Introduction
Colorectal cancer (CRC) remains a leading cause of
cancer-associated deaths worldwide with an incidence of over one
million newly diagnosed cases per year. Despite intensive research
and therapeutic efforts, the mortality rate of CRC is ~40–50%
(1). Furthermore, the rate of
metastatic cases is high (2).
Drug resistance is responsible for poor prognosis in many cancer
types (3). Thus, to identify
proteins which may have predictive value is of importance not only
in metastasized CRC, but also in other advanced epithelial cancers.
In this regard, the deregulation of DNA damage repair systems (i.e.
mismatch repair) represents an important aspect, since it
contributes to the resistance of cancer cells to conventional
chemotherapy.
One further repair protein is excision repair
cross-complementing 1 (ERCC1), which is also implicated in therapy
resistance. ERCC1 is a structure specific DNA repair endonuclease
responsible for 5′ incision (5′-endonuclease), a key enzyme in the
nucleotide excision repair (NER) pathway and is essential for
repair of platinum-DNA adducts, and is thus associated with therapy
resistance to platinum-containing compounds (3,4).
NER is responsible for repair of DNA damages caused by oxidative
and alkylating agents (3). ERCC1
was suggested as a promising marker in CRC (4). ERCC1-overexpressing cancer cells are
thought to be more resistant to platinum-based chemotherapy.
Increased ERCC1 mRNA levels were found to be associated with
resistance to platinum-based chemotherapy (i.e. ovarian, gastric,
cervical, colorectal and non-small cell lung cancer) suggesting
that platinum-paclitaxel chemotherapy would be more effective in
ERCC1-negative cancer (3). It is
known that ERCC1 protein expression, estimated by
immunohistochemistry, is an independent prognostic factor for
progression-free and overall survival in NSCLC patients treated
with platinum-based chemotherapy (5). Similar data could be achieved in CRC
(6). In several trials on CRC,
the ERCC1 expression level was proposed as a candidate marker for
predicting the efficacy of oxaliplatin therapy for metastatic
patients. In stage III colon cancer, ERCC1 expression is strongly
predictive in the selection of patients which will benefit from
additional oxaliplatin to 5-fluorouracil (5-FU) therapy (7).
Ribonucleoside-diphosphate reductase 1 (RRM1) gene
encodes the regulatory subunit of ribonucleotide reductase enzyme.
Ribonucleotide reductase, composed of regulatory subunit RRM1 and
the catalytic subunit RRM2, is a crucial enzyme in new DNA
synthesis, catalysing the biosynthesis of deoxyribonucleotides from
the corresponding ribonucleotides (8). RRM1 is a key molecule for
gemcitabine efficacy and is also involved in tumor progression.
High RRM1 expression in tumor tissue predicts significantly better
prognosis while only patients with low RRM1 benefit from
gemcitabine therapy. In turn, overexpression of RRM1 protein is
strongly associated with gemcitabine resistance (8). RRM1 expression was also reported to
correlate with the tumorigenic and metastatic potential in lung
cancer (8).
The cell cytoskeleton is built up from microtubules,
microfilaments and intermediate filaments. Various changes in the
microtubule network have been identified in a wide range of
cancers, i.e. altered expression of tubulin isotypes, alterations
in tubulin posttranslational modifications and changes in the
expression of microtubule-associated proteins (MAPs) (9). Class III β-tubulin (TUBB3) is one of
the main microtubule (MT) proteins and is primarily expressed in
neurons and Sertoli cells in the testis (10,11). In lung cancer, the TUBB3 protein
expression level was found to have no correlation with age, gender,
smoking status or recurrence pattern or response rate to
chemotherapy. The response rate in TUBB3-positive cases was 18%,
while the rate was 27% in negative cases (no significant
differences could be detected) (5). High TUBB3 expression levels are
associated with poor prognosis in many epithelial cancers.
Additionally, TUBB3 has been suggested to take part in disease
aggressiveness by acting as a survival factor for cancer cells
(12). In colorectal adenomas,
TUBB3 expression can be detected in up to 100% of high-grade
dysplasia. Expression of TUBB3 was found to have no association
with grade of dysplasia or other clinical data in preneoplastic
lesions of CRC but was associated with Dukes' stage (13). TUBB3 overexpression in colon
cancer cells may contribute to a higher stability of the
microtubular network which may explain the lower activity of
anti-microtubule agents (14). In
addition, high TUBB3 expression levels were localized to the
invasive edge in CRC; positive TUBB3 staining was observed in all
cases, yet this was most prominent at the invasive front with the
presence of tumor budding (12).
This preferential localization of TUBB3 at the invasive margin
raises the possibility that changes in tubulin isotypes can
modulate the invasive activity of cancer cells. Microtubules are
indispensable for the directional migration of cells. Tubulins, the
major constituent protein of microtubules, are built up from
heterodimers of α and β subunits (12). It is believed that tumor buds
consist of migrating cells and TUBB3 expression in these cells is
linked to their motility. Furthermore, TUBB3 is expressed in a
variety of tumors, particularly in those that are aggressive and
likely to metastasize, and were found to be more resistant to
several chemotherapy regimens (i.e. estramustine, Taxol, paclitaxel
and docetaxel) (12). As we
previously demonstrated, MMR protein expression is correlated with
the expression of the apoptosis repressor protein apoptosis
repressor protein (ARC). It is known that overexpression of ERCC1,
RRM1 and TUBB3 is linked to therapeutic resistance against
therapeutic regimens, which is also found in advanced (stage IV)
CRC (15). In this context, we
investigated the expression trend of ERCC1, RRM1 and TUBB3 proteins
and their correlation to ARC protein expression, which is known to
be upregulated in CRC and associated with therapeutic resistance
inhibiting both extrinsic and intrinsic apoptotic signaling.
Materials and methods
Tissue samples
Paraffin-embedded surgical specimens of liver
metastasis of CRC were selected from the archives of the Institute
of Pathology at the University Hospital of Heidelberg. One hundred
patients (64 male, 37 female; mean age 62 years) were included.
None of the patients had received neo-adjuvant chemotherapy. Tissue
samples were fixed in neutral-buffered formalin and embedded in
paraffin. Paraffin sections were cut (4 µm) and examined on
coated slide glass for immunohistochemistry. Further data, such as
age, sex, size and number of metastases were collected from
histological studies. Tissue samples were provided by the Tissue
Bank of the National Center for Tumor Diseases (NCT, Heidelberg,
Germany) in accordance with the regulations of the tissue bank and
the approval of the Ethics Committee of Heidelberg University
according to ethical standards formulated in the Declaration of
Helsinki 1975 (revised in 1983).
Tissue microarray
Tissue microarray (TMA) blocks were obtained from
paraffin-embedded human liver specimens with a tissue microarrayer
(Beecher Instruments, Sun Prairie, WI, USA). From each case, two
cores of tumor tissue with a diameter size of 1.6 mm were punched
and for orientation of the TMA slides two muscle cores were used.
Muscle punches served also as positive controls for ARC
immunostaining.
Immunohistochemistry
Four-micrometer-thick slides were obtained from the
TMA. Slides were then deparaffinised according to standard protocol
by xylene, and dehydrated with 95–96% ethanol, 70% ethanol and
distilled water. All slides were stained simultaneously using a
computer-controlled autostainer (Ventana BenchMark Ultra, Ventana
Medical Systems, Inc., Tucson, AZ, USA). Then primary antibodies
were used: ERCC1 (8F1, Neomarkers; dilution 1:100), RRM1 (Protein
Tech Europe; dilution 1:200) and TUBB3 (Tuj-1/TubIII/4G3, Covalab;
dilution 1:2,000). Primary antibodies were incubated according to
routine staining protocols for diagnostic purpose. To detect
immunoreactions, Ultraview Universal DAB dectection kit (Ventana
Medical Systems, Inc.) and 3,3′-diaminobenzidine were used. A
counterstain was performed with hematoxylin and bluing reagent and
all slides were covered. For MMR proteins, p53 and ARC, the
staining methods were performed as previously published (16).
Evaluation of immunohistochemistry
For semi-quantitative assessment of staining
intensity, we adjusted a previously published scoring system for
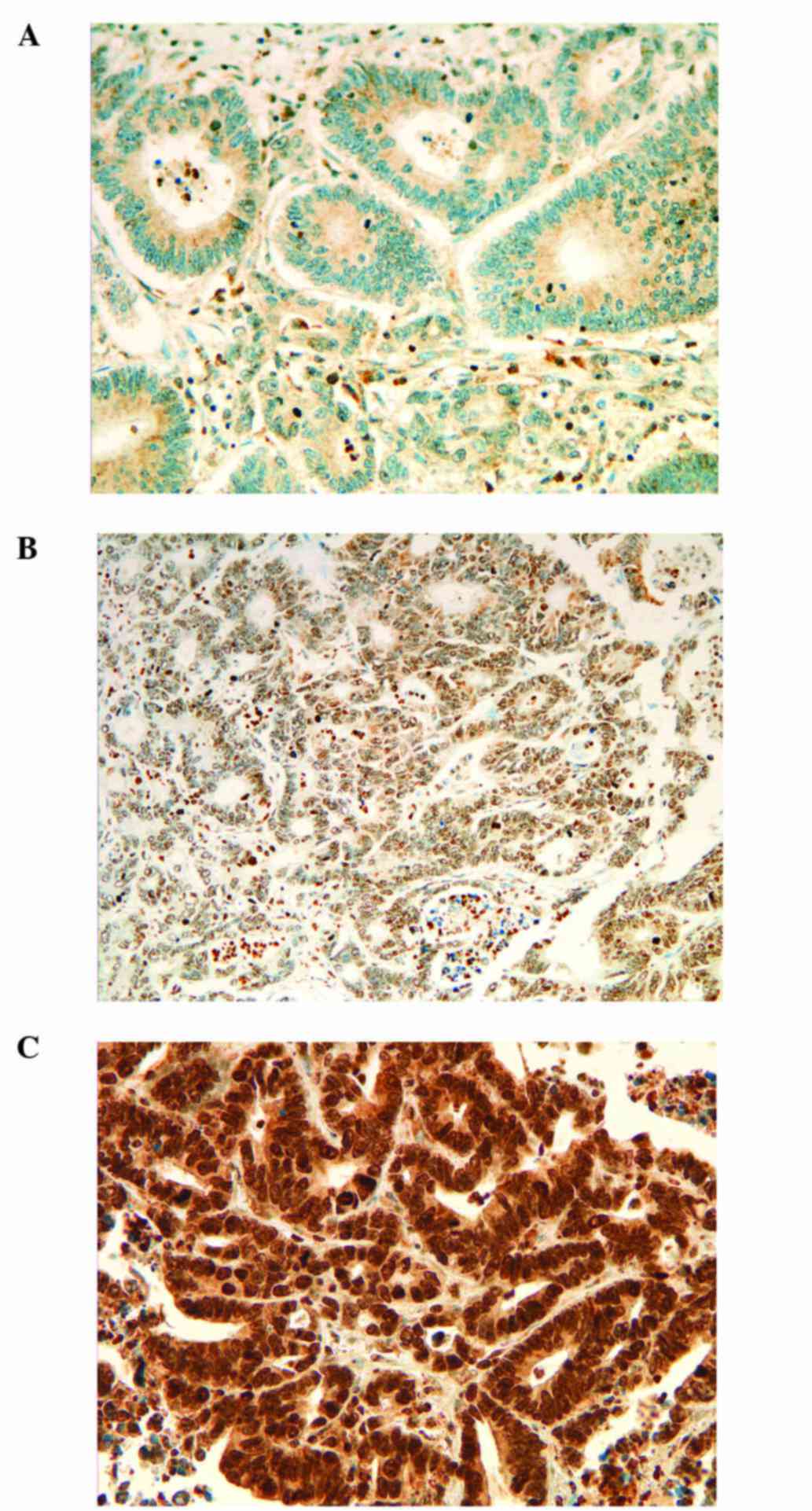
each protein and fitted to TMA dots (17,18). ERCC1 and RRM1 immunostainings were
scored using a three-graded scale: score 0, no expression
detectable or faint partial expression in <10% of the tumor
cells; score 1, weak to moderate expression of the entire tumor
tissue; score 2, strong positivity in the entire tumor tissue.
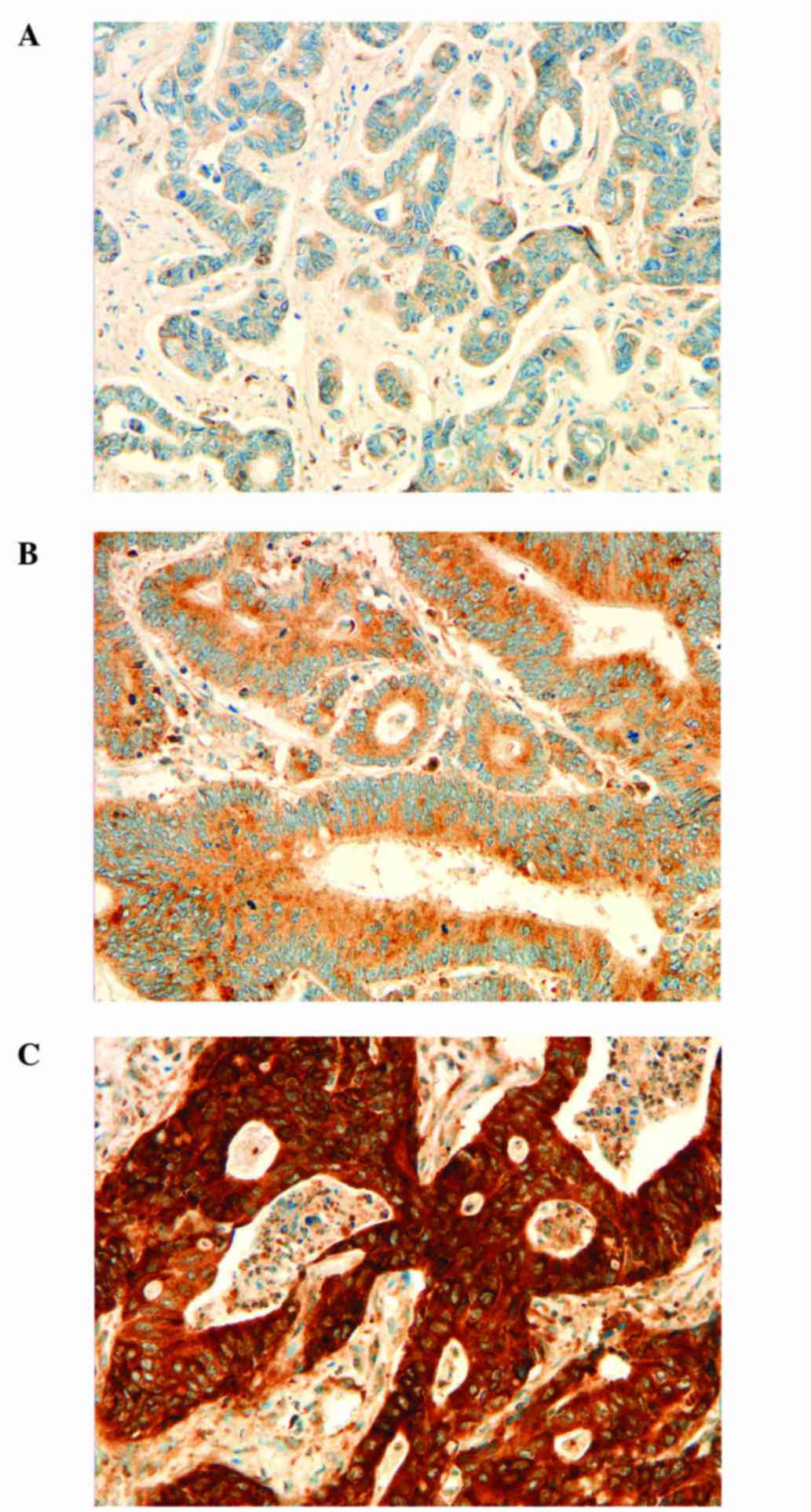
For TUBB3, a modified three-graded score was
established: score 0, no expression detectable or faint partial
expression in <10% of the tumor cells; score 1, diffuse and
strong positive staining associated to invasive front and tumor
budding, central tumor regions negative or with weaker intensity
than at the invasive front; score 2, strong positivity in the
entire tumor tissue.
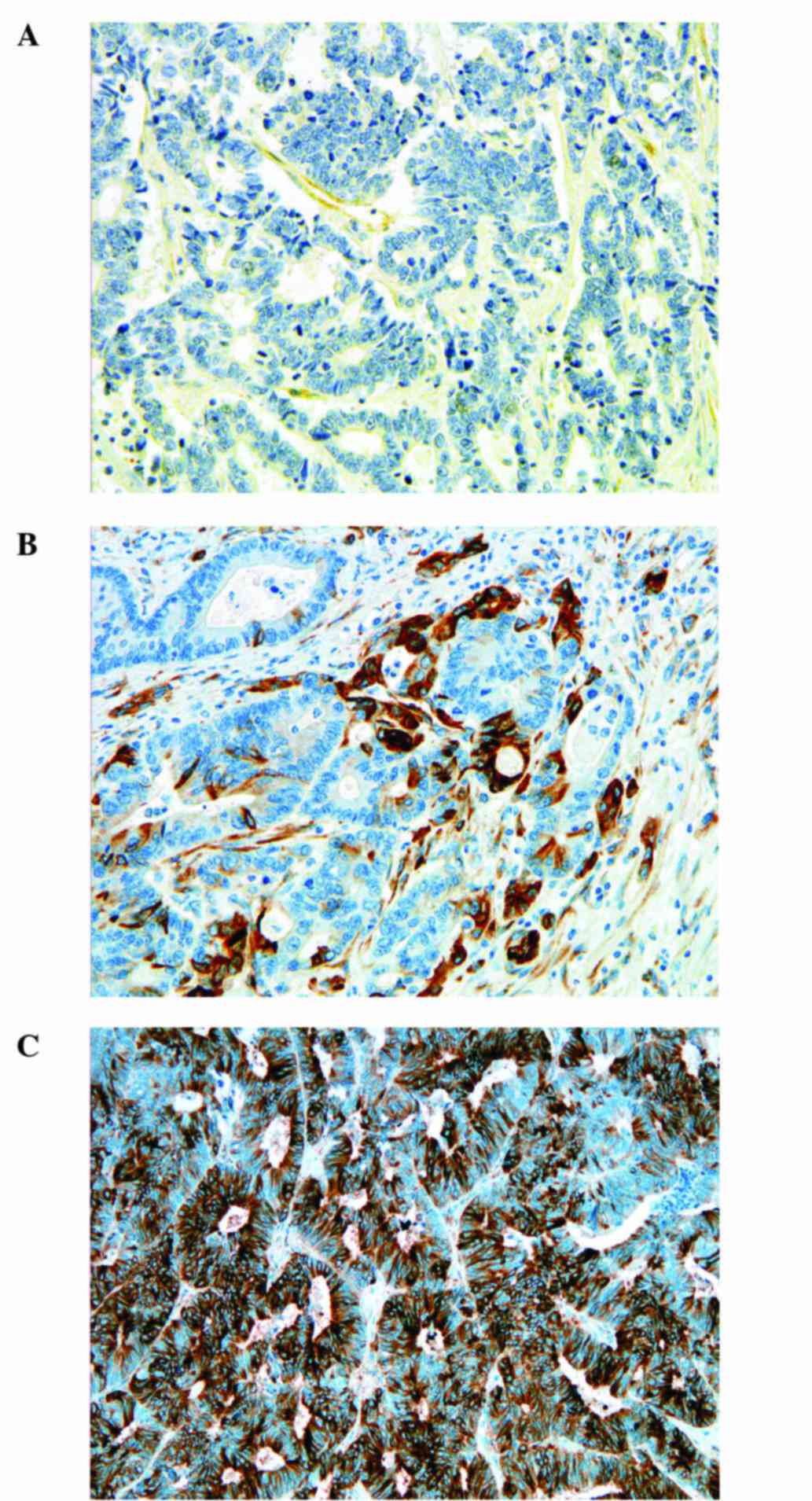
For MSI proteins, the staining was evaluated
according to Bethesda guidelines (19). Immunostaining for p53 was scored
using a three-graded scale: score 0, weak staining in <10% of
the tumor cells, score 1, moderate staining in up to 75% of the
tumor cells and score 2, strong nuclear staining in >75% of the
tumor cells. The results of MMR, p53 and ARC immunohistochemistry
for this collective have already been published (16).
The immunostained tissue microarray sections were
evaluated and scored under a light microscope independently by two
pathologists in a blinded manner. Discordant cases were reviewed
and re-evaluated based on a consensus opinion.
Statistical analysis
The statistical analyses were performed with SAS
software (SAS Institute, Cary, NC, USA). Associations between
clinical data, ARC, MMR proteins, ERCC1, TUBB3 and RRM1 were
estimated by Pearson's correlation and linear regression test. The
statistical significance was set at p<0.05 and p<0.01.
Results
Distribution of ERCC1, RRM1 and TUBB3
protein expression in the collective
The results of the immunohistochemistry for ERCC1,
RRM1 and TUBB3 are listed in Table
I. For ERCC1 we found 29.8% of the cases to be negative (score
0). Positive ERCC1 staining was detected in 70.2% of the cases
(score 1, 30.8% and score 2, 39.4%). For RRM1, the distribution was
different. Only 11 cases out of 95 valid cases (11.6%) were found
to be negative (score 0). Eighty-four cases (88.4%) showed positive
staining for RRM1, 51 cases showed a high expression level (score
2, 53.7%).
 | Table IResults of the immunohistochemistry
for ERCC1, RRM1 and TUBB3. |
Table I
Results of the immunohistochemistry
for ERCC1, RRM1 and TUBB3.
| Proteins | Immunoreactive score
0 n (%) | Immunoreactive score
1 n (%) | Immunoreactive score
2 n (%) | Valid cases n
(%) |
|---|
| ERCC1 | 28 (29.8) | 29 (30.8) | 37 (39.4) | 94 (100) |
| RRM1 | 11 (11.6) | 33 (34.7) | 51 (53.7) | 95 (100) |
| TUBB3 | 35 (35) | 52 (52) | 13 (13) | 100 (100) |
TUBB3 staining showed an interesting distribution.
Most of the cases showed pronounced positivity at the invasive
margin (52%). Thirty-five cases (35%) had negative staining and
only 13% had a diffuse positive staining reaction for TUBB3.
Representative images of the staining scores for ERCC1, RRM1 and
TUBB3 are shown in Figs.
1Figure 2–3.
Statistically significant correlation
between ERCC1, RRM1, TUBB3 and MMR proteins, but not with p53
Regarding MMR proteins we found statistically
significant correlations between MMR proteins and ERCC1, RRM1 and
TUBB3. In turn, none of the three markers demonstrated a
correlation with the p53 expression level. MLH1 and MSH2 proteins
showed a positive statistically significant correlation with ERCC1
(p<0.000 and p=0.008, respectively). This means that loss of
MLH1 and MSH2 is associated with lower expression or loss of ERCC1
in colorectal liver metastasis. A similar correlation was detected
between MSH2, MSH6 and RRM1 (p=0.005 for MSH2 and p=0.011 for
MSH6). Higher RRM1 expression levels were detected at intact
expression of MSH2 and MSH6.
Notably, TUBB3 expression showed a strong positive
correlation with MLH1 and MSH2 (p=0.019 and p=0.012, respectively).
The detailed correlations are documented in Table II.
 | Table IIStatistical correlations between
ERCC1, RRM1 and TUBB3 with mismatch repair proteins and p53. |
Table II
Statistical correlations between
ERCC1, RRM1 and TUBB3 with mismatch repair proteins and p53.
| Protein | MLH1 | MSH2 | MSH6 | PMS2 | p53 |
|---|
| ERCC1 | | | | | |
| Correlation
coefficient |
0.541a |
0.273a | 0.186 | 0.168 | 0.164 |
| Significance
(2-sided) | 0.000 | 0.008 | 0.072 | 0.106 | 0.116 |
| No. of valid
cases | 71 | 94 | 94 | 94 | 93 |
| RRM1 | | | | | |
| Correlation
coefficient | 0.104 |
0.283a |
0.261b | 0.198 | 0.146 |
| Significance
(2-sided) | 0.387 | 0.005 | 0.011 | 0.055 | 0.160 |
| No. of valid
cases | 71 | 95 | 95 | 95 | 94 |
| TUBB3 | | | | | |
| Correlation
coefficient |
0.271b |
0.252b | 0.025 | 0.033 | 0.035 |
| Significance
(2-sided) | 0.019 | 0.012 | 0.808 | 0.748 | 0.736 |
| No. of valid
cases | 75 | 99 | 99 | 99 | 98 |
Cytoplasmic ARC staining intensity is
strongly correlated with TUBB3 and RRM1 expression levels
In negative RRM1 cases, the ARC cytoplasmic
expression was also low (score 0/1) (6/10, 60%). Cases with
moderate RRM1 expression (score 1) also had in the majority of
cases a low level of ARC expression (19/33, 57.6%). Fourteen of 33
cases (42.4%) with moderate RRM1 expression had a high level of
cytoplasmic ARC (score 2/3). Cancers expressing RRM1 at high levels
(score 2, 51 cases) showed, in the majority of cases, elevated
cytoplasmic ARC levels [low ARC level in only 12 cases (12/51,
23.53%), high ARC level in 39 cases (39/51, 76.47%)]. In
conclusion, a high level of RRM1 expression in most of the cases
occurred simultaneously with elevated, high level cytoplasmic ARC
protein expression (score 2/3 in 76.47% of the valid cases).
Cytoplasmic ARC protein expression showed a positive, statistically
significant correlation with RRM1 expression levels (p<0.000).
Ninty-eight cases were valid for both proteins (ARC cytoplasmic and
TUBB3). In TUBB3 negative cases (score 0), cytoplasmic expression
of ARC was detected in 15 cases (15/35, 42.85%). In TUBB3 score 1
cases, it was even higher (35/51, 68.62%). In strongly diffuse
positive TUBB3 cases, the highest cytoplasmic ARC expression was
found (9/12, 75%). We found a progressive staining intensity for
cytoplasmic ARC regarding TUBB3 status. This association was also
significant (p<0.001). The distribution of the statistical
results is listed in Table
III.
 | Table IIIResults of the statistical analysis
between apoptosis repressor ARC protein and ERCC1, RRM1 and
TUBB3. |
Table III
Results of the statistical analysis
between apoptosis repressor ARC protein and ERCC1, RRM1 and
TUBB3.
| Protein | Cytoplasmic ARC
expression | Nuclear ARC
expression |
|---|
| ERCC1 | | |
| Correlation
coefficient | −0.053 | −0.020 |
| Significance
(2-sided) | 0.613 | 0.851 |
| Number of valid
cases | 93 | 93 |
| RRM1 | | |
| Correlation
coefficient |
0.378a | 0.147 |
| Significance
(2-sided) | 0.000 | 0.156 |
| Number of valid
cases | 94 | 94 |
| TUBB3 | | |
| Correlation
coefficient |
0.323a | −0.048 |
| Significance
(2-sided) | 0.001 | 0.641 |
| Number of valid
cases | 98 | 98 |
Correlations between ERCC1, RRM1, TUBB3
and clinical data
Concerning clinical parameters such as age, sex of
the patients, grade of the tumor and the number and size of
metastases, there was no significant correlation with ERCC1 or RRM1
expression (Table IV). Regarding
patient age, there was a strong negative correlation with TUBB3
expression level (p=0.008). In addition, the TUBB3 expression level
was also positively associated with tumor grade (p=0.047) and TUBB3
expression was also correlated with the RRM1 expression level
(p=0.022).
 | Table IVResults of the statistical analysis
between ERCC1, RRM1 and TUBB3 and clinical data. |
Table IV
Results of the statistical analysis
between ERCC1, RRM1 and TUBB3 and clinical data.
| Protein | Age | Sex | Tumor grade | No. of
metastases |
|---|
| ERCC1 | | | | |
| Correlation
coefficient | 0.106 | −0.205 | −0.010 | −0.030 |
| Significance
(2-sided) | 0.320 | 0.054 | 0.930 | 0.781 |
| No. of valid
cases | 90 | 89 | 82 | 86 |
| RRM1 | | | | |
| Correlation
coefficient | 0.004 | 0.001 | 0.151 | 0.027 |
| Significance
(2-sided) | 0.971 | 0.995 | 0.174 | 0.803 |
| No. of valid
cases | 91 | 90 | 83 | 87 |
| TUBB3 | | | | |
| Correlation
coefficient |
−0.269a | −0.139 |
0.213b | 0.026 |
| Significance
(2sided) | 0.008 | 0.178 | 0.047 | 0.807 |
| No. of valid
cases | 96 | 95 | 87 | 92 |
Discussion
Apoptotic signaling is one of the most important
processes in therapeutic resistance. In addition to known
regulatory proteins, there are many others, which can influence the
apoptotic process, and some can thus enhance or inhibit therapeutic
effects. ERCC1, RRM1 and TUBB3 are known to have therapeutic
predictive value in the current therapy of metastasized CRC
(3,5,6).
In the present study, we investigated the expression levels of
ERCC1, RRM1 and TUBB3 in liver metastasis of CRC and analysed their
associations to sex, age, tumor grade, mucin production, tumor size
and number of metastases. Furthermore, we investigated their
correlation to MMR proteins, p53 and apoptosis repressor ARC.
In our collective, ERCC1 protein loss was detected
in one-third of the cases (29.8%). A proportion of 70.2% showed a
positive reaction and score 2 (strong nuclear expression) was
confirmed in 39.4% of all valid cases. In another study, which
investigated stage III CRC, ERCC1 and MSI levels were found to be
positive in 55 and 17%, respectively (7). According to literature data, score 2
cases do not benefit from platinum-based chemotherapy. On the other
hand, one-third of cases (loss of ERCC1) have a better prognosis
following platinum-based chemotherapy (15). Cases with moderate ERCC1
expression (score 1), in our opinion, require further
investigation. It must be evaluated in functional studies, whether
a mild loss in ERCC1 expression is enough to sensitize cancer cells
to platinum-based chemotherapy. According to our scoring system, we
detected certain negative and positive cases, thus allowing a
prediction for platinum-based chemotherapy in two-thirds of the
patients after a single immunohistochemistry.
We found a statistically significant positive
correlation between ERCC1 and MLH1, MSH2 (p=0.000 and p=0.008,
respectively). The frequent loss of ERCC1 and MLH1 both could be
explained by methylation. Similar correlations were found in
mesothelioma (20). In NSCLC,
ERCC1 nuclear staining was noted in 45.59% of the cases, and TUBB3
cytoplasmic staining was noted in 65.44% of the cases detected.
ERCC1 and TUBB3 double-negative cases exhibited a better
therapeutic response to platinum-based therapy (3). We found 11 cases (11.7% of valid
cases) as double-negative for ERCC1 and TUBB3. Analogous to other
epithelial neoplasia, 11.7% of the metastatic cases could benefit
much more from platinum-based therapy, than the others. Negative
expression of ERCC1 and TUBB3 was found to be associated with a
significantly higher response rate, longer median progression-free
survival and overall survival after platinum-paclitaxel treatment
(3). One study found that
patients with advanced CRC with high expression of ERCC1 are not
indicated for oxaliplatin-based chemotherapy (17), whereas patients with low levels of
ERCC1 expression have been reported to have an improved response
and a longer overall survival in metastatic CRC treated with FOLFOX
combination (18). In this study,
a low level of ERCC1 was detected in 90% of the cases (18). One study, in accordance with other
study results, demonstrated the potential utility of ERCC1
expression as a prognostic and possibly predictive biomarker in
metastasized CRC. It was able to identify a population with poor
prognosis, as well as a population with a markedly high response
rate to FOLFOX combination therapy (18). The cases were evaluated according
to a pre-established cut-off for ERCC1 (17). As noted, the expression levels of
ERCC1 are not always consistent with sensitivity to platinum-based
treatment. We assume that the cut-off level was set too high and
suggest to define more than only two groups (positive and
negative). Therefore, we propose the use of large prospective or
retrospective studies with standard chemotherapy to analyse the
expression of predictive markers, such as ERCC1, RRM1 or TUBB3 (and
its interaction partners) to set a cut-off for cases, which can
benefit from a therapeutic regimen. Too high or too low cut-off
levels result in subsets of patients, who may suffer from long-term
toxicity with no benefit of treatment, and patient groups that do
not receive the optimal therapy regimen.
RRM1 overexpression is associated with gemcitabine
resistance. RRM1 is a key molecule for gemcitabine efficacy and is
also involved in tumor progression. High RRM1 expression in tumor
tissue predicts significantly better prognosis, whereas only
patients with low RRM1 benefit from gemcitabine therapy. In turn,
overexpression of RRM1 protein is strongly associated with
gemcitabine resistance (8). RRM1
expression was also reported to correlate with tumorigenic and
metastatic potential in lung cancer (8). According to our data, only 11.6% of
the cases with loss of RRM1 expression had a positive response to
gemcitabine. In other words, patients with high RRM1 protein
expression in tumor tissue should be treated with alternative
drugs, i.e. oxaliplatin, 5-FU and leucovorin (CONKO-003) instead of
gemcitabine (8). As mentioned
above, score 1 cases need further functional analysis and lower
RRM1 activity may also be sufficient not to overcome
gemcitabine-induced DNA damage leading to the death of tumor
cells.
Loss of MSH2 and MSH6 expression is associated with
lower levels of RRM1 protein (p=0.005 and p=0.011, respectively).
For this association the following mechanisms could be responsible:
i) RRM1 is a key enzyme in the synthesis of new DNA, thus defected
MMR proteins, i.e. MSH2 and MSH6 lead to DNA damage which can
downregulate the new DNA synthesis, leading to cell cycle arrest.
One possible connection between RRM1 and the MMR system is through
DNA damage. It leads to cell cycle arrest, which results in lower
RRM1 expression level or ii) there is a possible connection through
transforming growth factor-β (TGF-β); in normal cells TGF-β can
activate the MSH2 promoter (through Smad p53 dependent mechanism),
whereas at the posttranscriptional level, miR-21 induced by TGF-β
targets MSH2 transcript and suppresses its expression. In contrast,
in cancer cells p53 is inactivated and miR-21 is overexpressed,
thus TGF-β fails to activate the MSH2 promoter resulting in genomic
instability (21).
Cytoplasmic ARC protein expression showed a
positive, statistically significant correlation with RRM1
expression levels (p<0.000). Which mechanism leads to higher
RRM1 expression with ARC overexpression or in turn how ARC
expression can induce RRM1 overexpression should be elucidated in
functional studies, but it is known that RRM1-overexpressing cells
have an increased level of apoptosis (22), thus it is possible that a certain
overexpression level can induce apoptotic signaling, which in turn
induces ARC expression to suppress apoptosis induction. This
possibility is further strengthened by the positive correlation
between RRM1 and TUBB3 found in our collective (p=0.022).
The main staining pattern for TUBB3 was expression
at the invasive front, similar to primary CRC studied previously
(12). TUBB3 expression was not
detected in 35% of the valid cases. These cases are potential
candidates for taxane-based chemotherapy with highly predicted
response. In our collective, we found a statistically significant
correlation between MLH1, MSH2 and TUBB3 (p=0.019 and p=0.012,
respectively), which further strengthens the evidence of the
regulatory role of mismatch repair proteins in apoptosis. In the
case of sufficient MLH1 and MSH2 expression, TUBB3 is significantly
highly expressed to suppress the activities of MLH1 and MSH2.
These results can indicate that a defected MMR
system would induce TUBB3 overexpression leading to MT
rearrangement, which can influence apoptosis (i.e. activating
pro-apoptotic signaling proteins). Microtubules (MTs) have an
important role in apoptosis, i.e. survivin is believed to regulate
apoptosis by controlling microtubule polymerization. Thus, the
disruption of normal MT function (either increasing or decreasing
MT length) may trigger apoptosis. MT system (and thus TUBB3) has an
important role in the regulation of DNA damage-induced apoptosis.
DNA damage (i.e. γ-radiation) induces α-, β- and γ-tubulin
production and polymerization, and stimulates MT reorganization
(23). One explanation is that
DNA damage through cyclin B1 and cdc2 kinase activation leads to
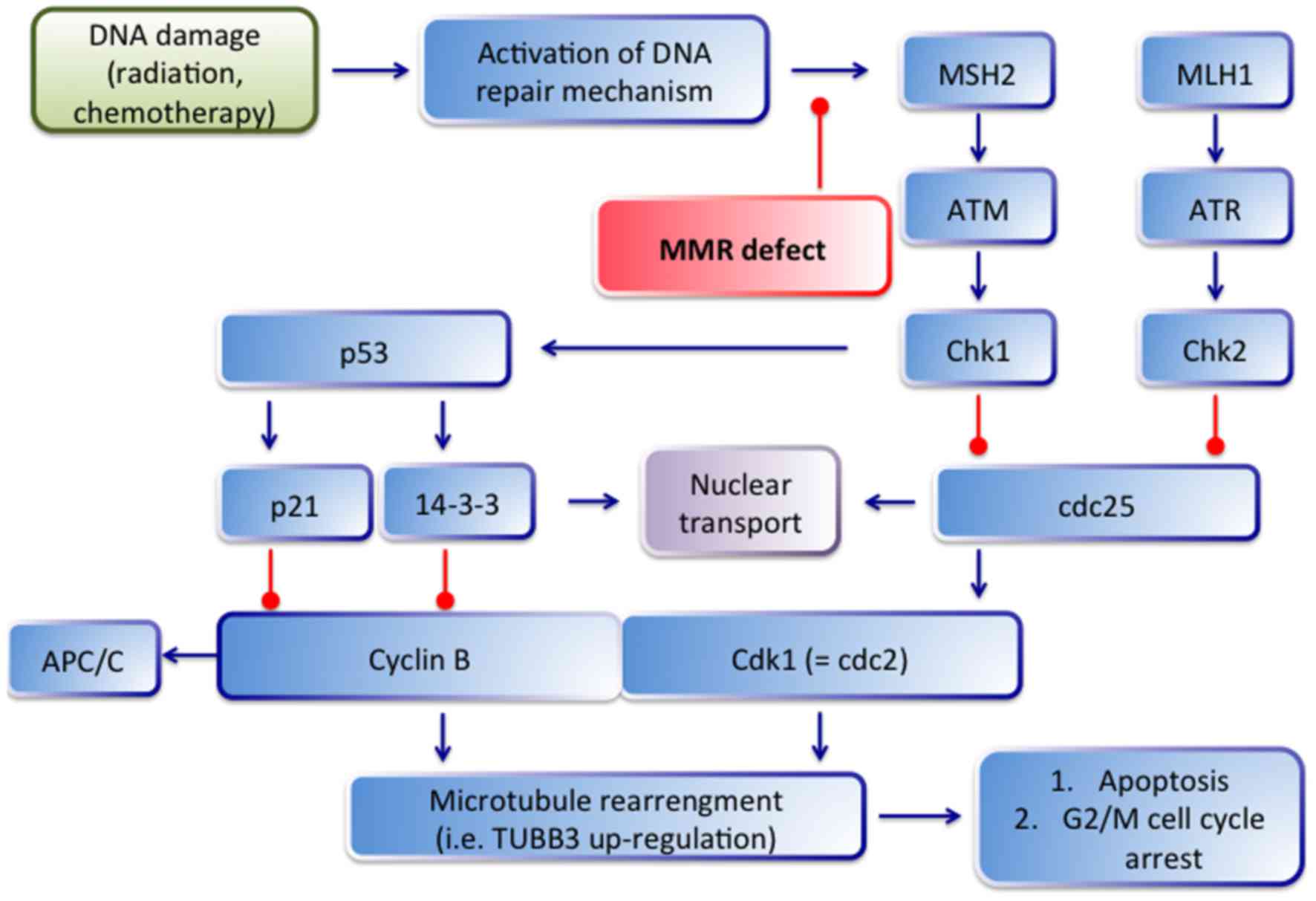
tubulin polymerization and to release of apoptosis (23). The possible connection between
TUBB3, DNA damage and mismatch repair are depicted in Fig. 4. After DNA damage, the ATM/ATR
signaling pathway is activated and phosphorylates (and activates)
Chk1 and Chk2, which subsequently phosphorylate cdc25 (23). Phosporylated cdc25 is sequestered
in the cytoplasm by 14-3-3 proteins, which hinder the activation of
cyclin B1/Cdk1 complex by cdc25 resulting in G2/M cell cycle
arrest. In the case of MMR loss (i.e. in our cases, the loss of
MLH1 and MSH2) the ATM/ATR system cannot be activated by MMR
proteins and finally do not lead to cell cycle arrest.
Consequently, microtubule rearrangement and TUBB3 upregulation is
lacking. This correlation between MLH1/MSH2 and TUBB3 was
statistically significant in our collective (p=0.019 and p=0.012,
respectively).
Furthermore, MLH1 and MSH2 are responsible for
resistance to cisplatin or methylating agents. The defective MMR
system cannot recognize the cisplatin-induced DNA damage resulting
in cell survival and therapeutic resistance (24,25). Taken together, cases with defected
MMR system (micro-satellite instable cancer) and with a high
expression level of TUBB3 are potentially resistant not only to
taxanes, but also to platinum-based therapy. Thus, we favor, in the
case of MSI, immunohistochemical testing also for TUBB3 to exclude
taxane resistance. The interactions between DNA repair systems, MT
and apoptotic proteins (i.e. ARC) should be further investigated to
elucidate the resistance mechanism of tumor cells and the survival
regulatory mechanism.
In addition and alternatively to the above mentioned
mechanisms, the statistically significant correlation between TUBB3
and cytoplasmic ARC expression (p=0.001) can also be explained.
Survival feedback mechanism can induce ARC expression to suppress
pro-apoptotic signaling, thus cancer cells can survive despite of
DNA damage (i.e. microsatellite instability). In our previous
study, we found a strong correlation between ARC expression level
and MSH2 status (16). TUBB3
overexpression can stabilize the MT system and make cancer cells
resistant against anti-microtubule agents. Direct interaction
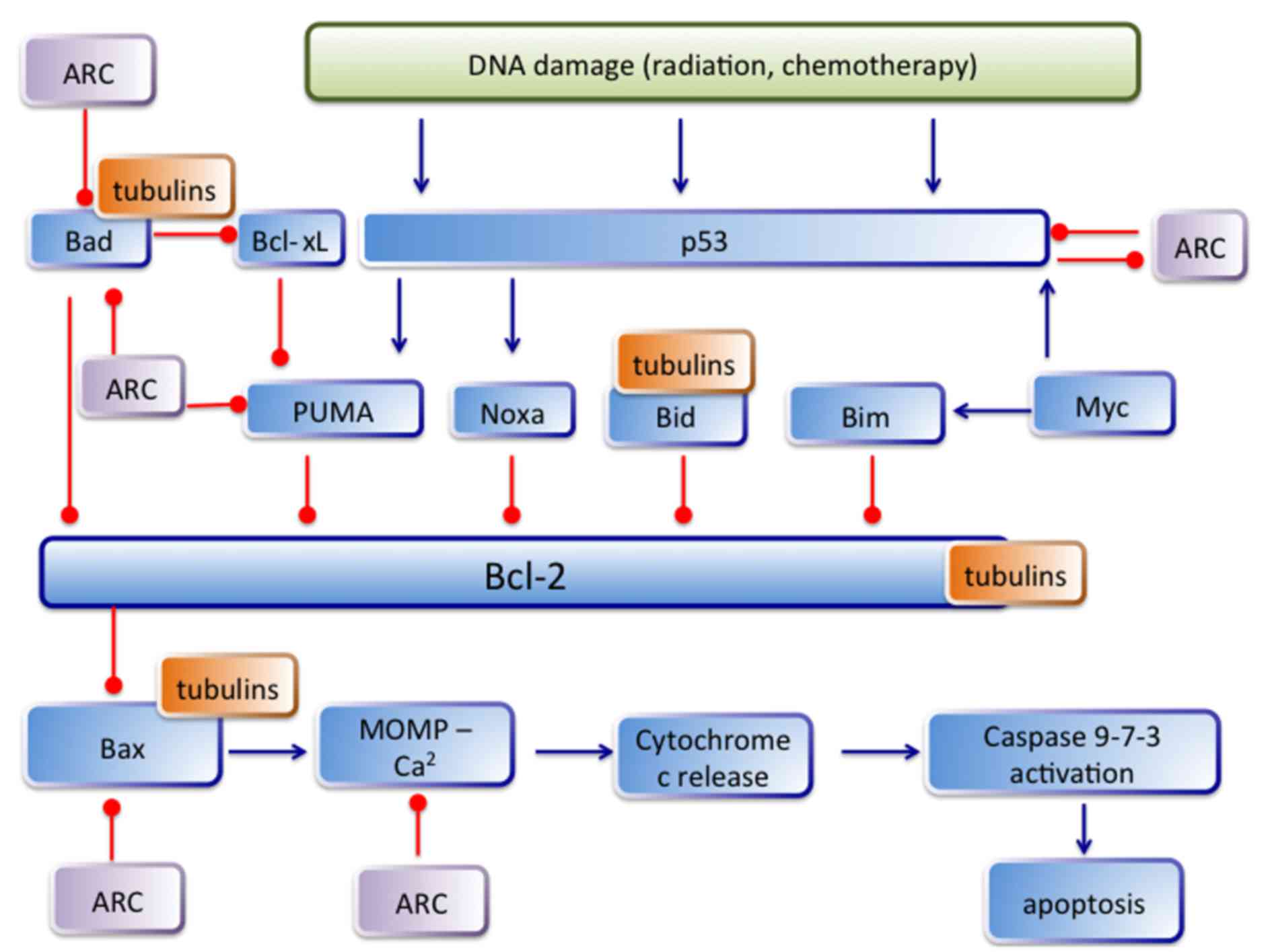
between tubulin with several members of the Bcl-2 family has been
described. Bcl-2, Bid and Bad were found to inhibit the assembly,
whereas Bak and Bax promote tubulin polymerization. Thereby,
tubulin is localized not only in the cytoplasm, but also binds to
mitochondria (associated with VDAC in mitochondrial membrane). Both
pro- and anti-apoptotic proteins bind to tubulin and those of lower
affinity are more easily released following a conformational change
induced by a ligand. Thus, Bcl-2, Bid and Bad may remain bound,
while Bax would be released changing the ratio of free pro- and
anti-apoptotic proteins. Furthermore, in the case of TUBB3
overexpression pro- and anti-apoptotic proteins stay bound, but
tubulin ligands can change the affinity towards proteases. In
addition, Bcl-2 protects against acetylation of tubulin and Bcl-2
is able to normalize the level of acetylated tubulin (26). The interaction between TUBB3 and
apoptotic proteins (especially between ARC and TUBB3) seems to be
more complex. There are many common interaction partners of ARC and
TUBB3 and which protein effects will dominate depends on
intracellular circumstances. The known interactions between
apoptotic proteins, including ARC and TUBB3 are depicted in
Fig. 5. Despite the increasing
number of studies that highlight the importance of TUBB3 in tumor
cells, its mode of action still needs to be fully determined. It
appears that the intrinsic apoptotic pathway is involved as
evidenced by increased caspase-3/7 activity (27). Evidence in other cell types
suggests that TUBB3 may be part of a cell survival pathway. For
instance, its expression level can be modulated by different types
of cell stress, i.e. hypoxia (anti-VEGFR therapy) and nutrient
deprivation (28,29). To confirm the interaction between
ARC and TUBB3, functional studies are needed.
Testing the expression of ERCC1, RRM1 and TUBB3 is
crucial and necessary before treatment for gemcitabine, cisplatin
and 5-FU. As known, MSI tumors will not benefit from 5-FU treatment
and, to this analogy, the testing for ERCC1, RRM1 and TUBB3 before
platinum-based, gemcitabine and taxane therapy, respectively. There
are a lack of diagnostic tests to determine which chemotherapy
regimen offers the greatest chance for response in an individual
patient (18). For metastatic
CRC, the current treatment paradigm consists of 5-FU-based regimens
in combination with either oxaliplatin (FOLFOX) or irinotecan
(FOLFIRI), potentially combined with therapy targeting either EGFR
or VEGFR inhibitor (30). Large,
prospective clinical trials have shown that the response rates for
either FOLFOX or FOLFIRI are only approximately 55% (15). Thus, there is an urgent need for
reliable predictive markers before therapeutic decision in
metastasized CRC.
In conclusion, we found statistically significant
correlations between MMR proteins and ERCC1, RRM1 and TUBB3.
Furthermore, we found a statistically significant correlation
between the apoptosis repressor protein ARC and RRM1 and TUBB3.
Taken together, regarding these proteins, there is a high
therapeutic resistance potential in CRC metastasis. Thus we propose
to test the known associated predictive proteins, before any
therapeutic option is offered. Further functional studies need to
declare the exact regulatory mechanism between RRM1, TUBB3 and ARC,
as exact relations among these proteins cannot be measured by means
of immunohistochemistry alone. The assessment of the abovementioned
markers may be a helpful tool to design chemotherapy protocols for
CRC liver metastasis and to define patients who may expect a
greater clinical benefit. Selection of chemotherapeutic drugs
according to their predicted efficacy should be a part of future
therapeutic decisions and prospective studies. A prospective
validation of these markers is warranted.
References
|
1
|
Siegel R, Desantis C and Jemal A:
Colorectal cancer statistics, 2014. CA Cancer J Clin. 64:104–117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Binefa G, Rodríguez-Moranta F, Teule A and
Medina-Hayas M: Colorectal cancer: From prevention to personalized
medicine. World J Gastroenterol. 20:6786–6808. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Z, Qing Y, Guan W, Li M, Peng Y, Zhang
S, Xiong Y and Wang D: Predictive value of APE1, BRCA1, ERCC1 and
TUBB3 expression in patients with advanced non-small cell lung
cancer (NSCLC) receiving first-line platinum-paclitaxel
chemotherapy. Cancer Chemother Pharmacol. 74:777–786. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ruzzo A, Graziano F, Loupakis F, Rulli E,
Canestrari E, Santini D, Catalano V, Ficarelli R, Maltese P,
Bisonni R, et al: Pharmacogenetic profiling in patients with
advanced colorectal cancer treated with first-line FOLFOX-4
chemotherapy. J Clin Oncol. 25:1247–1254. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Azuma K, Sasada T, Kawahara A, Takamori S,
Hattori S, Ikeda J, Itoh K, Yamada A, Kage M, Kuwano M, et al:
Expression of ERCC1 and class III beta-tubulin in non-small cell
lung cancer patients treated with carboplatin and paclitaxel. Lung
Cancer. 64:326–333. 2009. View Article : Google Scholar
|
|
6
|
Shirota Y, Stoehlmacher J, Brabender J,
Xiong YP, Uetake H, Danenberg KD, Groshen S, Tsao-Wei DD, Danenberg
PV and Lenz HJ: ERCC1 and thymidylate synthase mRNA levels predict
survival for colorectal cancer patients receiving combination
oxaliplatin and fluorouracil chemotherapy. J Clin Oncol.
19:4298–4304. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li P, Fang YJ, Li F, Ou QJ, Chen G and Ma
G: ERCC1, defective mismatch repair status as predictive biomarkers
of survival for stage III colon cancer patients receiving
oxaliplatin-based adjuvant chemotherapy. Br J Cancer.
108:1238–1244. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Akita H, Zheng Z, Takeda Y, Kim C, Kittaka
N, Kobayashi S, Marubashi S, Takemasa I, Nagano H, Dono K, et al:
Significance of RRM1 and ERCC1 expression in resectable pancreatic
adenocarcinoma. Oncogene. 28:2903–2909. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Parker AL, Kavallaris M and McCarroll JA:
Microtubules and their role in cellular stress in cancer. Front
Oncol. 4:1532014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo J, Qiang M and Ludueña RF: The
distribution of β-tubulin isotypes in cultured neurons from
embryonic, newborn, and adult mouse brains. Brain Res. 1420:8–18.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Verdier-Pinard P, Pasquier E, Xiao H, Burd
B, Villard C, Lafitte D, Miller LM, Angeletti RH, Horwitz SB and
Braguer D: Tubulin proteomics: Towards breaking the code. Anal
Biochem. 384:197–206. 2009. View Article : Google Scholar
|
|
12
|
Portyanko A, Kovalev P, Gorgun J and
Cherstvoy E: beta(III)-tubulin at the invasive margin of colorectal
cancer: Possible link to invasion. Virchows Arch. 454:541–548.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Giarnieri E, De Francesco GP, Carico E,
Midiri G, Amanti C, Giacomelli L, Tucci G, Gidaro S, Stroppa I,
Gidaro G, et al: Alpha- and beta-tubulin expression in rectal
cancer development. Anticancer Res. 25:3237–3241. 2005.PubMed/NCBI
|
|
14
|
Carles G, Braguer D, Dumontet C, Bourgarel
V, Gonçalves A, Sarrazin M, Rognoni JB and Briand C:
Differentiation of human colon cancer cells changes the expression
of beta-tubulin isotypes and MAPs. Br J Cancer. 80:1162–1168. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Colucci G, Gebbia V, Paoletti G, Giuliani
F, Caruso M, Gebbia N, Cartenì G, Agostara B, Pezzella G, Manzione
L, et al: Gruppo Oncologico Dell'Italia Meridionale: Phase III
randomized trial of FOLFIRI versus FOLFOX4 in the treatment of
advanced colorectal cancer: A multicenter study of the Gruppo
Oncologico Dell'Italia Meridionale. J Clin Oncol. 23:4866–4875.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tóth C, Meinrath J, Herpel E, Derix J,
Fries J, Buettner R, Schirmacher P and Heikaus S: Expression of the
apoptosis repressor with caspase recruitment domain (ARC) in liver
metastasis of colorectal cancer and its correlation with DNA
mismatch repair proteins and p53. J Cancer Res Clin Oncol.
142:927–935. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grimminger PP, Shi M, Barrett C, Lebwohl
D, Danenberg KD, Brabender J, Vigen CL, Danenberg PV, Winder T and
Lenz HJ: TS and ERCC-1 mRNA expressions and clinical outcome in
patients with metastatic colon cancer in CONFIRM-1 and -2 clinical
trials. Pharmacogenomics J. 12:404–411. 2012. View Article : Google Scholar
|
|
18
|
Choueiri MB, Shen JP, Gross AM, Huang JK,
Ideker T and Fanta P: ERCC1 and TS Expression as Prognostic and
Predictive Biomarkers in Metastatic Colon Cancer. PLoS One.
10:e01268982015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Umar A, Boland CR, Terdiman JP, Syngal S,
de la Chapelle A, Rüschoff J, Fishel R, Lindor NM, Burgart LJ,
Hamelin R, et al: Revised Bethesda Guidelines for hereditary
nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite
instability. J Natl Cancer Inst. 96:261–268. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ting S1, Mairinger FD, Hager T, Welter S,
Eberhardt WE, Wohlschlaeger J, Schmid KW and Christoph DC: ERCC1,
MLH1, MSH2, MSH6, and betaIII-tubulin: resistance proteins
associated with response and outcome to platinum-based chemotherapy
in malignant pleural mesothelioma. Clin Lung Cancer. 14:558–567.e3.
2013. View Article : Google Scholar
|
|
21
|
Yu Y, Wang Y, Ren X, Tsuyada A, Li A, Liu
LJ and Wang SE: Context-dependent bidirectional regulation of the
MutS homolog 2 by transforming growth factor β contributes to
chemoresistance in breast cancer cells. Mol Cancer Res.
8:1633–1642. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ohtaka K, Kohya N, Sato K, Kitajima Y, Ide
T, Mitsuno M and Miyazaki K: Ribonucleotide reductase subunit M1 is
a possible chemoresistance marker to gemcitabine in biliary tract
carcinoma. Oncol Rep. 20:279–286. 2008.PubMed/NCBI
|
|
23
|
Porter LA and Lee JM: Alpha-, beta-, and
gamma-tubulin polymerization in response to DNA damage. Exp Cell
Res. 270:151–158. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hassen S, Ali N and Chowdhury P: Molecular
signaling mechanisms of apoptosis in hereditary non-polyposis
colorectal cancer. World J Gastrointest Pathophysiol. 3:71–79.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Martin LP, Hamilton TC and Schilder RJ:
Platinum resistance: The role of DNA repair pathways. Clin Cancer
Res. 14:1291–1295. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Knipling L and Wolff J: Direct interaction
of Bcl-2 proteins with tubulin. Biochem Biophys Res Commun.
341:433–439. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McCarroll JA, Sharbeen G, Liu J, Youkhana
J, Goldstein D, McCarthy N, Limbri LF, Dischl D, Ceyhan GO, Erkan
M, et al: βIII-tubulin: A novel mediator of chemoresistance and
metastases in pancreatic cancer. Oncotarget. 6:2235–2249. 2015.
View Article : Google Scholar
|
|
28
|
Raspaglio G, Filippetti F, Prislei S,
Penci R, De Maria I, Cicchillitti L, Mozzetti S, Scambia G and
Ferlini C: Hypoxia induces class III beta-tubulin gene expression
by HIF-1alpha binding to its 3′ flanking region. Gene. 409:100–108.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Raspaglio G, De Maria I, Filippetti F,
Martinelli E, Zannoni GF, Prislei S, Ferrandina G, Shahabi S,
Scambia G and Ferlini C: HUR regulates beta-tubulin isotype
expression in ovarian cancer. Cancer Res. 70:5891–5900. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Van Cutsem E, Cervantes A and Nordlinger
B: Metastatic colorectal cancer: ESMO Clinical Practice Guidelines
for diagnosis, treatment and follow-up. Ann Oncol. 25(Suppl 3):
iii1–iii9. 2014. View Article : Google Scholar : PubMed/NCBI
|