Introduction
Diabetic nephropathy (DN) is the leading cause of
end-stage renal disease (ESRD). Currently, there is no effective
treatment for DN, and the prevention of the occurrence and
progression of DN has become a serious medical challenge. Intensive
insulin treatment has been shown in large prospective randomized
studies to delay the onset and progression of DN in patients with
type 1 and 2 diabetes (1) by
lowering blood glucose levels and thus preventing
hyperglycemia-associated damage (2). However, the use of insulin may
result in iatrogenic hyperinsulinemia (3–6),
which can also lead to kidney damage (4,5).
In the kidneys, nicotinamide adenine dinucleotide
phosphate (NADPH) oxidase is the predominant source of reactive
oxygen species (ROS). NADPH oxidase 4 (Nox4) is the key subunit of
NADPH oxidase expressed in mesangial cells, and Nox4-derived ROS
are the major contributor to renal morphological changes and
functional abnormalities in DN (7). It has been reported that in
vitro insulin treatment results in a rapid increase in
H2O2 generation within podocytes, as well as
an increase in the surface expression of Nox4 in cultured podocytes
(8). High concentrations of
insulin promoted pancreatic stellate cell proliferation and
extracellular signal-regulated kinase 1/2 (ERK1/2) phosphorylation,
which resulted in pancreatic islet fibrosis (9). High concentrations of insulin may
also contribute to renal extracellular matrix (ECM) accumulation in
diabetes (10). These results
suggested that high concentrations of insulin could promote
oxidative stress, cell proliferation, and ECM accumulation in
vitro. Oxidative stress serves an important role in DN.
Regarding the role of ROS in the activation of transforming growth
factor-β (TGF-β) signalling, it has been observed that NADPH
oxidase exerts a key role in the TGF-β1-mediated activation of
kidney myofibroblast and fibronectin extra domain A expression
through the Smad3 and ERK signalling pathways (11). NADPH oxidase-mediated renal
oxidative stress promotes albuminuria in DN (12). In addition, inhibition of Nox4
oxidase has been demonstrated to reduce whole kidney and glomerular
hypertrophy in the diabetic cortex and glomeruli (13), suggesting that Nox4 may have a
direct involvement in renal hypertrophy, perhaps via a mechanism
involving Nox4-derived, ROS-mediated Akt/PKB and ERK1/2 activation
(13). The detrimental role of
NADPH oxidase in the development of DN has been further confirmed
by a study in which treatment with NADPH oxidase inhibitors
markedly attenuated the progression of nephropathy by reducing the
occurrence of albuminuria and preventing the development of
glomerulosclerosis through a reduction of renal oxidative stress
(14). The inflammation pathways
also serve central roles in the progression of DN (15). Inflammation increases insulin
resistance by activating the ERK pathway (16). However, at the present time, a
complete understanding of renal oxidative stress, inflammation and
ERK protein expression in the context of iatrogenic
hyperinsulinemic diabetic rats, or how to intervene in these
processes, has yet to be fully elucidated.
Canonical transient receptor potential cation
channels (TRPCs), as members of the transient receptor potential
(TRP) superfamily, are Ca2+-permeable cation channels
widely expressed in a series of tissues and cells (17). The TRPC family comprises seven
members, designated as TRPC1-7 (18). Among these, TRPC6 is closely
associated with kidney disease (19). In vitro, the effects of
insulin on TRPC6 were mimicked by treating podocytes with
H2O2 (8).
However, the effects of intensive insulin therapy on TRPC6 in
iatrogenic hyperinsulinemic diabetic rats remain unclear, and the
present study was designed in an attempt to address this issue.
Astragaloside IV (AS-IV), a purified small molecular
saponin, is one of the main active ingredients of Radix Astragali,
which has been reported to possess comprehensive biological
properties, including antioxidant, anti-inflammatory, and
immunoregulatory effects (20).
It has been reported that AS-IV significantly inhibits renal
oxidative stress and apoptosis in acute kidney injury rodent models
(21). Previous work in our
laboratory demonstrated that AS-IV prevents damage to human
mesangial cells through inhibition of the NADPH
oxidase/ROS/Akt/nuclear factor-κB (NF-κB) pathway under high
glucose conditions (7). However,
the effects of AS-IV on iatrogenic hyperinsulinemic streptozotocin
(STZ)-induced diabetic rats under intensive insulin therapy have
not yet been elucidated. The purpose of the present study was to
examine the hypothesis that AS-IV prevents iatrogenic
hyperinsulinemia due to kidney injury in diabetic rats by
suppressing oxidative stress and inflammation, and regulating TRPC6
in the field of DN.
Materials and methods
Animals
The present study was approved by the Animal Care
and Use Committee of Anhui Medical University, and all studies were
conducted in accordance with guidelines for the Care and Use of
Laboratory Animals adopted by the Committee on the Care and Use of
Laboratory Animals at Anhui Medical University. Age- and
weight-matched male Sprague-Dawley rats weighing 180-200 g were
purchased from the Experimental Animal Center of Anhui Medical
University (certificate no. SCXK 2014-0007). Rats were housed in
individual cages in a temperature-controlled room with a 12/12-h
light-dark cycle (starting at 09:00 a.m.) and allowed to
acclimatize to the environment for 1 week prior to initiation of
the various interventions.
Induction of diabetes mellitus
Freshly prepared STZ (Sigma Chemical Co.; now Merck
KGaA, Darmstadt, Germany) was dissolved in 0.01 M citrate buffer
(pH 4.5) and administered intraperitoneally to the rats at 55
mg/kg. The age-matched control group received citrate buffer only.
On the third or fourth day following STZ administration, blood
glucose levels were measured using an ACCU-CHEK performa glucometer
(Roche Diagnostics GmbH, Mannheim, Germany) with a drop of blood
extracted from the tail vein. Rats with blood glucose levels
>16.7 mmol/l were considered diabetic (22).
Experimental study design
At 1 week following STZ injection, the diabetic rats
were treated with the long-acting insulin analog, Levemir (Novo
Nordisk, Bagsvaerd, Denmark) subcutaneously (s.c.) for 4 weeks at 6
U/day. In addition, age-matched control rats (control, n=8) were
treated with saline s.c. for the same period. Subsequently, at 8:00
a.m., 4 weeks after the induction of diabetes, the morning insulin
concentration was determined from tail vein samples, obtained under
sodium pentobarbital anesthesia from all rats. Insulin levels
>30 µU/ml in the diabetic model rats were considered to
represent the condition of hyperinsulinemia (23). These diabetic rats with
hyperinsulinemia were randomly divided into the hyperinsulinemic
(HINS; n=8), the AS-IV-2.5 (i.e., treated with 2.5 mg/kg AS-IV
daily; n=8), the AS-IV-5 (treated with 5 mg/kg AS-IV daily; n=8),
the AS-IV-10 (treated with 10 mg/kg AS-IV daily; n=8) and the
Tempol (n=8) groups. Diabetic rats in the HINS group continued with
s.c. insulin treatment for 12 weeks. The AS-IV-2.5, AS-IV-5 and
AS-IV-10 groups also continued to receive s.c. insulin for 12
weeks, but AS-IV 2.5, 5 and 10 mg/kg/day intragastric (i.g.)
administration protocols were also continued for 12 weeks in each
corresponding group. Diabetic rats in the Tempol group were given
Tempol (Merck KGaA) in their drinking water (1 mmol/l) for 12 weeks
(Tempol group; n=8). The control group was treated with saline i.g.
over the course of the same time period.
Determination of blood glucose levels and
serum levels of glycated hemoglobin (HbA1c) and insulin
At 9:00 a.m., morning blood glucose concentrations
were determined at 1- or 2 to 4-week intervals in all groups using
an ACCU-CHEK per forma glucometer (Roche Diagnostics GmbH) on blood
samples obtained from tail veins. HbA1c and serum
insulin levels were determined on blood samples obtained from tail
veins under sodium pentobarbital anesthesia at the end of the
12-week study period using a Variant-II analyser (Bio-Rad,
Laboratories, Inc., Hercules, CA, USA) and a sensitive rat antibody
radioimmunoassay (EMD Millipore, Billerica, MA, USA). Subsequent
biochemical markers were detected as described below.
Determination of biochemical markers and
urinary albumin
Serum creatinine (CREA), blood urea nitrogen (BUN),
alanine transaminase (ALT) and aspartate aminotransferase (AST)
levels were determined on the blood samples obtained from the
abdominal aorta using an Olympus auto analyser (AU640; Olympus,
Tokyo, Japan). All rats were kept in individual metabolic cages for
24 h urine collection at the end of 4, 8 and 12 weeks of drug
treatment. Urinary albumin concentrations were measured using a
turbidimetric inhibition immunoassay kit (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China), according to the
manufacturer's protocol.
Determination of oxidative stress-related
markers, pro-inflammatory cytokines and ECM
After blood sampling, the kidneys were quickly
removed from the animals, and the surrounding fat was cleaned. The
renal tissue was sheared from the edge of the upper pole of the
kidney and homogenized in 9X volume of ice-cold normal saline.
Subsequently, the homogenates were centrifuged (4,000 rev/min, 2683
× g at 4°C for10 min). Levels of malondialdehyde (MDA), glutathione
peroxidase (GSH-Px), superoxide dismutase activity (SOD),
interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α) and type IV
collagen (COl-IV) in the kidney were measured according to the
manufacturer's protocol (Nanjing Jiancheng Bioengineering
Institute). Levels of laminin (LN) in the kidney were measured
according to a different manufacturer's protocol (Nanjing SenBeijia
Biological Technology Co., Ltd., Nanjing, China). Levels of IL-1β,
TNF-α, COl-IV and LN were detected using the enzyme-linked
immunosorbent assay (ELISA) method. Levels of MDA (thiobarbituric
acid method), GSH-Px (colorimetric method), SOD (hydroxylamine
method) were also detected using the manufacturer's kits (Nanjing
Jiancheng Bioengineering Institute).
Routine histology
Rat kidneys were excised to determine the
morphological changes in the kidney. Renal cortical tissue slices
were obtained (5-µm thick), and portions were fixed in
neutral buffered formalin for routine paraffin embedment and
subsequent tissue staining by hematoxylin and eosin (H&E) and
Jones' periodic acid Schiff (PAS) for bright-field microscopic
evaluation. The slides were examined under a light microscope at a
magnification of x400 by a pathologist blind to the experimental
profile. In PAS sections, the purple color in glomeruli was defined
as 'positive', and the positive area of each glomerulus was
measured using the JD801 morphological microscope image analysis
system (JEDA Science-Technology Development Co., Ltd., Jiangsu,
China). The positive score of the glomeruli one rat was calculated
by averaging positive scores of 30 glomeruli from the same rat
(24). Six rats were examined in
each group.
Electron microscopy
To determine the structural changes in podocyte
morphology, electron microscopic morphometric evaluation was
performed using routine protocols. Renal cortex samples were cut
into 1 mm3 pieces, incubated on ice, immediately fixed
in 2.5% glutaraldehyde solution, and then embedded. Ultrathin
sections were examined under a transmission electron microscope
(TEM). The remaining cortex was sliced and frozen by immersion in
liquid nitrogen for subsequent detection by western blot analysis,
as described below.
Western blot analysis
Kidney cortex was homogenized in lysis buffer on ice
with a homogenizer. The supernatants were collected after
centrifuging at 10,000 rev./min at 16,770 × g for 15 min at 4°C.
Protein concentration in the supernatant was measured using the
Bradford acid (BCA) protein assay (Pierce; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Equal amounts of protein
extracts were fractionated on 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and then
transferred onto polyvinylidene difluoride (PVDF) membranes (EMD
Millipore). After having been blocked with 5% non-fat milk in
Tris-buffered saline with Tween-20 (TBST, pH 7.6) for 1 h at room
temperature, the membranes were incubated with the indicated
primary antibodies [anti-Nox4 (1:1,000, BS6796), anti-ERK1/2
(1:1,000, BS3628), anti-phospho-ERK1/2 (1:1,000, BS5016) (all from
Bioworld Technology, Inc., St. Louis Park, MN, USA); and anti-TRPC6
(1:1,000, ab12249) and anti-β-actin (1:1,000, ab8227) (both from
Abcam, Cambridge, MA, USA)] overnight at 4°C. The membranes were
rinsed three times with TBST and incubated with the respective
secondary antibodies (1:10,000 dilutions of each antibody) for 1 h
at room temperature. The protein bands were visualized with
SuperSignal™ West Femto Maximum Sensitivity Substrate (Thermo
Fisher Scientific Inc.) and captured using a Bioshine ChemiQ 4600
Mini Chemiluminescence imaging system (Ouxiang Scientific
Instrument Co. Ltd., Shanghai, China). The optical density of each
band was quantified using ImageJ software (National Institutes of
Health, Bethesda, MD, USA) and normalized to the intensity of
β-actin.
Statistical analysis
Data are presented as the means ± standard
deviation. Each experiment was repeated at least three times
independently. Statistical analysis was performed using SPSS 16.0
for Windows (SPSS, Inc., Chicago, IL, USA). Statistical differences
among groups were analysed by one-way ANOVA. Pairwise comparisons
were made using the Bonfferoni test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of AS-IV on blood glucose and
serum levels of insulin and HbA1c
To determine the effects of AS-IV on blood glucose,
serum insulin, and HbA1c, all groups except the control
group were subjected to intensive insulin treatment (Fig. 1A and B). Regular checks of blood
glucose levels demonstrated that diabetic rats had good glycemic
control. No statistically significant differences were observed in
blood glucose or HbA1c among the six groups (P>0.05).
These results also implied that AS-IV and Tempol did not lead to an
alteration in the blood glucose levels. Compared with the control
group, STZ-induced diabetic rats exhibited very high insulin levels
(P<0.05) (Fig. 1B). This
reveals that the long-term use of insulin may lead to iatrogenic
hyperinsulinemia, and that AS-IV and Tempol had no impact on
insulin levels.
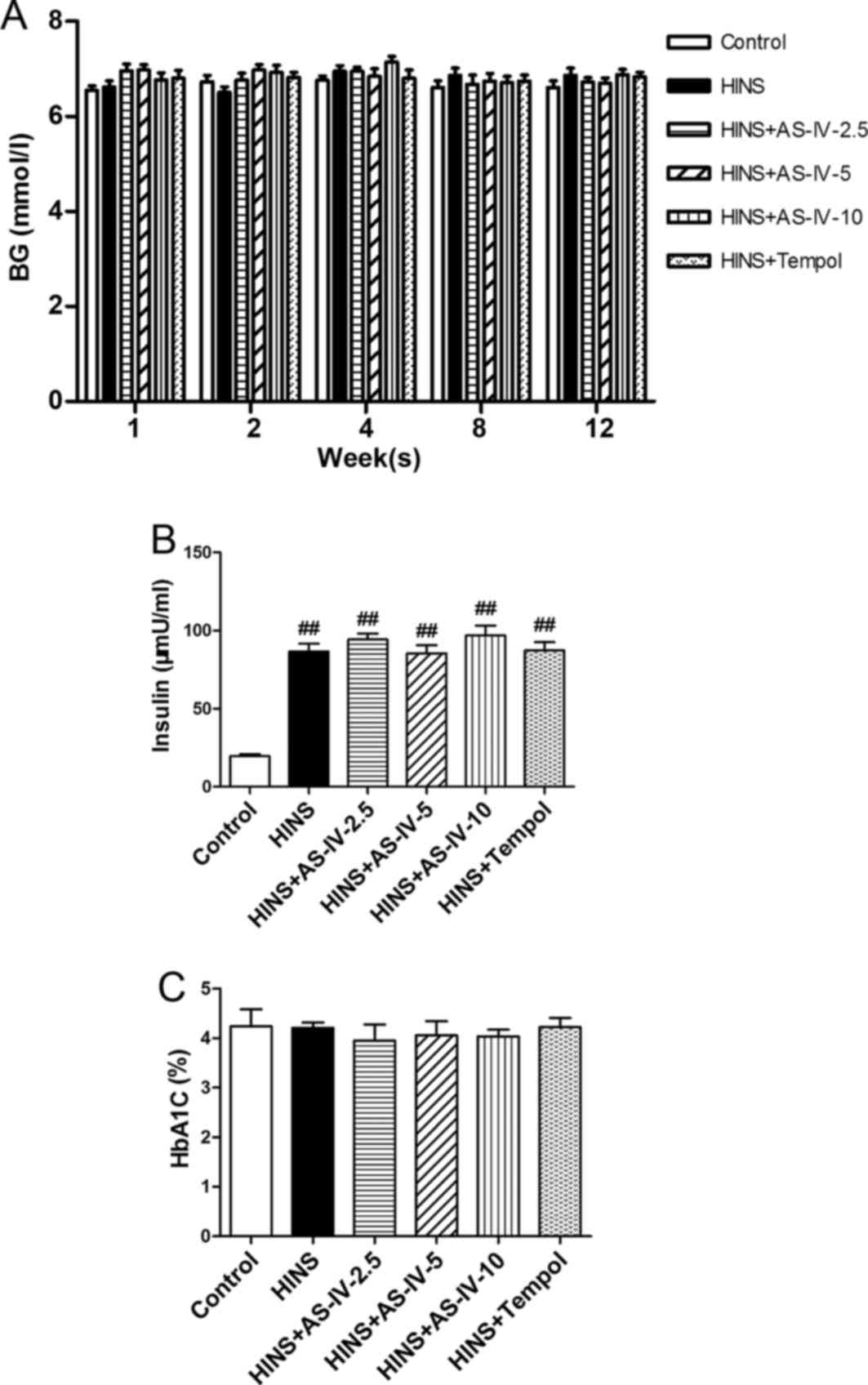 | Figure 1Effects of AS-IV on BG, serum insulin
and HbA1C in iatrogenic hyperinsulinemic diabetic rats.
(A) BG level of all the groups were examined after 1, 2, 4, 8 and
12 weeks, showing that AS-IV did not affect the levels. (B) Serum
insulin level of all groups after 12 weeks. (C) HbA1C
level of all groups after 12 weeks, showing that all groups
exhibited a good glycemic control. As AS-IV had no impact on the
insulin level, the long-term use of insulin may therefore lead to
iatrogenic hyperinsulinemia. Results are expressed as the means ±
standard deviation (n=8/each group). Refer to the Materials and
methods section for details of the assignment of the groups.
##P<0.01 vs. control. AS-IV, astragaloside IV; BG, blood
glucose; HbA1C, glycated hemoglobin; HINS,
hyperinsulinemic. |
Effects of AS-IV on serum levels of
biochemical markers and urinary albumin
No statistically significant differences in the
levels of CREA, BUN, ALT or AST among the six groups were
identified (P>0.05) (Fig.
2A–D). This suggested that the AS-IV doses used in the
experiment did not damage renal or liver function. Compared with
the control group, diabetic rats had higher levels of albuminuria
(P<0.05). Treatment with AS-IV significantly ameliorated
albuminuria in iatrogenic hyperinsulinemic rats. This protective
effect was evident at a dose as low as 5 mg/kg/day (Fig. 2E). AS-IV reduced albuminuria
time-dependently in diabetic rats, which was evident at least 8
weeks after AS-IV treatment. Treatment with AS-IV (at the doses of
5 and 10 mg/kg/day), or Tempol for 12 weeks, decreased the severity
of albuminuria (Fig. 2E).
Diabetic rats treated with AS-IV or Tempol were revealed to differ
from normal control rats at each time-point. Thus, AS-IV or Tempol
treatment was shown to ameliorate iatrogenic hyperinsulinemic
kidney damage, although without restoring levels to the baseline.
Even in the model group, the urinary albumin excretion rate was far
from reaching clinical diagnostic criteria for DN.
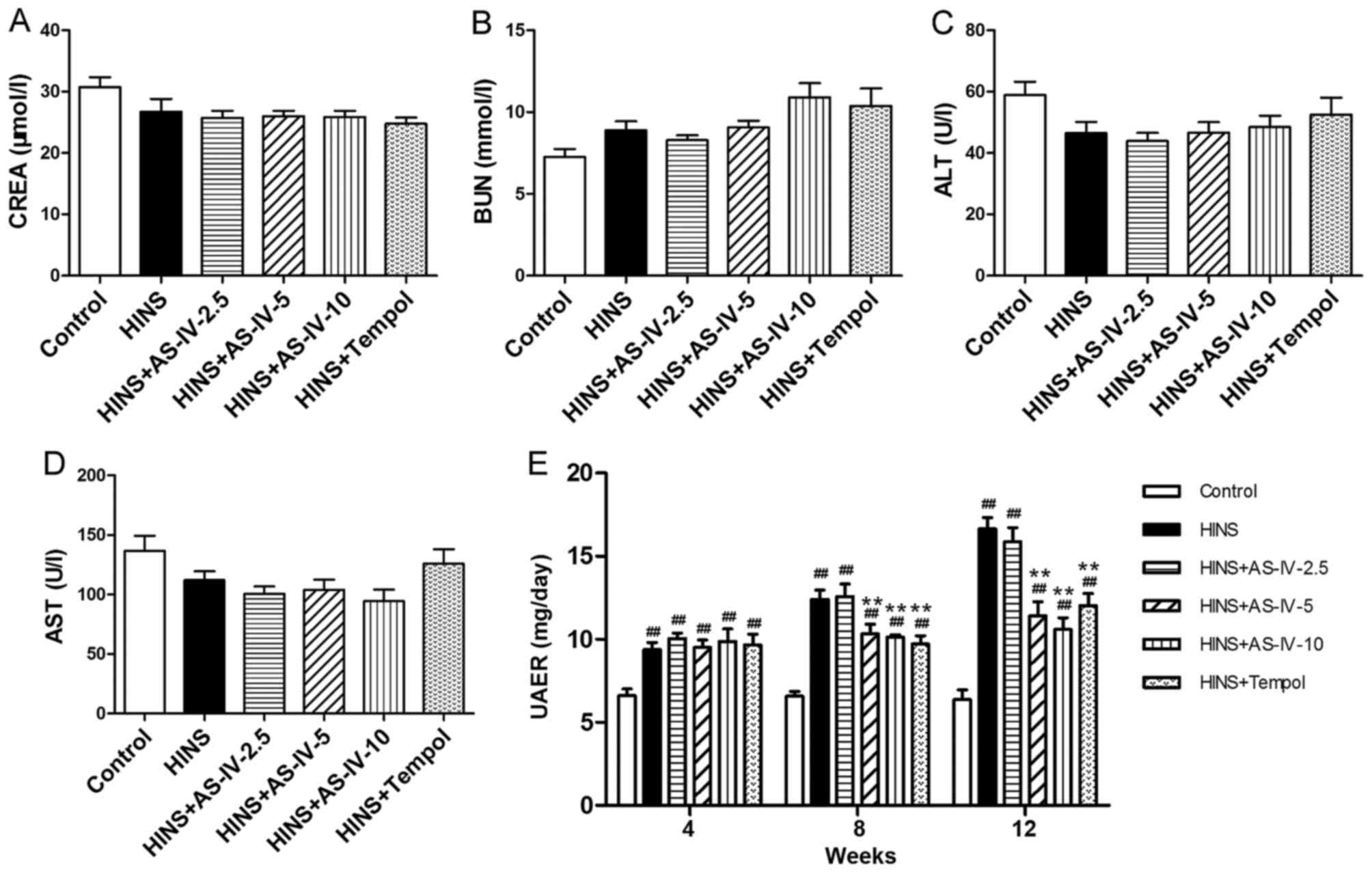 | Figure 2Effects of AS-IV on CREA, BUN, ALT,
AST and UAER in iatrogenic hyperinsulinemic diabetic rats. Shown
are the levels of (A) CREA, (B) BUN, (C) ALT and (D) AST of all the
groups after 12 weeks. Different doses of AS-IV used in these
experiments did not lead to renal or liver dysfunction. (E) Effects
of AS-IV on albuminuria in diabetic rats after 4, 8 and 12 weeks.
AS-IV ameliorated albuminuria of the iatrogenic hyperinsulinemic
diabetic rats. Results are expressed as the means ± standard
deviation (n=8/each group). Refer to the Materials and methods
section for etails of the assignment of the groups.
##P<0.01 vs. control; **P<0.01 vs.
HINS. AS-IV, astragaloside IV; BUN, blood urea nitrogen; ALT,
alanine transaminase; AST, aspartate aminotransferase; HINS,
hyperinsulinemic; UAER, urinary albumin excretion rate. |
Effects of AS-IV on oxidative
stress-associated markers
To assess the antioxidant effects of AS-IV, the
oxidative stress-associated markers, MDA, GSH-Px and SOD, were
measured. Compared with the control group, MDA levels were
increased in the HINS and AS-IV-2.5 groups (P<0.01) (Fig. 3A), whereas GSH-Px and SOD levels
were reduced in the HINS, AS-IV-2.5 and AS-IV-5 groups (P<0.01)
(Fig. 3B and C). These results
suggested that marked oxidative stress was occurring in the
iatrogenic hyperinsulinemic rats, in addition to their having a
reduced antioxidant capacity. However, as shown in Fig. 3A-C, AS-IV significantly reduced
the elevation of renal tissue levels of MDA, and increased the
elevation of tissue levels of GSH-Px and SOD, effects that were
evident at a dose as low as 2.5 mg/kg/day for MDA and GSH-Px (but
not for SOD; the increase for SOD was observed at a dose of AS-IV
as low as 5 mg/kg/day). These results demonstrated the marked
antioxidative capacity of AS-IV. Tempol was also able to reduce
MDA, and increase GSH-Px and SOD.
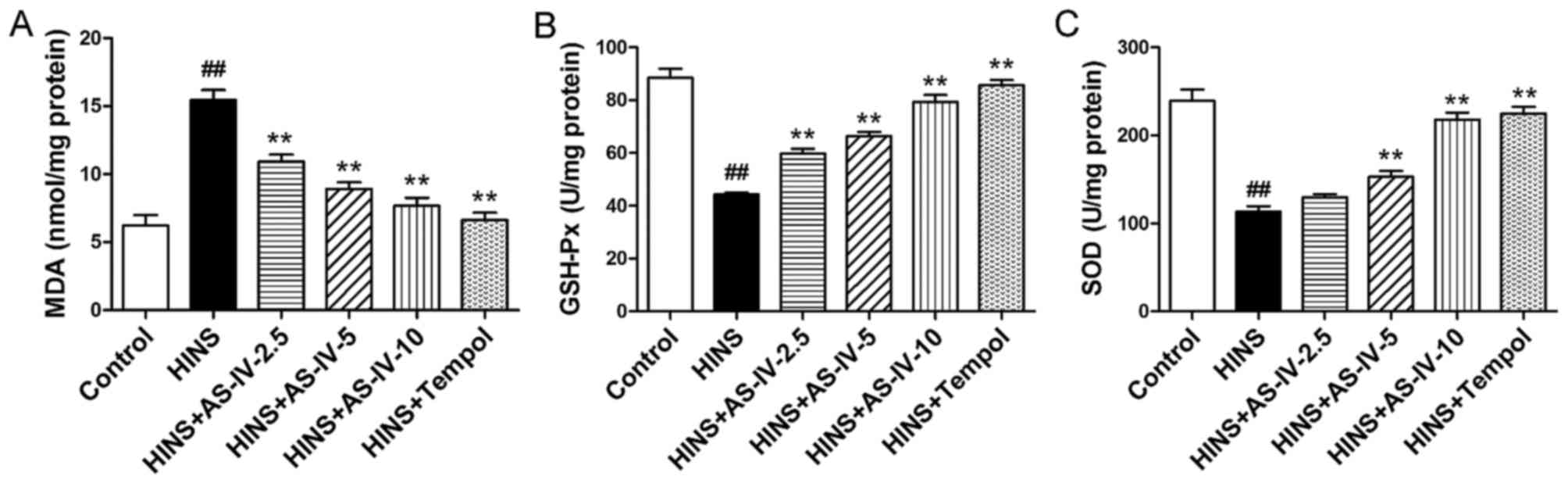 | Figure 3Effects of AS-IV on MDA, GSH-Px and
SOD in iatrogenic hyperinsulinemic diabetic rats. Shown are the
levels of (A) MDA, (B) GSH-Px and (C) SOD in the renal cortex of
all groups after 12 weeks. AS-IV significantly reduced the
elevation of renal tissue levels of MDA, and increased the
elevation of the tissue levels of GSH-Px and SOD, effects that were
evident at a dose as low as 2.5 mg/kg/day for MDA and GSH-Px, but
not for SOD (the increase for SOD was observed at a dose of AS -IV
as low as 5 mg/kg/day). Results are expressed as the means ± SD
(n=8/each group). Refer to the Materials and methods section for
details of the assignment of the groups. ##P<0.01 vs.
control; **P<0.01 vs. HINS. AS-IV, astragaloside IV;
MDA, malondialdehyde; GSH-Px, glutathione peroxidase; SOD,
superoxide dismutase; HINS, hyperinsulinemic. |
AS-IV decreases the secretion of
pro-inflammatory cytokines, and decreases abnormal ECM
synthesis
The effects of AS-IV on pro-inflammatory cytokines,
such as IL-1β and TNF-α, were examined (Fig. 4A and B). The tissue level of IL-1β
in the control group was significantly lower compared with that in
the HINS, AS-IV-2.5 and AS-IV-5 groups (P<0.01; Fig. 4A). TNF-α levels in the normal
control group were significantly lower compared with those in the
other groups, with the exception of the Tempol group (P<0.01;
Fig. 4B). Increases in the levels
of pro-inflammatory cytokines are able to promote ECM accumulation
and to mediate tissue injury. However, AS-IV significantly reduced
the renal tissue levels of IL-1β and TNF-α, which was evident at a
dose as low as 2.5 mg/kg/day for TNF-α Fig. 4B) and 5 mg/kg/day for IL-1β
(Fig. 4A). Tissue levels of
Col-IV and LN in the normal control group were identified to be
significantly lower compared with those in the HINS group
(P<0.01) (Fig. 4C and D).
Increases in the levels of Col-IV and LN are indicative of an
increase in the synthesis of renal ECM. AS-IV significantly reduced
renal tissue levels of Col-IV and LN, which was evident at a dose
as low as 5 mg/kg/day. Thus, AS-IV may attenuate basement membrane
thickening in iatrogenic hyperinsulinemic rats. Tempol was also
able to reduce renal tissue levels of IL-1β, TNF-α, Col-IV and
LN.
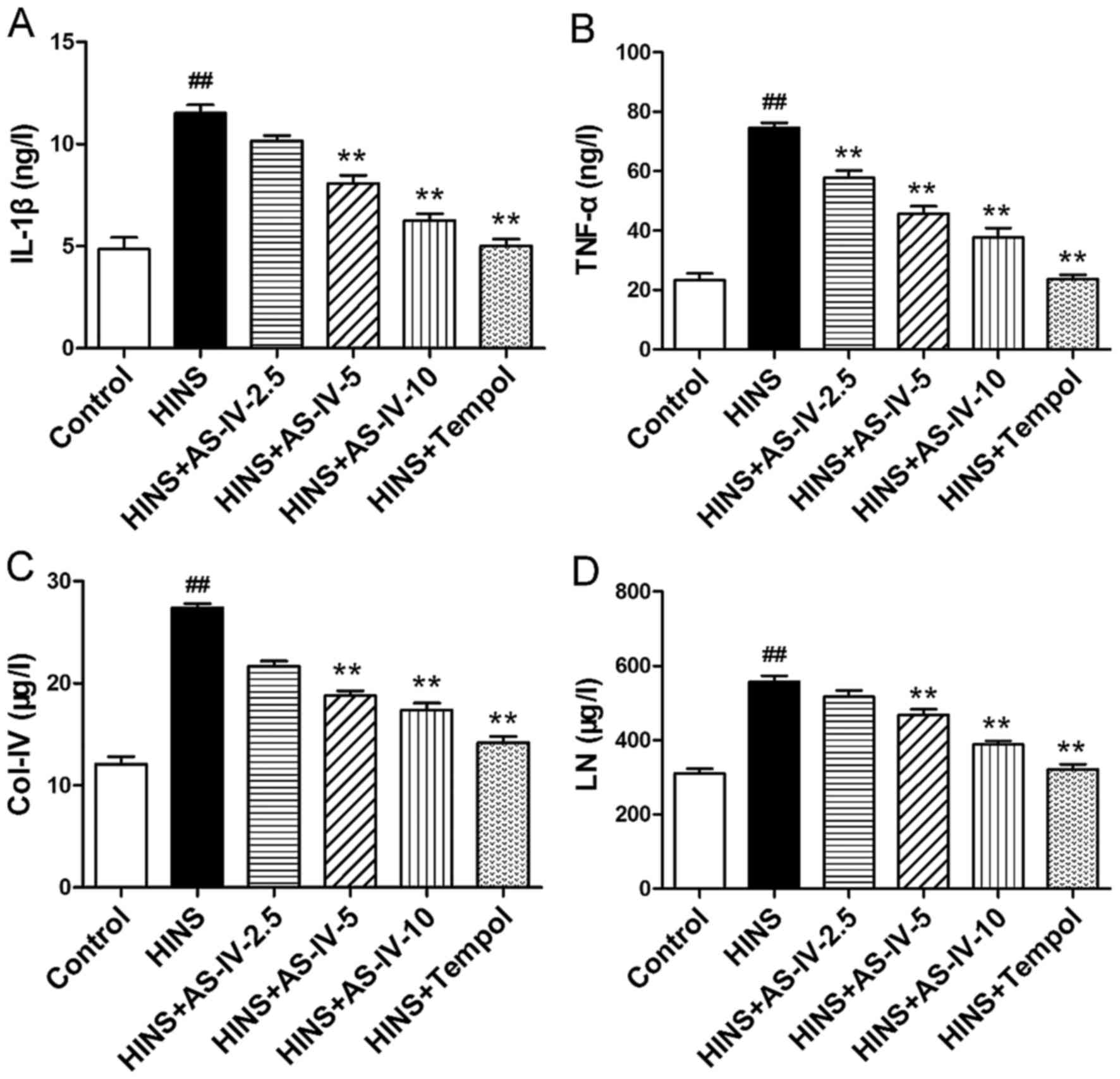 | Figure 4Effects of AS-IV on IL-1β, TNF-α,
Col-IV and LN in iatrogenic hyperinsulinemic diabetic rats. Shown
are the renal cortex levels of (A) IL-1β, (B) TNF-α, (C) Col-IV and
(D) LN of all groups after 12 weeks. AS-IV significantly reduced
the renal tissue levels of IL-1β, TNF-α, Col-IV and LN, which was
evident at a dose as low as 2.5 mg/kg/day (TNF-α) and 5 mg/kg/day
(IL-1β, Col-IV and LN). Results are expressed as the means ±
standard deviation (n=8/each group). Refer to the Materials and
methods section for details of the assignment of the
groups.##P<0.01 vs. control; **P<0.01
vs. HINS. AS -IV, astragaloside IV; HINS, hyperinsulinemic; IL-1β,
interleukin-1β; TNF-α, tumor necrosis factor-α; Col-IV, type IV
collagen; LN, laminin. |
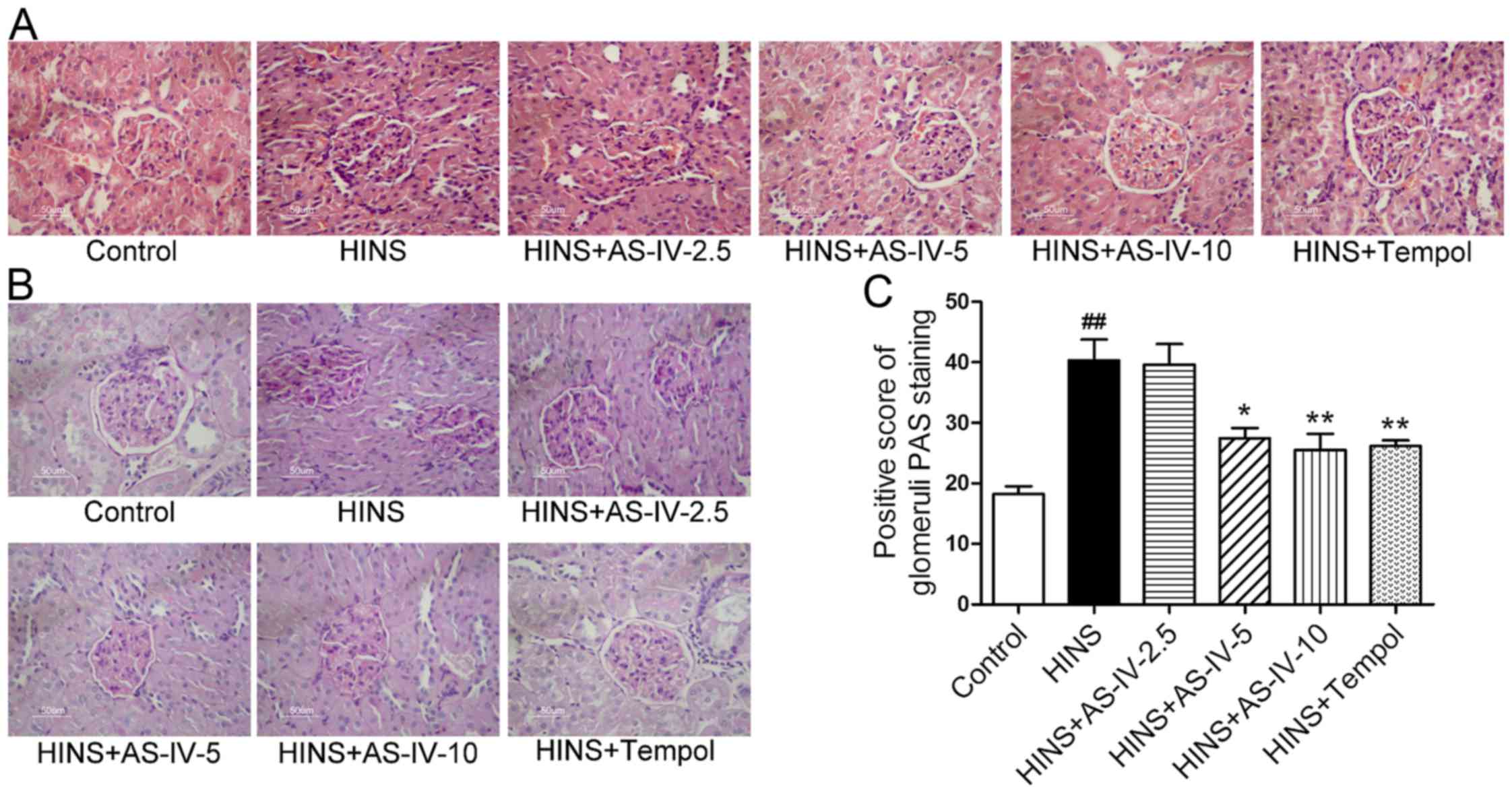
AS-IV alleviates the histopathological
alterations of kidneys in iatrogenic hyperinsulinemic rats
At 12 weeks following the successful modeling of
iatrogenic hyperinsulinemia, the HINS group exhibited modest levels
of mesangial cell proliferation compared with the normal control
rats (Fig. 5A and B). However,
AS-IV and Tempol treatment was able to prevent mesangial cell
proliferation to a significant degree when compared with the HINS
group. This trend was evident at a dose as low as 5 mg/kg/day.
Fig. 5A and B show representative
histopathological changes in the kidney, with H&E and PAS
staining in each group at week 12 following AS-IV treatment. The
HINS group revealed a modest level of proliferation of mesangial
cells, whereas almost no changes were observed in the control,
AS-IV-5, AS-IV-10 and Tempol groups.
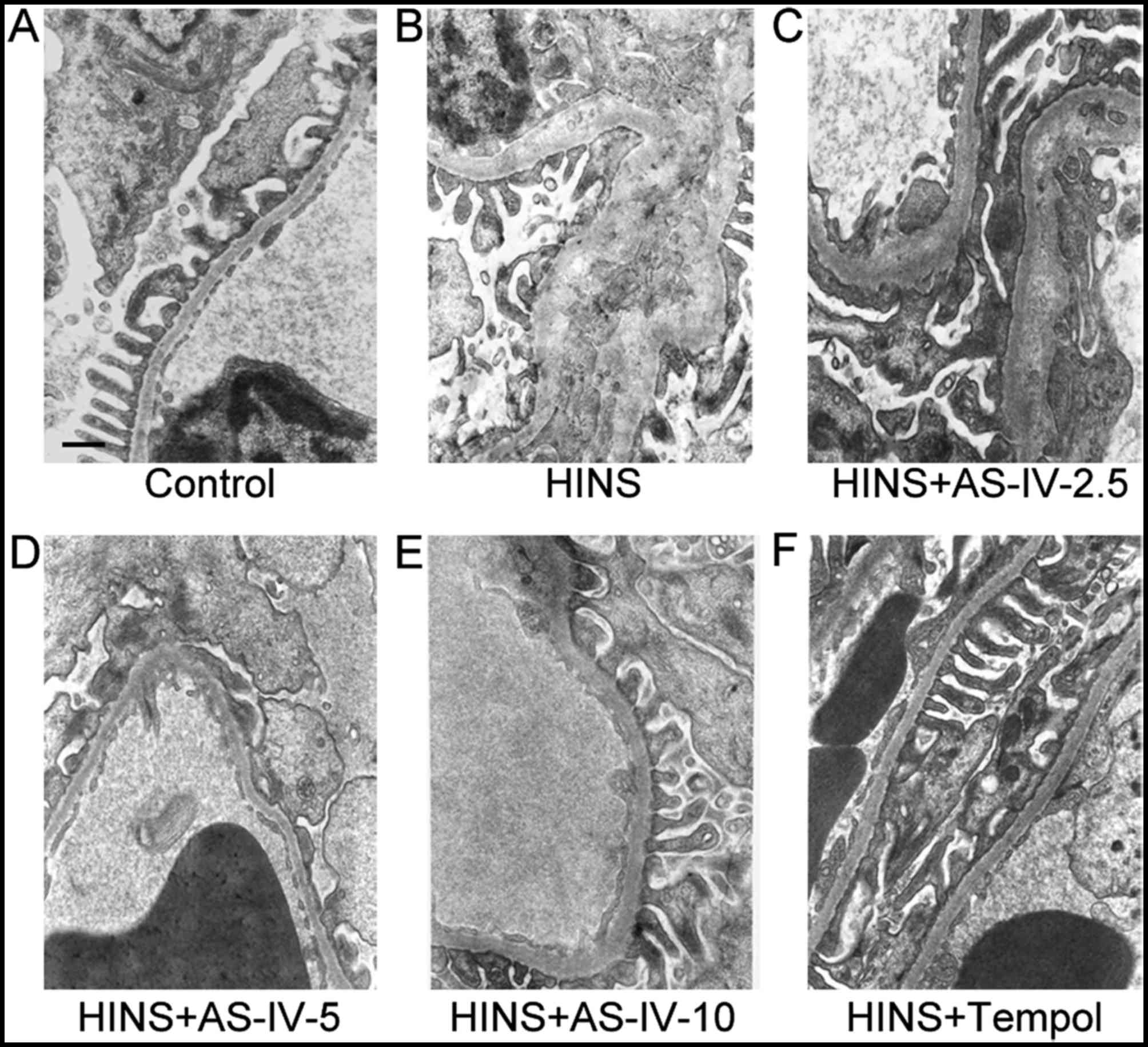
AS-IV alleviates basement membrane
thickening and podocyte foot process effacement of kidneys in
iatrogenic hyperinsulinemic rats
As shown in Fig.
6, the observation of basement membrane and podocyte
ultrastructure by electron microscopy revealed clear basement
membrane thickening and minor podocyte foot process effacement in
the HINS group, whereas the hyperinsulinemic rats treated with
AS-IV revealed a marked decrease in podocyte foot process
effacement and basement membrane thickening, which was evident at a
dose as low as 5 mg/kg/day.
AS-IV suppresses the activation of Nox4
and ERK1/2 induced by iatrogenic hyperinsulinemia, and upregulates
the expression of TRPC6 in the kidney
Having shown that AS-IV exerted antioxidative and
anti-inflammatory effects, subsequently the underlying mechanism
was investigated. In the kidney, particularly in mesangial cells,
Nox4 is the predominant source of ROS. The ERK pathway is an
important signalling pathway with regard to the proliferation of,
and ECM accumulation in, renal tissue. The role of the NADPH
oxidase/ERK1/2 pathway was therefore investigated. The protein
expression levels of Nox4, ERK1/2, p-ERK1/2 and TRPC6 were examined
by western blotting (Fig. 7A),
and the results were analyzed semi-quantitatively. The HINS group
exhibited elevated levels of Nox4 and phosphorylated ERK1/2 in the
kidneys compared with the normal control group (P<0.01)
(Fig. 7B). Treatment with AS-IV,
however, significantly inhibited Nox4 expression and
phosphorylation of ERK1/2 in the kidneys from the iatrogenic
hyperinsulinemic model rats (Fig. 7B
and C), which was evident at a dose as low as 2.5 and 5
mg/kg/day, respectively. TRPC6 is known as a
Ca2+-conductive cation channel that regulates the
contractile function of mesangial cells. Therefore, in the present
study, the effects of AS-IV on the expression level of TRPC6
protein in the rat model of iatrogenic hyperinsulinemia were
examined. We had investigated the expression of NADPH oxidase and
TRPC6 preliminarily, trying to find the relationship between them.
The results presented the relationship between them preliminarily
in Fig. 7, and we are
implementing cell experiments to confirm that. As illustrated in
Fig. 7D, compared with the normal
control group, the HINS group exhibited lower levels of TRPC6
protein (P<0.01). Treatment with AS-IV significantly enhanced
expression of the TRPC6 protein in the kidneys, which was evident
at a dose as low as 2.5 mg/kg/day. This dose coincided with that
causing inhibition of the Nox4 expression.
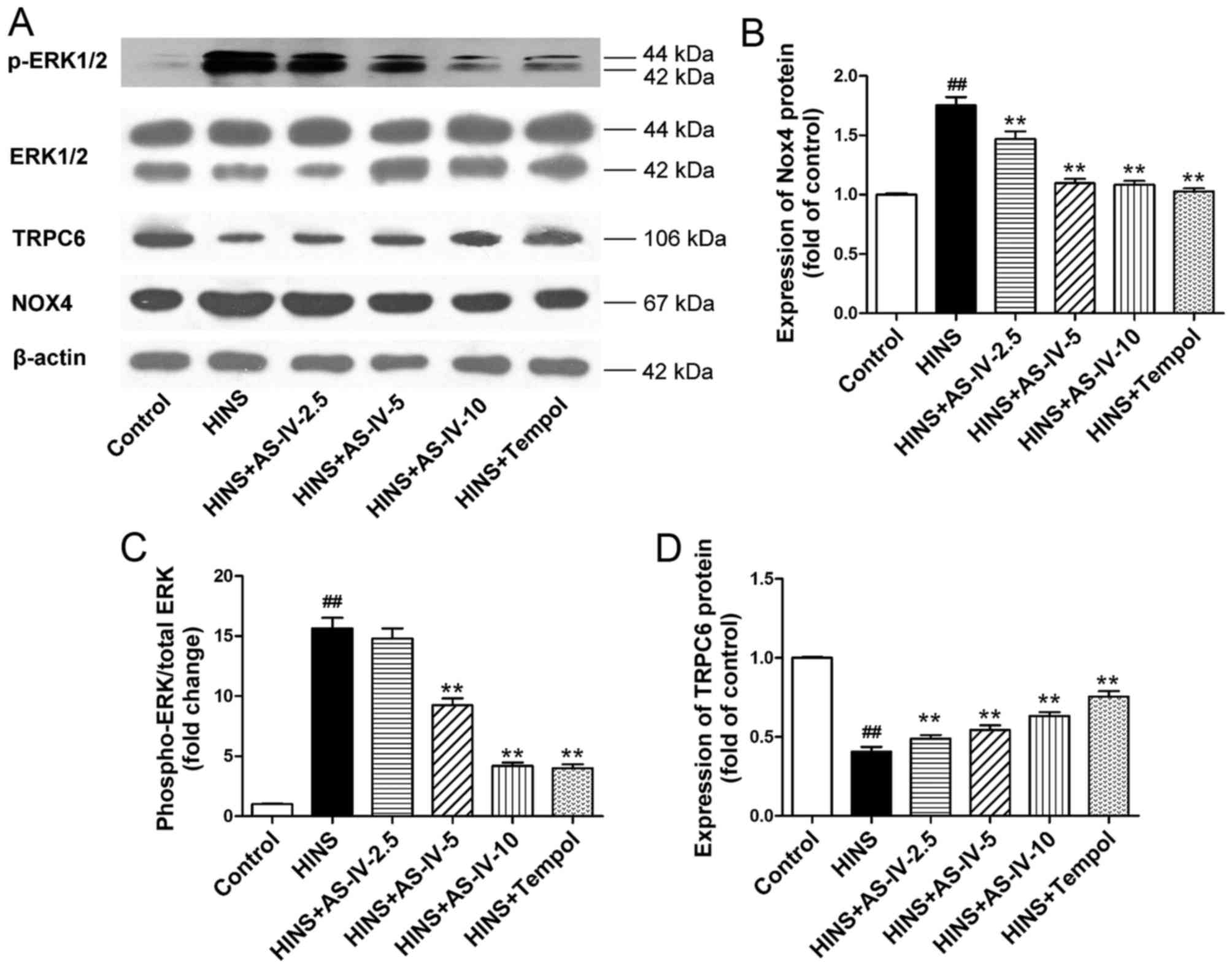 | Figure 7AS-IV regulates the protein
expression of Nox4, ERK1/2, p-ERK1/2 and TRPC6 in iatrogenic
hyperinsulinemic diabetic rats. (A) The protein expression of Nox4,
ERK1/2, p-ERK1/2, TRPC6 and β-actin was examined by western
blotting, and the results of semi-quantitative analysis of the data
for (B) Nox4, (C) p-ERK1/2/ERK1/2 and (D) TRPC6 are also shown. β
-actin was used as a loading control. AS-IV significantly inhibited
the expression of Nox4 and phosphorylation of ERK1/2 in the kidneys
from the iatrogenic hyperinsulinemic model rats, an effect that was
evident at a dose of AS -IV as low as 2.5 and 5 mg/kg/day for Nox4
and p -ERK1/2/ERK1/2, respectively. AS-IV significantly enhanced
expression of the TRPC6 protein in the kidneys, which was evident
at a dose as low as 2.5 mg/kg/day. Results are expressed as the
means ± standard deviation (n=8/each group). The experiments were
performed in triplicate. ##P<0.01 vs. control;
**P<0.01 vs. HINS. AS-IV, astragaloside IV; HINS,
hyperinsulinemic; Nox4, NADPH oxidase 4; ERK1/2, extracellular
signal-regulated kinase 1/2; p -, phosphorylated; TRPC6, transient
receptor potential cation channel 6. |
Discussion
The results presented in the current study support
the hypothesis that AS-IV prevents iatrogenic hyperinsulinemia due
to kidney injury in STZ-induced diabetic rats through the
suppression of oxidative stress, inhibiting the production of
pro-inflammatory factors. This leads to a prevention of mesangial
cell proliferation, basement membrane thickening and podocyte foot
process effacement, thereby ameliorating albuminuria.
DN is an important complication associated with
diabetes mellitus, and has become a common cause of end-stage renal
failure among patients undergoing chronic hemodialysis therapy.
Controlling the blood glucose level is the first issue of concern.
An insufficient control may lead to the rapid onset of DN. However,
patients with diabetes mellitus are able to achieve good blood
glucose control by using insulin, and yet, ultimately, the onset of
DN cannot be avoided. This demonstrates that, despite our ability
to tightly control the blood glucose level, development of DN is
not determined by a single factor associated with blood glucose.
Certain researchers have gone so far as to propose that intensive
insulin treatment does not protect renal function (25). Though not all patients with
diabetes mellitus have hyperinsulinemia, patients with type 1
diabetes (3), and type 2 diabetic
patients who are using insulin (26,27), have been observed to have
iatrogenic hyperinsulinemia. With respect to the establishment of a
rat model, the clinical processes of patients with diabetes
mellitus with iatrogenic hyperinsulinemia were simulated in the
present study. First, the model of diabetic rats was established.
Secondly, iatrogenic hyperinsulinemic diabetic rats were induced by
subcutaneous injection of insulin. The main purpose in the
establishment of this model was to obtain effective control of the
blood glucose level, in order to study the impact of iatrogenic
hyperinsulinemia on the kidney. As described in the Results
section, AS-IV did not change blood glucose or insulin levels. This
suggested that AS-IV was not able to exert any impact on the blood
glucose level: Only insulin itself could. More importantly, our rat
model simulated intensive insulin treatment. The goal of intensive
glycemic control is to regulate blood glucose and HbA1c
levels within the normal range (28). According to a preliminary
experiment, Levemir at a dose of 6 U/day was able to control the
blood glucose level well. Therefore, the hyperinsulinemic rats had
almost normal blood glucose and HbA1c levels compared
with normal control rats. The difference between hyperinsulinemic
rats and normal control rats was attributable solely to their
insulin levels. In order to minimize the interfering factors, a
high-sugar and high-fat manufacturing model was not employed.
Furthermore, nephrotoxicity is not likely to have resulted from the
use of STZ, since the dose of STZ (55 mg/kg) used to induce
diabetes has been reported to have minimal kidney toxicity in
experimental animals (29).
Iatrogenic or exogenous hyperinsulinemia is an unavoidable
consequence of the direct administration of insulin into the
general circulation (6).
Therefore, in our model, the generation of iatrogenic
hyperinsulinemia that was observed was expected.
Radix Astragali, the dried root of Astragalus
membranaceus (Fisch.) Bunge, has long been used in traditional
Chinese medicine for the treatment of diabetes. AS-IV is a novel
saponin extracted from Radix Astragali, which prevents damage to
human mesangial cells and umbilical vein endothelial cells by
suppressing oxidative stress, inflammation and cell proliferation,
as demonstrated in previous studies by our group (7,30).
High concentrations of insulin are able to promote cell
proliferation, ECM expansion and oxidative stress in vitro.
This therefore suggested the use of AS-IV in vivo to
intervene with renal damage caused by iatrogenic hyperinsulinemia,
as long as it proved to be effective.
The most important clinical manifestation of DN is
albuminuria (31). In the current
study, the amounts of albumin in the urine of diabetic rats were
low. Insulin has a therapeutic effect on diabetes mellitus. If
appreciable levels of albuminuria were to have been observed, it
was possible that our study could have been implemented for a
longer period of time. Though the overall level of kidney damage
was minor, rats were observed during the very early period of DN.
Direct observation of histological sections stained with H&E
and PAS revealed basement membrane thickening in the HINS group.
Subsequently, electron microscopy (magnification, x15,000) was used
to observe basement membrane thickening and podocyte foot process
effacement. AS-IV was able to prevent these pathological changes,
and these were the most intuitive results obtained in this study,
providing important evidence for renal function protection mediated
by AS-IV, results which are consistent with other studies (21,32).
Subsequently, the mechanism underlying the changes
described above was sought after. Oxidative stress and inflammation
are the two predominant factors of DN (33). First, in a high-insulin
environment, levels of oxidative stress may be increased (34), and endogenous hyperinsulinemia can
exacerbate oxidative stress (35). Piwkowska et al (36) demonstrated that high insulin
levels increase the glomerular barrier albumin permeability via a
protein kinase G type I (PKGI)-dependent mechanism involving
NAD(P)H-dependent generation of superoxide anion in podocytes.
Markers of oxidative stress, including ROS and reduced levels of
antioxidants, have been identified in renal tissues in human and
experimental models of diabetes (37). The excessive production of ROS is
an important mechanism underlying the pathogenesis of
diabetes-associated DN. The level of MDA provides a good index of
intensified oxidative stress in tissues exhibiting enhanced
peroxidation processes (38). SOD
and GSH-Px constitute the principal components of the cohort of
cellular antioxidants, and their deficiencies can cause oxidative
stress. The SOD enzyme system is a primary determinant of
superoxide removal (39). Changes
in the levels of the above indicators were observed in the present
study, implying that renal oxidative stress was present. The
antioxidant effect mediated by AS-IV was evident in the iatrogenic
hyperinsulinemic rats. Secondly, inflammation exerts a pivotal role
in the pathogenesis of DN. Hyperinsulinemia may increase
inflammation and pro-inflammatory cytokine release (40). Certain pro-inflammatory cytokines,
including IL-1 and TNF-α, have been reported to participate in the
pathogenesis of DN (41). ROS
generation was also noted to be associated with increased levels of
TNF-α and increased glomerular lesions in experimental diabetic
rats (42), suggesting a role for
free radical generation in association with TNF-α in the induction
and progression of diabetes-associated renal injury. Taken
together, the results in the present study suggested that renal
inflammation was present. The anti-inflammatory effect of AS-IV was
evident in the iatrogenic hyperinsulinemic rats, results which are
consistent with the study of Gui et al (32).
Col-IV and LN are important components of the
basement membrane, and important indicators of ECM accumulation.
Excessive deposition of Col-IV is an established feature associated
with diabetic glomerulopathy (43), whereas aberrant expression of LN
is a critical pathological characteristic of DN (44).
Oxidative stress is able to promote anti-glomerular
basement membrane glomerulonephritis in renin-angiotensin system
activation in the kidney (45),
and LN production in renal mesangial cells (46). However, it was reported that
inflammation could promote LN expression (47). It was also reported that high
insulin levels are able to promote ECM synthesis in vitro
(48). The ERK pathway is an
important signalling pathway involved with the proliferation of
renal tissue (49) and with the
ECM synthesis that occurs during scar formation (50). Hyperinsulinemia is able to
activate the ERK pathway (51).
In the kidney, oxidative stress can activate the ERK pathway
(52), and inflammation may also
activate the ERK pathway in the kidney (53). Therefore, it was hypothesized
that, in the iatrogenic hyperinsulinemic environment, oxidative
stress and inflammation in kidney tissues were enhanced, and the
two processes served to co-activate the ERK pathway, which resulted
in cell proliferation and increased ECM deposition. The possible
mechanisms in operation in the hyperinsulinemic environment of
mesangial cells and podocytes are as follows: First, from our
previous study, high insulin levels induce an increase in
Nox4-sourced ROS production, and ROS further promote mesangial cell
proliferation and increases in ECM deposition (54). Secondly, as for the podocytes, it
was reported that insulin increases the glomerular filtration
barrier permeability through PKGIα-dependent mobilization of large
conductance calcium-activated potassium (BKCa) channels (55). Thus, effacement of the podocyte
foot processes occurs, and albuminuria appears. Based on the
results in the present study, AS-IV exerts clear anti-inflammatory
effects and inhibits oxidative stress, thereby inhibiting mesangial
cell proliferation and basement membrane thickening. However, the
observed effects of mesangial cell proliferation and basement
membrane thickening levels were modest. This may be due to the
relatively short experimental period. In establishing the
experimental design, it was a concern that extended s.c. injections
of insulin could induce exogenous insulin resistance, or even
subcutaneous insulin resistance.
TRPC6 is an important protein in the maintenance of
normal renal function (56). It
protects against renal ischemia-reperfusion injury (57). In the present study, the
expression of TRPC6 was reduced in the iatrogenic hyperinsulinemic
environment. This reduction may have been associated with the
oxidative stress environment produced by iatrogenic
hyperinsulinemia, and this result was consistent with our previous
study (24). The possible reason
has yet to be elucidated, although the mechanism may be that TRPC6
protein is decreased by ROS (58). The possible mechanism will be
explored further in our subsequent studies. AS-IV is able to reduce
the degree of the TRPC6 protein decreases in iatrogenic
hyperinsulinemia by upregulating the expression of TRPC6.
The study drug selected for the present study was a
traditional Chinese medicine monomer, and its pharmacological
mechanism has yet to be fully elucidated. Previous studies have
shown that it has anti-inflammatory effects and inhibits oxidative
stress. It has been reported that the NADPH oxidase inhibitor,
apocynin, protects the kidney against DN in Otsuka Long Evans
Tokushima Fatty (OLETF) rats (14). As observed previously for
anti-oxidative stress drugs, Tempol exerts anti-inflammatory and
antioxidant effects, and it has also been reported to have
kidney-protective effects (24).
Tempol was therefore selected as a positive control drug.
In conclusion, AS-IV prevents kidney injury in
iatrogenic hyperinsulinemic diabetic rats by suppressing oxidative
stress, inhibiting IL-1β and TNF-α overproduction, downregulating
ERK1/2 activation, and upregulating TRPC6 expression in the field
of DN. The findings in the present study should provide a method
for delaying the occurrence of DN under intensive insulin
treatment.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (no. 81173624), the Nature
Science Foundation of Anhui Province (no. 11040606M201), the
College Natural Science Research Project of Anhui province (no.
KJ2016SD35), and the International Scientific and Technological
Cooperative Project of Anhui province (no. 1230603007). The authors
would like to thank Dr Li Gui and Dake Huang from the Synthetic
Laboratory of Anhui Medical University for their helpful technical
assistance.
References
|
1
|
American Diabetes Association: Standards
of medical care in diabetes - 2011. Diabetes Care. 34(Suppl 1):
S11–S61. 2011. View Article : Google Scholar
|
|
2
|
Choudhury D, Tuncel M and Levi M: Diabetic
nephropathy - - a multifaceted target of new therapies. Discov Med.
10:406–415. 2010.PubMed/NCBI
|
|
3
|
Wang MY, Yu X, Lee Y, McCorkle SK, Clark
GO, Strowig S, Unger RH and Raskin P: Iatrogenic hyperinsulinemia
in type 1 diabetes: Its effect on atherogenic risk markers. J
Diabetes Complications. 27:70–74. 2013. View Article : Google Scholar
|
|
4
|
Mariappan MM, DeSilva K, Sorice GP,
Muscogiuri G, Jimenez F, Ahuja S, Barnes JL, Choudhury GG, Musi N,
DeFronzo R, et al: Combined acute hyperglycemic and
hyperinsulinemic clamp induced profibrotic and proinflammatory
responses in the kidney. Am J Physiol Cell Physiol. 306:C202–C211.
2014. View Article : Google Scholar :
|
|
5
|
Ngubane PS, Hadebe SI, Serumula MR and
Musabayane CT: The effects of transdermal insulin treatment of
streptozotocin-induced diabetic rats on kidney function and renal
expression of glucose transporters. Ren Fail. 37:151–159. 2015.
View Article : Google Scholar
|
|
6
|
Draznin B, Miles P, Kruszynska Y, Olefsky
J, Friedman J, Golovchenko I, Stjernholm R, Wall K, Reitman M,
Accili D, et al: Effects of insulin on prenylation as a mechanism
of potentially detrimental influence of hyperinsulinemia.
Endocrinology. 141:1310–1316. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun L, Li W, Li W, Xiong L, Li G and Ma R:
Astragaloside IV prevents damage to human mesangial cells through
the inhibition of the NADPH oxidase/ROS/Akt/NF-κB pathway under
high glucose conditions. Int J Mol Med. 34:167–176. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim EY, Anderson M and Dryer SE: Insulin
increases surface expression of TRPC6 channels in podocytes: Role
of NADPH oxidases and reactive oxygen species. Am J Physiol Renal
Physiol. 302:F298–F307. 2012. View Article : Google Scholar :
|
|
9
|
Hong OK, Lee SH, Rhee M, Ko SH, Cho JH,
Choi YH, Song KH, Son HY and Yoon KH: Hyperglycemia and
hyperinsulinemia have additive effects on activation and
proliferation of pancreatic stellate cells: Possible explanation of
islet-specific fibrosis in type 2 diabetes mellitus. J Cell
Biochem. 101:665–675. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mariappan MM, Feliers D, Mummidi S,
Choudhury GG and Kasinath BS: High glucose, high insulin, and their
combination rapidly induce laminin-beta1 synthesis by regulation of
mRNA translation in renal epithelial cells. Diabetes. 56:476–485.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bondi CD, Manickam N, Lee DY, Block K,
Gorin Y, Abboud HE and Barnes JL: NAD(P)H oxidase mediates
TGF-beta1-induced activation of kidney myofibroblasts. J Am Soc
Nephrol. 21:93–102. 2010. View Article : Google Scholar :
|
|
12
|
Asaba K, Tojo A, Onozato ML, Goto A, Quinn
MT, Fujita T and Wilcox CS: Effects of NADPH oxidase inhibitor in
diabetic nephropathy. Kidney Int. 67:1890–1898. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gorin Y, Block K, Hernandez J, Bhandari B,
Wagner B, Barnes JL and Abboud HE: Nox4 NAD(P)H oxidase mediates
hypertrophy and fibronectin expression in the diabetic kidney. J
Biol Chem. 280:39616–39626. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nam SM, Lee MY, Koh JH, Park JH, Shin JY,
Shin YG, Koh SB, Lee EY and Chung CH: Effects of NADPH oxidase
inhibitor on diabetic nephropathy in OLETF rats: The role of
reducing oxidative stress in its protective property. Diabetes Res
Clin Pract. 83:176–182. 2009. View Article : Google Scholar
|
|
15
|
Mora C and Navarro JF: Inflammation and
pathogenesis of diabetic nephropathy. Metabolism. 53:265–266;
author reply 266-267. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang Y, Zeng J, Chen G, Xie X, Guo W and
Tian W: Periodontitis contributes to adipose tissue inflammation
through the NF-kappaB, JNK and ERK pathways to promote insulin
resistance in a rat model. Microbes Infect. 18:804–812. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Smyth JT, Hwang SY, Tomita T, DeHaven WI,
Mercer JC and Putney JW: Activation and regulation of
store-operated calcium entry. J Cell Mol Med. 14:2337–2349. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nilius B and Owsianik G: The transient
receptor potential family of ion channels. Genome Biol. 12:2182011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma R, Du J, Sours S and Ding M:
Store-operated Ca2+ channel in renal microcirculation
and glomeruli. Exp Biol Med (Maywood). 231:145–153. 2006.
View Article : Google Scholar
|
|
20
|
Ren S, Zhang H, Mu Y, Sun M and Liu P:
Pharmacological effects of astragaloside IV: A literature review. J
Tradit Chin Med. 33:413–416. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gui D, Huang J, Liu W, Guo Y, Xiao W and
Wang N: Astragaloside IV prevents acute kidney injury in two rodent
models by inhibiting oxidative stress and apoptosis pathways.
Apoptosis. 18:409–422. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Danda RS, Habiba NM, Rincon-Choles H,
Bhandari BK, Barnes JL, Abboud HE and Pergola PE: Kidney
involvement in a nongenetic rat model of type 2 diabetes. Kidney
Int. 68:2562–2571. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Banse HE, Frank N, Kwong GP and McFarlane
D: Relationship of oxidative stress in skeletal muscle with obesity
and obesity-associated hyperinsulinemia in horses. Can J Vet Res.
79:329–338. 2015.PubMed/NCBI
|
|
24
|
Luan J, Li W, Han J, Zhang W, Gong H and
Ma R: Renal protection of in vivo administration of tempol in
streptozotocin-induced diabetic rats. J Pharmacol Sci. 119:167–176.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chowdhury TA, O'Toole S and Yaqoob MM:
Managing blood glucose levels in patients with diabetes and renal
impairment. Br J Hosp Med (Lond). 77:C10–C13. 2016. View Article : Google Scholar
|
|
26
|
Velussi M, Cernigoi AM, De Monte A, Dapas
F, Caffau C and Zilli M: Long-term (12 months) treatment with an
anti-oxidant drug (silymarin) is effective on hyperinsulinemia,
exogenous insulin need and malondialdehyde levels in cirrhotic
diabetic patients. J Hepatol. 26:871–879. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Müssig K, Staiger H, Kantartzis K,
Fritsche A, Kanz L and Häring HU: Type 2 diabetes mellitus and risk
of malignancy: Is there a strategy to identify a subphenotype of
patients with increased susceptibility to endogenous and exogenous
hyperinsulinism? Diabet Med. 28:276–286. 2011.PubMed/NCBI
|
|
28
|
Gerstein HC, Bosch J, Dagenais GR, Díaz R,
Jung H, Maggioni AP, Pogue J, Probstfield J, Ramachandran A, Riddle
MC, et al ORIGIN Trial Investigators: Basal insulin and
cardiovascular and other outcomes in dysglycemia. N Engl J Med.
367:319–328. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Koulmanda M, Qipo A, Chebrolu S, O'Neil J,
Auchincloss H and Smith RN: The effect of low versus high dose of
streptozotocin in cynomolgus monkeys (Macaca fascilularis). Am J
Transplant. 3:267–272. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ma Y and Li W, Yin Y and Li W: AST IV
inhibits H2O2-induced human umbilical vein
endothelial cell apoptosis by suppressing Nox4 expression through
the TGF-β1/Smad2 pathway. Int J Mol Med. 35:1667–1674. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gluhovschi C, Gluhovschi G, Petrica L,
Timar R, Velciov S, Ionita I, Kaycsa A and Timar B: Urinary
biomarkers in the assessment of early diabetic nephropathy. J
Diabetes Res. 2016:46261252016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gui D, Huang J, Guo Y, Chen J, Chen Y,
Xiao W, Liu X and Wang N: Astragaloside IV ameliorates renal injury
in streptozotocin-induced diabetic rats through inhibiting
NF-κB-mediated inflammatory genes expression. Cytokine. 61:970–977.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Voroneanu L, Nistor I, Dumea R, Apetrii M
and Covic A: Silymarin in Type 2 Diabetes Mellitus: A systematic
review and meta-analysis of randomized controlled trials. J
Diabetes Res. 2016:51474682016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Abhijit S, Bhaskaran R, Narayanasamy A,
Chakroborty A, Manickam N, Dixit M, Mohan V and Balasubramanyam M:
Hyperinsulinemia-induced vascular smooth muscle cell (VSMC)
migration and proliferation is mediated by converging mechanisms of
mitochondrial dysfunction and oxidative stress. Mol Cell Biochem.
373:95–105. 2013. View Article : Google Scholar
|
|
35
|
Xu L and Badr MZ: Enhanced potential for
oxidative stress in hyperinsulinemic rats: imbalance between
hepatic peroxisomal hydrogen peroxide production and decomposition
due to hyperinsulinemia. Horm Metab Res. 31:278–282. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Piwkowska A, Rogacka D, Kasztan M,
Angielski S and Jankowski M: Insulin increases glomerular
filtration barrier permeability through dimerization of protein
kinase G type Iα subunits. Biochim Biophys Acta. 1832:791–804.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee HB, Yu MR, Yang Y, Jiang Z and Ha H:
Reactive oxygen species-regulated signaling pathways in diabetic
nephropathy. J Am Soc Nephrol. 14(Suppl 3): S241–S245. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kedziora-Kornatowska K, Szram S,
Kornatowski T, Szadujkis-Szadurski L, Kedziora J and Bartosz G: The
effect of verapamil on the antioxidant defence system in diabetic
kidney. Clin Chim Acta. 322:105–112. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fridovich I: Superoxide radical and
superoxide dismutases. Annu Rev Biochem. 64:97–112. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu S, Zhang Q, Chen C, Ge D, Qu Y, Chen
R, Fan YM, Li N, Tang WW, Zhang W, et al: Hyperinsulinemia enhances
interleukin-17-induced inflammation to promote prostate cancer
development in obese mice through inhibiting glycogen synthase
kinase 3-mediated phosphorylation and degradation of interleukin-17
receptor. Oncotarget. 7:13651–13666. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hasegawa G, Nakano K, Sawada M, Uno K,
Shibayama Y, Ienaga K and Kondo M: Possible role of tumor necrosis
factor and interleukin-1 in the development of diabetic
nephropathy. Kidney Int. 40:1007–1012. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xiao H, Li Y, Qi J, Wang H and Liu K:
Peroxynitrite plays a key role in glomerular lesions in diabetic
rats. J Nephrol. 22:800–808. 2009.PubMed/NCBI
|
|
43
|
Pugliese G, Pricci F, Pugliese F, Mene P,
Lenti L, Andreani D, Galli G, Casini A, Bianchi S, Rotella CM, et
al: Mechanisms of glucose-enhanced extracellular matrix
accumulation in rat glomerular mesangial cells. Diabetes.
43:478–490. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Setty S, Michael AA, Fish AJ, Michael
Mauer S, Butkowski RJ, Virtanen I and Kim Y: Differential
expression of laminin isoforms in diabetic nephropathy and other
renal diseases. Mod Pathol. 25:859–868. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kinoshita Y, Kondo S, Urushihara M, Suga
K, Matsuura S, Takamatsu M, Shimizu M, Nishiyama A, Kawachi H and
Kagami S: Angiotensin II type I receptor blockade suppresses
glomerular renin-angiotensin system activation, oxidative stress,
and progressive glomerular injury in rat anti-glomerular basement
membrane glomerulonephritis. Transl Res. 158:235–248. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Singh LP, Cheng DW, Kowluru R, Levi E and
Jiang Y: Hexosamine induction of oxidative stress, hypertrophy and
laminin expression in renal mesangial cells: Effect of the
anti-oxidant alpha-lipoic acid. Cell Biochem Funct. 25:537–550.
2007. View Article : Google Scholar
|
|
47
|
Ji K and Tsirka SE: Inflammation modulates
expression of laminin in the central nervous system following
ischemic injury. J Neuroinflammation. 9:1592012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mariappan MM, Shetty M, Sataranatarajan K,
Choudhury GG and Kasinath BS: Glycogen synthase kinase 3beta is a
novel regulator of high glucose- and high insulin-induced
extracellular matrix protein synthesis in renal proximal tubular
epithelial cells. J Biol Chem. 283:30566–30575. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Brenner BM, Cooper ME, de Zeeuw D, Keane
WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z and
Shahinfar S; RENAAL Study Investigators: Effects of losartan on
renal and cardiovascular outcomes in patients with type 2 diabetes
and nephropathy. N Engl J Med. 345:861–869. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hu X, Wang H, Liu J, Fang X, Tao K, Wang
Y, Li N, Shi J, Wang Y, Ji P, et al: The role of ERK and JNK
signaling in connective tissue growth factor induced extracellular
matrix protein production and scar formation. Arch Dermatol Res.
305:433–445. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fernandez-Twinn DS, Blackmore HL, Siggens
L, Giussani DA, Cross CM, Foo R and Ozanne SE: The programming of
cardiac hypertrophy in the offspring by maternal obesity is
associated with hyperinsulinemia, AKT, ERK, and mTOR activation.
Endocrinology. 153:5961–5971. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
He T, Guan X, Wang S, Xiao T, Yang K, Xu
X, Wang J and Zhao J: Resveratrol prevents high glucose-induced
epithelial-mesenchymal transition in renal tubular epithelial cells
by inhibiting NADPH oxidase/ROS/ERK pathway. Mol Cell Endocrinol.
402:13–20. 2015. View Article : Google Scholar
|
|
53
|
Ma JQ, Ding J, Xiao ZH and Liu CM:
Puerarin ameliorates carbon tetrachloride-induced oxidative DNA
damage and inflammation in mouse kidney through ERK/Nrf2/ARE
pathway. Food Chem Toxicol. 71:264–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li G and Li W, Duan W and Li W: Protective
effects of As-IV on high insulin induced human mesangial cells
injury and its mechanisms. Acta Univ Medicinalis Anhui.
50:1629–1633. 2015.In Chinese.
|
|
55
|
Piwkowska A, Rogacka D, Audzeyenka I,
Kasztan M, Angielski S and Jankowski M: Insulin increases
glomerular filtration barrier permeability through PKGIα-dependent
mobilization of BKCa channels in cultured rat podocytes. Biochim
Biophys Acta. 1852:1599–1609. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Reiser J, Polu KR, Möller CC, Kenlan P,
Altintas MM, Wei C, Faul C, Herbert S, Villegas I, Avila-Casado C,
et al: TRPC6 is a glomerular slit diaphragm-associated channel
required for normal renal function. Nat Genet. 37:739–744. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Shen B, He Y, Zhou S, Zhao H, Mei M and Wu
X: TRPC6 may protect renal ischemia-reperfusion injury through
inhibiting necroptosis of renal tubular epithelial cells. Med Sci
Monit. 22:633–641. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Graham S, Gorin Y, Abboud HE, Ding M, Lee
DY, Shi H, Ding Y and Ma R: Abundance of TRPC6 protein in
glomerular mesangial cells is decreased by ROS and PKC in diabetes.
Am J Physiol Cell Physiol. 301:C304–C315. 2011. View Article : Google Scholar : PubMed/NCBI
|