Introduction
Glioblastoma (GBM) is the most common primary brain
tumour in adults and is usually lethal. Standard treatment for GBM
includes tumour resection [from 78% to nearly 100% of the
contrast-enhanced tumour volume in magnetic resonance imaging
(MRI)] followed by concomitant chemotherapy, particularly with
temozolomide, and radiation therapy (1,2).
However, despite these multimodal treatments, the mean survival
time for patients with GBM is 12–14 months (2,3).
Because of its aggressive infiltrative behaviour, most GBMs rapidly
invade neighbouring brain structures and have a high recurrence
rate (4). Up to 85% of
recurrences are within the previous radiation treatment field
(5).
5-Aminolevulinic acid (5-ALA) is a natural
biochemical precursor of heme that is converted by the heme
synthesis pathway into protoporphyrin IX (PpIX), a photosensitizing
porphyrin. Following systemic administration, 5-ALA metabolism
leads to the relative accumulation of PpIX in tumour cell
mitochondria (6,7). In particular, 5-ALA induces a high
accumulation of PpIX in glioma cells; thus, florescence-guided
resection using 5-ALA in high-grade glioma treatment has been
useful in determining tumour borders, facilitating tumour resection
compared to conventional microsurgery (8–10).
Although several porphyrin compounds, including hematoporphyrin
derivatives (HpD) and Photofrin, can act as radiosensitizers, the
radiosensitizing activity of 5-ALA remains controversial (11–13). We previously demonstrated the
radiosensitizing effects of 5-ALA in experimental gliomas in
vitro and in vivo (14–16). Other studies similarly reported
that radiotherapy combined with 5-ALA strongly inhibits tumour
growth in mouse melanoma, colon cancer, and prostate cancer models
(17–19). A recent DNA microarray study
further demonstrated that 5-ALA enhanced gene expression induced by
ionizing irradiation (IR) in cancer cells, such as those involved
in cell cycle arrest (20).
The mechanism underlying the radiosensitizing effect
of 5-ALA remains unclear. In general, IR causes the ionization and
excitation of water, leading to reactive oxygen species (ROS)
production (particularly hydroxyl radical). Primary ROS induce
double-strand breaks (DSB) in nuclear DNA and reproductive cell
death (21,22). A previous study reported that
production of ROS, such as superoxide, singlet oxygen, and hydroxyl
radical, was increased in PpIX solutions under IR exposure
(23). Consistently, we confirmed
that the production of primary ROS was increased, and the
subcellular localization of primary ROS coincided with
5-ALA-induced PpIX in glioma cells after 5-ALA treatment under IR
exposure (14). Thus,
5-ALA-induced PpIX may be a key mediator and enhance the production
of primary ROS under IR exposure, thereby increasing reproductive
cell death in tumours. Otherwise, this initial reaction during
exposure to IR occurs only for a short period of time (21). Furthermore, we observed strong
inhibition of in vivo tumour growth under IR exposure with
5-ALA administration (15).
Tumour specimens at 16 days after IR with 5-ALA administration
exhibited strong aggregation of cytotoxic Iba-1-positive
macrophages with phagocytosis (15). Even after considering the
immunological effects, whether tumour growth in glioma is strongly
inhibited by short-term ROS-induced DSB in nuclear DNA and
subsequent reproductive cell death alone remains to be
determined.
Recent findings demonstrated that IR increases
mitochondrial membrane potential, respiration, and ATP production
and causes ROS production in the mitochondria of tumour cells
(24–26). These ROS (particularly superoxide
and hydrogen peroxide) are secondary species produced by primary
ROS (particularly hydroxyl radical) via water radiolysis (25,27). Importantly, this secondary process
occurs in mitochondria of tumour cells and induces long-lasting ROS
production >24 h after IR exposure (24,28). During these processes, impaired
mitochondria that were damaged by oxidative stress from primary ROS
cause several changes, such as synthesis of new mitochondria
(increased mitochondrial mass), synthesis of new mitochondrial DNA
(mitochondrial polyploidization), and instability of the
mitochondrial electron transport chain (ETC) complex, to cope with
their own oxidative damage. However, these impaired mitochondria
also produce large amounts of secondary ROS and propagate oxidative
damage to the surrounding normal mitochondria and nucleus. This
intermitochondrial communication amplifies the damage signal and
production of secondary ROS, permanently damages the remaining
normal mitochondria and nuclear DNA, and eventually leads to cell
death (25). Previously, we
demonstrated that the delayed intracellular production of ROS 12 h
after IR exposure was enhanced in glioma cells after 5-ALA
treatment, particularly in the cytoplasm (16). In addition, 5-ALA treatment can
cause marked accumulation of PpIX in glioma cell mitochondria and
improve mitochondrial dysfunction by improving cytochrome c
oxidase activity to restore oxidative phosphorylation, thereby
disrupting the Warburg effect in tumour cells (29). Thus, we hypothesized that 5-ALA,
by inducing PpIX accumulation within tumour cell mitochondria, may
cause mitochondrial changes following IR exposure, such as
increased mitochondrial mass and altered activity of the ETC
complex in gliomas, leading to cell death.
In the present study, we assessed the
radiosensitizing effect of 5-ALA using ciprofloxacin (CPFX), which
is a known enhancer of 5-ALA-induced PpIX accumulation (31), in glioma cells. We evaluated the
subcellular localization of secondary ROS and mitochondria by
confocal laser scanning microscopy and the delayed changes in
mitochondrial mass and ETC complex activity after IR exposure with
different amounts of 5-ALA-induced PpIX in glioma cells. Finally,
we considered the possible mechanisms underlying the
radiosensitizing effect of 5-ALA based on the effects observed for
mitochondria.
Materials and methods
Chemicals
5-ALA was provided by SBI Pharmaceuticals Co., Ltd.
(Tokyo, Japan) and dissolved in fresh culture medium at a final
concentration of 1 mM for intracellular ROS imaging and 0.3 mM for
other in vitro experiments. CPFX was purchased from WAKO
Pure Chemical Co. (Osaka, Japan). CM-H2DCFDA (DCFD), MitoTracker
Green FM, and Mito Tracker Deep Red FM were purchased from
Sigma-Aldrich K.K. (Tokyo, Japan) and Invitrogen (Carlsbad, CA,
USA). DCFD was dissolved in Hank's Balanced Salt Solution (HBSS)
with calcium and magnesium and without red phenol 1X (Invitrogen)
at a final concentration of 10 μM. MitoTracker Green FM and
MitoTracker Deep Red FM were dissolved in fresh culture medium at a
final concentration of 50 nM. Other materials were of the highest
grade available.
Culture and treatment of cells
A rat glioma cell line (9L) and a human glioma cell
line (U251) were used (16). The
9L cells were cultured for several days in RPMI-1640, and U251
cells were cultured in Dulbecco's modified Eagle's medium (DMEM)
with 10% fetal bovine serum (FBS) at 37°C before use. These cell
lines were maintained in a humidified incubator with 5%
CO2 at 37°C. Cells were passaged in the exponential
growth phase using a 0.05% trypsin solution containing 0.5 mM
ethylenediaminetetraacetic acid. Cells at 70% confluency were used
in the subsequent experiments. 5-ALA was dissolved in RPMI-1640
(9L) or DMEM (U251) with 10% FBS to achieve a final concentration
of 0.3 or 1 mM and incubated with 9L or U251 cells for 4 h. These
5-ALA concentrations and incubation time were used in the
subsequent experiments. CPFX was dissolved in water and then
diluted to the appropriate concentration in RPMI-1640 (9L) or DMEM
(U251) with 10% FBS and incubated with 9L or U251 cells for 24 h.
For cotreatment with CPFX and 5-ALA, the cells were initially
incubated with CPFX medium for 24 h, washed with phosphate-buffered
saline (PBS), and immediately incubated in complete medium
containing 0.3 mM 5-ALA for 4 h (30,31).
MTT assay
Cells were seeded in 96-well plates at a density of
5×103 cells/well and incubated in medium containing
various concentration of CPFX for 24 h (30,31). The MTT assay was conducted using a
Cell Titer 96R Non-Radioactive Cell Proliferation assay (Promega
K.K., Tokyo, Japan). After exposure to CPFX, the cells were washed
with PBS, and MTT solution was added. The cells were incubated with
MTT solution for 4 h, the reaction was terminated with Stop
solution, and the cells were incubated another 4 h. The absorbance
of the coloured solutions was measured at 550/655 nm on Microplate
Manager software (version 5.2.1; Bio-Rad Laboratories, Hercules,
CA, USA). The results for cell viability are presented relative to
control cells.
Flow cytometric analyses
After each period of incubation, the cells were
detached from the substratum by trypsinization and collected by
centrifugation (400 × g for 3 min at 4°C). Immediately afterwards,
the cells were resuspended in cold PBS/FBS and analysed using a
flow cytometer (EC800; Sony Biotechnology, Tokyo, Japan). Overall,
3×106 cells in each sample were evaluated. Analyses of
flow cytometric data were carried out using FlowJo (Tree Star Inc.,
Ashland, OR, USA). The median fluorescence intensity (MFI) of
various parameters in treated glioma cells relative to that of the
control cells was calculated for each cell line as we described
previously (16).
Evaluation of PpIX fluorescence intensity
in glioma cells
Cells were seeded in 100-mm culture dishes and
cultured in complete medium containing 0.3 mM 5-ALA for 4 h (5-ALA
group) or CPFX for 24 h (CPFX group) and then washed with PBS. The
cells in the 5-ALA with CPFX group were incubated with 5 μM
CPFX for 24 h, washed with PBS, and immediately incubated in
complete medium containing 0.3 mM 5-ALA for 4 h (30,31). After culture, we immediately
assessed PpIX fluorescence using a flow cytometer (excitation, 488
nm; emission, 640/30 nm band-pass filter). Control cells were not
exposed to 5-ALA or CPFX. Analyses of flow cytometric data were
carried out using FlowJo. The MFI of PpIX for treated cells
relative to that of the control cells was calculated for each cell
line.
Evaluation of cell responses to IR
Cells were seeded at a density of 100 cells (0 Gy)
or 400 cells (8 Gy) for 9L and 400 cells (0 Gy) or 4,000 cells (8
Gy) for U251 per 60-mm culture dish based on preliminary
measurements to determine the optimal cell concentration for each
irradiation dose. The cells in each group were treated as described
above and then washed with PBS (30,31). Cells exposed only to IR without
5-ALA or CPFX were prepared for comparison. The culture dishes were
stored in a light-protected humidified chamber to avoid activation
of 5-ALA-induced PpIX. After the medium was replaced with 3 ml
fresh culture medium, the cells were irradiated using a
Gamma-irradiator (Gammacell 40 Extractor; Nordion International,
Inc., Kanata, ON, Canada) at 8 Gy (0.71 Gy/min). During IR, the
culture dishes were kept in a dark container at room temperature.
The response of the cells to IR was evaluated using a standard
colony-forming assay (14).
Briefly, after 12 days of irradiation treatment, the cells were
fixed and stained using a Diff Quick Staining kit (Sysmex Co.,
Kobe, Japan). Two culture dishes were prepared for each dose point,
and three independent experiments were performed. Only colonies
containing ≥50 cells were scored. Plating efficiency was determined
for unirradiated controls treated in the same manner and maintained
under the same conditions. The surviving fraction was then
calculated and presented relative to control cells without 5-ALA or
CPFX treatment and IR exposure.
Detection of subcellular localization of
ROS and mitochondria 12 h after IR in 9L cells
Intracellular production of ROS was detected using
the oxidant-sensitive fluorescent probe DCFD, and mitochondria were
detected using Mito Tracker Deep Red FM with a confocal
laser-scanning microscope (LMS5 Pascal; Carl Zeiss, Jena, Germany)
(16). Cells were seeded in 35-mm
glass-bottom dishes (Asahi Techno Glass, Tokyo, Japan). After each
period of incubation with 1 mM 5-ALA, cells were washed with PBS
and exposed to 10 Gy IR. At 12 h after IR, the cells were washed
twice with PBS, incubated with 50 nM Mito Tracker Deep Red FM for
30 min, washed again with PBS, and immediately incubated with 10
μM DCFD for 15 min. After washing twice with PBS, the cells
were observed immediately. Mito Tracker Deep Red fluorescence
(excitation, 488/633 nm; emission, 650 nm band-pass filter) and
DCFD fluorescence (excitation, 488/633 nm; emission, 505–530-nm
band-pass filter) were imaged on a confocal laser-scanning
microscope. In addition, control cells without 5-ALA treatment and
with or without IR were prepared for comparison. All procedures
were carried out in the dark.
Evaluation of intracellular ROS levels
after IR in glioma cells
Intracellular production of ROS 12 h after IR was
assessed using DCFD, an oxidant-sensitive fluorescent probe, using
a flow cytometer (16). Cells in
the 5-ALA group, the CPFX group, and the 5-ALA with CPFX group were
seeded in 100-mm culture dishes and prepared as described above.
Immediately thereafter, the cells were irradiated with 8 Gy
γ-irradiation at room temperature in the dark using a γ-irradiator.
At 12 h after exposure to IR, the cells were incubated with 10
μM DCFD for 15 min at 37°C and washed twice with PBS. DCFD
fluorescence was then analysed using a flow cytometer (excitation,
488 nm; emission, 525/50 nm band-pass filter) as described above.
Control cells did not receive 5-ALA and CPFX treatment or IR
exposure. Cells exposed to IR without 5-ALA or CPFX treatment were
also prepared for comparison. Analyses of flow cytometric data were
carried out using FlowJo. The MFI of DCFD for treated cells
relative to that of control cells was calculated for each cell
line.
Evaluation of mitochondrial mass after IR
in glioma cells
Mitochondrial mass was measured by staining cells
with MitoTracker Green FM (24).
The cells were seeded in 100-mm culture dishes as described above
and then irradiated with 8 Gy γ-irradiation at room temperature in
the dark. Immediately and at 12 h after IR, the cells were
incubated with 50 nM Mito Tracker Green FM for 30 min at 37°C and
then washed twice with PBS. Mito Tracker Green FM fluorescence was
analysed using a flow cytometer (excitation, 488 nm; emission,
525/50 nm band-pass filter). Control cells were not exposed to IR
or 5-ALA and CPFX. Analyses of flow cytometric data were carried
out using FlowJo. The MFI of Mito Tracker Green FM for treated
cells relative to that of the control cells was calculated for each
cell line.
Evaluation of mitochondrial ETC activity
after IR by western blot analysis
Mitochondrial ETC activity was evaluated by western
blotting using Total OXPHOS Rodent WB Antibody Cocktail (32,33). Cells were seeded in 100-mm culture
dishes and treated as described above. Thereafter, the cells were
irradiated with 8 Gy in the dark using a γ-irradiator. Before,
immediately after, and 12 h after IR, the cells were washed with
PBS and lysed in 0.4 ml lysis buffer [RIPA buffer:Halt protease
inhibitor cocktail (1:100; Thermo Fisher Scientific, Rockford, IL,
USA)]. Protein (20 μg) was separated by poly-acrylamide gel
electrophoresis (4–15%) and transferred to PVDF membranes (Bio-Rad
Laboratories). The membranes were blocked for 1 h with 1% bovine
serum albumin (Sigma-Aldrich) in Tris-buffered saline, pH 7.6,
containing 0.1% Tween-20 (T-TBS) and washed once with T-TBS. The
membranes were incubated for 1 h with 1:800 Total OXPHOS Rodent WB
Antibody Cocktail (ab110413) or 1:1,000 rabbit anti-β-actin
polyclonal antibody (ab8227) (both from Abcam) at room temperature,
washed three times for 10 min in T-TBS, and incubated for 1 h with
1:50,000 HRP-linked sheep anti-mouse IG (NA931) or 1:50,000
HRP-linked donkey anti-rabbit IG (NA934) (both from GE Healthcare,
Logan, UT, USA) at room temperature. The blots were visualized
using ImmunoStar® LD (Wako, Tokyo, Japan) with a
C-DiGit™ Blot Scanner (LI-COR, Lincoln, NE, USA), quantitatively
analysed using the public domain software ImageJ 1.46r (National
Institutes of Health, Bethesda, MD, USA), and normalized to actin
for each experiment. The ratio of blots was compared to that of
control bands. Control blots were not exposed to IR, 5-ALA or
CPFX.
Statistical analyses
Data are presented as means ± SE and were analysed
with Fisher's protected least significant difference test.
P<0.05 was considered statistically significant.
Results
Cytotoxic influence of CPFX on viability
of glioma cell lines
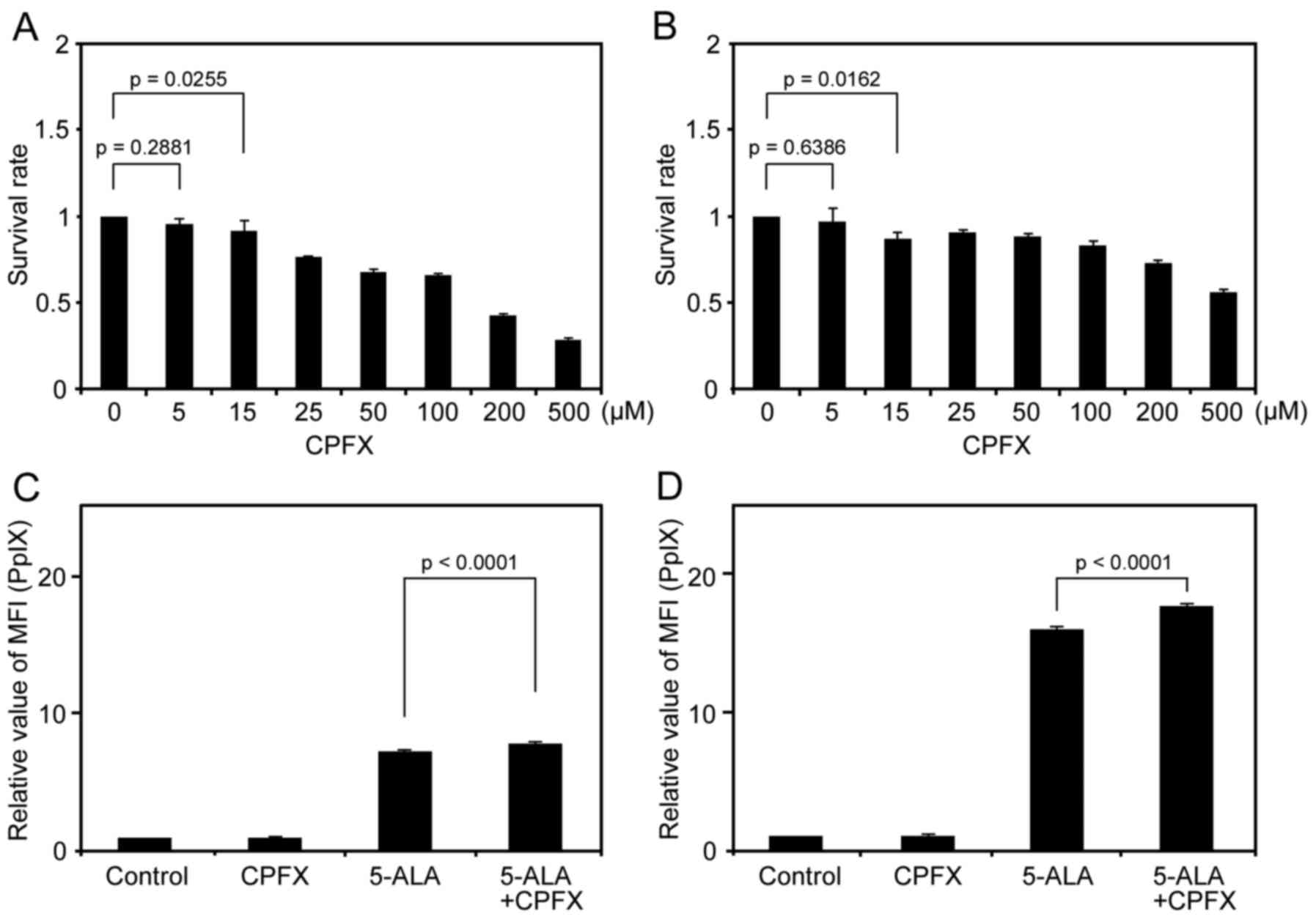
First, we evaluated an appropriate concentration of
CPFX for 9L and U251 cells using an MTT assay at 5, 15, 25, 50,
100, 200 and 500 μM CPFX. Cytotoxicity was not observed at 5
μM CPFX for 9L and U251 cells (p=0.2881 and 0.6386,
respectively). However, at 15 μM CPFX, the survival rate of
cells significantly decreased compared to controls in the two cell
lines (p=0.0255 in 9L, and p=0.0162 in U251) (Fig. 1A and B). To avoid CPFX
cytotoxicity for glioma cells, we therefore used 5 μM CPFX
for all subsequent experiments.
Low-dose CPFX enhances 5-ALA-induced PpIX
accumulation and leads to increased cell death after IR in glioma
cells
We examined the influence of low-dose CPFX on the
intracellular accumulation of 5-ALA-induced PpIX in glioma cells
using flow cytometric analyses. The MFI of PpIX in 5-ALA-treated
cells was obviously increased compared with the control cells in
the two cell lines, consistent with our previous results (Fig. 1C and D) (16). In addition, the MFI of PpIX in
cells treated with 5-ALA and low-dose CPFX was significantly
increased compared with that for cells treated only with 5-ALA for
the two cell lines (Fig. 1C and
D). The relative MFI of PpIX (mean ± SE) in 9L cells was
7.28±0.05 and 7.89±0.06 in the 5-ALA group and the 5-ALA with CPFX
group, respectively (p<0.0001). Similarly, the relative MFI of
PpIX (mean ± SE) in U251 cells was 16.0±0.13 and 17.6±0.20 in the
5-ALA group and the 5-ALA with CPFX group, respectively
(p<0.0001). By contrast, the relative MFI of PpIX (mean ± SE) in
9L and U251 cells was 1.03±0.01 and 1.03±0.01, respectively, in the
CPFX group, and there were no significant differences compared to
controls for either cell line (p=0.4852 and 0.8427,
respectively).
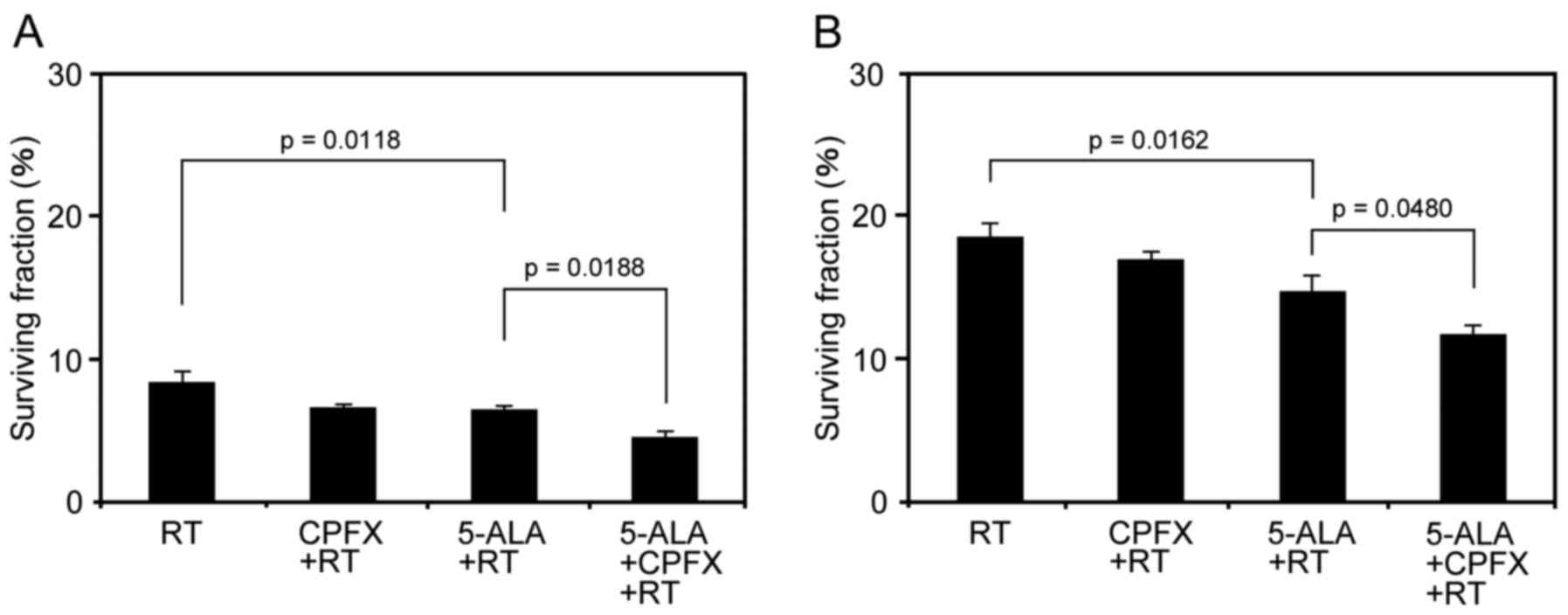
Next, we tested the influence of IR under the
production of 5-ALA-induced PpIX enhanced by low-dose CPFX
treatment in 9L and U251 cells. In 9L cells, the surviving fraction
(%) in the RT group, RT with 5-ALA treatment group, and RT with
5-ALA and CPFX treatment group was 8.38±0.70, 6.38±0.33, and
4.57±0.37, respectively (Fig.
2A). The surviving fraction in the RT with 5-ALA treatment
group was significantly lower than that in the RT group (p=0.0118).
Moreover, RT with 5-ALA and CPFX treatment significantly decreased
the surviving fraction compared to that for RT with 5-ALA treatment
(p=0.0188) (Fig. 2A). In U251
cells, the surviving fraction (%) in the RT group, the RT with
5-ALA treatment group, and the RT with 5-ALA and CPFX treatment
group was 18.50±1.05, 14.7±1.16 and 11.7±0.62, respectively
(Fig. 2B). The surviving fraction
in the RT with 5-ALA treatment group was significantly lower than
that in the RT group (p=0.0162). Similar to the results with 9L
cells, RT with 5-ALA and CPFX treatment significantly decreased the
surviving fraction compared to that for RT with 5-ALA treatment
(p=0.0480) in U251 cells (Fig.
2B). The surviving fraction in the RT with CPFX treatment group
(6.65±0.15) was significantly decreased compared to that in the RT
group in 9L cells (p=0.0234), but there was no significant
difference between RT with CPFX (16.95±0.58) and only RT in U251
cells (p=0.2567).
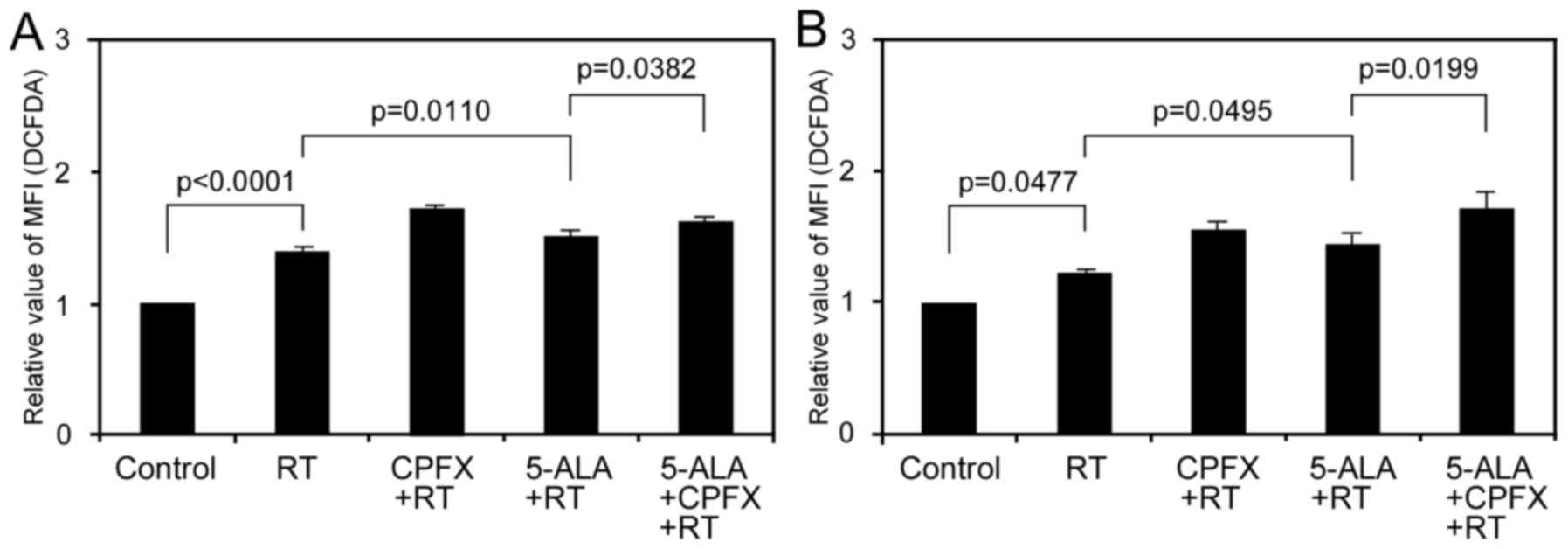
Differences in the amount of
5-ALA-induced PpIX accumulation influence delayed production of
intracellular ROS after IR in glioma cells
To evaluate the influence of different amounts of
5-ALA-induced PpIX accumulation on the delayed production of
intracellular ROS after IR, we evaluated ROS production 12 h after
IR in glioma cells treated with low-dose CPFX using flow cytometric
analysis. In 9L cells, the relative MFI of DCFD in the RT, RT with
CPFX treatment, RT with 5-ALA treatment, and RT with 5-ALA and CPFX
treatment groups was 1.40±0.04, 1.72±0.02, 1.522±0.39 and
1.62±0.04, respectively (Fig.
3A). Although delayed ROS production with RT only significantly
increased compared to the control (p<0.0001), that in the RT
with 5-ALA treatment group also significantly increased compared to
that in the RT group (p=0.0110), consistent with our previous
results (16). Moreover, delayed
ROS production in the RT with 5-ALA and CPFX treatment group
significantly increased compared to that in the RT with 5-ALA
treatment group (p=0.0382). In the U251 cells, the relative MFI of
DCFD in the RT, RT with CPFX treatment, RT with 5-ALA treatment,
and RT with 5-ALA and CPFX treatment groups was 1.23±0.02,
1.56±0.06, 1.45±0.09 and 1.72±0.13, respectively (Fig. 3B). Similar to the effects in 9L
cells, although delayed ROS production with RT only significantly
increased compared to control (p=0.0477), that in the RT with 5-ALA
treatment group also significantly increased compared to that in
the RT group (p=0.0495) in the U251 cells, consistent with our
previous results (16).
Additionally, delayed ROS production in the RT with 5-ALA and CPFX
treatment group also significantly increased compared to that in
the RT with 5-ALA treatment group (p=0.0199). By contrast, delayed
ROS production for RT with CPFX treatment significantly increased
compared to that for RT alone in the two cell lines (p<0.001 in
9L, p=0.0048 in U251).
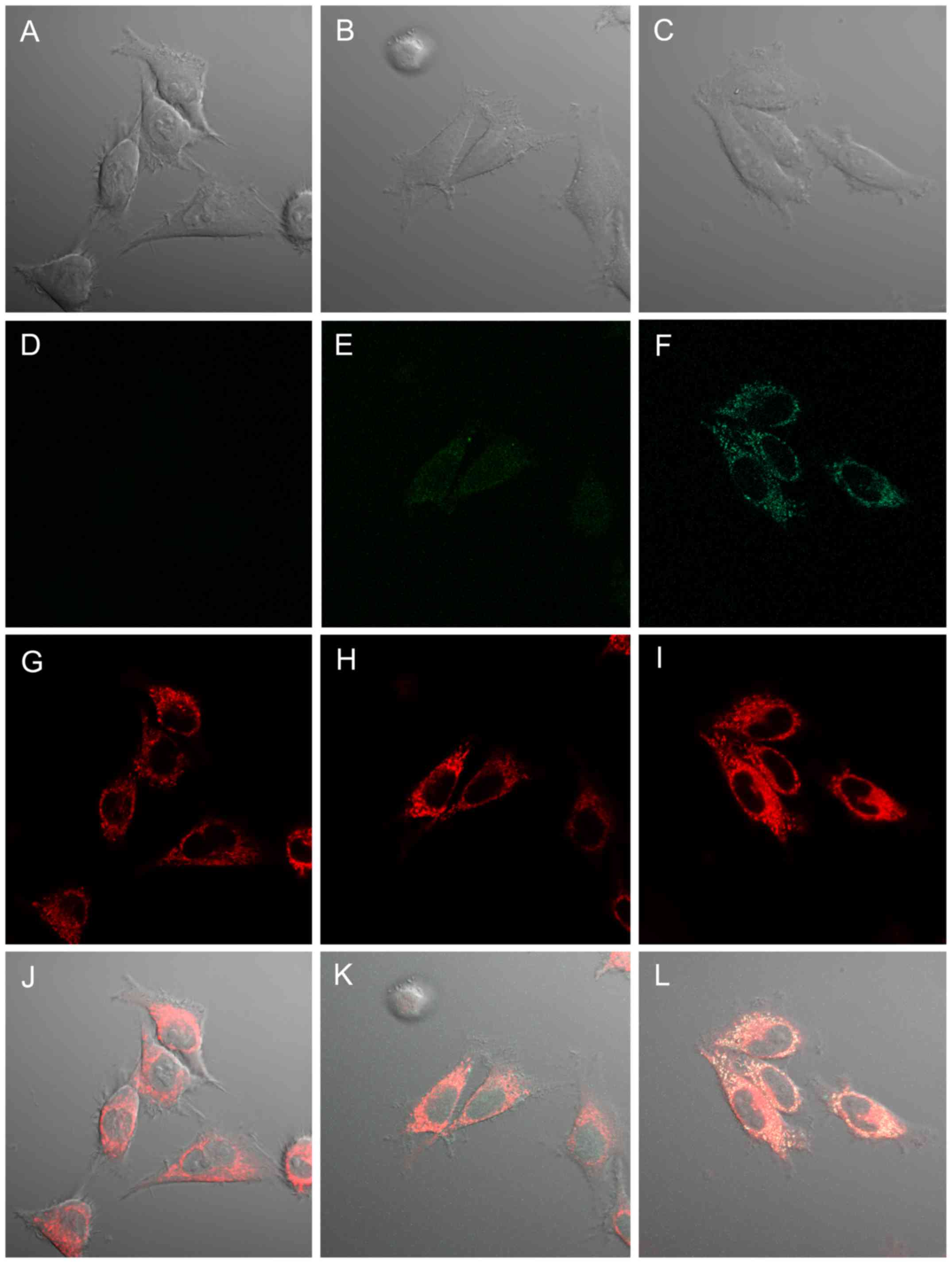
Subcellular localization of delayed
intracellular ROS production and mitochondria after IR in glioma
cells
In a previous study, we detected delayed
intracellular ROS production enhanced by IR with 5-ALA treatment
mainly in the cytoplasm of glioma cells (16). To determine the more precise
localization of delayed ROS production, we observed delayed ROS
production in 9L cells 12 h after IR using the oxidant-sensitive
probe DCFD and mitochondrial staining (MitoTracker Deep Red FM) by
confocal laser-scanning microscopy (Fig. 4). In preliminary experiments, we
confirmed no PpIX fluorescence in 9L cells 12 h after 5-ALA
treatment under our imaging conditions. Mitochondrial fluorescence
was clearly observed in 9L cells without 5-ALA treatment and IR
exposure as a control (Fig. 4A, D and
J). In addition, no interaction between mitochondria and DCFD
fluorescence was observed following our imaging study (Fig. 4D and G). Mitochondrial
fluorescence in 9L cells 12 h after RT was increased compared to
that in the control (Fig. 4D and
E). Moreover, mitochondrial fluorescence in 9L cells 12 h after
RT with 5-ALA treatment obviously increased compared to other
groups (Fig. 4F). In addition,
slight DCFD fluorescence in 9L cells 12 h after RT was observed in
the nucleus and cytoplasm (Fig.
4H). DCFD fluorescence in 9L cells 12 h after RT with 5-ALA
treatment obviously increased compared to other groups and was
localized mainly in the cytoplasm, consistent with our previous
results (16) (Fig. 4I). Furthermore, the enhanced DCFD
fluorescence in RT with 5-ALA treatment coincided with
mitochondrial fluorescence (Fig.
4L).
Differences in amount of 5-ALA-induced
PpIX accumulation influence mitochondrial mass after IR in glioma
cells
To evaluate the influence of different amounts of
5-ALA-induced PpIX accumulation on mitochondrial mass after IR, we
evaluated mitochondrial mass after IR in glioma cells treated with
low-dose CPFX using flow cytometric analysis. The mitochondrial
mass of glioma cells was obviously changed between just after and
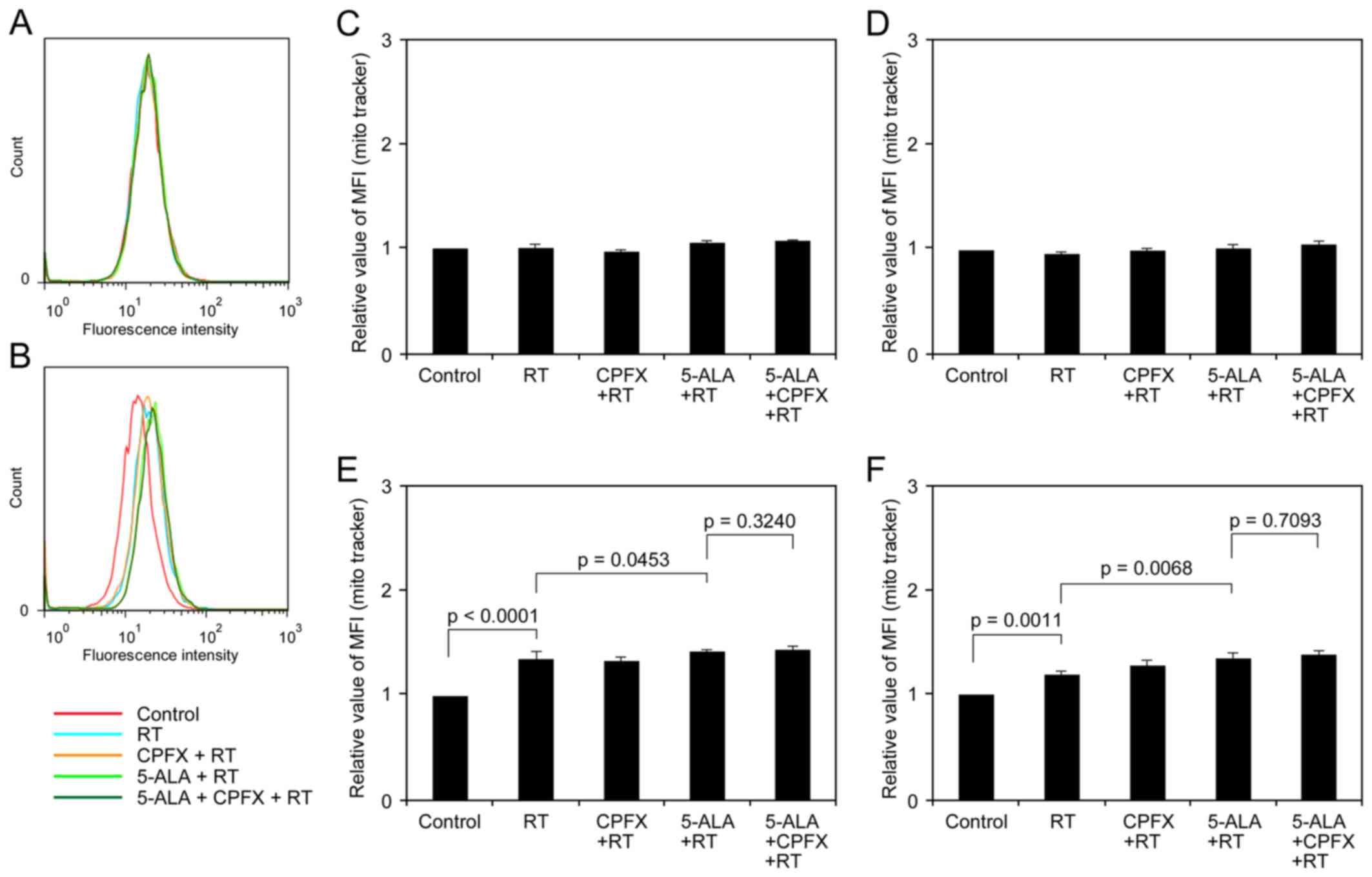
12 h after IR exposure (Fig. 5A and
B). In both glioma cell lines just after IR, the differences
among treatment groups were not marked (Fig. 5C and D). In the 9L and U251 cells
12 h after IR, the relative MFI of mitochondria in cells with RT
was 1.36±0.02 and 1.20±0.04, respectively (Fig. 5E and F), significant increases
compared to the control (p<0.0001 and p=0.0011, respectively).
The cells receiving RT with 5-ALA treatment also exhibited
significantly increased mitochondrial mass compared to the cells
receiving RT in the two cell lines (1.42±0.02, p=0.0453 in 9L;
1.36±0.05, p=0.0068 in U251). Although the cells receiving RT with
5-ALA and CPFX treatment exhibited increased mitochondrial mass
compared to the cells receiving RT with 5-ALA treatment in the two
cell lines (1.44±0.03 in 9L, 1.38±0.04 in U251), the differences
were not significant (p=0.3240 and 0.7093, respectively).
IR with 5-ALA treatment increases
mitochondrial complex III activity in glioma cells
Since delayed ROS production in tumour cells after
IR was associated with mitochondrial ETC activity (24–27) we evaluated mitochondrial ETC
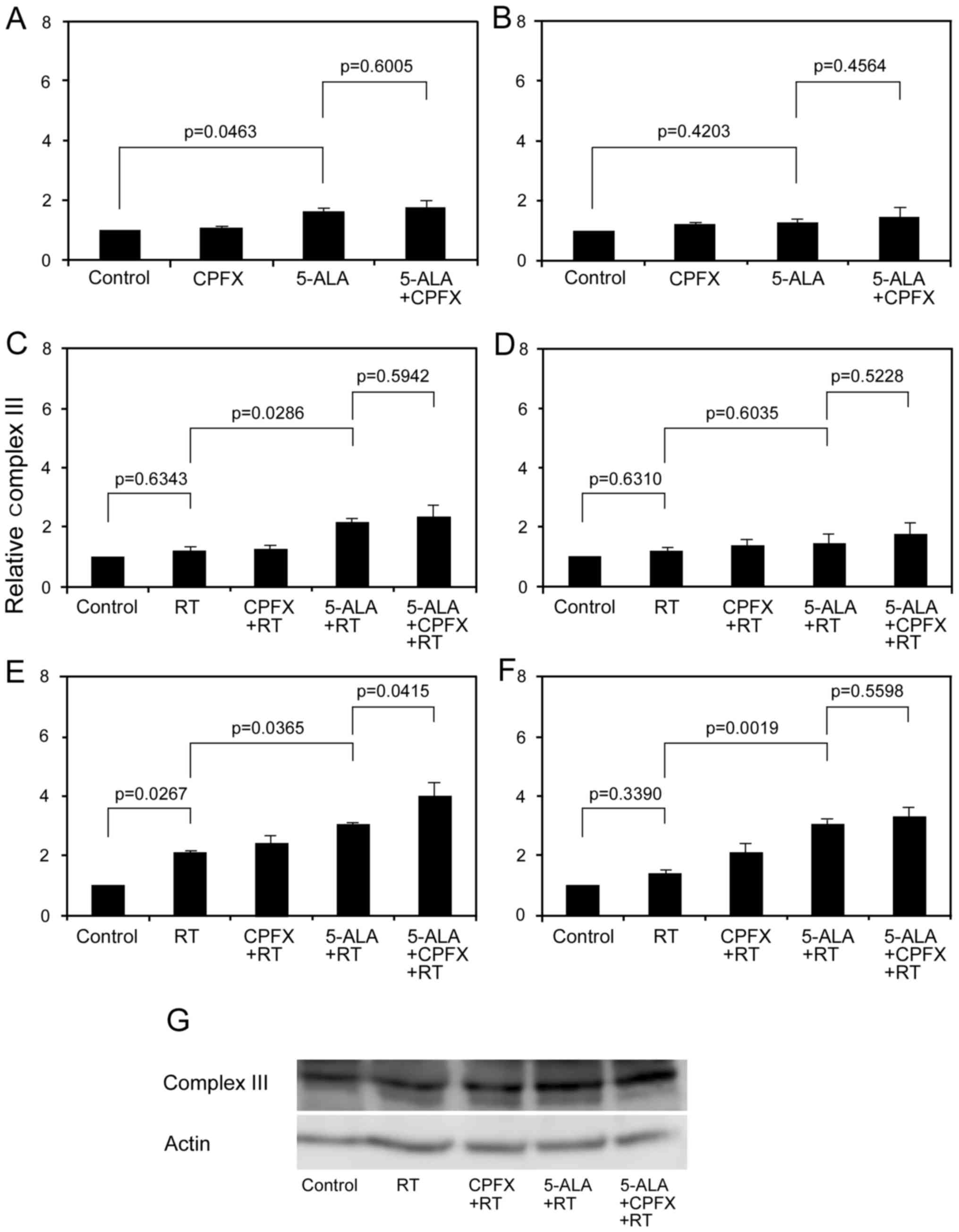
activity using Total OXPHOS Rodent WB Antibody Cocktail (32,33). In glioma cells before IR, the
relative complex III value in CPFX, 5-ALA, and 5-ALA with CPFX
treatment groups was 1.02±0.09, 1.61±0.13 and 1.75±0.26,
respectively, in 9L cells and 1.14±0.02, 1.23±0.13 and 1.44±0.28,
respectively, in U251 cells (Fig. 6A
and B). 5-ALA treatment increased complex III activity in
glioma cells with some variations (p=0.0463 in 9L, p=0.4203 in
U251). Complex III activity in glioma cells 12 h after RT in each
group increased compared to that just after RT (Fig. 6C–G). In glioma cells just after
IR, the relative complex III value in the RT, CPFX, 5-ALA, and
5-ALA with CPFX treatment groups was 1.18±0.16, 1.24±0.15,
2.13±0.13 and 2.33±0.40, respectively, in 9L cells and 1.22±0.13,
1.37±0.23, 1.44±0.30 and 1.74±0.39, respectively, in U251 cells
(Fig. 6C and D). Complex III
activity in 9L cells receiving 5-ALA treatment just after RT
obviously increased compared to those receiving only RT (p=0.0286).
In glioma cells 12 h after IR, the relative complex III value in
RT, CPFX, 5-ALA, and 5-ALA with CPFX treatment groups was
2.07±0.07, 2.38±0.31, 3.06±0.04 and 4.02±0.42, respectively, in 9L
cells and 1.40±0.14, 2.08±0.31, 3.05±0.18 and 3.28±0.34,
respectively, in U251 cells (Fig. 6E
and F). At 12 h after RT, complex III activity in the RT group
increased compared to control in 9L and U251 cells with some
variations (p=0.0267 in 9L, p=0.3390 in U251). However, complex III
activity in the RT with 5-ALA treatment group significantly
increased compared to that in the RT group in the two cell lines
(p=0.0365 in 9L, p=0.0019 in U251). Complex III activity for RT
with 5-ALA and CPFX treatment increased compared to that for RT
with 5-ALA treatment in 9L cells (p=0.0415) and U251 cells
(p=0.5598) (Fig. 6E–G). Although
we similarly evaluated other mitochondrial complexes (I, II, IV and
V), there were no apparent differences (data not shown).
Discussion
5-ALA enhances mitochondrial stress by IR
and leads to increased cell death with mitochondrial changes in
glioma cells
We previously demonstrated that 5-ALA enhances the
delayed production of ROS after IR mainly in the cytoplasm,
supposed as the mitochondria, of glioma cells (14,15,24). In the present study, to elucidate
the radiosensitizing effect of 5-ALA in glioma cells, we
investigated the cell response after IR with different amounts of
5-ALA-induced PpIX accumulation in glioma cells, focusing on
mitochondria. We confirmed that the enhancement of delayed ROS
production and cell death via IR was proportionate to 5-ALA-induced
PpIX accumulation in glioma cells and that delayed ROS production
occurred within the increased mitochondria of glioma cells.
Moreover, IR with 5-ALA treatment increased the mitochondrial mass
and mitochondrial complex III activity at 12 h after irradiation,
leading to cell death proportionate to 5-ALA-induced PpIX
accumulation in glioma cells. To the best of our knowledge, this is
the first study to investigate the radiosensitizing effect of 5-ALA
with a focus on the response of mitochondria in glioma cells.
Most anticancer drugs induce targeted cell death, at
least in part, through the generation of elevated amounts of
intracellular ROS (34). Thus,
non-toxic materials that selectively upregulate intracellular ROS
to induce lethal effects for tumour cells are desirable. In
general, the biological consequences of IR leading to cell death
are highly influenced by the activation of the DNA damage response
mechanism, particularly for nuclear DSBs (35,36). However, the number of studies
investigating the effects of IR on the mitochondria is far less
than that on the cell nucleus (25). Mitochondria occupy a fairly
substantial fraction of cell volume (4–25% depending on the cell),
which renders them a likely target of radiation traversal through
the cell (37). Mitochondrial DNA
only accounts for approximately 0.25% of the total cellular DNA,
but all of the mitochondrial DNA (except the D-loop) consists of
genes for protein synthesis (38,39). In addition, mitochondrial DNA is
considered more prone to oxidative damage because it lacks histone
protection and an efficient DNA repair system (40,41). Thus, recent studies suggest that
mitochondria are specific targets in the tumouricidal efficacy of
radiation therapy (25–27).
The increase in delayed ROS production and
mitochondrial mass was hypothesized to arise from the propagation
of oxidative stress from impaired mitochondria initially damaged by
IR, so-called 'amplification of intermitochondrial communication'
(25). In the present study, we
confirmed that IR without 5-ALA treatment increased delayed ROS
production and mitochondrial mass at the late period in glioma
cells, consistent with previous studies (24,28). However, 5-ALA-induced PpIX
accumulation obviously enhanced delayed ROS production and
mitochondrial mass in glioma cells. Additionally, the enhancement
of these delayed ROS production obviously occurred in the
'mitochondria' and not in the 'nucleus'. Considering the
characteristics of ROS (very short lifetime and limited diffusion
distance) and the distance between impaired mitochondria,
surrounding normal mitochondria, and the nucleus, renders this
distribution of delayed ROS production reasonable (21). Thus, the direct biological effects
of delayed ROS on the nucleus may be limited, at least in the late
period after IR with 5-ALA treatment.
The precise mechanism by which 5-ALA-induced PpIX
enhances delayed ROS production remains unclear. Mitochondria play
important roles in ROS production during IR exposure (27). In addition, mitochondria consume
approximately 90% of physiological oxygen and are the richest
source of ROS (42,43). In the present study, we observed
that 5-ALA-induced PpIX enhanced complex III activity 12 h after IR
in glioma cells. Thus, during amplification of intermitochondrial
communication at the late period after IR with 5-ALA treatment,
electron leakage may occur in the ETC complex of mitochondria
damaged by oxidative stress, consequently elevating delayed ROS
production (25,27). PpIX enhances ROS production in
solution via water radiolysis induced by IR (23). We also observed that 5-ALA-induced
PpIX increased primary ROS production by IR and localized with
these ROS in glioma cells (14).
By contrast, although glioma cells were treated with 5-ALA after
IR, delayed ROS production was not so elevated in our previous
study (16). Taken together, for
cells that accumulate large amounts of 5-ALA-induced PpIX in
mitochondria, IR can initially induce strong oxidative stress to
mitochondria and subsequently enhance delayed ROS production by
amplifying intermitochondrial communication, consequently inducing
strong cell death in glioma cells. Thus, we suggest that 5-ALA
enhances the mitochondrial stress induced by IR, thereby acting as
a radiosensitizer in gliomas.
Low-dose CPFX enhances 5-ALA
radiosensitization effects by increasing PpIX accumulation in
glioma cells
CPFX is known to enhance 5-ALA-induced PpIX in
tumour cells, and a previous study demonstrated that 100 μM
CPFX enhanced 5-ALA-induced PpIX accumulation in human epithelial
cervical cancer (HeLa) and epidermoid carcinoma (A431) cells
(31). CPFX also has anticancer
effects in several tumour cell lines with some variations (44). Therefore, we first evaluated the
cytotoxicity of CPFX for glioma cells and chose 5 μM as the
appropriate concentration of CPFX for glioma cells (Fig. 1). Despite the low dose, CPFX
increased 5-ALA-induced PpIX accumulation without its own
cytotoxicity and enhanced cell death by IR in glioma cells. Recent
studies demonstrated that the accumulation of 5-ALA-induced PpIX in
tumour cells varies depending on the tumour cell types and local
cellular environment via four possible processes: i) enzyme
activity of heme synthesis (45),
ii) membrane transporters (influx of 5-ALA, efflux of PpIX)
(46–48), iii) iron metabolism (49,50) and iv) turnover rate of heme
synthesis (51). In the final
step of heme synthesis in mitochondria, iron (Fe2+) is
inserted into PpIX by ferrochelatase to form heme (7). CPFX acts as an iron-chelator,
decreasing iron utilization for heme biosynthesis in the
mitochondria of tumour cells, consequently leading to elevated
5-ALA-induced PpIX accumulation (31). In addition, CPFX itself acts as a
radiosensitizer in tumour cells (30). CPFX promotes p53 phosphorylation
(cell-cycle arrest), reduces Bcl-2 production (anti-apoptotic
effect), and leads to increased cell death in tumour cells
(30). Our results also confirm
the radiosensitizing effect of CPFX in glioma cells, although it
was weak compared to that of 5-ALA. Although the biological effect
of CPFX on mitochondria, such as increasing mitochondrial mass and
mitochondrial complex activity, was decreased, CPFX treatment
delayed ROS production after IR in glioma cells with some
variations. Thus, CPFX itself may affect the nucleus and enhance
delayed ROS production in cellular organelles except for the
mitochondria.
5-ALA as a candidate drug for direct
mitochondrial targeting in cancer therapy using IR
In the past, cancer drugs mainly targeted specific
molecular signals associated with cell proliferation, cell death,
cellular differentiation, and tumour angiogenesis (52–55). However, these molecular pathways
are multifaceted, and an alternative anticancer strategy targeting
tumour metabolism is required (56). Mitochondria are the centre arena
of cell metabolism, such as ATP production, lethal signal
transduction, and intracellular ROS production. Thus, emerging
studies have begun to investigate mitochondrial metabolism as a
specific target for cancer therapy (57,58). 5-ALA can certainly accumulate PpIX
in the mitochondria of tumour cells (6,7).
In addition, 5-ALA is already used in clinical applications, such
as fluorescence-guided resection for malignant gliomas, without
adverse effects (8–10). In the present study, we
demonstrated that 5-ALA obviously enhanced delayed ROS production
within mitochondria, leading to enhanced mitochondrial changes
induced by IR. Importantly, these processes occur in the specific
mitochondrial arena. Thus, we suggest that 5-ALA has potential as a
drug for the direct targeting of mitochondria in cancer therapy
under IR exposure.
The radiosensitizing effects of porphyrin compounds
such as Photofrin and HpD have been reported (59–61). A recent study demonstrated the use
of radiotherapy with tumour-specific folic acid-conjugated
carboxymethyl lauryl chitosan/superparamagnetic iron oxide micelles
to effectively deliver porphyrin compounds (chlorin e6) to cancer
cells (62). Although 5-ALA is
well-known as a specific reagent for photodynamic therapy and
fluorescence-guided resection in neurosurgery (8,63),
5-ALA is not itself a porphyrin compound but a prodrug that is
converted into PpIX within tumour cell mitochondria (7). For radiotherapy combined with 5-ALA,
we consider that it is important to increase the PpIX concentration
within tumour cells, specifically in mitochondria, just before IR
exposure. We previously confirmed that cell death by single-dose IR
with 5-ALA treatment was weak, but multi-dose IR with repeated
5-ALA treatment enhanced cell death in experimental glioma
(14,15). Previous findings demonstrated that
IR decreases the activity of mitochondrial aconitase and iron
regulatory protein-1, which are essential for the function of
iron-sulphur clusters in mitochondria (26). Thus, IR may insult utilization of
iron on heme synthesis at mitochondria and consequently enhance
5-ALA-induced PpIX accumulation within mitochondria. Tumour cells
after IR exposure exhibit increased 5-ALA-induced PpIX accumulation
(12,16). For multi-dose (fractionated) IR,
the PpIX concentration within tumour cells may be altered with each
fraction. In addition, we previously confirmed 5-ALA-induced PpIX
as a potential biomarker for malignant gliomas via MRI in a
prospective clinical case study (64). Therefore, we suggest that
modulation of the intensity and field of the radiation beam based
on 5-ALA-induced PpIX tumour concentrations evaluated by MRI for
each patient and for each fraction can increase the therapeutic
effect in tumours and decrease the side effects of IR, such as
radiation necrosis, brain oedema, and leukoencephalopathy, on
normal surrounding tissue by avoiding excessive IR exposure.
Further investigation into the biological effects of IR on glioma
cells based on the 5-ALA-induced PpIX concentration is
required.
Interactions between 5-ALA and ultrasound, so-called
sonodynamic therapy, and hyperthermia have been previously reported
as cancer therapy (65,66). These external energy exposures may
affect mitochondrial accumulation of 5-ALA-induced PpIX and
revealed that the antitumour effect is the same as that of IR.
Since their introduction in the late 1990s, cancer stem cells
(CSCs) have been shown to be more radioresistant/chemo-resistant
than non-stem cells via enhanced DNA-repair capacity, ROS defences,
and self-renewal potential (67–69). Although mitochondria are the most
prominent source of intracellular ROS, low levels of ROS have been
involved in cancer cell stemness (70). However, a recent study
demonstrated that photodynamic therapy (PDT) using 5-ALA reduced
the self-renewal property and stemness signature expression in CSCs
(71). In addition, PDT with
5-ALA enhanced the sensitivity of CSCs to chemotherapy (cisplatin)
by suppressing ABCG2 (the efflux transporter of PpIX) and led to
reduced tumourigenicity of CSCs. Thus, CSCs may accumulate
5-ALA-induced PpIX within mitochondria such as non-progenitor
tumour cells. If this occurs, radiotherapy with 5-ALA constitutes
an effective cancer treatment for CSCs. Therefore, it is crucial to
investigate how to combine existing therapy with 5-ALA and to
extend the application of 5-ALA, so called 'drug repositioning', in
future studies.
In conclusion, 5-ALA is a useful tool for
fluorescence-guided resection in malignant gliomas as a live
molecular marker. Furthermore, 5-ALA selectively accumulates PpIX
in mitochondria and induces strong oxidative stress in glioma cell
mitochondria under IR. Thus, 5-ALA shows potential as a
mitochondria-targeting drug in cancer therapy.
Acknowledgments
We appreciate Dr Hitoshi Nakagawa and Dr Masahiro
Ishizuka (SBI Pharmaceuticals Co., Ltd., Minato-ku, Tokyo, Japan)
for advice regarding western blotting for OXPHOS. This study was
supported by JSPS KAKENHI (grant no. 25462282).
References
|
1
|
Sanai N, Polley MY, McDermott MW, Parsa AT
and Berger MS: An extent of resection threshold for newly diagnosed
glioblastomas. J Neurosurg. 115:3–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al European Organisation for Research and Treatment of Cancer
Brain Tumor and Radiotherapy Groups; National Cancer Institute of
Canada Clinical Trials Group: Radiotherapy plus concomitant and
adjuvant temozolomide for glioblastoma. N Engl J Med. 352:987–996.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Westphal M, Ram Z, Riddle V, Hilt D and
Bortey E; Executive Committee of the Gliadel Study Group: Gliadel
wafer in initial surgery for malignant glioma: Long-term follow-up
of a multicenter controlled trial. Acta Neurochir (Wien).
148:269–275. 2006. View Article : Google Scholar
|
|
4
|
Claes A, Idema AJ and Wesseling P: Diffuse
glioma growth: A guerilla war. Acta Neuropathol. 114:443–458. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Minniti G, Amelio D, Amichetti M, Salvati
M, Muni R, Bozzao A, Lanzetta G, Scarpino S, Arcella A and Enrici
RM: Patterns of failure and comparison of different target volume
delineations in patients with glioblastoma treated with conformal
radiotherapy plus concomitant and adjuvant temozolomide. Radiother
Oncol. 97:377–381. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ishizuka M, Abe F, Sano Y, Takahashi K,
Inoue K, Nakajima M, Kohda T, Komatsu N, Ogura S and Tanaka T:
Novel development of 5-aminolevurinic acid (ALA) in cancer
diagnoses and therapy. Int Immunopharmacol. 11:358–365. 2011.
View Article : Google Scholar
|
|
7
|
Yamamoto J: A role of 5-aminolevulinic
acid for treating malignant gliomas: Clinical implications and
future prospects (Review). ALA-Porphyrin Sci. 4:3–12. 2016.
|
|
8
|
Stummer W, Pichlmeier U, Meinel T,
Wiestler OD, Zanella F and Reulen HJ; ALA-Glioma Study Group:
Fluorescence-guided surgery with 5-aminolevulinic acid for
resection of malignant glioma: A randomised controlled multicentre
phase III trial. Lancet Oncol. 7:392–401. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yamamoto J, Kitagawa T, Akiba D and
Nishizawa S: 5-Aminolevulinic acid-induced fluorescence in
cerebellar primary central nervous system lymphoma: A case report
and literature review. Turk Neurosurg. 25:796–800. 2015.PubMed/NCBI
|
|
10
|
Yamamoto J, Takahashi M, Idei M, et al: A
pitfall of fluorescence-guided surgery with 5-aminolevulinic acid
for the treatment of malignant brain tumor -case report-.
ALA-Porphyrin Sci. 1:61–66. 2012.
|
|
11
|
Luksiene Z, Berg K and Moan J: Combination
of photodynamic therapy and X-irradiation: A study on 5-ALA
radiomodifying properties. SPIE. 2325:306–311. 1994.
|
|
12
|
Berg K, Luksiene Z, Moan J and Ma L:
Combined treatment of ionizing radiation and photosensitization by
5-aminolevulinic acid-induced protoporphyrin IX. Radiat Res.
142:340–346. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schaffer M, Schaffer PM, Corti L, Gardiman
M, Sotti G, Hofstetter A, Jori G and Dühmke E: Photofrin as a
specific radiosensitizing agent for tumors: Studies in comparison
to other porphyrins, in an experimental in vivo model. J Photochem
Photobiol B. 66:157–164. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamamoto J, Ogura S, Tanaka T, Kitagawa T,
Nakano Y, Saito T, Takahashi M, Akiba D and Nishizawa S:
Radiosensitizing effect of 5-aminolevulinic acid-induced
protoporphyrin IX in glioma cells in vitro. Oncol Rep.
27:1748–1752. 2012.PubMed/NCBI
|
|
15
|
Yamamoto J, Ogura S, Shimajiri S, Nakano
Y, Akiba D, Kitagawa T, Ueta K, Tanaka T and Nishizawa S:
5-Aminolevulinic acid-induced protoporphyrin IX with multi-dose
ionizing irradiation enhances host antitumor response and strongly
inhibits tumor growth in experimental glioma in vivo. Mol Med Rep.
11:1813–1819. 2015.
|
|
16
|
Kitagawa T, Yamamoto J, Tanaka T, Nakano
Y, Akiba D, Ueta K and Nishizawa S: 5-Aminolevulinic acid strongly
enhances delayed intracellular production of reactive oxygen
species (ROS) generated by ionizing irradiation: Quantitative
analyses and visualization of intracellular ROS production in
glioma cells in vitro. Oncol Rep. 33:583–590. 2015.
|
|
17
|
Takahashi J, Misawa M, Murakami M, Mori T,
Nomura K and Iwahashi H: 5-Aminolevulinic acid enhances cancer
radiotherapy in a mouse tumor model. Springerplus. 2:6022013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kamada Y, Murayama Y, Harada K, Nishimura
M, Kondo Y, Konishi H, Morimura R, Komatsu S, Shiozaki A, Kuriu Y,
et al: Radiosensitizing effect of 5-aminolevulinic acid (5-ALA) in
Colon cancer. Gan To Kagaku Ryoho. 41:1608–1610. 2014.In
Japanese.
|
|
19
|
Wang D, Cvetkovic B, Gupta R, Chen L, Ma
CMC, Zhang Q and Zeng J: Radiatoin therapy combined with
5-amiolevulinic acid: A preliminary study with an in vivo mouse
model implanted with human PC-3 tumor cells. Int J Radiat Oncol
Biol Phys. 93:e5222015. View Article : Google Scholar
|
|
20
|
Takahashi J, Misawa M and Iwahashi H:
Transcriptome analysis of porphyrin-accumulated and
x-ray-irradiated cell cultures under limited proliferation and
non-lethal conditions. Microarrays (Basel). 4:25–40. 2015.
View Article : Google Scholar
|
|
21
|
Riley PA: Free radicals in biology:
Oxidative stress and the effects of ionizing radiation. Int J
Radiat Biol. 65:27–33. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hall E and Giaccia A: Physics and
chemistry of radiation absorption. Radiology for the Radiologist
Lippipncott. Williams and Wilkins; Philadelphia, PA: pp. 5–15.
2006
|
|
23
|
Takahashi J and Misawa M: Characterization
of reactive oxygen species generated by protoporphyrin IX under
X-ray irradiation. Radiat Phys Chem. 78:889–898. 2009. View Article : Google Scholar
|
|
24
|
Yamamori T, Yasui H, Yamazumi M, Wada Y,
Nakamura Y, Nakamura H and Inanami O: Ionizing radiation induces
mitochondrial reactive oxygen species production accompanied by
upregulation of mitochondrial electron transport chain function and
mitochondrial content under control of the cell cycle checkpoint.
Free Radic Biol Med. 53:260–270. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kam WW and Banati RB: Effects of ionizing
radiation on mitochondria. Free Radic Biol Med. 65:607–619. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Azzam EI, Jay-Gerin JP and Pain D:
Ionizing radiation-induced metabolic oxidative stress and prolonged
cell injury. Cancer Lett. 327:48–60. 2012. View Article : Google Scholar
|
|
27
|
Richardson RB and Harper ME: Mitochondrial
stress controls the radiosensitivity of the oxygen effect:
Implications for radiotherapy. Oncotarget. Feb 15–2016.Epub ahead
of print.
|
|
28
|
Saenko Y, Cieslar-Pobuda A, Skonieczna M
and Rzeszowska- Wolny J: Changes of reactive oxygen and nitrogen
species and mitochondrial functioning in human K562 and HL60 cells
exposed to ionizing radiation. Radiat Res. 180:360–366. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sugiyama Y, Hagiya Y, Nakajima M, Ishizuka
M, Tanaka T and Ogura S: The heme precursor 5-aminolevulinic acid
disrupts the Warburg effect in tumor cells and induces
caspase-dependent apoptosis. Oncol Rep. 31:1282–1286. 2014.
|
|
30
|
Kiang JG, Garrison BR, Smith JT and
Fukumoto R: Ciprofloxacin as a potential radio-sensitizer to tumor
cells and a radio-protectant for normal cells: Differential effects
on γ-H2AX formation, p53 phosphorylation, Bcl-2 production, and
cell death. Mol Cell Biochem. 393:133–143. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ohgari Y, Miyata Y, Chau TT, Kitajima S,
Adachi Y and Taketani S: Quinolone compounds enhance
delta-aminolevulinic acid-induced accumulation of protoporphyrin IX
and photosensitivity of tumour cells. J Biochem. 149:153–160. 2011.
View Article : Google Scholar
|
|
32
|
Kanzleiter T, Rath M, Penkov D, Puchkov D,
Schulz N, Blasi F and Schürmann A: Pknox1/Prep1 regulates
mitochondrial oxidative phosphorylation components in skeletal
muscle. Mol Cell Biol. 34:290–298. 2014. View Article : Google Scholar :
|
|
33
|
Thomas MM, Trajcevski KE, Coleman SK,
Jiang M, Di Michele J, O'Neill HM, Lally JS, Steinberg GR and Hawke
TJ: Early oxidative shifts in mouse skeletal muscle morphology with
high-fat diet consumption do not lead to functional improvements.
Physiol Rep. 2:22014. View Article : Google Scholar
|
|
34
|
Wondrak GT: Redox-directed cancer
therapeutics: Molecular mechanisms and opportunities. Antioxid
Redox Signal. 11:3013–3069. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lomax ME, Folkes LK and O'Neill P:
Biological consequences of radiation-induced DNA damage: Relevance
to radiotherapy. Clin Oncol (R Coll Radiol). 25:578–585. 2013.
View Article : Google Scholar
|
|
36
|
Barendsen GW: The relationships between
RBE and LET for different types of lethal damage in mammalian
cells: Biophysical and molecular mechanisms. Radiat Res.
139:257–270. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Leach JK, Van Tuyle G, Lin PS,
Schmidt-Ullrich R and Mikkelsen RB: Ionizing radiation-induced,
mitochondria-dependent generation of reactive oxygen/nitrogen.
Cancer Res. 61:3894–3901. 2001.PubMed/NCBI
|
|
38
|
Clayton DA: Transcription and replication
of mitochondrial DNA. Hum Reprod. 15(Suppl 2): 11–17. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Anderson S, Bankier AT, Barrell BG, de
Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA,
Sanger F, et al: Sequence and organization of the human
mitochondrial genome. Nature. 290:457–465. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Richter C, Park JW and Ames BN: Normal
oxidative damage to mitochondrial and nuclear DNA is extensive.
Proc Natl Acad Sci USA. 85:6465–6467. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Larsen NB, Rasmussen M and Rasmussen LJ:
Nuclear and mitochondrial DNA repair: Similar pathways?
Mitochondrion. 5:89–108. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cadenas E and Davies KJ: Mitochondrial
free radical generation, oxidative stress, and aging. Free Radic
Biol Med. 29:222–230. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Babior BM: NADPH oxidase: An update.
Blood. 93:1464–1476. 1999.PubMed/NCBI
|
|
44
|
Kloskowski T, Gurtowska N, Nowak M,
Joachimiak R, Bajek A, Olkowska J and Drewa T: The influence of
ciprofloxacin on viability of A549, HepG2, A375.S2, B16 and C6 cell
lines in vitro. Acta Pol Pharm. 68:859–865. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Teng L, Nakada M, Zhao SG, Endo Y,
Furuyama N, Nambu E, Pyko IV, Hayashi Y and Hamada JI: Silencing of
ferrochelatase enhances 5-aminolevulinic acid-based fluorescence
and photodynamic therapy efficacy. Br J Cancer. 104:798–807. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tran TT, Mu A, Adachi Y, Adachi Y and
Taketani S: Neurotransmitter transporter family including SLC6A6
and SLC6A13 contributes to the 5-aminolevulinic acid (ALA)-induced
accumulation of protoporphyrin IX and photodamage, through uptake
of ALA by cancerous cells. Photochem Photobiol. 90:1136–1143.
2014.PubMed/NCBI
|
|
47
|
Chua C, Zaiden N, Chong KH, See SJ, Wong
MC, Ang BT and Tang C: Characterization of a side population of
astrocytoma cells in response to temozolomide. J Neurosurg.
109:856–866. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Matsumoto K, Hagiya Y, Endo Y, Nakajima M,
Ishizuka M, Tanaka T and Ogura S: Effects of plasma membrane ABCB6
on 5-aminolevulinic acid (ALA)-induced porphyrin accumulation in
vitro: Tumor cell response to hypoxia. Photodiagn Photodyn Ther.
12:45–51. 2015. View Article : Google Scholar
|
|
49
|
Hayashi M, Fukuhara H, Inoue K, Shuin T,
Hagiya Y, Nakajima M, Tanaka T and Ogura S: The effect of iron ion
on the specificity of photodynamic therapy with 5-aminolevulinic
acid. PLoS One. 10:e01223512015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sawamoto M, Imai T, Umeda M, Fukuda K,
Kataoka T and Taketani S: The p53-dependent expression of frataxin
controls 5-aminolevulinic acid-induced accumulation of
protoporphyrin IX and photo-damage in cancerous cells. Photochem
Photobiol. 89:163–172. 2013. View Article : Google Scholar
|
|
51
|
Kim JE, Cho HR, Xu WJ, Kim JY, Kim SK, Kim
SK, Park SH, Kim H, Lee SH, Choi SH, et al: Mechanism for enhanced
5-aminolevulinic acid fluorescence in isocitrate dehydrogenase 1
mutant malignant gliomas. Oncotarget. 6:20266–20277. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Uhm JH, Ballman KV, Wu W, Giannini C,
Krauss JC, Buckner JC, James CD, Scheithauer BW, Behrens RJ, Flynn
PJ, et al: Phase II evaluation of gefitinib in patients with newly
diagnosed Grade 4 astrocytoma: Mayo/North Central Cancer Treatment
Group Study N0074. Int J Radiat Oncol Biol Phys. 80:347–353. 2011.
View Article : Google Scholar
|
|
53
|
Curry WT and Lim M: Immunomodulation:
checkpoint blockade etc. Neuro Oncol. 17(Suppl 7): vii26–vii31.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Norden AD, Drappatz J and Wen PY: Novel
anti-angiogenic therapies for malignant gliomas. Lancet Neurol.
7:1152–1160. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Cloughesy T, Finocchiaro G, Belda-Iniesta
C, Recht L, Brandes AA, Pineda E, Mikkelsen T, Chinot OL, Balana C,
Macdonald DR, et al: Randomized, double-blind, placebo-controlled,
multicenter phase II study of onartuzumab plus bevacizumab versus
placebo plus bevacizumab in patients with recurrent glioblastoma:
efficacy, safety, and hepatocyte growth factor and
O6-methylguanine-DNA methyltransferase biomarker analyses. J Clin
Oncol. Dec 5–2016.Epub ahead of print.
|
|
56
|
Pan JG and Mak TW: Metabolic targeting as
an anticancer strategy: Dawn of a new era? Sci STKE.
2007:pe142007.PubMed/NCBI
|
|
57
|
Fulda S, Galluzzi L and Kroemer G:
Targeting mitochondria for cancer therapy. Nat Rev Drug Discov.
9:447–464. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Weinberg SE and Chandel NS: Targeting
mitochondria metabolism for cancer therapy. Nat Chem Biol. 11:9–15.
2015. View Article : Google Scholar :
|
|
59
|
Kostron H, Swartz MR, Miller DC and
Martuza RL: The interaction of hematoporphyrin derivative, light,
and ionizing radiation in a rat glioma model. Cancer. 57:964–970.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kulka U, Schaffer M, Siefert A, Schaffer
PM, Olsner A, Kasseb K, Hofstetter A, Dühmke E and Jori G:
Photofrin as a radiosensitizer in an in vitro cell survival assay.
Biochem Biophys Res Commun. 311:98–103. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Schaffer M, Ertl-Wagner B, Schaffer PM,
Kulka U, Jori G, Wilkowski R, Hofstetter A and Dühmke E:
Feasibility of photofrin II as a radiosensitizing agent in solid
tumors - preliminary results. Onkologie. 29:514–519.
2006.PubMed/NCBI
|
|
62
|
Chen HP, Tung FI, Chen MH and Liu TY: A
magnetic vehicle realized tumor cell-targeted radiotherapy using
low-dose radiation. J Control Release. 226:182–192. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yamamoto J, Yamamoto S, Hirano T, Li S,
Koide M, Kohno E, Okada M, Inenaga C, Tokuyama T, Yokota N, et al:
Monitoring of singlet oxygen is useful for predicting the
photodynamic effects in the treatment for experimental glioma. Clin
Cancer Res. 12:7132–7139. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Yamamoto J, Kakeda S, Yoneda T, et al:
Improving contrast enhancement in magnetic resonance imaging using
5-aminolevulinic acid-induced protoporphyrin IX for high-grade
gliomas: A prospective case study and clinical implications. Oncol
Lett. In press.
|
|
65
|
Gao Z, Zheng J, Yang B, Wang Z, Fan H, Lv
Y, Li H, Jia L and Cao W: Sonodynamic therapy inhibits angiogenesis
and tumor growth in a xenograft mouse model. Cancer Lett.
335:93–99. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Takahashi K, Hasegawa T, Ishii T, Suzuki
A, Nakajima M, Uno K, Yasuda I, Kishi A, Sadamoto K, Abe F, et al:
Antitumor effect of combination of hyperthermotherapy and
5-aminolevulinic acid (ALA). Anticancer Res. 33:2861–2866.
2013.PubMed/NCBI
|
|
67
|
Rycaj K and Tang DG: Cancer stem cells and
radioresistance. Int J Radiat Biol. 90:615–621. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Krause M, Dubrovska A, Linge A and Baumann
M: Cancer stem cells: Radioresistance, prediction of radiotherapy
outcome and specific targets for combined treatments. Adv Drug
Deliv Rev. Feb 12–2016.Epub ahead of print. PubMed/NCBI
|
|
69
|
Tannock IF: Cancer: Resistance through
repopulation. Nature. 517:152–153. 2015. View Article : Google Scholar
|
|
70
|
Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie
MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, et al: Association
of reactive oxygen species levels and radioresistance in cancer
stem cells. Nature. 458:780–783. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Yu CH and Yu CC: Photodynamic therapy with
5-aminolevulinic acid (ALA) impairs tumor initiating and
chemo-resistance property in head and neck cancer-derived cancer
stem cells. PLoS One. 9:e871292014. View Article : Google Scholar : PubMed/NCBI
|