Introduction
Alcohol overconsumption may lead to the occurrence
and development of various alcohol-associated liver diseases,
including alcoholic steatohepatitis, alcohol-related fibrosis and
cirrhosis. Among all hepatic diseases, alcoholic liver disease
(ALD) is the second most common cause of morbidity and mortality
after viral hepatitis. In addition, the number of ALD cases has
been reported to increase annually, and therefore it is considered
a critical health problem in China (1,2).
Despite the high incidence of alcoholic hepatitis, there is
currently no effective treatment available; therefore, novel
therapeutic strategies are urgently required. However, achieving
this goal has been limited by a poor understanding regarding the
pathogenesis underlying alcoholic hepatitis (3,4).
Numerous mechanisms underlying alcohol
exposure-induced hepatocellular dysfunction and ALD progression
have been proposed, as follows: i) Activation of alcohol
dehydrogenase and aldehyde dehydrogenase, which results in
overproduction of acetate (5);
ii) upregulation of cytochrome P450 2E1 (CYP2E1) expression, which
induces alcoholic oxidative stress and hepatotoxicity (6); iii) increased intestinal
permeability and serum lipopolysaccharide concentration (7,8);
iv) increased production of tumor necrosis factor (TNF)-α and other
proinflammatory cytokines, which exacerbates hepatic inflammation
(9); v) abnormal lipid metabolism
(6); and vi) activation of
Kupffer cells and hepatic stellate cells (HSCs), which may lead to
collagen synthesis and liver fibrosis (10). However, which factor serves the
predominant role remains to be elucidated.
Ginseng is a traditional Chinese medicine that is
used as an alternative medicine worldwide, which may affect
numerous biological processes, including inflammatory responses,
cell proliferation and apoptosis. The key molecules responsible for
the pharmacological effects of ginseng are ginsenosides; among
them, ginsenoside Rg1 (G-Rg1) is the most abundant ingredient of
Panax ginseng. Previous studies have reported that G-Rg1
exerts regulatory effects on reactive oxygen stress, aging,
angiogenesis and neuronal differentiation (11–14). In addition, numerous studies have
explored the hepatoprotective effects of G-Rg1, and have indicated
that G-Rg1 may inhibit the production of proinflammatory factors,
including TNF-α (15), and
substantially delay the progression of liver fibrosis (16). Recently, G-Rg1 was revealed to
promote glucocorticoid (GC) receptor-mediated suppression of
nuclear factor (NF)-κB activation, in order to exert its
anti-inflammatory properties (17), and to protect against hepatic
ischemia/reperfusion injury (18,19), carbon tetrachloride
(CCl4)-induced liver injury (20) and hepatic fibrosis (21,22). Similarly, our previous study
demonstrated that G-Rg1 significantly reduced liver damage in a
murine model of acute liver failure (ALF) via the suppression of
NF-κB activation and the inhibition of TNF-α-induced,
caspase-dependent hepatocellular apoptosis (23). Similar to ALF, other hepatic
disorders, including ALD, are accompanied by abundant production of
inflammatory cytokines (9). To
the best of our knowledge, inflammatory cytokine production is
orchestrated by two signals, as follows: i) NF-κB activation, which
subsequently promotes the transcription of numerous
inflammation-associated genes, including IL-1β, IL-6 and TNF-α, and
provides the basis for generation of these cytokines; ii)
activation of inflammasomes by diverse stimulators, namely
pathogen-associated molecular patterns and damage-associated
molecular patterns, which in turn activate the cysteine protease
caspase-1 and promote maturation of proinflammatory cytokines
(24). Furthermore, excessive
inflammation may lead to a marked loss of hepatocytes. Therefore,
it may be hypothesized that G-Rg1 serves anti-inflammatory and
anti-apoptotic roles in alcoholic hepatitis, which may be
beneficial for its treatment.
The present study aimed to assess the potential of
G-Rg1 as a novel therapy for the treatment of alcoholic hepatitis
by evaluating its hepatoprotective effects on an alcohol-induced
L-O2 cell injury model and on a rat model of alcoholic hepatitis.
Furthermore, the molecular mechanisms underlying the
hepatoprotective effects of G-Rg1 against damage induced by ethanol
and its metabolites were investigated in vitro and in
vivo. The present data may provide novel evidence regarding the
development of preventive and therapeutic strategies for ALD.
Materials and methods
Reagents
G-Rg1 (purity ≥98.55%) in powder form was purchased
from Jicui Biotechnology Co., Ltd. (Yunnan, China). Dexamethasone
(DEX), the nonfluorescent dye dichlorodihydrofluorescein diacetate
(H2DCFDA) and 5,5'-dithiobis(2-nitrobenzoic acid) (DTNB)
were obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Ethanol (40%, wt/vol) was purchased from Beijing Dingguo Changsheng
Biotechnology Co., Ltd. (Beijing, China). Assay kits for alanine
aminotransferase (ALT; C009-3), aspartate aminotransferase (AST;
C010-3) and total bilirubin (TBIL; C019-1) were purchased from
Nanjing Jiancheng Bioengineering Institute (Nanjing, China). ELISA
kits for the detection of IL-1 (SEA563Ra), IL-6 (SEA079Ra) and
TNF-α (SEA133Ra) were purchased from USCN Life Sciences, Inc.
(Wuhan, China). PrimeScript reverse transcription (RT) Master Mix
kit was purchased from Takara Biotechnology Co., Ltd. (Dalian,
China). SYBR-Green Universal quantitative polymerase chain reaction
(qPCR) Master Mix kit was purchased from Bio-Rad Laboratories, Inc.
(Hercules, CA, USA). Antibodies against NF-κB (13757-1), CYP2E1
(H00001571-A01), caspase-1 (ABP56678), caspase-3 (ABP50018),
caspase-8 (ABP50858) and β-actin (A01011) were purchased from Abcam
(Cambridge, UK). ApopTag® Plus Peroxidase In Situ
Apoptosis kit was purchased from EMD Millipore (Billerica, MA,
USA).
Cells and cell culture
L-O2 cells (Chongqing Bopei Biotechnology Co., Ltd.,
Chongqing, China) were cultured in Dulbecco's modified Eagle's
medium (DMEM; Gibco; Life Technologies, Grand Island, NY, USA)
supplemented with 10% (v/v) fetal bovine serum (Gibco; Life
Technologies) and 1% penicillin/streptomycin (100 U/ml) at 37°C in
a humidified CO2 chamber until cells reached 90%
confluence. Subsequently, cells were collected and plated at
6x103 cells/ well in 96-well microplates, after which
they were divided into the following groups: Control group, which
was treated with saline; model group, which was treated with 40%
ethanol for 48 h; and G-Rg1 group, which was pretreated with 0.5,
1.0 and 1.5 mg/ml G-Rg1 for 2 h, and was then treated with 40%
ethanol for 48 h; each group consisted of triplicate wells. Cells
and culture supernatants were collected after treatment.
Animals and treatment
Rats were housed under standard laboratory
conditions for 1 week prior to experimentation, and all animals
received humane care in compliance with the Guide for the Care and
Use of Laboratory Animals published by the National Institutes of
Health (NIH Publication No. 85-23, revised in 1996). Rats were
maintained under standard conditions (23+2°C, 55+5% humidity and 12
h light-dark cycle) in an air-conditioned room. Rats were allowed
free access to a standard rodent diet and water. The present study
was approved by the Ethics Committee of Chongqing Medical
University (Chongqing, China).
A total of 80 female Sprague-Dawley rats (weight,
200–220 g; age, 7–8 weeks) were purchased from the Animal Facility
at Chongqing Medical University. The rats were randomly divided
into the following four groups (n=20/group): Control, model, G-Rg1
and DEX groups. The alcoholic hepatitis model was established by
intragastric administration of ethanol (4 g/ kg body weight,
twice/day) for 12 consecutive weeks (23,24). Following establishment of the
animal model, the G-Rg1 and DEX groups were intragastrically
administered G-Rg1 (40 mg/ kg body weight) or DEX (1 mg/kg) once a
day for 5 days, respectively. The control group was treated with an
equal volume of saline and was not treated with ethanol. After
administration of the indicated treatments, all rats were
sacrificed, and blood and liver samples were collected for further
analysis.
Electron microscopy
After 48 h of cell culture, cells were treated with
trypsin, and adherent cells were washed and resuspended in
centrifuge tubes, which were centrifuged at 10,000 × g for 10 min.
Subsequently, 2.5% glutaraldehyde solution was slowly added along
the tube wall, and the ultrastructure of the cells was observed by
electron microscopy (S-3000N; Hitachi, Tokyo, Japan).
Biochemical parameter assessment
ALT, AST and TBIL activities in cell culture
supernatants and rat serum were measured according to the
manufacturer's protocols. Collected blood samples were placed at
4°C for 2 h and centrifuged at 4,000 × g for 15 min at 4°C to
obtain the serum.
ELISA
ELISA kits specific for IL-1, IL-6 and TNF-α were
conducted according to the manufacturer's protocols, and were
performed in triplicate for each sample. Cell culture supernatants
and serum were used for ELISA.
Reactive oxygen species (ROS) and
gluthathione peroxidase (GSH-Px) detection
ROS were measured using a nonfluorescent dye,
H2DCFDA. Briefly, fluorescence measurements were made in
6-well plates (10,000 cells/well). Cells in each group were treated
for 48 h, and the cells were washed with 1X PBS and treated with 10
µM H2DCDDA in the dark at 37°C for 20 min.
Dichlorofluorescein fluorescence in each well was measured at 37°C
using a Fluoroskan fluorescence plate reader (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at an excitation wavelength of
488 nm and an emission wavelength of 538 nm.
The GSH-Px content of the cells was measured using
DTNB, according to the method described by Sedlak and Lindsay
(27). Briefly, the cell
supernatants (500 µl) were mixed with 2 ml 0.2 M phosphate buffer
and 10 µl 0.01 M DTNB in methanol. Absorbance of the mixture was
measured at 412 nm.
RT-qPCR assay
Total RNA was extracted from the liver samples using
TRIzol™ reagent (15596-026; Invitrogen, Carlsbad, CA, USA) and
reverse-transcribed into cDNA using a PrimeScript RT master mix kit
according to the manufacturer's protocol. RT-qPCR reactions were
conducted in a total volume of 20 µl [0.5 µl primer (20 µmol/l), 1
µl cDNA, 8 µl H2O and 10 µl 2X SYBR-Green qPCR mix] with
SYBR-Green Universal qPCR master mix on an ABI 7500 thermocycler
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The thermal
cycling conditions were as follows: 94°C for 2 min, followed by 35
cycles at 94°C for 30 sec, 55°C for 30 sec and 72°C for 30 sec, and
a final extension step at 72°C 10 min. The relative expression
values were normalized to those of GAPDH, and mRNA fold changes
were determined using the 2−ΔΔCq method (28). Primers used for PCR were as
follows: GAPDH, forward 5'-acagcaa-cagggtggtggac-3', reverse
5'-tttgagggtgcagcgaactt-3'; NF-κB, forward 5'-aat
ttggcttcctttcttggct-3', reverse 5'-ctgcgataccttaatga-cagcg-3'.
Western blot analysis
Western blot analysis was performed as described
previously (23). Liver tissues
were homogenized in RIPA lysis buffer (WB-0071), which was
purchased from Beijing Dingguo Changsheng Biotechnology Co., Ltd. .
Briefly, total cell lysates were extracted according to a standard
protocol, and protein concentration was determined using a
bicinchoninic acid assay kit. Subsequently, the samples were
subjected to 10% SDS-PAGE and proteins (50 µg proteins per sample)
were transferred onto a polyvinylidene fluoride membrane (EMD
Millipore), which was blocked, for 1 h at room temperature, with 5%
skim milk in Tris-buffered saline containing 0.5% Tween-20 .
Subsequently, the membrane was incubated with primary antibodies
(1:1,000 dilution) specific to NF-κB, CYP2E1, caspase-1, -3 or -8
overnight at 4°C, and subsequently incubated with horseradish
peroxidase (HRP)-conjugated secondary antibodies (1:1,000 dilution;
705-035-003; Jackson Immunoresearch, West Grove, PA, USA) for 2 h
at 37°C. HRP signals were detected using an
electroche-miluminescence detection system (Intron Biotechnology,
Inc., Seongnam, South Korea). For semi-quantification, band
densities were determined using Bio-Rad Quantity One software
(version 4.6.9; Bio-Rad Laboratories, Inc.), and values were
normalized to β-actin.
Immunohistochemistry and
histopathology
Liver tissues from the right lobe were fixed in 4%
buffered paraformaldehyde for 24 h at 4°C, embedded in paraffin and
cut into 4 µm sections using a rotary microtome (Leica RM 2135;
Meyer Instruments, Houston, TX, USA). Subsequently, the sections
underwent hematoxylin and eosin (H&E) and immunohistochemical
staining. As previously described (23), sections were dewaxed with xylene
and rehydrated, and then microwave antigen retrieval was performed
at 95°C for 15 min using 0.01 M citric acid buffer (pH 6.0). The
sections were then incubated with a 3% hydrogen peroxide solution
(SP KIT-A1; Beijing Dingguo Changsheng Biotechnology Co., Ltd.) for
10 min at room temperature. Following several washes in PBS (pH
7.4), sections were blocked with 10% goat serum and then incubated
overnight with primary antibodies [caspase-3 (1:50; 19677-1-AP;
Proteintech, Chicago, IL, USA), Casp8 (1:500; ab25901; Abcam),
NF-κB p65 (1:1500; ab16502; Abcam)] overnight at 4°C. After several
washes with PBS, biotinylated goat anti-rabbit IgG (1:200; IB-0061;
Beijing Dingguo Changsheng Biotechnology Co., Ltd.) was added at
37°C for 30 min. Horseradish peroxidase-labeled streptavidin
(1:400; IH-0061; Beijing Dingguo Changsheng Biotechnology Co.,
Ltd.) was added after several washes with PBS, followed by 30 min
of incubation at 37°C. After additional washes with PBS,
immunoreactivity was detected with a diaminobenzidine (DAB)
staining kit (AR-0611; Beijing Dingguo Changsheng Biotechnology
Co., Ltd.), and sections were counterstained with H&E. To
evaluate the histopathological alterations, samples were examined
under a light microscope (Olympus BX51, Hamburg, Germany), and an
arbitrary scope was viewed under ×40–400 magnifications; the scores
from ≥ 3 fields/liver section were determined to obtain the mean
value. The scores of hepatic inflammation were as follows: score 0,
absent; score 1, small amount of cells present at the junction of
the necrotic zone; score 2, normal amount of cells present; score
3, predominantly neutrophils present; score 4, predominantly
mononuclear cells present. Fibrosis extent was graded as follows:
score 0, absent; score 1, thin septa present; score 2, thin septa
present linking hepatic veins; score 3, broad/ well-developed
septa; score 4, cirrhosis. All specimens were scored by 3
pathologists, each one of whom was blinded to the scoring of the
others.
Flow cytometry for cell apoptosis
detection
Flow cytometric analysis was conducted according to
standard methods. Briefly, after culturing for 48 h, isolated L-O2
cells were adjusted to 1x106 cells/ml, suspended in 100
µl binding buffer and stained with FITC-conjugated Annexin V and
propidium iodide (Annexin V-FITC/PI Apoptosis Detection kit;
40302ES20; Qcbio Science & Technologies Co., Ltd., Shanghai,
China) for 30 min at room temperature in the dark. The samples were
then washed twice in PBS and were fixed in 1% paraformaldehyde.
Data acquisition was performed on a FACSCalibur flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA) in order to determine the
percentage of apoptotic cells.
Terminal
deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL)
staining
A TUNEL assay was performed using the
ApopTag® Plus Peroxidase In Situ apoptosis kit
(EMD Millipore) according to the manufacturer's protocol, as
described previously (23). Liver
tissues were used for TUNEL staining.
Statistical analyses
All quantitative values are presented as the mean ±
standard error of the mean and statistical analyses were performed
using GraphPad Prism 5.0 software (GraphPad Software, Inc., La
Jolla, CA, USA). Statistical significance between two groups was
compared using unpaired t-test and multiple comparisons were made
using one-way analysis of variance followed by Dunnett or Tukey's
post hoc tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
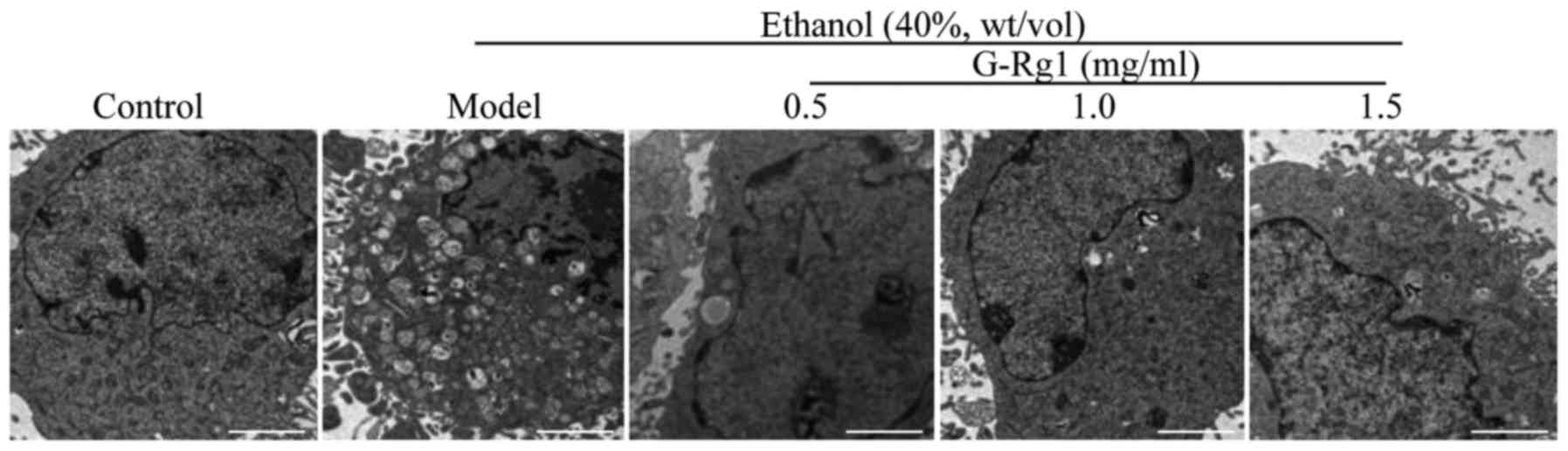
G-Rg1 improves the ultrastructure of
ethanol-exposed hepatocytes
Alcohol is able to induce pathological alterations
to the subcellular structures of hepatocytes (29). Therefore, the present study aimed
to determine whether G-Rg1 could protect hepatocytes from
alcohol-induced subcellular damage. L-O2 cells were cultured in
DMEM containing 40% ethanol in combination with 0.5, 1.0 or 1.5
mg/ml G-Rg1, and their ultra-structure was analyzed by electron
microscopy. Consistent with the results of Gao et al
(17), ethanol-exposed cells
exhibited marked alterations in the microstructure, including
swelling of cells, loose cell structure and enlarged mitochondria.
In addition, no intact endoplasmic reticulum was detectable;
however, numerous vacuoles were visible in the cytoplasm. Compared
with in the ethanol-treated group, microstructure was markedly
improved in cells treated with a low dose of G-Rg1 (0.5 mg/ml), in
which intact mitochondria and fewer vacuoles were detected. When
treated with higher doses of G-Rg1 (1.0 and 1.5 mg/ml), the
microstructure was almost the same as in the control cells
(Fig. 1). These results indicated
that G-Rg1 may protect organelles against ethanol-induced
damage.
G-Rg1 restores abnormal levels of
biochemical parameters in alcoholic hepatitis
The release of ALT, AST and TBIL is widely used as a
biomarker to evaluate hepatocellular damage. To further validate
the hepatoprotective activities of G-Rg1, the present study
determined the effects of G-Rg1 on the release of these
intracytoplasmic proteins in an in vitro ethanol-induced
cell injury model and in an in vivo rat hepatitis model.
L-O2 cells were cultured in DMEM supplemented with 40% ethanol, and
were treated with G-Rg1 as aforementioned, after which culture
supernatants were collected to measure ALT, AST and TBIL. As
expected, all three biochemical parameters were elevated in the
culture supernatant of ethanol-treated cells, indicating that
cellular damage occurred in this group. Conversely, the levels of
these parameters were significantly decreased when cells were
cotreated with G-Rg1 (Fig.
2A).
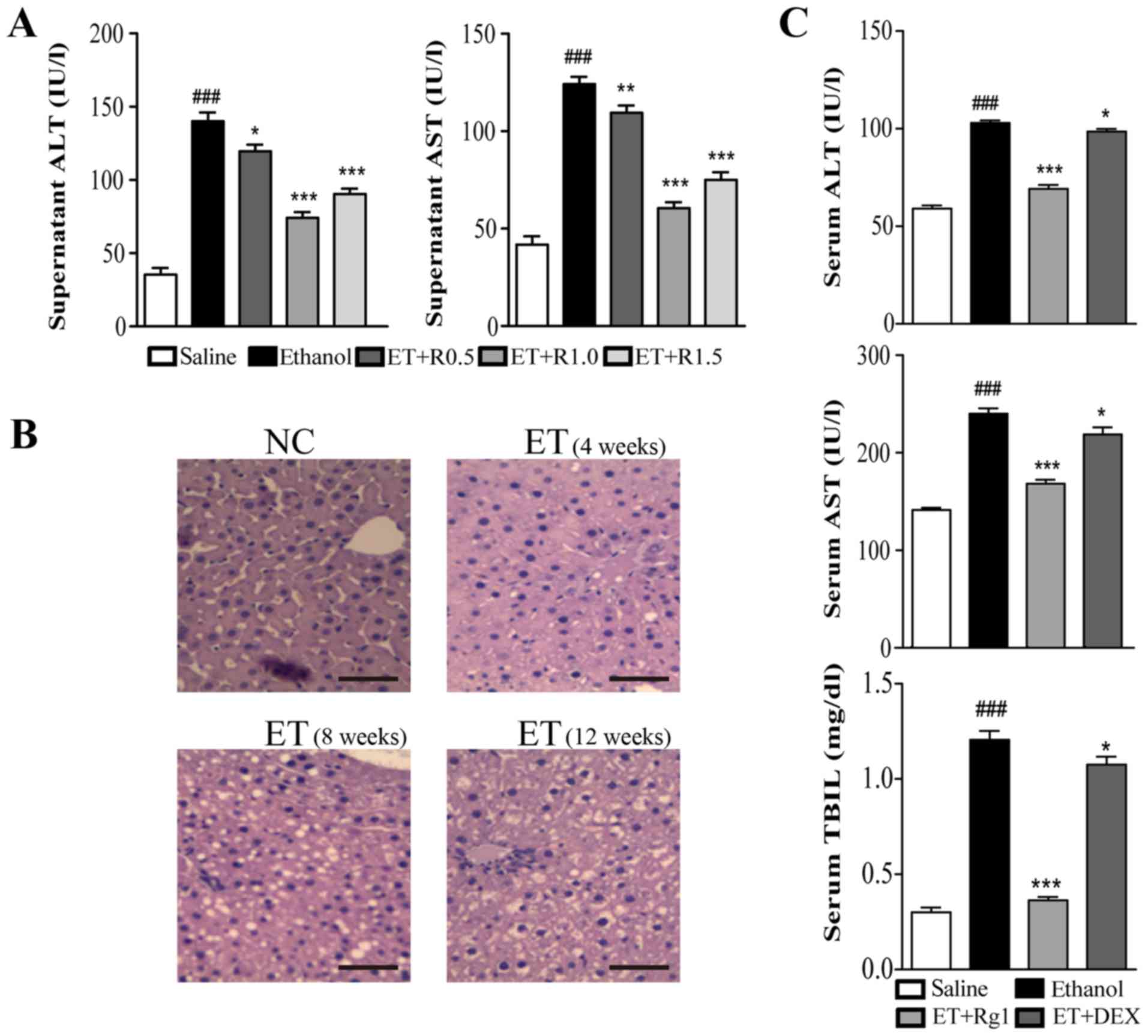 | Figure 2G-Rg1 decreases ethanol-induced
biochemical parameter elevation. (A) L-O2 cells were cultured in
medium containing ethanol, and were treated with G-Rg1. After a 48
h culture, AST and ALT in the supernatant were measured using assay
kits. (B and C) Rats were intragastrically injected with ethanol
for up to 12 weeks to establish the alcoholic liver injury model,
and were treated with G-Rg1 or DEX. Following treatment, rats were
sacrificed. (B) Liver biopsies were taken to assess ethanol-induced
histopathological alterations through hematoxylin and eosin
staining. (C) Serum levels of AST, ALT and TBIL were analyzed using
assay kits. Scale bar, 50 µm. Data are presented as the mean ±
standard error of the mean. ###P<0.001 vs. the
control group; *P<0.05, **P<0.01 and
***P<0.001 vs. the model/ethanol group. Data from at
least two independent experiments are presented. ALT, alanine
aminotransferase; AST, aspartate aminotransferase; DEX,
dexamethasone; ET, ethanol; G-Rg1, ginsenoside Rg1; NC, normal
control; R0.5/1.0/1.5, 0.5/1.0/1.5 mg/ml G-Rg1; TBIL, total
bilirubin. |
To assess the hepatoprotective effects of G-Rg1
in vivo, a rat model of alcoholic hepatitis was generated.
Sprague-Dawley rats were intragastrically administered alcohol for
12 consecutive weeks, and were administrated G-Rg1 once a day for a
further 5 days. Subsequently, rats were sacrificed, and liver
specimens were used to evaluate histological alterations by H&E
staining. In addition, serum samples were collected to analyze
biochemical parameters. In the liver tissue of normal rats,
hepatocytes were polygonal in shape, equal in size and were
arranged around the central vein to form lobules. Furthermore,
there were no visible lipid droplets in the tissue. Conversely, in
rats administered alcohol, hepatocytes exhibited irregular shapes,
and lipid vacuoles accumulated in response to alcohol
administration, indicating that the alcoholic hepatitis model was
successfully established (25,26,30) (Fig.
2B). In accordance with the pathological alterations in liver
morphology, serum levels of ALT, AST and TBIL were significantly
increased in ethanol-treated rats. However, in rats treated with
G-Rg1, these parameters were markedly decreased to levels similar
to those in normal rats, and were lower than those in DEX-treated
rats (Fig. 2C). Together, these
data suggested that G-Rg1 may protect hepatocytes from
alcohol-induced injury.
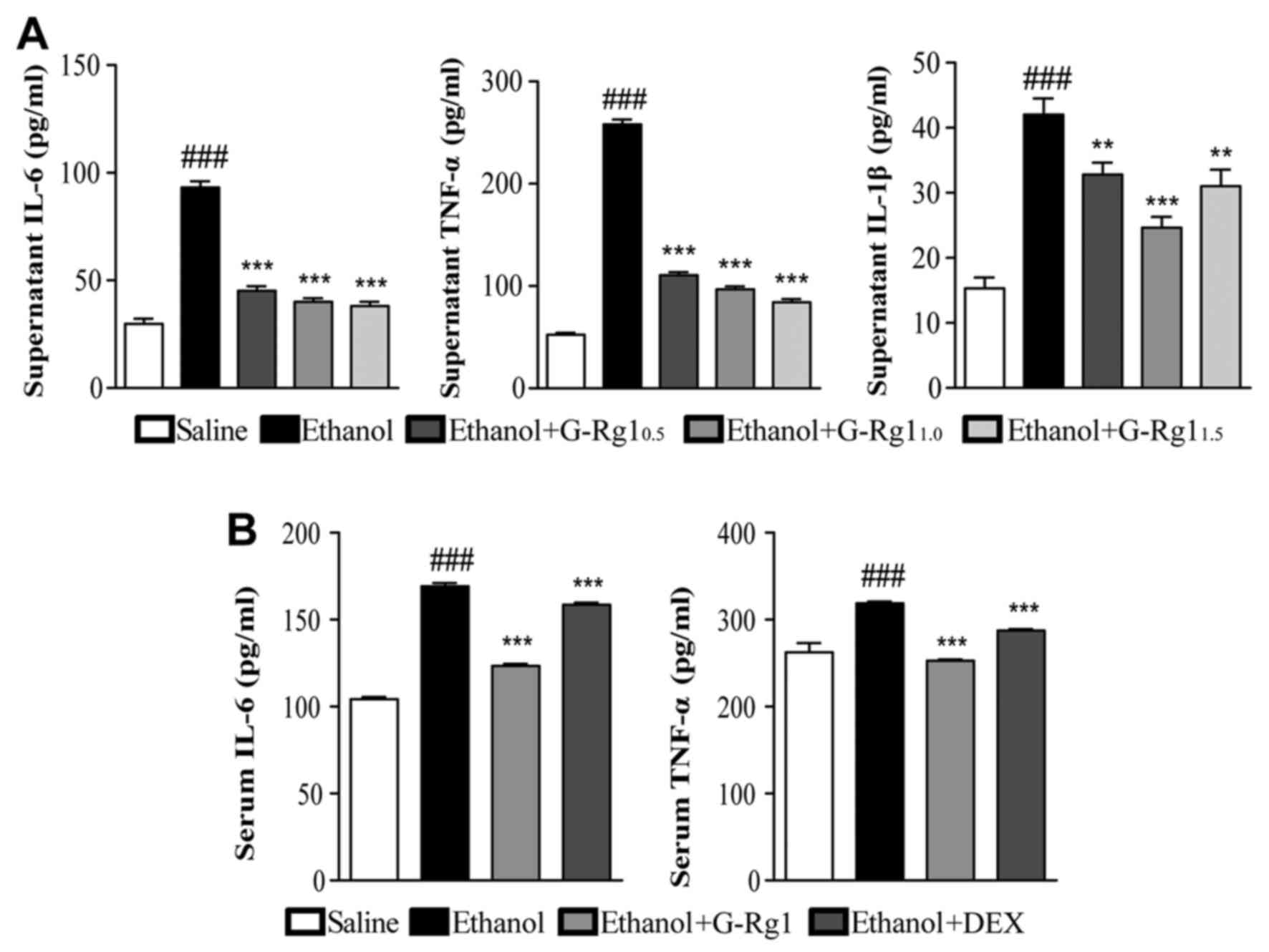
G-Rg1 decreases the production of
proinflammatory cytokines
Alcoholic hepatitis is accompanied with the
overproduction of proinflammatory cytokines, which may lead to
excessive inflammation and further aggravate hepatocellular damage
(9). To evaluate whether G-Rg1
regulates the production of proinflammatory cytokines, ELISA assays
were performed on culture supernatants from L-O2 cells treated with
ethanol with or without G-Rg1, and on serum samples from rats with
alcoholic hepatitis treated with or without G-Rg1.
The results of the present study demonstrated that
in the culture supernatants from cells exposed to ethanol, the
protein expression levels of IL-6, TNF-α and IL-1β were
significantly increased compared with in the normal control group.
Conversely, when cells were cotreated with G-Rg1, the upregulation
of these cytokines was largely abrogated, particularly with regards
to IL-6 and TNF-α (Fig. 3A).
Similar to the in vitro data, rats with alcoholic hepatitis
had higher serum levels of IL-6 and TNF-α compared with in normal
rats, and the elevated levels of these two cytokines were partially
reduced following treatment with G-Rg1 (Fig. 3B). Therefore, G-Rg1 may modulate
the production of proinflammatory cytokines, which may further
contribute to the hepatoprotective effects of G-Rg1.
G-Rg1 inhibits activation of the NF-κB
pathway
The NF-κB pathway is a key signaling pathway that is
involved in the regulation of immune and inflammatory responses,
which controls the expression of numerous proinflammatory cytokine
genes, including IL-1β, IL-6 and TNF-α. To better understand the
molecular mechanisms underlying the regulatory effects of G-Rg1 on
proinflammatory cytokine production, the present study aimed to
determine whether G-Rg1 could inhibit activation of the NF-κB
pathway. L-O2 cells were exposed to ethanol, and were treated with
G-Rg1, after which the protein expression levels of NF-κB were
detected by western blot analysis.
The results indicated that p65 expression was
increased in the lysates of ethanol-exposed cells compared with in
normal control cells; however, p65 expression was decreased when
cells were treated with a moderate dose of G-Rg1 (1.0 mg/ml)
(Fig. 4A), thus suggesting that
G-Rg1 may negatively regulate NF-κB activation. To validate the
effects of G-Rg1 on NF-κB activation in vivo, a rat model of
alcoholic hepatitis was established, and rats were treated with
G-Rg1 as aforementioned. Initially, the mRNA expression levels of
NF-κB p65 were detected in hepatocytes by RT-qPCR; as shown in
Fig. 4B, the mRNA expression
levels of NF-κB p65 were higher in model rats compared with in
normal control rats, whereas they were downregulated by G-Rg1
treatment. Subsequently, the protein expression levels of NF-κB
were determined by western blot analysis. The results demonstrated
that p65 was markedly increased in model rats. Conversely,
treatment with G-Rg1 resulted in p65 downregulation, to a similar
level as that in normal rats (Fig.
4C). To further confirm these results, immunohistochemistry was
performed on liver biopsies. Consistent with the western blotting
data, p65 expression was increased in liver biopsies from model
rats compared with in normal rats, and was markedly decreased by
G-Rg1 treatment (Fig. 4D). Taken
together, these results suggested that G-Rg1 may inhibit production
of proinflammatory cytokines via downregulation of NF-κB
activation.
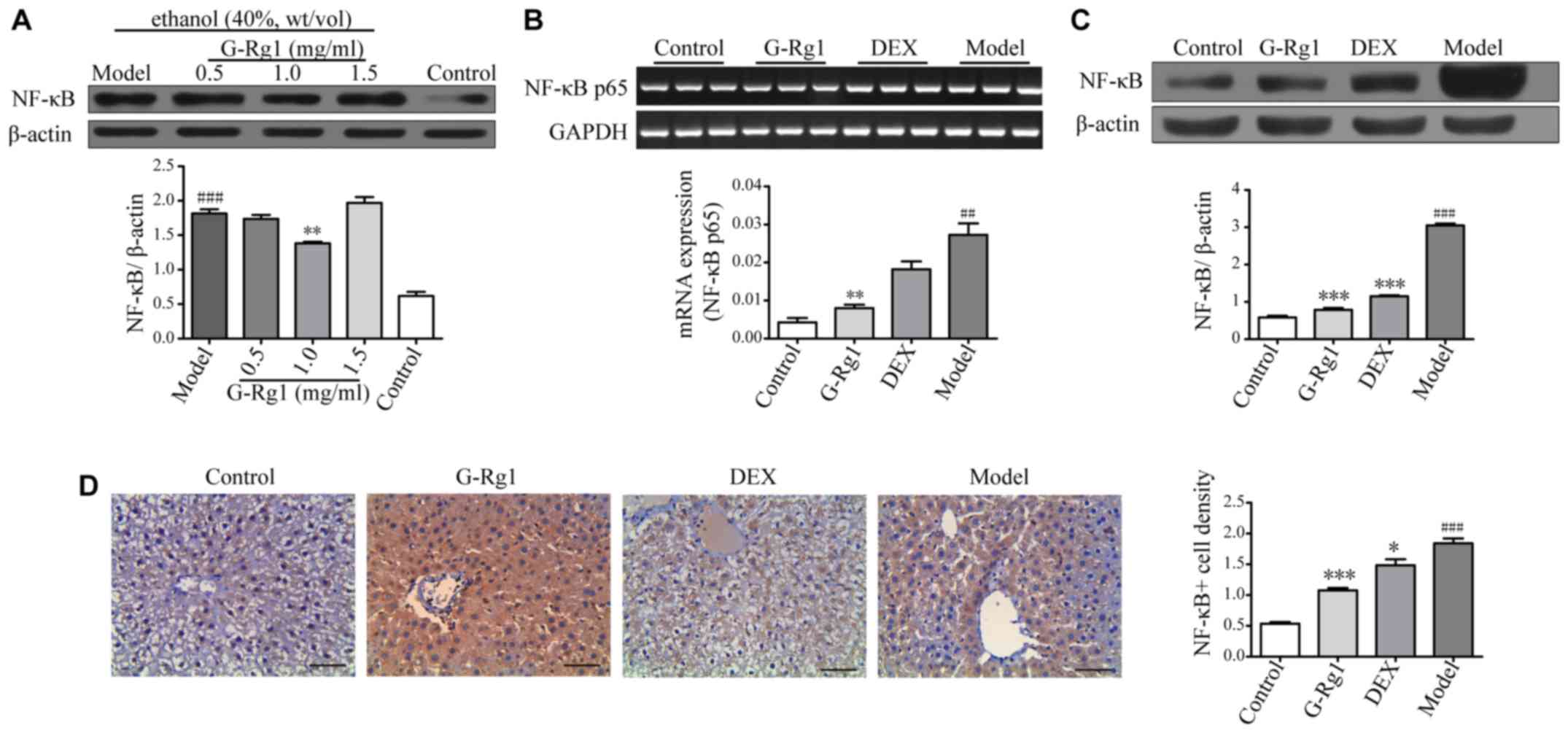 | Figure 4G-Rg1 suppresses activation of the
NF-κB pathway. (A) L-O2 cells were treated with ethanol, ethanol +
G-Rg1 (0.5, 1.0 and 1.5 mg/ml) or saline, and the protein
expression levels of NF-κB were determined by western blot
analysis, β-actin was used as a loading control. (B) Polymerase
chain reaction was used to detect NF-κB p65 expression in liver
tissues from normal control rats, and rats with alcoholic hepatitis
treated with or without G-Rg1 or DEX. GAPDH was used as an internal
control. (C) Western blot analysis and (D) immunohistochemical
staining for NF-κB expression in the liver tissue of rats. Scale
bar, 50 µm. Data are presented as mean ± standard error of the
mean. ##P<0.01 and ###P<0.001 vs. the
control/saline group; *P<0.05, **P<0.01
and ***P<0.001 vs. the model/ethanol group. Data from
at least three independent experiments are shown. DEX,
dexamethasone; G-Rg1, ginsenoside Rg1; NF-κB, nuclear factor-κB |
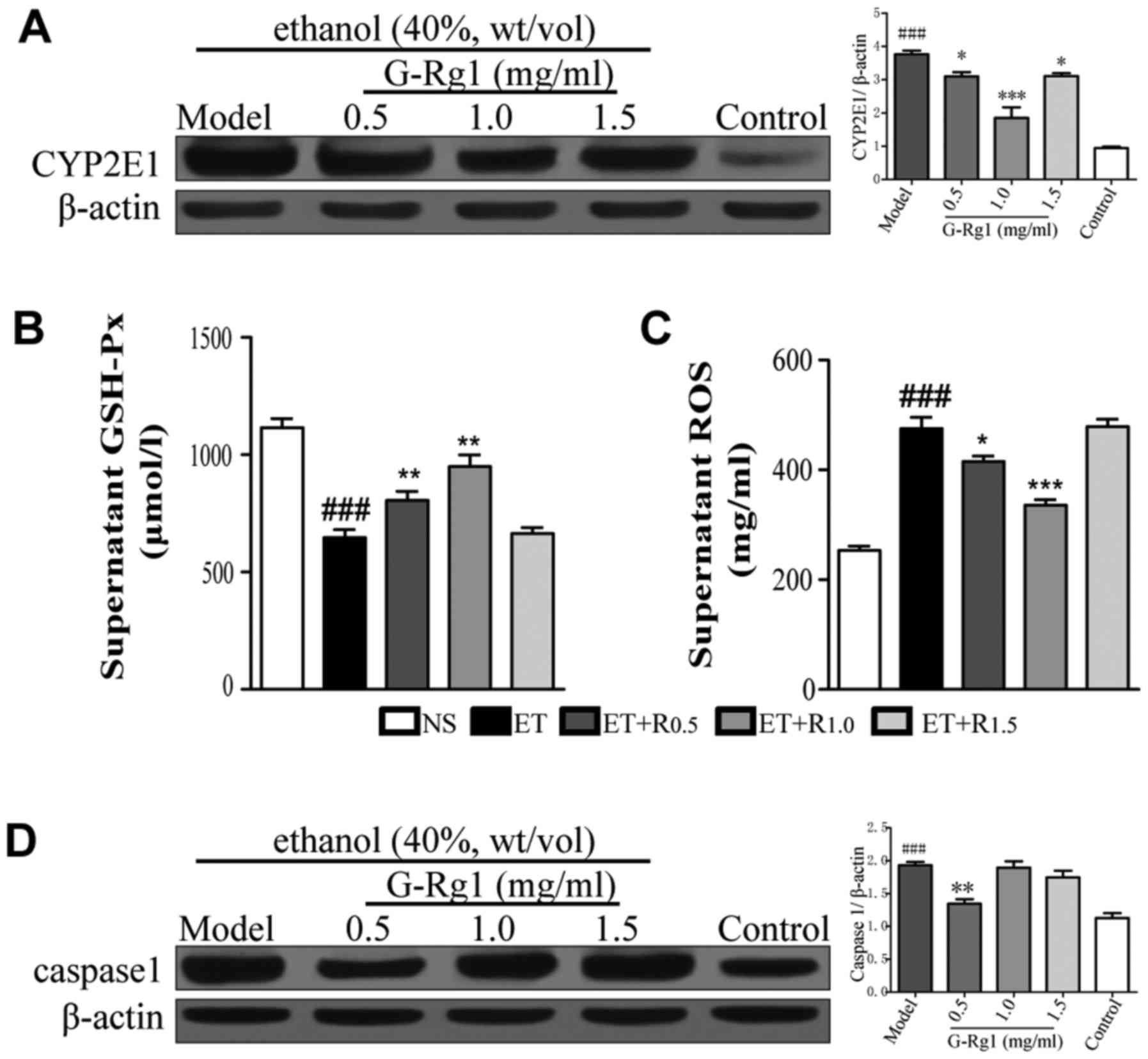
G-Rg1 protects hepatocytes by modulating
oxidative stress and inflammasome activation
As aforementioned, inflammatory cytokine production
is regulated by activation of NF-κB, which subsequently promotes
the transcription of IL-1β, IL-6 and TNF-α, and inflammasomes,
which in turn activate the cysteine protease caspase-1 and promote
the maturation of proinflammatory cytokines (24). During alcoholic hepatitis,
metabolism of alcohol increases the levels of CYP2E1, and decreases
the levels of antioxidant enzymes, including GSH-Px. As a result,
ROS levels are elevated, which finally leads to activation of
inflammasomes (31). To elucidate
whether the regulatory effects of G-Rg1 on proinflammatory cytokine
production are associated with inflammasome activation, the
expression levels of CYP2E1, GSH-Px, ROS and caspase-1 were
detected in ethanol-exposed cells that were treated with or without
G-Rg1.
The results demonstrated that CYP2E1 was upregulated
in response to ethanol exposure (Fig.
5A). Conversely, GSH-Px expression was decreased when cells
were exposed to ethanol (Fig.
5B). In accordance with these data, cellular ROS was increased
in ethanol-exposed cells (Fig.
5C). However, when cells were treated with a moderate dose of
G-Rg1 (1 mg/ml), CYP2E1 levels were markedly decreased, whereas
GSH-Px levels were significantly elevated. Accordingly, abnormal
ROS levels were reduced compared with in alcohol-exposed cells
(Fig. 5A–C). Notably, expression
of caspase-1, which is the essential effector molecule of
inflammasomes, was markedly increased in ethanol-exposed cells;
however, it was decreased following treatment with G-Rg1 (Fig. 5D). Consistent with these data, the
mature form of IL-1β, which is dependent on caspase-1 for
processing, exhibited higher levels in the culture supernatants of
ethanol-treated cells compared with in G-Rg1-treated cells
(Fig. 3A). These data suggested
that G-Rg1 may exert anti-inflammatory effects through inhibition
of inflammasome activation.
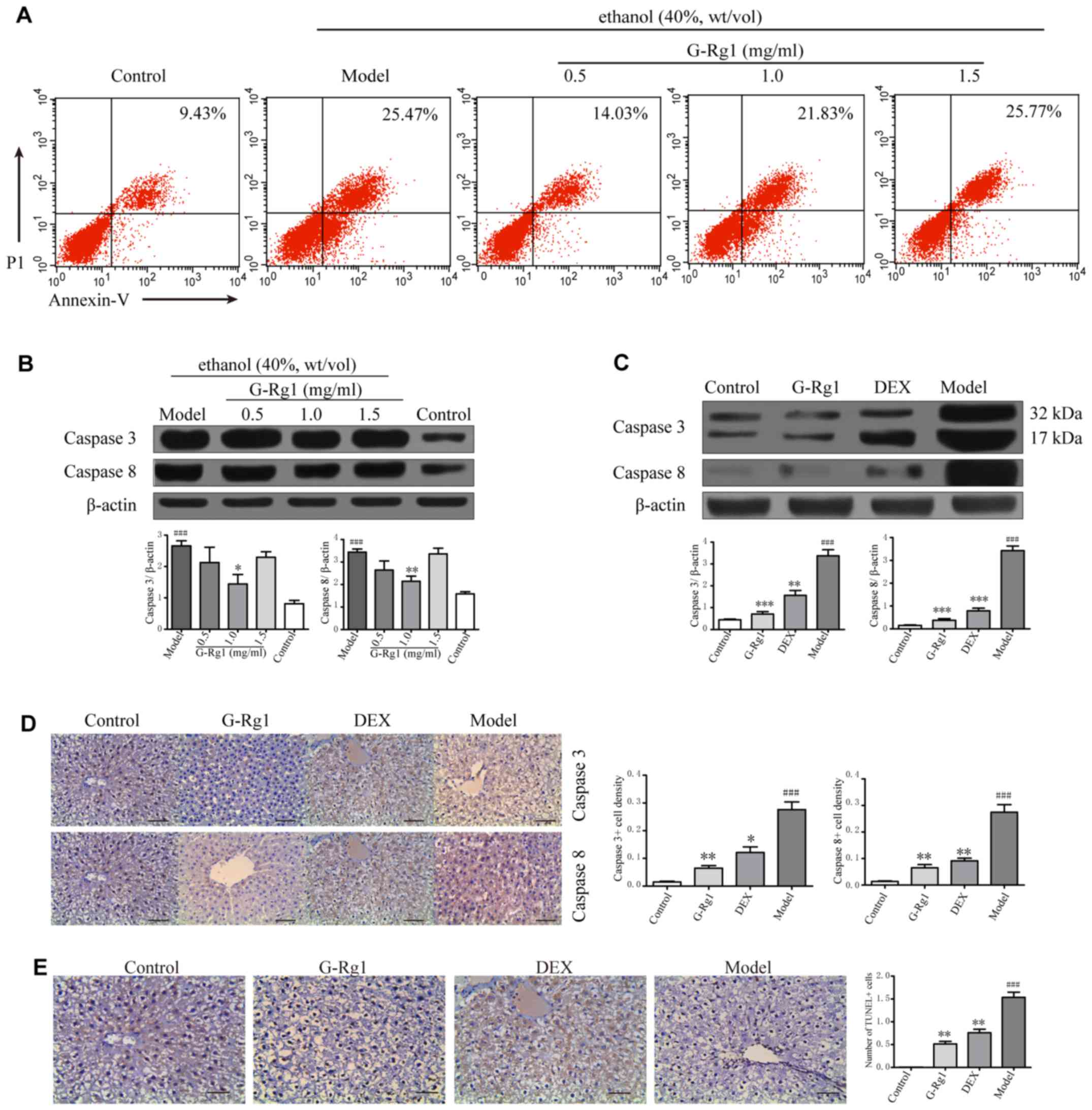
G-Rg1 reduces the apoptosis of
hepatocytes
Dysregulated proinflammatory cytokine production may
initiate excessive death of hepatocytes, which contributes to the
pathogenesis of ALD. Therefore, the present study aimed to
determine whether G-Rg1 could promote the survival of hepatocytes
in order to exert its hepatoprotective effects. Alcoholic cell
injury was induced in L-O2 cells, which were then treated with
G-Rg1; cell viability was assessed by flow cytometry, and caspase
expression was detected by western blot analysis.
The results indicated that ~25% of cells underwent
apoptosis in the ethanol-treated group, whereas the proportion of
apoptotic cells was decreased to ~14% in cells treated with 0.5
mg/ml G-Rg1 (Fig. 6A).
Corresponding to decreased cell apoptosis, a decline in the protein
expression levels of caspase-3 and -8 was detected in G-Rg1-treated
cells (Fig. 6B). To verify these
results in vivo, the expression levels of caspase-3 and -8
were detected in liver samples from rats with alcoholic hepatitis
that were treated with or without G-Rg1. Similar to the in
vitro findings, caspase-3 and -8 expression was increased in
model rats, as determined by western blot analysis and
immunohistochemistry. Conversely, the levels of caspase-3 and -8
were markedly decreased in G-Rg1-treated rats (Fig. 6C and D). Consequently, apoptosis
of hepatocytes was induced in model rats, whereas it was markedly
prevented by G-Rg1 treatment (Fig.
6E). These results indicated that G-Rg1 may alleviate alcoholic
hepatitis through suppression of hepatocyte death.
Discussion
Due to alcohol overconsumption, cases of ALD,
including alcohol-induced hepatic steatosis, hepatitis, progressive
fibrosis, cirrhosis and hepatocellular carcinoma, are increasing
and are considered one of the biggest public health burdens
worldwide (32–34). However, at present, there are no
strategies that effectively delay or reverse the progression of
ALD. Therefore, the identification of an agent able to prevent and
treat ALD is urgently required.
G-Rg1, which is the most abundant active ingredient
of P. ginseng, has been reported to possess hepatoprotective
effects in several liver injury models, including
ischemia/reperfusion injury, CCl4-induced acute liver
failure and hepatic fibrosis (35–37); however, the underlying molecular
mechanisms require further elucidation. Consistent with these
previous studies, the present study demonstrated that G-Rg1 could
protect against alcohol-induced liver damage, as evidenced by
improved hepatocyte microstructure, ameliorated hepatic
histopathological alterations, and reduced release of biochemical
parameters that are hallmarks of hepatocyte damage. Furthermore,
the present results suggested that G-Rg1 may protect against ALD
through two potential mechanisms: i) Inhibition of excessive
inflammatory responses and ii) reduction of hepatocyte apoptosis.
Since hepatic inflammation is closely associated with the loss of
hepatocytes, and homeostasis of hepatocytes is likely to be
maintained by the balance between renewal and death of these cells,
the present data thus provided an insight into the hepatoprotective
effects of G-Rg1.
Hepatic inflammation, which is associated with the
majority of acute and chronic liver disorders, largely determines
the outcome of liver damage (38). Mild and short-term inflammation is
in favor of repairing tissue damage and the re-establishment of
hepatic homeostasis. Conversely, excessive and persistent
inflammation may lead to a marked loss of hepatocytes, activation
of HSCs and liver fibrosis/cirrhosis (3,4).
Therefore, preventing excessive inflammation is the central issue
for the treatment of liver diseases. Anti-inflammatory drugs,
including GCs, are extensively used to treat inflammatory diseases.
G-Rg1 is a type of triterpene saponin, which are homologous to GCs
in structure; in the present study, G-Rg1 was revealed to inhibit
the production of proinflammatory cytokines. Similar to a previous
study (23), G-Rg1 suppressed
activation of NF-κB, which contributed to decreased transcription
of inflammation-associated genes. In addition, G-Rg1 modulated the
levels of CYP2E1, GSH-Px and ROS, which in turn affected the
activation of inflammasomes and the downstream effector molecule
caspase-1, and finally regulated the maturation of proinflammatory
cytokines. Notably, G-Rg1 was as potent as DEX in suppression of
proinflammatory cytokine production and NF-κB activation, whereas
much fewer side effects were reported by other studies (39,40). Therefore, the present study
provided information regarding the molecular mechanisms underlying
the anti-inflammatory effects of G-Rg1, and indicated that
exploring chemical components from traditional herbs may be a
promising direction for the development of novel drugs for the
treatment of inflammatory diseases, including ALD.
Although G-Rg1 was able to protect hepatocytes
against alcohol-induced injury, it should be noted that besides
hepatocytes, the liver is composed of diverse types of resident and
circulating immune cells. These cells include macrophages (Kupffer
cells), monocytes, neutrophils, dendritic cells, natural killer
cells, natural killer T cells and T lymphocytes. While cytokines
serve a crucial role in initiating and maintaining hepatic
inflammation and liver damage, the major source of these cytokines
is immune cells (41). Therefore,
immune cells should be dominant in determining the outcomes of
inflammatory responses in the liver, as well as the progression of
hepatic disorders. However, there is currently little information
regarding the regulatory effects of G-Rg1 on the functionality and
cellular behaviors of immune cells. Further studies to elucidate
these effects may help broaden current understanding regarding the
hepatoprotective effects of G-Rg1 and could be beneficial for the
treatment of liver diseases.
In conclusion, the present study demonstrated that
G-Rg1 was capable of protecting the liver from alcohol-induced
injury through inhibition of inflammatory responses and
hepatocellular apoptosis. These results may help to further
elucidate the molecular mechanisms underlying the hepatoprotective
effects of G-Rg1 and evaluate the potential of G-Rg1 as a candidate
agent for the prevention and treatment of ALD.
Acknowledgments
The authors would like to thank Dr Zhengyu Shi for
helping with the statistical analysis, and Dr Chengwei Liu for
providing technical support (both from Department of Infectious
Diseases, The First Affliated Hospital of Chongqing Medical
University, Chongqing, China). The present study was supported by
the Chongqing Science Foundation (grant no. KJ130332 to Wenxiang
Huang).
References
|
1
|
Chang B, Li B, Huang A, Sun Y, Teng G,
Wang X, Liangpunsakul S, Li J and Zou Z: Changes of four common
non-infectious liver diseases for the hospitalized patients in
Beijing 302 hospital from 2002 to 2013. Alcohol. 54:61–65. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang H, Ma L, Yin Q, Zhang X and Zhang C:
Prevalence of alcoholic liver disease and its association with
socioeconomic status in north-eastern China. Alcohol Clin Exp Res.
38:1035–1041. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iwaisako K, Brenner DA and Kisseleva T:
What's new in liver fibrosis? The origin of myofibroblasts in liver
fibrosis. J Gastroenterol Hepatol. 27(Suppl 2): 65–68. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schattenberg JM, Galle PR and Schuchmann
M: Apoptosis in liver disease. Liver Int. 26:904–911. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zakhari S and Li TK: Determinants of
alcohol use and abuse: Impact of quantity and frequency patterns on
liver disease. Hepatology. 46:2032–2039. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
You M and Crabb DW: Recent advances in
alcoholic liver disease II. Minireview: Molecular mechanisms of
alcoholic fatty liver. Am J Physiol Gastrointest Liver Physiol.
287:G1–G6. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nagata K, Suzuki H and Sakaguchi S: Common
pathogenic mechanism in development progression of liver injury
caused by non-alcoholic or alcoholic steatohepatitis. J Toxicol
Sci. 32:453–468. 2007. View Article : Google Scholar
|
|
8
|
Enomoto N, Ikejima K, Bradford BU, Rivera
CA, Kono H, Goto M, Yamashina S, Schemmer P, Kitamura T, Oide H, et
al: Role of Kupffer cells and gut-derived endotoxins in alcoholic
liver injury. J Gastroenterol Hepatol. 15(Suppl): D20–D25. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gao B: Cytokines, STATs and liver disease.
Cell Mol Immunol. 2:92–100. 2005.PubMed/NCBI
|
|
10
|
Friedman SL: Hepatic stellate cells:
Protean, multifunctional, and enigmatic cells of the liver. Physiol
Rev. 88:125–172. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xie XS, Liu HC, Wang FP, Zhang CL, Zuo C,
Deng Y and Fan JM: Ginsenoside Rg1 modulation on thrombospondin-1
and vascular endothelial growth factor expression in early renal
fibro-genesis in unilateral obstruction. Phytother Res.
24:1581–1587. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu L, Chen WF and Wong MS: Ginsenoside Rg1
protects dopaminergic neurons in a rat model of Parkinson's disease
through the IGF-I receptor signalling pathway. Br J Pharmacol.
158:738–748. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shen LH and Zhang JT: Ginsenoside Rg1
promotes proliferation of hippocampal progenitor cells. Neurol Res.
26:422–428. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shi AW, Wang XB, Lu FX, Zhu MM, Kong XQ
and Cao KJ: Ginsenoside Rg1 promotes endothelial progenitor cell
migration and proliferation. Acta Pharmacol Sin. 30:299–306. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu CF, Bi XL, Yang JY, Zhan JY, Dong YX,
Wang JH, Wang JM, Zhang R and Li X: Differential effects of
ginsenosides on NO and TNF-alpha production by LPS-activated N9
microglia. Int Immunopharmacol. 7:313–320. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu F, Zhang SS and Kang GF: Effects of
panax notoginseng saponins on the expression of tumor necrosis
factor alpha and secretion phospholipase A2 in rats with liver
fibrosis. Zhonghua Gan Zang Bing Za Zhi. 11:51–52. 2003.In Chinese.
PubMed/NCBI
|
|
17
|
Gao Y, Chu S, Li J, Li J, Zhang Z, Xia C,
Heng Y, Zhang M, Hu J, Wei G, et al: Anti-inflammatory function of
ginsenoside Rg1 on alcoholic hepatitis through glucocorticoid
receptor related nuclear factor-kappa B pathway. J Ethnopharmacol.
173:231–240. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tao T, Chen F, Bo L, Xie Q, Yi W, Zou Y,
Hu B, Li J and Deng X: Ginsenoside Rg1 protects mouse liver against
ischemia-reper-fusion injury through anti-inflammatory and
anti-apoptosis properties. J Surg Res. 191:231–238. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang XJ, He C, Li P, Su H and Wan JB:
Ginsenoside Rg1, a potential JNK inhibitor, protects against
ischemia/reperfusion-induced liver damage. J Funct Foods.
15:580–592. 2015. View Article : Google Scholar
|
|
20
|
Xin Y, Wei J, Chunhua M, Danhong Y,
Jianguo Z, Zongqi C and Jian-An B: Protective effects of
Ginsenoside Rg1 against carbon tetrachloride-induced liver injury
in mice through suppression of inflammation. Phytomedicine.
23:583–588. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Geng J, Peng W, Huang Y, Fan H and Li S:
Ginsenoside-Rg1 from Panax notoginseng prevents hepatic fibrosis
induced by thioacetamide in rats. Eur J Pharmacol. 634:162–169.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li JP, Gao Y, Chu SF, Zhang Z, Xia CY, Mou
Z, Song XY, He WB, Guo XF and Chen NH: Nrf2 pathway activation
contributes to anti-fibrosis effects of ginsenoside Rg1 in a rat
model of alcohol- and CCl4 -induced hepatic fibrosis.
Acta Pharmacol Sin. 35:1031–1044. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao J, Shi Z, Liu S, Li J and Huang W:
Ginsenosides Rg1 from Panax ginseng: A potential therapy for acute
liver failure patients. Evid Based Complement Alternat Med.
538059:2014. View Article : Google Scholar
|
|
24
|
Bieghs V and Trautwein C: The innate
immune response during liver inflammation and metabolic disease.
Trends Immunol. 34:446–452. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsukamoto H, Reidelberger RD, French SW
and Largman C: Long-term cannulation model for blood sampling and
intragastric infusion in the rat. Am J Physiol. 247:R595–R599.
1984.PubMed/NCBI
|
|
26
|
Tsukamoto H, Horne W, Kamimura S, Niemelä
O, Parkkila S, Ylä-Herttuala S and Brittenham GM: Experimental
liver cirrhosis induced by alcohol and iron. J Clin Invest.
96:620–630. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sedlak J and Lindsay RH: Estimation of
total, protein-bound, and nonprotein sulfhydryl groups in tissue
with Ellman's reagent. Anal Biochem. 25:192–205. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
29
|
Santos Rocha SW, Silva BS, Gomes FO,
Soares e Silva AK, Raposo C, Barbosa KP, Torres DO, dos Santos AC
and Peixoto CA: Effect of diethylcarbamazine on chronic hepatic
inflammation induced by alcohol in C57BL/6 mice. Eur J Pharmacol.
689:194–203. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Singal AK, Kodali S, Vucovich LA,
Darley-Usmar V and Schiano TD: Diagnosis and treatment of alcoholic
hepatitis: A systematic review. Alcohol Clin Exp Res. 40:1390–1402.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Szabo G and Csak T: Inflammasomes in liver
diseases. J Hepatol. 57:642–654. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Walker RK, Cousins VM, Umoh NA, Jeffress
MA, Taghipour D, Al-Rubaiee M and Haddad GE: The good, the bad, and
the ugly with alcohol use and abuse on the heart. Alcohol Clin Exp
Res. 37:1253–1260. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rehm J, Samokhvalov AV and Shield KD:
Global burden of alcoholic liver diseases. J Hepatol. 59:160–168.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu J, Wang X, Liu R, Liu Y, Zhang T, Fu H
and Hai C: Oleanolic acid co-administration alleviates
ethanol-induced hepatic injury via Nrf-2 and ethanol-metabolizing
modulating in rats. Chem Biol Interact. 221:88–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zou Y, Tao T, Tian Y, Zhu J, Cao L, Deng X
and Li J: Ginsenoside Rg1 improves survival in a murine model of
polymicrobial sepsis by suppressing the inflammatory response and
apoptosis of lymphocytes. J Surg Res. 183:760–766. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shen L and Zhang J: Ginsenoside Rg1
increases ischemia-induced cell proliferation and survival in the
dentate gyrus of adult gerbils. Neurosci Lett. 344:1–4. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Karikura M, Tanizawa H, Hirata T, Miyase T
and Takino Y: Studies on absorption, distribution, excretion and
metabolism of ginseng saponins. VIII. Isotope labeling of
ginsenoside Rb2. Chem Pharm Bull (Tokyo). 40:2458–2460. 1992.
View Article : Google Scholar
|
|
38
|
Kubes P and Mehal WZ: Sterile inflammation
in the liver. Gastroenterology. 143:1158–1172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cao YW, Jiang Y, Zhang DY, Wang M, Chen
WS, Su H, Wang YT and Wan JB: Protective effects of Penthorum
chinense Pursh against chronic ethanol-induced liver injury in
mice. J Ethnopharmacol. 161:92–98. 2015. View Article : Google Scholar
|
|
40
|
Ding RB, Tian K, Huang LL, He CW, Jiang Y,
Wang YT and Wan JB: Herbal medicines for the prevention of
alcoholic liver disease: A review. J Ethnopharmacol. 144:457–465.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Brenner C, Galluzzi L, Kepp O and Kroemer
G: Decoding cell death signals in liver inflammation. J Hepatol.
59:583–594. 2013. View Article : Google Scholar : PubMed/NCBI
|