Introduction
Acute pancreatitis (AP) remains a common acute
abdominal disease, which includes mild acute pancreatitis (MAP) and
severe acute pancreatitis (SAP) (1). Patients with MAP can exhibit an
improvement in severity or can even be cured by active treatment
within 1 week. However, SAP rapidly ensues, readily causing
systemic inflammatory response syndrome (SIRS) in the early stages
and subsequently, multiple organ dysfunction syndrome (MODS), with
a high fatality rate of up to 15–20%. Despite multiple basic and
clinical studies assessing SAP, no ideal therapeutic drug is yet
available (2,3).
Luteolin (Lut, 3, 4′, 5′, 7-tetrahydroxyflavone), a
natural flavonoid first isolated from Reseda odorata L. and
is widely found in many vegetables and herbal medicines, has long
been used in traditional Asian medicine for the treatment of
diseases associated with oxidative injury and acute inflammation,
such as endotoxemia, acute lung injury, acute myocardial infarction
and hepatitis (4–6). Luteolin displays specific
anti-inflammatory effects at micromolar concentrations, partly
explained by its antioxidant capacity, including the activation of
antioxidant enzymes, the suppression of nuclear factor-κB (NF-κB)
pathway activation and the inhibition of pro-inflammatory
substances (4). However, the role
and underlying pharmacological mechanisms of luteolin in diseases
are largely unknown.
Recently, it has been reported that luteolin is an
effective heme oxygenase-1 (HO-1) inducer and that it exerts
anti-inflammatory effects in macrophages in a dose-dependent
manner, leading to the suppression of inducible nitric oxide
synthase (iNOS)-derived nitric oxide (NO) production, suggesting
the potential therapeutic effects of luteolin in inflammatory
diseases (7). HO-1 is the
rate-limiting enzyme in heme degradation; it catalyzes the
oxidative degradation of heme to equimolar quantities of carbon
monoxide (CO), iron and biliverdin (8). Of note, HO-1 overexpression can be
applied in multiple clinical conditions, such as organ
transplantation, acute kidney injury, hypertension and
atherosclerosis (9–14). Importantly, HO-1 is known to
exhibit cytoprotective, anti-inflammatory, anti-proliferative,
antioxidant and anti-apoptotic activities, making it a promising
therapeutic target for the treatment of inflammatory diseases of
the gastrointestinal system (15). Panhematin leads to the rapid
induction and activation of pancreatic HO-1, and has potential for
use in the treatment of human pancreatitis (16). In addition, hemin-like compounds
or hemin-activated macrophages prevent AP via the upregulation of
HO-1 (17). In agreement with
these studies, stressful conditions, such as severe hypoxia,
hyperpyrexia and endotoxemia observed in patients with SAP can be
alleviated by the appropriate induction of HO-1 levels (18,19). Furthermore, HO-1 exerts protective
effects against cardiomyocytic apoptosis and oxidative stress by
inhibiting NF-κB activity (18,20,21).
Oxidative stress and the activation of NF-κB have
been suggested to play important roles in SAP (22–24). However, whether luteolin exerts
its anti-inflammatory and antioxidant effects by inducing HO-1
expression in SAP remains unknown. Therefore, in this study, we
aimed to assess the protective effects of luteolin in mice with SAP
induced by cerulein plus lipopolysaccharide (LPS), and unveil the
underlying mechanisms.
Materials and methods
Chemicals
Purified luteolin (>99%, CAS: 491-70-3) was
purchased from Shanghai Tauto Biotech Co., Ltd. (Shanghai, China)
and its quality was analyzed by high-performance liquid
chromatography (HPLC) (Fig. 1).
Cerulein, LPS and zinc protoporphyrin (ZnPP) were obtained from
Sigma-Aldrich (St. Louis, MO, USA). Luteolin was dissolved in 35%
propanediol to the concentration of 20 mg/ml. Cerulein and LPS were
dissolved in 0.9% NaCl to the concentration of 10 μg/ml and
1 mg/ml, respectively. The ZnPP solution was prepared as follows:
first, 25 mg ZnPP were dissolved in 3.3 ml NaOH (0.2 M) in a dark
room and 0.2 M HCl was added to adjust the pH to 7.0. Finally,
saline was added to 50 ml (0.5 mg/ml). The resulting solution was
stored away from light.
Animal model of SAP
Male Institute of Cancer Research (ICR) mice (8
weeks old; weighing 20–23 g) were purchased from Shanghai SLAC
Laboratory Animal Co., Ltd. (Shanghai, China). The animals were
maintained on a 12 h-light/12 h-dark cycle at 22°C, given water
ad libitum, fed standard laboratory chow and allowed to
acclimatize for a minimum of 1 week. All animal-related procedures
were approved by the Institutional Animal Care and Use Committee of
Shanghai Jiao Tong University (Shanghai, China). This study was
also performed under the permission of Science and Technology
Commission of Shanghai municipality with the permit no. SYXK
2013-0050.
To investigate the optimal dose of luteolin, we
first evaluated its pharmacological toxicity and half lethal dose
(LD50), and the effect of luteolin on cerulein plus
LPS-induced SAP in mice was determined at different doses,
including 25, 50 and 100 mg/kg. To investigate the effects of
luteolin treatment on SAP, 54 male ICR mice were divided into 3
groups (n=18 per group) as follows: the normal control (NC) group
(without SAP), the SAP control group (mice with SAP treated with
the vehicle) and the luteolin (Lut) group (SAP mice treated with
luteolin). The mice were fasted for 12 h with free access to
drinking water prior to the induction of SAP. SAP was induced by an
intraperitoneal injection of 50 μg/kg cerulein
(Sigma-Aldrich) in 0.9% NaCl, as previously described (25,26). After the final administration, the
animals were intraperitoneally injected with LPS (Sigma-Aldrich) at
5 mg/kg. The mice in the Lut group were intraperitoneally injected
with luteolin (Sigma-Aldrich) at 100 mg/kg 2 h prior to the
induction of SAP. The SAP control group mice were intraperitoneally
injected with equal volumes of 35% propanediol as the Lut group. In
the NC group, the mice were continuously administered the same
volume of saline 7 times. After modeling, the mice were still
fasted but were allowed free access to drinking. Each group was
subdivided into 3 subgroups to obtain serum and pancreatic tissues
at the time points of 1, 3 and 6 h post-modeling (n=6 per group).
Blood (1 ml) was collected by retro-orbital bleeding at 1, 3 and 6
h after modeling under anesthesia with phenobarbital sodium (50
mg/kg) intraperitoneally. Serum samples were obtained by
centrifugation (900 × g, 5 min) and stored at −20°C for ELISA,
amylase and lipase detection. Pancreatic tissues were extracted at
1, 3 and 6 h after modeling under anesthesia with phenobarbital
sodium (50 mg/kg) intraperitoneally, and the pancreas head and tail
were fixed in 4% paraformaldehyde and embedded in paraffin for
pathological analysis; the remaining tissue was stored in liquid
nitrogen.
In order to assess the potential effects of luteolin
on SAP via the induction of HO expression, another 40 ICR mice
(weighing 20–23 g) were divided into 5 groups (n=8 per group) as
follows: the NC group, SAP model with vehicle treatment (SAP
control) group, SAP model with ZnPP (Sigma-Aldrich) only treatment
(ZnPP) group, SAP model with luteolin treatment (Lut) group and SAP
model with luteolin plus ZnPP treatment (Lut + ZnPP) group. The SAP
model was established as described above. Lut + ZnPP group mice
were intraperitoneally injected with ZnPP and luteolin at 5 and 100
mg/kg, 2 h before SAP modeling. Lut group and ZnPP group mice were
intraperitoneally injected with 100 or 5 mg/kg ZnPP 2 h before SAP
modeling, respectively. SAP control groups were only treated with
the vehicle.
Analysis of pancreas wet/dry weight
ratio
The pancreatic tissue was removed from liquid
nitrogen and weighed to obtain the wet weight. Subsequently, the
pancreatic tissue was dried at 60°C for 48 h and weighed to obtain
the dry weight. Finally, the wet/dry weight ratios of the pancreas
were calculated.
Analysis of biochemical indexes in serum
and pancreas
The amylase and lipase activities were determined on
an automatic biochemical analyzer Hitachi 7600-110 (Hitachi, Tokyo,
Japan). Tumor necrosis factor α (TNFα), interleukin (IL)-6, HO-1
and IL-10 levels in serum were assessed using ELISA kits
(eBioscience, Inc., San Diego, CA, USA). Malondialdehyde (MDA),
superoxide dismutase (SOD) and myeloperoxidase (MPO) levels in the
pancreas were also evaluated using specific kits (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China) according to the
manufacturer's instructions.
Detection of HO-1 activity and CO content
in pancreatic tissue
HO-1 activity was assessed in pancreas samples as
previously reported (27,28). Briefly, tissue samples were
homogenized in cool phosphate-buffered saline (PBS; pH 7.4) and
centrifuged at 18,000 × g for 15 min at 4°C. The reaction system (1
ml) was composed of tissue homogenate (600 μl), 0.8 mmol/l
nicotin-amide adenine dinucleotide phosphate (NADPH), 1 mmol/l
glucose-6-phosphate, 0.2 U glucose 6-phosphate dehydrogenase and
2.5 mmol/l protohemin. After thorough mixing, the reaction was
incubated for 1 h at 37°C in a water bath with shaking in the dark;
chloroform was added to terminate the reaction. The amounts of
generated bilirubin were determined by absorbance at 464 and 530
nm. HO-1 activity was expressed as the amount of bilirubin in
nmol/h/mg protein. The CO content in the homogenates was determined
using a CO assay kit (Nanjing Jiancheng Bioengineering Institute)
according to the manufacturer's instructions. The results were
expressed as μmol CO/g tissue.
Histological analysis of pancreas
tissue
The embedded pancreatic tissues were sectioned at 5
μm, and stained with hematoxylin and eosin (H&E) for
histological analysis, including the determination of pancreatic
edema, inflammatory cell infiltration, bleeding and necrotic cell
number. The double blind method was carried out by two pathologists
at Shanghai No. 9 People's Hospital (Shanghai, China). Pancreatic
tissue damage was evaluated as previously described by Rongione
et al (29) and as shown
in Table I: pathological score =
edema score + necrosis score + inflammatory cellular infiltration
score + bleeding score. The higher the pathological score, the more
severe the tissue damage. Five slices were assessed in each
group.
 | Table IHistological scoring for acute
pancreatitis, as described by Rongione et al (25). |
Table I
Histological scoring for acute
pancreatitis, as described by Rongione et al (25).
| Edema | Necrosis | Inflammatory cell
infiltration | Bleeding |
|---|
| 1 = Focally
increased between lobules | 1 = Periductal
necrosis (<5%) | 1 = In ducts
(around ductal margins) | 1 = In the
parenchyma bleeding (0–25%) |
| 2 = Diffusely
increased between lobules | 2 = Focal necrosis
(5–20%) | 2 = In the
parenchyma (in <50% of the lobules) | 2 = In the
parenchyma bleeding (25–50%) |
| 3 = Acini disrupted
and separated | 3 = Diffuse
parenchymal necrosis (20–50%) | 3 = In the
parenchyma (in 50–75% of the lobules) | 3 = In the
parenchyma bleeding (50–75%) |
| 4 = Obvious lobules
separated | 4 = Diffuse
parenchymal necrosis >50% | 4 = In the
parenchyma (>75% of the lobules) | 4 = In the
parenchyma bleeding >75 |
Reverse transcription-quantitative PCR
(RT-qPCR)
RNA was extracted using TRIzol reagent (Invitrogen
Life Technologies, Carlsbad, CA, USA). cDNA was obtained using a
reverse transcription kit (Invitrogen Life Technologies), and qPCR
performed using SYBR-Green (Takara Bio, Inc., Tokyo, Japan) on an
ABI 7900 instrument (Applied Biosystems Foster City, CA, USA). The
TNFα, IL-6, IL-10 and HO-1 mRNA levels were detected and the
relative mRNA levels were normalized to β-actin using the ΔΔCt
method. The primers used are listed in Table II.
 | Table IIPrimers used in this study. |
Table II
Primers used in this study.
| Genes | Primers |
|---|
| TNFα | F:
5′-TCTCTTCAAGGGACAAGGCTG-3′ |
| R:
5′-ATAGCAAATCGGCTGACGGT-3′ |
| IL-6 | F:
5′-CTGGTCTTCTGGAGTTCCGTTTC-3′ |
| R:
5′-CATAGCACACTAGGTTTGCCGAG-3′ |
| HO-1 | F:
5′-GATAGAGCGCAACAAGCAGAA-3′ |
| R:
5′-CAGTGAGGCCCATACCAGAAG-3′ |
| IL-10 | F:
5′-CTTACTGACTGGCATGAGGATCA-3′ |
| R:
5′-GCAGCTCTAGGAGCATGTGG-3′ |
| β-actin | F:
5′-GTCCCTCACCCTCCCAAAAG-3′ |
| R:
5′-GCTCCCTCAAC-ACCTCAACCC-3′ |
Western blot analysis
The pancreatic tissue (50 mg) was pulverized after
being snap-frozen in liquid nitrogen. RIPA lysis buffer (Beyotime
Institute of Biotechnology, Suzhou, China) was then added for total
protein extraction. Pancreatic nucleoproteins were obtained using a
specific kit (Nanjing KeyGen Biotech., Co., Ltd., Nanjing, China).
Total protein concentrations were quantified using the BCA kit
(Nanjing KeyGen Biotech., Co., Ltd.). Primary antibodies against
HO-1 (sc-1796), inhibitor α of NF-κB (IκBα; sc-1643) (both from
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), NF-κB p65
(ab16502; Abcam, Cambridge, MA, USA), β-actin (sc-47778) and lamin
B (sc-6216) (both from Santa Cruz Biotechnology, Inc.) were used.
Donkey anti-goat IgG (Code no. 705-625-147), donkey anti-mouse IgG
(Code no. 715-625-150) and donkey anti-rabbit IgG (Code no.
711-625-152) secondary antibodies were from Jackson Laboratory (Bar
Harbor, ME, USA). Images were acquired on an Odyssey infrared
fluorescent scanning imaging system (LI-COR Biosciences, Inc.,
Lincoln, NE, USA). Grey values of bands were quantitatively
analyzed using Quantity One software, and relative protein levels
expressed as the target band value/internal reference band
value.
Immunohistochemical staining
Immunohistochemistry was employed to assess NF-κB
expression in the pancreatic tissue specimens as previously
described (30). The paraffin
-embedded tissue sections were deparaffinized and incubated with 3%
hydrogen peroxide for 10 min to block endogenous peroxidase.
Antigen retrieval was then carried out with citrate buffer (0.01 M,
pH 6.0) followed by blocking in goat serum. Following incubation
for 15 min at room temperature, anti-NF-κB p65 (ab16502; 250×
dilution) antibodies were added followed by overnight incubation at
4°C. The following day, biotin-labeled goat anti-rabbit IgG was
added followed by incubation at 37°C for 15 min. The slides were
then incubated at 37°C for 15 min in the presence of
peroxidase-conjugated streptavidin. The sections were stained with
3,3′-diaminobenzidine (DAB) and counterstained with hematoxylin.
Color separation was carried out with 1% hydrochloride and alcohol,
followed by a 20-min wash. Five high power fields (×400
magnification) were randomly selected in each sample for analysis.
Positive NF-κB signals appeared brown. The positive rate was
expressed as number of positive cells/number of total cells using
the Image-Pro Plus software version 6.0 (Media Cybernetics,
Rockville, MD, USA).
Statistical analyses
Data are presented as the means ± standard error of
the mean (SEM) and analyzed using the Statistical Package for the
Social Sciences (SPSS) 18.0 software (SPSS, Inc., Chicago, IL,
USA). An appropriate statistical test, either the Student's t-test
or one-way analysis of variance (ANOVA) with post-hoc Tukey's test,
was used to assess differences between groups. A value of P<0.05
was considered to indicate a statistically significant
difference.
Results
Luteolin markedly improves inflammatory
cell infiltration and necrosis in the pancreas
In order to assess the effects of luteolin on SAP
and determine an optimal dose with no observed adverse effect
(NOAE) in mice, we first evaluated its pharmacological toxicity and
LD50 value, which was 460 mg/kg (data not shown).
Subsequently, routine biochemical parameters of the liver and
kidneys were assessed following treatment with luteolin. No obvious
liver or kidney toxicity was observed in the mice treated with
luteolin at 100 mg/kg. However, treatment with 200 mg/kg of
luteolin induced toxicity in mice. We also observed that luteolin
protected the mice from cerulein plus LPS-induced SAP in a
dose-dependent manner (25, 50 and 100 mg/kg) (data not shown).
Therefore, the optimal dose of luteolin was 100 mg/kg, and this
used in the subsequent experiments.
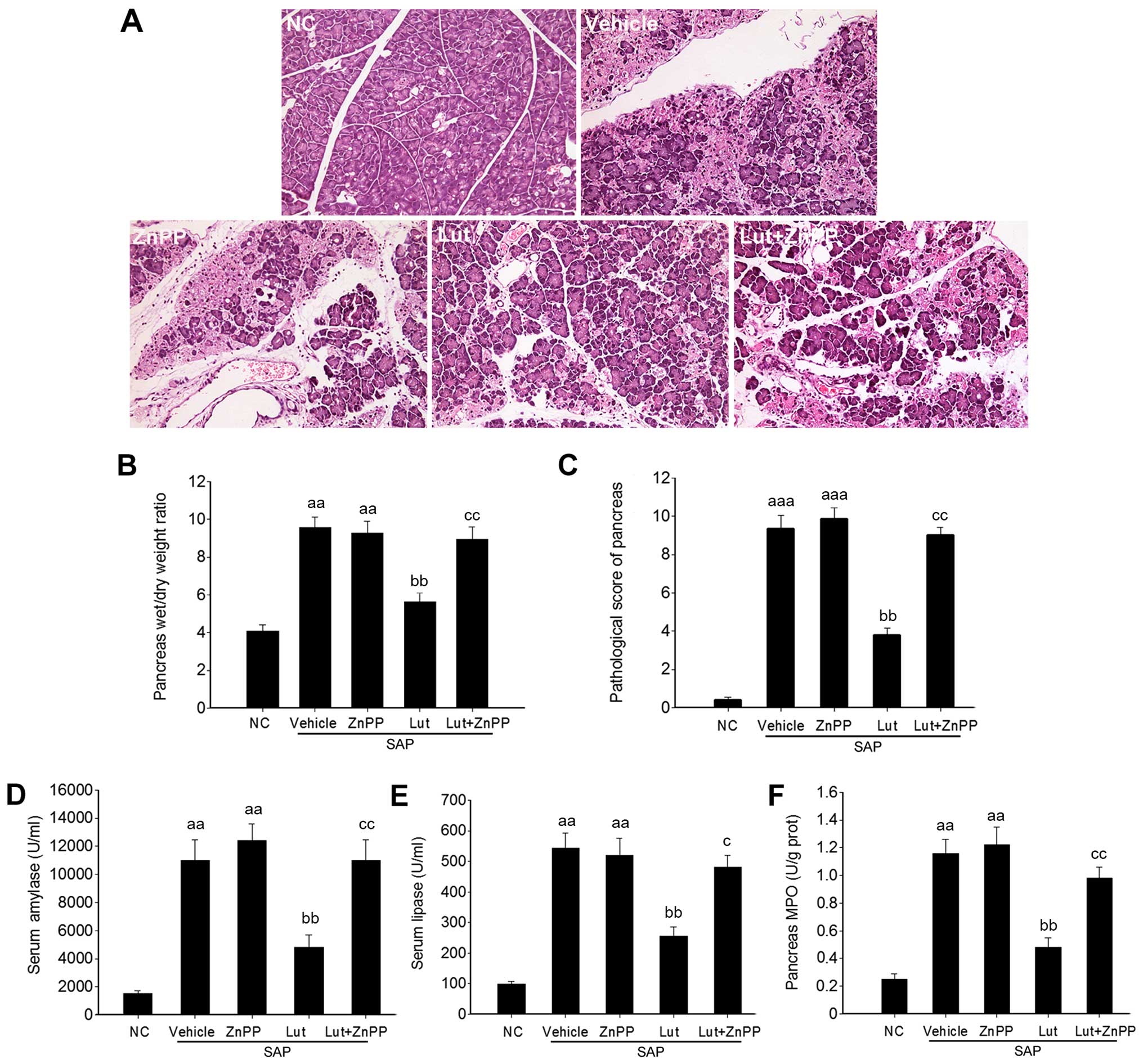
To investigate the potential protective role of
luteolin in SAP, the wet/dry weight ratios of the pancreas and
histological data of the pancreatic tissues and SAP-associated
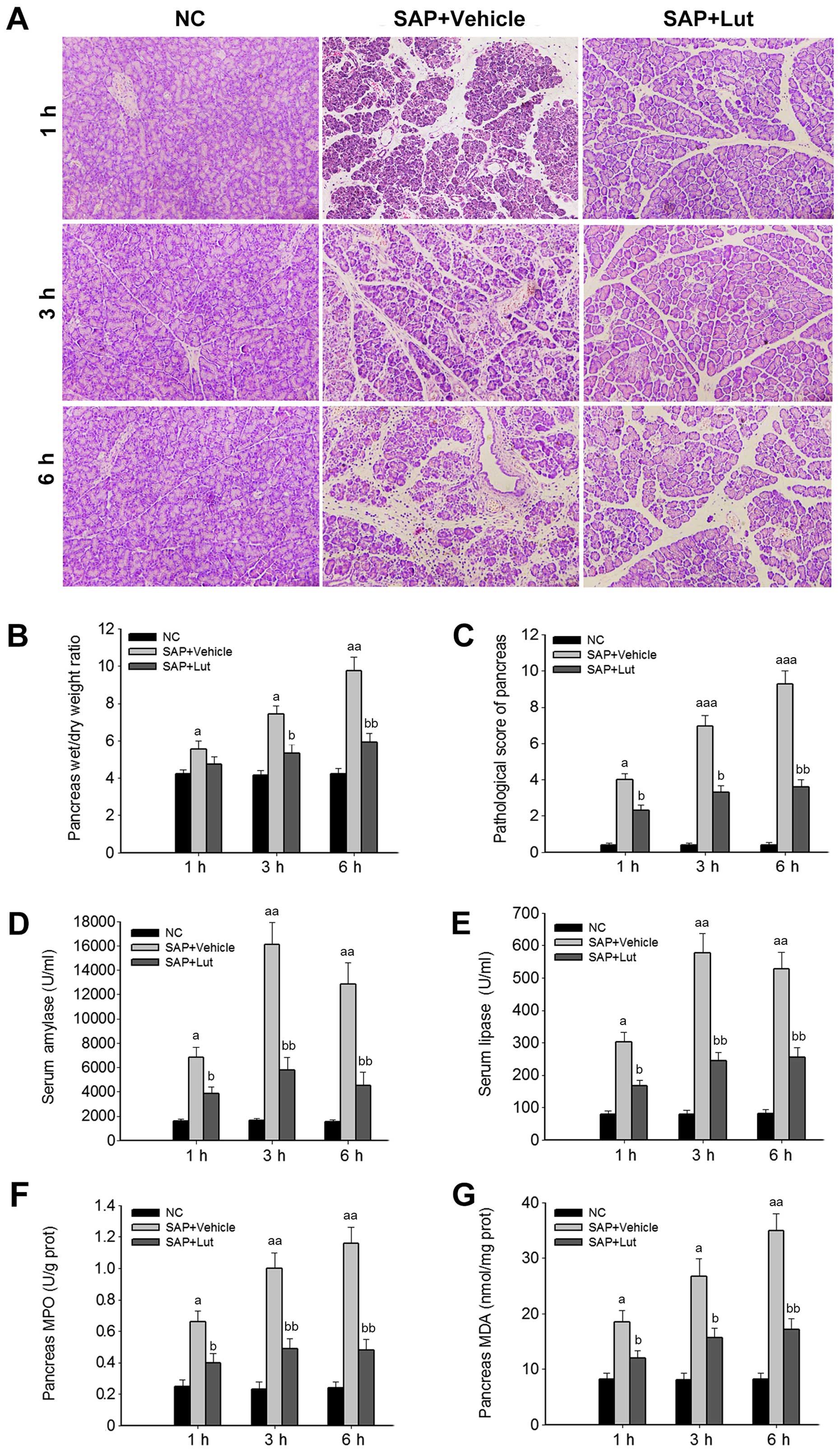
pathological markers were analyzed. The histological data revealed
inflammatory acinar cell infiltration and degenerative necrosis of
local acinar cells in the vehicle group at 1 h. Patchy necrosis of
acinar cells with processing lesions was observed at 3 h.
Disappearance of acinar cell structure with large necrotic areas
and bleeding around the pancreas was further noted at 6 h. However,
in the Lut group, pancreatic edema and inflammatory cell
infiltration were present at 3 and 6 h, but pancreatic acinar cell
necrosis was less severe (local acinar cell necrosis and unobvious
bleeding around the pancreas) compared with the SAP control group
(Fig. 2A). In addition, compared
with the SAP control group, treatment with luteolin significantly
reduced the wet/dry weight ratios of the pancreas at 3 and 6 h
(Fig. 2B). Accordingly, the
pathological scores of the pancreas in the Lut group were
significantly decreased compared with those obtained in the SAP
control group at 1, 3 and 6 h (Fig.
2C). In agreement with the changes observed in the pancreas,
the serum amylase and lipase amounts, and the MPO and MDA levels in
the pancreas were significantly suppressed in the Lut group
compared with the SAP control group values (Fig. 2D–G).
Treatment with luteolin significantly
decreases pro-inflammatory cytokine levels and increases the
amounts of HO-1 and IL-10 in serum and pancreas
Based on the above-mentioned results, the effects of
luteolin on the systemic inflammatory response were further
investigated. Considering that HO-1 is a potential target for the
treatment of SAP (18,19), the effects of luteolin on HO-1
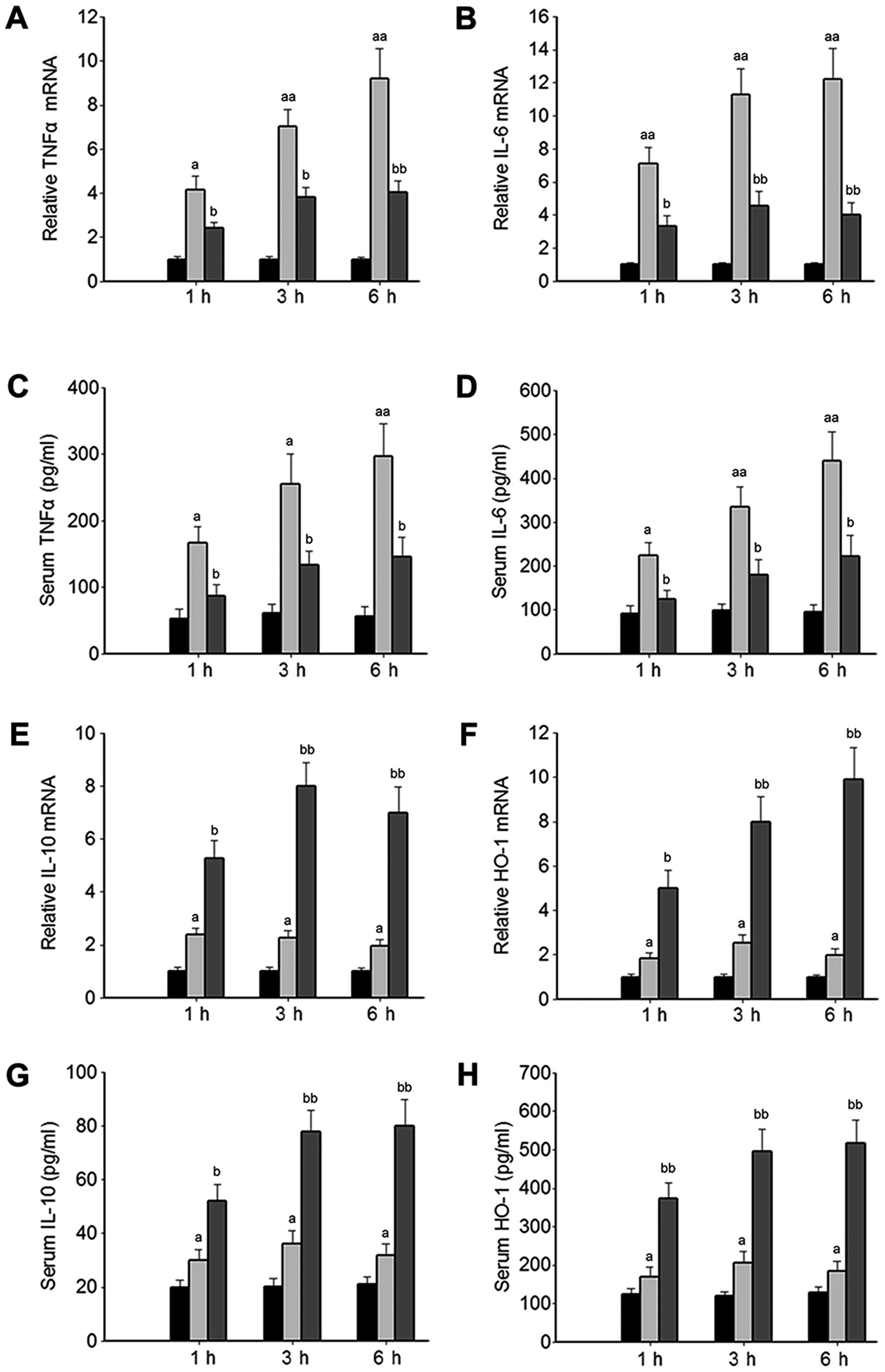
expression were analyzed. The levels of pro-inflammatory cytokines,
including TNFα and IL-6 in serum and pancreatic tissue were
significantly increased in the mice with SAP treated with the
vehicle (SAP control group) compared with those of the normal mice
(NC group), and were significantly inhibited in the mice with SAP
by treatment with luteolin (Lut group, Fig. 3A–D). Furthermore, the HO-1 and
IL-10 mRNA levels were significantly increased in the Lut group
compared with the SAP control group (Fig. 3E and F). Consistently, the levels
of HO-1 and IL-10 in serum were significantly elevated in the Lut
group compared with the SAP control group (Fig. 3G and H).
ZnPP effectively suppresses the enzymatic
activity, but not the expression of HO-1 in the pancreas
To determine whether the protective effects of
luteolin are dependent on HO-1, ZnPP, a selective HO-1 activity
competitive inhibitor (31,32), was used in this study. Firstly,
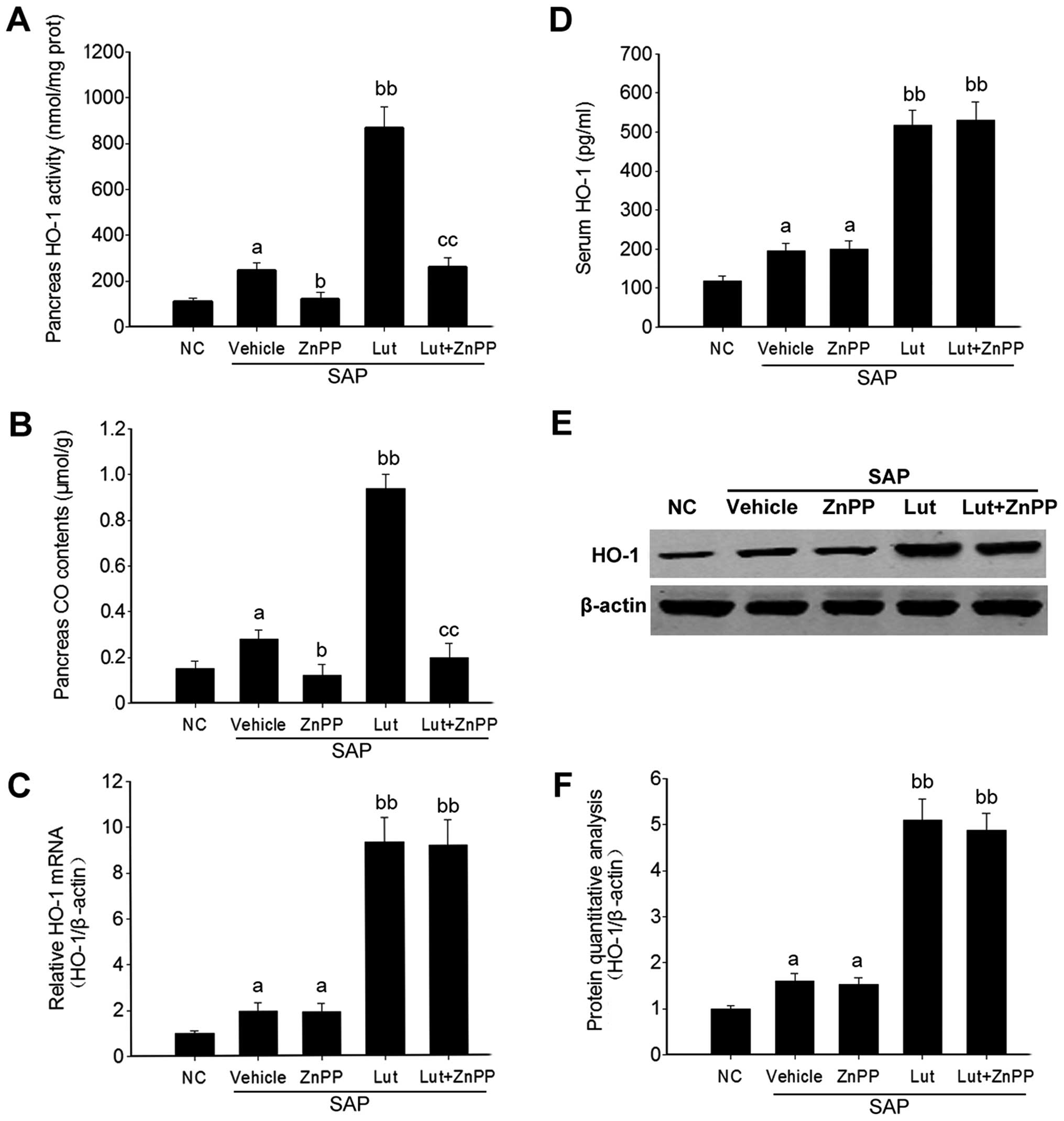
the inhibitory effect of ZnPP was determined. The results revealed
that HO-1 activity and the level of another catalyst, CO, were
significantly inhibited in the pancreatic tissues of mice in both
the ZnPP group and the Lut + ZnPP group (Fig. 4A and B). However, the mRNA and
protein levels of HO-1 in the pancreas, as well as the HO-1 level
in serum, were not significantly affected by ZnPP treatment
(Fig. 4C–F).
Inhibition of HO-1 abolishes the
protective effects of luteolin on cerulein plus LPS-induced SAP in
mice
Based on effective inhibition of HO-1 activity by
ZnPP, the protective effects of luteolin against SAP were further
investigated. As shown in Fig.
5A, tissue necrosis and inflammatory infiltration were more
severe in the mice in the Lut + ZnPP group than those in the Lut
group. The wet/dry ratios and pathological scores of the pancreas
were also significantly higher in the mice in the Lut + ZnPP group
than those in the Lut group (Fig. 5B
and C). In addition, the serum amylase and lipase levels, and
pancreatic MPO amounts were almost totally reversed in the mice in
the Lut + ZnPP compared with those in the Lut group (Fig. 5D–F). Although ZnPP inhibited HO-1
activity to a certain degree, which was similar to the level of
HO-1 activity in the vehicle group (Fig. 4A), ZnPP almost totally abolished
the effect of luteolin in terms of histological phenotype,
pathological scores and biochemical indexes (Fig. 5). In addition, treatment with ZnPP
only did not alter the phenotype of mice with SAP (Fig. 5).
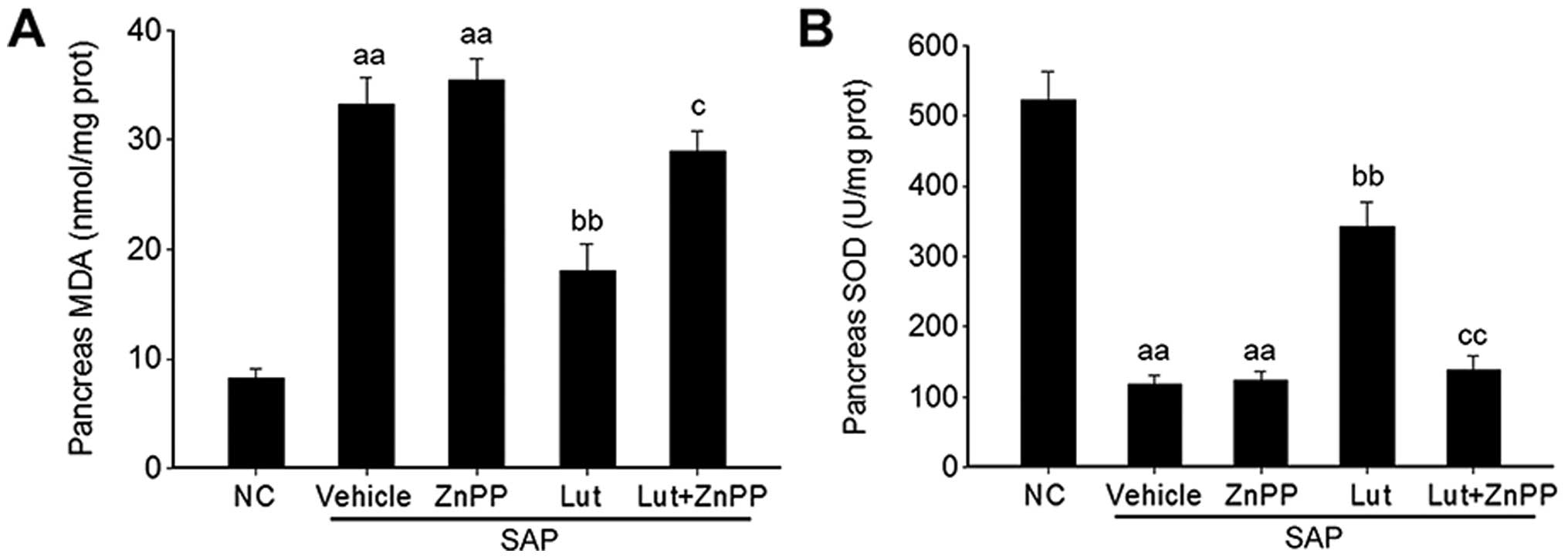
Luteolin improves the antioxidant
activity via HO-1 in the pancreas
Given that luteolin plays a key role in the
regulation of oxidative stress (18), the effects of luteolin and
luteolin plus ZnPP on the MDA level and SOD activity were
determined in order to explore the mechanisms through which
luteolin improves SAP through its antioxidant activity via the
induction of HO-1 expression. The MDA levels in the pancreas were
significantly increased in the mice with SAP treated with the
vehicle (SAP control group), and were significantly reduced by
treatment with luteolin (Lut group); the inhibitory effects of
luteolin on the MDA levels were abolished by treatment of the mice
with ZnPP (Lut + ZnPP group) (Fig.
6A). Conversely, SOD activity in the pancreas was significantly
decreased in the mice in the SAP control group, and was
significantly stimulated by treatment with luteolin (Lut group);
however, this effect was also abolished by treatment with ZnPP (Lut
+ ZnPP group) (Fig. 6B). In
addition, treatment with ZnPP only (ZnPP group) did not affect the
serum MDA levels or pancreatic SOD activity (Fig. 6A and B).
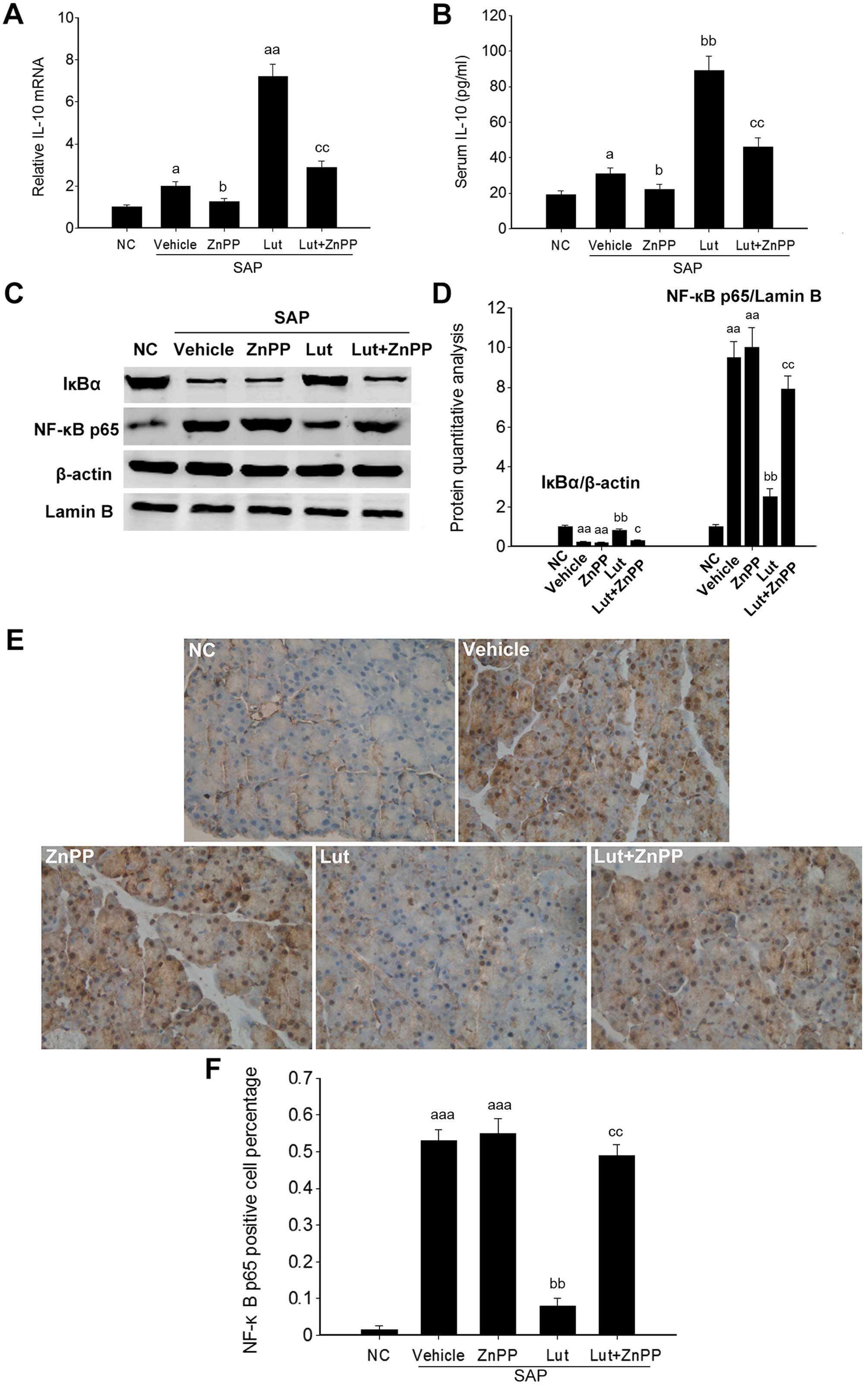
Luteolin improves the anti-inflammatory
activity via HO-1 in the pancreas
To further investigate the involvement of HO-1 in
the effects of luteolin on SAP, inflammatory markers were
determined in the pancreatic tissues of mice in the Lut and Lut +
ZnPP groups. The Pncreatic IL-10 mRNA levels and the serum IL-10
levels were significantly increased in the Lut group, and were
significantly decreased in the Lut + ZnPP group (Fig. 7A and B). Given that HO-1 plays a
role in regulating NF-κB signaling, the expression levels of two
key proteins of the NF-κB signaling pathway, including IκBα and
NF-κB p65 were analyzed. NF-κB p65 expression was significantly
increased in the pancreatic tissues of mice with SAP, and was
significantly suppressed by treatment with luteolin. Conversely,
the expression of IκBα, an inhibitory protein of NF-κB p65, was
significantly inhibited in the pancreatic tissues of mice with SAP,
and was significantly stimulated by treatment with luteolin
(Fig. 7C and D). The activation
of NF-κB is associated with its translocation from the cytoplasm to
the nucleus. Immunohistochemical analysis indicated that the NF-κB
p65 levels in the nucleus were markedly increased in the pancreatic
tissues of mice with SAP, and was significantly decreased in the
Lut group (Fig. 7E), which
suggested that luteolin effectively suppressed the activation of
NF-κB in the pancreas of mice with SAP. In addition, treatment with
ZnPP only did not affect the pancreatic IL-10 mRNA level and serum
IL-10 amounts, as well as the expression of IκBα and NF-κB p65
(Fig. 7).
Discussion
During the SAP inflammatory response, common signal
transduction pathways are induced by pro-inflammatory cytokines and
oxidative stress, and are activated by the mitogen-activated
protein kinase (MAPK) and NF-κB signaling pathways, resulting in
inflammatory cascade amplification (33). The improvement of oxidative stress
and the inflammatory response is the main mechanism of alleviating
SAP. In this study, luteolin protected mice from cerulein plus
LPS-induced SAP, via the HO-1 regulation of oxidative stress and
the inflammatory process.
In this study, the continuous administration of
cerulein combined with LPS was used to induce SAP, which shares the
pathological changes observed clinically in SAP; the model is
therefore widely used in mouse studies of SAP (25,34). In the present study, cerulein
combined with LPS effectively induced SAP in mice, which displayed
higher pathological scores in the pancreas, increased levels of
TNFα and IL-6, and enhanced NF-κB activity in the pancreas,
suggesting that the cerulein plus LPS-induced SAP model is an
effective tool for studying the mechanisms of action and treatment
effects in SAP.
Luteolin has been wildly used as a traditional Asian
medicine for the treatment of diseases associated with oxidative
injury and acute inflammation, such as endotoxemia, acute lung
injury, acute myocardial infarction and hepatitis (4–6).
However, the pharmacological toxicity of luteolin has not been
studied in detail. In this study, we firstly assessed the
pharmacological toxicity of luteolin. The results revealed that the
LD50 for luteolin was 460 mg/kg. Of note, 100 mg/kg
luteolin displayed no liver or kidney toxicity, suggesting a good
safety profile for luteolin (data not shown).
Luteolin has many known properties:
anti-inflammatory, antioxidant and anti-fibrotic effects (4,6,35).
The oral administration of luteolin (10 mg/kg) has been shown to
effectively suppress neutrophil infiltration, as well as the
increase in TNFα and IL-6 levels in bronchoalveolar lavage fluid
from bleomycin-instilled C57BL/6J mice (36). Luteolin injected intraperitoneally
at different doses of 10 or 25 mg/kg has also beehn shown to
significantly increase SOD1 activity and decrease MDA levels in
mice with permanent middle cerebral artery occlusion, which results
in alleviated neurological deficits, infarct volume and brain water
content (37). In the present
study, luteolin significantly alleviated SAP in mice at 100 mg/kg.
With regards to its anti-inflammatory activity, luteolin
significantly suppressed the cerulein plus LPS-induced increase in
TNFα and IL-6 levels in the pancreas and serum, and the IL-10
levels were significantly increased by treatment with luteolin.
Furthermore, luteolin significantly suppressed the cerulein plus
LPS-induced increase in MDA levels, and stimulated SOD1 activity in
the pancreas. Taken together, these results suggest that luteolin
is a protective agent for SAP, and HO-1 is a potential drug target
for the treatment of SAP. Given that a higher dose (100 mg/kg) of
luteolin was used in this study compared with previous studies (10
or 25 mg/kg) (38), lower doses
of luteolin and their effects on SAP warrant further
investigation.
During the occurrence of SAP, high oxygen free
radicals (OFRs) cause biomembrane lipid peroxidation, generating
MDA; proteins are further peroxidized, causing DNA damage and
resulting in apoptosis (39–41). OFR can also activate NF-κB,
enhancing TNFα and IL-6 levels. Furthermore, pro-inflammatory
cytokines combined with OFR trigger common signaling pathways
mainly by NF-κB activation, further causing inflammatory cascade
reactions (42–44). In this study, the MDA levels in
the model group increased at 6 h compared with the NC group,
suggesting an overt oxidative stress in SAP. Luteolin significantly
decreased the MDA levels; however, this effect was suppressed when
luteolin was administered in combination with ZnPP. This indicated
that the antioxidant properties of luteolin depended, at least in
part, on HO-1. On the other hand, SOD is an important OFR scavenger
(45). Not only has HO-1 been
described as an antioxidant, it also induces the expression of
other antioxidants, such as SOD and catalase to fight oxidative
stress (46,47). In this study, SOD activity in the
pancreatic tissue at 6 h in the model group was significantly
reduced compared with the NC group value. Luteolin overtly
increased SOD activity, which was reduced following combined
treatment with ZnPP, a HO-1 activity inhibitor. This suggested that
luteolin induces SOD expression by upregulating HO-1 expression,
playing an antioxidant role. Previous studies have indicated that
panhematin leads to the rapid induction and activation of
pancreatic HO-1, and hemin-like compounds or hemin-activated
macrophages upregulate HO-1 expression in the pancreas to prevent
AP via the upregulation of HO-1 (16,17). These findings indicated that HO-1
is a potential target for the treatment of SAP.
It is widely known that over-activated inflammatory
cells and factors are closely related to the damage caused by SAP,
and inflammatory factors are regulated by NF-κB at the gene level
(48). NF-κB is widely
distributed in multiple cell types, and is mainly composed of p50
and p65 subunits in the form of homo- or hetero-dimers. In SAP, a
high expression of NF-κB enhances pro-inflammatory cytokines, such
as TNFα and IL-6 to release. Moreover, TNFα and IL-6 increase NF-κB
activity by positive feedback, further enhancing the transcription
of related cytokines, which enhances the severity of the condition.
It has been demonstrated that limiting NF-κB activation
significantly reduces the severity of SAP (49). Antioxidants can inhibit NF-κB
activation effectively, including n-acetyl-cysteine, pyrrolidine
dithiocarbamate (PDTC) and ethyl acetonate, which have certain
therapeutic effects. However, NF-κB inhibitors with non-specific
effects will suppress other signaling pathways and have potential
toxicity, which limit their applications (50). As shown in this study, luteolin
significantly inhibited NF-κB p65 expression and stimulated IκBα
expression. The suppression of HO-1 activity using ZnPP abolished
the effects of luteolin NF-κB p65 and IκBα expression. Therefore,
luteolin inhibits NF-κB activity to exert anti-inflammatory effects
in a HO-1-dependent manner.
HO-1 and its catalysts involved in maintaining
cellular homeostasis play important protective roles by
counteracting oxidative damage, the inflammatory response and cell
apoptosis, and also regulate cell proliferation. It was recently
demonstrated that CO is also an endothelium-derived relaxing factor
involved in the regulation of several physiological and
pathological processes, with many effects, such as relaxing the
vascular smooth muscle, inhibiting platelet aggregation and
neutrophil adhesion, and regulating nerve, body fluid and endocrine
functions (51,52). CO at low concentrations
selectively inhibits the expression of pro inflammatory cytokines
and increases IL-10 levels through MAPK, which is activated by
mitogen (53,54). IL-10 is an important
anti-inflammatory cytokine generated by Th2-lymphocytes; it can
inhibit TNFα and IL-6 synthesis, and can protect against multiple
organ damage caused by SAP. In the early stages of SAP, IL-10
levels are slightly increased. However, this increase is much less
than that of pro-inflammatory cytokines, which enhances the
severity of the patient's condition. Therefore, finding an adequate
balance between pro- and anti-inflammatory cytokines in the
treatment of SAP may be a useful solution. In this study, IL-10
expression in the SAP group was slightly higher than that in the NC
group as detected by ELISA, in line with previous studies (55,56). Of note, luteolin significantly
increased the level of IL-10, which depends on HO-1 activity. These
results suggested that luteolin plays its protective role in SAP by
inducing IL-10 in a HO-1-dependent manner.
Accumulating evidence indicates that nuclear factor
(erythroid-derived 2)-like 2 (Nrf2) signaling is involved in the
protective effects of luteolin against oxidative stress (32,57–60). Furthermore, Nrf2, as the guardian
of redox homeostasis, activates a battery of antioxidant and
cytoprotective genes that share in common a cis-acting
enhancer sequence termed antioxidant response element (ARE) that
include HO-1 in response to oxidative stress (61). Thus, we hypothesized that luteolin
induces HO-1 expression in an Nrf2-dependent manner, and we aim to
investigate this hypothesis in future studies.
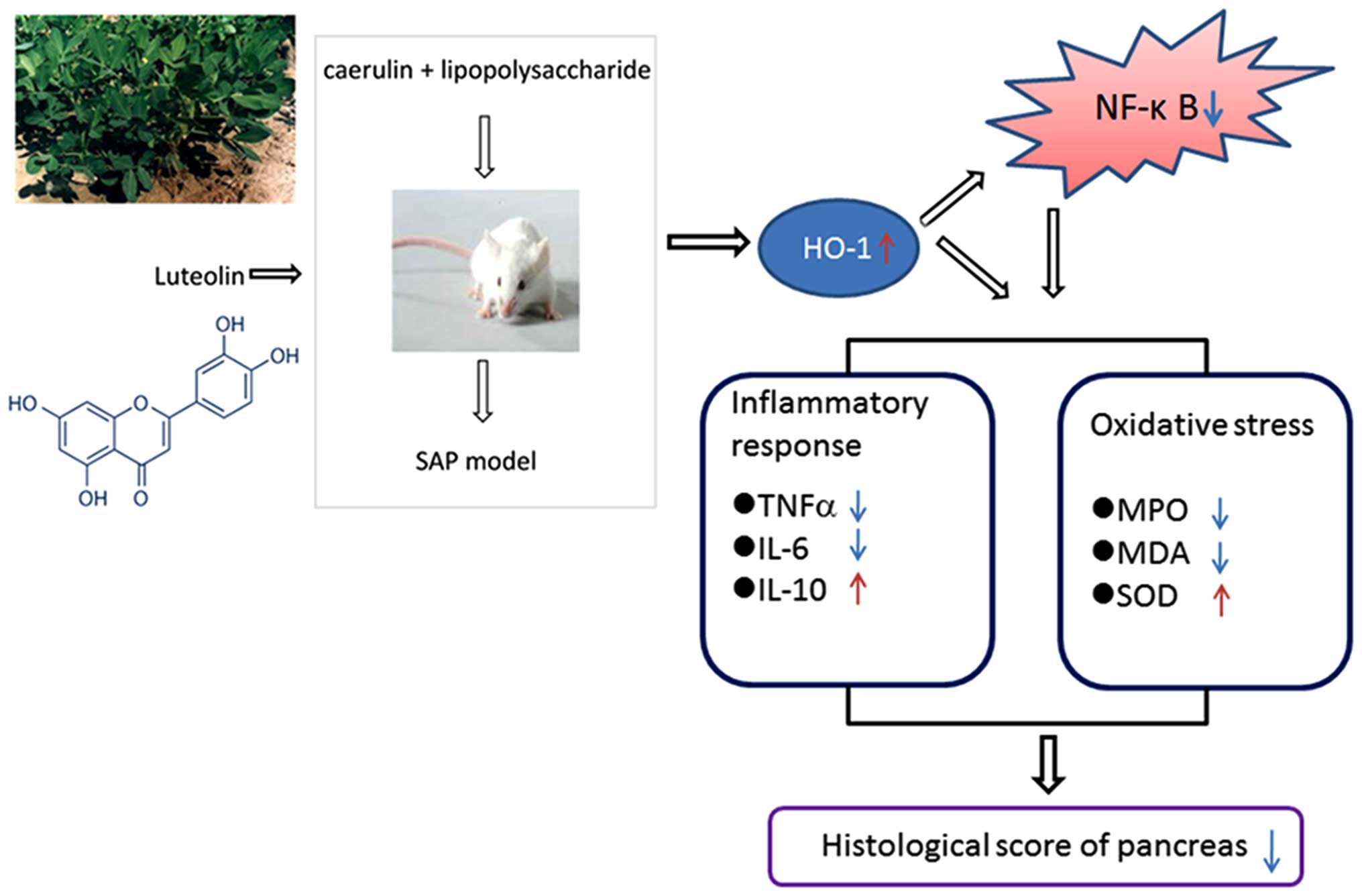
In conclusion, our data demonstrated that luteolin
significantly improved cerulein plus LPS-induced SAP in mice. These
effects depended on HO-1 induction by luteolin: increased HO-1
levels decreased NF-κB activity, increased anti-inflammatory and
antioxidant activities, reduced lipid peroxidation, and increased
IL-10 levels (Fig. 8). These
findings suggest that luteolin is a potential protective agent for
SAP.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81570580 and 81270928) and
Doctoral Innovation Fund of Shanghai Jiao Tong University School of
Medcine (grant no. BXJ201440).
References
|
1
|
Di Fabio F, Abu Hilal M and Johnson CD:
Acute pancreatitis: mild, severe or potentially fatal.
Pancreatology. 11:373–375. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rebours V: Acute pancreatitis: an overview
of the management. Rev Med Interne. 35:649–655. 2014.In French.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Talukdar R and Vege SS: Acute
pancreatitis. Curr Opin Gastroenterol. 31:374–379. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Seelinger G, Merfort I and Schempp CM:
Anti-oxidant, anti-inflammatory and anti-allergic activities of
luteolin. Planta Med. 74:1667–1677. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kuo MY, Liao MF, Chen FL, Li YC, Yang ML,
Lin RH and Kuan YH: Luteolin attenuates the pulmonary inflammatory
response involves abilities of antioxidation and inhibition of MAPK
and NFκB pathways in mice with endotoxin-induced acute lung injury.
Food Chem Toxicol. 49:2660–2666. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nabavi SF, Braidy N, Gortzi O,
Sobarzo-Sanchez E, Daglia M, Skalicka-Woźniak K and Nabavi SM:
Luteolin as an anti-inflammatory and neuroprotective agent: a brief
review. Brain Res Bull. 119:1–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sung J and Lee J: Anti-Inflammatory
activity of butein and luteolin through suppression of NFκB
activation and induction of heme oxygenase-1. J Med Food.
18:557–564. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maines MD: The heme oxygenase system: a
regulator of second messenger gases. Annu Rev Pharmacol Toxicol.
37:517–554. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu M, Wang J, Fang Q, Liu P, Chen S, Zhe
N, Lin X, Zhang Y, Zhao J and Zhou Z: High expression of heme
oxygenase-1 in target organs may attenuate acute graft-versus-host
disease through regulation of immune balance of TH17/Treg. Transpl
Immunol. 37:10–17. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu B, Song HL, Yang Y, Yin ML, Zhang BY,
Cao Y, Dong C and Shen ZY: Improvement of liver transplantation
outcome by heme oxygenase-1-transduced bone marrow mesenchymal stem
cells in rats. Stem Cells Int. 2016:92350732016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nath KA: Heme oxygenase-1 and acute kidney
injury. Curr Opin Nephrol Hypertens. 23:17–24. 2014. View Article : Google Scholar :
|
|
12
|
Lever JM, Boddu R, George JF and Agarwal
A: Heme oxygenase-1 in kidney health and disease. Antioxid Redox
Signal. 25:165–183. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Durante W: Targeting heme oxygenase-1 in
vascular disease. Curr Drug Targets. 11:1504–1516. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu ML, Ho YC and Yet SF: A central role of
heme oxygenase-1 in cardiovascular protection. Antioxid Redox
Signal. 15:1835–1846. 2011. View Article : Google Scholar
|
|
15
|
Chang M, Xue J, Sharma V and Habtezion A:
Protective role of hemeoxygenase-1 in gastrointestinal diseases.
Cell Mol Life Sci. 72:1161–1173. 2015. View Article : Google Scholar :
|
|
16
|
Habtezion A, Kwan R, Akhtar E, Wanaski SP,
Collins SD, Wong RJ, Stevenson DK, Butcher EC and Omary MB:
Panhematin provides a therapeutic benefit in experimental
pancreatitis. Gut. 60:671–679. 2011. View Article : Google Scholar
|
|
17
|
Nakamichi I, Habtezion A, Zhong B, Contag
CH, Butcher EC and Omary MB: Hemin-activated macrophages home to
the pancreas and protect from acute pancreatitis via heme
oxygenase-1 induction. J Clin Invest. 115:3007–3014. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nuhn P, Mitkus T, Ceyhan GO, Künzli BM,
Bergmann F, Fischer L, Giese N, Friess H and Berberat PO: Heme
oxygenase 1-generated carbon monoxide and biliverdin attenuate the
course of experimental necrotizing pancreatitis. Pancreas.
42:265–271. 2013. View Article : Google Scholar
|
|
19
|
Habtezion A, Kwan R, Yang AL, Morgan ME,
Akhtar E, Wanaski SP, Collins SD, Butcher EC, Kamal A and Omary MB:
Heme oxygenase-1 is induced in peripheral blood mononuclear cells
of patients with acute pancreatitis: a potential therapeutic
target. Am J Physiol Gastrointest Liver Physiol. 300:G12–G20. 2011.
View Article : Google Scholar :
|
|
20
|
Bellezza I, Tucci A, Galli F, Grottelli S,
Mierla AL, Pilolli F and Minelli A: Inhibition of NF-κB nuclear
translocation via HO-1 activation underlies α-tocopheryl succinate
toxicity. J Nutr Biochem. 23:1583–1591. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yeh CH, Chen TP, Wang YC, Lin YM and Lin
PJ: HO-1 activation can attenuate cardiomyocytic apoptosis via
inhibition of NF-kappaB and AP-1 translocation following cardiac
global ischemia and reperfusion. J Surg Res. 155:147–156. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
López Martín A and Carrillo Alcaraz A:
Oxidative stress and acute pancreatitis. Rev Esp Enferm Dig.
103:559–562. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rakonczay Z Jr, Hegyi P, Takács T,
McCarroll J and Saluja AK: The role of NF-kappaB activation in the
pathogenesis of acute pancreatitis. Gut. 57:259–267. 2008.
View Article : Google Scholar
|
|
24
|
Peng Y, Gallagher SF, Landmann R, Haines K
and Murr MM: The role of p65 NF-kappaB/RelA in pancreatitis-induced
Kupffer cell apoptosis. J Gastrointest Surg. 10:837–847. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Norkina O, Graf R, Appenzeller P and De
Lisle RC: Caerulein-induced acute pancreatitis in mice that
constitutively overexpress Reg/PAP genes. BMC Gastroenterol.
6:162006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Niederau C, Ferrell LD and Grendell JH:
Caerulein-induced acute necrotizing pancreatitis in mice:
protective effects of proglumide, benzotript, and secretin.
Gastroenterology. 88:1192–1204. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song HJ, Shin CY, Oh TY and Sohn UD: The
protective effect of eupatilin on indomethacin-induced cell damage
in cultured feline ileal smooth muscle cells: involvement of HO-1
and ERK. J Ethnopharmacol. 118:94–101. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Song HJ, Shin CY, Oh TY, Min YS, Park ES
and Sohn UD: Eupatilin with heme oxygenase-1-inducing ability
protects cultured feline esophageal epithelial cells from cell
damage caused by indomethacin. Biol Pharm Bull. 32:589–596. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rongione AJ, Kusske AM, Kwan K, Ashley SW,
Reber HA and McFadden DW: Interleukin 10 reduces the severity of
acute pancreatitis in rats. Gastroenterology. 112:960–967. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Van Laere SJ, Van der Auwera I, Van den
Eynden GG, Elst HJ, Weyler J, Harris AL, van Dam P, Van Marck EA,
Vermeulen PB and Dirix LY: Nuclear factor-kappaB signature of
inflammatory breast cancer by cDNA microarray validated by
quantitative real-time reverse transcription-PCR,
immunohistochemistry, and nuclear factor-kappaB DNA-binding. Clin
Cancer Res. 12:3249–3256. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fujioka K, Kalish F, Wong RJ and Stevenson
DK: Inhibition of heme oxygenase activity using a microparticle
formulation of zinc protoporphyrin in an acute hemolytic newborn
mouse model. Pediatr Res. 79:251–257. 2016. View Article : Google Scholar
|
|
32
|
Sun GB, Sun X, Wang M, Ye JX, Si JY, Xu
HB, Meng XB, Qin M, Sun J, Wang HW, et al: Oxidative stress
suppression by luteolin-induced heme oxygenase-1 expression.
Toxicol Appl Pharmacol. 265:229–240. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu HS, Pan CE, Liu QG, Yang W and Liu XM:
Effect of NF-kappaB and p38 MAPK in activated monocytes/macrophages
on pro-inflammatory cytokines of rats with acute pancreatitis.
World J Gastroenterol. 9:2513–2518. 2003.PubMed/NCBI
|
|
34
|
Wang X, Wang B and Wu J:
Pancreatitis-associated protein-I mRNA expression in mouse pancreas
is upregulated by lipopolysaccharide independent of
cerulein-pancreatitis. J Gastroenterol Hepatol. 16:79–86. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Domitrović R, Jakovac H, Tomac J and Sain
I: Liver fibrosis in mice induced by carbon tetrachloride and its
reversion by luteolin. Toxicol Appl Pharmacol. 241:311–321. 2009.
View Article : Google Scholar
|
|
36
|
Chen CY, Peng WH, Wu LC, Wu CC and Hsu SL:
Luteolin ameliorates experimental lung fibrosis both in vivo and in
vitro: implications for therapy of lung fibrosis. J Agric Food
Chem. 58:11653–11661. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Qiao H, Dong L, Zhang X, Zhu C, Zhang X,
Wang L, Liu Z, Chen L, Xing Y, Wang C and Li Y: Protective effect
of luteolin in experimental ischemic stroke: upregulated SOD1, CAT,
Bcl-2 and claudin-5, down-regulated MDA and Bax expression.
Neurochem Res. 37:2014–2024. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Qiao H, Zhang X, Zhu C, Dong L, Wang L,
Zhang X, Xing Y, Wang C, Ji Y and Cao X: Luteolin downregulates
TLR4, TLR5, NF-κB and p-p38MAPK expression, upregulates the p-ERK
expression, and protects rat brains against focal ischemia. Brain
Res. 1448:71–81. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ramudo L and Manso MA: N-acetylcysteine in
acute pancreatitis. World J Gastrointest Pharmacol Ther. 1:21–26.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Modzelewski B, Janiak A and Hollynski J:
Hyperlipoproteinemia in necrotizing pancreatitis. Pol Merkur
Lekarski. 18:415–417. 2005.In Polish. PubMed/NCBI
|
|
41
|
Booth DM, Mukherjee R, Sutton R and
Criddle DN: Calcium and reactive oxygen species in acute
pancreatitis: friend or foe? Antioxid Redox Signal. 15:2683–2698.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hackert T and Werner J: Antioxidant
therapy in acute pancreatitis: experimental and clinical evidence.
Antioxid Redox Signal. 15:2767–2777. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim H: Inhibitory mechanism of lycopene on
cytokine expression in experimental pancreatitis. Ann N Y Acad Sci.
1229:99–102. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ramudo L, De Dios I, Yubero S, Vicente S
and Manso MA: ICAM-1 and CD11b/CD18 expression during acute
pancreatitis induced by bile-pancreatic duct obstruction: effect of
N-acetylcysteine. Exp Biol Med (Maywood). 232:737–743. 2007.
|
|
45
|
Li YY, Li XL, Yang CX, Zhong H, Yao H and
Zhu L: Effects of tetrandrine and QYT on ICAM-1 and SOD gene
expression in pancreas and liver of rats with acute pancreatitis.
World J Gastroenterol. 9:155–159. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fan J, Xu G, Jiang T and Qin Y:
Pharmacologic induction of heme oxygenase-1 plays a protective role
in diabetic retinopathy in rats. Invest Ophthalmol Vis Sci.
53:6541–6556. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Turkseven S, Kruger A, Mingone CJ,
Kaminski P, Inaba M, Rodella LF, Ikehara S, Wolin MS and Abraham
NG: Antioxidant mechanism of heme oxygenase-1 involves an increase
in superoxide dismutase and catalase in experimental diabetes. Am J
Physiol Heart Circ Physiol. 289:H701–H707. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kim H, Seo JY and Kim KH: NF-kappaB and
cytokines in pancreatic acinar cells. J Korean Med Sci. 15(Suppl):
S53–S54. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jin S, Orabi AI, Le T, Javed TA, Sah S,
Eisses JF, Bottino R, Molkentin JD and Husain SZ: Exposure to
radiocontrast agents induces pancreatic inflammation by activation
of nuclear factor-κB, calcium signaling, and calcineurin.
Gastroenterology. 149:753–764. 2015. View Article : Google Scholar
|
|
50
|
Yang X, Jin H, Liu K, Gu Q and Xu X: A
novel peptide derived from human pancreatitis-associated protein
inhibits inflammation in vivo and in vitro and blocks NF-kappa B
signaling pathway. PLoS One. 6:e291552011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Constantin M, Choi AJ, Cloonan SM and
Ryter SW: Therapeutic potential of heme oxygenase-1/carbon monoxide
in lung disease. Int J Hypertens. 2012:8592352012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Barbagallo I, Marrazzo G, Frigiola A,
Zappala A and Li Volti G: Role of carbon monoxide in vascular
diseases. Curr Pharm Biotechnol. 13:787–796. 2012. View Article : Google Scholar
|
|
53
|
MacGarvey NC, Suliman HB, Bartz RR, Fu P,
Withers CM, Welty-Wolf KE and Piantadosi CA: Activation of
mitochondrial biogenesis by heme oxygenase-1-mediated NF-E2-related
factor-2 induction rescues mice from lethal Staphylococcus aureus
sepsis. Am J Respir Crit Care Med. 185:851–861. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Drechsler Y, Dolganiuc A, Norkina O,
Romics L, Li W, Kodys K, Bach FH, Mandrekar P and Szabo G: Heme
oxygenase-1 mediates the anti-inflammatory effects of acute alcohol
on IL-10 induction involving p38 MAPK activation in monocytes. J
Immunol. 177:2592–2600. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Vasseur P, Devaure I, Sellier J, Delwail
A, Chagneau-Derrode C, Charier F, Tougeron D, Tasu JP, Rabeony H,
Lecron JC and Silvain C: High plasma levels of the pro-inflammatory
cytokine IL-22 and the anti-inflammatory cytokines IL-10 and IL-1ra
in acute pancreatitis. Pancreatology. 14:465–469. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang F, Fei J, Zhao B, Chen E and Mao E:
Protective effect of adenoviral transfer of heme oxygenase-1 gene
on rats with severe acute pancreatitis. Am J Med Sci. 348:224–231.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Pandurangan AK, Kumar SA, Dharmalingam P
and Ganapasam S: Luteolin, a bioflavonoid inhibits
azoxymethane-induced colon carcinogenesis: involvement of iNOS and
COX-2. Pharmacogn Mag. 10(Suppl 2): S306–S310. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Pandurangan AK, Dharmalingam P, Sadagopan
SK and Ganapasam S: Luteolin inhibits matrix metalloproteinase 9
and 2 in azoxymethane-induced colon carcinogenesis. Hum Exp
Toxicol. 33:1176–1185. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Pandurangan AK, Ananda Sadagopan SK,
Dharmalingam P and Ganapasam S: Luteolin, a bioflavonoid inhibits
azoxymethane-induced colorectal cancer through activation of Nrf2
signaling. Toxicol Mech Methods. 24:13–20. 2014. View Article : Google Scholar
|
|
60
|
Huang CS, Lii CK, Lin AH, Yeh YW, Yao HT,
Li CC, Wang TS and Chen HW: Protection by chrysin, apigenin, and
luteolin against oxidative stress is mediated by the Nrf2-dependent
up-regulation of heme oxygenase 1 and glutamate cysteine ligase in
rat primary hepatocytes. Arch Toxicol. 87:167–178. 2013. View Article : Google Scholar
|
|
61
|
Innamorato NG, Rojo AI, García-Yagüe AJ,
Yamamoto M, de Ceballos ML and Cuadrado A: The transcription factor
Nrf2 is a therapeutic target against brain inflammation. J Immunol.
181:680–689. 2008. View Article : Google Scholar : PubMed/NCBI
|