Introduction
Osteoarthritis (OA) is a common chronic disease that
is characterized by articular cartilage degeneration and secondary
bone hyperplasia (1).
Correspondingly, OA is a major cause of joint pain and disability
in the aging population. At the onset of OA, articular cartilage is
affected. The integrity of joint structures, including subchondral
bone, synovium, menisci, ligaments, periarticular muscles and
nerves is then affected (2,3).
Eventually, the complete loss of articular cartilage can lead to
joint deformity and disability (2). Normal articular cartilage is a
closed tissue without vascularity, thereby preventing surveillance
by the body's immune system. However, in OA, the innate immune
system is activated and this plays a role in the induction of
inflammatory mediators and specific cellular infiltration (4). The Toll-like receptors (TLRs), a
type of pattern recognition receptor, and their signaling pathways
are particularly relevant in this process (5,6).
TLR4 is the main TLR expressed by chondrocytes (7), and it not only plays important roles
in activating immune responses, but is also involved in the
pathogenesis of inflammatory diseases. It has been reported that
TLR4 is more highly expressed in chondrocytes from patients with OA
than in chrondrocytes from OA-free subjects (8). Accordingly, the inhibition of TLR4
has been shown to reduce the upregulation of interleukin (IL)-1β
and to interfere with the progression of OA (9). Moreover, in a study on rheumatoid
arthritis, the inhibition of TLR4 signaling was shown to attenuate
articular damage by blocking the identification of endogenous TLR
ligands (10). By contrast, TLR4
activation can also potentially lead to a significant increase in
the release of proteoglycan and type II collagen degradation
products (11). As previously
demonstrated, in human OA-affected chondrocytes, a TLR-dependent
catabolic effect is elicited by alarmins (S100A8 and S100A9)
(8), thereby suggesting that an
anti-anabolic effect is mediated by TLR4 in articular chondrocytes
and this may suppress cartilage repair in OA (11). Taken together, these results
indicate that the modulation of TLR4-mediated signaling may provide
a potential therapeutic option for the treatment of OA.
Currently, the clinical management of OA is aimed at
reducing joint pain and inflammation, since there is no treatment
available to successfully restore cartilage (12). Therefore, recent research efforts
have focused on the development of novel biological therapies to
attenuate and/or reverse cartilage degradation at the molecular
level (13). Resveratrol
(trans-3,5,4′-trihydroxystilbene) is a natural polyphenolic
compound that is present in grapes, berries and peanuts (14) and has been shown to exert
protective effects against OA by mediating anti-apoptotic,
anti-inflammatory and anti-oxidative functions in chondrocytes and
animal models (13,15–19). It has also been reported that
resveratrol modulates several pathways, including the nuclear
factor-κB (NF-κB) (15,20), mitogen-activated protein kinase
(MAPK) (21), extracellular
signal-regulated kinase (ERK), p38 and AKT (22) signaling pathways. Among these, the
role of the NF-κB signaling pathway is of particular interest. The
activation of TLR4 has been shown to induce NF-κB-dependent
apoptosis and the expression of pro-inflammatory cytokines
(23,24). However, the mechanisms through
which TLR4/NF-κB signaling contributes to the pathogenesis of OA
are not yet fully understood. The classic TLR4 signaling pathway
involves both myeloid differentiation factor 88 (MyD88)-dependent
and -independent pathways that mediate signaling via TIR
domain-containing adaptor-inducing interferon-β (TRIF) (25). Previously, we demonstrated that
resveratrol prevents IL-1β-induced inflammation in human articular
chondrocytes, in part by inhibiting the TLR4/MyD88/NF-κB signaling
pathway (26). By contrast,
several other studies have reported that resveratrol specifically
targets the TRIF complex of the TLR4 signaling pathway in some cell
types (27,28).
Therefore, the aim of the present study was to
determine whether the biological effects of resveratrol on
IL-1β-induced human articular chondrocytes involves both
TLR4/MyD88-dependent and -independent signaling pathways.
Materials and methods
Reagents and antibodies
Resveratrol, collagenase type II, and a protease
inhibitor cocktail were purchased from Sigma-Aldrich (St. Louis,
MO, USA). CellTiter 96® Aqueous One Solution reagent was
purchased from Promega (Madison, WI, USA). Dulbecco's modified
Eagle's medium (DMEM)/Ham's F-12 medium (1:1), and fetal calf serum
(FCS) were obtained from HyClone/Thermo Fisher Scientific, Inc.
(Logan, UT, USA). IL-1β was purchased from PeproTech (Rocky Hill,
NJ, USA). Enzyme-linked immunosorbent assay (ELISA) kits for matrix
metalloproteinase (MMP)-13 and IL-6 were obtained from Wuhan Boster
Biotechnology, Ltd. (Wuhan, China). RNAiso Plus and a RT-PCR kit
were purchased from Takara Biotechnology Co., Ltd. (Dalian, China).
A BCA kit was obtained from Beyotime Institute of Biotechnology
(Shanghai, China). Polyclonal anti-β-actin (sc-477787), anti-TLR4
(sc-293072), anti-MyD88 (sc-74532), and anti-TNF
receptor-associated factor 6 (TRAF6; sc-8409) antibodies were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Antibodies specific for phospho-IL-1 receptor-associated kinase 4
(p-IRAK4) (Thr345/Ser346) (Cat. no. 11927) and anti-TRIF (Cat. no.
4596) were obtained from Cell Signaling Technology, Inc. (Beverly,
MA, USA). Anti-mouse and anti-rabbit secondary antibodies were
purchased from Pierce (Rockford, IL, USA). Enhanced
chemiluminescence reagent was obtained from Amersham Biosciences
(Buckinghamshire, UK).
Isolation and culture of
chondrocytes
This study was approved by the Ethics Committee at
the Shengjing Hospital China Medical University (Shenyang, China)
and informed consent was obtained from all participants.
Chondrocyte isolation and culture were performed as previously
described (26). Briefly, knee
articular cartilage was obtained from 16 patients with OA (aged
50–70 years) that were undergoing joint replacement surgery.
Cartilage slices were digested with 0.25% trypsin for 1 h and then
with 0.04% collagenase type II in a 37°C water bath overnight.
Cells were seeded in 25 cm2 flasks (1–2×105
cells/ml) containing DMEM/Ham's F-12 medium supplemented with 10%
FCS, penicillin (100 U/ml) and streptomycin (100 U/ml), and the
cells were grown at 37°C with 5% CO2. Third passage
chondrocytes were used in the experiments.
Identification of cell phenotype
The chondrocytes were seeded on glass coverslips and
fixed with 4% paraformaldehyde for 20 min. The cells were then
incubated with 3% H2O2 for 10 min to inhibit
endogenous peroxidase activity, and were subsequently blocked with
goat serum for 15 min at room temperature followed by incubation
with type II collagen antibody (cat. no. A00517; Wuhan Boster
Biotechnology, Ltd.) (1:100) overnight at 4°C. The following day,
the coverslips were washed and incubated with the appropriate
biotin-conjugated anti-rabbit secondary antibodies (cat. no.
BM2004; Wuhan Boster Biotechnology, Ltd.) for 15 min at room
temperature, then were incubated with streptavidin-biotin complex
(SABC, SA1021; Wuhan Boster Biotechnology, Ltd.) for an additional
15 min. The cells were stained with 3,3′-diaminobenzidine (DAB;
ZSGB-Bio, Beijing, China) for 10 min at room temperature followed
by hematoxylin (Tianjin Guangfu Fine Chemical Research Institute,
Tianjin, China) staining and gradient alcohol dehydration. For the
detection of proteoglycans, the cells on coverslips were stained
with 1% toluidine blue (Tianjin Guangfu Fine Chemical Research
Institute) for 30 min and were then fixed with 4%
paraformaldehyde.
Cell viability assay
Chondrocytes (5×103 cells/well) were
seeded in triplicate in 96-well plates and were cultured in the
presence or absence of 10 ng/ml IL-1β and various concentrations of
resveratrol (0, 6.25, 12.5, 25, 50, 100, and 200 µM) for 24,
48, or 72 h at 37°C, 5% CO2. Cell viability was
determined by MTS assay. Briefly, 20 µl of CellTiter
96® Aqueous One Solution reagent was pipetted into each
well of the 96-well assay plates containing the samples in 100
µl of culture medium. The plates were then incubated at 37°C
in a humidified, 5% CO2 atmosphere. After 4 h, the
absorbance values at 490 nm were recorded using a microplate reader
(Multiskan MK3; Thermo Fisher Scientific, Inc.) and the data were
expressed as the means ± standard deviation (SD) of 3 independent
experiments.
Treatment of chondrocytes
Chondrocytes grown in a monolayer were incubated
with serum-starved medium (0.5% FCS). After 1 h, the chondrocytes
were stimulated with 10 ng/ml IL-1β for 1 h before various
concentrations of resveratrol (0, 6.25, 12.5, 25, 50, 100, and 200
µM) were added to the cells followed by incubation for 24
h.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted using RNAiso Plus reagent,
according to the manufacturer's instructions. The expression levels
of TLR4, MyD88, IRAK4, TRIF and
TRAF6 were quantitatively measured using a 7500 real-time
PCR detection system (Applied Biosystems, Carlsbad, CA, USA). Each
PCR reaction mixture (total volume, 20 µl) included 10
µl of 2X SYBR-Green Master mix (Takara Biotechnology Co.,
Ltd.), 0.8 µl of forward and reverse primers (10
µmol/µl; Table I),
0.4 µl of Rox Reference Dye II (50X; Takara Biotechnology
Co., Ltd.) and 2 µl of cDNA. The PCR protocol included 40
cycles with denaturation at 95°C for 5 sec and an annealing and
extension temperature of 60°C for 34 sec. The detection of
endogenous β-actin was used as a control. Data were analyzed using
the 2−ΔΔCT method, as previously described (29).
 | Table IThe primer sequences. |
Table I
The primer sequences.
| Genes | Primer sequences
(5′-3′) | Products
(bp) |
|---|
| β-actin | F:
CACACTGTGCCCATCTACGA | 101 |
| R:
CTCAGTGAGGATCTTCATGAGGTAGT | |
| TLR4 | F:
AGGACTGGGTAAGGAATGAGC | 148 |
| R:
ATCACCTTTCGGCTTTTATGG | |
| MyD88 | F:
CACTCAGCCTCTCTCCAGGT | 178 |
| R:
AGTCTTCAGGGCAGGGACA | |
| IRAK4 | F:
GCCGCTTCTACAAAGTATGG | 83 |
| R:
CCATCACTTTGTAGAAGCGGC | |
| TRIF | F:
TCCAAATACCAAGCCGTG | 149 |
| R:
TCTGTTCCGATGATGATTCC | |
| TRAF6 | F:
CTGGAAGCCCTAAGACAAAGA | 191 |
| R:
GGCAAGGAAAGGCACTGTT | |
Western blot analysis
Following isolation of the proteins from the cells
using RIPA buffer containing PMSF (Dingguo Changsheng
Biotechnology, Beijing, China), cell lysates were centrifuged
(12,000 × g, 15 min, 4°C) and the supernatants were collected. The
concentrations of total proteins were determined using a BCA kit.
Protein samples were boiled for 5 min in 1X SDS sample buffer [50
mM Tris-HCl (pH 6.8), 20% glycerol, 2% SDS, 0.02% bromophenol blue]
containing 2% 2-mercaptoethanol. Extracted proteins were subjected
to SDS-PAGE and were then transferred to polyvinylidene difluoride
membranes for 3 h at 4°C. The membranes were incubated with
anti-TLR4, anti-MyD88, and anti-TRAF6 antibodies (1:300), or with
anti-p-IRAK4 and anti-TRIF antibodies (1:1,000), overnight at 4°C.
After the membranes were washed, they were incubated with the
appropriate HRP-conjugated secondary antibodies (1:5,000) for 45
min at room temperature. Enhanced chemiluminescence reagents were
used to visualize antibody binding which was subsequently
quantified using Scion Image 4.0 software (http://www.bbioo.com/download/58-196-1.html).
ELISA
The concentrations of MMP-13 and IL-6 were detected
in the culture supernatants using commercially available ELISA kits
(Wuhan Boster Biotechnology, Ltd.), according to the manufacturer's
instructions. Data are expressed as the means ± SD and are
representative of 3 independent experiments.
Statistical analysis
All data are expressed as the means ± SD and were
analyzed using one-way analysis of variance (ANOVA) with SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA). A p-value <0.05 was
considere to indicate a statiscially significant difference.
Results
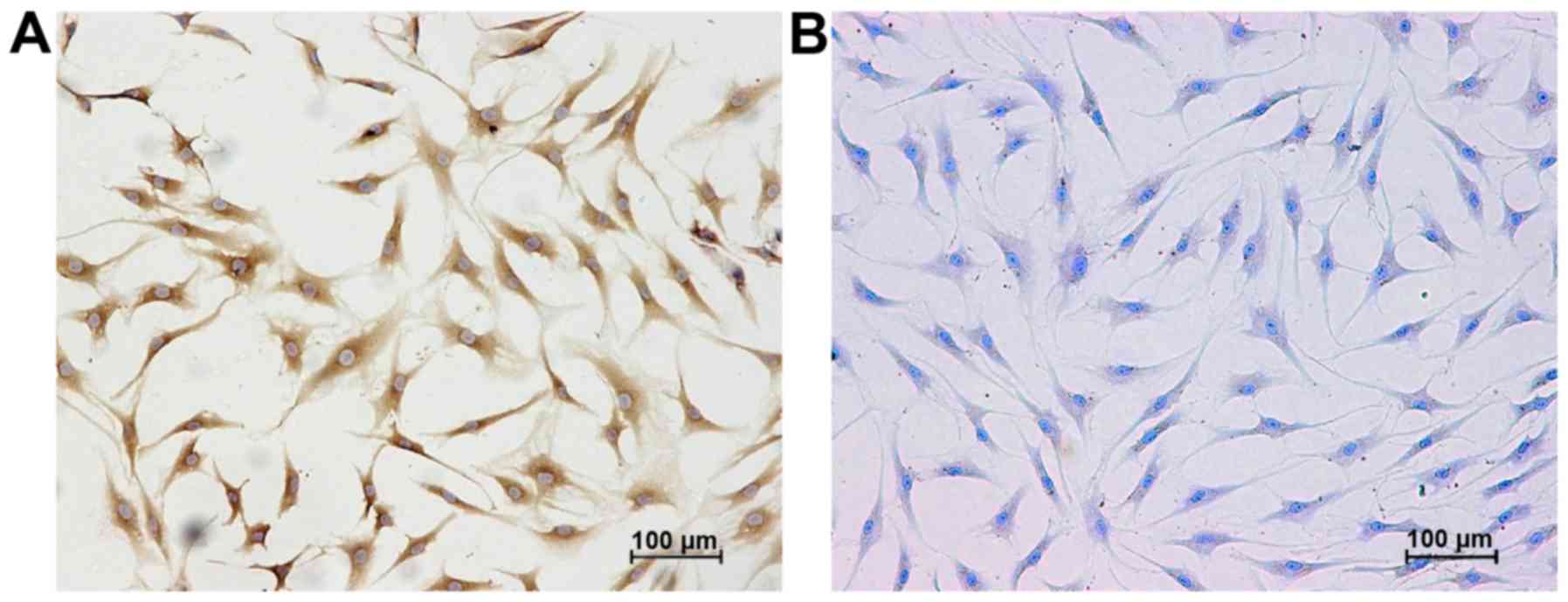
Identification of chondrocyte
phenotype
Typically, chondrocytes produce large amounts of
type II collagen, which compose the extracellular matrix (ECM)
along with proteoglycans. Therefore, immunocytochemical methods and
toluidine blue staining were used to detect the expression of type
II collagen and proteoglycans, respectively, in the third passage
chondrocytes that were obtained from the knee articular cartilage
of patietns with OA. All the cultured cells exhibited positive
staining for type II collagen and proteoglycans, thereby
demonstrating that chondrocytes were successfully isolated from the
knee articular cartilage samples (Fig. 1).
IL-1β decreases cell viability, while
resveratrol counteracts these effects of IL-1β in the isolated
human articular chondrocytes
As shown by MTS assays that were performed to
monitor cell viability, treatment with resveratrol was found to
increase chondrocyte cell viability at concentrations of 6.25 and
12.5 µM after 24, 48 and 72 h. By contrast, exposure to
IL-1β (10 ng/ml) inhibited chondrocyte cell viability. However,
resveratrol at concentrations ranging from 6.25 to 50 µM
resulted in a major increase in cell viability, which was
suppressed by exposure to IL-1β (Fig.
2).
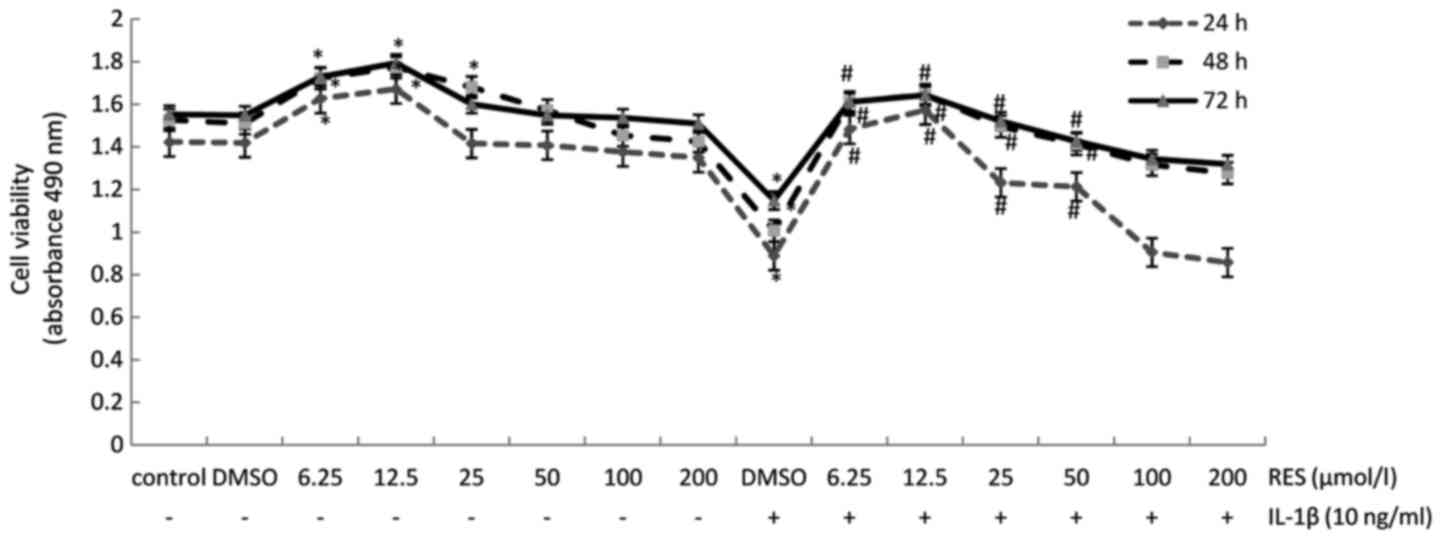 | Figure 2Resveratrol improves the viability of
chondrocytes that was inhibited by interleukin (IL)-1β. Cultured
human articular chondrocytes were treated with various
concentrations of resveratrol (0, 6.25, 12.5, 25, 50, 100, and 200
µM) with or without 10 ng/ml IL-1β for 24, 48, or 72 h. Cell
viability was subsequently examined by MTS assays. All samples were
analyzed in triplicate and the data are expressed as the means ± SD
from 3 independent experiments. *p<0.05 and
#p<0.05, significant difference vs. control and
IL-1β, respectively. |
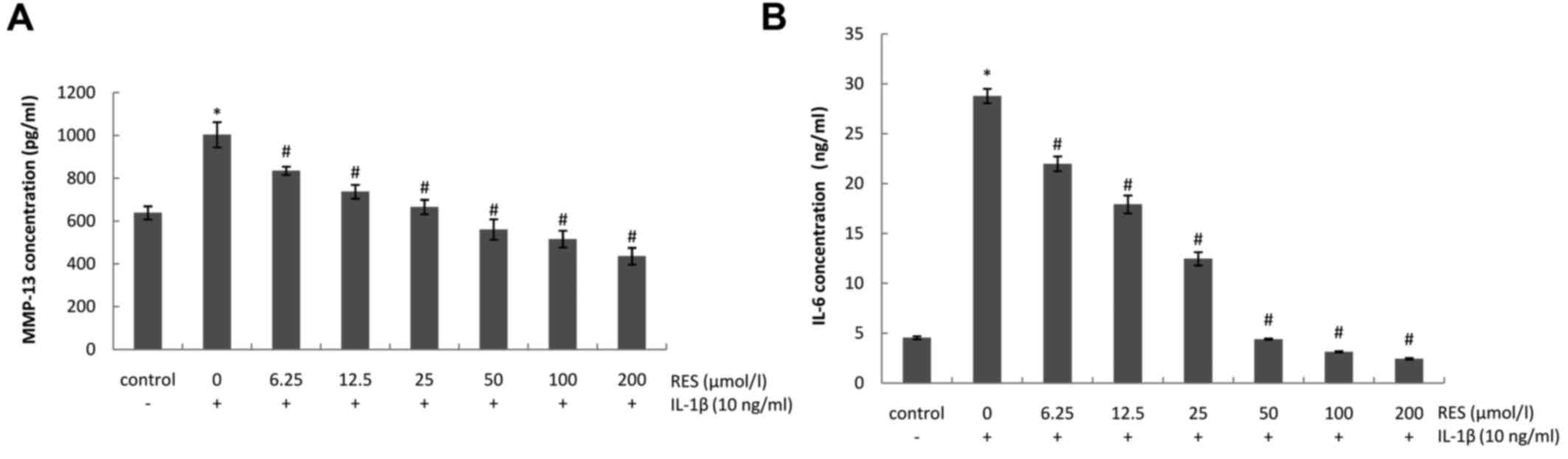
Resveratrol inhibits the IL-1β-induced
production of MMP-13 and IL-6
To determine whether resveratrol exerts
anti-catabolic and anti-inflammatory effects on IL-1β-stimulated
chondrocytes, the expression levels of MMP-13 and IL-6 were
detected in chondrocyte culture supernatants. As shown in Fig. 3, the levels of MMP-13 and IL-6
were significantly upregulated in the presence of IL-1β (10 ng/ml).
By contrast, treatment with resveratrol (6.25–200 µM)
effectively antagonized these catabolic and inflammatory effects in
a dose-dependent manner.
Resveratrol suppresses downstream targets
of both TLR4/MyD88-dependent and -independent signaling pathways
activated by IL-1β in human articular chondrocytes
To determine whether TLR4/MyD88-dependent and
-independent signaling pathways are activated in the presence of
IL-1β, and to determine whether resveratrol can inhibit their
activation, chondrocytes were incubated with IL-1β (10 ng/ml) for 1
h, were then incubated with or without various concentrations of
resveratrol (6.25–200 µM) for 24 h. The results of RT-qPCR
and western blot analysis were subsequently performed to detect the
mRNA and protein levels of various downstream targets of the TLR4
signaling pathway. Exposure to IL-1β induced a marked increase in
the mRNA and protein expression levels of the TLR4/MyD88-dependent
pathway targets, MyD88, p-IRAK4 and TRAF6, as well as in those of
the MyD88-independent pathway target, TRIF. By contrast,
resveratrol had an opposite effect, decreasing the levels of these
targets (Fig. 4), thereby
suggesting that resveratrol exerts an anti-osteoarthritic effect
that involves both TLR4/MyD88-dependent and -independent signaling
pathways.
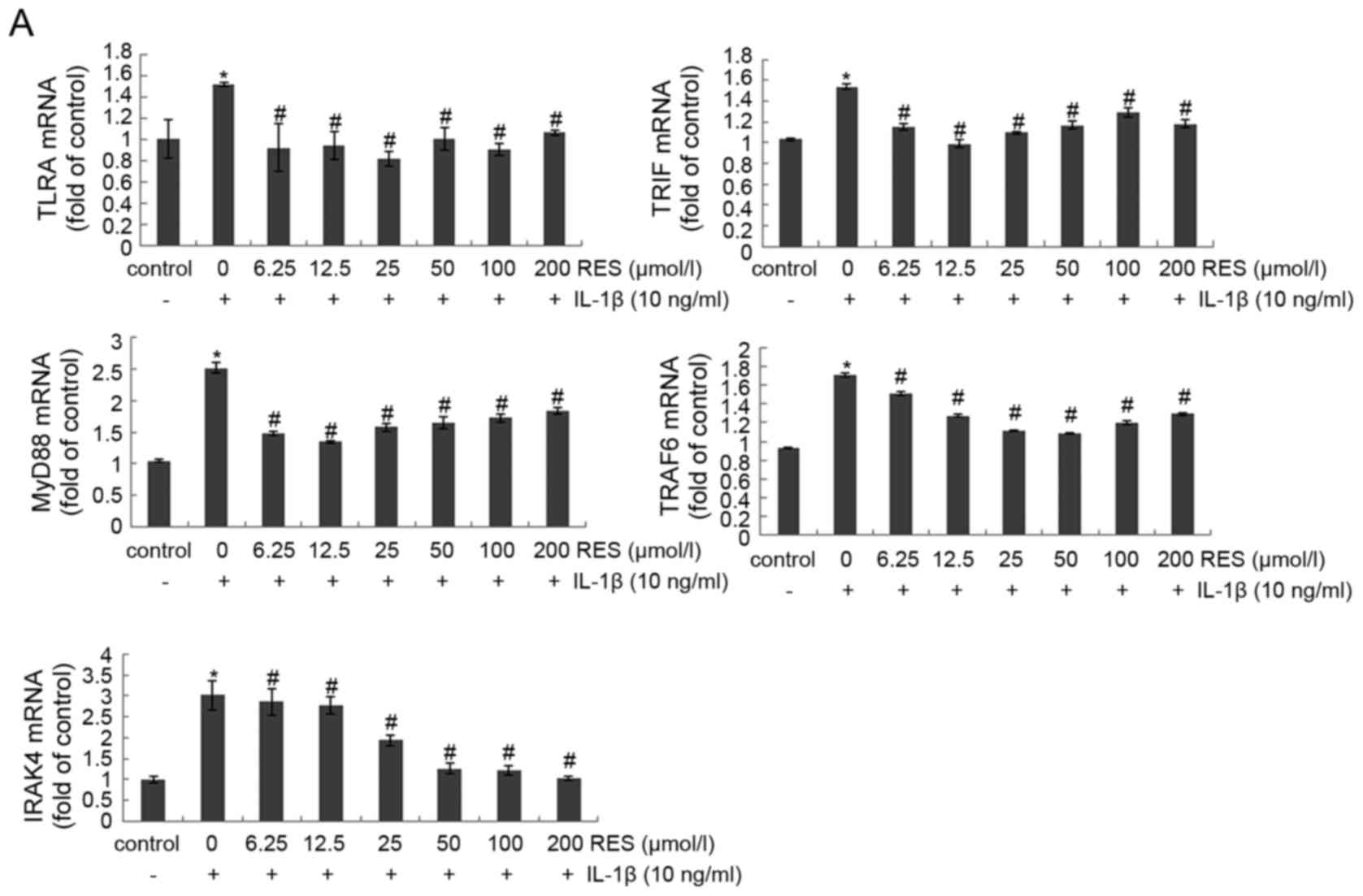 | Figure 4Resveratrol inhibits multiple
downstream targets of Toll-like receptor 4 (TLR4)/myeloid
differentiation factor 88 (MyD88)-dependent and -independent
signaling pathways that were activated by interleukin (IL)-1β.
Serum-starved (0.5% FCS) human articular chondrocytes were
incubated with 10 ng/ml IL-1β for 1 h and were subsequently
co-treated with various concentrations of resveratrol (0, 6.25,
12.5, 25, 50, 100, and 200 µM) and 10 ng/ml IL-1β. After 24
h, total RNA was collected to perform RT-qPCR and cell lysates were
collected for western blot analysis and densitometric analysis.
Detection of β-actin was used as a control. The results represent
duplicates of 3 independent experiments that were performed. The
levels of (A) mRNA and (B) protein are shown, respectively, for the
following targets: TLR4, MyD88, phospho-IL-1 receptor-associated
kinase 4 (p-IRAK4), TIR domain-containing adaptor-inducing
interferon-β (TRIF), and TRAF6. *p<0.05 and
#p<0.05, significant difference vs. control and
IL-1β, respectively. |
Discussion
In normal human joints, articular chondrocytes
maintain a dynamic equilibrium between the synthesis and
degradation of ECM components (e.g., type II collagen and
proteoglycans). However, in OA, perturbations in the normal
metabolic properties of chondrocytes result in the destruction of
the ECM due to the release of enzymes, such as MMPs (e.g., MMP-1,
-3 and -13) and aggrecanases (13). Correspondingly, the overproduction
of matrix-degrading enzymes by chondrocytes has been shown to play
a central role in matrix degeneration in arthritic cartilage
(30,31). Moreover, catabolic mediators, such
as IL-1β and IL-6 have been shown to upregulate these enzymes in
cartilage (32,33). In the present study, IL-1β
significantly increased the concentrations of MMP-13 and IL-6 that
were present in the supernatants assayed, indicating that the
equilibrium between the synthesis and degradation of the ECM had
been disturbed. Furthermore, the induction of excessive levels of
IL-6 by IL-1β can further degrade cartilage in part by inducing the
expression of MMP-13 (34). By
contrast, resveratrol was found to reduce the production of MMP-13
and IL-6 in a dose-dependent manner, thereby implying that
resveratrol can prevent IL-1β-induced chondrocyte damage by
reducing the production of MMP-13 and IL-6. Resveratrol has
previously been shown to antagonize the catabolic factor-mediated
upregulation of multiple matrix degrading enzymes, thereby
providing evidence that resveratrol has the capacity to slow the
catabolic processes that are involved in cartilage degeneration
(13). Thus, the findings of the
present study suggest that resveratrol counteracts potent
inflammatory catabolic factors in chondrocytes, and this confers
chondroprotection in OA.
Resveratrol is an anti-inflammatory dietary
phytochemical that antagonizes the catabolic effects of TNF-α and
IL-1β by inhibiting the NF-κB pathway in a variety of tissues
(35,36). In our previous study, resveratrol
was found to exert a protective effect against the IL-1β-induced
inflammatory response in human OA-affected chondrocytes, and this
was partly mediated by the TLR4/MyD88/NF-κB signaling pathway
(26). The results of a recent
study also suggested that MyD88-dependent TLR2/TLR4 signaling may
drive the catabolic response of chondrocytes (37). In the present study, both the mRNA
and protein levels of TLR4 were increased in chondrocytes
stimulated with IL-1β, while treatment with resveratrol (12.5–200
µM) decreased TLR4 expression. These results are consistent
with those of our previous study, and in combination, these results
suggest that the TLR4/MyD88 pathway is involved in the pathogenesis
of OA. Sebai et al demonstrated that resveratrol may mediate
antioxidant properties in either a MyD88-dependent manner that does
not involve IRAK1, yet possibly involves IRAK2 or IRAK4, or in a
TRIF pathway-dependent manner (27). In the present study, we examined
whether IRAK4 contributes to the upregulation of TLR4/MyD88
signaling by IL-1β and the downregulation of this signaling pathway
by resveratrol. Our results indicated that IL-1β upregulated
p-IRAK4 protein expression, while resveratrol reversed this effect.
The present data also confirm the involvement of the
MyD88-dependent signaling pathway in the anti-catabolic and
anti-inflammatory effects of resveratrol on IL-1β-induced OA.
Another interesting finding was that the
MyD88-independent pathway (also known as the TRIF signaling
pathway) is involved in mediating the protective effects of
resveratrol on IL-1β-induced human articular chondrocytes.
Initially it appeared that MyD88 was required for the signal
transduction events engaged by TLR activation. Certain TLR
agonists, such as lipopolysaccharide (LPS), have been reported to
generate signals dependent on TRIF (38) in the absence of MyD88 (39). The present results demonstrated
that TRIF expression was significantly upregulated following
exposure to IL-1β, whereas the addition of resveratrol reduced TRIF
expression. Taken together, these results indicate that
MyD88-independent signaling is initiated during OA, and resveratrol
exerts protective effects on OA by inhibiting this pathway.
TRAF6 is a component of the MyD88-dependent path way
that activates NF-κB and controls the expression of genes, such as
TNFα and IL-6 (40,41). Crosstalk between TRIF and TRAF6
has also been reported in LPS-induced signaling (39). It has been demonstrated that
resveratrol suppresses LPS-induced TRAF6 expression and
ubiquitination and attenuates LPS-induced TLR4-TRAF6, MAPK and Akt
pathways as part of its anti-inflammatory effects (25). In the present study, TRAF6
expression was significantly upregulated following exposure to
IL-1β, while the addition of resveratrol reduced the expression
levels of TRAF6, thereby indicating that both TRAF6-sensitive (and
potentially MyD88-dependent) and TRAF6-insensitive (and potentially
MyD88-independent and TRIF-dependent) mechanisms are involved in
the pathogenesis of OA. Thus, resveratrol appears to exert a
protective effect against OA by inhibiting both MyD88-dependent and
-independent pathways.
In conclusion, the results of the present study
demonstrate that resveratrol exerts protective effects against
matrix degradation and inflammation in OA-affected chondrocytes by
inhibiting both TLR4/MyD88-dependent and -independent signaling
pathways. Thus, resveratrol represents a potential treatment
strategy for OA. However, there are several questions that remain
to be answered. First, it has to be determined whether the major
TLR4 signaling pathway is MyD88-dependent or -independent in
resveratrol-treated OA. A genome-wide approach will be critical for
defining the individual contributions, and possible redundancies,
of these different pathways (39). Second, it also has to be
determined whether crosstalk between TLR4 and other signaling
pathways, such as the ERK/MAPK pathway and the PI3K/Akt pathway
occurs. The results of the present study demonstrated that
different concentrations of resveratrol led to the inhibition of
TLR4 and its downstream signaling targets to varying degrees,
thereby implying that other signaling pathways may also contribute
to the effects of resveratrol. Finally, in vivo studies are
required in ordre to determine whether the present results are
relevant to animal models of OA and have the potential to be
translated to patients clinically.
Acknowledgments
This study was supported by grants from the National
Natural Scientific Foundation of China (no. 81372971) and Liaoning
Natural Scientific foundation (no. 201202267), P.R. China.
References
|
1
|
Goldring SR: The role of bone in
osteoarthritis pathogenesis. Rheum Dis Clin North Am. 34:561–571.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sofat N, Ejindu V and Kiely P: What makes
osteoarthritis painful? The evidence for local and central pain
processing. Rheumatology (Oxford). 50:2157–2165. 2011. View Article : Google Scholar
|
|
3
|
Man GS and Mologhianu G: Osteoarthritis
pathogenesis - a complex process that involves the entire joint. J
Med Life. 7:37–41. 2014.PubMed/NCBI
|
|
4
|
Scanzello CR, Plaas A and Crow MK: Innate
immune system activation in osteoarthritis: is osteoarthritis a
chronic wound? Curr Opin Rheumatol. 20:565–572. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ting JP, Duncan JA and Lei Y: How the
noninflammasome NLRs function in the innate immune system. Science.
327:286–290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McCormack WJ, Parker AE and O'Neill LA:
Toll-like receptors and NOD-like receptors in rheumatic diseases.
Arthritis Res Ther. 11:2432009. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Haglund L, Bernier SM, Onnerfjord P and
Recklies AD: Proteomic analysis of the LPS-induced stress response
in rat chondrocytes reveals induction of innate immune response
components in articular cartilage. Matrix Biol. 27:107–118. 2008.
View Article : Google Scholar
|
|
8
|
Schelbergen RF, Blom AB, van den Bosch MH,
Slöetjes A, Abdollahi-Roodsaz S, Schreurs BW, Mort JS, Vogl T, Roth
J, van den Berg WB and van Lent PL: Alarmins S100A8 and S100A9
elicit a catabolic effect in human osteoarthritic chondrocytes that
is dependent on Toll-like receptor 4. Arthritis Rheum.
64:1477–1487. 2012. View Article : Google Scholar
|
|
9
|
Abdollahi-Roodsaz S, Joosten LA, Roelofs
MF, Radstake TR, Matera G, Popa C, van der Meer JW, Netea MG and
van den Berg WB: Inhibition of Toll-like receptor 4 breaks the
inflammatory loop in autoimmune destructive arthritis. Arthritis
Rheum. 56:2957–2967. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shotorbani SS, Su ZL and Xu HX: Toll-like
receptors are potential therapeutic targets in rheumatoid
arthritis. World J Biol Chem. 2:167–172. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bobacz K, Sunk IG, Hofstaetter JG, Amoyo
L, Toma CD, Akira S, Weichhart T, Saemann M and Smolen JS:
Toll-like receptors and chondrocytes: the
lipopolysaccharide-induced decrease in cartilage matrix synthesis
is dependent on the presence of toll-like receptor 4 and
antagonized by bone morphogenetic protein 7. Arthritis Rheum.
56:1880–1893. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sgaglione NA: Biologic approaches to
articular cartilage surgery: future trends. Orthop Clin North Am.
36:485–495. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Im HJ, Li X, Chen D, Yan D, Kim J, Ellman
MB, Stein GS, Cole B, Kc R, Cs-Szabo G and van Wijnen AJ:
Biological effects of the plant-derived polyphenol resveratrol in
human articular cartilage and chondrosarcoma cells. J Cell Physiol.
227:3488–3497. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yadav M, Jain S, Bhardwaj A, Nagpal R,
Puniya M, Tomar R, Singh V, Parkash O, Prasad GB, Marotta F and
Yadav H: Biological and medicinal properties of grapes and their
bioactive constituents: an update. J Med Food. 12:473–484. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eo SH, Cho H and Kim SJ: Resveratrol
inhibits nitric oxide-induced apoptosis via the NF-kappa B pathway
in rabbit articular chondrocytes. Biomol Ther (Seoul). 21:364–370.
2013. View Article : Google Scholar
|
|
16
|
Csaki C, Keshishzadeh N, Fischer K and
Shakibaei M: Regulation of inflammation signalling by resveratrol
in human chondrocytes in vitro. Biochem Pharmacol. 75:677–687.
2008. View Article : Google Scholar
|
|
17
|
Shakibaei M, John T, Seifarth C and
Mobasheri A: Resveratrol inhibits IL-1 beta-induced stimulation of
caspase-3 and cleavage of PARP in human articular chondrocytes in
vitro. Ann N Y Acad Sci. 1095:554–563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Elmali N, Esenkaya I, Harma A, Ertem K,
Turkoz Y and Mizrak B: Effect of resveratrol in experimental
osteoarthritis in rabbits. Inflamm Res. 54:158–162. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Elmali N, Baysal O, Harma A, Esenkaya I
and Mizrak B: Effects of resveratrol in inflammatory arthritis.
Inflammation. 30:1–6. 2007. View Article : Google Scholar
|
|
20
|
Lei M, Wang JG, Xiao DM, Fan M, Wang DP,
Xiong JY, Chen Y, Ding Y and Liu SL: Resveratrol inhibits
interleukin 1β-mediated inducible nitric oxide synthase expression
in articular chondrocytes by activating SIRT1 and thereby
suppressing nuclear factor-κB activity. Eur J Pharmacol. 674:73–79.
2012. View Article : Google Scholar
|
|
21
|
Shakibaei M, Mobasheri A and Buhrmann C:
Curcumin synergizes with resveratrol to stimulate the MAPK
signaling pathway in human articular chondrocytes in vitro. Genes
Nutr. 6:171–179. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eo SH, Cho HS and Kim SJ: Resveratrol
regulates type II collagen and COX-2 expression via the ERK, p38
and Akt signaling pathways in rabbit articular chondrocytes. Exp
Ther Med. 7:640–648. 2014.PubMed/NCBI
|
|
23
|
Han KJ, Su X, Xu LG, Bin LH, Zhang J and
Shu HB: Mechanisms of the TRIF-induced interferon-stimulated
response element and NF-kappaB activation and apoptosis pathways. J
Biol Chem. 279:15652–15661. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Baumgarten G, Knuefermann P, Nozaki N,
Sivasubramanian N, Mann DL and Vallejo JG: In vivo expression of
proinflammatory mediators in the adult heart after endotoxin
administration: the role of toll-like receptor-4. J Infect Dis.
183:1617–1624. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jakus PB, Kalman N, Antus C, Radnai B,
Tucsek Z, Gallyas F Jr, Sumegi B and Veres B: TRAF6 is functional
in inhibition of TLR4-mediated NF-κB activation by resveratrol. J
Nutr Biochem. 24:819–823. 2013. View Article : Google Scholar
|
|
26
|
Liu L, Gu H, Liu H, Jiao Y, Li K, Zhao Y,
An L and Yang J: Protective effect of resveratrol against
IL-1β-induced inflammatory response on human osteoarthritic
chondrocytes partly via the TLR4/MyD88/NF-κB signaling pathway: an
'in vitro study'. Int J Mol Sci. 15:6925–6940. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sebai H, Ristorcelli E, Sbarra V,
Hovsepian S, Fayet G, Aouani E and Lombardo D: Protective effect of
resveratrol against LPS-induced extracellular lipoperoxidation in
AR42J cells partly via a Myd88-dependent signaling pathway. Arch
Biochem Biophys. 495:56–61. 2010. View Article : Google Scholar
|
|
28
|
Jung DY, Lee H, Jung BY, Ock J, Lee MS,
Lee WH and Suk K: TLR4, but not TLR2, signals autoregulatory
apoptosis of cultured microglia: a critical role of IFN-beta as a
decision maker. J Immunol. 174:6467–6476. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
30
|
Vincenti MP and Brinckerhoff CE:
Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in
arthritis: integration of complex signaling pathways for the
recruitment of gene-specific transcription factors. Arthritis Res.
4:157–164. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yammani RR, Carlson CS, Bresnick AR and
Loeser RF: Increase in production of matrix metalloproteinase 13 by
human articular chondrocytes due to stimulation with S100A4: role
of the receptor for advanced glycation end products. Arthritis
Rheum. 54:2901–2911. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Im HJ, Pacione C, Chubinskaya S, Van
Wijnen AJ, Sun Y and Loeser RF: Inhibitory effects of insulin-like
growth factor-1 and osteogenic protein-1 on fibronectin fragment-
and interleukin-1beta-stimulated matrix metalloproteinase-13
expression in human chondrocytes. J Biol Chem. 278:25386–25394.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Muddasani P, Norman JC, Ellman M, van
Wijnen AJ and Im HJ: Basic fibroblast growth factor activates the
MAPK and NFkappaB pathways that converge on Elk-1 to control
production of matrix metalloproteinase-13 by human adult articular
chondrocytes. J Biol Chem. 282:31409–31421. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Legendre F, Bogdanowicz P, Boumediene K
and Pujol JP: Role of interleukin 6 (IL-6)/IL-6R-induced signal
tranducers and activators of transcription and mitogen-activated
protein kinase/extracellular. J Rheumatol. 32:1307–1316.
2005.PubMed/NCBI
|
|
35
|
Estrov Z, Shishodia S, Faderl S, Harris D,
Van Q, Kantarjian HM, Talpaz M and Aggarwal BB: Resveratrol blocks
interleukin-1beta-induced activation of the nuclear transcription
factor NF-kappaB, inhibits proliferation, causes S-phase arrest,
and induces apoptosis of acute myeloid leukemia cells. Blood.
102:987–995. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kundu JK, Shin YK and Surh YJ: Resveratrol
modulates phorbol ester-induced pro-inflammatory signal
transduction pathways in mouse skin in vivo: NF-kappaB and AP-1 as
prime targets. Biochem Pharmacol. 72:1506–1515. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu-Bryan R and Terkeltaub R: Chondrocyte
innate immune myeloid differentiation factor 88-dependent signaling
drives procatabolic effects of the endogenous Toll-like receptor
2/Toll-like receptor 4 ligands low molecular weight hyaluronan and
high mobility group box chromosomal protein 1 in mice. Arthritis
Rheum. 62:2004–2012. 2010.PubMed/NCBI
|
|
38
|
Weighardt H, Jusek G, Mages J, Lang R,
Hoebe K, Beutler B and Holzmann B: Identification of a TLR4- and
TRIF-dependent activation program of dendritic cells. Eur J
Immunol. 34:558–564. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Björkbacka H, Fitzgerald KA, Huet F, Li X,
Gregory JA, Lee MA, Ordija CM, Dowley NE, Golenbock DT and Freeman
MW: The induction of macrophage gene expression by LPS
predominantly utilizes Myd88-independent signaling cascades.
Physiol Genomics. 19:319–330. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jiang Z, Mak TW, Sen G and Li X: Toll-like
receptor 3-mediated activation of NF-kappaB and IRF3 diverges at
Toll-IL-1 receptor domain-containing adapter inducing IFN-beta.
Proc Natl Acad Sci USA. 101:3533–3538. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sato S, Sugiyama M, Yamamoto M, Watanabe
Y, Kawai T, Takeda K and Akira S: Toll/IL-1 receptor
domain-containing adaptor inducing IFN-beta (TRIF) associates with
TNF receptor-associated factor 6 and TANK-binding kinase 1, and
activates two distinct transcription factors, NF-kappa B and
IFN-regulatory factor-3, in the Toll-like receptor signaling. J
Immunol. 171:4304–4310. 2003. View Article : Google Scholar : PubMed/NCBI
|