Introduction
Diabetes mellitus (DM) is a metabolic disorder
characterized by chronic hyperglycemia resulting from a defect in
insulin metabolism and impaired function of carbohydrate, lipid and
protein metabolism that lead to long-term complications. DM is
associated with the generation of reactive oxygen species (ROS),
which cause oxidative damage, particularly to the liver, kidney,
eyes, small and large blood vessels, immune and gastrointestinal
systems (1–3). The liver, an insulin-dependent
organ, plays a pivotal role in glucose and lipid homeostasis, and
is severely affected during the progression of DM (4,5).
Furthermore, the liver is the focal organ involved in oxidizing and
detoxifying processes, as well as free radical reactions (6-8).
Accumulating literature reviews suggest that enhanced oxidative
stress and significant reduced antioxidant defenses are considered
to play an important role in diabetic liver injury (9,10).
Zanthoxylum bungeanum (Z. bungeanum) belongs
to the Zanthoxylum genus of the Rutaceae family. The fruit
of Z. bungeanum, locally called 'Huajiao', is one of the
best-known and widely used plants in traditional Chinese medicine.
Young Z. bungeanum leaves have been used as foodstuffs, and
mature Z. bungeanum leaves are considered carminative,
stimulant and sudorific (11).
The previous studies revealed that Z. bungeanum leaves are
rich in flavonoids with good radical scavenging abilities (12,13). Some studies have indicated that
hyperoside is the major flavonoid of Z. bungeanum leaves
(12–15). Pharmacological investigations have
demonstrated that hyperoside have diverse biological activities
such as antioxidant (12,16), anticancer (17), anti-inflammatory (18), anticoagulant (19) and cardioprotective activities
(20). Moreover, hyperoside has
been found to play crucial roles in rat lens aldose reductase
inhibition (21). Previously, it
has been reported that hyperoside has a pivotal role in blood
glucose level in streptozotocin-induced hyperglycemia by improving
the function of pancreatic islets, increasing glycolysis and
decreasing gluconeogenesis (22).
Hyperoside may be one of the primary glucosidase inhibitor
constituents of Agrimonia pilosa Ledeb (23). However, only a few researchers
have paid attention to investigating the preventive effects of
hyperoside from Z. bungeanum leaves on diabetic liver
injury. Currently, there are no available studies on the effects of
hyperoside on diabetes induced by a high-carbohydrate/high-fat diet
(HFD) and alloxan in mice, at least to the best of our knowledge.
Since flavonol supplements in dietary food may evoke protective
effects under oxidative stress, the present study determined the
protective effects of hyperoside from Z. bungeanum leaves
(HZL) on hyperglycemia and the liver damaged induced by HFD diet
and alloxan in mice.
Materials and methods
Reagents
Leaves of Z. bungeanum were collected in
Shaanxi, China in August 2015 and identified by experts in the
College of Forestry, Northwest A&F University (Xian, China).
Alloxan was purchased from Sigma-Aldrich; Merck KGaA (Darmstadt,
China). Blood glucose was meas ured using kits from Shanghai
Rongsheng Biotechnology Co., Ltd. (Shanghai, China). The levels of
total cholesterol (TC, cat. no. A111-1), triglyceride (TG, cat. no.
A110-1), low-density lipoprotein cholesterol (LDL-C, cat. no.
A113-1), high-density lipoprotein cholesterol (HDL-C, cat. no.
A112-1), nitric oxide (NO, cat. no. A012-1), malondialdehyde (MDA,
cat. no. A003-1), and the activities of
Na+/K+ ATPase (cat. no. A070-2), inducible
nitric oxide synthase (iNOS, cat. no. A014-1-1), plasma alanine
aminotransferase (ALT, cat. no. A009-2), aspartate aminotransferase
(AST, cat. no. C010-2), superoxide dismutase (SOD, cat. no.
A001-1-1), catalase (CAT, cat. no. A007-1-1), glutathione
peroxidase (GPx, cat. no. A005) were detected using kits from the
Nanjing Jiancheng Bioengineering Research Institute (Nanjing,
China). Insulin levels were determined using a radioimmunoassay kit
from Biosino Bio-Technology and Science, Inc. (Beijing, China).
Rabbit anti-active p65, p-p65, p38, p-p38, JNK, p-JNK, ERK, p-ERK
activating transcription factor 3 (ATF3), Bcl-2, Bax, cytochrome
c (Cyt c), caspase-9, and caspase-3 polyclonal antibodies
were all purchased from Cell Signaling Technology, Inc. (Danvers,
MA, USA); β-actin antibodies (cat. no. SC10731) were purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). All the chemicals
were of analytical grade. Hyperoside was isolated from Z.
bungeanum leaves. Z. bungeanum leaves were soaked in a
70% ethanol solvent (1:10, w/v) for 2.5 h and sonicated in an
ultrasonic bath at 200 kHz at 55°C for 45 min. Samples were
filtered, concentrated and dried using a rotary evaporator. The
remaining ethanol crude extracts was further fractioned by column
chromatography on silica gel (silica gel 200-300 mesh, 120×10 cm
i.d., flow rate 10 ml/min), successively eluting with petroleum
ether, chloroform, ethyl acetate, acetone and methanol. A fraction
of ethyl acetate was further separated and purified by
high-performance liquid chromatography. The chromatographic
conditions used were as follows: the column was a SB-C18 (250 mm ×
4.6 mm i.d., 5 μm) at ambient temperature. The injection
volume was 20 μl and the detection wavelength was 254 nm.
The mobile phase consisted of water with 0.5% trifluoroacetic acid
(solvent A) and acetonitrile with 0.5% trifluoroacetic acid
(solvent B). The flow rate was 0.8 ml/min. The gradient program was
set as follows: from 0 to 30 min, eluent B was increased from 15 to
35%; from 30 to 35 min, eluent B was increased from 35 to 65%; and
from 35 to 55 min, eluent B was increased from 65 to 100% and then
maintained at 100% for 10–20 min. Samples were filtered through a
0.22 μm membrane filter prior to injection. The eluent was
concentrated using the rotary vacuum evaporator and vacuum-dried to
obtain hyperoside from HZL. The structure of the isolate was
determined by reverse phase high-performance liquid chromatography
in comparison with authentic hyperoside (National Institute for the
Control of Pharmaceutical and Biological Products, Beijing,
China).
Animals, diets and experimental
design
Male Chinese Kunming mice (6-week-old, weighing 20±2
g, n=90) were obtained from the Experimental Animal Center of Xi'an
Jiaotong University (Xi'an, China) and were allowed to acclimatize
for 1 week before being randomly assigned to different experimental
groups. All of the mice were maintained on a 12 h light/dark cycle
on a standard chow diet until experimental analysis. The
experimental animal protocol was approved by the Experimental
Animal Ethics Committee of Xi'an Jiaotong University. The
experimental procedures were carried out in accordance with
international guidelines for the Care and Use of Experimental
Animals.
After adaptation for 1 week, mice were randomly
divided into 6 groups (n=15 in each group, 5 mice/cage) as follows:
i) normal group; ii) normal + 200 mg/kg body weight (BW)/day HZL
(HHZL) groups; iii) DM group: HFD-alloxan treatment (HFD, 52.6%
standard laboratory chow, 10% lard, 15% sucrose, 15% yolk powder,
5% casein, 1.2% cholesterol, 0.2% bile salt, 0.6% calcium
bicarbonate); iv) DM + 50 mg/kg BW/day HZL (LHZL); v) DM + 100
mg/kg BW/day HZL (MHZL); and vi) DM + 200 mg/kg BW/day HZL (HHZL).
HZL was suspended in 1% Tween-80 in water prior to administration.
The mice in groups 2 and 4–6 received daily administrations by
gastric gavage of different doses HZL, and mice in groups 1 and 3
were administered the vehicle (1% Tween-80 in water, 10 ml/kg BW)
once a day for 4 weeks. After 4 weeks of dietary manipulation, the
groups of mice fed the HFD were injected intraperitoneally with
0.04% alloxan dissolved in sterile normal saline in a dose of 100
mg/kg BW The mice were allowed to continue to feed on their
respective diets till the experimental tenure. Water and food were
available to the animals ad libitum. Body weight and food
intake were recorded weekly. Two weeks after alloxan injection, the
animals were anesthetized with isoflurane (3%) after fasting for 8
h, and blood samples were obtained by cardiac puncture. The mice
were then sacrificed by cervical dislocation and liver were
collected, weighed, frozen in liquid nitrogen and stored at −80°C
for further analysis.
Biochemical analyses of blood
samples
Plasma was obtained by centrifuging the blood at
2,000 × g for 15 min at 4°C. Fasting blood glucose level was
monitored periodically during the treatment with the tail prick
method using kits based on the glucose oxidase method. The blood
glucose level was expressed as mmol/l. Plasma insulin, TC, TG,
HDL-C and LDL-C levels were measured by adhering tothe commercial
kits' instructions.
The oral glucose tolerance test (OGTT) was performed
at 14 days following alloxan treatment. Briefly, after fasting for
8 h, blood was collected from the tail veins of all mice (0 min).
Immediately after blood collection, all mice received an
intraperitoneal injection of glucose (2 g/kg BW). Blood samples
were successively collected at the indicated time intervals (0, 30,
60 and 120 min), and blood glucose levels were determined as
mentioned above. The area under the curve (AUC) was calculated as
the area under the glucose curve from 0 to 120 min multiplied by
the minutes at the measured time-points.
Liver oxidative stress levels test
Liver were thawed, weighed and homogenized with
Tris-HCl (5 mM containing 2 mM EDTA, pH 7.4). Homogenates were
centrifuged (1,500 × g, 10 min, at 4°C) and the supernatant was
used immediately for the assays. Na+/K+
ATPase activity, ALT activity, AST activities, lipid peroxidation
product MDA level, SOD activity, CAT activity, GPx activity, iNOS
activity and NO content were measured by following the commercial
kits' instructions.
Western blot analysis of proteins
Whole protein lysates of liver tissue were extracted
using radioimmunoprecipitation assay buffer [25 mM Tris-HCl (pH
7.6), 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS;
Thermo Fisher Scientific, Inc., Waltham, MA, USA] supplemented with
1% protease inhibitor cocktail and 1% phenylmethylsulfonyl
fluoride. Protein concentrations were measured using a BCA Protein
Assay kit (A045-3; Nanjing Jiancheng Bioengineering Research
Institute). Aliquots containing 50 μg of protein were loaded
onto a into 8% SDS-PAGE gel, immunoblotted onto a polyvinylidene
difluoride membrane (Takara Biotechnology Co., Ltd., Dalian,
China), blocked for 1 h at room temperature with 5% bovine serum
albumin in TBS buffer, and then incubated overnight (4°C) with
respective primary antibodies for anti-p65 (cat. no. 3034),
anti-p-p65 (cat. no. 3033), anti-p38 (cat. no. 9212), anti-p-p38
(cat. no. 9216), anti-JNK (cat. no. 9252), anti-p-JNK (cat. no.
9255), anti-ERK1/2 (cat. no. 4695), anti-p-ERK1/2 (cat. no. 4370),
anti-Bcl-2 (cat. no. 2876), anti-Bax (cat. no. 2772s), anti-Cyt c
(cat. no. 4280s), anti-caspase-9 (cat. no. 95083) and
anti-caspase-3 (cat. no. 9662s) (diluted 1:1,000; all from Cell
Signaling Technology, Inc.) and anti-ATF3 (cat. no. ab7005, diluted
1:1,000; Abcam, Cambridge, UK). After being washed with TBS-T,
membrane was incubated with secondary horseradish
peroxidase-conjugated anti-rabbit IgGs (cat. no. ZB2301, diluted
1:2,000; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.,
Beijing, China) for 2 h at room temperature. The bound complexes
were detected by incubating with a chemiluminescence solution
purchased from EMD Millipore (cat. no. WBKLS0050; Billerica, MA,
USA) according to the manufacturer's instructions.
Chemiluminescence was imaged on a Fujifilm LAS-3000 system
(Fujifilm, Tokyo, Japan). The immunoblot bands were quantified by
densitometry analysis, and the ratio to β-actin was calculated and
presented, setting the values of normal diet fed mice as 1.
Liver histological analysis
Liver tissues were fixed with 10% formalin and
processed for paraffin embedding. Tissue sections were cut to 5
μm and stained with hematoxylin and eosin for assessing
histopathological changes. The degree of injury was assessed
semi-quantitatively by the following criteria and scored based on
the area affected: i) increased eosinophilic staining of the
hepatocytes and the accumulation of erythrocytes in the sinusoids
(0, none; 1, <25%; 2, 25 to <50%; 3, 50 to <75%; and 4, 75
to 100%); ii) cellular vacuolization (0, none; 1, <25%; and 2,
>25%); and iii) cell lysis (0, none; 1, <25%; and 2,
>25%). The final score of each sample is the summarization of
the three parameters.
Transmission electron microscope
A portion of liver (~1 mm3) from control
and experimental groups of mice were fixed in 3% glutaraldehyde in
sodium phosphate buffer (200 mM, pH 7.4) for 3 h at 4°C. Tissue
samples were washed with the same buffer, post-fixed in 1% osmium
tetroxide and sodium phosphate buffer (200 mM, pH 7.4) for 1 h at
4°C. The samples were again washed with the same buffer for 3 h at
4°C, dehydrated with graded series of ethanol and embedded in
araldite. Thin sections were cut with LKBUM4 ultramicrotome using a
diamond knife (Diatome Ltd., Nidau, Switzerland), mounted on a
copper grid and stained with 2% uranyl acetate and Reynolds lead
citrate. The grids were examined under a Hitachi H-7650
transmission electron microscope (Hitachi, Ltd., Tokyo, Japan).
Statistical analysis
All data are expressed as the means ± standard
deviation of at least three independent determinations for each
experiment. Statistical analyses were performed using SPSS version
13.0 (SPSS, Inc., Chicago, IL, USA). A one-way analysis of variance
(ANOVA) with Duncan's multiple range test was used to examine
differences between groups.
Results
Hypoglycemic and lipid metabolic effects
of HZL
Hypoglycemic effect of HZL on the body weight,
fasting blood sugar levels and insulin levels of normal and
diabetic mice was presented in Fig.
1. As shown in Fig. 1A–E,
HHZL did not cause a significant (P>0.05) change in body weight,
blood sugar levels and insulin levels in normal mice. Body weight,
fasting blood glucose levels and insulin levels in the mice with
HFD-alloxan-induced diabetes were significantly increased
(P<0.01, P<0.01 and P<0.01, respectively) in comparison to
the normal mice during 6 weeks. The administration of 200, 100 and
50 mg/kg BW HZL (HHZL, MHZL and LHZL) in the diabetic groups led to
a significant decrease (P<0.01, P<0.01 and P<0.05,
respectively) in body weight as compared with untreated diabetic
mice in 6 weeks. In serum glucose level, administration HHZL, MHZL
and LHZL in diabetic treated groups lead to significant decrease
(P<0.01, P<0.01 and P<0.01, respectively) as compared with
untreated diabetic mice at 6 weeks. In OGTT, compared with diabetic
mice, HZL lowered the blood glucose after glucose loading and
reduced the AUC (Fig. 1D),
indicating that HZL improved the glucose tolerance. Also, a
noticeable decrease (P<0.01, P<0.01 and P<0.01,
respectively) was observed in serum insulin level in HHZL, MHZL and
LHZL groups than the diabetic treated group at 6 weeks. Fig. 1F–I showed the mean values of TC,
TG, LDL-C and HDL-C levels of both control and experimental groups
after 6 weeks. An increased HDL-C level (P<0.01) and a decreased
LDL-C level (P<0.05) were observed in HHZL-treated normal mice,
albeit with marginal change of TC and TG levels (P>0.05).
HFD-alloxan-treatment resulted in obvious development of DM in
mice, characterized by elevated blood TC, TG and LDL-C level
(P<0.01, P<0.01 and P<0.01, respectively), and decreased
HDL-C level (P<0.01). The mice with HFD-alloxan-induced diabetes
that were treated with HHZL, MHZL and LHZL exhibited lowered TC, TG
(P<0.01, P<0.01 P>0.05 for TC; P<0.01, P<0.01,
P<0.01 for TG, respectively) and largely restored HDL-C levels
(P<0.01, P<0.01 and P<0.01, respectively), compared with
the untreated diabetic mice. In all HZL treatment groups, no
significant change was observed in LDL-C levels (P>0.05).
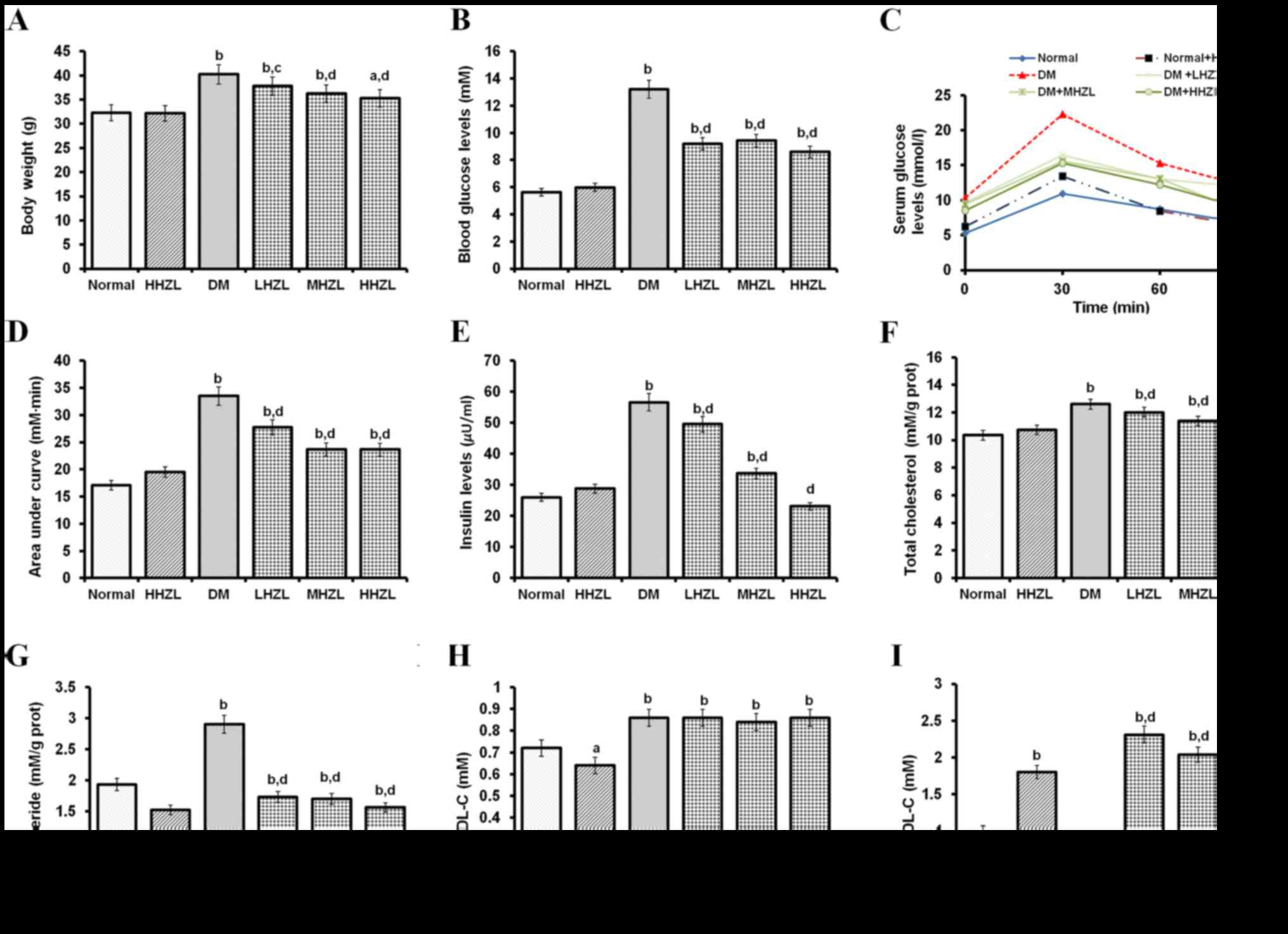 | Figure 1Hypoglycemic and lipid metabolic
effects of HZL on diabetic mice. (A) Body weight; (B) blood glucose
levels; (C) OGTT; (D) AUC; (E) insulin levels; (F) TC; (G) TG; (H)
LDL-C levels; and (I) HDL-C levels. Data are presented as mean ±
standard deviation. aP<0.05, bP<0.01
vs. normal group; cP<0.05, dP<0.01 vs. DM group.
HZL, hyperoside from Z. bungeanum leaves; OGTT, oral glucose
tolerance test; AUC, area under the curve; TC, total cholesterol;
TG, triglyceride; DM, diabetes mellitus; LHZL, DM + 50 mg/kg BW/day
HZL; MHZL, DM + 100 mg/kg BW/day HZL; HHZL, DM + 200 mg/kg BW/day
HZL; LDL-C, low-density lipoprotein cholesterol; HDL-C,
high-density lipoprotein cholesterol. |
Effects of HZL on hepatic dysfunction,
oxidative stress markers and antioxidant enzymes
Fig. 2A–C present
the mean values of Na+/K+ ATPase, AST and ALT
activities in the livers of mice in both normal and experimental
groups 6 weeks post-treatment. HHZL treatment markedly attenuated
hepatic dysfunction in diabetic animals, as evidenced by the
lowering of the elevated levels of AST and ALT (P<0.01,
P<0.01, respectively) and the restoration of the undermined
Na+/K+ ATPase activities (P<0.01).
Fig. 2D–F present the mean values
of oxidative stress markers in both normal and experimental groups
after 6 weeks in the liver. In diabetic mice the MDA levels, NO
contents and iNOS activity were significantly increased (P<0.01,
P<0.01 and P<0.01, respectively). Treatment with HZL
significantly reversed the MDA and NO productions and iNOS activity
in diabetic mice. In the present study, HFD-alloxan induction
caused an obvious loss in antioxidant enzyme (SOD, GPx and SOD)
activities in the liver, a representative sign of DM. Nevertheless,
HZL treatment markedly increased the activities of SOD, GSH and CAT
(P<0.01, P<0.01 and P<0.05, respectively) in a
dose-dependent manner (Fig.
2G–I).
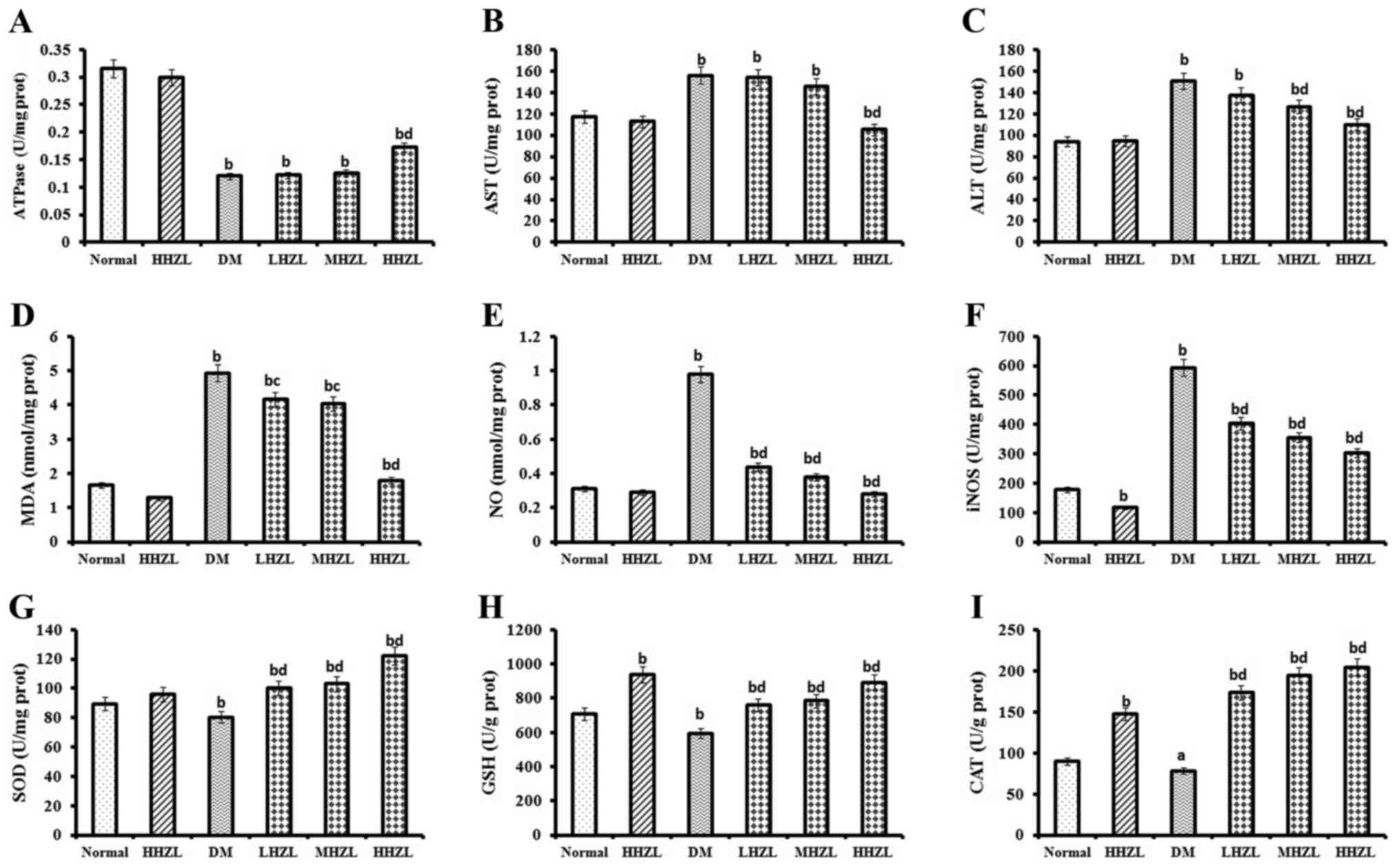 | Figure 2Effects of HZL on hepatic
dysfunction, oxidative stress markers and antioxidant enzyme
activity in the liver of normal and diabetic mice. (A)
Na+/K+ ATPase activity; (B) AST activity; (C)
ALT activity; (D) MDA level; (E) NO level; (F) iNOS activity; (G)
SOD activity; (H) GSH activity; and (I) CAT activity. Data are
presented as mean ± standard deviation. aP<0.05,
bP<0.01 vs. normal group; cP<0.05,
dP<0.01 vs. DM group. HZL, hyperoside from Z.
bungeanum leaves; AST, aspartate aminotransferase; ALT, alanine
aminotransferase; MDA, malondialdehyde; NO, nitric oxide; iNOS,
inducible nitric oxide synthase; SOD, superoxide dismutase; GPx,
glutathione peroxidase. |
Hepatic apoptosis-related protein
expressions
Following this, the authors determined the
mechanisms behind the protective effects of HZL against
HFD-alloxan-induced damage to liver. As shown in Fig. 3, HFD-alloxan treatment caused
significantly increase in ATF3, p-p65, p-p38, p-Erk1/2 and p-JNK
protein levels (P<0.01, P<0.01, P<0.01, P<0.01 and
P<0.01, respectively) compared with the normal mice. Whereas
administration of HZL cause dose-dependent decrease the protein
levels of ATF3 (Fig. 3A and B),
p-p65, p-p38, p-Erk1/2 and p-JNK (Fig. 3C–G) compared with the untreated
diabetic mice. Those results were consistent with the change of
p65, p38, Erk1/2 and JNK expression levels.
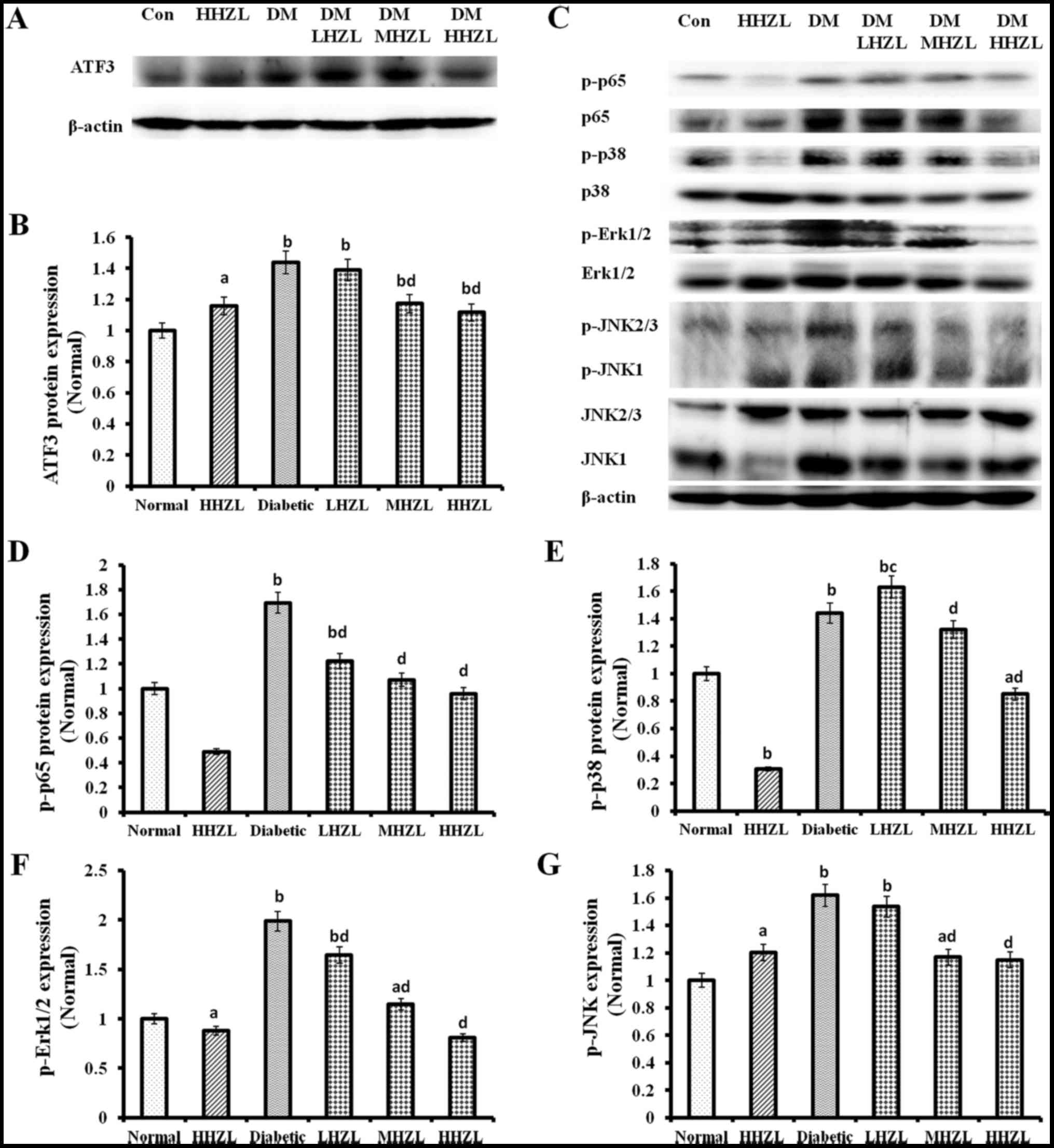 | Figure 3The protein expression of ATF3 and
the phosphorylation p65, p38, ERK1/2 and JNK1/2/3 in the liver of
normal and diabetic mice. (A and C) Western blotting and
quantitative analysis of renal (B) ATF3; (D) p-p65; (E) p-p38; (F)
p-Erk1/2; and (G) p-JNK expression. Data are presented as mean ±
standard deviation. aP<0.05, bP<0.01
vs. normal group; cP<0.05, dP<0.01 vs.
DM group. ATF3, activating transcription factor 3; HZL, hyperoside
from Z. bungeanum leaves; DM, diabetes mellitus; LHZL, DM +
50 mg/kg BW/day HZL; MHZL, DM + 100 mg/kg BW/day HZL; HHZL, DM +
200 mg/kg BW/day HZL; Con, control. |
Being considered to be one of meaningful events
during hepatic damage caused by DM, apoptosis of hepatocytes was
examined in the present study. Analysis of caspase-apoptosis
pathway revealed that treatment with HFD-alloxan led to a marked
increase in Bax and Cyt c, and a decrease in Bcl-2 levels, and thus
enhanced the cleavage of caspase-9 and caspase-3 (P<0.01,
P<0.01, P<0.01, P<0.01 and P<0.01, respectively;
Fig. 4). Not surprisingly, HZL
treatment markedly decreased apoptotic signaling by lowering the
protein levels of Bax (Fig. 4C),
Cyt c (Fig. 4E and F), elevating
expression of Bcl-2 (Fig. 4A and
B) and inhibiting cleavage of caspase-9 (Fig. 4D) and caspase-3 (Fig. 4G and H).
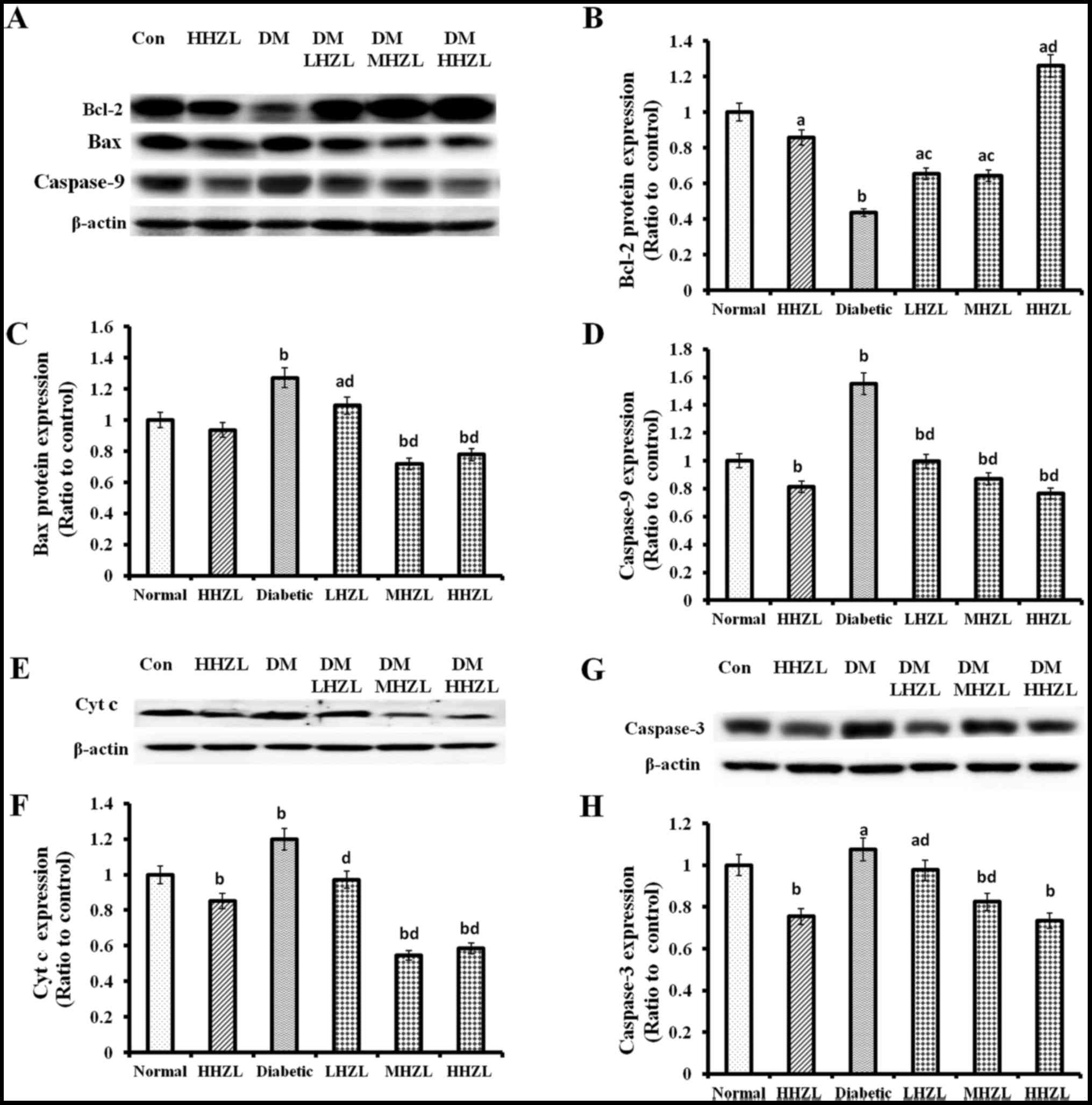 | Figure 4Bcl-2, Bax, Cyt c, caspase-9 and
caspase-3 protein expression in the liver of normal and diabetic
mice. (A, E and G) Western blotting and quantitative analysis of
renal (B) Bcl-2; (C) Bax; (D) caspase-9; (F) Cyt c; and (H)
caspase-3 expression. Data are presented as mean ± standard
deviation. aP<0.05, bP<0.01 vs. normal
group; cP<0.05, dP<0.01 vs. DM group.
HZL, hyperoside from Z. bungeanum leaves; DM, diabetes
mellitus; LHZL, DM + 50 mg/kg BW/day HZL; MHZL, DM + 100 mg/kg
BW/day HZL; HHZL, DM + 200 mg/kg BW/day HZL; Con, control. |
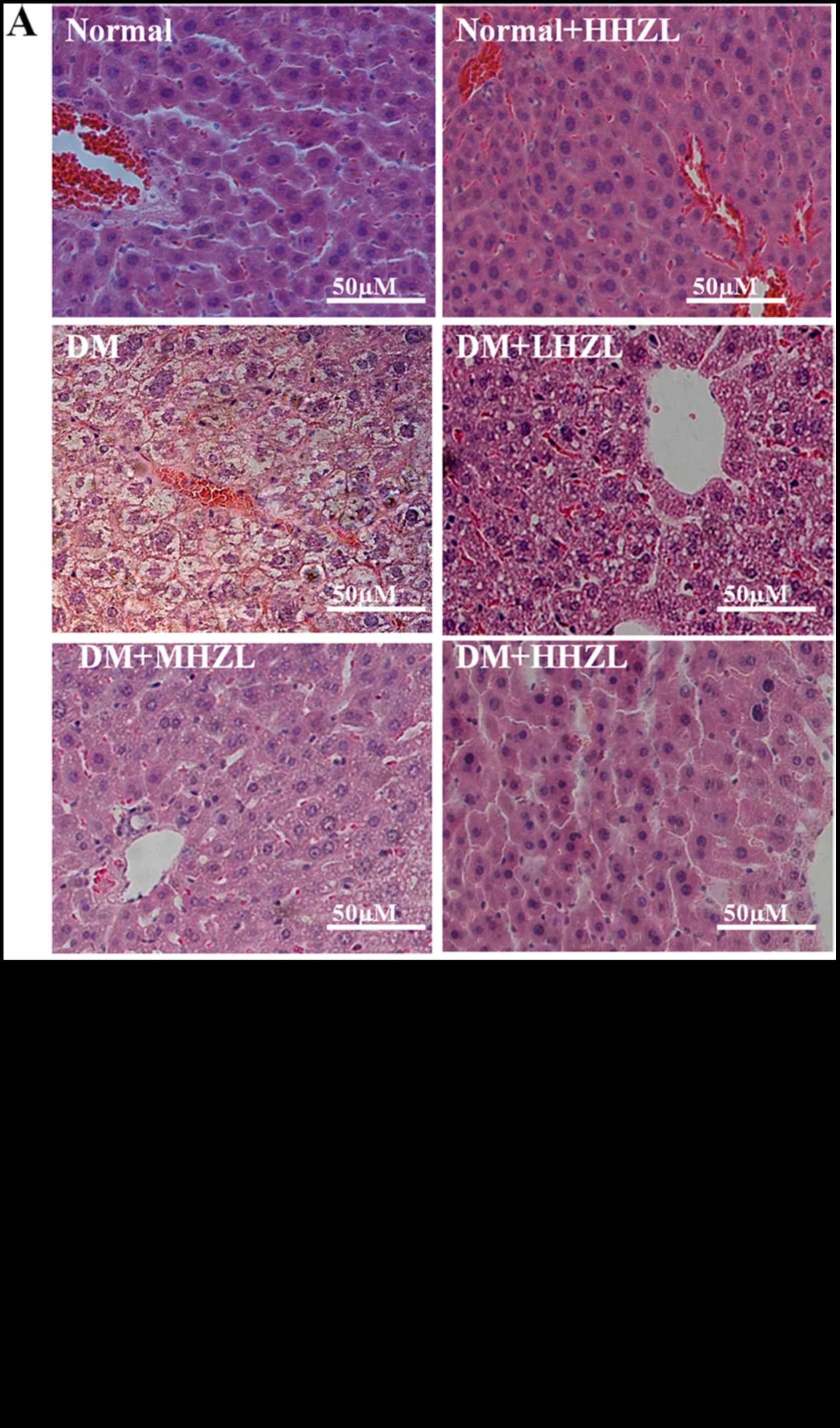
Histopathological analysis
The liver tissue sections indicated that healthy
control mice had normal hepatic cells with well-preserved
cytoplasm, nucleus and central vein (Fig. 5). In the diabetic group, the loss
of hepatic architecture was observed, accompanied by lymphocytic
inflammation and focal necrosis of hepatic cells. HZL treatment
markedly restored the structural integrity of the damaged cells.
Nevertheless, total loss of hepatic architecture and partially
lymphocytic inflammation was observed in LHZL and MHZL group.
Overall, status of liver tissues in the HHZL group was better than
the LHZL and MHZL group and had almost returned to the normal level
(Fig. 5A and B).
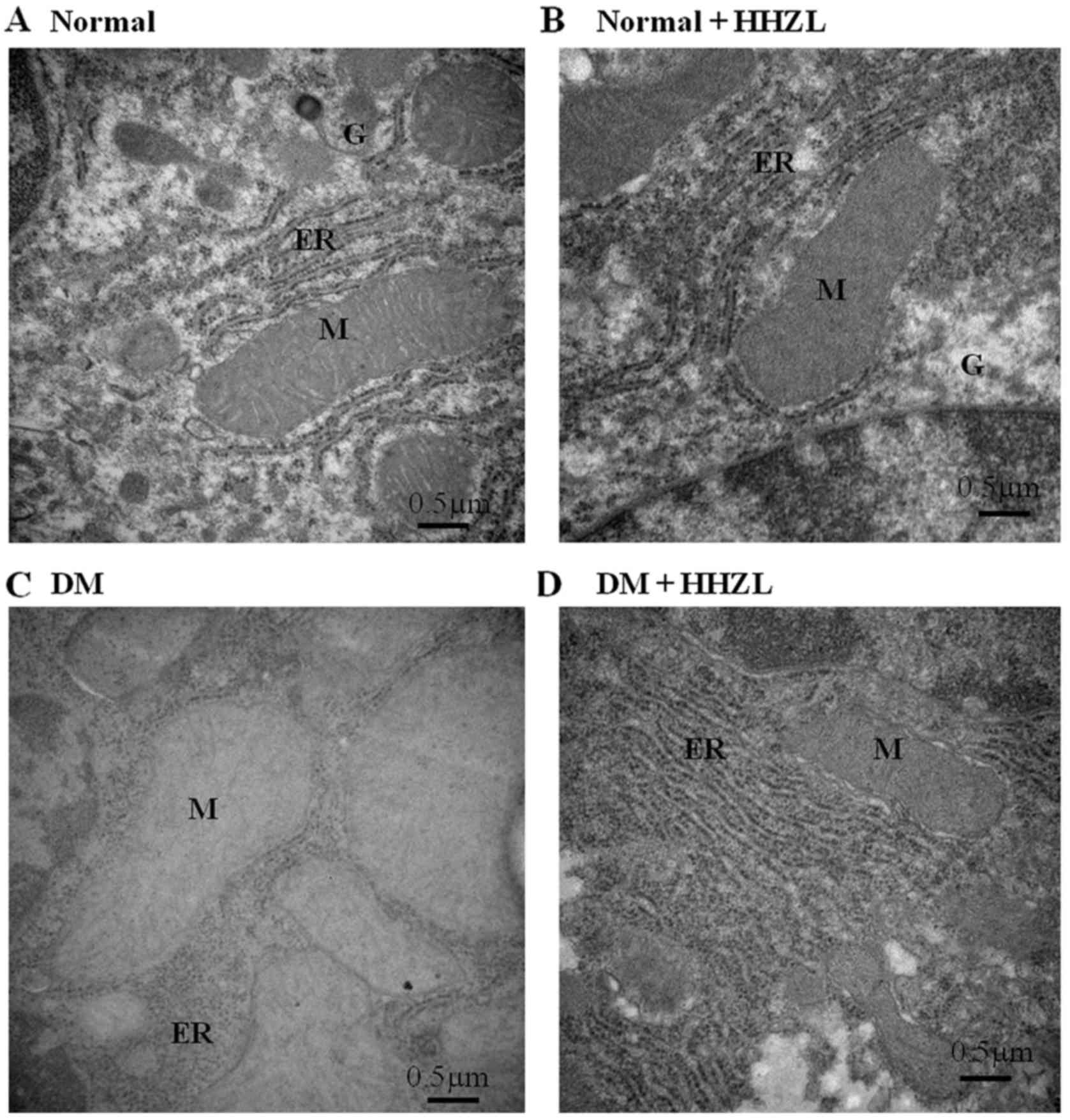
The ultrastructural changes occurred in hepatic of
mice are presented in Fig. 6.
Fig. 6A represents the EM image
of hepatic of control mice, showing the intact cellular organelles
such as mitochondria, endoplasmic reticulum (ER) and Golgi complex,
and HZL treatment alone no obvious influence on hepatocytes
(Fig. 6B). The EM image of
hepatocytes from diabetic mice (Fig.
6C) exhibited a noticeable destruction of hepatic accompanied
with loss of mitochondrial cristae, vacuolization with ballooning
appearance of mitochondria, as well as dilation of the rough ER,
which could be considerably prevented by HZL treatment during the
progression of DM induced by HFD-alloxan, as evidenced by minimal
nuclear membrane damage, minimal loss in the cristae with weak
swelling of mitochondria and no vacuolarization of cytoplasmic
region of hepatic (Fig. 6D).
Discussion
DM is currently considered a worldwide epidemic and
finding effective therapeutic strategies against this disease is
highly important. In the present study, the authors investigated
the effects of the potent antioxidants hyperoside isolated from
Z. bungeanum leaves in mice with HFD-alloxan-induced
diabetes. The daily administration of HHZL (200 mg/kg BW) for 42
days led to significant decline in LDL-C levels, iNOS activity,
cleavage of caspase-9 and caspase-3, as well as evident increases
in HDL-C levels, GPx activity, CAT activity and protein levels of
ATF3 comparison with normal groups. Insignificant difference in
body weight gain, blood glucose levels, insulin levels, TC levels,
Na+/K+ ATPase activity, AST activity, ALT
activity, MDA levels, NO levels, SOD activity, as well as
phosphorylation of p65, p38 and Erk1/2 were observed. These results
indicated that hyperoside isolated from Z. bungeanum leaves
was no toxic effects on normal mice.
A previous study demonstrated that hyperoside can
decrease blood glucose levels in streptozotocin-induced hyper
glycemia by improving the function of pancreatic islets and
increasing glycolysis and decreasing gluconeogenesis (22). In the present study, HFD-alloxan
led to a significant increase in body weight, blood glucose levels
and insulin levels in the experimental mice. Body weight gain,
blood glucose levels and insulin levels were reduced in the
HZL-treated groups in a dose-dependent manner when compared with
the diabetic mice. These results supported the notion that HZL may
be developed as an anti-hyperglycemic agent for the treatment of
DM.
The mice with HFD-alloxan-induced diabetes showed
abnormalities in lipid metabolism, as evidenced by increased TG, TC
and LDL-C levels and decreased HDL-C levels, similar to the
characteristics of human type 2 diabetes (24,25). Hypertriglyceridemia may occur due
to increased absorption and formation of triglycerides in the form
of chylomicrons following consumption of a diet rich in fat or
through increased endogenous production of TG-enriched hepatic VLDL
and decreased TG uptake in peripheral tissues (26). In the present study, a decrease in
TC, TG and LDL-C levels and an increase in HDL-C levels were
observed in the serum of HZL-treated diabetic mice in a
dose-dependent manner, suggesting that HZL regulated hepatic lipid
metabolism.
In experimental diabetes, alloxan exerts its toxic
effe cts on liver and other organs in addition to pancreatic
β-cells (27,28). The higher levels of ALT observed
in the diabetic mice indicates a certain degree of hepatic damage.
In this study, the diabetic hyperglycemia produced elevation of
hepatic AST and ALT levels, which were considered as typical signs
of liver dysfunction. HHZL decreased the elevated levels of AST and
ALT in diabetic mice. In experimental diabetes, changes in
Na+/K+ ATPase activity have been reported in
different tissues. The diabetic mice had significantly decreased
activities of Na+/K+ ATPase in erythrocytes
and tissues (29,30). A significant increase in
Na+/K+ ATPase activity was observed in
HZL-treated diabetic mice. These results, concomitant with the
effect on histological changes, indicated that HZL protects against
diabetic liver injury.
Oxidative stress is considered to be the main factor
contributing to development of diabetic complications and tissue
injury (31). The insulin
insufficiency and hyperglycemia further augment liver damage
through ROS mediated lipid peroxidation of hepatocellular membrane
(32,33). Hepatic
Na+/K+ ATPase activity was reduced, which may
also be due to the membrane peroxidative damage induced by
increased lipid peroxidation status (34). In diabetic mice, the authors
observed a significant increase in MDA levels, whereas HZL
treatment significantly reduced MDA levels in diabetic mice. Excess
NO by iNOS has been reported to induce deleterious effects in the
liver (35). These data showed
that treatment with HZL caused marked reduction in NO production
and iNOS activity in diabetic liver. These results indicated that
the protective effect of HZL be related to antioxidant
activity.
Antioxidant therapy is considered to be a
significant pharmacological prelude for the management of diabetes,
as their benefits are not only attributed to its radical quenching
but also to their ability to interact with numerous basic cellular
activities. In this context, several studies have reported the
declined activities of these antioxidant enzymes in the diabetic
liver and HZL with antioxidant activities has been shown to restore
activities of these antioxidant enzymes in liver of diabetic mice.
These findings suggested that the HZL may exert its hepatic protect
effect through the enhancement of cellular antioxidant system.
ATF3 is an adaptive response transcription factor
for various cell types to cope with extra and/or intracellular
changes, including cytokines, chemokines, growth factors, hormones,
hypoxia, DNA damage and nutrient deprivation. Expression of its
corresponding gene is induced by oxidative stress signals in a
variety of tissues, including the liver (36). ATF3 has been proven to regulate
apoptotic cell death in response to oxidant stress through
suppressing NF-κB-dependent transcription of anti-apoptotic genes
(37,38), triggering the MAPKs (39,40), mediating the caspase activation
(41). Researchers also have
demonstrated that oxidative stress mediates the activation of MAPK,
NF-κB and caspase-dependent signaling pathways (42). Overexpression of activated NF-κB,
MAPK and caspase proteins is considered as an important factor
contributing to the development of diabetic liver injury (43–45). NF-κB, as an oxidative
stress-responsive transcription factor, enhances the inducible
nitric oxide synthase expression leading to the elevated generation
of NO (46). Meanwhile, NF-κB is
an appropriate target to treat hepatocellular toxicity (47). ROS exert a significant effect on
hepatocyte apoptosis in diabetes (48). Literature also suggests that
extensive cell apoptosis eventually resulting in loss of function
in tissues is associated with upregulation of Bax, and release of
Cyt c (49). Cyt c released from
mitochondria leads to activation of caspase-9 that induced
apoptosis, which has been implicated in the pathogenesis of
diabetic liver injury. For all these reasons, the authors evaluated
the phosphorylation of p65/NF-κB, MAPK (including p38, JNK and
ERK), expression of apoptosis related proteins such as Bcl-2, Bax,
Cyt c and activation of caspase-9 and caspase-3 in diabetic induced
liver injury and evaluated the inhibitory effects of HZL on these
risk factors. The results of western blot analysis revealed that
HFD-alloxan treatment promoted the phosphorylation of p65, p38, JNK
and ERK proteins, whereas HZL administration decreased this
phosphorylation. These results indicated that the protect effects
of HZL may be associated with the suppression of NF-κB and MAPK
signaling pathways in the livers of diabetic mice. Furthermore, HZL
treatment of diabetic mice significantly suppressed hepatic protein
levels of Bax, Cyt c, caspase-9 and caspase-3, although there were
no changes in Bcl-2 protein levels among all experimental groups.
The results presented here suggested that HZL could prevent
apoptosis-induced hepatic damage, at least in part, through the
amelioration of oxidative stress-induced diabetic liver injury.
Recently, the effect of hyperoside on alleviating
diabetes and diabetic complications has been receiving increasing
attention (50–52). The authors further studied the
effect of HZL in islet cells, and results show that HZL could
inhibit HFD-alloxan-induced islet cells injury by downregulating
p65/NF-κB and ERK/MAPK signals and inhibiting apoptosis
(unpublished data). In addition, a study by Martin et al
(53) showed that Cocoa phenolic
extract including hyperoside protects pancreatic β-cells against
oxidative stress. Instreptozotocin-induced type 2 diabetic rats,
hyperoside could directly lower glucose levels by improving the
function of pancreatic islets and increasing glycolysis and
decreasing gluconeogenesis (22).
Moreover, many studies have shown that quercetin as aglycone of
hyperoside prevented and protected streptozotocin-induced oxidative
stress and β-cell damage in the rat pancreas (54–57). These results theoretically support
the notion that HZL protect the islet cells from the injury caused
by oxidative stress. Furthermore, studies on the effect of HZL in
islet cells are required to get a deeper understanding of molecular
mechanisms of the HZL hyperglycemic effect.
In conclusion, the present study demonstrated that
hyperosides from Z. bungeanum leaves exerted significant
anti-hyperglycemic and hepatocyte-protective effects in mice with
HFD- and alloxan-induced diabetes. The observed hepatocyte
protection, as well as the antioxidant potential of hyperoside was
partially responsible for its anti-diabetogenic properties. Its
hepatocyte-protective mechanisms involved the inhibition of the
NF-κB, MAPK and caspase-dependent apoptotic pathways. These maybe
helpful to understand the role of Z. bungeanum leaves in the
clinical treatment of DM and its secondary complications.
Acknowledgments
The present study was financed by the Special Fund
for Forest Scientific Research in the Public Welfare (grant no.
201304811), the National Natural Science Foundation of China (grant
no. 31101266) and the Scientific and Technical Foundation of
Shaanxi Province (grant no. 2014JM41005). The present study was
partly supported by the Institute of Mitochondrial Biology and
Medical, Xi'an Jiaotong University (Xi'an, China).
Abbreviations:
|
HZL
|
hyperoside from Zanthoxylum
bungeanum leaves
|
|
DM
|
diabetes mellitus
|
|
HFD
|
high-carbohydrate/high-fat diet
|
|
TC
|
total cholesterol
|
|
TG
|
triglyceride
|
|
LDL-C
|
low-density lipoprotein
cholesterol
|
|
HDL-C
|
high-density lipoprotein
cholesterol
|
|
ALT
|
alanine aminotransferase
|
|
AST
|
aspartate aminotransferase
|
|
NO
|
nitric oxide
|
|
iNOS
|
inducible nitric oxide synthase
|
|
SOD
|
superoxide dismutase
|
|
GPx
|
glutathione peroxidase
|
|
MDA
|
malondialdehyde
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
OGTT
|
oral glucose tolerance test
|
|
ROS
|
reactive oxygen species
|
References
|
1
|
Bullon P, Newman HN and Battino M:
Obesity, diabetes mellitus, atherosclerosis and chronic
periodontitis: a shared pathology via oxidative stress and
mitochondrial dysfunction? Periodontol. 2000. 64:139–153. 2014.
View Article : Google Scholar
|
|
2
|
Crujeiras AB, Díaz-Lagares A, Carreira MC,
Amil M and Casanueva FF: Oxidative stress associated to
dysfunctional adipose tissue: a potential link between obesity,
type 2 diabetes mellitus and breast cancer. Free Radic Res.
47:243–256. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stefanović A, Kotur-Stevuljević J, Spasić
S, Bogavac-Stanojević N and Bujisić N: The influence of obesity on
the oxidative stress status and the concentration of leptin in type
2 diabetes mellitus patients. Diabetes Res Clin Pract. 79:156–163.
2008. View Article : Google Scholar
|
|
4
|
Shima T, Uto H, Ueki K, Takamura T, Kohgo
Y, Kawata S, Yasui K, Park H, Nakamura N, Nakatou T, et al:
Clinicopathological features of liver injury in patients with type
2 diabetes mellitus and comparative study of histologically proven
nonalcoholic fatty liver diseases with or without type 2 diabetes
mellitus. J Gastroenterol. 48:515–525. 2013. View Article : Google Scholar
|
|
5
|
Takeuchi J, Takada A, Nakada Y, Sawae G
and Okumura Y: Clinical and experimental studies of liver injury in
diabetes mellitus. II. Experimental studies. Acta Hepatosplenol.
17:228–240. 1970.PubMed/NCBI
|
|
6
|
Casas-Grajales S and Muriel P:
Antioxidants in liver health. World J Gastrointest Pharmacol Ther.
6:59–72. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shin SM, Yang JH and Ki SH: Role of the
Nrf2-ARE pathway in liver diseases. Oxid Med Cell Longev.
763257:2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stadler K, Jenei V, von Bölcsházy G,
Somogyi A and Jakus J: Increased nitric oxide levels as an early
sign of premature aging in diabetes. Free Radic Biol Med.
35:1240–1251. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lucchesi AN, Freitas NT, Cassettari LL,
Marques SF and Spadella CT: Diabetes mellitus triggers oxidative
stress in the liver of alloxan-treated rats: a mechanism for
diabetic chronic liver disease. Acta Cir Bras. 28:502–508. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu X, Zhang J, Ming Y, Chen X, Zeng M and
Mao Y: The aggravation of mitochondrial dysfunction in nonalcoholic
fatty liver disease accompanied with type 2 diabetes mellitus.
Scand J Gastroenterol. 50:1152–1159. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiong QB and Shi DW: Morphological and
histological studies of Chinese traditional drug 'hua jiao'
(pericarpium zanthoxyli) and its allied drugs. Yao Xue Xue Bao.
26:938–947. 1991.In Chinese.
|
|
12
|
Yang LC, Li R, Tan J and Jiang ZT:
Polyphenolics composition of the leaves of Zanthoxylum bungeanum
Maxim. grown in Hebei, China, and their radical scavenging
activities. J Agric Food Chem. 61:1772–1778. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Y, Luo Z, Wang D, He F and Li D:
Phytochemical profiles and antioxidant and antimicrobial activities
of the leaves of Zanthoxylum bungeanum. Scientific World Journal.
2014:1810722014.PubMed/NCBI
|
|
14
|
Zhang Y, Luo Z and Wang D: Efficient
quantification of the phenolic profiles of Zanthoxylum bungeanum
leaves and correlation between chromatographic fingerprint and
antioxidant activity. Nat Prod Res. 29:2024–2029. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Wang D, Yang L, Zhou D and Zhang
J: Purification and characterization of flavonoids from the leaves
of Zanthoxylum bungeanum and correlation between their structure
and antioxidant activity. PLoS One. 9:e1057252014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sukito A and Tachibana S: Isolation of
hyperoside and isoquercitrin from Camellia sasanqua as antioxidant
agents. Pak J Biol Sci. 17:999–1006. 2014. View Article : Google Scholar
|
|
17
|
Li FR, Yu FX, Yao ST, Si YH, Zhang W and
Gao LL: Hyperin extracted from Manchurian rhododendron leaf induces
apoptosis in human endometrial cancer cells through a mitochondrial
pathway. Asian Pac J Cancer Prev. 13:3653–3656. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ku SK, Kwak S, Kwon OJ and Bae JS:
Hyperoside inhibits high-glucose-induced vascular inflammation in
vitro and in vivo. Inflammation. 37:1389–1400. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ku SK, Kim TH, Lee S, Kim SM and Bae JS:
Antithrombotic and profibrinolytic activities of
isorhamnetin-3-O-galactoside and hyperoside. Food Chem Toxicol.
53:197–204. 2013. View Article : Google Scholar
|
|
20
|
Li ZL, Hu J, Li YL, Xue F, Zhang L, Xie
JQ, Liu ZH, Li H, Yi D H, Liu JC, et al: The effect of hyperoside
on the functional recovery of the ischemic/reperfused isolated rat
heart: potential involvement of the extracellular signal-regulated
kinase 1/2 signaling pathway. Free Radic Biol Med. 57:132–140.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jung HA, Islam MD, Kwon YS, Jin SE, Son
YK, Park JJ, Sohn HS and Choi JS: Extraction and identification of
three major aldose reductase inhibitors from Artemisia montana.
Food Chem Toxicol. 49:376–384. 2011. View Article : Google Scholar
|
|
22
|
Verma N, Amresh G, Sahu PK, Mishra N, Rao
ChV and Singh AP: Pharmacological evaluation of hyperin for
antihyperglycemic activity and effect on lipid profile in diabetic
rats. Indian J Exp Biol. 51:65–72. 2013.PubMed/NCBI
|
|
23
|
Liu X, Zhu L, Tan J, Zhou X, Xiao L, Yang
X and Wang B: Glucosidase inhibitory activity and antioxidant
activity of flavonoid compound and triterpenoid compound from
Agrimonia pilosa Ledeb. BMC Complement Altern Med. 14:122014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Friedman MI: Insulin-induced hyperphagia
in alloxan-diabetic rats fed a high-fat diet. Physiol Behav.
19:597–599. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang LQ, Wei W, Chen LM and Liu S: Effects
of berberine on diabetes induced by alloxan and a
high-fat/high-cholesterol diet in rats. J Ethnopharmacol.
108:109–115. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dixon JL, Shen S, Vuchetich JP, Wysocka E,
Sun GY and Sturek M: Increased atherosclerosis in diabetic
dyslipidemic swine: protection by atorvastatin involves decreased
VLDL triglycerides but minimal effects on the lipoprotein profile.
J Lipid Res. 43:1618–1629. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Buko V, Lukivskaya O, Nikitin V, Tarasov
Y, Zavodnik L, Borodinsky A, Gorenshtein B, Janz B, Gundermann KJ
and Schumacher R: Hepatic and pancreatic effects of
polyenoylphosphatidylcholine in rats with alloxan-induced diabetes.
Cell Biochem Funct. 14:131–137. 1996.PubMed/NCBI
|
|
28
|
Zhang X, Liang W, Mao Y, Li H, Yang Y and
Tan H: Hepatic glucokinase activity is the primary defect in
alloxan-induced diabetes of mice. Biomed Pharmacother. 63:180–186.
2009. View Article : Google Scholar
|
|
29
|
Al-Numair KS, Veeramani C, Alsaif MA and
Chandramohan G: Influence of kaempferol, a flavonoid compound, on
membrane-bound ATPases in streptozotocin-induced diabetic rats.
Pharm Biol. 53:1372–1378. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ramesh B and Pugalendi KV: Influence of
umbelliferone on membrane-bound ATPases in streptozotocin-induced
diabetic rats. Pharmacol Rep. 59:339–348. 2007.PubMed/NCBI
|
|
31
|
Çelık VK, Şahın ZD, Sari İ and Bakir S:
Comparison of oxidant/antioxidant, detoxification systems in
various tissue homogenates and mitochondria of rats with diabetes
induced by streptozocin. Exp Diabetes Res. 2012:3868312012.
View Article : Google Scholar
|
|
32
|
Alevizos I, Misra J, Bullen J, Basso G,
Kelleher J, Mantzoros C and Stephanopoulos G: Linking hepatic
transcriptional changes to high-fat diet induced physiology for
diabetes-prone and obese-resistant mice. Cell Cycle. 6:1631–1638.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Feng W, Zhao T, Mao G, Wang W, Feng Y, Li
F, Zheng D, Wu H, Jin D, Yang L, et al: Type 2 diabetic rats on
diet supplemented with chromium malate show improved
glycometabolism, glycometabolism-related enzyme levels and lipid
metabolism. PLoS One. 10:e01259522015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Banks MA, Porter DW, Martin WG and
Castranova V: Effects of in vitro ozone exposure on peroxidative
damage, membrane leakage, and taurine content of rat alveolar
macrophages. Toxicol Appl Pharmacol. 105:55–65. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bahmani F, Tajadadi-Ebrahimi M, Kolahdooz
F, Mazouchi M, Hadaegh H, Jamal AS, Mazroii N, Asemi S and Asemi Z:
The consumption of synbiotic bread containing Lactobacillus
sporogenes and inulin affects nitric oxide and malondialdehyde in
patients with type 2 diabetes mellitus: randomized, double-blind,
placebo-controlled trial. J Am Coll Nutr. 35:506–513. 2016.
View Article : Google Scholar
|
|
36
|
Allen-Jennings AE, Hartman MG, Kociba GJ
and Hai T: The roles of ATF3 in glucose homeostasis. A transgenic
mouse model with liver dysfunction and defects in endocrine
pancreas. J Biol Chem. 276:29507–29514. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hua B, Tamamori-Adachi M, Luo Y, Tamura K,
Morioka M, Fukuda M, Tanaka Y and Kitajima S: A splice variant of
stress response gene ATF3 counteracts NF-kappaB-dependent
anti-apoptosis through inhibiting recruitment of CREB-binding
protein/300 coactivator. J Biol Chem. 281:1620–1629. 2006.
View Article : Google Scholar
|
|
38
|
Jung DH, Kim KH, Byeon HE, Park HJ, Park
B, Rhee DK, Um SH and Pyo S: Involvement of ATF3 in the negative
regulation of iNOS expression and NO production in activated
macrophages. Immunol Res. 62:35–45. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lu D, Chen J and Hai T: The regulation of
ATF3 gene expression by mitogen-activated protein kinases. Biochem
J. 401:559–567. 2007. View Article : Google Scholar :
|
|
40
|
Inoue K, Zama T, Kamimoto T, Aoki R, Ikeda
Y, Kimura H and Hagiwara M: TNFalpha-induced ATF3 expression is
bidirectionally regulated by the JNK and ERK pathways in vascular
endothelial cells. Genes Cells. 9:59–70. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mashima T, Udagawa S and Tsuruo T:
Involvement of transcriptional repressor ATF3 in acceleration of
caspase protease activation during DNA damaging agent-induced
apoptosis. J Cell Physiol. 188:352–358. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ma JQ, Ding J, Zhang L and Liu CM: Ursolic
acid protects mouse liver against CCl4-induced oxidative stress and
inflammation by the MAPK/NF-κB pathway. Environ Toxicol Pharmacol.
37:975–983. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kohl T, Gehrke N, Schad A, Nagel M, Wörns
MA, Sprinzl MF, Zimmermann T, He YW, Galle PR, Schuchmann M, et al:
Diabetic liver injury from streptozotocin is regulated through the
caspase-8 homolog cFLIP involving activation of JNK2 and
intrahepatic immunocompetent cells. Cell Death Dis. 4:e7122013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sawant SP, Dnyanmote AV and Mehendale HM:
Mechanisms of inhibited liver tissue repair in toxicant challenged
type 2 diabetic rats. Toxicology. 232:200–215. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Devi SS and Mehendale HM: The role of
NF-kappaB signaling in impaired liver tissue repair in
thioacetamide-treated type 1 diabetic rats. Eur J Pharmacol.
523:127–136. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Farombi EO, Shrotriya S and Surh YJ:
Kolaviron inhibits dimethyl nitrosamine-induced liver injury by
suppressing COX-2 and iNOS expression via NF-kappaB and AP-1. Life
Sci. 84:149–155. 2009. View Article : Google Scholar
|
|
47
|
Muriel P: NF-kappaB in liver diseases: a
target for drug therapy. J Appl Toxicol. 29:91–100. 2009.
View Article : Google Scholar
|
|
48
|
Bhattacharya S, Gachhui R and Sil PC: The
prophylactic role of D-saccharic acid-1,4-lactone against
hyperglycemia-induced hepatic apoptosis via inhibition of both
extrinsic and intrinsic pathways in diabetic rats. Food Funct.
4:283–296. 2013. View Article : Google Scholar
|
|
49
|
Rashid K, Das J and Sil PC: Taurine
ameliorate alloxan induced oxidative stress and intrinsic apoptotic
pathway in the hepatic tissue of diabetic rats. Food Chem Toxicol.
51:317–329. 2013. View Article : Google Scholar
|
|
50
|
Zhou L, An XF, Teng SC, Liu JS, Shang WB,
Zhang AH, Yuan YG and Yu JY: Pretreatment with the total flavone
glycosides of flos Abelmoschus manihot and hyperoside prevents
glomerular podocyte apoptosis in streptozotocin-induced diabetic
nephropathy. J Med Food. 15:461–468. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
An X, Zhang L, Yuan Y, Wang B, Yao Q, Li L
and Zhang J, He M and Zhang J: Hyperoside pre-treatment prevents
glomerular basement membrane damage in diabetic nephropathy by
inhibiting podocyte heparanase expression. Sci Rep. 7:64132017.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang Z, Sethiel MS, Shen W, Liao S and
Zou Y: Hyperoside downregulates the receptor for advanced glycation
end products (RAGE) and promotes proliferation in ECV304 cells via
the c-Jun N-terminal kinases (JNK) pathway following stimulation by
advanced glycation end-products in vitro. Int J Mol Sci.
14:22697–22707. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Martín MA, Ramos S, Cordero-Herrero I,
Bravo L and Goya L: Cocoa phenolic extract protects pancreatic beta
cells against oxidative stress. Nutrients. 5:2955–2968. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Coskun O, Kanter M, Korkmaz A and Oter S:
Quercetin, a flavonoid antioxidant, prevents and protects
streptozotocin-induced oxidative stress and beta-cell damage in rat
pancreas. Pharmacol Res. 51:117–123. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Adewole SO, Caxton-Martins EA and Ojewole
JA: Protective effect of quercetin on the morphology of pancreatic
beta-cells of streptozotocin-treated diabetic rats. Afr J Tradit
Complement Altern Med. 4:64–74. 2006.PubMed/NCBI
|
|
56
|
Youl E, Bardy G, Magous R, Cros G, Sejalon
F, Virsolvy A, Richard S, Quignard JF, Gross R, Petit P, et al:
Quercetin potentiates insulin secretion and protects INS-1
pancreatic β-cells against oxidative damage via the ERK1/2 pathway.
Br J Pharmacol. 161:799–814. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Bardy G, Virsolvy A, Quignard JF, Ravier
MA, Bertrand G, Dalle S, Cros G, Magous R, Richard S and Oiry C:
Quercetin induces insulin secretion by direct activation of L-type
calcium channels in pancreatic beta cells. Br J Pharmacol.
169:1102–1113. 2013. View Article : Google Scholar : PubMed/NCBI
|