Introduction
Diabetes mellitus, a complex metabolic disease, has
become one of the most serious threats to global public health with
an estimated worldwide prevalence of 285 million cases in the adult
population (1). In both type 1
diabetes (T1D) and type 2 diabetes (T2D), an inadequate mass of
functional pancreatic β-cells is the major determinant for the
onset of hyperglycemia and the development of overt disease
(2,3). A variety of pharmacological
treatments for diabetes, including insulin therapy, exhibit limited
ability to mimic the physiology of insulin secretion and are
frequently associated with severe hypoglycemic comas (4). To overcome the limitations of
traditional treatment, replenishing the lost insulin-producing
cells by transplantation or by the expansion of existing pancreatic
β-cells is the preferred approach for achieving glucose homeostasis
(5).
Recent advances in the identification of stem cells
that possess the potential to differentiate into insulin-producing
cells and improve pancreatic regeneration provide future treatment
options (6). Mesenchymal stem
cells (MSCs) have received attention as they can be easily isolated
from bone marrow and rapidly expanded ex vivo (7) and do not induce major toxicity
following transplantation (8).
MSC transplantation can improve the metabolic profiles of diabetic
animal models (9,10), and the co-infusion of
insulin-secreting adipose-derived MSCs with bone marrow-derived
hematopoietic stem cells has been shown to control hyperglycemia in
patients with T1D (11); however,
the mechanisms underlying these beneficial effects remain poorly
understood. As the number of MSCs that differentiate into
functionally competent β-cells in vivo is too low to support
a physiological change (~1.7–3% of infused MSCs) (12), there may be another mechanism
underlying their therapeutic effects. MSCs may contribute to tissue
regeneration through their immunomodulatory potential (13,14). Furthermore, MSCs secrete
anti-inflammatory cytokines and inhibit the expression of
pro-inflammatory cytokines by immune cells (15,16). Finally, MSCs produce trophic
factors, such as epidermal growth factor (EGF), hepatocyte growth
factor (HGF), insulin-like growth factor-1 (IGF-1) and basic
fibroblast growth factor (bFGF) (17,18).
Betatrophin, also known as lipasin (19) or angiopoietin-like 8 (20), was recently described as a potent
stimulator of mouse β-cell proliferation (21). Its transient overexpression in the
liver induces β-cell proliferation and improves glucose tolerance
in young adult mice (21).
However, betatrophin knockout mice do not display an altered
glucose homeostasis (22). In
patients with T2D, betatrophin levels are associated with measures
of insulin resistance; however, studies assessing its level in
individuals with T2D have provided conflicting results, with some
reporting its increase in patients with T2D (23), while others have shown that it is
decreased in these same patients (24). As the mechanisms through which
betatrophin improves diabetes mellitus remains unknown (25), in this study, we aimed to evaluate
the in vitro and in vivo effects of
lentivirus-induced betatrophin overexpression in adipose-derived
MSCs (ADMSCs). The biological characteristics and differentiation
potential of the betatrophin-overexpressing ADMSCs (ADMSCs-BET)
were assessed in vitro. Furthermore, their effects on
pancreatic β-cells were examined via co-culture with pancreatic
islets and transplantation into mice with streptozotocin
(STZ)-induced diabetes. Our findings have the potential to form the
basis for further clinical analysis of combining MSC
transplantation with gene therapy as a novel therapy for the
treatment of diabetes.
Materials and methods
Adenoviruses and gene transfer
Human ADMSCs were obtained from the Typical Culture
Preservation Commission Cell Bank, Chinese Academy of Sciences
(Shanghai, China). The ADMSCs were cultured in MesenPro RS medium
(Life Technologies, Carlsbad, CA, USA). A cDNA fragment containing
the full-length coding regions of betatrophin was obtained from the
cDNA library of human vascular endothelial cells (the Typical
Culture Preservation Commission Cell Bank) by a RT-PCR method,
using a reverse transcription PCR kit (Toyobo Co., Ltd, Osaka,
Japan). The recombinant adenovirus, expressing either
β-galactosidase (LacZ) or betatrophin, was generated using cosmid
cassettes and the adenovirus DNA-terminal protein complex method
(COS/TPC method), with an Adenovirus Expression vector kit (Takara,
Osaka, Japan). For lentivirus-mediated gene transfer, the MSCs were
exposed to lentiviral vectors at a multiplicity of infection (MOI)
of approximately 10–20 for 12 h.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells and the mouse
livers using TRIzol reagent (Life Technologies). Reverse
transcription was performed using MMLV reverse transcriptase
(Thermo Fisher Scientific, Waltham, MA, USA), and the PCR reaction
was performed using the SYBR-Green PCR Master Mix (Life
Technologies). The PCR primers used in this study are listed in
Table I. Each reaction mixture
was incubated at 50°C for 2 min and 95°C for 5 min followed by 40
cycles of 15 sec at 95°C and 35 sec at 60°C using an ABI 7500
Sequence detection system (Applied Biosystems, Foster City, CA,
USA). The corresponding relative mRNA expression was normalized to
β-actin and was calculated using the 2−ΔΔCq method.
 | Table IPCR primers used for RT-qPCR. |
Table I
PCR primers used for RT-qPCR.
| Gene | Primer sequences
(5′→3′) |
|---|
|
Betatrophin |
ATGGGATCCATGCCAGTGCCTGCTCTGTGCCTG
ATGGTCGACTCAGGCTGGGAGCGCCGCTGTGTG |
| Insulin |
CCGTCGTGAAGTGGAG
CAGTTGGTAGAGGGAGCAG |
|
Glucagon |
AGCTGCCTTGTACCAGCATT
TGCTCTCTCTTCACCTGCTCT |
| Glut2 |
TTACTCTCCATTTCAGTCCTTTGT
TAGAGCAGCTCTTTATTCCAGATTT |
| Foxa2 |
CCCCTGAGTTGGCGGTGGT
TTGCTCACGGAAGAGTAGCC |
| Nkx2.2 |
ACCACAGTCCATGCCATCAC
TGCCCGCCTGGAAGGTGGCG |
| Pax-4 |
CGACAAGATTTGCCATGGAT
CAACCTTTGGAAAAACCAACA |
| Pax-6 |
CAGCTTGGTGGTGTCTTTGTC
CCCTCGGATAATAATCTGTCTCG |
| NeoD |
ATCAGCCCACTCTCGCTGTA
GCCCCAGGGTTATGAGACTAT |
| Ngn3 |
CCGGACATCTCCCCATACGAAG
ACACCAGTGCTCCGGCTCT |
| MafB |
AGCAGGGTTTGAAATTGATCC
GCATGGTGCTTGCAGTTTTA |
| MafA |
ATCATCACTCTGCCCACCAT
AGTCGGATGACCTCCTCCTT |
| β-actin |
GAGACCTTCAACACCCCAGC
ATGTCACGCACGATTTCCC |
Western blot analysis
Cell lysates (50 µg) were separated by 12%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and blotted onto polyvinylidene fluoride (PVDF)
membranes (Amersham Biosciences, Uppsala, Sweden). After blocking
with 5% skim milk, the membranes were then blotted with the
indicated primary antibodies: mouse anti-human betatrophin antibody
(1:200; AG-25B-0033-C100; Adipogen, San Diego, CA, USA), rabbit
anti-human insulin (1:200; Cat. no. 3014; Cell Signaling
Technology, Danvers, MA, USA), active caspase-3 (1:200; Cat. no.
9661S; Cell Signaling Technology), mouse anti-human glyceraldehyde
3-phosphate dehydrogenase (GAPDH) (1:5,000; ab110305; Abcam,
Cambridge, UK), or mouse anti-human β-actin (1:10,000; A5441-100UL;
Sigma-Aldrich, St. Louis, MO, USA). After washing, the membranes
were incubated with the horseradish peroxidase (HRP)-conjugated
secondary antibody at room temperature for 1 h: either goat
anti-rabbit IgG (1:8,000; AS10 668; Epitomics Inc., Burlingame, CA,
USA) or rabbit anti-mouse IgG (1:10,000; A9044-2ML; Sigma-Aldrich).
The protein intensity was determined by ECL chemiluminescence
reagent (PerkinElmer Life Sciences, Inc., Waltham, MA, USA), and
their intensities were quantitatively measured by densitom-etry
(LabWorks, UVP Inc., Upland, CA, USA).
Cell cycle analysis
The ADMSCs were seeded at 0.2×106
cells/25 cm2 flask. At 80–90% confluence, the cells were
harvested for cell cycle analysis. Briefly, the cells were washed
and fixed in 70% ethanol overnight at −20°C. The fixed cells were
then washed and incubated in 100 µg/ml propidium iodide (PI)
and 20 ng/ml RNAase (both from Sigma-Aldrich) in phosphate-buffered
saline (PBS) for 30 min. Cell cycles were assessed by flow
cytometry, and analysis was performed using FCS Express V3 software
(BD Biosciences, San Jose, CA, USA).
Flow cytometry
The ADMSCs were analyzed for the surface expression
of a battery of markers at passage 4 (P4). Anti-mouse antibodies
purchased from BD Biosciences included CD45 (FITC-conjugated, Cat.
no. 553099), CD14 (FITC-conjugated, Cat. no. 553739), CD90
(APC-conjugated, Cat. no. 553007) and CD73 (PE-conjugated, Cat. no.
550741). Antibodies against CD105 (PE-conjugated, Cat. no.
25-1057-41) were purchased from eBioscience (San Diego, CA, USA). A
total of 1×106 cells were incubated with
fluorescent-conjugated antibodies for 30 min at room temperature
followed by analysis using a Becton-Dickinson FACSCalibur (BD
Biosciences, Franklin Lakes, NJ, USA) and CellQuest Pro
software.
To assess apoptosis, the ADMSC/islet co-cultures
were dissociated with Liberase (Roche Life Science, Basel,
Switzerland) and the stained with FITC Annexin V and PI (BD
Biosciences) followed by flow cytometric analysis.
ADMSC differentiation assays
To examine whether betatrophin overexpression alters
the differentiation capacity of ADMSCs (ADMSCs-BET), the cells were
induced to undergo adipogenic, osteogenic and chondrogenic
differentiation. Adipogenic and osteogenic differentiation were
induced as previously described (26). The acquisition of the adipogenic
phenotype was determined by staining the monolayers with a 0.5% Oil
Red O solution (O1391-250ML; Sigma-Aldrich). ADMSC colonies that
underwent adipogenic differentiation contained cells with numerous
lipid vesicles of various sizes. Osteogenic mineralization was
assessed by staining with 40 mM Alizarin Red, pH 4.1
(Sigma-Aldrich).
For chondrogenic differentiation, the pellet culture
system described by Sekiya et al (27) was used. ADMSC pellets were
cultured in chondrogenic differentiation medium, which consisted of
DMEM supplemented with 500 ng/ml bone morphogenic protein-6 (BMP-6;
R&D Systems, Minneapolis, MN, USA), 10 ng/ml tumor growth
factor-β3 (TGF-β3), 10−7 M dexamethasone, 50
µg/ml ascorbate 2-phosphate, 40 µg/ml proline, 100
µg/ml pyruvate and 50 mg/ml ITS + premix (Becton-Dickinson:
6.25 µg/ml insulin, 6.25 µg/ml transferrin, 6.25
ng/ml selenous acid, 1.25 mg/ml bovine serum albumin, 5.35 mg/ml
linoleic acid). The medium was replaced every 2–3 days for 21 days.
Pellets were then fixed in formalin, embedded in paraffin and
sectioned, and the sections were stained with Toluidine blue
(198161; Sigma-Aldrich).
Collection of human islets
Human islets were collected from patients who
underwent pancreaticoduodenal surgery at the Eastern Hepatobiliary
Hospital, Shanghai, China between September 2012 and January 2013.
Six male patients (age range, 52–63 years) were recruited. Patients
with known distal metastasis and portal vein tumor thrombus were
excluded; pancreatic involvement was not observed during surgery.
As cholangiocarcinoma invades the distal common bile duct,
duodenopancreatectomy (Whipple's surgery) was also required. This
study was approved by the Institutional Review Board of the Second
Military Medical University, and all participants provided informed
consent.
Co-culture of ADMSCs with islets
The ADMSCs or ADMSCs-BET were seeded at the density
of 1.0×106 cells to a 100 mm low adherence culture dish
(Corning, Corning, NY, USA) with 500 human islets in 10 ml culture
medium, as previously described (28). Islets cultured alone were used as
controls. The culture medium consisted of DMEM low glucose (5.6 mM
glucose) with 1% fetal bovine serum (FBS), 20 ng/ml EGF, 20 ng/ml
bFGF, 10 mM HEPES, 100 units penicillin/1,000 units streptomycin
and 71.5 µM β-mercaptoethanol for 48 h. DMEM low glucose,
FBS, EGF, bFGF and penicillin/streptomycin were purchased from Life
Technologies. HEPES and β-mercaptoethanol were obtained from
Sigma-Aldrich.
Immunohistochemical staining
For immunohistochemical staining, pancreatic tissue
sections were blocked by incubation in 2.5% bovine serum albumin
for 20 min at room temperature. The sections were then incubated
with mouse anti-vimentin (1:400; ab92547), rabbit anti-insulin
(1:500; ab63820) or anti-Ki67 (1:500; ab15580) (all from Abcam)
antibodies overnight at 4°C, followed by fluorescein
isothiocyanate-conjugated anti-mouse (SA1062) or anti-rabbit
(SA1064) secondary antibodies (1:200; Boster, Wuhan, China) for 2 h
at room temperature. The nuclei were stained with
4,6-diamidino-2-phenylindole (DAPI) for a further 40–60 min. The
tissue sections were assessed using a fluorescence microscope
(Leitz, Wetzlar, Germany). Image acquisition was performed with the
SPOT RT Imaging system (Diagnostic Instruments, Sterling Heights,
MI, USA). In addition, the ratio of β-cells per islet (i.e., the
number of β-cells over the total number of islet cells) was
measured using Image-Pro 6.0 Image-Pro Plus version 6.0
(MediaCybernetics, Rockville, MD, USA).
Insulin secretion assays
Glucose-independent insulin secretion was triggered
by membrane depolarization that was induced by a combination of 30
mM KCl (A610440-0500; Sangon Biotech Co., Ltd., Shanghai, China)
and 30 mM Arg (A600205-0100; Sangon Biotech Co., Ltd.) at 20 and 50
min after the experiment began, as previously described (29). Glucose-stimulated insulin
secretion (GSIS) was used to assess human islet function in the
presence of 60 and 300 mg/l glucose. A total of 20 µl was
removed at 0, 10, 20, 30 up to 90 min, and secreted insulin level
was measured by enzyme-linked immunosorbent assay (ELISA; Crystal
Chem, Downers Grove, IL, USA) following the manufacturer's
instructions.
Measurement of the adenosine triphosphate
(ATP)/adenosine diphosphate (ADP) ratio
The ADP/ATP ratio was determined using the ADP/ATP
Ratio Assay kit (Cat. no. MAK135; Sigma-Aldrich) according to the
manufacturer's instructions. Briefly, the cells (4,000/well) were
seeded in a 96-well, flat-bottom, white plate with clear bottoms.
The ATP level was assayed by a luminometric method. After removing
the culture medium, 100 µl of nucleotide releasing buffer
was added and incubated for 5 min. The samples were mixed with 100
µl of a commercially available lyophilized ATP monitoring
reagent containing firefly luciferase and luciferin at first
reconstituted in an imidazole buffer (100 mM, pH 7.75). The emitted
light was measured using a BioTek luminometer. The ADP/ATP ratio of
the 3 different groups was detected. All assays were performed in
triplicate.
Quantification of secreted proteins
The levels of interleukin (IL)-4, IL-10, IL-13,
IL1-β, tumor necrosis factor-α (TNF-α), NADP-cytochrome P450
reductase (NCP1), X-linked inhibitor of apoptosis protein (XIAP),
B-cell lymphoma-extra large (Bcl-xL) and B-cell lymphoma-2 (Bcl-2)
secreted by the ADMSCs in co-culture with pancreatic islets were
measured using the RayBio human growth factor antibody array
(RayBiotech, Norcross, GA, USA). ADMSCs were maintained in MesenPro
medium (Invitrogen, Carlsbad, CA, USA) for 2 days, and the
conditioned medium was then collected for growth factor array
analysis according to the manufacturer's instructions. MesenPro
medium served as the control.
Induction of diabetes and ADMSC
transplantation
This study was approved by the Institutional Animal
Care and Use Committee of the Second Military Medical University.
T1D was induced in 10-week-old BALB/c mice (n=40, weighting 25–27
g, obtained from the Shanghai SLAC Laboratory Animal Co., Ltd,
Shanghai, China) by an intraperitoneal injection of STZ
Sigma-Aldrich) at a dose of 60 mg/kg body weight in 25 mM sodium
citrate (pH 4.5), as previously described (30). The onset of diabetes was defined
as elevated blood glucose values of >200 mg/dl found at two
consecutive tests at 3-day intervals. Blood glucose was measured
after an 8-h fast.
The diabetic mice were further divided into the
following 3 groups (n=8 per group) as follows: the diabetic
(control) group, the diabetic mice with ADMSC transplantation
group, and the diabetic mice with ADMSC-BET transplantation group.
For ADMSC transplantation, 1×106 cells in 0.2 ml of PBS
were injected into the diabetic mice via the tail vein 7 weeks
after the STZ injection. In the control group, 0.2 ml of PBS was
injected into the diabetic mice via the tail vein at the same time
points as those for ADMSC transplantation.
Quantification of betatrophin and
C-peptide
The animals were sacrificed at 28 days, and the
levels of betatrophin in homogenized liver samples and plasma were
then quantified using an EIA kit (Phoenix Pharmaceuticals, Belmont,
CA, USA) following the manufacturer's instructions. The plasma
level of C-peptide was measured by an EIA kit (BioVision, San
Francisco, CA, USA) following the manufacturer's instructions.
Statistical analysis
Values are expressed as the means ± standard
deviation (SD). Differences between groups were examined by one-way
ANOVA, followed by Bonferroni post-hoc tests. Statistical
assessments were evaluated at a two-sided significance level of
0.05, and performed using IBM SPSS software, version 22 (IBM Corp.,
Armonk, NY, USA).
Results
Generation and characterization of
ADMSCs-BET
We overexpressed betatrophin in ADMSCs using a
lentivirus. A significantly higher mRNA and protein expression
level was detected in the ADMSCs-BET compared with the control
group consisting of ADMSCs transfected with the vehicle [the
recombinant adenovirus, expressing β-galactosidase (LacZ)]
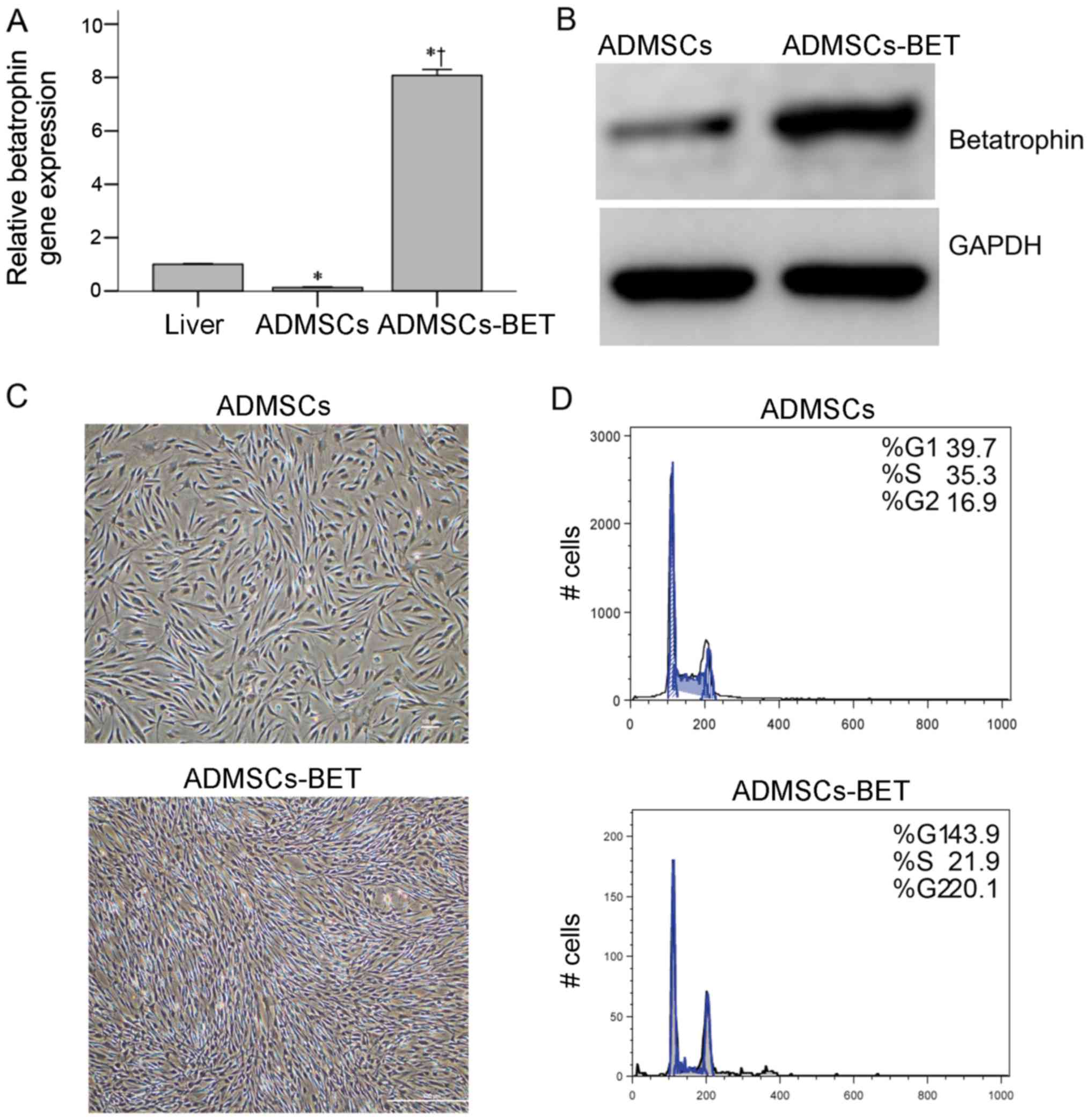
(Fig. 1A and B, respectively). As
compared to mouse hepatic tissue, in which betatrophin is primarily
expressed, there was a significantly higher betatrophin mRNA
expression in the ADMSCs-BET (1.0±0.024 vs. 8.08±0.23, P<0.001).
These data demonstrate the successful lentiviral-mediated
overexpression of betatrophin in ADMSCs.
For clinical application, ADMSCs-BET should have the
biological properties of ADMSCs. As shown in Fig. 1C, the ADMSCs-BET and ADMSCs
exhibited a similar fibroblast-like morphology. Similar cell cycle
progression was also observed with 52.2 and 42% of the ADMSCs and
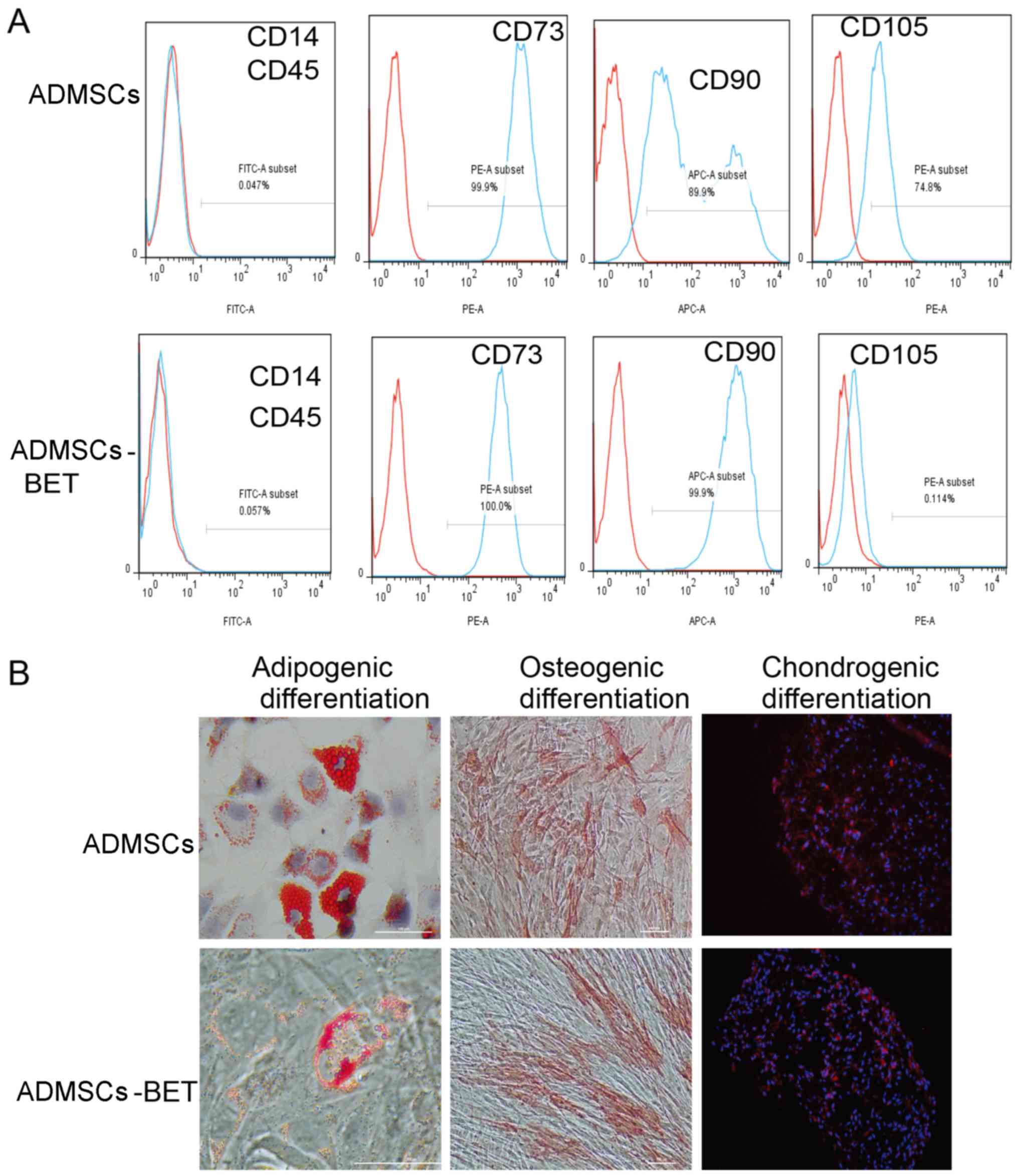
ADMSCs-BET in the S + G2/M phases, respectively (Fig. 1D). As the surface marker profile
and differentiation potential are thought to be minimal criteria
for MSCs (31), we tested an
array of surface markers by flow cytometry. As shown in Fig. 2A, both the ADMSCs and ADMSC-BET
were positive for CD73, CD90 and CD105, and negative for CD14 and
CD45. Furthermore, both the ADMSCs and ADMSCs-BET had the capacity
to differentiate into adipogenic, osteogenic and chondrogenic
lineages in vitro (Fig.
2B), as has also been demonstrated by previous studies
(32,33). Thus, the overexpression of
betatrophin did not alter the biological characteristics of the
ADMSCs.
Islet-MSC-BET co-culture enhances islet
viability and β-cell insulin secretion
To identify whether betatrophin overexpression
provides additional benefits beyond ADMSCs on the viability and
function of islets, ADMSCs-BET or ADMSCs were co-cultured with
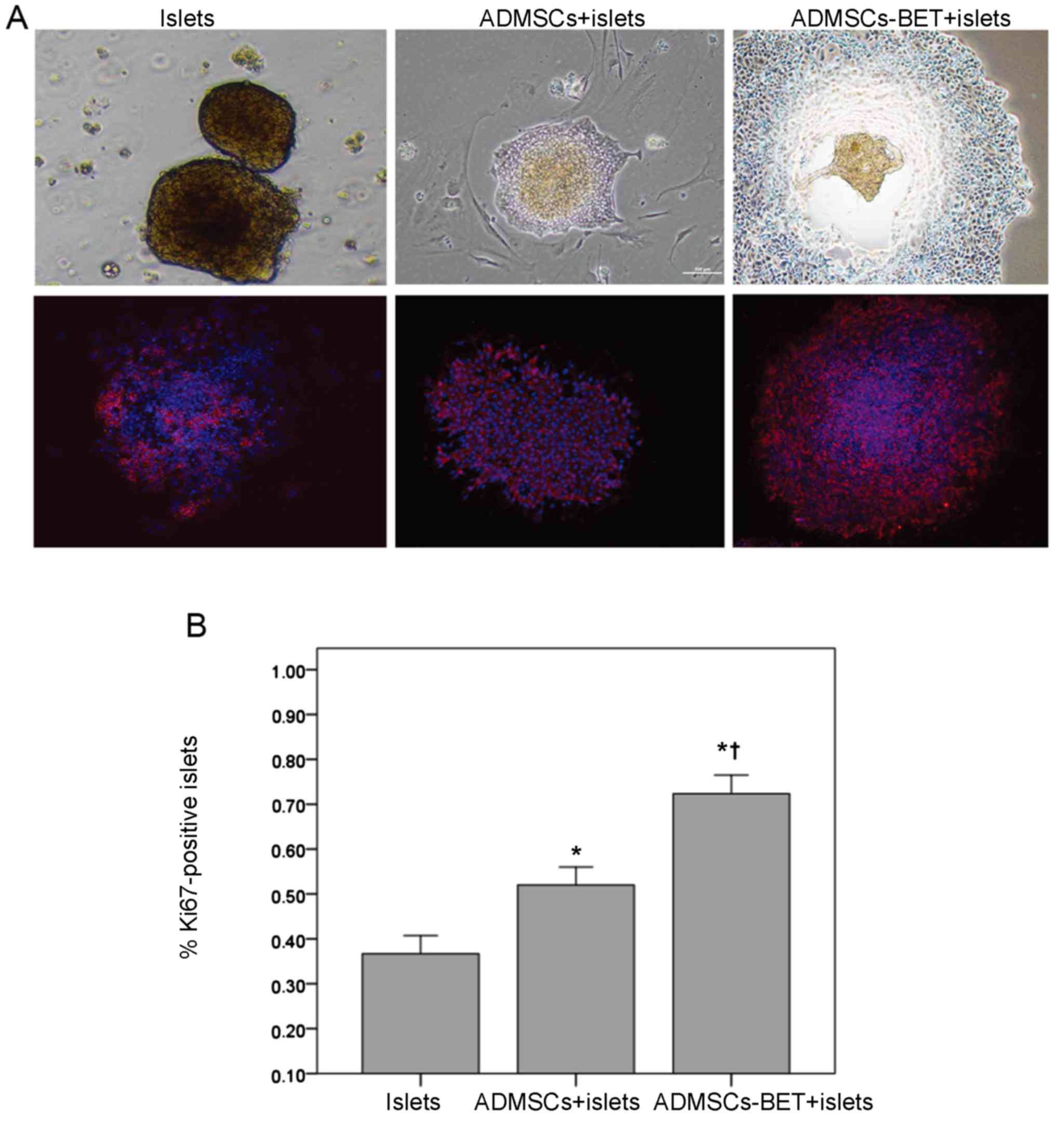
human islets as previously described (34). Co-culture of the islets with
ADMSCs-BET induced a marked increase in the size of the islets, as
well as the formation of new islet-like aggregates of cells; no
such changes were observed with the ADMSCs alone (Fig. 3, top row). Furthermore, the
expression of Ki67 antigen, a nuclear marker of cell proliferation,
was substantially increased in the islets co-cultured with
ADMSCs-BET compared with the islets co-cultured with ADMSCs or
cultured alone (Fig. 3, bottom
row). The proportion of Ki67-positive cells in the ADMSCs-BET +
islet group was significantly greater than that of the other 2
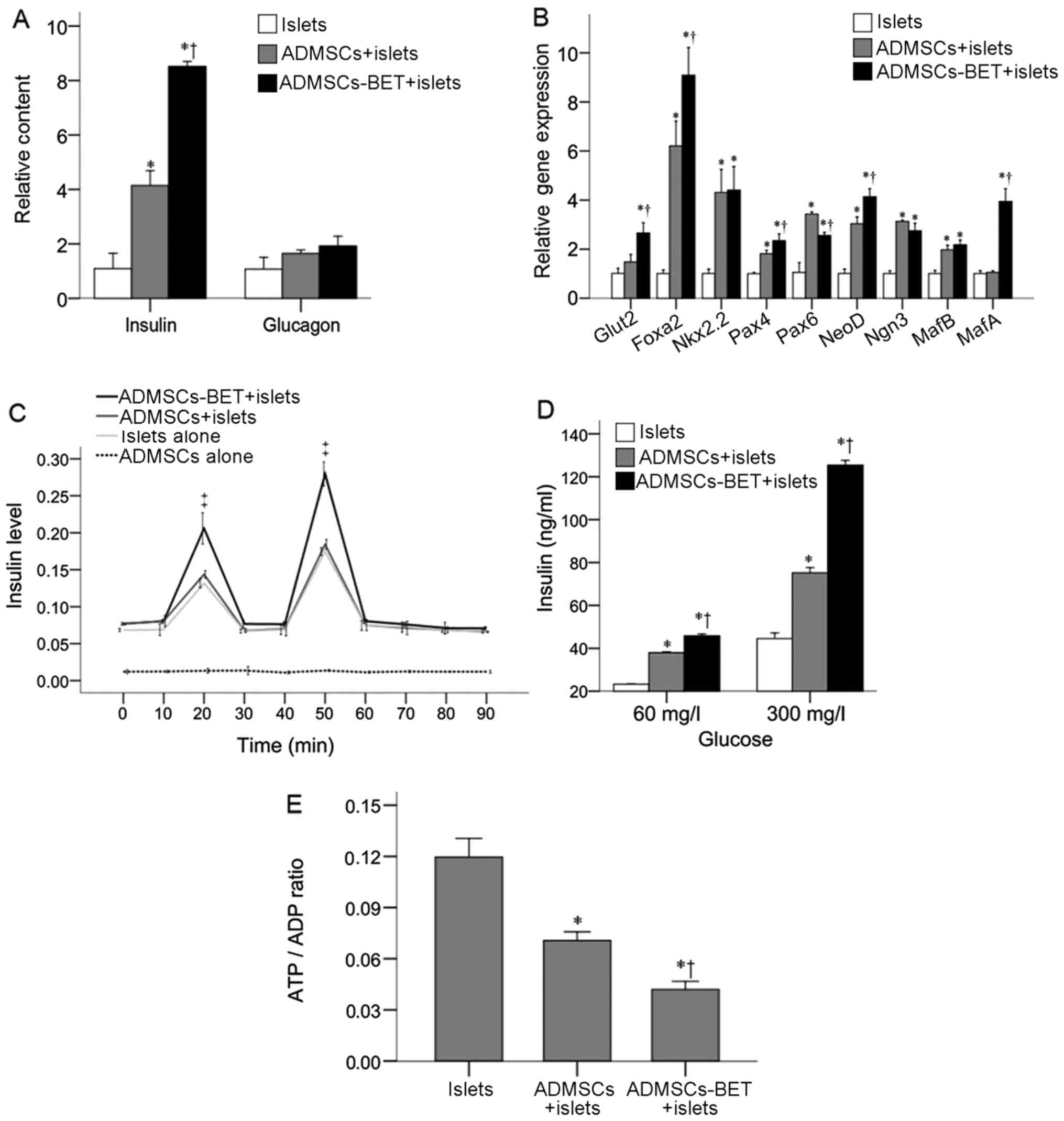
groups (p<0.0167). The analysis of insulin mRNA levels revealed
that the islets co-cultured with ADMSCs expressed markedly higher
levels than the islets cultured alone (4.15±0.54 vs. 1.10±0.56,
respectively; P<0.001) (Fig.
4A). The insulin mRNA levels of the islets co-cultured with
ADMSCs-BET were significantly higher than those cultured with
ADMSCs or alone (8.52±0.19 vs. 4.15±0.54 and 1.10±0.56,
respectively; both P<0.001). By contrast, all 3 groups expressed
similar glucagon mRNA levels (P>0.05).
The mRNA expression of a panel of regulatory factors
related to insulin transcription was then analyzed. Co-culture with
both ADMSCs and ADMSCs-BET significantly upregulated the Foxa2,
Nkx2.2, Pax4, Pax6, NeuroD and MafB mRNA levels (all P≤0.002)
(Fig. 4B). In addition, the
Foxa2, Pax4 and neuroD mRNA levels were further increased following
co-culture with ADMSCs-BET compared to co-culture with ADMSCs (all
P≤0.013). Of note, the Pax6 mRNA levels were decreased with
ADMSCs-BET co-culture as compared to co-culture with ADMSCs
(P=0.005); however, the mRNA levels of Glut2 and MafA were only
induced following co-culture with ADMSCs-BET (P≤0.001).
As shown in Fig.
4C, KCl and Arg induced strong and equivalent insulin secretion
by islets co-cultured with ADMSCs and ADMSCs-BET; however, the
insulin levels in the ADMSCs-BET + islet group were significantly
higher than those in the ADMSCs + islet group and islet alone group
at 20 and 50 min (P<0.001). The analysis of GSIS in the presence
of 60 and 300 mg/l glucose revealed that the islets cultured with
both ADMSCs and ADMSCs-BET secreted significantly more insulin than
the islets cultured alone (P<0.001) (Fig. 4D). Furthermore, ADMSCs-BET induced
significantly greater glucose-stimulated insulin secretion compared
to the ADMSCs (P<0.001).
The analysis of the ADP/ATP ratio revealed that
co-culture with ADMSCs and ADMSCs-BET produced lower ADP/ATP ratios
than the islets cultured alone (both P<0.001) (Fig. 4E). The ADMSCs-BET were
significantly more effective in reducing the ADP/ATP ratio
(P=0.003). Taken together, these results suggest that the islets
cultured with ADMSCs-BET displayed an enhanced viability and
insulin secretory function and reduced ADP/ATP ratios beyond those
observed with ADMSC co-culture.
Expression of betatrophin enhances the
anti-inflammatory and anti-apoptotic potential of ADMSCs
Pro-inflammatory cytokines can cause impaired
function, and ultimately the cell death of islets by apoptosis or
necrosis in T1D, and inflammation may participate in the
pathogenesis of T2D (35). In
addition, inflammation-induced apoptosis plays a significant role
in the loss of islet function immediately after islet
transplantation (36,37). Moreover, anti-inflammatory
cytokines are protective against pancreatic β-cell death (38,39). Thus, we measured the levels of
several inflammatory- and apoptosis-related proteins in the
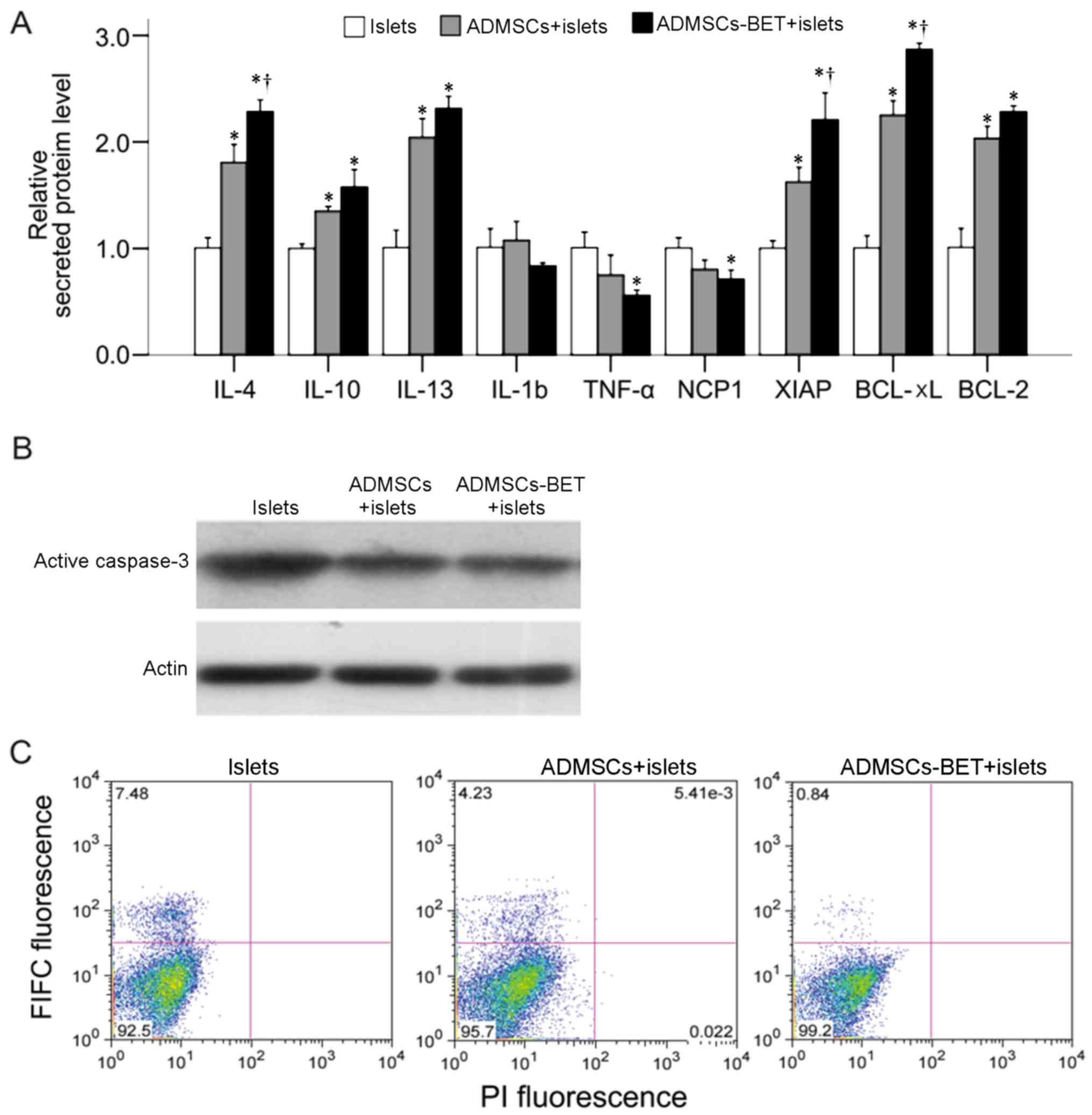
supernatant of the co-culture system. As shown in Fig. 5A, the L-4, IL-10 and IL-13 protein
levels were significantly increased when the islets were
co-cultured with either ADMSCs or ADMSCs-BET; the IL-4 levels in
the ADMSCs-BET group were significantly higher compared with those
in the ADMSCs group (all P<0.05). Whereas no differences in
IL-1B levels were observed among the 3 groups, the TNF-α and NCP1
levels were significantly decreased in the islets co-cultured with
ADMSCs-BET as compared to the control group (both P<0.05). In
addition, the XIAP, Bcl-xL and Bcl-2 levels were significantly
increased in the islets co-cultured with ADMSCs or ADMSCs-BET (all
P<0.05); however, the XIAP and Bcl-xL levels were significantly
higher in the ADMSCs-BET group as compared to the ADMSCs group
(both P<0.05). Furthermore, western blot analysis revealed that
the level of active caspase-3 was reduced in the islets co-cultured
with ADMSCs-BET (Fig. 5B), and a
decreased amount of apoptotic cells (green fluorescence) was
detected in the islets co-cultured with ADMSCs-BET, as shown by
Annexin V FITC and PI staining (Fig.
5C). Taken together, ADMSCs have anti-apoptotic and
anti-inflammatory effects on islets, and the transduction of
betatrophin in ADMSCs appears to provide additional benefits.
Infusion of ADMSCs-BET attenuates
hyperglycemia in mice with STZ-induced diabetes
To assess the function of ADMSCs in vivo, a
single dose of 1×106 ADMSCs or ADMSCs-BET was injected
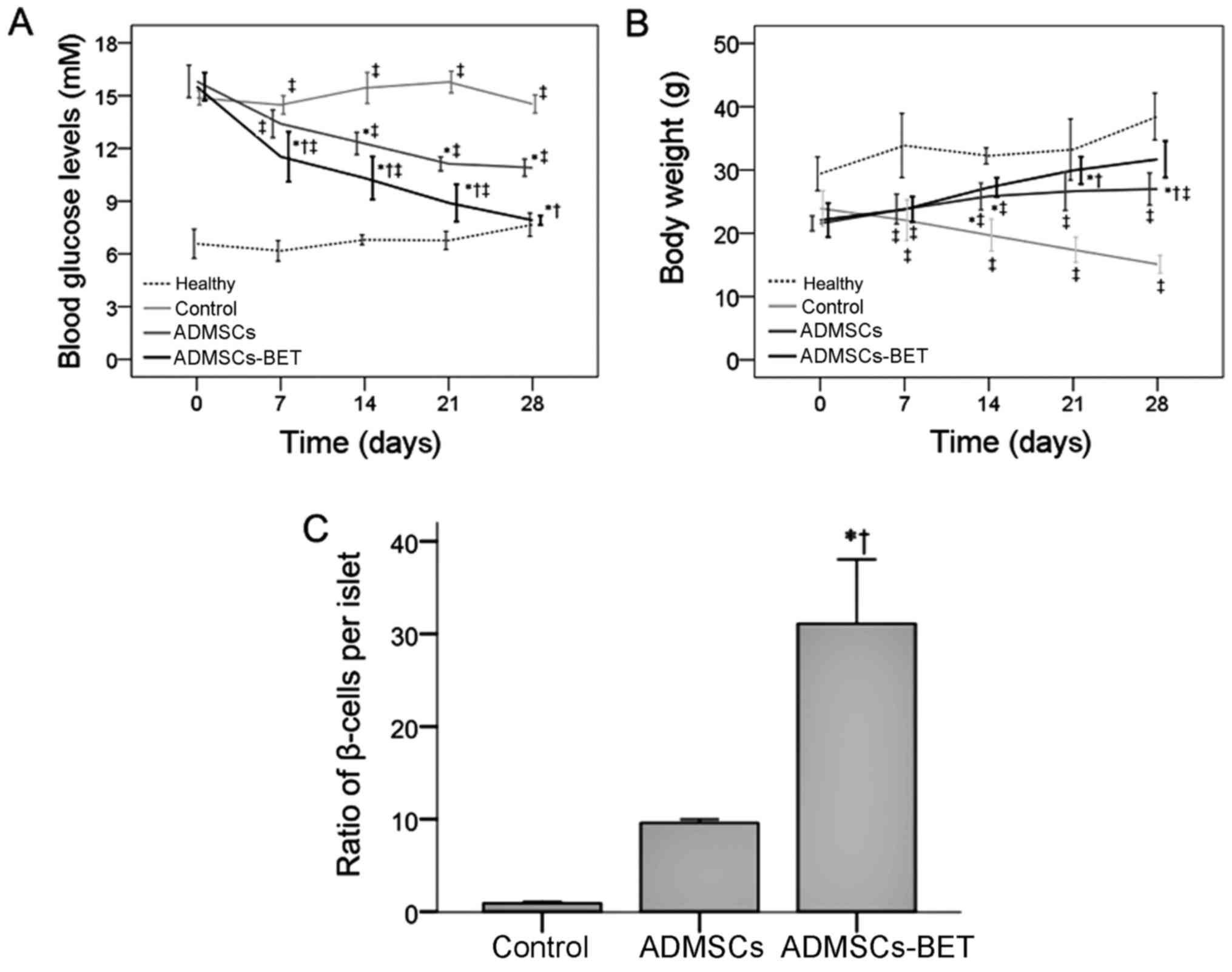
into mice with STZ-induced diabetes via the tail vein. As shown in
Fig. 6A, the transplantation of
both ADMSCs and ADMSCs-BET significantly reduced the fasting blood
glucose levels compared with the control group at days 14, 21 and
28 (all P<0.05); however, the reduction in blood glucose levels
in mice receiving ADMSCs-BET was significantly greater compared
with the mice receiving ADMSCs (all P<0.05). Consistent with
these results, ADMSCs-BET transplantation led to a significant
restoration of the ratio of β-cells/islets (Fig. 6C).
The analysis of the mean body weight revealed that
whereas the control group experienced a decrease in body weight
over the course of the study, the ADMSCs and ADMSCs-BET groups had
significantly increased body weights on days 7–28 (all P<0.05)
(Fig. 6B). Furthermore, mice in
the ADMSCs-BET group had significantly higher body weights compared
with those in the ADMSCs group on days 21 and 28 (both
P<0.05).
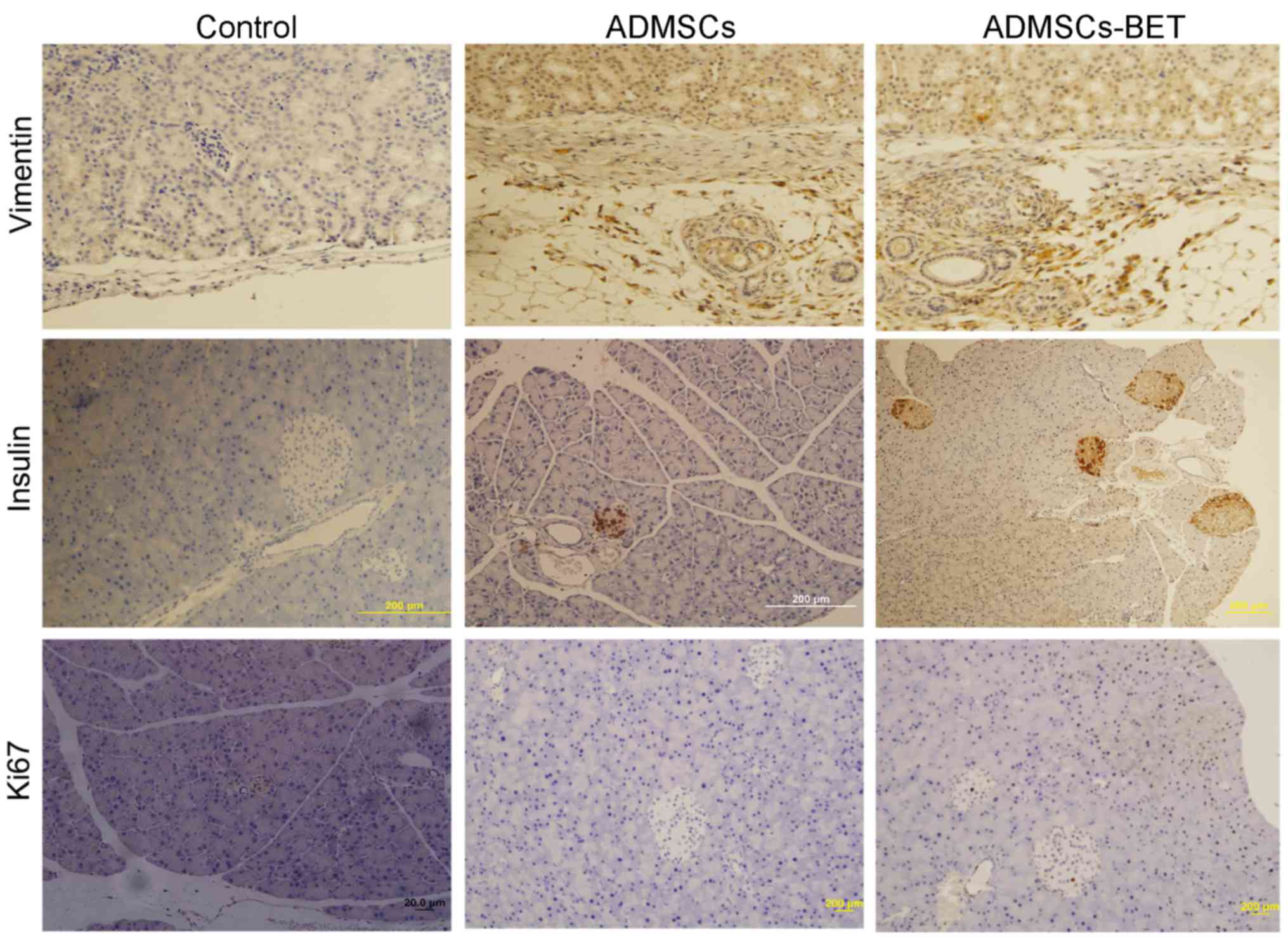
As shown in Fig.
7, immunohistochemical analysis of pancreatic tissue revealed
an increase in the number of Ki67-positive and insulin-positive
cells in the ADMSCs and ADMSCs-BET groups, suggesting that their
transplantation increased β-cell proliferation, resulting in
reduced blood glucose levels in mice with STZ-induced diabetes.
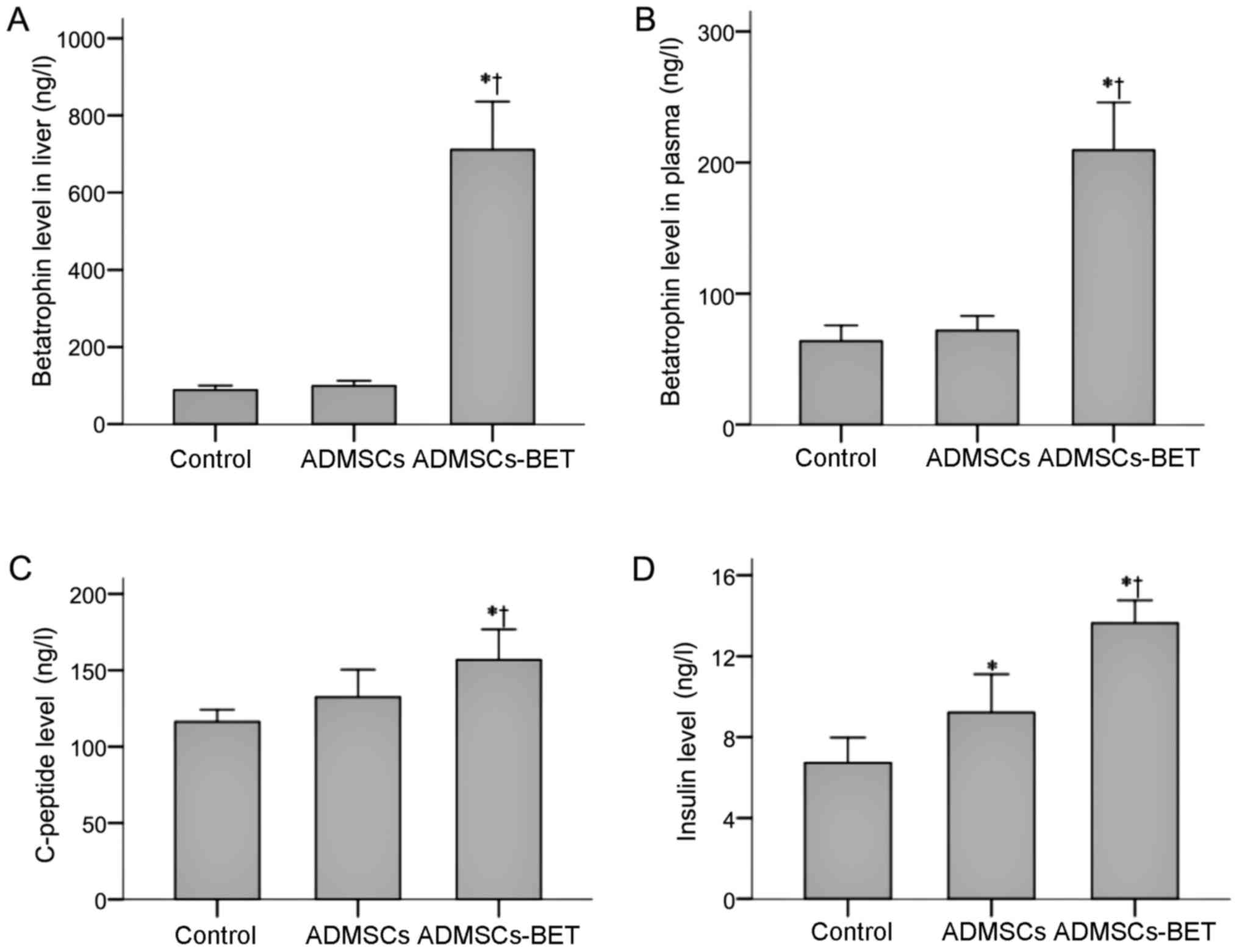
To confirm that mice transplanted with ADMSCs-BET
actually had increased betatrophin levels, we evaluated betatrophin
levels in the liver and plasma, which were both significantly
higher in the ADMSCs-BET group compared to the other 2 groups (all
P<0.001) (Fig. 8A and B). No
significant difference was observed between the control and ADMSCs
groups.
As shown in Fig.
8C, the plasma C-peptide levels of the ADMSCs-BET group were
significantly higher than those in the control and ADMSCs groups on
day 28 (156.85±19.92 vs. 116.22±8.01 and 132.45±18.01,
respectively; P<0.001 and P=0.007, respectively). Furthermore,
although the plasma insulin levels of the ADMSCs group were
significantly higher than those in the control group (9.22±1.89 vs.
6.73±1.24, respectively; P=0.003) (Fig. 8D), they were highest in the
ADMSCs-BET group than in the control or ADMSCs groups (13.64±1.12
vs. 6.73±1.24 and 9.22±1.89, respectively; P<0.001).
Discussion
In vivo studies and clinical trials have
demonstrated that MSCs are capable of reducing blood glucose levels
in animal models or in humans with T1D or T2D (40,41). Furthermore, MSCs are ideal
cellular delivery vehicles for gene delivery (42,43) without the limitations of viral
vector-based gene therapy, such as insertional mutagenesis and the
generation of innate, and specific anti-viral immune responses
against viral proteins (44,45). In the present study, ADMSCs stably
overexpressing betatrophin augmented the therapeutic effects of
MSCs in mice with STZ-induced diabetes. Although betatrophin
overexpression did not affect ADMSC proliferation, differentiation
and morphology, it provided strong paracrine effects and conferred
additional cyto-protective effects in vitro and in
vivo, leading to greater β-cell number, increased insulin
production and reduced glucose levels.
The application of gene-modified hMSCs expressing
human insulin by a retroviral vector has been reported to be able
to ameliorate diabetes in rats (46). The same group later reported the
reversal of hyperglycemia after the administration of
insulin-expressing mMSCs to the liver (47). Similarly, we showed that the
transplantation of ADMSCs overexpressing betatrophin improved
diabetes in mice with STZ-induced diabetes. Betatrophin is a novel
β-cell mitogen reported by Yi et al (21) that enhances endogenous β-cell
replication and improves glucose tolerance and insulin secretion
following hydrodynamic tail injections of plasmids encoding mouse
and human betatrophin into mice with insulin resistance induced by
the insulin receptor antagonist, S961. However, the effects of
betatrophin on human islets were not analyzed (21). Follow-up studies by Jiao et
al (48), which injected S961
in an attempt to increase hepatic betatrophin expression in mice,
demonstrated that despite the induction of vigorous replication in
mouse β-cells transplanted into renal subcapsular tissues of SCID
mice, endogenous betatrophin failed to induce replication in
transplanted human β-cells. In the present study, the impact of
human ADMSCs-BET was analyzed using co-culture experiments with
human islets and in vivo experiments with mice with
STZ-induced diabetes. Exposure to ADMSCs-BET increased β-cell
proliferation and insulin levels compared to the control. This
findings is different from that reported in the study by Cox et
al (49), in which tail vein
injections of betatrophin DNA into mice of different ages did not
alter β-cell proliferation. It is possible that the inconsistent
responses of human β-cells to elevated betatrophin may be due to
the models employed (i.e., S961-induced insulin resistance vs.
STZ-induced diabetes), particularly since S961-induced insulin
resistance may be transient and temporary. In addition, the
proliferative capability of the islets may vary among mice of
different age groups, which may bias the results. Finally, while
increased β-cell proliferation in response to betatrophin may
represent the underlying mechanism through which it impacts β-cell
activity, further studies are necessary to fully elucidate the
impact of betatrophin overexpression in human β-cells.
MSCs were initially thought to promote tissue
regeneration via direct cell replacement given their capacity to
differentiate into a variety of mesenchymal cell types (50). Indeed, autologous MSC
transdifferentiation to functional β-cells has been thought to have
the potential to overcome the limitation of the source and the
viability of transplanted islets (51). Most protocols applied to induce
MSCs along the β-cell lineage by adding a combination of soluble
factors known to influence pancreatic development to the culture
medium. The combined suppression or overexpression of certain
transcriptional factors was also reported to cause the
differentiation of bone marrow MSCs into insulin-producing cells
(52,53), suggesting that genetic
modifications of MSCs could be performed in vitro to improve
MSC efficacy. This is similar to the present study in which
ADMSC-BET transplantation induced the ratio of β-cells per islet,
as well as the number of insulin-producing cells over the control
and ADMSC groups. Two reasons may explain the increased ratio in
the experimental groups: i) an immunoregulatory effect of MSCs that
may alleviate islet inflammation, reducing β-cell apoptosis and ii)
β-cell proliferation by betatrophin (54). However, further studies are
necessary to determine the mechanisms involved.
In the present study, the expression of both Ngn3
and Pax6 was increased in the ADMSCs + islet and ADMSCs-BET + islet
groups. Ngn3 is a marker for islet progenitor cells, and both Ngn3
and Pax6 are important transcription factors in islet development
and differentiation (55,56). In addition, Pax6 is important for
α-cell differentiation and maturation, which regulates glucagon at
the transcriptional level (57,58). Importantly, under conditions that
induce extreme β-cell loss, α-cells can convert to β-cells
(59,60). The increased Ngn3 and Pax6
expression in the ADMSCs + islet and ADMSCs-BET + islet groups
suggests that the ADMSCs may have differentiated toward islet
cells. Furthermore, the expression level of Pax6 in ADMSCs-BET +
islet group was significantly lower than that in the ADMSCs + islet
group, which may be due to differentiation towards β-cells driven
by betatrophin.
Over the past decade, accumulating evidence has
indicated that in addition to their role as building blocks for new
β-cells, MSCs ameliorate hyperglycemia in diabetic subjects via
their immunomodulatory (41)
properties by secreting a diverse array of paracrine factors
(61,62). In the present study, islets
cultured with ADMSCs-BET had increased IL-4, IL-10 and IL-13 levels
and reduced TNF-α and NCP1 levels. We hypothesize that the
additional benefits observed with betatrophin overexpression may
result from the combination of betatrophin and growth factors
secreted by MSCs and/or from the immunomodulatory and
anti-apoptotic properties of the ADMSCs. The anti-inflammatory
capacity of the ADMSCs observed in the present study was consistent
with a previous study by Kuo et al (63), which showed that ADMSCs
accelerated diabetic wound healing by reducing the pro-inflammatory
environment and increasing growth factor expression.
Wang et al (64) reported that human-derived MSCs can
localize to the liver and pancreas following their transplantation
into rats with STZ-induced T1DM and may not cause immune rejection.
In addition to animal models of diabetes, human-derived MSC
transplantation has also been conducted in a rat meniscus injury
model (65), as well as other
models (66), and these studies
suggest that it does not cause immune rejection, which may be due
to the low antigenicity of MSCs. Furthermore, as autoimmunity, a
key factor in T1DM pathogenesis, may not be observed in severe
combined immunodeficient mice (SCID) mice, neither SCID mice nor
immunosuppressants were used in the present study.
In conclusion, combining cell transplantation with
gene therapy may represent a useful therapeutic tool for the
treatment of diabetes. Specifically, betatrophin-overexpressing
ADMSCs increased human islet viability and β-cell insulin secretion
in vitro and in vivo, which may be mediated by the
enhanced anti-inflammatory and anti-apoptotic potential of ADMSCs
with betatrophin overexpression.
Abbreviations:
|
ADMSCs
|
adipose-derived mesenchymal stem
cells
|
|
bFGF
|
basic fibroblast growth factor
|
|
Bcl-xL
|
B-cell lymphoma-extra large, Bcl-2,
B-cell lymphoma 2
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
EGF
|
epidermal growth factor
|
|
GSIS
|
glucose- stimulated insulin
secretion
|
|
HGF
|
hepatocyte growth factor
|
|
IGF-1
|
insulin-like growth factor-1
|
|
MSCs
|
mesenchymal stem cells
|
|
MOI
|
multiplicity of infection
|
|
PI
|
propidium iodide
|
|
SD
|
standard deviation
|
|
STZ
|
streptozotocin
|
|
T2D
|
type 2 diabetes
|
|
T1D
|
type 1 diabetes
|
|
XIAP
|
X-linked inhibitor of apoptosis
protein
|
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81100585, 81100586,
81170718, 81270890, 81300672 and 81301722), the Natural Science
Foundation of Shanghai Municipality, China (nos. 11nm0504200,
13ZR1410300 and 13ZR1414700), the Youth Foundation of Shanghai
Municipal Health Bureau, China (nos. 20114Y115 and 2012J022A), and
the Scientific Research Foundation for Junior Teachers of Medicine
in the Second Military Medical University, China (no.
2011QN20).
References
|
1
|
Shaw JE, Sicree RA and Zimmet PZ: Global
estimates of the prevalence of diabetes for 2010 and 2030. Diabetes
Res Clin Pract. 87:4–14. 2010. View Article : Google Scholar
|
|
2
|
Atkinson MA and Eisenbarth GS: Type 1
diabetes: New perspectives on disease pathogenesis and treatment.
Lancet. 358:221–229. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nyenwe EA, Jerkins TW, Umpierrez GE and
Kitabchi AE: Management of type 2 diabetes: Evolving strategies for
the treatment of patients with type 2 diabetes. Metabolism.
60:1–23. 2011. View Article : Google Scholar
|
|
4
|
Mishra PK, Singh SR, Joshua IG and Tyagi
SC: Stem cells as a therapeutic target for diabetes. Front Biosci
(Landmark Ed). 15:461–477. 2010. View
Article : Google Scholar
|
|
5
|
Couri CE and Voltarelli JC: Stem
cell-based therapies and immunomodulatory approaches in newly
diagnosed type 1 diabetes. Curr Stem Cell Res Ther. 6:10–15. 2011.
View Article : Google Scholar
|
|
6
|
Ezquer FE, Ezquer ME, Parrau DB, Carpio D,
Yañez AJ and Conget PA: Systemic administration of multipotent
mesenchymal stromal cells reverts hyperglycemia and prevents
nephropathy in type 1 diabetic mice. Biol Blood Marrow Transplant.
14:631–640. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Deans RJ and Moseley AB: Mesenchymal stem
cells: Biology and potential clinical uses. Exp Hematol.
28:875–884. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Conget P, Rodriguez F, Kramer S, Allers C,
Simon V, Palisson F, Gonzalez S and Yubero MJ: Replenishment of
type VII collagen and re-epithelialization of chronically ulcerated
skin after intra-dermal administration of allogeneic mesenchymal
stromal cells in two patients with recessive dystrophic
epidermolysis bullosa. Cytotherapy. 12:429–431. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barry FP and Murphy JM: Mesenchymal stem
cells: Clinical applications and biological characterization. Int J
Biochem Cell Biol. 36:568–584. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tang DQ, Cao LZ, Burkhardt BR, Xia CQ,
Litherland SA, Atkinson MA and Yang LJ: In vivo and in vitro
characterization of insulin-producing cells obtained from murine
bone marrow. Diabetes. 53:1721–1732. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thakkar UG, Trivedi HL, Vanikar AV and
Dave SD: Insulin-secreting adipose-derived mesenchymal stromal
cells with bone marrow-derived hematopoietic stem cells from
autologous and allogenic sources for type 1 diabetes mellitus.
Cytotherapy. 17:940–947. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Heineman FW and Balaban RS: Phosphorus-31
nuclear magnetic resonance analysis of transient changes of canine
myocardial metabolism in vivo. J Clin Invest. 85:843–852. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ben-Ami E, Berrih-Aknin S and Miller A:
Mesenchymal stem cells as an immunomodulatory therapeutic strategy
for autoimmune diseases. Autoimmun Rev. 10:410–415. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maccario R, Podestà M, Moretta A, Cometa
A, Comoli P, Montagna D, Daudt L, Ibatici A, Piaggio G, Pozzi S, et
al: Interaction of human mesenchymal stem cells with cells involved
in alloantigen-specific immune response favors the differentiation
of CD4+ T-cell subsets expressing a
regulatory/suppressive phenotype. Haematologica. 90:516–525.
2005.PubMed/NCBI
|
|
15
|
Rasmusson I, Ringdén O, Sundberg B and Le
Blanc K: Mesenchymal stem cells inhibit lymphocyte proliferation by
mitogens and alloantigens by different mechanisms. Exp Cell Res.
305:33–41. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rasmusson I: Immune modulation by
mesenchymal stem cells. Exp Cell Res. 312:2169–2179. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Phinney DG and Prockop DJ: Concise review:
mesenchymal stem/multipotent stromal cells: the state of
transdifferentiation and modes of tissue repair - current views.
Stem Cells. 25:2896–2902. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Caplan AI and Dennis JE: Mesenchymal stem
cells as trophic mediators. J Cell Biochem. 98:1076–1084. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang R: Lipasin, a novel
nutritionally-regulated liver-enriched factor that regulates serum
triglyceride levels. Biochem Biophys Res Commun. 424:786–792. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Quagliarini F, Wang Y, Kozlitina J,
Grishin NV, Hyde R, Boerwinkle E, Valenzuela DM, Murphy AJ, Cohen
JC and Hobbs HH: Atypical angiopoietin-like protein that regulates
ANGPTL3. Proc Natl Acad Sci USA. 109:19751–19756. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yi P, Park JS and Melton DA: Betatrophin:
A hormone that controls pancreatic β cell proliferation. Cell.
153:747–758. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y, Quagliarini F, Gusarova V, Gromada
J, Valenzuela DM, Cohen JC and Hobbs HH: Mice lacking ANGPTL8
(Betatrophin) manifest disrupted triglyceride metabolism without
impaired glucose homeostasis. Proc Natl Acad Sci USA.
110:16109–16114. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen X, Lu P, He W, Zhang J, Liu L, Yang
Y, Liu Z, Xie J, Shao S, Du T, et al: Circulating betatrophin
levels are increased in patients with type 2 diabetes and
associated with insulin resistance. J Clin Endocrinol Metab.
100:E96–E100. 2015. View Article : Google Scholar
|
|
24
|
Gómez-Ambrosi J, Pascual E, Catalán V,
Rodríguez A, Ramírez B, Silva C, Gil MJ, Salvador J and Frühbeck G:
Circulating betatrophin concentrations are decreased in human
obesity and type 2 diabetes. J Clin Endocrinol Metab.
99:E2004–E2009. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kugelberg E: Diabetes: Betatrophin -
inducing β-cell expansion to treat diabetes mellitus? Nat Rev
Endocrinol. 9:3792013. View Article : Google Scholar
|
|
26
|
Izadpanah R, Joswig T, Tsien F, Dufour J,
Kirijan JC and Bunnell BA: Characterization of multipotent
mesenchymal stem cells from the bone marrow of rhesus macaques.
Stem Cells Dev. 14:440–451. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sekiya I, Colter DC and Prockop DJ: BMP-6
enhances chondrogenesis in a subpopulation of human marrow stromal
cells. Biochem Biophys Res Commun. 284:411–418. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yeung TY, Seeberger KL, Kin T, Adesida A,
Jomha N, Shapiro AM and Korbutt GS: Human mesenchymal stem cells
protect human islets from pro-inflammatory cytokines. PLoS One.
7:e381892012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Isaac R, Boura-Halfon S, Gurevitch D,
Shainskaya A, Levkovitz Y and Zick Y: Selective serotonin reuptake
inhibitors (SSRIs) inhibit insulin secretion and action in
pancreatic β cells. J Biol Chem. 288:5682–5693. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bivalacqua TJ, Usta MF, Kendirci M,
Pradhan L, Alvarez X, Champion HC, Kadowitz PJ and Hellstrom WJ:
Superoxide anion production in the rat penis impairs erectile
function in diabetes: Influence of in vivo extracellular superoxide
dismutase gene therapy. J Sex Med. 2:187–198. 2005. View Article : Google Scholar
|
|
31
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop DJ and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The International Society for Cellular
Therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kögler G, Sensken S, Airey JA, Trapp T,
Müschen M, Feldhahn N, Liedtke S, Sorg RV, Fischer J, Rosenbaum C,
et al: A new human somatic stem cell from placental cord blood with
intrinsic pluripotent differentiation potential. J Exp Med.
200:123–135. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Niemeyer P, Krause U, Fellenberg J, Kasten
P, Seckinger A, Ho AD and Simank HG: Evaluation of mineralized
collagen and alpha-tricalcium phosphate as scaffolds for tissue
engineering of bone using human mesenchymal stem cells. Cells
Tissues Organs. 177:68–78. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rackham CL, Dhadda PK, Chagastelles PC,
Simpson SJ, Dattani AA, Bowe JE, Jones PM and King AJ:
Pre-culturing islets with mesenchymal stromal cells using a direct
contact configuration is beneficial for transplantation outcome in
diabetic mice. Cytotherapy. 15:449–459. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Donath MY and Shoelson SE: Type 2 diabetes
as an inflammatory disease. Nat Rev Immunol. 11:98–107. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Barshes NR, Wyllie S and Goss JA:
Inflammation-mediated dysfunction and apoptosis in pancreatic islet
transplantation: Implications for intrahepatic grafts. J Leukoc
Biol. 77:587–597. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Biarnés M, Montolio M, Nacher V, Raurell
M, Soler J and Montanya E: Beta-cell death and mass in
syngeneically transplanted islets exposed to short- and long-term
hyperglycemia. Diabetes. 51:66–72. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zaccone P, Phillips J, Conget I, Gomis R,
Haskins K, Minty A, Bendtzen K, Cooke A and Nicoletti F:
Interleukin-13 prevents autoimmune diabetes in NOD mice. Diabetes.
48:1522–1528. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kaminski A, Kaminski ER and Morgan NG:
Pre-incubation with interleukin-4 mediates a direct protective
effect against the loss of pancreatic beta-cell viability induced
by proinflammatory cytokines. Clin Exp Immunol. 148:583–588. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jiang R, Han Z, Zhuo G, Qu X, Li X, Wang
X, Shao Y, Yang S and Han ZC: Transplantation of placenta-derived
mesenchymal stem cells in type 2 diabetes: A pilot study. Front
Med. 5:94–100. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Abdi R, Fiorina P, Adra CN, Atkinson M and
Sayegh MH: Immunomodulation by mesenchymal stem cells: A potential
therapeutic strategy for type 1 diabetes. Diabetes. 57:1759–1767.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhu YG, Qu JM, Zhang J, Jiang HN and Xu
JF: Novel interventional approaches for ALI/ARDS: Cell-based gene
therapy. Mediators Inflamm. 2011:5601942011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Devaney J, Contreras M and Laffey JG:
Clinical review: gene-based therapies for ALI/ARDS: where are we
now? Crit Care. 15:2242011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nayak S and Herzog RW: Progress and
prospects: Immune responses to viral vectors. Gene Ther.
17:295–304. 2010. View Article : Google Scholar
|
|
45
|
Raper SE, Chirmule N, Lee FS, Wivel NA,
Bagg A, Gao GP, Wilson JM and Batshaw ML: Fatal systemic
inflammatory response syndrome in a ornithine transcarbamylase
deficient patient following adenoviral gene transfer. Mol Genet
Metab. 80:148–158. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lu Y, Wang Z and Zhu M: Human bone marrow
mesenchymal stem cells transfected with human insulin genes can
secrete insulin stably. Ann Clin Lab Sci. 36:127–136.
2006.PubMed/NCBI
|
|
47
|
Xu J, Lu Y, Ding F, Zhan X, Zhu M and Wang
Z: Reversal of diabetes in mice by intrahepatic injection of
bone-derived GFP-murine mesenchymal stem cells infected with the
recombinant retrovirus-carrying human insulin gene. World J Surg.
31:1872–1882. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jiao Y, Le Lay J, Yu M, Naji A and
Kaestner KH: Elevated mouse hepatic betatrophin expression does not
increase human β-cell replication in the transplant setting.
Diabetes. 63:1283–1288. 2014. View Article : Google Scholar :
|
|
49
|
Cox AR, Lam CJ, Bonnyman CW, Chavez J,
Rios JS and Kushner JA: Angiopoietin-like protein 8
(ANGPTL8)/betatrophin overexpression does not increase beta cell
proliferation in mice. Diabetologia. 58:1523–1531. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Volarevic V, Arsenijevic N, Lukic ML and
Stojkovic M: Concise review: Mesenchymal stem cell treatment of the
complications of diabetes mellitus. Stem Cells. 29:5–10. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sun Y, Chen L, Hou XG, Hou WK, Dong JJ,
Sun L, Tang KX, Wang B, Song J, Li H, et al: Differentiation of
bone marrow-derived mesenchymal stem cells from diabetic patients
into insulin-producing cells in vitro. Chin Med J (Engl).
120:771–776. 2007.
|
|
52
|
Li HT, Jiang FX, Shi P, Zhang T, Liu XY,
Lin XW and Pang XN: In vitro reprogramming of rat bone
marrow-derived mesenchymal stem cells into insulin-producing cells
by genetically manipulating negative and positive regulators.
Biochem Biophys Res Commun. 420:793–798. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Guo QS, Zhu MY, Wang L, Fan XJ, Lu YH,
Wang ZW, Zhu SJ, Wang Y and Huang Y: Combined transfection of the
three transcriptional factors, PDX-1, NeuroD1, and MafA, causes
differentiation of bone marrow mesenchymal stem cells into
insulin-producing cells. Exp Diabetes Res.
2012:6720132012.PubMed/NCBI
|
|
54
|
Wang Y, Chen X, Cao W and Shi Y:
Plasticity of mesenchymal stem cells in immunomodulation:
Pathological and therapeutic implications. Nat Immunol.
15:1009–1016. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Laakso M: Not for the eyes only: AX6 and
glucose metabolism. Diabetologia. 52:381–384. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lyttle BM, Li J, Krishnamurthy M, Fellows
F, Wheeler MB, Goodyer CG and Wang R: Transcription factor
expression in the developing human fetal endocrine pancreas.
Diabetologia. 51:1169–1180. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
St-Onge L, Sosa-Pineda B, Chowdhury K,
Mansouri A and Gruss P: Pax6 is required for differentiation of
glucagon-producing alpha-cells in mouse pancreas. Nature.
387:406–409. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Grapp M, Teichler S, Kitz J, Dibaj P,
Dickel C, Knepel W and Krätzner R: The homeodomain of AX6 is
essential for AX6-dependent activation of the rat glucagon gene
promoter: Evidence for a PH0 -like binding that induces an active
conformation. Biochim Biophys Acta. 1789:403–412. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Thorel F, Népote V, Avril I, Kohno K,
Desgraz R, Chera S and Herrera PL: Conversion of adult pancreatic
alpha-cells to beta-cells after extreme beta-cell loss. Nature.
464:1149–1154. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Shen J, Cheng Y, Han Q, Mu Y and Han W:
Generating insulin-producing cells for diabetic therapy: Existing
strategies and new development. Ageing Res Rev. 12:469–478. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
English K: Mechanisms of mesenchymal
stromal cell immunomodulation. Immunol Cell Biol. 91:19–26. 2013.
View Article : Google Scholar
|
|
62
|
Tolar J, Le Blanc K, Keating A and Blazar
BR: Concise review: Hitting the right spot with mesenchymal stromal
cells. Stem Cells. 28:1446–1455. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kuo YR, Wang CT, Cheng JT, Kao GS, Chiang
YC and Wang CJ: Adipose-derived stem cells accelerate diabetic
wound healing through the induction of autocrine and paracrine
effects. Cell Transplant. 25:71–81. 2016. View Article : Google Scholar
|
|
64
|
Wang H, Qiu X, Ni P, Qiu X, Lin X, Wu W,
Xie L, Lin L, Min J, Lai X, et al: Immunological characteristics of
human umbilical cord mesenchymal stem cells and the therapeutic
effects of their transplantion on hyperglycemia in diabetic rats.
Int J Mol Med. 33:263–270. 2014.
|
|
65
|
Horie M, Choi H, Lee RH, Reger RL,
Ylostalo J, Muneta T, Sekiya I and Prockop DJ: Intra-articular
injection of human mesenchymal stem cells (MSCs) promote rat
meniscal regeneration by being activated to express Indian hedgehog
that enhances expression of type II collagen. Osteoarthritis
Cartilage. 20:1197–1207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lin CS, Lin G and Lue TF: Allogeneic and
xenogeneic transplantation of adipose-derived stem cells in
immunocompetent recipients without immunosuppressants. Stem Cells
Dev. 21:2770–2778. 2012. View Article : Google Scholar : PubMed/NCBI
|