Introduction
Grape seed proanthocyanidin (GSP), referred to as
condensed tannins, includes a high content of flavonoids. The free
radical-scavenging abilities of GSP were found to reduce the risk
of cancer (1), blood clotting
(2) and cardiovascular disease
(3). Proanthocyanidins are
compounds naturally found in vegetables, bark, fruits and seeds;
grape seeds are a particularly rich source of proanthocyanidins in
both quantity and variety (4). A
variety of proanthocyanidins have multiple functions such as
antibacterial, antiviral (5),
anticarcinogenic (5,6) and anti-inflammatory activity
(7). GSP is sold in the market as
a dietary supplement because of its potential antioxidant activity,
together with its low toxicity and lack of genotoxic potential
(8). However, there have been no
studies on the anti-inflammatory effects of GSP in human hepatic
stellate cells (HSCs).
Chronic liver disease is often caused by
inflammation. In all disease stages, inflammation is observed and
is characterized by the development of hepatocellular carcinoma,
cirrhosis and fibrosis, which are mainly of viral or autoimmune
origin, or are caused by alcohol abuse (9). HSCs are the major players in liver
inflammation and fibrogenesis. For example, HSC activation and the
subsequent matrix secretion by activated HSCs induce liver
fibrosis, leading to cirrhosis in chronic liver injury (10). The reciprocal relationship between
HSCs and precancerous hepatocytes or hepatoma cells promotes
tumorigenesis, migration and invasion of cancer cells, and
formation of metastasis (11).
Specifically, both activated and proliferating HSCs play key roles
in the inflammation-fibrosis-carcinoma axis, whereas apoptotic HSCs
promote fibrosis resolution (11). The human HSC cell line, LX-2,
exhibits the typical characteristics of HSCs under primary culture.
Thus, LX-2 cells are considered a novel tool for analyzing hepatic
fibrosis (12).
After liver injury, recruited inflammatory cells
accumulate in the damaged site. A wide repertoire of
pro-inflammatory and anti-inflammatory compounds including
chemokines, cytokines and growth factors mediate the inflammatory
response of immune cells during the process of fibrosis (13). HSCs also play an active role in
the progression of inflammation by interacting with various immune
cells (14). Almost all
inflammatory stimuli converge on HSCs. As inflammation is an
important factor in the pathogenesis of liver fibrosis, managing
inflammatory responses is an important strategy for treating
hepatic fibrosis (15).
Medicinal plants produce compounds that suppress
inflammation, suggesting that their extracts could be used for the
treatment of symptoms of fibrosis. For example, these extracts
reduce liver fibrosis by decreasing hepatic secretion of
inflammatory cytokines at the protein and mRNA levels in the liver
(16). Specifically, vegetal
compounds target pro-inflammatory cytokines and chemokines such as
interleukin-1β (IL-1β), IL-2, IL-6, IL-8, interferon-γ (IFN-γ) and
tumor necrosis factor-α (TNF-α) (17). Moreover, liver inflammation is
suppressed by upregulation of the hepatic levels of
anti-inflammatory cytokines (IL-4, IL-10 and IL-13), together with
inhibition of the expression of inducible nitric oxide synthase
(iNOS) and cyclooxygenase-2 (COX-2) (16). Toll-like receptors (TLRs) 2 and 4
are central mediators of inflammation during liver fibrosis. TLR
ligands consist of pathogen-associated molecular patterns (PAMPs)
and damage-associated molecular patterns (DAMPs) (18). The high mobility group box 1
(HMGB1)-TLR2/TLR4-NF-κB signaling pathway is a potential
therapeutic target for suppression of inflammation in liver
fibrosis. In the present study, we investigated the underlying
molecular mechanisms and prophylactic effects of GSP on
lipopolysaccharide (LPS)-stimulated LX-2 cells.
Materials and methods
Materials
GSP from Vitis vinifera was kindly supplied
by Hanlim Pharmaceutical (Seoul, Korea). GSP contains
proanthocyanidins as a major component (80%), as well as several
catechin monomers (19). GSP was
solubilized in phosphate-buffered saline (PBS). Dulbecco's modified
Eagle's medium (DMEM) and other related products were purchased
from Gibco (Grand Island, NY, USA). Fetal bovine serum (FBS) was
obtained from PAA Laboratories (Linz, Austria). Sigma-Aldrich (St.
Louis, MO, USA) was the supplier of all other chemicals of
analytical grade. R&D Systems (Minneapolis, MN, USA) was the
supplier of the antibody against iNOS (MAB9502) and the
enzyme-linked immunosorbent assay (ELISA) kit for IL-8 (D8000C).
Antibodies against COX-2 (#4842) and β-actin (#4970) as well as
horseradish peroxidase-conjugated anti-mouse (#7076) and
anti-rabbit (#7074) IgG were purchased from Cell Signaling
Technology (Beverly, MA, USA). Anti-phospho or total antibodies to
JNK (#9258, #9251 for total and phospho form), Akt (#4691, #4060
for total and phospho form), ERK (#4695, #4370 for total and
phospho form), p38 (#8690, #4511 for total and phospho form), IκBα
(#9242, #9246 for total and phospho form) and NF-κB (#8242, #3031
for total and phospho form) were also purchased from Cell Signaling
Technology.
Cell culture
The LX-2 cell line is an immortalized human HSC
line, and was provided by Dr Scott L. Friedman (Mount Sinai
Hospital, New York, NY, USA). LX-2 cells were grown as previously
described (12). They were grown
in monolayers with DMEM supplemented with 2% (v/v) heat-inactivated
FBS, 100 µg/ml streptomycin and 100 U/ml penicillin at 37°C
in a humidified atmosphere of 5% CO2 in air. Then, after
rinsing with PBS, they were starved by incubation in a serum-free
medium for 24 h. The treated LX-2 cells were exposed to LPS (1
µg/ml) with or without GSP.
Cell viability
The anti-proliferative effects of GSP on LX-2 cells
were determined as follows. Cells were grown on 6-well plates
(1×105/well) for 24 or 48 h, after which the indicated
concentration of GSP was added; controls received 0.01% PBS. After
the indicated incubation times, the cells in each well were
harvested with trypsin-EDTA solution (JBI, Seoul, Korea) and washed
once with PBS containing 5% FBS. Then, the number of cells was
counted using an ADAM-MC cell counter (NanoEnTeK, Seoul, Korea),
and the effects of GSP on cell proliferation were examined by the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay (20). A total of
1.5×104 LX-2 cells/well were plated in 96-well culture
plates and incubated for 24 h. Then, GSP concentrations of 1–100
µg/ml were added to the cell culture; cells were incubated
for an additional 24 and 48 h. At predetermined times following
treatment with GSP, the medium was replaced with MTT (20 µl,
5 mg/ml) in each well. Incubation was continued at 37°C for an
additional 2 h, after which the plate contents were centrifuged and
the supernatants were disposed of. Formazan precipitates were
dissolved with 200 µl dimethyl sulphoxide. The absorbance
was obtained at 570 nm in comparison to 650 nm as a blank using an
E-max microplate reader (Molecular Devices, Sunnyvale, CA, USA).
The change in percentage of cell proliferation in each well was
expressed in comparison to the non-GSP-treated controls. All
experiments were repeated three times independently.
RNA isolation, cDNA synthesis and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA from the cells was obtained with TRIzol
reagent (Invitrogen, Carlsbad, CA, USA). First-strand cDNA was
synthesized by reverse transcription in a 20-µl reaction
mixture containing 1 mM dNTPs, 1 µg RNA, 1X reaction buffer,
5 µM random primers, and 20 units of AMV reverse
transcriptase (Promega, Madison, WI, USA). The individual sequences
for the gene-specific primers used were as follows: IL-6,
5′-GTCTTGCCTGCTGCCTTC-3′ and 5′-AGTGCCTCTTTGCTGCTTTC-3′ (194 bp);
IL-8, 5′-GACATACTCCAAACCTTTCCAC-3′ and 5′-CTTCTCCACAAACCTCTGC-3′
(160 bp); IL-1β, 5′-TGATGGCTTATTACAGTGGCAATG-3′ and
5′-GTAGTGGTGGTCGGAGATTCG-3′ (140 bp); TLR-2,
5′-TCTCCCATTTCCGTCTTTTT-3′ and 5′-GGTCTTGGTGTTCATTATCTTC-3′ (125
bp); TLR-4, 5′-GAAGCTGGTGGCTGTGGA-3′ and 5′-TGATGTAGAACCCGCAAG-3′
(213 bp); β-actin, 5′-GCGAGAAGATGACCCAGATC-3′ and
5′-GGATAGCACAGCCTGGATAG-3′ (77 bp); NOD1,
5′-GTCACTGAGGTCCATCTGAAC-3′ and 5′-CATCCACTCCTGGAAGAACCT-3′ (363
bp); NOD2, 5′-CATGTGCTGCTACGTGTTCTC-3′ and
5′-CCTGCCACAATTGAAGAGGTG-3′ (226 bp); iNOS,
5′-TGGATGCAACCCCATTGTC-3′ and 5′-CCCGCTGCCCCAGTTT-3′ (59 bp);
COX-2, 5′-CAAATCCTTGCTGTTCCCACCCAT-3′ and
5′-GTGCACTGTGTTTGGAGTGGGTTT-3′ (173 bp). Quantitative PCR (qPCR)
was carried out using a StepOnePlus real-time PCR system (Applied
Biosystems, Foster City, CA, USA). PCR was carried out with 1
µl cDNA in 20 µl reaction mixtures consisting of 1
µl primers, 10 µl Power SYBR-Green PCR Master Mix and
7 µl PCR-grade water. The amplification protocols included
an initial denaturation step (95°C, 10 min), 40 subsequent cycles
of denaturation (95°C, 15 sec), and an annealing step (60°C, 1
min). The crossing point value (ΔCT) of each cDNA was applied to
the formula 2−(target gene - β-actin) to quantify the
relative amounts of each cDNA.
Western blot analysis
Treated cells were washed with cold PBS to be lysed
with lysis buffer (Cell Signaling Technology). Bicinchoninic acid
(BCA) protein assay was employed to determine the total protein
concentration, in accordance with the manufacturer's instructions.
Protein (30 µg) was mixed with loading buffer, boiled for 5
min, and loaded onto 8–12% polyacrylamide gels. After
electrophoresis, the proteins were transferred to PVDF membranes.
After blocking the membranes with 5% non-fat dried milk for 1 h,
they were incubated for 1 h in a solution of Tris-buffered saline
with 0.05% Tween-20 (TBS-T) containing primary antibody at a
dilution rate of 1:500–1:1,000. After washing with TBS-T, the
membranes were soaked in TBS-T solution containing horseradish
peroxidase-conjugated secondary antibodies at a dilution of 1:2,500
for 1 h. After a second wash with TBS-T, target protein bands were
visualized using an Enhanced Chemiluminescence kit (Thermo
Scientific, Rockford, IL, USA). The target protein expression was
visualized using a Davinch-Chemi Chemiluminescence Imaging system
(Davinch-K Co., Seoul, Korea).
Measurement of IL-8
LX-2 cells were seeded in 6-well plates at a density
of 6×105 cells/well and cultured with various doses of
GSP for 4 h at 37°C before challenge with LPS (1 µg/ml) for
24 h. After treatment, culture media were collected and subjected
to centrifugation at 1,000 × g for 15 min. The supernatants were
analyzed with IL-8 ELISA kits (R&D Systems).
Statistical analysis
All values are expressed as the mean ± standard
deviation (SD). SigmaPlot version 10 software (Systat Software
Inc., Chicago, IL, USA) was used for statistical analyses.
Student's t-test or one-way ANOVA was used for the determination of
statistical significance of differences between the LPS-treated and
GSP plus LPS-treated cells. In all analyses, a p-value of <0.05
was considered statistically significant.
Results
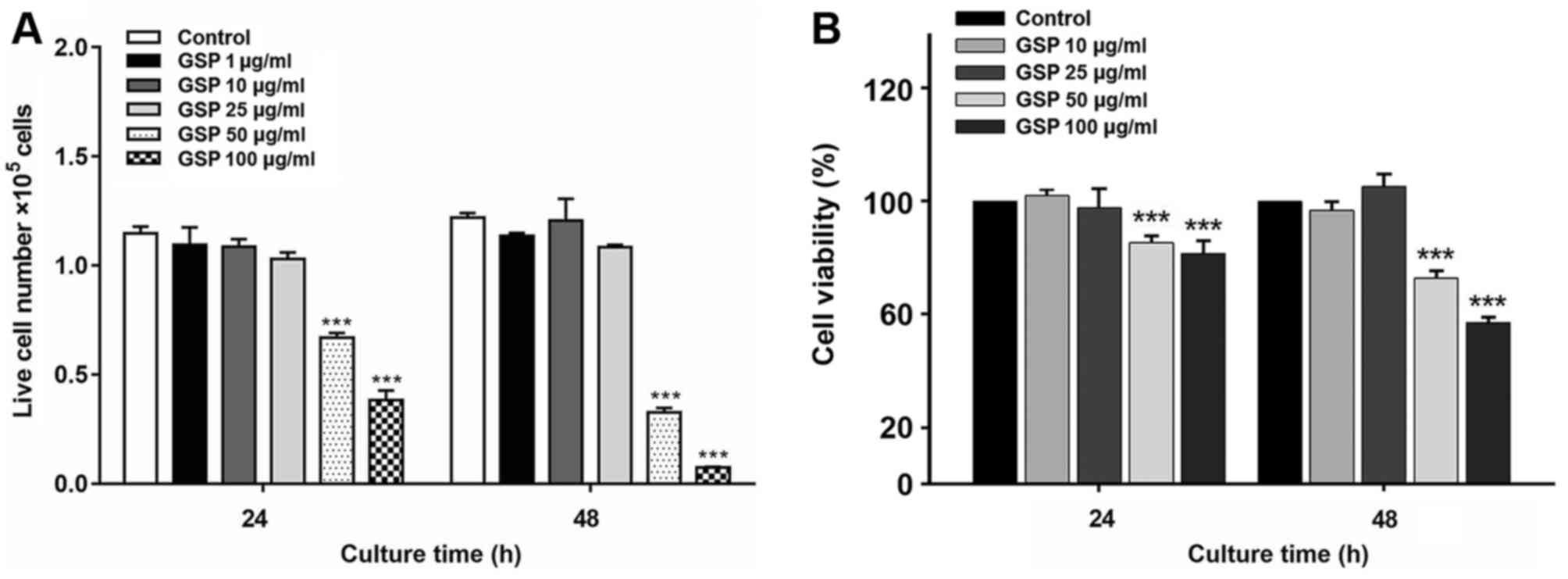
Effects of GSP on the viability of
LPS-induced human HSCs
The cytotoxic effects of GSP on LX-2 cells were
evaluated by exposing the cells to varying concentrations of GSP
for 24 and 48 h. The data are expressed as the number of cells
(Fig. 1A) and the percentage of
cell viability (Fig. 1B) compared
to the controls. GSP did not exhibit cytotoxicity towards LX-2
cells at doses of 10 and 25 µg/ml, which were the doses used
for treatment with GSP in subsequent experiments.
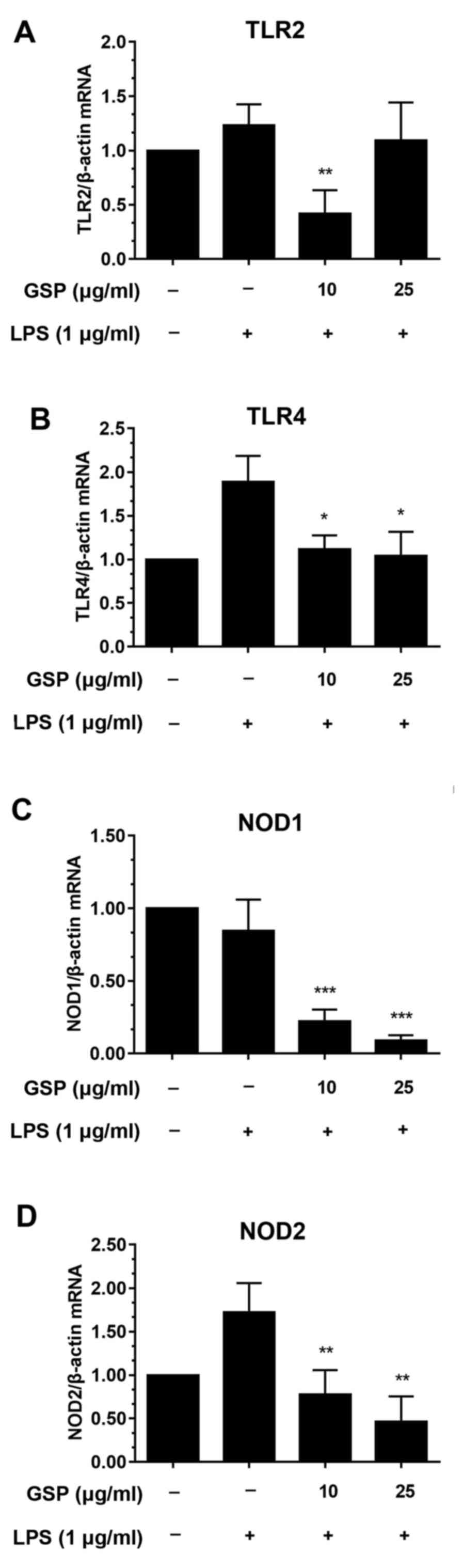
Effects of GSP on the mRNA levels of
NOD1, NOD2, TLR2 and TLR4 in LPS-stimulated LX-2 cells
The effects of GSP on the mRNA expression of NOD1,
NOD2, TLR2 and TLR4 were investigated by qPCR. TLR2 and TLR4 are
central intermediaries of inflammation in liver fibrosis. In
particular, TLR4 may be involved in the increase in inflammation
and fibrosis of the liver (21).
In this study, LX-2 cells were pre-incubated with GSP before LPS
treatment. LPS treatment caused significant increases in mRNA
levels of TLR4 (Fig. 2B) and NOD2
(Fig. 2D) compared to the
controls. The mRNA levels of NOD2 and TLR4 were decreased in the
cells pretreated with GSP. In contrast, mRNA levels of TLR2 and
NOD1 were not significantly affected by LPS stimulation (Fig. 2A and C). Decreases in mRNA levels
following GSP pretreatment were likely only due to the drug, and
were independent of LPS stimulation.
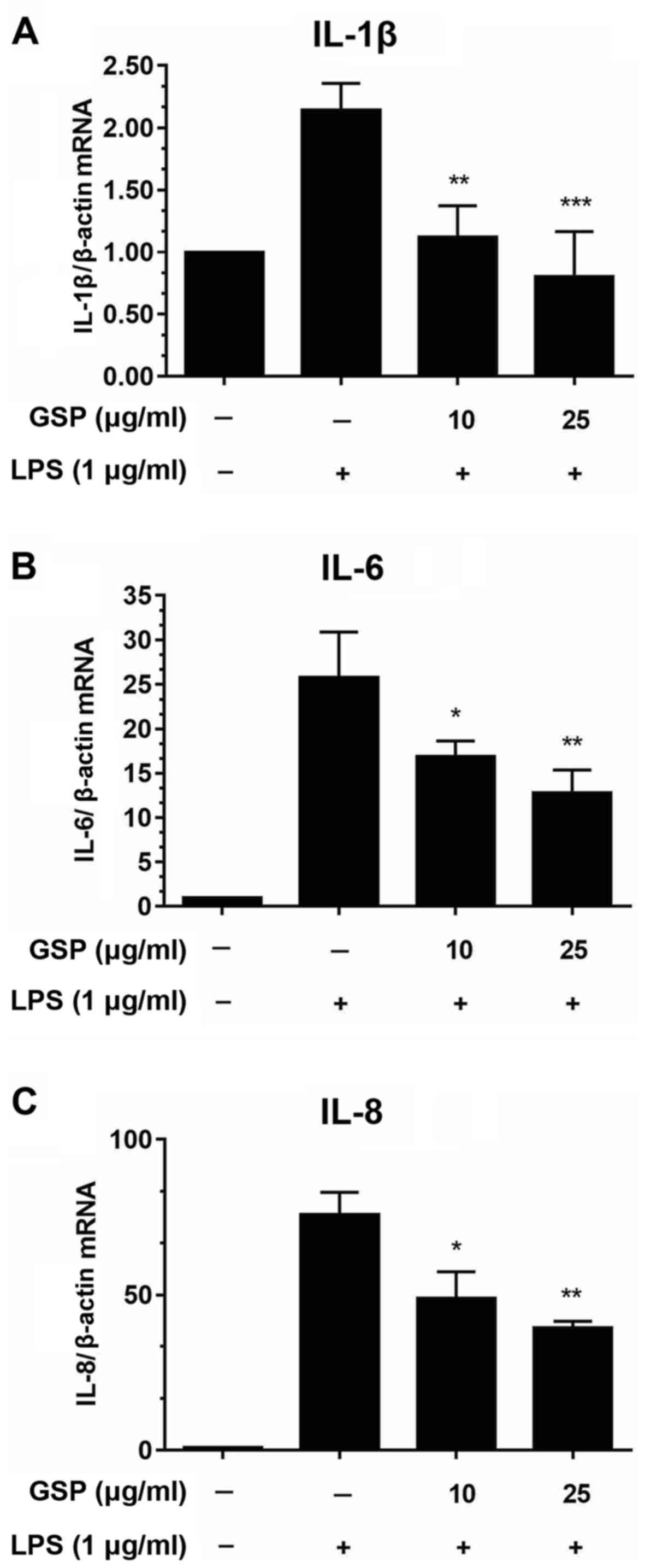
Effects of GSP on mRNA levels of IL-1β,
IL-6 and IL-8 in LPS-stimulated LX-2 cells
We performed qPCR to evaluate the mRNA expression
levels of pro-inflammatory cytokines including IL-1β, IL-6 and IL-8
in LPS-stimulated HSCs. As shown in Fig. 3, significant increases in cytokine
expression were induced by LPS treatment; however, pretreatment
with GSP decreased the mRNA expression levels. These findings
indicated that GSP regulates immune responses by reducing the mRNA
expression levels of pro-inflammatory genes.
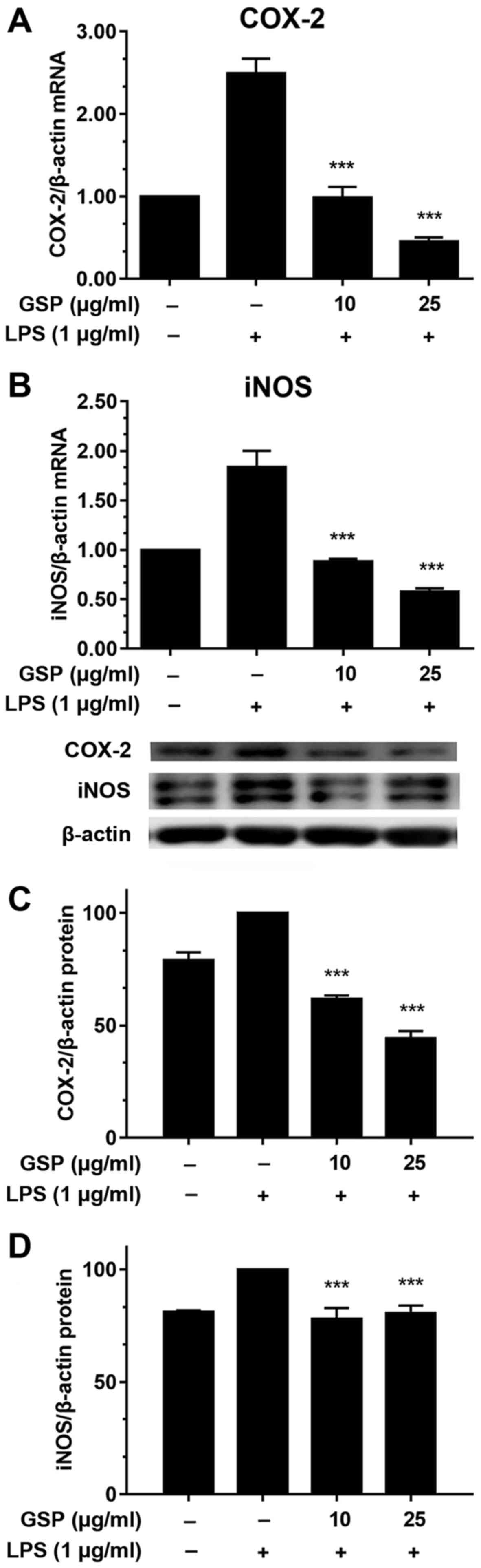
Effects of GSP on mRNA and protein
expression of COX-2 and iNOS in LPS-induced LX-2 cells
Next, we performed qPCR and western blot analyses to
investigate the effects of GSP on the expression levels of COX-2
and iNOS. Fig. 4A and B show that
LPS treatment noticeably increased the mRNA expression levels of
COX-2 and iNOS. In contrast, GSP pretreatment decreased the mRNA
and protein levels of COX-2 and iNOS in the LPS-induced LX-2 cells
(Fig. 4C and D).
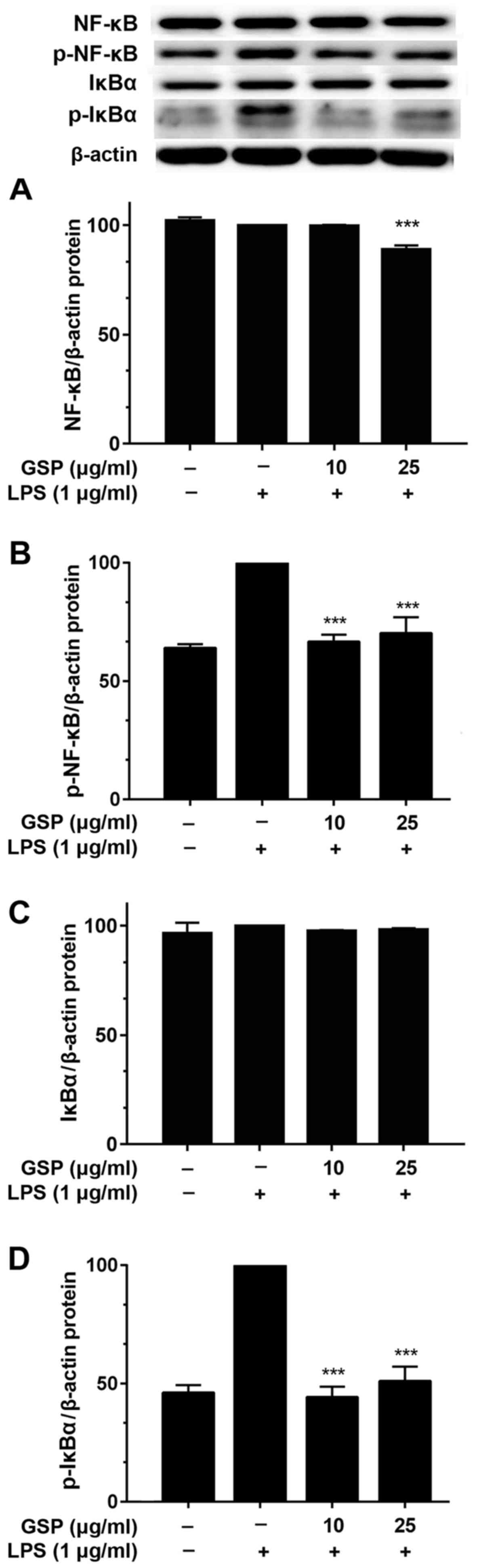
Effects of GSP on phosphorylation of
NF-κB in LPS-induced LX-2 cells
All major pro-inflammatory mediators are induced via
activation of NF-κB (22).
Therefore, we hypothesized that the aforementioned findings were
related to the NF-κB signaling pathway. Thus, the effects of GSP on
IκBα phosphorylation and NF-κB p65 activation were investigated.
After pretreatment of LX-2 cells with GSP for 24 h and stimulation
with LPS for 2 h, western blotting was performed for IκBα and NF-κB
p65. Fig. 5 shows that the total
protein expression levels of IκBα and NF-κB p65 were unaffected by
treatment with LPS and pretreatment with GSP (Fig. 5A and C). However, while LPS
treatment increased the protein levels of phosphorylated IκBα and
NF-κB p65, pretreatment with GSP significantly decreased levels of
both these proteins (Fig. 5B and
D).
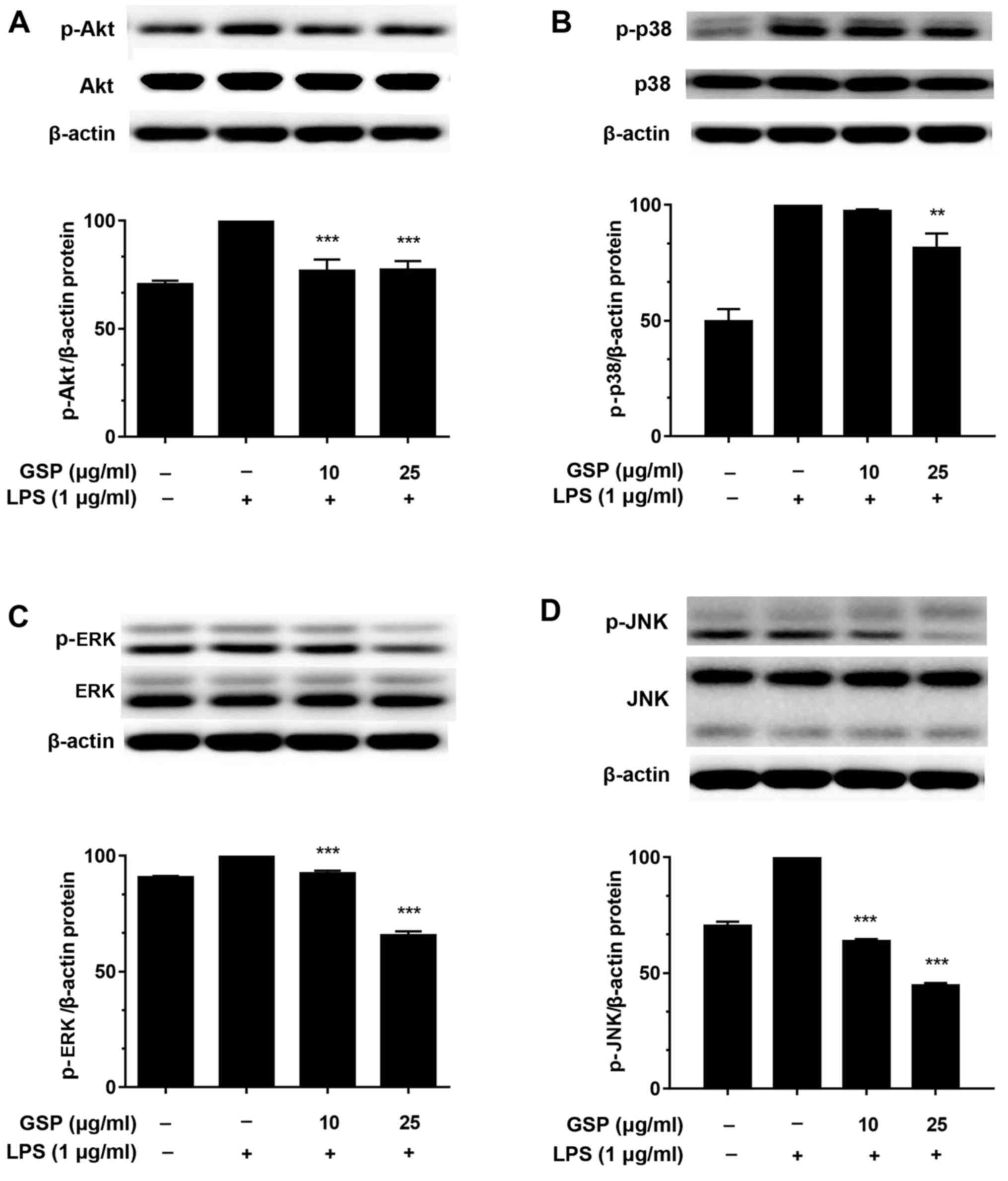
Effects of GSP on phosphorylation of Akt
and MAPKs in LPS-induced LX-2 cells
To investigate whether inhibition of the
inflammatory response by GSP occurs via the PI3K/Akt and MAPK
pathways, we studied the effects of GSP on LPS-stimulated
phosphorylation of upstream kinases such as Akt, ERK, p38, and JNK
in LX-2 cells. Total protein was extracted 2 h after LPS
stimulation, and the expression of ERK, Akt, p38, JNK, and their
phosphorylated proteins was examined. The treatment of LX-2 cells
with LPS induced increases in expression levels of phosphorylated
Akt, ERK, p38 and JNK (Fig. 6).
However, while the protein level of each kinase stayed constant,
the expression level decreased with pre-incubation with GSP. These
results suggest that GSP pretreatment interferes with key signaling
pathways, including Akt and p38 MAPK. These results indicate that
activation of pro-inflammatory mediators is suppressed by GSP
pretreatment of HSCs.
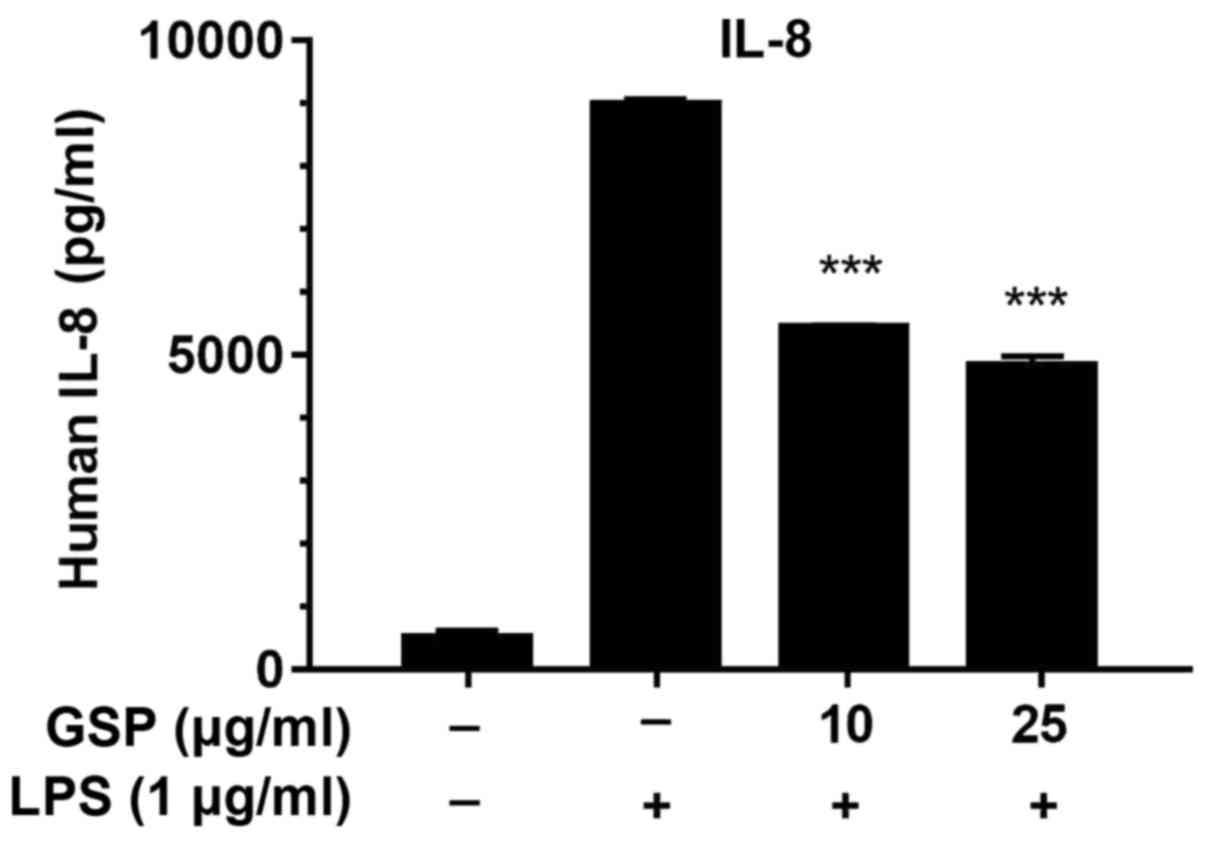
Effects of GSP on IL-8 cytokine
production in LPS-induced LX-2 cells
To further analyze the anti-inflammatory effects of
GSP, we used ELISA to measure the production of IL-8 in
LPS-stimulated LX-2 cells. The expression level of IL-8 was
markedly increased after LPS treatment, but was significantly
decreased in the GSP-treated cells (Fig. 7). The inhibitory effects of GSP on
IL-8 protein production was correlated with the suppression of IL-8
mRNA expression (Fig. 3C). The
results above indicate that the anti-inflammatory effects of GSP
occur via suppression of pro-inflammatory genes or proteins related
to the MAPK, Akt and NF-κB signaling pathways in LPS-stimulated
cells.
Discussion
Inflammation is heavily involved in liver fibrosis.
HSCs play an active role in this process via interactions with
diverse types of immune cells (14). Inflammation may often coexist with
liver fibrosis after liver insult. During inflammatory progression,
lymphocytes and neutrophils may invade the liver and have direct
effects on HSCs. The immune cells are affected by activated HSCs,
which secrete chemokines and cytokines such as TGF-β, IL-10 and
IL-6 (23). Medicinal plants
reduce liver fibrosis and inflammation by downregulating hepatic
expression and secretion of inflammatory cytokines. Inflammatory
cytokines targeted by plant-derived compounds include TNF-α, IL-1α,
IL-1β, IL-2, IL-4, IL-6, IL-12, IL-18 and IFN-γ. Pro-inflammatory
cytokines can regulate the synthesis of a wide variety of acute
phase proteins in the liver. They are also involved in the
pathogenesis of liver cirrhosis and fibrosis (24). It is possible that during the
inflammatory response, chemoattraction of various immune cells is
promoted by human HSCs. That is, activated HSCs can evoke
neutrophil chemotaxis after secreting IL-8, a neutrophil
chemoattractant, and macrophage inhibitory protein-2 (MIP-2)
(25,26). Accordingly, both the inflammatory
response and liver fibrosis are affected by HSCs. Previous studies
indicate that HSCs are a major factor in liver fibrosis and hepatic
inflammation. Thus, inhibition of the secretion of pro-inflammatory
cytokines by HSCs is an important strategic element for inhibiting
inflammation and fibrosis of the liver.
Our results demonstrated that GSP noticeably
suppressed the expression of IL-1β, IL-6 and IL-8 (Fig. 3). Inflammatory stimuli (such as
LPS treatment in HSCs) induce excessive production of cytokines,
which intensifies the immune response and subsequent inflammation
(27). Therefore,
anti-inflammatory therapies often target pro-inflammatory
cytokines, supporting our findings that GSP has anti-inflammatory
activity via inhibition of the protein expression of IL-1β, IL-6
and IL-8 (Figs. 3 and 7).
TLRs, particularly TLR2 and TLR4, are central
mediators of inflammation during liver fibrosis. LPS signals are
transmitted via TLR2 and TLR4 to the intracellular compartment, and
thus, the two transmembrane receptors play important roles in the
immune system (28). In this
study, we demonstrated that GSP regulates TLR4 gene transcription
in activated human HSCs (Fig.
2B). GSP could be employed for the inhibition of LPS/TLR4
signaling to prevent liver fibrosis. These results also suggest
that GSP plays a regulatory role in inflammation at the level of
TLR transcription. NODs and TLRs are a central part of the
mammalian innate immune response (29). For example, NOD1 and NOD2 are
primarily involved in mediating antibacterial defenses (30). We found that GSP downregulated
LPS-induced NOD2 expression (Fig.
2D). These results suggest that both ligands for NOD2 and TLR4
may synergistically improve innate immune responses for the
induction of the inflammatory response in HSCs (Fig. 2B and D).
iNOS and COX-2 play critical roles in the
pathogenesis of certain types of human cancer and inflammatory
disorders (31). Therefore,
anti-inflammatory agents that inhibit iNOS and COX-2 genes may be
used as potential therapeutics to treat inflammatory and infectious
diseases. The results of this study indicate that GSP can
efficiently inhibit the expression of iNOS and COX-2 (Fig. 4). NF-κB, a transcription factor,
plays a role in the regulation of the expression of inflammatory
mediators such as IL-1β, IL-6, iNOS and COX-2 (22). When activated by stimuli such as
LPS, NF-κB dissociates from IκBα to become an active form, and IκBα
is degraded. In this study, we found that GSP attenuated
LPS-stimulated phosphorylation of NF-κB in HSCs by blocking IκBα
phosphorylation. The MAPK pathway is involved in the expression and
regulation of inflammatory mediators including iNOS and COX-2, as
well as in the activation of NF-κB (32). Since GSP inhibits NF-κB
activation, we proposed the hypothesis that the MAPK pathway is
involved in the attenuation of inflammatory mediators. This study
showed that GSP decreased LPS-stimulated activation of MAPK in HSCs
(Figs. 5 and 6). We believe that GSP exerts its
anti-inflammatory effects partly by the attenuation of MAPK and
NF-κB activation.
LPS is a strong activator of the PI3K/Akt and MAPK
pathways. It was recently shown that the PI3K/Akt pathway plays an
important role in the regulation of LPS-induced acute inflammatory
responses (33). However, its
role in the regulation of NF-κB transactivation is still unclear.
In this study, LPS-stimulated phosphorylation of p38 MAPK and Akt
was suppressed following GSP treatment. This suggests that the
suppression of LPS-induced secretion of pro-inflammatory cytokines
in HSCs may be correlated with the suppression of p38 MAPK
phosphorylation by GSP (Fig. 6).
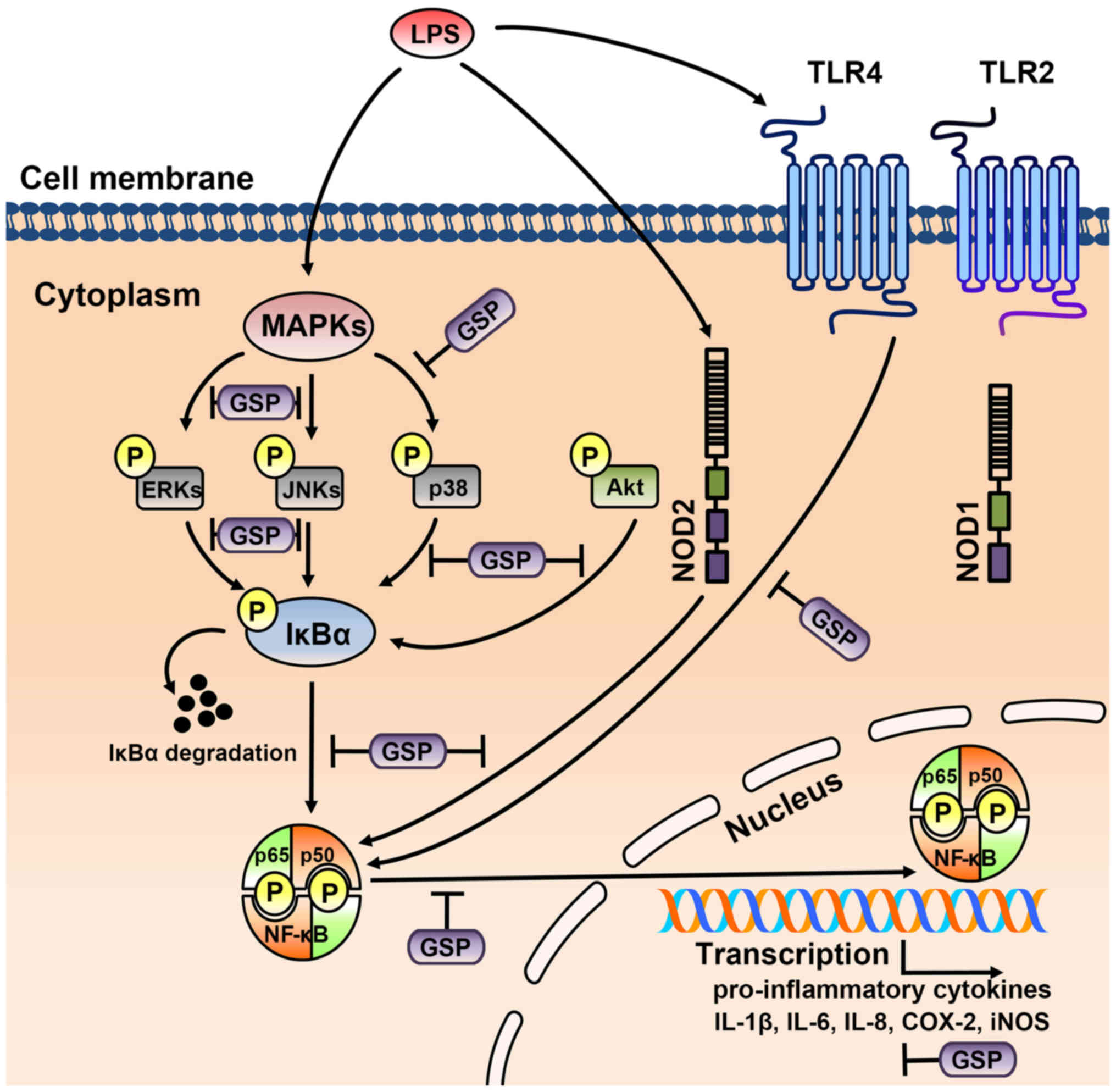
Our current findings suggest that GSP may block LPS-induced NF-κB
activation by inhibiting the phosphorylation of MAPKs and Akt,
subsequently decreasing the protein levels of iNOS, COX-2 and other
related inflammatory cytokines. In addition, our results also
suggest that innate immune responses may be initiated by TLR4
ligands and further increased by NOD2 ligands, resulting in the
stimulation of an inflammatory response in human HSCs (Fig. 8).
In conclusion, the results of this study
demonstrated that GSP suppresses many inflammatory events,
including pro-inflammatory cytokine secretion in LPS-stimulated
HSCs. That is, GSP plays a critical role in suppressing the
expression of COX-2 and iNOS, as well as the expression of
pro-inflammatory cytokines such as IL-8. These inhibitory effects
appear to occur via inhibition of IκBα phosphorylation and
subsequent suppression of NF-κB activation, p38 MAP kinase, and Akt
signaling. Hence, GSP may be a potential anti-inflammatory agent
with which to treat liver disease.
Acknowledgments
The present study was financially supported by a
grant (B110053) from the Korean Health Technology R&D Project,
Ministry of Health and Welfare, Republic of Korea. This study was
also supported by a Korea University Grant.
References
|
1
|
Bagchi D, Bagchi M, Stohs SJ, Das DK, Ray
SD, Kuszynski CA, Joshi SS and Pruess HG: Free radicals and grape
seed proanthocyanidin extract: Importance in human health and
disease prevention. Toxicology. 148:187–197. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Murphy KJ, Chronopoulos AK, Singh I,
Francis MA, Moriarty H, Pike MJ, Turner AH, Mann NJ and Sinclair
AJ: Dietary flavanols and procyanidin oligomers from cocoa
(Theobroma cacao) inhibit platelet function. Am J Clin Nutr.
77:1466–1473. 2003.PubMed/NCBI
|
|
3
|
Steinberg FM, Bearden MM and Keen CL:
Cocoa and chocolate flavonoids: Implications for cardiovascular
health. J Am Diet Assoc. 103:215–223. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maffei Facinó R, Carini M, Aldini G, Berti
F, Rossoni G, Bombardelli E and Morazzoni P: Procyanidines from
Vitis vinifera seeds protect rabbit heart from ischemia/reperfusion
injury: Antioxidant intervention and/or iron and copper
sequestering ability. Planta Med. 62:495–502. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
De Bruyne T, Pieters L, Witvrouw M, De
Clercq E, Vanden Berghe D and Vlietinck AJ: Biological evaluation
of proanthocyanidin dimers and related polyphenols. J Nat Prod.
62:954–958. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ye X, Krohn RL, Liu W, Joshi SS, Kuszynski
CA, McGinn TR, Bagchi M, Preuss HG, Stohs SJ and Bagchi D: The
cytotoxic effects of a novel IH636 grape seed proanthocyanidin
extract on cultured human cancer cells. Mol Cell Biochem.
196:99–108. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li WG, Zhang XY, Wu YJ and Tian X:
Anti-inflammatory effect and mechanism of proanthocyanidins from
grape seeds. Acta Pharmacol Sin. 22:1117–1120. 2001.PubMed/NCBI
|
|
8
|
Ray S, Bagchi D, Lim PM, Bagchi M, Gross
SM, Kothari SC, Preuss HG and Stohs SJ: Acute and long-term safety
evaluation of a novel IH636 grape seed proanthocyanidin extract.
Res Commun Mol Pathol Pharmacol. 109:165–197. 2001.
|
|
9
|
Seki E and Schwabe RF: Hepatic
inflammation and fibrosis: Functional links and key pathways.
Hepatology. 61:1066–1079. 2015. View Article : Google Scholar :
|
|
10
|
Iredale JP: Hepatic stellate cell behavior
during resolution of liver injury. Semin Liver Dis. 21:427–436.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang BB, Cheng JY, Gao HH, Zhang Y, Chen
ZN and Bian H: Hepatic stellate cells in
inflammation-fibrosis-carcinoma axis. Anat Rec (Hoboken).
293:1492–1496. 2010. View
Article : Google Scholar
|
|
12
|
Xu L, Hui AY, Albanis E, Arthur MJ,
O'Byrne SM, Blaner WS, Mukherjee P, Friedman SL and Eng FJ: Human
hepatic stellate cell lines, LX-1 and LX-2: New tools for analysis
of hepatic fibrosis. Gut. 54:142–151. 2005. View Article : Google Scholar
|
|
13
|
Henderson NC and Iredale JP: Liver
fibrosis: Cellular mechanisms of progression and resolution. Clin
Sci (Lond). 112:265–280. 2007. View Article : Google Scholar
|
|
14
|
Yi HS and Jeong WI: Interaction of hepatic
stellate cells with diverse types of immune cells: Foe or friend? J
Gastroenterol Hepatol. 28(Suppl 1): 99–104. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fallowfield JA: Therapeutic targets in
liver fibrosis. Am J Physiol Gastrointest Liver Physiol.
300:G709–G715. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Duval F, Moreno-Cuevas JE, González-Garza
MT, Maldonado-Bernal C and Cruz-Vega DE: Liver fibrosis and
mechanisms of the protective action of medicinal plants targeting
inflammation and the immune response. Int J Inflamm.
2015:9434972015. View Article : Google Scholar
|
|
17
|
Moon JE, Kim DM and Kim JY:
Anti-inflammatory effect of Rhus verniciflua stokes extract in the
murine macrophage cell line, Raw264.7. J Korean Soc Appl Biol Chem.
58:481–486. 2015. View Article : Google Scholar
|
|
18
|
Piccinini AM and Midwood KS: DAMPening
inflammation by modulating TLR signaling. Mediators Inflamm.
21:20102010.
|
|
19
|
Gabetta B, Fuzzati N, Griffini A, Lolla E,
Pace R, Ruffilli T and Peterlongo F: Characterization of
proanthocyanidins from grape seeds. Fitoterapia. 71:162–175. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seki E, De Minicis S, Osterreicher CH,
Kluwe J, Osawa Y, Brenner DA and Schwabe RF: TLR4 enhances TGF-beta
signaling and hepatic fibrosis. Nat Med. 13:1324–1332. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hambleton J, Weinstein SL, Lem L and
DeFranco AL: Activation of c-Jun N-terminal kinase in bacterial
lipopolysaccharide-stimulated macrophages. Proc Natl Acad Sci USA.
93:2774–2778. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hellerbrand C, Wang SC, Tsukamoto H,
Brenner DA and Rippe RA: Expression of intracellular adhesion
molecule 1 by activated hepatic stellate cells. Hepatology.
24:670–676. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kayano K and Okita K: Does IL-6 regulate
liver fibrosis/cirrhosis directly and indirectly? J Gastroenterol.
35:250–251. 2000. View Article : Google Scholar
|
|
25
|
Czaja MJ, Geerts A, Xu J, Schmiedeberg P
and Ju Y: Monocyte chemoattractant protein 1 (MCP-1) expression
occurs in toxic rat liver injury and human liver disease. J Leukoc
Biol. 55:120–126. 1994.PubMed/NCBI
|
|
26
|
Maher JJ, Lozier JS and Scott MK: Rat
hepatic stellate cells produce cytokine-induced neutrophil
chemoattractant in culture and in vivo. Am J Physiol.
275:G847–G853. 1998.PubMed/NCBI
|
|
27
|
Reitamo S, Remitz A, Tamai K and Uitto J:
Interleukin-10 modulates type I collagen and matrix metalloprotease
gene expression in cultured human skin fibroblasts. J Clin Invest.
94:2489–2492. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Underhill DM and Ozinsky A: Toll-like
receptors: Key mediators of microbe detection. Curr Opin Immunol.
14:103–110. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Inohara N and Nuñez G: NODs: Intracellular
proteins involved in inflammation and apoptosis. Nat Rev Immunol.
3:371–382. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee MS and Kim YJ: Signaling pathways
downstream of pattern-recognition receptors and their cross talk.
Annu Rev Biochem. 76:447–480. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Surh YJ, Chun KS, Cha HH, Han SS, Keum YS,
Park KK and Lee SS: Molecular mechanisms underlying chemopreventive
activities of anti-inflammatory phytochemicals: Down-regulation of
COX-2 and iNOS through suppression of NF-kappa B activation. Mutat
Res. 480–481:243–268. 2001. View Article : Google Scholar
|
|
32
|
Uto T, Suangkaew N, Morinaga O, Kariyazono
H, Oiso S and Shoyama Y: Eriobotryae folium extract suppresses
LPS-induced iNOS and COX-2 expression by inhibition of NF-kappaB
and MAPK activation in murine macrophages. Am J Chin Med.
38:985–994. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schabbauer G, Tencati M, Pedersen B,
Pawlinski R and Mackman N: PI3K-Akt pathway suppresses coagulation
and inflammation in endotoxemic mice. Arterioscler Thromb Vasc
Biol. 24:1963–1969. 2004. View Article : Google Scholar : PubMed/NCBI
|