Introduction
Astrocytic brain tumors, the most common type of
primary malignant brain tumors, are typically characterized by
diffuse infiltrative growth and a low survival rate (1–3).
Despite improvements in GBM therapy consisting of surgical
resection and chemoradiotherapy with temozolomide (TMZ), only 10%
of these patients survive 5 years after diagnosis and the majority
of patients have a median survival of only 12–14 months (4–6).
Thus, in order to identify potential diagnostic and therapeutic
targets, it is urgent to understand the underlying molecular events
which lead to the progression of glioma.
Deltex-3-like (DTX3L), also known as B-lymphoma and
BAL-associated protein (BBAP), belongs to the Deltex (DTX) family
(7,8). As an E3 ligase, DTX3L has been
reported to regulate ESCRT-0 ubiquitination by inhibiting the
activity of AIP4 (9). In
addition, DTX3L exerts an effect on the DNA damage response pathway
by modulating monoubiquitination of histone H4 (10). DTX3L was also identified as a
binding partner of B aggressive lymphoma 1 (BAL1), a risk-related
gene and protein in diffuse large B cell lymphoma (DLBCL) (11). Furthermore, it has been reported
that DTX3L is overexpressed in melanoma and regulates melanoma
metastasis via the FAK/PI3K/AKT pathway (12). Recently, DTX3L was also shown to
play a role in prostate cancer cells via inhibiting IRF1 expression
and its depletion was found to be related with tumor cell
proliferation (13). All of these
studies demonstrate that DTX3L is a novel biomarker with which to
predict the progression of tumors. Nevertheless, to the best of our
knowledge, the function of DTX3L has not yet been reported in
gliomas.
In the present study, we evaluated the expression of
DTX3L in human glioma tissues and explored its role in the
migration and apoptosis of glioma cells. These findings may be
useful to identify a new therapeutic target for glioma.
Materials and methods
Primary human glioma specimens
All human glioma specimens were collected from 96
patients between 2007 and 2012 at a single institution, the
Department of Pathology, Affiliated Hospital of Nantong University.
All tumors were from patients with newly diagnosed gliomas who had
received no therapy before sample collection. Normal brain tissues
were obtained from patients undergoing surgery for epilepsy. All
tissue samples were obtained using protocols approved by the Ethics
Committee of the Affiliated Hospital of Nantong University. Tissues
were frozen immediately after surgery and stored at −80°C until
use. For immunoblot analysis, all the tissues were frozen in liquid
nitrogen immediately after surgery. The specimens for histological
examination were fixed in formalin and embedded in paraffin for
sectioning.
Immunohistochemical staining
Paraffin sections (5-μm-thick) from tissues
were dewaxed in xylene, rehydrated in graded alcohol and endogenous
peroxidase activity was blocked by infiltrating in 0.3% hydrogen
peroxide. Then, to enhance the accessibility of the antigen, the
sections were treated with 0.1 M citrate buffer (pH 6.0) and heated
to 121°C in an autoclave for 3 min. After cooling, hydrogen
peroxide (0.3%) was used to block endogenous peroxide activity for
20 min. Next the sections were rinsed in phosphate-buffered saline
(PBS) (pH 7.2) and then incubated with the DTX3L (cat. no.
sc-100627; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and
Ki-67 (cat. no. sc-23900; Santa Cruz Biotechnology, Inc.)
antibodies for 2 h at room temperature. All slides were processed
using immunoglobulin IgG as a secondary antibody. After being
washed in PBS, the peroxidase reaction was visualized by incubating
the sections with DAB (0.02% diaminobenzidine tetrahydrochloride,
0.1% phosphate buffer solution and 3% H2O2).
Finally, the sections were counterstained with hematoxylin,
dehydrated with graded alcohol and mounted in resin mount. A
microscope was used to observe the stained sections. The
immunostaining was evaluated separately by two independent
pathologists. For the assessment of DTX3L and Ki-67, five
high-power fields were randomly chosen and at least 300 cells were
counted in each section. The percentage of tumor cells that stained
positive was scored as follows: 1, 0–49% positive tumor cells; 2,
50–74% positive tumor cells; and 3, 75–100% positive tumor cells.
The intensity of staining was estimated and scored as follows: 0,
no staining; 1, weak staining; 2, moderate staining; and 3, strong
staining. Then, we combined scores from the two scales and
classified each section as follows: 0–4.5 was classified as low
expression and 4.5–9 was classified as high expression.
Western blot analysis
Proteins isolated from tissues and cell samples were
promptly homogenized in lysis buffer which contained 1 M Tris-HCl
pH 7.5, 1% Triton X-100, 1% Nonidet P-40 (NP-40), 10% sodium
dodecyl sulfate (SDS), 0.5% sodium deoxycholate, 0.5 M EDTA, 10
μg/ml leupeptin, 10 μg/ml aprotinin, and 1 mM PMSF
and then centrifuged for 30 min at 10,000 × g to collect the
supernatant liquid. The supernatants were stored at −80°C until
use. The protein concentrations were measured with a Bio-Rad
protein assay (Bio-Rad, Hercules, CA, USA). Equal amount of protein
from each sample was separated by SDS-polyacrylamide gel
electrophoresis (PAGE) and then transferred onto a PVDF membrane
(Millipore, Bedford, MA, USA). After being blocked in 5% non-fat
milk in TBST [150 mM NaCl, 20 mM Tris (pH 7.4) and 0.05% Tween-20]
for 2 h, the membranes were first incubated with primary antibodies
overnight at 4°C. The primary antibodies used in this report
included the followed: anti-glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) (cat. no. sc-47724; 1:3,000), anti-DTX3L
(cat. no. sc-100627; 1:500), E-cadherin (cat. no. sc-8426; 1:500),
vimentin (cat. no. sc-6260; 1:1,000) (all from Santa Cruz
Biotechnology, Inc.), anti-caspase-3 (cleaved) (cat. no. 9661;
1:1,000), and anti-PARP (cleaved) (cat. no. 5625; 1:1,000) (both
from Cell Signaling Technology, Danvers, MA, USA). After washing
for three times, 5 min each, the membranes were then incubated with
horseradish peroxidase-conjugated human anti-mouse (cat. no.
A11126) or anti-rabbit (cat. no. 71-2700) antibodies (1:1,000;
Pierce, Rockford, IL, USA) as the secondary antibody at room
temperature for 2 h. Finally, the membranes were detected with ECL
detection systems. The experiments were implemented in three
independent reactions.
Cell cultures and transient
transfection
Normal human gliocyte HEB cells and human
glioblastoma cell lines including U251MG, U87MG and A172 were
obtained from the Cell Library of the Chinese Academy of Sciences.
Cells were cultured in Dulbecco's modified Eagle's medium (DMEM)
(Gibco-BRL, Grand Island, NY, USA) with 10% fetal bovine serum
(FBS), 2 mM L-glutamine, and 100 U/ml penicillin-streptomycin
mixture (Gibco-BRL) at 37°C in a humidified incubator containing 5%
CO2. The medium was changed every 2–3 days, and cultures
were split using 0.25% trypsin. Lipofectamine 2000 (Invitrogen,
Carlsbad, CA, USA) was used to transfect the U251MG cells with
siRNAs according to the manufacturer's instructions. Four siRNAs
targeting the DTX3L gene were designed and synthesized, and western
blot analysis was used to identify the most effective siRNA for the
further experiments. The sequences are as follows:
5′-CGTATTAGGAGTCTCAGAT-3′, 5′-GATGGACATTGATAGCGAT-3′,
5′-TGATTTAATGCCAGTCTAA-3′ and 5′-CAATTACATGATGAATGTA-3′. Cells were
transfected with DTX3L-siRNA or control-siRNA and then collected
after 24–48 h for the following assays.
Multicellular tumor spheroid formation
assays
U251MG cells transfected with DTX3L siRNA and
control siRNA were seeded at a density of 4×105
cells/well in 6-well plates. Then, the cells were washed with PBS
and stained with 0.5% (w/v) crystal violet in 70% ethanol after
being cultured for 4–6 days. Finally, the amount of multicellular
tumor spheroids from the indicated fields (>10) was measured
under a light microscope.
Cell growth assay
To evaluate the effect of transfection of DTX3L
(control or siRNA), the cells were plated in 96-well plates at a
density of 2×104/well in a volume of 100 μl in
medium with 10% FBS. Each well was transfected for the indicated
times after 24 h. Cell Counting Kit-8 (Dojindo, Kumamoto, Japan)
reagents were added to each well for 2-h incubation at 37°C, after
which absorbance at the wavelength of 450 nm was measured in an
automated plate reader.
Wound-healing assays
U87MG cells were seeded at a density of
2×106 in 6-well plates and incubated in DMEM
supplemented with 10% FBS for 24 h. The cells were then transfected
with DTX3L siRNA and control siRNA for 36 h and subsequently serum
starved for 12 h. A straight scratch was made in each well with a
10-μl micropipette tip at the same time. After being washed
three times with PBS, the cells were cultured in 5% FBS-DMEM in a
humidified atmosphere of 5% CO2 at 37°C, and images of
the width of the wound were captured under a microscope at 0-, 24-
and 48-h time-points.
Transwell migration assay
U87MG cells were transfected with DTX3L siRNA and
control siRNA for 48 h and then resuspended in DMEM supplemented
with 0.1% bovine serum albumin. Cells at a density of
1×105 were added to the upper chambers of 24-well
Transwell plates (8-μm pore size; Corning, Inc., Corning,
NY, USA), and complete medium was added to the lower chambers.
After being incubated for 36 h at 37°C, the cells remaining in the
upper chamber (non-migrated) were removed, and the ones on the
bottom chamber (migrated) were fixed and stained with crystal
violet to visualize the nuclei. The number of cells that had
migrated through the polycarbonate membrane was counted under ×200
magnification. All experiments were performed in triplicate and
repeated twice.
Annexin V analysis
U87MG cells were transfected with the indicated
siRNA or plasmid and subsequently treated with TMZ for 4 h as
indicated. Then after being separated with trypsin, the cells were
washed three times and stained with FITC-Annexin V (Biosource,
Camarillo, CA, USA) for 15 min and then finally detected on a
FacsCalibur (BD Biosciences, San Jose, CA, USA).
Statistical analysis
All of the numerical data were analyzed using the
SPSS 17.0 software package (SPSS, Inc., Chicago, IL, USA). The
statistical significance of the correlations between DTX3L and
clinicopathologic features were analyzed by the Chi-square
(χ2) test. Univariate survival analysis was performed
using the Kaplan-Meier method and curves were compared using the
log-rank test. Multivariate survival analysis was carried out using
Cox's proportional hazards regression model and the risk ratio and
its 95% confidence interval were recorded for every marker. P-value
<0.05 was considered to indicate a statistically significant
difference. All values are presented as mean ± SEM and each
experiment was carried out three times.
Results
Identification of DTX3L expression in
glioma
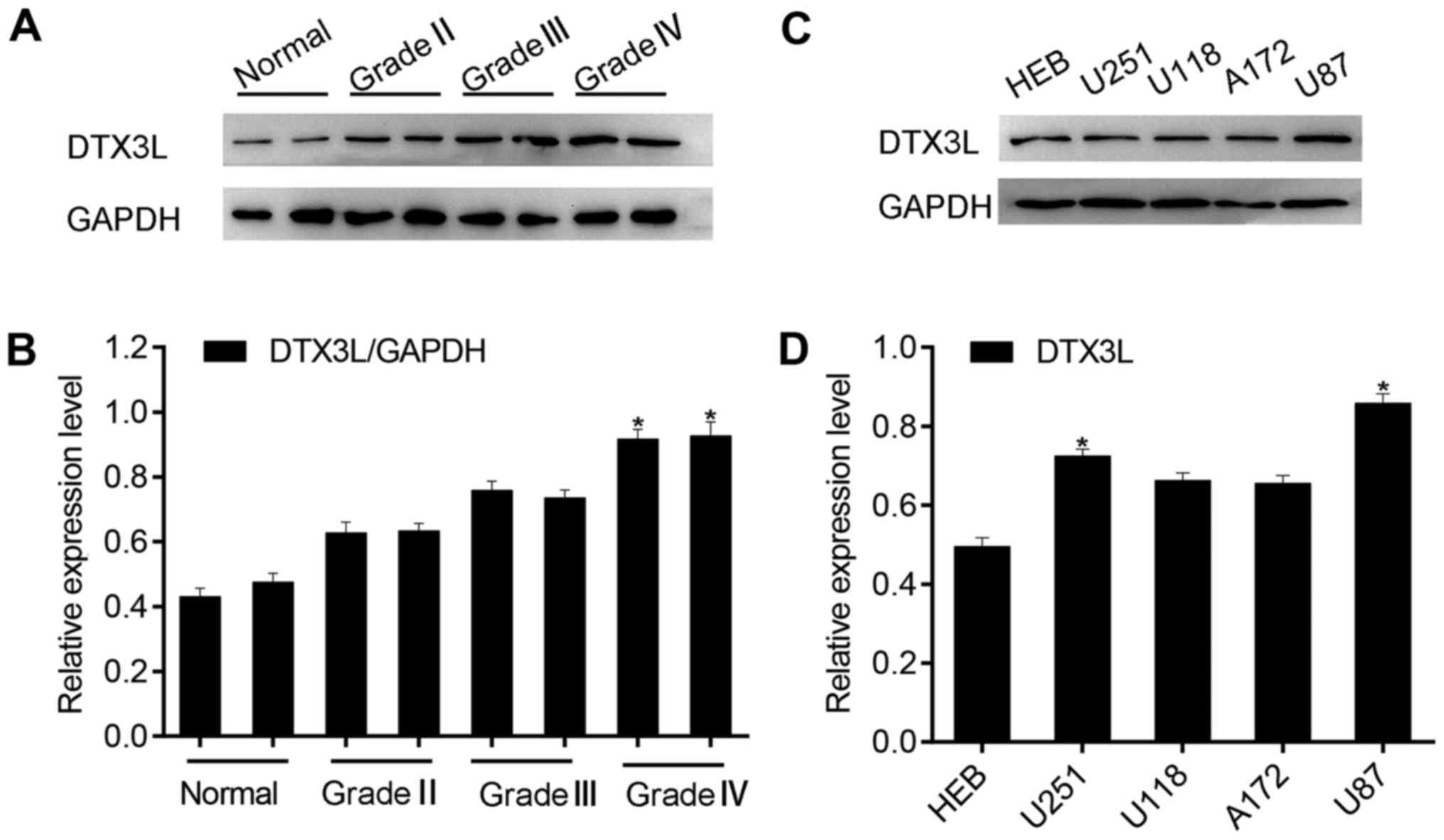
To reveal the potential role of DTX3L in glioma
progression, we firstly used western blotting to analyze the
expression of DTX3L in a panel of glioma specimens, including two
normal brain tissue samples and six glioma samples. We noted that
DTX3L protein expression was significantly increased in high-grade
glioma tissues compared with that observed in the low-grade glioma
tissues and normal brain tissues (Fig. 1A and B). Then, we also examined
the expression of DTX3L in normal human gliocyte HEB cells and
glioma cell lines, U87MG, U251MG, A172 and U118. As expected, DTX3L
was highly expressed in the glioma cell lines (Fig. 1C and D) compared to that noted in
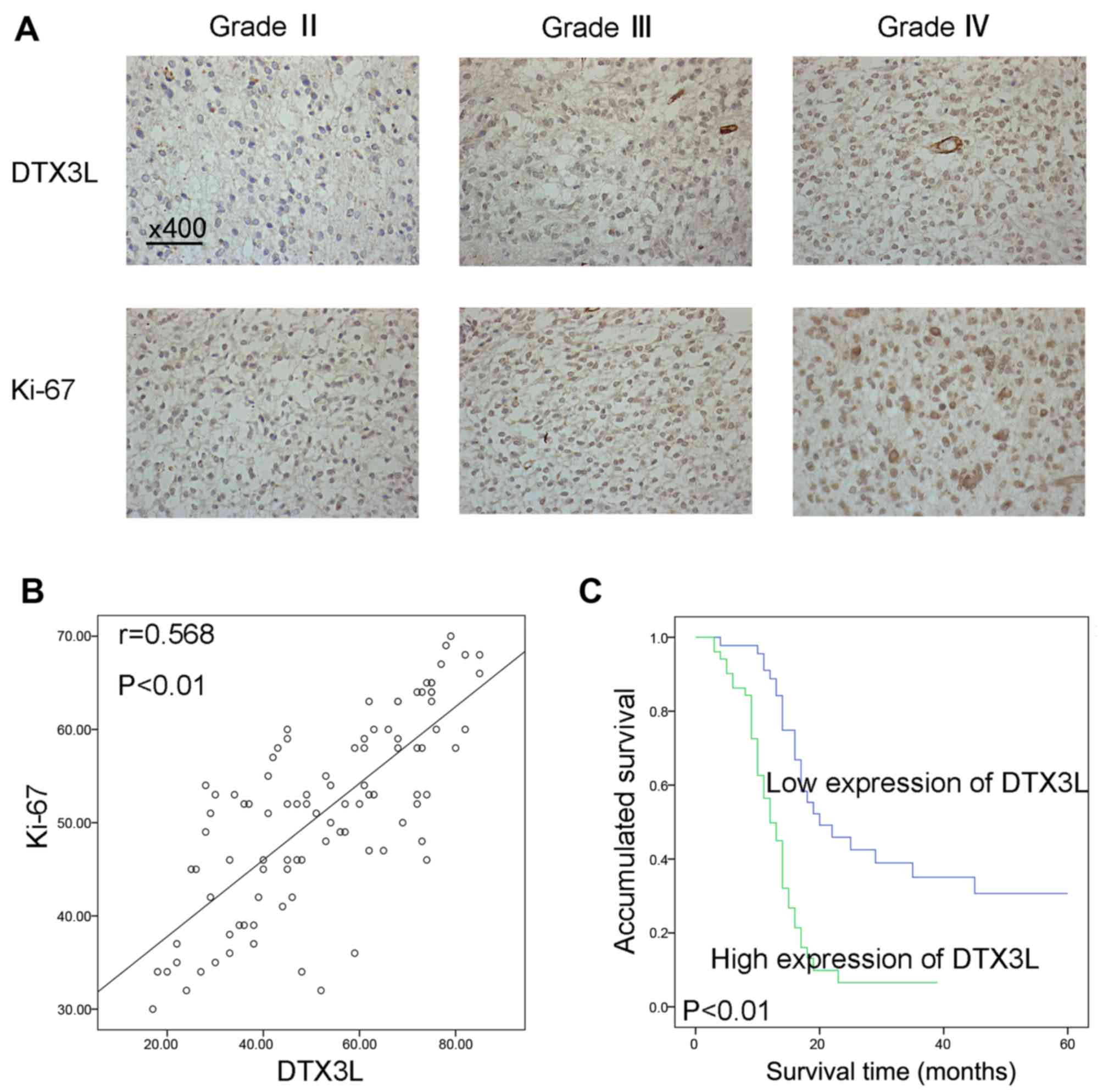
the HEB cells. Next, immunohistochemistry was used to analyze DTX3L
expression and Ki-67 expression in 96 glioma samples to confirm the
association of DTX3L with glioma progression. DTX3L expression was
increased as the degree of malignancy was increased (Fig. 2A). In addition, Spearman's
correlation coefficient demonstrated a positive correlation between
DTX3L and Ki-67 expression (r=0.568, P<0.01) (Fig. 2B).
DTX3L expression and patient
survival
To further explore the correlation between
clinicopathologic and pathophysiological features of glioma and
DTX3L expression, the immunohistochemical results of the 96 glioma
specimens are listed in Table I.
We found that DTX3L expression was significantly correlated with
clinicopathologic grade (P=0.002) and Ki-67 expression (P=0.027).
However, no significant correlation was observed between DTX3L
expression and patient age, sex, tumor location, type of surgery,
or tumor size in the 96 glioma cases. Multivariate Cox regression
analysis indicated that DTX3L expression is an independent
predictor of survival (P=0.022) (Table II). Furthermore, Kaplan-Meier
survival curves indicated that upregulation of DTX3L was
significantly correlated with a shortened overall survival
(P<0.01) (Fig. 2C).
 | Table ICorrelation between DTX3L expression
and the clinicopathological characteristics of the 96 glioma
specimens. |
Table I
Correlation between DTX3L expression
and the clinicopathological characteristics of the 96 glioma
specimens.
| Variables | Total | DTX3L expression
| χ2
value | P-value |
|---|
| Low (score
<5) | High (score ≥5) |
|---|
| Age (years) |
| <50 | 48 | 25 | 23 | 1.046 | 0.306 |
| ≥50 | 48 | 20 | 28 | | |
| Sex |
| Female | 39 | 19 | 20 | 0.090 | 0.765 |
| Male | 57 | 26 | 31 | | |
| Tumor location |
| Frontal | 30 | 18 | 12 | 3.895 | 0.420 |
| Parietal | 12 | 6 | 6 | | |
| Occipital | 13 | 6 | 7 | | |
| Temporal | 28 | 10 | 18 | | |
| Unknown | 13 | 5 | 8 | | |
| Surgery |
| Biopsy | 17 | 8 | 9 | 0.014 | 0.993 |
| Total
resection | 55 | 26 | 29 | | |
| Subtotal
section | 24 | 11 | 13 | | |
| Tumor size
(cm) |
| <4 | 54 | 28 | 26 | 1.228 | 0.268 |
| ≥4 | 42 | 17 | 25 | | |
| WHO grade |
| II | 28 | 19 | 9 | 12.268 | 0.002a |
| III | 43 | 21 | 22 | | |
| IV | 25 | 5 | 20 | | |
| Ki-67
expression |
| Low | 44 | 26 | 18 | 4.868 | 0.027a |
| High | 52 | 19 | 33 | | |
 | Table IIContribution of various potential
prognostic factors to survival by Cox regression analysis of 96
glioma specimens. |
Table II
Contribution of various potential
prognostic factors to survival by Cox regression analysis of 96
glioma specimens.
|
Characteristics | Hazard ratio | 95% CI | P-value |
|---|
| Age (years) | 1.271 | 0.753–2.146 | 0.370 |
| Sex | 1.597 | 0.886–2.878 | 0.119 |
| Tumor location | 0.985 | 0.820–1.184 | 0.874 |
| Tumor size | 1.054 | 0.607–1.830 | 0.852 |
| Surgery | 1.074 | 0.712–1.619 | 0.734 |
| WHO grade | 1.832 | 1.269–2.647 | 0.001a |
| DTX3L
expression | 2.038 | 1.110–3.744 | 0.022a |
| Ki-67
expression | 2.150 | 1.149–4.022 | 0.017a |
Knockdown of DTX3L by RNA
interference
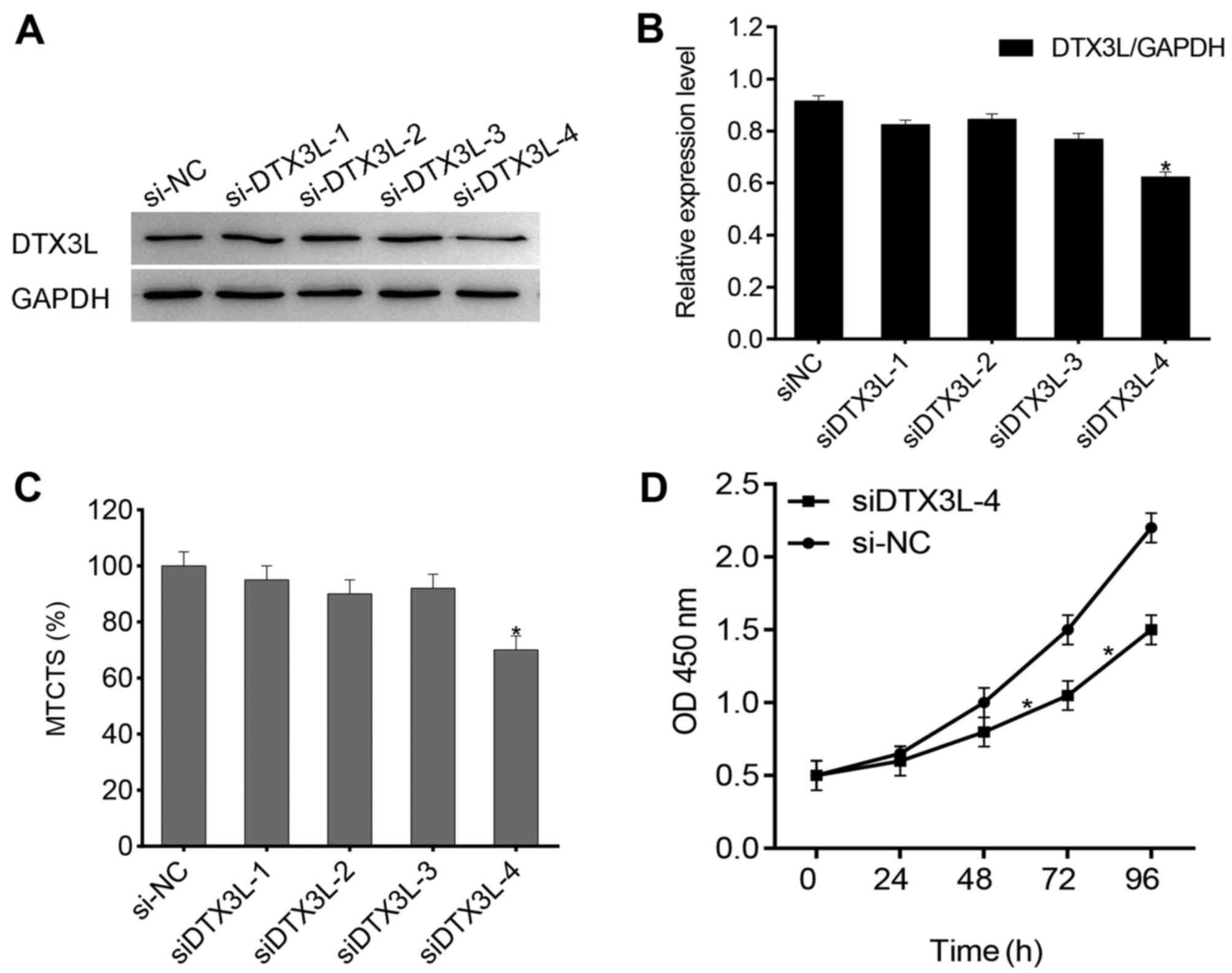
We selected U87MG cells for the siRNA experiment, as
this cell line expresses a high level of endogenous DTX3L. To
further study the potential function of DTX3L in glioma cell
proliferation, we used four siRNAs against DTX3L expression to test
their interference efficiency and the result showed that DTX3L
protein levels were significantly decreased in the cells
transfected with siDTX3L-1 compared with the level noted in the
cells transfected with the control siRNA (Fig. 3A and B). It has been reported that
U87MG cells have the ability to spontaneously form multicellular
tumor spherioids (14,15); thus we speculated that DTX3L has
an effect on tumor spheroid formation. We transfected U87MG cells
with DTX3L-siRNA or control-siRNA. Knockdown of DTX3L resulted in
an obvious decrease in multicellular tumor spheroids (Fig. 3C). In addition, we examined the
effect of DTX3L silencing on the cell growth rate. As expected,
knockdown of DTX3L was able to inhibit the cell growth rate
(Fig. 3D). Taken together, we
demonstrated that DTX3L silencing coutributed to a reduction in the
proliferation of the glioma cells.
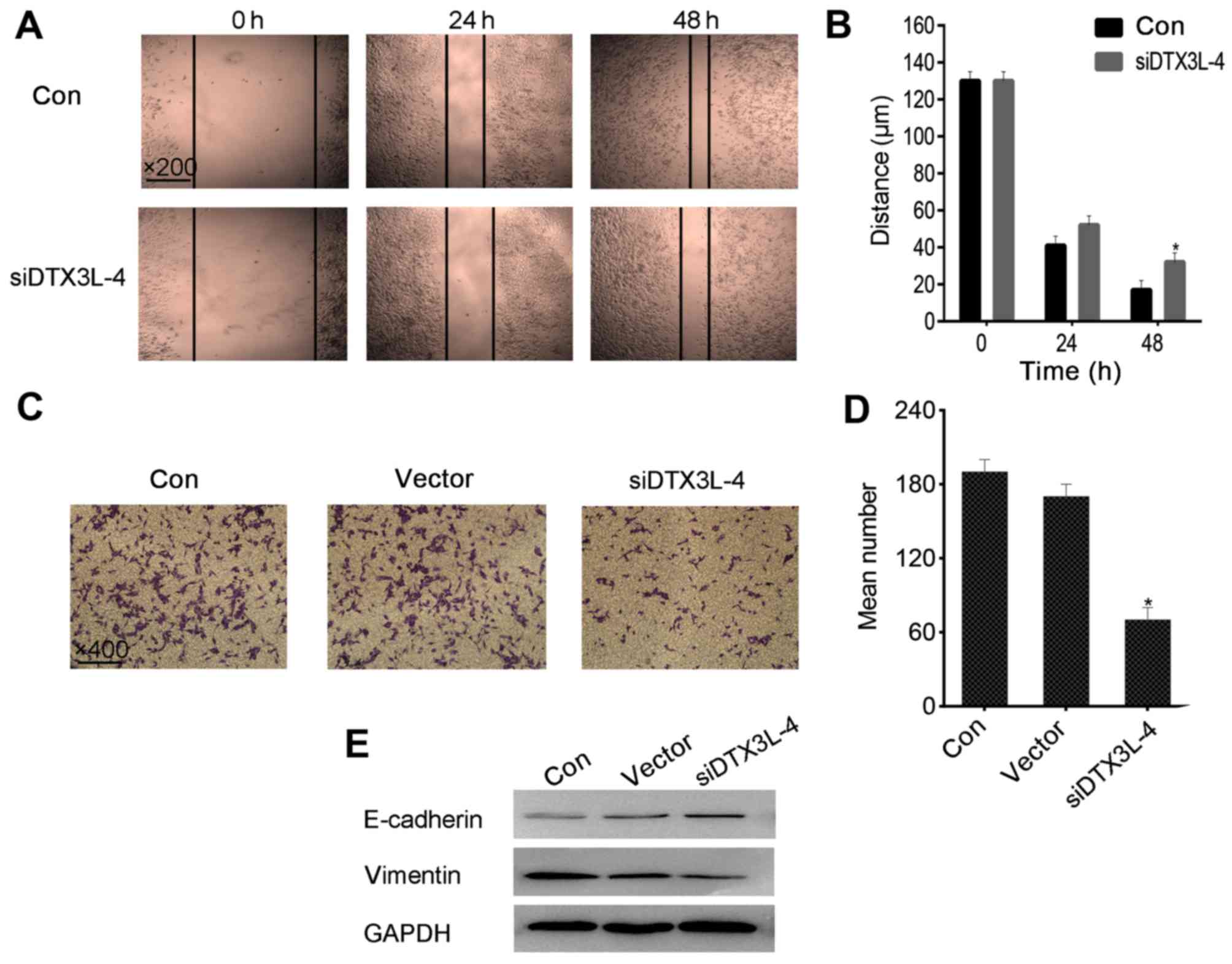
Decreased cell invasion in the
DTX3L-depleted glioma cells
Since a previous study reported that DTX3L
significantly contributes to the migration and invasion of melanoma
cells (12), we also investigated
whether silencing of DTX3L could inhibit glioma cell migration and
invasion. We analyzed the potential ability of DTX3L to induce cell
motility using wound-healing and Transwell assays. In the
wound-healing assay, the cell migration was reduced in the
DTX3L-siRNA group compared with that in the control group, which
indicated that the migratory ability of the U87MG cells transfected
with DTX3L-siRNA was much weaker than that in the cells transfected
with the control-siRNA (Fig. 4A and
B). In addition, the results of the Transwell migration assay
also showed that the number of U87MG cells which passed through the
membrane onto the lower chamber was markedly less in the the
DTX3L-siRNA-transfected cells compared to that noted in the
control-siRNA-transfected cells (Fig.
4C and D). Next, in order to validate these results, we
assessed the protein levels of E-cadherin and vimentin, which are
markers of epithelial-mesenchymal transition (EMT) (16,17) and contribute to tumor metastasis,
in the DTX3L-siRNA group and the negative control group by western
blot analysis. As shown in Fig.
4C, in the DTX3L-siRNA group, E-cadherin expression was
upregulated, while vimentin expression exhibited an opposite trend.
These observations confirm that DTX3L was associated with glioma
migration and invasion.
Loss of DTX3L expression sensitizes
glioma cells to a chemotherapeutic drug
TMZ is the main therapeutic strategy for patients
suffering from gliomas (18,19). In this study, we analyzed whether
loss of DTX3L expression could enhance the cytotoxic effects of TMZ
in U87MG cells. We first transfected U87MG cells with DTX3L-siRNA
or control-siRNA and subsequently treated the U87MG cells with TMZ.
Next western blot analysis was performed to detect the cleavage of
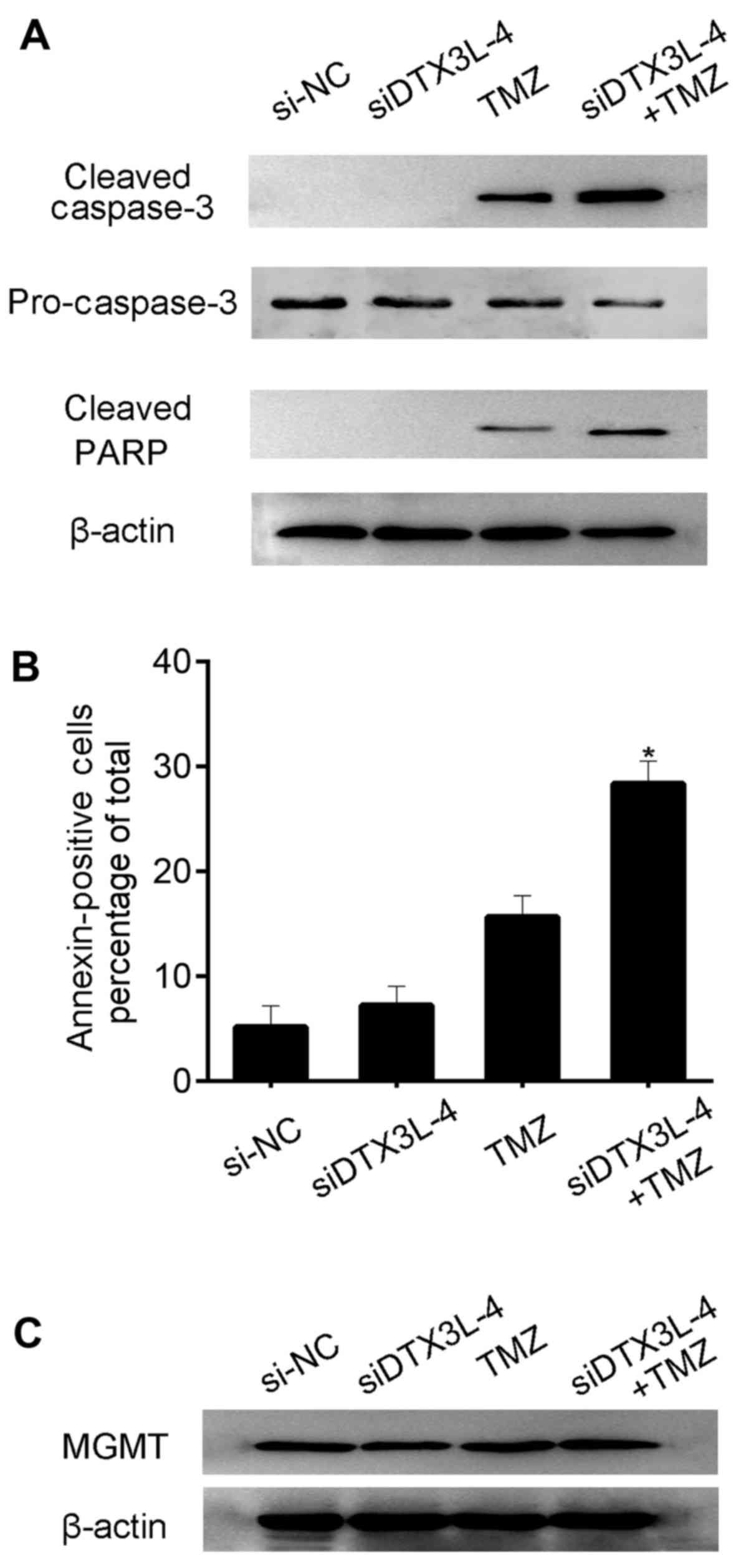
caspase-3 and PARP in the U87MG cells. As expected, knockdown of
DTX3L contributed to increased expression of caspase-3 and PARP
(Fig. 5A). Meanwhile, the
percentages of Annexin V-positive U87MG cells in the different
treatment groups were determined. The percentage of apoptotic cells
was increased in the DTX3L-siRNA + TMZ group compared with that in
the DTX3L-siRNA and TMZ treatment alone groups. Our data revealed
that knockdown of DTX3L expression by siRNA facilitated glioma cell
apoptosis induced by TMZ (Fig.
5B). Since enzyme O6-methylguanine-DNA
methyltransferase (MGMT) has been reported to suppress TMZ
sensitivity, we further examined the protein level of MGMT. The
results showed that MGMT was not apparently alter in the experiment
(Fig. 5C).
Discussion
Malignant gliomas are the most prevalent brain
tumors with a poor survival despite maximal therapy (20,21). Although traditional treatments for
glioma such as surgical resection, chemotherapy and radiotherapy
have a beneficial effect on patient survival, the prognosis of
glioma patients remains poor (4,21,22). This poor prognosis is mainly due
to loss of control of the primary tumor and widespread invasion of
tumor cells throughout the normal brain (21,23). Therefore, elucidation of the
molecular biological mechanisms underlying glioma pathogenesis are
urgently needed.
DTX3L is a member of the Deltex (DTX) family (DTX1,
DTX2 and DTX3) (7). Much research
suggests that DTX3L is involved in the occurrence and development
of a variety of solid cancers (12,13). DTX3L, as a component of the
BAL/DTX3L complex, has been reported to be involved in host
response diffuse large B-cell lymphomas by regulating the
nucleocytoplasmic trafficking of transcription factors (24). An additional study found that
DTX3L participates in the DNA damage response pathway following
exposure to genotoxic agents via the post-translational
modification of histone H4 (10).
A proteomic study showed that DTX3L has an effect on tumorigenesis
via influencing the nuclear activities of STAT1 by antagonistically
regulating the tyrosine phosphorylation of STAT1 on Y701 in
cooperation with ARTD9 (13).
Moreover, elevated DTX3L was found to contribute to cellular
metastasis during melanoma pathogenesis (12). On the basis of these findings, we
inferred that DTX3L may be involved in the progression of tumors.
Thus, in the present study, we explored the potential function of
DTX3L in glioma progression.
Recent studies have reported that aberrant
functioning of DTX3L is considered to cause cell migration and
dysregulated cell growth (12,14). In this study, we showed that
depletion of DTX3L by RNA interference suppressed glioma cell
migration and the DTX3L protein level was negatively correlated
with E-cadherin expression, which is a marker of
epithelial-mesenchymal transition (EMT) and contributes to cancer
metastasis (25). As known,
during carcinogenesis, tumor cell resistance to apoptosis
contributes to the development and progression of cancers (26). TMZ is considered as a first-line
chemotherapeutic drug for the treatment of glioma patients
(27). Accordingly, we aimed to
ascertain whether DTX3L has an effect on sensitizing glioma cells
to TMZ-induced apoptosis. As expected, our data revealed that
knockdown of DTX3L expression by siRNA facilitated glioma cell
apoptosis induced by TMZ. In conclusion, this is the first study to
demonstrate that the DTX3L level is significantly increased in
human glioma tissues and DTX3L upregulation is correlated with
glioma grade and poor prognosis of glioma. Furthermore, the
silencing of DTX3L by RNA interference inhibited the growth and
migration of glioma cells as well as induced cell apoptosis. Based
on these findings, we propose that DTX3L may serve as a potential
prognostic biomarker and a therapeutic target for glioma. However,
the detailed mechanism underlying the association between DTX3L and
the development of glioma needs further investigation.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China Grants (nos. 81272789 and
81572491).
References
|
1
|
Roy LO, Poirier MB and Fortin D:
Transforming growth factor-beta and its implication in the
malignancy of gliomas. Target Oncol. 10:1–14. 2015. View Article : Google Scholar
|
|
2
|
Osswald M, Jung E, Sahm F, Solecki G,
Venkataramani V, Blaes J, Weil S, Horstmann H, Wiestler B, Syed M,
et al: Brain tumour cells interconnect to a functional and
resistant network. Nature. 528:93–98. 2015.PubMed/NCBI
|
|
3
|
Tao T, Cheng C, Ji Y, Xu G, Zhang J, Zhang
L and Shen A: Numbl inhibits glioma cell migration and invasion by
suppressing TRAF5-mediated NF-κB activation. Mol Biol Cell.
23:2635–2644. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bai RY, Staedtke V and Riggins GJ:
Molecular targeting of glioblastoma: Drug discovery and therapies.
Trends Mol Med. 17:301–312. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qazi MA, Vora P, Venugopal C, McFarlane N,
Subapanditha MK, Murty NK, Hassell JA, Hallett RM and Singh SK: A
novel stem cell culture model of recurrent glioblastoma. J
Neurooncol. 126:57–67. 2016. View Article : Google Scholar
|
|
6
|
Ding Z, Liu Y, Yao L, Wang D, Zhang J, Cui
G, Yang X, Huang X, Liu F and Shen A: Spy1 induces
de-ubiquitinating of RIP1 arrest and confers glioblastoma's
resistance to tumor necrosis factor (TNF-α)-induced apoptosis
through suppressing the association of CLIPR-59 and CYLD. Cell
Cycle. 14:2149–2159. 2015. View Article : Google Scholar :
|
|
7
|
Takeyama K, Aguiar RC, Gu L, He C, Freeman
GJ, Kutok JL, Aster JC and Shipp MA: The BAL-binding protein BBAP
and related Deltex family members exhibit ubiquitin-protein
isopeptide ligase activity. J Biol Chem. 278:21930–21937. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Mao D, Roswit WT, Jin X, Patel
AC, Patel DA, Agapov E, Wang Z, Tidwell RM, Atkinson JJ, et al:
PARP9-DTX3L ubiquitin ligase targets host histone H2BJ and viral 3C
protease to enhance interferon signaling and control viral
infection. Nat Immunol. 16:1215–1227. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Holleman J and Marchese A: The ubiquitin
ligase deltex-3l regulates endosomal sorting of the G
protein-coupled receptor CXCR4. Mol Biol Cell. 25:1892–1904. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yan Q, Dutt S, Xu R, Graves K, Juszczynski
P, Manis JP and Shipp MA: BBAP monoubiquitylates histone H4 at
lysine 91 and selectively modulates the DNA damage response. Mol
Cell. 36:110–120. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yan Q, Xu R, Zhu L, Cheng X, Wang Z, Manis
J and Shipp MA: BAL1 and its partner E3 ligase, BBAP, link
Poly(ADP-ribose) activation, ubiquitylation, and double-strand DNA
repair independent of ATM, MDC1, and RNF8. Mol Cell Biol.
33:845–857. 2013. View Article : Google Scholar :
|
|
12
|
Thang ND, Yajima I, Kumasaka MY, Iida M,
Suzuki T and Kato M: Deltex-3-like (DTX3L) stimulates metastasis of
melanoma through FAK/PI3K/AKT but not MEK/ERK pathway. Oncotarget.
6:14290–14299. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bachmann SB, Frommel SC, Camicia R,
Winkler HC, Santoro R and Hassa PO: DTX3L and ARTD9 inhibit IRF1
expression and mediate in cooperation with ARTD8 survival and
proliferation of metastatic prostate cancer cells. Mol Cancer.
13:1252014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kolchinsky A and Roninson IB: Drug
resistance conferred by MDR1 expression in spheroids formed by
glioblastoma cell lines. Anticancer Res. 17:3321–3327.
1997.PubMed/NCBI
|
|
15
|
Günther W, Pawlak E, Damasceno R, Arnold H
and Terzis AJ: Temozolomide induces apoptosis and senescence in
glioma cells cultured as multicellular spheroids. Br J Cancer.
88:463–469. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stoops SL, Waterson AG, An H, Deane N,
Daniels JS, Morrison R, Engers JL, Beauchamp D and Lindsley CW:
Discovery and characterization of a small molecule that restores
E-cadherin expression in cancer cell lines via a new mechanism.
Probe Reports from the NIH Molecular Libraries Program (Internet).
Bethesda (MD): National Center for Biotechnology Information (US);
2010–2012 Dec 13. (updated 2013 Mar 22). Available from: https://www.ncbi.nlm.nih.gov/books/NBK344134/.
|
|
17
|
Lindsay CR, Le Moulec S, Billiot F, Loriot
Y, Ngo-Camus M, Vielh P, Fizazi K, Massard C and Farace F: Vimentin
and Ki-67 expression in circulating tumour cells derived from
castrate-resistant prostate cancer. BMC Cancer. 16:1682016.
View Article : Google Scholar
|
|
18
|
Castro GN, Cayado-Gutiérrez N, Zoppino FC,
Fanelli MA, Cuello-Carrión FD, Sottile M, Nadin SB and Ciocca DR:
Effects of temozolomide (TMZ) on the expression and interaction of
heat shock proteins (HSPs) and DNA repair proteins in human
malignant glioma cells. Cell Stress Chaperones. 20:253–265. 2015.
View Article : Google Scholar :
|
|
19
|
Zhuang D, Liu Y, Mao Y, Gao L, Zhang H,
Luan S, Huang F and Li Q: TMZ-induced PrPc/par-4 interaction
promotes the survival of human glioma cells. Int J Cancer.
130:309–318. 2012. View Article : Google Scholar
|
|
20
|
Ogbomo H, Cinatl J Jr, Mody CH and Forsyth
PA: Immunotherapy in gliomas: Limitations and potential of natural
killer (NK) cell therapy. Trends Mol Med. 17:433–441. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rich JN and Bigner DD: Development of
novel targeted therapies in the treatment of malignant glioma. Nat
Rev Drug Discov. 3:430–446. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ding Z, Liu X, Liu Y, Zhang J, Huang X,
Yang X, Yao L, Cui G and Wang D: Expression of far upstream element
(FUSE) binding protein 1 in human glioma is correlated with c-Myc
and cell proliferation. Mol Carcinog. 54:405–415. 2015. View Article : Google Scholar
|
|
23
|
Chen J, Sun J, Yang L, Yan Y, Shi W, Shi
J, Huang Q, Chen J and Lan Q: Upregulation of B23 promotes tumor
cell proliferation and predicts poor prognosis in glioma. Biochem
Biophys Res Commun. 466:124–130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Juszczynski P, Kutok JL, Li C, Mitra J,
Aguiar RC and Shipp MA: BAL1 and BBAP are regulated by a gamma
interferon-responsive bidirectional promoter and are overexpressed
in diffuse large B-cell lymphomas with a prominent inflammatory
infiltrate. Mol Cell Biol. 26:5348–5359. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu H, Tian Y, Yuan X, Liu Y, Wu H, Liu Q,
Wu GS and Wu K: Enrichment of CD44 in basal-type breast cancer
correlates with EMT, cancer stem cell gene profile, and prognosis.
Onco Targets Ther. 9:431–444. 2016.PubMed/NCBI
|
|
26
|
Li RY, Chen LC, Zhang HY, Du WZ, Feng Y,
Wang HB, Wen JQ, Liu X, Li XF, Sun Y, et al: MiR-139 inhibits Mcl-1
expression and potentiates TMZ-induced apoptosis in glioma. CNS
Neurosci Ther. 19:477–483. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yan Y, Xu Z, Dai S, Qian L, Sun L and Gong
Z: Targeting autophagy to sensitive glioma to temozolomide
treatment. J Exp Clin Cancer Res. 35:232016. View Article : Google Scholar : PubMed/NCBI
|