Introduction
Metastasis is the leading cause of cancer-related
mortality (1). The regulation of
metastasis by tumor cells does not occur autonomously, but it
instead involves a dynamic crosstalk between tumor cells and host
cells, which is increasingly recognized as a key indicator of
malignant progression (2). There
is a substantial amount of data and information available support
the hypothesis that platelets play a critical role in promoting
hematogenous tumor metastasis. The link between platelets and
cancer progression was first proposed in the mid-19th century by
Trousseau, who diagnosed himself and his patients with excessive
blood clotting caused by an occult carcinoma that led to the
inflammation of blood vessels (3). There are long-term clinical data to
indicate that the platelet count and blood hypercoagulable state
may be important prognostic factors in many types of cancer,
including breast cancer (4),
cervical carcinoma (5,6) and lung cancer (7). In the circulation, platelets
released from megakaryocytes bind to circulating tumor cells,
forming platelet-cell microemboli. This type of microemboli has
been shown to enhance tumor cell migration and motility, allow
tumor cells to evade immune cell monitoring and blood flow shear
destruction and promote angiogenesis (8).
Among the multitude of different signaling molecules
found in the blood, transforming growth factor-β (TGF-β) is known
to aggregate metastasis by promoting epithelial-mesenchymal
transition (EMT) and the invasiveness of primary carcinomas
(9). TGF-β1, an ubiquitous
cytokine, induces cancer cells to proliferate and promotes them to
form an invasive phenotype (10,11). TGF-β ligands are secreted from
cells in three isoforms (TGF-β1, TGF-β2, TGF-β3), with the latency
associated protein (LAP), which makes these isoforms inactive. This
latent TGF-β complex contains another protein known as the latent
binding protein, which assists in the extracellular localization of
the latent complex. TGF-β1 is activated in vivo by the
proteolytic cleavage of LAP at a low pH or from interactions with
other proteins, such as thrombospondins and αvβ6 integrin (12). Active TGF-β1 is released as a
dimer and is involved in numerous regulatory activities that
influence development, tissue repair, immune defense, inflammation
and tumorigenesis (13). Released
TGF-β1 binds to the TGFβRI/II complex and the Smad signaling
pathway is activated by the phosphorylation and activation of
downstream pathways, including mitogen-activated protein kinase
(MAPK) (14), nuclear factor
(NF)-κB (15) and Rho-GTPase
(16), which regulate tumor
extracellular matrix remodeling, inflammatory responses and
angiogenesis that promote tumor metastasis (17,18). Clinical data have indicated that
patients with various types of cancer, such as breast cancer,
prostate cancer and gastric cancer have elevated blood levels of
TGF-β1, and its local expression level has been shown to positively
correlate with tumor size, histological grade and the number of
metastases (19–22). The development of therapies that
target platelet-mediated TGF-β1 in the tumor microenvironment may
provide promising treatments for preventing tumor metastasis.
Chemicals in the diet are increasingly being
recognized as essential factors for cancer chemoprevention and
treatment (23). The
identification of new drugs from plants has a long and successful
history. In particular, traditional Chinese medicine has been
widely used for thousands of years to promote blood circulation for
inhibiting tumor metastasis (24). Diallyl trisulfide (DATS) is a
fat-soluble compound that is the major biological component of
garlic - a commonly used remedy to promote blood circulation
(25). It can be isolated,
purified and obtained by chemical synthesis. Clinical studies have
indicated that garlic has a strong effect on the coagulation system
and can significantly inhibit the induction of platelet activation
and aggregation by regulating a variety of active agents, including
thrombin, adenosine diphosphate (ADP), platelet-activating factor
(PAF) and collagen (26–28). Moreover, the use of garlic as a
chemopreventive agent has gained interest in the field of cancer
prevention and treatment (29,30). Currently, studies on the antitumor
activity of garlic have focused on the inhibition of tumor
proliferation, blocking the cell cycle and inducing apoptosis;
however, there have been relatively fewer investigations carried
out into its role in tumor metastasis (31–34). Despite this situation, there are
some preclinical studies that have indeed demonstrated that animals
administered garlic have exhibited reduced rates of metastasis
(35–37).
In this study, we examined the effects of DATS on
activated platelet-induced tumor metastasis in vitro. Our
data indicates that DATS suppresses breast cancer cells migration
and invasion by inhibiting the release of TGF-β1 in the
platelet-tumor cell system.
Materials and methods
Chemicals and reagents
DATS/DADS/DAS (Helin Co., Ltd., China) was isolated
from garlic extract (Helin Co., Ltd.) with the purity of 97% as
determined by HPLC. It was dissolved at a concentration of 1 M in
100% DMSO as a stock solution, stored at −20°C, and diluted with
culture medium before each experiment to a final DMSO concentration
of 0.1%. IL-15 medium (Gibco, Invitrogen Life Technologies, Inc.,
Carlsbad, CA, USA) was supplemented with 2%
penicillin/streptomycin. Heat-inactivated fetal bovine serum (FBS)
was obtained from Sijiqing Biotech Co., Ltd. (Hanzhou, China).
Human platelets were purchased from Blood Center of Jiangsu, China.
Thromboxane B2 (TXB2) and 6-keto-PGF1α radioimmunoassay (RIA) kits
were purchased from Beijing North Institute of Biological
Technology (Beijing, China). Transwell filter discs (8 µm)
for migration assay by Corning were from Fisher Scientific (Nepean,
ON, Canada). Rat tail collagen was prepared by the Galenical
Pharmacy Institute of Nanjing University of Chinese Medicine,
Nanjing, China. Recombinant human TGF-β1 protein was from PeproTech
(Princeton, NJ, USA) and human TGF-β1 neutralizing antibody was
from R&D Systems (Minneapolis, MN, USA).
Cell culture
The MDA-MB-231 human breast cancer cell line was
obtained from the American Type Culture Collection (ATCC;
Rockville, MD, USA) and was grown to a monolayer culture in IL-15
medium supplemented with 10% heat-inactivated FBS,
penicillin/streptomycin at 37°C with 5% CO2. The cells
were not used >15 to 20 passages after the initiation of
culture.
Blood collection
Freshly drawn venous blood from healthy volunteers
was collected into 130 mM aqueous trisodium citrate anticoagulant
solution (9:1). The donors claimed to not have taken drugs known to
interfere with platelet functions during 2 weeks prior to blood
collection and gave their informed consent. This study was approved
by the Ethics Commitee of Nanjing University of Chinese Medicine.
Citrated blood samples were centrifuged at 150 rpm for 15 min to
obtain platelet-rich plasma, followed by a second centrifugation at
1,500 rpm for 15 min to obtain platelet-poor plasma (used as a
blank value).
Platelet aggregation assay
Dissolved DATS was prepared using a stock solution
of 10 µM DATS with PBS. In the next step, 50 µl of
the stock solution were incubated with 450 µl of
platelet-rich plasma for 5 min. Platelet aggregation was then
measured in a four-channel aggregometer (Chrono-Log Corporation,
Havertown, PA,USA) using the turbidimetric method according to the
manufacturer's instructions. Follwoing 5 min of pre-incubation at
37°C, the platelets were stimulated by the addition of ADP (10
µmol/l), PAF (5 nmol/l) or thrombin (0.1 U). The extent of
platelet aggregation was determined by the area under the
aggregation curve from 0 to 5 min following exposure to the
stimulants, the platelet aggregation rate was expressed as a
percentage of the area value, and the full platelet aggregation was
always expressed as 100%. Due to the fact that DATS exerts a
significant inhibitory effect on PAF-induced platelet aggregation,
we therefore investigated the concentration-response curve of DATS
from 0.01 to 10 µM.
RIA
The washed platelets (3.0×108/ml),
pre-incubated with DATS at 37°C for 30 min, were treated with PAF
(5 nmol/l) for 5 min at 37°C. Incubation was terminated by the
addition of 50 µM indomethacin and 2 mM EDTA, the mixture
was centrifuged at 14,000 × g for 2 min at 4°C and the TXB2 and
6-keto-PGF1α contents of samples were determined using the
[125I]TXB2 and [125I]6-keto-PGF1α RIA
kits.
TGF-β1 ELISA
TGF-β1 levels were detected in the conditioned
medium from tissue culture (40 h), washed platelets or
platelet-rich plasma using the Quantikine TGF-β1 immunoassay kit
(R&D Systems) following the manufacturer's instructions.
Westen blot analysis
Whole-cell lysates were prepared with RIPA buffer
containing protease and phosphatase inhibitors. Nuclear and
cytoplasmic cell extracts were prepared using the NE-PER Nuclear
and Cytoplasmic Extraction kit (Thermo Fisher Scientific Inc.,
Rockford, IL, USA). Equal amounts of cell lysates (50 µg)
were loaded on 8 or 10% SDS-PAGE and transferred onto PVDF
membranes. After the membranes were blocked, they were incubated
with monoclonal antibodies against p-Smad, Smad (1:1,000; Cell
Signaling Technology, Danvers, MA, USA), GPADH (1:5,000; Bioworld
Technology, Louis Park, MN, USA) followed by incubation with
horseradish peroxidase-conjugated IgGs (1:10,000; Bioworld
Technology). Target proteins were developed with an ECL detection
agent (Millipore, Braunschweig, Germany) and visualized with the
ChemiDoc XRS system (Bio-Rad, Hercules, CA, USA).
Wound healing mobility assay
The MDA-MB-231 cells (5×105) were seeded
into a 6-well plate and allowed to grow to a confluent monolayer in
complete medium. The medium was replaced with serum-free medium
containing 1×107 platelets treated with PAF (5 nmol/l)
for 1 h at 37°C. The monolayers were disrupted (i.e., wounded) with
P200 micropipette tips and any cellular debris present was removed
by washing with sterile PBS.
Cell monolayers were then incubated with the medium
containing various concentrations of DATS for 24 h at 37°C. Images
of the exact wound areas were acquired using an inverted microscope
(Zeiss, Jena, Germany) and the number of cells in the scraped zone
of each well was counted at the indicated time points using an
inverted microscope (Zeiss) (0 and 24 h after scraping). The number
of cells in the scraped zone of each well was counted 3 times and
the counts were averaged.
Boyden chamber migration assay
Cell motility was tested in a Transwell Boyden
chamber (Corning Costar, Cambridge, MA, USA) using a polycarbonate
filter (8 µm pores). The MDA-MB-231 cells (3×105)
resuspended in 90 µl IL-15 medium medium containing various
concentrations of DATS were carefully transferred into the upper
chamber. The lower chamber was filled with 600 µl 10% FBS
medium containing 3×107 PAF-activated platelets to
attract cells in the upper chamber. The Transwell Boyden chamber
was then incubated at 37°C for 6 h. After the gentle remo val of
the filter from the chamber, the cells on the upper side of the
filter were removed by wiping with a cotton swab. The filter was
fixed with 5% glutaraldehyde at 40°C for 10 min and stained with
0.1% crystal violet stain solution (c0121; Beyotime, Shanghai,
China). The cells on the lower surface of the filter, which
penetrated the pore of the filter, were fixed onto a glass slide.
Cells in 5 randomly selected microscopic fields (using an inverted
microscope; Zeiss) (magnification, ×400) of the lower surface were
counted. This experiment was performed independently 3 times.
Collagen invasion assay
In vitro invasion assay was performed under
the same conditions as the Transwell chamber motility assay except
that the upper surface of the filter was coated with rat tail
collagen. Rat tail collagen was maintained in a stock solution of 5
mg/ml and stored at -20°C. The rat tail collagen was mixed with 10×
IL-15 medium and 1 M NaOH at a ratio of 1.37:0.22:0.1 at 40°C. A
total of 70 µl of the complex was then added to the upper
chamber and incubated at 37°C for 30 min. An additional 100
µl of IL-15 medium was added to the surface of the collagen
and was incubated at 37°C for a further 30 min, after which the
medium was removed. The MDA-MB-231 cells (3×105) in 90
µl medium treated with various concentrations of DATS were
carefully transferred onto the collagen in the upper chambers. The
lower chamber was filled with 600 µl medium supplemented
with 10% FBS to attract cells in the upper chamber. Following 24 h
of incubation at 37°C, the filter of the chamber was gently removed
and the cells on the upper side of the filter were wiped. The
filter was then fixed with 5% glutaraldehyde at 4°C for 10 min and
stained with 0.1% crystal violet staining solution. The stained
cells were counted in 5 randomly selected microscopic fields
(magnification, ×400). This experiment was performed independently
3 times.
Statistical analysis
The results were analyzed using a two-tailed
Student's t-test using SPSS 11.0 software (Aspire Software
International, Leesburg, VA, USA) and thye results were considered
significant between two samples at a value of P<0.05.
Results
TGF-β1 is critical for activated
platelet-induced metastasis in vitro
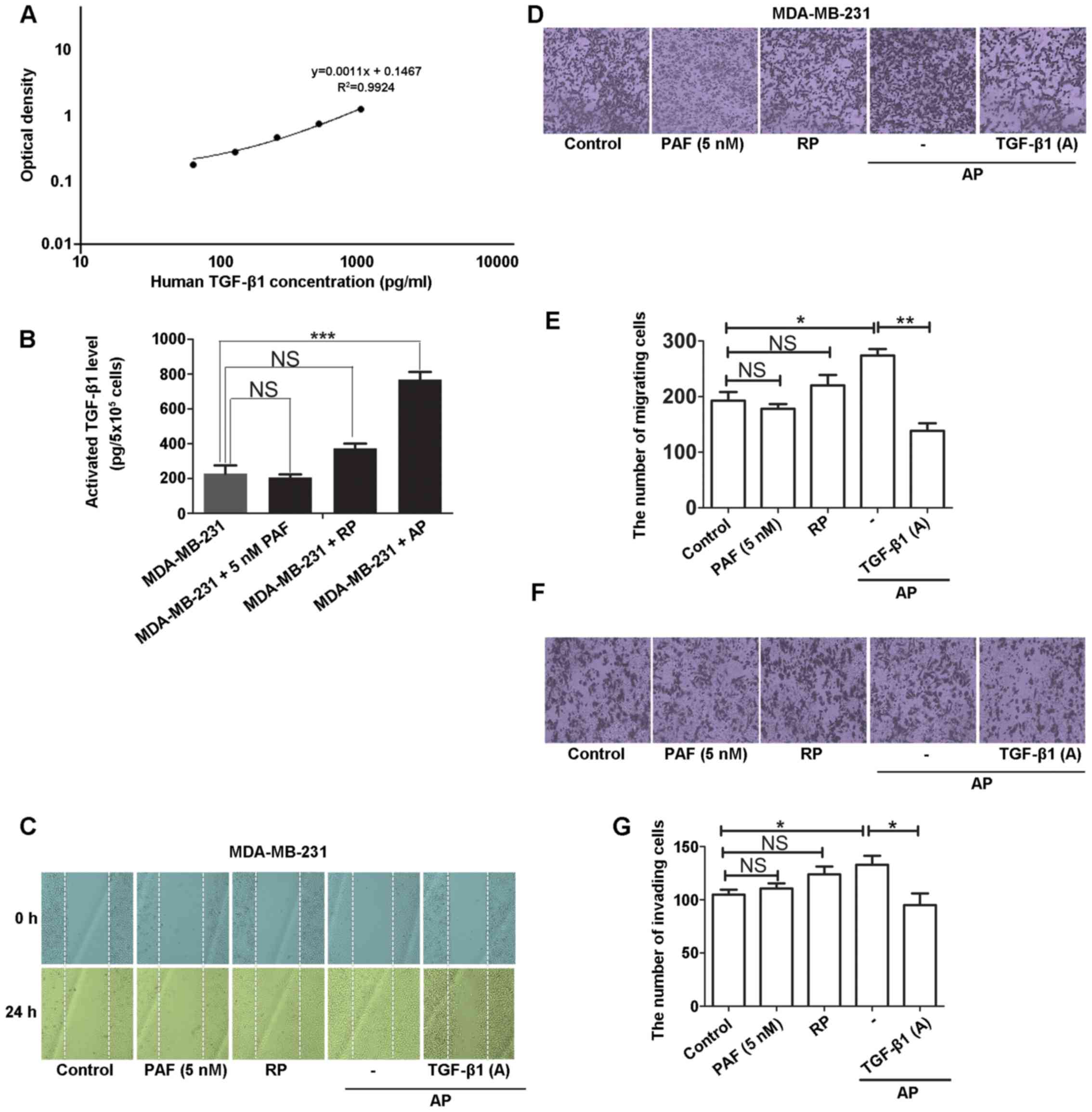
To examine the effects of activated platelets on
tumor cell migration and invasion, we used PAF as a platelet
agonist. The MDA-MB-231 human breast cancer cells were incubated
with activated platelets for 24 h and the levels of TGF-β1 in the
platelet-tumor cell system were measured by ELISA. The results
revealed that the activated platelets rather than PAF or resting
platelets (RP) promoted the release of TGF-β1 in MDA-MB-231 cells
(Fig. 1A and B). Moreover, the
activated platelets induced the horizontal (Fig. 1C) and vertical migration (Fig. 1D and E) and invasion (Fig. 1F and G) of MDA-MB-231 cells, all
of which were attenuated in the presence of the TGF-β1 neutralizing
antibody. These results indicate that only activated platelets have
the potential to trigger the malignant biological behaviors of
tumor cells and the release of TGF-β1 plays an essential role in
facilitating these malignant behaviors in the platelet-tumor cell
system.
Release of TGF-β1 in the activated
platelet-tumor cell system is decreased by DATS
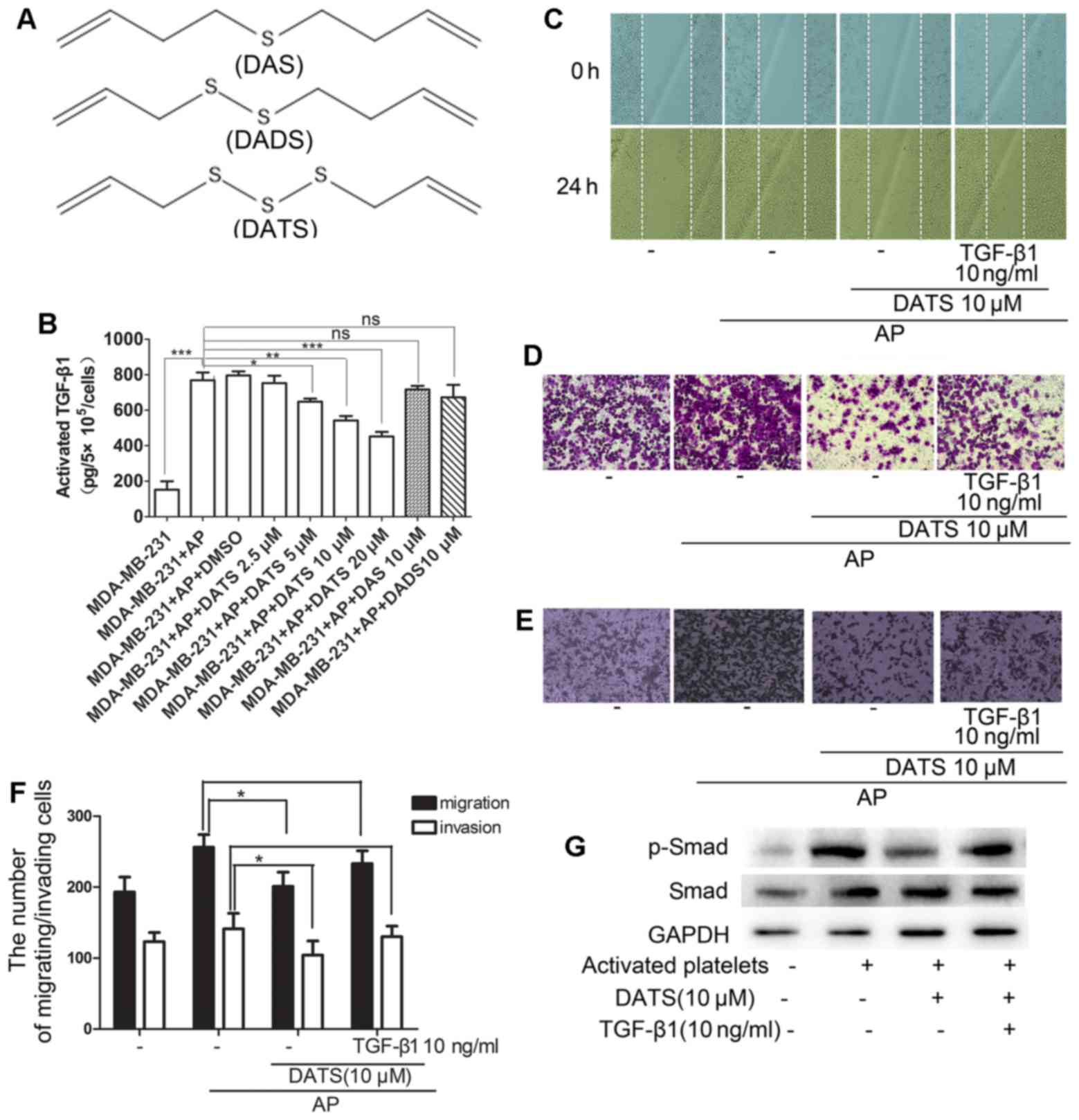
The number of sulfur atoms in allyl-sulfides is an
important factor to determine the chemical and biological
activities of garlic-derived organosulfides. As shown in Fig. 2A, DATS has more sulfur atoms
compared with diallyl sulfide (DAS) and diallyl disulfide (DADS).
In Fig. 1B, we demonstrated that
MDA-MB-231 cells exposed to activated platelets stimulated by PAF
secreted increased pro-metastatic factor TGF-β1. To this end,
various concentrations of DATS (0–20 µM) and 10 µM
DADS/DAS were added to the activated platelet-tumor cell system and
incubated for 24 h at 37°C. It was found that DATS attenuated the
activated TGF-β1 level in the cell culture supernatant in a
dose-dependent manner. However, 10 µM DADS/DAS had no
obvious effect on the release of TGF-β1 (Fig. 2B).
DATS inhibits the activated
platelet-induced migration and invasion of MDA-MB-231 cells by
reducing the release of TGF-β1
Since the level of TGF-β1 was decreased following
treatment with DATS, we hypothesized that the MDA-MB-231 cells
stimulated with the activated platelets would yield a net decrease
in metastatic potential when treated with DATS. Indeed, we found
that 10 µM DATS inhibited the horizontal (Fig. 2C) and vertical migration (Fig. 2D) and invasion (Fig. 2E) of MDA-MB-231 cells induced by
activated platelets using wound healing and Transwell Boyden
chamber assays. Furthermore, the addition of exogenous rTGF-β1
resulted in a reversed effect on the inhibition by DATS (Fig. 2C–F). Of note, it was shown that 10
µM DATS also suppressed the phosphorylation of Smad, a
pivotal molecule of EMT that is closely associated with metastasis,
induced by activated platelets and 10 ng/ml rTGF-β1 reversed this
effect (Fig. 2G).
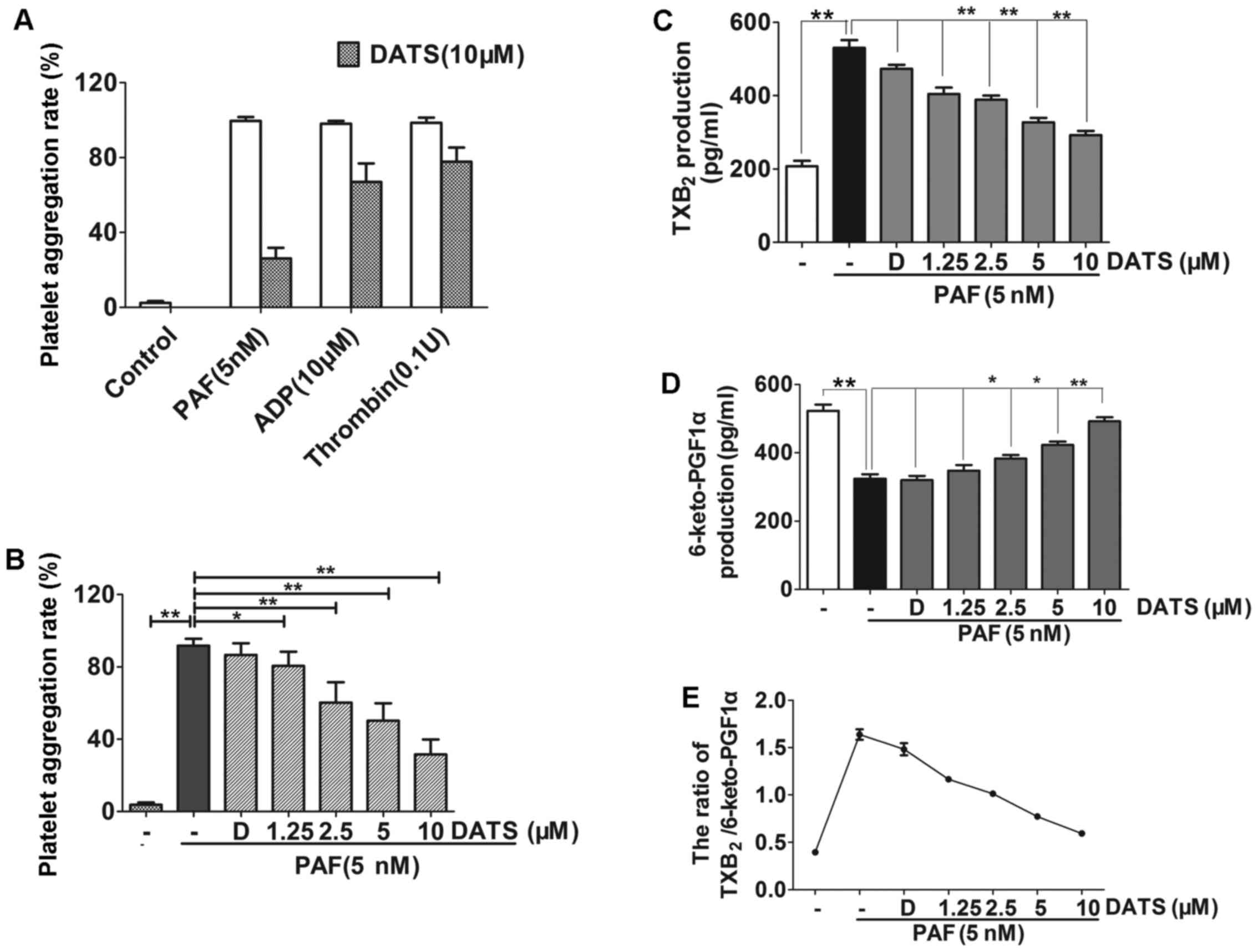
DATS exerts an inhibitory effect on
platelet aggregation and activation
To determine whether DATS influences platelet
function in the blood circulatory system, the platelets were
incubated with various concentrations of DATS while being stirred.
DATS inhibited platelet aggregation in a dose-dependent manner.
Treatments of the cells with 5 and 10 µM DATS with the
platelets for 10 min decreased platelet aggregation by 55 and 65%
(Fig. 3A and B),
respectively.
Thromboxane A2 (TXA2) and prostacyclin I2
(PGI2) are two metabolites that are associated with platelet
activation. An RIA kit was used to examine the effects of DATS on
the levels of TXA2 (measured as TXB2), PGI2 (as 6-keto-PGF1α) and
the thrombogenic ratio (TXB2/6-keto-PGF1α) of platelet excreta in
human platelets activated by PAF. Significant changes in the levels
of TXB2 and 6-keto-PGF1α and the TXB2/6-keto-PGF1α ratio were
observed in the presence of DATS. DATS significantly reduced the
level of TXB2, and increased the level of 6-keto-PGF1α in a
dose-dependent manner, leading to a marked decrease in the
TXB2/6-keto-PGF1α ratio (Fig.
3C–E).
Discussion
Metastasis is a complex multi-step process involving
tumor cell migration and invasion. Accumulating evidence has
indicated that hematogenous metastasis is facilitated by tumor
cell-platelet emboli formation, and the platelet-tumor cell
interaction is considered to be crucial for the process of tumor
metastasis (38–40). In addition, it is commonly
accepted that blood stasis is highly associated with the
progression of tumor metastasis (41). Blood coagulation and tumor
malignant biological behaviors interact bidirectionally, by which
tumor burden is aggregated to supply more procoagulants and in turn
act as strong promoters of cancer growth and spread (42–44).
To enhance the understanding of breast cancer cell
metastasis and the role of platelets therein, we established a
model in which the malignant biological behaviors of MDA-MB-231
cells can be induced by PAF-activated platelets. In this model, we
detected the interaction between platelets and tumor cells and
investigated the key factors that mediate tumor cell migration and
invasion in a tumor cell-activated platelet system. Various
signaling molecules, including TGF-β, P-selectin, VEGF and
angiopoietin that are abundant in platelets, play important roles
in modulating tumor cell motility (45–47). In this study, it was found that
the release of TGF-β1 was markedly increased in the activated
platelet-tumor cell system. More importantly, our data indicated
that the blockage of TGF-β1 resulted in a significant reductions in
the malignant biological behaviors of MDA-MB-231 cells. We
therefore revealed the fact that TGF-β1 is likely to be the
critical molecule that mediates the bidirectional interactions
between tumor cells and platelets. Given the central role of TGF-β1
in the EMT process, we speculate that the downstream signaling of
TGF-β1, including the pivotal transcriptional factors Snail and
Twist may be influenced accordingly and the balance of N-cadherin
and E-cadherin is inclined to be the former in the platelet-tumor
cell system. Collectively, the development of new drugs that not
only inhibit the aggregation and activation of platelets, blocking
the formation of thrombus, but also suppressing the metastasis of
tumor cells is likely to become a novel and potent strategy of
anticancer investigation.
Epidemiological and experimental studies have
provided evidence in support of the association between garlic
intake and reduced cancer risk (29,48). Studies over the past decade have
also shown that garlic has a specific activity in treating
cardiovascular diseases (49),
and the effect correlates with the inhibition of platelet
activation in the circulatory system (50). We thus attempted to elucidate the
effects of a series of garlic organic sulfides (DAS/DADS/DATS) on
the activated platelet-induced metastasis of MDA-MB-231 human
breast cancer cells. DATS is a lipo-soluble compound from garlic
extract with the most sulfur atoms and has been proven to be the
most effective compound among these garlic organic sulfides. Our
results revealed that DATS rather than the other two organic
sulfides decreased the release of TGF-β1 at 10 µM in the
platelet-tumor cell system. We postulated that the sulfur atoms may
be the critical functional group for the antitumor effects of
garlic organic sulfides, which still requires further confirmation.
Of note, a potential study to address the effect of sulfur atom in
tumor progress and platelet activities can be proposed by
synthesizing compounds composed of different numbers of sulfur
atoms. The effects of these compounds on an array of tumor
malignant biological behaviors, including proliferation, migration
and invasion can be evaluated in the presence of activated
platelets.
As a lipophilic compound extracted from garlic, DATS
has been shown to be a novel anticancer agent. Numerous studies
have indicated that DATS has strong anti-proliferative and
pro-apoptotic activities in many cell lines (51–53). DATS-rich garlic oil benefits blood
anti-coagulation factors and further prevents the development of
thrombus. DATS also exhibited the greatest inhibitory effect on
ADP-induced platelet aggregation compared to the other two organic
sulfides in our study. Of note, the P2Y12 receptor,
activated by ADP, exerts great influence on platelet activation by
inducing a number of intracellular signaling events downstream of
the Gi pathway that contribute to fibrinogen receptor
activation. Given the inhibition of ADP-induced platelet
aggregation by DATS, it is reasonable to detect
P2Y12-mediated downstream signaling in the future.
Moreover, since thrombin-induced platelet activation involves the
cleavage of protease-activated receptors (PARs) 1 and 4, it may be
also worthwhile examining whether DATS can regulate PAR signaling
pathways, which may provide us with more detailed indications of
the DATS-mediated inhibitory effect on platelet activation.
Notably, the present study demonstrateed that 10 µM is an
effective dose for DATS, which is consistent with previous studies
(54–56). The results of dose-response
experiments of DATS on platelet activation and aggregation (data
not shown) also confirmed the effective consumption of garlic to
show the impact on platelets.
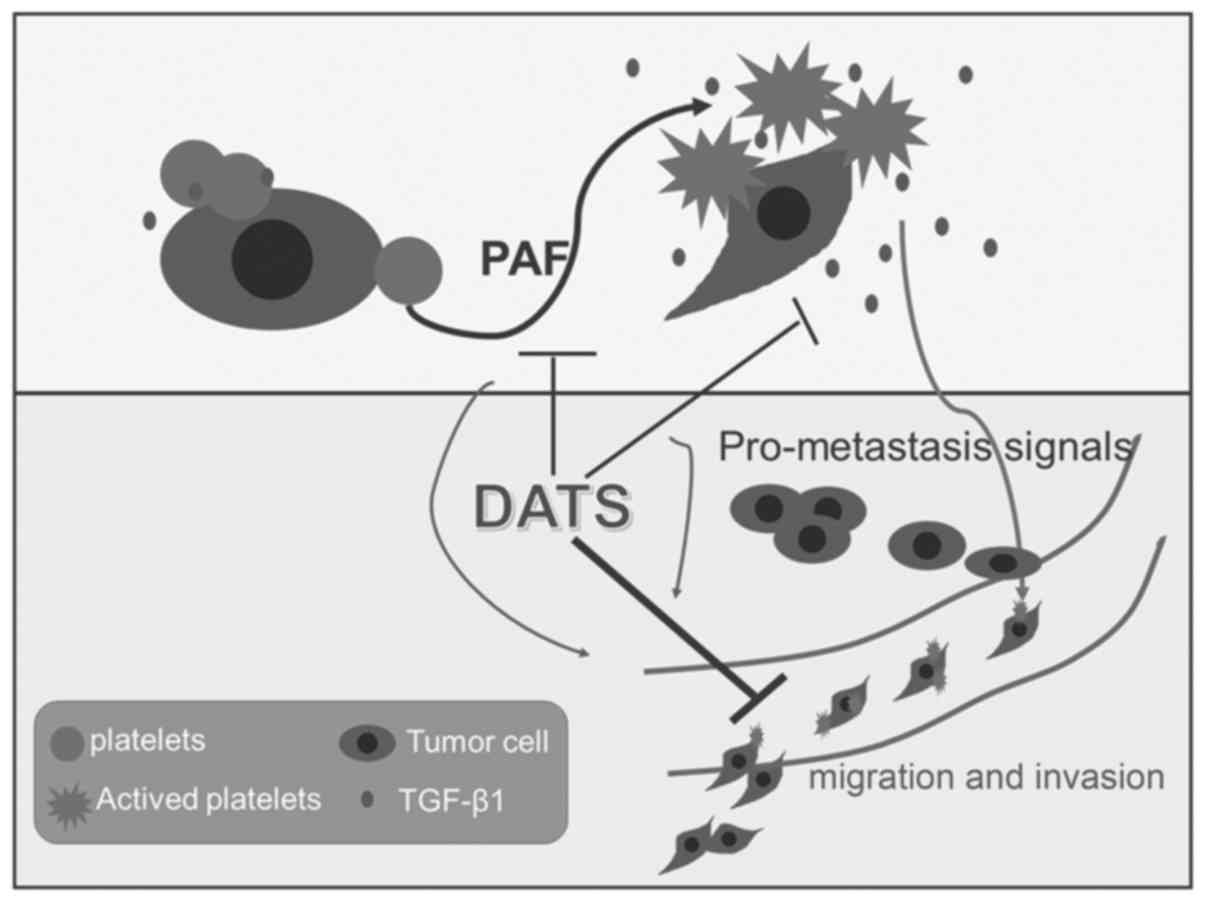
Collectively, our study has indicated that DATS can
act both on platelets and tumor cells, and it exerts great
influence on platelet activities, including reducing platelet
activation and aggregation induced by PAF. It also plays a
significant role in diminishing the release of TGF-β1 from tumor
cells, which can be recognized as the critical step for tumor
hematogenous metastasis (Fig. 4).
This observation is of great importance due to the fact that tumor
progression and platelet aggregation form a vicious circle in the
process of their interactions. They produce synergistic malignant
effects in hematogenous metastasis, which incurs increased
difficulties in the treatment of cancer. To this end, DATS acts as
a potent compound that targets both tumor cells and platelet
activation and aggregation, which to a certain extent indicates an
effective method with which to prevent tumor progression and limit
the interactions between tumor cells and platelets. Taken together,
our study provides definitive evidence that DATS plays a pivotal
role in decreasing platelet activities and reveals a novel
mechanism of this garlic ingredient in inhibiting tumor
hematogenous metastasis.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (nos. 81173174 and 81202655), National
Key Technology Research and Development Program (no. 2008BAI51B02),
Ph.D. Programs Foundation of Ministry of Education of China (no.
20113237110008), Chinese Postdoctoral Science Foundation
(2014M551639), Postdoctoral Science Foundation of Jiangsu Province
(1401138C), Doctoral Innovation Project of Jiangsu Province
(KYLX_0977) and Jiangsu College Graduate Research and Innovation
Projects (no. KYLX_0977; CXZZ13_0627). The funders had no role in
study design, data collection and analysis, decision to publish, or
preparation of the manuscript.
References
|
1
|
Zhang XH: Why cancer cells metastasize?
Med Hypotheses. 80:669–671. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Labelle M and Hynes RO: The initial hours
of metastasis: the importance of cooperative host-tumor cell
interactions during hematogenous dissemination. Cancer Discov.
2:1091–1099. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mezouar S, Mege D, Darbousset R, Farge D,
Debourdeau P, Dignat-George F, Panicot-Dubois L and Dubois C:
Involvement of platelet-derived microparticles in tumor progression
and thrombosis. Semin Oncol. 41:346–358. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stravodimou A and Voutsadakis IA:
Pretreatment thrombocytosis as a prognostic factor in metastatic
breast cancer. Int J Breast Cancer. 2013:2895632013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang PL, Cheng YB and Kuerban G: The
clinical characteristic differences between thrombosis-related
edema and lymphedema following radiotherapy or chemoradiotherapy
for patients with cervical cancer. J Radiat Res (Tokyo).
53:125–129. 2012. View Article : Google Scholar
|
|
6
|
Holmes CE, Levis JE and Ornstein DL:
Activated platelets enhance ovarian cancer cell invasion in a
cellular model of metastasis. Clin Exp Metastasis. 26:653–661.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu JF, Cai L, Zhang XW, Wen YS, Su XD,
Rong TH and Zhang LJ: High plasma fibrinogen concentration and
platelet count unfavorably impact survival in non-small cell lung
cancer patients with brain metastases. Chin J Cancer. 33:96–104.
2014. View Article : Google Scholar :
|
|
8
|
Gupta GP and Massagué J: Platelets and
metastasis revisited: a novel fatty link. J Clin Invest.
114:1691–1693. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Smith AL, Robin TP and Ford HL: Molecular
pathways: targeting the TGF-β pathway for cancer therapy. Clin
Cancer Res. 18:4514–4521. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Perera M, Tsang CS, Distel RJ, Lacy JN,
Ohno-Machado L, Ricchiuti V, Samaranayake LP, Smejkal GB, Smith MG,
Trachtenberg AJ, et al: TGF-beta1 interactome: metastasis and
beyond. Cancer Genomics Proteomics. 7:217–229. 2010.PubMed/NCBI
|
|
11
|
Ma J, Gao HM, Hua X, Lu ZY and Gao HC:
Role of TGF-β1 in human colorectal cancer and effects after
cantharidinate intervention. Asian Pac J Cancer Prev. 15:4045–4048.
2014. View Article : Google Scholar
|
|
12
|
Hyytiäinen M, Penttinen C and Keski-Oja J:
Latent TGF-beta binding proteins: extracellular matrix association
and roles in TGF-beta activation. Crit Rev Clin Lab Sci.
41:233–264. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meindl-Beinker NM, Matsuzaki K and Dooley
S: TGF-β signaling in onset and progression of hepatocellular
carcinoma. Dig Dis. 30:514–523. 2012. View Article : Google Scholar
|
|
14
|
Bakkebø M, Huse K, Hilden VI, Smeland EB
and Oksvold MP: TGF-β-induced growth inhibition in B-cell lymphoma
correlates with Smad1/5 signalling and constitutively active p38
MAPK. BMC Immunol. 11:572010. View Article : Google Scholar
|
|
15
|
Binker MG, Binker-Cosen AA, Gaisano HY, de
Cosen RH and Cosen-Binker LI: TGF-β1 increases invasiveness of
SW1990 cells through Rac1/ROS/NF-κB/IL-6/MMP-2. Biochem Biophys Res
Commun. 405:140–145. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Morita T, Mayanagi T and Sobue K: Dual
roles of myocardin-related transcription factors in epithelial
mesenchymal transition via slug induction and actin remodeling. J
Cell Biol. 179:1027–1042. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wilkins-Port CE, Higgins SP, Higgins CE,
Kobori-Hotchkiss I and Higgins PJ: Complex regulation of the
pericellular proteolytic microenvironment during tumor progression
and wound repair: functional interactions between the serine
protease and matrix metalloproteinase cascades. Biochem Res Int.
2012:4543682012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hawinkels LJ, Verspaget HW, van der
Reijden JJ, van der Zon JM, Verheijen JH, Hommes DW, Lamers CB and
Sier CF: Active TGF-beta1 correlates with myofibroblasts and
malignancy in the colorectal adenoma-carcinoma sequence. Cancer
Sci. 100:663–670. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Donovan MJ and Cordon-Cardo C: Genomic
analysis in active surveillance: predicting high-risk disease using
tissue biomarkers. Curr Opin Urol. 24:303–310. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Joseph JV, Balasubramaniyan V, Walenkamp A
and Kruyt FA: TGF-β as a therapeutic target in high grade gliomas -
promises and challenges. Biochem Pharmacol. 85:478–485. 2013.
View Article : Google Scholar
|
|
21
|
Han H, Cao FL, Wang BZ, Mu XR, Li GY and
Wang XW: Expression of angiogenesis regulatory proteins and
epithelial-mesenchymal transition factors in platelets of the
breast cancer patients. ScientificWorldJournal. 2014:8782092014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pak KH, Kim DH, Kim H, Lee do H and Cheong
JH: Differences in TGF-β1 signaling and clinicopathologic
characteristics of histologic subtypes of gastric cancer. BMC
Cancer. 16:602016. View Article : Google Scholar
|
|
23
|
Surh YJ and Ferguson LR: Dietary and
medicinal antimutagens and anticarcinogens: molecular mechanisms
and chemopreventive potential - highlights of a symposium. Mutat
Res. 523–524:1–8. 2003. View Article : Google Scholar
|
|
24
|
Mousa SA: Antithrombotic effects of
naturally derived products on coagulation and platelet function.
Methods Mol Biol. 663:229–240. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chan KC, Yin MC and Chao WJ: Effect of
diallyl trisulfide-rich garlic oil on blood coagulation and plasma
activity of anticoagulation factors in rats. Food Chem Toxicol.
45:502–507. 2007. View Article : Google Scholar
|
|
26
|
Khatua TN, Adela R and Banerjee SK: Garlic
and cardioprotection: insights into the molecular mechanisms. Can J
Physiol Pharmacol. 91:448–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Allison GL, Lowe GM and Rahman K: Aged
garlic extract and its constituents inhibit platelet aggregation
through multiple mechanisms. J Nutr. 136(Suppl 3): 782S–788S.
2006.PubMed/NCBI
|
|
28
|
Rahman K and Billington D: Dietary
supplementation with aged garlic extract inhibits ADP-induced
platelet aggregation in humans. J Nutr. 130:2662–2665.
2000.PubMed/NCBI
|
|
29
|
Trio PZ, You S, He X, He J, Sakao K and
Hou DX: Chemo-preventive functions and molecular mechanisms of
garlic organosulfur compounds. Food Funct. 5:833–844. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chandra-Kuntal K, Lee J and Singh SV:
Critical role for reactive oxygen species in apoptosis induction
and cell migration inhibition by diallyl trisulfide, a cancer
chemopreventive component of garlic. Breast Cancer Res Treat.
138:69–79. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li Y, Zhang J, Zhang L, Si M, Yin H and Li
J: Diallyl trisulfide inhibits proliferation, invasion and
angiogenesis of osteosarcoma cells by switching on suppressor
microRNAs and inactivating of Notch-1 signaling. Carcinogenesis.
34:1601–1610. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lai KC, Hsu SC, Kuo CL, Yang JS, Ma CY, Lu
HF, Tang NY, Hsia TC, Ho HC and Chung JG: Diallyl sulfide, diallyl
disulfide, and diallyl trisulfide inhibit migration and invasion in
human colon cancer colo 205 cells through the inhibition of matrix
metalloproteinase-2, -7, and -9 expressions. Environ Toxicol.
28:479–488. 2013. View Article : Google Scholar
|
|
33
|
Singh SV, Powolny AA, Stan SD, Xiao D,
Arlotti JA, Warin R, Hahm ER, Marynowski SW, Bommareddy A, Potter
DM and Dhir R: Garlic constituent diallyl trisulfide prevents
development of poorly differentiated prostate cancer and pulmonary
metastasis multiplicity in TRAMP mice. Cancer Res. 68:9503–9511.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xiao D, Herman-Antosiewicz A, Antosiewicz
J, Xiao H, Brisson M, Lazo JS and Singh SV: Diallyl
trisulfide-induced G(2)-M phase cell cycle arrest in human prostate
cancer cells is caused by reactive oxygen species-dependent
destruction and hyperphosphorylation of Cdc 25 C. Oncogene.
24:6256–6268. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shankar S, Chen Q, Ganapathy S, Singh KP
and Srivastava RK: Diallyl trisulfide increases the effectiveness
of TRAIL and inhibits prostate cancer growth in an orthotopic
model: molecular mechanisms. Mol Cancer Ther. 7:2328–2338. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Powolny AA and Singh SV: Multitargeted
prevention and therapy of cancer by diallyl trisulfide and related
Allium vegetable-derived organosulfur compounds. Cancer Lett.
269:305–314. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ng KT, Guo DY, Cheng Q, Geng W, Ling CC,
Li CX, Liu XB, Ma YY, Lo CM, Poon RT, et al: A garlic derivative,
S-allylcysteine (SAC), suppresses proliferation and metastasis of
hepatocellular carcinoma. PLoS One. 7:e316552012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Belloc C, Lu H, Soria C, Fridman R,
Legrand Y and Menashi S: The effect of platelets on invasiveness
and protease production of human mammary tumor cells. Int J Cancer.
60:413–417. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nierodzik ML, Plotkin A, Kajumo F and
Karpatkin S: Thrombin stimulates tumor-platelet adhesion in vitro
and metastasis in vivo. J Clin Invest. 87:229–236. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jurasz P, Alonso-Escolano D and Radomski
MW: Platele-cancer interactions: mechanisms and pharmacology of
tumour cell-induced platelet aggregation. Br J Pharmacol.
143:819–826. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Qian YF and Wang XJ: Effects of
blood-activating and stasis-resolving drugs on tumor formation and
metastasis. J Tradit Chin Med. 29:301–310. 2009. View Article : Google Scholar
|
|
42
|
Gil-Bernabé AM, Ferjancic S, Tlalka M,
Zhao L, Allen PD, Im JH, Watson K, Hill SA, Amirkhosravi A, Francis
JL, et al: Recruitment of monocytes/macrophages by tissue
factor-mediated coagulation is essential for metastatic cell
survival and premetastatic niche establishment in mice. Blood.
119:3164–3175. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Im JH, Fu W, Wang H, Bhatia SK, Hammer DA,
Kowalska MA and Muschel RJ: Coagulation facilitates tumor cell
spreading in the pulmonary vasculature during early metastatic
colony formation. Cancer Res. 64:8613–8619. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
McEachron TA, Pawlinski R, Richards KL,
Church FC and Mackman N: Protease-activated receptors mediate
crosstalk between coagulation and fibrinolysis. Blood.
116:5037–5044. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Battinelli EM, Markens BA and Italiano JE
Jr: Release of angiogenesis regulatory proteins from platelet alpha
granules: modulation of physiologic and pathologic angiogenesis.
Blood. 118:1359–1369. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hara T, Shimizu K, Ogawa F, Yanaba K,
Iwata Y, Muroi E, Takenaka M, Komura K, Hasegawa M, Fujimoto M, et
al: Platelets control leukocyte recruitment in a murine model of
cutaneous arthus reaction. Am J Pathol. 176:259–269. 2010.
View Article : Google Scholar :
|
|
47
|
Xu L, Tong R, Cochran DM and Jain RK:
Blocking platelet-derived growth factor-D/platelet-derived growth
factor receptor beta signaling inhibits human renal cell carcinoma
progression in an orthotopic mouse model. Cancer Res. 65:5711–5719.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fleischauer AT and Arab L: Garlic and
cancer: a critical review of the epidemiologic literature. J Nutr.
131:1032S–1040S. 2001.PubMed/NCBI
|
|
49
|
Ginter E and Simko V: Garlic (Allium
sativum L.) and cardiovascular diseases. Bratisl Lek Listy.
111:452–456. 2010.PubMed/NCBI
|
|
50
|
Allison GL, Lowe GM and Rahman K: Aged
garlic extract inhibits platelet activation by increasing
intracellular cAMP and reducing the interaction of GPIIb/IIIa
receptor with fibrinogen. Life Sci. 91:1275–1280. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li W, Tian H, Li L, Li S, Yue W, Chen Z,
Qi L, Hu W, Zhu Y, Hao B, et al: Diallyl trisulfide induces
apoptosis and inhibits proliferation of A549 cells in vitro and in
vivo. Acta Biochim Biophys Sin (Shanghai). 44:577–583. 2012.
View Article : Google Scholar
|
|
52
|
Watanabe K, Hosono T, Watanabe K,
Hosono-Fukao T, Ariga T and Seki T: Diallyl trisulfide induces
apoptosis in Jurkat cells by the modification of cysteine residues
in thioredoxin. Biosci Biotechnol Biochem. 78:1418–1420. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ma HB, Huang S, Yin XR, Zhang Y and Di ZL:
Apoptotic pathway induced by diallyl trisulfide in pancreatic
cancer cells. World J Gastroenterol. 20:193–203. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sakamoto K, Lawson LD and Milner JA: Allyl
sulfides from garlic suppress the in vitro proliferation of human
A549 lung tumor cells. Nutr Cancer. 29:152–156. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li J, Liu W, Zhao K, Zhang Y, Li X, Yang
Q, Li Z and Li J: Diallyl trisulfide reverses drug resistance and
lowers the ratio of CD133+ cells in conjunction with
methotrexate in a human osteosarcoma drug-resistant cell subline.
Mol Med Rep. 2:245–252. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tsai CY, Wang CC, Lai TY, Tsu HN, Wang CH,
Liang HY and Kuo WW: Antioxidant effects of diallyl trisulfide on
high glucose-induced apoptosis are mediated by the
PI3K/Akt-dependent activation of Nrf2 in cardiomyocytes. Int J
Cardiol. 168:1286–1297. 2013. View Article : Google Scholar : PubMed/NCBI
|