Introduction
A number of factors have been implicated in the
development of osteoarthritis (OA), such as age, obesity, trauma
and genetics. However, the pathogenesis of OA remains poorly
understood. Mild OA frequently manifests with symptoms of
inflammation, severe or chronic pain, edema and stiffness. In
severe cases, patients are unable to move due to joint deformity.
Idiopathic and secondary OA causes great suffering and severely
affects the quality of life of patients (1). The study of OA has led to the
realization that the pathological changes occurring in OA mainly
include the degeneration of articular cartilage, subchondral bone
destruction and synovial tissue hyperplasia.
Arthritis is usually characterized by dysfunctions
in cytokines, immune factors and neurotransmitters (2). Cytokines, such as interleukin
(IL)-1β are key inducers of OA, which involves the excessive
production of catabolic enzymes and leads to the metabolic
imbalance of chondrocytes. These catabolic enzymes abnormally
destroy the matrix and physiological function of articular
cartilage (3–5). It has been shown that that
chondrocytes obtained from patients with OA actively produce
prostaglandins (PGs), tumor necrosis factor-α (TNF-α), IL-1β and
IL-6 (6). The synovial membrane
also produces inflammatory cytokines, which diffuse into the
cartilage and manifest structural alterations associated with the
development and progression of OA (7). In joint inflammation, prostaglandin
E2 (PGE2), one of the PG subtypes,
degenerates cartilage tissue via matrix metalloproteinases (MMPs)
(8); MMP-13 is specifically
expressed in cartilage tissues from patients with OA (9). Additionally, substance P (SP), an
important neuropeptide, plays an important role in the regulation
of arthritis-induced pain and inflammation (10).
At present, the treatment of OA includes pain
control, treatment with anti-inflammtory agents and the
re-establishment of joint mobility. Non-steroidal anti-inflammatory
drugs (NSAIDs) are widely used in the treatment of OA. However,
researchers have found that NSAIDs often lead to a greater risk of
complications. Other medications, such as glucosamine
hydrochloride, chondroitin sulfate and hyaluronic acid, are able to
increase joint lubrication and joint activity, although their
effects are not convincing. The exploration of potential analgesic
and anti-inflammatory drugs has gradually emerged as a new focus of
research in OA.
As is well known, the purinergic membrane receptor
super-family may be divided into the P1 and P2 receptor families.
The P2 family, which can be further divided into the P2X and P2Y
subtypes, is closely related to OA-induced pain (11,12). Importantly, a previous study
reported that P2 receptor signaling is altered in OA, and that it
is associated with an elevated ATP level (12). P2X (P2X1–8) are
ligand-gated ion channel receptors, whereas P2Y are G-protein
coupled receptors. The expression of several P2X members has been
identified in cartilage tissues, including P2X2,
P2X3, P2X4 and P2X7 (13,14). P2X receptors have also been
identified in peripheral glial cells and are known to play a role
in neuropathic pain. It has been demonstrated that P2X7
receptor (P2X7R) is involved in regulating inflammation
and pain (15). Moreover,
P2X7R upregulation in macrophages and P2X7R
upregulation in spinal cord following peripheral nerve damage has
been reported (16,17). Pain sensitivity has also been
linked to P2X7R gene polymorphisms in women with
post-mastectomy pain syndrome (PMPS) and OA (11,18,19). Evidence suggests that
P2X7R is an important therapeutic target in the
treatment of rheumatoid arthritis (RA) (20,21). Although accumulating evidence has
indicated that P2X7R may be closely associated with pain
in OA, the effects of P2X7R and the underlying
mechanisms involved in the development and progression of OA and
the damage to cartilage tissue, however, remain unclear.
The administration of monosodium iodoacetate (MIA)
leads to some OA-related pathological alterations, including the
degeneration and necrosis of chondrocytes and the damage to
cartilage tissue (22).
Beyreuther et al demonstrated that OA was induced in the
knee joints of rats upon MIA injection for 5 days (23). In our study, an animal model of OA
was established by administering MIA to Wistar rats. In addition,
we examined the effects of AZD9056, a P2X7R antagonist,
on the cartilage tissue of rats with OA.
Our study demonstrates the role of P2X7R
in MIA-induced OA. Our results demonstrate that the inhibition of
P2X7R exerts protective effects against OA, and our
finidngs may provide the basis of a promising targeted therapeutic
approach for the treatment of articular cartilage
degradation-related diseases, such as OA.
Materials and methods
Antibodies and reagents
All materials were purchased from Gibco (Rockville,
MD, USA), unless otherwise stated. MIA, AZD9056 (a P2X7R
selective antagonist) and helenalin [a nuclear factor-κB (NF-κB)
signaling pathway inhibitor] were purchased from Sigma-Aldrich (St.
Louis, MO, USA). The following antibodies were used: rabbit
anti-P2X7R (ab48871), anti-MMP-13 (ab39012), anti-SP (ab67006),
anti-PGE2 (ab2318), anti-IL-1β (ab9787), anti-IL-6
(ab6672), anti-TNF-α (ab9635), anti-inhibitor of NF-κB kinase
(IKK)α (ab4111), anti-phosphorylated (p)-IKKα (ab38515), anti-IKKβ
(ab124957), anti-p-IKKβ (ab59195), anti-NF-κB p65 (ab16502) and
anti-p-NF-κB p65 (ab86299) antibodies were purchased from Abcam
(Cambridge, UK); rabbit anti-inhibitor of NF-κB (IκB)α (sc-371) and
anti-p-IκBα (sc-101713) antibodies were purchased from Santa Cruz
Biotechnology (Santa Cruz, CA, USA).
Anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH, G9545)
antibodies were obtained from Sigma. HRP-conjugated goat
anti-rabbit antibodies (ab97051) were purchased from Abcam.
Animals
Male Wistar rats (9–14 weeks old, weighing 293–411
g; Shanghai SLAC Laboratory Animal Co., Ltd., Shanghai, China,
n=93) were used. Each rat was housed alone in a plastic box under a
12-hour light dark cycle (light from 06:00 a.m to 6:00 p.m).
Humidity (55±10%) and room temperature (19–23°C) were governed to
be constant. Food and water were available ad libitum. All
rats were handled for several 10-min sessions daily for 2 weeks so
that they could adjust to the testing environment. All animal
experiments were conducted according to the Committee of the
Chinese Academy of Sciences, and the methods complied with the code
of conduct for conscious animal pain published by the International
Association for the Study of Pain (IASP). The protocol of the
experiments was approved by the local ethics committee of Weinan
Central Hospital (approval number no. WNZXHLAC019).
Induction of OA and general grouping of
animals
The rats were anesthetized with isoflurane (2% in
O2) and received a single intra-articular injection of
MIA (5 mg/kg), as previously described (24) in sterile 0.9% saline (MIA, n=30;
18 rats for behavioral tests, 3 rats for western blot analysis on
day 7, 3 rats for western blot analysis on day 14, 3 rats for
ELISA, 3 rats for RT-qRCR on day 7; after the behavioral tests on
day 21, the 18 rats were also sacrificed and used for western blot
analysis and ELISA). MIA solution was administered through the
infrapatellar ligament of the left hind knee using a 26G needle.
Non-osteoarthritic rats, including a blank control group (control,
n=15; 9 rats for behavioral tests, 3 rats for ELISA, 3 rats for
RT-qPCR; after the behavioral tests, the 9 rats were also
sacrificed and used for western blot analysis and ELISA) and a
solvent group (vehicle, n=18; 9 rats for behavioral tests, 3 rats
for western blot analysis on at day 7, 3 rats for western blot
analysis on day 14; 3 rats for ELISA; after the behavioral tests on
day 21, the 9 rats were sacrificed and used for western blot
analysis and ELISA), were also employed. In the present study, we
sought to examine the role of P2X7R in mediating the development
and progression of OA. The other treatment groups were as follows:
the AZD9056 (12.5 mg/kg) [as previously described (20,25)] treatment group (MIA + AZD; AZD9056
was injected every 2 days for 7 days at 2 weeks after MIA
injection, n=24, 18 rats for behavioral tests, 3 rats for western
blot analysis on day 14, 3 rats for ELISA; after the behavioral
tests on day 21, the 18 rats were sacrificed and used for western
blot analysis and ELISA), and helenalin (0.1 mg/kg) [as previously
described (26)] treatment group
(MIA + HEL; helenalin was injected every 2 days for 7 days at 2
weeks after MIA injection, n=6, 3 rats for western blot analysis on
day 14 and 3 rats for western blot analysis on day 21). Testing was
done by a researcher blinded to the randomized treatments.
Reverse transcription-quantitative PCR
(RT-qPCR)
The rats used for RT-qPCR were sacrificed by spinal
dislocation and the skin of the limbs was disinfected. Under
aseptic conditions, the femur was intercepted 5 cm above the
femoral condyle, the tibia was cut out under 3 mm of the tibial
plateau, and the knee joint was carefully removed. The surrounding
soft tissue was removed and the aponeuroses on both sides of the
patella were cut open, and the knee joint was openened. A no. 15
conventional scalpel blade was used to cut off the cartilage on the
articular surface.
Total RNA from the rat left knee joint cartilage was
extracted using Unizol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA) according to the manufacturer's instructions.
Approximately 5 μg of RNA from each sample was used as a
template for cDNA synthesis with a reverse transcription kit
(Fermentas, St. Leon-Rot, Germany). Subsequently, SYBR-Green Master
mix (Life Technologies, Carlsbad, CA, USA) was used for the
quantitative analysis of gene expression. Amplification involved a
denaturation step (95°C for 5 min, 1 cycle), and amplification and
quantification were repeated for 40 cycles (95°C for 5 sec and 60°C
for 1 min, respectively). The primers used for amplification are
listed in Table I. The data of
the relative gene expression levels were calculated using the
2−ΔΔCt method and are presented as the fold change of
transcripts for genes. The sample mean value was calculated and
expressed as the cycle threshold (Ct). mRNA expression was
calculated as the difference (ΔCt) between the Ct value of the
target gene and the Ct value of the inner control.
2−ΔΔCt means the fold change in the target gene
expression, as previously described (27,28). GAPDH was used as internal control
for normalization in RT-qPCR.
 | Table ISequences of primers used for
RT-qPCR. |
Table I
Sequences of primers used for
RT-qPCR.
| Gene | Forward primer
(5′→3′) | Reverse primer
(5′→3′) |
|---|
|
P2X1R | AGG CTC AGG CTG TCA
TTG TC | GTG GCA CAA GAA CAC
ACG AC |
|
P2X2R | TTC GAC AAG GTG CGT
ACT CC | GTC CCA TAT GCT GGC
CAA GT |
|
P2X3R | GAA GGG TAC TGC GTC
AAC CA | AGC GCC TAA CCA TGG
CTT TC |
|
P2X4R | GTG GCG GAC TAT GTG
ATT CCA | TGA CAG ACG CAG TAG
CCA TC |
|
P2X5R | CCG TGA CCT GAT GAA
AGC CT | CAT CTC GTT GGC CTC
AAC CT |
|
P2X6R | ATG TGG CTG ACT TCG
TGA GG | GAG CAG TCA GAG CCT
TTC GT |
|
P2X7R | AAC AGC CAA TGA GTC
CGA GG | TAG GGA CGG CTC AGT
GGT TA |
|
P2X8R | CAT GCC ATG GGG CAG
GTG TCC TGG AAG | GGC TTG GAT CTT TTC
TCC AC |
| GAPDH | AGG AGC AAT GAT CTT
GAT CTT | TGC CAA CAC AGT GCT
GTC T |
Behavioral assessments
On the one hand, pain-related behaviors induced by
MIA were tested at different time points; the test for the hindlimb
weight-bearing asymmetry and paw withdrawal thresholds were
evaluated at 3, 7, 10, 14, 15, 17, 19 and 21 days after the
injection of MIA or the vehicle, as previously described (24). On the other hand, the
intra-articular injection of AZD9056 was administered at 14, 16, 18
and 20 days after the injection of MIA. Subsequently, in the
presence of AZD9056, the test for the hindlimb weight-bearing
asymmetry was evaluated at 15, 17, 19 and 21 days and paw
withdrawal thresholds were evaluated at 14, 15, 17, 19 and 21 days
after the injection of MIA. Weight-bearing asymmetry was presented
as the percentage in the weight distribution of the left and right
hindlimb, as previously described (29). Baseline levels were measured
immediately prior to the intra-articular injection.
Specifically, for the test of paw withdrawal
threshold, 30 min before the test, the rats were placed in a
transparent organic glass box (20×20×30 cm3) on steel
wire mesh (1×1 cm2). Paw withdrawal threshold was tested
by using Von Frey monofilaments (58011, Stoelting Co., USA) (from
0.6 to 26 g). When the bilateral hind limbs came into contact with
the steel wire mesh and the rat was settled, the paw withdrawal
threshold test was performed. Von Frey monofilaments were applied,
in ascending order of bending force (the maximum value is 26 g).
Von Frey monofilaments contact and push the plantar surface of the
left hind paw; the monofilament is bent at an appropriate degree
and is held for 2–3 sec. The lowest weight (g) of monofilament that
elicited a withdrawal reflex was recorded as the paw withdrawal
threshold.
For the test of hind paw weight-bearing asymmetry,
LE7900 - Incapacitance Tester (NatureGene Corp., Medford, NJ, USA)
was used. The rats were kept in an upright position while the hind
paws rested on the separate small electronic balance of the
Incapacitance Tester so that the weight distributed on the right
and left hind paws could be measured. Once the rat was settled,
three consecutive readings (each measured over 3 seconds) were
recorded. The average of a total of 3 readings was determined for
each hind limb for each rat and used for subsequent analyses.
Weight of test hindlimb (%) = [readings of right weight-bearing
(no-injection side) - readings of left weight-bearing (injection
side)]/[the readings of total weight-bearing of both hind
limbs]×100.
Evaluation of knee edema size
The knee edema sizes at 3, 7, 10, 14, 15, 17, 19 and
21 days after the injection of MIA were assessed in randomly
selected rats. The AZD9056-treated rats were evaluated at 14, 15,
17, 19 and 21 days after the injection of MIA. Knee diameter was
measured using calibrated digital calipers, and differences in the
diameter between the right and left knees were determined by the
value of the left (MIA-injected) side minus the value of the right
side, as previously described (30).
Measurement of cytokine levels
The rats were anaesthetized with 1% mebumal sodium
(Sigma-Aldrich). Blood samples were obtained from the carotid
artery and centrifuged at 3,500 × g for 15 min. The supernatant was
then collected and stored at −80°C for the analysis of serum
cytokine levels. The levels of pro-inflammatory cytokines (IL-1β,
IL-6 and TNF-α) were analyzed using enzyme-linked immunosorbent
assay (ELISA) kits (Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA) according to the manufacturer's instructions.
Western blot analysis
The protein expression levels of P2X7R,
MMP-13, SP, PGE2, IL-1β, IL-6 and TNF-α were detected in
the knee joint cartilage tissues of rats with OA by western blot
analysis. The expression levels of IKKα, IKKβ, IκBα, NF-κB p65 and
their corresponding phosphorylated forms was also detected.
Briefly, the minced, homogenized and frozen left side cartilage
tissues in each group were triturated in lysis buffer. The debris
were removed by centrifugation at 12,000 x g for 5 min at 4°C. The
total protein concentration in the supernatant was measured using a
BCA protein assay kit (Beyotime Institute of Biotechnology,
Nanjing, China). A total of 20–30 μg of protein was loaded
on 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) gels and then electroblotted onto polyvinylidene
fluoride (PVDF) membranes. The membranes were blocked by 3% non-fat
milk for 1.5 h at 37°C, and then incubated with primary antibodies
against P2X7R (1:800), MMP-13 (1:1,000), SP (1:1,200),
PGE2 (1:1,000), IL-1β (1:500), IL-6 (1:500), TNF-α
(1:500), IKKα (1:600), p-IKKα (1:500), IKKβ (1:1,000), p-IKKβ
(1:400), IκBα (1:800), p-IκBα (1:500), NF-κBp65 (1:600), p-NF-κBp65
(1:500) and GAPDH (1:2,000) at 4°C overnight. Following incubation
with HRP-conjugated secondary antibody for 1.5 h at room
temperature, protein bands were visualized using an enhanced
chemiluminescence detection system. Densitometry values were
analyzed using Image-Pro Plus 6.0 software (Media Cybernetics,
Inc., Rockville, MD, USA), which were then normalized to GAPDH.
Statistical analysis
The data are presented as the means ± standard
deviation (SD). Statistical analysis was carried out using one-way
analysis of variance (ANOVA) followed by Bonferroni tests for
multiple groups, or Student's t-tests for differences between 2
groups using SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA).
Differences with a P-value <0.05 were regarded as statistically
significant.
Results
Induction of OA by an intra-articular
injection of MIA
We first investigated whether the model of OA model
was successfully established. The weight-bearing asymmetry and paw
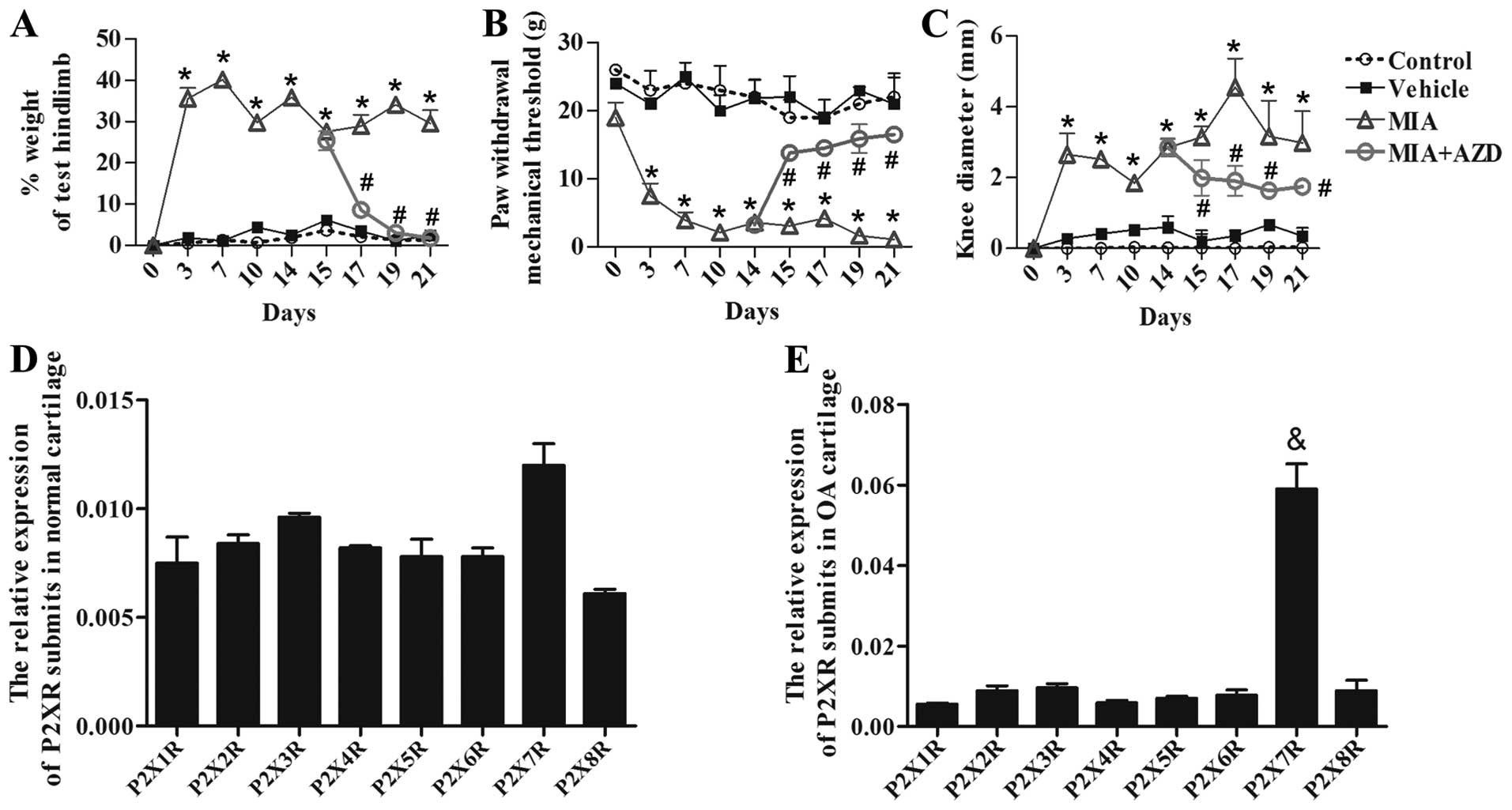
withdrawal thresholds were evaluated. As shown in Fig. 1A and B, the two indexes remained
unaltered in the vehicle group; however, in the rats administered
MIA, the weight-bearing asymmetry remained >27.6 % of the high
level from days 3 to 21 (Fig. 1A;
p<0.05), and the paw withdrawal thresholds were markedly reduced
compared with those of the control and vehicle group rats from days
3 to 21 (Fig. 1B; p<0.05). In
addition, notable increases in knee edema size were observed from
days 3 to 21 after the injection of MIA (Fig. 1C; p<0.05).
P2X7R expression is elevated
in rats with OA
To determine whether P2X7R is involved in
regulating OA development, we analyzed the expression of
P2X1–8R in rat cartilage tissues from rats with OA by
RT-qPCR. The results revealed that the mRNA expression of the mRNA
expression of P2X7R was slightly higher than that of the
other subtypes in normal rats (Fig.
1D). However, in rats with OA, the expression of
P2X7R was significantly increased following the
injection of MIA (Fig. 1E;
p<0.05); however, the expression of other subtypes of P2X
receptors was not significantly altered (Fig. 1E). These results suggest that
P2X7R plays an important role in the development of
OA.
Effects of P2X7R antagonist on OA-induced
pain and inflammation
The P2X7R antagonist, AZD9056, was then
used to further examine the role of P2X7R in OA. As
shown in Fig. 1A, weight-bearing
asymmetry was markedly reduced after day 15 by continuous treatment
with AZD9056 compared with MIA administration alone (p<0.05).
The paw withdrawal thresholds were also almost restored in a
time-dependent manner by continuous treatment with AZD9056
(Fig. 1B; p<0.05). Edema is
the clinical symptom of tissue inflammation. Treatment with AZD9056
resulted in a statistically significant decrease in knee edema size
compared with MIA administration alone (Fig. 1C; p<0.05). These results
demonstrated that the P2X7R antagonist, AZD9056, exerted
pain-alleviating and anti-inflammatory effects in our rat model of
MIA-induced OA.
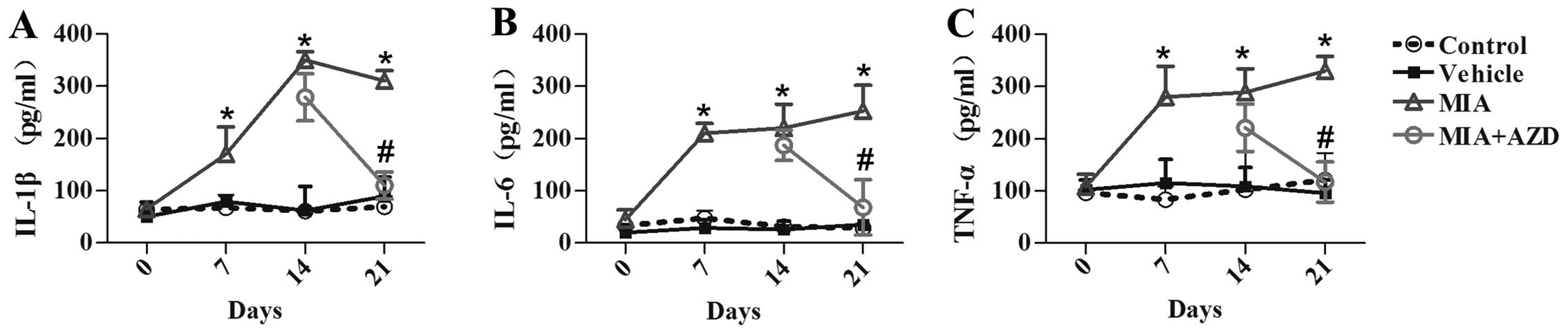
Effects of AZD9056 on cytokine levels in
serum
The cytokine levels in serum were then evaluated in
order to further investigate the anti-inflammatory effects of
AZD9056. As shown in Fig. 2,
TNF-α, IL-6 and IL-1β were highly expressed in the serum of rats
with OA compared with those in the control group (p<0.05).
However, the levels of IL-1β, IL-6 and TNF-α were notably decreased
following the intra-articular injection of AZD9056 (Fig. 2A–C; p<0.05).
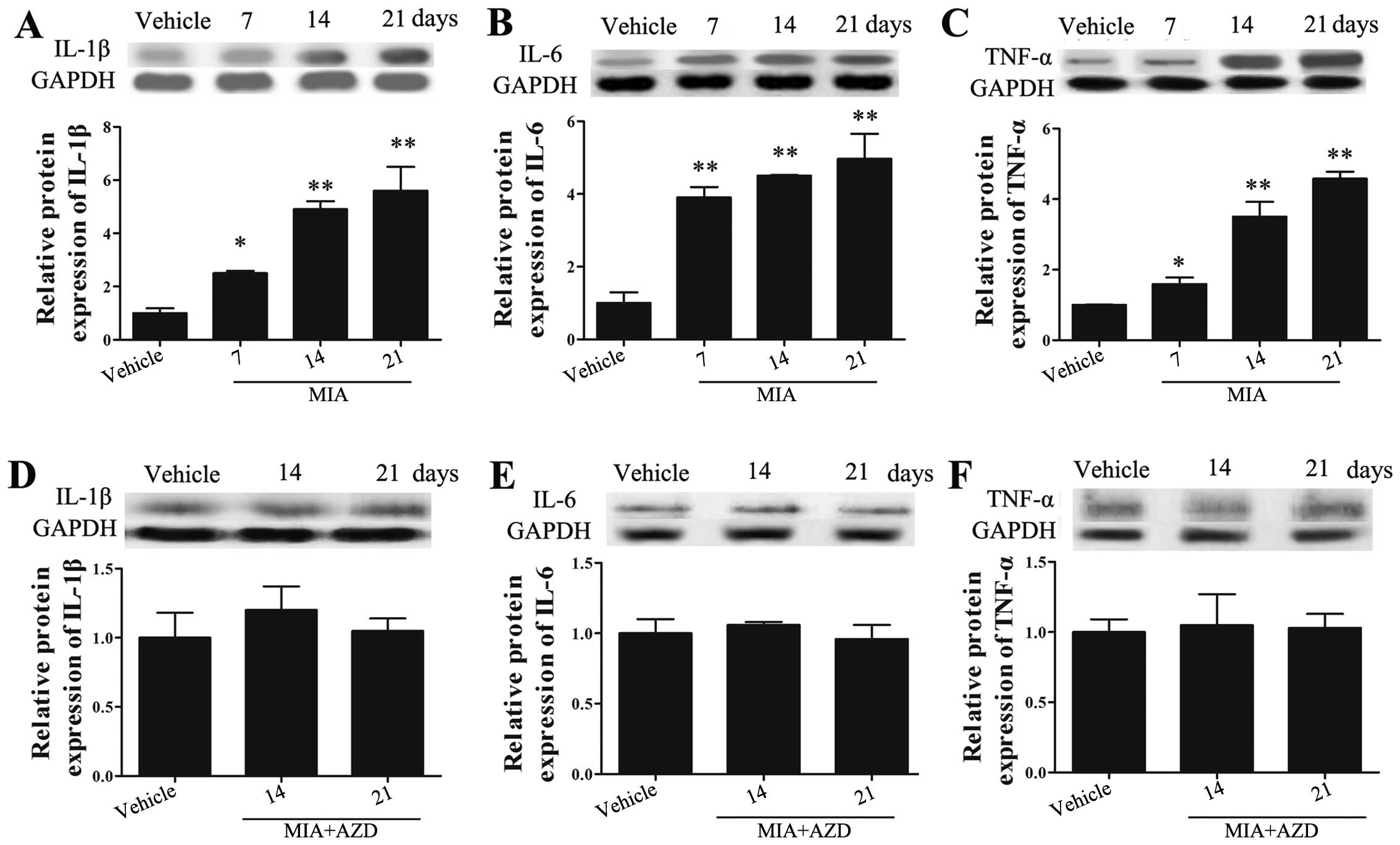
Effects of AZD9056 on cytokine levels in
knee joint cartilage tissues from rats with OA
We then examined the expression levels of cytokines
in the knee joint cartilage tissues of rats with OA by western blot
analysis. As shown in Fig. 3A–C,
the levels of IL-1β, IL-6 and TNF-α in the knee joint cartilage
tissues of rats with OA were continuously increased compared with
those in the rats in the vehicle group (p<0.05). We then wished
to examine the effects of AZD9056 on the levels of inflammatory
factors in the knee joint cartilage tissues of rats with OA. As
shown in Fig. 3D–F, the protein
expression levels of IL-1β, IL-6 and TNF-α in the knee joint
cartilage tissues following treatment with AZD9056 were reversed
and downregulated (compare values in Fig. 3A–C for MIA with those in Fig. 3D–F MIA + AZD on days 14 and 21).
These results suggested that treatment with AZD9056 exerted an
inhibitory effect on inflammation.
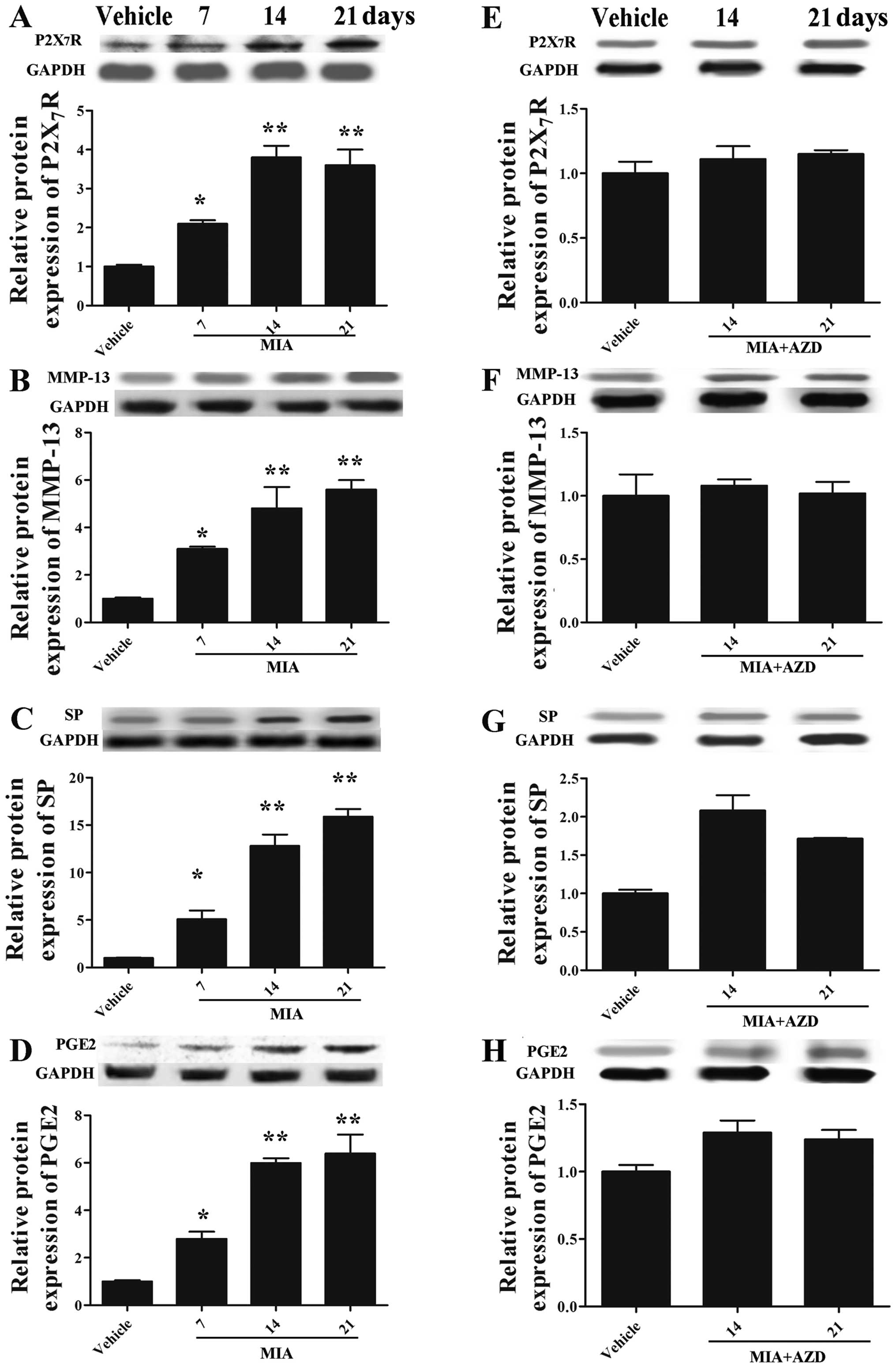
AZD9056 reverses the MIA-induced increase
in the expression of P2X7R, MMP-13, SP and
PGE2
We examined the expression of P2X7R,
MMP-13, SP and PGE2 in order to evaluate the effects of
AZD9056 on OA development. As shown in Fig. 4A–D, MIA markedly increased the
expression levels of P2X7R, MMP-13, SP and
PGE2 in the cartilage tissues (p<0.05), while this
trend was reversed by treatment with AZD9056 (Fig. 4E–H) (compare values in Fig. 4A–D for MIA with those in Fig. 4E–H MIA + AZD on days 14 and 21).
These results collectively demonstrated that AZD9056 played an
important role in inhibiting OA development, suggesting that
P2X7R is a key regulator of OA.
Administration of MIA activates the NF-κB
signaling pathway
To explore whether the NF-κB signaling pathway is
involved in regulating the development of OA, we detected the
expression of IKKα, IKKβ, IκBα, NF-κB p65, which are the effectors
of the NF-κB signaling pathway, and their corresponding
phosphorylated forms (Fig. 5).
The results of western blot analysis revealed that the levels of
these signaling molecules were upregulated in the joint cartilage
tissues of rats with OA (Fig.
5A–H; p<0.05). Specifically, the expression of IKKα
(Fig. 5A and E), IκBα (Fig. 5C and G) and NF-κBp 65 (Fig. 5D and H) was significantly
increased on days 7, 14 and 21 after the MIA injection, and that of
IKKβ (Fig. 5B and F) was notably
upregulated on dayd 14 and 21 (p<0.05). Moreover, the
phosphorylated forms of IKKα (Fig. 5A
and I), IKKβ (Fig. 5B and J),
IκBα (Fig. 5C and K) and NF-κB
p65 (Fig. 5D and L) were also
markedly upregulated in the rats with OA (p<0.05). These results
suggested that the NF-κB signaling pathway was activated upon MIA
stimulation in the model of OA.
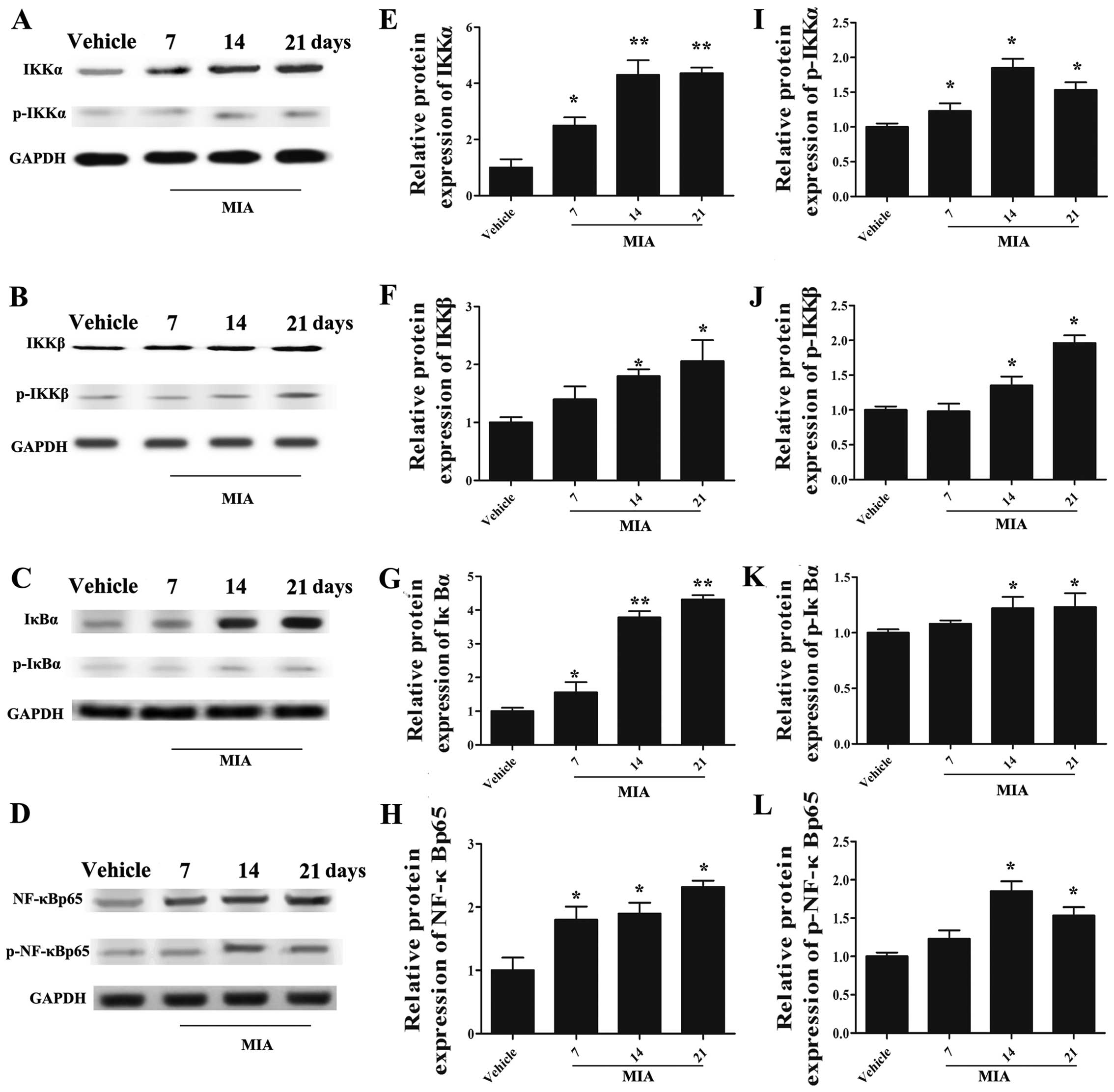 | Figure 5Expression of [inhibitor of nuclear
factor-κB (NF-κB) kinase (IKK)α], IKKβ, inhibitor of NF-κB (IκB)α,
NF-κBp65, p-IKKα, p-IKKβ, p-IκBα and p-NF-κB p65 in rats with
monosodium iodoacetate (MIA)-induced osteoarthritis (OA). The
protein levels of IKKα, IKKβ, IκBα, NF-κB p65 and those of their
phosphorylation forms were markedly incrased by MIA injection
compared with the vehicle group. (A) The protein bands of IKKα and
p-IKKα and (E and I) corresponding densitometric analysis, (B) IKKβ
and p-IKKβ and (F and J) corresponding densitometric analysis, (C)
IκBα and p-IκBα and (G and K) corresponding densitometric analysis,
(D) NF-κB p65 and p-NF-κB p65 and (H and L) corresponding
densitometric analysis are shown. n=3, *p<0.05 and
**p<0.01 vs. vehicle. |
AZD9056 inhibits the activation of the
NF-κB signaling pathway
To further delineate the role of P2X7R in
mediating the NF-κB pathway, the P2X7R antagonist,
AZD9056, was used. The results of western blot analysis (Fig. 6A–D) revealed that treatment with
AZD9056 inhibited the expression of IKKα, IKKβ, IκBα and NF-κB p65
(Fig. 6A–H), as well as that of
their corresponding phosphorylated forms (Fig. 6A–D and I–L) in the presence of
MIA, indicating that AZD9056 suppressed NF-κB signaling (compare
values in Fig. 5 for MIA with
those in Fig. 6 MIA + AZD on days
14 and 21). Thus, the above results confirm the role of
P2X7R in regulating NF-κB signaling.
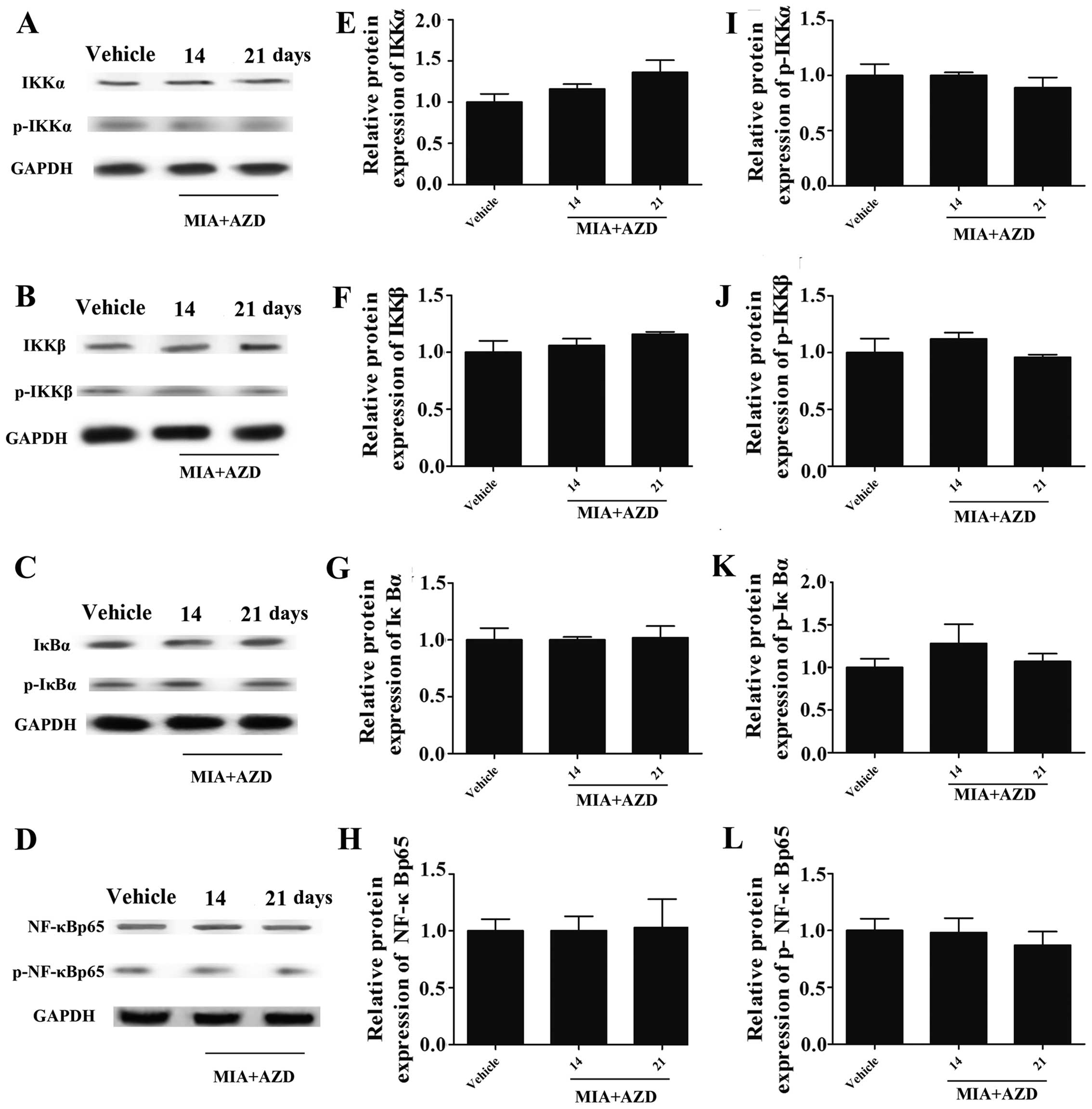 | Figure 6Expression of [inhibitor of nuclear
factor-κB (NF-κB) kinase (IKK)α], IKKβ, inhibitor of NF-κB (IκB)α,
NF-κBp65, p-IKKα, p-IKKβ, p-IκBα and p-NF-κB p65 in rats with
monosodium-iodoacetate (MIA)-induced osteoarthritis (OA) under
AZD9056 treatment conditions. (A-D) The protein bands analyzed by
western blot analysis. (E) After the AZD9056 injection, the
expression of IKKα, (F) IKKβ, (G) IκBα, (H) NF-κBp65 and (I-L)
their phosphorylation forms returned to levels similar to those of
the vehicle group. n=3. |
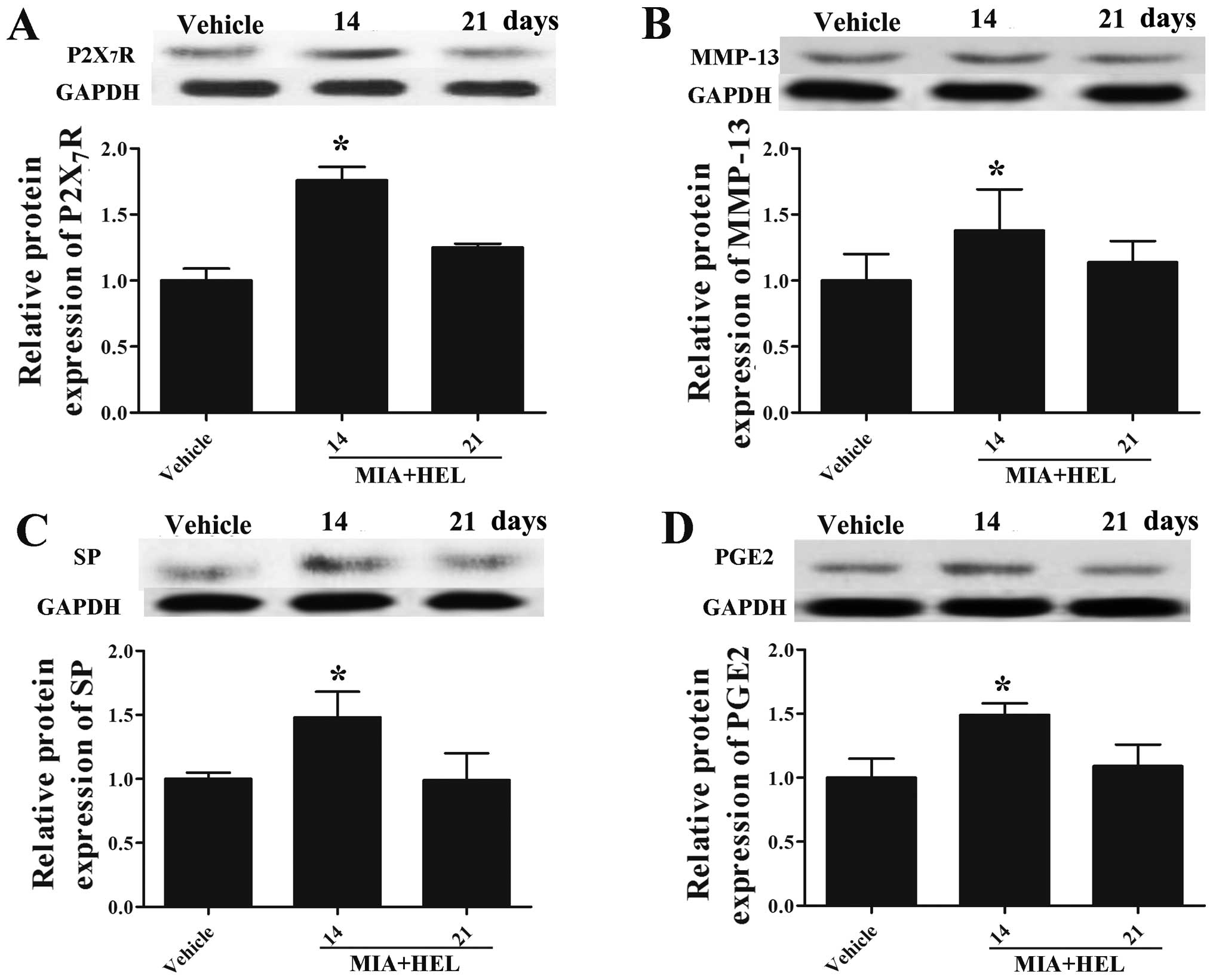
Helenalin reverses the MIA-induced
expression of P2X7R, MMP-13, SP and PGE2
To further investigate the role of NF-κB signaling
in the development of OA, the NF-κB signaling pathway inhibitor,
helenalin, was used to evaluate the expression of P2X7R,
MMP-13, SP and PGE2. As shown in Fig. 7, in the presence of helenalin, the
protein expression levels of P2X7R (Fig. 7A), MMP-13 (Fig. 7B), SP (Fig. 7C) and PGE2 (Fig. 7D), which had been increased
following the MIA injection (Fig.
4A–D) were returned to levels similar to those of the vehicle
group on the 21st day (compare values in Fig. 4A–D for MIA with those in Fig. 7 MIA + HEL on day 21). Due to the
delayed drug effects of helenalin, the levels of P2X7R,
MMP-13, SP and PGE2 remained high on the 14th day
compared to the vehicle (p<0.05). These results provide further
evidence that the activation of NF-κB signaling participates in OA
development, indicating that P2X7R is a regulator of OA
by targeting the NF-κB signaling pathway.
Discussion
In the present study, we discovered the following
results: i) the mRNA expression of P2X7R was slightly
higher than that of the other subtypes in normal rats.
Correspondingly, the mRNA expression of P2X7R was
markedly increased in the rats with MIA-induced OA; ii) the
P2X7R antagonist, AZD9056, relieved hindlimb
weight-bearing asymmetry and increased paw withdrawal thresholds,
and also reduced the swelling of the knee joint in the rats with
OA; iii) AZD9056 reversed the upregulated expression of IL-β, IL-6
and TNF-α in both serum and the knee articular cartilage tissues of
rats with OA; iv) further experiments indicated that the expression
of P2X7R, MMP-13, SP and PGE2 was increased
in the knee joint cartilage tissues of rats with OA, but this trend
was significantly reversed by AZD9056; v) the expression of IKKα,
IKKβ, IκBα and NF-κBp65 and that of their corresponding
phosphorylation forms was significantly increased in the knee
cartilage tissues of rats with OA, and AZD9056 reversed these
effects; and vi) the NF-κB pathway inhibitor, helenalin, reversed
the increased expression of P2X7R, SP, PGE2
and MMP-13 induced by MIA in rats with OA. Thus, we discovered an
important role of P2X7R in rats with OA, and also found
that a P2X7R antagonist can be used to regulate OA by
targeting the NF-κB pathway.
AZD9056, an adamantane amide, is regarded as a
selective P2X7R antagonist. In the present study,
AZD9056 was shown to effectively relieve OA-induced pain, as shown
by the results of behavioral tests. Moreover, the sustained use of
AZD9056 played a role in eliminating edema induced by OA,
indicating that AZD9056 had a prominent suppressive effect on
inflammation. Similarly, recent evidence from another study
indicated that AZD9056 possessed anti-inflammatory and
pain-relieving properties (25).
It was also indicated that AZD9056 inhibited inflammatory factors,
protected the synovial tissue and prevented the degradation of the
cartilage matrix in mammals with RA (25). However, OA differs from RA, as RA
is an autoimmune disease, but OA is usually caused by the 'wear and
tear' of joints. However, there is evidence to suggest that OA is
associated with arthritis (11).
OA frequently leads to inflammation and the degeneration of
articular cartilage, the destruction of subchondral bone and
hyperplasia of synovial tissue (31). In addition, it has been reported
that chondrocytes have a direct effect in inflammatory pain through
the activation of neurons (4,31).
It has also been shown that chondrocytes obtained from patients
with OA actively produce inflammatory cytokines, such as nitric
oxide (NO), PG, IL-1β, TNF-α, IL-6, and IL-8 (6). Moreover, the activation of
P2X7R in mouse mast cells has been shown to increase the
expression of IL-4, IL-6, IL-13 and TNF-α (32). Another study demonstrated that the
activation of P2X7R in rat immune cells promoted the
release of IL-6, IL-1β and TNF-α (33). However, the blockade of TNF-α
(using adalimumab) and the silencing of TNF-α has been shown to
lead to a prolonged inhibitory effect on inflammation in animal
models (34–36). Our data demonstrated that the
increasing trend in the expression of IL-1β, IL-6, and TNF-α in the
knee joint cartilage tissue of rats with OA was reversed by the
P2X7R antagonist, AZD9056. These results suggest that
P2X7R plays an important role in the modulation of
OA-induced inflammation in cartilage.
Inflammatory cytokines affect a range of ion
channels; however, the role of ion channels in OA development
remains unclear. However, P2X7R has been reported to be
associated with joint inflammation and OA (11). SP is a neuropeptide which has been
reported to exert anti-inflammatory and analgesic effects via its
antagonists in OA or RA (10,37,38). Furthermore, PGE2 has
been reported to promote cartilage degeneration by increasing the
production and secretion of proteinases, such as MMPs and
aggrecanases (8). MMP-13, which
is the main expressed MMP in OA-affected cartilage, is specifically
expressed in OA-affected cartilage but is not present in normal
adult cartilage; during the process of OA, MMP-13 is one of the
most effective collagenase II and is considered to be the marker of
cartilage degeneration (9,39–41).
The activation of MMPs increases the degradation of cartilage
matrix and cartilage cell apoptosis, eventually leading to
cartilage damage (40,42). In this study, we confirmed the
increased expression of MMP-13 in knee joint tissues from rats with
OA and this was reversed by AZD9056. The results suggest that
AZD9056 may play protective role in cartilage in OA. Our findings
strongly suggest that antagonists of P2X7R have
potential for clinical and therapeutic use in the alternative
management of OA. However, further studies are required to confirm
our results.
In terms of arthritis and relative inflammatory
diseases, the NF-κB pathway is among the most attractive targets
for such therapeutic intervention. In previous studies, the NF-κB
pathway has been shown to be activated when chondrocytes are
stimulated with IL-1β (43–45). It has been found that
P2X7R triggers the activation of the NF-κB signaling
pathway, and the activation of P2X7R is believed to have
a close association with inflammatory diseases, such as arthritis
(46). These findings raise the
possibility that the modulation of the NF-κB pathway is a viable
path for improving the treatment efficacy of OA. In the present
study, we found that P2X7R regulated OA by targeting the
NF-κB signaling pathway. Firstly, our results demonstrated that the
levels of IKKα, IKKβ, IκBα, NF-κB p65 and their phosphorylated
forms were upregulated in rats with OA. However, it was shown that
the protein expression of these molecules was reversed by AZD9056.
According to previously published results, inactive NF-κB is
present in the cytoplasm as a heterotrimer complex consisting of
two subunits, p65 and p50, bound to an additional inhibitory
subunit IκBα, which prevents NF-κB from entering the nuclei. In
particular, purinergic signals activate NF-κB through P2X7R by
selectively targeting NF-κB p65 (47). Consistent with these results, we
found that the blockade of P2X7R downregulated NF-κB p65
and the corresponding phosphorylation level. Another study
demonstrated that in complete Freund's adjuvant (CFA)-induced
arthritis, IKKα and IKKβ were activated by P2X7R,
causing the activation of NF-κB and the phosphorylation of IκBα
(48). These reports and our
results demonstrate that P2X7R is involved in the
regulation of the NF-κB signaling pathway in cartilage tissues in
OA.
Secondly, we aimed to inhibit NF-κB signaling using
the NF-κB signaling inhibitor, helenalin. Our results indicated
that helenalin suppressed the expression of P2X7R,
MMP-13, SP and PGE2 in the left side knee joint
cartilage tissues of rats with OA. Existing evidence indicates that
the P2X7R-mediated signal is necessary for the
activation of the NF-κB pathway (49–52). The above-mentioned results suggest
that the effects of helenalin are closely related to
P2X7R. In general, our data suggested that
P2X7R regulates OA by targeting the NF-κB pathway, which
is considered one of the most attractive targets for the
prevention, treatment and prognosis of arthritis and cartilage
degenerative diseases.
In conclusion, in this study, P2X7R was
identified not only as a regulator of OA-induced pain and
inflammation, but that it can also influence the expression of
MMP-13 and NF-κB signaling in OA-affected cartilage tissues,
providing evidence that P2X7R may serve as a potential
therapeutic target for the management of OA-related cartilage
degenerative diseases.
References
|
1
|
Sinkov V and Cymet T: Osteoarthritis:
understanding the pathophysiology, genetics, and treatments. J Natl
Med Assoc. 95:475–482. 2003.PubMed/NCBI
|
|
2
|
Cutolo M and Straub RH: Recent aspects of
gonadal hormone and neurotransmitter interactions with synovial and
immune cells: implications in rheumatoid arthritis. Ann Rheum Dis.
59:657–661. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goldring MB: Osteoarthritis and cartilage:
the role of cytokines. Curr Rheumatol Rep. 2:459–465. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Largo R, Alvarez-Soria MA, Díez-Ortego I,
Calvo E, Sánchez-Pernaute O, Egido J and Herrero-Beaumont G:
Glucosamine inhibits IL-1beta-induced NFkappaB activation in human
osteoarthritic chondrocytes. Osteoarthritis Cartilage. 11:290–298.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fan Z, Bau B, Yang H, Soeder S and Aigner
T: Freshly isolated osteoarthritic chondrocytes are catabolically
more active than normal chondrocytes, but less responsive to
catabolic stimulation with interleukin-1beta. Arthritis Rheum.
52:136–143. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pelletier JP, Martel-Pelletier J and
Abramson SB: Osteoarthritis, an inflammatory disease: potential
implication for the selection of new therapeutic targets. Arthritis
Rheum. 44:1237–1247. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pelletier JP, Fernandes JC, Jovanovic DV,
Reboul P and Martel-Pelletier J: Chondrocyte death in experimental
osteoarthritis is mediated by MEK 1/2 and p38 pathways: role of
cyclooxygenase-2 and inducible nitric oxide synthase. J Rheumatol.
28:2509–2519. 2001.PubMed/NCBI
|
|
8
|
Bar-Or D, Rael LT, Thomas GW and Brody EN:
Inflammatory pathways in knee osteoarthritis: potential targets for
treatment. Curr Rheumatol Rev. May 21–2015.Epub ahead of print.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li NG, Shi ZH, Tang YP, Wang ZJ, Song SL,
Qian LH, Qian DW and Duan JA: New hope for the treatment of
osteoarthritis through selective inhibition of MMP-13. Curr Med
Chem. 18:977–1001. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lam FF and Ng ES: Substance P and
glutamate receptor antagonists improve the anti-arthritic actions
of dexamethasone in rats. Br J Pharmacol. 159:958–969. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Staunton CA, Lewis R and Barrett-Jolley R:
Ion channels and osteoarthritic pain: potential for novel
analgesics. Curr Pain Headache Rep. 17:3782013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Millward-Sadler SJ, Wright MO, Flatman PW
and Salter DM: ATP in the mechanotransduction pathway of normal
human chondrocytes. Biorheology. 41:567–575. 2004.PubMed/NCBI
|
|
13
|
Knight MM, McGlashan SR, Garcia M, Jensen
CG and Poole CA: Articular chondrocytes express connexin 43
hemichannels and P2 receptors - a putative mechanoreceptor complex
involving the primary cilium? J Anat. 214:275–283. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Varani K, De Mattei M, Vincenzi F, Tosi A,
Gessi S, Merighi S, Pellati A, Masieri F, Ongaro A and Borea PA:
Pharmacological characterization of P2X1 and
P2X3 purinergic receptors in bovine chondrocytes.
Osteoarthritis Cartilage. 16:1421–1429. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bravo D, Maturana CJ, Pelissier T,
Hernández A and Constandil L: Interactions of pannexin 1 with NMDA
and P2X7 receptors in central nervous system
pathologies: possible role on chronic pain. Pharmacol Res.
101:86–93. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dray A and Read SJ: Arthritis and pain.
Future targets to control osteoarthritis pain. Arthritis Res Ther.
9:2122007. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang K, Zhuang Y, Yan M, Chen H, Ge AQ,
Sun L and Miao B: Effects of riluzole on P2X7R
expression in the spinal cord in rat model of neuropathic pain.
Neurosci Lett. 618:127–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ursu D, Ebert P, Langron E, Ruble C,
Munsie L, Zou W, Fijal B, Qian YW, McNearney TA, Mogg A, et al:
Gain and loss of function of P2X7 receptors: mechanisms,
pharmacology and relevance to diabetic neuropathic pain. Mol Pain.
10:372014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sorge RE, Trang T, Dorfman R, Smith SB,
Beggs S, Ritchie J, Austin JS, Zaykin DV, Vander Meulen H, Costigan
M, et al: Genetically determined P2X7 receptor pore formation
regulates variability in chronic pain sensitivity. Nat Med.
18:595–599. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McInnes IB, Cruwys S, Bowers K and
Braddock M: Targeting the P2X7 receptor in rheumatoid arthritis:
biological rationale for P2X7 antagonism. Clin Exp Rheumatol.
32:878–882. 2014.PubMed/NCBI
|
|
21
|
Portales-Cervantes L, Niño-Moreno P,
Doníz-Padilla L, Baranda-Candido L, García-Hernández M,
Salgado-Bustamante M, González-Amaro R and Portales-Pérez D:
Expression and function of the P2X(7) purinergic receptor in
patients with systemic lupus erythematosus and rheumatoid
arthritis. Hum Immunol. 71:818–825. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guzman RE, Evans MG, Bove S, Morenko B and
Kilgore K: Mono-iodoacetate-induced histologic changes in
subchondral bone and articular cartilage of rat femorotibial
joints: an animal model of osteoarthritis. Toxicol Pathol.
31:619–624. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Beyreuther B, Callizot N and Stöhr T:
Antinociceptive efficacy of lacosamide in the monosodium
iodoacetate rat model for osteoarthritis pain. Arthritis Res Ther.
9:R142007. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sagar DR, Staniaszek LE, Okine BN,
Woodhams S, Norris LM, Pearson RG, Garle MJ, Alexander SP, Bennett
AJ, Barrett DA, et al: Tonic modulation of spinal hyperexcitability
by the endocannabinoid receptor system in a rat model of
osteoarthritis pain. Arthritis Rheum. 62:3666–3676. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Keystone EC, Wang MM, Layton M, Hollis S
and McInnes IB; D1520C00001 Study Team: Clinical evaluation of the
efficacy of the P2X7 purinergic receptor antagonist
AZD9056 on the signs and symptoms of rheumatoid arthritis in
patients with active disease despite treatment with methotrexate or
sulphasalazine. Ann Rheum Dis. 71:1630–1635. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lyss G, Knorre A, Schmidt TJ, Pahl HL and
Merfort I: The anti-inflammatory sesquiterpene lactone helenalin
inhibits the transcription factor NF-kappaB by directly targeting
p65. J Biol Chem. 273:33508–33516. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
28
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bove SE, Calcaterra SL, Brooker RM, Huber
CM, Guzman RE, Juneau PL, Schrier DJ and Kilgore KS: Weight bearing
as a measure of disease progression and efficacy of
anti-inflammatory compounds in a model of monosodium
iodoacetate-induced osteoarthritis. Osteoarthritis Cartilage.
11:821–830. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fernihough J, Gentry C, Malcangio M, Fox
A, Rediske J, Pellas T, Kidd B, Bevan S and Winter J: Pain related
behaviour in two models of osteoarthritis in the rat knee. Pain.
112:83–93. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Konttinen YT, Sillat T, Barreto G, Ainola
M and Nordström DC: Osteoarthritis as an autoinflammatory disease
caused by chondrocyte-mediated inflammatory responses. Arthritis
Rheum. 64:613–616. 2012. View Article : Google Scholar
|
|
32
|
Lister MF, Sharkey J, Sawatzky DA,
Hodgkiss JP, Davidson DJ, Rossi AG and Finlayson K: The role of the
purinergic P2X7 receptor in inflammation. J Inflamm (Lond).
4:52007. View Article : Google Scholar
|
|
33
|
Gourine AV, Poputnikov DM, Zhernosek N,
Melenchuk EV, Gerstberger R, Spyer KM and Gourine VN: P2 receptor
blockade attenuates fever and cytokine responses induced by
lipopolysaccharide in rats. Br J Pharmacol. 146:139–145. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Catal F, Mete E, Tayman C, Topal E,
Albayrak A and Sert H: A human monoclonal anti-TNF alpha antibody
(adalimumab) reduces airway inflammation and ameliorates lung
histology in a murine model of acute asthma. Allergol Immunopathol
(Madr). 43:14–18. 2015. View Article : Google Scholar
|
|
35
|
Johnsen-Soriano S, Sancho-Tello M, Arnal
E, Díaz-Llopis M, Navea A, Miranda M, Bosch-Morell F and Romero FJ:
Comparison of the acute effects of anti-TNF-alpha drugs on a
uveitis experimental model. Ocul Immunol Inflamm. 18:208–215. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Grounds MD, Davies M, Torrisi J,
Shavlakadze T, White J and Hodgetts S: Silencing TNFalpha activity
by using Remicade or Enbrel blocks inflammation in whole muscle
grafts: an in vivo bioassay to assess the efficacy of anti-cytokine
drugs in mice. Cell Tissue Res. 320:509–515. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hong HS and Son Y: Substance P ameliorates
collagen II-induced arthritis in mice via suppression of the
inflammatory response. Biochem Biophys Res Commun. 453:179–184.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim SJ, Kim JE, Kim SH, Kim SJ, Jeon SJ,
Kim SH and Jung Y: Therapeutic effects of neuropeptide substance P
coupled with self-assembled peptide nanofibers on the progression
of osteoarthritis in a rat model. Biomaterials. 74:119–130. 2016.
View Article : Google Scholar
|
|
39
|
Malemud CJ, Islam N and Haqqi TM:
Pathophysiological mechanisms in osteoarthritis lead to novel
therapeutic strategies. Cells Tissues Organs. 174:34–48. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Schlomann U, Wildeboer D, Webster A,
Antropova O, Zeuschner D, Knight CG, Docherty AJ, Lambert M,
Skelton L, Jockusch H and Bartsch JW: The metalloprotease
disintegrin ADAM8. Processing by autocatalysis is required for
proteolytic activity and cell adhesion. J Biol Chem.
277:48210–48219. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
van den Berg WB: Osteoarthritis year 2010
in review: pathomechanisms. Osteoarthritis Cartilage. 19:338–341.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chubinskaya S, Kuettner KE and Cole AA:
Expression of matrix metalloproteinases in normal and damaged
articular cartilage from human knee and ankle joints. Lab Invest.
79:1669–1677. 1999.
|
|
43
|
Csaki C, Mobasheri A and Shakibaei M:
Synergistic chondroprotective effects of curcumin and resveratrol
in human articular chondrocytes: inhibition of IL-1beta-induced
NF-kappaB-mediated inflammation and apoptosis. Arthritis Res Ther.
11:R1652009. View
Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen YJ, Tsai KS, Chan DC, Lan KC, Chen
CF, Yang RS and Liu SH: Honokiol, a low molecular weight natural
product, prevents inflammatory response and cartilage matrix
degradation in human osteoarthritis chondrocytes. J Orthop Res.
32:573–580. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ding Q, Zhong H, Qi Y, Cheng Y, Li W, Yan
S and Wang X: Anti-arthritic effects of crocin in
interleukin-1β-treated articular chondrocytes and cartilage in a
rabbit osteoarthritic model. Inflamm Res. 62:17–25. 2013.
View Article : Google Scholar
|
|
46
|
He C, Chen X, Zhao C, Qie Y, Yan Z and Zhu
X: Eleutheroside E ameliorates arthritis severity in
collagen-induced arthritis mice model by suppressing inflammatory
cytokine release. Inflammation. 37:1533–1543. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ferrari D, Wesselborg S, Bauer MK and
Schulze-Osthoff K: Extracellular ATP activates transcription factor
NF-kappaB through the P2Z purinoreceptor by selectively targeting
NF-kappaB p65. J Cell Biol. 139:1635–1643. 1997. View Article : Google Scholar
|
|
48
|
Chang X, He H, Zhu L, Gao J, Wei T, Ma Z
and Yan T: Protective effect of apigenin on Freund's complete
adjuvant-induced arthritis in rats via inhibiting P2X7/NF-κB
pathway. Chem Biol Interact. 236:41–46. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Korcok J, Raimundo LN, Ke HZ, Sims SM and
Dixon SJ: Extracellular nucleotides act through P2X7 receptors to
activate NF-kappaB in osteoclasts. J Bone Miner Res. 19:642–651.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kahlenberg JM, Lundberg KC, Kertesy SB, Qu
Y and Dubyak GR: Potentiation of caspase-1 activation by the P2X7
receptor is dependent on TLR signals and requires NF-kappaB-driven
protein synthesis. J Immunol. 175:7611–7622. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Genetos DC, Karin NJ, Geist DJ, Donahue HJ
and Duncan RL: Purinergic signaling is required for fluid shear
stress-induced NF-κB translocation in osteoblasts. Exp Cell Res.
317:737–744. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kim JE, Kim DS, Jin Ryu H, Il Kim W, Kim
MJ, Won Kim D, Young Choi S and Kang TC: The effect of P2X7
receptor activation on nuclear factor-κB phosphorylation induced by
status epilepticus in the rat hippocampus. Hippocampus. 23:500–514.
2013. View Article : Google Scholar : PubMed/NCBI
|