Introduction
With a complex pathophysiology, ischemic stroke is
damaging disease posing a significant threat to quality of life,
and is the second leading cause of mortality and disability
worldwide (1). As a contributor
to mortality rates and loss of central nerve cells in the infarct
region, ischemic cerebral artery thrombosis and embolus-induced
ischemic cerebrovascular occlusion are encountered most frequently
in cases of ischemic stroke (2).
On the occurrence of initial ischemic injury, swelling of the
damaged region of the brain may lead to secondary damage. The
primary cause of neurological deficits is the neuronal damage
caused by cerebral ischemia/reperfusion injury. Therefore, in cases
of cerebral ischemia injury, the promotion of neuranagenesis is
important as a measure of recovery of cerebral function. At
present, despite numerous investigations into the pathological
mechanism of ischemic stroke, few effective therapies are available
for patients with the disease.
It has been found that cerebral ischemia can limit
the supply of glucose and oxygen to brain tissue, which is
considered to be a causal factor for neuron degeneration, the onset
of ischemic stroke and Alzheimer's-type changes in the aged brain
(3–5). As a hypoxic environment can have a
detrimental effect on cell survival, hypoxic-ischemic cerebral
injury can be utilized as a model for acquired neurodegenerative
conditions with extensive parenchymal involvement. In practice, the
model of oxygen-glucose deprivation (OGD) is used for
investigations of cerebral ischemia injury in vitro, as its
conditions can be easily controlled (6). In in vitro investigations,
glucose-free culture medium is used for experiments involving
hypoxic conditions, with an oxygen content of <1%, to simulate
the status of cerebral ischemia and hypoxia caused by a short
supply of oxygen and glucose. Reperfusion, comprising the
resupplying of oxygen and glucose, is used to simulate the state of
blood flow recovery in brain tissue. The OGD/reperfusion (OGD/R)
model has been considered an ideal model for use in in vitro
investigations of ischemia/reperfusion injury, as the conditions of
the model can be easily controlled.
Neural stem cells (NSCs) are self-renewing
multipotent cells, which can generate neurons, astrocytes and
oligodendrocytes. The NSCs exist in the subventricular zone (SVZ)
of the lateral ventricle and the dentate gyrus subgranular zone of
the hippocampus throughout life (7,8).
The NSCs have neuroprotective functions due to their
anti-inflammatory, glial scar-inhibitory, and anti-apoptotic
effects; accordingly, they promote the recovery of patients with
neurological disease (9). These
characteristics of NSCs show that the NSCs can affect the
regeneration of damaged brain tissues and possess therapeutic
potential, which is promising for the treatment of pathological
processes of disorders or injuries of the central nervous system.
Although the characteristics of NSCs have been investigated
extensively for over a decade (10), to enable the use of NSCs for
accurate and safe therapies, key issues remain to be resolved,
including the effective promotion, proliferation and induction of
complete differentiation into neurons, of the NSCs.
It has been found that vascular endothelial growth
factor (VEGF) is an important signaling molecule in angiogenesis
and neurogenesis (11). Previous
studies have shown that VEGF mRNA can be expressed in the ischemic
cortex and hippocampus of rats with transient global cerebral
ischemia, and the mechanism may be associated with the expression
of hypoxia-inducible factor-1α (HIF-1α) by cerebral injury and the
regulatory downstream gene expression of VEGF (12,13). The mRNA expression of VEGF can
promote the migration and proliferation of microvascular
endothelial cells in the damaged region to form new blood vessels,
improve blood supply and reduce cerebral ischemic injury; the
regulatory downstream gene expression of VEGF can exert effects on
neurotrophic activities to induce the proliferation and
differentiation of NSCs and repair brain damage (14–17). Therefore, the HIF-1α-VEGF pathway
may be a component of the pathogenetic mechanism of cerebral
ischemic injury.
Ginsenoside is the major active component of
ginseng, and it has been shown to be safe and effective in the
treatment of acute ischemic stroke (18,19). It has been reported that
ginsenoside has effects on improving neurological outcome,
decreasingthe infarct area, ameliorating mitochondrial dysfunction,
reducing oxidative damage, promoting glutamate clearance and
inhibiting mitochondrial-nuclear translocation of
apoptosis-inducing factor, in rats with middle cerebral artery
occlusion (MCAO) injury (20–25). It has also been reported that
ginsenoside can promote the differentiation and proliferation of
NSCs (26). However, whether its
therapeutic effect on cerebral ischemia and hypoxia injury is
associated with the HIF-1α-VEGF pathway remains to be
elucidated.
The aim of the present study was to establish an
OGD/R model of NSCs to investigate the synergistic effects of
oxygen and glucose withdrawal, and the duration of OGD/R (2, 4 and
6 h) on the proliferation, differentiation and outgrowth of NSCs.
This was assessed by determining the protein levels of VEGF and
HIF-1α, and the growth status of the NSCs to confirm the effect of
ginsenoside in the promotion of NSC proliferation and
differentiation by regulating the HIF-1α-VEGF pathway.
Materials and methods
Chemicals and reagents
Fetal bovine serum (FBS), DMEM/F12, basic fibroblast
growth factor, penicillin-streptomycin liquid and trypsin were
obtained from Invitrogen; Thermo Fisher Scientific, Inc. (Waltham,
MA, USA); polylysine, mouse anti-bromodeoxyuridine (BrdU) antibody
(cat. no. ab8152), rabbit anti-nestin antibody (cat. no. ab11306),
mouse anti-neuron-specific class III β-tubulin (tuj-1) antibody
(cat. no. ab52623), mouse anti-vimentin antibody (cat. no. ab8978),
goat anti-rabbit IgG antibody (cat. no. ab6939), and goat anti-rat
IgG antibody (cat. no. ab6717) were obtained from Abcam (Cambridge,
MA, USA); 2-(4-amidinophenyl)-6-indolecarbamidine (DAPI) staining
solution and HEPES were obtained from Solarbio (Beijing, China);
dimethylsulfoxide (DMSO) was obtained from Amresco LLC (Solon, OH,
USA); and β-glycerin sodium and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were obtained from Beyotime (Beijing, China).
Animals
A total of 4 pregnant female Sprague-Dawley rats of
SPF grade were purchased from Sibeifu Experimental Animal Science
and Technology Co., Ltd. (Beijing, China; no. SCXK2011-0004) on
embryonic day 17 (E17). All animals were housed individually at
22±2°C and a relative humidity of 50±10% with a 12 h light/12 h
dark cycle. Food and water were given ad libitum throughout
the experiment. All procedures in the present study were performed
in accordance with the institutional guidelines and ethics of
Beijing University of Chinese Medicine (Beijing, China). All
surgical procedures were performed under anesthesia and all efforts
were made to minimize suffering.
Isolation and culture of NSCs
The E17 fetuses were removed from the female rats in
advanced pregnancy individually following anesthetization,
following which the fetuses were immediately decapitated, and the
brain and its surrounding meninges were immediately removed. The
primary cell cultures were established from the hippocampal tissues
of the fetuses. The dissociated embryonic tissue was digested with
0.05% trypsin for 25 min at 37°C, and the suspension of NSCs was
passed through a 200-mesh sieve following washing with DMEM-F12 and
NSC complete culture solution. Following cell counting with a light
microscope (magnifcation, ×40), the harvested NSCs were grown in an
incubator (Thermo Fisher Scientific, Inc.) with 5% CO2
and at a constant temperature of 37°C. Following culture for 5–7
days, spheres of NSCs were formed in the suspension, which were
mechanically dissociated into individual cells. The suspension of
NSCs was then digested with 0.05% trypsin for 2 min at 37°C, and
cultured to a clonal density of 1×105 cells/ml in 5%
CO2 at 37°C.
MTT method
The MTT method was used to identify the optimal
concentration of ginsenoside and duration of OGD/T on the
proliferation and differentiation of the NSCs. Following isolation,
the single-cell suspension (1×105 cells/ml) was cultured
in a 96-well plate coated with poly-lysine (0.01%, wt/vol).
Following culture in a humidified 5% CO2/95% air
incubator at 37°C for 24 h, ginsenoside at 10 concentrations (0.25,
0.5, 1, 2.5, 6.25, 12.5, 25, 50, 100 and 200 μg/ml) were
respectively added into the plate wells for 3 days. In the control
group, ginsenoside was added into complete medium only. The optical
density (OD) values of each well were detected on a microplate
reader at a wavelength of 570 nm, and the optimal concentration was
calculated according to the OD values.
The single-cell suspension (5×105
cells/ml) was cultured in 96-well plates coated with poly-lysine
(0.01%, wt/vol) with the optimal dose of ginsenoside. The cells
were cultured for eight periods of time (1, 3, 6, 12, 24, 48, 72
and 96 h) in the control group and ginsenoside group. The OD values
of each well were detected on a microplate spectrophotometer, and
the optimal time-period was calculated according to the OD
values.
OGD/R model establishment and ginsenoside
administration
Three groups, namely the control group, vehicle
group and ginsenoside-treated group, were included in the present
study. The OGD/R model was established as reported previously
(27,28) with minor modification. The cells
in the control group were incubated with glucose Earle's balanced
salt solution (BSS), and the cells in the vehicle group and
ginsenoside-treated group were incubated with glucose-free Earle's
BSS. The cells were then immediately transferred to a humidified
anaerobic chamber for 4 h with 94% N2, 5% CO2
and 1% O2 at 37°C. During the OGD process, ginsenoside
(1 μg/ml) was added to the Earle's BSS in the
ginsenoside-treated group. For terminating cell OGD and perfusion,
the cells were then cultured in neurobasal medium and the
supplements under a humidified atmosphere of 5% CO2 and
95% air at 37°C, during which ginsenoside (1 μg/ml) was
added to the medium in the ginsenoside-treated group again. The
cells were then used for the subsequent experiments of western blot
analysis, enzyme-linked immunosorbent assay (ELISA) and
double-labeling immunofluorescence.
Western blot analysis
Western blot analysis was performed on the extracts
prepared from the different groups of NSCs, and the cell lysates
collected and analyzed for evaluating the expression of HIF-1α.
PMSF (2 μl; 0.25 mol/l), cytoplasmic extraction reagent
(CER)I, CERII and nuclear extraction reagent were added in sequence
to the drying cell lysates, and the nucleoprotein was collected
from the supernatant following repeated vortexing and
centrifugation (16,000 × g, 4°C, 10 min).
The concentrations of the nucleoprotein were
determined with BCA protein assay kit. The nucleoprotein (30
μg) was then resolved by sodium dodecyl sulfate-PAGE, and
then transferred onto a PVDF membrane (EMD Millipore, Billerica,
MA, USA). The membrane was incubated with primary antibodies HIF-1α
(1:500, cat. no. ab113642; Abcam, Beijing, China) in
saline/Tween-20 buffered by 1% bovine serum albumin (Thermo Fisher
Scientific, Inc.)/phosphate at 4°C overnight, following the
incubation with secondary antibodies goat anti-mouse IgG (1:30,
cat. no. SA00001-1; ProteinTech, Hubei, China) at room temperature
for 2 h. The protein signals were detected using the enhanced
chemiluminescence method.
ELISA
The cell-free supernatants of the treated and
untreated NSC suspensions in each group were collected 72 h
following treatment with ginsenoside, and were used to measure the
production of VEGF using an ELISA kit according to the
manufacturer's protocol. The levels of VEGF were determined and
calculated according to the results of the OD value at 450 nm.
Immunofluorescence staining
The design of the fluorescent labeling experiment
for each group is shown in Table
I. The cells (1×105/ml) were plated onto coverslips
for 24 h, and the coverslips were respectively coated with
poly-D-lysine and immunofluorescent labeling for nestin/BrdU,
nestin/vimentin, and nestin/tuj-1, in order to detect the
self-renewal and proliferation, astrocytic differentiation, and
neuronal differentiation of the NSCs. The primary antibodies used
comprised nestin and BrdU antibodies (1:300 and 1:400,
respectively), nestin and vimentin antibodies (1:400 and 1:500,
respectively), and nestin and tuj-1 antibodies (1:400 each). Goat
anti-rabbit IgG-Cy3 (1:50) and goat anti-mouse IgG-FITC (1:50) were
used as the secondary antibodies. Counterstaining of cell nuclei
was then performed with DAPI (100 ng/ml) for 10 min. Viable cells
were then counted with laser-scanning confocal microscopy (Olympus,
Tokyo, Japan).
 | Table ITreatment groups and durations of
OGD/R. |
Table I
Treatment groups and durations of
OGD/R.
| Fluorescent-labeled
antibodies | Group | Time of OGD
(h) | Time of simulated
reperfusion (h) | Total OGD/R time
(h) |
|---|
| Nestin/BrdU | Control | 0 | 6 | 6 |
| 0 | 8 | 8 |
| 0 | 10 | 10 |
| Vehicle | 4 | 2 | 6 |
| 4 | 4 | 8 |
| 4 | 6 | 10 |
|
Ginsenoside-treated | 4 | 2 | 6 |
| 4 | 4 | 8 |
| 4 | 6 | 10 |
| Nestin/tuj-1 | Control | 0 | 6 | 6 |
| 0 | 8 | 8 |
| 0 | 10 | 10 |
| Vehicle | 4 | 2 | 6 |
| 4 | 4 | 8 |
| 4 | 6 | 10 |
|
Ginsenoside-treated | 4 | 2 | 6 |
| 4 | 4 | 8 |
| 4 | 6 | 10 |
|
Nestin/vimentin | Control | 0 | 6 | 6 |
| 0 | 8 | 8 |
| 0 | 10 | 10 |
| Vehicle | 4 | 2 | 6 |
| 4 | 4 | 8 |
| 4 | 6 | 10 |
|
Ginsenoside-treated | 4 | 2 | 6 |
| 4 | 4 | 8 |
| 4 | 6 | 10 |
Image analysis and statistical
analysis
Image-Pro Plus software (version 6.0; Media
Cybernetics, Inc., Rockville, MD, USA) was used to analyze the
number, density and OD of the positive cells in the fluorescence
images. All data were processed with the use of SPSS 20.0 (IBM
SPSS, Armonk, NY, USA). The data are expressed as the mean ±
standard deviation. The significance of variables was determined
using a paired-sample t-test. P<0.05 was considered to indicate
a statistically significant difference.
Results
Identification of NSCs
Initially, the cells in the suspension prepared
during the dissection of the fetuses were single, small and
transparent, and were round or elliptical in shape with little
protuberance and a glossy appearance. Cell clone spheres began to
form at 24 h post-dissection. They became round in shape following
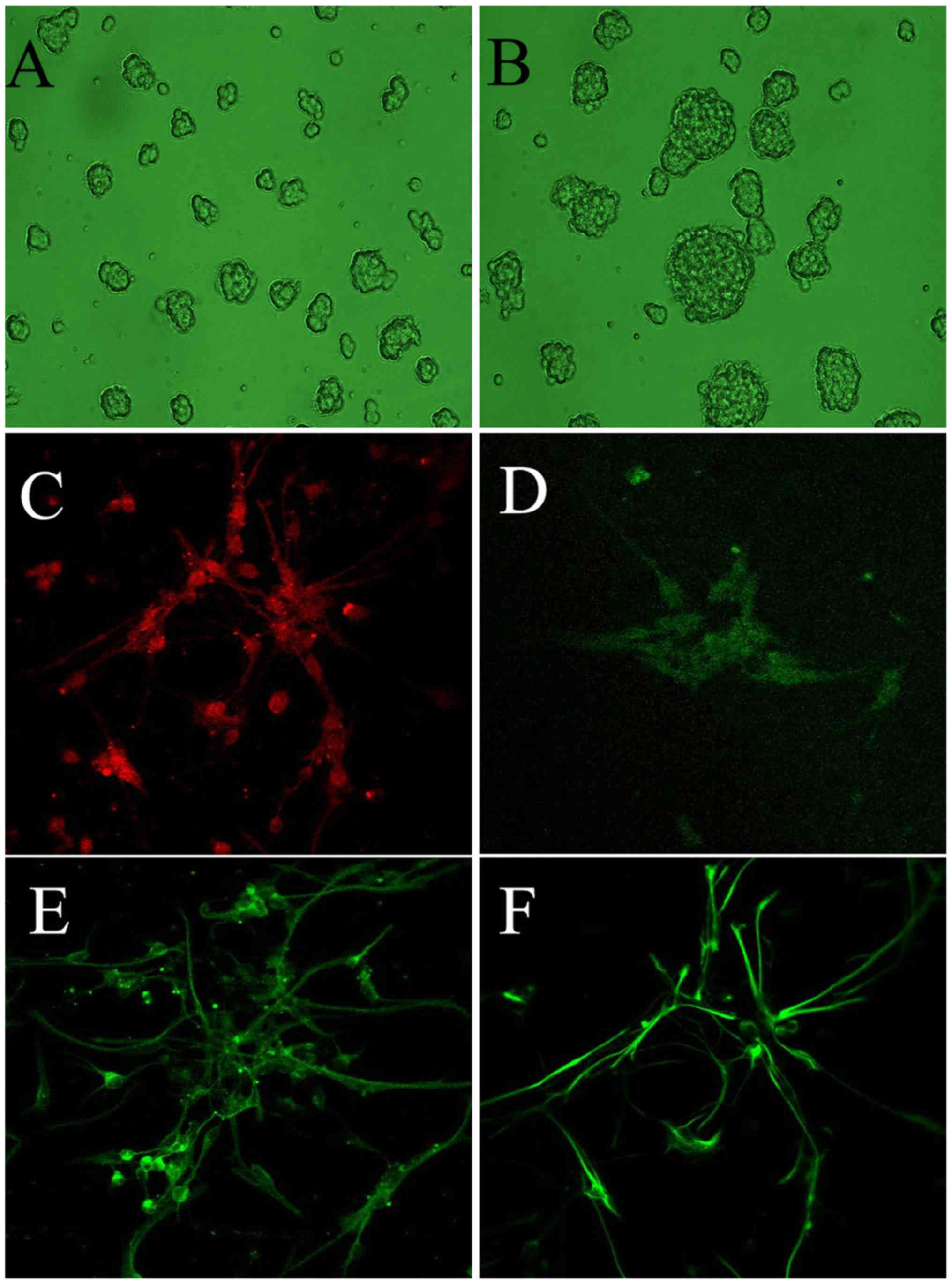
3–4 days and were passaged at 7–8 days (Fig. 1). The primary neurospheres had a
regular morphology without irregularly sized processes, and their
volumes increased with culture duration, the same as was observed
in the passaged cell clone spheres.
The expression of nestin was measured following 4
days of cell culture (Fig. 1C)
The nestin-positive cells varied in volume, with or without
apophyses, and positive red staining was observed in the cytoplasm.
The stained cells were round or oval in shape, and their nuclear
zones were unstained and often shifted to one side of the cell.
Positive dark green staining was observed in the
nuclear region, which confirmed the expression of BrdU, whereas
less staining was observed in the cytoplasm (Fig. 1D) The BrdU-positive cells were
found to be small in volume with apophyses.
A green color was observed in the cytoplasm, which
confirmed the expression of tuj-1 (Fig. 1E). The nuclear region of
tuj-1-positive cells was unstained and often shifted to one side of
the cell. The tuj-1-positive cells were small in volume, round in
shape and featured apophyses.
The expression of vimentin, confirmed by green
staining, was observed in the cytoplasm (Fig. 1F). The nuclear zones of the
vimentin-positive cells were unstained and often shifted to one
side of the cell.
The above-mentioned results indicated that the NSCs
were identified by the positive expression of nestin, NSC
proliferation was assessed according to the positive expression of
BrdU, and NSC differentiation was identified by the enhanced
expression of tuj-1 and vimentin.
Determination of the optimal dose and
OGD/R duration for the effect of ginsenoside on NSC proliferation
and differentiation
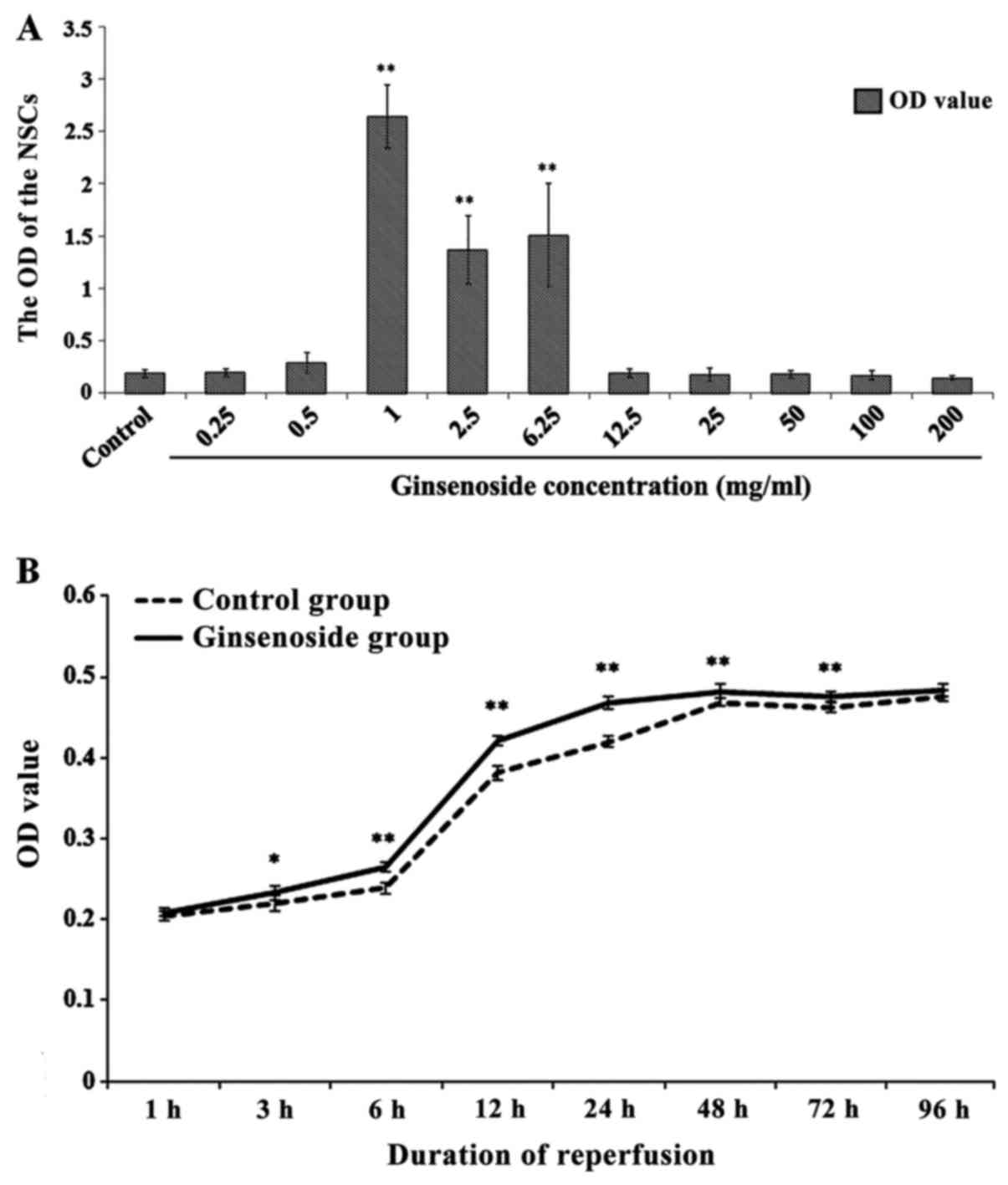
The effects of ginsenoside on the proliferation and
differentiation of NSCs, which were cultured with different
concentrations of ginsenoside for 3 days, are shown in Fig. 2A. It was found that, compared with
the control group, the OD values of the NSCs were significantly
increased by ginsenoside at concentrations of 1, 2.5 and 6.25
μg/ml (P<0.01), according to the MTT assay. In addition,
the OD value of the NSCs reached its highest level at a
concentration of 1 μg/ml, which suggested that this may be
the optimal dose for affecting the proliferation and
differentiation of the NSCs.
The effect of ginsenoside at a dose of 1
μg/ml with different OGD/R durations on the proliferation
and differentiation of NSCs is shown in Fig. 2B. Compared with that in the
control group, the OD value of the NSCs was significantly higher
between 3 and 72 h in the ginsenoside-treated group, which
indicated that ginsenoside promoted the proliferation and
differentiation of NSCs in this period of time; however, it was
found that this effect was optimal within 24 h, following which it
weakened with time.
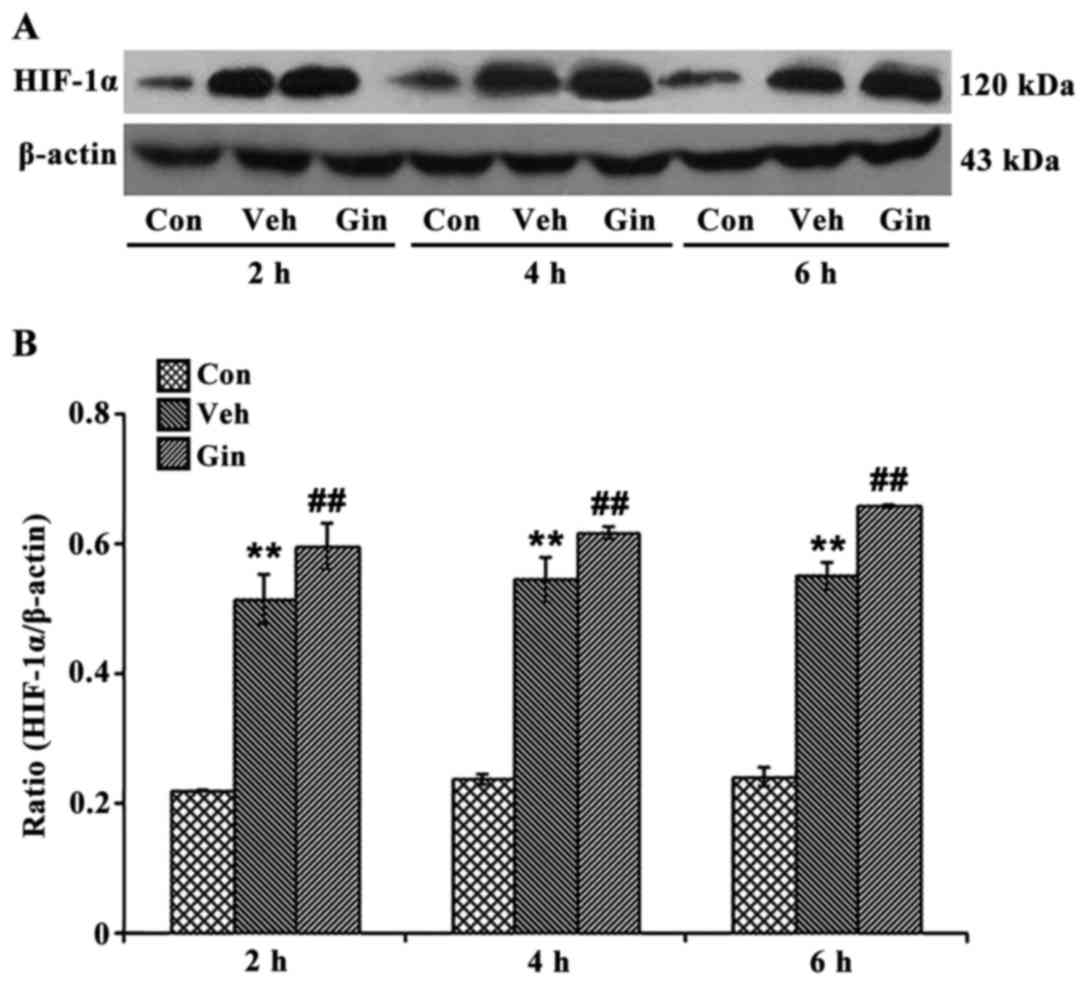
Effect of ginsenoside on the protein
expression of HIF-1
The expression of HIF-1α in the ginsenoside-treated
group was confirmed using western blot analysis (Fig. 3). The results of the western blot
analysis showed that, compared with the vehicle group, the
expression of HIF-1α in the OGD/R-treated NSCs in the
ginsenoside-treated group was significantly increased (P<0.05).
The difference between the expression levels of HIF-1α in the NSCs
of the vehicle and ginsenoside-treated groups gradually increased
with the duration of simulated reperfusion, and were highest at 6
h.
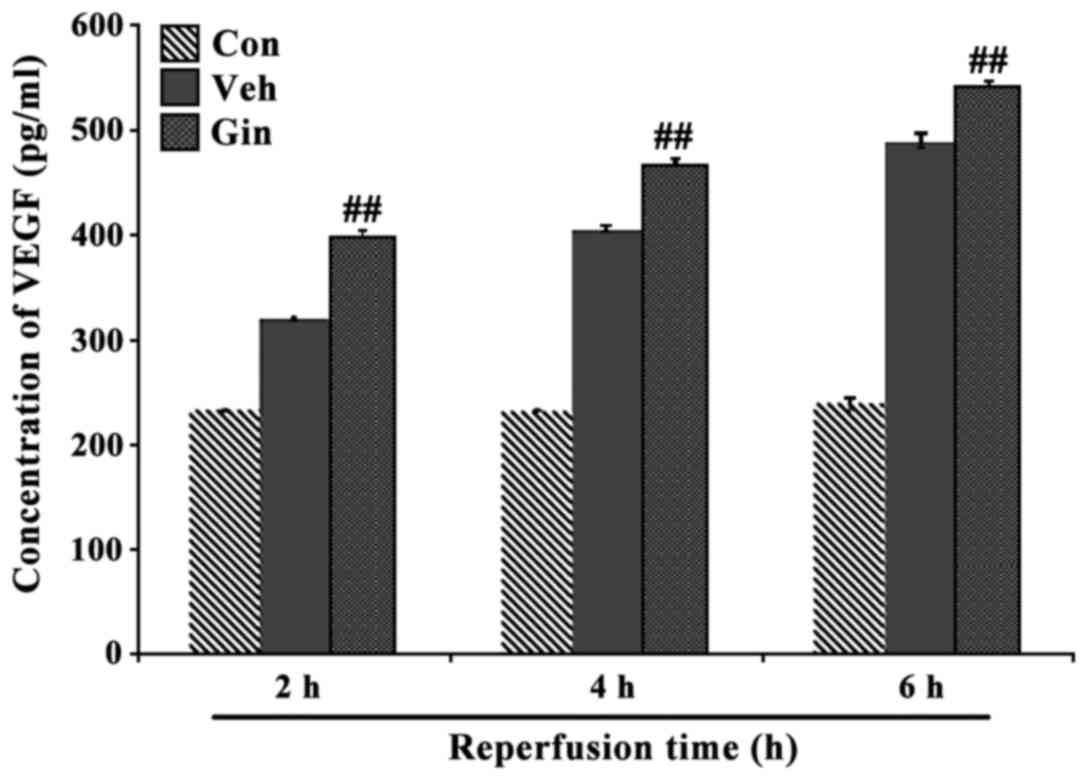
Effect of ginsenoside on the protein
expression of VEGF
The results of the ELISA showed that the protein
expression of VEGF was at a low level in the NSCs of the control
group (Fig. 4), however, the
level was markedly upregulated when the cells were damaged by
hypoxia (P<0.05). The protein levels of VEGF in the NSCs of the
ginsenoside-treated group were significantly increased with the
duration of simulated reperfusion (P<0.05), showing the same
results as with HIF-1α.
Effect of ginsenoside on the
proliferation and differentiation of NSCs
The results of the immunofluorescence staining
experiments of the effects of ginsenoside on the proliferation and
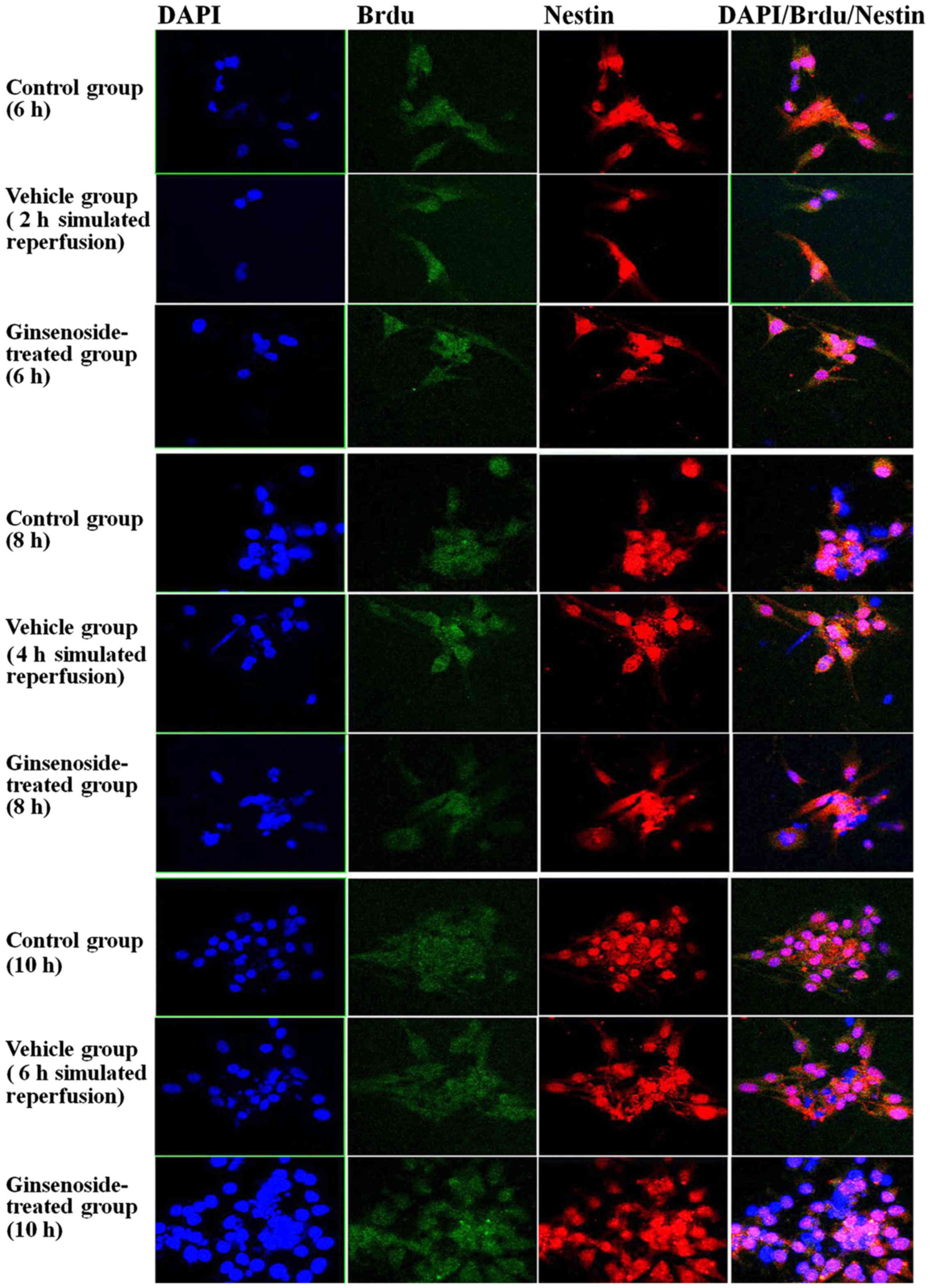
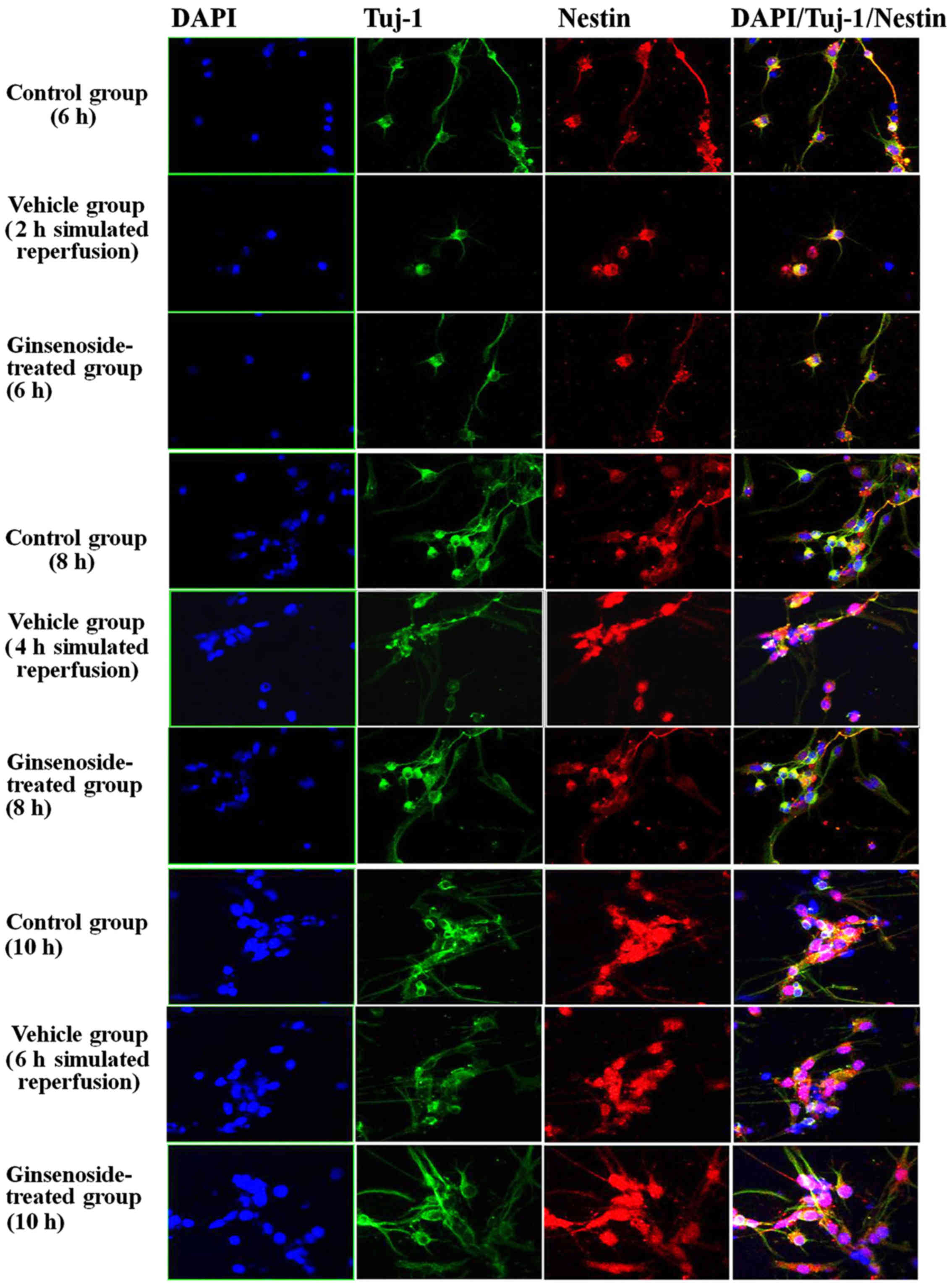
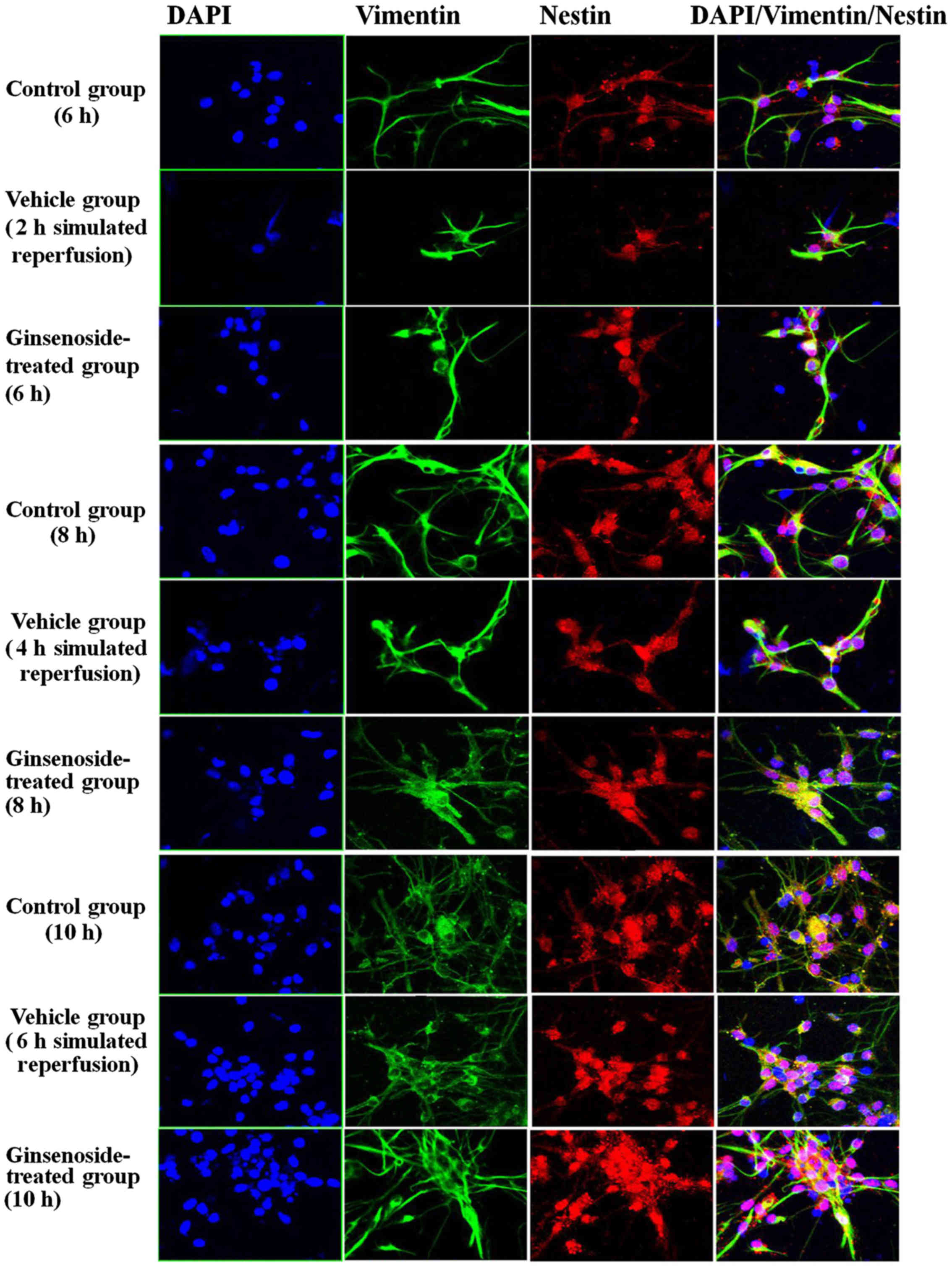
differentiation of NSCs are shown in Figs. 5Figure 6–7, which include a series of findings.
Firstly, it was found that the expression of nestin-positive cells
was in the cytoplasm, and the body of the cell was round or oval in
shape with no or few neurites; the number of the nestin-positive
cells in the vehicle group was lower, compared with that in the
control group, and the cells had no or few neurites. However, it
was found that, in the ginsenoside-treated group, the number of
nestin-positive cells increased and the majority of the cells
contained neurites. In the ginsenoside-treated group, the BrdU
staining was present mainly in the nuclei of the nestin-positive
cells with minimal staining in the cytoplasm. The cells were also
smaller in volume and contained neurites. Secondly, it was found
that the number of BrdU-positive cells decreased and the cells
contained no or few protrusions in the vehicle group, compared with
those in the control group. The number of BrdU-positive cells was
increased and the cells contained increased neurites. The tuj-1
staining was observed mainly in the cytoplasm and the cells were
smaller in volume with neurites. In the vehicle group, the number
of tuj-1-positive cells was reduced and the volumes of the cells
were smaller, compared with those in the ginsenoside-treated group,
in which the volume of cells and the number of neurites were
increased, with aggregation of vimentin staining in the cytoplasm.
Thirdly, it was found that, in the vehicle group, the number of
vimentin-positive cells was decreased and the cells were smaller in
volume with fewer neurites, compared with those in the control
group. However, in the ginsenoside-treated group, the number of
vimentin-positive cells and the volume of the cells were increased
and the cells contained more neurites.
The statistical results showed that, compared with
those in the control group, the number of positive cells, OD and
area density were significantly decreased between 2 and 4 h of
reoxygenation (P<0.01) in the vehicle group, whereas the
positive cell number, OD and area density were significantly
increased (P<0.01) in the ginsenoside-treated group, compared
with those in the vehicle group. The statistical results also
showed that the number of positive cells, OD and area density were
decreased at 6 h of reoxygenation (P>0.05) in the vehicle group,
whereas the number of positive cells, OD and density were increased
significantly (P<0.01), compared with those in the vehicle
groups (Figs. 8Figure 9–10).
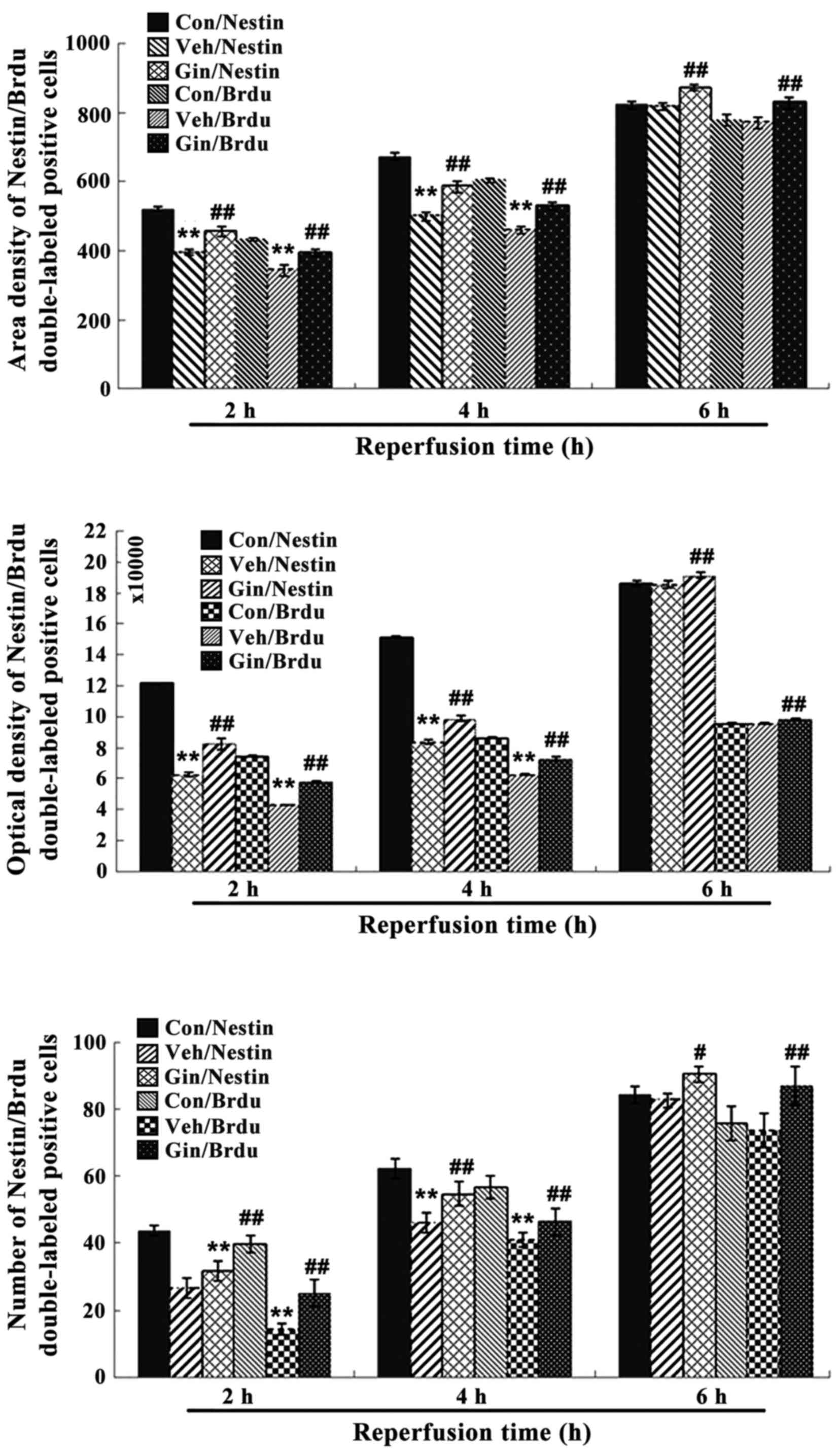 | Figure 8Nestin/BrdU double-labeled
immunochemical staining results following 2, 4 and 6 h of
reperfusion. Results for area density, optical density and positive
number are shown. **P<0.01 vs. Con;
#P<0.05 and ##P<0.01 vs. Veh. Compared
with those in the control group, the number of positive cells,
optical density and area density were decreased significantly
between 2 and 4 h of reoxygenation (P<0.01) in the vehicle group
and were significantly increased (P<0.01) in the
ginsenoside-treated group, compared with those in the vehicle
group. The statistical results showed that the number of positive
cells, optical density and area density were decreased following 6
h of reoxygenation (P>0.05) in the vehicle group, but were
significantly increased (P<0.01) in the ginsenoside-treated
group, compared with those in the vehicle groups. Veh. Con, control
group; Veh, vehicle group; Gin, ginsenoside-treated group; BrdU,
bromodeoxyuridine. |
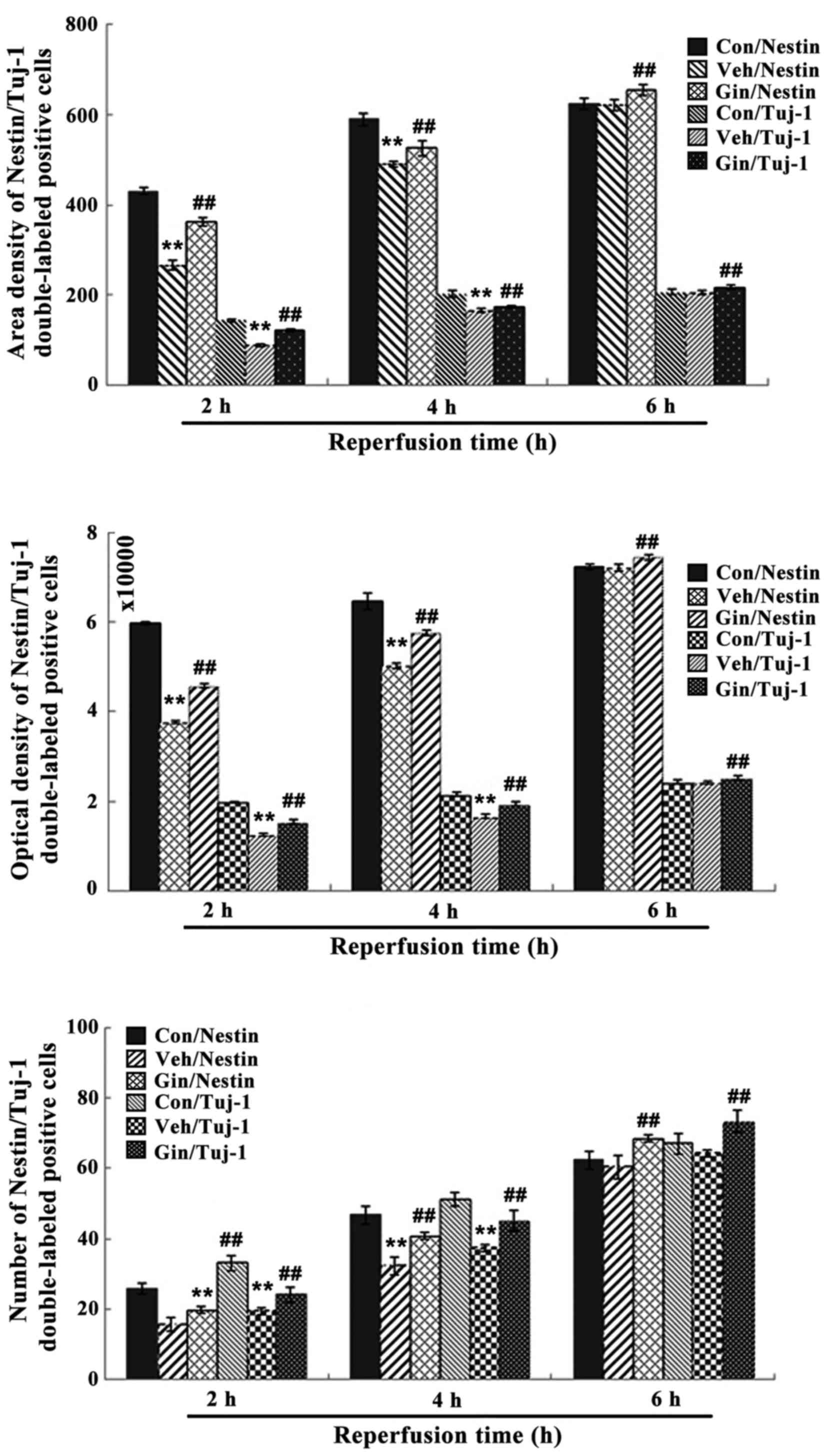 | Figure 9Nestin/tuj-1 double-labeled
immunochemical staining results of the control group, vehicle group
and ginsenoside-treated group following 2, 4 and 6 h. of
reperfusion. **P<0.01 vs. Con; ##P<0.01
vs. Veh. Compared with those in the control group, the number of
positive cells, optical density and area density were significantly
decreased between 2 and 4 h of reoxygenation (P<0.01) in the
vehicle group, but were increased significantly (P<0.01) in the
ginsenoside-treated group, compared with those in the vehicle
group. The number of positive cells, optical density and area
density were decreased following 6 h of reoxygenation (P>0.05)
in the vehicle group, but were increased significantly (P<0.01)
in the ginsenoside-treated group, compared with those in the
vehicle group. Veh. Con, control group; Veh, vehicle group; Gin,
ginsenoside-treated group; BrdU, bromodeoxyuridine; tuj-1,
neuron-specific class III β-tubulin. |
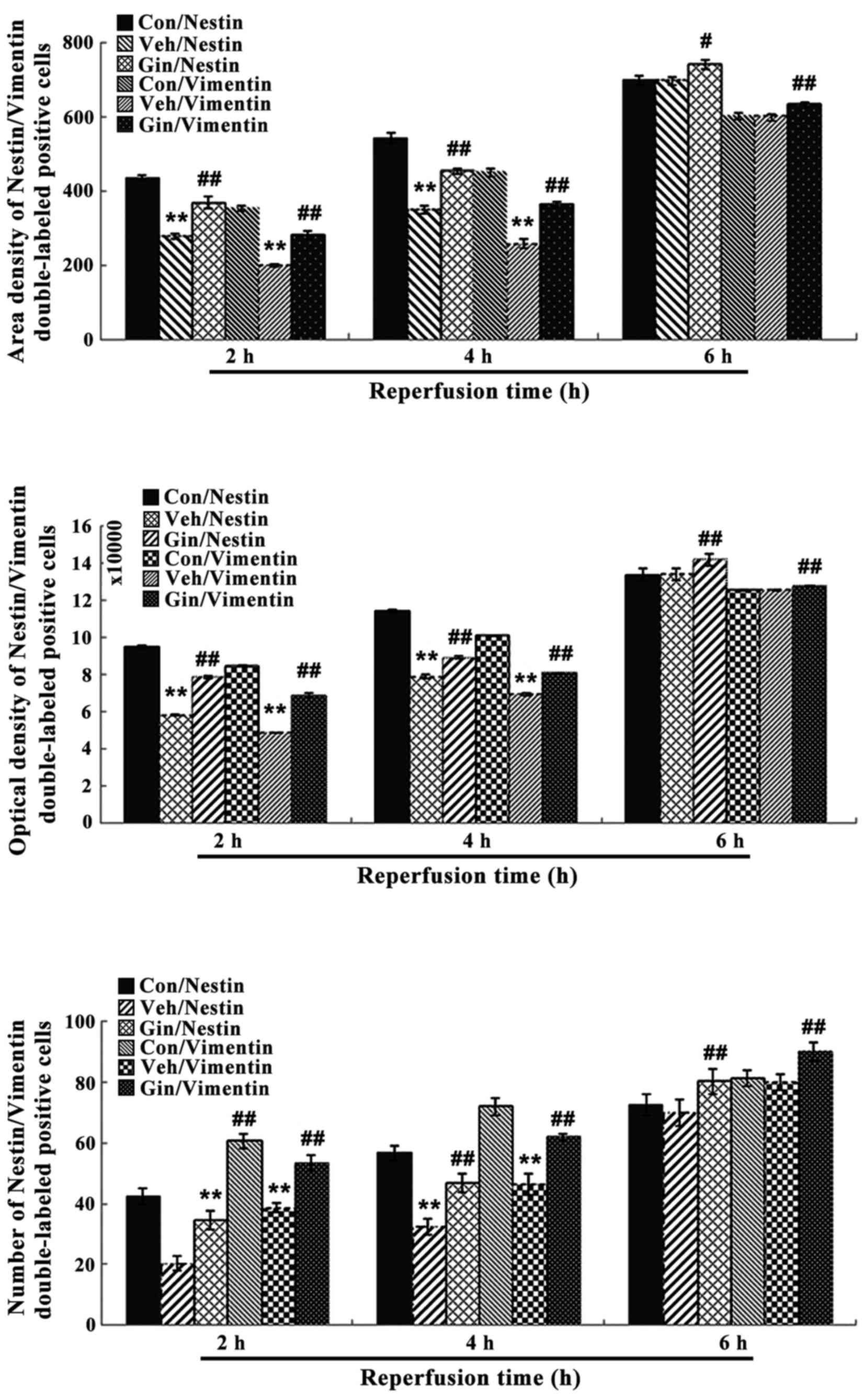 | Figure 10Nestin/vimentin double-labeled
immunochemical staining results of the control group, vehicle
group, and ginsenoside-treated group following 2, 4 and 6 h of
reperfusion. **P<0.01 vs. Con; #P<0.05;
##P<0.01 vs. Veh. Compared with those in the control
group, the number of positive cells, optical density and area
density were decreased significantly between 2 and 4 h of
reoxygenation (P<0.01) in the vehicle group, but were increased
significantly (P<0.01) in the ginsenoside-treated group,
compared with those in the vehicle group. The number of positive
cells, optical density and area density were decreased following 6
h of reoxygenation (P>0.05) in the vehicle group, but were
increased significantly (P<0.01) in the ginsenoside-treated
group, compared with those in the vehicle group. Veh. Con, control
group; Veh, vehicle group; Gin, ginsenoside-treated group; BrdU,
bromodeoxyuridine. |
Discussion
Characterized by a loss of neurons and glial cells
in the brain, stroke, or traumatic brain injury, can lead to the
death of brain cells. NSCs can proliferate and migrate into the
injured region of the brain, and differentiate into the
corresponding nerve cells to become involved in the formation of
neural circuits, and the promotion of structural and functional
repair of the injured brain (29,30). NSCs may also be involved in
treatment of the brain degeneration associated with certain
diseases, including Parkinson's disease and Alzheimer's disease
(31–33). Ginsenoside can exert effects
promoting the proliferation and differentiation of NSCs (34), however, the molecular mechanism of
this effect remains to be elucidated.
Acting upstream of the Wnt/β-catenin pathway, HIF-1α
may contribute to the production of VEGF (35), which is an important signaling
molecule in angiogenesis and neurogenesis (11). As a master regulator of cellular
adaptation to hypoxia, HIF-1α is considered as a potent therapeutic
target in cerebral ischemia. HIF-1α can be activated by the
reduction in cellular oxygen supply caused by MCAO, followed by the
secretion of its downstream protein, VEGF; this is a critical
factor in angiogenesis, and can be produced and secreted by various
types of cell to increase capillary permeability and stimulate the
proliferation of endothelial cells (36–38). A previous study using a neonatal
hypoxia/ischemia model also confirmed the effects of ginsenoside on
inhibiting apoptosis, and increasing the expression of HIF-1α and
VEGF in injured brain tissue. Tang et al concluded that Rg1
has a neuroprotective role in brain repair following neonatal
hypoxia/ischemia, and that HIF-1α is a potential target for
therapeutic intervention in neonates with hypoxic/ischemic brain
injury (39). As a type of
intermediate filament protein, nestin is a specific marker for
embryonic NSCs. Previous in vitro studies have shown that
NSCs are characterized by the expression of nestin, capacity for
continuous proliferation, self-renewal, and multidirectional
differentiation under specific conditions (16,40–42). The results of the present study
showed that ginsenoside at certain concentrations increased the
number of nestin-expressing NSCs, which suggested that ginsenoside
may promote the survival, proliferation and self-renewal of NSCs by
inducing the production of undifferentiated and nestin-positive
cells. The results from the experiments on ginsenoside-treated
BrdU-positive NSCs suggested that ginsenoside accelerated the
proliferation of NSCs. The results of the experiments on rats in
the present study also suggested that ginsenoside promoted the
differentiation of embryonic cortical NSCs into tuj-1-positive
neurons and vimentin-positive astrocytes.
The increased number of positive NSCs, higher OD
values and area density in the ginsenoside-treated group in the
present study suggested that ginsenoside may protect the nerve
cells from injury induced by OGD/R. The increase in the number of
positive NSCs, in addition to the increased duration for
reoxygenation may be due to the hypoxia-induced activation of
HIF-1α and the formation of HIF-1, which is the result of the
migration of activated HIF-1α to the nucleus and combing with
HIF-1β. HIF-1 may regulate the production of VEGF protein following
transcription and translation. The stability of VEGF protein can
increase under anoxic conditions, and the combination of its
receptors and specific ligands can promote angiogenesis,
proliferation and migration of nerve cells, neurotrophic activity
and nerve regeneration. VEGF can also promote NSC survival in the
brain and stimulate neurogenesis in vitro and in vivo
(15–17). The change in the number of
positive NSCs in the ginsenoside-treated group in the present study
showed that ginsenoside may increase the differentiation and
proliferation of NSCs. The increase in the expression levels of
VEGF and HIF-1α by ginsenoside indicated that ginsenoside may exert
cerebral protection by promoting the protein expression of HIF-1α,
followed by initiating the downstream VEGF pathway at the early
phase of OGD/R.
In conclusion, the results from the experiments in
the present study suggested that ginsenoside may maintain the
replication of NSCs, promote NSC proliferation, promote their
differentiation into neurons and astrocytes. This indicated that
ginsenoside exerted a protective effect on the NSCs injured by
ischemia-reperfusion through the HIF-1α-VEGF pathway. The results
of the present study provide a theoretical basis for the possible
treatment of ischemic stroke with ginsenoside, and provide scope
for investigating the mechanisms of traditional Chinese medicine
preparations in treating diseases associated with the nervous
system in novel drugs.
Acknowledgments
This study was funded by the National Natural
Science Foundation of China (grant no. 81373830).
References
|
1
|
Shi Q, Zhang P, Zhang J, Chen X, Lu H,
Tian Y, Parker TL and Liu Y: Adenovirus-mediated brain-derived
neurotrophic factor expression regulated by hypoxia response
element protects brain from injury of transient middle cerebral
artery occlusion in mice. Neurosci Lett. 465:220–225. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mehta SL, Manhas N and Raghubir R:
Molecular targets in cerebral ischemia for developing novel
therapeutics. Brain Res Brain Res Rev. 54:34–66. 2007. View Article : Google Scholar
|
|
3
|
Peruzzotti-Jametti L, Donegá M, Giusto E,
Mallucci G, Marchetti B and Pluchino S: The role of the immune
system in central nervous system plasticity after acute injury.
Neuroscience. 283:210–221. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kalluri HS, Eickstaedt J and Dempsey RJ:
Oxygen glucose deprivation inhibits the growth and ERK
phosphorylation of neural progenitor cells in vitro. Neurosci Lett.
426:145–148. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lipton P: Ischemic cell death in brain
neurons. Physiol Rev. 79:1431–1568. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
de Pablo Y, Nilsson M, Pekna M and Pekny
M: Intermediate filaments are important for astrocyte response to
oxidative stress induced by oxygen-glucose deprivation and
reperfusion. Histochem Cell Biol. 140:81–91. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reynolds BA and Weiss S: Generation of
neurons and astrocytes from isolated cells of the adult mammalian
central nervous system. Science. 255:1707–1710. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alvarez-Buylla A and Lim DA: For the long
run: Maintaining germinal niches in the adult brain. Neuron.
41:683–686. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bacigaluppi M, Pluchino S,
Peruzzotti-Jametti L, Kilic E, Kilic U, Salani G, Brambilla E, West
MJ, Comi G, Martino G, et al: Delayed post-ischaemic
neuroprotection following systemic neural stem cell transplantation
involves multiple mechanisms. Brain. 132:2239–2251. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jin K, Mao X, Xie L, Galvan V, Lai B, Wang
Y, Gorostiza O, Wang X and Greenberg DA: Transplantation of human
neural precursor cells in Matrigel scaffolding improves outcome
from focal cerebral ischemia after delayed postischemic treatment
in rats. J Cereb Blood Flow Metab. 30:534–544. 2010. View Article : Google Scholar :
|
|
11
|
Sun J, Sha B, Zhou W and Yang Y:
VEGF-mediated angiogenesis stimulates neural stem cell
proliferation and differentiation in the premature brain. Biochem
Biophys Res Commun. 394:146–152. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Blancher C, Moore JW, Talks KL, Houlbrook
S and Harris AL: Relationship of hypoxia-inducible factor
(HIF)-1alpha and HIF-2alpha expression to vascular endothelial
growth factor induction and hypoxia survival in human breast cancer
cell lines. Cancer Res. 60:7106–7113. 2000.
|
|
13
|
Ben-Yosef Y, Lahat N, Shapiro S, Bitterman
H and Miller A: Regulation of endothelial matrix
metalloproteinase-2 by hypoxia/reoxygenation. Circ Res. 90:784–791.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Olsson AK, Dimberg A, Kreuger J and
Claesson-Welsh L: VEGF receptor signalling - in control of vascular
function. Nat Rev Mol Cell Biol. 7:359–371. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun Y, Jin K, Xie L, Childs J, Mao XO,
Logvinova A and Greenberg DA: VEGF-induced neuroprotection,
neurogenesis, and angiogenesis after focal cerebral ischemia. J
Clin Invest. 111:1843–1851. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jin K, Zhu Y, Sun Y, Mao XO, Xie L and
Greenberg DA: Vascular endothelial growth factor (VEGF) stimulates
neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA.
99:11946–11950. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wada T, Haigh JJ, Ema M, Hitoshi S,
Chaddah R, Rossant J, Nagy A and van der Kooy D: Vascular
endothelial growth factor directly inhibits primitive neural stem
cell survival but promotes definitive neural stem cell survival. J
Neurosci. 26:6803–6812. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu X, Wang L, Wen A, Yang J, Yan Y, Song
Y, Liu X, Ren H, Wu Y, Li Z, et al: Ginsenoside-Rd improves outcome
of acute ischaemic stroke - a randomized, double-blind,
placebo-controlled, multicenter trial. Eur J Neurol. 19:855–863.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu X, Xia J, Wang L, Song Y, Yang J, Yan
Y, Ren H and Zhao G: Efficacy and safety of ginsenoside-Rd for
acute ischaemic stroke: A randomized, double-blind,
placebo-controlled, phase II multi-center trial. Eur J Neurol.
16:569–575. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ye R, Yang Q, Kong X, Han J, Zhang X,
Zhang Y, Li P, Liu J, Shi M, Xiong L, et al: Ginsenoside Rd
attenuates early oxidative damage and sequential inflammatory
response after transient focal ischemia in rats. Neurochem Int.
58:391–398. 2011. View Article : Google Scholar
|
|
21
|
Ye R, Zhang X, Kong X, Han J, Yang Q,
Zhang Y, Chen Y, Li P, Liu J, Shi M, et al: Ginsenoside Rd
attenuates mitochondrial dysfunction and sequential apoptosis after
transient focal ischemia. Neuroscience. 178:169–180. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ye R, Kong X, Yang Q, Zhang Y, Han J and
Zhao G: Ginsenoside Rd attenuates redox imbalance and improves
stroke outcome after focal cerebral ischemia in aged mice.
Neuropharmacology. 61:815–824. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ye R, Kong X, Yang Q, Zhang Y, Han J, Li
P, Xiong L and Zhao G: Ginsenoside Rd in experimental stroke:
Superior neuroprotective efficacy with a wide therapeutic window.
Neurotherapeutics. 8:515–525. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu G, Wu Z, Yang F, Zhao H, Liu X, Deng Y,
Shi M and Zhao G: Ginsenoside Rd blocks AIF mitochondrio-nuclear
translocation and NF-κB nuclear accumulation by inhibiting
poly(ADP-ribose) polymerase-1 after focal cerebral ischemia in
rats. Neurol Sci. 34:2101–2106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang X, Shi M, Bjørås M, Wang W, Zhang G,
Han J, Liu Z, Zhang Y, Wang B, Chen J, et al: Ginsenoside Rd
promotes glutamate clearance by up-regulating glial glutamate
transporter GLT-1 via PI3K/AKT and ERK1/2 pathways. Front
Pharmacol. 4:1522013. View Article : Google Scholar :
|
|
26
|
Si YC, Li Q, Xie CE, Niu X, Xia XH and Yu
CY: Chinese herbs and their active ingredients for activating xue
(blood) promote the proliferation and differentiation of neural
stem cells and mesenchymal stem cells. Chin Med. 9:132014.
View Article : Google Scholar
|
|
27
|
Lin D, Li G and Zuo Z: Volatile anesthetic
post-treatment induces protection via inhibition of glycogen
synthase kinase 3β in human neuron-like cells. Neuroscience.
179:73–79. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yan W, Fang Z, Yang Q, Dong H, Lu Y, Lei C
and Xiong L: SirT1 mediates hyperbaric oxygen
preconditioning-induced ischemic tolerance in rat brain. J Cereb
Blood Flow Metab. 33:396–406. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gage FH: Mammalian neural stem cells.
Science. 287:1433–1438. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Eriksson PS, Perfilieva E, Björk-Eriksson
T, Alborn AM, Nordborg C, Peterson DA and Gage FH: Neurogenesis in
the adult human hippocampus. Nat Med. 4:1313–1317. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bayer SA, Yackel JW and Puri PS: Neurons
in the rat dentate gyrus granular layer substantially increase
during juvenile and adult life. Science. 216:890–892. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Temple S and Alvarez-Buylla A: Stem cells
in the adult mammalian central nervous system. Curr Opin Neurobiol.
9:135–141. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kuhn HG and Svendsen CN: Origins,
functions, and potential of adult neural stem cells. BioEssays.
21:625–630. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang B, Feng G, Tang C, Wang L, Cheng H,
Zhang Y, Ma J, Shi M and Zhao G: Ginsenoside Rd maintains adult
neural stem cell proliferation during lead-impaired neurogenesis.
Neurol Sci. 34:1181–1188. 2013. View Article : Google Scholar
|
|
35
|
Lee SH, Kim MH and Han HJ: Arachidonic
acid potentiates hypoxia-induced VEGF expression in mouse embryonic
stem cells: Involvement of Notch, Wnt, and HIF-1alpha. Am J Physiol
Cell Physiol. 297:C207–C216. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Land SC and Tee AR: Hypoxia-inducible
factor 1alpha is regulated by the mammalian target of rapamycin
(mTOR) via an mTOR signaling motif. J Biol Chem. 282:20534–20543.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tokuda H, Adachi S, Matsushima-Nishiwaki
R, Kato K, Natsume H, Otsuka T and Kozawa O: Enhancement of basic
fibroblast growth factor-stimulated VEGF synthesis by Wnt3a in
osteoblasts. Int J Mol Med. 27:859–864. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ferrara N: Vascular endothelial growth
factor: Basic science and clinical progress. Endocr Rev.
25:581–611. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tang B, Wang D, Li M, Wu Q, Yang Q, Shi W
and Chen C: An in vivo study of hypoxia-inducible factor-1α
signaling in ginsenoside Rg1-mediated brain repair after
hypoxia/ischemia brain injury. Pediatr Res. 81:120–126. 2017.
View Article : Google Scholar
|
|
40
|
Si YC, Zhang JP, Xie CE, Zhang LJ and
Jiang XN: Effects of Panax notoginseng saponins on proliferation
and differentiation of rat hippocampal neural stem cells. Am J Chin
Med. 39:999–1013. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jori FP, Galderisi U, Piegari E, Cipollaro
M, Cascino A, Peluso G, Cotrufo R, Giordano A and Melone MA:
EGF-responsive rat neural stem cells: Molecular follow-up of neuron
and astrocyte differentiation in vitro. J Cell Physiol.
195:220–233. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Meng XT, Li C, Dong ZY, Liu JM, Li W, Liu
Y, Xue H and Chen D: Co-transplantation of bFGF-expressing amniotic
epithelial cells and neural stem cells promotes functional recovery
in spinal cord-injured rats. Cell Biol Int. 32:1546–1558. 2008.
View Article : Google Scholar : PubMed/NCBI
|