1. Introduction
Cancer, characterized by uncontrolled cell growth,
is considered to be the second leading cause of mortality (30% of
total mortality) worldwide, which seriously threatens public health
(1). A report released by the
World Health Organization on the global situation of cancer in 2012
indicated that the annual new cancer cases reached 14,000,000
globally, with 8,200,000 deaths, and the number of new cases is
expected to increase to 24,000,000 in the year 2035 (2). The global cancer burden is currently
growing at an alarming rate, and there is no effective available
treatment to date to curb the spread of cancer.
At present, the clinical management of cancer always
involves several conventional modalities. Surgical resection is an
effective method for the treatment of tumors in the early stages
and for the clinical treatment of local tumors. However, surgery is
often ineffective once tumors have spread or are diagnosed at an
advanced and/or late stage of the disease. The prognosis remains
poor due to tumor recurrence, diffusion and even metastasis
following surgery (3).
Radiotherapy is more effective than surgery in many cases. However,
its application is limited by tumor metastasis and various
side-effects. Recently, biotherapies such as immunotherapy, gene
therapy and monoclonal antibody therapy have become a hotspot and
provide a new method with which to prevent and treat tumors. Since
many aspects are still unclear, tumor biotherapy has not been
widely used (4). Chemotherapy is
currently the most commonly used treatment option for cancer.
However, the administration of traditional chemotherapeutic agents
at high doses always induce significant non-selective toxicities,
such as a reduction in bone density and immunosuppression, while
low or moderate doses of these agents usually does not exert
significant antitumor effects (5); in addition, there is the issue of
acquired drug resistance. Therefore, the development of novel
agents which can selectively induce cancer cell death without
threatening normal cells is of utmost importance.
It has been generally recognized that natural
products play a unique therapeutic role in the treatment of a
number of diseases. Ginseng, the root of Panax ginseng C.A.
Meyer, has been widely used in East Asia countries for thousands
years as a natural tonic (6).
Ginsenosides, extracted from Panax ginseng C.A. Meyer, are
the main active components with a wide range of pharmacological
activities. More than 100 types of ginsenosides have been isolated
and determined from ginseng (7).
Among these ginsenosides, ginsenoside Rg3 has been shown to have
significant physiological activites (8), such as hepatoprotection,
neuroprotection, cardiovascular-protection, promotion of immunity,
as well as anti-fatigue, antioxidant, and most importantly,
antitumor effects (9–14). There is increasing evidence
indicating that ginsenoside Rg3 exerts antitumor effects in a
number of cancer models, such as lung, liver and breast cancer
(15–17). Furthermore, ginsenoside Rg3 may be
a beneficial supplement, and the combined administration of
ginsenoside Rg3 and conventional chemotherapeutic drugs may be more
effective than either one being administered alone. The use of
ginsenoside Rg3 in cancer therapy may aid in the prevention of
toxicity and morbidity associated with conventional chemotherapy,
even though the underlying mechanisms have not yet been fully
elucidated (18). Thus, in this
review, we aimed to provide a systematic summary on the
cancer-preventive effects of ginsenoside Rg3.
2. Chemical structure
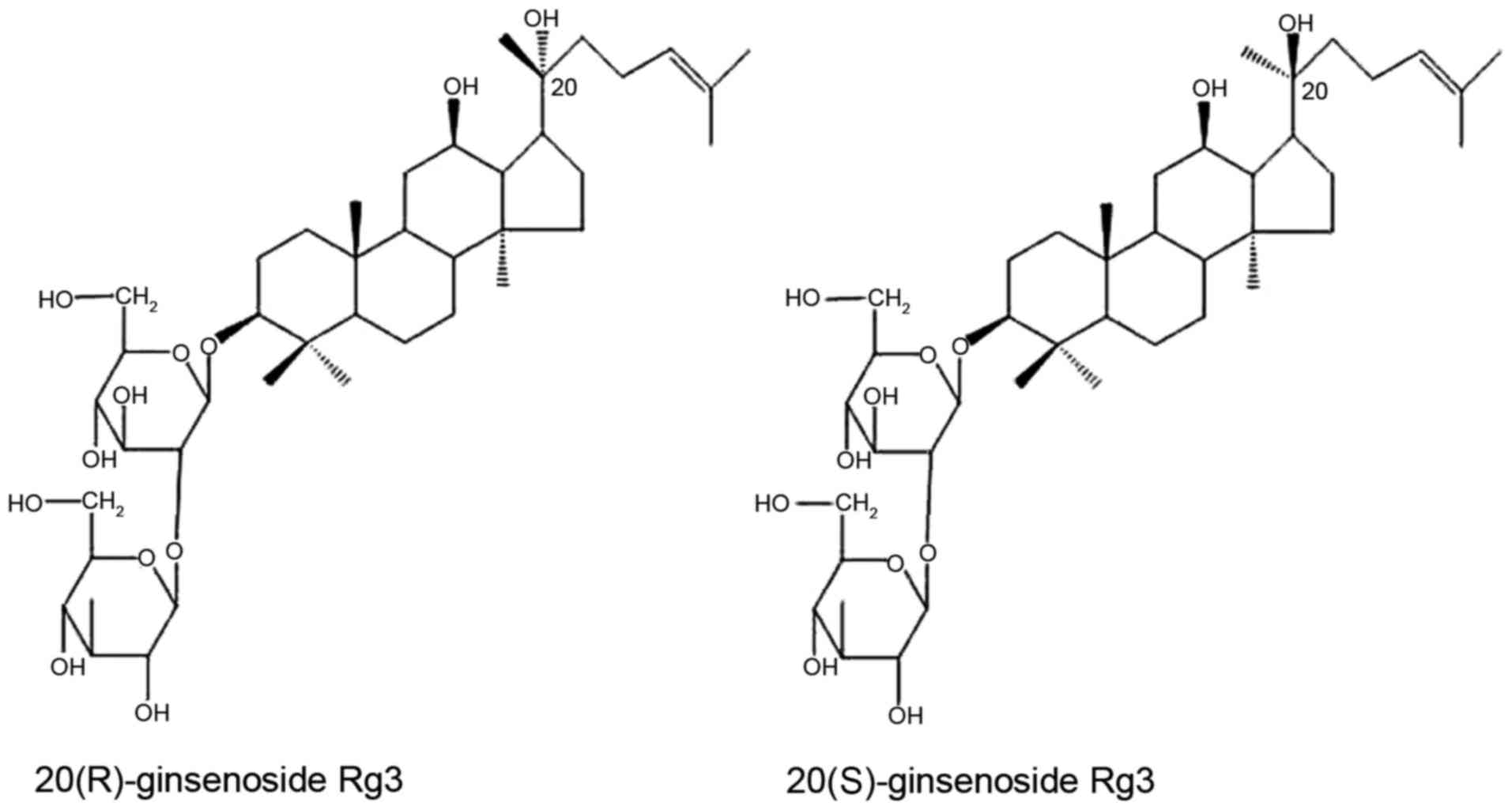
Ginsenoside Rg3 is a type of tetracyclic
triterpenoid saponin, rich in red ginseng. The major ginsenosides,
such as Rb1, Rb2, and Rd can be readily converted into ginsenoside
Rg3, the production of which may increase from 0.37 to 1.32% (w/w)
by heating (19). Due to the
different spatial structures from C20 positions, there are two
enantiomers, 20(R) and 20(S)-isomer (Fig. 1) (20). With differential configuration,
their antitumor activities exhibit certain differences (21).
3. Effects of Rg3 in cancer models
Natural products have always been a very good source
of drugs against cancer and have gained much attention lately. For
example, paclitaxel is one of the most important antitumor natural
agents isolated from the bark of Pacific yew tree (22). A study carried out in 1978
demonstrated the inhibition or prevention of carcinogenesis induced
by various chemical carcinogens for ginseng (23). Ginsenoside Rg3 has been found to
be an effective ingredient contributing to the anti-carcinogenic
activity of ginseng. To provide a summary of the antitumor effects
of ginsenoside Rg3, we collected data from previous scientific
studies published over the last 10 years. Ginsenoside Rg3 has been
shown to possess significant anticancer activity and it may be used
alone (Table I) or as a
supplement to chemotherapeutic drugs in order to improve the
therapeutic efficacy and minimize or eliminate drug-induced
toxicity and chemotherapeutic resistance (Table II).
 | Table ISummary of the anticancer activities
of ginsenoside Rg3. |
Table I
Summary of the anticancer activities
of ginsenoside Rg3.
| Cancer | Ginsenoside
Rg3 | Observation | Cell type | Effects | Mechanisms of
action | Refs. |
|---|
| Breast cancer | Rg3 | In vitro (30
µM) | MDA-MB-231 | Induction of
apoptosis | Inhibition of
mutant p53 and NF-κB signaling via possibly inactivation of ERK and
Akt to activate mitochondrial death pathway | (17,35) |
| Breast cancer |
20(S)-ginsenoside
Rg3 | In vitro
(100–300 µM) | MCF-7
MDA-MB-231 | Inhibition of
proliferation | Arrested the cells
in the G1-phase | (46) |
| Breast cancer |
20(S)-ginsenoside
Rg3 | In vitro
(20–60 µg/ml) | MDA-MB-231 | Inhibition of
metastasis | Inhibition of the
expression of CXCR4 | (48) |
| Colon cancer |
20(S)-ginsenoside
Rg3 | In vitro
(10–100 µM) | HT-29 | Inhibition of
proliferation, induction of apoptosis | Activation of
caMKKβ/AMPK mediated apoptosis mainly by regulating the
mitochondrial pathway involving p53/Bcl-2/Bax/cytochrome
c/caspase-3, caspase-9/PARP | (36) |
| Colon cancer |
20(S)-ginsenoside
Rg3 | In vitro
(≥100 µM) | HT-29 | Inhibition of
proliferation, induction of apoptosis | Reduced PCNA, STRAP
and other protein related to mitosis and DNA repair, downregulated
Rho-GDI while upregulated TM1, GSTP1 and Annexin | (42) |
| Colon cancer |
20(S)-ginsenoside
Rg3 | In vitro
(100–300 µM) | HCT116 | Inhibition of
proliferation | Mostly by
regulating the Eph/ephrin pathway and gene expression of AKAPA8L
and PITPNA | (44) |
| Colon cancer | Rg3 | In vitro
(above 100 µM)
in vivo (20 mg/kg) | HCT116
SW480 | Inhibition of
proliferation, inhibition of tumor growth in xenograft model | Blocked the nuclear
translocation of β-catenin and then inhibited β-catenin/Tcf signal
pathway | (43) |
| Colon cancer | Rg3 | In vitro
(200 µM) | SW480 | Inhibition of
metastasis | Inhibited NF-κB
signaling pathway and the NF-κB-regulated gene expression such as
c-Myc, COX-2 and MMP-9 | (52) |
Esophageal
Carcinoma
Renal cancer | Rg3 | In vitro
(25–200 µM) | Eca-109 786–0 | Inhibition of
proliferation and angiogenesis | Suppressed VEGF
expression by blocking multiple signaling pathways including
HIF-1α, COX-2, NF-κB, STAT3 and MAPKs | (56) |
| Gallbladder |
20(S)-ginsenoside
Rg3 | In vitro
(25–400 µM)
in vivo (20 and 40 mg/kg) | GBC-SD
NOZ | Cycle arrest,
induction of apoptosis, inhibition of the growth of xenografts | Suppressed MDM2 to
activate the p53 pathway, induced cycle arrest at
G0/G1 and mitochondrial-dependent
apoptosis | (34) |
| Gallbladder | Rg3 | In vitro
(10–100 µg/ml)
in vivo (20 mg/kg) | GBC-SD
QBC939
Mz-ChA-1 | Induction of
apoptosis, inhibition of the growth of tumor xenografts | Activation of ER
stress to regulate apoptosis related proteins such as caspase-12,
CHOP | (38) |
| Gastric cancer |
20(S)-ginsenoside
Rg3 | In vitro
(25–100 µM) | AGS | Induction of
apoptosis, inhibition of proliferation | Upregulation of
caspase-3, caspase-8, caspase-9 and Bax while downregulation of
Bcl-2 | (32) |
| Gastric cancer | Rg3 | In vitro (50
µg/ml) | SGC-7901 | Induction of
apoptosis | Inhibition of the
expression of FUT4 via SP1 and HSF1 transcriptional regulation and
eventually, activation of caspase-3, caspase-8 and caspase-9 | (37) |
| Gastric cancer | Rg3 | In vitro
(200–500 µM) | AGS | Induction of
apoptosis | Blocked TRPM7
channel activity | (39) |
| Glioblastoma
multiforme | Rg3 | In vitro
(≥10 µM) | U87MG | Induction of
apoptosis | Suppression of
MEK/MAPK signaling pathway, activation of ROS by antioxidant enzyme
system | (30) |
| Glioma |
20(S)-ginsenoside
Rg3 | In vitro (20
µM) | U87 | Inhibition of
proliferation | Induced cell
senescence by activating Akt and p53/p21 dependent signaling
pathways | (45) |
| Lung cancer | Rg3 | In vitro
(50–100 µM)
in vivo (30 mg/kg) | A549 | Induction of
apoptosis, inhibition of proliferation, inhibition of tumor
growth | Inhibited EGFR
activation and its downstream signal transduction, induced
caspase-dependent apoptotic pathway | (15) |
| Lung cancer |
20(R)-ginsenoside
Rg3 | In vitro (25
and 50 µg/ml) | A549 | Inhibition of
metastasis | Inhibited TGF-β1
induced EMT via downregulation of Snail and inactivation of MMP-2,
p38 MAPK and Smad2 | (53) |
| Liver cancer |
20(S)-ginsenoside
Rg3 | In vitro
(≥10 µM) | Hep3B | Induction of
apoptosis | Upregulation of
ROS, Bax, the release of cytochrome c and caspase-3,
down-regulation of Bcl-2, MMP | (16) |
| Liver cancer | Rg3 | In vitro
(50–200 µg/ml)
in vivo (3.0 mg/kg) | Hep1–6
HepG2 | Induction of
apoptosis, inhibition of tumor growth and increased survival time
of tumor-bearing mice | Decrease of Bcl-2
and Bcl-xL while increase of Bax, caspase-3 and the release of
cytochrome c | (29) |
| Liver cancer | Rg3 | In vitro
(25–100 µg/ml) | SMMC-7721
HepG2 | Inhibition of
proliferation, induction of apoptosis | Induction of
caspase-dependent endogenous apoptotic pathway | (33) |
| Melanoma |
20(R)-ginsenoside
Rg3 | In vitro
(25–100 µg/ml)
in vivo (20 mg/kg) | A375 | Inhibition of,
proliferation inhibition of the growth of tumor xenografts | Reduction of FUT4
and LeY to inhibit EGFR/MAPK signaling pathway | (41) |
| Melanoma |
20(R)-ginsenoside
Rg3 | In vitro (50
µg/ml)
in vivo (20 mg/kg) | A375
C8161 | Inhibition of
proliferation, inhibition of xenograft tumor volume and weight | Decreased the
expression of HDAC3, increasing p53 acetylation and transcription
activity, induced cell cycle arrest at
G0/G1 | (47) |
| Melanoma |
20(S)-ginsenoside
Rg3 | In vitro
(40–100 µg/ml)
in vivo (1.5 mg/kg) | B16 | Inhibition of
proliferation, induction of apoptosis, inhibition of metastasis,
increased the survival time | Regulation of cell
cycle and the expression of caspase and Bcl-2, inhibition of
angiogenesis | (31) |
| Melanoma |
20(S)-ginsenoside
Rg3 | In vitro
(25–100 µM) | B16F10 | Inhibition of
metastasis | Inhibited MMP-13
expression by the p38 MAPK signaling pathway | (51) |
| Ovarian cancer |
20(S)-ginsenoside
Rg3 | In
vitro
in vivo | SKOV-3 (160
µg/ml)
3AO (80 µg/ml) | Induction of
apoptosis, inhibition of proliferation, inhibition of the growth of
tumor xenografts | Suppression of
Warburg effect though the STAT3/HK2 pathway | (91) |
| Ovarian cancer |
20(S)-ginsenoside
Rg3 | In vitro
(25–100 µg/ml) | HO-8910 | Induction of
apoptosis | Regulation of
PI3K/Akt and XIAP pathways to activate caspase-3 and caspase-9 | (28) |
| Ovarian cancer |
20(S)-ginsenoside
Rg3 | In
vitro
in vivo (5 mg/kg) | SKOV-3 (80
µg/ml)
3AO (160 µg/ml) | Inhibition of
metastasis, inhibition of tumor growth and metastasis | Blocked
hypoxia-induced EMT by decreasing HIF-1α expression | (54) |
| Ovarian cancer | Rg3 | In vitro
(2.5, 5.0 µg/ml)
in vivo (0.3–3 mg/kg) | SKOV-3 | Inhibition of
metastasis, and angiogenesis, inhibition of lung metastasis of
ovarian cancer | Inhibition of
angiogenesis, tumor cell invasive ability, as well as the
expression of MMP-9 | (50) |
| Pancreatic
cancer | Rg3 | In vitro
(25–200 µM)
in vivo (5, 10, 20 mg/kg) | SW-1990 | Inhibition of VM
and the growth of tumor xenografts | Inhibition of the
expression of particular genes including VE-cadherin, EphA2, MMP-2
and MMP-9 | (60) |
| Prostate
cancer |
20(S)-ginsenoside
Rg3 | In vitro
(above 100 µM) | PC3
LNCaP | Inhibition of
proliferation | Inhibition of DNA
synthesis and cancer cells attachment, regulation of three MAP
kinase activity, including ERKs, p38 MAP kinases and JNK | (40) |
| Prostate
cancer |
20(S)-ginsenoside
Rg3 | In vitro (1
and 10 µM) | PC-3M | Inhibition of
migration | Transcriptionally
inhibited the expression of AQP1 via the p38 MAPK pathway | (55) |
 | Table IICombination therapy with ginsenoside
Rg3 and conventional chemotherapeutic drugs. |
Table II
Combination therapy with ginsenoside
Rg3 and conventional chemotherapeutic drugs.
| Drug | Cancer | Observation | Cell type | Activity | Mechanisms | Refs. |
|---|
|
As2O3 | Lung cancer | In
vitro
in vivo | NCI-H1299 | Enhanced the
antitumor efficacy, prolonged the survival time of tumor-bearing
mice | Promoted
proliferation inhibition and apoptosis induction | (62) |
| Cisplatin | Bladder cancer | In
vitro | T24R2 | Enhanced the
antitumor activity | Stimulated cell
arrest at G2/M, activated intrinsic apoptotic pathway | (61) |
| Cisplatin | Colon cancer | In vivo | | Augmented the
anti-neoplastic activity of cisplatin, decreased cisplatin-induced
tissue damage in the kidney and liver, inhibited resistance to
chemotherapeutics | Reduction of the
level of nuclear Nrf2, HO-1/NQO-1 and ROS | (72) |
|
Cyclophosphamide | | In vivo | | Suppressed
CP-induced side effects | Inhibited
CP-induced oxidative stress by regulating the activities of SOD and
GPx, and MDA contents | (6) |
|
Cyclophosphamide | Ovarian cancer | In vivo | | Reinforced each
other's antitumor activity, decreased CP-induced side-effects | Not
investigated | (64) |
|
Cyclophosphamide | Lewis lung
cancer | In vivo | | Augmented the
antitumor and anti-angiogenesis activity, reduced drug-induced
toxicity and prolonged the survival time | Inhibited the
expression of Ki-67, VEGF, Bcl-2 and p53 | (65) |
| Docetaxel | Prostate
cancer | In
vitro | LNCaP
PC-3
DU145 | Augmented
susceptibility of cancer cells to docetaxel | Inactivation of
NF-κB and then regulation of its target gene expression such as Bax
and caspase-3 | (70) |
| Docetaxel | Colon cancer | In
vitro | HCT116
SW620 | Potentiated
sensitivity of cancer cells to docetaxel | Inhibition of NF-κB
to regulate the expression of pro-apoptotic protein and
anti-apoptotic protein | (71) |
| Doxorubicin | Hepatocellular
carcinoma | In
vitro
In vivo | SK-Hep1
HepG2
Huh-7
Hep3B | Sensitized
doxorubicin-induced cancer cell death | Inhibited autophagy
possibly by blocking lysosomal function via regulating gene
expression such as CHOP | (74) |
| Doxorubicin | | In
vivo
in vitro | CMEC | Suppressed
doxorubicin induced cardiotoxicity | Improved cardiac
function and endothelial dysfunction at least partially by
activating the Nrf2/ARE and Akt pathway | (63) |
| Gemcitabine | Lung cancer | In vivo | | Inhibited
gemcitabine induced side-effects, enhanced each other's antitumor
activity | Not
investigated | (18) |
| Paclitaxel and
cisplatin | Esophageal squamous
cell carcinoma | In vivo | | Enhanced the
antitumor efficacy of chemotherapy | Lowered the tumor
microvascular density and the Ki-67 expression | (66) |
| Paclitaxel | Breast cancer | In
vitro
in vivo | Caco-2 | Increased the
bioavailability and antitumor effect of paclitaxel lowered
paclitaxel induced toxicity | Inhibited P-gp in
tumor tissue, accelerated the distribution of paclitaxel to tissues
and avoided a high plasma concentration | (67) |
| TRAIL | Hepatocellular
carcinoma | In
vitro
in vivo | HepG2, SK-Hep1,
Huh-7, Hep3B | Sensitized
TRAIL-induced HCC cell apoptosis | Increased of TRAIL
receptors DR5 expression by CHOP upregulation | (73) |
Treatment with Rg3 significantly has been shown to
inhibit the growth of cancer in various cancer models. Ginsenoside
Rg3 has been shown to exert cancer-preventive effects in both in
vitro and in vivo studies in a dose- and time-dependent
manner. The protective effects of Rg3 are mainly related to the
induction of apoptosis, and the inhibition of proliferation,
metastasis and angiogenesis (Table
I). Table II displays the
combined treatment effects of both Rg3 and several existing
chemical drugs on cancer models. Ginsenoside Rg3 has been shown to
enhance the antitumor effects of conventional chemotherapeutic
agents and to reduce drug-induced toxicity and chemotherapeutic
resistance in vitro and in vivo. Combination
therapies using chemotherapeutic agents and ginsenoside may be an
innovative and promising therapeutic strategy for the treatment of
human cancer. However,the pharmacodynamic interactions between
chemotherapeutic drugs and Rg3 warrant further investigation.
In addition, the hepatic arterial administration of
Rg3 combined with local transarterial embolization (TAE) was shown
to more effectively inhibited VX2 liver tumor growth than any
mono-therapies, with the inhibition of angiogenesis and the
induction of caspase-dependent apoptosis (24).
4. Suggested mechanisms of action of
Rg3
Cancer is a class of diseases involving genetic
damage which alters several intracellular biochemical signals and
eventually results in uncontrolled cell growth (25). Drugs with antitumor activity, such
as Rg3 may be able to alter these abnormal alterations through
certain mechanisms. Although the anticancer activities of Rg3 have
been widely investigated, the exact molecular mechanisms are not
yet clear. The possible mechanisms of action of Rg3 based on the
existing studies are described as follows:
Induction of apoptosis
Apoptosis, known as programmed cell death, is one of
the principal mechanisms which maintains cellular homeostasis. In
human cancers, tumor cells proliferate more rapidly than normal
cells and always lose appropriate apoptotic control. This
disruption of growth balance can promote tumor development
(26). As shown in Table I, Rg3 can induce apoptosis via two
major pathways: the mitochondrial-dependent intrinsic apoptotic
pathway and the death receptor-dependent extrinsic pathway.
Mitochondrial-dependent apoptosis involves a reduction in
mitochondrial membrane potential, which results in the release of
cytochrome c from the mitochondria, and the activation of
caspase-9 and caspase-3, and finally, apoptosis (27). It has been shown that
20(S)-ginsenoside Rg3-induced apoptosis is dependent on the
activation of caspase-3 and caspase-9, which is mediated by the
PI3K pathway in HO-8910 human ovarian cancer cells (28). Reactive oxygen species (ROS) and
Bcl-2 family members play an important role in the mitochondrial
apoptotic pathway. Ginsenoside Rg3 has been shown to activate
intracellular ROS generation and/or increase the ratio of Bax to
Bcl-2 protein to induce mitochondrial-dependent apoptosis (16,29–33). p53 and nuclear factor (NF)-κB, two
nuclear transcription factors, play a critical role in
mitochondrial membrane potential through the regulation of the
expression of apoptosis-related genes, such as inhibitors of
apoptosis proteins (IAPs), X-linked inhibitor of apoptosis protein
(XIAP) and Bcl-2. p53 is an important pro-apoptotic factor, while
NF-κB is a type of anti-apoptotic factor. 20(S)-ginsenoside
Rg3 exerts cytotoxic effects by activating the p53 pathway and
subsequently inducing mitochondrial-dependent apoptosis (34). Moreover, ginsenoside Rg3 has been
shown to inhibit mutant p53 and NF-κB signaling, possibly via the
inactivation of extracellular signal-regulated kinase (ERK) and Akt
to activate the mitochondrial death pathway, including the
reduction of the ratio of Bcl-2/Bax expression, the disruption of
mitochondrial membrane potential, the activation of caspase-3 and
ROS generation (17,35). p53 and Bax have also been shown to
play a role in the 20(S)-Rg3-induced apoptosis of HT-29
colon cancer cells via the activation of 5′ AMP-activated protein
kinase (36).
The extrinsic apoptotic pathway is activated by
specific ligands, such as Fas ligand, tumor necrosis factor-α
(TNF-α), and tumor necrosis factor-related apoptosis-inducing
ligand (TRAIL). These receptors can activate a caspase-8-dependent
cascade to induce apoptosis (21). Another previous study demonstrated
that ginsenoside Rg3 induced apoptosis through the activation of
caspase-8 in the human gastric cancer cell line, AGS (37). The increased expression of Fas has
also been observed in Rg3-treated A549 cells (15). In addition, the occurence of
extrinsic apoptosis with the activation of endoplasmic reticulum
stress mediates ginseng Rg3-induced apoptosis (38).
In addition, ion channels are crucial to tumor
growth and cancer cell survival. Kim et al suggested that
the blockade of TRPM7 channels played an important role in the
Rg3-induced apoptosis of AGS cells, a human gastric adenocarcinoma
cell line (39).
Inhibition of proliferation
Ginsenoside Rg3 has been shown to exert significant
inhibitory effects on cancer cell proliferation. In LNCaP and PC3
human prostate carcinoma cell lines, 20(S)-ginsenoside Rg3
was shown to exhibit good growth inhibitory activity, which may be
associated with the modulation of mitogen-activated protein (MAP)
kinases (40). Rg3 inhibited EGFR
activation and its downstream signal transduction to suppress the
growth and proliferation of lung cancer cells A549 (15). Another study on melanoma suggested
that 20(R)-ginsenoside Rg3 inhibited cancer cell
proliferation by the deactivation of EGFR and the decrease of
fucosyltransferase IV (FUT4)/Lewis Y (LeY) expression (41). In addition, the protein expression
of proliferating cell nuclear antigen (PCNA), serine/threonine
kinase receptor associated protein (STRAP) and that of proteins
related to mitosis and DNA repair was shown to be suppressed in
20(S)-ginsenoside Rg3-treated human colon cancer cell lines
(42). Ginsenoside Rg3 has also
been shown to inhibit the nuclear translocation of β-catenin, an
oncogene, and the proliferation of colon cancer cell lines
(43). Microarray hybridization
analysis has shown 20(S)-ginsenoside Rg3 inhibits the
proliferation of HCT116 cells, mostly by regulating the Eph/ephrin
pathway and the gene expression of anchor protein 8-like (AKAPA8L)
and phosphatidylinositol transfer protein alpha (PITPNA) (44). Additionally, chronic treatment
with 20(S)-ginsenoside Rg3 at a sub-apoptotic concentration
has been shown to induce senescence-like growth arrest in human
glioma cells through the Akt and p53/p21 pathways (45).
Cell cycle arrest involves a set of events,
resulting in cell growth inhibition. 20(S)-ginsenoside Rg3
has been shown to have an anti-proliferative activity in MCF-7
human breast cancer cells by arresting the cell cycle at the
G1-phase (46). p53, a tumor
suppressor, plays an important role in mediating cell cycle arrest
and the DNA damage response or apoptosis, as well as p21, a cell
cycle inhibitor, downstream of p53. 20(S)-ginsenoside Rg3
has been found to have the ability to inhibit mouse double minute 2
homolog (MDM2), a negative regulator of p53, to activate the
p53/p21 pathway and subsequently induce cycle arrest at the
G0/G1 phase in gallbladder cancer cells
(34). Moreover, was previously
demonstrated that in both A375 and C816 melanoma cell lines,
20(R)-ginsenoside Rg3 inhibited proliferation and induced
cell cycle arrest at the G0/G1 phase by
decreasing the expression of histone deacetylase (HDAC)3 and
increasing p53 acetylation and transcriptional activity (47).
Inhibition of metastasis
Metastasis is a complex process through which cancer
cells spread from a primary site to form tumors at other distant
parts of the body. Tumor metastasis is a major cause of tumor
recurrence and mortality, in many types of cancer, such as breast
cancer (48). Matrix
metalloproteinases (MMPs), which can degrade the extracellular
matrix and basement membrane, play an important role in tumor
metastasis, and MMP-2 and MMP-9 are of particular importance in
these events (49). As previously
demonstrated, the Rg3-induced downregulation of MMP-9 appears to be
associated with the decreased invasive capacity of SKOV-3 ovarian
cancer cells (50). The
suppression of MMP-13 by the p38 MAP kinase signaling pathway has
been shown to play a role in the inhibition of the metastasis of
B16F10 cells by 20(S)-ginsenoside Rg3 in (51). In addition, ginsenoside Rg3 has
been shown to inhibit the migration of SW480 colon cancer cells by
suppressing NF-κB activity and the expression of NF-κB-regulated
gene products, including MMP-9, cyclooxygenase (COX)-2 and c-Myc
(52).
EMT is also an important mechanism involved in
cancer metastasis. 20(R)-ginsenoside Rg3 and
20(S)-ginsenoside Rg3 possess the ability to inhibit tumor
metastasis by the suppression of EMT. In a previous study,
20(R)-ginsenoside Rg3 suppressed lung cancer migration
or/and invasion by inhibiting TGF-β1-induced EMT accompanied by the
inactivation of MMP-2, p38 MAPK and Smad2 (53). In another study,
20(S)-ginsenoside Rg3 effectively suppressed hypoxia-induced
EMT, inhibiting ovarian cancer metastasis (54).
In the breast cancer cell line, MDA-MB-231,
20(S)-ginsenoside Rg3 was reported to exert anti-metastatic
effects by inhibiting the expression of C-X-C chemokine receptor
type 4 (CXCR4), which is a vital molecule in migration (48). Moreover, 20(S)-ginsenoside
Rg3 has been shown to decrease the incidence of metastasis by
inhibiting the expression of aquaporin 1 (AQP1) in PC-3M prostate
cancer cells (55).
Inhibition of angiogenesis
Tumor angiogenesis is a process involving the
formation of new blood vessels, which is essential to tumor growth
and metastasis by supplying oxygen and nutrients. Rg3 has been
found to inhibit tumor angiogenesis via the suppression of vascular
endothelial growth factor (VEGF) expression by blocking multiple
hypoxia-induced and angiogenesis-related signaling pathways in the
human esophageal carcinoma cell line, Eca-109, and in the renal
cell carcinoma cell line, 786-0 cells (56). In addition, both in vitro
and in vivo experiments have demonstrated that
20(R)-ginsenoside Rg3 significantly inhibits human umbilical
vein endothelial cell (HUVEC) proliferation and VEGF- and basic
fibroblast growth factor (bFGF)-stimulated angiogenesis (57). Endothelial progenitor cells (EPCs)
are closely related to tumor angiogenesis by promoting angiogenic
factors, such as VEGF. In human umbilical cord blood (hUCB)-derived
CD34-positive stem cells, treatment with Rg3 was shown to inhibit
EPC differentiation and tube formation via the VEGF-dependent
Akt/endothelial nitric oxide synthase (eNOS) signaling pathway
(58). Rg3 also was found to
effectively inhibit EPC proliferation and VEGF-induced angiogenesis
in vivo through multiple signaling cascades, such as p38 MAP
kinase (59).
Furthermore, vasculogenic mimicry (VM), a novel
tumor microcirculation system different from classical
endothelium-dependent angiogenesis, plays a critical role in tumor
progression. Guo et al found that ginsenoside Rg3
effectively inhibited VM formation in pancreatic cancer by
inhibiting the expression of particular genes, including
VE-cadherin, EphA2, MMP-2 and MMP-9, both in vitro and in
tumor xenografts (60).
Inhibition of multidrug resistance (MDR)
and increase of chemosensitivity
Some studies have suggested that combination
treatment may be more lead to an improved treatment efficacy with
decreased toxicity by exerting synergistic effects, although the
exact mechanisms involved remain unclear (18,61). For example,
As2O3 in combination with Rg3 has been shown
to significantly inhibit NCI-H1299 lung cancer cell proliferation
and to prolong the survival of tumor-bearing mice (62). Rg3 has been shown to suppress
doxorubicin-induced cardio-toxicity both in vitro and in
vivo, possibly by activating the NF-E2-related factor 2
(Nrf2)/antioxidant responsive element (ARE). and PI3/Akt pathways
(63). 20(S)-ginsenoside
Rg3 has also been shown to protect normal cells against
cyclophosphamide (CP)-induced genotoxity through antioxidant
activity (6). In Lewis lung
carcinoma and ovarian cancer models, the co-administration of
ginsenoside Rg3 was shown to effectively enhance the inhibitory
effects of CP on tumors and to reduce the occurrence of
side-effects (64,65). Ginsenoside Rg3 has also been shown
to improve the antitumor activity of paclitaxel and cisplatin in an
animal model of esophageal squamous cell carcinoma (66). In addition,
20(S)-ginsenoside Rg3 increases the oral bioavailability of
paclitaxel and then improves the antitumor activity of paclitaxel
(67).
Rg3 enhances the chemosensitivity of cancer cells to
chemical drugs or/and can help cells to overcome MDR, which may
partly explain the mechanisms of combination therapy. Previous
studies have indicated that 20(S)-ginsenoside Rg3 is a
highly effective modulating agent in reversing MDR in
drug-resistant P388 leukemia cells and human fibrocarcinoma KBV20C
cells specifically (68,69). Combination therapy using Rg3 and
the conventional chemotherapeutic agent, docetaxel, has been shown
to significantly enhance the sensitivity of cancer cells and to
decrease drug resistance in prostate cancer and colon cancer cells
via the inhibition of NF-κB (70,71). Moreover, Rg3 has been shown to
enhance the susceptibility of the colon to cisplatin by inhibiting
HO-1/NQO-1 expression. Moreover, Rg3 also decreased
cisplatin-induced tissue damage in the kidneys and liver by
preventing cisplatin-induced intracellular ROS generation (72). 20(S)-ginsenoside Rg3 has
also been shown to increase TRAIL receptor DR5 expression and
sensitivity to TRAIL in hepatocellular carcinoma (HCC) cells, such
as HepG2 (73).
20(S)-ginsenoside Rg3 is also capable of sensitizing
doxorubicin-treated liver cancer cells death by inhibiting
doxorubicin-induced autophagy possibly by blocking lysosomal
function (74).
Promotion of immunity
Rg3 also plays a role in the improvement of host
immunity in tumor-bearing animal models. A previous study suggested
that a ginsenoside Rg3-fortified red ginseng preparation
significantly suppressed tumor growth in H460 tumor-bearing mice by
immunopotentiation (75). In
addition, treatment with Rg3, has been shown to markedly enhance
(P<0.05) cellular immunity by stimulating ConA-induced
lymphocyte proliferation and the secretion of cytokines
[interleukin (IL)-2, interferon (IFN)-γ] in the immune organs and
serum of hepatoma H22-bearing mice (76). Moreover, Rg3 has been shown to
augment innate immunity and IFN-β expression via the upregulation
of DDX3 and the activation of the TANK-binding kinase 1
(TBK1)/inhibitor-κB kinase ε (IKKε)/interferon regulatory factor 3
(IRF3) pathway (77). Therefore,
Rg3 may be a potent immunomodulatory candidate for the treatment of
cancer.
5. Clinical studies
Although many studies have demonstrated a clear
anticancer activity of Rg3 in various cancer cell lines or/and
xenograft tumor models, the efficacy of Rg3 in human cancer
requires further investigation. Shenyi capsule (ginsenoside Rg3
monomer preparation), a class I new drug in traditional Chinese
medicine, is being used clinically in the treatment of various
types of cancer, such as lung cancer, breast cancer and
gastrointestinal tumors in China (78). A prospective, randomized,
controlled study using 133 non-small cell lung cancer cases
suggested that Shenyi capsule, particularly in combination with
chemotherapy, improved the post-operative lifespan of patients
mainly by enhancing the immune function and inhibiting angiogensis
(79). Another randomized trial
of 60 advanced esophageal cancer cases found that the combination
of chemotherapy with Shenyi capsule was effective in improving the
quality of life of patients and 1 year survival rates. In addition,
a meta-analysis involving 274 female breast cancer patients in
China also showed that the combination treatment group exhibited
significantly attenuated leucopenia. However, there were no
significant differences in the total response rate compared with
the chemotherapy groups (80,81).
In general, the published clinical studies suggested
that Rg3 was a good antitumor agent by improving the immune
function and the quality of life of cancer patients. However, there
has been no strong evidence to confirm the efficacy of Rg3.
Further, large controlled and highly qualified clinical trials are
required in order to better guide clinical applications.
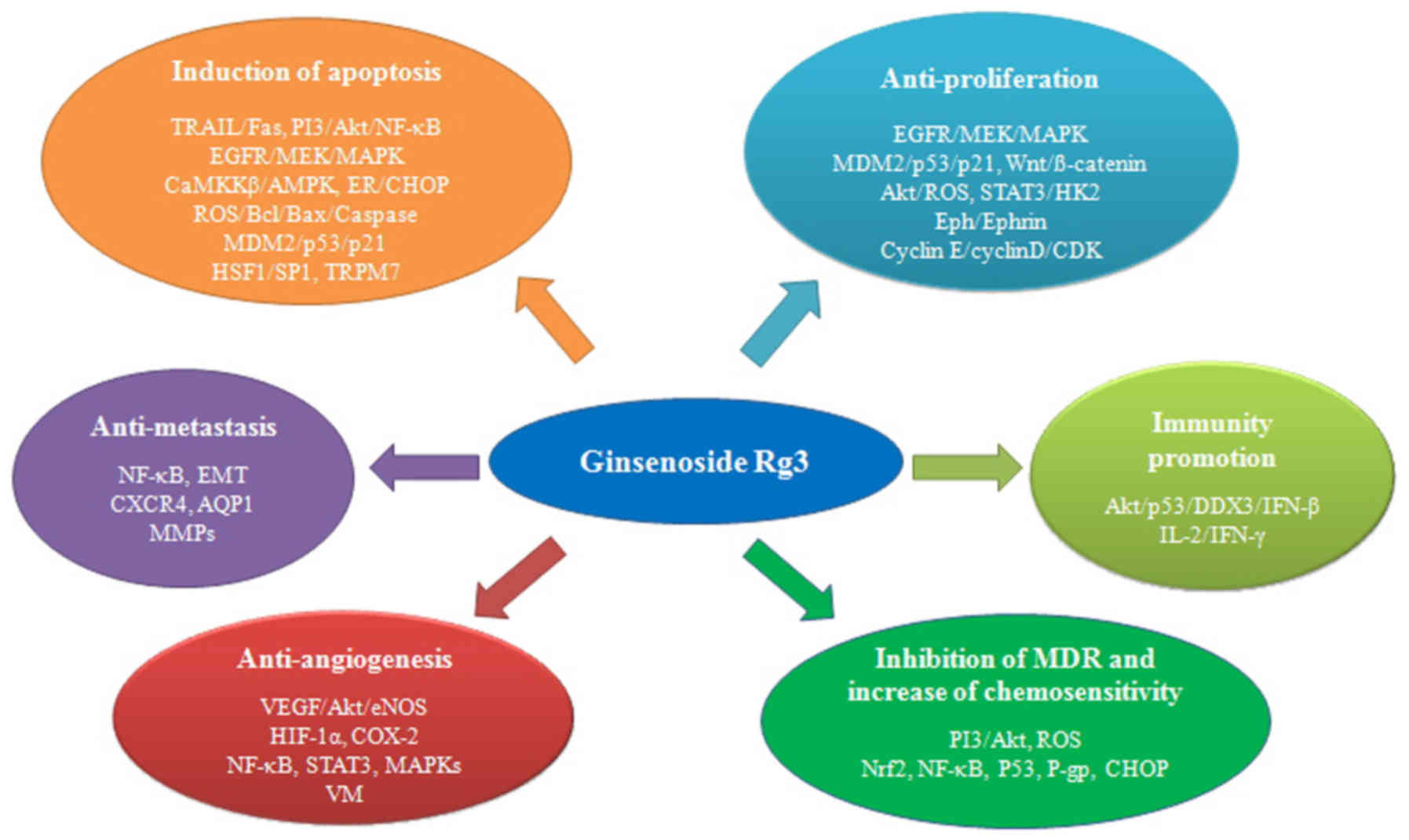
6. Conclusions
Ginsenoside Rg3 displays significant antitumor
activities in several types of cancer both in vitro and
in vivo. The molecular mechanisms of tumor inhibition
employed by ginsenoside Rg3 mainly involve the induction of
apoptosis, and the inhibition of proliferation, metastasis and
angiogensis, and the promotion of immunity (Fig. 2). Furthermore, experiments have
demonstrated that Rg3 is relatively safe for use in the marrow,
heart, lung, liver, kidney and nervous system (82). Long-term toxicological studies on
beagle dogs and rats also confirmed that 20(S)-ginsenoside
Rg3 was non-toxic and well-tolerated (83,84). Ginsenoside Rg3 has also been shown
to significantly decrease some environmental carcinogen-induced DNA
damage, such as N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) and
benzo[a] pyrene (85,86). These results make ginsenoside Rg3
an attractive candidate for cancer prevention. Combination therapy
with conventional cancer treatments, such as chemotherapy and
surgery has been suggested to be more effective. Rg3 improves the
chemosensitivity of tumor cells to chemical drugs, reverses MDR and
decreases toxicity. Additionally, Rg3 combined with TAE, more
effectively inhibits tumor growth. Apart from these, a recent study
found that 20(S)-ginsenoside Rg3 induced the apoptosis of
HepG2 cells, accompanied by the induction of autophagy via
mitochondrial and Ca2+-related pathways, which decreased
the survival of cancer cells. Thus, co-treatment with autophagy
inhibitors and Rg3 would be beneficial (87). Clinical trials still demonstrate
that ginsenoside Rg3 is an effective antitumor agent. Therefore,
ginsenoside Rg3 is gaining more attention as an anticancer drug due
to its favorable safety and efficacy.
However, there are still several aspects limiting
the use of Rg3: i) its mechanisms of action have not yet been fully
established. For example, ginsenoside Rg3 induces strong
genotoxicity and DNA damage in human osteosarcoma cells. However,
whether these genotoxic effects are directly associated with cell
cycle arrest and apoptosis is not clear (85). Further studies are warranted to
determine the mechanisms through which the different signaling
pathways are orchestrated and and those through which the
synergistic antitumor activities of ginsenosides and conventional
cancer treatments are realized in order to fully understand the
benefits of Rg3. ii) Its poor aqueous solubility and low oral
bioavailability (88). Although
studies have been performed to explore a method to solve this
problem, for example, 20(S)-ginsenoside Rg3-loaded magnetic
human serum albumin nanospheres [20(S)-Rg3/HSAMNP] were
created and were shown to markedly enhance the efficiency of HeLa
cervical cancer cell inhibition when combined with hyperthermia
(89), and liposomal ginsenoside
Rg3 showed increased anticancer activity in vitro compared
to the Rg3 solution (90), it is
necessary to evaluate the efficacy of these optimized therapy in
vivo. It may be possible to develop novel Rg3 analogues with
improved efficacy, pharmacokinetics and bioavailability profiles.
iii) Currently, the evidence of Rg3 efficacy is not yet conclusive
in humans. There is a significant need to perform further, larger
cohort clinical studies to confirm its efficacy for better
application in the clinic.
In conclusion, Rg3 has great potential for use as a
broad-spectrum anticancer drug and an effective adjuvant to cancer
therapies in the future.
Acknowledgments
This study was supported by funds from the National
Natural Science Foundation of China (81502515 and 81503332),
Shanghai Natural Science Foundation (no. 13ZR1431900), Shanghai
Municipal Health and Family Planning Commission (nos. 20134173,
20134090 and ZYXK2012010), Key Disciplines Group Construction
Project of Pudong Health Bureau of Shanghai (no. PWZxq2014-12), and
the Open Research Fund of State Key Laboratory Breeding Base of
Systematic Research, Development and Utilization of Chinese
Medicine Resources (no. ME2016012).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stewart B and Wild C: World Cancer Report
2014. International Agency for Research on Cancer, World Health
Organization; Lyon, France: 2014
|
|
3
|
Cabrera R and Nelson DR: Review article:
the management of hepatocellular carcinoma. Aliment Pharmacol Ther.
31:461–476. 2010. View Article : Google Scholar
|
|
4
|
Fletcher A, Choudhury A and Alam N:
Metastatic bladder cancer: a review of current management. ISRN
Urol. 2011:5452412011.PubMed/NCBI
|
|
5
|
Baldo BA and Pham NH: Adverse reactions to
targeted and non-targeted chemotherapeutic drugs with emphasis on
hypersensitivity responses and the invasive metastatic switch.
Cancer Metastasis Rev. 32:723–761. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang QH, Wu CF, Duan L and Yang JY:
Protective effects of ginsenoside Rg(3) against
cyclophosphamide-induced DNA damage and cell apoptosis in mice.
Arch Toxicol. 82:117–123. 2008. View Article : Google Scholar
|
|
7
|
Lü JM, Yao Q and Chen C: Ginseng
compounds: an update on their molecular mechanisms and medical
applications. Curr Vasc Pharmacol. 7:293–302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu L, Zhu XM, Wang QJ, Zhang DL, Fang ZM,
Wang CY, Wang Z, Sun BS, Wu H and Sung CK: Enzymatic preparation of
20(S, R)-protopanaxadiol by transformation of 20(S, R)-Rg3 from
black ginseng. Phytochemistry. 71:1514–1520. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gum SI and Cho MK: The amelioration of
N-acetyl-p-benzoquinone imine toxicity by ginsenoside Rg3: the role
of Nrf2-mediated detoxification and Mrp1/Mrp3 transports. Oxid Med
Cell Longev. 2013:9579472013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He B, Chen P, Yang J, Yun Y, Zhang X, Yang
R and Shen Z: Neuroprotective effect of 20(R)-ginsenoside Rg(3)
against transient focal cerebral ischemia in rats. Neurosci Lett.
526:106–111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kwok HH, Guo GL, Lau JK, Cheng YK, Wang
JR, Jiang ZH, Keung MH, Mak NK, Yue PY and Wong RN: Stereoisomers
ginsenosides-20(S)-Rg3 and -20(R)-Rg3 differentially induce
angiogenesis through peroxisome proliferator-activated
receptor-gamma. Biochem Pharmacol. 83:893–902. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wei X, Chen J, Su F, Su X, Hu T and Hu S:
Stereospecificity of ginsenoside Rg3 in promotion of the immune
response to ovalbumin in mice. Int Immunol. 24:465–471. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu Y, Zhang P, Wang C, Shan Y, Wang D,
Qian F, Sun M and Zhu C: Effect of ginsenoside Rg3 on tyrosine
hydroxylase and related mechanisms in the forced swimming-induced
fatigue rats. J Ethnopharmacol. 150:138–147. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wei X, Su F, Su X, Hu T and Hu S:
Stereospecific antioxidant effects of ginsenoside Rg3 on oxidative
stress induced by cyclophosphamide in mice. Fitoterapia.
83:636–642. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Joo EJ, Chun J, Ha YW, Ko HJ, Xu MY and
Kim YS: Novel roles of ginsenoside Rg3 in apoptosis through
downregulation of epidermal growth factor receptor. Chem Biol
Interact. 233:25–34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park HM, Kim SJ, Kim JS and Kang HS:
Reactive oxygen species mediated ginsenoside Rg3- and Rh2-induced
apoptosis in hepatoma cells through mitochondrial signaling
pathways. Food Chem Toxicol. 50:2736–2741. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim BM, Kim DH, Park JH, Na HK and Surh
YJ: Ginsenoside Rg3 induces apoptosis of human breast cancer
(MDA-MB-231) cells. J Cancer Prev. 18:177–185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu TG, Huang Y, Cui DD, Huang XB, Mao SH,
Ji LL, Song HB and Yi C: Inhibitory effect of ginsenoside Rg3
combined with gemcitabine on angiogenesis and growth of lung cancer
in mice. BMC Cancer. 9:2502009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim WY, Kim JM, Han SB, Lee SK, Kim ND,
Park MK, Kim CK and Park JH: Steaming of ginseng at high
temperature enhances biological activity. J Nat Prod. 63:1702–1704.
2000. View Article : Google Scholar
|
|
20
|
Kim IW, Sun WS, Yun BS, Kim NR, Min D and
Kim SK: Characterizing a full spectrum of physico-chemical
properties of (20S)- and (20R)-ginsenoside Rg3 to be proposed as
standard reference materials. J Ginseng Res. 37:124–134. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nag SA, Qin JJ, Wang W, Wang MH, Wang H
and Zhang R: Ginsenosides as anticancer agents: in vitro and in
vivo activities, structure-activity relationships, and molecular
mechanisms of action. Front Pharmacol. 3:252012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wani MC, Taylor HL, Wall ME, Coggon P and
McPhail AT: Plant antitumor agents VI The isolation and structure
of taxol, a novel antileukemic and antitumor agent from Taxus
brevifolia. J Am Chem Soc. 93:2325–2327. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yun TK, Lee YS, Lee YH, Kim SI and Yun HY:
Anticarcinogenic effect of Panax ginseng C.A. Meyer and
identification of active compounds. J Korean Med Sci. 16(Suppl):
S6–S18. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu Y, Zhang C, Liu L and Li X: Hepatic
arterial administration of ginsenoside Rg3 and transcatheter
arterial embolization for the treatment of VX2 liver carcinomas.
Exp Ther Med. 5:761–766. 2013.PubMed/NCBI
|
|
25
|
Amelio I, Melino G and Knight RA: Cell
death pathology: cross-talk with autophagy and its clinical
implications. Biochem Biophys Res Commun. 414:277–281. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Karmakar S, Banik NL, Patel SJ and Ray SK:
Curcumin activated both receptor-mediated and mitochondria-mediated
proteolytic pathways for apoptosis in human glioblastoma T98G
cells. Neurosci Lett. 407:53–58. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang JH, Nao JF, Zhang M and He P:
20(s)-ginsenoside Rg3 promotes apoptosis in human ovarian cancer
HO-8910 cells through PI3K/Akt and XIAP pathways. Tumour Biol.
35:11985–11994. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiang JW, Chen XM, Chen XH and Zheng SS:
Ginsenoside Rg3 inhibit hepatocellular carcinoma growth via
intrinsic apoptotic pathway. World J Gastroenterol. 17:3605–3613.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Choi YJ, Lee HJ, Kang DW, Han IH, Choi BK
and Cho WH: Ginsenoside Rg3 induces apoptosis in the U87MG human
glioblastoma cell line through the MEK signaling pathway and
reactive oxygen species. Oncol Rep. 30:1362–1370. 2013.PubMed/NCBI
|
|
31
|
Chen J, Peng H, Ou-Yang X and He X:
Research on the antitumor effect of ginsenoside Rg3 in B16 melanoma
cells. Melanoma Res. 18:322–329. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Park EH, Kim YJ, Yamabe N, Park SH, Kim
HK, Jang HJ, Kim JH, Cheon GJ, Ham J and Kang KS: Stereospecific
anticancer effects of ginsenoside Rg3 epimers isolated from
heat-processed American ginseng on human gastric cancer cell. J
Ginseng Res. 38:22–27. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang C, Liu L, Yu Y, Chen B, Tang C and
Li X: Antitumor effects of ginsenoside Rg3 on human hepatocellular
carcinoma cells. Mol Med Rep. 5:1295–1298. 2012.PubMed/NCBI
|
|
34
|
Zhang F, Li M, Wu X, Hu Y, Cao Y, Wang X,
Xiang S, Li H, Jiang L, Tan Z, et al: 20(S)-ginsenoside Rg3
promotes senescence and apoptosis in gallbladder cancer cells via
the p53 pathway. Drug Des Devel Ther. 9:3969–3987. 2015.PubMed/NCBI
|
|
35
|
Kim BM, Kim DH, Park JH, Surh YJ and Na
HK: Ginsenoside Rg3 inhibits constitutive activation of NF-κB
signaling in human breast cancer (MDA-MB-231) cells: ERK and Akt as
potential upstream targets. J Cancer Prev. 19:23–30. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yuan HD, Quan HY, Zhang Y, Kim SH and
Chung SH: 20(S)-ginsenoside Rg3-induced apoptosis in HT-29 colon
cancer cells is associated with AMPK signaling pathway. Mol Med
Rep. 3:825–831. 2010.
|
|
37
|
Aziz F, Wang X, Liu J and Yan Q:
Ginsenoside Rg3 induces FUT4-mediated apoptosis in H. pylori
CagA-treated gastric cancer cells by regulating SP1 and HSF1
expressions. Toxicol In Vitro. 31:158–166. 2016. View Article : Google Scholar
|
|
38
|
Wu K, Li N, Sun H, Xu T, Jin F and Nie J:
Endoplasmic reticulum stress activation mediates Ginseng
Rg3-induced anti-gallbladder cancer cell activity. Biochem Biophys
Res Commun. 466:369–375. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim BJ, Nah SY, Jeon JH, So I and Kim SJ:
Transient receptor potential melastatin 7 channels are involved in
ginsenoside Rg3-induced apoptosis in gastric cancer cells. Basic
Clin Pharmacol Toxicol. 109:233–239. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim HS, Lee EH, Ko SR, Choi KJ, Park JH
and Im DS: Effects of ginsenosides Rg3 and Rh2 on the proliferation
of prostate cancer cells. Arch Pharm Res. 27:429–435. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shan X, Aziz F, Tian LL, Wang XQ, Yan Q
and Liu JW: Ginsenoside Rg3-induced EGFR/MAPK pathway deactivation
inhibits melanoma cell proliferation by decreasing FUT4/LeY
expression. Int J Oncol. 46:1667–1676. 2015.PubMed/NCBI
|
|
42
|
Lee SY, Kim GT, Roh SH, Song JS, Kim HJ,
Hong SS, Kwon SW and Park JH: Proteomic analysis of the anti-cancer
effect of 20S-ginsenoside Rg3 in human colon cancer cell lines.
Biosci Biotechnol Biochem. 73:811–816. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
He BC, Gao JL, Luo X, Luo J, Shen J, Wang
L, Zhou Q, Wang YT, Luu HH, Haydon RC, et al: Ginsenoside Rg3
inhibits colorectal tumor growth through the down-regulation of
Wnt/β-catenin signaling. Int J Oncol. 38:437–445. 2011. View Article : Google Scholar
|
|
44
|
Luo X, Wang CZ, Chen J, Song WX, Luo J,
Tang N, He BC, Kang Q, Wang Y, Du W, et al: Characterization of
gene expression regulated by American ginseng and ginsenoside Rg3
in human colorectal cancer cells. Int J Oncol. 32:975–983.
2008.PubMed/NCBI
|
|
45
|
Sin S, Kim SY and Kim SS: Chronic
treatment with ginsenoside Rg3 induces Akt-dependent senescence in
human glioma cells. Int J Oncol. 41:1669–1674. 2012.PubMed/NCBI
|
|
46
|
Wang CZ, Aung HH, Zhang B, Sun S, Li XL,
He H, Xie JT, He TC, Du W and Yuan CS: Chemopreventive effects of
heat-processed Panax quinquefolius root on human breast cancer
cells. Anticancer Res. 28:2545–2551. 2008.PubMed/NCBI
|
|
47
|
Shan X, Fu YS, Aziz F, Wang XQ, Yan Q and
Liu JW: Ginsenoside Rg3 inhibits melanoma cell proliferation
through down-regulation of histone deacetylase 3 (HDAC3) and
increase of p53 acetylation. PLoS One. 9:e1154012014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen XP, Qian LL, Jiang H and Chen JH:
Ginsenoside Rg3 inhibits CXCR4 expression and related migrations in
a breast cancer cell line. Int J Clin Oncol. 16:519–523. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xu TM, Cui MH, Xin Y, Gu LP, Jiang X, Su
MM, Wang DD and Wang WJ: Inhibitory effect of ginsenoside Rg3 on
ovarian cancer metastasis. Chin Med J (Engl). 121:1394–1397.
2008.
|
|
51
|
Lee SG, Kang YJ and Nam JO:
Anti-metastasis effects of ginsenoside Rg3 in B16F10 Cells. J
Microbiol Biotechnol. 25:1997–2006. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Junmin S, Hongxiang L, Zhen L, Chao Y and
Chaojie W: Ginsenoside Rg3 inhibits colon cancer cell migration by
suppressing nuclear factor kappa B activity. J Tradit Chin Med.
35:440–444. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kim YJ, Choi WI, Jeon BN, Choi KC, Kim K,
Kim TJ, Ham J, Jang HJ, Kang KS and Ko H: Stereospecific effects of
ginsenoside 20-Rg3 inhibits TGF-β1-induced epithelial-mesenchymal
transition and suppresses lung cancer migration, invasion and
anoikis resistance. Toxicology. 322:23–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu T, Zhao L, Zhang Y, Chen W, Liu D, Hou
H, Ding L and Li X: Ginsenoside 20(S)-Rg3 targets HIF-1α to block
hypoxia-induced epithelial-mesenchymal transition in ovarian cancer
cells. PLoS One. 9:e1038872014. View Article : Google Scholar
|
|
55
|
Pan XY, Guo H, Han J, Hao F, An Y, Xu Y,
Xiaokaiti Y, Pan Y and Li XJ: Ginsenoside Rg3 attenuates cell
migration via inhibition of aquaporin 1 expression in PC-3M
prostate cancer cells. Eur J Pharmacol. 683:27–34. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chen QJ, Zhang MZ and Wang LX: Gensenoside
Rg3 inhibits hypoxia-induced VEGF expression in human cancer cells.
Cell Physiol Biochem. 26:849–858. 2010. View Article : Google Scholar
|
|
57
|
Yue PY, Wong DY, Wu PK, Leung PY, Mak NK,
Yeung HW, Liu L, Cai Z, Jiang ZH, Fan TP and Wong RN: The
angiosuppressive effects of 20(R)- ginsenoside Rg3. Biochem
Pharmacol. 72:437–445. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kim JW, Jung SY, Kwon YH, Lee SH, Lee JH,
Lee BY and Kwon SM: Ginsenoside Rg3 inhibits endothelial progenitor
cell differentiation through attenuation of VEGF-dependent Akt/eNOS
signaling. Phytother Res. 26:1286–1293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kim JW, Jung SY, Kwon YH, Lee JH, Lee YM,
Lee BY and Kwon SM: Ginsenoside Rg3 attenuates tumor angiogenesis
via inhibiting bioactivities of endothelial progenitor cells.
Cancer Biol Ther. 13:504–515. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Guo JQ, Zheng QH, Chen H, Chen L, Xu JB,
Chen MY, Lu D, Wang ZH, Tong HF and Lin S: Ginsenoside Rg3
inhibition of vasculogenic mimicry in pancreatic cancer through
downregu-lation of VE cadherin/EphA2/MMP9/MMP2 expression. Int J
Oncol. 45:1065–1072. 2014.PubMed/NCBI
|
|
61
|
Lee YJ, Lee S, Ho JN, Byun SS, Hong SK,
Lee SE and Lee E: Synergistic antitumor effect of ginsenoside Rg3
and cisplatin in cisplatin resistant bladder tumor cell line. Oncol
Rep. 32:1803–1808. 2014.PubMed/NCBI
|
|
62
|
Che JB, Liu ZH, Ma HB, Li Y, Zhao H, Li
XH, Liu WC and Shi GN: Influence of As O inhibition of lung cancer
NCI-H1299 cells and on subsistence 2 3 combined with ginsenosides
Rg3 on of nude mice bearing hepatoma. Asian Pac J Trop Med.
7:772–775. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wang X, Chen L, Wang T, Jiang X, Zhang H,
Li P, Lv B and Gao X: Ginsenoside Rg3 antagonizes
adriamycin-induced cardiotoxicity by improving endothelial
dysfunction from oxidative stress via upregulating the Nrf2-ARE
pathway through the activation of akt. Phytomedicine. 22:875–884.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Xu TM, Xin Y, Cui MH, Jiang X and Gu LP:
Inhibitory effect of ginsenoside Rg3 combined with cyclophosphamide
on growth and angiogenesis of ovarian cancer. Chin Med J (Engl).
120:584–588. 2007.
|
|
65
|
Zhang Q, Kang X and Zhao W: Antiangiogenic
effect of low-dose cyclophosphamide combined with ginsenoside Rg3
on Lewis lung carcinoma. Biochem Biophys Res Commun. 342:824–828.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Chang L, Huo B, Lv Y, Wang Y and Liu W:
Ginsenoside Rg3 enhances the inhibitory effects of chemotherapy on
esophageal squamous cell carcinoma in mice. Mol Clin Oncol.
2:1043–1046. 2014.PubMed/NCBI
|
|
67
|
Yang LQ, Wang B, Gan H, Fu ST, Zhu XX, Wu
ZN, Zhan DW, Gu RL, Dou GF and Meng ZY: Enhanced oral
bioavailability and anti-tumour effect of paclitaxel by
20(s)-ginsenoside Rg3 in vivo. Biopharm Drug Dispos. 33:425–436.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kwon HY, Kim EH, Kim SW, Kim SN, Park JD
and Rhee DK: Selective toxicity of ginsenoside Rg3 on multidrug
resistant cells by membrane fluidity modulation. Arch Pharm Res.
31:171–177. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Park JD, Rhee DK and Lee YH: Biological
activities and chemistry of saponins from Panax ginseng C.A. Meyer.
Phytochem Rev. 4:159–175. 2005. View Article : Google Scholar
|
|
70
|
Kim SM, Lee SY, Cho JS, Son SM, Choi SS,
Yun YP, Yoo HS, Yoon DY, Oh KW, Han SB and Hong JT: Combination of
ginsenoside Rg3 with docetaxel enhances the susceptibility of
prostate cancer cells via inhibition of NF-kappaB. Eur J Pharmacol.
631:1–9. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kim SM, Lee SY, Yuk DY, Moon DC, Choi SS,
Kim Y, Han SB, Oh KW and Hong JT: Inhibition of NF-kappaB by
ginsenoside Rg3 enhances the susceptibility of colon cancer cells
to docetaxel. Arch Pharm Res. 32:755–765. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lee CK, Park KK, Chung AS and Chung WY:
Ginsenoside Rg3 enhances the chemosensitivity of tumors to
cisplatin by reducing the basal level of nuclear factor erythroid
2-related factor 2-mediated heme oxygenase-1/NAD(P)H quinone
oxidore-ductase-1 and prevents normal tissue damage by scavenging
cisplatin-induced intracellular reactive oxygen species. Food Chem
Toxicol. 50:2565–2574. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Lee JY, Jung KH, Morgan MJ, Kang YR, Lee
HS, Koo GB, Hong SS, Kwon SW and Kim YS: Sensitization of
TRAIL-induced cell death by 20(S)-ginsenoside Rg3 via CHOP-mediated
DR5 upregulation in human hepatocellular carcinoma cells. Mol
Cancer Ther. 12:274–285. 2013. View Article : Google Scholar
|
|
74
|
Kim DG, Jung KH, Lee DG, Yoon JH, Choi KS,
Kwon SW, Shen HM, Morgan MJ, Hong SS and Kim YS: 20(S)-Ginsenoside
Rg3 is a novel inhibitor of autophagy and sensitizes hepatocellular
carcinoma to doxorubicin. Oncotarget. 5:4438–4451. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Park D, Bae DK, Jeon JH, Lee J, Oh N, Yang
G, Yang YH, Kim TK, Song J, Lee SH, et al: Immunopotentiation and
antitumor effects of a ginsenoside Rg3-fortified red ginseng
preparation in mice bearing H460 lung cancer cells. Environ Toxicol
Pharmacol. 31:397–405. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Wu R, Ru Q, Chen L, Ma B and Li C:
Stereospecificity of ginsenoside Rg3 in the promotion of cellular
immunity in hepatoma H22-bearing mice. J Food Sci. 79:H1430–H1435.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Choi YJ, Kang LJ and Lee SG: Stimulation
of DDX3 expression by ginsenoside Rg3 through the Akt/p53 pathway
activates the innate immune response via TBK1/IKKε/IRF3 signalling.
Curr Med Chem. 21:1050–1060. 2014. View Article : Google Scholar
|
|
78
|
Cheng Y and Hua HQ: Clinical research
progress in anti-tumor effects of ginesenoside Rg3. Medical
recapitulate. 21:2938–2940. 2015.
|
|
79
|
Lu P, Su W, Miao ZH, Niu HR, Liu J and Hua
QL: Effect and mechanism of ginsenoside Rg3 on postoperative life
span of patients with non-small cell lung cancer. Chin J Integr
Med. 14:33–36. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Huang JY, Sun Y, Fan QX and Zhang YQ:
Efficacy of Shenyi capsule combined with gemcitabine plus cisplatin
in treatment of advanced esophageal cancer: a randomized controlled
trial. Zhong Xi Yi Jie He Xue Bao. 7:1047–1051. 2009.In Chinese.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wang XS, Wu Q and Liang L: Combined Shen
yi-Jiao nang and chemotherapy in treatment of breast cancer: a
systematic review. Pract J Clin Med. 9:192–195. 2012.
|
|
82
|
Coon JT and Ernst E: Panax ginseng: a
systematic review of adverse effects and drug interactions. Drug
Saf. 25:323–344. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Liu JP, Lu D, Nicholson RC, Li PY and Wang
F: Toxicity of a novel anti-tumor agent 20(S)-ginsenoside Rg3: a
26-week intramuscular repeated administration study in Beagle dogs.
Food Chem Toxicol. 49:1718–1727. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Liu JP, Lu D, Nicholson RC, Zhao WJ, Li PY
and Wang F: Toxicity of a novel anti-tumor agent 20(S)-ginsenoside
Rg3: a 26-week intramuscular repeated administration study in rats.
Food Chem Toxicol. 50:3388–3396. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zhang YH, Li HD, Li B, Jiang SD and Jiang
LS: Ginsenoside Rg3 induces DNA damage in human osteosarcoma cells
and reduces MNNG-induced DNA damage and apoptosis in normal human
cells. Oncol Rep. 31:919–925. 2014.
|
|
86
|
Poon PY, Kwok HH, Yue PY, Yang MS, Mak NK,
Wong CK and Wong RN: Cytoprotective effect of 20S-Rg3 on benzo[a]
pyrene-induced DNA damage. Drug Metab Dispos. 40:120–129. 2012.
View Article : Google Scholar
|
|
87
|
Cheong JH, Kim H, Hong MJ, Yang MH, Kim
JW, Yoo H, Yang H, Park JH, Sung SH, Kim HP and Kim J:
Stereoisomer-specific anticancer activities of ginsenoside Rg3 and
Rh2 in HepG2 cells: disparity in cytotoxicity and
autophagy-inducing effects due to 20(S)-epimers. Biol Pharm Bull.
38:102–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wang Y, Jin Y, Zhou C, Qu H and Cheng Y:
Discovering active compounds from mixture of natural products by
data mining approach. Med Biol Eng Comput. 46:605–611. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Yang R, Chen D, Li M, Miao F, Liu P and
Tang Q: 20(s)-ginsenoside Rg3-loaded magnetic human serum albumin
nanospheres applied to HeLa cervical cancer cells in vitro. Biomed
Mater Eng. 24:1991–1998. 2014.PubMed/NCBI
|
|
90
|
Yu H and Teng L, Meng Q, Li Y, Sun X, Lu
J, J Lee R and Teng L: Development of liposomal ginsenoside Rg3:
formulation optimization and evaluation of its anticancer effects.
Int J Pharm. 450:250–258. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Li J, Liu T, Zhao L, Chen W, Hou H, Ye Z
and Li X: Ginsenoside 20(S) Rg3 inhibits the Warburg effect through
STAT3 pathways in ovarian cancer cells. Int J Oncol. 46:775–781.
2015.
|