Introduction
Angiogenesis is an essential process for embryonic
development and tissue repair, whereas abnormal angiogenesis is a
fundamental characteristic in the pathophysiology of ocular
diseases, such as retinopathy of prematurity (ROP), diabetic
retinopathy and choroidal neovascularization (CNV), usually
associated with age-related macular degeneration. Retinochoroidal
neovascularization diseases can lead to blindness in developed
countries (1). Among the several
different therapies that are currently utilized, anti-vascular
endothelial growth factor (VEGF) therapy has undoubtedly appeared
to be effective (2). However,
according to the SEVEN-UP study (seven-year outcome in
ranibizumab-treated patients in ANCHOR, MARINA and HORIZON: a
multicenter cohort), best-corrected visual acuity (BCVA) in 34% of
the eyes examined declined by 15 letters or more, with an overall
mean decline of 8.6 letters, at a mean of 7.3 years after entering
the ANCHOR or MARINA trial (3).
Similar outcomes were also observed in patients enrolled in the
comparison of AMD treatment trials (CATT) study (4). Therefore, investigating additional
VEGF-independent pathways that trigger abnormal angiogenesis in the
eye seems critical.
Increasing evidence has suggested that macrophages
play a significant role in both physiological and pathological
angiogenesis (5–7). Macrophages were initially believed
to be solely pro-inflammatory and destructive phagocytes until
demonstrated to be able to convert to a pro-healing phenotype
(8). Recently, it was reported
that macrophages represent approximately 50% of the tumor mass and
play vital roles in the regulation of angiogenesis and tumor
progression (9). Researchers have
demonstrated that cells of the monocyte-macrophage lineage are
characterized by diversity and plasticity, as they can shift
between different activation modes driven by the local
microenvironment and have divergent functions. Unique stimuli endow
macrophages with distinct molecular phenotypes and effector
functions (10). M1 macrophages
were believed to produce high levels of oxidative metabolites and
pro-inflammatory cytokines, and combat invading pathogens and tumor
cells, while M2 macrophages promote angiogenesis and remold the
matrix, orchestrating homeostasis following the inflammatory
response, which was confirmed to be associated with the resolution
of chronic leg ulcers (11),
atherosclerotic lesions (12) and
traumatic spinal cord injury (10). Yet, the roles of macrophage
populations in angiogenesis remain controversial and are poorly
understood (13). Some studies
have found that decreased numbers of M1/M2 ratios correlate with
biomaterial vascularization (14–18), while others have revealed that
increased numbers of M1/M2 ratios contribute to enhanced
vascularization (19–21). Furthermore, it has been
demonstrated that macrophages are necessary and sufficient to
induce the regression of lens vasculature during development and to
inhibit abnormal blood vessels in eyes affected by age-related
macular degeneration (AMD) (22,23). However, alternative lines of
evidence have implicated the promoting role of macrophages in
abnormal blood vessel growth in AMD (24,25).
Although CD11c was a marker traditionally associated
with dendritic cells (DCs), a recent study found it to be expressed
by some macrophages (26).
Another recent study by Vianello et al on epicardial adipose
tissue considered CD11c-positive cells as M1 state macrophages
(27), and the study by Shu et
al on the prognostic value of polarized macrophages in patients
with hepatocellular carcinoma following curative resection also
labeled M1 macrophages with CD11c (28). The study by Lumeng et al
reported that CD11c-positive macrophages express pro-inflammatory
cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin
(IL)-6, while CD11c-negative ones express arginase 1 and IL-10.
They defined the former as M1 and the latter as M2. They also found
that most of the CD11c-positive macrophages were CD206-negative,
and that most of the CD206-positive macrophages were
CD11c-negative, suggesting two distinct subsets of macrophages
(29). Therefore, in the present
study, we aimed to distinguish retinal macrophages using
CD11c+F4/80+ as an M1 marker and
CD206+F4/80+ as an M2 marker in order to
evaluate the phenotypic distribution, gene expression and effector
function of retinal macrophages under the condition of
oxygen-induced ischemic retinopathy.
Materials and methods
Mice and ethics statement
All mice used in this study were pathogen-free (SPF)
C57BL/6 mice and kept under the conditions in compliance with the
ARVO Statement for the use of Animals in Ophthalmic and Vision
Research, and the National Institutes of Health Guide for the Care
and Use of Laboratory Animals with the approval (SYXK-2012–0026) of
the Scientific Investigation Board of Shanghai Jiaotong University
School of Medicine, Shanghai, China. All efforts were made to
minimize animal suffering.
Mouse model of oxygen-induced retinopathy
(OIR)
OIR was induced by exposure to high concentrations
of oxygen, followed by the return to normal room air; this leads to
ischemia. C57BL/6 pups (n=320) were exposed to 75% oxygen at
post-natal day (P)7 with their nursing mother and returned to room
air at P12, as previously described (30). The oxygen concentration was
continuously monitored and controlled with a controller
(BioSpherix, Lacona, NY, USA). Age-matched controls were kept in
room air.
Wholemount immunofluorescence staining of
mouse retinas
Platelet endothelial cell adhesion molecule
(PECAM)-1, CD11c and CD206 were selectively stained by in
vivo immunostaining as previously described (31). Briefly, the mice were administered
an intraocular injection of 1 µl primary anti-PECAM-1 (BD
Biosciences, San Jose, CA, USA), CD11c (ab11029; Abcam, Cambridge,
UK) and CD206 (MCA2235A; AbD Serotec, Kidlington, UK) antibodies
under a dissecting microscope with a Harvard Pump Microinjection
system (Harvard Apparatus, Holliston, MA, USA) using pulled glass
micropipettes (32) and then
euthanized 12 h later. The eyes were enucleated and fixed in 4%
formalin for 5 h. Retinas were dissected, washed and incubated with
secondary antibody [Alexa 555-conjugated goat anti-mouse IgG
(sc-362267; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA)
and Alexa 488-conjugated anti-rat IgG (4416s; Cell Signaling
Technology, Inc., Danvers, MA, USA)] at room temperature for 45 min
and then flat mounted (4 mice were enrolled in both the OIR and
normal control groups for each time point). For anti-mouse F4/80
and FITC-isolectin B4 immunostaining, dissected retinas were
incubated with PE-labeled rat anti-mouse F4/80 antibody
(12-4801-82; eBioscience, Vienna, Austria) or FITC-isolectin B4
(FL-1201; Vector Laboratories, Inc., Burlingame, CA, USA) at room
temperature for 45 min following fixation in 4% formalin for 5 h.
The samples were imaged with a confocal microscope (Zeiss LSM510
laser scanning confocal microscope; Zeiss, Oberkochen, Germany) or
a fluorescence microscope and captured with a digital still camera
(Nikon Instruments, Inc., New York, NY, USA). Three-dimensional
surface rendering of high-resolution confocal z-stacks was carried
out with Volocity software (Improvision) (6 mice were enrolled in
both the OIR and normal control groups for quantitative wholemount
staining and 4 mice were enrolled in both groups for
immunofluorescence staining assays at each time point).
Immunohistochemical staining of inducible
nitric oxide synthase (iNOS), arginase 1 and isolectin B4
C57BL/6 mice with or without oxygen-induced ischemic
retinopathy were euthanized at P13 and P18, and the eyes were
rapidly removed and frozen in optimum cutting temperature embedding
compound (Miles Diagnostics, Elkhart, IN, USA). The frozen sections
(10-µm-thick) were thawed, air-dried and fixed in 4%
pre-chilled paraformaldehyde (PFA). The sections were respectively
incubated in 5% bovine serum albumin followed by overnight
incubation at 4°C with monoclonal rat anti-mouse iNOS antibody
(13120s; Cell Signaling Technology, Inc.) and polyclonal rabbit
anti-mouse arginase 1 antibody (GTX109242; GeneTex, Irvine, CA,
USA). The sections were then respectively incubated in Alexa
555-conjugated anti-rat IgG (sc-3740), or Alexa 555-conjugated
anti-goat IgG (sc-362264) (both from Santa Cruz Biotechnology,
Inc.) and FITC-labeled isolectin B4 (FL-1201; Vector Laboratories
Inc.) for 45 min at room temperature. Sections were finally stained
with DAPI (sc-3598; Santa Cruz Biotechnology, Inc.) for 15 min at
room temperature to display the nuclei. The sections were
thoroughly washed with phosphate-buffered saline containing 0.25%
Triton X-100 (PBST) between all incubations. The sections were
examined under a Nikon microscope and captured as digital files
using a Nikon Digital Still Camera DXM 1200 (Nikon Instruments,
Inc.) (3 mice were enrolled in both the OIR and normal control
groups for each time point).
Reverse transcription-quantitative PCR
(RT-qPCR)
RNA was isolated from the retinas of mice with OIR
and age-matched controls using TRIzol reagent (Invitrogen,
Carlsbad, CA, USA) in accordance with the manufacturer's
instructions, and as previously described (33). Each sample of total RNA was
pre-treated with DNase I (Promega, Fitchburg, WI, USA), and 2
µg of each was reverse transcribed into complementary DNA
(cDNA) using M-MLV Transcriptase and oligo(dT) primers (Promega),
according to the manufacturer's instructions. Quantitative PCR
(qPCR) was performed as previously described (34). In brief, each PCR reaction was
carried out in a 20 µl volume using iQ SYBR-Green Supermix
(Roche, Basel, Switzerland) in ABI 7500, and normalized to
house-keeping gene, cyclophilin A, which has been reported to be
stable under many different conditions and used as a normalization
control in previous studies on retinal neovascularization (35–37); 10 mice were enrolled in both the
OIR and normal control groups for each time point. Two eyes from
one mouse were considered as one sample. The ΔΔCT method was used
for relative quantification. Primer sequences used were designed as
follows: murine CD11c forward, 5′-GTGCCCATCAGTTCCTTACA-3′ and
reverse, 5′-GAGAAGAACTGTGGAGCTGAC-3′; TNF-α forward,
5′-GAACTGGCAGAAGAGGCACT-3′ and reverse, 5′-AGGGTCTGGGCCATAGAACT-3′;
CD206 forward, 5′-GGAATCAAGGGCACAGAGTTA-3′ and reverse,
5′-ATTGTGGAGCAGATGGAA-3′; F4/80 forward,
5′-CGTCAGGTACGGGATGAATATAAG-3′ and reverse,
5′-CTATGCCATCCACTTCCAAGAT-3′; iNOS forward,
5′-TCTCCCTTTCCTCCCTTCTT-3′ and reverse, 5′-AAAC
TCAACCTCCTGACTGAAG-3′; CD163 forward, 5′-CAGACTGGTTGGAGGAGAAATC-3′
and reverse, 5′-TGACTT GTCTCTGGAAGCTG-3′; monocyte chemoattractant
protein-1 (MCP-1) forward, 5′-CTCGGACTGTGATGCCTTAAT-3′ and reverse,
5′-TAAATGCAAGGTGTGGATCCA-3′); CD16 forward,
5′-CGGGATGTTTGGTTCTTCAATC-3′ and reverse,
5′-CATACAGAGAGAGTGAGTGCAAG-3′; cyclophilin A forward,
5′-CAGACGCCACTGTCGCTTT-3′ and reverse,
5′-TGTCTTTGGAACTTTGTCTGCAA-3′.
Isolation of mouse retina
CD11b+ cells
The retinas of normal mice and those with OIR were
carefully dissected out and digested in pre-warmed 16.5 U/ml papain
solution (Worthington, Freehold, NJ, USA) for 30 min with gentle
pipetting, and the cell digestion suspension was then transferred
and passed through cell strainers (BD Falcon, Franklin Lakes, NJ,
USA) to obtain single cell suspension. The cells were spinned down
at 900 rpm. After gently removing the supernatant, the cell pellet
was suspended with 90 µl MACS buffer (BD Biosciences, San
Jose, CA, USA), mixed well with 10 µl anti-mouse CD11b
magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany),
incubated at 4°C for 20 min, washed once and resuspended in 500
µl MACS buffer and loaded on a pre-moistured MS column (BD
Biosciences, San Jose, CA, USA) to sort for CD11b+ cells
according to the manufacturer's instructions. The selected cells
were collected and proceeded to flow cytometric analysis.
Flow cytometric analysis
CD11b+ cells from the retinas of the mice
with OIR or the normal mice were resuspended in MACS buffer (BD
Biosciences, San Jose, CA, USA) and incubated with PE-conjugated
anti-mouse CD11c (12-0114-82; eBioscience), PE-conjugated
anti-mouse F4/80 (12-4801-82; eBioscience) and Alexa Fluor
647-conjugated CD206 (MCA2235A647; AbD Serotec) and the matching
control isotype IgG (MCA421; AbD Serotec) for 30 min at 4°C. The
cells were then washed and rinsed again and incubated with
secondary antibodies for 30 min at 4°C. The cells were then washed
and re-suspended in FACS buffer (BD Biosciences, San Jose, CA, USA)
and analyzed by flow cytometry (BD FACSCalibur flow cytometer; BD
Biosciences, Heidelberg, Germany). M1 macrophages were identified
as F4/80-positive/CD11c-positive/CD206-negative and M2 macrophages
were identified as F4/80-positive/CD11c-negative/CD206-positive.
Data analysis was performed using FlowJo software (Tree Star,
Ashland, OR, USA) (6 mice were enrolled in both the OIR and normal
control groups for each time point).
Microarray analysis
The high-throughput screening of differential mRNA
expression between the mice with OIR and the normal mice were
obtained using Affymetrix GeneChip Mouse Genome 430 2.0 arrays.
Genes were identified as differentially expressed if they exhibited
a fold change of at least 1.5 and a P-value <0.05. Two retinas
from one mouse were considered as one sample (6 mice were enrolled
in both the OIR and normal control groups).
Statistical analysis
Quantitative data are presented as the mean values ±
standard deviation (SD). Statistical significance was determined by
the two tailed Student's t-test and one-way ANOVA with
Student-Newman-Keuls method for multiple comparisons. Differences
were considered to be statistically significant at P-values of
0.05, 0.01 and 0.001. Statistical analysis was performed using SAS
9.0 software.
Results
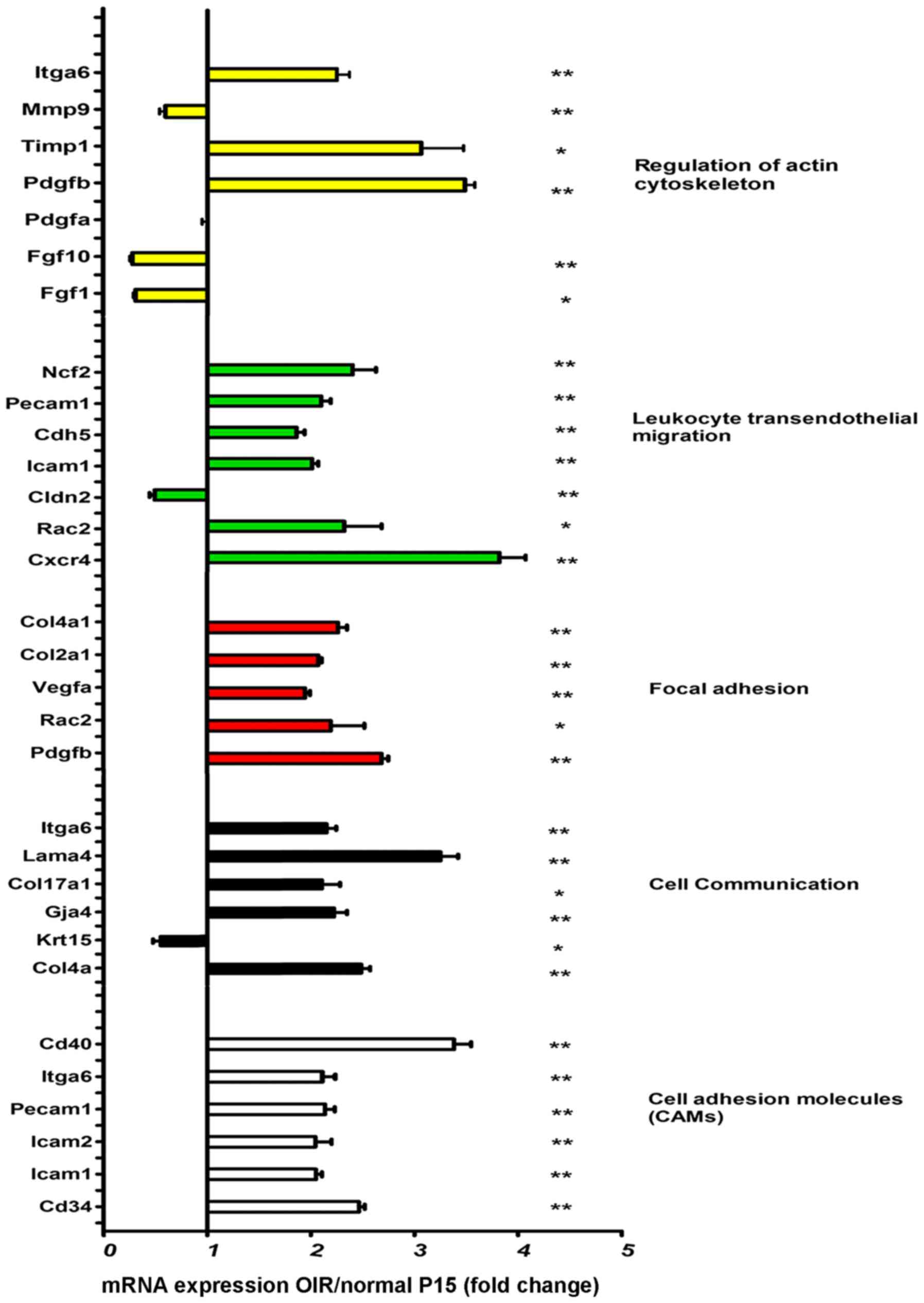
High levels of mRNAs associated with
leukocyte transendothelial migration and cell adhesion in retinas
of mice with OIR
The expression changes associated with leukocyte
transendothelial migration, cell adhesion and cell communication
molecules were assessed at mRNA level by RT-qPCR. Specifically, we
measured the expression ratio in the retinas of mice with OIR to
that of the normal controls at P15. The significant upregulation of
most leukocyte transendothelial migration and cell adhesion
molecules was observed in the mice with OIR compared to the normal
controls (Fig. 1). Precipitated
by hyperoxia, vaso-obliteration occurred. When the mice were
returned to room air following exposure to hyperoxia, this caused
relative ischemia in the non-perfused retina, and thus
neovascularization occurred (30).
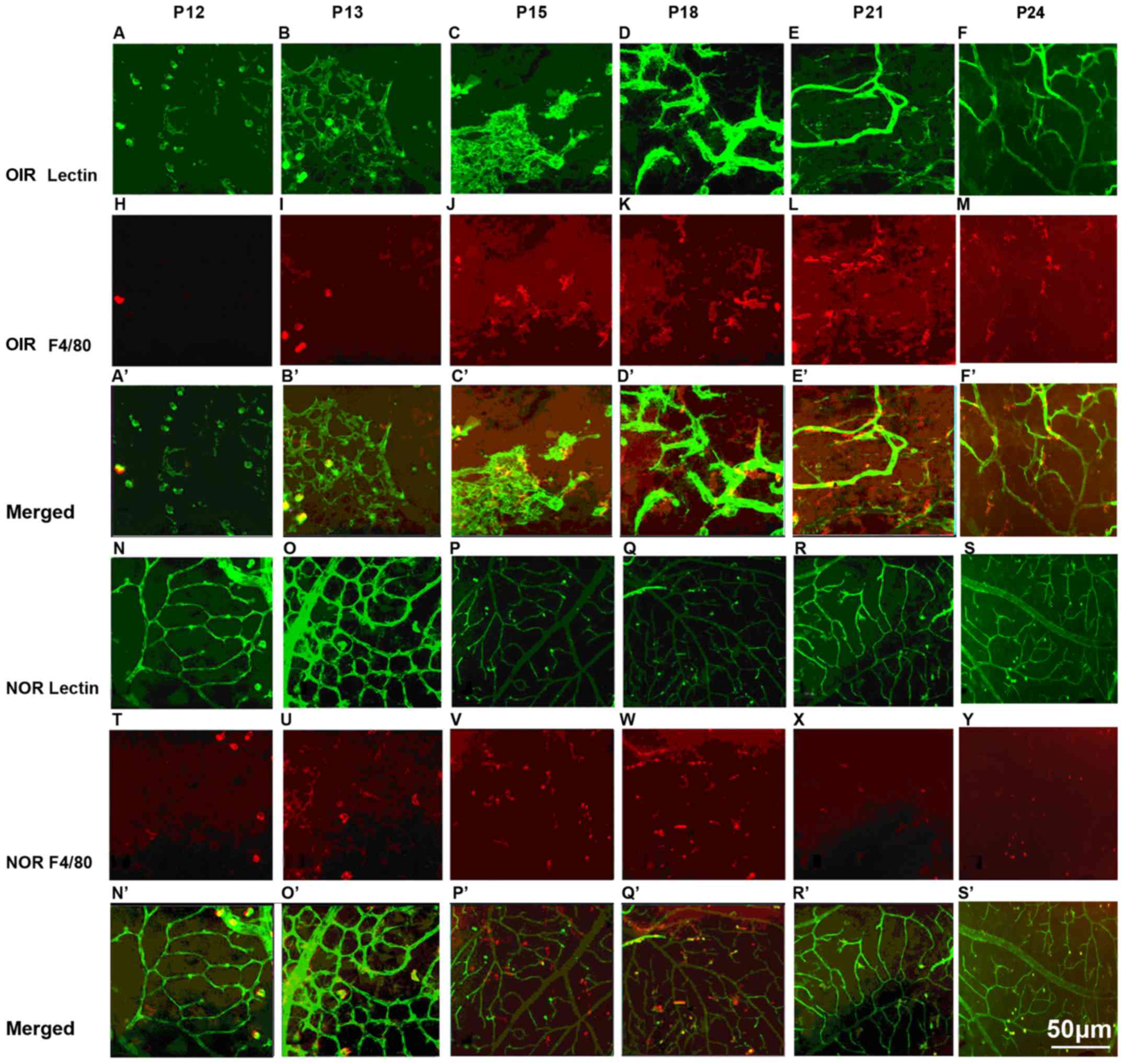
Detection of ascendant macrophage influx
with the progression of retinal neovascularization (RNV)
The retinas from the mice with OIR and the
age-matched controls were dissected, fixed and immunofluorescence
stained with FITC-isolectin B4 and PE-labeled F4/80 antibody
(Fig. 2). The wholemounts of
retinas from mice with oxygen-induced ischemic retinopathy
exhibited increased RNV from P13 to P24, with a significant
increase at P18, peaking at P21 and subsequently decreasing.
Macrophage density increased significantly at P18 and P21 compared
to the controls and subsequently decreased. There seemed to be an
increasing number of macrophages with the progression of
angiogenesis over time.
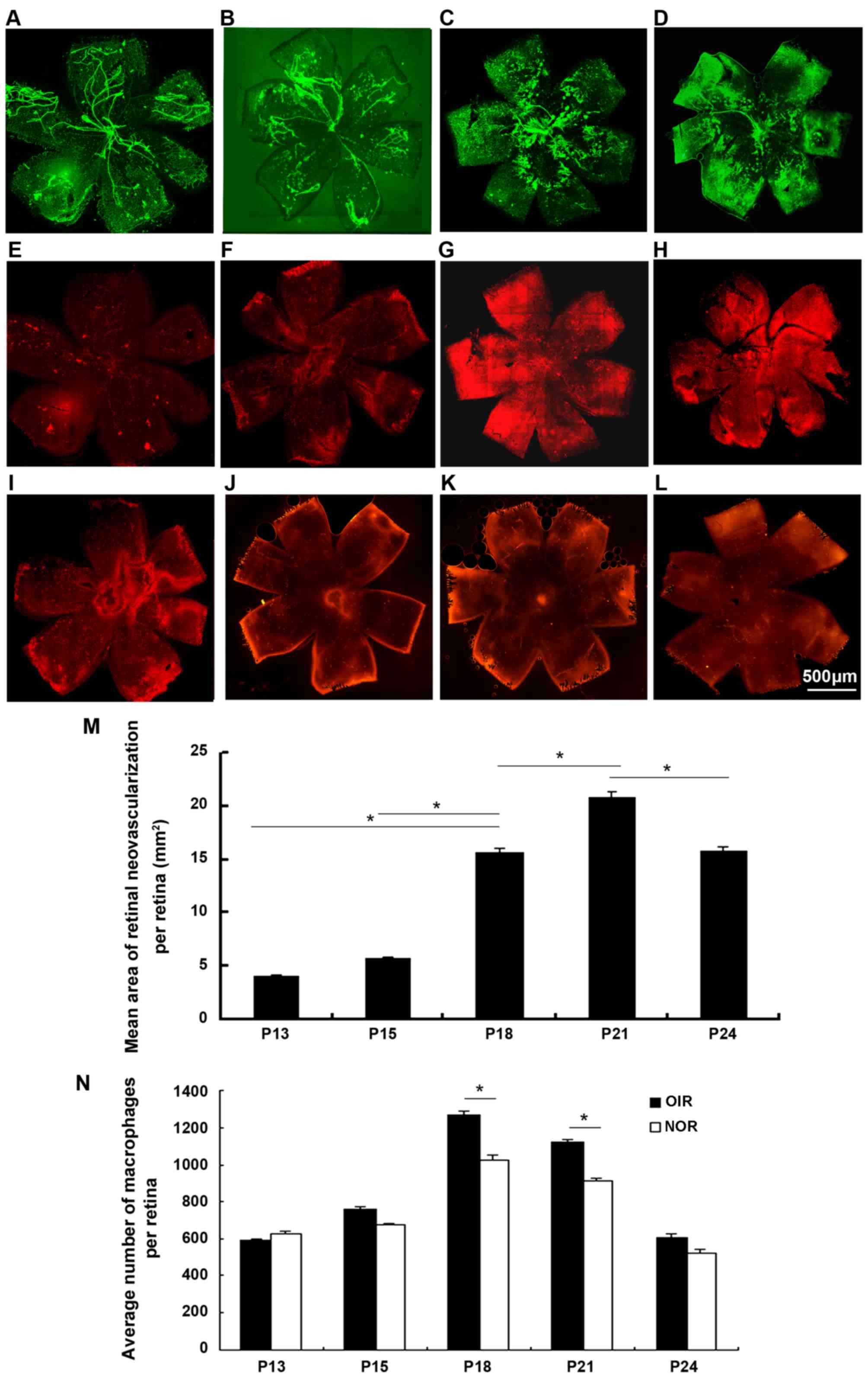 | Figure 2Immunofluorescence staining of
retinal wholemounts for vasculature and macrophages in retinas of
mice with OIR from P13 to P24 [lectin, green (A–D); F4/80, red
(E–H)] and age-matched controls [F4/80, red (I–L)]. Retinas from
mice with OIR were stained with FITC-isolectin B4 and PE-labled
anti-mouse F4/80 antibody for 45 min at room temperature. We
observed an increasing area of retinal neovascularization (RNV)
from P13 to P24, accompanied by the ascending influx of
macrophages. Both RNV and macrophages on the surface of the retinas
from mice with OIR were estimated at P13 (A and E), P18 (B and F),
P21 (C and G) and P24 (D and H). Macrophages influx of the
age-matched controls was also examined at P13 (I), P18 (J), P21 (K)
and P24 (L). RNV significantly increased from P18, peaking at P21,
and then decreased [(M) P<0.05], accompanied by a significant
macrophage influx ascending at P18 and P21 compared to the controls
and then decreasing (N) (n=6 mice/group). OIR, oxygen-induced
retinopathy; NOR, normal; P, post-natal day.
*P<0.05. |
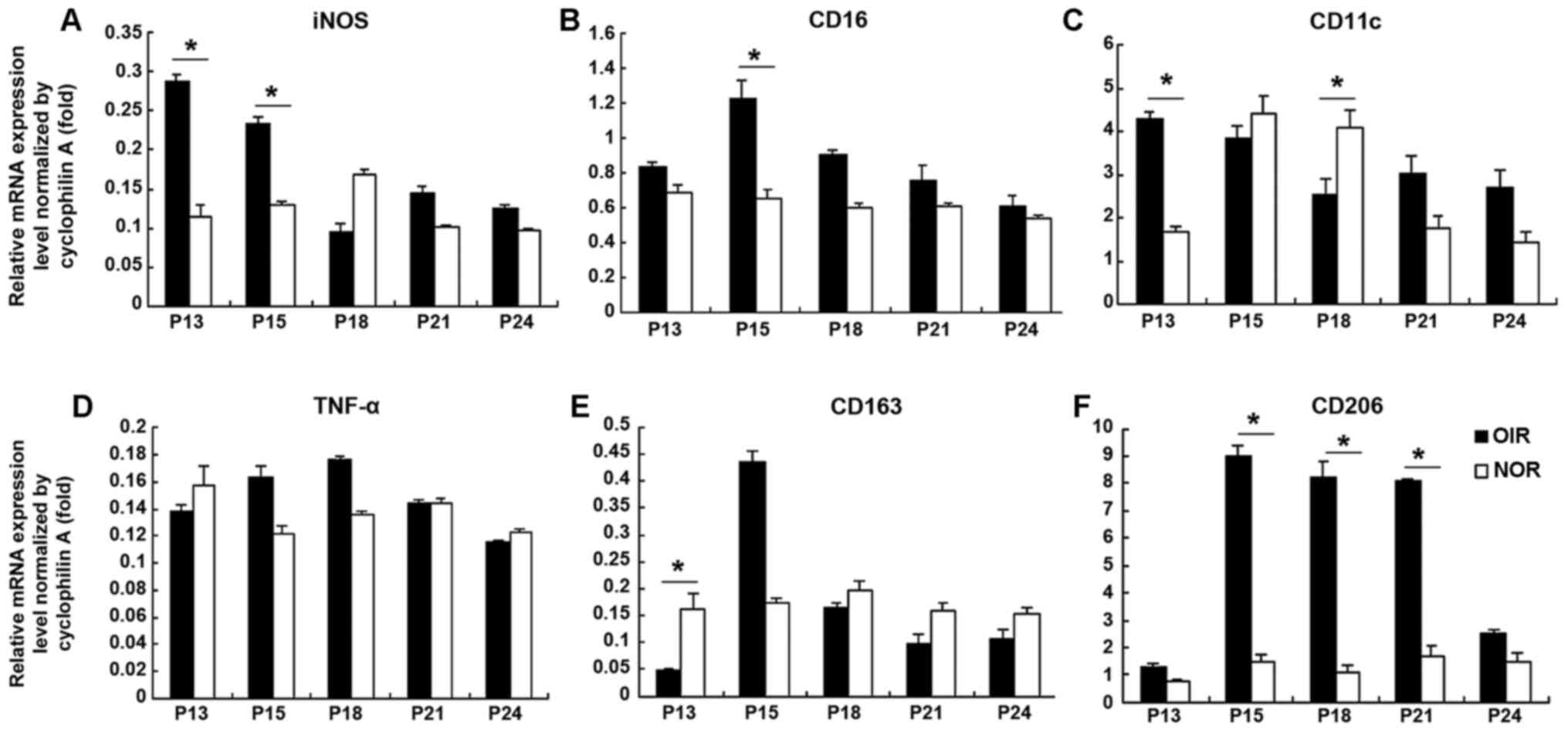
Decrease in the levels of M1
polarization-associated genes and increase in the levels of M2
polarization-associated genes in retinas from mice with OIR from
P13 to P24
The retinas from mice with OIR were pooled for RNA
extraction from P13 to P24 with the age-matched normal retinas as
controls. RNA was then reverse transcribed into cDNA and specific
M1 and M2 macrophage polarization-associated genes were evaluated
by qPCR (Fig. 3). The expression
levels of M1 macrophage polarization-associated genes [iNOS
(Fig. 3A), CD16 (Fig. 3B)] were significantly upregulated
(approximately 2–3-fold of the controls) in the mice with OIR at
P15. Although relatively high levels of TNF-α (Fig. 3D) and CD163 (Fig. 3E) (an M2 macrophage
polarization-associated marker) were observed, no statistical
significance was obtained. An increased expression of CD11c
(Fig. 3C) was observed in the
mice with OIR soon after returning to room air at P13
(approximately 2.5-fold compared to the normal controls); however,
CD11c expression decreased at P18 and reached levels close to those
of the normal controls. The CD206 expression level in the mice with
OIR was upregulated from P13 until P21 (approximately 8-fold
compared to the normal controls at P18 and P21), but decreased at
P24, suggesting a switch towards M2 polarization during the
process.
Close association of macrophages with RNV
at different time points
Retina flat mounts from the mice with OIR and the
controls were immunofluorescence stained with PE-labeled F4/80
anti-body and FITC-isolectin B4. In the mice with oxygen-induced
ischemic retinopathy, the macrophages were located in close
proximity to the area affected by RNV, as shown by
immunofluorescence staining of the retinas (Fig. 4A–M). Compared with the moderate
number of macrophages observed in the retinas of normal mice
(Fig. 4N–Y), the retinas from the
mice with ischemic retinopathy exhibited a high density of
macrophages neighboring the RNV (Fig.
4A′–F′ and N′–S′), indicating a potential role of macrophages
in RNV.
Polarization of macrophages towards the
M2 subtype during the progression of RNV
The numbers of macrophages in the retinas of mice
with OIR and normal mice were analyzed by flow cytometry at
different time points. F4/80-positive cells were gated out from the
live cells and we used CD11c and CD206 as markers to identify M1 or
M2 macrophages. F4/80+, CD11c+ and
CD206− cells are marked as M1-positive cells, and
F4/80+, CD11c− and CD206+ cell are
marked as M2-positive cells. The cell distribution patterns in the
retinas from the normal mice, and the mice with OIR during the
early stage (P13) and later stage (P21) are shown in Fig. 5B. The M2 macrophage number
(Fig. 5E) in the mice with OIR at
P12 and P13 was lower than that of the normal group; however, it
then increased sharply after the the mice with OIR were returned to
normal air, and remained at a relatively high level at P15–P21,
returning to normal levels at P24. The M1 macrophage number
(Fig. 5D) increased at P13, and
rapidly decreased at P18; at P24, it returned to relatively normal
levels in the mice with OIR. The F4/80+ macrophage
numbers (Fig. 5C) in the mice
with OIR rapidly increased from P15 until later on. A sharp
decrease in the M1/M2 ratio of macrophages was thus suggested in
the mice with OIR, indicating that there was a shift in macrophage
polarization towards the M2 subtype. At P24, the M1, M2, M1/M2
distribution pattern returned to normal.
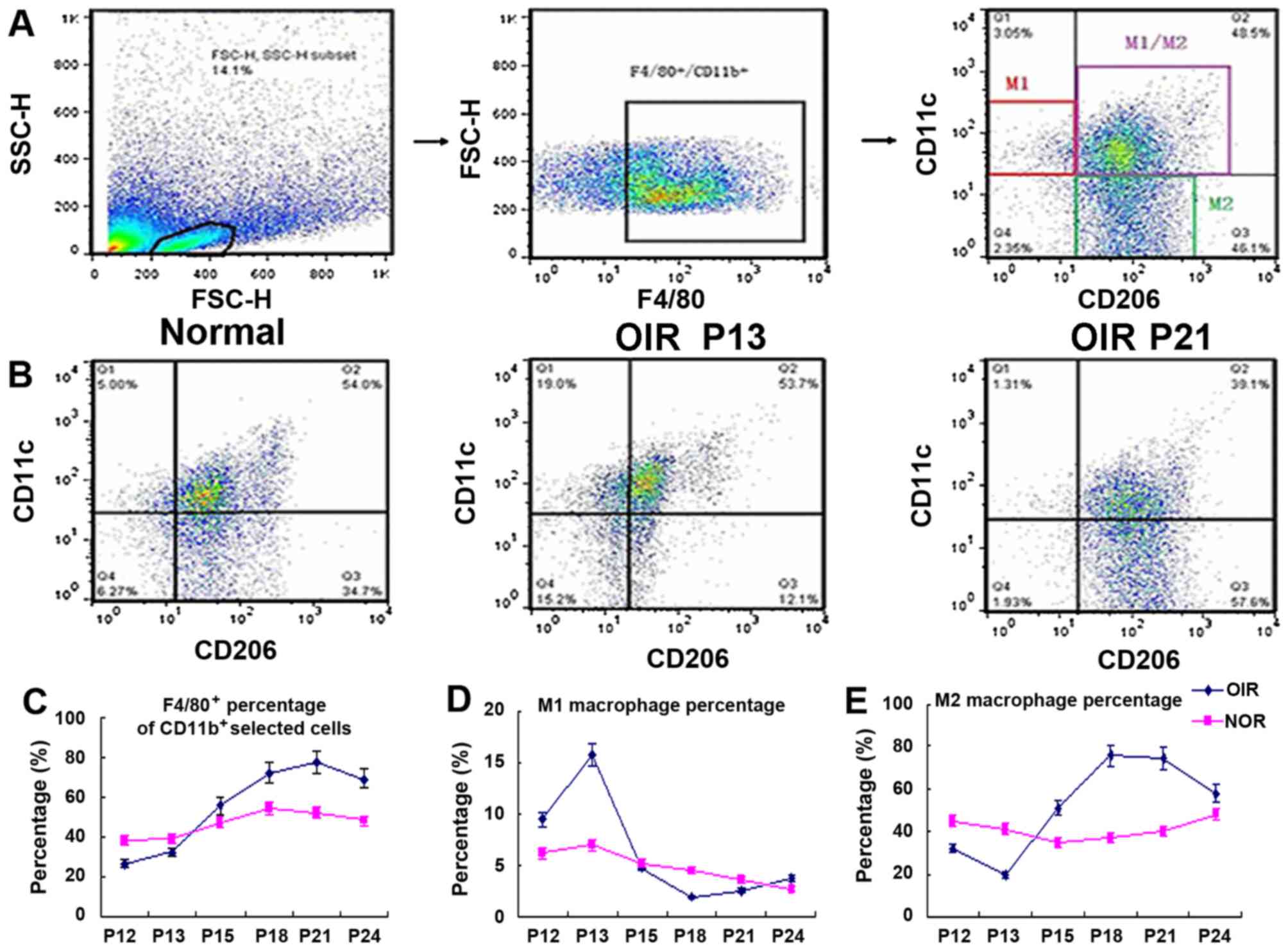 | Figure 5Flow cytometric analysis of mice with
OIR at different time points. (A) The selection of M1- or
M2-positive cells. In brief, we first gated out the live cells, and
then gated out the F4/80-positive cells. In this subgroup, we used
CD11c and CD206 as markers to identify M1 or M2 macrophages.
F4/80+, CD11c+, CD206− cells are
markered as M1 positive cells, F4/80+,
CD11c−, CD206+ cells are marked as
M2-positive cells. (B) The cell distribution pattern in normal mice
and mice with OIR in the early stage (P13) and later stage (P21).
(C–E) The percentage of total F4/80+ macrophages, the M1
macrophages, and the M2 macro-phage in mice with OIR and the
age-matched controls. M2 macrophage number (E) in the mice with OIR
at P12 and P13 was lower than that of the normal group, but then
increased sharply after the mice with OIR were returned to normal
air, and remained at a relatively high level at P15–P21, returning
to normal levels at P24. The M1 macrophage number (D) increased at
P13, and rapidly decreased at P18, and at P24 it returned to
relatively normal levels. The total number of F4/80+
macrophages (C) in mice with OIR at P12 and P13 was lower than that
of the age-matched control mice, but it rapidly increased at P15
and onwards; we observed a sharp decrease in the M1/M2 cells; the
macrophages in the mice with OIR were polarized towards the M2
subtype macrophage. At P24, the M1, M2, M1/M2 distribution pattern
returned to normal (n=6 mice/group). OIR, oxygen-induced
retinopathy; NOR, normal; P, post-natal day. |
Contributions of M1 and M2 macrophages to
the different steps of RNV
Arginase 1 and iNOS were stained to indicate M2 and
M1 polarized macrophages as previously reported (38). A relatively high expression level
of iNOS was observed at P13 in the eyes of the mice with OIR, while
a relatively high expression level of arginase 1 was identified at
P18 (Fig. 6, arrowheads) Although
a close association between iNOS and arginase 1 expression was
identified with vascular formation, co-localization was not
identified in these sections. Furthermore, differential expression
patterns of iNOS and arginase 1 during the progression of
retinopathy may indicate the different roles that M1 and M2
macrophages play in this process.
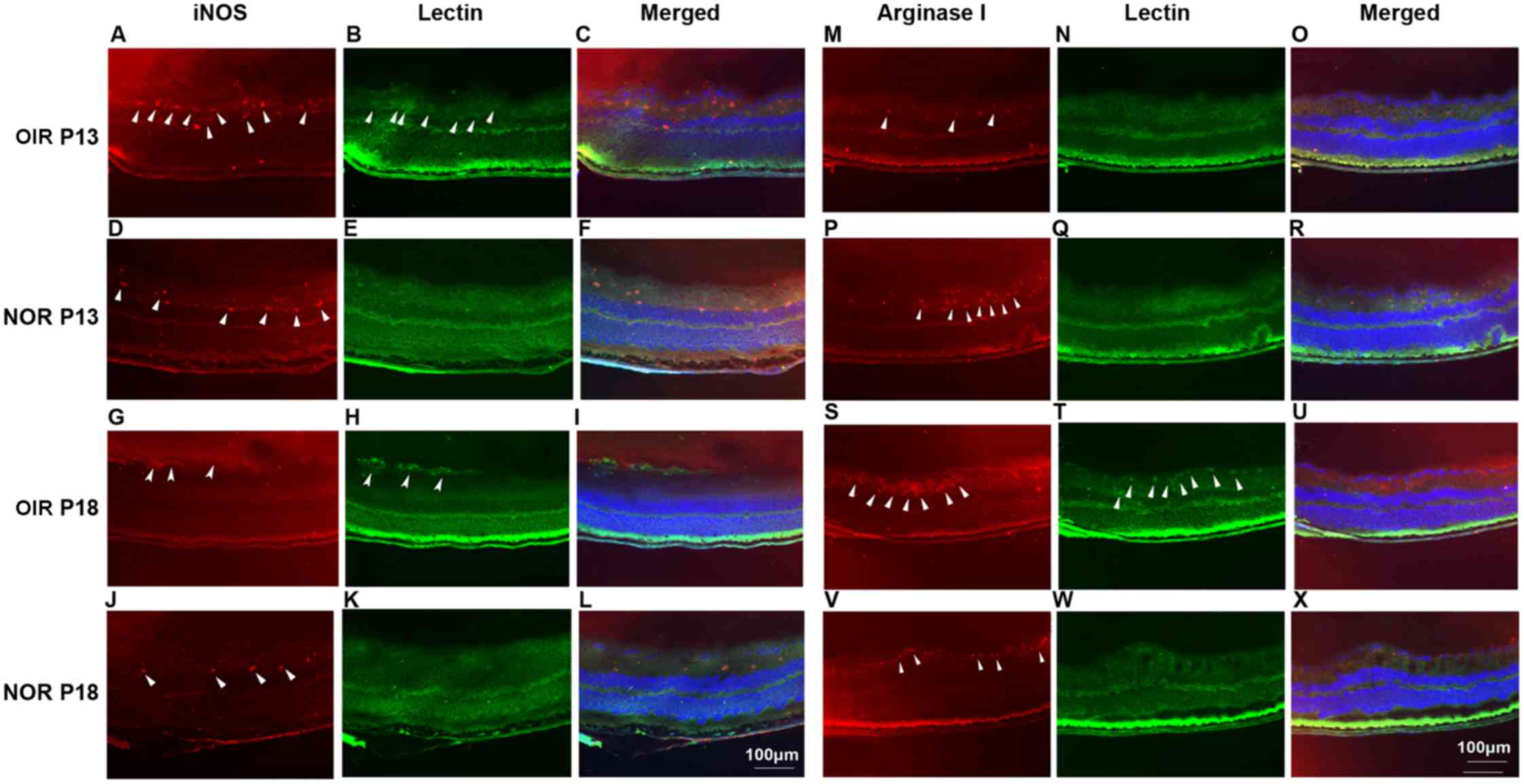 | Figure 6Immunofluorescence staining of
iNOS/arginase 1 (red) and isolectin B4 (green) in the eyes of
normal mcie and mice with OIR at P13 and P18. C57BL/6 mice with or
without ischemic retinopathy were euthanized at P13 and P18, and
their eyes were rapidly removed and frozen sections were created.
After staining with primary anti-mouse iNOS (A, D, G and J) and
arginase 1 (M, P, S and V) antibody, followed by Alexa-555 labeled
secondary antibody, and FITC-isolectin B4 (B, E, H and K; N, Q, T
and W) the sections were examined under fluorescent microscopy.
Merged images are shown in panels C, F, I and L for iNOS and lectin
B4 and in panels O, R, U and X for arginase 1 and lectin B4. Nuclei
were stained with DAPI (blue) (n=3 mice/group). OIR, oxygen-induced
retinopathy; NOR, normal; P, post-natal day. |
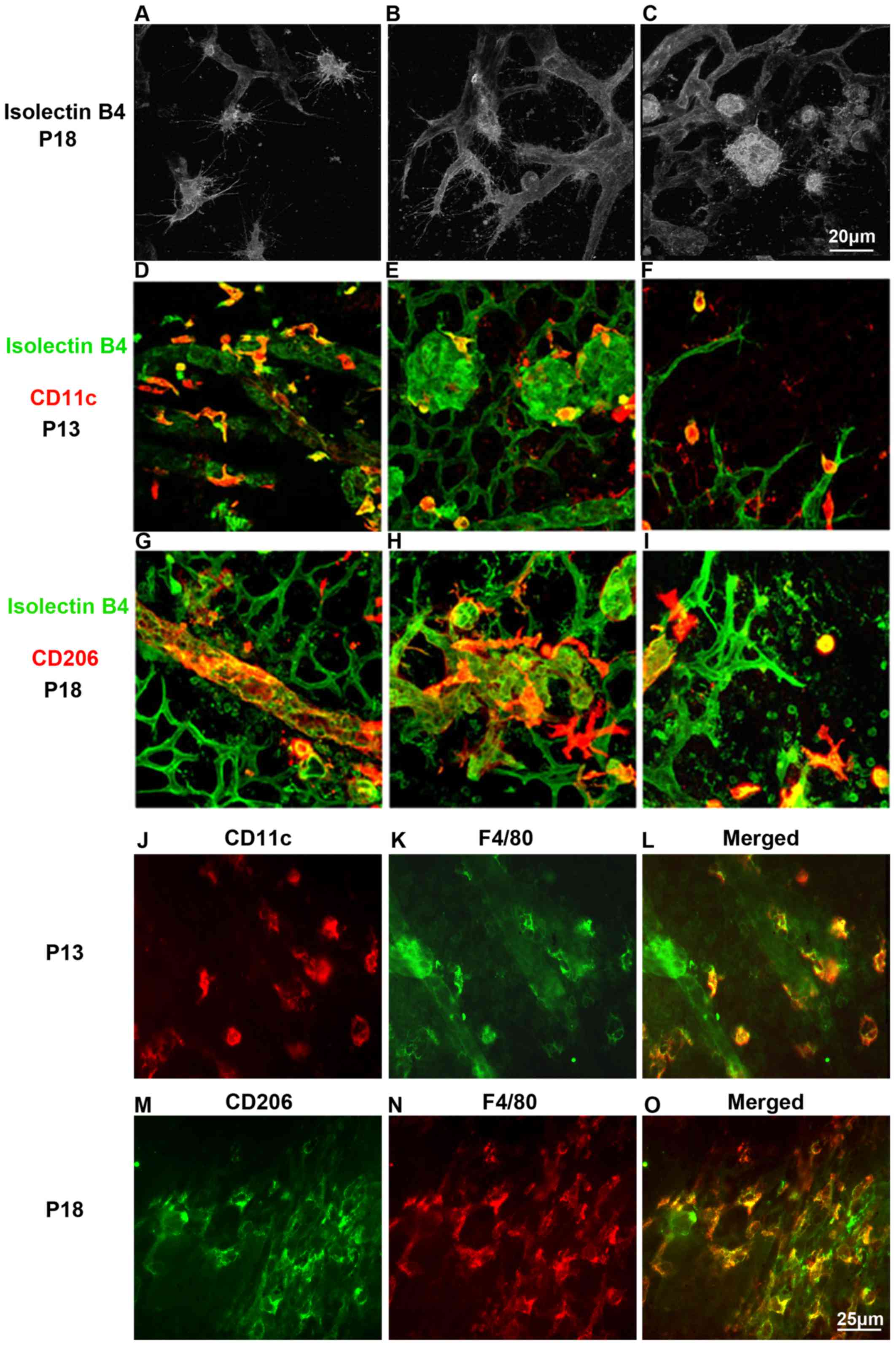
Differential effects of M1, M2
macrophages during the proces of RNV
To explore the phenotypic and functional differences
of M1 and M2 macrophages, the retinas of mice with OIR at P13 and
P18 were analyzed. Retinal flat mounts were immunofluorescence
stained with isolectin B4 and CD11c or CD206 (Fig. 7). Laser scanning confocal imaging
revealed the co-localization of CD11c-positive cells or
CD206-positive cells and endothelial cells. F4/80-positive cells
were generally stained with CD11c at P13 and with CD206 at P18
(Fig. 7J–O). This illustrated
that CD11c-positive cells interacted with endothelial tip cells at
the vascular front at early stage, while CD206-positive cells
embraced the emerging vessels and bridged the neighboring vessel
sprouts, suggesting a promotive function for tip cell fusion at the
later stage.
Discussion
It has been well established that blood vessels grow
into networks through a process involving sprouting, anastomosis
and maturation (39). Immune
vascular interactions can play an important role in regulating
angiogenesis in the eye (22,40,41). Accumulating evidence has
implicated a critical role for macrophages in this process. Gao
et al (42) studied the
role of macrophages in vasculogenesis of RNV in a mouse model of
OIR by depleting macrophages using an intra-peritoneal injections
of of clodronate-liposomes at P9, P11, P13 and P15. They found that
macrophage depletion (the quantities of retinal macrophages were
reduced by approximately 80% and the mRNA expression of F4/80 also
decreased at P17) did not affect the vaso-obliterative phase, but
reduced the retinal avascular area and neovascular tufts during the
neovascularization phase in OIR. Their findings demonstrated that
the depletion of macrophages markedly decreased OIR severity,
angiogenic cytokines and extracellular matrix degradation at P17,
causing growth restriction of pathologic RNV (42). It has been demonstrated that
cytokines can influence the macrophage-mediated regulation of
angiogenesis. In a model of laser-induced CNV, mice that lacked
IL-10 (IL-10−/−) were significantly impaired in their
ability to generate CNV. In the eye, IL-10 promotes angiogenesis by
altering macrophage function. The polarization of macrophages can
play a pivotal role in determining the ultimate effector function
of these cells (43).
The present study demonstrated that the retinas of
mice with OIR expressed high levels of mRNAs associated with
leukocyte transendothelial migration and cell adhesion, indicating
an active interplay between inflammation and angiogenesis under
conditions of OIR in this animal model. Sato et al analyzed
comprehensive gene-expression profiles in murine OIR and indicated
that genes associated with inflammation expressed high values from
the beginning to the late stages of OIR, which preceded the
angiogenesis and the upregulation of angiogenic genes (44). Furthermore, macrophage activation
signatures defined in vitro have been reported to be highly
influenced by factors often overlooked in vivo, such as cell
migration, adhesion and chemoattractants (45). This expression pattern suggests an
increase influx of macrophages along with the neovascularization
process that was demonstrated by our immunofluorescence retinal
staining, suggesting a close correlation between macrophages and
the neovascularization process.
In order to investigate the phenotype of the
increased inflammatory cells and their correlation with RNV, we
performed qPCR for inflammation-associated genes and
immunofluorescence staining for retinal vessels together with
macrophages from P12 to P24. The results of qPCR suggested a
significant increase in the expression of the macrophage marker,
F4/80, accompanied with upregulated expression levels of
M1-associated genes. The staining results indicated a close
association of macrophages with protruding bulbous networks of
neovascularization in ischemic retinas from the mice. Further
assays to classify the infiltrated macrophages demonstrated
enhanced M1 phenotype polarization at P13 and enhanced M2 phenotype
polarization at P15–P18, indicating a shift in the macrophage
polarization towards the M2 subtype. In addition, Spiller et
al demonstrated that M1 macrophages appear at early stages of
wound healing (1–3 days) and are later replaced by M2 macrophages
(4–7 days) (46). Taking into
consideration the findings of Sato et al that
inflammation-associated genes are upregulated prior to the
formation of neovascularization, at P12 and P13, and
angiogenesis-associated genes were mostly upregulated at P16 and
P17, when neovascularization became most noticeable (44), our results showed good consistency
with the respective roles of M1 and M2 macrophages previously
reported.
The distinct features of M1 and M2 macrophages
phenotypically and functionally in RNV has attracted increasing
interest. Marchetti et al demonstrated that human umbilical
cord blood-derived myeloid progenitor cells differentiated in
vivo into M2 macrophages and induced resident macrophages to M2
polarization. These M2 polarized macrophages prevented
neovascularization and maintained the stabilization of the ischemic
retina by modulating the inflammatory responses, reducing oxidative
stress and promoting tissue repair (47). On the other hand, the study by
Zhou et al showed that M2 macrophages, rather than M1
macrophages, played an important role in promoting pathological
neovascularization and inhibiting physiological revascularization
(48). In this study, to
investigate the potential role of the two phenotypes, retina flat
mounts of OIR mice were stained and scanned by confocal microscopy.
The results illustrated that M1 macrophages interacted with
endothelial tip cells, while M2 macrophages promoted cell fusion
that facilitated anastomosis. Similar findings were also observed
by Fantin et al in the nervous system (49). The ablation of macrophages
resulted in reduced vessel intersections, providing evidence that
macrophages play an active role in vessel anastomosis (49). Caicedo et al found
extensive macrophage recruitment in the retina under CNV, with
infiltrating macrophages predominated over resident microglia,
suggesting that macrophages were closely associated with retinal
blood vessels (50). Cao et
al found an increased number of M2 macrophages compared to M1
macrophages in normal aging eyes (51). Dace et al demonstrated that
IL-10 and hypoxia unmasks the pro-angiogenic phenotype in a
macrophage (23). These mouse
model experiments demonstrated that macrophages had shown 'wound
healing' and 'angiogenesis' gene expression signatures (52).
This study demonstrated the specific contributing
roles of M1, M2 macrophages in different steps of RNV following
OIR. During this angiogenic process, M1 macrophages dominated the
first 2–3 days following OIR, while M2 macrophages represented the
overwhelming macrophage subset thereafter, which is in accordance
with the studies of post-myocardial infarction and wound healing
process (38,53,54). Recently, Ma et al
investigated subretinal fluid and surgically dissected retrolental
membranes from patients with advanced ROP and demonstrated that the
microenvironment in eyes with advanced ROP is both pro-angiogenic
and pro-inflammatory, with the preponderance of M1 over M2
(55). In addition, the M1
macrophage-secreted cytokines, TNF-α and VEGF, facilitate these
cells to promote blood vessel sprouting by interaction with
endothelial tip cells, whereas M2 macrophages promote anastomosis,
which has been reported to be associated with Notch1 signaling;
however, the secreted factors remain to be clarified (56). These results, together with the
continued presence of M1 and M2 macrophages contributing to
neovascularization suggest the coordinated involvement of both
subsets of macrophages guides retinal new blood vessel
formation.
The present study provides an outlook of macrophage
polarization during OIR, in an aim to shed light on the therapeutic
potential target of macrophages in the treatment of neovascular eye
diseases in addition to anti-VEGF therapy. However, there are still
disadvantages in this study. First, there are different subgroups
within M2 macrophages. In addition to traditional M2 macrophages,
called M2a, macrophages stimulated with IL-10 are classified as
M2c. Yet the different function of these two subsets in RNV remains
unclear. Further studies are necessary to investigate their
distinctions in this process. Second, clearly it is the combined
effect of cytokine profiles that drives the angiogenic phenotype of
macrophages. For instance, TNF-α has been shown to be
pro-angiogenic in cancers, but it is also secreted at functionally
significant levels by the anti-angiogenic M1 macrophages (57). CD163 was considered as the M2
phenotype, but CD163+ macrophages have been reported to
secret inflammatory cytokines in response to biomaterials in
vitro and patients with psoriasis (58,59). As a result, the interplay between
M1 and M2 macrophages in angiogenesis, particularly the combined
effects of timing, calls for further attention. In addition, other
animal models in which retinopathy is not healed by
neovascularization need to be investigated to study further the
macrophage response.
We are now at a circumstance where identifying
additional VEGF-independent pathways that trigger abnormal
angiogenesis in the eye is critical. Ma et al suggested that
anti-inflammatory therapy and the promotion of M2 activation over
M1 activity should be included in addition to the surgical removal
of the fibrovascular membranes and anti-angiogenic therapy to cope
with advanced ROP based on their findings (55). Furthermore, it is also important
to identify safe and effective modalities of the targeted delivery
of therapeutic agents to the posterior compartment of the eye to
maximize sustained treatment effects and minimize local or systemic
adverse events. Macrophages, as the members of the innate immune
system, are believed to be the potential target for researchers to
exert control and to modulate ocular neovascularization.
In conclusion, the findings of this study
demonstrate that M1 and M2 macrophages play an active role in OIR
by contributing to different steps of RNV. Thus, tissue macrophages
may be considered as a potential target for the anti-angiogenic
therapy of ocular neovascularization diseases.
Acknowledgments
The study was supported by grants from the National
Nature Science Foundation of China, 81470639 and 81570853; the
Shanghai Nature Science Foundation Grant 14411968400; the Shanghai
Charity Cancer Research Center Program 2013, and 2015 Doctoral
Innovation Fund Projects BXJ201414 from Shanghai Jiaotong
University School of Medicine, China. The authors would like to
thank Professor Honglin Wang for assisting with the flow cytometry
experiment.
Glossary
Abbreviations
Abbreviations:
|
NV
|
neovascularization
|
|
RNV/CNV
|
retinal/choroidal
neovascularization
|
|
Mf
|
macrophage
|
|
ROP
|
retinopathy of prematurity
|
|
Pn
|
post-natal day n
|
References
|
1
|
Hiratsuka S, Minowa O, Kuno J, Noda T and
Shibuya M: Flt-1 lacking the tyrosine kinase domain is sufficient
for normal development and angiogenesis in mice. Proc Natl Acad Sci
USA. 95:9349–9354. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sadiq MA, Hanout M, Sarwar S, Hassan M, Do
DV, Nguyen QD and Sepah YJ: Platelet derived growth factor
inhibitors: A potential therapeutic approach for ocular
neovascularization. Saudi J Ophthalmol. 29:287–291. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rofagha S, Bhisitkul RB, Boyer DS, Sadda
SR and Zhang K; SEVEN-UP Study Group: Seven-year outcomes in
ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a
multicenter cohort study (SEVEN-UP). Ophthalmology. 120:2292–2299.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Comparison of Age-related Macular
Degeneration Treatments Trials (CATT) Research Group; Martin DF,
Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, Toth C,
Redford M and Ferris FL III: Ranibizumab and bevacizumab for
treatment of neovascular age-related macular degeneration: two-year
results. Ophthalmology. 119:1388–1398. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Newman AC and Hughes CC: Macrophages and
angiogenesis: a role for Wnt signaling. Vasc Cell. 4:132012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ligresti G, Aplin AC, Zorzi P, Morishita A
and Nicosia RF: Macrophage-derived tumor necrosis factor-alpha is
an early component of the molecular cascade leading to angiogenesis
in response to aortic injury. Arterioscler Thromb Vasc Biol.
31:1151–1159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu H, Xu JB, He YL, Peng JJ, Zhang XH,
Chen CQ, Li W and Cai SR: Tumor-associated macrophages promote
angiogenesis and lymphangiogenesis of gastric cancer. J Surg Oncol.
106:462–468. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stein M, Keshav S, Harris N and Gordon S:
Interleukin 4 potently enhances murine macrophage mannose receptor
activity: a marker of alternative immunologic macrophage
activation. J Exp Med. 176:287–292. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ding Y, Song N and Luo Y: Role of bone
marrow-derived cells in angiogenesis: Focus on macrophages and
pericytes. Cancer Microenviron. 5:225–236. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kigerl KA, Gensel JC, Ankeny DP, Alexander
JK, Donnelly DJ and Popovich PG: Identification of two distinct
macrophage subsets with divergent effects causing either
neurotoxicity or regeneration in the injured mouse spinal cord. J
Neurosci. 29:13435–13444. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ashcroft GS, Jeong MJ, Ashworth JJ,
Hardman M, Jin W, Moutsopoulos N, Wild T, McCartney-Francis N, Sim
D, McGrady G, et al: Tumor necrosis factor-alpha (TNF-α) is a
therapeutic target for impaired cutaneous wound healing. Wound
Repair Regen. 20:38–49. 2012. View Article : Google Scholar
|
|
12
|
Khallou-Laschet J, Varthaman A, Fornasa G,
Compain C, Gaston AT, Clement M, Dussiot M, Levillain O,
Graff-Dubois S, Nicoletti A, et al: Macrophage plasticity in
experimental atherosclerosis. PLoS One. 5:e88522010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kitajewski J: Wnts heal by restraining
angiogenesis. Blood. 121:2381–2382. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Badylak SF, Valentin JE, Ravindra AK,
McCabe GP and Stewart-Akers AM: Macrophage phenotype as a
determinant of biologic scaffold remodeling. Tissue Eng Part A.
14:1835–1842. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brown BN, Valentin JE, Stewart-Akers AM,
McCabe GP and Badylak SF: Macrophage phenotype and remodeling
outcomes in response to biologic scaffolds with and without a
cellular component. Biomaterials. 30:1482–1491. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fishman JM, Lowdell MW, Urbani L, Ansari
T, Burns AJ, Turmaine M, North J, Sibbons P, Seifalian AM, Wood KJ,
et al: Immunomodulatory effect of a decellularized skeletal muscle
scaffold in a discordant xenotransplantation model. Proc Natl Acad
Sci USA. 110:14360–14365. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Madden LR, Mortisen DJ, Sussman EM, Dupras
SK, Fugate JA, Cuy JL, Hauch KD, Laflamme MA, Murry CE and Ratner
BD: Proangiogenic scaffolds as functional templates for cardiac
tissue engineering. Proc Natl Acad Sci USA. 107:15211–15216. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang L, Cao Z, Bai T, Carr L, Ella-Menye
JR, Irvin C, Ratner BD and Jiang S: Zwitterionic hydrogels
implanted in mice resist the foreign-body reaction. Nat Biotechnol.
31:553–556. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bota PC, Collie AM, Puolakkainen P, Vernon
RB, Sage EH, Ratner BD and Stayton PS: Biomaterial topography
alters healing in vivo and monocyte/macrophage activation in vitro.
J Biomed Mater Res A. 95:649–657. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tolg C, Hamilton SR, Zalinska E, McCulloch
L, Amin R, Akentieva N, Winnik F, Savani R, Bagli DJ, Luyt LG, et
al: A RHAMM mimetic peptide blocks hyaluronan signaling and reduces
inflammation and fibrogenesis in excisional skin wounds. Am J
Pathol. 181:1250–1270. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tous E, Weber HM, Lee MH, Koomalsingh KJ,
Shuto T, Kondo N, Gorman JH III, Lee D, Gorman RC and Burdick JA:
Tunable hydrogel-microsphere composites that modulate local
inflammation and collagen bulking. Acta Biomater. 8:3218–3227.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Apte RS, Richter J, Herndon J and Ferguson
TA: Macrophages inhibit neovascularization in a murine model of
age-related macular degeneration. PLoS Med. 3:e3102006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dace DS, Khan AA, Kelly J and Apte RS:
Interleukin-10 promotes pathological angiogenesis by regulating
macrophage response to hypoxia during development. PLoS One.
3:e33812008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Espinosa-Heidmann DG, Suner IJ, Hernandez
EP, Monroy D, Csaky KG and Cousins SW: Macrophage depletion
diminishes lesion size and severity in experimental choroidal
neovascularization. Invest Ophthalmol Vis Sci. 44:3586–3592. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sakurai E, Anand A, Ambati BK, van Rooijen
N and Ambati J: Macrophage depletion inhibits experimental
choroidal neovascularization. Invest Ophthalmol Vis Sci.
44:3578–3585. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gautier EL, Shay T, Miller J, Greter M,
Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, et
al: Immunological Genome Consortium: Gene-expression profiles and
transcriptional regulatory pathways that underlie the identity and
diversity of mouse tissue macrophages. Nat Immunol. 13:1118–1128.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vianello E, Dozio E, Arnaboldi F, Marazzi
MG, Martinelli C, Lamont J, Tacchini L, Sigrüner A, Schmitz G and
Corsi Romanelli MM: Epicardial adipocyte hypertrophy: Association
with M1-polarization and toll-like receptor pathways in coronary
artery disease patients. Nutr Metab Cardiovasc Dis. 26:246–253.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shu QH, Ge YS, Ma HX, Gao XQ, Pan JJ, Liu
D, Xu GL, Ma JL and Jia WD: Prognostic value of polarized
macrophages in patients with hepatocellular carcinoma after
curative resection. J Cell Mol Med. 20:1024–1035. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lumeng CN, DelProposto JB, Westcott DJ and
Saltiel AR: Phenotypic switching of adipose tissue macrophages with
obesity is generated by spatiotemporal differences in macrophage
subtypes. Diabetes. 57:3239–3246. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Smith LE, Wesolowski E, McLellan A, Kostyk
SK, D'Amato R, Sullivan R and D'Amore PA: Oxygen-induced
retinopathy in the mouse. Invest Ophthalmol Vis Sci. 35:101–111.
1994.PubMed/NCBI
|
|
31
|
Shen J, Xie B, Dong A, Swaim M, Hackett SF
and Campochiaro PA: In vivo immunostaining demonstrates macrophages
associate with growing and regressing vessels. Invest Ophthalmol
Vis Sci. 48:4335–4341. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mori K, Duh E, Gehlbach P, Ando A,
Takahashi K, Pearlman J, Mori K, Yang HS, Zack DJ, Ettyreddy D, et
al: Pigment epithelium-derived factor inhibits retinal and
choroidal neovascularization. J Cell Physiol. 188:253–263. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shen J, Yang X, Xie B, Chen Y, Swaim M,
Hackett SF and Campochiaro PA: MicroRNAs regulate ocular
neovascularization. Mol Ther. 16:1208–1216. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fujimura S, Takahashi H, Yuda K, Ueta T,
Iriyama A, Inoue T, Kaburaki T, Tamaki Y, Matsushima K and Yanagi
Y: Angiostatic effect of CXCR3 expressed on choroidal
neovascularization. Invest Ophthalmol Vis Sci. 53:1999–2006. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dong A, Shen J, Zeng M and Campochiaro PA:
Vascular cell-adhesion molecule-1 plays a central role in the
proangiogenic effects of oxidative stress. Proc Natl Acad Sci USA.
108:14614–14619. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xin X, Rodrigues M, Umapathi M,
Kashiwabuchi F, Ma T, Babapoor-Farrokhran S, Wang S, Hu J, Bhutto I
and Welsbie DS: Hypoxic retinal Muller cells promote vascular
permeability by HIF-1-dependent up-regulation of angiopoietin-like
4. Proc Natl Acad Sci USA. 110:E3425–E3434. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xie B, Shen J, Dong A, Swaim M, Hackett
SF, Wyder L, Worpenberg S, Barbieri S and Campochiaro PA: An Adam15
amplification loop promotes vascular endothelial growth
factor-induced ocular neovascularization. FASEB J. 22:2775–2783.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Arnold L, Henry A, Poron F, Baba-Amer Y,
van Rooijen N, Plonquet A, Gherardi RK and Chazaud B: Inflammatory
monocytes recruited after skeletal muscle injury switch into
antiinflammatory macrophages to support myogenesis. J Exp Med.
204:1057–1069. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Herbert SP and Stainier DY: Molecular
control of endothelial cell behaviour during blood vessel
morphogenesis. Nat Rev Mol Cell Biol. 12:551–564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dace DS and Apte RS: Effect of senescence
on macrophage polarization and angiogenesis. Rejuvenation Res.
11:177–185. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kelly J, Ali Khan A, Yin J, Ferguson TA
and Apte RS: Senescence regulates macrophage activation and
angiogenic fate at sites of tissue injury in mice. J Clin Invest.
117:3421–3426. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gao X, Wang YS, Li XQ, Hou HY, Su JB, Yao
LB and Zhang J: Macrophages promote vasculogenesis of retinal
neovascularization in an oxygen-induced retinopathy model in mice.
Cell Tissue Res. 364:599–610. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kelly J, Ali Khan A, Yin J, Ferguson TA
and Apte RS: Senescence regulates macrophage activation and
angiogenic fate at sites of tissue injury in mice. J Clin Invest.
117:3421–3426. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sato T, Kusaka S, Hashida N, Saishin Y,
Fujikado T and Tano Y: Comprehensive gene-expression profile in
murine oxygen-induced retinopathy. Br J Ophthalmol. 93:96–103.
2009. View Article : Google Scholar
|
|
45
|
Martinez FO and Gordon S: The M1 and M2
paradigm of macrophage activation: Time for reassessment.
F1000Prime Rep. 6:132014. View
Article : Google Scholar : PubMed/NCBI
|
|
46
|
Spiller KL, Anfang RR, Spiller KJ, Ng J,
Nakazawa KR, Daulton JW and Vunjak-Novakovic G: The role of
macrophage phenotype in vascularization of tissue engineering
scaffolds. Biomaterials. 35:4477–4488. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Marchetti V, Yanes O, Aguilar E, Wang M,
Friedlander D, Moreno S, Storm K, Zhan M, Naccache S, Nemerow G, et
al: Differential macrophage polarization promotes tissue remodeling
and repair in a model of ischemic retinopathy. Sci Rep. 1:762011.
View Article : Google Scholar :
|
|
48
|
Zhou Y, Yoshida S, Nakao S, Yoshimura T,
Kobayashi Y, Nakama T, Kubo Y, Miyawaki K, Yamaguchi M, Ishikawa K,
et al: M2 macrophages enhance pathological neovascularization in
the mouse model of oxygen-induced retinopathy. Invest Ophthalmol
Vis Sci. 56:4767–4777. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fantin A, Vieira JM, Gestri G, Denti L,
Schwarz Q, Prykhozhij S, Peri F, Wilson SW and Ruhrberg C: Tissue
macrophages act as cellular chaperones for vascular anastomosis
downstream of VEGF-mediated endothelial tip cell induction. Blood.
116:829–840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Caicedo A, Espinosa-Heidmann DG, Piña Y,
Hernandez EP and Cousins SW: Blood-derived macrophages infiltrate
the retina and activate Muller glial cells under experimental
choroidal neovascularization. Exp Eye Res. 81:38–47. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cao X, Shen D, Patel MM, Tuo J, Johnson
TM, Olsen TW and Chan CC: Macrophage polarization in the maculae of
age-related macular degeneration: A pilot study. Pathol Int.
61:528–535. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Rae F, Woods K, Sasmono T, Campanale N,
Taylor D, Ovchinnikov DA, Grimmond SM, Hume DA, Ricardo SD and
Little MH: Characterisation and trophic functions of murine
embryonic macrophages based upon the use of a Csf1r-EGFP transgene
reporter. Dev Biol. 308:232–246. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Troidl C, Möllmann H, Nef H, Masseli F,
Voss S, Szardien S, Willmer M, Rolf A, Rixe J, Troidl K, et al:
Classically and alternatively activated macrophages contribute to
tissue remodelling after myocardial infarction. J Cell Mol Med.
13:3485–3496. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yan X, Anzai A, Katsumata Y, Matsuhashi T,
Ito K, Endo J, Yamamoto T, Takeshima A, Shinmura K, Shen W, et al:
Temporal dynamics of cardiac immune cell accumulation following
acute myocardial infarction. J Mol Cell Cardiol. 62:24–35. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ma J, Mehta M, Lam G, Cyr D, Ng TF, Hirose
T, Tawansy KA, Taylor AW and Lashkari K: Influence of subretinal
fluid in advanced stage retinopathy of prematurity on proangiogenic
response and cell proliferation. Mol Vis. 20:881–893.
2014.PubMed/NCBI
|
|
56
|
Outtz HH, Tattersall IW, Kofler NM,
Steinbach N and Kitajewski J: Notch1 controls macrophage
recruitment and Notch signaling is activated at sites of
endothelial cell anastomosis during retinal angiogenesis in mice.
Blood. 118:3436–3439. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sethi G, Sung B and Aggarwal BB: TNF: A
master switch for inflammation to cancer. Front Biosci.
13:5094–5107. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bartneck M, Heffels KH, Pan Y, Bovi M,
Zwadlo-Klarwasser G and Groll J: Inducing healing-like human
primary macrophage phenotypes by 3D hydrogel coated nanofibres.
Biomaterials. 33:4136–4146. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Fuentes-Duculan J, Suárez-Fariñas M, Zaba
LC, Nograles KE, Pierson KC, Mitsui H, Pensabene CA, Kzhyshkowska
J, Krueger JG and Lowes MA: A subpopulation of CD163-positive
macrophages is classically activated in psoriasis. J Invest
Dermatol. 130:2412–2422. 2010. View Article : Google Scholar : PubMed/NCBI
|