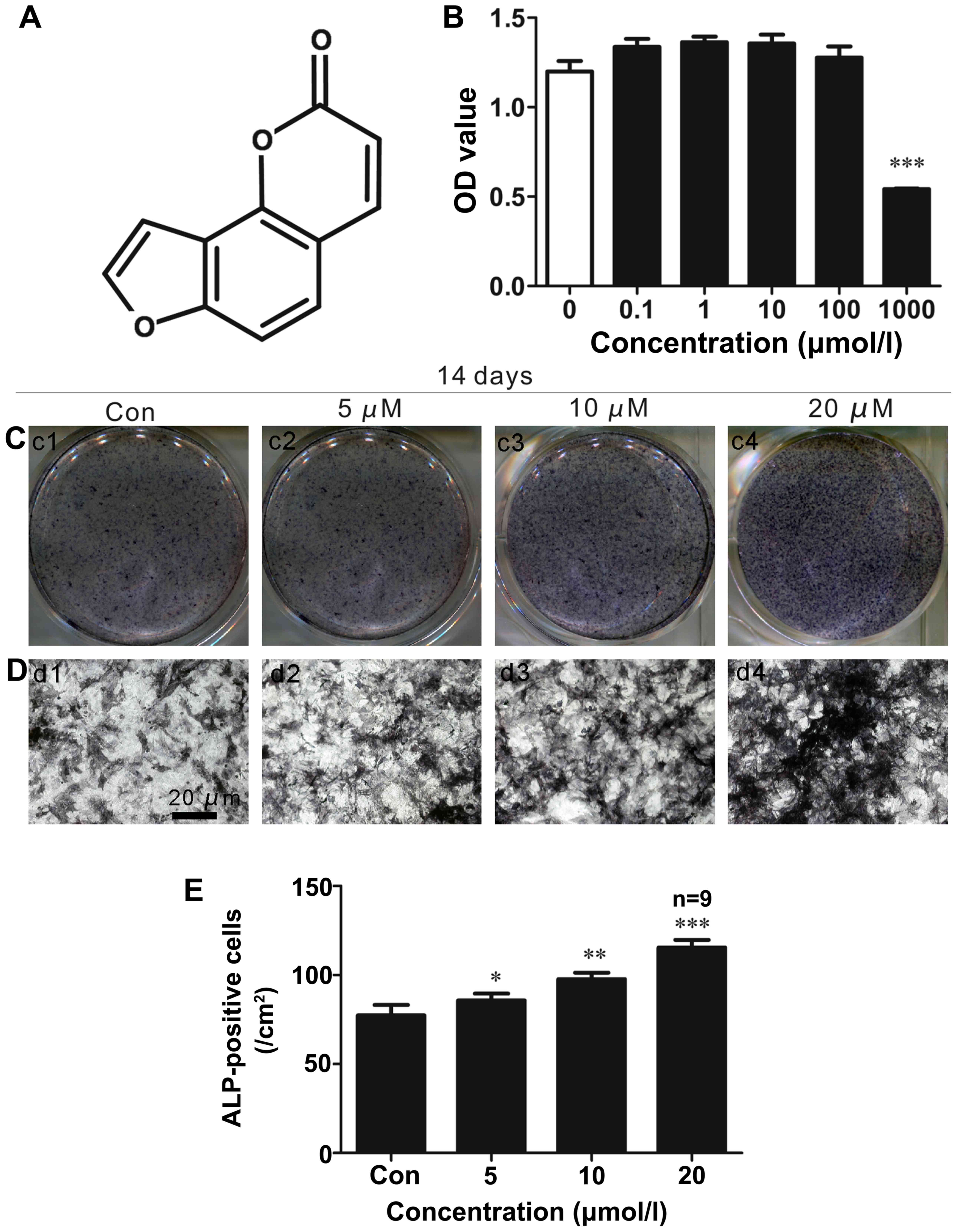
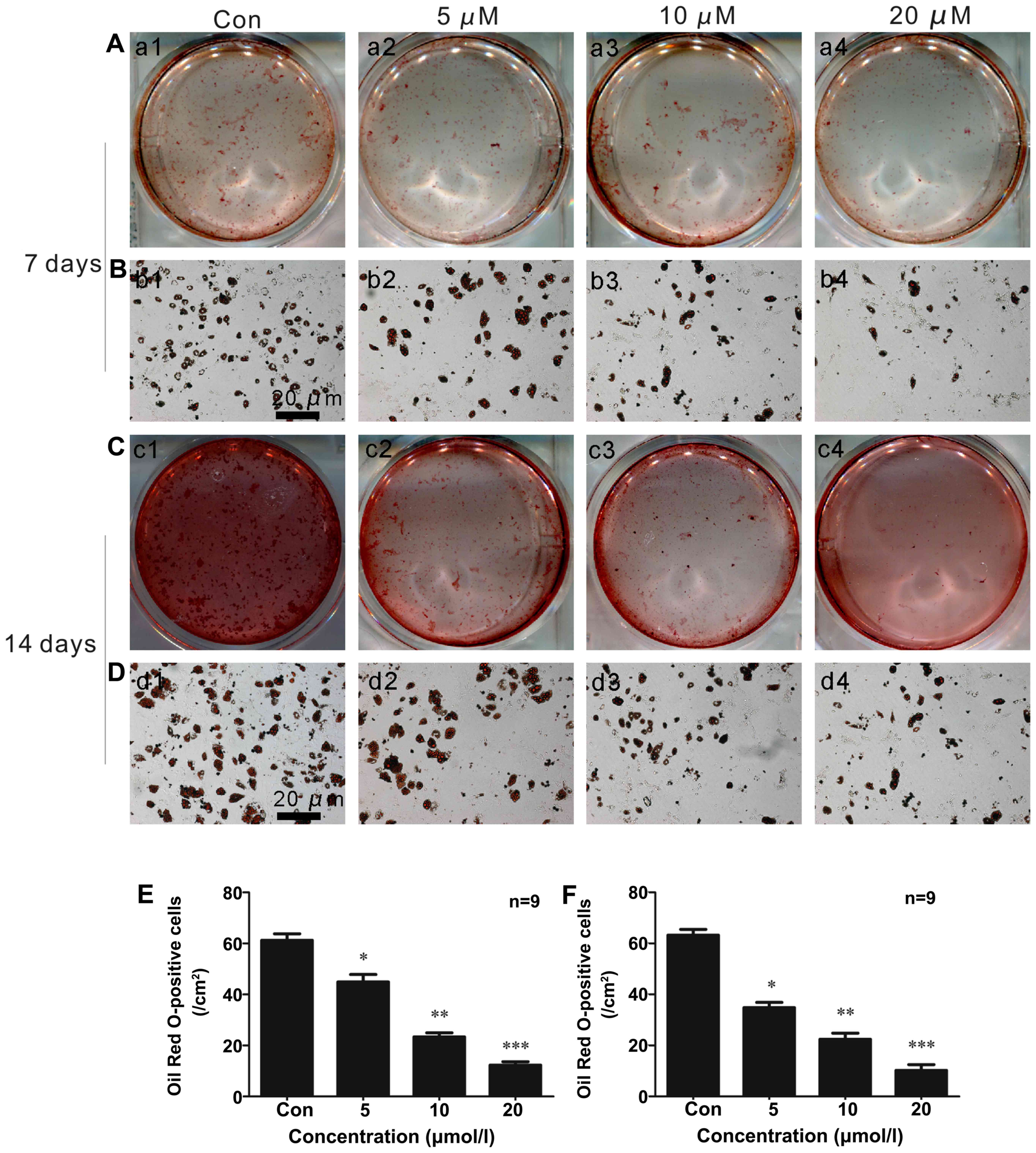
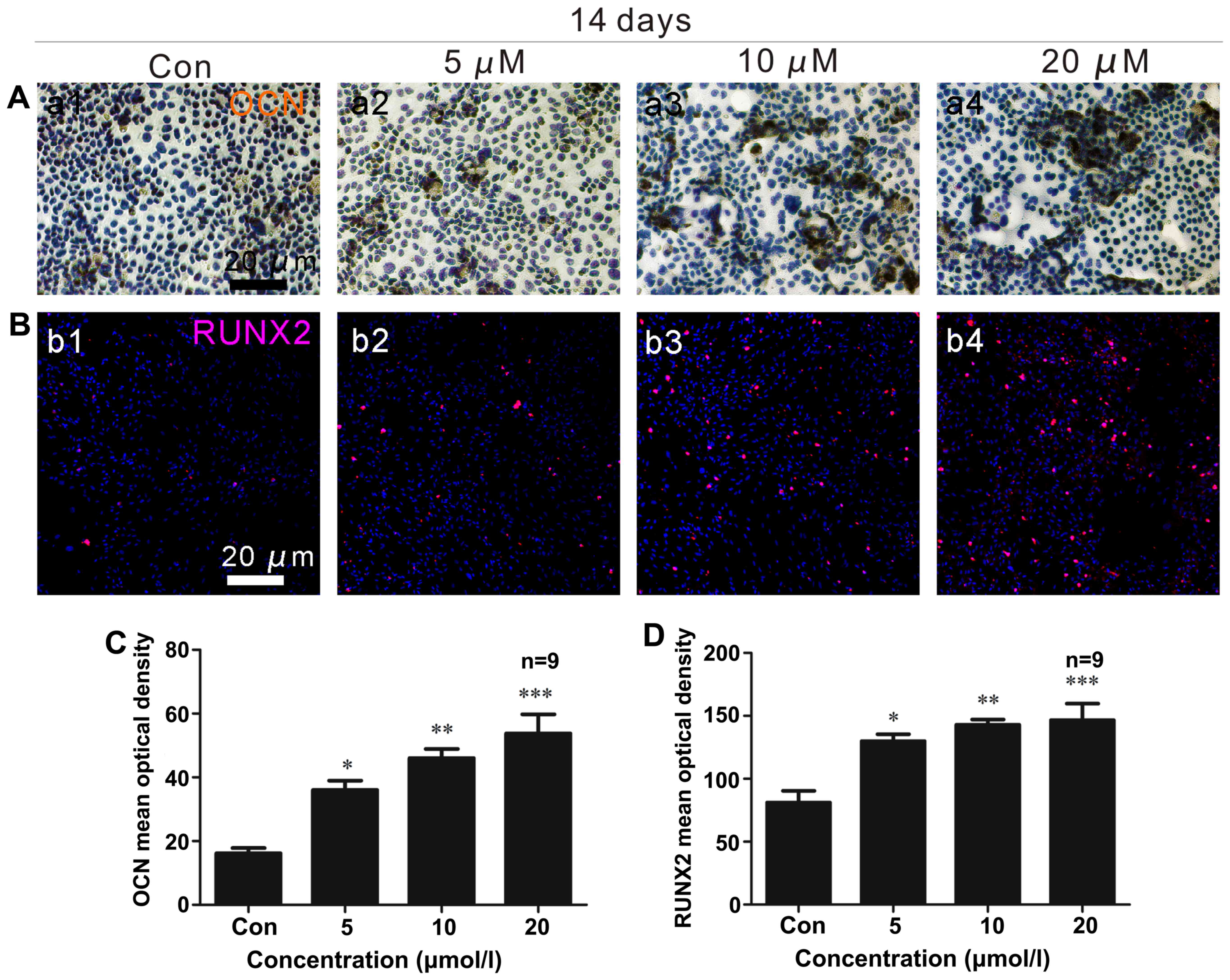
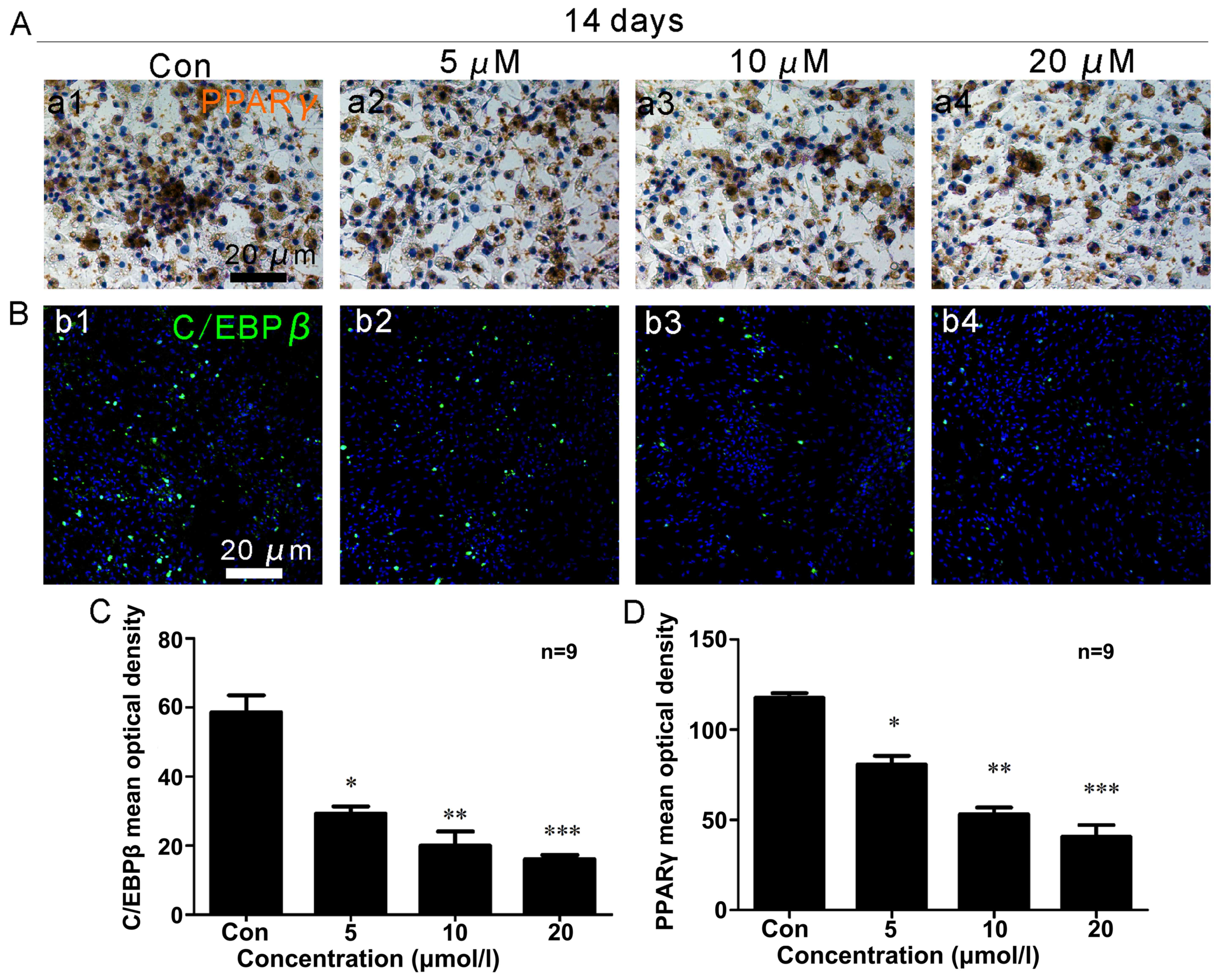
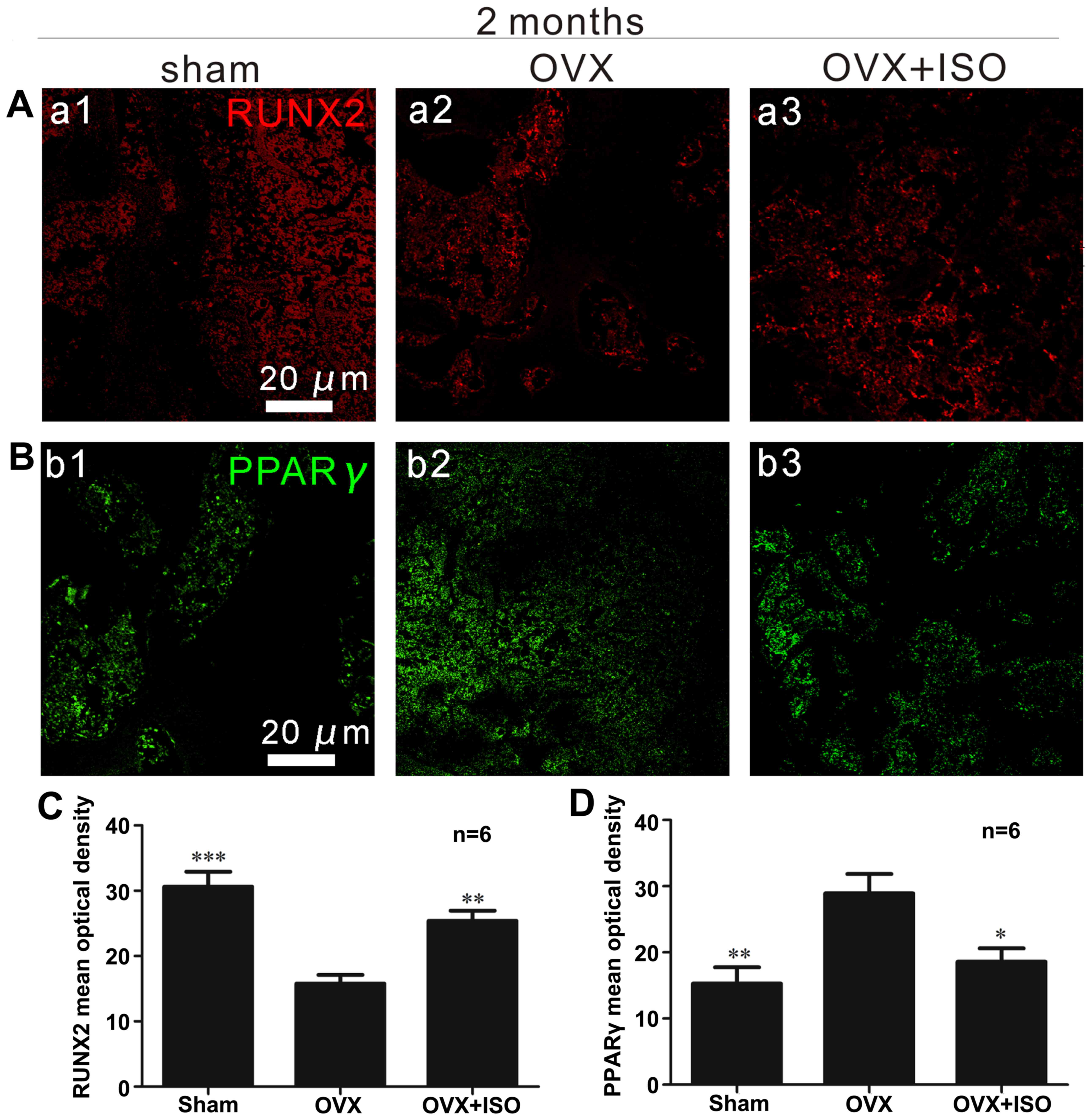
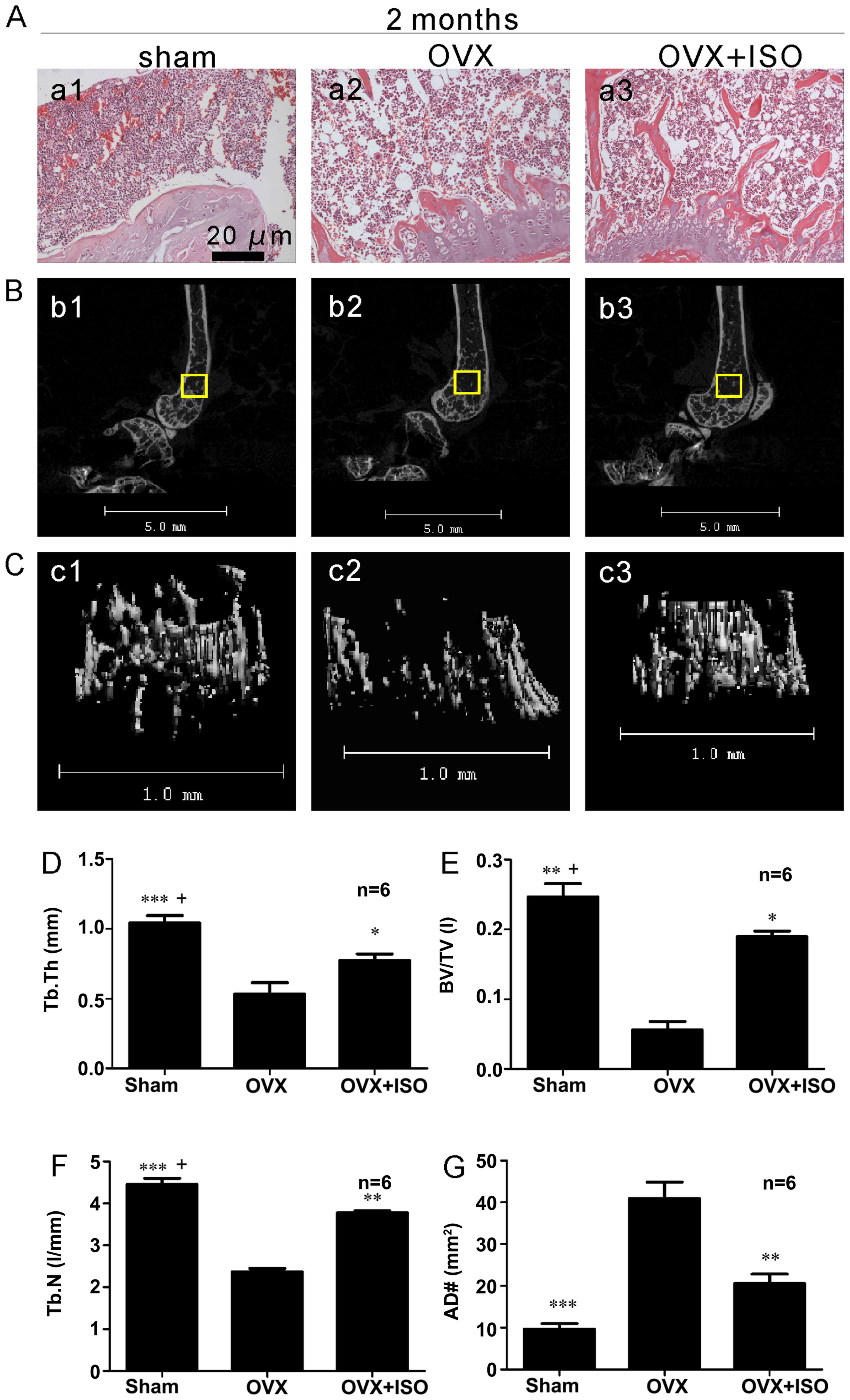
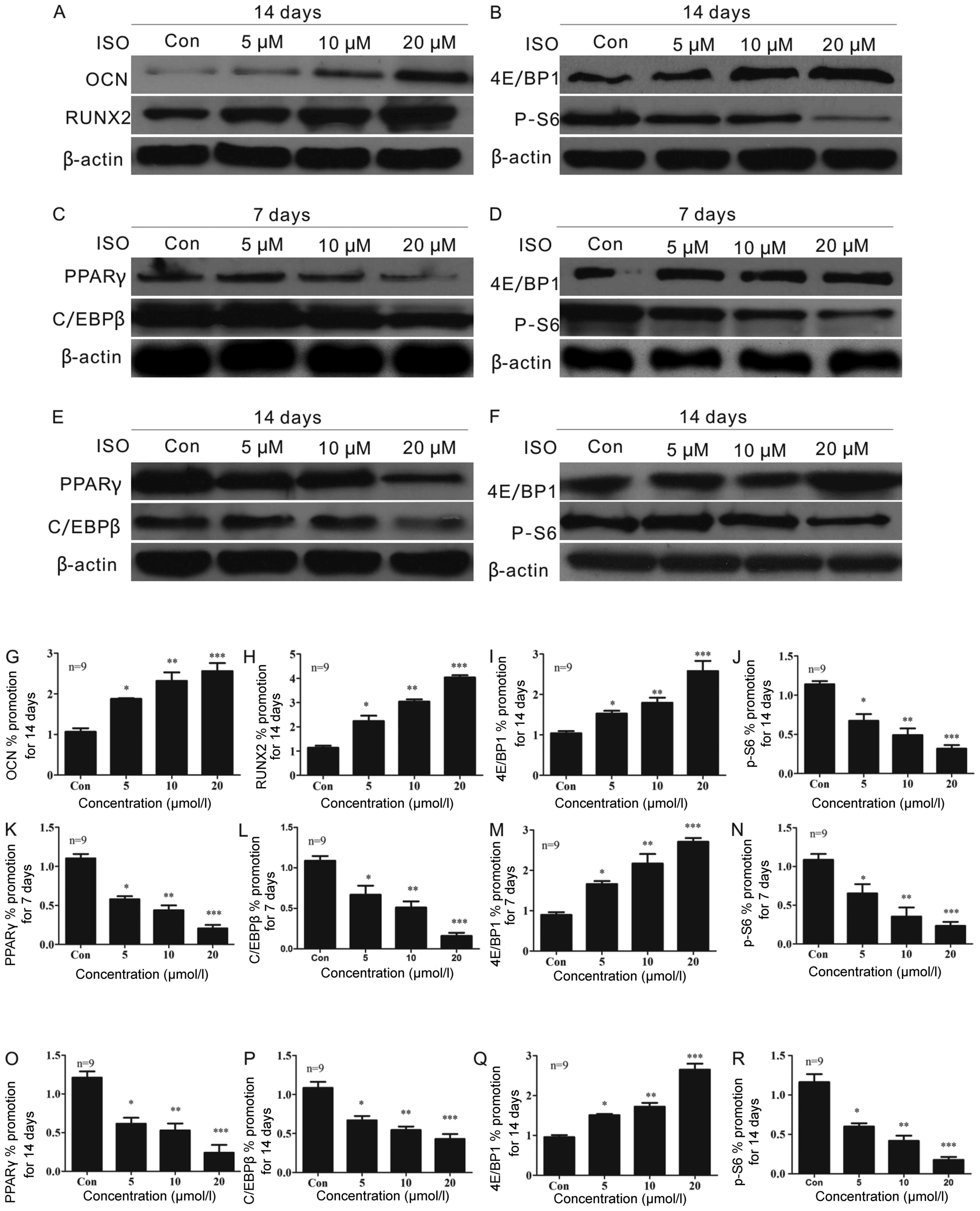
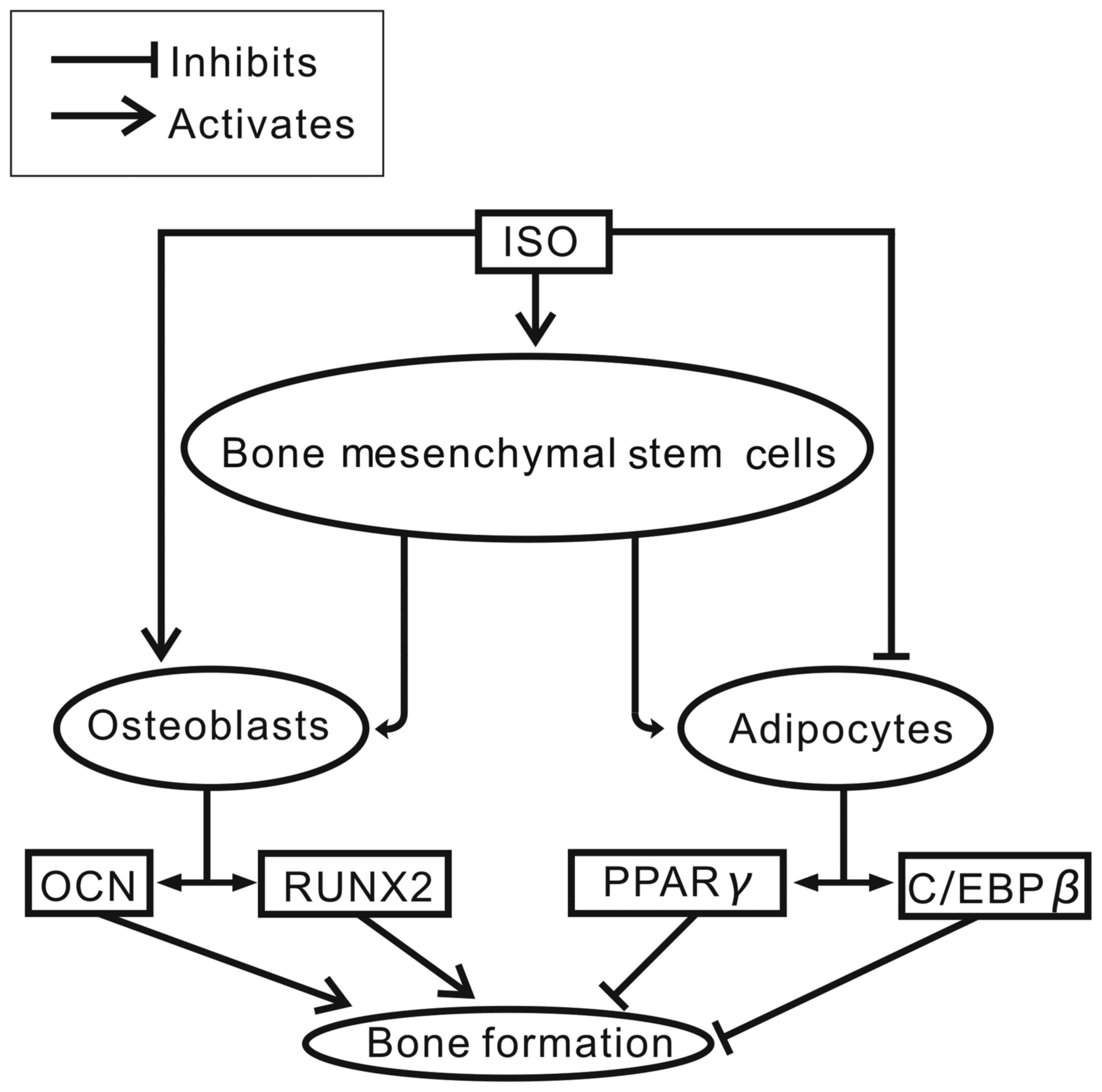
|
1
|
Schurman L, Bagur A, Claus-Hermberg H,
Messina OD, Negri AL, Sánchez A, González C, Diehl M, Rey P, Gamba
J, et al: Guías 2012 para el diagnóstico, la prevención y el
tratamiento de la osteoporosis. Medicina (B Aires). 73:55–74.
2013.In Spanish.
|
|
2
|
Wang ZQ, Li JL, Sun YL, Yao M, Gao J, Yang
Z, Shi Q, Cui XJ and Wang YJ: Chinese herbal medicine for
osteoporosis: A systematic review of randomized controlled trails.
Evid Based Complement Alternat Med. 2013:3562602013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tastekin N and Zateri C: Probable
osteosarcoma risk after prolonged teriparatide treatment: comment
on the article by Saag et al. Arthritis Rheum. 62:1837–1838. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nuttall ME and Gimble JM: Is there a
therapeutic opportunity to either prevent or treat osteopenic
disorders by inhibiting marrow adipogenesis. Bone. 27:177–184.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Burkhardt R, Kettner G, Böhm W,
Schmidmeier M, Schlag R, Frisch B, Mallmann B, Eisenmenger W and
Gilg T: Changes in trabecular bone, hematopoiesis and bone marrow
vessels in aplastic anemia, primary osteoporosis, and old age: A
comparative histomorphometric study. Bone. 8:157–164. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Griffith JF, Yeung DK, Antonio GE, Wong
SY, Kwok TC, Woo J and Leung PC: Vertebral marrow fat content and
diffusion and perfusion indexes in women with varying bone density:
MR evaluation. Radiology. 241:831–838. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Majors AK, Boehm CA, Nitto H, Midura RJ
and Muschler GF: Characterization of human bone marrow stromal
cells with respect to osteoblastic differentiation. J Orthop Res.
15:546–557. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rodríguez JP, Montecinos L, Ríos S, Reyes
P and Martínez J: Mesenchymal stem cells from osteoporotic patients
produce a type I collagen-deficient extracellular matrix favoring
adipogenic differentiation. J Cell Biochem. 79:557–565. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sekiya I, Larson BL, Vuoristo JT, Cui JG
and Prockop DJ: Adipogenic differentiation of human adult stem
cells from bone marrow stroma (MSCs). J Bone Miner Res. 19:256–264.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song L and Tuan RS: Transdifferentiation
potential of human mesenchymal stem cells derived from bone marrow.
FASEB J. 18:980–982. 2004.PubMed/NCBI
|
|
11
|
Park SR, Oreffo RO and Triffitt JT:
Interconversion potential of cloned human marrow adipocytes in
vitro. Bone. 24:549–554. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Beresford JN, Bennett JH, Devlin C, Leboy
PS and Owen ME: Evidence for an inverse relationship between the
differentiation of adipocytic and osteogenic cells in rat marrow
stromal cell cultures. J Cell Sci. 102:341–351. 1992.PubMed/NCBI
|
|
13
|
Gimble JM: The function of adipocytes in
the bone marrow stroma. New Biol. 2:304–312. 1990.PubMed/NCBI
|
|
14
|
Tavassoli M: Marrow adipose cells and
hemopoiesis: An interpretative review. Exp Hematol. 12:139–146.
1984.PubMed/NCBI
|
|
15
|
Elbaz A, Wu X, Rivas D, Gimble JM and
Duque G: Inhibition of fatty acid biosynthesis prevents adipocyte
lipotoxicity on human osteoblasts in vitro. J Cell Mol Med.
14:982–991. 2010. View Article : Google Scholar :
|
|
16
|
Dong X, Bi L, He S, Meng G, Wei B, Jia S
and Liu J: FFAs-ROS-ERK/P38 pathway plays a key role in adipocyte
lipotoxicity on osteoblasts in co-culture. Biochimie. 101:123–131.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Greco EA, Lenzi A and Migliaccio S: The
obesity of bone. Ther Adv Endocrinol Metab. 6:273–286. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pae HO, Cho H, Oh GS, Kim NY, Song EK, Kim
YC, Yun YG, Kang CL, Kim JD, Kim JM and Chung HT: Bakuchiol from
Psoralea corylifolia inhibits the expression of inducible nitric
oxide synthase gene via the inactivation of nuclear transcription
factor-kappaB in RAW 264.7 macrophages. Int Immunopharmacol.
1:1849–1855. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu H, Zhang L, Liu D, Tang P and Song F:
Isolation and purification of psoralen and isopsoralen and their
efficacy and safety in the treatment of osteosarcoma in nude rats.
Afr Health Sci. 14:641–647. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tang DZ, Yang F, Yang Z, Huang J, Shi Q,
Chen D and Wang YJ: Psoralen stimulates osteoblast differentiation
through activation of BMP signaling. Biochem Biophys Res Commun.
405:256–261. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wong RW and Rabie AB: Effect of psoralen
on bone formation. J Orthop Res. 29:158–164. 2011. View Article : Google Scholar
|
|
22
|
Tsai MH, Huang GS, Hung YC, Bin L, Liao LT
and Lin LW: Psoralea corylifolia extract ameliorates experimental
osteoporosis in ovariectomized rats. Am J Chin Med. 35:669–680.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ming L, Ge B, Chen K, Ma H and Zhai Y:
Effects of isopsoralen on bone marrow stromal stem cells
differentiate and proliferate in vitro. Zhongguo Zhong Yao Za Zhi.
36:2124–2128. 2011.In Chinese. PubMed/NCBI
|
|
24
|
Ming LG, Cheng KM, Ge BF, Ma HP and Zai
YK: Effect of isopsoralen on the proliferation and differentiate of
osteoblasts in vitro. Zhong Yao Cai. 34:404–408. 2011.In Chinese.
PubMed/NCBI
|
|
25
|
Lee SK, Kalinowski JF, Jacquin C, Adams
DJ, Gronowicz G and Lorenzo JA: Interleukin-7 influences osteoclast
function in vivo but is not a critical factor in
ovariectomy-induced bone loss. J Bone Miner Res. 21:695–702. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bouxsein ML, Boyd SK, Christiansen BA,
Guldberg RE, Jepsen KJ and Muller R: Guidelines for assessment of
bone microstructure in rodents using micro-computed tomography. J
Bone Miner Res. 25:1468–1486. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rossini M, Gatti D and Adami S:
Involvement of WNT/β-catenin signaling in the treatment of
osteoporosis. Calcif Tissue Int. 93:121–132. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cybulski N, Polak P, Auwerx J, Rüegg MA
and Hall MN: mTOR complex 2 in adipose tissue negatively controls
whole-body growth. Proc Natl Acad Sci USA. 106:9902–9907. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xiang X, Yuan F, Zhao J, Li Z, Wang X,
Guan Y, Tang C, Sun G, Li Y and Zhang W: Deficiency in pulmonary
surfactant proteins in mice with fatty acid binding protein
4-Cre-mediated knockout of the tuberous sclerosis complex 1 gene.
Exp Physiol. 98:830–841. 2013. View Article : Google Scholar :
|
|
30
|
Polak P, Cybulski N, Feige JN, Auwerx J,
Rüegg MA and Hall MN: Adipose-specific knockout of raptor results
in lean mice with enhanced mitochondrial respiration. Cell Metab.
8:399–410. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lim SH, Ha TY, Kim SR, Ahn J, Park HJ and
Kim S: Ethanol extract of Psoralea corylifolia L. and its main
constituent, bakuchiol, reduce bone loss in ovariectomised
Sprague-Dawley rats. Br J Nutr. 101:1031–1039. 2009. View Article : Google Scholar
|
|
32
|
Wang D, Li F and Jiang Z: Osteoblastic
proliferation stimulating activity of Psoralea corylifolia extracts
and two of its flavonoids. Planta Med. 67:748–749. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yuan X, Bi Y, Yan Z, Pu W, Li Y and Zhou
K: Psoralen and isopsoralen ameliorate sex hormone
deficiency-induced osteoporosis in female and male mice. Biomed Res
Int. 2016:68694522016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wood RJ: Vitamin D and adipogenesis: New
molecular insights. Nutr Rev. 66:40–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Moerman EJ1, Teng K, Lipschitz DA and
Lecka-Czernik B: Aging activates adipogenic and suppresses
osteogenic programs in mesenchymal marrow stroma/stem cells: the
role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling
pathways. Aging Cell. 3:379–389. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yoshida CA, Furuichi T, Fujita T, Fukuyama
R, Kanatani N, Kobayashi S, Satake M, Takada K and Komori T:
Core-binding factor beta interacts with Runx2 and is required for
skeletal development. Nat Genet. 32:633–638. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Peng S, Zhang G, He Y, Wang X, Leung P,
Leung K and Qin L: Epimedium-derived flavonoids promote
osteoblastogenesis and suppress adipogenesis in bone marrow stromal
cells while exerting an anabolic effect on osteoporotic bone. Bone.
45:534–544. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang Z, Huang JH, Liu SF, Zhao YJ, Shen
ZY, Wang YJ and Bian Q: The osteoprotective effect of psoralen in
ovariectomy-induced osteoporotic rats via stimulating the
osteoblastic differentiation from bone mesenchymal stem cells.
Menopause. 19:1156–1164. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Duque G: Bone and fat connection in aging
bone. Curr Opin Rheumatol. 20:429–434. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chan GK and Duque G: Age-related bone
loss: Old bone, new facts. Gerontology. 48:62–71. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Verma S, Rajaratnam JH, Denton J, Hoyland
JA and Byers RJ: Adipocytic proportion of bone marrow is inversely
related to bone formation in osteoporosis. J Clin Pathol.
55:693–698. 2005. View Article : Google Scholar
|
|
42
|
Rodríguez JP, Garat S, Gajardo H, Pino AM
and Seitz G: Abnormal osteogenesis in osteoporotic patients is
reflected by altered mesenchymal stem cells dynamics. J Cell
Biochem. 75:414–423. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Owen M: Marrow stromal stem cells. J Cell
Sci Suppl. 10:63–76. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gasparrini M, Rivas D, Elbaz A and Duque
G: Differential expression of cytokines in subcutaneous and marrow
fat of aging C57BL/6J mice. Exp Gerontol. 44:613–618. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ecklund K, Vajapeyam S, Feldman HA, Buzney
CD, Mulkern RV, Kleinman PK, Rosen CJ and Gordon CM: Bone marrow
changes in adolescent girls with anorexia nervosa. J Bone Miner
Res. 25:298–304. 2010. View Article : Google Scholar
|
|
46
|
Fournier C, Perrier A, Thomas M, Laroche
N, Dumas V, Rattner A, Vico L and Guignandon A: Reduction by
strontium of the bone marrow adiposity in mice and repression of
the adipogenic commitment of multipotent C3H10T1/2 cells. Bone.
50:499–509. 2012. View Article : Google Scholar
|
|
47
|
Syed FA, Oursler MJ, Hefferanm TE,
Peterson JM, Riggs BL and Khosla S: Effects of estrogen therapy on
bone marrow adipocytes in postmenopausal osteoporotic women.
Osteoporos Int. 9:1323–1330. 2008. View Article : Google Scholar
|