Introduction
Periplaneta americana (Linnaeus) (Blattodea:
Blattidae), the largest insect member of the family Blattidae, is
one of the most ancient insect groups with the strongest vitality.
We explored its bioactive substances and the unique physiological
mechanism. P. americana has been used in traditional Chinese
medicine (TCM) to eliminate stagnant blood, clear hematocele,
detoxify and promote urination and detumescence (1). P. americana has also been
exploited as an alternative naturopathic remedy for impaired
healing of ulcers, burn wounds, tuberculosis, ulcerative colitis,
heart disease and cancer (2–6).
The major focus of this study is on the promotion of wound
healing.
In the early 1980s, W11-a12,
also known as Kangfuxin, was refined from the alcohol extract of
P. americana (PAE), and was effective against thermal
lesions, such as burns and scalds (1,7).
PAE derived from P. americana dry worm contains peptides,
polyols, epidermal growth factors, sticky sugar acids, amino acids
and other active substances (8).
Recent studies have explored the pharmacological effects and
mechanisms of PAE in wound healing. Histopathological studies
suggest that the necrotic tissue and inflammatory exudate induced
by thermal lesions or chemical damages were significantly reduced
after PAE treatment in rat and rabbit models (9). In addition, increased epithelial
repair area, follicular regeneration, early fibrous tissue repair
and rapid decrease of inflammatory cells were detected in the
PAE-treated groups. PAE also promoted the synthesis and secretion
of extracellular cell matrix (ECM) in the wound (10); activated cellular functions by
accelerating the opening of macrophage ion channels (11); increased the number of
neutrophils, improved the granulocyte chemotactic function and
spontaneous movement, resulting in wound repair and clearance
(12,13).
However, wound healing is a complex process of cell
proliferation, migration, matrix synthesis and contraction, and
involves various types of cells and regulatory mechanisms. Resident
cells (keratinocytes, fibroblasts and endothelial cells) and
inflammatory cells participate in wound healing (2,14).
Evidence has revealed that several signaling pathways are
associated with wound healing via triggering their target gene
expression, such as the Janus-activated kinase/signal transducer
and activator of transcription 3 (JAK/STAT3) signaling (15–18). In wound healing, cytokines
contribute to activate STATs and the activated JAK//STAT3
pathway controls the proliferation and differentiation
necessary for wound healing (19,20). Furthermore, through activation of
JAK/STAT3 signaling cascades, the cytokine induces anti-apoptotic
pathways and anti-microbial molecules to help prevent tissue damage
and aid in their repair (21–23). In addition, a study demonstrated a
critical role for STAT3 in the migration but not proliferation of
keratinocytes in wound healing (24). The pivotal roles of Smad3
signaling in cutaneous wound healing have been well documented
(15,16). Smad3 binds with a Smad mediator
(SMAD4) to form a complex, moving into the nucleus and regulates
the expression of genes including those involved in keratinocyte
migration, fibroblast infiltration and extracellular matrix
construction (25,26). Additionally, Smad3 could balance
the reepithelialization and fibrogenesis of the repaired tissues
(27,28).
Previous studies have mainly focused on the effect
of PAE on cellular repair (10–13). The resident biological functions
and cellular signal transduction pathways underlying the healing
effect of PAE are not fully established. In the present study, we
investigated the biological function and mechanisms of PAE in human
keratinocyte cells and rat skin injury models to facilitate the
clinical application of PAE.
Materials and methods
Cell lines
The spontaneously immortalized human keratinocyte
HaCaT cell line was obtained from the American Type Culture
Collection (ATCC, Rockville, MD, USA) and was cultured in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal calf serum (FCS) and 1% penicillin-streptomycin. Culture
conditions were maintained constant at 37°C in a 5% CO2
humidified atmosphere.
Preparation of PAE
P. americana was obtained from the Good
Agricultural Practice (GAP) breeding base (Sichuan, China). P.
americana (200 g) powder was extracted with 90% EtOH (1.2
liters) twice at 80°C. After solvent evaporation, the ethanol
extract was recovered. The extract (20 g) was suspended in water
(200 ml) at 80°C and filtered through a 0.22-mm filter membrane in
appropriate concentrations and stored at −20°C until use.
HPLC-diode array detector (HPLC-DAD) was used for the study of
P. americana extract. Diamonsil C18 (250×4.6 mm; 5
μm) was selected as the chromatography column. The optimized
mobile phase consisted of solvent A (3% v/v methanol in water
containing 0.07% v/v acetic acid) and solvent B (methanol). The
following gradient was used to represent time (min)/mobile phase A
(%)/mobile phase B (%): 0.0/100/0, 10/100/0, 20/70/30, 21/50/50,
35/0/100, at a flow rate of 0.6 ml/min at 25°C, and 254 nm in 10
μl injection volume.
In vitro proliferation, migration and
wound healing assays
Proliferation was determined using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. Cells were seeded in a volume of 200 μl (2,000
cells/well) on 96-well plates after cultivation with or without
PAE. The culture medium containing serum was replaced by MTT every
24 h. A final MTT concentration of 0.5 mg/ml was added to the wells
followed by incubation for 4 h at 37°C. The supernatant was
discarded and replaced with dimethyl sulfoxide (DMSO) (150
μl/well). The optical densities (OD) were measured at 570 nm
with a microplate reader (Bio-Rad, Hercules, CA, USA). The
experiment was repeated in triplicate.
Cell migration was analyzed in a 24-well plate with
Millicell Cell Culture Insert, using an 8-μm pore size
polycarbonate membrane (Millipore Corporation, Billerica, MA, USA).
After 48 h of cultivation in a medium with or without PAE (0.3125
mg/ml), the cells (2×104/well) were transferred into the
upper chamber with the non-coated membrane and suspended in 200
μl of serum-free DMEM. In the lower chamber, 600 μl
of the medium supplemented with 10% fetal bovine serum was added.
After incubation for 24 h, the chambers were fixed with 4%
paraformaldehyde for 20 min, and stained with hematoxylin for 15
min. Images were captured with an optical microscope.
The wound healing assay was performed by seeding the
cell cultures on a 6-well plate and grown to 90% confluency,
followed by 48 h of starvation in serum-free medium with or without
PAE (0.3125 mg/ml). The culture medium was removed and the
monolayers were scratched using a 200-μl pipette to create a
uniform cell-free wound area. Debris was removed by gently washing
with sterile phosphate-buffered saline (PBS). Cell migration into
the wound area was monitored and photographed at 0, 24 and 48 h
using an optical microscope.
Western blot analysis and antibodies
Cells were lysed with radioimmunoprecipitation assay
(RIPA) buffer and phosphatase inhibitors. The protein concentration
was measured using the bicinchoninic acid (BCA)-protein
quantification assay (Beyotime Biotechnology, Shanghai, China).
Normalized lysates (30 μg) were separated by electrophoresis
on 8–12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) gel and transferred to a PVDF membrane (Millipore
Corporation). The membrane was blocked for 1 h at room temperature
and incubated overnight at 4°C with primary antibody. After three
washes with TBST, the membrane was incubated with horseradish
peroxidase-(HRP)-conjugated IgG. Signals were visualized with
enhanced chemiluminescence (ECL; Millipore Corporation). Primary
antibodies against Smad3 (#9523), phospho-Stat3 (#9145), Stat3
(#12640), JAK1 (#3344), JAK2 (#3230) (dilution, 1:1,000; Cell
Signaling Technology, Beverly, MA, USA), phospho-Smad3 (ab52903),
phospho-JAK2 (ab32101) (dilution, 1:1,000; Abcam, Cambridge, MA,
USA), phospho-JAK1 (sc-101716; dilution, 1:1,000; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) were used.
Immunofluorescence staining
Each group of HaCaT cells was washed with PBS
followed by fixation with 4% paraformaldehyde (pH 7.4) in 6-well
plates. Cells were incubated with 0.5% Triton X-100 for 30 min at
room temperature, and treated with 5% BSA for 1 h. Cells were
incubated with the following primary antibodies: Smad3 (#9523;
dilution, 1:100), phospho-Stat3 (#9145; dilution, 1:100), Stat3
(#12640; dilution, 1:500) (all from Cell Signaling Technology), and
phospho-Smad3 (ab52903; dilution, 1:100; Abcam) overnight at 4°C.
The cells were incubated with the corresponding fluorescent
dye-conjugated secondary antibodies (#4412; dilution, 1:200; Cell
Signaling Technology) at 37°C for 1 h, and protected from light.
The cells were visualized via fluorescence microscopy.
Rat skin injury models
Healthy adult C57 male mice were purchased from the
West China School of Preclinical and Forensic Medicine, Sichuan
University, Sichuan, China. All the experiments were conducted
according to the Guide for the Care and Use of Laboratory Animals
at the Animal Experimental Center of Sichuan University. We
obtained ethical approval of the Medical Ethics Committee of
Sichuan University with approval no. K2016033. Thermal injuries
were created with a solid aluminum bar measuring 10 mm in diameter,
and pre-heated in boiling water at a temperature of 100°C. The bar
was maintained symmetrically in contact with the skin on the dorsal
flank for 15 sec (29). The right
skin of the dorsal flank was wiped with PAE (original fluid, 5
mg/ml), while the left was treated with normal saline as the
untreated control. The daily treatment regimen lasted for 21 days.
After injury, to minimize the suffering of the animals, each mouse
was housed individually and fed with sterilized food and tap water
to prevent infection. The wound healing rates were measured on days
0, 7, 14 and 21 after injury, and the complete wound healing time
was calculated as follows: Healing rate = (original wound area -
non-healing wound area)/original wound area (30). Mice were sacrificed after 3
weeks.
Immunohistochemistry
All the immunohistochemical assays were conducted
following the manufacturer's instructions. Briefly, the skin
tissues were fixed in formalin and embedded in paraffin.
Consecutive paraffin sections (4-μm) of the tissue samples
were prepared and incubated overnight at 4°C with primary
antibodies followed by incubation with peroxidase-labeled polymer
conjugated to goat anti-rabbit immunoglobulins (EnVision/HRP; Dako,
Glostrup, Denmark). Primary antibodies against Smad3 (#9523),
phospho-Stat3 (#9145), Stat3 (#12640), JAK1 (#3344), JAK2 (#3230)
(dilution, 1:1,000; all from Cell Signaling Technology),
phospho-Smad3 (ab52903), phospho-JAK2 (ab32101) (dilution, 1:1,000;
both from Abcam), phospho-JAK1 (sc-101716; dilution 1:1,000; Santa
Cruz Biotechnology, Inc.) were used.
Statistical analysis
Statistical analyses were performed using SPSS 19.0
(SPSS, Inc., Chicago, IL, USA) or Prism 5.0 (GraphPad Software, La
Jolla, CA, USA). Quantitative data were analyzed using a two-tailed
Student's t-test, and one-way analysis of variance (ANOVA) followed
by Dunnett's multiple comparison post-test. Differences were
considered statistically significant at P<0.05, P<0.05 and
P<0.01.
Results
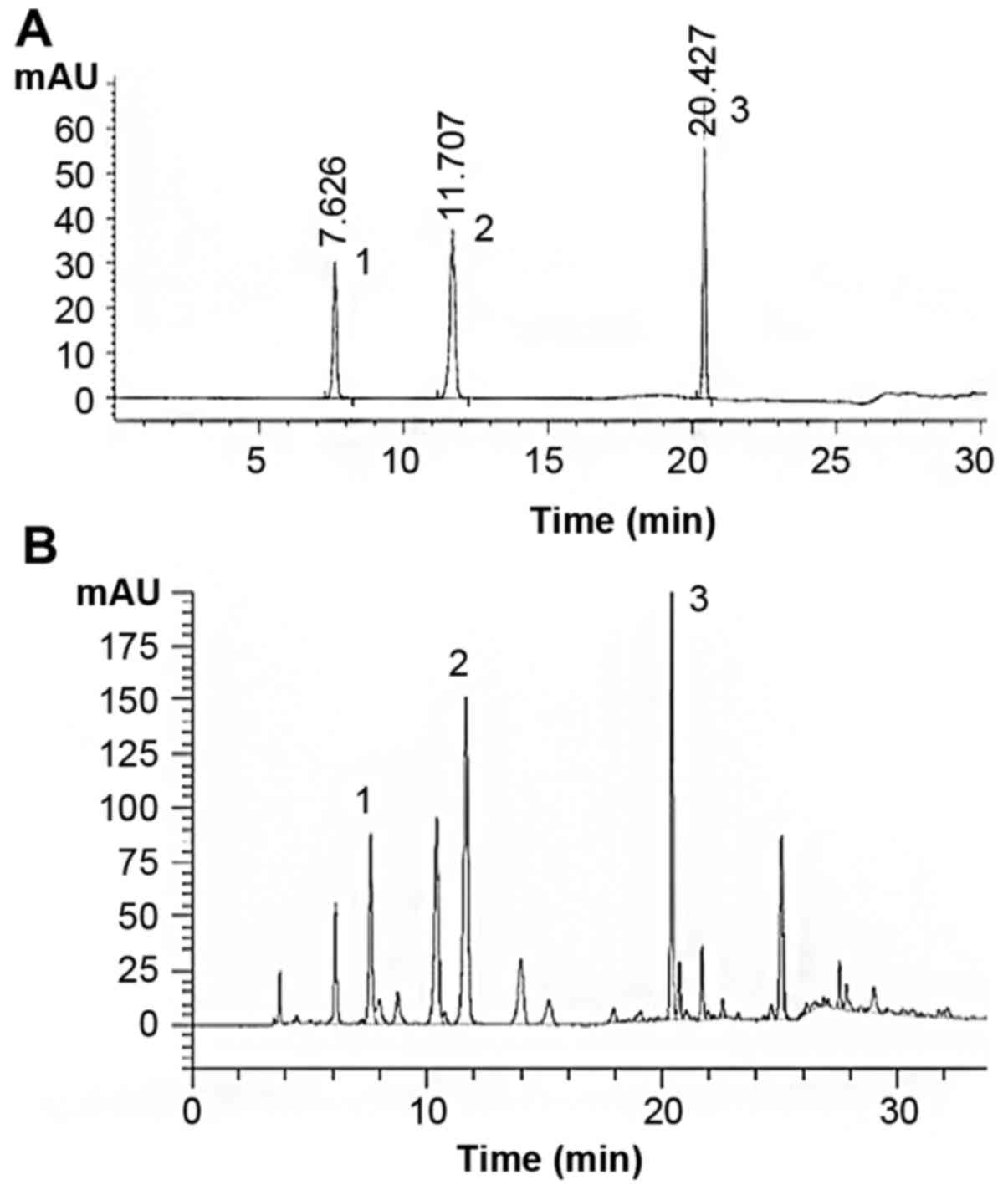
Chromatographic separation of PAE
PAE contained polyalcohols, amino acids, pyrimidines
and proteoglycans. Analysis of PAE revealed the presence of uracil
(retention time 7.626 min, 1.145 mg/g), hypoxanthine (retention
time 11.707 min, 4.253 mg/g), and inosine (retention time 20.427
min, 8.156 mg/g) (Fig. 1).
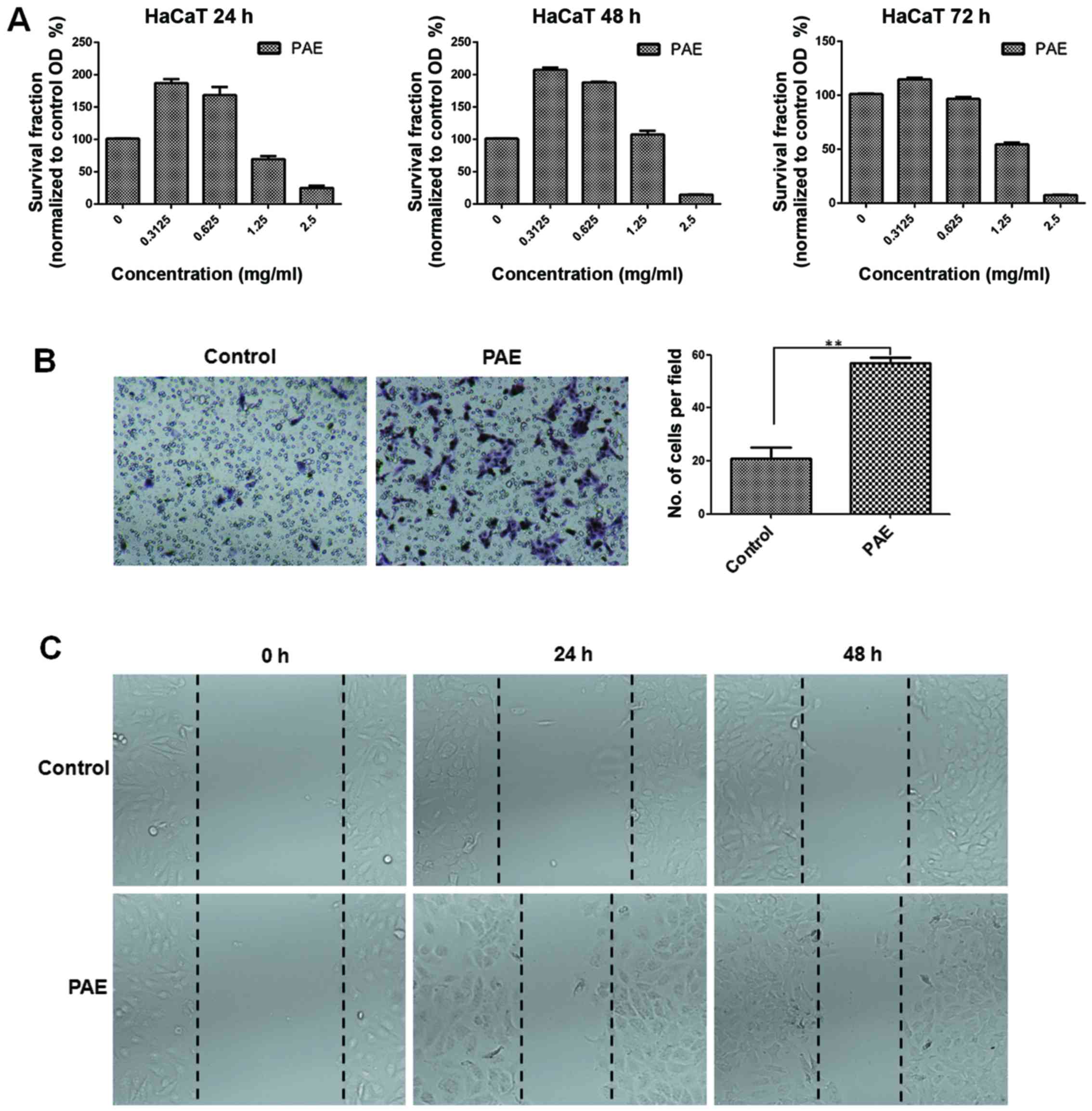
PAE increases cell proliferation and
migration of human keratinocytes in vitro
The MTT assay was used to confirm the proliferation
of HaCaT cells in the presence of PAE at three time-points. The low
(0.3125 mg/ml) dose of the extract induced optimal growth in HaCaT
cells at 24, 48 and 72 h, respectively (p<0.05) (Fig. 2A). Conversely, high doses of PAE
(1.25 and 2.5 mg/ml) inhibited cell proliferation. Furthermore, the
treatment with the extract caused obvious proliferation after 48 h
of treatment. Transwell and wound healing assays were used to
determine HaCaT cell migration in skin. Fig. 2B shows that compared with the
control group, cell migration in the PAE-treated group increased
significantly (p<0.01). Furthermore, consistent with the results
from the Transwell assay, data from the wound healing assay also
showed significantly improved migration of wound closure in the PAE
treatment group compared with the control group in the HaCaT cells
(p<0.01) (Fig. 2C).
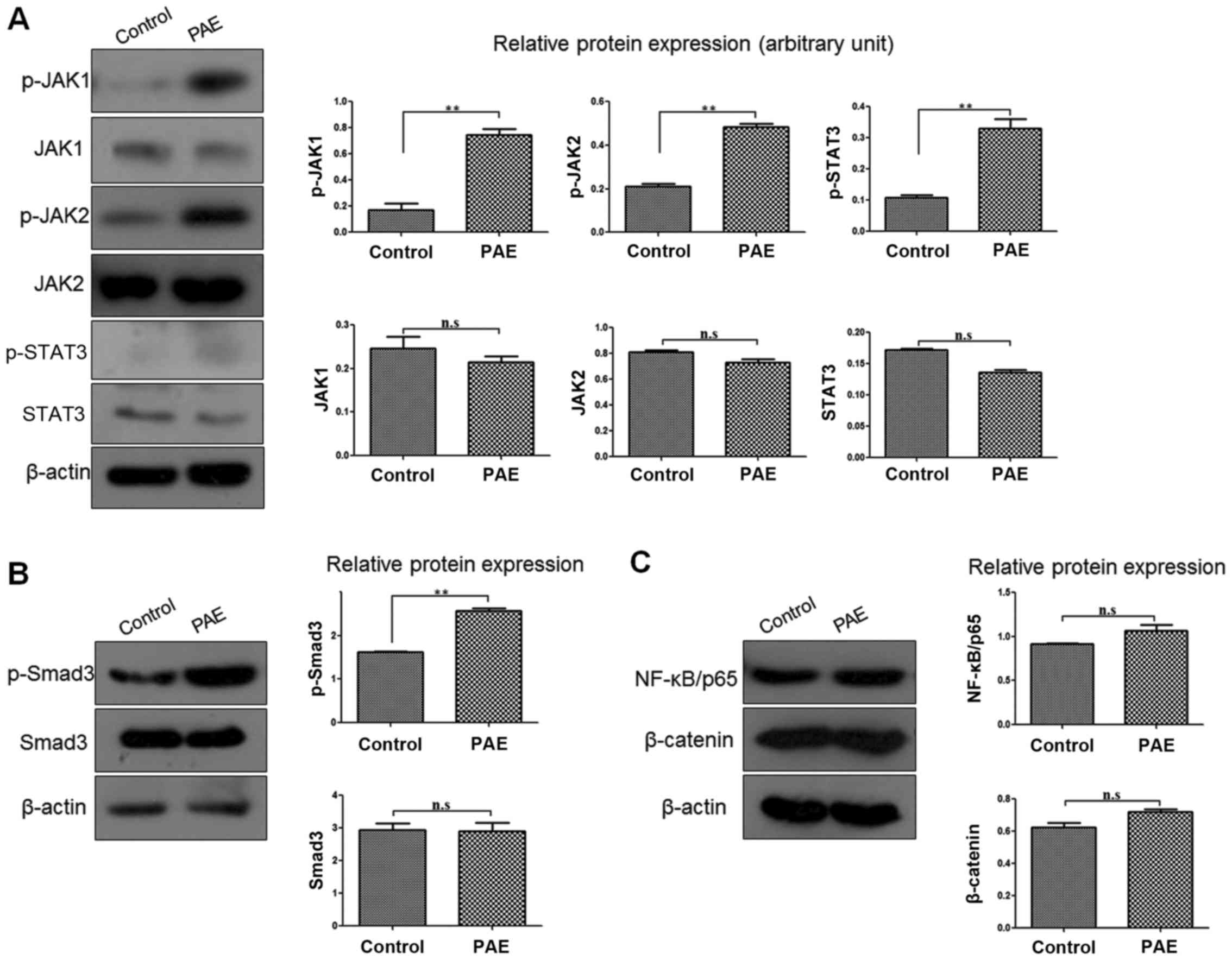
PAE enhances the JAK/STAT3 signaling
pathway and Smad3 activities in human keratinocytes
To better understand the signaling pathway or
mechanism underlying the PAE-mediated regulation of HaCaT cells,
the signaling molecules associated with proliferation and migration
were screened. After PAE treatment, the expression levels of
p-JAK1, p-JAK2 and its downstream molecule p-STAT3 were markedly
upregulated in the HaCaT cells, while the total protein levels
(JAK1, JAK2 and STAT3) were unchanged (p<0.01) (Fig. 3A). In addition, phosphorylation of
Smad3 (p-Smad3) was also significantly increased, while Smad3 was
not affected (p<0.01) (Fig.
3B). However, nuclear factor-κB (NF-κB)/p65 and β-catenin
expression was not distinctly different between the PAE treatment
and the control groups of HaCaT cells (p>0.05) (Fig. 3C).
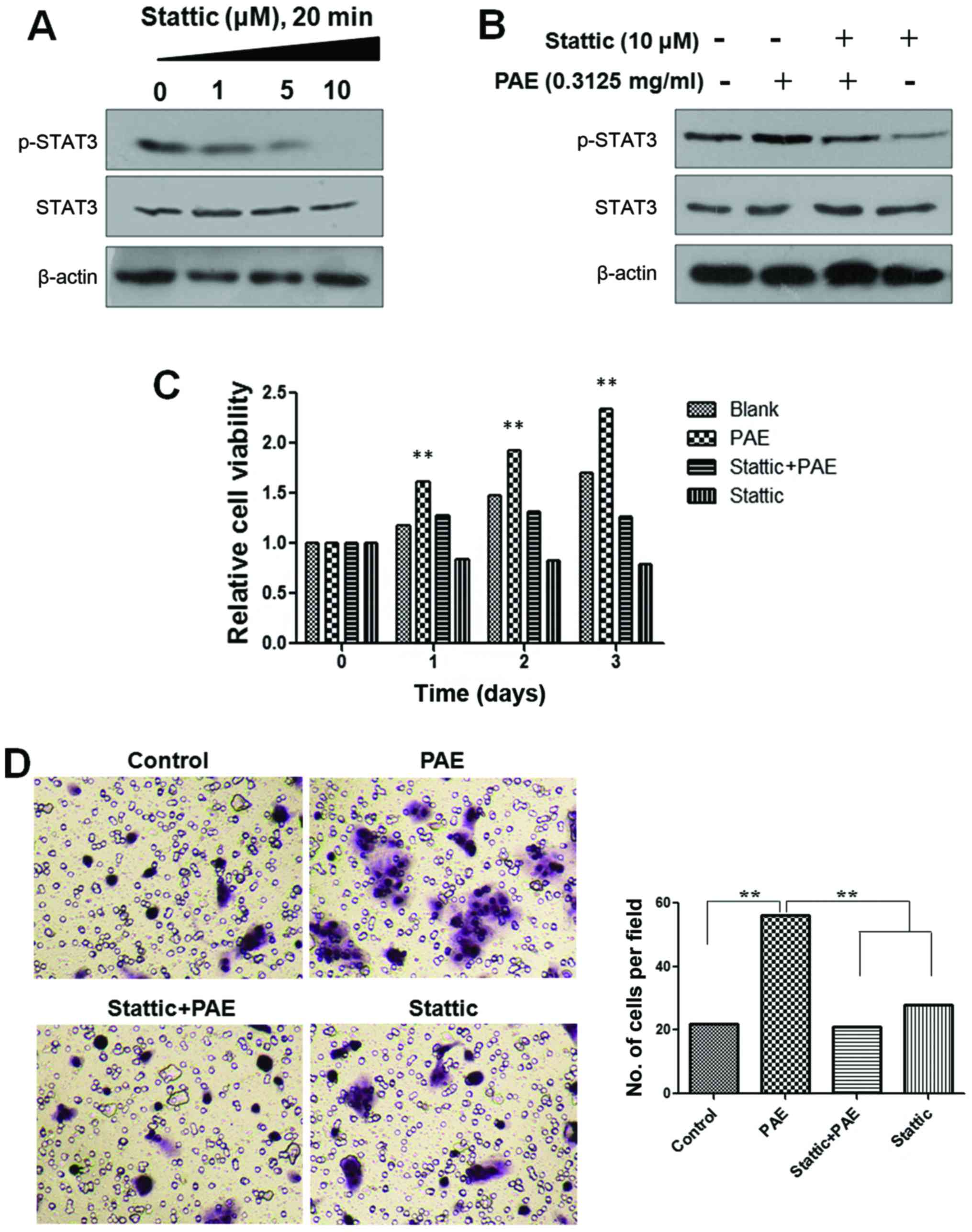
Effects of STAT3 inhibitor on
PAE-mediated proliferation and migration of HaCaT cells
To further confirm that PAE increases cell
proliferation and migration of human keratinocytes via JAK/STAT3
signaling, the inhibitor of STAT3 was used to verify the effects of
PAE on cell proliferation and migration. A STAT3 inhibitor,
stattic, was used to block STAT3 activation. Phosphorylation of
STAT3 was decreased after treatment with stattic in a
dose-dependent manner in the HaCaT cells. However, the total
expression of STAT3 was not changed. Furthermore, when cells were
treated with PAE, after inhibition of STAT3 phosphorylation by
stattic, the p-STAT3 levels remained unchanged (Fig. 4A and B). Furthermore, as shown in
Fig. 4C and D, PAE-induced cell
growth and migration were abrogated by pretreatment with static in
the HaCaT cells. The result confirmed that the PAE extract promoted
HaCaT cell proliferation and migration by activating the JAK/STAT3
signaling pathway.
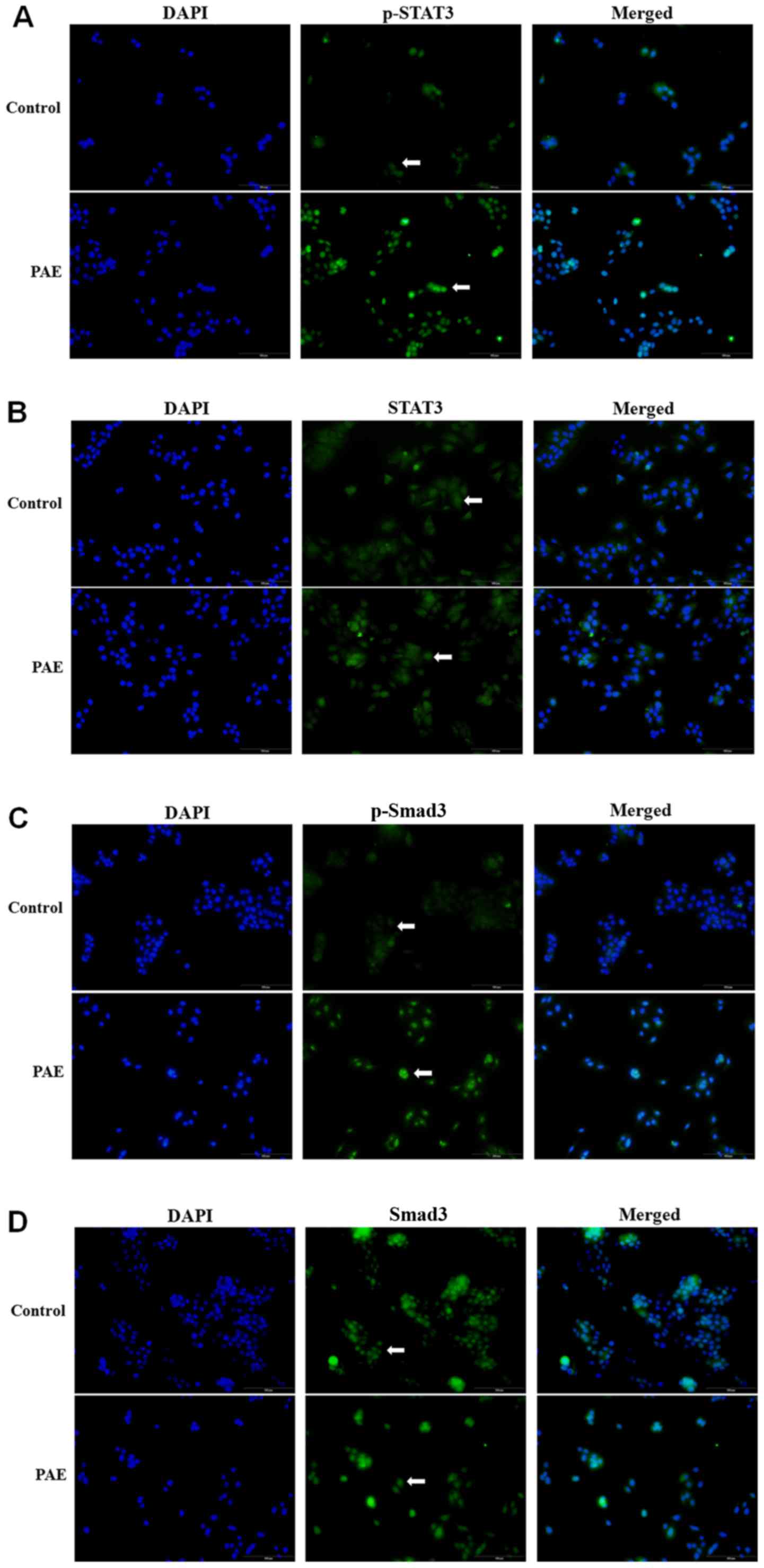
PAE increases phosphorylation of STAT3
and Smad3 expression and alters their distribution
The distribution pattern of STAT3 and Smad3 was
investigated using fluorescence immunostaining. The results
demonstrated that PAE treatment increased STAT3 phosphorylation in
the cytoplasm followed by nuclear translocation (Fig. 5A). By contrast, the total
expression of STAT3 was constant irrespective of PAE stimulation in
the HaCaT cells (Fig. 5B).
Furthermore, phosphorylation of Smad3 (p-Smad3) was also
significantly increased in PAE-treated cells, while total Smad3
remained unchanged (Fig. 5C and
D). The altered phosphorylation of STAT3 and Smad3 suggested
activation of the JAK/STAT3 and Smad3 signaling cascade by PAE in
HaCaT cells.
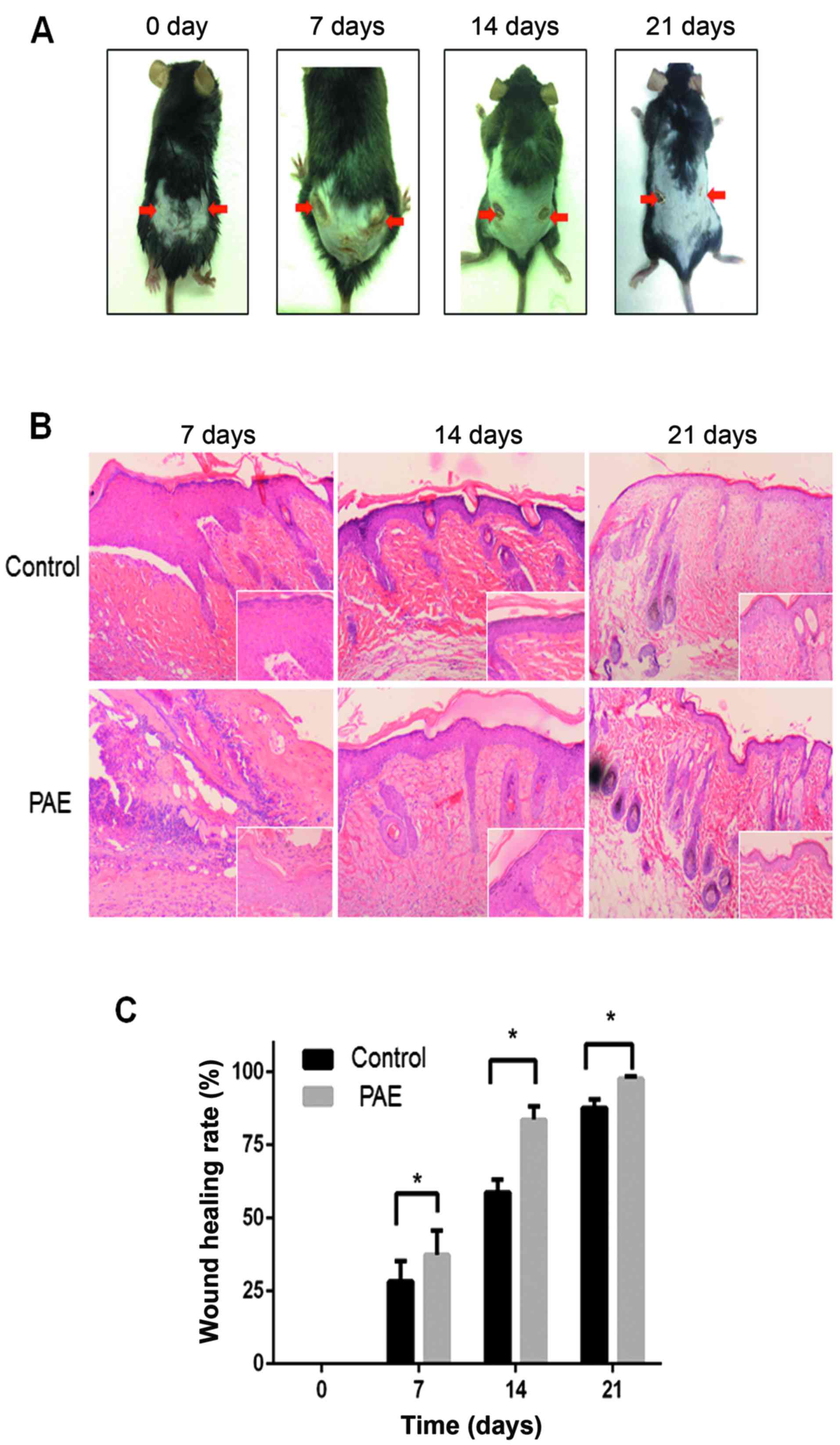
PAE promotes wound healing in vivo
To establish the in vivo effect of PAE on
cutaneous wound healing, we established a mouse model of deep
second-degree thermal burn (16).
Skin lesions were not prominent initially, but eventually appeared
and worsened. The results demonstrated that the burn wound in the
PAE (original fluid, 50 mg/ml)-treated side (right side) showed
significant fibroblast proliferation and fibrosis resulting in scar
tissue formation compared with the control (left side) (Fig. 6A). Histopathological analysis
revealed an increase in epithelial repair, follicle regeneration
and additional fibrous tissues in the PAE-treated groups (Fig. 6B). The healing rates and times of
skin wounds without and with PAE treatment were sequentially
recorded and evaluated. The wound healing rates were measured on
days 0, 7, 14 and 21 after burn, and the complete wound healing
time was calculated. The healing rate of the treated group was
significantly higher than that of the control group at different
time-points (p<0.05) (Table I
and Fig. 6C). The results suggest
that PAE accelerates the healing of burn wounds.
 | Table IHealing rates at different
time-points after burn (%). |
Table I
Healing rates at different
time-points after burn (%).
| Group | 7 day | 14 day | 21 day |
|---|
| Control | 28.17±7.03 | 58.73±4.43 | 87.72±2.88 |
| Treatment | 37.34±8.39a | 83.61±4.61a | 97.69±0.82a |
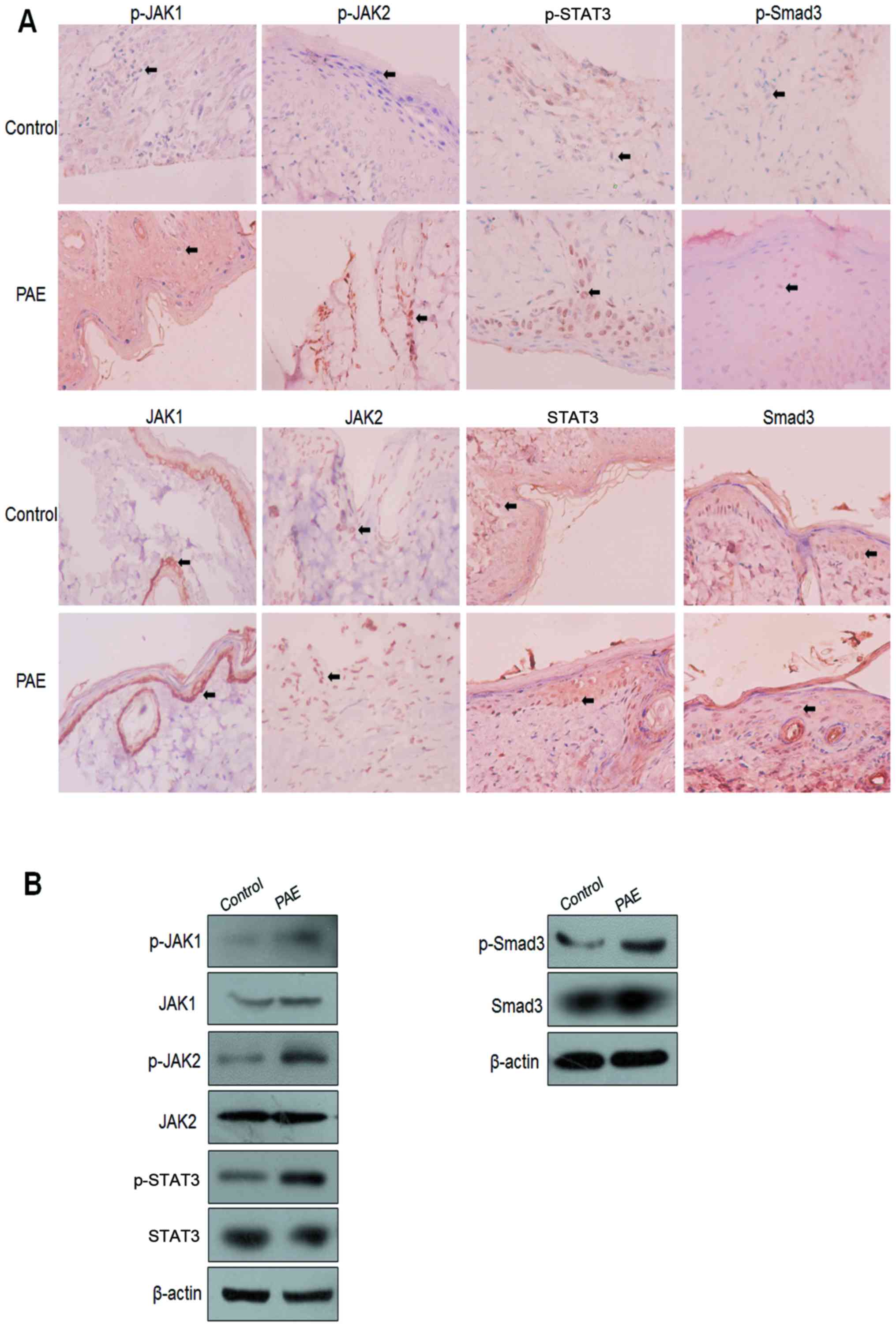
JAK/STAT3 signaling and Smad3 activities
in PAE-mediated wound healing
To further confirm the signaling cascade and
mechanism of PAE-induced regulation of wound healing,
immunohistochemical (IHC) and western blot analysis were used to
assay the JAK/STAT3 signaling and phosphorylation of Smad3 in burn
wound specimens. Enhanced nuclear staining of p-JAK1, p-JAK2,
p-STAT3 and p-Smad3 was detected in the PAE treatment group
compared with the control group. By contrast, the nuclear
expression of JAK1, JAK2, STAT3 and Smad3 proteins showed no
significant difference between the control wounds and the treated
wounds (Fig. 7A). Consistent with
the IHC findings, the western blot analysis further validated the
increased expression of p-JAK1, p-JAK2, p-STAT3, p-Smad3, but not
JAK1, JAK2, STAT3 and Smad3 proteins (Fig. 7B).
Discussion
The beneficial effects of Periplaneta
americana extracts (PAEs) on skin wound healing have been
adequately demonstrated, suggesting their potential role as
bioactive agents for alternative treatment of skin wounds (1,2).
However, the role of PAE in the regulation of target cells and the
molecular mechanisms of wound healing in the skin are still
unclear. In the present study, we extracted and purified PAE from
P. americana to explore the underlying mechanism. We
established skin wound healing models in vitro and in
vivo to demonstrate that PAE promoted the proliferation and
migration of human keratinocyte HaCaT cells and wound healing in a
mouse model. The effects of PAE were mediated via the JAK/STAT3 and
Smad3 signaling pathways in vitro and in vivo.
Wound healing comprises four distinct but
overlapping phases: hemostasis, inflammation, proliferation and
remodeling (2,31). Keratinocytes are a major skin
component and an important determinant of wound healing efficiency
(15,32,33). In this study, we found that PAE
promoted the proliferation and migration of human immortalized
keratinocyte HaCaT cells. To confirm the pharmacological effects of
PAE and establish the optimal extraction process, the human
immortalized keratinocyte line HaCaT was used as an in vitro
wound healing model for the evaluation of the cellular and
molecular effects of PAE. The results showed an increase in
proliferation and migration of keratinocytes after treatment with
PAE (0.3125 mg/ml) for 48 h. In addition, PAE significantly
increased the healing rates and time by enhancing the epithelial
repair, follicle regeneration and fibrous tissue proliferation in
cutaneous thermal burns in vivo.
Although the effects of PAE on wound healing have
long been recognized, the underlying molecular mechanisms remain
largely unknown. Studies suggest the role of numerous signaling
pathways in cutaneous wound healing (15–18). In this study, the expression of
JAK/STAT3 signaling and the activation of Smad3, NF-κB/p65 and
β-catenin in vitro and in wound tissues in vivo were
evaluated by western blot analysis and validated
immunohistochemically. We found that the expression and nuclear
translocation of p-JAK1, p-JAK2 and its downstream p-STAT3 were
markedly upregulated in the HaCaT cells and wound tissues after PAE
treatment. In addition, the levels of p-Smad3 expression were
increased in the treated cells and tissues, suggesting the active
role of these signaling pathways in PAE-mediated wound healing.
However, NF-κB and Wnt signaling appeared to be minimally activated
irrespective of PAE treatment because of limited expression of
NF-κB/P65 and β-catenin upregulation, or nuclear translocation.
STAT3 is a member of the latent transcription factor
family that acts as a downstream effector of cytokine and growth
factor receptor signaling. The canonical JAK/STAT signaling pathway
involves the activation of JAK or growth factor receptor kinases,
phosphorylation of STAT proteins, their dimerization and
translocation into the nucleus whereas STATs act as transcription
factors with pleiotropic downstream effects (34). The pivotal roles of JAK/STAT3
signaling and TGF-β/Smad3 in cutaneous wound healing are well known
(15,35–37). The proliferation and
differentiation of keratinocytes during wound healing are regulated
by cytokines and chemokines, which are secreted by resident and
inflammatory cells and activate the transcription factor STAT3
(38,39). Moreover, the specific ablation of
STAT3 in the follicular and interfollicular keratinocytes resulted
in impaired wound healing (24).
Our results suggested that the enhanced phosphorylation and
activation of JAK/STAT3 and Smad3 by the active ingredients in PAE
accelerates wound healing efficiency. However, the composition of
active ingredients and the downstream factors need to be defined to
elucidate the mechanism of PAE.
Consequently, PAE promoted wound healing in
vitro and in vivo experimental models, which were
strongly correlated with the activation of JAK/STAT3 and Smad3
signaling. Pretreatment with stattic inhibited HaCaT cell
proliferation and migration induced by PAE. Furthermore, the
enhanced activities of JAK/STAT3 and Smad3 pathways may represent
major molecular targets of PAE, in accelerated wound healing.
Abbreviations:
|
PAEs
|
Periplaneta americana
extracts
|
|
JAK/STAT3
|
Janus-activated kinase/signal
transducer and activator of transcription 3
|
|
IHC
|
immunohistochemical staining
|
|
TCM
|
traditional Chinese medicine
|
|
ECM
|
extracellular cell matrix
|
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (no. 81403194).
References
|
1
|
Luo TS, Gao MT, Ma FF, Liu GM and Zhang
CG: Research advances in pharmacological action and clinical
application of Periplaneta americana. Agric Sci Technol.
13:888–892. 2012.
|
|
2
|
Chen XH, Ran XZ, Sun RS, Shi CM, Su Y, Guo
CH and Cheng TM: Protective effect of an extract from Periplaneta
americana on hematopoiesis in irradiated rats. Int J Radiat Biol.
85:607–613. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yan MC, Yang H and Yang RM: Observation of
the clinical effects of W11-a12. Yunnan Med.
3:138–140. 1987.
|
|
4
|
Pan XL: The observation and nurse care
with Kangfuxin local medicine - change in treating diabetes
acromelic gangrene. J Nurse Train. 11:26–27. 1996.
|
|
5
|
Wang HL and Liu J: Clinical observation of
patients burned at III degree in small proportion of total surface
area treated with Kangfuxin. J Binzhou Med Coll. 22:3681999.
|
|
6
|
Lin Q, Cao D, Yang YQ, et al: Study on
action of Kangfuxin solution on experimental gastric ulcer. Chin
Trad Pat Med. 23:122–124. 2001.
|
|
7
|
Du GM and Li WL: The clinical and
laboratory studies about effects of Kangfuxin on superoxide
dismutase and cell immune function of old persons. Yunnan J Chin
Trad Med. 10:32–33. 1989.
|
|
8
|
Man HX, Huang L, Na KG, et al: Research
progress of chemical component and biological activity in medicinal
Periplaneta americana. Anti-infect Pharmacol. 11:403–407. 2014.
|
|
9
|
Wang ZY, Huang XH, Xie YK, Chen S and Wang
S: Influence of Kangfuxin liquid on the wound healing of
experimental animal burns and scalds. J Tradit Chin Med.
52:1316–1317. 2011.
|
|
10
|
Shu CX, Cheng TM and Yan GM: Effects of
whole body irradiation on several components of skin wound
extracellular matrix and the repair-promoting action of
W11-a12. Chin J Traumatol. 11:604–607.
2001.
|
|
11
|
Shu CX, Ye BL, Cheng TM and Xiao JS:
Effects of irradiation and W11-a12 on
anion-selective channel of mouse peritoneal macrophage. Acta Acade
Med Milita Tertiae. 23:290–292. 2001.
|
|
12
|
Chen XH, Cheng TM and Ai GP: Effects of
systemic irradiation and W11-a12 on
neutrophils in wounds. Acta Acade Med Milita Tertiae. 23:287–289.
2001.
|
|
13
|
Chen XH, Sun RS, Cheng TM, et al:
Neutrophil apoptosis in wound of irradiated rats and effects of
W11-a12 in accelerating wound healing. Chin J
Clin Rehabil. 9:108–110. 2005.
|
|
14
|
Amadeu TP, Seabra AB, de Oliveira MG and
Monte-Alto-Costa A: Nitric oxide donor improves healing if applied
on inflammatory and proliferative phase. J Surg Res. 149:84–93.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li PN, Li H, Zhong LX, Sun Y, Yu LJ, Wu
ML, Zhang LL, Kong QY, Wang SY and Lv DC: Molecular events
underlying maggot extract promoted rat in vivo and human in vitro
skin wound healing. Wound Repair Regen. 23:65–73. 2015. View Article : Google Scholar
|
|
16
|
Pakyari M, Farrokhi A, Maharlooei MK and
Ghahary A: Critical role of transforming growth factor beta in
different phases of wound healing. Adv Wound Care (New Rochelle).
2:215–224. 2013. View Article : Google Scholar
|
|
17
|
Ren X, Ge M, Qin X, Xu P, Zhu P, Dang Y,
Gu J and Ye X: S100a8/NF-κB signal pathway is involved in the
800-nm diode laser-induced skin collagen remodeling. Lasers Med
Sci. 31:673–678. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi Y, Shu B, Yang R, Xu Y, Xing B, Liu J,
Chen L, Qi S, Liu X, Wang P, et al: Wnt and Notch signaling pathway
involved in wound healing by targeting c-Myc and Hes1 separately.
Stem Cell Res Ther. 6:1202015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tokumaru S, Sayama K, Yamasaki K,
Shirakata Y, Hanakawa Y, Yahata Y, Dai X, Tohyama M, Yang L,
Yoshimura A, et al: SOCS3/CIS3 negative regulation of STAT3 in
HGF-induced keratinocyte migration. Biochem Biophys Res Commun.
327:100–105. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yasukawa H, Ohishi M, Mori H, Murakami M,
Chinen T, Aki D, Hanada T, Takeda K, Akira S, Hoshijima M, et al:
IL-6 induces an anti-inflammatory response in the absence of SOCS3
in macrophages. Nat Immunol. 4:551–556. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lejeune D, Dumoutier L, Constantinescu S,
Kruijer W, Schuringa JJ and Renauld JC: Interleukin-22 (IL-22)
activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a
rat hepatoma cell line. Pathways that are shared with and distinct
from IL-10. J Biol Chem. 277:33676–33682. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wolk K, Witte E, Witte K, Warszawska K and
Sabat R: Biology of interleukin-22. Semin Immunopathol. 32:17–31.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu R, Ding Y, Zhu L, Qu Y, Zhang C, Liu L
and Chen L: IL-22 mediates the oral mucosal wound healing via STAT3
in keratinocytes. Arch Oral Biol. 72:14–20. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sano S, Itami S, Takeda K, Tarutani M,
Yamaguchi Y, Miura H, Yoshikawa K, Akira S and Takeda J:
Keratinocyte-specific ablation of Stat3 exhibits impaired skin
remodeling, but does not affect skin morphogenesis. EMBO J.
18:4657–4668. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Penn JW, Grobbelaar AO and Rolfe KJ: The
role of the TGF-β family in wound healing, burns and scarring: A
review. Int J Burns Trauma. 2:18–28. 2012.
|
|
26
|
Hong HJ, Jin SE, Park JS, Ahn WS and Kim
CK: Accelerated wound healing by smad3 antisense
oligonucleotides-impregnated chitosan/alginate polyelectrolyte
complex. Biomaterials. 29:4831–4837. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Biernacka A, Dobaczewski M and
Frangogiannis NG: TGF-β signaling in fibrosis. Growth Factors.
29:196–202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Werner S, Krieg T and Smola H:
Keratinocyte-fibroblast interactions in wound healing. J Invest
Dermatol. 127:998–1008. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tavares Pereira DS, Lima-Ribeiro MH, de
Pontes-Filho NT, Carneiro-Leão AM and Correia MT: Development of
animal model for studying deep second-degree thermal burns. J
Biomed Biotechnol. 2012:4608412012. View Article : Google Scholar :
|
|
30
|
Zhang J, La X, Fan L, Li P, Yu Y, Huang Y,
Ding J and Xing Y: Immunosuppressive effects of mesenchymal stem
cell transplantation in rat burn models. Int J Clin Exp Pathol.
8:5129–5136. 2015.PubMed/NCBI
|
|
31
|
Diegelmann RF and Evans MC: Wound healing:
An overview of acute, fibrotic and delayed healing. Front Biosci.
9:283–289. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hata S, Okamura K, Hatta M, Ishikawa H and
Yamazaki J: Proteolytic and non-proteolytic activation of
keratinocyte-derived latent TGF-β1 induces fibroblast
differentiation in a wound-healing model using rat skin. J
Pharmacol Sci. 124:230–243. 2014. View Article : Google Scholar
|
|
33
|
Yamaoka H, Sumiyoshi H, Higashi K, Nakao
S, Minakawa K, Sumida K, Saito K, Ikoma N, Mabuchi T, Ozawa A, et
al: A novel small compound accelerates dermal wound healing by
modifying infiltration, proliferation and migration of distinct
cellular components in mice. J Dermatol Sci. 74:204–213. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Levy DE and Darnell JE Jr: Stats:
Transcriptional control and biological impact. Nat Rev Mol Cell
Biol. 3:651–662. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhu BM, Ishida Y, Robinson GW,
Pacher-Zavisin M, Yoshimura A, Murphy PM and Hennighausen L: SOCS3
negatively regulates the gp130-STAT3 pathway in mouse skin wound
healing. J Invest Dermatol. 128:1821–1829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Murray PJ: The JAK-STAT signaling pathway:
Input and output integration. J Immunol. 178:2623–2629. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tokumaru S, Sayama K, Shirakata Y,
Komatsuzawa H, Ouhara K, Hanakawa Y, Yahata Y, Dai X, Tohyama M,
Nagai H, et al: Induction of keratinocyte migration via
transactivation of the epidermal growth factor receptor by the
antimicrobial peptide LL-37. J Immunol. 175:4662–4668. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Avitabile S, Odorisio T, Madonna S,
Eyerich S, Guerra L, Eyerich K, Zambruno G, Cavani A and Cianfarani
F: Interleukin-22 promotes wound repair in diabetes by improving
keratinocyte pro-healing functions. J Invest Dermatol.
135:2862–2870. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nelson AM, Katseff AS, Ratliff TS and
Garza LA: Interleukin 6 and STAT3 regulate p63 isoform expression
in keratinocytes during regeneration. Exp Dermatol. 25:155–157.
2016. View Article : Google Scholar :
|