Introduction
Doxorubicin (DOXO) is a widely used chemotherapeutic
agent that is effective in treating hematological and solid tumor
malignancies, including leukemia, breast cancer, lung carcinoma and
kidney cancer (1). Despite its
high efficacy, the clinical application of DOXO has been greatly
restricted due to its side effects (2). Among the adverse effects, the major
limiting factor for therapy with DOXO is cardiotoxicity, which is
characterized by dilated cardiomyopathy that can develop for up to
several years following cessation of treatment (3,4).
Thus, cardiotoxicity dramatically limits the use of DOXO as a
chemotherapeutic agent.
Cell-based therapies have huge potential for
treating DOXO-induced cardiotoxicity. Mesenchymal stem cell (MSC)
transplantation is regarded as a promising option due to its
regenerative effects and immunological safety (5,6).
However, DOXO has toxic effects on cultured MSCs, resulting in
decreased proliferation and an impaired capacity for
differentiation (5,6). Furthermore, DOXO reduces the
survival, induces the apoptosis and impairs the paracrine function
of MSCs (7), indicating the
induction of cellular senescence (8). Therefore, rejuvenating the activity
of MSCs in the presence of DOXO could improve their therapeutic
potential for the treatment of DOXO-induced cardiotoxicity.
Macrophage migration inhibitory factor (MIF) is a
pleiotropic cytokine that maintains homeostasis by regulating
physiological signaling pathways (9). MIF is thought to serve a fundamental
role in cellular senescence; a previous study reported that in aged
hearts, MIF secretion was significantly reduced and resulted in the
dysregulation of glucose uptake during ischemia/reperfusion
(10). In addition, it has been
demonstrated that increasing the activity of MIF attenuates
ischemia/reperfusion-induced injuries (11). Furthermore, MIF contributes to
cell survival and proliferation, and has been identified to prevent
cellular senescence (12). With
respect to MSCs, MIF is a potential candidate for preventing
naturally-occurring and hypoxia-induced senescence (13,14). The present study investigated
whether MIF could rejuvenate MSCs in the presence of DOXO and thus
enhance their function. MIF could then be applied to rejuvenate
MSCs and reduce DOXO-associated cardiomyopathy.
Previous study has demonstrated that MIF acts
through the phosphatidylinositol 3-kinase (PI3K)-RAC-α
serine/threonine-protein kinase (Akt) signaling pathway to promote
cellular resistance to glucose deprivation, ischemia, hypoxia,
oxidative and senescence (13,15). Activation of the PI3K-Akt
signaling pathway can slow down the process of senescence, and has
been investigated in MSCs as a therapeutic target forage-associated
diseases (12,13). Conversely, the downregulation of
Akt expression can accelerate cellular senescence in MSCs (16). DOXO has been identified to impair
Akt expression, leading to the senescence of human cardiac
progenitor cells (17). In the
present study, whether the activation of the PI3K-Akt signaling
pathway by MIF could restore the proliferation and function of
senescent MSCs in the presence of DOXO was investigated.
Cellular senescence can be triggered by multiple
mechanisms, including those resulting in the production of reactive
oxygen species (ROS) and oxidative stress (18). DOXO induces cellular injury
primarily through the generation of ROS, which are a common
mediator of cellular senescence (19,20). It has also been demonstrated that
MIF exerts a protective effect on MSCs by inhibiting ROS generation
(15). Given the involvement of
ROS in the senescence of MSCs (21), the present study hypothesized that
modulating oxidative stress would rejuvenate senescent MSCs.
Therefore, the present study investigated whether MIF could
attenuate oxidative stress and promote the rejuvenation of
senescent MSCs induced by DOXO.
Materials and methods
Animals
A total of 96 male Sprague Dawley rats (eight groups
with n=12/group) weighing 60–80 g (age, 105.25±11.94 days) were
purchased from the Laboratory Animals Center of Wenzhou Medical
University (Wenzhou, China). The rats were cared for in accordance
with published guidelines from the US National Institutes of Health
(Bethesda, MD, USA) (22). The
rats were raised apart and kept at a temperature of 21±2°C, with a
relative humidity of 30–70% and a 12-h light/dark cycle. The rats
had access to food and water ad libitum. All study
procedures were approved by the Wenzhou Medical University
Institutional Animal Care and Use Committee (Wenzhou, China).
Reagents
Dulbecco's modified Eagle's medium (DMEM) and fetal
bovine serum (FBS) were purchased from HyClone (GE Healthcare Life
Sciences, Logan, UT, USA). TRIzol reagent was obtained from Thermo
Fisher Scientific, Inc. (Waltham, MA, USA). Rabbit monoclonal
anti-rat antibodies directed against Akt (cat. no. 9272),
phosphorylated (p)-Akt (S473, cat. no. 4060; T308, cat. no. 13038)
and β-actin (cat. no. 4970) were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA) and the horseradish
peroxidase-conjugated anti-rabbit secondary antibody (cat. no.
sc-2357) was obtained from Santa Cruz Biotechnology, Inc. (Dallas,
TX, USA). Sigma-Aldrich (Merck KGaA, Darmstadt, Germany) provided
the enzyme-linked immunosorbent assay (ELISA) kits for vascular
endothelial growth factor (VEGF; cat. no. RAB0512), basic
fibroblast growth factor (bFGF; cat. no. RAB1139), hepatocyte
growth factor (HGF; cat. no. RAB1145) and insulin-like growth
factor (IGF; cat. no. RAB1146), and the MTT and dimethyl sulfoxide
(DMSO). The small interfering (si)RNAs targeting Akt transcripts
were prepared by Thermo Fisher Scientific, Inc. Cell proliferation
was assessed using the Cell Counting Kit-8 (CCK-8; HaiGene
Technology, Harbin, China) assay. Rat recombinant MIF was obtained
from Propec-Tany TechnoGene, Ltd. (East Brunswick, NJ, USA).
Cell culture and treatment
Bone marrow-derived MSCs were isolated and
identified using a standard protocol as previously described
(23). Briefly, MSCs were
isolated from the bone marrow of the Sprague Dawley rats and
cultured in DMEM supplemented with 10% FBS at 37°C in 5%
CO2. The culture medium was changed every 2–3 days. All
experiments were performed using MSCs from the third passage. The
MSCs were pretreated with DOXO (5 µmol/l; Sigma-Aldrich;
Merck KGaA) for 1 h at 37°C, as previously described (7). Prior to subsequent tests, the MSCs
were washed with phosphate-buffered saline (PBS). The concentration
of treatment with MIF was 100 ng/ml, and treatment was for 1 h at
37°C.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Gene expression levels were analyzed by RT-qPCR.
Briefly, total cellular RNA was isolated using TRIzol reagent and
reverse transcribed using the Transcriptor First Stand cDNA
Synthesis kit (Roche Applied Science, Mannheim, Germany) according
to the manufacturer's protocol. qPCR was performed using the Fast
Start Universal SYBR Master (Sigma-Aldrich; Merck KGaA) according
to the manufacturer's protocol. The thermocycling conditions were
as follows: 40 cycles of amplification at 95°C for 15 sec, followed
by 64°C for 20 sec and 72°C for 25 sec. The threshold number of
cycles (Cq) was set within the exponential phase of the reaction,
and the ΔCq value for each target gene was calculated by
subtracting the Cq value for the internal control, glyceraldehyde
3-phosphate dehydrogenase (GAPDH), from that of the target gene.
Relative gene expression levels were calculated by comparing the
ΔCq values between the control and experimental conditions for each
target using the following equation: Relative gene expression =
2−(ΔCq sample−ΔCq control) (24). The primer pairs used to detect the
mRNA levels of target genes are listed in Table I.
 | Table ISequences of the primers used for
reverse transcription-quantitative polymerase chain reaction
analysis. |
Table I
Sequences of the primers used for
reverse transcription-quantitative polymerase chain reaction
analysis.
| Gene | Primer (5′-3′)
|
|---|
| Forward | Reverse |
|---|
| MIF |
ATGAACTTTCTGCTGTCTTG |
TCACCGCCTCGGCTTGTCA |
| Telomere
length |
GGTTTTTGAGGGTGAGGGTGAGGGTGAGGGTGA |
TCCCGACTATCCCTATCCCTATCCCTATCCCTATCC |
| Akt |
TCGTGTGGCAAGATGTGTATGAGA |
CAGGCGCGTGTGGTGAT |
| p53 |
TCTGTCATCTTCCGTCCCTTCTC |
CCGTGCACATAACAGACTTGGCT |
| p16 |
GGTCACCGACAGGCATAACTTC |
AAAGGAGGGCTGAGGCCTAA |
| GAPDH |
GGCTCTCTGCTCCTCCCTGTT |
GGCTCTCTGCTCCTCCCTGTT |
Cell proliferation assay
The rate of cell proliferation was estimated using a
CCK-8 assay according to the manufacturer's protocol. Briefly,
1×104 MSCs/well were cultured in 96-well plates. When
the cells reached 80–90% confluence, they were incubated with CCK-8
solution for 1 h at 37°C, after which the absorbance of each well
at 450 nm was recorded. The MSCs were treated with DOXO (5
µmol/l/day) and the proliferation rate was measured each day
for the following 7 days.
MTT assay
The MTT assay was used to determine cell viability.
A total of 1×104 MSCs/well were cultured in 96-well
plates. When the cells reached 80–90% confluence, they were treated
with DOXO (5 µmol/l) for 24 h at 37°C. Then, a total of 300
µl of MTT reagent was added to each well 2 h prior to
harvesting. The supernatant was removed and the cells were
incubated with 400 µl of DMSO for 10 min. The absorbance of
the wells at 540 nm was recorded using a microplate reader.
ELISAs
The concentrations of MIF, VEGF, bFGF, HGF and IGF
secreted by MSCs were assessed by ELISA according to the
manufacturer's protocol, as previously described (15). The absorbance of each well was
quantified at 450 nm.
ROS measurement
Levels of intracellular ROS were determined using
2,7-dichlorodihydrofluorescein diacetate (Beyotime Institute of
Biotechnology, Haimen, China), following the manufacturer's
protocol. The fluorescent intensity of the cells was measured using
a fluorescence spectrophotometer, with excitation and emission
wavelengths of 488 and 525 nm, respectively.
Superoxide dismutase (SOD) activity
assay
SOD activity in the MSCs was determined using a
colorimetric assay kit (SOD activity assay kit; cat. no. ab65354;
Abcam, Cambridge, UK) according to the manufacturer's protocol.
Briefly, protein was isolated from MSCs using cell lysis buffer
(Beyotime Institute of Biotechnology) and SOD activity was measured
in 10 µg of total protein extract. Absorbance was measured
at 450 nm.
Lipid peroxidation assay
Lipid peroxidation was monitored using a Lipid
Peroxidation (MDA) assay kit (Colorimetric/Fluorometric; Abcam) to
measure the formation of malondialdehyde (MDA) according to the
manufacturer's protocol. Briefly, MSCs (1×106 cells)
were homogenized on ice in 300 µl of MDA lysis buffer (with
3 µl of 100X butyl-ated hydroxytoluene), then centrifuged
(13,000 × g for 10 min at 4°C) to remove insoluble material. The
supernatant (200 µl) was added to 600 µl of
thiobarbituric acid and incubated at 95°C for 60 min. The samples
were then cooled to room temperature in an ice bath for 10 min and
their absorbance at 532 nm was measured spectrophotometrically.
Relative telomere length measurement
Quantification of the relative telomere length in
MSCs was performed using qPCR based on a previously established
method (25), using the
aforementioned RT-qPCR protocol and GAPDH as the normalizing gene.
The primer pairs used to detect telomere length were as follows:
Forward, 5′-GGTTTTTGAGGGTGAGGGTGAGGGTGAGGGTGA-3′ and reverse,
5′-TCCCGACTATCCCTATCCCTATCCCTATCCCTATCC-3′.
Relative telomerase activity
measurement
The telomerase activity of MSCs was examined using
the TeloTAGGG Telomerase PCR ELISAPLUS kit (Sigma-Aldrich; Merck
KGaA) according to the manufacturer's protocol. Cell lysis buffer
provided in the kit was used to lyse the MSCs. After incubation for
30 min on ice, cell lysates were centrifuged for 20 min at 4°C
(12,000 × g). A total of 3 µg of cell extract was used for
each telomeric repeat amplification reaction and 3 µl of
inactivated cell lysate was used for telomeric repeat amplification
protocol (TRAP) reactions. Each TRAP reaction was performed with
amplification of an internal control from the kit. The amplified
products were immobilized on streptavidin-coated microtiter plates
via biotin-streptavidin interaction. Subsequently, amplification
was detected by adding 100 µl anti-digoxigenin antibodies
conjugated to horseradish peroxidase (provided in the kit) to each
well, and incubating the plate at 15–25°C for 30 min while shaking
at 300 rpm. After the addition of the peroxidase substrate
(3,3′,5,5′-tetramethylbenzidine), the amount of TRAP product was
determined by measuring the absorbance at 450 nm using a microplate
reader.
Western blot analysis
Western blotting was performed as previously
described (15). Briefly, cells
were washed twice with ice-cold PBS and lysed with cell lysis
buffer [20 mM Tris (pH 7.5), 150 mM NaCl, 1% Triton X-100, sodium
pyrophosphate, β-glycerophosphate, EDTA,
Na3VO4 and leupeptin; Beyotime Institute of
Biotechnology]. The lysates were centrifuged for 5 min at 12,000 ×
g (4°C) and the resulting supernatant contained the total cellular
protein. A BCA assay was used for protein quantification. For each
sample, 20 µg total protein/lane was resolved by 5–10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and transferred onto polyvinylidene difluoride
membranes. The membranes were blocked for 1 h at 37°C with 5%
skimmed milk in Tris-buffered saline containing 0.1% Tween-20, and
then incubated overnight at 4°C with the primary antibodies
directed against Akt, p-Akt and β-actin (1:1,000). The membranes
were washed, and then incubated for 1 h at 37°C with the
horseradish peroxidase-conjugated secondary antibodies and
developed using chemiluminescent substrate (BeyoECL Plus; Beyotime
Institute of Biotechnology). The stained protein bands were
visualized using a ChemiDoc XRS system and quantified using
Quantity One software v. 4.5.2 (both from Bio-Rad Laboratories,
Inc., Hercules, CA, USA).
Knockdown of gene expression using
siRNA
MSCs were transfected using X-tremeGENE HP DNA
Transfection reagent (Roche Life Sciences) according to the
manufacturer's protocol. Briefly, MSCs (1×105
cells/well) were cultured in 6-well plates for 24 h, then treated
with the transfection reagent at a reagent-to-siRNA weight ratio of
3:1 for 20 min, followed by the addition of a mixture containing
100 nM siRNA and incubation in 2 ml DMEM for 48 h at 37°C.
Scrambled non-targeting siRNA (siRNA-NT) was used as a negative
control. The efficiency of Akt knockdown was determined by RT-qPCR,
as described above. The sequences of the siRNAs used were as
follows: siRNA-Akt, GAUCUCCUCAUCAUCUGGATT; and siRNA-NT,
UUCUCCGAACGUGUCACGUTT.
Statistical analysis
Data were expressed as the mean ± standard deviation
from three independent experiments. Differences among groups were
tested by one-way analysis of variance followed by Tukey's multiple
comparisons test. Comparisons between two groups were evaluated
using the paired Student's t-test. All statistical analyses were
performed using SPSS software (version 19.0; IBM Corp., Armonk, NY,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
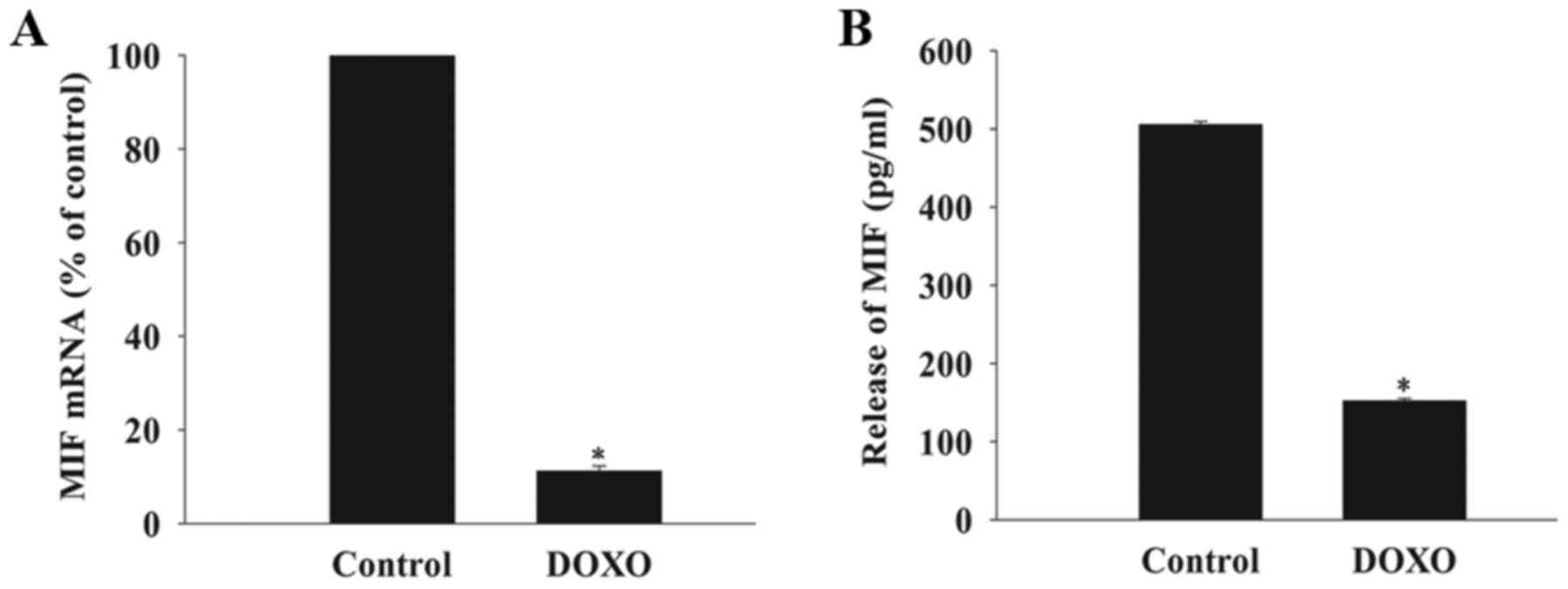
DOXO decreases the expression and release
of MIF in MSCs
The basal mRNA expression and release of MIF from
MSCs was measured after exposure to DOXO at a concentration of 5
µmol/l using RT-qPCR analysis and an ELISA. This revealed
that the baseline expression of MIF mRNA and release of MIF from
cells exposed to DOXO was significantly decreased compared with the
control group (Fig. 1).
DOXO induces the senescence of MSCs
DOXO has been demonstrated to be cytotoxic to MSCs
(5), so whether DOXO could induce
the senescence of MSCs was investigated in the present study. When
MSCs were treated with DOXO at a concentration of 5 µmol/l,
the proliferation rate decreased significantly, starting
immediately after treatment and lasting for 7 days (Fig. 2A). Furthermore, DOXO treatment
significantly decreased MSC viability compared with the control
group, as measured by the MTT assay (Fig. 2B).
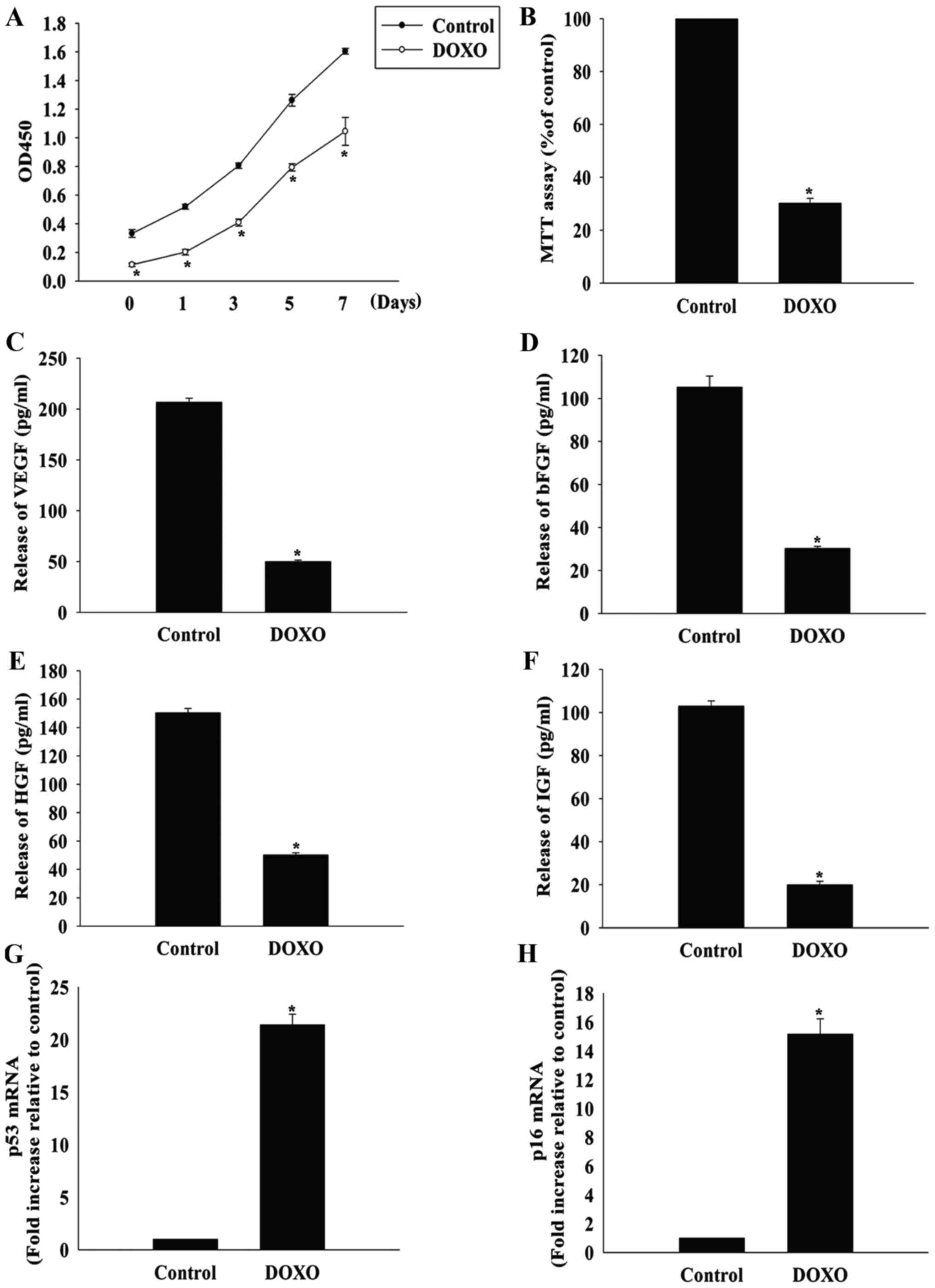 | Figure 2DOXO induces the senescence of MSCs.
(A) Proliferation growth curves of MSCs incubated with or without
DOXO were determined using the Cell Counting Kit-8 assay after 1,
3, 5 and 7 days of treatment. (B) MSC viability after DOXO
treatment was analyzed using the MTT assay. The relative
concentration of (C) VEGF, (D) bFGF, (E) HGF and (F) IGF was
calculated using ELISAs in MSCs with or without DOXO treatment.
Reverse transcription-quantitative polymerase chain reaction
analysis of (G) p53 and (H) p16 mRNA levels in MSCs treated with
DOXO. *P<0.05 vs. the control group. DOXO,
doxorubicin; MSC, mesenchymal stem cell; VEGF, vascular endothelial
growth factor; bFGF, basic fibroblast growth factor; HGF,
hepatocyte growth factor; IGF, insulin-like growth factor; p53,
cellular tumor antigen p53; ELISA, enzyme-linked immunosorbent
assay. |
MSCs secrete a variety of cytokines and growth
factors that can function in a paracrine and autocrine manner, and
their trophic effects on MSCs are known to be impaired by
senescence (14). As expected,
the release of VEGF, bFGF, HGF and IGF was significantly lower in
MSCs treated with 5 µmol/l DOXO compared with the control
group (Fig. 2C–F). Furthermore,
the expression of the senescence-associated genes cellular tumor
antigen p53 and p16 was significantly increased in the DOXO
treatment group compared with the control group (Fig. 2G and H).
Exogenous MIF rescues MSCs from
DOXO-induced senescence
A previous study has revealed that exogenous MIF
(100 ng/ml) increases the survival and rejuvenation of MSCs
(15). The present study
identified that 100 ng/ml MIF pretreatment in the presence of DOXO
significantly increased MSC proliferation (Fig. 3A) and cell viability (Fig. 3B) compared with the DOXO group. In
addition, MIF increased trophic effects, as indicated by the
significantly increased levels of secreted VEGF, bFGF, HGF and IGF
compared with the group treated with DOXO alone (Fig. 3C–F). Furthermore, MIF treatment
significantly increased telomere length and restored telomerase
activity, which were decreased by exposure to DOXO (Fig. 3G and H).
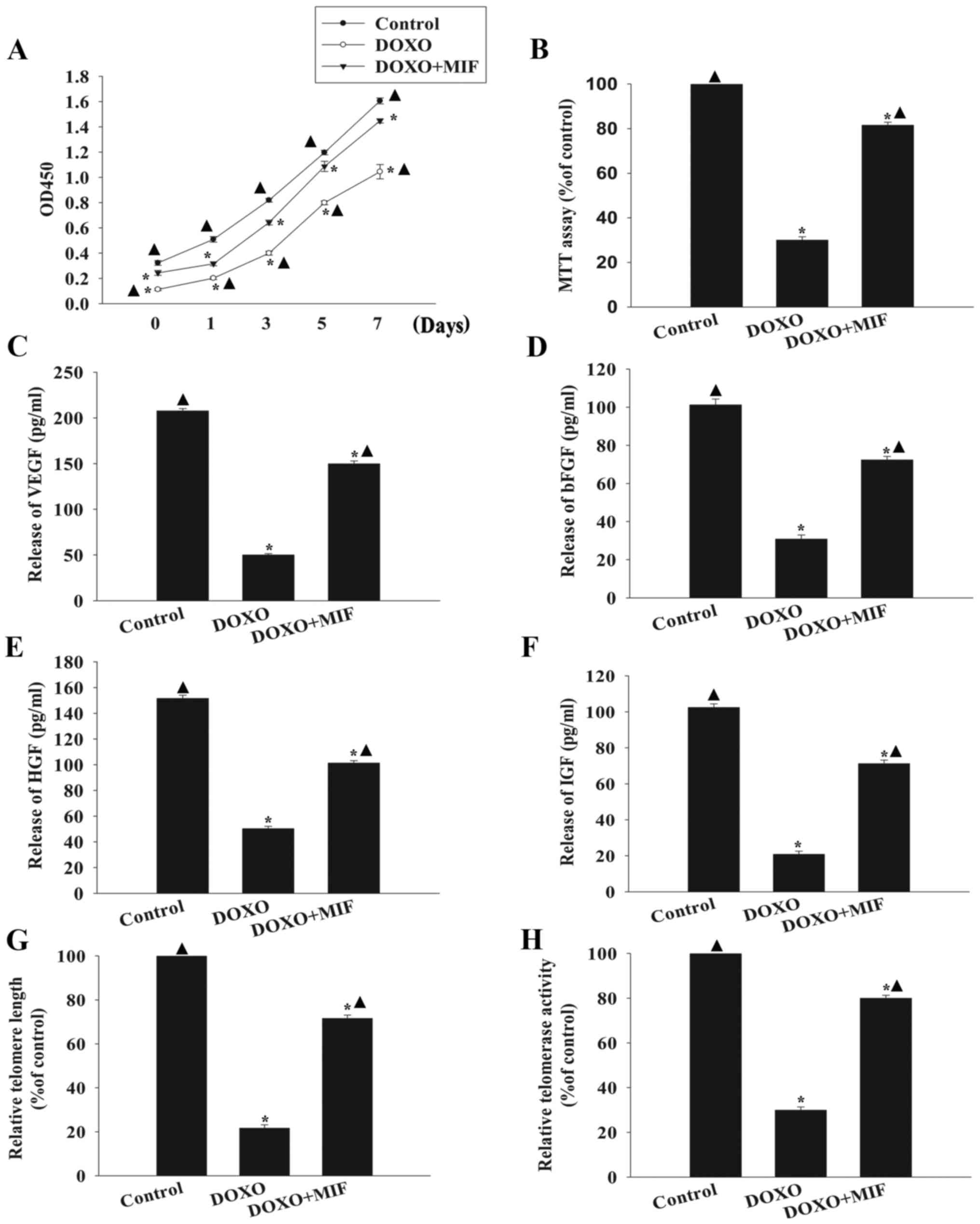 | Figure 3Exogenous MIF rescues MSCs from
DOXO-induced senescence. (A) Proliferation growth curves for
untreated, DOXO-treated and DOXO+MIF-treated MSCs were determined
using the Cell Counting Kit-8 assay. (B) MSC viability was analyzed
using the MTT assay in untreated MSCs, MSCs treated with DOXO and
MSCs treated with MIF in presence of DOXO. The relative
concentration of (C) VEGF, (D) bFGF, (E) HGF and (F) IGF was
calculated using ELISAs. (G) Analysis of telomere length through
mRNA levels was performed using reverse transcription-quantitative
polymerase chain reaction analysis. (H) Relative telomerase
activity was measured using the telomeric repeat amplification
protocol assay. *P<0.05 vs. the control group;
▲P<0.05 vs. the DOXO group. MIF, macrophage migration
inhibitory factor; MSC, mesenchymal stem cell; DOXO, doxorubicin;
VEGF, vascular endothelial growth factor; bFGF, basic fibroblast
growth factor; HGF, hepatocyte growth factor; IGF, insulin-like
growth factor; OD, optical density; ELISA, enzyme-linked
immunosorbent assay. |
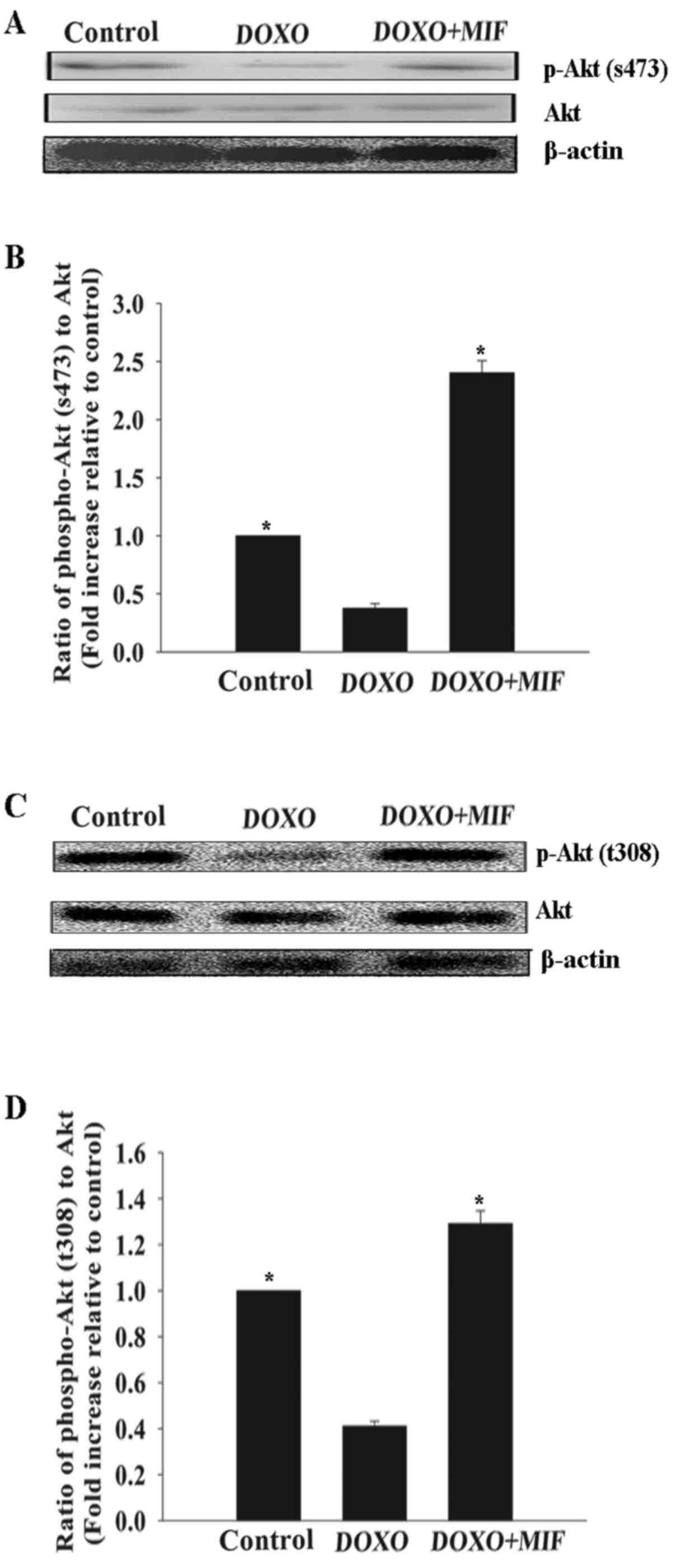
MIF exerts its anti-senescent effect
through the PI3K-Akt signaling pathway
The PI3K-Akt signaling pathway promotes survival and
rejuvenation in numerous cell types (13). Therefore, whether this pathway
mediated the anti-senescent effect of MIF in MSCs was investigated.
Compared with the control group, treatment with DOXO at a
concentration of 5 µmol/l significantly decreased the
phosphorylation of Akt, which was restored by MIF (Fig. 4).
To further confirm the role of the PI3K-Akt pathway
in the anti-senescent effect of MIF, Akt was silenced using siRNA
and the senescence of the MSCs was examined in the presence of DOXO
with or without MIF. The knockdown of Akt significantly decreased
the expression of Akt compared with the control group (Fig. 5A). Silencing Akt significantly
attenuated the anti-senescent effect of MIF, as demonstrated by the
decreased proliferation (Fig.
5B), viability (Fig. 5C) and
hormone secretion (Fig. 5D–G) of
MSCs following the knockdown. Furthermore, silencing Akt
significantly shortened telomere length (Fig. 5H) and decreased telomerase
activity (Fig. 5I). By contrast,
siRNA-NT had no significant effect on any of these factors.
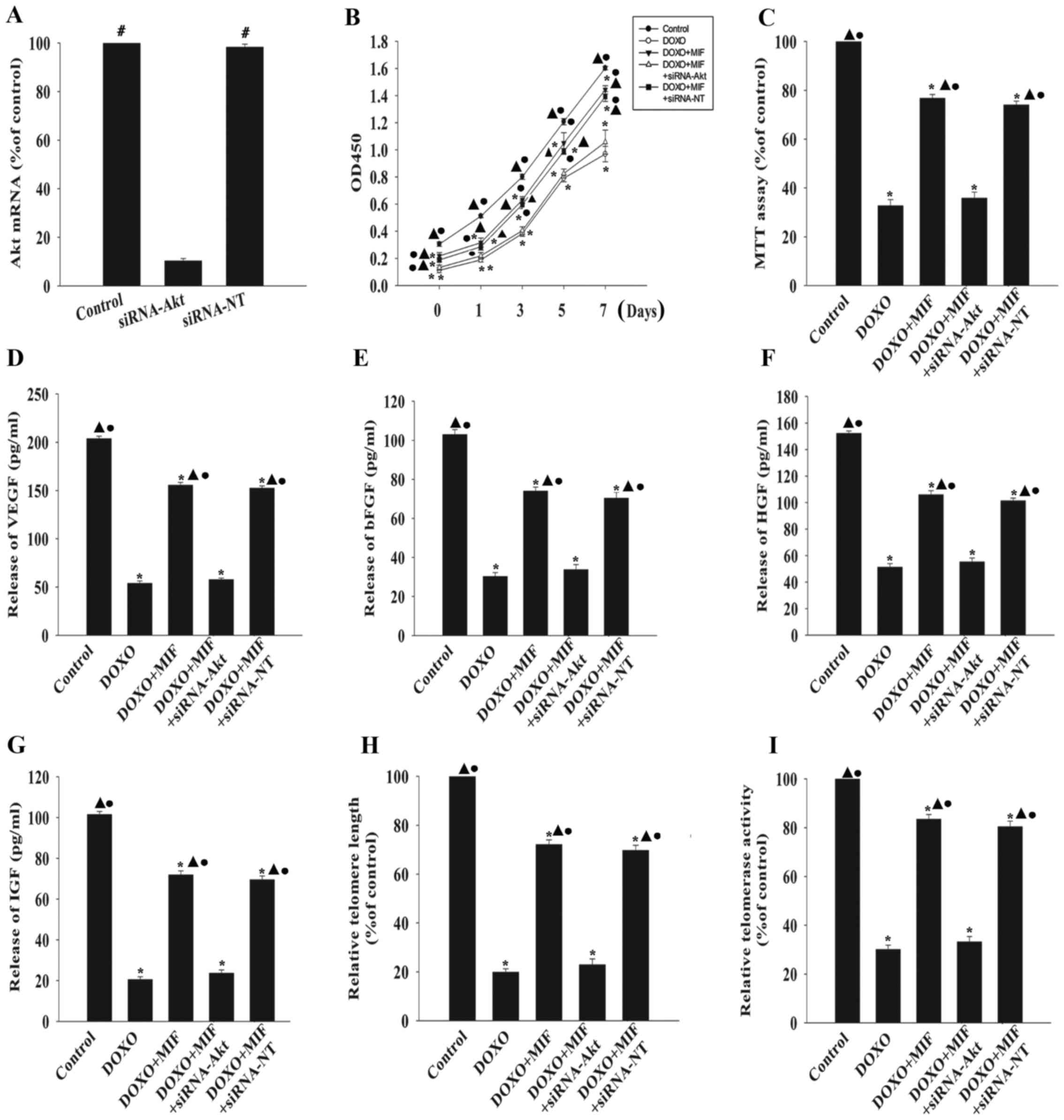 | Figure 5MIF exerts its anti-senescent effect
through the phosphatidylinositol 3-kinase-Akt signaling pathway.
(A) MSCs were transfected with siRNA directed against Akt or with
siRNA-NT as a control, and the expression of Akt mRNA was
determined by RT-qPCR. #P<0.05 vs. the siRNA-Akt
group. To determine if Akt was involved in the anti-senescent
effect of MIF, MSCs were transfected with siRNA directed against
Akt or with siRNA-NT as a control, then treated with MIF in the
presence of DOXO. (B) Proliferation growth curves of the MSCs were
determined using the Cell Counting Kit-8 assay. (C) Cell viability
was analyzed using the MTT assay. The relative concentration of (D)
VEGF, (E) bFGF, (F) HGF and (G) IGF were analyzed using ELISAs. (H)
Analysis of telomere length through mRNA levels was performed using
RT-qPCR. (I) Relative telomerase activity was measured using the
telomeric repeat amplification protocol assay.
*P<0.05 vs. the control group; ▲P<0.05
vs. the DOXO group. ●P<0.05 vs. the
DOXO+MIF+siRNA-Akt group. MIF, macrophage migration inhibitory
factor; MSC, mesenchymal stem cell; DOXO, doxorubicin; p-,
phosphorylated; Akt, RAC-α serine/threonine-protein kinase; si,
small interfering; NT, non-targeting; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; ELISA,
enzyme-linked immunosorbent assay. |
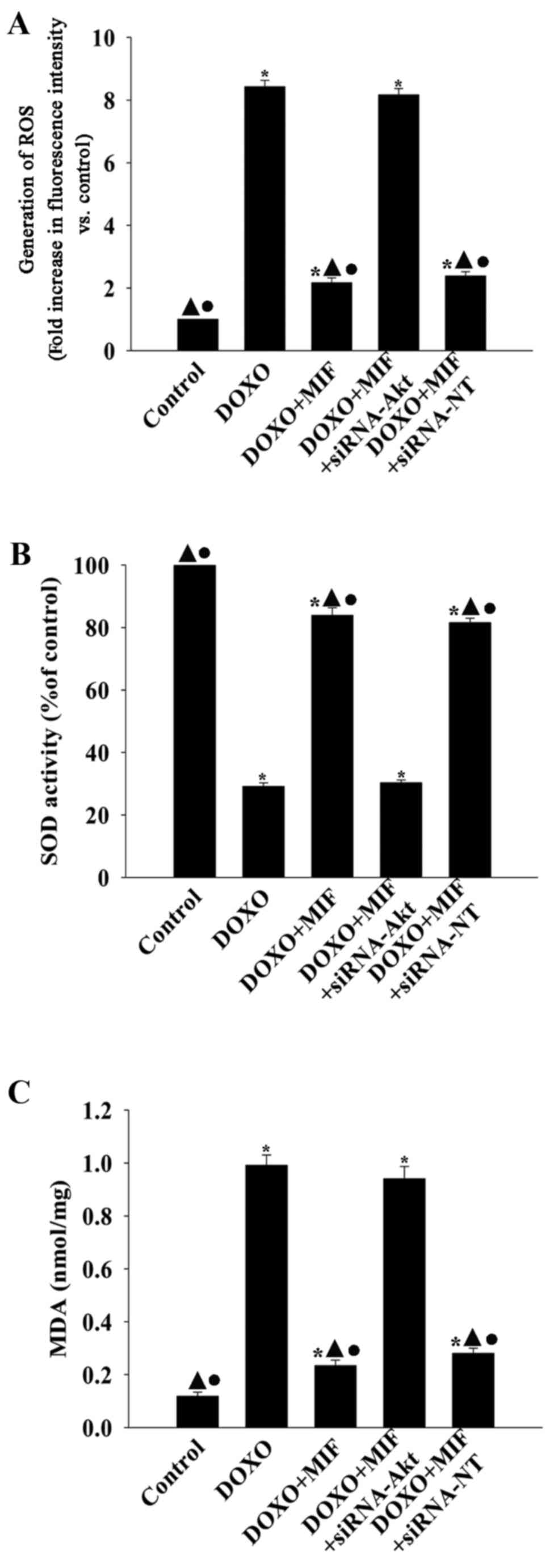
MIF exhibits anti-senescent effects via
inhibiting oxidative stress
To determine whether the anti-senescent effect of
MIF in the presence of DOXO involved the amelioration of oxidative
stress, the generation of ROS, activation of SOD and lipid
peroxidation were examined (Fig.
6). Compared with the control group, DOXO significantly
increased the generation of ROS and MDA activation, while
decreasing the activation of SOD. However, when treated with MIF,
the generation of ROS and MDA was significantly decreased and the
activation of SOD was significantly increased. siRNA directed
against Akt significantly blocked the inhibitory effect of MIF on
oxidative stress, resulting in the increased generation of ROS and
MDA, while decreasing the activation of SOD.
Discussion
Chemotherapy is an essential tool in cancer
treatment; however, as the number of cancer survivors has
increased, concerns about the effects of chemotherapy on the
quality of life and lifespan of patients have been highlighted
(26). DOXO, which belongs to the
anthracycline family, has been proven to be an effective treatment
for numerous types of cancer, including breast, lung, stomach,
bladder and skin cancer. However, despite its high efficacy as a
therapeutic agent against these tumors, DOXO is often accompanied
by unavoidable adverse effects, such as cardiovascular toxicity
(27,28). The spectrum of short- and
long-term cardio-toxic effects induced by DOXO ranges from
cardiomyocyte senescence and subclinical ventricular dysfunction to
severe cardiomyopathy and heart failure, necessitating cardiac
transplantation and potentially resulting in mortality (28,29). Several studies have revealed the
potential mechanisms by which DOXO causes these effects, including
the disruption of energy metabolism and apoptosis, increasing the
production of ROS and inducing mitochondrial injury (30,31). Therefore, enhancing the
therapeutic effect of DOXO while decreasing its adverse cardiotoxic
effects is an important objective (32,33).
Among the strategies that have been investigated to
prevent or repair cardiac injury, MSC transplantation is promising
candidate due to the regenerative properties, paracrine function
and immune modulatory effects of MSCs (6). A previous study reported that the
intramuscular injection of MSCs improved cardiac function in a
DOXO-induced dilated cardiomyopathy (DCM) rat model and attenuated
DCM-associated mitochondria impairment (34). In addition, the transplantation of
MSCs protects cardiomyocytes against DOXO-induced cardiomyopathy
through paracrine effects (33).
However, although MSCs exhibit therapeutic benefits against
DOXO-induced cardiomyopathy, DOXO can cause MSC injury. It has been
reported that DOXO impaired the proliferation, decreased the
production of connexin 43 and hindered the capacity of MSCs to
respond to cardio-myogenic differentiation stimuli (5). A previous study also revealed that
DOXO induced MSC apoptosis and impaired the paracrine effects of
MSCs (7). The present study
identified that DOXO induced MSC senescence, rapidly resulting in a
lower proliferation rate. This is in agreement with previous study,
which revealed that the combination of DOXO and low intensity
ultrasound significantly decreased the survival rate of C6 cells
(35). Treatment with DOXO also
impaired the viability and decreased the paracrine effects of MSCs
in the present study, indicating that MSCs cannot inhibit
DOXO-induced cardiomyopathy.
MIF is a proinflammatory cytokine, which was
originally identified to serve an important role in chronic
inflammatory diseases (36).
Previous studies have suggested that MIF is involved in cell
proliferation, survival and senescence (9,14).
In regards to senescence, it has been demonstrated that MIF
expression is reduced in aged hearts (37). A previous study also revealed that
cardiomyocytes in MIF-deficient mice exhibited contractile defects
in response to starvation and underwent increased apoptosis during
ischemia/reperfusion (10).
Mechanisms underlying this MIF-dependent anti-senescent effect are
associated with the modulation of senescence via signaling
pathways, genes and enzymes (38). It has been demonstrated that MIF
maintains neural stem cell properties, and promotes cell survival
and proliferation (12). MIF also
rejuvenates MSCs under hypoxic conditions (13). Previous study by our group
demonstrated that MIF could rejuvenate aged MSCs, leading to
increased proliferation rates, improved paracrine effects and an
improved resistance to hypoxia/ischemia-induced apoptosis (14). A recent study revealed that
overexpression of the MIF gene in induced pluripotent stem
cell-derived MSCs improved DOXO-induced cardiomyopathy (33). The present study identified that
in the presence of DOXO, pretreatment with MIF could drive MSCs
into a rejuvenated state, in which the cells exhibited increased
proliferation, viability and paracrine effects. Furthermore, MIF
increased telomere length and telomerase activity, which were
impaired by DOXO treatment.
In addition to its role in regulating cell survival,
proliferation and glucose metabolism, the PI3K-Akt signaling
pathway regulates cellular senescence through the functional
modulation of various gene products (39,40). Akt is a stress-signaling kinase
and key regulator of energy metabolism pathways that protect cells
against stress-induced injury and death (13). DOXO has been revealed to impair
this signaling pathway to induce cardiomyopathy (41). The use of exogenous factors, such
as brain-derived neurotrophic factor, as well as modulating the
expression of endogenous genes, such as sirtuin 1 (SIRT1), can
activate Akt to exert a protective effect against DOXO-induced
cardiomyopathy (17,41). The PI3K-Akt signaling pathway has
been revealed to exert an anti-senescent effect on MSCs under
hypoxic conditions, and MIF can activate this pathway (13). The results of the current study
demonstrated that MIF activated the Akt pathway and reduced the
DOXO-induced senescence of MSCs, as indicated by the increased
proliferation, viability, paracrine effects, telomere length and
telomerase activity observed following MIF treatment. By contrast,
silencing Akt using siRNA attenuated the anti-senescent effect of
MIF, suggesting that MSC rejuvenation by MIF is dependent upon the
PI3K-Akt signaling pathway.
Oxidative stress is closely associated with the
induction of cellular senescence (42). This is accompanied by ROS
generation, through increased oxidative enzyme activity and
decreased antioxidative enzyme activity (43). DOXO has been associated with
cellular DNA damage and endoplasmic reticulum stress, as well as
inducing cellular senescence during cardiomyopathy through
oxidative stress (7,8,44).
Previous studies have demonstrated that MIF is an effective
antioxidant agent (45), which by
modulating oxidative stress promotes the survival of MSC under
hypoxia/serum deprivation conditions (15). Consistent with these results, the
present study identified that DOXO induced oxidative stress,
increased the generation of ROS and MDA, and decreased the activity
of SOD. Conversely, MIF had an antioxidant effect on the
DOXO-treated MSCs. Previous study has revealed that by activating
Akt-dependent pro-survival and antiapoptotic signaling, SIRT1
enhances oxidative stress defenses, and prevents the senescence and
growth arrest of human cardiac progenitor cells treated with DOXO
(17). The results of the current
study indicated that MIF reduced DOXO-induced oxidative stress
through the PI3K-Akt signaling pathway, which was supported by the
fact that silencing Akt abolished the antioxidative effects of
MIF.
In conclusion, the present study demonstrated that
DOXO treatment induced MSC senescence and that pretreatment with
MIF could rejuvenate senescent MSCs. The results of the present
study suggest that MIF can rescue MSCs from DOXO-induced senescence
via regulation of oxidative stress through the PI3K-Akt signaling
pathway. These findings highlight potentially novel therapeutic
strategies for rejuvenating and protecting MSCs from injury induced
by DOXO, and provide a mechanistic understanding for the clinical
exploitation of MIF and MSCs in cardiac regeneration therapies for
DOXO-induced cardiomyopathy.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81500261 to M.H.),
the Science and Technology Planning Project of Wenzhou (grant no.
Y20160125 to M.H.) and the Medical Science and Technology Project
of Zhejiang Province (grant no. 2018236627 to M.H.).
References
|
1
|
Rochette L, Guenancia C, Gudjoncik A,
Hachet O, Zeller M, Cottin Y and Vergely C:
Anthracyclines/trastuzumab: New aspects of cardiotoxicity and
molecular mechanisms. Trends Pharmacol Sci. 36:326–348. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Butany J, Ahn E and Luk A: Drug-related
cardiac pathology. J Clin Pathol. 62:1074–1084. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Piegari E, De Angelis A, Cappetta D, Russo
R, Esposito G, Costantino S, Graiani G, Frati C, Prezioso L,
Berrino L, et al: Doxorubicin induces senescence and impairs
function of human cardiac progenitor cells. Basic Res Cardiol.
108:3342013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Du WW, Yang W, Chen Y, Wu ZK, Foster FS,
Yang Z, Li X and Yang BB: Foxo3 circular RNA promotes cardiac
senescence by modulating multiple factors associated with stress
and senescence responses. Eur Heart J. 38:1402–1412. 2017.
|
|
5
|
Oliveira MS, Carvalho JL, Campos ACDA,
Gomes DA, de Goes AM and Melo MM: Doxorubicin has in vivo
toxicological effects on ex vivo cultured mesenchymal stem cells.
Toxicol Lett. 224:380–386. 2014. View Article : Google Scholar
|
|
6
|
Ezquer F, Gutiérrez J, Ezquer M, Caglevic
C, Salgado HC and Calligaris SD: Mesenchymal stem cell therapy for
doxorubicin cardiomyopathy: Hopes and fears. Stem Cell Res Ther.
6:1162015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang F, Chen H, Liu Y, Yin K, Wang Y, Li
X, Wang G, Wang S, Tan X, Xu C, et al: Doxorubicin caused apoptosis
of mesenchymal stem cells via p38, JNK and p53 pathway. Cell
Physiol Biochem. 32:1072–1082. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Capasso S, Alessio N, Squillaro T, Di
Bernardo G, Melone MA, Cipollaro M, Peluso G and Galderisi U:
Changes in autophagy, proteasome activity and metabolism to
determine a specific signature for acute and chronic senescent
mesenchymal stromal cells. Oncotarget. 6:39457–39468. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miller EJ, Li J, Leng L, McDonald C,
Atsumi T, Bucala R and Young LH: Macrophage migration inhibitory
factor stimulates AMP-activated protein kinase in the ischaemic
heart. Nature. 451:578–582. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu X, Pacheco BD, Leng L, Bucala R and Ren
J: Macrophage migration inhibitory factor plays a permissive role
in the maintenance of cardiac contractile function under starvation
through regulation of autophagy. Cardiovasc Res. 99:412–421. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang J, Tong C, Yan X, Yeung E, Gandavadi
S, Hare AA, Du X, Chen Y, Xiong H, Ma C, et al: Limiting cardiac
ischemic injury by pharmacological augmentation of macrophage
migration inhibitory factor-AMP-activated protein kinase signal
transduction. Circulation. 128:225–236. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ohta S, Misawa A, Fukaya R, Inoue S,
Kanemura Y, Okano H, Kawakami Y and Toda M: Macrophage migration
inhibitory factor (MIF) promotes cell survival and proliferation of
neural stem/progenitor cells. J Cell Sci. 125:3210–3220. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Palumbo S, Tsai TL and Li WJ: Macrophage
migration inhibitory factor regulates AKT signaling in hypoxic
culture to modulate senescence of human mesenchymal stem cells.
Stem Cells Dev. 23:852–865. 2014. View Article : Google Scholar
|
|
14
|
Xia W, Zhang F, Xie C, Jiang M and Hou M:
Macrophage migration inhibitory factor confers resistance to
senescence through CD74-dependent AMPK-FOXO3a signaling in
mesenchymal stem cells. Stem Cell Res Ther. 6:822015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xia W, Xie C, Jiang M and Hou M: Improved
survival of mesenchymal stem cells by macrophage migration
inhibitory factor. Mol Cell Biochem. 404:11–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Okada M, Kim HW, Matsu-ura K, Wang YG, Xu
M and Ashraf M: Abrogation of age-induced MicroRNA-195 rejuvenates
the senescent mesenchymal stem cells by reactivating telomerase.
Stem Cells. 34:148–159. 2016. View Article : Google Scholar :
|
|
17
|
De Angelis A, Piegari E, Cappetta D, Russo
R, Esposito G, Ciuffreda LP, Ferraiolo FA, Frati C, Fagnoni F,
Berrino L, et al: SIRT1 activation rescues doxorubicin-induced loss
of functional competence of human cardiac progenitor cells. Int J
Cardiol. 189:30–44. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuilman T, Michaloglou C, Mooi WJ and
Peeper DS: The essence of senescence. Genes Dev. 24:2463–2479.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
De Falco E, Carnevale R, Pagano F,
Chimenti I, Fianchini L, Bordin A, Siciliano C, Monticolo R,
Equitani F, Carrizzo A, et al: Role of NOX2 in mediating
doxorubicin-induced senescence in human endothelial progenitor
cells. Mech Ageing Dev. 159:37–43. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ghosh AK, Rai R, Park KE, Eren M, Miyata
T, Wilsbacher LD and Vaughan DE: A small molecule inhibitor of
PAI-1 protects against doxorubicin-induced cellular senescence:
Molecular basis. Oncotarget. 7:72443–72457. 2016.PubMed/NCBI
|
|
21
|
Ho JH, Chen YF, Ma WH, Tseng TC, Chen MH
and Lee OK: Cell contact accelerates replicative senescence of
human mesenchymal stem cells independent of telomere shortening and
p53 activation: roles of Ras and oxidative stress. Cell Transplant.
20:1209–1220. 2011. View Article : Google Scholar
|
|
22
|
Liu WH, Liu JJ, Wu J, Zhang LL, Liu F, Yin
L, Zhang MM and Yu B: Novel mechanism of inhibition of dendritic
cells maturation by mesenchymal stem cells via interleukin-10 and
the JAK1/STAT3 signaling pathway. PLoS One. 8:e554872013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hou M, Cui J, Liu J, Liu F, Jiang R, Liu
K, Wang Y, Yin L, Liu W and Yu B: Angiopoietin-like 4 confers
resistance to hypoxia/serum deprivation-induced apoptosis through
PI3K/Akt and ERK1/2 signaling pathways in mesenchymal stem cells.
PLoS One. 9:e858082014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) μethod. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Crepin T, Carron C, Roubiou C, Gaugler B,
Gaiffe E, Simula- Faivre D, Ferrand C, Tiberghien P, Chalopin JM,
Moulin B, et al: ATG-induced accelerated immune senescence:
Clinical implications in renal transplant recipients. Am J
Transplant. 15:1028–1038. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bray F, Jemal A, Grey N, Ferlay J and
Forman D: Global cancer transitions according to the Human
Development Index (2008–2030): A population-based study. Lancet
Oncol. 13:790–801. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Horie T, Ono K, Nishi H, Nagao K,
Kinoshita M, Watanabe S, Kuwabara Y, Nakashima Y, Takanabe-Mori R,
Nishi E, et al: Acute doxorubicin cardiotoxicity is associated with
miR-146a-induced inhibition of the neuregulin-ErbB pathway.
Cardiovasc Res. 87:656–664. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Volkova M and Russell R III: Anthracycline
cardiotoxicity: Prevalence, pathogenesis and treatment. Curr
Cardiol Rev. 7:214–220. 2011. View Article : Google Scholar
|
|
29
|
Franco VI, Henkel JM, Miller TL and
Lipshultz SE: Cardiovascular effects in childhood cancer survivors
treated with anthracyclines. Cardiol Res Pract. 2011:1346792011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fan GC, Zhou X, Wang X, Song G, Qian J,
Nicolaou P, Chen G, Ren X and Kranias EG: Heat shock protein 20
interacting with phosphorylated Akt reduces doxorubicin-triggered
oxidative stress and cardiotoxicity. Circ Res. 103:1270–1279. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fisher PW, Salloum F, Das A, Hyder H and
Kukreja RC: Phosphodiesterase-5 inhibition with sildenafil
attenuates cardiomyocyte apoptosis and left ventricular dysfunction
in a chronic model of doxorubicin cardiotoxicity. Circulation.
111:1601–1610. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Piegari E, Russo R, Cappetta D, Esposito
G, Urbanek K, Dell'Aversana C, Altucci L, Berrino L, Rossi F and De
Angelis A: MicroRNA-34a regulates doxorubicin-induced
cardiotoxicity in rat. Oncotarget. 7:62312–62326. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang Y, Liang X, Liao S, Wang W, Wang J,
Li X, Ding Y, Liang Y, Gao F, Yang M, et al: Potent paracrine
effects of human induced pluripotent stem cell-derived mesenchymal
atem xells attenuate doxorubicin-induced cardiomyopathy. Sci Rep.
5:112352015. View Article : Google Scholar
|
|
34
|
Mao C, Hou X, Wang B, Chi J, Jiang Y,
Zhang C and Li Z: Intramuscular injection of human umbilical
cord-derived mesenchymal stem cells improves cardiac function in
dilated cardiomyopathy rats. Stem Cell Res Ther. 8:182017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang Z, Xu K, Bi Y, Yu G, Wang S, Qi X
and Zhong H: Low intensity ultrasound promotes the sensitivity of
rat brain glioma to Doxorubicin by down-regulating the expressions
of p-glucoprotein and multidrug resistance protein 1 in vitro and
in vivo. PLoS One. 8:e706852013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Baugh JA, Chitnis S, Donnelly SC, Monteiro
J, Lin X, Plant BJ, Wolfe F, Gregersen PK and Bucala R: A
functional promoter polymorphism in the macrophage migration
inhibitory factor (MIF) gene associated with disease severity in
rheumatoid arthritis. Genes Immun. 3:170–176. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ma H, Wang J, Thomas DP, Tong C, Leng L,
Wang W, Merk M, Zierow S, Bernhagen J, Ren J, et al: Impaired
macrophage migration inhibitory factor (MIF)-AMPK activation and
ischemic recovery in the senescent heart. Circulation. 122:282–292.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Maity A and Koumenis C: HIF and MIF - a
nifty way to delay senescence? Genes Dev. 20:3337–3341. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ibrahim MX, Sayin VI, Akula MK, Liu M,
Fong LG, Young SG and Bergo MO: Targeting isoprenylcysteine
methylation ameliorates disease in a mouse model of progeria.
Science. 340:1330–1333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li W, Croce K, Steensma DP, McDermott DF,
Ben-Yehuda O and Moslehi J: Vascular and metabolic implications of
novel targeted cancer therapies: Focus on kinase inhibitors. J Am
Coll Cardiol. 66:1160–1178. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hang P, Zhao J, Sun L, Li M, Han Y, Du Z
and Li Y: Brain-derived neurotrophic factor attenuates
doxorubicin-induced cardiac dysfunction through activating Akt
signalling in rats. J Cell Mol Med. 21:685–696. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Henson SM, Lanna A, Riddell NE, Franzese
O, Macaulay R, Griffiths SJ, Puleston DJ, Watson AS, Simon AK,
Tooze SA, et al: p38 signaling inhibits mTORC1-independent
autophagy in senescent human CD8+ T cells. J Clin
Invest. 124:4004–4016. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Finkel T and Holbrook NJ: Oxidants,
oxidative stress and the biology of ageing. Nature. 408:239–247.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee KH, Cho H, Lee S, Woo JS, Cho BH, Kang
JH, Jeong YM, Cheng XW and Kim W: Enhanced-autophagy by exenatide
mitigates doxorubicin-induced cardiotoxicity. Int J Cardiol.
232:40–47. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sobierajski J, Hendgencotta U, Luedike P,
Rammos C, Bernhagen J, Kelm M and Rassaf T: Time dependent
regulation of MIF by S-nitros(yl)ation reduces apoptosis in
myocardial I/R-injury. Eur Heart J. 31:S18–S19. 2013.
|