Introduction
Extrinsic aging is caused by environmental oxidative
factors primarily resulting from exposure to ultraviolet (UV)
radiation (1,2). Skin degeneration by UV radiation is
a cumulative process, and the degeneration rate depends on the
frequency, duration and intensity of solar exposure, as well as the
natural protection provided by skin pigmentation (2). UVB radiation penetrates the
superficial layers of the skin down to the basal layer of the
epidermis, where it generates harmful reactive oxygen and nitrogen
species (ROS and RNS, respectively) and subsequently induces
inflammation and sunburn and precipitates skin aging (3).
Oxidative stress plays a synergistic role in
UV-induced skin damage. ROS can be induced by UV radiation, and the
accumulation of ROS eventually causes inflammation and wrinkle
formation in the skin (4).
Increased ROS levels induce the production of matrix
metalloproteinases (MMPs) and lead to skin damage, subsequently
causing skin photoaging (5).
Thus, MMPs are used as major markers of UVB-induced wrinkle
formation and skin inflammation; additionally, their expression can
result in increased skin aging, including the formation of deep
wrinkles and thickening of the dermis and epidermis (6).
UVB-induced inflammatory responses involve an
increase in pro-inflammatory cytokines, including tumor necrosis
factor-α (TNF-α), interleukin-1β (IL-1β) and IL-6, all of which
accelerate skin damage and result in MMP activation (7,8).
There is considerable evidence that UV-induced oxidative stress
mediates the phosphorylation or activation of protein kinases such
as mitogen-activated protein kinases (MAPKs) through a series of
cascades (9). UVB-induced MAPK
phosphorylation has been implicated in various skin diseases,
including skin cancer and photoaging (10).
Garcinia mangostana (G. mangostana) L.
(mangosteen) is native to Southeast Asia, and its fruits, which are
famous for their remarkably pleasant flavor, have become a popular
botanical dietary supplement because of their perceived role in
promoting overall health (11,12). Various parts of G.
mangostana, mostly the fruit hull, bark and roots, have been
used in Southeast Asia for hundreds of years in herbal medicine to
treat a wide variety of medical conditions, including diarrhea,
infected wounds and abdominal pain (11,13). Mangosteen fruits are rich in
phenolic acids, xanthones, anthocyanins and condensed tannins,
including α-mangostin, β-mangostin, γ-mangostin and gartanin
(14,15). The most abundant xanthones in
mangosteen fruits are α-mangostin and γ-mangostin, also other
xanthones in mangosteen include β-mangostin, gartanin and among
others (15). α-mangostin is the
major compound that used as traditional medicine for various
activities containing anti-inflammatory, antioxidant and
antibacterial effects (16,17). Gartanin is a polyphenolic
xanthones was observed to have anticancer activities on human
bladder cancer cells (18). To
the best of the authors' knowledge, anti-wrinkle activity of
Garcinia mangostana L. extracts (GME) and its compounds by
oral administration in hairless mice was not studied.
In a previous study, mangosteen was reported to
contain a variety of bioactive compounds with potential
applications as therapeutic agents or functional food additives
(19,20). In the current study, the authors
isolated α-mangostin, β-mangostin and γ-mangostin, as well as
gartanin from GME and investigated whether these compounds could
protect skin from UVB-induced damage.
Materials and methods
Preparation of α-mangostin, β-mangostin,
γ-mangostin and gartanin
The compounds α-mangostin, β-mangostin, γ-mangostin
and gartanin, which were used in the present in vitro study,
were obtained from a previous study (21). Large-scale isolation of
α-mangostin for the in vivo experiments was conducted as
follows: Dried pericarps from G. mangostana (7.29 kg) were
extracted three times with ethanol at room temperature to obtain
351 g of solid extract. This extract was suspended in water and
then aliquoted into hexane, chloroform, ethyl acetate, n-butanol
and water, resulting in hexane-soluble (12 g), chloroform-soluble
(213 g), ethyl acetate-soluble (15 g), n-butanol-soluble (81 g) and
aqueous (126 g) extracts. The chloroform-soluble extract (200 g)
was chromatographed on a silica gel column (10×60 cm, 2,000 g)
using a gradient mixture of hexane and EtOAc (100:0 to 2:1)
followed by a gradient mixture of CHCl3 and MeOH (20:1
to 1:1) to produce 21 fractions (GMC-A to GMC-U). The GMC-I
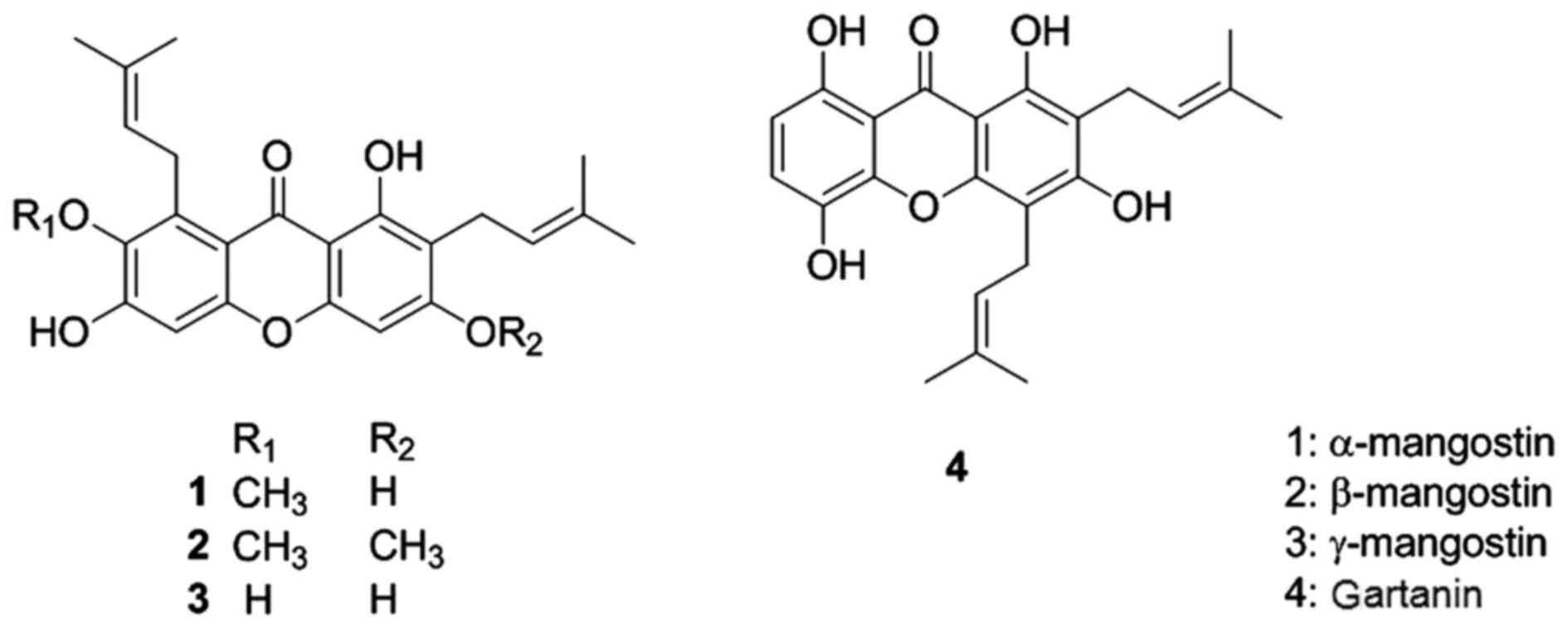
fraction yielded 45 g of α-mangostin. The structures of
α-mangostin, β-mangostin, γ-mangostin and gartanin are presented in
Fig. 1.
Cell culture and UVB irradiation
An immortalized, non-tumorigenic human keratinocyte
cell line (HaCaT) was maintained in Dulbecco's modified eagle's
medium (Life Technologies, Gibco-BRL Products, Rockville, MD, USA)
supplemented with 10% fetal bovine serum (FBS) and 1% antibiotics
at 37°C in a humidified incubator containing 5% CO2.
Cells (1×104) were seeded, allowed to adhere for 24 h,
and treated with various concentrations of GME and the purified
compounds prior to exposure to UVB radiation at a dose of 20
mJ/cm2. Control cells received neither GME nor purified
compounds and were not exposed to UVB radiation. Immediately
following UVB irradiation, the cell viability was assessed by
incubating the cells with MTS (Promega, Madison, WI, USA) for 1 h,
and the MTS reduction to formazan was measured according to the
manufacturer's instructions. The absorbance of the samples was
measured at 490 nm using a microplate fluorimeter (Molecular
Devices, LLC, Sunnyvale, CA, USA).
Determination of MMP-1 and MMP-9
secretion using enzyme-linked immunosorbent assay (ELISA)
The MMP-1 and MMP-9 levels in the culture media of
HaCaT cells (5×104) exposed to UVB radiation were
determined using human total MMP-1 and MMP-9 ELISA kits (R&D
Systems, Minneapolis, MN, USA) according to the manufacturer's
instructions. Briefly, HaCaT cells were seeded in 96-well plates
and treated with GME or the purified compounds. Following exposure
to UVB radiation, the cell culture supernatants were collected and
centrifuged at 189 × g for 5 min. The levels of MMP-1 and MMP-9
were quantified using colorimetric analysis using a microplate
fluorimeter (Molecular Devices, LLC).
Experimental animals
HR-1 hairless male mice (weighing 20–23 g, 6 weeks
old; Center Laboratory Animal Inc., Seoul, Korea) were stabilized
for one week before the study. The animals were maintained at a
temperature of 24°C and 50% relative humidity with 12 h light/dark
cycles and provided free access to food and water. All animal
procedures were performed in accordance to the guidelines for the
Care and Use of Laboratory Animals developed by the Institute of
Laboratory Animal Resources of the National Research Council, and
were approved by the Institutional Animal Care and Use Committee of
Daejeon University in Daejeon, Korea. The mice were divided into
control (n=5), UVB-irradiated vehicle (n=5) and UVB-irradiated
α-mangostin (n=5) groups. The mice from the α-mangostin group were
orally administered 0.1 ml water containing 100 mg/kg α-mangostin
daily prior to UVB irradiation. Normal drinking water was supplied
to the animals in the vehicle group, whereas the unexposed control
group was not UV irradiated and received untreated water.
UVB irradiation
Mice were subjected to UVB irradiation using an
UVM-225D Mineralight UV Display lamp (UVP, Inc., Upland, CA, USA),
which emitted radiation at a wavelength of 302 nm. The UV intensity
was measured using an HD2102-2 UV meter (Delta Ohm, Padova, Italy).
For the in vivo experiments, UVB radiation was applied to
the backs of the mice three times a week for 12 weeks. The
radiation dose was progressively increased from 60
mJ/cm2 per exposure at week 1 (one minimal erythematous
dose = 60 mJ/cm2) to 90 mJ/cm2 per exposure
at week 7.
Histological examination
Isolated mouse skin tissue was fixed in 10% neutral
buffered formalin, embedded in paraffin, and sliced into 5
µm sections, which were stained with hematoxylin and eosin.
The epidermal thickness was measured under light microscopy with an
eyepiece micrometer.
Antioxidant enzyme activities
Superoxide dismutase (SOD) and catalase (CAT)
activities were measured using a colorimetric assay kit (SOD: cat.
no. 706002, CAT: cat. no. 707002; Cayman Chemical Co., Ann Arbor,
MI, USA) according to the manufacturer's protocol. For protein
extraction, skin tissue was homogenized in cold lysis RIPA buffer
(Thermo Fisher Scientific, San Jose, CA, USA). The absorbance was
measured at 450 and 540 nm to determine the SOD and CAT activity,
respectively, using a plate reader (Molecular Devices, LLC).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from skin tissue from
UVB-irradiated mice using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) according to the manufacturer's
protocol. RT-qPCR was performed using TaqMan assays (Applied
Biosystems; Thermo Fisher Scientific, Inc.) specific for
involucrin, loricrin, filaggrin, IL-1β, IL-6 and TNF-α on a
QuantStudio™ 6 Flex Real-Time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). Each sample was assayed in
triplicate, and the relative mRNA expression levels were calculated
using the ΔΔCt method and normalized to the β-actin mRNA levels in
each sample (22).
Determination of MMP-1 and MMP-9
secretion by ELISA
MMP-1 and MMP-9 levels in the skin tissue after UVB
irradiation were determined using total MMP-1 (cat. no. DMP100) and
MMP-9 (cat. no. DMP900) ELISA kits according to the manufacturer's
instructions. The levels of MMP-1 and MMP-9 were quantified by
colorimetric analysis.
Western blotting
The protein content in the supernatant was
determined using a Bio-Rad protein assay kit (Bio-Rad, Hercules,
CA, USA). The protein lysate was separated using sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 10% gel,
and the proteins were transferred to polyvinylidene difluoride
membranes and then blocked with blocking buffer (ATTO, Tokyo,
Japan), which were incubated with specific primary antibodies
targeting MMP-1 (1:1,000 dilution, ab137332; Abcam, Cambridge, MA,
USA), MMP-9 (1:1,000 dilution, cat. no. 3852), p-ERK (1:1,000
dilution, cat. no. 4370), ERK (1:1,000 dilution, cat. no. 9102),
p-p38 (1:1,000 dilution, cat. no. 9211), p38 (1:1,000 dilution,
cat. no. 9212), p-JNK (1:1,000 dilution, cat. no. 9251), JNK
(1:1,000 dilution, cat. no. 9252), and β-actin (1:1,000 dilution,
cat. no. 4970) (all from Cell Signaling Technology, Beverly, MA,
USA) at 4°C overnight. The blots were washed for 10 min three times
with phosphate-buffered saline (PBS) containing 0.1% Tween-20 and
then incubated for 2 h with the appropriate anti-rabbit secondary
antibody (1:5,000 dilution, SC-2004; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA). Protein bands were detected using an
enhanced chemiluminescence solution (Bio-Rad) western blot
detection system (LAS-4000; Fuji, Tokyo, Japan).
Statistic analysis
All measurements were completed in triplicate, and
the data are presented as the mean ± standard error of the mean.
The results were subjected to analysis of variance using Tukey's
multiple comparison test to analyze the differences, and P<0.05
was considered to indicate a statistically significant
difference.
Results
Protective effects of GME and purified
compounds against UVB-induced damage in HaCaT cells
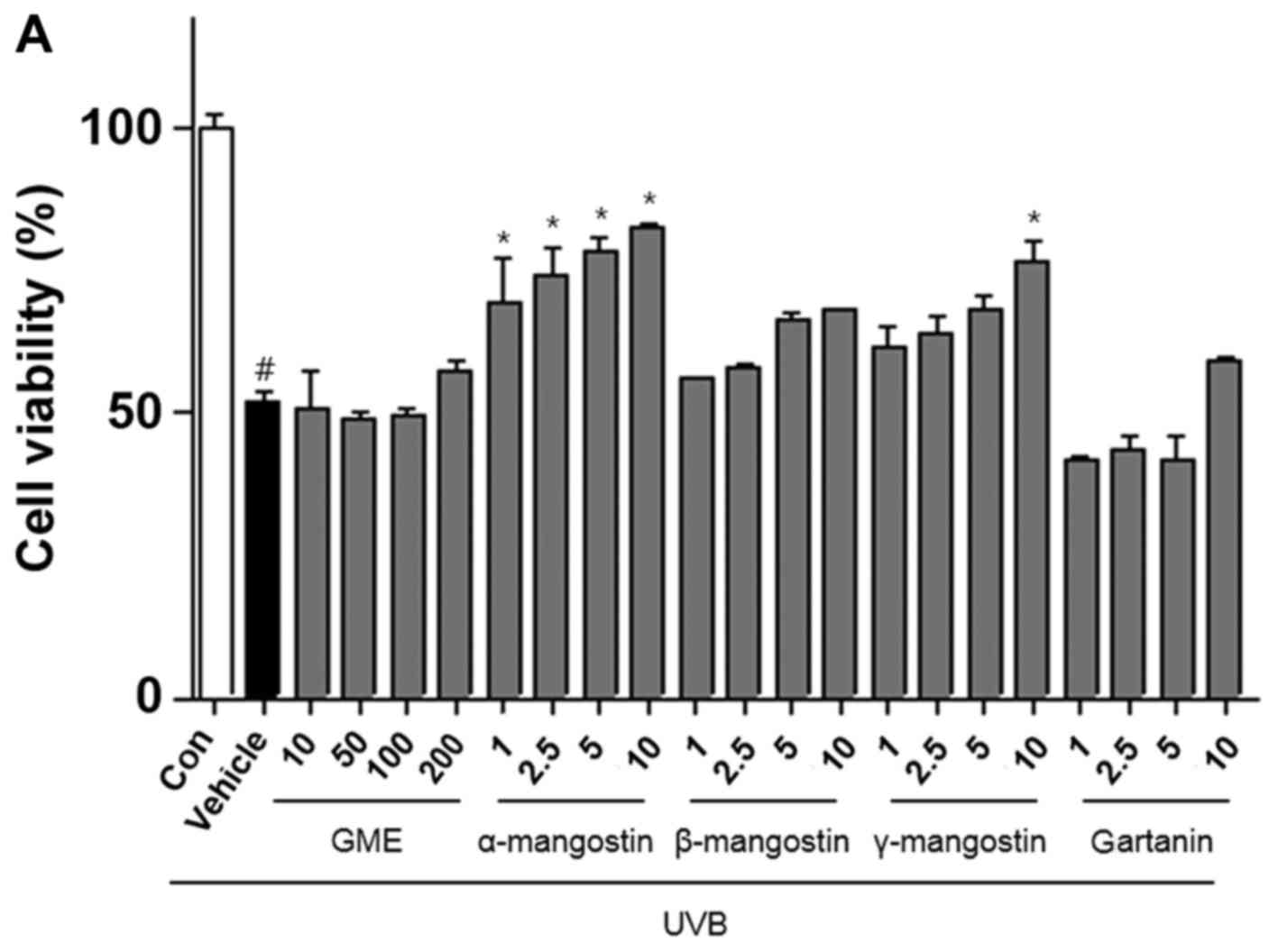
The authors investigated the effect of GME and the
purified compounds on HaCaT cell proliferation after UVB exposure.
Cell viability was reduced to 51.7% by UVB irradiation in untreated
cells but increased to 82.6% (Fig.
2A) in the presence of α-mangostin.
Effects of GME and purified compounds on
UVB-induced secretion of MMP-1 and MMP-9
The authors evaluated the effect of GME and the
purified compounds on UVB-induced MMP expression in HaCaT cells.
UVB radiation resulted in a marked increase in MMP-1 and MMP-9
levels compared with non-irradiated cells (Fig. 2B and C). The ELISA analysis
revealed that GME and the individual compound treatments reduced
the MMP-1 and MMP-9 protein levels in the culture media of HaCaT
cells in a dose-dependent manner.
Effects of GME and compounds on
involucrin, filaggrin, and loricrin levels in UVB-irradiated HaCaT
cells
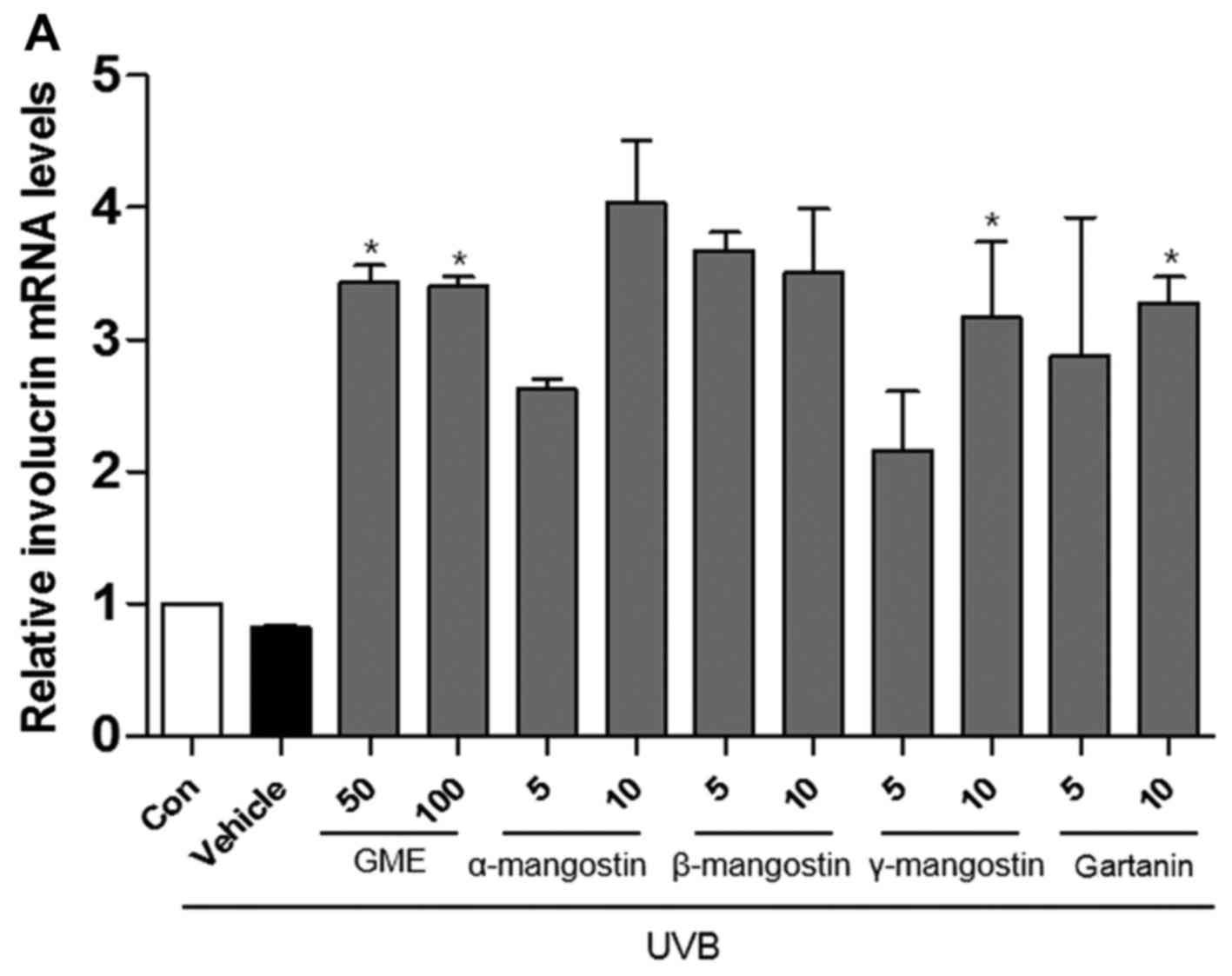
The results indicated that the expression of
involucrin, filaggrin and loricrin was decreased in UVB-irradiated
HaCaT cells compared with non-irradiated cells. As presented in
Fig. 3, the mRNA expression
levels of involucrin, filaggrin and loricrin in UVB-irradiated
cells treated with GME or the purified compounds were increased
compared to the untreated cells exposed to UVB.
α-mangostin inhibits UVB-induced wrinkle
formation in hairless mice
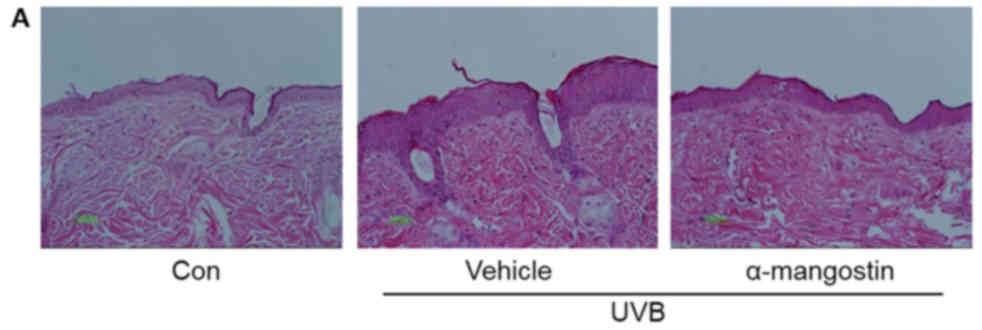
Because α-mangostin was the most effective compound
in the in vitro studies, the authors investigated the effect
of α-mangostin on UVB-induced wrinkle formation by repeatedly
exposed hairless mouse skin to UVB for 12 weeks. Histological
evaluation shows that this exposure induced skin wrinkles, which
was blocked by α-mangostin (Fig.
4). To evaluate the effect of α-mangostin on skin thickening,
the epidermal thickness was measured. The epidermal thickness of
the UVB-irradiated α-mangostin-treated group was 118.7 vs. 55.0
µm in the UVB-irradiated vehicle-treated group.
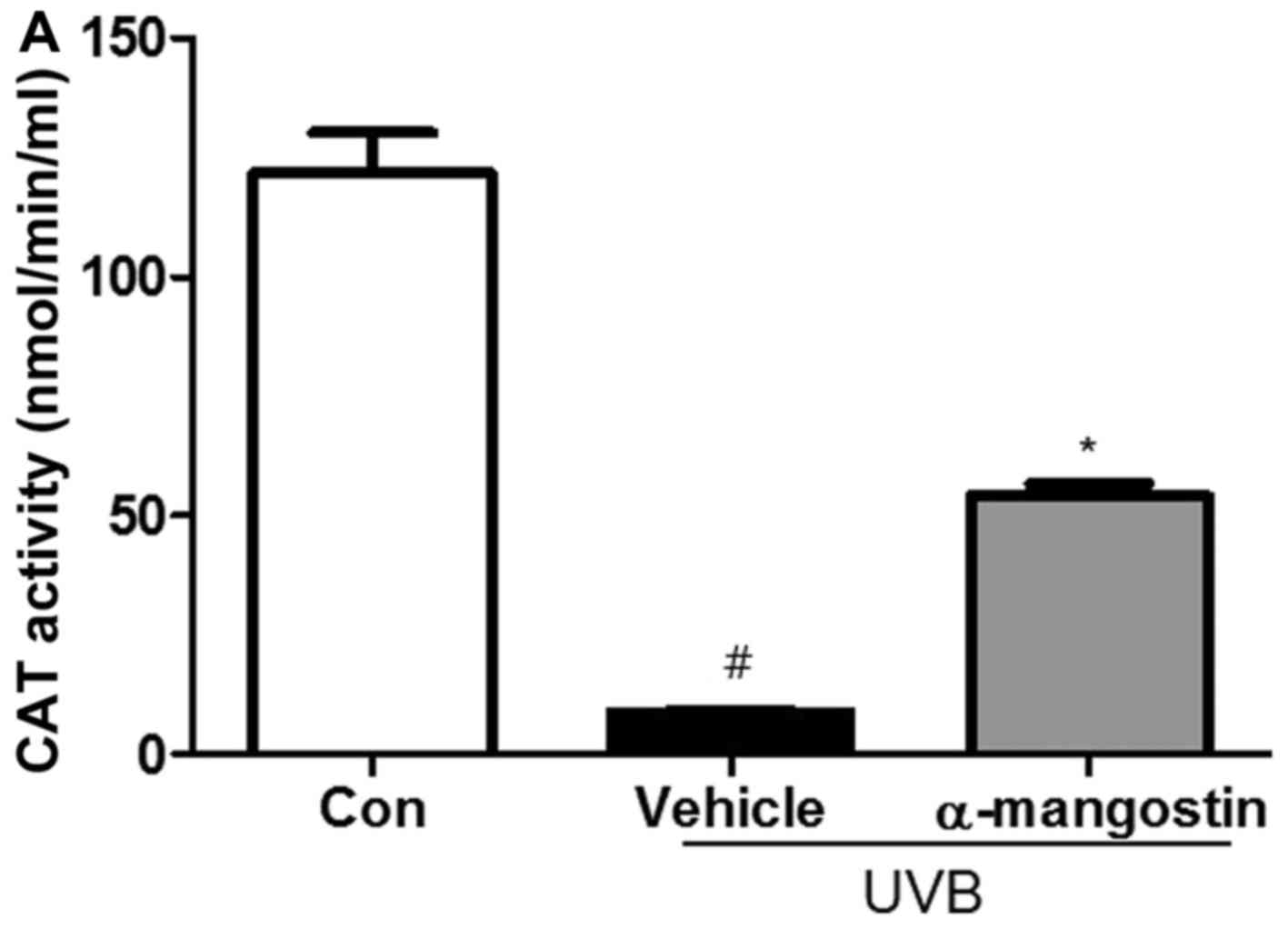
Effects of α-mangostin on the activity of
antioxidant enzymes in UVB-irradiated hairless mice
To investigate whether the radical-scavenging
activity of α-mangostin was mediated by antioxidant enzymes, the
activities of well-known antioxidant enzymes were examined in
hairless mice exposed to UVB radiation. The SOD activity was
decreased in the UVB-irradiated hairless mice compared to unexposed
control mice (Fig. 5) but was
enhanced by α-mangostin treatment in a dose-dependent manner.
Furthermore, compared with the control group, the UVB-irradiated
group exhibited decreased CAT activity, but treatment with
α-mangostin ameliorated this loss of CAT activity. These results
indicated that α-mangostin could protect the activities of
antioxidant enzymes, which scavenge free radicals, and thereby
further inhibit UVB-induced oxidative stress.
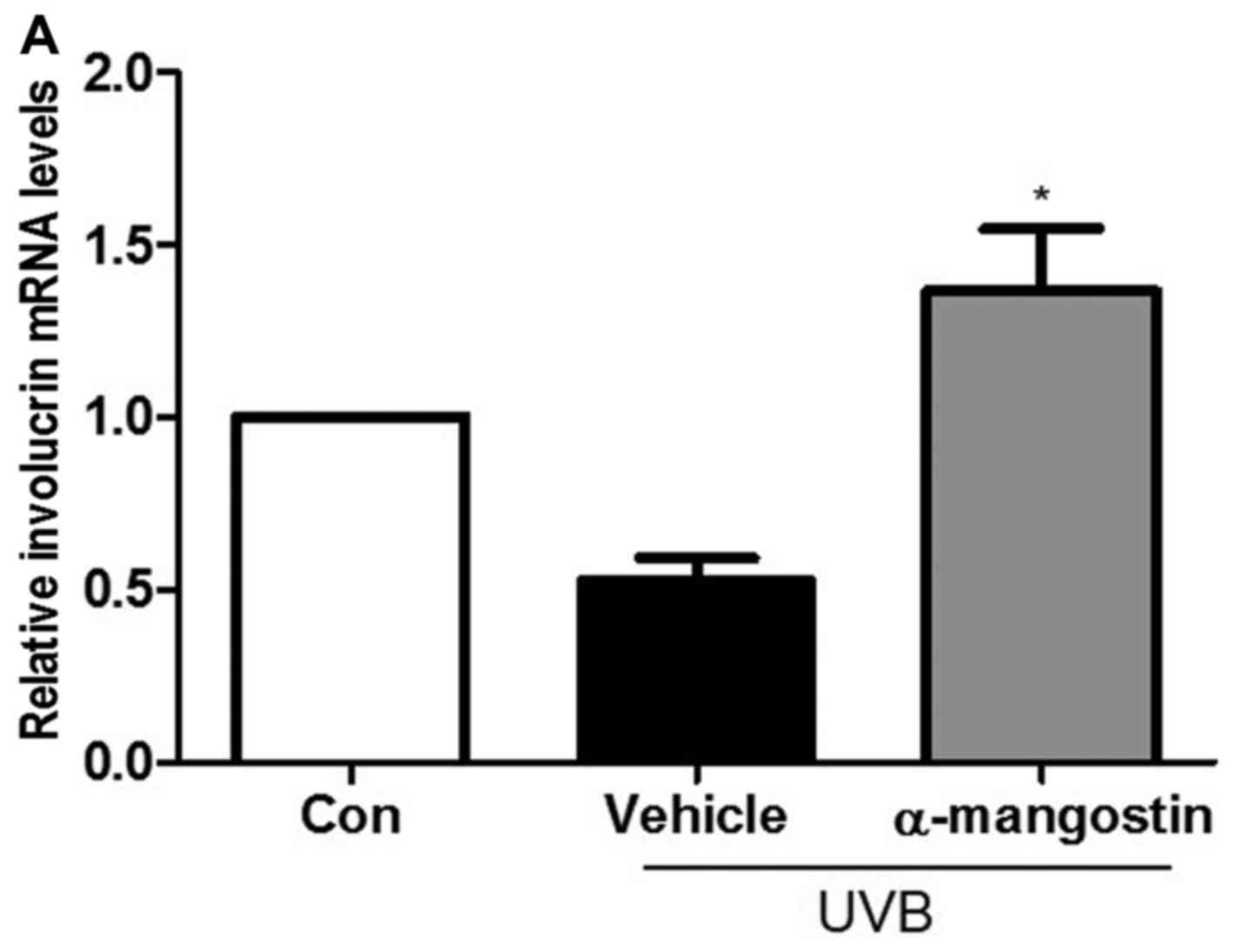
Effects of α-mangostin on involucrin,
filaggrin, and loricrin expression in UVB-irradiated hairless
mice
The authors indicated that the expression of
involucrin, filaggrin and loricrin decreased in the UVB-irradiated
group compared to the unexposed control group. As shown in Fig. 6, the mRNA expression levels of
involucrin, filaggrin and loricrin in the UVB-irradiated hairless
mice treated with α-mangostin were increased compared to the
UVB-irradiated vehicle-treated mice.
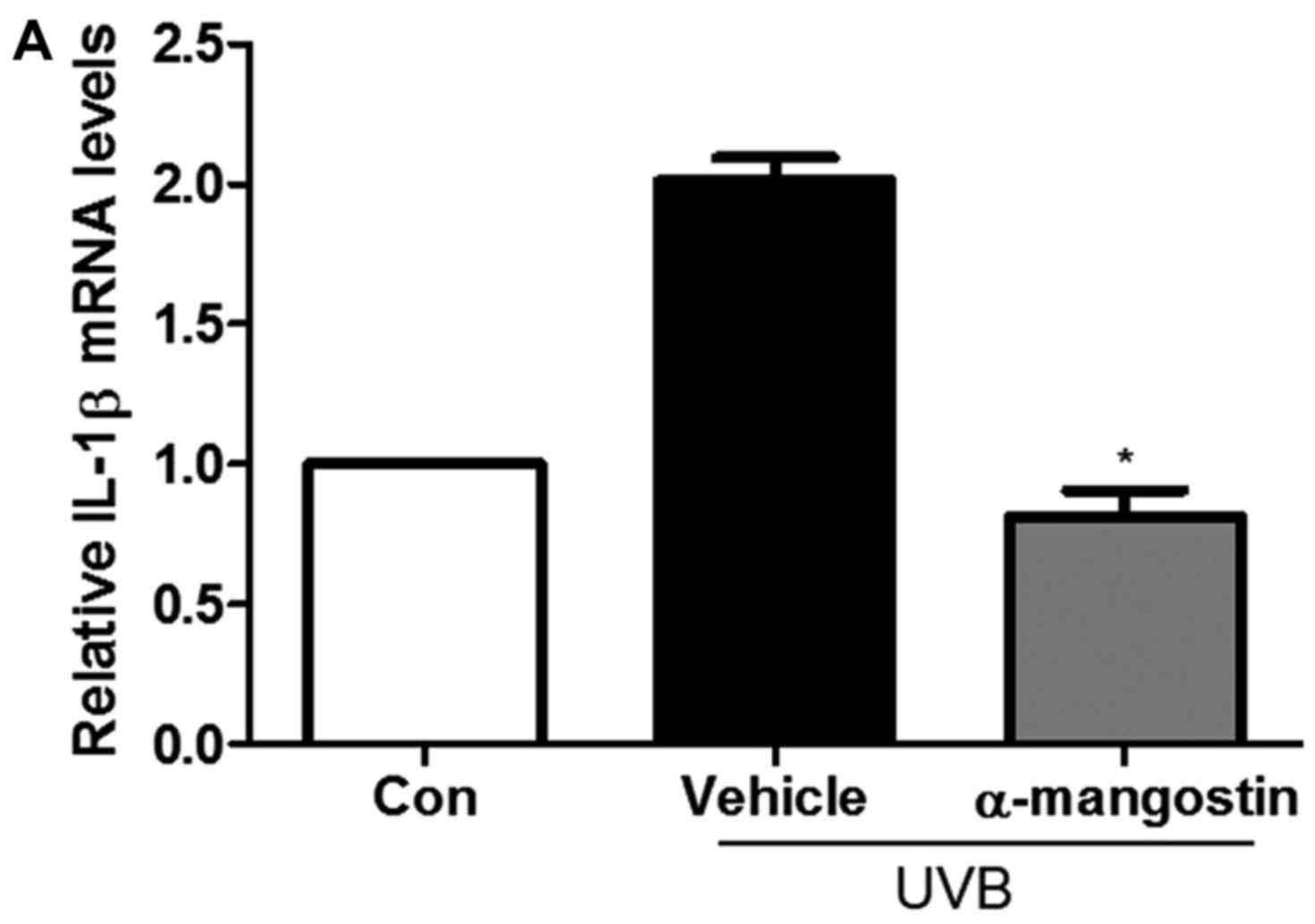
Effect of α-mangostin on the release of
inflammatory cytokines in hairless mice
To assess the regulatory effect of α-mangostin on
the production of pro-inflammatory cytokines in UVB-irradiated
hairless mice, the mRNA expression of pro-inflammatory cytokines
was investigated. As presented in Fig. 7, the IL-1β, IL-6 and TNF-α mRNA
levels were increased in mice exposed to UVB compared to the
unexposed control; however, this increase was suppressed by
treatment with α-mangostin. This suggested that α-mangostin exerted
a photoprotective effect by reducing the inflammatory response to
UVB radiation.
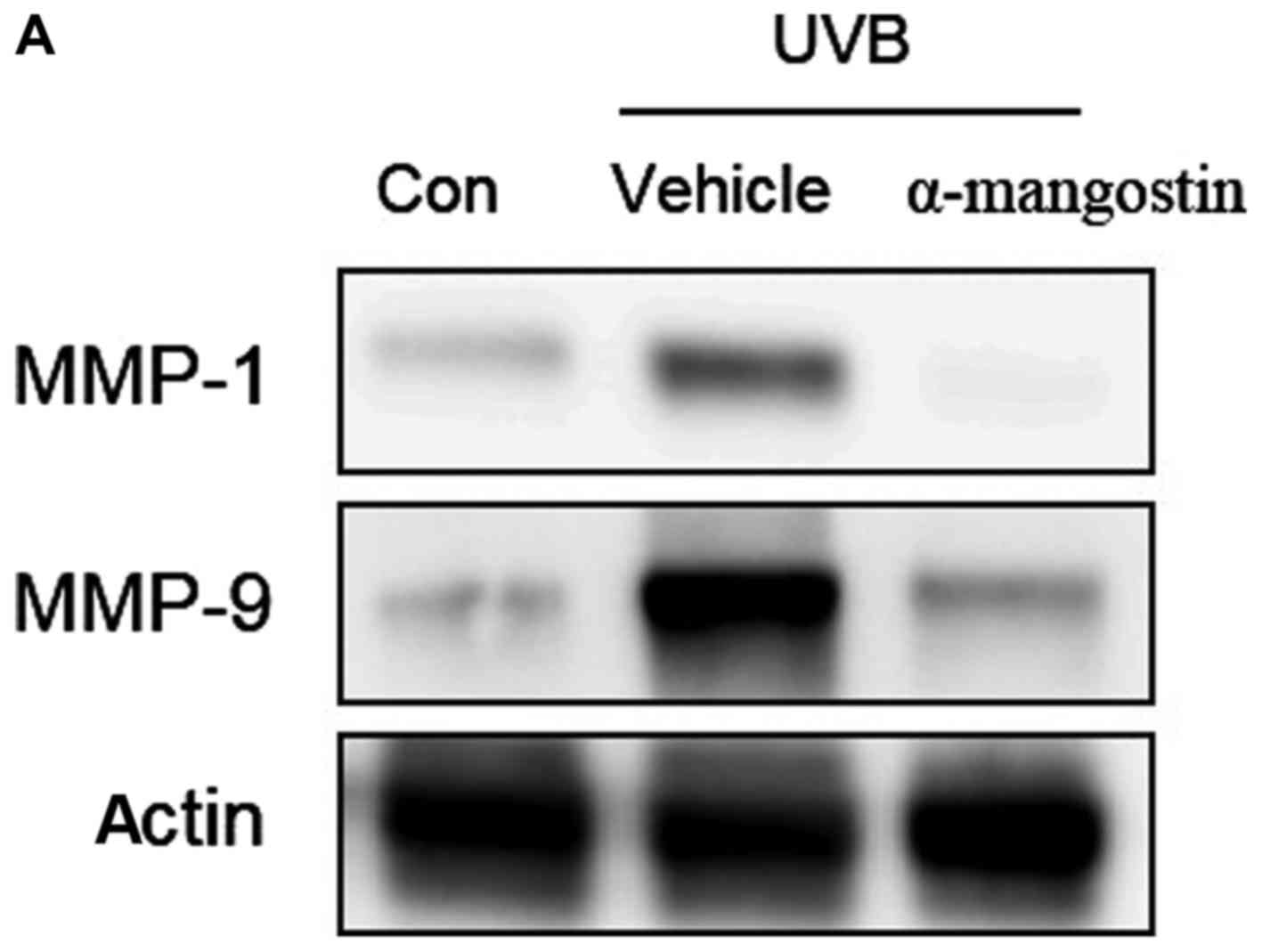
Effects of α-mangostin on MAPK
phosphorylation in UVB-irradiated hairless mice
Using western blotting, the authors assessed the
effect of oral administration of α-mangostin on the activation of
MAPK family proteins and downstream MMP-1 and MMP-9 proteins in
hairless mouse skin exposed to UVB irradiation (Fig. 8A). UVB irradiation increased the
skin expression of MMP-1 and MMP-9 levels in hairless mice.
Treatment with α-mangostin reduced the expression of MMP-1 and
MMP-9 (Fig. 8B and C). The
authors examined whether α-mangostin could suppress the UVB-induced
phosphorylation of MAPKs, including ERK, p38 and JNK. As presented
in Fig. 8D, UVB irradiation
resulted in the phosphorylation of ERK, p38 and JNK, whereas
treatment of mice with α-mangostin prior to UVB irradiation
inhibited the UVB-mediated phosphorylation of these MAPKs.
Discussion
Acute UV exposure on human skin causes sunburn,
altered pigmentation, inflammation, immune suppression, and dermal
connective tissue damage (3). UV
irradiation of the skin enhances collagenase activity and
contributes to wrinkle formation via collagen degradation in the
dermal extracellular matrix (ECM) (23). The authors investigated the
effects of UVB exposure on skin aging in a hairless mouse model,
specifically, its effects on the development of skin wrinkles. It
was identified that extended UVB radiation induced skin wrinkles in
hairless mice after 12 weeks.
Previous pharmacological studies of G.
mangostana suggested numerous beneficial effects of the
mangosteen, such as anti-acne, anti-adipogenic, antibacterial,
anticancer, anti-obesity and antioxidative effects (21,24,25). Thus, the effects of α-mangostin on
skin aging were investigated in the same hairless mouse model,
especially regarding the development of skin wrinkles.
UVB-induced wrinkle formation is mediated by a
complex mechanism involving ECM damage, cell death and the
consequent inflammatory responses in both the epidermis and dermis
(26). Histological and
ultrastructural studies have revealed that photodamaged skin is
associated with increased epidermal thickness and alterations in
connective tissue organization (27). Epidermal thickness is often used
as a parameter to quantitatively evaluate skin photoaging because
epidermal hypertrophy is thought to cause wrinkle formation
(28). The thickness of the basal
membrane in photodamaged skin is increased, possibly reflecting
damage of basal keratinocytes. Furthermore, the distribution of
melanocytes along the basal membrane is irregular, and these cells
vary widely in size, dendricity and pigmentation (29). Also, a cornified cell envelope,
which is composed of involucrin, loricrin and filaggrin is a
component of differentiated epidermal keratinocytes and
corneocytes, and it is important in the skin barrier and moisture
(30). The correct formation of
the cornified cell envelope is essential for the barrier function
of the skin then barrier inhibits the TEWL and associated loss of
solutes (31). In the current
study, the mRNA expression levels of involucrin, filaggrin and
loricrin in UVB-irradiated cells treated with GME or the purified
compounds were increased compared to cells only receiving UVB
irradiation. In addition, the epidermal thickness of the dorsal
skin of the mice was increased upon UVB exposure but was
significantly reduced in mice administered α-mangostin prior to UVB
exposure.
UV irradiation of the skin increases the levels of
hydrogen peroxide, other ROS, and antioxidant enzymes (32). Increased ROS production alters the
structure and function of multiple genes and protein, leading to
skin damage (11). Although ROS
are part of normal regulatory circuits and the cellular redox state
is tightly controlled by antioxidants, an increased ROS load and
the loss of cellular redox homeostasis can promote carcinogenesis
and photoaging (33). In the
present study, the results revealed that α-mangostin could protect
the activities of antioxidant enzymes, which scavenge free
radicals, thereby further inhibiting UVB-induced oxidative
stress.
Upregulated MMP-1 expression following UV
irradiation results in collagen degradation, the histopathological
hallmark of photoaging (34). A
wealth of evidence has indicated that MMP induction plays a major
role in the pathogenesis of photoaging (35). MMP-1 initiates the degradation of
type I and III collagens and further degrades the collagen
fragments generated by collagenases (6). Chronic UV irradiation of human skin
results in elevated MMP expression, leading to marked degenerative
changes in the upper dermal connective tissue via degradation of
elastic and collagen fibers (36). In the current study, treatment
with either GME or the purified compounds reduced the MMP-1 and
MMP-9 expression levels.
UV radiation stimulates and activates various cells
to produce and release cytokines, which likely play a significant
role in the process of photoaging (37), and produces free radicals that
release pro-inflammatory cytokines and growth factors, which
activate proteases that degrade collagen and elastin. This
eventually results in structural and functional changes in the ECM
(38). UVB radiation triggers
cutaneous inflammatory responses by directly inducing epidermal
keratinocytes to produce specific pro-inflammatory cytokines,
including TNF-α, IL-1β and IL-6 and cyclooxygenase-2 (39,40). In the current study, the effect of
GME was examined and purified compounds on HaCaT cells as well as
of α-mangostin on UVB-induced pro-inflammatory cytokine production
in UVB-irradiated hairless mouse skin; the results indicated that
the inhibitory effect of α-mangostin on the production of
inflammatory mediators was accompanied by reduced mRNA expression
levels of IL-1β, IL-6 and TNF-α.
UVB irradiation has been shown to stimulate ROS
production and activate MAPKs (41), including ERK1/2, JNK and p38 MAPK
(42). In the study, α-mangostin
inhibited the UVB-induced activation of JNK, p38 and ERK.
In conclusion, GME and the purified compounds from
G. mangostana L. could effectively reduce skin damage.
Specifically, α-mangostin ameliorated wrinkling processes induced
by UVB irradiation of a hairless mouse model, as indicated by
histological examination, increased SOD and CAT activities, and
decreased MMP expression; furthermore, α-mangostin could reduce the
protein levels of pro-inflammatory cytokine and multiple MAPKs.
Acknowledgments
The present study was supported by grants from the
Korea Institute of Oriental Medicine (grant no. K15301) and the
National Research Foundation of Korea funded by the Korean
government (grant no. NRF-2015R1A2A2A01006736).
References
|
1
|
Helfrich YR, Sachs DL and Voorhees JJ:
Overview of skin aging and photoaging. Dermatol Nurs. 20:177–183;
quiz 184. 2008.PubMed/NCBI
|
|
2
|
Kammeyer A and Luiten RM: Oxidation events
and skin aging. Ageing Res Rev. 21:16–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dupont E, Gomez J and Bilodeau D: Beyond
UV radiation: A skin under challenge. Int J Cosmet Sci. 35:224–232.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wenk J, Brenneisen P, Meewes C, Wlaschek
M, Peters T, Blaudschun R, Ma W, Kuhr L, Schneider L and
Scharffetter-Kochanek K: UV-induced oxidative stress and
photoaging. Curr Probl Dermatol. 29:83–94. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Quan T, Qin Z, Xia W, Shao Y, Voorhees JJ
and Fisher GJ: Matrix-degrading metalloproteinases in photoaging.
Invest Dermatol Symp Proc. 14:20–24. 2009. View Article : Google Scholar
|
|
6
|
Amano S, Ogura Y, Akutsu N, Matsunaga Y,
Kadoya K, Adachi E and Nishiyama T: Protective effect of matrix
metalloproteinase inhibitors against epidermal basement membrane
damage: Skin equivalents partially mimic photoageing process. Br J
Dermatol. 153(Suppl 2): 37–46. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brink N, Szamel M, Young AR, Wittern KP
and Bergemann J: Comparative quantification of IL-1beta, IL-10,
IL-10r, TNFalpha and IL-7 mRNA levels in UV-irradiated human skin
in vivo. Inflamm Res. 49:290–296. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Campanini MZ, Pinho-Ribeiro FA, Ivan AL,
Ferreira VS, Vilela FM, Vicentini FT, Martinez RM, Zarpelon AC,
Fonseca MJ, Faria TJ, et al: Efficacy of topical formulations
containing Pimenta pseudocaryophyllus extract against UVB-induced
oxidative stress and inflammation in hairless mice. J Photochem
Photobiol B. 127:153–160. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Son Y, Cheong YK, Kim NH, Chung HT, Kang
DG and Pae HO: Mitogen-activated protein kinases and reactive
oxygen species: how can ROS activate MAPK pathways? J Signal
Transduct. 2011:7926392011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bickers DR and Athar M: Oxidative stress
in the pathogenesis of skin disease. J Invest Dermatol.
126:2565–2575. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Obolskiy D, Pischel I, Siriwatanametanon N
and Heinrich M: Garcinia mangostana L.: A phytochemical and
pharmacological review. Phytother Res. 23:1047–1065. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kinghorn AD, Chai HB, Sung CK and Keller
WJ: The classical drug discovery approach to defining bioactive
constituents of botanicals. Fitoterapia. 82:71–79. 2011. View Article : Google Scholar
|
|
13
|
Pedraza-Chaverri J, Cárdenas-Rodríguez N,
Orozco-Ibarra M and Pérez-Rojas JM: Medicinal properties of
mangosteen (Garcinia mangostana). Food Chem Toxicol. 46:3227–3239.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zadernowski R, Czaplicki S and Naczk M:
Phenolic acid profiles of mangosteen fruits (Garcinia mangostana).
Food Chem. 112:685–689. 2009. View Article : Google Scholar
|
|
15
|
Han AR, Kim JA, Lantvit DD, Kardono LB,
Riswan S, Chai H, Carcache de Blanco EJ, Farnsworth NR, Swanson SM
and Kinghorn AD: Cytotoxic xanthone constituents of the stem bark
of Garcinia mangostana (mangosteen). J Nat Prod. 72:2028–2031.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gopalakrishnan C, Shankaranarayanan D,
Kameswaran L and Nazimudeen SK: Effect of mangostin, a xanthone
from Garcinia mangostana Linn. in immunopathological and
inflammatory reactions. Indian J exp Biol. 18:843–846.
1980.PubMed/NCBI
|
|
17
|
Ibrahim MY, Hashim NM, Mariod AA, Mohan S,
Abdulla MA, Abdelwahab SI and Arbab IA: α-Mangostin from Garcinia
mangostana Linn: An updated review of its pharmacological
properties. Arab J Chem. 9:317–329. 2016. View Article : Google Scholar
|
|
18
|
Liu Z, Antalek M, Nguyen L, Li X, Tian X,
Le A and Zi X: The effect of gartanin, a naturally occurring
xanthone in mangosteen juice, on the mTOR pathway, autophagy,
apoptosis, and the growth of human urinary bladder cancer cell
lines. Nutr Cancer. 65(Suppl 1): 68–77. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pothitirat W, Chomnawang MT, Supabphol R
and Gritsanapan W: Comparison of bioactive compounds content, free
radical scavenging and anti-acne inducing bacteria activities of
extracts from the mangosteen fruit rind at two stages of maturity.
Fitoterapia. 80:442–447. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Naczk M, Towsend M, Zadernowski R and
Shahidi F: Protein- binding and antioxidant potential of phenolics
of mangosteen fruit (Garcinia mangostana). Food Chem. 128:292–298.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pothitirat W, Chomnawang MT, Supabphol R
and Gritsanapan W: Free radical scavenging and anti-acne activities
of mangosteen fruit rind extracts prepared by different extraction
methods. Pharm Biol. 48:182–186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
23
|
Fisher GJ, Kang S, Varani J, Bata-Csorgo
Z, Wan Y, Datta S and Voorhees JJ: Mechanisms of photoaging and
chronological skin aging. Arch Dermatol. 138:1462–1470. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Balunas MJ, Su B, Brueggemeier RW and
Kinghorn AD: Xanthones from the botanical dietary supplement
mangosteen (Garcinia mangostana) with aromatase inhibitory
activity. J Nat Prod. 71:1161–1166. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sakagami Y, Iinuma M, Piyasena KG and
Dharmaratne HR: Antibacterial activity of alpha-mangostin against
vancomycin resistant enterococci (VRe) and synergism with
antibiotics. Phytomedicine. 12:203–208. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Matsuda M, Hoshino T, Yamakawa N, Tahara
K, Adachi H, Sobue G, Maji D, Ihn H and Mizushima T: Suppression of
UV-induced wrinkle formation by induction of HSP70 expression in
mice. J Invest Dermatol. 133:919–928. 2013. View Article : Google Scholar
|
|
27
|
Rittié L and Fisher GJ: UV-light-induced
signal cascades and skin aging. Ageing Res Rev. 1:705–720. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gilchrest BA: Skin aging and photoaging.
Dermatol Nurs. 2:79–82. 1990.PubMed/NCBI
|
|
29
|
Hendi A, Brodland DG and Zitelli JA:
Melanocytes in long-standing sun-exposed skin: Quantitative
analysis using the MART-1 immunostain. Arch Dermatol. 142:871–876.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee WJ, Park KH, Cha HW, Sohn MY, Park KD,
Lee SJ and Kim DW: The expression of involucrin, loricrin, and
filaggrin in cultured sebocytes. Ann Dermatol. 26:134–137. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hänel KH, Cornelissen C, Lüscher B and
Baron JM: Cytokines and the skin barrier. Int J Mol Sci.
14:6720–6745. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
D'Orazio J, Jarrett S, Amaro-Ortiz A and
Scott T: UV radiation and the skin. Int J Mol Sci. 14:12222–12248.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Burke KE: Photoaging: The role of
oxidative stress. G Ital Dermatol Venereol. 145:445–459.
2010.PubMed/NCBI
|
|
34
|
Fisher GJ, Wang ZQ, Datta SC, Varani J,
Kang S and Voorhees JJ: Pathophysiology of premature skin aging
induced by ultraviolet light. N Engl J Med. 337:1419–1428. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhuang Y and Lyga J: Inflammaging in skin
and other tissues - the roles of complement system and macrophage.
Inflamm Allergy Drug Targets. 13:153–161. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chung JH and Eun HC: Angiogenesis in skin
aging and photoaging. J Dermatol. 34:593–600. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kondo S: The roles of cytokines in
photoaging. J Dermatol Sci. 23(Suppl 1): S30–S36. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fisher GJ: The pathophysiology of
photoaging of the skin. Cutis. 75(Suppl 2): 5–9. 2005.PubMed/NCBI
|
|
39
|
Jung E, Lee J, Baek J, Jung K, Lee J, Huh
S, Kim S, Koh J and Park D: Effect of Camellia japonica oil on
human type I procollagen production and skin barrier function. J
ethnopharmacol. 112:127–131. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Urbanski A, Schwarz T, Neuner P, Krutmann
J, Kirnbauer R, Köck A and Luger TA: Ultraviolet light induces
increased circulating interleukin-6 in humans. J Invest Dermatol.
94:808–811. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Peus D, Vasa RA, Beyerle A, Meves A,
Krautmacher C and Pittelkow MR: UVB activates ERK1/2 and p38
signaling pathways via reactive oxygen species in cultured
keratinocytes. J Invest Dermatol. 112:751–756. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Roux PP and Blenis J: ERK and p38
MAPK-activated protein kinases: A family of protein kinases with
diverse biological functions. Microbiol Mol Biol Rev. 68:320–344.
2004. View Article : Google Scholar : PubMed/NCBI
|