Introduction
Inflammation, a cause of various diseases, may be
accompanied by oxidative damage, and the synergistic effect of
these conditions may increase with the worsening of numerous
diseases. Lipopolysaccharide (LPS), a pathogenic endotoxin present
in the outer membrane of gram-negative bacteria, promotes the
inflammatory reaction and oxidative stress, and is commonly used to
generate models of disease for evaluating the pharmacodynamic
efficacy of drugs (1,2). In particular, macrophages are
activated when exposed to inflammatory stimuli such as LPS,
resulting in excessive production of pro-inflammatory mediators and
cytokines as well as reactive oxygen species (ROS) (3–5).
Nitric oxide (NO) and prostaglandin E2 (PGE2)
are representative pro-inflammatory mediators. NO regulated by
inducible NO synthase (iNOS) reacts with peroxides to promote
oxidative stress and inflammatory processes (6,7).
PGE2, another important mediator synthesized from
arachidonic acid metabolites, is catalyzed by cyclooxygenase-2
(COX-2) during the inflammatory response (6,8,9).
In addition, major pro-inflammatory cytokines, such as tumor
necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), are
overexpressed in macrophages stimulated by LPS and contribute to
the pathogenesis of various inflammatory diseases (10,11). Accumulating evidence suggests that
LPS causes overproduction of pro-inflammatory mediators and
cytokines through the activation of nuclear factor-κB (NF-κB)
associated with the mitogen-activated protein kinases (MAPKs) and
phosphatidylinositol-3 kinase (PI3K)/Akt pathways (12–14). In addition, inflammatory stress is
known to increase reactive oxygen species (ROS) production and
reduce the production of antioxidant enzymes that protect tissues
from oxidative damage (15,16). In particular, the induction of
phase II detoxifying enzymes and cytoprotective genes, such as heme
oxygenase-1 (HO-1), is mediated by NF-erythroid 2-related factor 2
(Nrf2), which has a central role in protecting cells against
inflammation and oxidative damage (16–18).
The fruit of Schisandra chinensis (Turcz.)
Baill. and Schisandra sphenanthera Rehd. et Wils. have been
widely used for treating several diseases in Asia for thousands of
years (19,20). Numerous studies have attempted to
identify the key phytochemicals present in these two fruits; to
date, several lignan compounds have been identified as the major
bioactive components (21,22).
Among them, schisandrin A, a dibenzocyclooctadiene derivative, has
been indicated to have a role in the inhibition of inflammation and
elimination of free radicals. For instance, the schisandrin
A-enriched extract of Schisandra sphenanthera fruit has been
reported to exert anti-inflammatory effects by inhibiting
PGE2 production and suppressing COX-2 expression in
HaCaT keratinocytes irradiated with ultraviolet B light (23). Schisandrin A also demonstrated a
protective effect on primary cortical neurons against
L-glutamate-induced neurotoxicity (24). Furthermore, by reducing the
intracellular calcium concentration and causing the release of
lactate dehydrogenase, it significantly improved the viability of
primary cortical cells in the oxygen-glucose
deficiency/reoxygenation model (25). In addition, schisandrin A has
proven beneficial in preventing cell damage in the pathogenesis of
central nervous system diseases, including ischemia, and regulated
inflammation- and apoptosis-associated proteins in SH-SY5Y cells
following glucose deprivation injury (26). Schisandrin A has also been
reported to inhibit inflammation-induced neuronal damage by
decreasing the production of NO, TNF-α and IL-6 induced by LPS in
microglial cells; furthermore, various signaling pathways,
including the NF-κB pathway, were indicated to be involved in this
process, suggesting that schisandrin A is a promising candidate for
treating inflammatory neurode-generative diseases (27). Similarly, this compound exhibited
anti-inflammatory activity in LPS-stimulated RAW 264.7 cells; in
particular, it increased glutathione S-transferase activity and
decreased glutathione levels, thereby suppressing edema; this
indicates that the anti-inflammatory action of schisandrin A is
closely associated with its antioxidant effect (28). Furthermore, several previous
studies have reported that lignan-like substances with a structure
similar to that of schisandrin A possess anti-inflammatory
activities and excellent Nrf2-induction or ROS-scavenging abilities
(29–34); however, the underlying mechanisms
associated with the anti-inflammatory and antioxidant activity of
schisandrin A have remained to be fully elucidated. Therefore, the
present study evaluated the protective effect of schisandrin A and
the underlying mechanisms associated with inflammation and
oxidative stress in RAW 264.7 macrophages exposed to LPS derived
from Escherichia coli (E. coli).
Materials and methods
Cell culture and LPS stimulation
The RAW 264.7 murine macrophage cell line was
obtained from the Korea Cell Line Bank (Seoul, Korea) and
maintained in Dulbecco's modified Eagle's medium (DMEM) containing
10% (v/v) fetal bovine serum, L-glutamine (2 mM), penicillin (100
U/ml) and streptomycin (100 U/ml) (all from WelGENE Inc., Daegu,
Korea) at 37°C in a humidified atmosphere containing 5%
CO2 and 95% air. Schisandrin A was purchased from
Sigma-Aldrich (cat. no. SML0054; Merck KGaA, Darmstadt, Germany),
dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich; Merck KGaA)
and final concentrations were adjusted by dilution with complete
culture medium. The DMSO concentration did not exceed 0.05% (i.e.,
a non-cytotoxic range). To stimulate the cells, the medium was
replaced with fresh DMEM, and LPS (E. coli Serotype 055:B5;
cat. no. L2880; Sigma-Aldrich; Merck KGaA) was added in the
presence or absence of schisandrin A for the indicated periods.
Assessment of cell viability
To evaluate the cytotoxicity of schisandrin A, RAW
264.7 cells were seeded in 96-well plates at a density of
1×103 cells/well. The cells were treated with various
concentrations of schisandrin A for 1 h prior to incubation with
LPS (100 ng/ml) for 24 h. After the incubation was complete, images
of cells from each well were captured under a phase-contrast
microscope (Carl Zeiss, Oberkochen, Germany). Subsequently, MTT
(Sigma-Aldrich; Merck KGaA) was added to each well at 0.5 mg/ml,
followed by incubation at 37°C in the dark. After 3 h of
incubation, the MTT solution was removed and 200 μl 5% DMSO
was added to dissolve the crystals. The viable cells were detected
by reading the absorbance of formazan at 540 nm using an
enzyme-linked immunosorbent assay (ELISA) microplate reader
(Dynatech Laboratories, Chantilly, VA, USA). The optical density of
the formazan formed in the control (untreated) cells was considered
to represent 100% viability.
Measurement of NO and PGE2
production
RAW 264.7 cells were pretreated with schisandrin A
for 1 h; subsequently, they were stimulated with LPS for 24 h.
Controls were maintained under the same culture conditions;
however, they were not pre-incubated or stimulated. NO levels were
indirectly determined by measuring the stable NO catabolite nitrite
in the medium utilising the Griess reaction. In brief, the
conditioned medium (100 μl) was mixed with the same volume
of Griess reagent (Sigma-Aldrich; Merck KGaA) and incubated for 10
min at room temperature. The optical density at 540 nm was measured
using an ELISA microplate reader and the nitrite concentration was
calculated according to a standard curve generated from known
concentrations of sodium nitrite. The PGE2 concentration
in the conditioned medium was measured using a commercial
PGE2 ELISA kit (cat. no. 514010; Cayman Chemical Co.,
Ann Arbor, MI, USA) according to the manufacturer's
instructions.
ELISA for pro-inflammatory cytokines
The generation of pro-inflammatory cytokines TNF-α
and IL-6β was measured using ELISA kits. The RAW 264.7 cells were
pre-incubated with schisandrin A for 1 h, followed by LPS
stimulation for 24 h, and cytokine contents in the cell-free
supernatants were measured using cytokine sandwich ELISA kits (cat.
nos. MTA00B and MLB00C; R&D Systems, Minneapolis, MN, USA)
according to the manufacturer's protocols.
Reverse transcription-polymerase chain
reaction (RT-PCR) assay
The RAW 264.7 cells were pretreated with various
concentrations of schisandrin A for 1 h, followed by treatment with
LPS (100 ng/ml) for 24 h. Controls were maintained under the same
culture conditions, but were not pre-incubated or stimulated. Total
RNA was extracted using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), according to the
manufacturer's instructions. The complementary (c)DNA of each
sample was prepared using 2 μg RNA, 1 μl Moloney's
murine leukemiavirus reverse transcriptase, 1 mM deoxynucleoside
triphosphate and 1 μl oligo(dT) according to the
manufacturer's standardized protocol. DNA amplification was
performed in AccuPower® PCR PreMix (Bioneer Corp.,
Daejeon, Korea). iNOS, COX-2, TNF-α and IL-1β genes were amplified
from the cDNA using PCR (Eppendorf, Hamburg, Germany). After
amplification, the PCR products were electrophoresed on 1% agarose
gels and visualized following staining with ethidium bromide
(Sigma-Aldrich; Merck KGaA) for 10 min at room temperature under
ultraviolet irradiation using the Gel Documentation System
(CHEMI-SMART 2026M.WL; Vilber Lourmat, Marne-la-Valle, France).
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a
loading control. The PCR primers were as follows: iNOS forward,
5′-ATG TCC GAA GCA AAC ATCAC-3′ and reverse, 5′-TAA TGT CCA GGA AGT
AGG TG-3′; COX-2 forward, 5′-CAG CAA ATC CTT GCT GTT CC-3′ and
reverse, 5′-TGG GCA AAG AAT GCA AAC ATC-3′; TNF-α forward, 5′-TCT
CAT CAG TTC TAT GGC CC-3′ and reverse, 5′-GGG AGT AGA CAA GGT ACA
AC-3′; IL-1β forward, 5′-GGG CTG CTT CCA AAC CTT TG-3′ and reverse,
5′-GCT TGG GAT CCA CAC TCT CC-3′ and GAPDH forward, 5′-AGG CCG GTG
CTG AGT ATG TC-3′ and reverse, 5′-TGC CTG CTT CAC CAC CTT CT-3′
(Bioneer Corp.). The PCR reaction was initiated at 94°C for 2 min,
followed by 25 cycles of 94°C for 30 sec, annealing temperature for
30 sec and 72°C for 30 sec, and a final extension step at 72°C for
5 min. The annealing temperatures were 63°C for iNOS, COX-2, TNF-α
and IL-1β, and 61°C for GAPDH.
Protein isolation and western blot
analysis
The RAW 264.7 cells were incubated with schisandrin
A at the indicated concentrations for 1 h prior to stimulation with
LPS (100 ng/ml) for 24 h. As described previously (35), the cells were collected, lysed
with a cell lysis buffer and the protein concentration was
determined using the Bradford Protein assay kit (Bio-Rad
Laboratories, Hercules, CA, USA). In a parallel experiment,
cytoplasmic and nuclear extracts were prepared using an NE-PER
Nuclear and Cytoplasmic Extraction reagents kit (Pierce; Thermo
Fisher Scientific, Inc.) following the manufacturer's instructions.
For western blotting, equal amounts of protein samples (30
μg/lane) were subjected to 10–13% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then
electrophoretically transferred onto polyvinylidene difluoride
membranes (Schleicher & Schuell, Keene, NH, USA). Subsequently,
the membranes were blocked with 5% non-fat dry milk in
Tris-buffered saline containing 0.1% Triton X-100 (TBST) for 1 h
and probed with specific primary antibodies (Table I) at 4°C overnight. After washing
with TBST, the membranes were incubated with the appropriate
horseradish peroxidase (HRP)-conjugated secondary antibodies
(dilution, 1:500; cat. no. sc-2004, goat anti-rabbit IgG-HRP;
sc-2005, goat anti-mouse IgG-HRP; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) for 2 h at room temperature. The protein bands
were detected to X-ray film using an enhanced chemiluminescence kit
(cat. no. RPN 2232; GE Healthcare Life Sciences, Little Chalfont,
UK) according to the manufacturer's instructions.
 | Table IList of antibodies used in the
present study. |
Table I
List of antibodies used in the
present study.
| Antibody | Dilution | Cat. no. | Species of
origin | Supplier |
|---|
| iNOS | 1:1,000 | BD-610328 | Rabbit
polyclonal | BD Biosciences |
| COX-2 | 1:500 | 160126 | Rabbit
polyclonal | Cayman
Chemical |
| IL-1β | 1:1,000 | sc-7884 | Rabbit
polyclonal | Santa Cruz
Biotechnology, Inc. |
| TNF-α | 1:1,000 | 3707S | Rabbit
polyclonal | Cell Signaling
Technology, Inc. |
| NF-κB p65 | 1:1,000 | sc-109 | Rabbit
polyclonal | Santa Cruz
Biotechnology, Inc. |
| IκB-α | 1:1,000 | sc-371 | Rabbit
polyclonal | Santa Cruz
Biotechnology, Inc. |
| Lamin B | 1:1,000 | sc-6216 | Goat
polyclonal | Santa Cruz
Biotechnology, Inc. |
| JNK | 1:1,000 | 9252S | Rabbit
polyclonal | Cell Signaling
Technology, Inc. |
| p-JNK | 1:1,000 | 9255 | Mouse
monoclonal | Cell Signaling
Technology, Inc. |
| ERK | 1:1,000 | sc-154 | Rabbit
polyclonal | Santa Cruz
Biotechnology, Inc. |
| p-ERK | 1:1,000 | 9106S | Mouse
monoclonal | Cell Signaling
Technology, Inc. |
| p38 MAPK | 1:1,000 | sc-728 | Rabbit
polyclonal | Santa Cruz
Biotechnology, Inc. |
| p-p38 MAPK | 1:1,000 | 9211S | Rabbit
polyclonal | Cell Signaling
Technology, Inc. |
| PI3K | 1:1,000 | 4257 | Rabbit
polyclonal | Cell Signaling
Technology, Inc. |
| p-PI3K | 1:1,000 | 4228 | Rabbit
polyclonal | Cell Signaling
Technology, Inc. |
| Akt | 1:1,000 | sc-8312 | Rabbit
polyclonal | Santa Cruz
Biotechnology, Inc. |
| p-Akt | 1:1,000 | sc-101629 | Rabbit
polyclonal | Santa Cruz
Biotechnology, Inc. |
| Nrf2 | 1:1,000 | sc-13032 | Rabbit
polyclonal | Santa Cruz
Biotechnology, Inc. |
| p-Nrf2 | 1:2,000 | Ab76026 | Rabbit
polyclonal | Abcam, Inc. |
| HO-1 | 1:1,000 | 374090 | Rabbit
polyclonal | Calbiochem,
Inc. |
| Keap1 | 1:1,000 | sc-15246 | Goat
polyclonal | Santa Cruz
Biotechnology, Inc. |
| Actin | 1:1,000 | sc-1615 | Goat
polyclonal | Santa Cruz
Biotechnology, Inc. |
Immunofluorescence staining for NF-κB
nuclear translocation
The effect of schisandrin A on LPS-induced nuclear
translocation of NF-κB was also assessed using immunofluorescence
microscopy. The RAW 264.7 cells were first grown on glass
coverslips for 24 h at 37°C in a humidified atmosphere containing
5% CO2 and subsequently incubated with 200 μM
schisandrin A for 1 h prior to treatment with 100 ng/ml LPS for 30
min in the same culture conditions. The cells were fixed in 3.7%
paraformaldehyde for 15 min, permeabilized with 0.2% Triton X-100
in phosphate-buffered saline (PBS) for 15 min and blocked for 10
min at room temperature with PBS containing 5% bovine serum albumin
(Sigma-Aldrich; Merck KGaA). The cells were then stained with the
primary antibody against NF-κB p65 (dilution, 1:100) overnight at
4°C. Subsequently, cells were incubated with a
fluorescein-conjugated anti-rat immunoglobulin G (dilution, 1:100;
cat. no. 31629; Molecular Probes; Thermo Fisher Scientific, Inc.)
in the dark for 40 min at 37°C. Nuclei were sequentially stained
with DAPI solution (2.5 μg/ml; Sigma-Aldrich; Merck KGaA).
The slides were then mounted and fluorescence images were captured
using a fluorescence microscope (Carl Zeiss).
Determination of intracellular ROS
The intracellular ROS production was monitored using
5,6-carboxy-2′7′-dichloroflu-orescin diacetate (DCF-DA), as
previously described (36). This
substrate freely permeates the cells, and upon incorporation, it is
oxidized to fluorescent DCF. In brief, RAW 264.7 cells were
pretreated with 200 μM schisandrin A for 1 h and then
stimulated with or without 100 ng/ml LPS. After 6 h incubation, the
cells were stained with 10 μM DCF-DA (Molecular Probes;
Thermo Fisher Scientific, Inc.) for 30 min at 37°C in the dark. The
cells were collected, washed with PBS twice, and a total of 10,000
events were then immediately analyzed using a flow cytometer (BD
Biosciences, San Jose, CA, USA). To confirm the involvement of
elevated ROS in the LPS-induced inflammatory response, the cells
were pre-incubated with N-acetyl cysteine (NAC; Sigma-Aldrich;
Merck KGaA), an established antioxidant, for 1 h prior to LPS
treatment.
Statistical analysis
Each experiment was performed in triplicate and
values are expressed as the mean ± standard deviation. Statistical
analysis was performed using GraphPad Prism software (version 5.03;
GraphPad Software, Inc., La Jolla, CA, USA). Differences between
groups were assessed using analysis of variance followed by Tukey's
post hoc test or by the unpaired Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
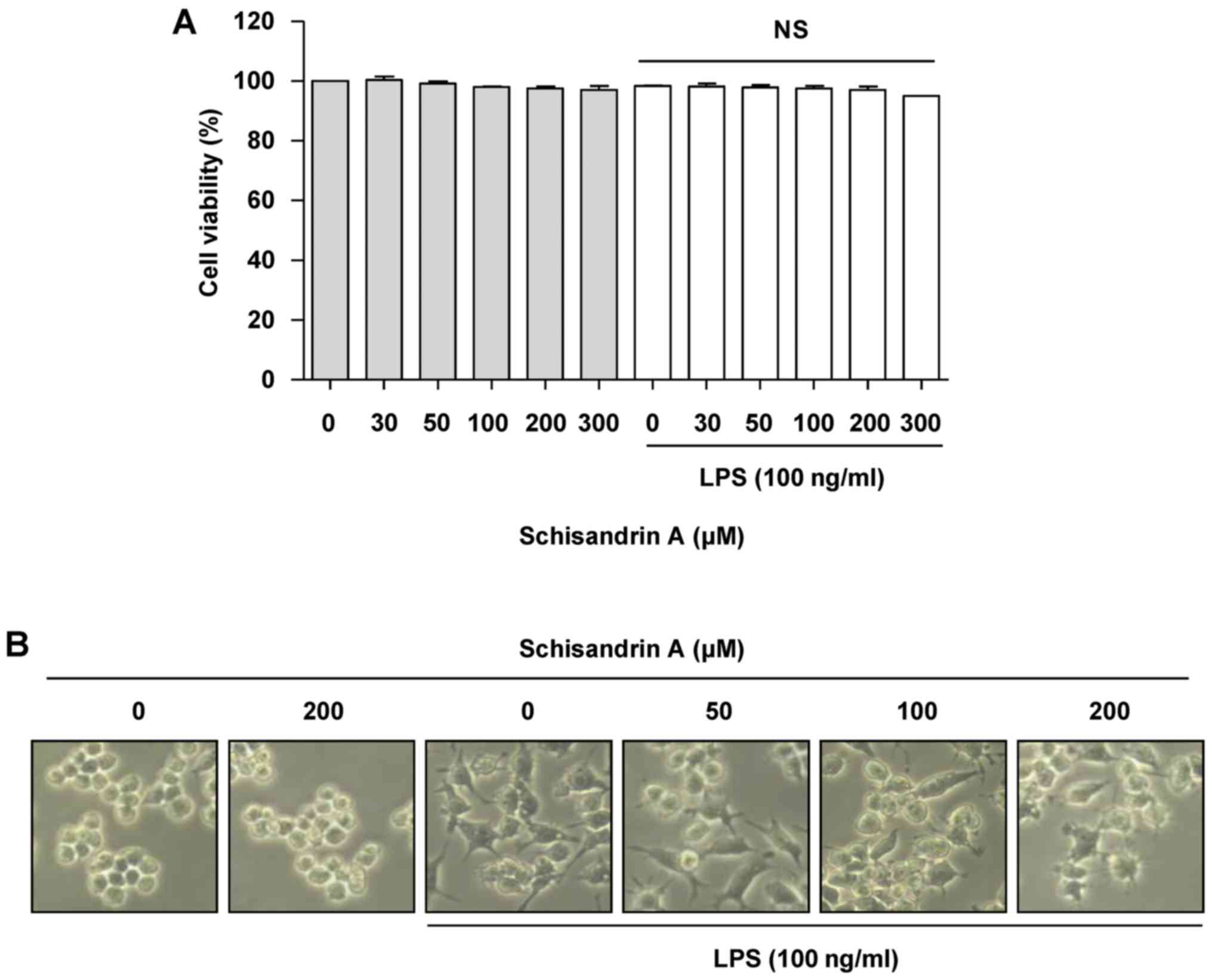
Effect of schisandrin A on the viability
of RAW 264.7 macrophages
The RAW 264.7 cells were treated with different
concentrations of schisandrin A for 1 h prior to incubation with
100 ng/ml LPS. An MTT assay was performed to select the
concentration range of schisandrin A that would not affect the cell
viability. Fig. 1A demonstrates
that schisandrin A at up to 300 μM in the presence, as well
as in the absence of LPS did not affect the viability of RAW 264.7
cells. In addition, morphological changes in the RAW 264.7 cells
treated with LPS alone were slightly alleviated by pretreatment
with schisandrin A (Fig. 1B).
Accordingly, the anti-inflammatory effects of schisandrin A at
concentrations of <200 μM on RAW 264.7 macrophages were
assessed.
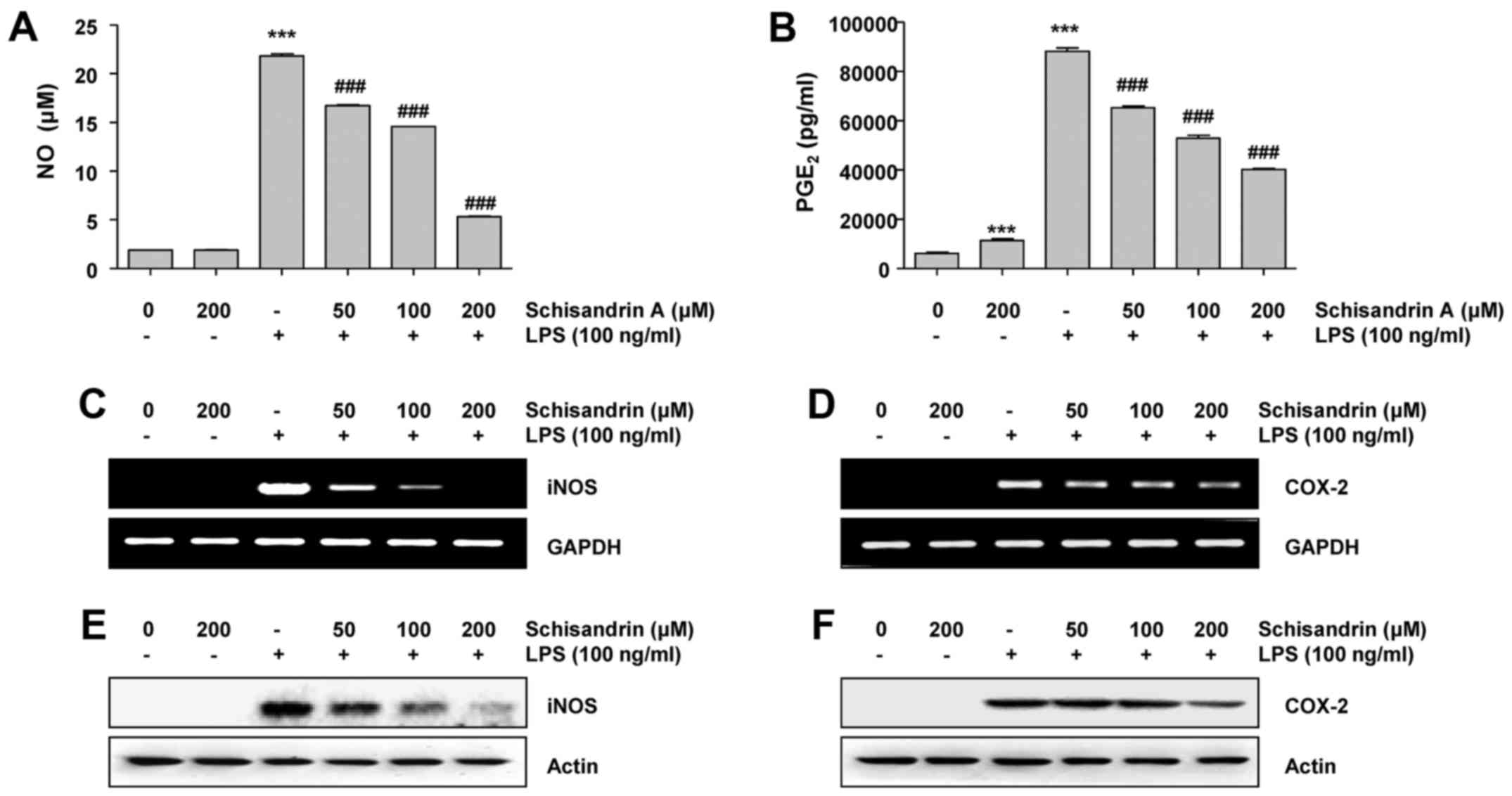
Effects of schisandrin A on LPS-induced
production of pro-inflammatory mediators in RAW 264.7
macrophages
To examine the effects of schisandrin A on the
LPS-induced production of representative pro-inflammatory mediators
NO and PGE2 in RAW 264.7 cells, the cells were
pretreated with various concentrations of schisandrin A (50, 100
and 200 μM) for 1 h. Thereafter, they were stimulated for 24
h with 100 ng/ml LPS. The NO and PGE2 levels in the
cellular supernatants were assessed using Griess reagent and ELISA,
respectively. Fig. 1 indicate
that the stimulation of RAW 264.7 cells with LPS alone
significantly increased the NO and PGE2 concentrations
in the culture medium; which was reduced by schisandrin A in a
dose-dependent manner. Therefore, it was investigated whether the
inhibitory effects of schisandrin A on NO and PGE2
production were associated with the regulation of their
synthesizing enzymes, iNOS and COX-2, respectively. It was revealed
that iNOS as well as COX-2 mRNA and protein expression levels were
markedly elevated in the LPS-stimulated RAW 264.7 cells. However,
schisandrin A significantly reduced the LPS-induced expression of
iNOS as well as COX-2 mRNA and protein in a concentration-dependent
manner (Fig. 2C–F). These results
suggest that schisandrin A inhibits NO and PGE2
production via downregulation of iNOS as well as COX-2 mRNA and
protein levels.
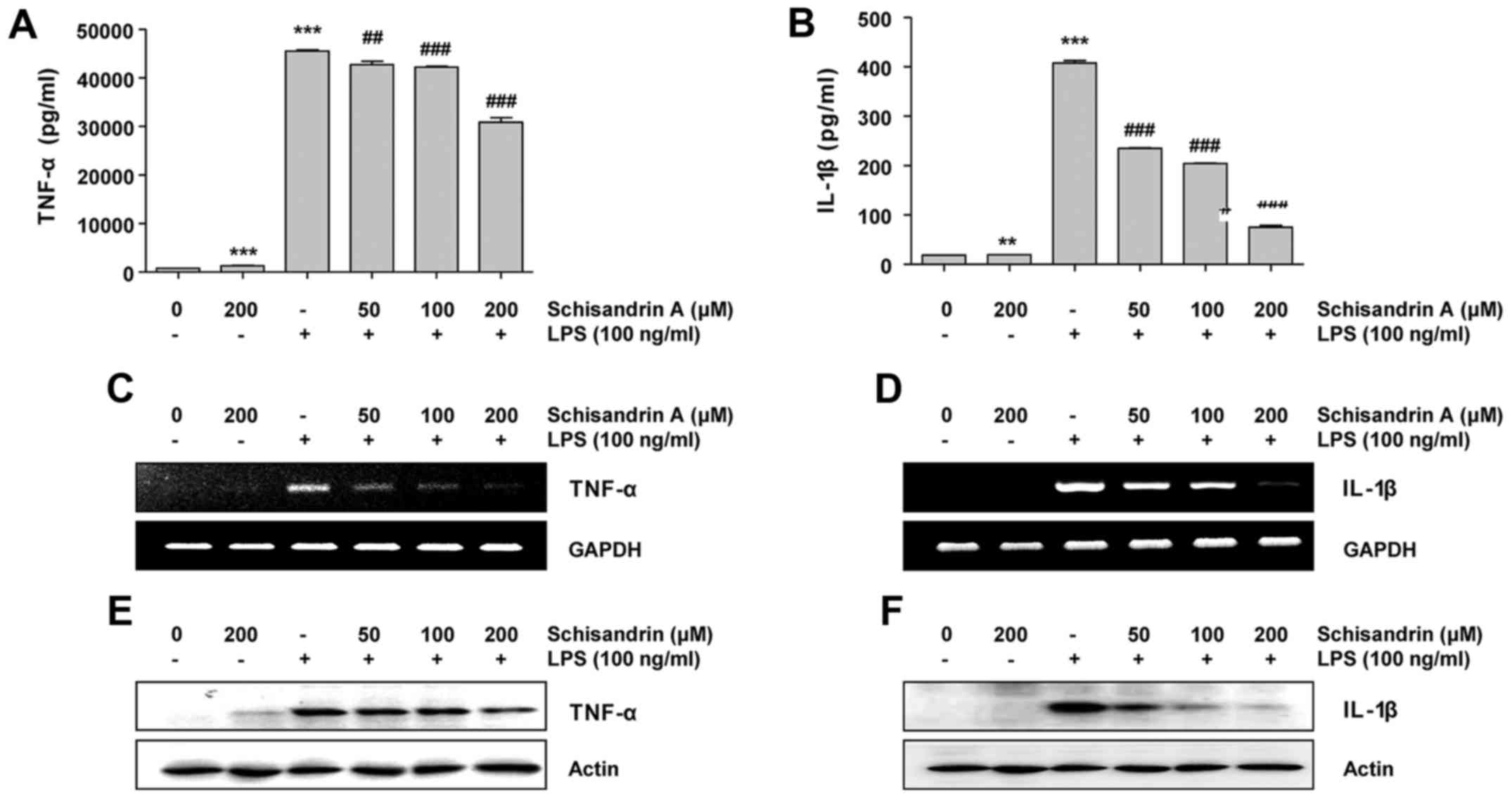
Effects of schisandrin A on LPS-induced
production of proinflammatory cytokines in RAW 264.7
macrophages
With the production of pro-inflammatory mediators,
pro-inflammatory cytokines also have a crucial role in initiating
the inflammatory response (10,11). Therefore, it was assessed whether
schisandrin A inhibits the production of pro-inflammatory
cytokines, including TNF-α and IL-1β, in LPS-treated RAW 264.7
cells. The results indicate that pretreatment with schisandrin A
prior to the LPS challenge significantly reduced the TNF-α
concentration in the culture medium of RAW 264.7 cells in a
concentration-dependent manner. Furthermore, this was associated
with the downregulation of its mRNA and protein expression.
Similarly, schisandrin A effectively inhibited LPS-induced IL-1β
secretion, as well as mRNA and protein expression (Fig. 3). These results suggest that
schisandrin A also modulates inflammatory cytokine expression at
the transcriptional level.
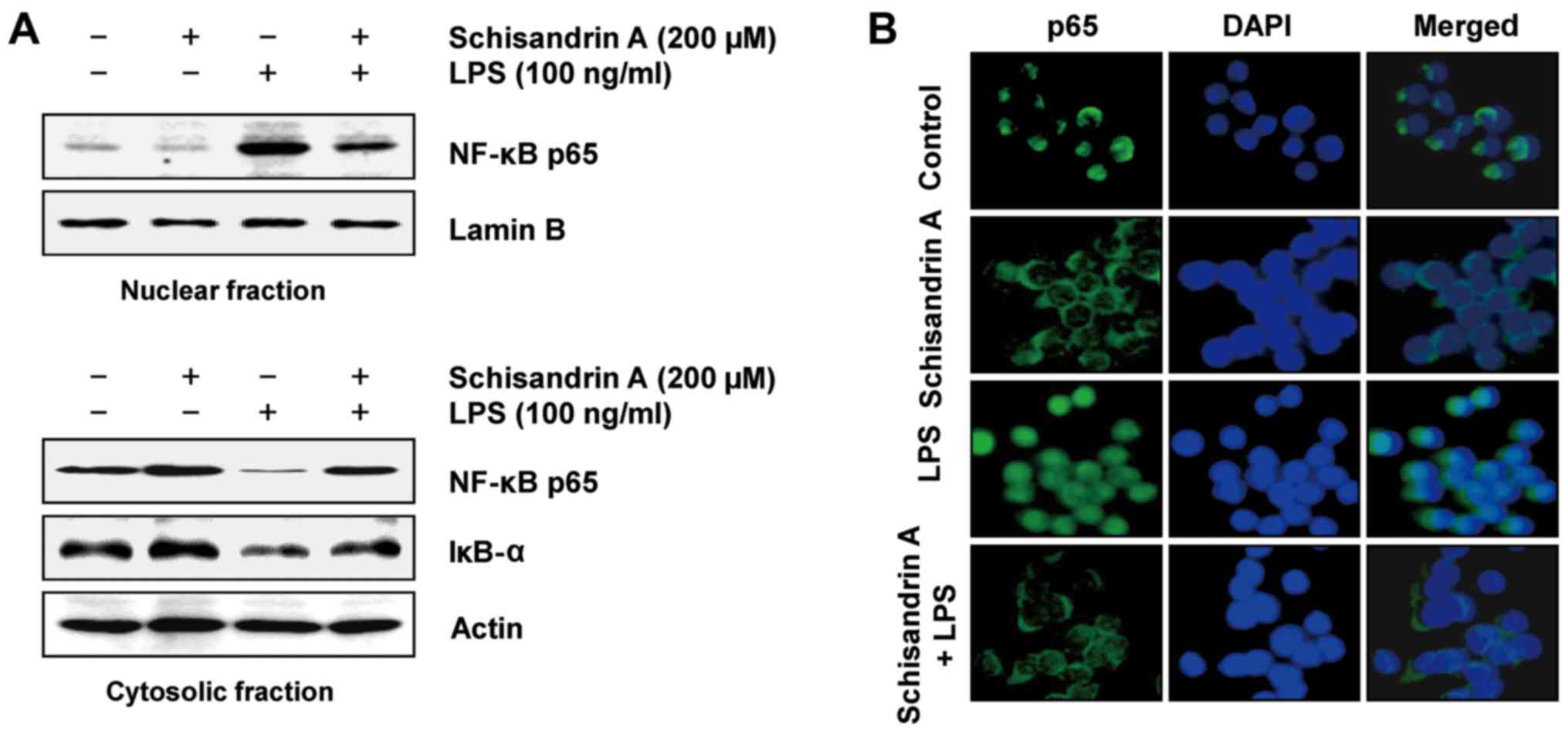
Schisandrin A inhibits LPS-induced
nuclear translocation of NF-κB and degradation of IκB-α in RAW
264.7 macrophages
The nuclear translocation of transcription factor
NF-κB is considered a prerequisite for the transcription of genes
associated with inflammatory processes (12,13); therefore, the ability of
schisandrin A to inhibit NF-κB translocation was investigated.
Fig. 4 indicates that LPS
stimulation markedly promoted the nuclear translocation of NF-κB
p65 with a concurrent downregulation of IκB-α. However, schisandrin
A pretreatment markedly inhibited the translocation of NF-κB to the
nucleus and restored the depleted IκB-α in the LPS-stimulated
cells. In addition, LPS enhanced the nuclear accumulation of NF-κB
p65, as evidenced by immunofluorescence staining; however, this was
effectively reduced by schisandrin A pretreatment. These
observations suggest that schisandrin A acts as a negative
regulator of LPS-stimulated NF-κB activation in RAW 264.7
macrophages.
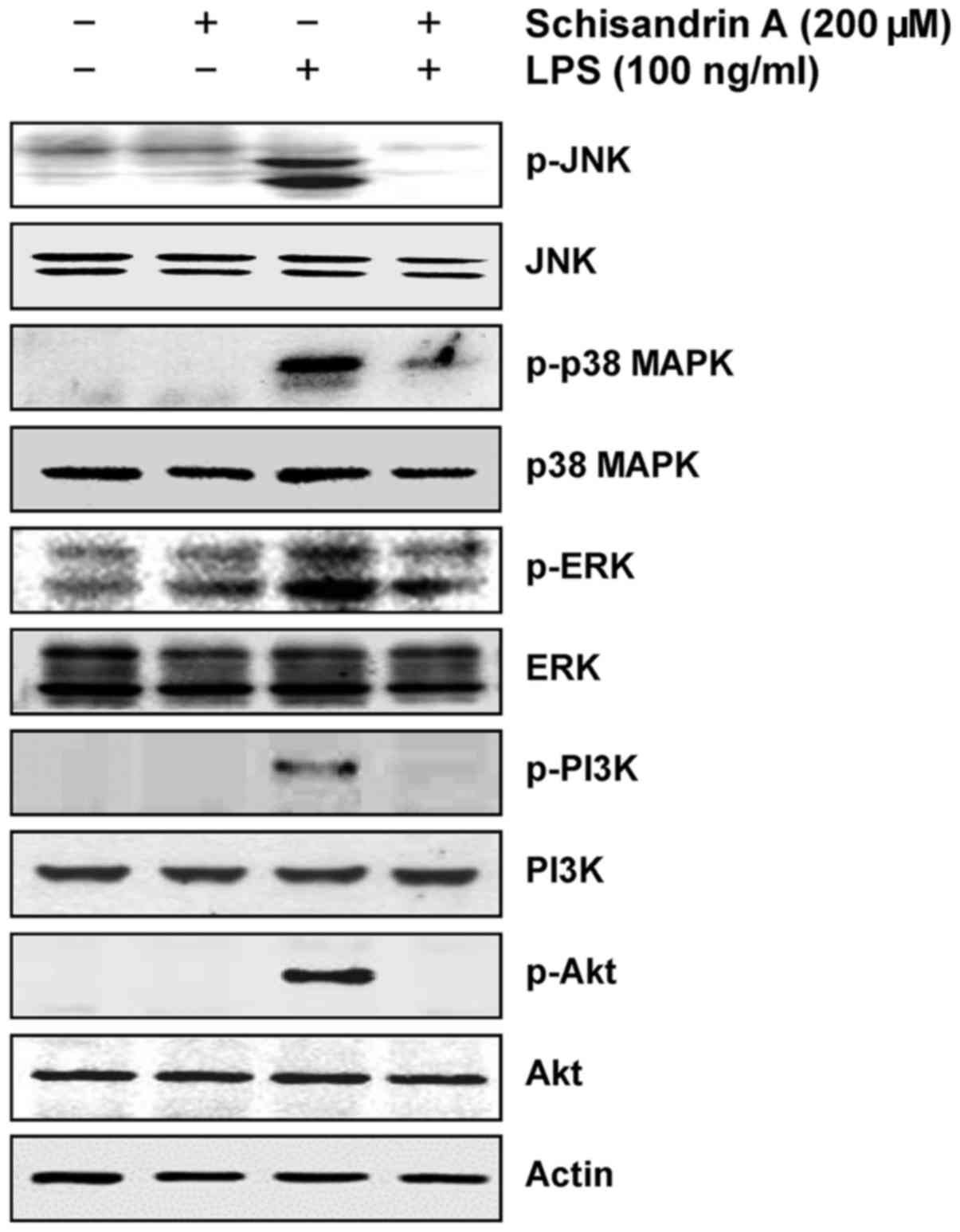
Inhibition of LPS-induced activation of
MAPK and PI3K/Akt pathways by schisandrin A in RAW 264.7
macrophages
Previous studies have established that the MAPKs and
PI3K/Akt signaling pathways are involved in LPS-induced
inflammation in macrophages (13,14). Therefore, the possible involvement
of these signaling pathways in schisandrin A-mediated inhibition of
LPS-induced inflammatory responses was examined in the present
study. The immunoblotting results indicated that LPS treatment
substantially promoted the phosphorylation of three MAPKs, c-Jun
N-terminal kinase (JNK), p38 MAPK and extracellular
signal-regulated kinase (ERK), as well as PI3K and Akt (Fig. 5); however, these effects were
completely abrogated by pretreatment with schisandrin A. Thus,
schisandrin A suppresses the inflammatory response by inactivating
the MAPK and PI3K/Akt signaling pathways in LPS-stimulated RAW
264.7 macrophages.
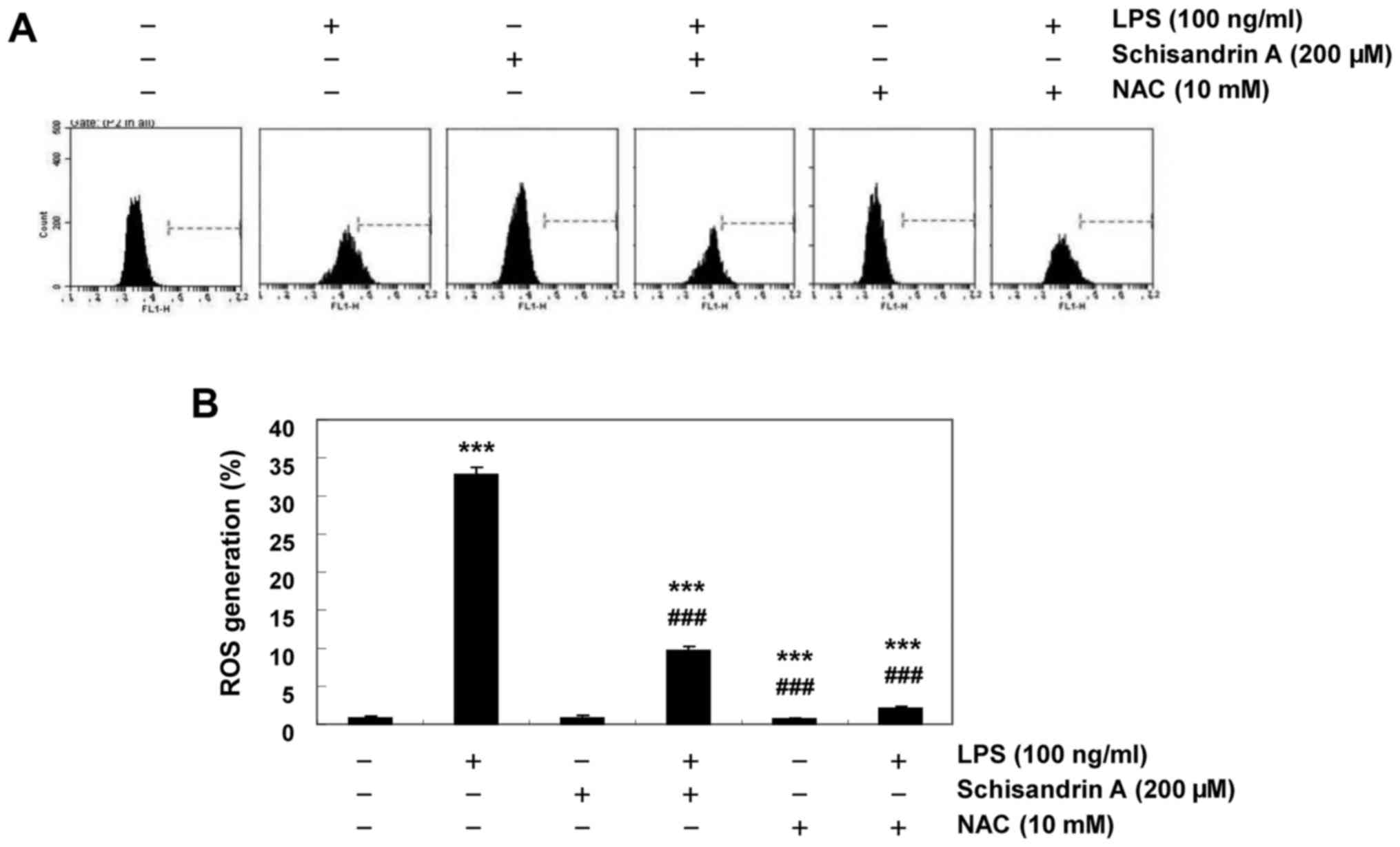
Schisandrin A inhibits LPS-induced ROS
production in RAW 264.7 macrophages
Previous studies have demonstrated that inflammation
is partly mediated by oxidative stressors and that LPS-activated
macrophages increase ROS accumulation (4,5).
Thus, the protective effect of schisandrin A on intracellular ROS
formation in LPS-stimulated RAW 264.7 cells was investigated using
the DCF-DA assay. Fig. 6
indicates that following LPS treatment, the DCF fluorescence
intensity was significantly increased. Furthermore, pretreatment
with schisandrin A or NAC, an antioxidant, significantly reversed
the LPS-induced increases in the ROS content. These results
confirmed that the anti-inflammatory effect of schisandrin A may be
associated with its antioxidant activity.
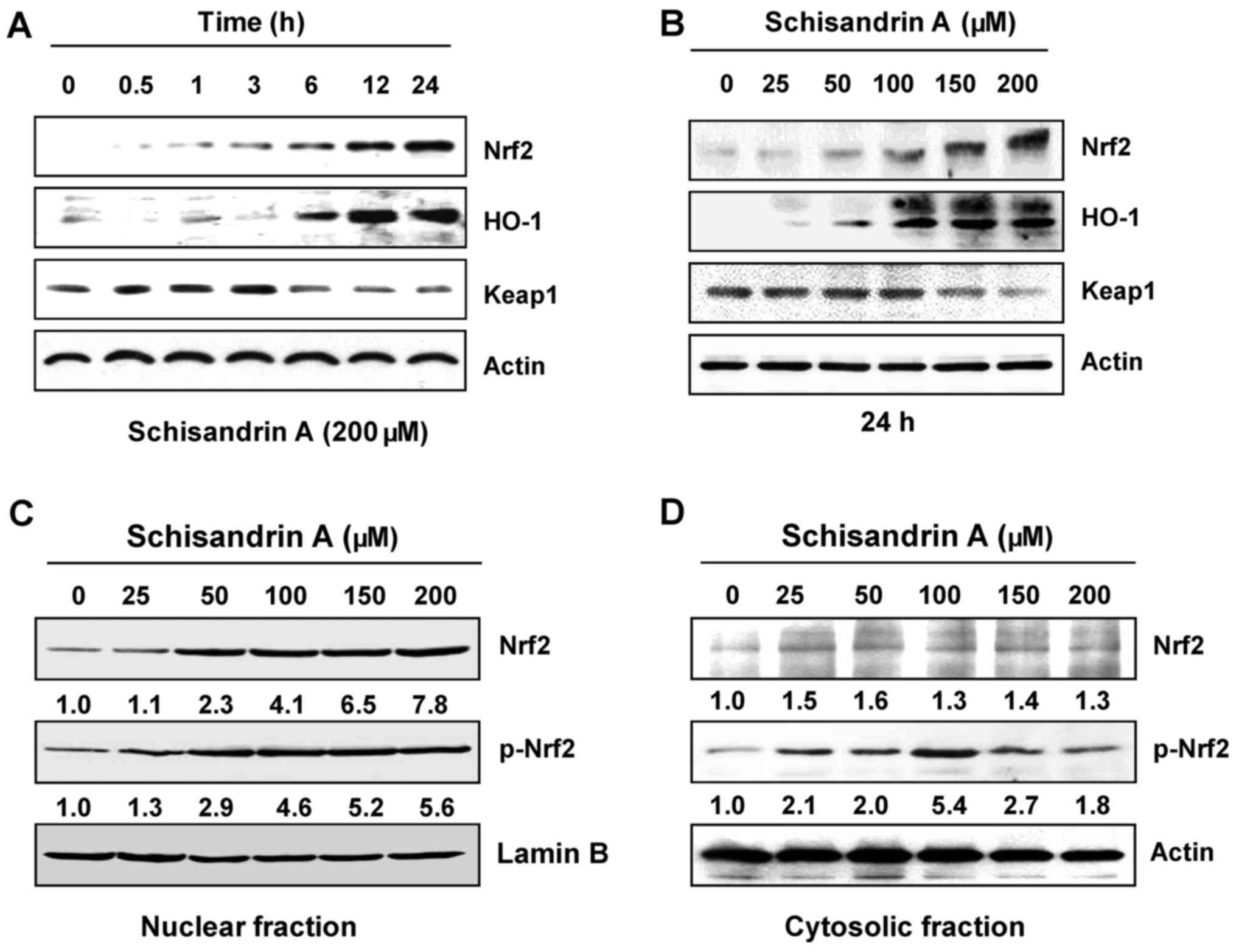
Induction of Nrf2 and HO-1 expression by
schisandrin A in RAW 264.7 macrophages
The Nrf2/HO-1 signaling system has a vital role in
intracellular antioxidant systems (16,18). The present study further
investigated whether Nrf2/HO-1 signaling is involved in the
antioxidant effect of schisandrin A. Western blot analysis
indicated that with the increase in the treatment duration and
concentration of schisandrin A, the expression of Nrf2 gradually
increased; simultaneously, the expression of Kelch-like
ECH-associated protein 1 (Keap1), a negative regulator of Nrf2,
decreased. In addition, schisandrin A effectively increased HO-1
expression (Fig. 7A and B),
indicating that the antioxidant effect of schisandrin A is
associated with the activation of the Nrf2/HO-1 signaling pathway.
Furthermore, immunoblot analysis using the nuclear and cytosolic
fractions of the RAW 264.7 cells revealed that the amount of total
and phosphorylated Nrf2 protein in the nucleus was markedly
increased following schisandrin A treatment (Fig. 7C and D).
Discussion
Macrophages have a critical role in initiating
inflammation and the innate and adaptive immune response. For this
purpose, macrophages undergo a series of processes involving the
activation and release of several specific effector proteins,
including pro-inflammatory mediators and cytokines, when stimulated
by LPS, a major component of the outer membrane of Gram-negative
bacteria (1,2). This process may be accompanied by
oxidative stress, an important target for anti-inflammatory drugs.
Therefore, nutraceutical agents that modulate inflammatory
mediators in activated macrophages have been the focus of recent
research and may prove useful in the treatment of inflammatory
diseases with minimal adverse effects.
The present study demonstrated the in vitro
anti-inflammatory properties of schisandrin A in LPS-stimulated RAW
264.7 cells and proposed an underlying mechanism of action. LPS
derived from E. coli was used to generate the most widely
used experimental model to date for assessing the capacity of drugs
to inhibit the inflammatory response (1,2).
As in previous similar studies, it has been shown that LPS
treatment increased the production of pro-inflammatory mediators,
including NO and PGE2, in RAW 264.7 cells (3–6);
however, pretreatment with schisandrin A inhibited their production
in a concentration-dependent manner without affecting the cell
viability. It was also revealed that LPS-induced mRNA and protein
expression of iNOS as well as COX-2 was effectively inhibited by
schisandrin A in a dose-dependent manner. Although appropriate NO
levels influence numerous physiological conditions, excessive
levels of iNOS-induced NO in macrophages is closely associated with
several inflammatory diseases (6,7).
In addition, PGE2 produced from arachidonic acid by the
action of prostanase-forming enzyme COX-2 is mainly generated in
response to various inflammatory stimuli, and COX-2 overexpression
promotes inflammatory signaling cascades (6,8).
During the inflammatory response, activated macrophages also
produce pro-inflammatory cytokines, including TNF-α and IL-1β,
which influence acute as well as chronic inflammation; therefore,
reduction of these cytokines may delay the inflammatory response
(10,11). In the present study, alongside the
inhibition of LPS-induced NO and PGE2 generation,
LPS-activated TNF-α and IL-1β protein secretion and gene expression
were also effectively inhibited by schisandrin A.
To further elucidate the schisandrin A-mediated
anti-inflammatory mechanisms, the present study examined whether
schisandrin A affects the activation of NF-κB, an important
transcription factor involved in controlling DNA transcription and
cytokine production in response to inflammation (12,13). Under basal conditions, NF-κB is
present in the cytoplasm as a heterodimer with its specific
inhibitor IκB-α. IκB is rapidly phosphorylated and degraded by the
ubiquitination-mediated 26S proteasome when macrophages are induced
by inflammatory stimuli, including LPS (4,5,12).
The liberated NF-κB then translocates to the nucleus and binds to
the NF-κB binding site to initiate the transcription of various
inflammatory genes, leading to the development and progression of
inflammation (13,14). Thus, blocking the translocation of
NF-κB to the nucleus is considered an important target for agents
developed to inhibit the inflammatory response. In the present
study, it was observed that schisandrin A suppresses LPS-induced
IκB degradation, thus maintaining NF-κB in the cytoplasm and
decreasing nuclear translocation to ultimately reduce the
expression of the above-mentioned inflammatory genes.
It has been reported that the activation of NF-κB by
LPS is followed by a series of events, leading to the activation of
signaling pathways, including MAPKs and PI3K/Akt that are crucial
for regulating inflammation and producing inflammatory factors
(13,14). To investigate whether the MAPK
signaling pathways are involved in the schisandrin A-induced
inhibition of the inflammatory response, the effects of schisandrin
A on the activation of the three MAPKs (JNK, p38 MAPK and ERK) were
investigated, revealing that the LPS-induced phosphorylation of all
the three MAPKs was markedly suppressed by schisandrin A
pretreatment. In addition, schisandrin A also reduced the
LPS-induced phosphorylation PI3K and Akt, a dominant effector of
PI3K signaling (13,14), in RAW 264.7 cells. This implied
that schisandrin A reduced LPS-induced inflammation, at least
partially, by inhibiting the MAPK and PI3K/Akt signaling pathways,
which in turn blocked NF-κB inactivation.
While the production of an abnormally high amounts
of ROS stimulates the recruitment of additional macrophages to the
inflammation site for the release of pro-inflammatory mediators and
cytokines (1,37,38), LPS is known to rapidly stimulate
ROS production in macrophages and induce the secretion of
inflammatory mediators and cytokines (37,39). The present study demonstrated that
in RAW 264.7 macrophages stimulated by LPS, schisandrin A
significantly attenuated ROS generation. Although the exact
mechanisms of the association between the production of ROS and the
activation of NF-κB, MAPKs, and PI3K/Akt requires further
investigation, the present results indicate that inhibition of ROS
generation is an important component of the anti-inflammatory
effects of schisandrin A.
Nrf2 is a key coordinator for improving the
intracellular responses to several oxidative and inflammatory
insults. Furthermore, the beneficial effects of Nrf2 in various
diseases have been previously reported (16,40). Under normal redox conditions, Nrf2
is bound to Keap1, a representative repressor protein of Nrf2, in
the cytoplasm, and is easily degraded through the
ubiquitin-proteasome pathway. However, when exposed to a stressor
or inducer, Nrf2 dissociates from Keap1, migrates to the nucleus,
and is sequestered to the antioxidant response elements of
cytoprotective and antioxidant enzymes to promote gene expression
(13,18). The results of the present study
indicated that Nrf2 expression increased considerably with the
increase in the concentration and duration of schisandrin A
treatment. In addition, a concomitant decrease in Keap1 accompanied
by the translocation of Nrf2 into the nuclei was observed.
Furthermore, the expression of HO-1, a representative target gene
of Nrf2, was also significantly elevated by schisandrin A
treatment. HO-1 catalyzes the decomposition of heme to iron, carbon
monoxide and biliverdin, which in turn is converted to bilirubin by
biliverdin reductase (40–42).
The products of this enzymatic reaction have an important
biological role in antioxidant and cytoprotective processes through
eliminating ROS to suppress cell damage and death (17,42,43). Although further study assessing
the association between the inhibition of ROS production and the
activation of Nrf2/HO-1 signaling is warranted, the present results
suggest that schisandrin A-mediated induction of Nrf2 and HO-1 may
contribute to the inhibition of the inflammatory response to LPS by
decreasing oxidative stress in RAW 264.7 macrophages.
The fruit of Schizandra chinensis contains a
variety of lignan compounds, including schisandrin A, and has long
been used in the treatment of gastrointestinal disorders,
respiratory insufficiency, cardiovascular disease, physical fatigue
and weakness, excessive sweating and insomnia in East-Asia
(22,44). In Russian traditional medicine, it
has also been used as a tonic and a natural medicine with
restorative and anti-aging effects, as well as the ability to
improve vitality and mental wellbeing (19). Although clinical studies on the
anti-inflammatory effects of the fruit of Schizandra
chinensis are limited, it has been reported to have beneficial
effects on necrotizing inflammatory variables in patients with
chronic hepatitis C (45).
Therefore, the value of studies evaluating the various
pharmacological effects of lignan compounds derived from the fruit
of Schizandra chinensis, including the present study, are
important, and the present results suggested that schisandrin A may
be beneficial in patients with persistent and systemic
inflammation.
In conclusion, the results of the present study
indicated that schisandrin A exerts anti-inflammatory effects by
inhibiting the ROS production and reducing the activity of the MAPK
and PI3K/Akt pathways, resulting in the inhibition of NF-κB,
particularly in association with the activation of Nrf2/HO-1
signaling. This, in turn, inhibited the transcriptional activity of
the target genes of NF-κB, including inflammatory mediators and
cytokines, in a model of LPS-activated RAW 264.7 macrophages.
Although further investigation of the precise underlying mechanisms
is required, it is concluded that schisandrin A may be a natural
bioactive compound that exerts anti-inflammatory effects by
modulating multiple signaling pathways and may be a useful agent
for treating inflammation-mediated disease states.
Acknowledgments
This study was supported by the High Value-added
Food Technology Development Program (grant no. 314043-3), the
Ministry of Agriculture, Food and Rural Affairs, the International
Science and Business Belt Program through the Ministry of Science,
ICT and Future Planning (grant no. 2016K000297) and Blue-Bio
Industry Regional Innovation Center (RIC; grant no. RIC08-06-07) at
Dongeui University as an RIC program under the Ministry of Trade,
Industry and Energy and Busan city.
References
|
1
|
Cuschieri J and Maier RV: Oxidative
stress, lipid rafts, and macrophage reprogramming. Antioxid Redox
Signal. 9:1485–1497. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lucas K and Maes M: Role of the Toll like
receptor (TLR) radical cycle in chronic inflammation: Possible
treatments targeting the TLR4 pathway. Mol Neurobiol. 48:190–204.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leiro J, Alvarez E, Arranz JA, Laguna R,
Uriarte E and Orallo F: Effects of cis-resveratrol on inflammatory
murine macrophages: Antioxidant activity and down-regulation of
inflammatory genes. J Leukoc Biol. 75:1156–1165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Su YW, Chiou WF, Chao SH, Lee MH, Chen CC
and Tsai YC: Ligustilide prevents LPS-induced iNOS expression in
RAW 264.7 macrophages by preventing ROS production and
down-regulating the MAPK, NF-κB and AP-1 signaling pathways. Int
Immunopharmacol. 11:1166–1172. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hong SH, Jeong HK, Han MH, Park C and Choi
YH: Esculetin suppresses lipopolysaccharide-induced inflammatory
mediators and cytokines by inhibiting nuclear factor-κB
translocation in RAW 264.7 macrophages. Mol Med Rep. 10:3241–3246.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Murakami A and Ohigashi H: Targeting NOX,
INOS and COX-2 in inflammatory cells: Chemoprevention using food
phytochemicals. Int J Cancer. 121:2357–2363. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang GY, Taboada S and Liao J: Induced
nitric oxide synthase as a major player in the oncogenic
transformation of inflamed tissue. Methods Mol Biol. 512:119–156.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Norberg JK, Sells E, Chang HH, Alla SR,
Zhang S and Meuillet EJ: Targeting inflammation: Multiple
innovative ways to reduce prostaglandin E2. Pharm Pat
Anal. 2:265–288. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim J, Kim J and Bae JS: ROS homeostasis
and metabolism: A critical liaison for cancer therapy. Exp Mol Med.
48:e2692016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Klapproth JM and Sasaki M: Bacterial
induction of proinflammatory cytokines in inflammatory bowel
disease. Inflamm Bowel Dis. 16:2173–2179. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Striz I, Brabcova E, Kolesar L and
Sekerkova A: Cytokine networking of innate immunity cells: A
potential target of therapy. Clin Sci (Lond). 126:593–612. 2014.
View Article : Google Scholar
|
|
12
|
Endale M, Park SC, Kim S, Kim SH, Yang Y,
Cho JY and Rhee MH: Quercetin disrupts tyrosine-phosphorylated
phosphatidylinositol 3-kinase and myeloid differentiation factor-88
association, and inhibits MAPK/AP-1 and IKK/NF-κB-induced
inflammatory mediators production in RAW 264.7 cells.
Immunobiology. 218:1452–1467. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang BP, Lin CH, Chen HM, Lin JT, Cheng
YF and Kao SH: AMPK activation inhibits expression of
proinflammatory mediators through downregulation of PI3K/p38 MAPK
and NF-κB signaling in murine macrophages. DNA Cell Biol.
34:133–141. 2015. View Article : Google Scholar
|
|
14
|
Jung JS, Choi MJ, Lee YY, Moon BI, Park JS
and Kim HS: Suppression of lipopolysaccharide-induced
neuroinflammation by morin via MAPK, PI3K/Akt, and PKA/HO-1
signaling pathway modulation. J Agric Food Chem. 65:373–382. 2017.
View Article : Google Scholar
|
|
15
|
Nakajima S and Kitamura M: Bidirectional
regulation of NF-κB by reactive oxygen species: A role of unfolded
protein response. Free Radic Biol Med. 65:162–174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang Y, Li W, Su ZY and Kong AN: The
complexity of the Nrf2 pathway: Beyond the antioxidant response. J
Nutr Biochem. 26:1401–1413. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Loboda A, Damulewicz M, Pyza E, Jozkowicz
A and Dulak J: Role of Nrf2/HO-1 system in development, oxidative
stress response and diseases: An evolutionarily conserved
mechanism. Cell Mol Life Sci. 73:3221–3247. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kang KA and Hyun JW: Oxidative stress,
Nrf2, and epigenetic modification contribute to anticancer drug
resistance. Toxicol Res. 33:1–5. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Panossian A and Wikman G: Pharmacology of
Schisandra chinensis Bail.: An overview of Russian research and
uses in medicine. J Ethnopharmacol. 118:183–212. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liang CQ, Luo RH, Yan JM, Li Y, Li XN, Shi
YM, Shang SZ, Gao ZH, Yang LM, Zheng YT, et al: Structure and
bioactivity of triterpenoids from the stems of Schisandra
sphenanthera. Arch Pharm Res. 37:168–174. 2014. View Article : Google Scholar
|
|
21
|
Xiao WL, Huang SX, Wang RR, Zhong JL, Gao
XM, He F, Pu JX, Lu Y, Zheng YT, Zheng QT, et al: Nortriterpenoids
and lignans from Schisandra sphenanthera. Phytochemistry.
69:2862–2866. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chun JN, Cho M, So I and Jeon JH: The
protective effects of Schisandra chinensis fruit extract and its
lignans against cardiovascular disease: A review of the molecular
mechanisms. Fitoterapia. 97:224–233. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huyke C, Engel K, Simon-Haarhaus B, Quirin
KW and Schempp CM: Composition and biological activity of different
extracts from Schisandra sphenanthera and Schisandra chinensis.
Planta Med. 73:1116–1126. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim SR, Lee MK, Koo KA, Kim SH, Sung SH,
Lee NG, Markelonis GJ, Oh TH, Yang JH and Kim YC:
Dibenzocyclooctadiene lignans from Schisandra chinensis protect
primary cultures of rat cortical cells from glutamate-induced
toxicity. J Neurosci Res. 76:397–405. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang CP, Li GC, Shi YW, Zhang XC, Li JL,
Wang ZW, Ding F and Liang XM: Neuroprotective effect of schizandrin
A on oxygen and glucose deprivation/reperfusion-induced cell injury
in primary culture of rat cortical neurons. J Physiol Biochem.
70:735–747. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
E Q, Tang M, Zhang X, Shi Y, Wang D, Gu Y,
Li S, Liang X, Wang Z and Wang C: Protection of seven
dibenzocyclooctadiene lignans from Schisandra chinensis against
serum and glucose deprivation injury in SH-SY5Y cells. Cell Biol
Int. 39:1418–1424. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song F, Zeng K, Liao L, Yu Q, Tu P and
Wang X: Schizandrin A inhibits microglia-mediated
neuroninflammation through inhibiting TRAF6-NF-κB and Jak2-Stat3
signaling pathways. PLoS One. 11:e01499912016. View Article : Google Scholar
|
|
28
|
Leong PK, Wong HS, Chen J, Chan WM, Leung
HY and Ko KM: Differential action between schisandrin A and
schisandrin B in eliciting an anti-inflammatory action: The
depletion of reduced glutathione and the induction of an
antioxidant response. PLoS One. 11:e01558792016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Checker R, Patwardhan RS, Sharma D, Menon
J, Thoh M, Bhilwade HN, Konishi T and Sandur SK: Schisandrin B
exhibits anti-inflammatory activity through modulation of the
redox-sensitive transcription factors Nrf2 and NF-κB. Free Radic
Biol Med. 53:1421–1430. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park SY, Park SJ, Park TG, Rajasekar S,
Lee SJ and Choi YW: Schizandrin C exerts anti-neuroinflammatory
effects by upregulating phase II detoxifying/antioxidant enzymes in
microglia. Int Immunopharmacol. 17:415–426. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xie Y, Hao H, Wang H, Guo C, Kang A and
Wang G: Reversing effects of lignans on CCl4-induced hepatic CYP450
down regulation by attenuating oxidative stress. J Ethnopharmacol.
155:213–221. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ba Q, Cui C, Wen L, Feng S, Zhou J and
Yang K: Schisandrin B shows neuroprotective effect in
6-OHDA-induced Parkinson's disease via inhibiting the negative
modulation of miR-34a on Nrf2 pathway. Biomed Pharmacother.
75:165–172. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dong Q, Hou H, Wu J and Chen Y: The
Nrf2-ARE pathway is associated with Schisandrin b attenuating
benzo(a)pyrene-Induced HTR cells damages in vitro. Environ Toxicol.
31:1439–1449. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gao C, Chen H, Niu C, Hu J and Cao B:
Protective effect of Schizandrin B against damage of UVB irradiated
skin cells depend on inhibition of inflammatory pathways.
Bioengineered. 8:36–44. 2017. View Article : Google Scholar
|
|
35
|
Lee IC, Lee SM, Ko JW, Park SH, Shin IS,
Moon C, Kim SH and Kim JC: Role of mitogen-activated protein
kinases and nuclear factor-kappa B in
1,3-dichloro-2-propanol-induced hepatic injury. Lab Anim Res.
32:24–33. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim HK: Adenophora remotiflora protects
human skin keratinocytes against UVB-induced photo-damage by
regulating antioxidative activity and MMP-1 expression. Nutr Res
Pract. 10:371–376. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hernández-Ledesma B, Hsieh CC and de Lumen
BO: Antioxidant and anti-inflammatory properties of cancer
preventive peptide lunasin in RAW 264.7 macrophages. Biochem
Biophys Res Commun. 390:803–808. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mittal M, Siddiqui MR, Tran K, Reddy SP
and Malik AB: Reactive oxygen species in inflammation and tissue
injury. Antioxid Redox Signal. 20:1126–1167. 2014. View Article : Google Scholar :
|
|
39
|
Kasahara E, Sekiyama A, Hori M, Hara K,
Takahashi N, Konishi M, Sato EF, Matsumoto S, Okamura H and Inoue
M: Mitochondrial density contributes to the immune response of
macrophages to lipopolysaccharide via the MAPK pathway. FEBS Lett.
585:2263–2268. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee DH, Park JS, Lee YS, Sung SH, Lee YH
and Bae SH: The hypertension drug, verapamil, activates Nrf2 by
promoting p62-dependent autophagic Keap1 degradation and prevents
acetaminophen-induced cytotoxicity. BMB Rep. 50:91–96. 2017.
View Article : Google Scholar :
|
|
41
|
Pittalà V, Salerno L, Romeo G, Modica MN
and Siracusa MA: A focus on heme oxygenase-1 (HO-1) inhibitors.
Curr Med Chem. 20:3711–3732. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ryter SW and Choi AM: Targeting heme
oxygenase-1 and carbon monoxide for therapeutic modulation of
inflammation. Transl Res. 167:7–34. 2016. View Article : Google Scholar
|
|
43
|
Motterlini R and Foresti R: Heme
oxygenase-1 as a target for drug discovery. Antioxid Redox Signal.
20:1810–1826. 2014. View Article : Google Scholar
|
|
44
|
Szopa A, Ekiert R and Ekiert H: Current
knowledge of Schisandra chinensis (Turcz.) Baill. (Chinese magnolia
vine) as a medicinal plant species: A review on the bioactive
components, pharmacological properties, analytical and
biotechnological studies. Phytochem Rev. 16:195–218. 2017.
View Article : Google Scholar :
|
|
45
|
Melhem A, Stern M, Shibolet O, Israeli E,
Ackerman Z, Pappo O, Hemed N, Rowe M, Ohana H, Zabrecky G, et al:
Treatment of chronic hepatitis C virus infection via antioxidants:
Results of a phase I clinical trial. J Clin Gastroenterol.
39:737–742. 2005. View Article : Google Scholar : PubMed/NCBI
|