Introduction
Exposure to traumatic events is a risk factor for
anxiety disorders (1). Acute
stress-induced anxiety helps to maintain arousal and vigilance
during dangerous conditions; however, anxiety is unfavorable for
the recovery of a subset of patients that have experienced
emergency major surgery or trauma (2,3).
Anxiety emerges sometimes in the aftermath of major surgery or
incident injury. This has been increasingly established in
traumatic events research, which is a growing area of focus
(3–5).
Several approaches have been used to solve this
issue, including psychotherapy and anti-depressant medications
(6), with little success.
Furthermore, the application of certain medications during the
peri-operative period sometimes produces undesired reactions
(7), such as a risk for bleeding
(8). The dysfunction of the
hypothalamic pituitary adrenal (HPA) axis is one of the most widely
accepted hypothesis of anxiety (9), which is a central system in
maintaining neuroendocrine equilibrium.
The HPA axis is composed of corticotropin-releasing
hormone (CRH) that is secreted from the hypothalamus and
successively stimulates adrenocorticotropic hormone (ACTH)
secretion from the pituitary gland. Glucocorticoid hormones [human,
cortisol; rodent, corticosterone (CORT)] are then secreted from the
cortex of the adrenal glands. The HPA axis has an established
association with anxiety, including physiological homeostasis and
the stress response (10,11). Disturbances in this system result
in severe hormonal imbalances, which may be strongly implicated in
the pathology of major depressive disorder (9); however, the potential role of the
HPA axis in trauma-induced anxiety remains unknown.
MicroRNAs (miRNAs or miRs) are small, non-coding
RNAs that typically bind to specific sequences in the
3′-untranslated regions (3′-UTRs) of targeted mRNAs to negatively
fine-tune protein expression. miRNAs have recently emerged as a key
regulator of metabolism and many are considered to be biomarkers of
depression or anxiety (12).
miRNAs play an important role in mediating anxiety via
glucocorticoid (GC) hormone signaling. There is evidence that
miR-124a may mediate anxiety via GC/glucocorticoid hormone receptor
(GR) signaling (13) and that
miR-608 may affect anxiety and CORT (14); however, the molecular mechanisms
through which major surgery induces anxiety and the involvement of
miRNAs in the hypothalamus in mediating the HPA axis require
further investigation.
This study aimed to investigate the role of the HPA
axis in trauma-induced, anxiety-like behavior and to evaluate
whether miRNAs in the hypothalamus are involved in a rodent
model.
Materials and methods
Animals
All animal experiments performed on rats were
conducted in accordance with NIH Guidelines (NIH Publications no.
8023, revised in 1978) and approved by the Animal Use and Care
Committee of Fudan University, Shanghai, China. Adult male
Sprague-Dawley (SD) rats (n=51; weighing, 200±20 g) and 1-day-old
neonatal SD rats (n=100) were purchased from the Experimental
Animal Center of the Chinese Academy of Sciences (Shanghai, China)
and housed in a quiet room with a 12:12 light/dark cycle with ad
libitum access to food and water. Room temperature was
maintained at 25±2°C.
Surgery
The adult male Sprague-Dawley rats in the model
group were subjected to hepatectomies under anesthesia by sodium
pentobarbital (30 mg/kg). Briefly, an approximately 7-cm-long
surgical incision was made from the xiphoid process to the pubic
symphysis along the abdomen. From this incision, 10% of the liver
was removed from the right lobe. The incision was then closed
following exhaustive hemostasis. All rats were kept warm under a
22°C environment during the hepatectomy and covered with a sterile
gauze after hepatectomy. All surgeries were performed between 8:00
and 10:00 a.m. The rats in the sham-operated (sham) group were
administered only anesthesia and only an incision was made. No
intervention process was performed on the rats in the intact
group.
Animal groups
In the first set of animal experiments the SD rats
were divided into the intact, sham and model group. There were 7
rats in each group. In the second set of animal experiments, the SD
rats were divided into the hepatectomy + vehicle and hepatectomy +
NBI-27914 group. There were 7 rats in each group. In the third set
of animal experiments, the SD rats were divided into the miR-NC-A,
miR-34b-A, hepatectomy + miR-NC-A and hepatectomy + miR-34b-A
group. There were 4 rats in each group.
Tissue collection
The rats in each group were sacrificed by
decapitation. Their brains were immediately removed and the
hypothalamus was separated from the brain. All samples were
snap-frozen in liquid nitrogen and then stored at −80°C until
further processing.
Radioimmunoassay (RIA)
Blood samples were collected by decapitation at the
time of sacrifice and the serum was separated by centrifugation.
The concentrations of CRH, ACTH and CORT were determined by RIA
kits that were purchased from the Beijing Sinouk Institute of
Biological Technology (Beijing, China). All the samples were
assayed together, with each sample analyzed in duplicate.
Cell culture
Primary cultures of fetal hypothalamic neuronal
cells were prepared from 1-day-old neonatal Sprague-Dawley rats, as
previously described (15). In
brief, their brains were immediately removed, and the hypothalami
were separated and placed in ice-cold Hanks' balanced salt solution
(HBSS, 14065056; Gibco Waltham, MA, USA). The hypothalamic tissue
was placed in 0.125% pancreatic enzymes (25200056; Gibco) for 20
min after being removed from the vessel. The hypothalamic cells
were harvested by centrifugation (1,000 rpm, 5 min, 4°C) following
filtration using a strainer (352350; BD Falcon, Franklin Lakes, NJ,
USA). The cells were resuspended by neurobasal medium (21103049;
Gibco) that was supplemented with 10% fetal bovine serum (FBS;
12657-029; Gibco) and plated on culture plates (353047; BD Falcon)
at approximately 16,000 cells/cm2 and then incubated at
37°C in an atmosphere of 95% air, 5% CO2 overnight. We
changed the culture solution to neurobasal medium that contained 1%
B27 (17504-044; Life Technologies, Waltham, MA, USA) the following
day and we then changed half the medium every 3 days. The cells
were maintained for 1 week with this medium before experimental
use.
293T cells (http://www.cellbank.com.cn/) were grown in Dulbecco's
modified Eagle's medium (DMEM; 12491-015; Invitrogen, Carlsbad, CA,
USA) containing 10% FBS. The cells were also incubated at 37°C in a
humidified atmosphere of 5% CO2.
Pharmacological applications and miRNA
transfection
Drugs were injected into the paraventricular nucleus
(PVN) in vivo according to the rat brain in stereotaxic
coordinates third at AP 1.5 mm, DV 0.4 mm, H 7.8 mm using a drug
delivery system from RWD Life Science, Shenzhen, China (RWD, 62037,
62137, 62237). This system was fixed into the rat brain 10 days
before an experiment to avoid unnecessary disturbances. The rats
were administered injections of the vehicle (0.5 µl 0.9%
saline) or 5-chloro-4-[N-(cyclo-propyl)
methyl-N-propylamino]-2-m ethyl-6-(2,4,6-trichlor-ophenyl)
amino-pyridine (NBI-27914: 5 nmol; 184241-44-9; Sigma, St. Louis,
MO, USA) which was dissolved in the vehicle before the surgery.
miRNA mimics for miR-351, miR-34b, miR-34c, miR-24,
miR-204, miR-214, miR-27a, miR-122, miR-150, miR-216a and miR-218,
agomirs (miR–34b) and their negative controls (miR04201-1-10) were
commercially synthesized from RuiBio, Guangzhou, China. These miRNA
mimics and control were transfected into the hypothalamic neurons
and 293T cells using transfection reagent (FuGENE® HD
Transfection Reagent, E2312; Promega Madison, WI, USA), according
to the manufacturer' s instructions in vitro. miR-34b agomir
and its negative control were injected into the PVN using the drug
delivery system at 1 week and 3 days before the experiment, each at
a concentration of 5 nmol.
Following transfection, the hypothalamics neurons
from primary culture were incubated with the vehicle or 10
µM forskolin, and these cells were harvested at 2, 4 and 24
h after the forskolin application.
Open field test (OFT) and elevated plus
maze test
One day after surgery, all the rats were transferred
to a quiet room to assess their anxiety activity. Each rat was
placed in the center of a black polycarbonate box (100×100×48 cm,
length x width x height), which had been cleared by 75% ethanol to
reduce the inference of odors. Their locomotor activity, times of
crossing the central area and forelegs lifting from the floor were
recorded for 5 min using a video camera that was mounted 100 cm
above the arena.
One hour after OFT, the anxiety-like behavior of the
rats was determined using the elevated plus maze (EPM) test. It was
a cross-shaped platform constituted of 4 arms (50×10 cm) and was 50
cm elevated above ground. The rats were placed in the central area
(10×10 cm) of the maze cleared by 75% ethanol towards an open arm.
Their behavior were recorded for 5 min using a video camera that
was mounted 100 cm above the arena. All data were analyzed using
Xinruan software (XR-Xmaze+; Xinruan, Shanghai,
China).
Real-time-polymerase chain reaction
(RT-PCR)
For the analysis of mRNA expression, total
hypothalamic RNA was extracted using TRIzol reagent (15596-026;
Invitrogen) according to the manufacturer' s instructions. The
purity and integrity of the RNA were examined spectroscopically
before obtaining cDNA using the GoScript™ Reverse Transcription
system (M1705; Promega). The reactions were set up with 10
µl SYBR-Green Real Master Mix (Promega), 1.6 µl
primer mixture (200 nM), and 1.6 µl cDNA template. The
thermal cycling conditions were as follows: 95°C, 3 min for
denaturation, followed by 38 cycles of 95°C, 10 sec, and 60°C, 30
sec, and 72°C for 30 sec. After the cycles, a melting curve
analysis was performed to ensure the purity of PCR products.
For the analysis of miRNA expression, miRNA was
extracted using the miRcute miRNA assay (DP501; Tiangen Biotech,
Beijing, China). The purity and integrity of the RNA were also
examined spectroscopically before obtaining cDNA using the
GoScript™ Reverse Transcription system (M1705; Promega).
The primers that were used for the analysis of mRNA
and miRNA expression (Table I)
were designed and synthesized by Invitrogen and purified by high
performance liquid chromatography (HPLC). All experiments were run
in triplicate and relative mRNA and miRNA levels were analyzed by
means of the 2−ΔΔCt method and normalized to
glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and U6 RNA,
respectively.
 | Table IPrimers for RT-PCR. |
Table I
Primers for RT-PCR.
| Gene symbol | Primer
sequences |
|---|
| CRH | F: CTC TCT GGA TCT
CAC CTT CCA C |
| R: CTA AAT GCA GAA
TCG TTT TGG C |
| CRHR1 | F: TGG AAC CTC ATC
TCG GCT TT |
| R: GTG AGC TGG ACC
ACA AAC CA |
| CRHR2 | F: TTC CTG CTG CAA
CTC ATC GA |
| R: GCG GCA CCA GAC
CTC ATT |
| AVP | F: TGC CTG CTA CTT
CCA GAA CTG C |
| R: AGG GGA GAC ACT
GTC TCA GCT C |
| AVPR1a | F: GCG GAA AGA CAG
CGT CCT CGC GAC A |
| R: GCT CAT GCT ATC
GGA GTC ATC CTT GGC GAA T |
| AVPR1b | F: AGA TTC TAC CAA
TGT GGC TTT C |
| R: ATG GTG GCT CAA
GGA ACG |
| rno-miR-34b | F: ACA CTC CAG CTG
GGA GGC AGT GTA ATT AGC |
| R: TGG TGT CGT GGA
GTC G |
| U6 | F: CTC GCT TCG GCA
GCA CA |
| R: AAC GCT TCA CGA
ATT TGC GT |
| GAPDH | F: GTA TGA CTC TAC
CCA CGG CAA GT |
| R: TTC CCG TTG ATG
ACC AGC TT |
Western blot analysis
The hypothalamic tissue was isolated from the rat
brains and homogenized in RIPA buffer (9806; Cell Signaling
Technology, Danvers, MA, USA). Following calibration by the BCA
protein assay kit (23225; Pierce Pharmaceuticals, Waltham, MA, USA)
the hypothalamic supernatant was denatured for 10 min at 100°C in a
solution of 4X Laemmli sample buffer (161-0747; Bio-Rad, Hercules,
CA, USA). The proteins were separated using Bio-Rad equipment
(PowerPac Universal; Bio-Rad) and transferred onto polyvinylidene
fluoride (PVDF) membranes (ISEQ00010; Merck Millipore, Darmstadt,
Germany). The PVDF membranes were then incubated in 5% non-fat milk
for 1 h at room temperature and incubated at 4°C in primary
antibodies overnight (CRHR1: AP01194PU-N, 1:500; Acris Antibodies
GmbH, Herford, Germany).
After washing in buffer (TBS-0.1% Tween-20), the
membranes were incubated with HRP-conjugated rabbit anti-goat IgG
(H+L) (SA00001-4; Proteintech, Chicago, IL, USA), diluted at
1:10,000, for 2 h at 4°C. Target protein signals were detected
using an ECL detection kit (Immobilon Western Chemiluminescent
Horseradish Peroxidase Substrate p90720; Millipore) and exposed
using an Image Quant LAS 4000 mini (GE Healthcare, Buckinghamshire,
UK). The signals were quantified using Quantity One software. The
results for signal intensity were expressed in arbitrary
densitometric units, after normalizing to GAPDH (ab181602; Abcam,
Cambridge, UK) as an internal standard.
Immunofluorescence (IF)
After being cultured for 1 week, the cells were
washed with hanks balanced salt solution (HBSS: C0218; Beyotime,
Jiangsu, China) and fixed using 4% paraformaldehyde (PFA) in 0.01 M
phosphate-buffered saline (PBS) at room temperature for 10 min.
They were then blocked with 10% FBS at 37°C for 1 h before being
incubated in a mouse polyclonal antibody against NeuN (ABN78;
1:1,000; Millipore) diluted in PBS containing 1% bovine serum
albumin (BSA: 9048-46-8; Amersco, Solon, OH, USA), 0.02% sodium
azide and 0.03% Triton X-100 at 4°C overnight. After rinsing, the
cells were incubated in secondary antibody solutions [1:500, Alexa
Flour 488 donkey anti-mouse IgG (H+L) antibody, A11032; Life
Technologies] for 1 h at room temperature. The cells were analyzed
using a Fluorescence microscope (Leica, Wetzlar, Germany).
Luciferase reporter assay
A forward primer (GCTAGCTGCAAGCTCACTGACGAGCC) and
reverse primer (TCTAGAGGACTGGACCATTCTAACCC) were used to amplify
the segment of CRHR1 3′-UTR sequence by RT-PCR. The Dual-Luciferase
reporter genes were constructed using the psiCHECKTM-2 vector
(Promega) and the 3′-UTR sequences of rat CRHR1. The sequences were
introduced between the NotI and XhoI sites to
Renilla luciferase 3′-UTR. The Firefly luciferase vector was
used for internal reference. Constructs with mutated 3′-UTR of
CRHR1 were used as negative controls.
The 293T cells were cultured in DMEM that was
supplemented with 10% FBS. A total of 4×104 cells/well
were seeded onto 24-well plates. Following 24 h in culture, the
cells were transfected using transfection reagent
(FuGENE® HD Transfection Reagent, E2312; Promega) with a
mixture containing 200 ng/ml of the Dual-Luciferase reporter
plasmid and 40 nM miR-34b mimic or NC mimic. The cells transfected
with the vectors containing a mutation in the 3′-UTR of CRHR1
(p-Luc-3_-UTR MUT CRHR1) served as controls for normalization. When
the cells were transfected for 24 h, the luciferase activity was
measured using a Dual-Lucferase® Reporter assay system
(E1910; Promega). All transfections were repeated independently 3
times.
Statistical analysis
The data are presented as the mean ± standard
deviation (SD) and were analyzed using SPSS 17.0 software. For
statistical comparisons, the values were subjected to a one-way
ANOVA followed by Tukey's test among the groups. A P-value of
<0.05 was considered to indicate a statistically significant
difference.
Results
Surgery-induced anxiety-like behavior and
hyperactivity of the HPA axis
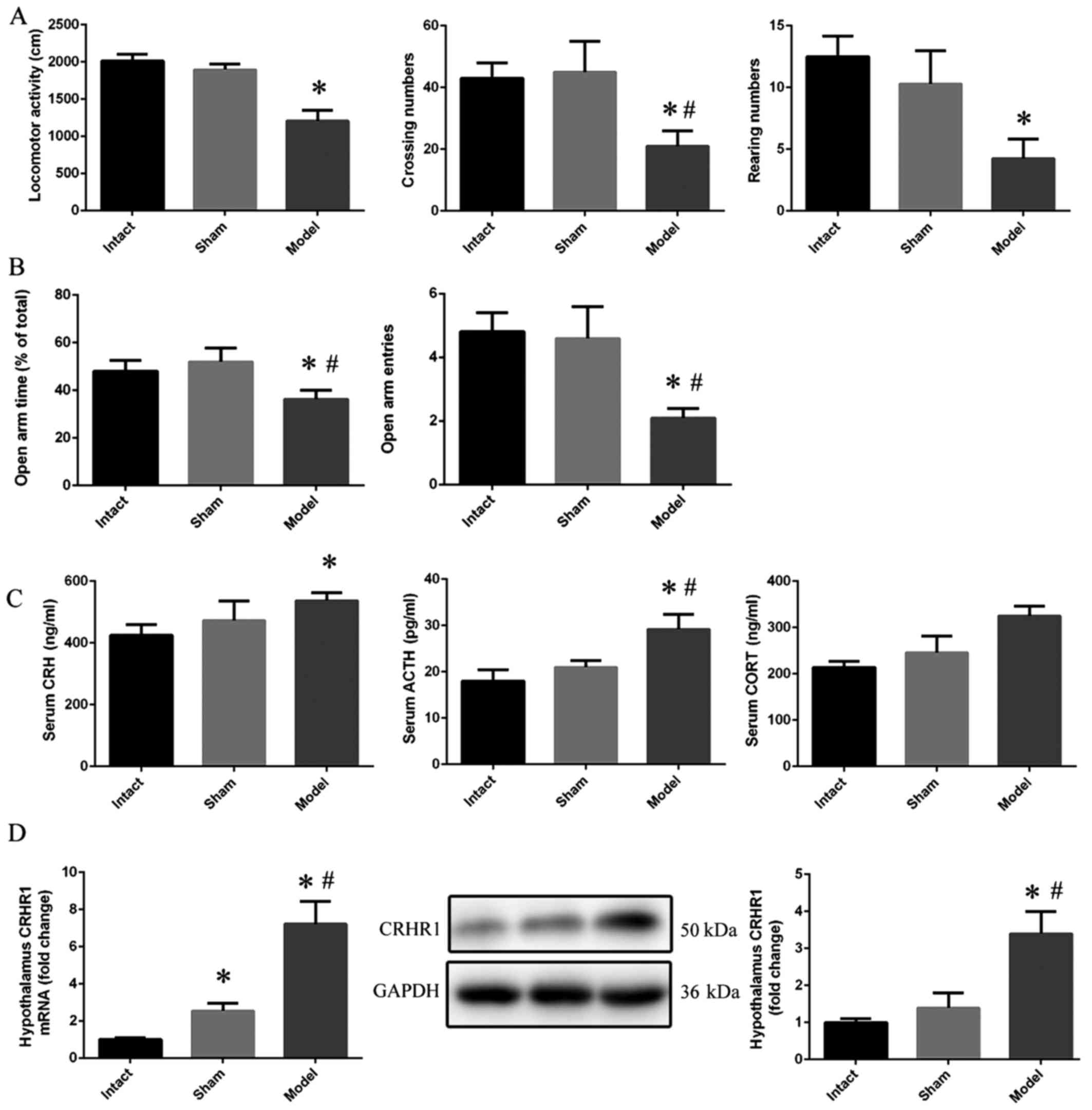
The results of the OFT revealed a significant
decrease in locomotor activity in the model group compared with the
intact group (p<0.05). Moreover, the crossing numbers
(p<0.05) and rearing numbers (p<0.01) in the model group also
exhibited a statistically significant decreasing trend when
compared with the intact group (Fig.
1A). In the EPM test, the rats in the model group demonstrated
a lower open arm time (p<0.05) and open arm entries (p<0.01)
compared with those in the intact and sham group (Fig. 1B). Moreover, the concentrations of
serum CRH (p<0.05), ACTH (p<0.01) and CORT (p<0.001)
increased significantly in the model group when compared with the
intact group (Fig. 1C). In
addition, the mRNA (p<0.001) and protein (p<0.01) expression
of CRHR1 in the model group was upregulated when compared with the
intact group (Fig. 1D).
Surgery-induced anxiety and hyperactivity
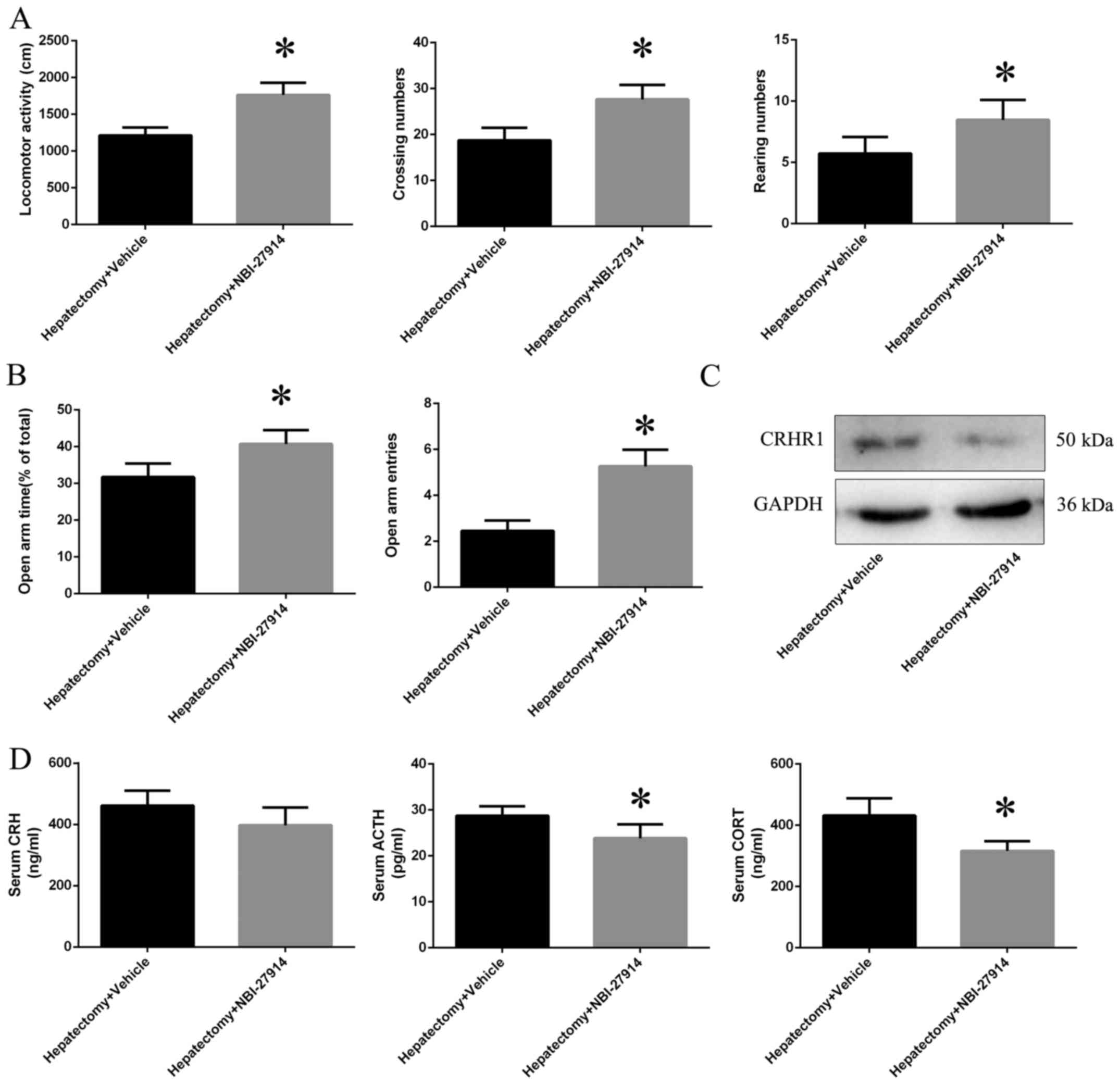
of the HPA axis is blocked by NBI-27914
Increases in locomotor activity (p<0.01),
crossing numbers (p<0.05) and rearing numbers (p<0.01) in the
OFT were observed in the hepatectomy + NBI-27914 group when
compared with the hepatectomy + vehicle group (Fig. 2A); we also observed increases in
both the open arm time (p<0.05) and entries (p<0.01)
(Fig. 2B). Moreover, CRHR1
protein expression level was downregulated in the hepatectomy +
NBI-27914 group (p<0.01) compared with the hepatectomy + vehicle
group (Fig. 2C). The
concentrations of serum ACTH (p<0.05) and CORT (p<0.01) were
decreased in the hepatectomy + NBI-27914 group when compared with
the hepatectomy + vehicle group; however, there was no difference
in the levels of CRH (p>0.05) between the hepatectomy + vehicle
and hepatectomy + NBI-27914 group (Fig. 2D).
Forskolin induces CRH and AVP signaling
in primary hypothalamic neurons
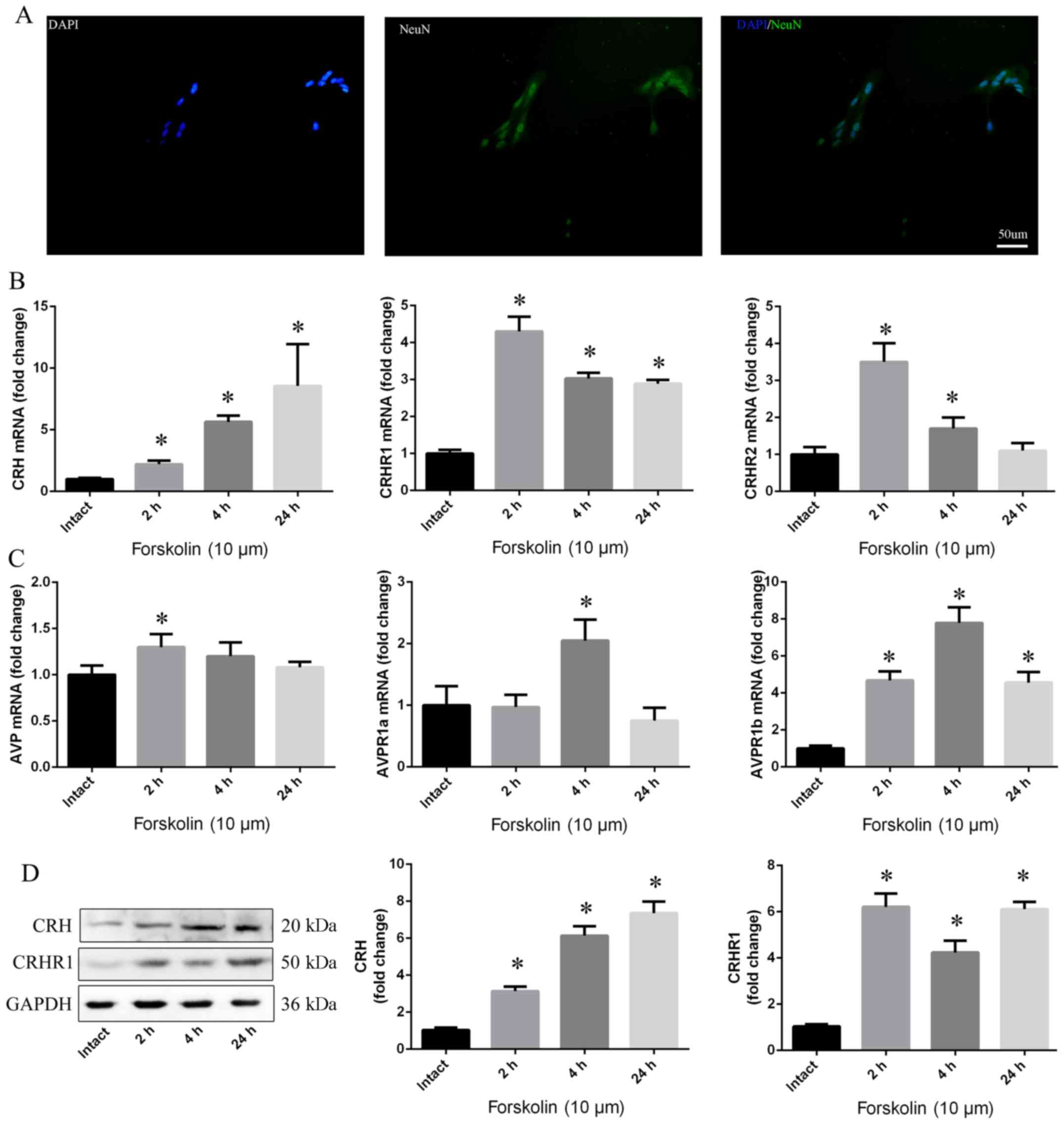
After 7 days in culture, hypo-thalamic neuronal
cells were observed by NeuN using immunofluorescence, showing that
approximately 90% of the cells were neurons (Fig. 3A). The mRNA levels of CRH, CRHR1,
CRHR2, AVP, AVPR1a and AVPR1b were enhanced in response to
forskolin, which was observed after 2, 4 and 24 h (p<0.05)
(Fig. 3B and C). Moreover, a
similar trend in CRH and CRHR1 protein levels was observed in the
hypothalamic neurons (p<0.05) (Fig. 3D).
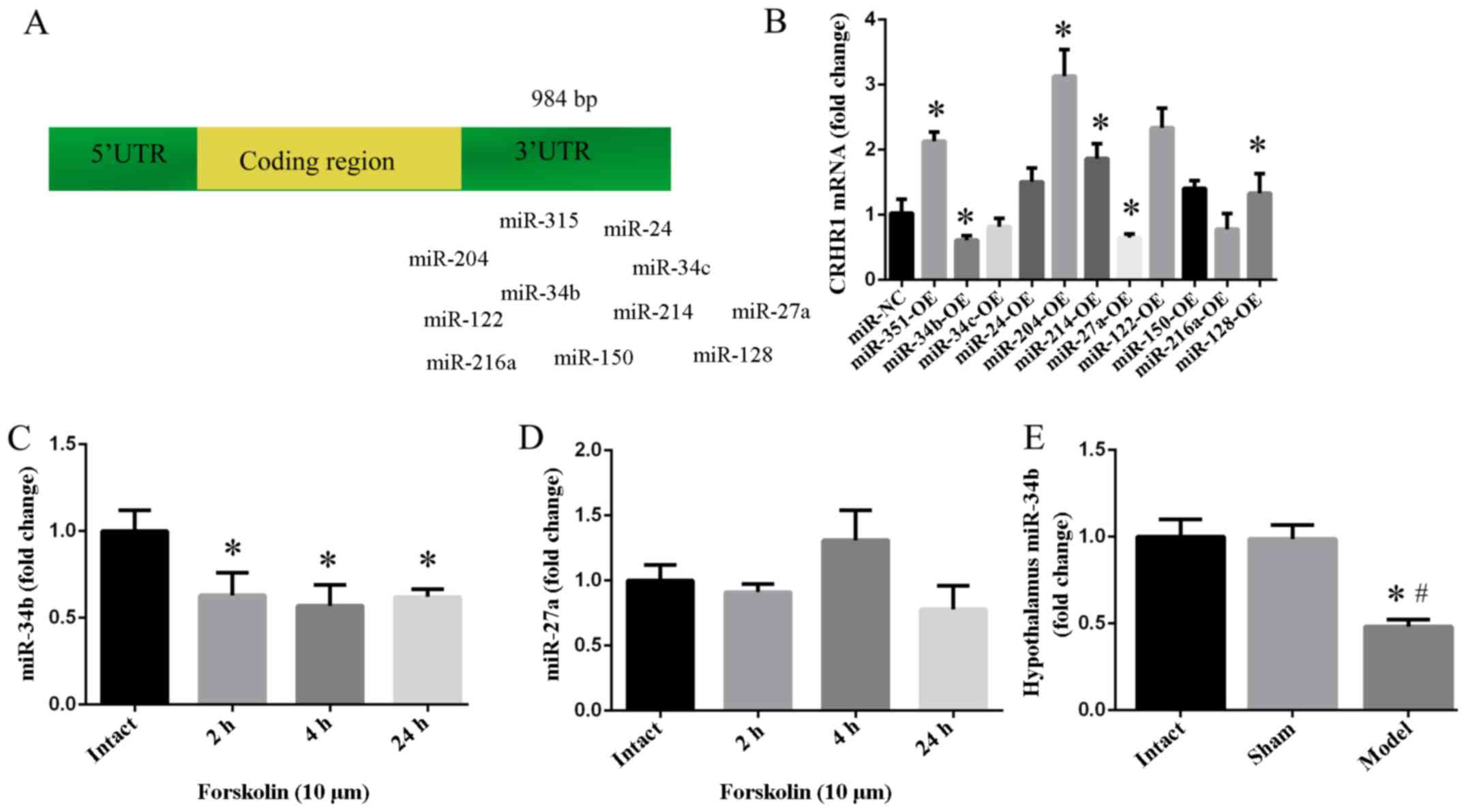
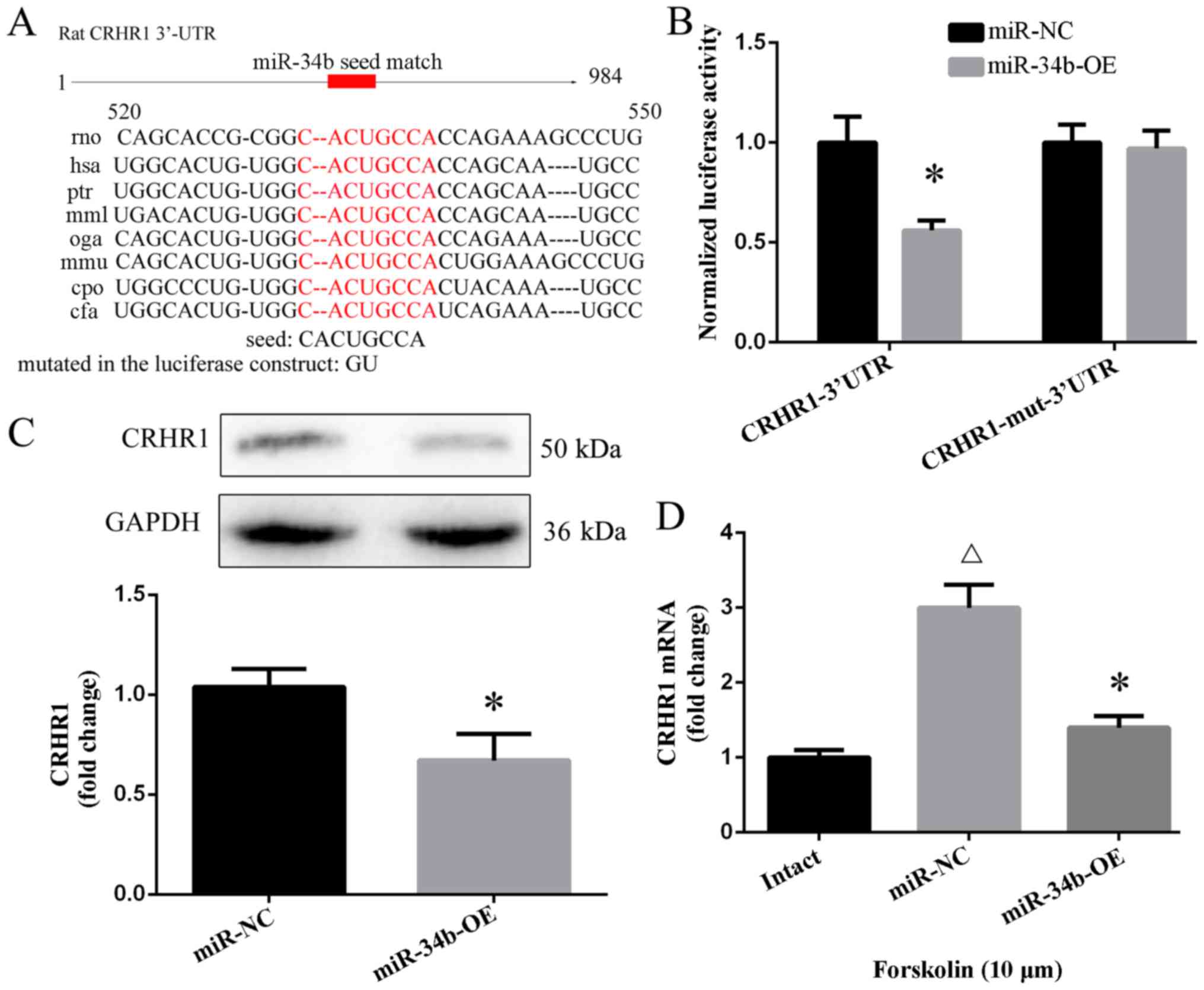
CRHR1 is a target of miR-34b
From previous bioinformatic analyses, particularly
research from http://www.targetscan.org/vert_61/ and http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2/,
miR-24, miR-34b, miR-34c, miR-27a, miR-122, miR-128, miR-150,
miR-204, miR-214 and miR-216a were identified as candidate miRNAs
for binding to the 3′-UTR of CRHR1 mRNA (Fig. 4A). Following the overexpression of
these miRNAs, CRHR1 mRNA was found to be decreased following the
administration of miR-27a (p<0.05) and miR-34b (p<0.05)
(Fig. 4B). miR-34b was observed
to be decreased in response to forskolin whithin 1 day (p<0.05)
(Fig. 4C), while there was no
significant effect on miR-27a (p>0.05) (Fig. 4D). Compared with the intact group,
hypothalamic miR-34b in the model group exhibited a decreasing
trend (p<0.01) (Fig. 4E).
The genes with a mutation in the seed region of the
3′-UTR of CRHR1 were unable to bind to miR-34b (Fig. 5A), thereby not eliminating CRHR1
mRNA. The luciferase activity of the cells transfected with miR-34b
expression and p-Luc-3′-UTR CRHR1 was decreased by 50% when
compared with the cells that were co-transfected with miR-NC mimic
and p-Luc-3′-UTR CRHR1 (p<0.05). The negative control construct
of mutations in the 3′-UTR of CRHR1 showed no obvious change in
luciferase activity (p>0.05) (Fig.
5B). The overexpression of miR-34b downregulated the CRHR1
protein levels (p<0.05) (Fig.
5C). Moreover, there was an increase in the CRHR1 mRNA level by
forskolin in the primary hypothalamus after 1 day (p<0.01),
while the overexpression of miR-34b attenuated this increase in the
CRHR1 mRNA level compared with the miR-NC group (p<0.01)
(Fig. 5D).
Surgery-induced anxiety and hyperactivity
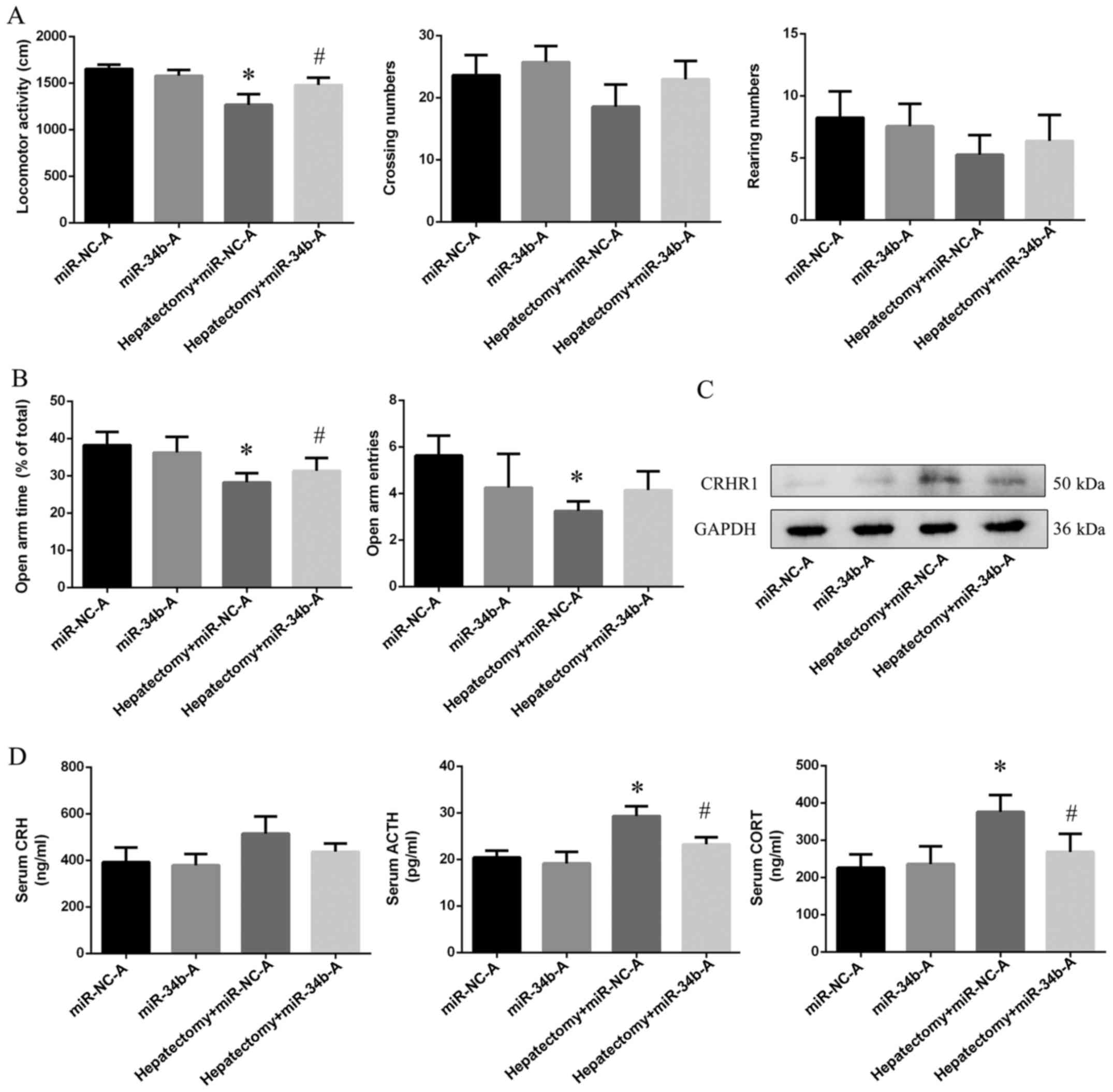
of the HPA axis is blocked by the overexpression of miR-34b
Using cerebral stereo-taxis, miR-34b agomiR was used
to induce the overexpression of miR-34b in the hypothalamus before
surgery. As shown by the results of OFT, the locomotor activity
(p<0.01) was decreased in the hepatectomy + miR-NC-A group
compared with the miR-NC-A group. The overexpression of miR-34b
using agomir in the rats in the hepatectomy + miR-34b-A group
increased their locomotor activity (p<0.01) compared with the
rats in the hepatectomy + miR-NC-A group (Fig. 6A). Furthermore, both the open arm
time (p<0.01) and entries (p<0.05) were decreased in the
hepatectomy + miR-NC-A group compared with the miR-NC-A group, and
the open arm time was increased (p<0.05) in the hepactomy +
miR-34b-A group compared with the hepatectomy + miR-NC-A group
(Fig. 6B). In addition, we found
that CRHR1 expression in the hepatectomy + miR-NC-A group was
increased compared with the miR-NC-A group (p<0.001), and was
decreased in the hepatectomy + miR-NC-A group (p<0.05) compared
with the hepatectomy + miR-34b-A group (Fig. 6C).
The ACTH (p<0.05) and CORT (p<0.01) levels
were upregulated in the hepatectomy + miR-NC-A group when compared
with the miR-NC-A group. Moreover, the levels of ACTH (p<0.05),
CORT (p<0.05) in the hepatectomy + miR-34b-A group were
decreased when compared with those in the hepatectomy + miR-NC-A
(Fig. 6D).
Discussion
Trauma affects a large number of individuals and
must be considered a significant public health issue.
Trauma-related disorders, particularly anxiety, are commonly
experienced by patients who are exposed to emergency trauma or
undergo surgery. Patients with post-surgery depression have been
found to be a host of poor surgical recovery outcomes (2).
Anxiety is often characterized by a malfunction of
the HPA axis (16), which is a
hormonal pathway that is initiated by the release of CRH from the
PVN in the hypothalamus. Thus, the release of CRH stimulates the
secretion of ACTH into the bloodstream from the anterior pituitary
gland where axons from parvocellular neurons in the PVN project
through the median eminence. ACTH stimulates the synthesis and
secretion of cortisol from the adrenal glands. Glucocorticoids can
suppress CRH or ACTH gene expression in the hypothalamus or
pituitary gland. It has been shown that the HPA axis modulates
childhood trauma in the development of borderline personality
disorder (17). The HPA axis has
been shown to be associated with anxiety (18).
In the present study, a partial hepatectomy was used
as a type of stress with which to excessively activate the HPA
axis, as research has demonstrated an evident increase in CORT
levels in rodents following a hepatectomy (19,20) in order to facilitate liver
regeneration. We found that the rats subjected to hepatectomy were
anxious and experienced a dysfunction of the HPA axis, based upon
the results of the OFT, EPM test and RIA.
As the CRH system in the hypothalamus is the central
integrator in the HPA axis (21),
a dysregulated CRH/CRHR1 system is considered to be one of the most
common mechanisms that is associated with emotional disorders
(22). CRHR1 exaggerates the CRH
secretion in the hypothalamus as there is a positive ultrashort
loop feedback of the CRH system in the hypothalamus (23). CRHR1 in the PVN has been shown to
be involved in prenatal hypoxia exposure-induced anxiety in adult
male rat offspring (24). CRHR1
in the amygdala has been reported to be involved in anxiety induced
by environmental factors (25).
The CRHR1 gene determines stress vulnerability (26) and contributes significantly to the
depression severity rating (27).
Another study demonstrated that the methylation of CRHR1 in the
hypo-thalamus is linked to anxiety-like behavior (28). With increases in CRH, ACTH and
CORT levels, CRHR1 was increased both at the mRNA and protein
levels following surgery in the present study. Furthermore, the
rats also expressed anxiety-like behavior. NBI-27914 is a CRHR1
specific antagonist (29,30). In this study, we found that the
application of NBI-27914 in the hypothalamus decreased the
hyperactivity of the HPA axis and thus attenuated anxiety-like
behavior by the inhibition of CRHR1. These results indicate that
CRHR1 is involved in surgery-induced hyperactivity and anxiety.
miRNAs are small, non-coding RNAs that
post-transcriptionally regulate gene expression. Hundreds of miRNAs
have been identified throughout the brain and are involved in the
regulation of neuronal development, function and dysfunction
(31). miRNAs have been proven to
affect anxiety by altering synaptic plasticity (32). Serum levels of miRNA-132 and
miRNA-128 have been reported to affect anxiety by targeting BDNF
(33–35), which has been proven to mediate
anxiety through neuronal neurogenesis and differentiation. The
results from a previous study demonstrated that miR-134 mediate
homeostatic synaptic depression (36).
miR-1202 enriched in the human brain has been shown
to be associated with the pathophysiology of depression by
targeting metabotropic glutamate receptor 4 (37). miR-185 and miR-491 also
participate in the pathogenesis of depression (38). Furthermore, miRNAs that possess
polymorphisms may affect depression risk and treatment (39).
Nevertheless, limited research has been conducted
concerning the association between miRNAs and the HPA axis in
anxiety, particularly surgery-induced anxiety. The amygdala,
hippocampus and other limbic structures can affect the function of
the HPA axis (40).
Following bioinformatic analysis using TargetScan
and miRWalk, 11 miRNAs were found to be overexpressed in primary
hypothalamic neurons. miR-34b and miR-27a were found to have a
negative association with CRHR1 mRNA.
Forskolin was used to increase the expression of
cyclic AMP in vitro and activated the CRH system, which has
been proven to do so by previous studies (41,42). In this study, we found an evident
increase in activity in the CRH and AVP systems in primary
hypothalamic neurons following treatment with forskolin. Moreover,
accompanying this increasing trend in CRHR1 mRNA levels in the
hypothalamic neurons, miR-34b was decreased following treatment
with forskolin. Moreover, the overexpression of miR-34b decreased
the CRHR1 protein levels and the forskolin-induced increase in the
CRHR1 mRNA levels. A dual luciferase assay verified that miR-34b
decreased the CRHR1 mRNA level by binding to its 3′-UTR. The
overexpression of miR-34b by a miR-34b agomir that was injected
into the PVN decreased the mRNA and protein level of CRHR1 in the
hypothalamus and attenuated the dysfunction of the HPA axis and
anxiety-like behavior.
In brief, the results of this study demonstrated
that the HPA axis was hyperactive in rats following surgery and
anxiety-like behavior increased when compared with the intact (not
operated) rats. We characterized the role of the HPA axis and
miR-34b in the pathogenesis of trauma-induced anxiety and validated
that miR-34b is a novel miRNA that regulates the CRHR1 mRNA level
in the hypothalamus. We clarified that CRHR1 and its related miRNAs
play an important role in the regulation of the HPA axis.
Acknowledgments
This study was supported by the National Key Program
of Basic Science (973) 2013CB531906 and the National Natural
Science Fund of China (81573712, 81471370).
References
|
1
|
Hossain M, Zimmerman C, Abas M, Light M
and Watts C: The relationship of trauma to mental disorders among
trafficked and sexually exploited girls and women. Am J Public
Health. 100:2442–2449. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tully PJ, Winefield HR, Baker RA, Denollet
J, Pedersen SS, Wittert GA and Turnbull DA: Depression, anxiety and
major adverse cardiovascular and cerebrovascular events in patients
following coronary artery bypass graft surgery: A five year
longitudinal cohort study. Biopsychosoc Med. 9:142015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Castillo RC, Wegener ST, Heins SE,
Haythornthwaite JA and Mackenzie EJ: Longitudinal relationships
between anxiety, depression, and pain: Results from a two-year
cohort study of lower extremity trauma patients. Pain.
154:2860–2866. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Karanci AN and Dirik G: Predictors of pre-
and postoperative anxiety in emergency surgery patients. J
Psychosom Res. 55:363–369. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Norrholm SD and Ressler KJ: Genetics of
anxiety and trauma-related disorders. Neuroscience. 164:272–287.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Renoir T, Hasebe K and Gray L: Mind and
body: How the health of the body impacts on neuropsychiatry. Front
Pharmacol. 4:1582013. View Article : Google Scholar
|
|
7
|
Seitz DP, Bell CM, Gill SS, Reimer CL,
Herrmann N, Anderson GM, Newman A and Rochon PA: Risk of
perioperative blood transfusions and postoperative complications
associated with serotonergic antidepressants in older adults
undergoing hip fracture surgery. J Clin Psychopharmacol.
33:790–798. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jeong BO, Kim SW, Kim SY, Kim JM, Shin IS
and Yoon JS: Use of serotonergic antidepressants and bleeding risk
in patients undergoing surgery. Psychosomatics. 55:213–220. 2014.
View Article : Google Scholar
|
|
9
|
Pariante CM and Lightman SL: The HPA axis
in major depression: Classical theories and new developments.
Trends Neurosci. 31:464–468. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zuloaga DG, Jacobskind JS and Raber J:
Methamphetamine and the hypothalamic-pituitary-adrenal axis. Front
Neurosci. 9:1782015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jacobson L:
Hypothalamic-pituitary-adrenocortical axis: Neuropsychiatric
aspects. Compr Physiol. 4:715–738. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fan HM, Sun XY, Guo W, Zhong AF, Niu W,
Zhao L, Dai YH, Guo ZM, Zhang LY and Lu J: Differential expression
of microRNA in peripheral blood mononuclear cells as specific
biomarker for major depressive disorder patients. J Psychiatr Res.
59:45–52. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Durairaj RV and Koilmani ER: Environmental
enrichment modulates glucocorticoid receptor expression and reduces
anxiety in Indian field male mouse Mus booduga through upregulation
of microRNA-124a. Gen Comp Endocrinol. 199:26–32. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hanin G, Shenhar-Tsarfaty S, Yayon N, Yau
YH, Bennett ER, Sklan EH, Rao DC, Rankinen T, Bouchard C,
Geifman-Shochat S, et al: Competing targets of microRNA-608 affect
anxiety and hypertension. Hum Mol Genet. 23:4569–4580. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang YN, Lai CC, Chiu CT, Lin JJ and Wang
JY: L-ascorbate attenuates the endotoxin-induced production of
inflammatory mediators by inhibiting MAPK activation and NF-κB
translocation in cortical neurons/glia Cocultures. PLoS One.
9:e972762014. View Article : Google Scholar
|
|
16
|
Sotnikov S, Wittmann A, Bunck M, Bauer S,
Deussing J, Schmidt M, Touma C, Landgraf R and Czibere L: Blunted
HPA axis reactivity reveals glucocorticoid system dysbalance in a
mouse model of high anxiety-related behavior.
Psychoneuroendocrinology. 48:41–51. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Martín-Blanco A, Ferrer M, Soler J, Arranz
MJ, Vega D, Calvo N, Elices M, Sanchez-Mora C, García-Martinez I,
Salazar J, et al: The role of hypothalamus-pituitary-adrenal genes
and childhood trauma in borderline personality disorder. Eur Arch
Psychiatry Clin Neurosci. 266:307–316. 2016. View Article : Google Scholar
|
|
18
|
Li C, Liu Y, Yin S, Lu C, Liu D, Jiang H
and Pan F: Long-term effects of early adolescent stress:
Dysregulation of hypothalamic-pituitary-adrenal axis and central
corticotropin releasing factor receptor 1 expression in adult male
rats. Behav Brain Res. 288:39–49. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cipriano C, Giacconi R, Muzzioli M,
Gasparini N, Orlando F, Corradi A, Cabassi E and Mocchegiani E:
Metallothionein (I+II) confers, via c-myc, immune plasticity in
oldest mice: Model of partial hepatectomy/liver regeneration. Mech
Ageing Dev. 124:877–886. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Witek-Janusek L, Yu M and Marotta SF:
Hypoxic and nycthemeral responses by the adrenal cortex of
partially hepatectomized rats. Aviat Space Environ Med. 55:538–541.
1984.PubMed/NCBI
|
|
21
|
Bonfiglio JJ, Inda C, Refojo D, Holsboer
F, Arzt E and Silberstein S: The corticotropin-releasing hormone
network and the hypothalamic-pituitary-adrenal axis: Molecular and
cellular mechanisms involved. Neuroendocrinology. 94:12–20. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wasserman D, Wasserman J and Sokolowski M:
Genetics of HPA-axis, depression and suicidality. Eur Psychiatry.
25:278–280. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ono N, Samson WK, McDonald JK, Lumpkin MD,
Bedran de Castro JC and McCann SM: Effects of intravenous and
intraventricular injection of antisera directed against
corticotropin-releasing factor on the secretion of anterior
pituitary hormones. Proc Natl Acad Sci USA. 82:7787–7790. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fan JM, Wang X, Hao K, Yuan Y, Chen XQ and
Du JZ: Upregulation of PVN CRHR1 by gestational intermittent
hypoxia selectively triggers a male-specific anxiogenic effect in
rat offspring. Horm Behav. 63:25–31. 2013. View Article : Google Scholar
|
|
25
|
Sotnikov SV, Chekmareva NY, Schmid B,
Harbich D, Malik V, Bauer S, Kuehne C, Markt PO, Deussing JM,
Schmidt MV, et al: Enriched environment impacts
trimethylthiazoline-induced anxiety-related behavior and immediate
early gene expression: Critical role of Crhr1. Eur J Neurosci.
40:2691–2700. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Labermaier C, Kohl C, Hartmann J, Devigny
C, Altmann A, Weber P, Arloth J, Quast C, Wagner KV, Scharf SH, et
al: A polymorphism in the Crhr1 gene determines stress
vulnerability in male mice. Endocrinology. 155:2500–2510. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schatzberg AF, Keller J, Tennakoon L,
Lembke A, Williams G, Kraemer FB, Sarginson JE, Lazzeroni LC and
Murphy GM: HPA axis genetic variation, cortisol and psychosis in
major depression. Mol Psychiatry. 19:220–227. 2014. View Article : Google Scholar
|
|
28
|
Wang X, Meng FS, Liu ZY, Fan JM, Hao K,
Chen XQ and Du JZ: Gestational hypoxia induces sex-differential
methylation of Crhr1 linked to anxiety-like behavior. Mol
Neurobiol. 48:544–555. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Silberman Y and Winder DG: Corticotropin
releasing factor and catecholamines enhance glutamatergic
neurotransmission in the lateral subdivision of the central
amygdala. Neuropharmacology. 70:316–323. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lowery-Gionta EG, Navarro M, Li C, Pleil
KE, Rinker JA, Cox BR, Sprow GM, Kash TL and Thiele TE:
Corticotropin releasing factor signaling in the central amygdala is
recruited during binge-like ethanol consumption in C57BL/6J mice. J
Neurosci. 32:3405–3413. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Im HI and Kenny PJ: MicroRNAs in neuronal
function and dysfunction. Trends Neurosci. 35:325–334. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hu Z, Yu D, Gu QH, Yang Y, Tu K, Zhu J and
Li Z: miR-191 and miR-135 are required for long-lasting spine
remodelling associated with synaptic long-term depression. Nat
Commun. 5:32632014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li YJ, Xu M, Gao ZH, Wang YQ, Yue Z, Zhang
YX, Li XX, Zhang C, Xie SY and Wang PY: Alterations of serum levels
of BDNF-related miRNAs in patients with depression. PLoS One.
8:e636482013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Briones TL and Woods J: Chronic binge-like
alcohol consumption in adolescence causes depression-like symptoms
possibly mediated by the effects of BDNF on neurogenesis.
Neuroscience. 254:324–334. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yoneyama M, Tanaka M, Hasebe S, Yamaguchi
T, Shiba T and Ogita K: Beneficial effect of cilostazol-mediated
neuronal repair following trimethyltin-induced neuronal loss in the
dentate gyrus. J Neurosci Res. 93:56–66. 2015. View Article : Google Scholar
|
|
36
|
Fiore R, Rajman M, Schwale C, Bicker S,
Antoniou A, Bruehl C, Draguhn A and Schratt G: MiR-134-dependent
regulation of Pumilio-2 is necessary for homeostatic synaptic
depression. EMBO J. 33:2231–2246. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lopez JP, Lim R, Cruceanu C, Crapper L,
Fasano C, Labonte B, Maussion G, Yang JP, Yerko V, Vigneault E, et
al: miR-1202 is a primate-specific and brain-enriched microRNA
involved in major depression and antidepressant treatment. Nat Med.
20:764–768. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Serafini G, Pompili M, Hansen KF, Obrietan
K, Dwivedi Y, Shomron N and Girardi P: The involvement of microRNAs
in major depression, suicidal behavior, and related disorders: A
focus on miR-185 and miR-491-3p. Cell Mol Neurobiol. 34:17–30.
2014. View Article : Google Scholar
|
|
39
|
He Y, Zhou Y, Xi Q, Cui H, Luo T, Song H,
Nie X, Wang L and Ying B: Genetic variations in microRNA processing
genes are associated with susceptibility in depression. DNA Cell
Biol. 31:1499–1506. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Meyer DL, Davies DR, Barr JL, Manzerra P
and Forster GL: Mild traumatic brain injury in the rat alters
neuronal number in the limbic system and increases conditioned fear
and anxiety-like behaviors. Exp Neurol. 235:574–587. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kageyama K, Itoi K, Iwasaki Y, Niioka K,
Watanuki Y, Yamagata S, Nakada Y, Das G, Suda T and Daimon M:
Stimulation of corticotropin-releasing factor gene expression by
FosB in rat hypothalamic 4B cells. Peptides. 51:59–64. 2014.
View Article : Google Scholar
|
|
42
|
Nikodemova M, Kasckow J, Liu H,
Manganiello V and Aguilera G: Cyclic adenosine 3′,5′-monophosphate
regulation of corticotropin-releasing hormone promoter activity in
AtT-20 cells and in a transformed hypothalamic cell line.
Endocrinology. 144:1292–1300. 2003. View Article : Google Scholar : PubMed/NCBI
|