Introduction
Bone homeostasis is controlled by the balance
between resorption and bone formation, mediated by osteoblasts and
osteoclasts (1,2). The excessive bone loss results in
osteoporosis which is a common disease among women worldwide after
menopause (3). However, current
treatments for bone mass recovery are still limited. Osteoblasts
are a specialized subset of cells that play an important role in
bone formation (1). Osteoblasts
originate from mesenchymal stem cells that secrete alkaline
phosphatase (ALP) and bone matrix proteins, including osteopontin
(OPN) and collagen type Iα1 (COL1A1) (4). Targeting osteoblast differentiation
has become a promising therapeutic strategy for osteoporosis.
Osteoblast differentiation is mediated by a variety
of multiple factors, including microRNAs (miRNAs or miRs) (5). miRNAs are a group of small RNAs, ~22
nucleotides in length, which negatively regulate gene expression by
targeting the 3′-untranslated regions (3′-UTR) (6–8).
By post-transcriptionally modulating gene expression, miRNAs can
regulate numerous biological processes, including cell
proliferation, apoptosis and differentiation (6–8).
In recent years, a growing body of evidence has reported that
miRNAs play a critical role in regulating osteoblast
differentiation (9–12). Therefore, targeting osteoblast
differentiation by miRNAs may show great promise for the treatment
of bone loss diseases, such as osteoporosis.
Runt-related transcription factor 2 (Runx2) is a
master transcription factor for controlling osteoblast
differentiation (13–16). The signal transducer and activator
of transcription 1 (STAT1) has been found to be a critical
regulator for Runx2 (17). STAT1
can interact with Runx2 and thus restrain Runx2 in the cytoplasm
leading to the inhibition of osteoblast differentiation (18). STAT1 has been shown to promote
bone resorption in mice (19,20). Interestingly, B cell lymphoma 6
(Bcl6) has been reported as a transcriptional repressor for STAT1
(21). Bcl6 is primarily
expressed in B lymphocytes and plays an important role in
regulating B lymphocyte growth and development (22,23). It has been found that Bcl6
inhibits the expression of STAT1 and thus promotes osteoblast
differentiation (21). Therefore,
Bcl6/STAT1/Runx2 signaling plays an important role in bone
homeostasis targeting which may provide a novel strategy for the
control of osteoblast differentiation.
miR-10b has been suggested as a regulator for cell
differentiation (24,25). A recent study has reported that
miR-10b shows decreased expression during osteoblast
differentiation (26). However,
the precise effect of miR-10b on osteoblast differentiation remains
unknown. In this study, we aimed to investigate the potential role
of miR-10b and the potential underlying mechanism in regulating
osteoblast differentiation. We found that miR-10b was downregulated
during osteoblast differentiation. Overexpression of miR-10b
inhibited osteoblast differentiation, whereas suppression of
miR-10b promoted osteoblast differentiation. Bcl6 was identified as
a target gene of miR-10b in osteoblast differentiation. miR-10b
regulated Bcl6 expression as well as STAT1/Runx2 signaling.
However, the miR-10b suppression-induced effects were partially
reversed by Bcl6 knockdown. Taken together, our study suggests that
miR-10b contributes to osteoblast differentiation through targeting
Bcl6, providing novel insight into understanding the molecular
mechanism underlying osteoblast differentiation and suggesting a
potential target for inhibiting bone loss.
Materials and methods
Cell culture
Pre-osteoblast MC3T3-E1 cells were purchased from
the Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China) and cultured in Alpha Modified Eagle′s Medium
(α-MEM; Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine
serum (FBS; Gibco, Rockville, MD, USA) and 1%
penicillin/streptomycin (Sigma-Aldrich, St. Louis, MO, USA). The
cells were grown in a humidified atmosphere of 5% CO2 at
37°C. To induce osteoblast differentiation, cells were grown in
osteogenic differentiation medium (HyClone, Logan, UT, USA)
supplemented with 10% FBS, 50 µg/ml ascorbic acid and 10 mM sodium
β-glycerophosphate. The medium was refreshed every two days for the
induction of osteoblast differentiation (27).
Quantitative (real-time) polymerase chain
reaction (RT-qPCR)
Total RNAs or miRNAs were extracted by TRIzol
(Invitrogen) or mirVana miRNA isolation kit (Applied Biosystems,
Foster City, CA, USA), respectively. For mRNA detection, total RNAs
were reverse-transcribed into cDNA by M-MLV reverse transcriptase
(Takara, Dalian, China). For miRNA detection, miRNAs were
reverse-transcribed into cDNA by the TaqMan microRNA reverse
transcription kit (Applied Biosystems). qPCR was performed using
Power SYBR-Green PCR Master Mix on an Applied Biosystems AB7500
Real-Time PCR system (both from Applied Biosystems) following the
procedures: 94°C for 5 min, 30 cycles of two-step cycling program
(94°C for 10 sec, 60°C for 20 sec and 72°C for 30 sec), and 72°C
for 10 min. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and
small nuclear RNA U6 served as the internal controls. Relative gene
expression was quantified by using the 2−ΔΔCt method.
The primer sequences were as follows: miR-10b forward,
5′-TACCCTGTAGAACCGAATTTG-3′ and reverse, 3′-GTGCGTGTCGTGGAGTC-5′;
U6 forward, 5′-CGCTTCACGAATTTGCGT-3′ and reverse, 5′-CTCGCTTCG
CAGCACA-3′; Bcl6 forward, 5′-AGACGCACAGTGACAAACCATACA-3′ and
reverse, 5′-CTCCACAAATGTTACAGCGATAGG-3′; ALP forward,
5′-CACCATTTTTAGTACTGGCCATCG-3′ and reverse,
5′-GCTACATTGGTGTTGAGCTTTGG-3′; OPN forward,
5′-TCTCCTTGCGCCACAGAATG-3′ and reverse, 5′-TCCTTAGACTCACCGCTCTT-3′;
COL1A1 forward, 5′-CCCCGGTCAGAGAGGAGAAA-3′ and reverse, 5′-TCC
AGAAGGACCTTGTTTGC-3′; GAPDH forward, 5′-AATGG ATTTGGACGCATTGGT-3′
and reverse, 5′-TTTGCACTGGTACGTGTTGAT-3′.
Transfection
The miR-10b mimics, miR-10b inhibitor and negative
control (NC) were purchased from GenePharma (Shanghai, China) and
transfected into cells using Lipofectamine 2000 (Invitrogen)
according to the manufacturer's protocols. Bcl6 siRNA and NC siRNA
were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA) and transfected into cells as per the recommended methods. The
transfection efficiency was evaluated by RT-qPCR or western blot
analysis.
ALP activity assay
Cells were transfected with miR-10b mimics or
miR-10b inhibitor followed by the induction of osteoblast
differentiation for 6 days. Then, the cells were harvested and
detected by ALP assay kit. Briefly, the cells were lysed in lysis
buffer and the supernatants were collected and incubated with
SensoLyte p-nitrophenylphosphate at 37°C for 30 min. The
absorbance at a wavelength of 405 nm was detected by an
enzyme-linked immunosorbent assay (ELISA) reader (Bio-Rad,
Hercules, CA, USA).
Measurement of matrix mineralization
After the induction of osteoblast differentiation,
cells were harvested and fixed in 70% ethanol for 1 h. Then, cells
were incubated with 40 mM Alizarin Red S solution (Sigma-Aldrich)
for 10 min. The mineral deposits stained by Alizarin Red S were
isolated and dissolved in 0.1 N NaOH. The absorbance at a
wavelength of 540 nm was measured by an ELISA reader (Bio-Rad).
Dual-luciferase reporter assay
Bioinformatics analysis was performed by using
microRNA.org-Targets and Expression
(http://www.microrna.org/) and TargetScan
(http://www.targetscan.org/). The miR-10b
target region of Bcl6 3′-UTR was inserted into a pmirGLO luciferase
vector (Promega, Madison, WI, USA) to obtain wild-type pmirGLO-Bcl6
3′-UTR. Meanwhile, Bcl6 3′-UTR sequences containing the mutant
binding sites for miR-10b were cloned into a pmirGLO luciferase
vector (Promega) to obtain mutant-type pmirGLO-Bcl6 3′-UTR. To
confirm the interaction between miR-10b and Bcl6 3′-UTR, wild-type
or mutant-type pmirGLO-Bcl6 3′-UTR was co-transfected into MC3T3-E1
cells with miR-10b mimics or miR-10b inhibitors. After incubation
for 48 h, the cells were harvested and the relative luciferase
activity was measured by Dual-GLO Luciferase assay system
(Promega).
Western blot analysis
Cytosolic and nuclear fractions were extracted using
the nuclear extraction kit (Beyotime, Haimen, China) according to
the manufacturer's protocols. Briefly, cells were harvested and
washed with phosphate-buffered saline (PBS) followed by
centrifugation at 8,000 × g for 15 min at 4°C. The cell sediments
were treated with buffer A containing 1 mM pheylmethylsulfonyl
fluoride (PMSF) and incubated in an ice bath for 10 min. Afterward,
buffer B was added and incubated for 1 min followed by
centrifugation at 12,000 × g for 15 min at 4°C. The supernatants
containing cytoplasmic fractions were collected. The sediments were
collected and re-suspended in nuclear protein extraction agent and
subjected to an ice bath for 30 min with vortexing at an interval
of 2 min. After centrifugation (12,000 × g for 15 min at 4°C), the
supernatants containing nuclear protein was collected. Protein
concentration was measured by a BCA kit (Beyotime). Equal amounts
of proteins were loaded on 10% sodium dodecyl sulfate
polyacrylamide gels for separation. The separated proteins were
electro-blotted to a polyvinylidene fluoride membrane (Millipore,
Boston, MA, USA). The membrane was blocked with 3% non-fat milk in
Tris-buffered saline containing 0.1% Tween-20 (TBST) for 1 h at
37°C. Then, the membrane was blotted with primary antibodies at
appropriate dilutions at 4°C overnight. After washes with TBST, the
membrane was incubated with horseradish peroxidase-conjugated
secondary antibodies (1:2,000; goat anti-rabbit IgG; sc-2004; Santa
Cruz Biotechnology, Inc.) for 1 h at 37°C. The protein signals were
visualized using Pierce ECL Western Blotting kit (Pierce, Rockford,
IL, USA). Quantitative analysis of the protein bands was performed
by Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville,
MD, USA). The primary antibodies including anti-Bcl6 (sc-368),
anti-STAT1 (sc-346), anti-Runx2 (sc-10758), anti-GAPDH (sc-25778)
and anti-Lamin B (sc-6217) were purchased from Santa Cruz
Biotechnology, Inc.
Data analysis
All data are presented as means ± standard
deviation. The statistical analysis was performed by SPSS version
18.0 (SPSS Inc., Chicago, IL, USA). Differences were assessed by
one-way analysis of variance followed by a Bonferroni correction. A
p-value of <0.05 was regarded as indicative of statistically
significance.
Results
miR-10b is downregulated during
osteoblast differentiation
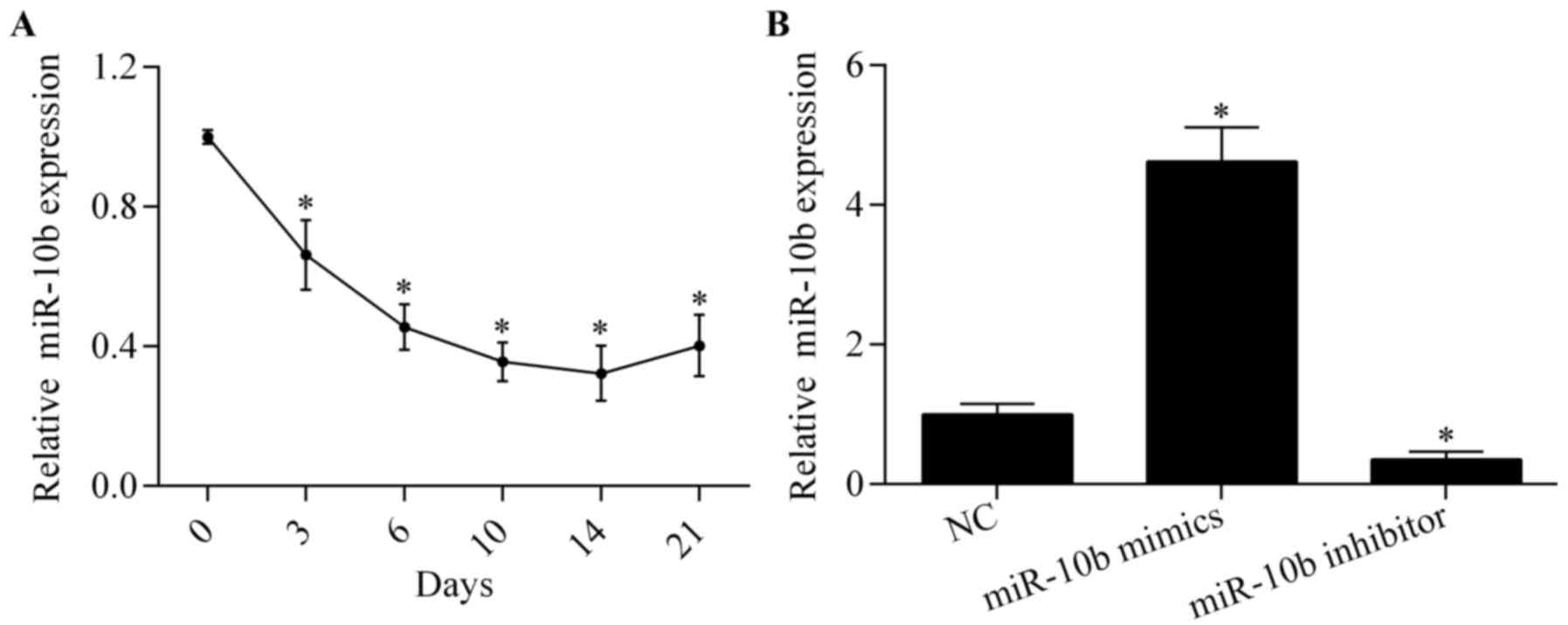
To investigate the potential role of miR-10b in
osteoblast differentiation, we examined the expression pattern of
miR-10b during osteoblast differentiation in MC3T3-E1 cells by
RT-qPCR. The results showed that miR-10b was significantly
downregulated post-osteoblast differentiation (Fig. 1A), indicating a critical role of
miR-10b involved in osteoblast differentiation.
miR-10b regulates osteoblast
differentiation
To explore the exact biological effect of miR-10b on
osteoblast differentiation, miR-10b was overexpressed or suppressed
by transfecting miR-10b mimics or miR-10b inhibitors, respectively
(Fig. 1B). We then examined the
effect of miR-10b overexpression or suppression on osteoblast
differentiation by evaluating ALP activity and matrix
mineralization. The results showed that both ALP activity (Fig. 2A) and matrix mineralization
(Fig. 2B) were markedly repressed
by miR-10b overexpression. Conversely, the suppression of miR-10b
significantly promoted ALP activity (Fig. 2A) and matrix mineralization
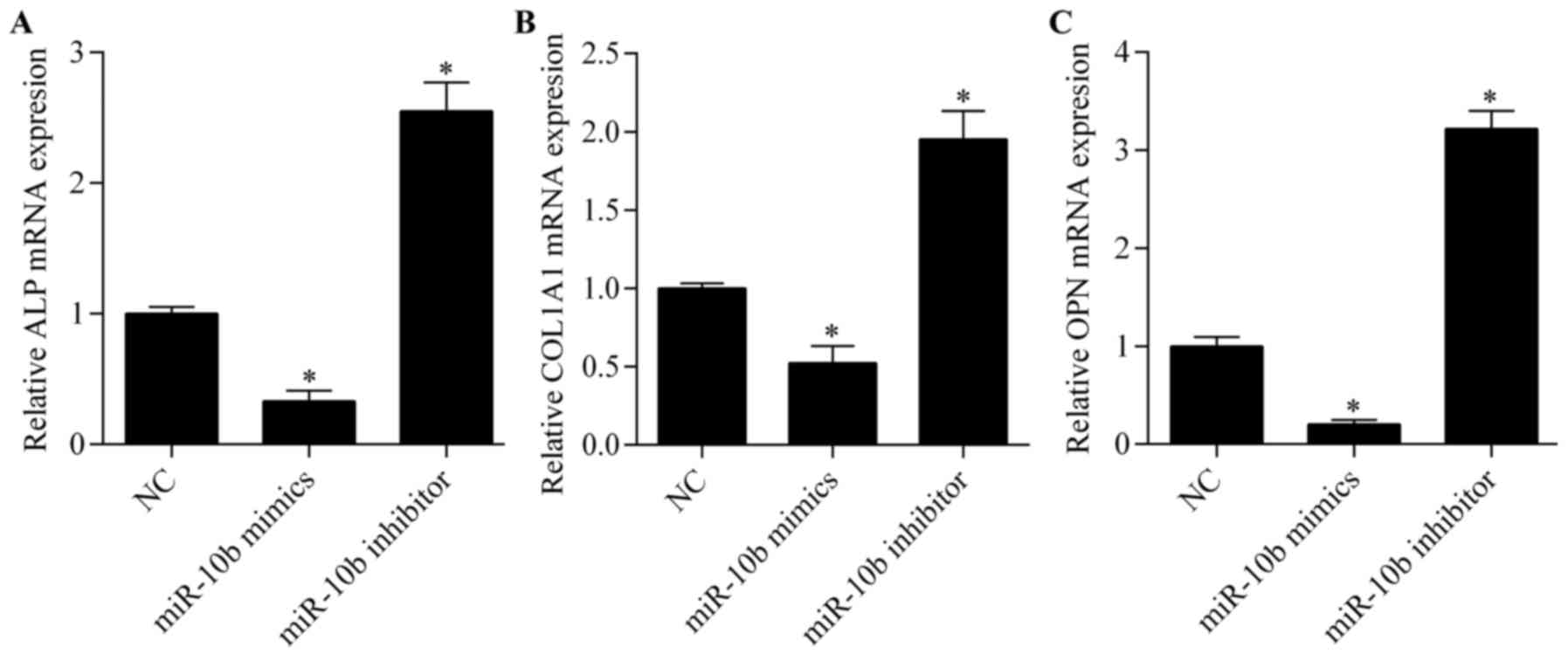
(Fig. 2B). Moreover, we detected
the expression of osteoclast marker genes, including ALP, COL1A1
and OPN by RT-qPCR. We found that the expression of these genes was
significantly suppressed by miR-10b overexpression, while miR-10b
suppression markedly elevated the expression of these genes
(Fig. 3). Overall, these results
suggest that miR-10b suppression promotes osteoblast
differentiation.
Bcl6 is a target gene of miR-10b in
osteoclasts
To investigate the underlying mechanism by which
miR-10b regulates osteoblast differentiation, we predicted the
potential target genes of miR-10b by bioinformatics analysis. Among
these putative target genes, Bcl6, a critical regulator of
osteoblast differentiation (21),
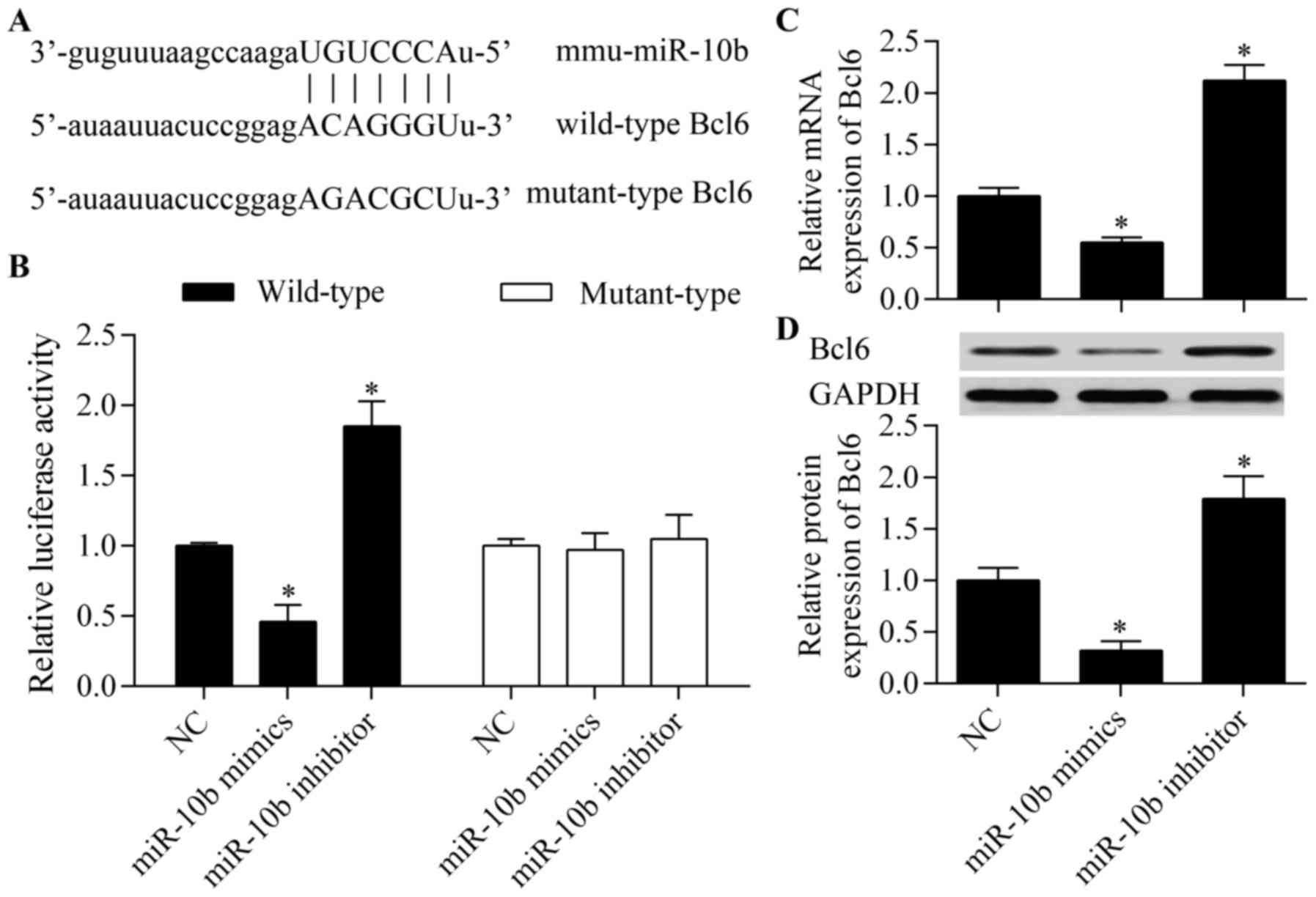
gained our interest for further analysis. The complementary
seed-matched wild-type or mutant-type binding sites between miR-10b
and Bcl6 3′-UTR are described in Fig.
4A. To verify the interaction between miR-10b and Bcl6 3′-UTR,
wild-type or mutant-type pmirGLO-Bcl6 3′-UTR was co-transfected
into MC3T3-E1 cells with miR-10b mimics or miR-10b inhibitor. The
results showed that miR-10b overexpression significantly inhibited
the luciferase reporter activity of wild-type luciferase vector
while miR-10b suppression increased the luciferase reporter
activity (Fig. 4B). However, no
obvious effect of miR-10b overexpression or suppression on
mutant-type luciferase vector was observed (Fig. 4B). These data indicated that
miR-10b directly targeted the 3′-UTR of Bcl6. To further confirm
that this interaction is effective, we then examined the effect of
miR-10b on Bcl6 expression. The results showed that miR-10b
significantly suppressed the mRNA (Fig. 4C) and protein (Fig. 4D) expression of Bcl6, whereas
miR-10b suppression increased Bcl6 expression. Taken together,
these results suggest that Bcl6 is the target of miR-10b in
osteoblasts.
miR-10b regulates STAT1/Runx2
signaling
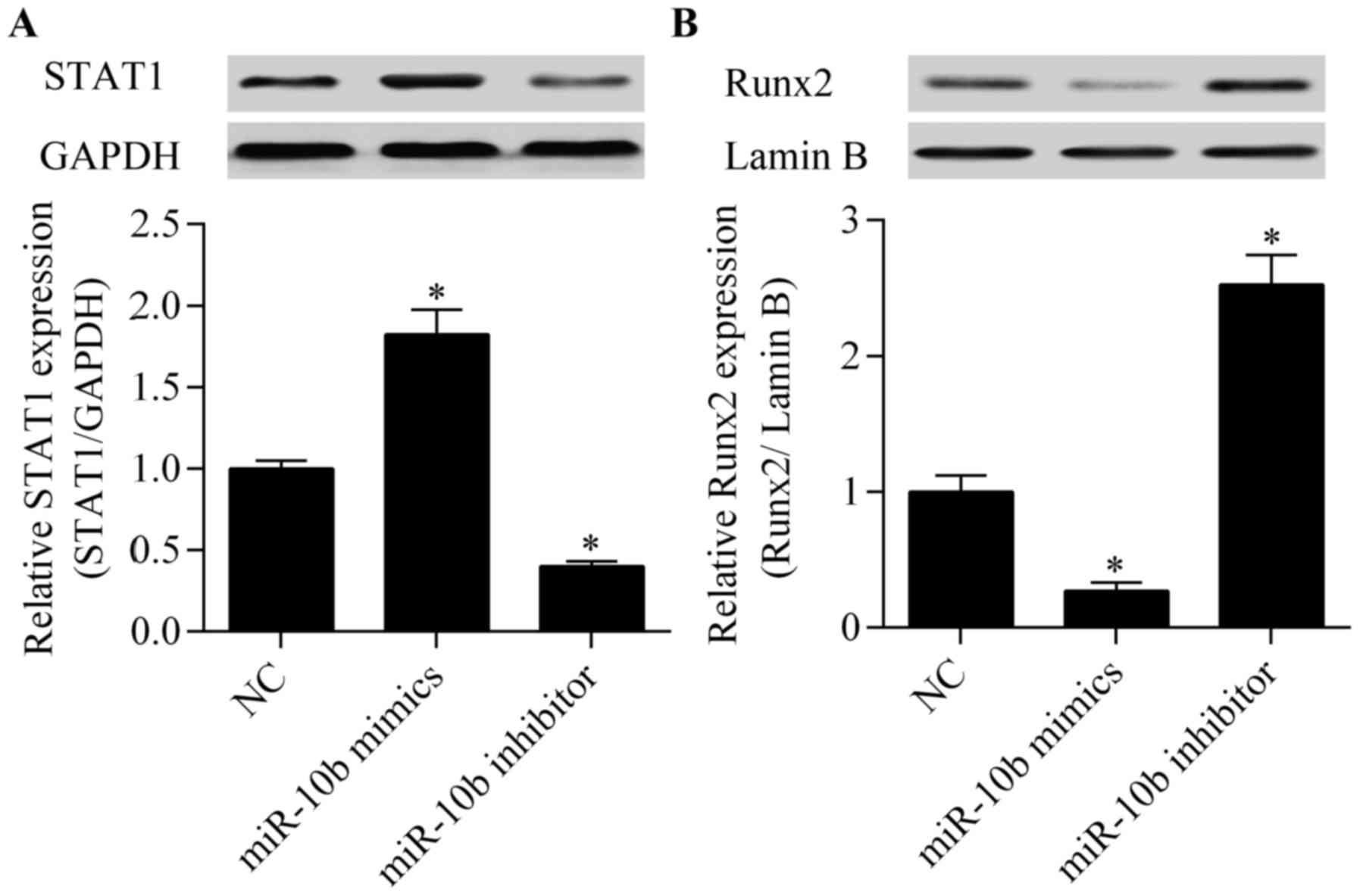
Considering the regulatory effect of miR-10b on Bcl6
expression, we detected the effect of miR-10b on downstream target
genes of Bcl6 involved in osteoblast differentiation. STAT1, which
is an important regulator for osteoblast differentiation (21), has been reported as a target gene
of Bcl6 (21). STAT1 negatively
regulates osteoblast differentiation by repressing Runx2 nuclear
translocation (18). We found
that the overexpression of miR-10b increased while the suppression
of miR-10b decreased STAT1 expression (Fig. 5A). Moreover, the Runx2 nuclear
translocation was significantly blocked by miR-10b overexpression
whereas miR-10b suppression promoted Runx2 nuclear translocation
(Fig. 5B). These results indicate
that miR-10b affects STAT1/Runx2 signaling.
miR-10b regulates osteoblast
differentiation through targeting Bcl6
To confirm that the regulatory effect of miR-10b on
osteoblast differentiation is regulated by targeting Bcl6, we
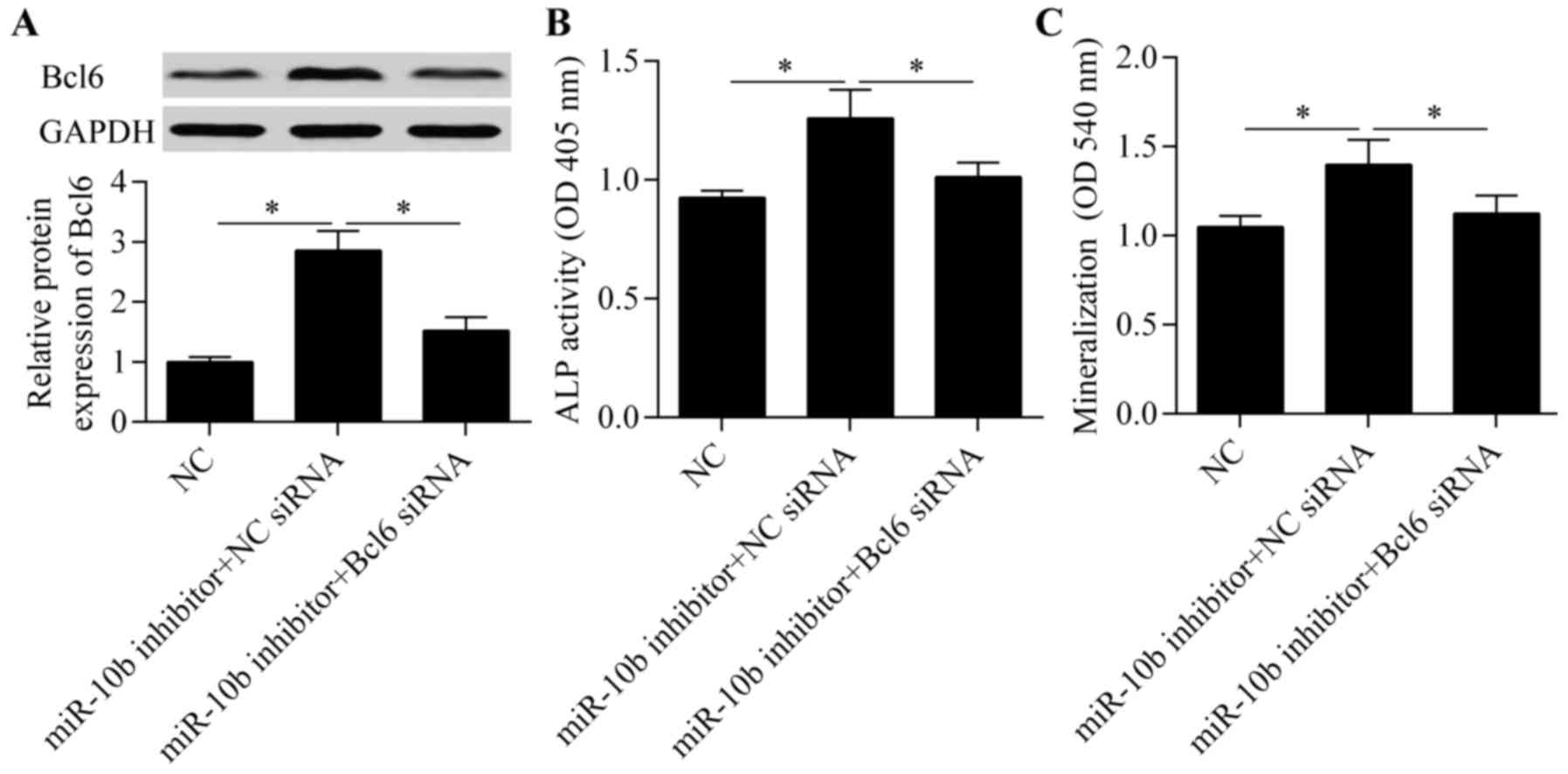
silenced Bcl6 expression along with miR-10b suppression. The
results showed that the promotive effect of miR-10b suppression on
Bcl6 expression was significantly blocked by Bcl6 knockdown
(Fig. 6A). As expected, the
osteoblast differentiation promoted by miR-10b suppression was
apparently abolished by Bcl6 knockdown (Fig. 6B and C). Moreover, the miR-10b
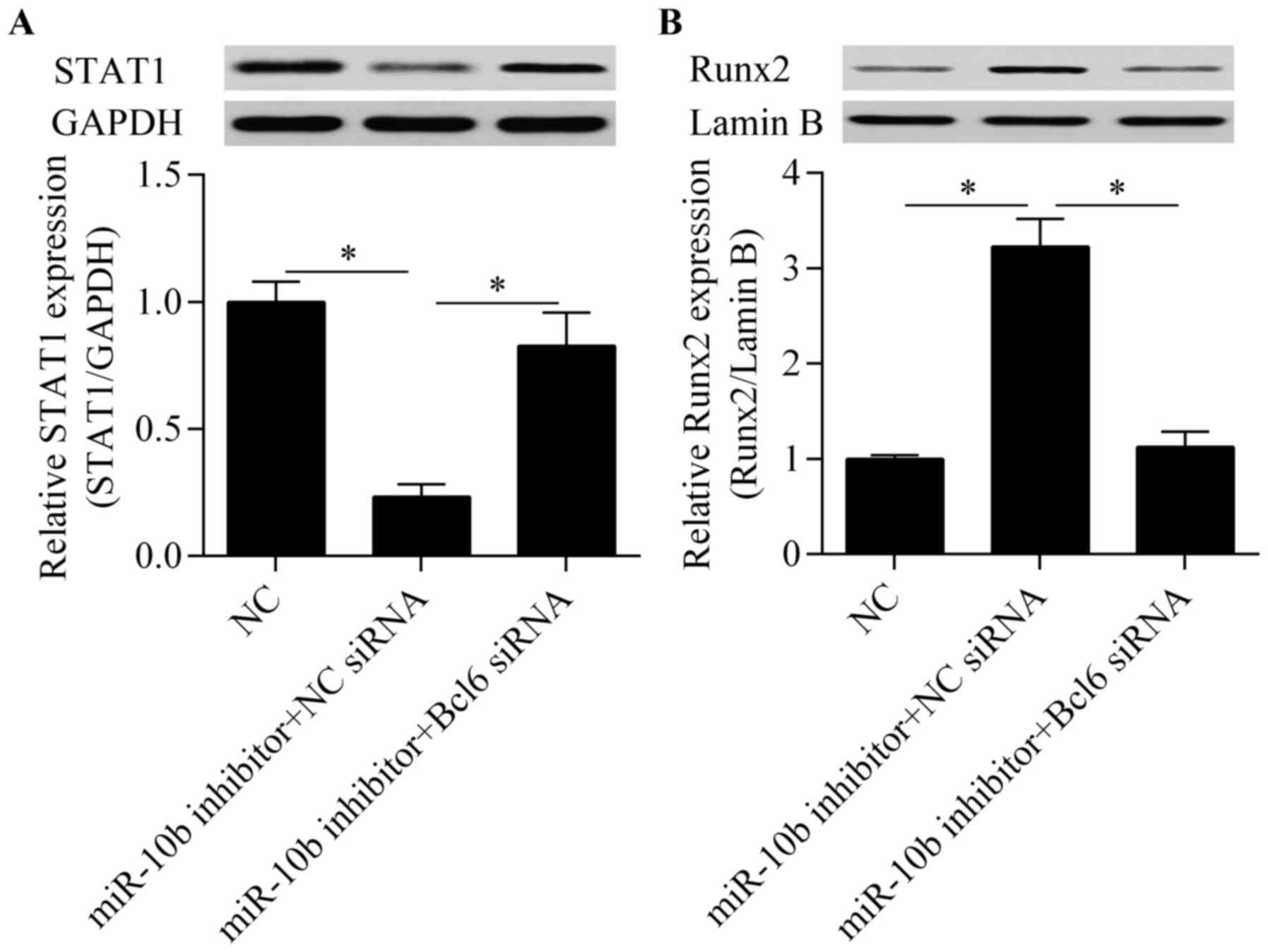
suppression-induced inhibitory effect on STAT1 expression (Fig. 7A) and the promotive effect on
Runx2 nuclear translocation (Fig.
7B) were significantly reversed by Bcl6 knockdown.
Discussion
A growing body of evidence has highlighted the
critical role of miRNAs in bone homeostasis (28). Osteoblasts secrete ALP and bone
matrix proteins to promote bone formation (4). Through targeting critical genes
involved in osteoblast differentiation, miRNAs regulate osteoblast
differentiation (9–12). Targeting osteoblast
differentiation by miRNAs has become a promising therapeutic
strategy for inhibiting bone loss. In this study, we showed that
miR-10b is a novel miRNA involved in regulating osteoblast
differentiation. We delineated that miR-10b regulates osteoblast
differentiation through targeting Bcl6, implying an important role
of miR-10b in bone homeostasis.
miR-10b has been widely studied in cancer (29,30), angiogenesis (31) and embryonic development (32) by focusing on different targets. It
has been found that miR-10b regulates myeloid differentiation and
neuroblastoma cell differentiation (24,25). Okamoto et al found that
miR-10b was significantly downregulated during osteoblast
differentiation (26). In line
with these findings, our results also showed decreased miR-10b
expression during osteoblast differentiation. Functional
experiments demonstrated that the overexpression of miR-10b
suppressed osteoblast differentiation while the suppression of
miR-10b promoted osteoblast differentiation. Our results suggest
that miR-10b is an osteoblast differentiation-related miRNA.
However, the underlying mechanism needs to be investigated.
To investigate the underlying mechanism by which
miR-10b regulates osteoblast differentiation, we aimed to identify
the functional target of miR-10b. Through bioinformatics analysis,
we found that Bcl6 is a putative target gene of miR-10b. Bcl6 has
been reported to positively regulate osteoblast differentiation
(21). Bcl6 is a transcriptional
repressor primarily expressed in B lymphocytes and regulates B
lymphocyte growth and development (22,23). Bcl6 participates in the regulation
of B-cell lymphomas and numerous types of human cancer (33,34). Bcl6 also regulates T follicular
helper cell differentiation (35,36) and germinal center formation
(37,38). A previous study showed that Bcl6
inhibits osteoclast differentiation (39). Bcl6 suppresses the expression of
nuclear factor of activated T cells c1 (NFATc1) which promotes
osteoclast differentiation (40–42). Importantly, Bcl6 also participates
in osteoblast differentiation (21). It has been reported that Bcl6
promotes osteoblast differentiation through the transcriptional
repression of STAT1 (21). STAT1
is a negative regulator of osteoblast differentiation (18–20). The lack of STAT1 promotes bone
formation and bone mass (18).
STAT1 inhibits osteoblast differentiation by blocking Runx2 nuclear
translocation (18). Targeting
the inhibition of STAT1 by various agents showed a promotive effect
on osteoblast differentiation (43–46). In this study, we demonstrated that
inhibition of STAT1 by miR-10b suppression-induced Bcl6 promoted
Runx2 nuclear translocation and osteoblast differentiation,
indicating a potential strategy for the control of osteoblast
differentiation by targeting STAT1.
Several studies have reported that Bcl6 is targeted
by various miRNAs (47,48). Bcl6 has been reported to be
targeted by miR-155 in macrophages involved in atherosclerosis
(49). miR-127 regulates breast
cancer cell proliferation and senescence by targeting Bcl6
(50). miR-187 suppresses lung
cancer development by targeting Bcl6 (51). Consistently, the targeting of Bcl6
by miR-187 also functions in regulating diffuse large B-cell
lymphoma cell apoptosis (52).
Here, our study for the first time reported that miR-10b is a novel
miRNA that targets and modulates Bcl6 expression in osteoblasts.
Taken together, these findings suggest that Bcl6 undergoes
epigenetic regulation in various cell types and pathological
processes.
Overall, this study showed that miR-10b participates
in osteoblast differentiation by targeting Bcl6 and STAT1/Runx2
signaling. Our findings provide novel insight into understanding
the molecular mechanism of osteoblast differentiation. miR-10b has
great potential to serve as an effective target for bone
formation.
Glossary
Abbreviations
Abbreviations:
|
miRNAs
|
microRNAs
|
|
UTR
|
untranslated regions
|
|
Bcl6
|
B cell lymphoma 6
|
|
Runx2
|
Runt-related transcription factor
2
|
|
STAT1
|
signal transducer and activator of
transcription 1
|
|
RT-qPCR
|
real-time quantitative polymerase
chain reaction
|
|
ALP
|
alkaline phosphatase
|
|
OPN
|
osteopontin
|
|
COL1A1
|
collagen type Iα1
|
References
|
1
|
Franceschi RT: The developmental control
of osteoblast-specific gene expression: Role of specific
transcription factors and the extracellular matrix environment.
Crit Rev Oral Biol Med. 10:40–57. 1999. View Article : Google Scholar
|
|
2
|
Park H, Noh AL, Kang JH, Sim JS, Lee DS
and Yim M: Peroxiredoxin II negatively regulates
lipopolysaccharide-induced osteoclast formation and bone loss via
JNK and STAT3. Antioxid Redox Signal. 22:63–77. 2015. View Article : Google Scholar :
|
|
3
|
Diddle AW and Smith IQ: Postmenopausal
osteoporosis: The role of estrogens. South Med J. 77:868–874. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Canalis E, Economides AN and Gazzerro E:
Bone morphogenetic proteins, their antagonists, and the skeleton.
Endocr Rev. 24:218–235. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang C, Geng J and Jiang S: MicroRNAs in
regulation of osteogenic differentiation of mesenchymal stem cells.
Cell Tissue Res. Jul 18–2016.Epub ahead of print.
|
|
6
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vishal M, Vimalraj S, Ajeetha R, Gokulnath
M, Keerthana R, He Z, Partridge NC and Selvamurugan N:
MicroRNA-590-5p stabilizes Runx2 by targeting Smad7 during
osteoblast differentiation. J Cell Physiol. 232:371–380. 2017.
View Article : Google Scholar
|
|
10
|
Yan J, Guo D, Yang S, Sun H, Wu B and Zhou
D: Inhibition of miR-222-3p activity promoted osteogenic
differentiation of hBMSCs by regulating Smad5-RUNX2 signal axis.
Biochem Biophys Res Commun. 470:498–503. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu Z, Wang Y, Sun Z, Wang H, Zhou H, Zhang
L, Zhang S and Cao X: miRNA-132-3p inhibits osteoblast
differentiation by targeting Ep300 in simulated microgravity. Sci
Rep. 5:186552015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fukuda T, Ochi H, Sunamura S, Haiden A,
Bando W, Inose H, Okawa A, Asou Y and Takeda S: MicroRNA-145
regulates osteoblastic differentiation by targeting the
transcription factor Cbfb. FEBS Lett. 589:3302–3308. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen D, Zhao M and Mundy GR: Bone
morphogenetic proteins. Growth Factors. 22:233–241. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Komori T, Yagi H, Nomura S, Yamaguchi A,
Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, et al:
Targeted disruption of Cbfa1 results in a complete lack of bone
formation owing to maturational arrest of osteoblasts. Cell.
89:755–764. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ducy P, Zhang R, Geoffroy V, Ridall AL and
Karsenty G: Osf2/Cbfa1: A transcriptional activator of osteoblast
differentiation. Cell. 89:747–754. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakashima K, Zhou X, Kunkel G, Zhang Z,
Deng JM, Behringer RR and de Crombrugghe B: The novel zinc
finger-containing transcription factor osterix is required for
osteoblast differentiation and bone formation. Cell. 108:17–29.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takayanagi H, Kim S, Koga T and Taniguchi
T: Stat1-mediated cytoplasmic attenuation in osteoimmunology. J
Cell Biochem. 94:232–240. 2005. View Article : Google Scholar
|
|
18
|
Kim S, Koga T, Isobe M, Kern BE, Yokochi
T, Chin YE, Karsenty G, Taniguchi T and Takayanagi H: Stat1
functions as a cytoplasmic attenuator of Runx2 in the
transcriptional program of osteoblast differentiation. Genes Dev.
17:1979–1991. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takayanagi H, Ogasawara K, Hida S, Chiba
T, Murata S, Sato K, Takaoka A, Yokochi T, Oda H, Tanaka K, et al:
T-cell-mediated regulation of osteoclastogenesis by signalling
cross-talk between RANKL and IFN-gamma. Nature. 408:600–605. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takayanagi H, Kim S, Matsuo K, Suzuki H,
Suzuki T, Sato K, Yokochi T, Oda H, Nakamura K, Ida N, et al: RANKL
maintains bone homeostasis through c-Fos-dependent induction of
interferon-beta. Nature. 416:744–749. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fujie A, Funayama A, Miyauchi Y, Sato Y,
Kobayashi T, Kanagawa H, Katsuyama E, Hao W, Tando T, Watanabe R,
et al: Bcl6 promotes osteoblastogenesis through Stat1 inhibition.
Biochem Biophys Res Commun. 457:451–456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chang CC, Ye BH, Chaganti RS and
Dalla-Favera R: BCL-6, a POZ/zinc-finger protein, is a
sequence-specific transcriptional repressor. Proc Natl Acad Sci
USA. 93:6947–6952. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jardin F, Ruminy P, Bastard C and Tilly H:
The BCL6 proto-oncogene: A leading role during germinal center
development and lymphomagenesis. Pathol Biol (Paris). 55:73–83.
2007. View Article : Google Scholar
|
|
24
|
Zou Q, Tan S, Yang Z, Wang J, Xian J,
Zhang S, Jin H, Yang L, Wang L and Zhang L: The human nucleophosmin
1 mutation A inhibits myeloid differentiation of leukemia cells by
modulating miR-10b. Oncotarget. 7:71477–71490. 2016.PubMed/NCBI
|
|
25
|
Foley NH, Bray I, Watters KM, Das S, Bryan
K, Bernas T, Prehn JH and Stallings RL: MicroRNAs 10a and 10b are
potent inducers of neuroblastoma cell differentiation through
targeting of nuclear receptor corepressor 2. Cell Death Differ.
18:1089–1098. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Okamoto H, Matsumi Y, Hoshikawa Y, Takubo
K, Ryoke K and Shiota G: Involvement of microRNAs in regulation of
osteoblastic differentiation in mouse induced pluripotent stem
cells. PLoS One. 7:e438002012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hassan MQ, Maeda Y, Taipaleenmaki H, Zhang
W, Jafferji M, Gordon JA, Li Z, Croce CM, van Wijnen AJ, Stein JL,
et al: miR-218 directs a Wnt signaling circuit to promote
differentiation of osteoblasts and osteomimicry of metastatic
cancer cells. J Biol Chem. 287:42084–42092. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Boyce BF, Rosenberg E, de Papp AE and
Duong LT: The osteoclast, bone remodelling and treatment of
metabolic bone disease. Eur J Clin Invest. 42:1332–1341. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang J, Wang B, Chen LQ, Yang J, Gong ZQ,
Zhao XL, Zhang CQ and Du KL: miR-10b promotes invasion by targeting
KLF4 in osteosarcoma cells. Biomed Pharmacother. 84:947–953. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Knirsh R, Ben-Dror I, Modai S, Shomron N
and Vardimon L: MicroRNA 10b promotes abnormal expression of the
proto-oncogene c-Jun in metastatic breast cancer cells. Oncotarget.
7:59932–59944. 2016.PubMed/NCBI
|
|
31
|
Wang X, Ling CC, Li L, Qin Y, Qi J, Liu X,
You B, Shi Y, Zhang J, Jiang Q, et al: MicroRNA-10a/10b represses a
novel target gene mib1 to regulate angiogenesis. Cardiovasc Res.
110:140–150. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Giusti J, Pinhal D, Moxon S, Campos CL,
Münsterberg A and Martins C: MicroRNA-10 modulates Hox genes
expression during Nile tilapia embryonic development. Mech Dev.
140:12–18. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Duan S, Cermak L, Pagan JK, Rossi M,
Martinengo C, di Celle PF, Chapuy B, Shipp M, Chiarle R and Pagano
M: FBXO11 targets BCL6 for degradation and is inactivated in
diffuse large B-cell lymphomas. Nature. 481:90–93. 2012. View Article : Google Scholar :
|
|
34
|
Wu Q, Liu X, Yan H, He YH, Ye S, Cheng XW,
Zhu GL, Wu WY, Wang XN, Kong XJ, et al: B-cell lymphoma 6 protein
stimulates oncogenicity of human breast cancer cells. BMC Cancer.
14:4182014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nance JP, Bélanger S, Johnston RJ, Hu JK,
Takemori T and Crotty S: Bcl6 middle domain repressor function is
required for T follicular helper cell differentiation and utilizes
the corepressor MTA3. Proc Natl Acad Sci USA. 112:13324–13329.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu X, Lu H, Chen T, Nallaparaju KC, Yan
X, Tanaka S, Ichiyama K, Zhang X, Zhang L, Wen X, et al:
Genome-wide analysis identifies Bcl6-controlled regulatory networks
during T follicular helper cell differentiation. Cell Rep.
14:1735–1747. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hu G and Zhao K: Looping around Bcl6 in
germinal center to sharpen B cell immunity. Immunity. 45:459–461.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ying Z, Mei M, Zhang P, Liu C, He H, Gao F
and Bao S: Histone arginine methylation by PRMT7 controls germinal
center formation via regulating Bcl6 transcription. J Immunol.
195:1538–1547. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Miyauchi Y, Ninomiya K, Miyamoto H,
Sakamoto A, Iwasaki R, Hoshi H, Miyamoto K, Hao W, Yoshida S,
Morioka H, et al: The Blimp1-Bcl6 axis is critical to regulate
osteoclast differentiation and bone homeostasis. J Exp Med.
207:751–762. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Park-Min KH, Lee EY, Moskowitz NK, Lim E,
Lee SK, Lorenzo JA, Huang C, Melnick AM, Purdue PE, Goldring SR, et
al: Negative regulation of osteoclast precursor differentiation by
CD11b and β2 integrin-B-cell lymphoma 6 signaling. J Bone Miner
Res. 28:135–149. 2013. View Article : Google Scholar
|
|
41
|
Swarnkar G, Shim K, Nasir AM, Seehra K,
Chen HP, Mbalaviele G and Abu-Amer Y: Myeloid deletion of nemo
causes osteopetrosis in mice owing to upregulation of
transcriptional repressors. Sci Rep. 6:298962016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Morita M, Yoshida S, Iwasaki R, Yasui T,
Sato Y, Kobayashi T, Watanabe R, Oike T, Miyamoto K, Takami M, et
al: Smad4 is required to inhibit osteoclastogenesis and maintain
bone mass. Sci Rep. 6:352212016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li J, He X, Wei W and Zhou X: MicroRNA-194
promotes osteoblast differentiation via downregulating STAT1.
Biochem Biophys Res Commun. 460:482–488. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xiao WZ, Gu XC, Hu B, Liu XW, Zi Y and Li
M: Role of microRNA-129-5p in osteoblast differentiation from bone
marrow mesenchymal stem cells. Cell Mol Biol (Noisy-le-grand).
62:95–99. 2016.
|
|
45
|
Tajima K, Takaishi H, Takito J, Tohmonda
T, Yoda M, Ota N, Kosaki N, Matsumoto M, Ikegami H, Nakamura T, et
al: Inhibition of STAT1 accelerates bone fracture healing. J Orthop
Res. 28:937–941. 2010.PubMed/NCBI
|
|
46
|
Yoshida K, Okamura H, Amorim BR, Hinode D,
Yoshida H and Haneji T: PKR-mediated degradation of STAT1 regulates
osteoblast differentiation. Exp Cell Res. 315:2105–2114. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tryndyak VP, Ross SA, Beland FA and
Pogribny IP: Downregulation of the microRNAs miR-34a, miR-127, and
miR-200b in rat liver during hepatocarcinogenesis induced by a
methyl-deficient diet. Mol Carcinog. 48:479–487. 2009. View Article : Google Scholar
|
|
48
|
Martín-Pérez D, Vargiu P, Montes-Moreno S,
León EA, Rodríguez-Pinilla SM, Lisio LD, Martínez N, Rodríguez R,
Mollejo M, Castellvi J, et al: Epstein-Barr virus microRNAs repress
BCL6 expression in diffuse large B-cell lymphoma. Leukemia.
26:180–183. 2012. View Article : Google Scholar
|
|
49
|
Nazari-Jahantigh M, Wei Y, Noels H, Akhtar
S, Zhou Z, Koenen RR, Heyll K, Gremse F, Kiessling F, Grommes J, et
al: MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in
macrophages. J Clin Invest. 122:4190–4202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen J, Wang M, Guo M, Xie Y and Cong YS:
miR-127 regulates cell proliferation and senescence by targeting
BCL6. PLoS One. 8:e802662013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sun C, Li S, Yang C, Xi Y, Wang L, Zhang F
and Li D: MicroRNA-187-3p mitigates non-small cell lung cancer
(NSCLC) development through Downregulation of BCL6. Biochem Biophys
Res Commun. 471:82–88. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Huang F, Jin Y and Wei Y: MicroRNA-187
induces diffuse large B-cell lymphoma cell apoptosis via targeting
BCL6. Oncol Lett. 11:2845–2850. 2016.PubMed/NCBI
|