood was collected on days 3, 5, and 14 (see
Fig. 1A), and serum AST, ALT and
T-Bil levels were assessed. All data are expressed as the means ±
SE (*P<0.05, **P<0.01 Ad.HB-EGF, Ad.HGF, a
combination of Ad.HB-EGF and Ad.HGF and Ad.LacZ each). Ad,
adenoviral vector; BDL, bile duct ligation; AST, aspartate
aminotransferase; ALT, alanine aminotransferase; T-Bil, total
bilirubin; HB-EGF, heparin-binding epidermal growth factor-like
growth factor; HGF, hepatocyte growth factor. |
On the other hand, serum T-Bil levels in mice
treated with control Ad.LacZ progressively increased to 14.1±1.7
mg/dl, 18.3±1.4 mg/dl and 23.0±1.8 mg/dl (108, 141 and 177 times
higher than normal levels) on days 3, 5 and 14 after BDL,
respectively (Fig. 2), suggesting
that BDL-induced cholestasis worsened over time. The increases in
serum T-Bil levels were significantly attenuated on days 3 and 5 by
injection of Ad.HB-EGF, Ad.HGF or both Ad.HB-EGF and Ad.HGF. The
further increases in T-Bil observed on day 14 were inhibited by
injection of Ad.HGF alone or Ad.HB-EGF and Ad.HGF.
Thus, HB-EGF alone produced potent cytoprotective
effects during acute hepatocyte injury after BDL, and the
anticholestatic effects of HGF were stronger than those of HB-EGF
during the chronic stage. These findings suggest that, following
BDL, HB-EGF and HGF are therapeutic predominantly during the acute
and chronic phases, respectively.
Gene therapy with HB-EGF and/or HGF
potently attenuates histologic liver injuries and hepatocyte
oncosis (necrosis)
Previous studies demonstrated that the
characteristic histopathologic feature in BDL-induced liver injury
is 'biliary infarct', defined as clusters of oncotic hepatocytes;
oncosis is a characteristic type of liver cell necrosis with
cytoplasmic swelling, disruption of plasma membrane integrity and
decreased nuclear staining in response to obstructive cholestasis.
Similar to previous studies (5–7),
histopathologic and morphometric analyses of livers 3 days after
BDL in mice treated with control Ad.LacZ revealed severe liver
injuries with prominent biliary infarcts (3.9±1.2%; percentage of
infarcted areas in the parenchymal areas) - defined as clusters of
swollen hepatocytes lacking nuclear staining (oncosis) - in the
periportal and/or the midzonal areas of the liver parenchyma
(Fig. 3). In actuality, the
hepatocytes showing the typical morphology of oncosis were
TUNEL-negative on day 3 (Fig.
4A). Larger areas were affected by the biliary infarcts
(12.1±2.7%) on day 14 (Fig. 3),
when the elevated serum AST and ALT levels had somewhat abated,
whereas serum T-Bil levels had increased (Fig. 2), suggesting that cholestatic
liver injuries worsened after BDL.
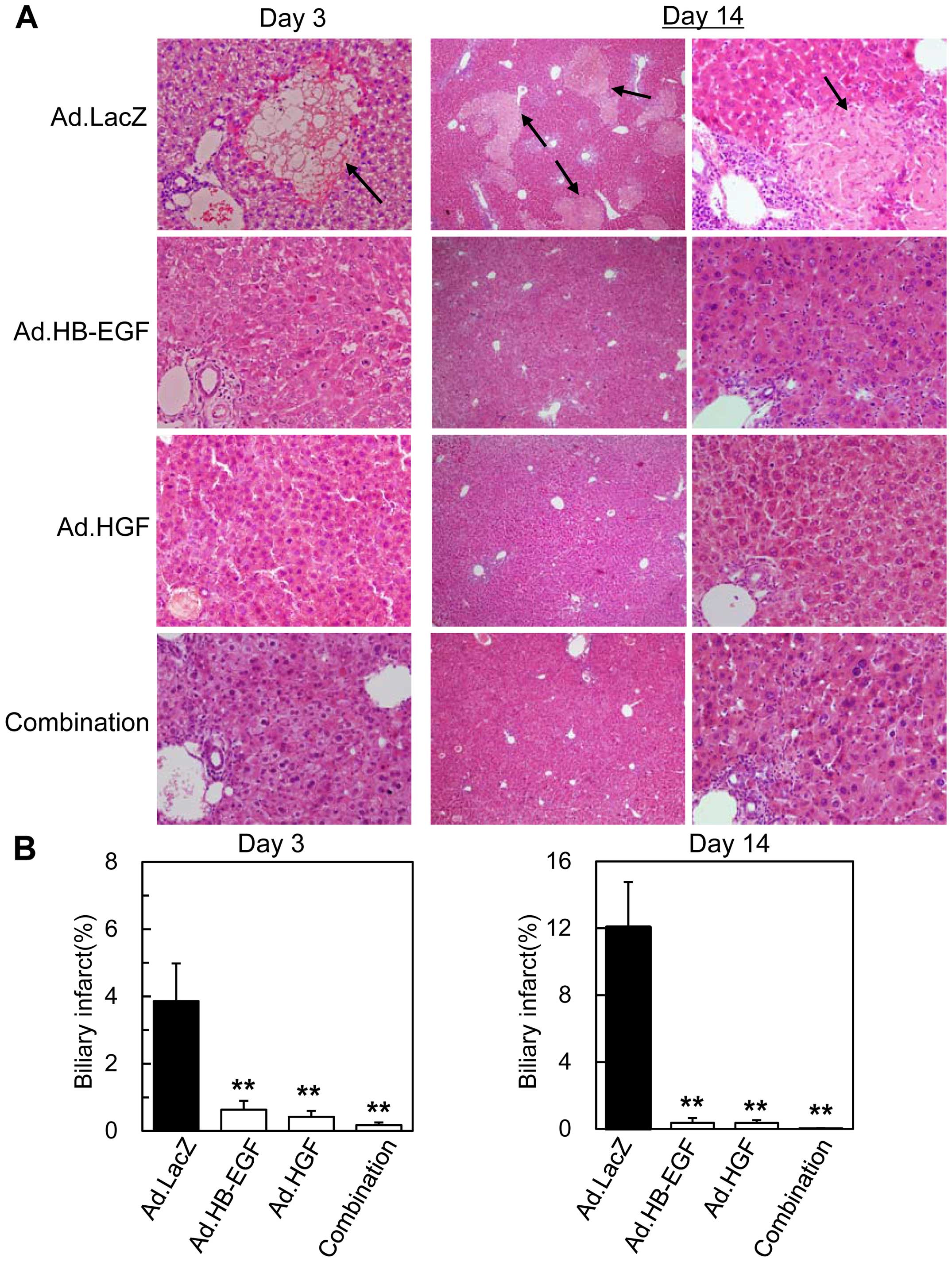 | Figure 3Liver histopathology after BDL and
gene therapy with HB-EGF and/or HGF. (A) Liver sections obtained
from mice 3 days (left panels, ×200 magnification) or 14 days
(middle and right panels, ×40 and ×200, respectively) after BDL and
injection of Ad.LacZ, Ad.HB-EGF, Ad.HGF, or the combination of
Ad.HB-EGF and Ad.HGF were stained with hematoxylin and eosin. Black
arrows indicate typical biliary infarcts. (B) Morphometric and
quantitative analyses of biliary infarcts on days 3 and 14. The
percentages of infarcted areas among the parenchymal areas were
calculated. All data are expressed as the means ± SE
(**P<0.01, Ad.HB-EGF, Ad.HGF, or a combination to the
two vs. control Ad.LacZ). No significant differences were observed
among the Ad.HB-EGF-, Ad.HGF-, and combination-treated samples.
BDL, bile duct ligation; HB-EGF, heparin-binding epidermal growth
factor-like growth factor; HGF, hepatocyte growth factor; Ad,
adenoviral vector. |
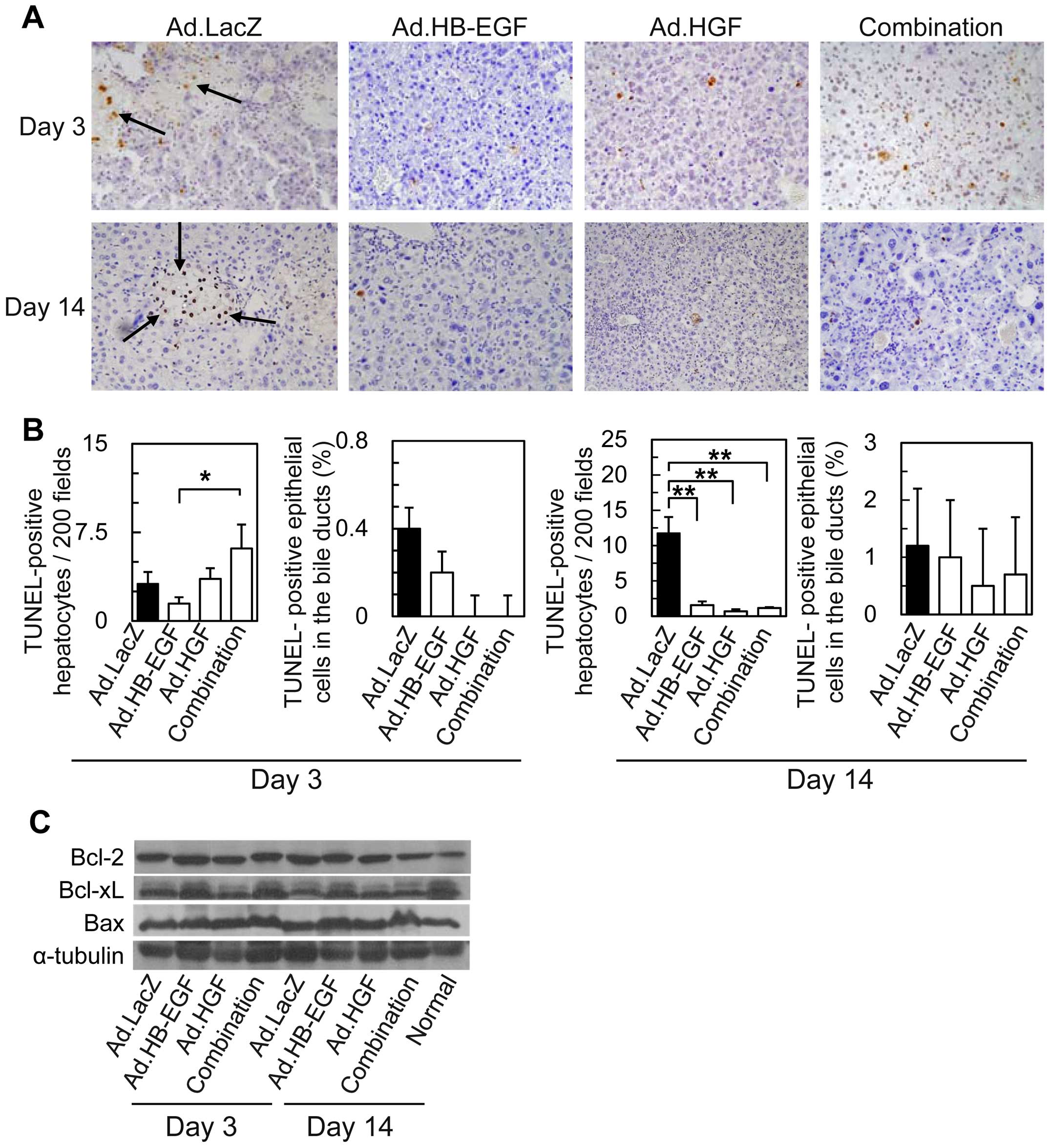 | Figure 4TUNEL staining of liver samples and
western blot analysis of Bcl/Bax-family proteins in livers after Ad
injection and BDL. (A) TUNEL staining of livers 3 and 14 days after
BDL and injection of Ad.LacZ, Ad.HB-EGF, Ad.HGF, or the combination
of Ad.HB-EGF and Ad.HGF (×200 magnification). The black arrows
indicate TUNEL-positive cells. (B) Morphometric and quantitative
analyses of TUNEL-positive hepatocytes and epithelial cells of the
interlobular bile ducts. All data are expressed as the means ± SE
(*P<0.05, **P<0.01 Ad.HB-EGF, Ad.HGF, a
combination of Ad.HB-EGF and Ad.HGF and Ad.LacZ each). No
significant differences in the numbers of TUNEL-positive
hepatocytes or epithelial cells in bile ducts were noted among
samples treated with Ad.HB-EGF, Ad.HGF, or the combination of
Ad.HB-EGF and Ad.HGF. (C) Western blot analysis of Bcl-2, Bcl-xL,
Bax, and α-tubulin (control) in mouse liver samples 3 and 14 days
after BDL and Ad injection. TUNEL, terminal deoxynucleotidyl
transferase mediated deoxyuridine triphosphate biotin nick-end
labeling; Ad, adenoviral vector; BDL, bile duct ligation; HB-EGF,
heparin-binding epidermal growth factor-like growth factor; HGF,
hepatocyte growth factor. |
By contrast, 3 and 14 days after BDL, minimal
histopathologic findings were observed in the livers of mice
treated with Ad.HB-EGF, Ad.HGF, or both Ads, including few biliary
infarcts (on day 3, 0.6±0.3%, 0.4±0.2%, and 0.2±0.1%, respectively;
on day 14, 0.4±0.3%, 0.4±0.2% and 0.04±0.02%, respectively). Thus,
HB-EGF and HGF potently inhibited BDL-induced liver injury based on
histopathologic analyses, which showed effects that were greater
than those observed using serum levels of liver enzymes; the same
tendency was observed in previous studies that examined these
growth factors in the treatment of acute liver injuries (17,18,29,30). More importantly, the data suggest
that HB-EGF and HGF are potently antioncotic (antinecrotic) for
hepatocytes.
Antiapoptotic effects of gene therapy
with HB-EGF and/or HGF in BDL-induced liver injury
To examine whether HB-EGF and HGF prevent
BDL-induced apoptosis of hepatocytes, apoptotic cells were detected
using in situ TUNEL assays (Fig. 4A) and morphometrically analyzed
(Fig. 4B). Previous studies
demonstrated that hepatocytes primarily die during acute
cholestasis via oncosis, although the apoptosis of hepatocytes is
also involved in BDL-induced liver injury (5,7–9).
Correspondingly, the majority of hepatocytes in biliary infarcts
from control Ad.LacZ-treated mice were TUNEL-negative, and few
hepatocytes (3.2±1.0 cells in 200 fields) around the periphery of
the biliary infarcts were TUNEL-positive 3 days after BDL (Fig. 4A and B). On day 14, the number of
TUNEL-positive hepatocytes increased to 3.7 times as many as that
observed on day 3 (Fig. 4B).
Ad.HB-EGF slightly reduced the number of
TUNEL-positive hepatocytes 3 days after BDL, although no
statistically significant difference was noted among the groups
(Fig. 4B). Fourteen days after
BDL, significantly (around 10-fold) fewer TUNEL-positive
hepatocytes were detected in the livers of mice treated with
Ad.HB-EGF, Ad.HGF, or a combination of Ad.HB-EGF and Ad.HGF
compared with Ad.LacZ-treated mice; differences among the three
treatment groups were not statistically significant.
On the other hand, in the mice treated with control
Ad.LacZ, <1% of epithelial cells in the interlobular bile ducts
were TUNEL-positive 3 days after BDL, with a slight increase to
1.2% after 14 days (Fig. 4B). The
treatments appeared to slightly reduce the percentage of
TUNEL-positive epithelial cells in the bile ducts, although no
statistically significant difference was noted among the
groups.
These data clearly indicate that HB-EGF and HGF
exert antiapoptotic and antioncotic effects on hepatocytes, even
though the expression of representative Bcl-2/Bax-family proteins -
Bcl-2, Bcl-xL and Bax - were not markedly affected by any of the
treatment approaches (Fig.
4C).
More potent inhibitory effects of HGF
than HB-EGF against liver fibrosis
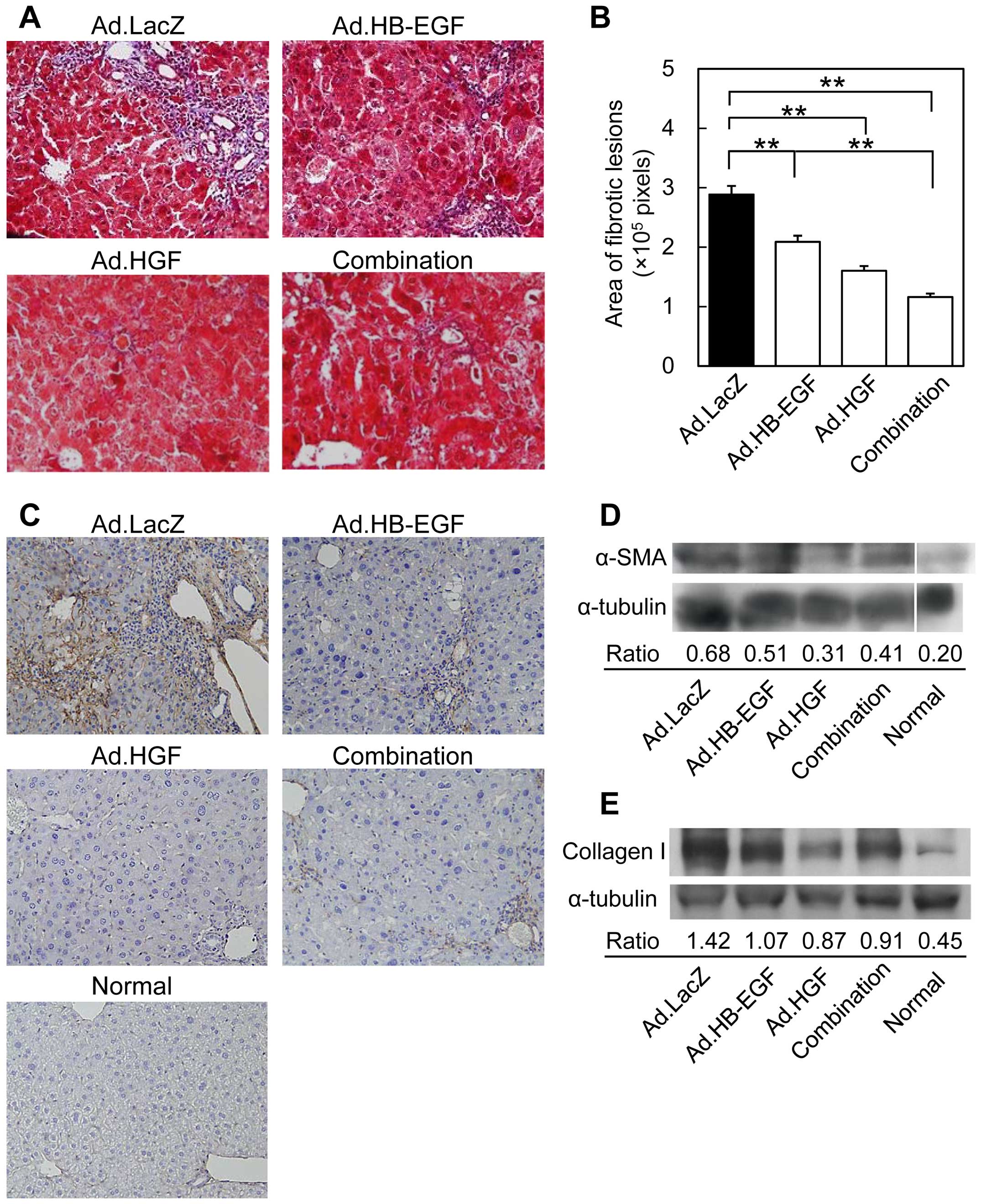
To examine the inhibitory effects of HB-EGF and HGF
on liver fibrosis, collagen fibers in the livers of mice 14 days
after BDL were stained with Masson's trichrome, and the fibrous
areas were quantified. In accordance with previous findings
(5), Ad.LacZ-treated control mice
showed severe fibrosis, including bridging by connective tissue
that linked different portal areas (Fig. 5A & B). Ad.HB-EGF treatment
resulted in significantly smaller fibrous areas with fainter and
thinner fibrous tissues. On the other hand, treatment with either
Ad.HGF or the combination of Ad.HB-EGF and Ad.HGF resulted in
larger decreases in the areas affected by fibrous tissues. This
result was supported by immunohistochemical staining of collagen
IV, which forms a basement membrane-like structure in the space of
Disse (Fig. 5C) (47).
To analyze activated hepatic stellate cells
(5,48), α-SMA protein levels were examined
by western blot analysis of liver samples obtained on day 14
(Fig. 5D). The expression of
α-SMA increased following BDL in Ad.LacZ-treated mice compared with
that in normal mice. Although α-SMA protein levels were slightly
lower in Ad.HB-EGF-treated mice, they were markedly attenuated by
Ad.HGF and the combination of Ad.HGF and Ad.HB-EGF. The same
tendency was noted for collagen I protein expression, increases of
which are a cardinal feature of liver fibrosis with elevated
deposition observed as hepatic stellate cells are activated to
become α-SMA-positive myofibroblasts (49). Taken together, these results
indicate that, compared with HB-EGF, HGF more potently inhibits
liver fibrosis.
HB-EGF and HGF additively induce liver
regeneration
We assessed the effects of HB-EGF, HGF and a
combination of the two growth factors on liver regeneration
following BDL by examining the number of Ki-67-positive cells
(Fig. 6). The numbers of
Ki-67-positive, regenerating hepatocytes 3 days after BDL
significantly increased by 2.1-, 2.4-, and 3.4-fold following
treatment with Ad.HB-EGF, Ad.HGF, and the combination,
respectively, compared with control Ad.LacZ; no significant
differences were noted among the three treatment groups. On the
other hand, the number of Ki-67-positive hepatocytes on day 14 in
control Ad.LacZ-treated mice was reduced by >90% in comparison
with the results observed on day 3. Although no significant
differences were noted among the groups treated with Ad.LacZ,
Ad.HB-EGF, or Ad.HGF, the combination of Ad.HB-EGF and Ad.HGF
significantly (2.6-fold) enhanced liver regeneration during the
chronic phase after BDL compared with control Ad.LacZ. By contrast,
no significant differences were noted among the groups in the
percentages of Ki-67-positive epithelial cells in the interlobular
bile ducts on day 3 or 14. These results suggest that HB-EGF and
HGF preferentially induce hepatocyte regeneration after BDL, and
that combination therapy additively increases and sustains the
regenerative activities of these growth factors.
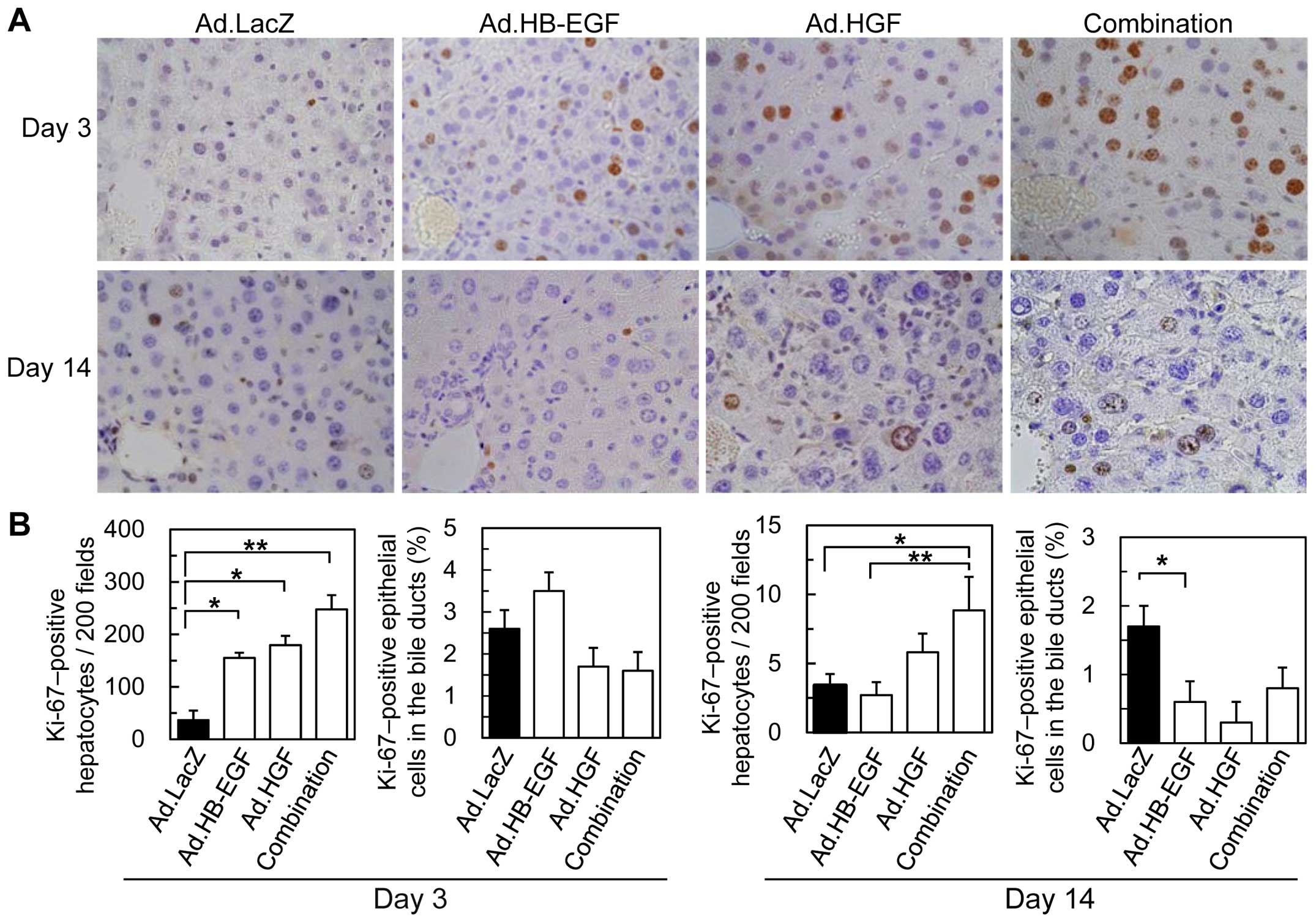 | Figure 6Immunohistochemical staining of Ki-67
in livers following BDL and Ad injections. (A) Representative
images of Ki-67 staining 3 and 14 days following BDL and injection
of Ad.LacZ, Ad.HB-EGF, Ad.HGF, or a combination of Ad.HB-EGF and
Ad.HGF. (B) Morphometric and quantitative analyses of Ki67-positive
cells. All data are expressed as the means ± SE
(*P<0.05, **P<0.01 Ad.HB-EGF, Ad.HGF, a
combination of Ad.HB-EGF and Ad.HGF and Ad.LacZ). BDL, bile duct
ligation; Ad, adenoviral vector; HB-EGF, heparin-binding epidermal
growth factor-like growth factor; HGF, hepatocyte growth
factor. |
Discussion
In this study, we, for the first time to the best of
our knowledge, examined the therapeutic effects of HB-EGF in
cholestatic liver injuries, and compared the benefits to those
associated with HGF. Notably, the protective effects of HB-EGF
against acute liver injury - assessed based on serum transaminase
levels - were greater than those of HGF, which is consistent with
previous findings regarding Fas-induced acute liver injury
(29). Unlike HGF, however, the
longer term anticholestatic and antifibrotic effects of HB-EGF
after BDL were moderate. These differences may reflect differences
in the biologic functions of these growth factors in the liver, an
idea that is supported by the different phenotypes observed in HGF
and HB-EGF null mice (50–52).
Our results also suggest that there is a time period during which
the effects of the growth factors for liver injury are most
pronounced. In fact, a previous study has shown that endogenous
HB-EGF mRNA levels increase more rapidly than those of endogenous
HGF mRNA during liver regeneration after partial hepatectomy
(53). In addition, we have
revealed that HB-EGF gene therapy is more protective and mitogenic
than HGF gene therapy for Fas-induced acute liver injury (29). Taken together, our data suggest
that HB-EGF and HGF have overlapping functions, including
antiapoptotic, antioncotic (antinecrotic), anticholestatic and
mitogenic activities in hepatocytes, whereas these growth factors
predominantly are acutely cytoprotective and chronically
antifibrotic, respectively.
In response to BDL-induced cholestatic liver injury,
the primary target cells for these growth factors may be
hepatocytes, because the cytoprotective and regenerative activities
were prominent in hepatocytes, whereas effects in the interlobular
bile ducts were not apparent. Taken together with the results of
previous studies (17,18,29,30), the results of this study suggest
that HGF and HB-EGF may protect hepatocytes against a range of
hepatotoxic stimuli, including the bile salt-induced toxicity
applied in this model (5,6). Other anti-cholestatic mechanisms,
including upregulation of basolateral bile acid/bilirubin
transporters and kidney excretion of bile salts/bilirubin, should
be carefully examined in future studies.
Furthermore, this study revealed, for the first time
to the best of our knowledge, that HB-EGF exerts potent antioncotic
(antinecrotic) effects on hepatocytes in vivo. Notably, the
expression of Bcl/Bax-family proteins, which were altered by HGF or
HB-EGF in previous studies to reduce Fas-induced hepatocyte
apoptosis (17,18,29,30), were not markedly affected in this
study. This likely reflects a smaller role for apoptosis relative
to oncosis in BDL-induced cholestatic liver injury, and suggests
that different cytoprotective mechanisms are responsible for
antiapoptotic and antioncotic (antinecrotic) effects. However,
further elucidation of the underlying molecular mechanism is
currently hampered by the paucity of overall molecular information
regarding oncotic processes.
As mentioned earlier, previous studies have yielded
controversial results regarding whether HB-EGF inhibits or
stimulates liver fibrosis in vivo, as well as the relative
antifibrotic effect of HB-EGF in comparison to that of HGF
(32–35). In this study, we showed that
HB-EGF exerts moderate but significant antifibrotic effects.
Sequential events that result in liver fibrosis have been
previously reported (5,49); during hepatic injury, hepatic
stellate cells are activated to become α-SMA-positive
myofibroblasts and produce collagen matrix, including predominantly
collagen I (5,49). Notably, the examination of areas
of liver fibrosis, deposition of collagen IV (another collagen
associated with liver fibrosis), and α-SMA and collagen I protein
expression demonstrated that HB-EGF and HGF exerted moderate and
strong antifibrotic effects, respectively. The moderate but
significant antifibrotic effects of HB-EGF may be clinically
meaningful because the induction of fibrosis by a cytoprotective
agent may diminish its therapeutic utility for chronic liver
diseases.
Clinically, these results suggest that HB-EGF or HGF
may be effective for cholestatic liver injury. Moreover,
combination therapy may be more beneficial based on the greater
anticholestatic and regenerative activities observed in hepatocytes
during the chronic phase. This study used an in vivo
adenoviral gene transduction strategy to investigate whether HB-EGF
has the potential to be used as a therapeutic agent for diverse
hepatic disorders, particularly those with complex pathogenesis,
including hepatocyte necrosis, liver fibrosis, and cholestatic
liver injuries, as well as the differences in the therapeutic
actions and pathophysiologic roles of HB-EGF and HGF (20,29,35,40,54,55). Future preclinical studies should
examine long-term effectiveness and optimize pharmacotherapeutic
protocols for each liver disease, including the timing of growth
factor administration, the effects of gene vs. protein therapy,
clinically appropriate vectors, and any treatment-related adverse
effects, including the potential for hepatocarcinogenesis.
Nevertheless, the dearth of pharmacologic agents that effectively
inhibit cholestatic liver injuries suggests that HB-EGF and/or HGF
should be developed as clinical therapies.
In summary, this study revealed that HB-EGF as well
as HGF inhibited BDL-induced cholestatic liver injury by
predominantly exerting acute cytoprotective and chronic
antifibrotic effects, respectively. Moreover, combining the growth
factors enhanced the anticholestatic effects and liver regeneration
during the chronic phase. These results provide a foundation for
novel pharmacotherapeutic approaches using HB-EGF and HGF, and aid
in elucidating the pathophysiologic roles of these hepatotrophic
growth factors.
Acknowledgments
We are grateful to Sayaka Yamashita and Eriko Kishi
for technical assistance in preparing the adenoviral vectors. K.
Kosai is the founder of WyK BiotechPharma Inc., but does not earn a
salary from the company.
Abbreviations:
|
HGF
|
hepatocyte growth factor
|
|
HB-EGF
|
heparin-binding epidermal growth
factor-like growth factor
|
|
Ads
|
adenoviral vectors
|
|
AST
|
aspartate aminotransferase
|
|
ALT
|
alanine aminotransferase
|
|
T-Bil
|
total bilirubin
|
|
TUNEL
|
terminal deoxynucleotidyl
transferase-mediated deoxyuridine triphosphate nick-end
labeling
|
|
α-SMA
|
α-smooth muscle actin
|
References
|
1
|
Heathcote EJ: Diagnosis and management of
cholestatic liver disease. Clin Gastroenterol Hepatol. 5:776–782.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
O'Leary JG and Pratt DS: Cholestasis and
cholestatic syndromes. Curr Opin Gastroenterol. 23:232–236. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kimura A, Yuge K, Kosai KI, Kage M,
Fujisawa T, Inoue T, Yamashita Y, Nakashima E and Kato H: Neonatal
cholestasis in two siblings: a variant of Dubin-Johnson syndrome? J
Paediatr Child Health. 31:557–560. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Trauner M, Fickert P, Halilbasic E and
Moustafa T: Lessons from the toxic bile concept for the
pathogenesis and treatment of cholestatic liver diseases. Wien Med
Wochenschr. 158:542–548. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Georgiev P, Jochum W, Heinrich S, Jang JH,
Nocito A, Dahm F and Clavien PA: Characterization of time-related
changes after experimental bile duct ligation. Br J Surg.
95:646–656. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fickert P, Trauner M, Fuchsbichler A,
Zollner G, Wagner M, Marschall HU, Zatloukal K and Denk H: Oncosis
represents the main type of cell death in mouse models of
cholestasis. J Hepatol. 42:378–385. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Majno G and Joris I: Apoptosis, oncosis,
and necrosis. An overview of cell death. Am J Pathol. 146:3–15.
1995.PubMed/NCBI
|
|
8
|
Canbay A, Higuchi H, Bronk SF, Taniai M,
Sebo TJ and Gores GJ: Fas enhances fibrogenesis in the bile duct
ligated mouse: a link between apoptosis and fibrosis.
Gastroenterology. 123:1323–1330. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miyoshi H, Rust C, Roberts PJ, Burgart LJ
and Gores GJ: Hepatocyte apoptosis after bile duct ligation in the
mouse involves Fas. Gastroenterology. 117:669–677. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Patkowski W, Skalski M, Zieniewicz K,
Nyckowski P, Smoter P and Krawczyk M: Orthotopic liver
transplantation for cholestatic diseases. Hepatogastroenterology.
57:605–610. 2010.PubMed/NCBI
|
|
11
|
O'Leary JG, Lepe R and Davis GL:
Indications for liver transplantation. Gastroenterology.
134:1764–1776. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Esquivel CO: Liver transplantation: Where
we are and where we are heading. Transplant Proc. 42:610–612. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miyazawa K, Tsubouchi H, Naka D, Takahashi
K, Okigaki M, Arakaki N, Nakayama H, Hirono S, Sakiyama O,
Takahashi K, et al: Molecular cloning and sequence analysis of cDNA
for human hepatocyte growth factor. Biochem Biophys Res Commun.
163:967–973. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakamura T, Nishizawa T, Hagiya M, Seki T,
Shimonishi M, Sugimura A, Tashiro K and Shimizu S: Molecular
cloning and expression of human hepatocyte growth factor. Nature.
342:440–443. 1989. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakamura T, Sakai K, Nakamura T and
Matsumoto K: Hepatocyte growth factor twenty years on: much more
than a growth factor. J Gastroenterol Hepatol. 26(Suppl 1):
188–202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ueki T, Kaneda Y, Tsutsui H, Nakanishi K,
Sawa Y, Morishita R, Matsumoto K, Nakamura T, Takahashi H, Okamoto
E and Fujimoto J: Hepatocyte growth factor gene therapy of liver
cirrhosis in rats. Nat Med. 5:226–230. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kosai K, Matsumoto K, Funakoshi H and
Nakamura T: Hepatocyte growth factor prevents endotoxin-induced
lethal hepatic failure in mice. Hepatology. 30:151–159. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kosai K, Matsumoto K, Nagata S, Tsujimoto
Y and Nakamura T: Abrogation of Fas-induced fulminant hepatic
failure in mice by hepatocyte growth factor. Biochem Biophys Res
Commun. 244:683–690. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kosai KI, Finegold MJ, Thi-Huynh BT,
Tewson M, Ou CN, Bowles N, Woo SL, Schwall RH and Darlington GJ:
Retrovirus-mediated in vivo gene transfer in the replicating liver
using recombinant hepatocyte growth factor without liver injury or
partial hepatectomy. Hum Gene Ther. 9:1293–1301. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yuge K, Takahashi T, Nagano S, Terazaki Y,
Murofushi Y, Ushikoshi H, Kawai T, Khai NC, Nakamura T, Fujiwara H
and Kosai K: Adenoviral gene transduction of hepatocyte growth
factor elicits inhibitory effects for hepatoma. Int J Oncol.
27:77–85. 2005.PubMed/NCBI
|
|
21
|
Ido A, Moriuchi A, Kim I, Numata M,
Nagata-Tsubouchi Y, Hasuike S, Uto H and Tsubouchi H:
Pharmacokinetic study of recombinant human hepatocyte growth factor
administered in a bolus intravenously or via portal vein. Hepatol
Res. 30:175–181. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ido A and Tsubouchi H: Translational
research to identify clinical applications of hepatocyte growth
factor. Hepatol Res. 39:739–747. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Z, Mizuno S and Nakamura T:
Antinecrotic and antiapoptotic effects of hepatocyte growth factor
on cholestatic hepatitis in a mouse model of bile-obstructive
diseases. Am J Physiol Gastrointest Liver Physiol. 292:G639–G646.
2007. View Article : Google Scholar
|
|
24
|
Suzumura K, Hirano T, Son G, Iimuro Y,
Mizukami H, Ozawa K and Fujimoto J: Adeno-associated virus
vector-mediated production of hepatocyte growth factor attenuates
liver fibrosis in mice. Hepatol Int. 2:80–88. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xia JL, Dai C, Michalopoulos GK and Liu Y:
Hepatocyte growth factor attenuates liver fibrosis induced by bile
duct ligation. Am J Pathol. 168:1500–1512. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kiso S, Kawata S, Tamura S, Higashiyama S,
Ito N, Tsushima H, Taniguchi N and Matsuzawa Y: Role of
heparin-binding epidermal growth factor-like growth factor as a
hepatotrophic factor in rat liver regeneration after partial
hepatectomy. Hepatology. 22:1584–1590. 1995.PubMed/NCBI
|
|
27
|
Higashiyama S, Abraham JA, Miller J,
Fiddes JC and Klagsbrun M: A heparin-binding growth factor secreted
by macrophage-like cells that is related to EGF. Science.
251:936–939. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kiso S, Kawata S, Tamura S, Umeki S, Ito
N, Tsushima H, Yamada A, Miyagawa J, Higashiyama S, Taniguchi N and
Matsuzawa Y: Effects of exogenous human heparin-binding epidermal
growth factor-like growth factor on DNA synthesis of hepatocytes in
normal mouse liver. Biochem Biophys Res Commun. 259:683–687. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Khai NC, Takahashi T, Ushikoshi H, Nagano
S, Yuge K, Esaki M, Kawai T, Goto K, Murofushi Y, Fujiwara T, et
al: In vivo hepatic HB-EGF gene transduction inhibits Fas-induced
liver injury and induces liver regeneration in mice: a comparative
study to HGF. J Hepatol. 44:1046–1054. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Khai NC, Sakamoto K, Takamatsu H,
Matsufuji H and Kosai K: Recombinant soluble form of
heparin-binding epidermal growth factor-like growth factor protein
therapy drastically inhibits Fas-mediated fulminant hepatic
failure: implications in clinical application. Hepatol Res.
41:594–596. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Higashiyama S, Iwabuki H, Morimoto C,
Hieda M, Inoue H and Matsushita N: Membrane-anchored growth
factors, the epidermal growth factor family: beyond receptor
ligands. Cancer Sci. 99:214–220. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang G, Besner GE and Brigstock DR:
Heparin-binding epidermal growth factor-like growth factor
suppresses experimental liver fibrosis in mice. Lab Invest.
92:703–712. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Peifley KA, Alberts GF, Hsu DK, Feng SL
and Winkles JA: Heparin-binding epidermal growth factor-like growth
factor regulates fibroblast growth factor-2 expression in aortic
smooth muscle cells. Circ Res. 79:263–270. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang D, Zhang J, Jiang X, Li X, Wang Y,
Ma J and Jiang H: Heparin-binding epidermal growth factor-like
growth factor: a hepatic stellate cell proliferation inducer via
ErbB receptors. J Gastroenterol Hepatol. 29:623–632. 2014.
View Article : Google Scholar
|
|
35
|
Ushikoshi H, Takahashi T, Chen X, Khai NC,
Esaki M, Goto K, Takemura G, Maruyama R, Minatoguchi S, Fujiwara T,
et al: Local overexpression of HB-EGF exacerbates remodeling
following myocardial infarction by activating noncardiomyocytes.
Lab Invest. 85:862–873. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shiota G, Wang TC, Nakamura T and Schmidt
EV: Hepatocyte growth factor in transgenic mice: effects on
hepatocyte growth, liver regeneration and gene expression.
Hepatology. 19:962–972. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Takayama H, LaRochelle WJ, Sharp R, Otsuka
T, Kriebel P, Anver M, Aaronson SA and Merlino G: Diverse
tumorigenesis associated with aberrant development in mice
overexpressing hepatocyte growth factor/scatter factor. Proc Natl
Acad Sci USA. 94:701–706. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Takahashi T, Kawai T, Ushikoshi H, Nagano
S, Oshika H, Inoue M, Kunisada T, Takemura G, Fujiwara H and Kosai
K: Identification and isolation of embryonic stem cell-derived
target cells by adenoviral conditional targeting. Mol Ther.
14:673–683. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen XH, Minatoguchi S, Kosai K, Yuge K,
Takahashi T, Arai M, Wang N, Misao Y, Lu C, Onogi H, et al: In vivo
hepatocyte growth factor gene transfer reduces myocardial
ischemia-reperfusion injury through its multiple actions. J Card
Fail. 13:874–883. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yuge K, Takahashi T, Khai NC, Goto K,
Fujiwara T, Fujiwara H and Kosai K: Intramuscular injection of
adenoviral hepatocyte growth factor at a distal site ameliorates
dextran sodium sulfate-induced colitis in mice. Int J Mol Med.
33:1064–1074. 2014.PubMed/NCBI
|
|
41
|
Kountouras J, Billing BH and Scheuer PJ:
Prolonged bile duct obstruction: a new experimental model for
cirrhosis in the rat. Br J Exp Pathol. 65:305–311. 1984.PubMed/NCBI
|
|
42
|
Chen SH, Chen XH, Wang Y, Kosai K,
Finegold MJ, Rich SS and Woo SL: Combination gene therapy for liver
metastasis of colon carcinoma in vivo. Proc Natl Acad Sci USA.
92:2577–2581. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen SH, Kosai K, Xu B, Pham-Nguyen K,
Contant C, Finegold MJ and Woo SL: Combination suicide and cytokine
gene therapy for hepatic metastases of colon carcinoma: sustained
antitumor immunity prolongs animal survival. Cancer Res.
56:3758–3762. 1996.PubMed/NCBI
|
|
44
|
Terazaki Y, Yano S, Yuge K, Nagano S,
Fukunaga M, Guo ZS, Komiya S, Shirouzu K and Kosai K: An optimal
therapeutic expression level is crucial for suicide gene therapy
for hepatic metastatic cancer in mice. Hepatology. 37:155–163.
2003. View Article : Google Scholar
|
|
45
|
Caruso M, Pham-Nguyen K, Kwong YL, Xu B,
Kosai KI, Finegold M, Woo SL and Chen SH: Adenovirus-mediated
interleukin-12 gene therapy for metastatic colon carcinoma. Proc
Natl Acad Sci USA. 93:11302–11306. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Peeters MJ, Patijn GA, Lieber A, Meuse L
and Kay MA: Adenovirus-mediated hepatic gene transfer in mice:
Comparison of intravascular and biliary administration. Hum Gene
Ther. 7:1693–1699. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Veidal SS, Karsdal MA, Nawrocki A, Larsen
MR, Dai Y, Zheng Q, Hägglund P, Vainer B, Skjøt-Arkil H and Leeming
DJ: Assessment of proteolytic degradation of the basement membrane:
a fragment of type IV collagen as a biochemical marker for liver
fibrosis. Fibrogenesis Tissue Repair. 4:222011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hamaoka M, Chinen I, Murata T, Takashima
S, Iwamoto R and Mekada E: Anti-human HB-EGF monoclonal antibodies
inhibiting ectodomain shedding of HB-EGF and diphtheria toxin
binding. J Biochem. 148:55–69. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tsukada S, Parsons CJ and Rippe RA:
Mechanisms of liver fibrosis. Clin Chim Acta. 364:33–60. 2006.
View Article : Google Scholar
|
|
50
|
Uehara Y, Minowa O, Mori C, Shiota K, Kuno
J, Noda T and Kitamura N: Placental defect and embryonic lethality
in mice lacking hepatocyte growth factor/scatter factor. Nature.
373:702–705. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Schmidt C, Bladt F, Goedecke S, Brinkmann
V, Zschiesche W, Sharpe M, Gherardi E and Birchmeier C: Scatter
factor/hepatocyte growth factor is essential for liver development.
Nature. 373:699–702. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Iwamoto R, Yamazaki S, Asakura M,
Takashima S, Hasuwa H, Miyado K, Adachi S, Kitakaze M, Hashimoto K,
Raab G, et al: Heparin-binding EGF-like growth factor and ErbB
signaling is essential for heart function. Proc Natl Acad Sci USA.
100:3221–3226. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kiso S, Kawata S, Tamura S, Higashiyama S,
Ito N, Tsushima H, Taniguchi N and Matsuzawa Y: Role of
heparin-binding epidermal growth factor-like growth factor as a
hepatotrophic factor in rat liver regeneration after partial
hepatectomy. Hepatology. 22:1584–1590. 1995.PubMed/NCBI
|
|
54
|
Esaki M, Takemura G, Kosai K, Takahashi T,
Miyata S, Li L, Goto K, Maruyama R, Okada H, Kanamori H, et al:
Treatment with an adenoviral vector encoding hepatocyte growth
factor mitigates established cardiac dysfunction in
doxorubicin-induced cardiomyopathy. Am J Physiol Heart Circ
Physiol. 294:H1048–H1057. 2008. View Article : Google Scholar
|
|
55
|
Li Y, Takemura G, Kosai K, Yuge K, Nagano
S, Esaki M, Goto K, Takahashi T, Hayakawa K, Koda M, et al:
Postinfarction treatment with an adenoviral vector expressing
hepatocyte growth factor relieves chronic left ventricular
remodeling and dysfunction in mice. Circulation. 107:2499–2506.
2003. View Article : Google Scholar : PubMed/NCBI
|