Introduction
Retinoblastoma is the most frequent intraocular
malignancy in children, with a worldwide incidence of
1/16,000-1/18,000 live births (1). Although current therapies have
achieved considerable improvements, there are still unsolved
issues: secondary tumor growth (2), optic nerve damage (3), and normal retina impairment caused
by nonspecific chemotherapy and radiation (4,5).
A novel therapy for tumors is gene therapy. Compared
with conventional therapies, gene therapy has exhibited several
advantages, such as specific regulation and a longstanding effect
in a controlled manner. There have been 2,388 clinical trials that
have investigated gene therapy for several tumors or cancers, such
as brain tumors, breast cancer, and prostate cancer. Among the 699
completed trials, many have shown the effectiveness of gene therapy
as a valuable supplement and even a novel approach to tumor/cancer
therapy.
Inactivation of the Rb1 gene alley, the major cause
of retinoblastoma, has made gene therapy a promising treatment for
retinoblastoma patients. In fact, several types of genes, including
the suicide gene (e.g., HSV-TK/GCV) (6,7),
anti-oncogenes (e.g., Rb1, P21 and P53) (8,9),
and anti-angiogenesis genes (e.g., sFlk21 and ExTek) (10,11), have been transfected into
retinoblastoma cells to inhibit their proliferation or to induce
their apoptosis.
Successful gene therapy is based on an appropriate
transgene vector; however, previous studies have mainly focused on
the function of transgenes rather than the vectors. A few types of
transgene vectors have been randomly adopted for retinoblastoma
gene transfection, including the adenoviral vector (6,7),
the liposome (12), and some
uncommon vectors such as the encephalomyocarditis virus (13). There is no systemic evaluation of
these vectors, and there is also a lack of evaluation of other
vectors that are utilized more often. Thus, it is crucial to
systematically optimize proper gene vectors for retinoblastoma
cells.
Generally, the present gene vectors are classified
as viral- and non-viral vectors. Various viral vectors have been
adopted to transfect the retina. Among them, vectors based on
retroviruses, recombinant adeno-associated viruses (rAAVs), and
lentiviruses (LVs) are the most commonly used. These vectors were
assumed to be effective in retinoblastoma transfection, considering
the retinal origin of retinoblastoma (14,15).
The retroviral vector has been considered as a
preferred gene transfer system. It can insert the transgene into
the dividing cell chromosome to ensure stable transmission to
daughter cells for long-term therapy. Researchers have utilized the
retroviral vector to restore the Rb1 gene into the retinoblastoma
cell line Y79 to prevent tumor cell proliferation (16). Unlike retroviruses, the rAAVs, a
parvovirus, can infect both dividing and non-dividing cells but do
not integrate into the host genome. They are divided into 12
serotypes (17), and serotype 2
is the most well-known and commonly used in retina research. rAAV2
was confirmed to efficiently transfect both retinal pigment
epithelium (RPE) cells (18) and
photoreceptors without obvious toxicity (19,20). In addition, it has been used in an
increasing number of clinical trials of retinal degeneration
diseases, such as Leber's congenital amaurosis type 2 (LCA2)
(21), age-related macular
degeneration (AMD) (22), and
choroideremia (23). The exchange
of capsids in rAAV serotypes results in distinct transduction in
various retinal cell types. The rAAV2/1, containing a rAAV2 genome
in one rAAV2 capsid, was reported to efficiently transfect the
retina (24). Another viral
vector that can transfect post-mitotic cells is the LV, which has a
larger payload capacity (~9 kb) than rAAV (~4.7 kb) (25). Moreover, it can drive prolonged
and stable transgene expression in the retina, brain, and even
various stem/progenitor cells by genome integration (26). It was published that >80%
photoreceptors expressed green fluorescent protein (GFP) for at
least 12 weeks following HIV-based LV vector transfection (27).
Non-viral vectors have several advantages over viral
vectors due to their low pathogenicity, low immunotoxicity, and
ease of production, despite their relatively lower transfection
efficiency. These vectors are broadly classified as naked/plasmid
DNA, physical-based and chemical-based vectors. The application of
naked/plasmid DNA is limited by low efficiency. Physical-based
vectors, such as electroporation and ultrasound microbubbles, are
primarily limited by cell damage and low efficiency. Conventional
chemical-based vectors, such as lipoplexes, have been used for
retina and RPE gene delivery (28), however, their transfection
efficiencies are very low due to their positive charges, which are
greatly affected by the negatively charged protein in serum. Here,
we adopted a neotype lipid vector, the X-treme HP reagent, for
WERI-Rb1 cell transfection because of its improved efficiency and
safety in several cell lines that are difficult to transfect, such
as human umbilical cord blood mesenchymal stem cells.
In addition to the vector, successful gene
transfection is also based on the cell culture system. Currently,
most of the transfection experiments have performed well in a
serum-free medium in vitro; however, a high transfection
efficiency in the presence of serum is desirable since serum
starvation can affect the cell cycle and viability. Moreover, serum
interference cannot be avoided in vivo. In addition to
serum, the cell culture status affects gene transduction efficiency
as well. Vectors perform differently in suspension or in adherent
cells. Retinoblastoma cells are usually suspended and form rosettes
or grape-like cell clusters, which hinder the attachment of
transgenes with the inner cells wrapping in clusters. In contrast,
an adherent culture may extend gene exposure and avoid a
chromosomal positional effect. Thus, we examined whether the
adherence of retinoblastoma cells would benefit transfection.
This study was designed to optimize a gene
transfection system specific for retinoblastoma. The classic
retinoblastoma cell line, WERI-Rb1 (W-RBCs), was selected as host
cells, and the GFP DNA was adopted as the reporter gene. We
systemically tested the efficacy and cytotoxicity of different
viral and non-viral vectors for GFP transfection and further
explored the potential effect of serum and the cell culture system
on GFP transfection. This study presents an efficient and low
cytotoxic system for retinoblastoma gene transfection and could
provide a promising transgene system for further gene therapy of
retinoblastoma.
Materials and methods
Cell culture
The human retinoblastoma cell line WERI-Rb1
(American Type Culture Collection, Manassas, VA, USA) was suspended
in RPMI-1640 medium (HyClone, Logan, UT, USA) with 10% fetal bovine
serum (FBS; Gibco, Carlsbad, CA, USA). Fresh medium was exchanged
24 h after thawing cells. Three to 4 days later, cells were
passaged to single cells by gentle mechanical dissociation and
reseeded at a density of 105 cells/ml. All cells were
cultured at 37°C in an atmosphere with 5% CO2 and
observed under an inverted microscope every other day.
An Ecopack2-293 packaging cell line (Clontech,
Mountain View, CA, USA) was cultured in a 293 culture medium,
consisting of Dulbecco's modified Eagle's medium (DMEM), 10% FBS,
L-glutamine (2 mM), non-essential amino acids (NEAA, 0.1 mM) (all
from Gibco), sodium pyruvate (1 mM), and penicillin/streptomycin
(100 μg/ml) (both from Sigma-Aldrich, St. Louis, MO, USA).
The cells were exchanged in fresh medium every 2–3 days and
passaged at 70–80% confluence.
Cell adherence
For adherent transfection, the culture dish was
pre-coated with 0.1 mg/ml poly D-lysine (PDL; Sigma-Aldrich) at
37°C. After 24 h, the PDL diluent was removed, and the dish was
washed with phosphate-buffered saline (PBS) once. The W-RBC
suspension was centrifuged at 157 × g for 10 min, and then the cell
precipitations were resuspended in a W-RBC culture medium, placed
onto PDL-coated plates, and cultured at 37°C in an atmosphere with
5% CO2.
Plasmid prep
GFP plasmid preps were performed using Qiagen
Plasmid Hispeed Midi kit (Qiagen, Valencia, CA, USA) following the
manufacturer's recommended protocol.
Retroviral packaging and transfection of
suspended W-RBCs
The Ecopack 2–293 cells with 70–80% cell fusion
cultured in a 60-mm dish were transfected using a transfection
mixture consisting of pVPack-GP GP (2.5 μg), pVPack-VSV-G
(1.5 μg), pMX-IRES-GFP (4 μg) (all from Agilent
Technologies, Santa Clara, CA, USA) and transfection reagent
Fugene-HD (20 μl). Fresh medium was exchanged 16 h after
incubation. After 48 h, the retrovirus containing supernatants was
collected and filtered using a 0.45-μm cellulose acetate
membrane (Sartorius, Göttingen, Germany) and then centrifuged
(5,000 × g) in 100 kDa ultrafiltration tube (Millipore, Temecula,
CA) for 30 min at 4°C. The preintegration complex (1 ml) was
collected and added along with polybrene (4 μg/ml) in 1 ml
of suspended W-RBCs at a cell density of 7.5×104/ml.
Twenty-four hours later, the cells were resuspended in fresh medium
and observed by an inverted microscope and fluorescence microscopy
(Carl Zeiss, Inc., Oberkochen, Germany).
Recombinant adeno-associated virus
transfection of suspended W-RBCs
The plasmids rAAV2-GFP (2.5×1011
μg/ml) and rAAV2/1-GFP (5×1011 μg/ml) were
constructed by Vector Gene Technology Company (VTGC, Beijing,
China). The transient transfection of GFP into W-RBCs by rAAV was
carried out according to the manufacturer's instructions. Briefly,
1.5×104 W-RBCs per milliliter of serum/antibiotic-free
medium were exposed to three different virus MOIs, respectively:
106, 105 and 104. Two hours later,
the cells were re-cultured in fresh medium at 37°C in an atmosphere
with 5% CO2. Cells were evaluated each day using an
inverted microscope, and GFP fluorescence was observed under
fluorescence microscopy (both from Carl Zeiss).
Lentiviral transfection of suspended
W-RBCs
Three hours prior to transfection, 5×104
W-RBCs were cultured in 1 ml serum/antibiotic-free medium. The LVs
(1.4×108 TU/ml) (VTGC) with different MOIs (2 and 10)
were respectively added into two cell groups in three
reduplications. Cells were cultured at 37°C in an atmosphere with
5% CO2 for 16 h and recultured in fresh medium. Cells
were evaluated each day using an inverted microscope, and GFP
fluorescence was observed under fluorescence microscopy (both from
Carl Zeiss).
X-treme HP transfection with/without
FBS
Transfection with X-treme Gene HP DNA transfection
reagent (Roche, Basel, Switzerland) was performed on both suspended
and adherent W-RBCs following manufacturer's instructions. Briefly,
DNA was diluted with a serum-free medium to a final concentration
of 1 μg plasmid DNA/100 μl medium (0.01
μg/μl). For the transfection with FBS, the X-treme
Gene HP DNA transfection reagent was pipetted directly into the
culture medium containing the diluted DNA with a ratio of 1:1 of
X-treme reagent (μl) to plasmid DNA (μg). For the
transfection without FBS, the cell culture medium was replaced with
a serum/antibiotic-free RPMI-1640 medium 3 h before adding the
reagent-DNA mixture. The transfection reagent and DNA complex were
incubated for 15 min at +15 to +25°C. The transfection complex was
then added into 5×104 suspended and adherent W-RBCs in a
dropwise manner. The removal of growth medium was not necessary.
Following transfection, the cells were incubated for 24 h before
consequent GFP expression measurement.
Transfection efficiency analysis
The analysis of the transfection efficiency was
carried out using the following 2 methods:
Fluorescence microscopy. GFP protein
expression of the transfected cells was observed on different days.
Fluorescence microscopy was performed using a fluorescence
microscope (Carl Zeiss), and images were recorded using AxioVision
software. GFP fluorescence was measured employing a wavelength
filter set at 10 (Carl Zeiss MicroImaging, Goettingen, Germany).
The results are expressed as the average percentage of GFP-positive
cells/image, as indicators of transfection efficiency. The
transfection efficiency of each protocol was compared.
Fluorescence-activated cell sorter (FACS)
analysis. GFP expression of the transfected cells was
investigated by a fluorescence-activated cell sorter to determine
the transfection efficiency of each protocol. Single transfected
W-RBCs and untransfected W-RBCs were respectively resuspended in
FACS analysis buffer (PBS, 0.5% BSA, 2 mM
EDTA-2Na-2H2O). The percentages of GFP+ cells
were assessed by comparing the different transfected groups to
untransfected cells by flow cytometry (FACSAria; BD Biosciences,
Franklin Lakes, NJ, USA).
Cell viability analysis
Viable cells were counted with a hemocytometer using
the standard trypan blue exclusion test (0.4% trypan blue;
Sigma-Adrich), as previously reported (29). Briefly, the W-RBC suspension (10
μl) was mixed with trypan blue (90 μl) and incubated
for 2 min at room temperature. Then, 10 μl of the cell
suspension was dropped on the hemocytometer, which counted the
viable and dead (blue) cells in 25 medium-sized squares. The total
number of viable cells was calculated by determining the number of
live cells × dilution factor × 104 × total volume (ml).
Viability (%) equals the number of live cells/(number of live cells
+ number of dead cells) ×100%.
Statistical analysis
Each experiment was performed in triplicate. All
data are represented as the mean ± standard deviation (SD).
Significance was assessed with the Student's t-test for the
comparison of two variables, or with a one-way or two-way ANOVA for
multivariable comparisons using SPSS 13.0 (IBM, Armonk, NY, USA). A
value of P<0.05 was considered significant.
Results
Recombinant adeno-associated virus
transduction of W-RBCs
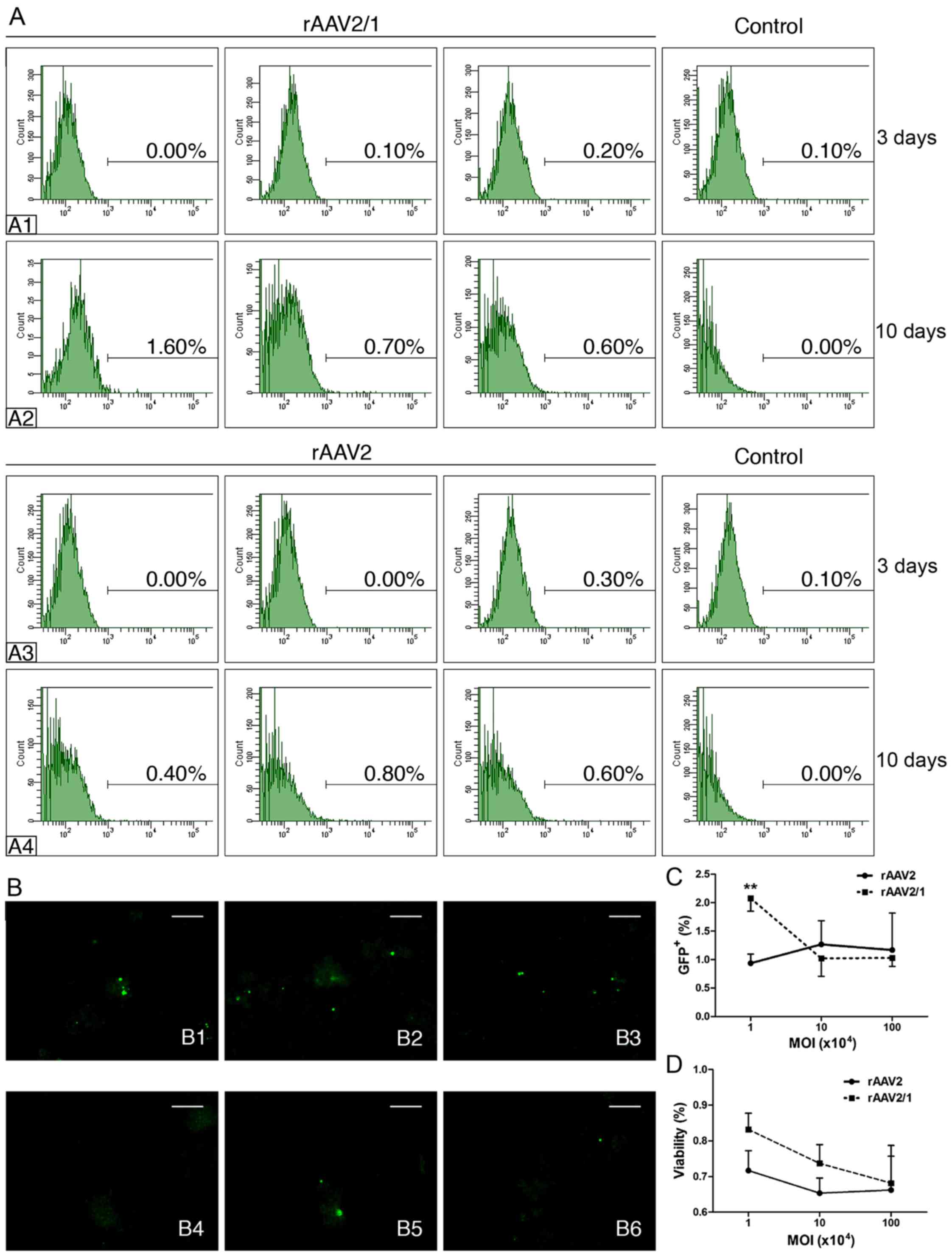
Two different rAAV vectors (rAAV2/1 and rAAV2) were
applied to test whether the rAAV vectors could efficiently
transfect W-RBCs. Three different MOIs (104,
105 and 106) were also compared in both rAAVs
for optimization. The transfected W-RBCs expressed negative GFP
during the first 48 h after transfection (data not shown). The flow
cytometric analysis showed a markedly low GFP+ cell
rate, which ranged from 0 to 0.3% in both AAVs with the three MOI
transfected groups at 72 h (Fig. 1-A1
and A3); however, on the 10th day, all transfected groups had
generally improved GFP expression. Specifically, the rAAV2 with the
105 MOI group presented more GFP+ cells
(0.8%) compared to the other two rAAV2 groups with different MOIs
(104, 0.4%; 106, 0.6%) (Fig. 1-A4) and the rAAV2/1 group with the
same MOI (105, 0.7%) (Fig.
1-A2). Notably, cells transfected with the lowest MOI
(104) by rAAV2/1 induced the highest GFP expression
(1.6%) among all experimental groups (Fig. 1-A2). The immunofluorescence assay
indicated that the rAAV2/1-mediated transfection induced more
W-RBCs to express GFP protein compared to rAAV2, despite having
three MOIs. The percentage of GFP+ cells in both
rAAVs-treated groups on the 10th day also confirmed that the
rAAV2/1 with the 104 MOI induced considerably more
GFP+ cells than the other groups. We further
investigated the cell viability by trypan blue staining 10 days
after transfection. We found that the cell survival rates of both
rAAV-treated groups declined in an MOI-dependent manner with a
higher cell survival at the lower MOI. Interestingly, the
rAAV2/1-treated groups, especially the group with 104
MOI, showed higher viability than the rAAV2-treated groups,
although there was no significant difference. These results showed
the relative effectiveness and the low cytotoxicity of rAAV2/1
transfection with 104 MOI; however, both rAAV vectors
showed poor efficacies.
Lentiviral transduction of W-RBCs
LVs are effective for transfecting both dividing and
non-dividing cells. Hence, we evaluated whether LVs could
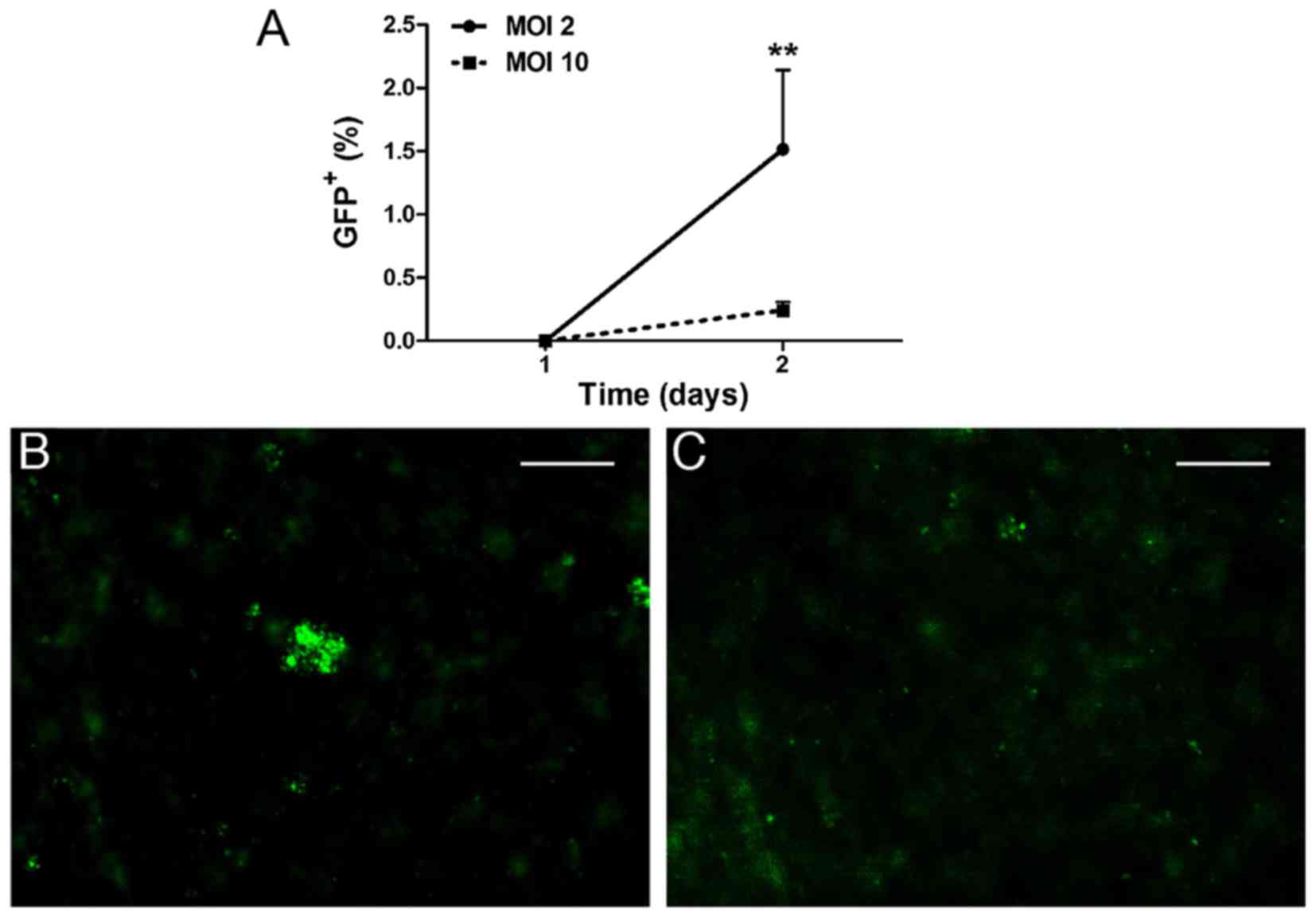
effectively drive the GFP plasmid into W-RBCs as well. Two
different MOIs (2 and 10) were adopted for optimization. The
transfected W-RBCs expressed no positive GFP on the 1st day after
transfection. Two days later, cells begun to express GFP protein,
and the MOI 2 group showed significantly more GFP+ cells
with an average percentage of 1.52%/image, which was considerably
more than the MOI 10 group (~0.24%/image) (Fig. 2A). The immunofluorescence also
exhibited a relatively higher intensity of GFP staining in the
cells treated by MOI 2 (Fig. 2B)
compared to MOI 10 (Fig. 2C).
Unfortunately, the efficiency of the LV vectors also remained at a
low level.
Optimization of vectors in suspended
W-RBCs
Given the poor performance of the rAAV and LV
vectors, retrovirus vectors and a non-virus reagent (X-treme HP)
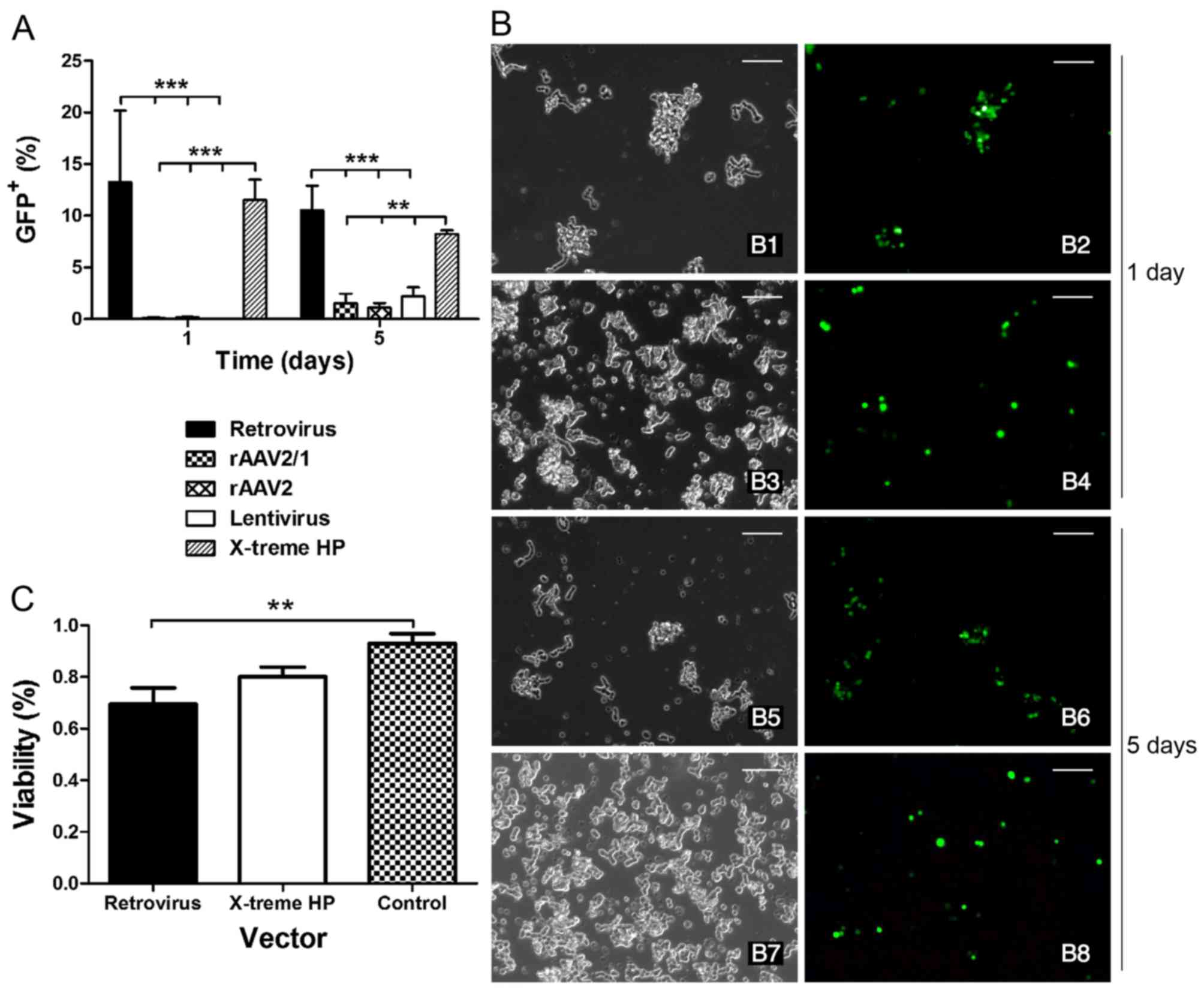
were investigated for optimization. The data showed that the number
of GFP+ cells was remarkably higher when transfected by
the retrovirus vectors and the X-treme HP reagent compared to the
other three virus vectors (rAAV2/1, rAAV2 and LVs) both on the 1st
and the 5th day (Fig. 3A).
Specifically, there were more GFP+ cells in the
retrovirus group than that in the X-treme HP group at the early
phase of transfection (1 day). Four days later, the number of cells
slightly decreased in both groups; however, no significant
difference was found between the two groups. The immunofluorescence
also showed a similar GFP intensity in both the retrovirus and
X-treme HP groups at day 1 and 5 (Fig. 3B). Furthermore, the cell viability
of X-treme HP on the 5th day presented a higher survival rate
(80.12%) compared to the retrovirus (69.56%), and there was a
significant difference in untransfected W-RBCs (92.93%) (Fig. 3C). Taken together, the retrovirus
and X-treme HP were more effective than rAAVs and LVs for W-RBC
transfection, but given the severe cytotoxicity and complicated
packaging of the retrovirus, the non-viral X-treme HP reagent was
more favorable for transfecting suspended W-RBCs.
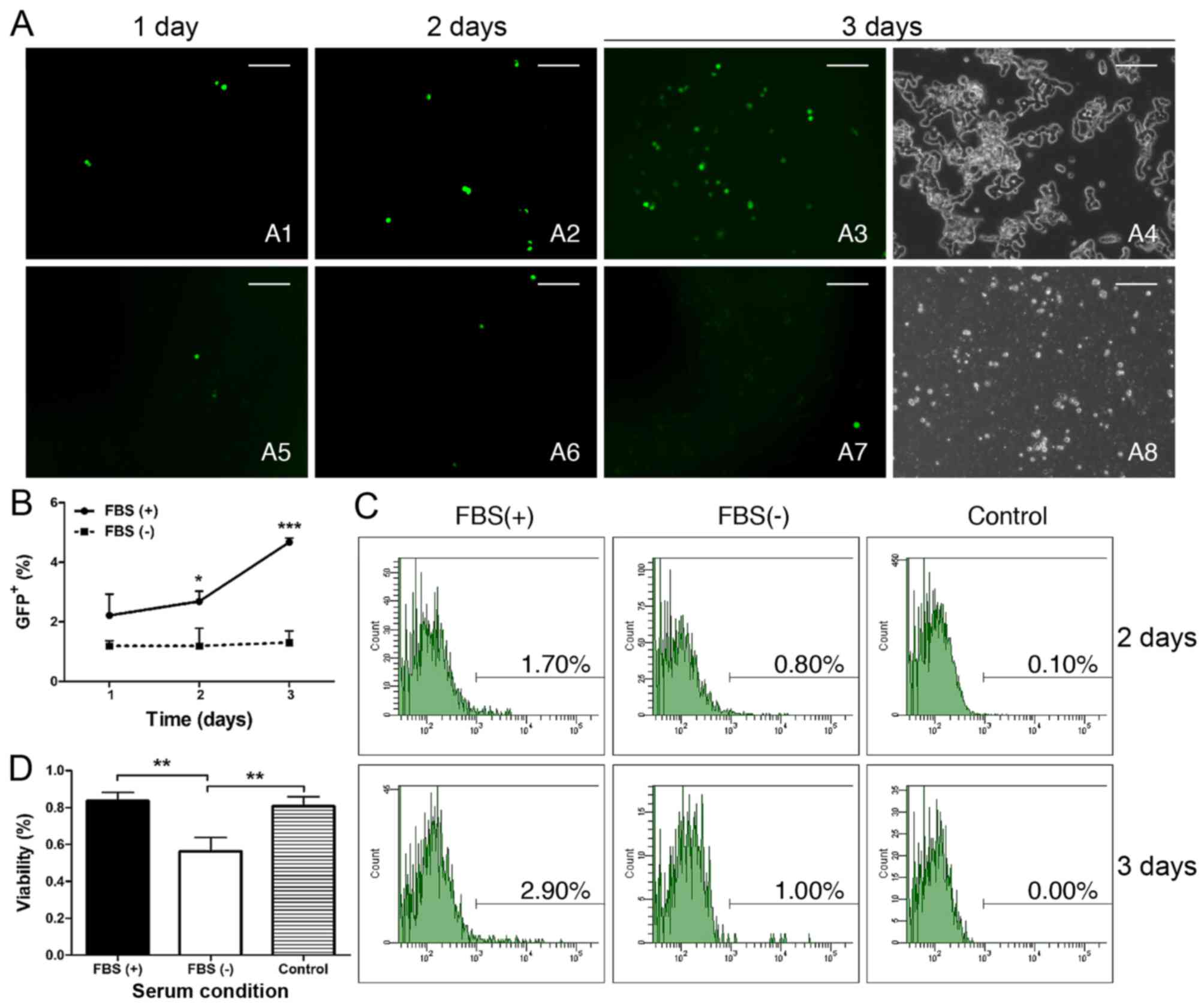
Comparison of serum-tolerant to
serum-free transfection in suspended W-RBCs by X-treme HP
reagent
We further explored whether there would be any
differences under serum-tolerant and serum-free conditions by
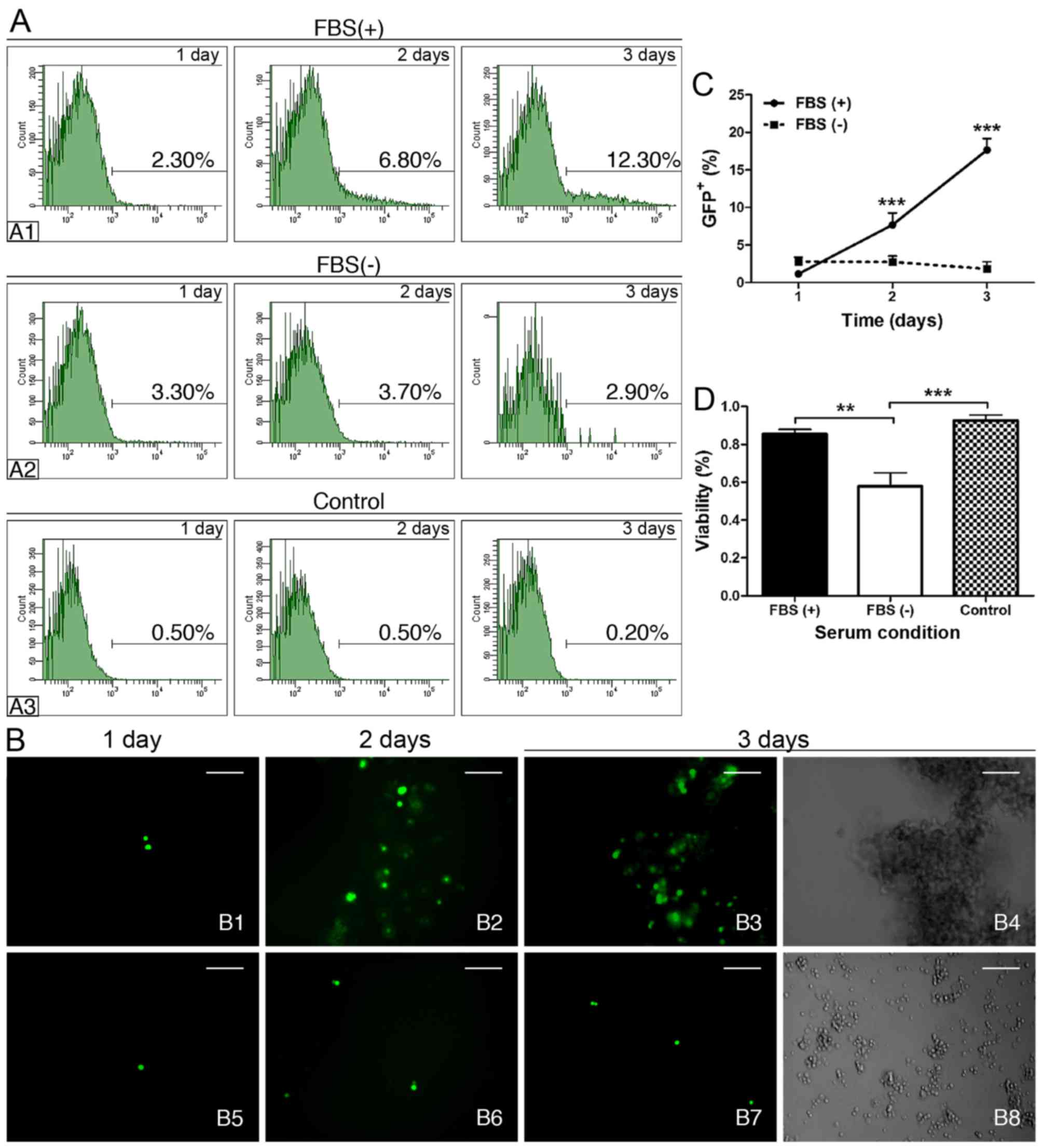
X-treme HP transfection. We found that the percentage of
GFP+ cells was 3.80% following serum-free transfection
after 24 h, and the percentage was higher than both the
serum-tolerant transfection (1.73%) and the untransfected control
(0.60%); however, only the latter showed a significant difference
(Table I). Interestingly, over
time, the serum-tolerant group (Fig.
4-A1) exhibited a progressive and significant increase in
GFP+ cells compared to the control (Fig. 4-A3) (Table I). Conversely, the GFP+
cells in the serum-free group showed no significant increase during
the 72 h (Fig. 4-A2 and Table I). Although the percentage of
GFP+ cells in the serum-free group was significantly
higher than the control from day 1 to 3, it was still markedly
lower than that in the serum-tolerant group on the 3rd day
(Fig. 4A and Table I). The immunofluorescence
(Fig. 4B) and cell quantitative
analysis (Fig. 4C) also presented
progressively increased GFP+ cells in the FBS(+) group
during the 3 days post-transfection, whereas the GFP+
cells of the FBS(−) group sustained a low level during the same
period. Moreover, there were significantly more cells that were
positively stained by trypan blue in the serum-free group compared
with that in the other two groups, which showed similar survival
rates (Fig. 4D). The serum
prevention of cytotoxicity was also observed in the phenotype of
W-RBCs. The serum-tolerant transfected cells generated large
grape-like cell clusters with the typical large stained nuclei of
retinoblastoma cells 3 days later (Fig. 4-B4), whereas the serum-free
transfected cells only grew small cell clusters with tiny soma,
high photopermeability, small nuclei and large cytoplasm (Fig. 4-B8). These results indicated that
serum benefited the X-treme HP transfection in suspended W-RBCs and
could also effectively preserve cells from potential cytotoxicity
during gene transduction.
 | Table IPercentage of GFP+ cells
in different serum conditions in suspended W-RBCs by X-treme HP
reagent as evaluated by FACS. |
Table I
Percentage of GFP+ cells
in different serum conditions in suspended W-RBCs by X-treme HP
reagent as evaluated by FACS.
| FBS(+) (%) | FBS(−) (%) | Control (%) |
|---|
| 1 day | 1.73±0.51 | 3.80±0.50a | 0.60±0.10 |
| 2 days | 5.23±1.37b | 4.73±1.31a | 0.73±0.21 |
| 3 days | 12.27±3.55c,d | 3.9±1.48a | 0.27±0.12 |
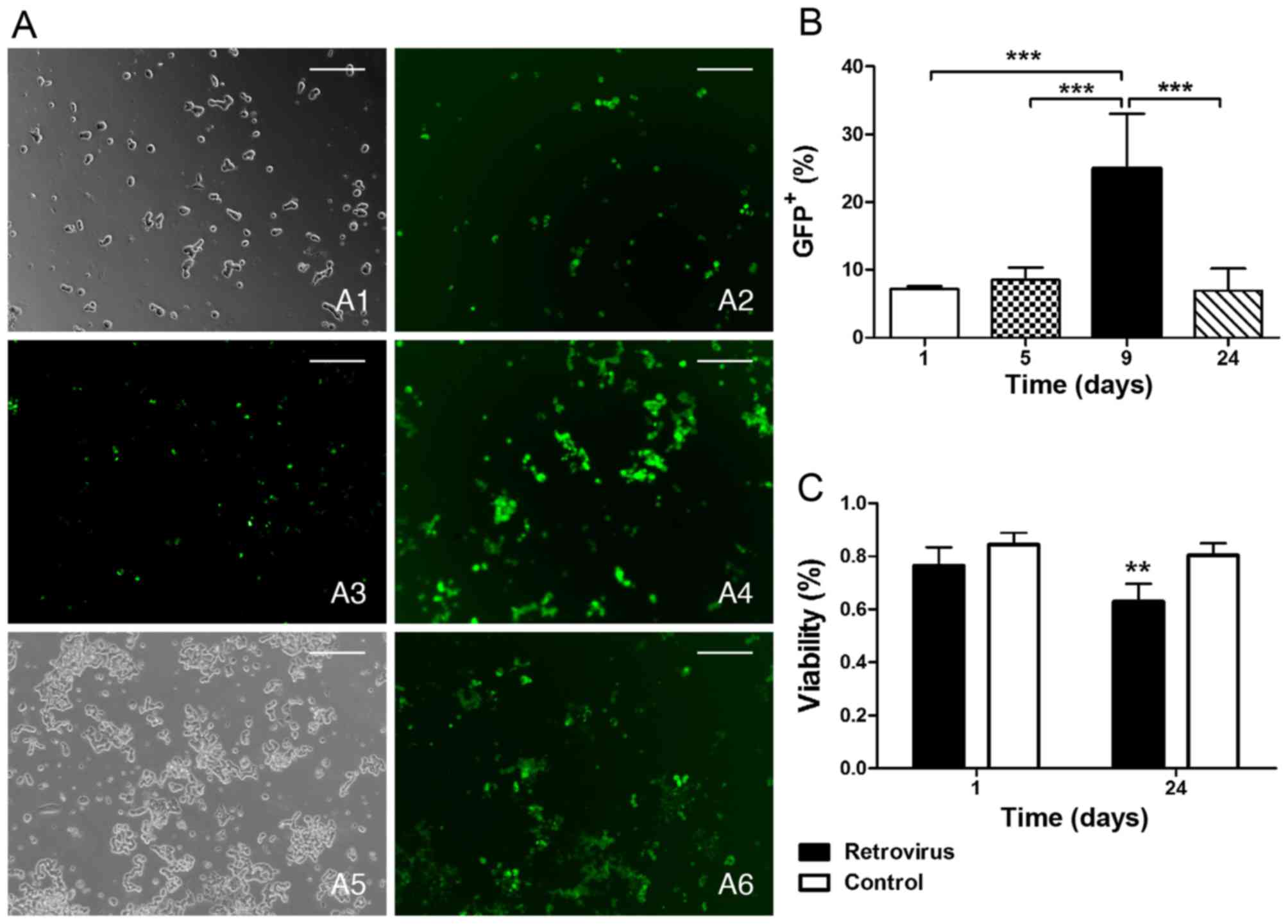
Transfection of adherent W-RBCs
Given the high efficiency of the retrovirus and
X-treme HP transfection in suspended W-RBCs, these two vectors were
also adopted for the transfection of adherent W-RBCs. The results
were as follows:
Retroviral transfection. We observed the GFP
expression during the post-transfection period ranging from 1 to 24
days and found that the cells expressing GFP protein remained at a
low level within 5 days and then reached a peak (~24.96%) when
extended to 9 days; however, for the long-term investigation (24
days), the number of GFP+ cells decreased to the primary
level (~6.94%) (Fig. 5A and B).
It is notable that the cell survival rate of the retrovirus vector
group was also found to significantly decrease (62.97%) 24 days
later compared with the blank control (80.36%), even though the
rate corresponded to the control on the 1st day (Fig. 5C).
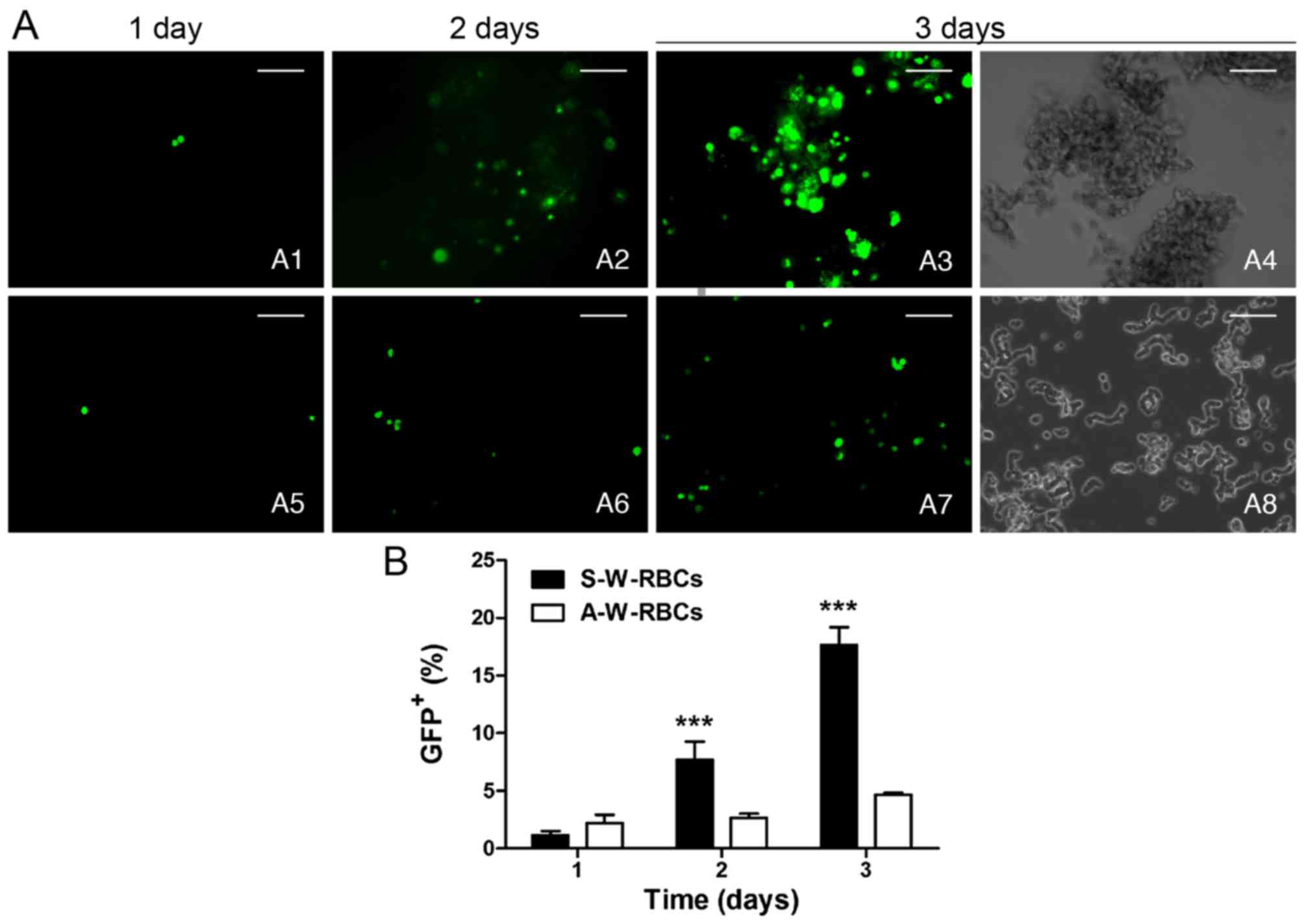
Comparison of serum-tolerant and serum-free
transfection in adherent W-RBCs by X-treme HP reagent. For the
X-treme HP transfection of adherent W-RBCs, the serum effect was
also evaluated. The immunofluorescence and GFP+ cell
quantification exhibited that the GFP expression of the
serum-tolerant group gradually increased from the 1st day to the
3rd day (Fig. 6-A1–A3 and B). In
contrast, there was no significant increase in GFP+
cells in the serum-free group, and it had remarkably fewer GFP
cells than the serum-tolerant group on day 2 and 3, respectively
(Fig. 6-A5–A7 and B). The FACS
also showed a tendency of increasing GFP+ cells from day
2 to 3, and the serum-tolerant cells expressed more GFP than the
serum-free cells and the blank control; however, a significant
difference was only observed between FBS(+) and the control on the
3rd day (Fig. 6C and Table II). For the cytotoxicity
analysis, the cells in serum-free conditions exhibited toxic
phenotypes, as mentioned (Fig.
6-A8), and a significantly lower survival rate than the
serum-tolerant group and the untreated control (Fig. 6D).
 | Table IIPercentage of GFP+ cells
in the different serum conditions in adherent W-RBCs by X-treme HP
reagent as evaluated by FACS. |
Table II
Percentage of GFP+ cells
in the different serum conditions in adherent W-RBCs by X-treme HP
reagent as evaluated by FACS.
| FBS(+) (%) | FBS(−) (%) | Control (%) |
|---|
| 2 days | 1.37±1.14 | 0.47±0.31 | 0.3±0.2 |
| 3 days | 3.57±2.27a | 2.10±1.91 | 0.3±0.1 |
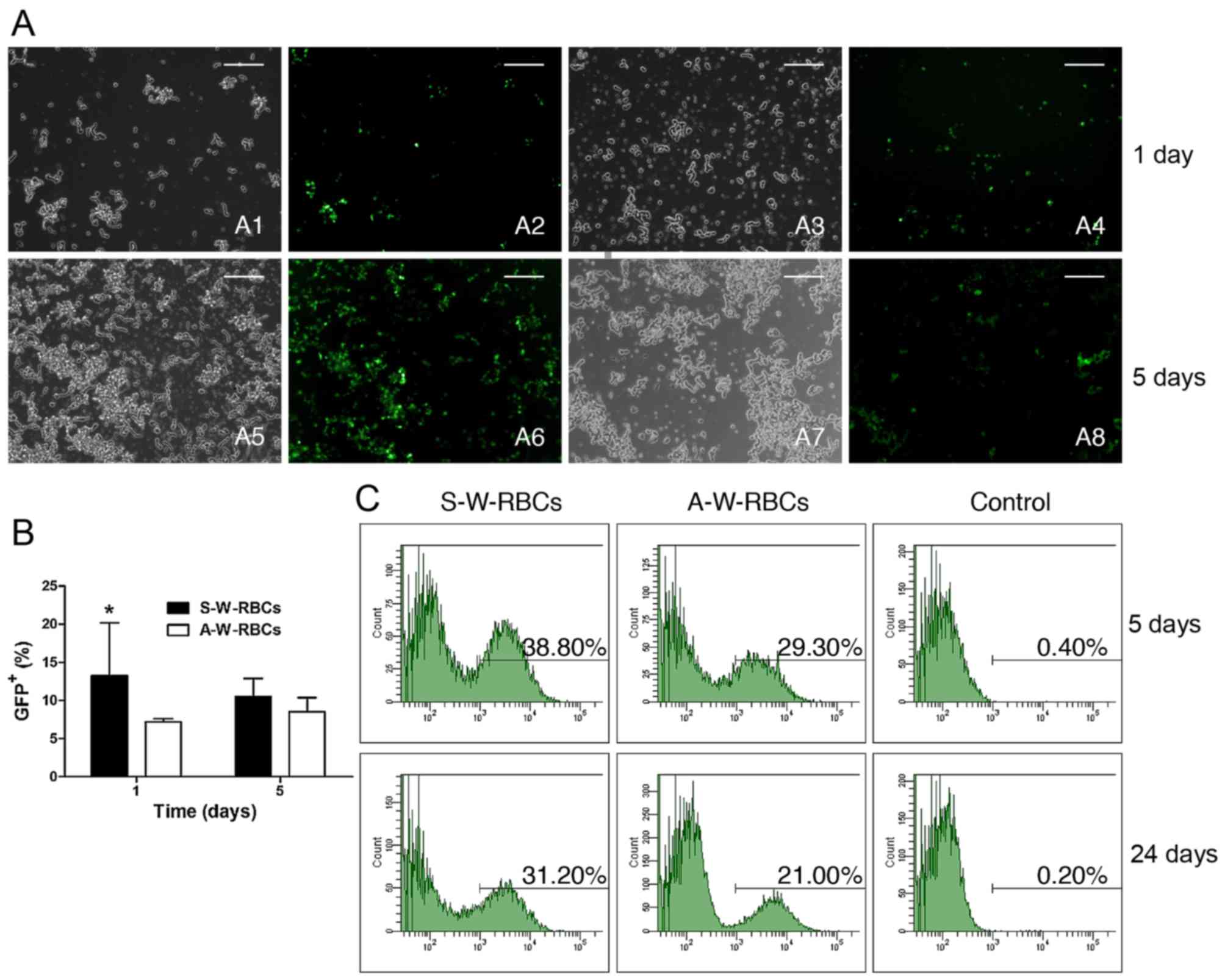
Comparison of suspended and adherent
transfection in W-RBCs
In order to examine whether the cell culture status
affects gene transfection, GFP expression was compared in both
suspended and adherent cultured W-RBCs and the results were as
follows:
Retroviral transfection. Fig. 7A and B shows a significant
difference in GFP expression between the two different cell culture
systems at day 1 and 5. The retrovirus transfection was much more
effective in the suspended W-RBCs (S-W-RBCs) than in the adherent
W-RBCs (A-W-RBCs) (Fig. 7A) based
on the fact that the GFP+ percentage of S-W-RBCs was
significantly more than that of the A-W-RBCs at day 1, and also
remained higher at day 5, although without statistical difference.
Flow cytometry was used to further investigate the GFP expression
when the observation period was extended to 24 days. There were
38.8% S-W-RBCs that expressed positive GFP on the 5th day, which
was more than the adherent (29.3%) and control (0.4%).
Interestingly, the GFP+ cell percentage of suspended
cells just slightly decreased to 31.2% 24 days later, which was
still much higher than the other two groups (Fig. 7C).
X-treme HP transfection. We confirmed that
serum could benefit X-treme HP transfection and prevent cell death;
hence, the serum-tolerant system was selected to compare X-treme HP
transfection in suspended and adherent W-RBCs. Both of the two
groups (suspended/adherent) had a progressive increase in GFP
expression from day 1 to 3, and the suspended groups presented a
higher increase compared with the adherent group. More cells
expressed GFP protein by suspended transfection than by adherent
transfection at day 2 and 3, which showed significant differences
(Fig. 8).
Discussion
Although comprehensive therapies contribute to a
significant improvement in the retinoblastoma survival rate, tumor
recurrence and extended impairment of neighboring tissues caused by
traditional therapies cannot be avoided. Gene therapy would be a
promising technique to targetly correct the gene defects of
retinoblastoma; however, little attention has been paid to optimize
the gene transfection system for retinoblastoma therapy in previous
studies. In this study, we systematically investigated three
critical elements that contribute to successful gene transfection:
the transgene vector, the serum condition, and the cell culture
status. We identified an optimized system that would be appropriate
for gene transfection of retinoblastoma cells.
Optimization of transgene vectors
Choosing an ideal vector was based on several
factors, including the delivery efficiency of therapeutic genes,
the avoidance of cytotoxicity, the maintenance of gene expression
for an appropriate duration needed to treat the disease, the
ability to target specific cells, and a low immune response.
Although previous studies adopted some vectors for retinoblastoma
gene therapy, these studies mainly focused on transgene effects
instead of the vector characteristics, as mentioned above.
Therefore, we compared two major factors, the efficacy and the
cytotoxicity, of different viral and non-viral vectors, which were
confirmed to be feasible for retina gene therapies, to identify
appropriate vectors with retinoblastoma tropism.
Most early studies adopted an adenovirus for gene
delivery to kill retinoblastoma cells. Nevertheless, adenovirus
vectors are immunogenic, and the transgene expression duration is
very short. In this study, rAAV vectors were adopted for a number
of features that could render them suitable for retinoblastoma gene
transfer instead of adenoviruses: i) the ability to transduce both
dividing and non-dividing cells; ii) a broad range of serotypes for
different retinal cell subset tropism; and iii) low pathogenicity
and immunogenicity (30,31). We found that the GFP expression
was negative in both the rAAV2 and rAAV2/1 vectors 2 days after
transfection. This delayed expression may result from the
transcription of rAAV DNA to an active double-stranded form after
infecting cells. This phenomenon has also been observed in mouse
subretinal rAAV transfection; the target gene showed effective
expression 2–3 weeks later (32).
Yet, we observed GFP+ cells on the 3rd day by flow
cytometry, although the percentage was low (<1%). The rAAV2 is
the most commonly used serotype in retina gene therapy and is
believed to be efficient in photoreceptor transfection (22,31), and rAAV2/1 (containing one rAAV2
capsid) was reported to mainly target RPE (24). Thus, rAAV2 is more likely to be
efficient than rAAV2/1 in retinoblastoma transduction given the
pre-photoreceptor origin of retinoblastoma (33); however, our results showed that
the rAAV2/1 with the lowest MOI (104) presented more
GFP+ cells and living cells than the rAAV2 groups on the
10th day. Hence, rAAV2/1 was considered to be a better vector for
W-RBCs as it rendered higher transgene expression with a lower dose
and a lower cytotoxicity. This unexpected outcome may be due to the
variability of retinoblastoma cells, which differ from normal
photoreceptors. Our findings indicated that the rAAV capsid is an
essential component for binding the vectors to target cells and for
transgene intracellular internalization.
rAAV vectors, however, exhibit a poor transfection
ability for W-RBCs, and also suffer from several drawbacks that
limit their application for retina gene therapy, including the
limited payload capacity (34),
the time required for the synthesis of double-stranded DNA
(35), the transient transfection
due to its non-insertional characteristic (34), and the cytotoxic T-cell (CTL)
responses caused by the pre-existing immunity to AAV capsids
(36,37). Thus, we tested another potential
post-mitotic cell vector, the LV vector, for retinoblastoma gene
transfection. LVs are attractive vectors because they can transit
much larger DNA than rAAV vectors and can integrate into the host
genome to transduce stably (38).
They can also avoid CTL responses because LV components have not
been pre-exposed in most human subjects (36). LV vectors have previously been
shown to be efficient and sustainable in gene transfection of human
RPE and photoreceptors (27,39). In this study, the GFP expression
following LV transfection was observed 2 days earlier than the
rAAV-mediated transfection. A relatively higher transfection
efficiency was found in the lower MOI LV group, but there was no
significant difference in GFP transfection efficiency between the
LV and rAAV vectors on either day 1 or 5.
As a classic transgene vector, the retroviral vector
was also utilized in this study because of the stable gene
integration of the host chromosome and the ability to transfect a
wide variety of mammalian cells (40). The data showed that GFP expression
was remarkably higher in the retrovirus transfer group compared to
both the rAAV and LV groups. This is interesting because rAAV and
LV vectors were assumed to be more efficient in gene transfection
than the retroviral vector because the retroviral vector only
infects dividing cells, whereas rAAV and LV vectors are able to
infect dividing, post-mitotic, and even terminal differentiated
cells. There seems to be three possible reasons. i) The retroviral
vector used in this study was pseudotyped with vesicular stomatitis
virus G protein (VSV-G), which traffics through different cell
compartments instead of normal receptor-mediated mechanisms
(39). Thus, the VSV-G
pseudotyped retroviral vector could infect almost all species and
could facilitate the nuclear entry of transgenes and extend the
intracellular half-life of the viral core. ii) WERI-Rb1 is a tumor
cell line with a high proliferation rate due to active mitosis;
thus, the non-dividing cells only account for a few percentages.
This would possibly limit the advantage of the non-dividing cell
potential vectors such as rAAVs and LVs. iii) One critical element
that contributes to transgene expression is the target cell type.
The rAAV vectors perform efficiently in normal photoreceptors, and
LV vectors are more active in degenerating photoreceptors. Although
retinoblastoma probably originates from pre-photoreceptors, the
gene mutations of retinoblastoma make it distinct from normal and
degenerative photoreceptors.
Another interesting finding was that there were
significantly more GFP+ cells following transfecting
with the X-treme HP reagent compared with rAAVs and LVs. Non-viral
vectors are generally assumed to have a much lower transgene
efficiency compared with viral vectors; however, our data showed
that the non-viral vector, X-treme HP, efficiently transduced GFP
plasmid DNA into W-RBCs similarly to the retroviral vector. Viral
vectors are often limited by their cytotoxicity, whereas non-viral
vectors are demonstrated to have relatively low cytotoxicity. In
this study, the cells in the X-treme HP group exhibited similar
survival rates as the untreated W-RBCs and a higher rate than the
retroviral-transfected groups, indicating that the X-treme HP
reagent has a lower cytotoxicity than its retroviral counterpart.
This is con-sistent with previous studies (41,42).
Collectively, the retroviral vectors and X-treme HP
were effective for W-RBC gene transfection; however, concerns about
the insertional oncogenesis, the risk of replication-competent
virus (RCV) generation (39), and
the inactivation of viral particles by the human complement system
(39) must be addressed before
clinical utilization of retroviral gene delivery. In addition, the
low cytotoxicity and the simple trans-fection procedure made
X-treme HP a more preferable vector for retinoblastoma gene
transfection.
Optimization of cell culture system
Although vectors play an important role in gene
transfection, unfortunately, there is no vector that can fulfill
all ideal vector properties. This has led to the study of other
factors, such as a suitable cell culture system for gene delivery.
We explored two elements of a cell culture system, which are
critical for gene transfection: the cell culture status and the
serum in the culture medium.
Retinoblastoma cells are usually suspended when
cultured, and gene expression studies are commonly carried out on
suspended cells; however, rosettes and grape-like cell clusters of
retinoblastoma could hinder the attachment of transgenes and the
inner cells which are stuck in cell clusters. The adherent culture
would facilitate the gene exposure and avoid the chromosomal
positional effect of the suspended culture; however, there is no
study that has compared the gene expression between suspended and
adherent retinoblastoma cells to our knowledge. We compared the GFP
expression of suspended W-RBCs with the adherent W-RBCs by both
retroviral vector and X-treme HP to identify their efficiency. The
data showed that the suspended W-RBCs expressed significantly more
GFP protein than the adherent W-RBCs by both the retroviral and the
X-treme HP-mediated transfection. Moreover, GFP expression
maintained a stable level after a 24-day duration in the suspended
cells, unlike the significant decrease in the adherent cells. These
data suggest that transgene expression was not similar in the
different cell culture statuses and that the suspended status was
more favorable for retinoblastoma gene therapy. This may be because
the adherent culture is a heterogeneous system in which at least
one surface of cells is attached on the plastic plate, which leads
to the inefficient attachment of the medium and the whole cells.
Hence, it is not capable of sufficient contact between transfection
complexes and cells unless the complexes are large enough to settle
onto the cells or unless they have high concentrations. In
contrast, the suspended cells can be exposed to transfection
complexes in a multidimensional manner and can also be easily
passaged.
Another vital element affecting of gene transfection
is the serum condition. Conventional liposome vectors exhibit an
efficient gene transfection ability in serum-free medium (41); however, the serum effect cannot be
avoided in future in vivo application. Given the efficiency
of GFP transfection in W-RBCs, the X-treme HP was adopted, and its
transduction response to serum was explored in this study. The data
presented a progressive increase in GFP+ cells when 10%
FBS was added into the X-treme HP transfection system in a period
of 3 days; however, the GFP+ cells were sustained at a
significantly lower level when the serum was not added to the
system. This phenomenon was observed in both suspended and adherent
W-RBCs. These findings indicated that the X-treme HP reagent had an
efficient serum-resistant ability despite its lipid component. In
addition, the remarkably high number of cells in the trypan blue
staining assay and the toxic cell phenotype in the serum-free group
revealed that the serum prevented the cells from possible
impairment during transfection. Thus, the improvement in cell
viability and the previously reported effect of the cell cycle of
the serum would further benefit the gene transfection efficiency
(43), and this is supported by
the fact that there were significantly more GFP+ cells
in the serum-tolerance group than in the serum-free group.
In conclusion, the suspended cell culture was
superior to the adherent culture for gene transfection in W-RBCs.
Moreover, the serum added to the transfection system did not only
protect cell viability but was also conducive for the transduction
of the target gene into W-RBCs.
In conclusion, this study provided an effective,
convenient, and low cytotoxic system for gene transfection in
W-RBCs. To the best of our knowledge, for the first time, we
systemically evaluated the influence of gene vectors, cell culture
status, and serum conditions on delivering target genes into
W-RBCs. This experimental system may be a promising transgene
system for the potential gene therapy of retinoblastoma; however,
future studies are needed to investigate the transfection system
in vivo for further application.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant 81371007, 81430009 and
81170846).
References
|
1
|
Bishop JO and Madson EC: Retinoblastoma.
Review of the current status. Surv Ophthalmol. 19:342–366.
1975.PubMed/NCBI
|
|
2
|
Shields CL, Meadows AT, Leahey AM and
Shields JA: Continuing challenges in the management of
retinoblastoma with chemotherapy. Retina. 24:849–862. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Garcia D and Quintyn JC: Treatment of
retinoblastoma by radiation therapy. Sixty-six years later. J Fr
Ophtalmol. 36:87–88. 2013.In French. View Article : Google Scholar
|
|
4
|
Kleinerman RA, Tucker MA, Abramson DH,
Seddon JM, Tarone RE and Fraumeni JF Jr: Risk of soft tissue
sarcomas by individual subtype in survivors of hereditary
retinoblastoma. J Natl Cancer Inst. 99:24–31. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mouw KW, Sethi RV, Yeap BY, MacDonald SM,
Chen YL, Tarbell NJ, Yock TI, Munzenrider JE, Adams J, Grabowski E,
et al: Proton radiation therapy for the treatment of
retinoblastoma. Int J Radiat Oncol Biol Phys. 90:863–869. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chévez-Barrios P, Chintagumpala M, Mieler
W, Paysse E, Boniuk M, Kozinetz C, Hurwitz MY and Hurwitz RL:
Response of retinoblastoma with vitreous tumor seeding to
adenovirus-mediated delivery of thymidine kinase followed by
ganciclovir. J Clin Oncol. 23:7927–7935. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hurwitz MY, Marcus KT, Chévez-Barrios P,
Louie K, Aguilar-Cordova E and Hurwitz RL: Suicide gene therapy for
treatment of retinoblastoma in a murine model. Hum Gene Ther.
10:441–448. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nichols KE, Walther S, Chao E, Shields C
and Ganguly A: Recent advances in retinoblastoma genetic research.
Curr Opin Ophthalmol. 20:351–355. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Giacinti C and Giordano A: RB and cell
cycle progression. Oncogene. 25:5220–5227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jia RB, Zhang P, Zhou YX, Song X, Liu HY,
Wang LZ, Luo M, Lu J, Ge SF and Fan XQ: VEGF-targeted RNA
interference suppresses angiogenesis and tumor growth of
retinoblastoma. Ophthalmic Res. 39:108–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jia RB, Fan XQ, Wang XL, Zhang XQ, Zhang P
and Lu J: Inhibition of VEGF expression by plasmid-based RNA
interference in the retinoblastoma cells. Zhonghua Yan Ke Za Zhi.
43:493–498. 2007.In Chinese. PubMed/NCBI
|
|
12
|
Chau KY and Ono SJ: Gene transfer into
retinoblastoma cells. Biotechniques. 26:444–446. 1999.PubMed/NCBI
|
|
13
|
Adachi M, Brooks SE, Stein MR, Franklin BE
and Caccavo FA: Destruction of human retinoblastoma after treatment
by the E variant of encephalomyocarditis virus. J Neurooncol.
77:233–240. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Di Polo A and Farber DB: Rod
photoreceptor-specific gene expression in human retinoblastoma
cells. Proc Natl Acad Sci USA. 92:4016–4020. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Herman MM, Perentes E, Katsetos CD, Darcel
F, Frankfurter A, Collins VP, Donoso LA, Eng LF, Marangos PJ,
Wiechmann AF, et al: Neuroblastic differentiation potential of the
human retinoblastoma cell lines Y-79 and WERI-Rb1 maintained in an
organ culture system. An immunohistochemical, electron microscopic,
and biochemical study. Am J Pathol. 134:115–132. 1989.PubMed/NCBI
|
|
16
|
Muncaster MM, Cohen BL, Phillips RA and
Gallie BL: Failure of RB1 to reverse the malignant phenotype of
human tumor cell lines. Cancer Res. 52:654–661. 1992.PubMed/NCBI
|
|
17
|
Daya S and Berns KI: Gene therapy using
adeno-associated virus vectors. Clin Microbiol Rev. 21:583–593.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Al-Saikhan FI: The gene therapy revolution
in ophthalmology. Saudi J Ophthalmol. 27:107–111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ali RR, Reichel MB, Thrasher AJ, Levinsky
RJ, Kinnon C, Kanuga N, Hunt DM and Bhattacharya SS: Gene transfer
into the mouse retina mediated by an adeno-associated viral vector.
Hum Mol Genet. 5:591–594. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Flannery JG, Zolotukhin S, Vaquero MI,
LaVail MM, Muzyczka N and Hauswirth WW: Efficient
photoreceptor-targeted gene expression in vivo by recombinant
adeno-associated virus. Proc Natl Acad Sci USA. 94:6916–6921. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maguire AM, Simonelli F, Pierce EA, Pugh
EN Jr, Mingozzi F, Bennicelli J, Banfi S, Marshall KA, Testa F,
Surace EM, et al: Safety and efficacy of gene transfer for Leber's
congenital amaurosis. N Engl J Med. 358:2240–2248. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maclachlan TK, Lukason M, Collins M,
Munger R, Isenberger E, Rogers C, Malatos S, Dufresne E, Morris J,
Calcedo R, et al: Preclinical safety evaluation of AAV2-sFLT01- a
gene therapy for age-related macular degeneration. Mol Ther.
19:326–334. 2011. View Article : Google Scholar
|
|
23
|
Lukason M, DuFresne E, Rubin H, Pechan P,
Li Q, Kim I, Kiss S, Flaxel C, Collins M, Miller J, et al:
Inhibition of choroidal neovascularization in a nonhuman primate
model by intravitreal administration of an AAV2 vector expressing a
novel anti-VEGF molecule. Mol Ther. 19:260–265. 2011. View Article : Google Scholar :
|
|
24
|
Auricchio A, Kobinger G, Anand V,
Hildinger M, O'Connor E, Maguire AM, Wilson JM and Bennett J:
Exchange of surface proteins impacts on viral vector cellular
specificity and transduction characteristics: The retina as a
model. Hum Mol Genet. 10:3075–3081. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pang J, Cheng M, Haire SE, Barker E,
Planelles V and Blanks JC: Efficiency of lentiviral transduction
during development in normal and rd mice. Mol Vis. 12:756–767.
2006.PubMed/NCBI
|
|
26
|
Mátrai J, Chuah MK and VandenDriessche T:
Recent advances in lentiviral vector development and applications.
Mol Ther. 18:477–490. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miyoshi H, Takahashi M, Gage FH and Verma
IM: Stable and efficient gene transfer into the retina using an
HIV-based lentiviral vector. Proc Natl Acad Sci USA.
94:10319–10323. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Garson K, Gamwell LF, Pitre EM and
Vanderhyden BC: Technical challenges and limitations of current
mouse models of ovarian cancer. J Ovarian Res. 5:392012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tosetti F, Venè R, Arena G, Morini M,
Minghelli S, Noonan DM and Albini A: N-(4-hydroxyphenyl)retinamide
inhibits retinoblastoma growth through reactive oxygen
species-mediated cell death. Mol Pharmacol. 63:565–573. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cepko CL: Emerging gene therapies for
retinal degenerations. J Neurosci. 32:6415–6420. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Day TP, Byrne LC, Schaffer DV and Flannery
JG: Advances in AAV vector development for gene therapy in the
retina. Adv Exp Med Biol. 801:687–693. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Janson CG, McPhee SW, Leone P, Freese A
and During MJ: Viral-based gene transfer to the mammalian CNS for
functional genomic studies. Trends Neurosci. 24:706–712. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu XL, Fang Y, Lee TC, Forrest D,
Gregory-Evans C, Almeida D, Liu A, Jhanwar SC, Abramson DH and
Cobrinik D: Retinoblastoma has properties of a cone precursor tumor
and depends upon cone-specific MDM2 signaling. Cell. 137:1018–1031.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Koirala A, Conley SM and Naash MI: A
review of therapeutic prospects of non-viral gene therapy in the
retinal pigment epithelium. Biomaterials. 34:7158–7167. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Boylan NJ, Kim AJ, Suk JS, Adstamongkonkul
P, Simons BW, Lai SK, Cooper MJ and Hanes J: Enhancement of airway
gene transfer by DNA nanoparticles using a pH-responsive block
copolymer of polyethylene glycol and poly-L-lysine. Biomaterials.
33:2361–2371. 2012. View Article : Google Scholar :
|
|
36
|
McIntosh JH, Cochrane M, Cobbold S,
Waldmann H, Nathwani SA, Davidoff AM and Nathwani AC: Successful
attenuation of humoral immunity to viral capsid and transgenic
protein following AAV-mediated gene transfer with a non-depleting
CD4 antibody and cyclosporine. Gene Ther. 19:78–85. 2012.
View Article : Google Scholar
|
|
37
|
VandenDriessche T: Muscling through AAV
immunity. Blood. 114:2009–2010. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Harvey AR, Kamphuis W, Eggers R, Symons
NA, Blits B, Niclou S, Boer GJ and Verhaagen J: Intravitreal
injection of adeno-associated viral vectors results in the
transduction of different types of retinal neurons in neonatal and
adult rats: A comparison with lentiviral vectors. Mol Cell
Neurosci. 21:141–157. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Daly G and Chernajovsky Y: Recent
developments in retroviral-mediated gene transduction. Mol Ther.
2:423–434. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu Y and Deisseroth A: Tumor vascular
targeting therapy with viral vectors. Blood. 107:3027–3033. 2006.
View Article : Google Scholar
|
|
41
|
Ramamoorth M and Narvekar A: Non viral
vectors in gene therapy- an overview. J Clin Diagn Res.
9:GE01–GE06. 2015.PubMed/NCBI
|
|
42
|
Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ,
Dorkin JR and Anderson DG: Non-viral vectors for gene-based
therapy. Nat Rev Genet. 15:541–555. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chan CL, Ewert KK, Majzoub RN, Hwu YK,
Liang KS, Leal C and Safinya CR: Optimizing cationic and neutral
lipids for efficient gene delivery at high serum content. J Gene
Med. 16:84–96. 2014. View Article : Google Scholar : PubMed/NCBI
|