Introduction
Colon cancer ranks as the second most common
malignant tumor in Western developed countries, and is a serious
threat to human life and health (1). With changes in living habits and
diet, the incidence of colon cancer in China has increased
substantially, and the incidence rate of colon cancer is double the
global average (2–4). Therefore, the effective prevention
and treatment of colon cancer has become a focus of medical
investigations (3,5,6).
In addition to traditional surgery, radiotherapy and chemotherapy,
the combining of traditional Chinese medicine and Western medicine
treatment strategies offer potential in the treatment of the colon
cancer (7,8). However, the need to identify of
efficient, low toxicity drugs remains. At present, natural medicine
has become a focus of clinical anticancer drug investigation, due
to its multi-target, multi-link and multi-channel antitumor
effects.
Codonopis bulleynana Forest ex Diels
(cbFeD) grows in the forest margin and bushes 700–200 m
above sea level in the Yunnan province in China (9). It has a wide range of
pharmacological effects, including its primary anticancer function.
Due to the characteristics of the fresh root of cbFeD being
unique as a national medicinal herb, few reports exist in
international journals, although there have been a wide range of
investigations in China. cbFeD can significantly increase
hemoglobin in elderly individuals with senile deficiency syndrome
safety (10). Studies
investigating the pharmacodynamics and acute toxicity of
cbFeD have shown that it can enhance gastrointestinal
peristalsis, improve tolerance to fatigue and hypoxia, and can
promote the recovery of hemoglobin, red blood cells, IgG and the
immunosuppressive effect in hemorrhagic blood-deficient mice
(11,12). cbFeD can enhance the immune
function of mice with xenograft tumors and enhance the phagocytic
functions of the reticuloendothelial system (13). It has been suggested that
cbFeD has a positive effect on chemotherapy, reducing
toxicity and enhancing immune function.
Previous studies have demonstrated that signaling
pathways, including autophagy, are involved in resistance to
chemotherapy or radiotherapy (14,15). Autophagy, or type 2 cell death, is
a regulated process, which is involved in the turnover of
long-lived proteins and whole organelles, or target-specific
organelles, including mitochondria and endoplasmic reticulum, to
eliminate superfluity or damaged organelles (16,17). Due to the ability of autophagy to
remove damaged proteins or organelles, it may paradoxically act as
a mechanism for promoting the survival of irradiated cells,
indicating that the inhibition of autophagy enhances radiation
treatment and increases its efficacy (18). The traditional Chinese Medicine
DangGuiBuXue Tang sensitizes colorectal cancer cells to
chemotherapy or radiotherapy by inducing autophagy (19). Autophagy is important in
cytotoxicity, infection and tumorigenesis. However, the functional
role of autophagy in cancer remains controversial. Certain studies
have demonstrated that autophagy inhibits the progression of
tumors, whereas others have demonstrated that autophagy promotes
cell death, particularly in cells resistant to apoptosis (20).
Oxaliplatin is a third-generation platinum
anticancer drug, following cisplatin and carboplatin. Oxaliplatin
induces autophagy and promotes the apoptosis of colon cancer cells
(21–23). Oxaliplatin inhibits colorectal
cancer growth and metastasis through inhibition of the nuclear
factor (NF)-κB pathway (24,25). Oxaliplatin was used as control in
the present study, in which the cytotoxic and antiproliferative
effects of cbFeD on human colon cancer HCT116 and SW480 cell
lines were examined. The present study also investigated the NF-κB
signaling pathway modulating apoptosis and autophagy in HCT116 and
SW480 cell in response to cbFeD treatment, and examined the
consequences of inhibiting the NF-κB signaling pathway during
cbFeD treatment using in vivo colon cancer models to
obtain therapeutic insights.
Materials and methods
Cell culture
The HCT116 and SW480 cell lines were obtained from
American Type Culture Collection (Manassas, VA, USA). The HCT116
and SW480 colon cancer cells were cultured in Dulbecco’s modified
Eagle’s medium (DMEM) supplemented with 10% FBS (cat. no. 10099158;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100
μg/ml of streptomycin and 100 U/ml of penicillin in a
humidified atmosphere of 5% CO2 at 37°C.
Preparation of drug-containing serum
The dry cbFeD was purchased from Yunnan
International Pty, Ltd. (Yunnan, China). Firstly, 1 kg of dry
cbFeD was soaked in cold water for 30 min, decocting twice
with 1:6 w/v distilled water for 1 h. Filtration was then performed
to the appropriate concentrations, the first decoction comprised in
1:10 w/v distilled water for 90 min and second comprised 1:8 w/v
distilled water for 60 min. A final quantity of 450 g dried powder
was obtained by spray drying at room temperature, which was then
sealed and stored in the dark at 4°C. The cbFeD powder was
dissolved in normal saline for the gavage experiments. The
drug-containing serum solutions were collected from mice following
exposure to the following treatments (once per day for 1 week):
Treatment with normal saline by gastrogavage (n=8; normal control
group); 5 g/kg of cbFeD by gastrogavage (n=8; low
cbFeD group); 10 g/kg of cbFeD by gastrogavage (n=8;
mid cbFeD group), 20 g/kg of cbFeD by gastrogavage
(n=8; high cbFeD group); 5 mg/kg oxaliplatin by gastrogavage
(n=8; oxaliplatin group). The blood samples were obtained from the
abdominal aorta following treatment, following which the serum was
isolated by centrifuging at 1,800 × g for 10 min at 4°C and stored
at −80°C for the follow-up experiments.
Experimental groups
The drug-containing serum solutions from the mice
were used to treat the HCT116 and SW480 cells. The five treatment
groups comprised the normal control group, the low cbFeD
group, the mid cbFeD group, the high cbFeD group, and
the oxaliplatin group. For detecting the role of p65, the inhibitor
pyrrolidine dithiocarbamate (PDTC) was added to a proportion of the
high cbFeD group cells.
Cell counting kit-8 (CCK-8) assay
Cell proliferation was determined using the
colorimetric water-soluble tetrazolium salt assay using a CCK-8 kit
(Beyotime Institute of Biotechnology, Co., Ltd., Haimen, China). In
brief, the cells at a density of 2×103 cells per well
was seeded in 96-well plates and incubated with the low, mid, or
high dose of cbFeD, with or without oxaliplatin, for 24, 48
and 72 h. Following treatment, the culture medium was removed and
replaced with 100 μl of fresh medium containing 10 μl
of CCK-8 solution in each well and the cells were incubated at 37°C
for 2 h. The number of viable cells was determined by reading the
absorbance at 450 nm using a Thermo Platemicroplate reader (Rayto
Life and Analytical Science Co., Ltd., Shenzhen, China).
Cell cycle assay
Following treatment, the cells were harvested and
resuspended at a density of 1×106 cells/ml. The cells
were fixed with ice-cold 70% ethanol for at least 30 min. Cell
cycle was analyzed using a flow cytometer with propidium iodide
(PI) as a specific fluorescent dye probe. The PI fluorescence
intensity of 10,000 cells was measured for each sample using a
Becton-Dickinson FACSCalibur flow cytometer (BD Biosciences,
Franklin Lakes, NJ, USA).
Cell apoptosis assay
Following treatment, cell apoptosis was assessed
using an Annexin V-FITC apoptosis detection kit (BD Pharmingen, San
Diego, CA, USA). In brief, Annexin V-FITC (5 μl) and PI (5
μl) were added to 100 μl cells at a concentration of
1×106 cells/ml, and incubated in the dark for 15 min at
room temperature. Subsequently, binding buffer was added and
apoptosis was analyzed using flow cytometry (BD Biosciences).
Western blot analysis
The cells were harvested using protein extraction
solution (Intron Biotechnology, Sungnam, Korea), and incubated for
30 min at 4°C. Following removal of cell debris, the supernatants
were collected by centrifuging at 13,000 × g for 15 min at 4°C and
the protein concentrations were determined using a Bio-Rad protein
assay reagent, according to the manufacturer’s protocol.
Subsequently, 30 μg proteins were separated by 10% SDS-PAGE,
and then transferred onto PVDF membranes. The blots were incubated
with 4% bovine serum albumin (BSA; 1:200; cat. no. C0258; Beyotime
Institute of Biotechnology, Co., Ltd.) blocking solution and
primary antibodies against p65 (1:500; cat. no. ab16502), IκBα
(1:400; cat. no. ab5076), LC3B-I/II (1:1,000; cat. no ab48394),
Beclin-1 (1:500; cat. no. ab62557), GAPDH (1:1,000; cat. no.
ab8245) (all from Abcam, Cambridge, MA, USA) and p-IκBα (1:400;
cat. no. YK7674; Shanghai Yuanmu Biotechnology Co., Ltd., Shanghai,
China) overnight at 4°C. Following being washed three times with
TBST, the blots were incubated with horseradish
peroxidase-conjugated secondary antibody (1:1,000; cat. no,
AB501-01A; Novoprotein Scientific, Summit, NJ, USA) for 1 h at room
temperature. Following being washed three times with TBST, the
blots were determined using an enhanced chemiluminescence kit
(Amersham; GE Healthcare Life Sciences, Chalfont, UK).
Confocal microscopy
Immunofluorescence staining for p65 and LC3B-II was
performed to precisely evaluate their expression in the cells.
Following treatment, the cells were washed with ice-cold PBS and
fixed in ice-cold acetone for 15 min. The cells were then blocked
in 10% normal goat serum (cat. no. C0265; Beyotime Institute of
Biotechnology, Co., Ltd.) at 37°C for 1 h, following which they
were washed by PBS and incubated at 37°C with anti-p65 (1:200; cat.
no. 710048) or anti-LC3B-II antibody (1:200; cat. no. 700712) (both
Thermo Fisher Scientific, Inc.) for 1 h. Following three washes,
the cells were incubated with FITC-conjugated goat anti-rabbit
secondary antibodies (1:200; cat. no. A0562; Beyotime Institute of
Biotechnology, Co., Ltd.) for 1 h at 37°C. The slides were mounted
with 4′,6-diamidino-2-phenylindole (DAPI). Confocal images were
captured (26) using a confocal
microscope (Olympus Corp., Tokyo, Japan) at the excitation and
emission wavelengths of 495 and 517 nm for FITC, 649 and 680 nm for
Cy5, and 358 and 463 nm for DAPI nuclear staining,
respectively.
Detection of autophagic vacuoles
The basic evidence for the induction of autophagy in
cells is the formation of acidic vesicular organelles (AVOs)
(27). Acridine orange was used
to stain AVOs in autophagic cells. Following treatment, the cells
were washed in PBS and incubated with AO (1 μg/ml) for 15
min at 25°C (28). The cells were
again washed with PBS, and AVO formation in the HCT116 and SW480
cells was observed using flow cytometry.
The presence of autophagic vesicles in HCT116 and
SW480 cells was also detected using transmission electron
microscopy (28). The cells were
fixed in 2.5% glutaraldehyde and 2% paraformaldehyde in 0.1 M
phosphate buffer (pH 7.4) for 1 h at 4°C. Following rinsing in PBS,
the cells were post-fixed in osmium tetroxide (1%) for 2 h,
dehydrated in graded acetone and embedded in araldite CY212. Semi
thin sections, 1 cm2 and 1-μm-thick were cut and
stained with 0.5% toluidine blue for 5 min. Ultrathin sections
(50–70-nm-thick) were stained with 2% uranyl acetate and Reynold’s
lead citrate, and observed with a transmission electron
microscope.
Xenograft tumors
A total of 18 female Balb/c athymic nude mice (5–6
weeks old, body weight 19–22 g) (Vital River Laboratories, Beijing,
China) were housed at 25°C in 40–70% humidity, in a 12 h light/dark
cycle with free access to food and water and were subcutaneously
injected in the right flank with 2.0×106 SW480 cells in
0.1 ml PBS. When tumors had formed, the tumor volume (V) was
measured using calipers daily and calculated using the following
formula: V=(L×W2)/2, where L was the length and W was
the width of the tumor. The mice were randomly divided into three
groups (n=5): Normal control group mice treated with normal saline
via gavage; high dose of cbFeD (20 g/kg) mice treated with a high
dose of cbFeD via gavage; oxaliplatin group mice with colon cancer
treated with oxaliplatin (5 mg/kg via gavage). Growth curves were
plotted using the average tumor volume within each experimental
group every week. After six weeks, the mice were sacrificed, and
the dissected tumors were collected and prepared for subsequent
analyses. All animal experiments were approved by the Animal Center
of Southwest Forestry University (Kunming, China). All experimental
procedures involving animals were performed according to the
institutional ethical guidelines for animal experiments and in
accordance with the Guide for the Care and Use of Laboratory
Animals.
IHC staining
The mice samples were fixed in 4% paraformaldehyde
and endogenous enzymes were inactivated by 3% hydrogen peroxide for
10 min. Antigen retrieval was prepared by immersion in citrate
buffer solution and heated at a high heat in a microwave oven for 4
min, followed by cooling and washing with PBS for 5 min. The
samples were blocked in 5% BSA for 20 min at room temperature and
were incubated with 50 μl primary anti body against LC3,
Beclin-1 and p65 overnight at 4°C, followed by incubation with
Alexa Fluor 549 secondary antibodies (1:400; cat. no. 331594;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 1 h at 4°C.
Following nuclear staining, the slides were exposed to a 70, 80,
90, 100% alcohol gradient of (5 min each), and mounted with neutral
gum on dry slides. Images were captured under an optical
microscope.
Statistical analysis
The results are presented as the mean ± standard
deviation. The statistical analysis was performed using GraphPad
Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA). For
comparisons Dunnett t-test or two-way analysis of variance was
used. P<0.05 was considered to indicate a statistically
significant difference.
Results
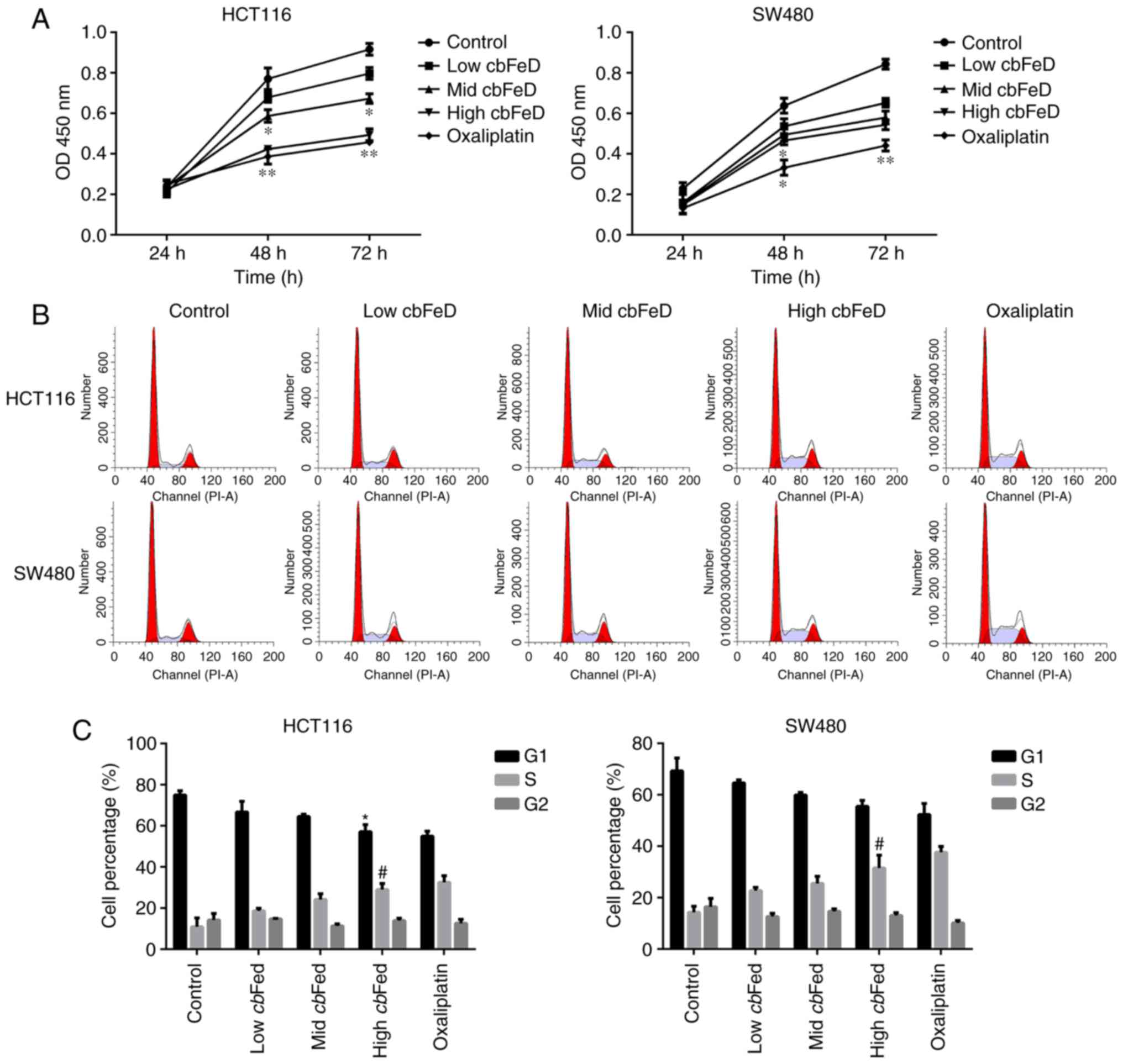
Effects of cbFeD on the proliferation and
cell cycle of HCT116 and SW480 cells
The cbFeD-containing serum solutions were
prepared from mice by gastrogavage with saline (control), or 5
(low), 10 (mid) and 20 (high) g/kg of cbFeD. To determine
the role of cbFeD on cell proliferation, cell cycle and cell
apoptosis, the HCT116 and SW480 cells were treated with these
cbFeD-containing serum solutions. The results showed that
cbFeD inhibited the proliferation of HCT116 and SW480 cells
at 48 and 72 h. The cell proliferation rate was decreased with
increasing concentrations of cbFeD-containing serum
solutions, suggesting that cbFeD inhibited the cell
proliferation in a dose-dependent manner (Fig. 1A). The cell proliferation in the
high cbFeD group was similar to that in the oxaliplatin
group. Similarly, cbFeD decreased the proportion of cells in
the G1 phase cells, but increased the proportion of cells in the S
phase, suggesting that cbFeD induced S phase arrest
(Fig. 1B and C), which was
similar to the effect of oxaliplatin. Therefore, cbFeD
inhibited cell proliferation in a dose-dependent manner and induced
cell cycle arrest at the S phase.
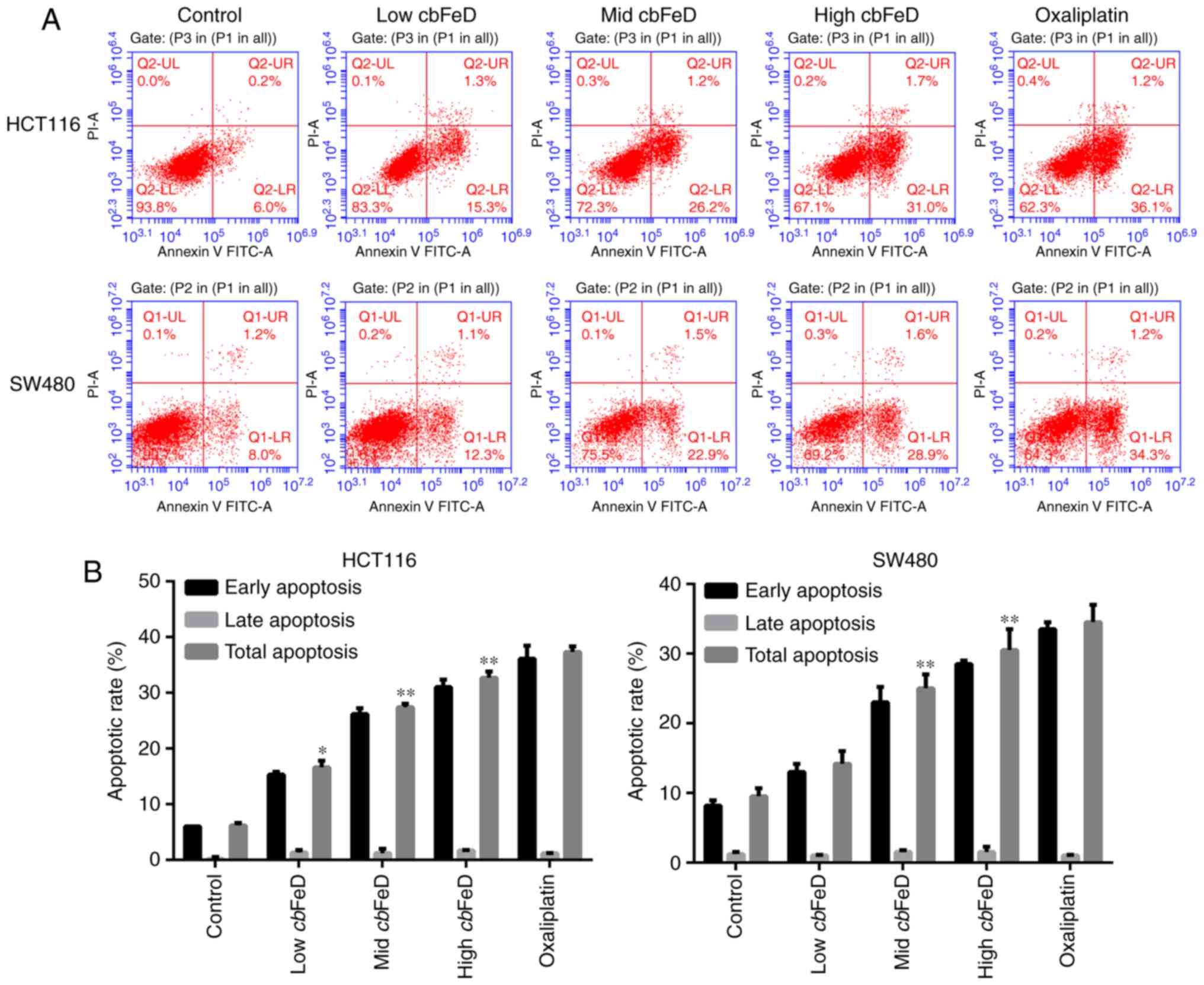
Effects of cbFeD on the apoptosis of
HCT116 and SW480 cells
The apoptotic rates of HCT116 and SW480 cells
following cbFeD treatment were also analyzed (Fig. 2A and B). The apoptotic rates of
the HCT116 cells treated with low, mid, and high doses of
cbFeD were 16.6, 27.4 and 32.7%, respectively, whereas the
apoptotic rate of the HCT116 cells in the control group was only
6.2%. cbFeD treatment produced the same effects on the SW480
cells. The results suggested that the apoptotic rate induced by
cbFeD was increased with dose. Treatment of the cells with
oxaliplatin confirmed the apoptosis of the HCT116 and SW480
cells.
Expression of p65, IκBα, p-IκBα, LC3B-I,
LC3B-II and Beclin-1 in HCT116 and SW480 cells
To determine the effects of cbFeD on the
NF-κB signaling pathway and autophagy, the expression levels of
p65, IκBα, p-IκBα, LC3B-I, LC3B-II and Beclin-1 in HCT116 and SW480
cells were detected using western blot analysis (Fig. 3). In the HCT116 and SW480 cells,
cbFeD inhibited the expression of IκBα, but enhanced the
expression of p-IκBα in a dose-dependent manner. In addition, the
expression of autophagic markers, including the ratio of LC3B-II
and LC3B-I, and Beclin-1 were significantly decreased, suggesting
cbFeD inhibited autophagy. The expression of p65 in the
plasma was decreased, however, nuclear expression was increased,
suggesting that cbFeD promoted the translocation of p65 into
cell nuclei. Therefore, cbFeD may inhibit autophagy, but
activate the NF-κB signaling pathway.
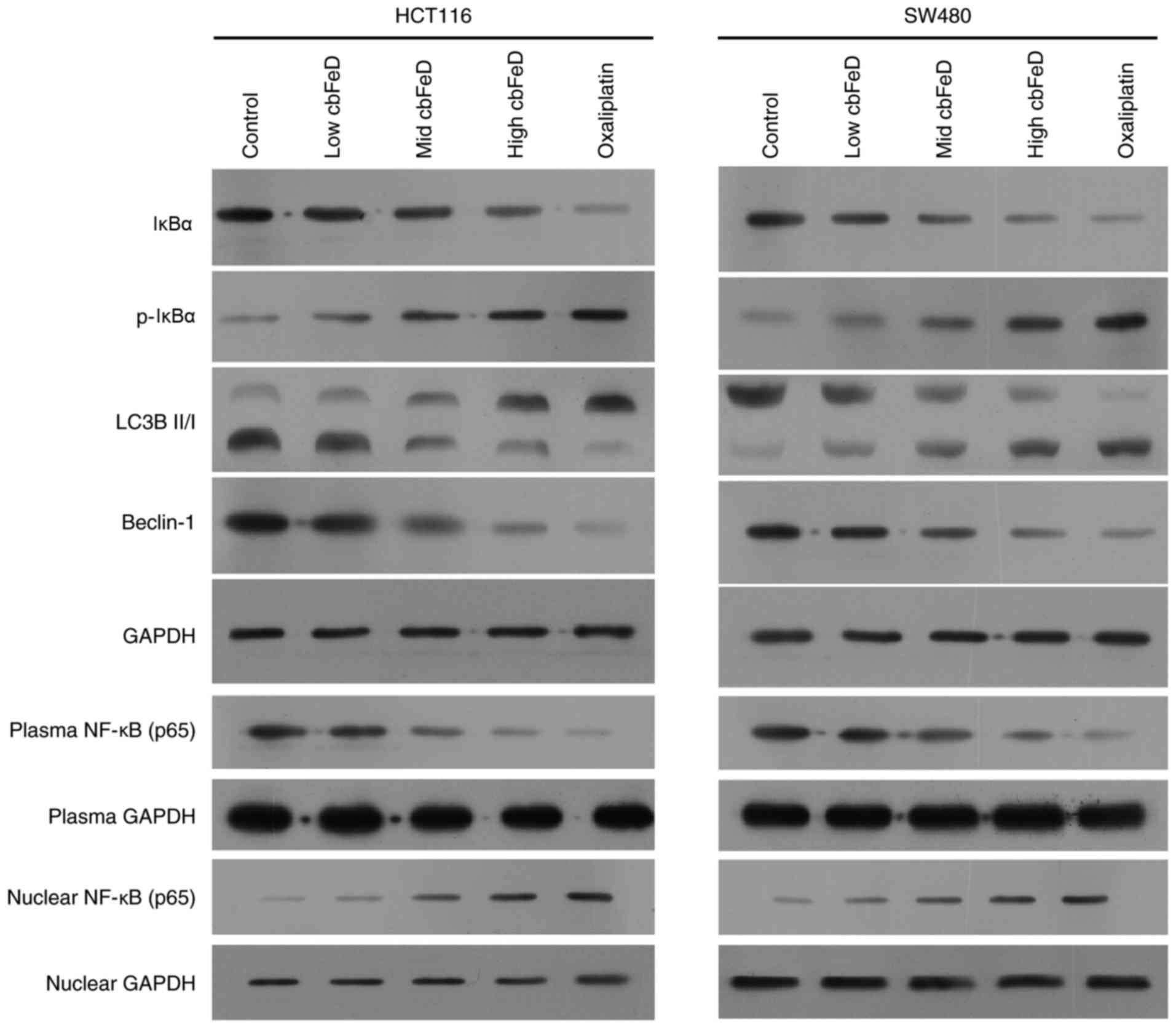 | Figure 3Effects of cbFeD on the
expression of p65, IκBα, p-IκBα, LC3B-I, LC3B-II and Beclin-1 in
HCT116 and SW480 cells. Cells were treated with
cbFeD-containing serum solutions prepared from mice treated
by gastrogavage with saline (control), or 5 (low), 10 (mid) or 20
(high) g/kg of cbFeD, and then the protein was extracted
from cell lysate, cell plasma and cell nuclei. Expression levels of
IκBα, p-IκBα, LC3B-I, LC3B-II and Beclin-1 in cell lysates of (A)
HCT116 and (B) SW480 cells were detected, and expression levels of
p65 in cell plasma and cell nuclei were detected using western blot
analysis. Oxaliplatin was used as a control. cbFeD,
Codonopis bulleynana Forest ex Diels; NF-κB, nuclear
factor-κB; IκB, inhibitor of nuclear factor-κB; p-, phosphorylated;
LC3, microtubule-associated protein 1 light chain 3. |
Effects of cbFeD on the distribution of
p65 in HCT116 and SW480 cells
To further confirm the activation of the NF-κB
signaling pathway by cbFeD, immunofluorescence staining of
p65 was performed. As shown in Fig.
4, p65 was present at the highest level in the cell plasma of
the HCT116 and SW480 cells in the normal group. However, following
treatment with a low dose of cbFeD, the p65 gradually
entered the nuclei in the HCT116 and SW480 cells. Similar changes
were observed in the mid and high dose cbFeD groups, with a
higher dose resulting in a lower concentration of p65 in the cell
plasma. This activation of the NF-κB signaling pathway was also
observed following oxaliplatin treatment. Therefore, cbFeD
activated the NF-κB signaling pathway in a dose-dependent
manner.
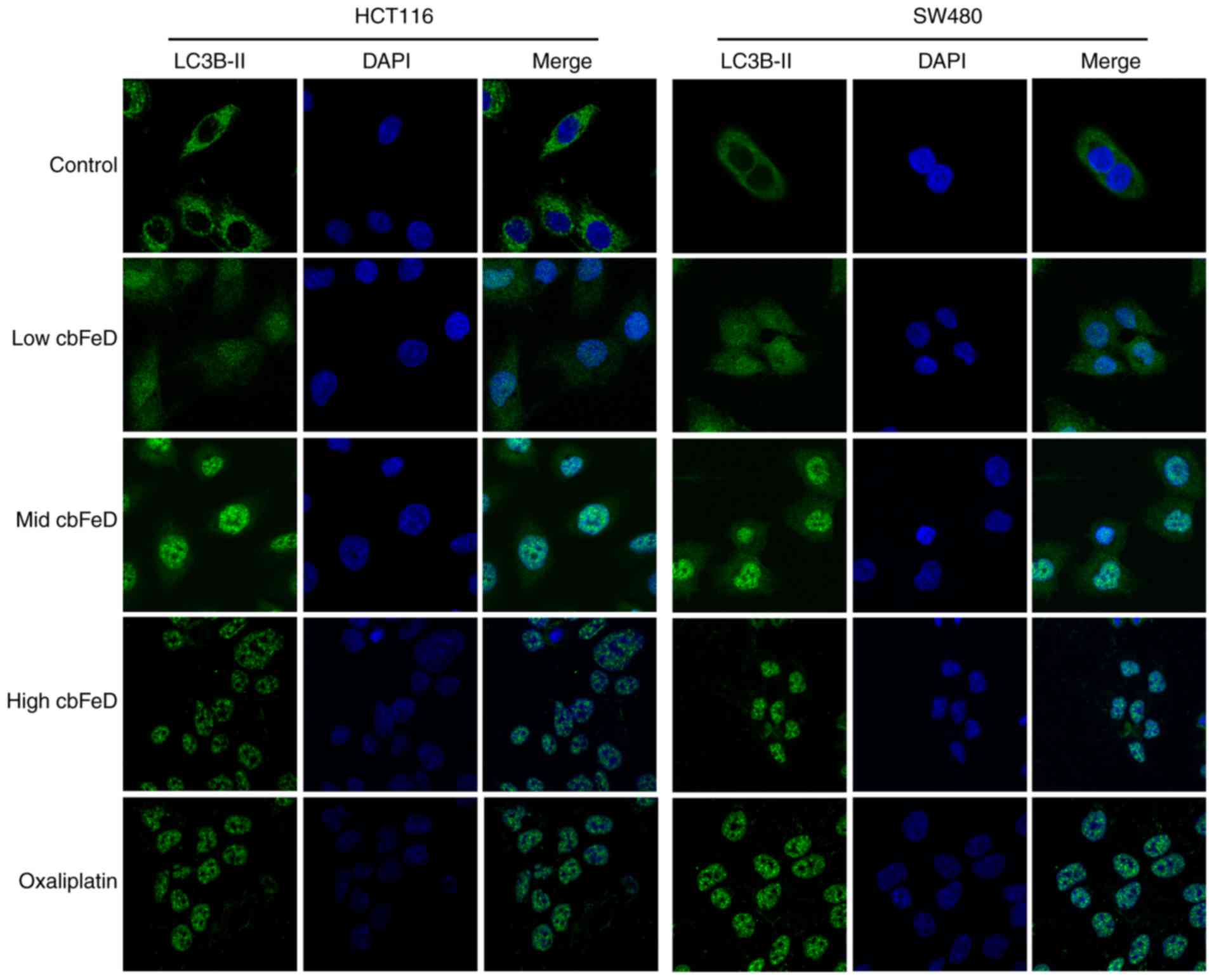
Effects of cbFeD on the distribution of
LC3B-II in HCT116 and SW480 cells
To examine the inhibition of autophagy by
cbFeD, immunofluorescence staining of LC3B-II was performed.
As shown in Fig. 5, in the normal
group, high expression levels of LC3B-II were found in the HCT116
and SW480 cells. However, the expression of LC3B-II was gradually
reduced with increased doses of cbFeD. The inhibition of
autophagy by cbFeD was also observed in the oxaliplatin
group. Therefore, cbFeD inhibited the autophagy of HCT116
and SW480 cells in a dose-dependent manner.
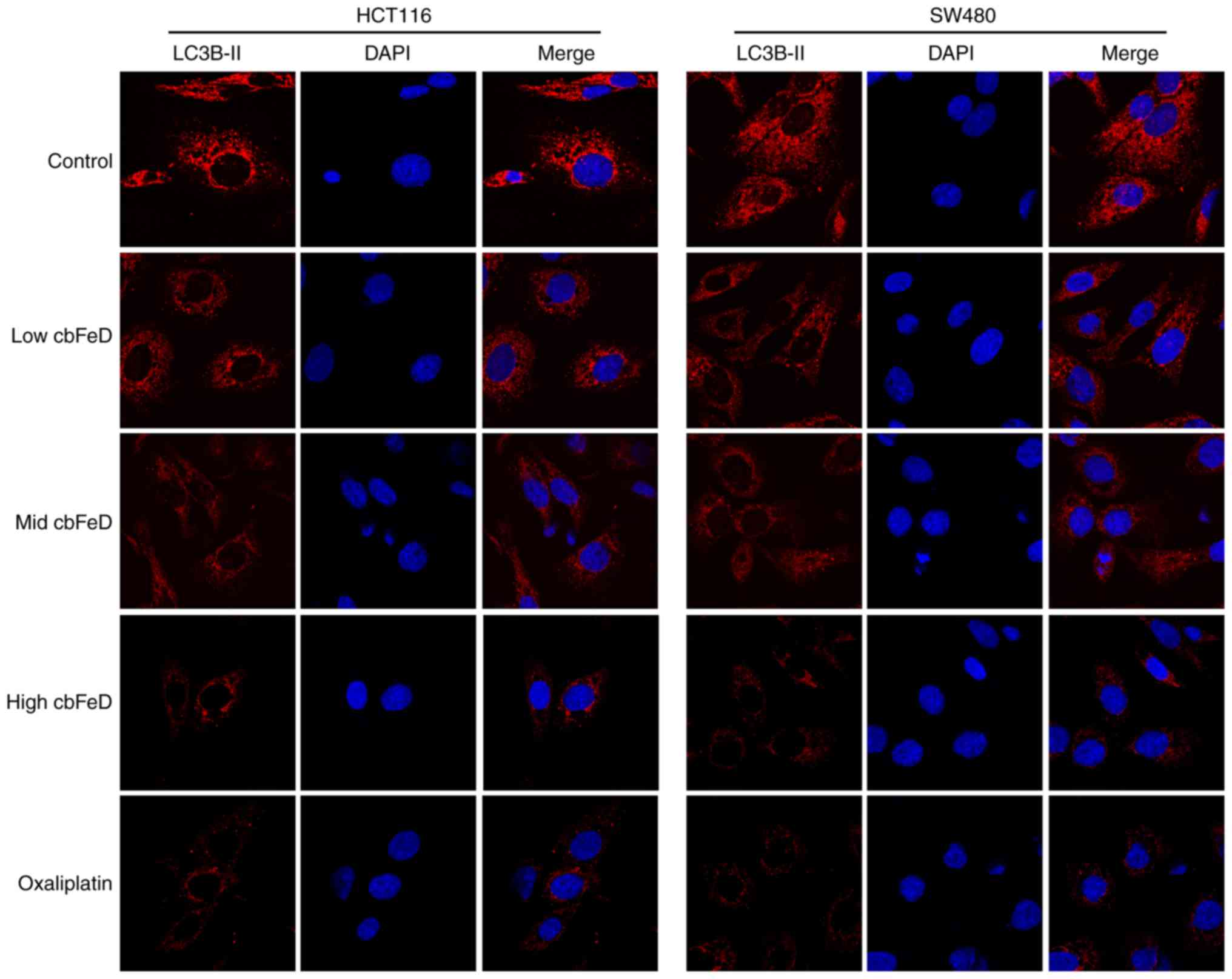 | Figure 5Immunofluorescence images of LC3B-II
in HCT116 and SW480 cells. Cells were treated with
cbFeD-containing serum solutions prepared from mice treated
by gastrogavage with saline (control), or 5 (low), 10 (mid) or 20
(high) g/kg of cbFeD, followed by immunofluorescence
staining of LC3B-II (magnification, ×200). Red, LC3B-II; blue,
DAPI; Merge, LC3B-II+DAPI. cbFeD, Codonopis
bulleynana Forest ex Diels; LC3, LC3, microtubule-associated
protein 1 light chain 3. |
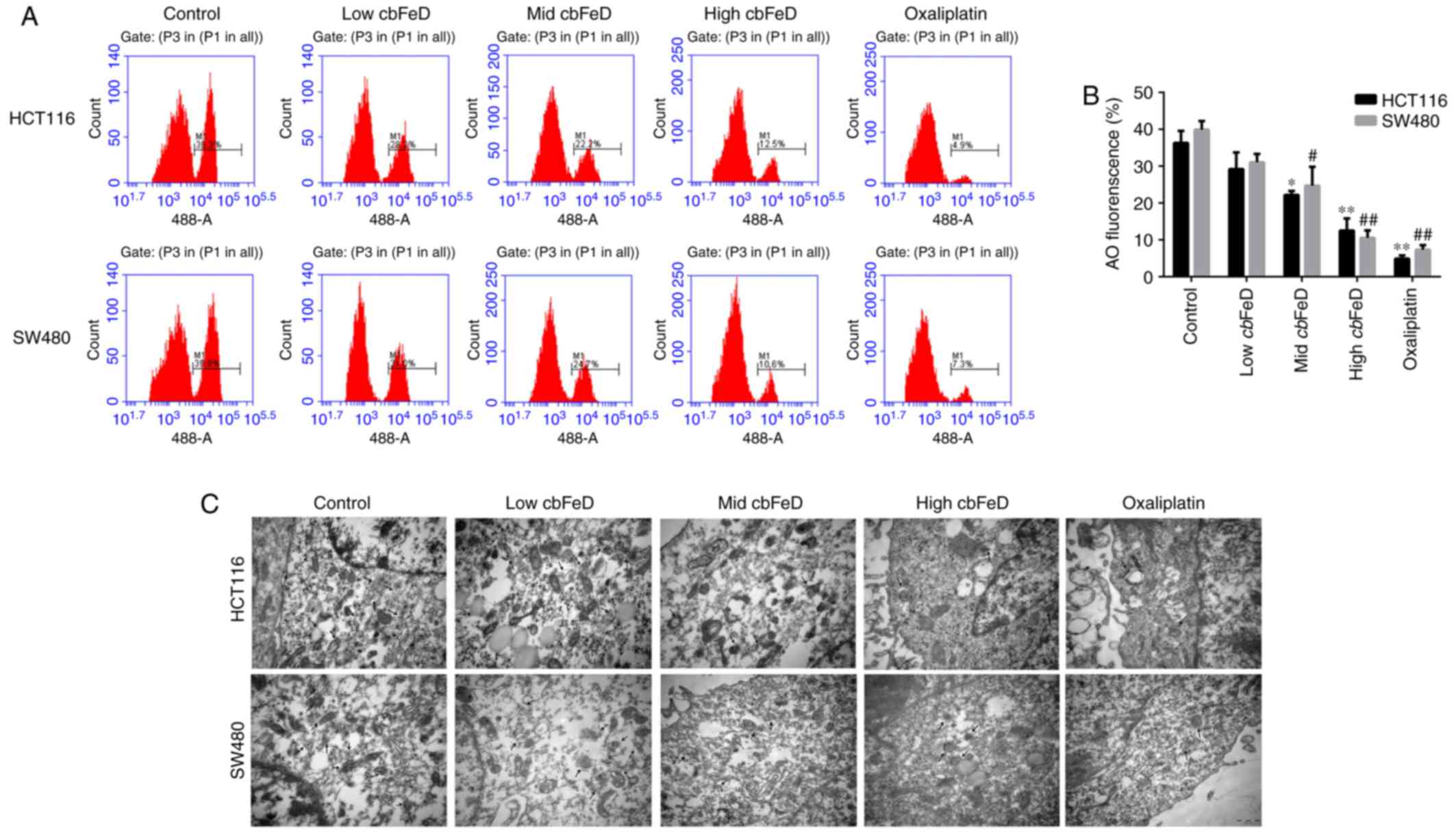
Effects of cbFeD on autophagic cells
The role of cbFeD in autophagy was further
confirmed by AO staining and electron microscopy. As shown in
Fig. 6A and B, the fluorescence
intensity of AO gradually decreased with cbFeD treatment in
a dose-dependent manner. Consistent with the AO staining, the
results of the electron microscopy showed fewer autophagic vesicles
in the cbFeD groups, compared with the number the control
groups in the HCT116 and SW480 cells (Fig. 6C). Fewer autophagic vesicles were
observed in cells at a higher cbFeD. The results of the AO
staining and electron microscopy were consistent in the cells
treated with oxaliplatin. Therefore, cbFeD inhibited
autophagy in a dose-dependent manner.
PDTC inhibits the effect of cbFeD in
HCT116 and SW480 cells
To investigate the effect of activation of the NF-κB
signaling pathway by cbFeD in HCT116 and SW480 cells and its
association with autophagy, PDTC was used as an inhibitor of p65 to
treat the cells pretreated with cbFeD (Fig. 7). Following treatment with PDTC,
the expression of IκBα was increased and the expression of p-IκBα
was reduced (Fig. 7A). The
expression of p65 in cell plasma was increased but the expression
in cell nuclei was decreased, suggesting that PDTC inhibited
activation of the NF-κB signaling pathway. Following inactivation
of the NF-κB signaling pathway, the ratio of LC3B-II and LC3B-I,
and the expression of Beclin-1 were increased. The AO staining also
showed that PDTC enhanced the production of autophagic vacuole in
the HCT116 and SW480 cells (Fig.
7B). The inactivation of the NF-κB signaling pathway and
production of autophagic vacuoles were also observed in the
oxaliplatin treatment group. Therefore, cbFeD inhibited
autophagy via activation of the NF-κB signaling pathway.
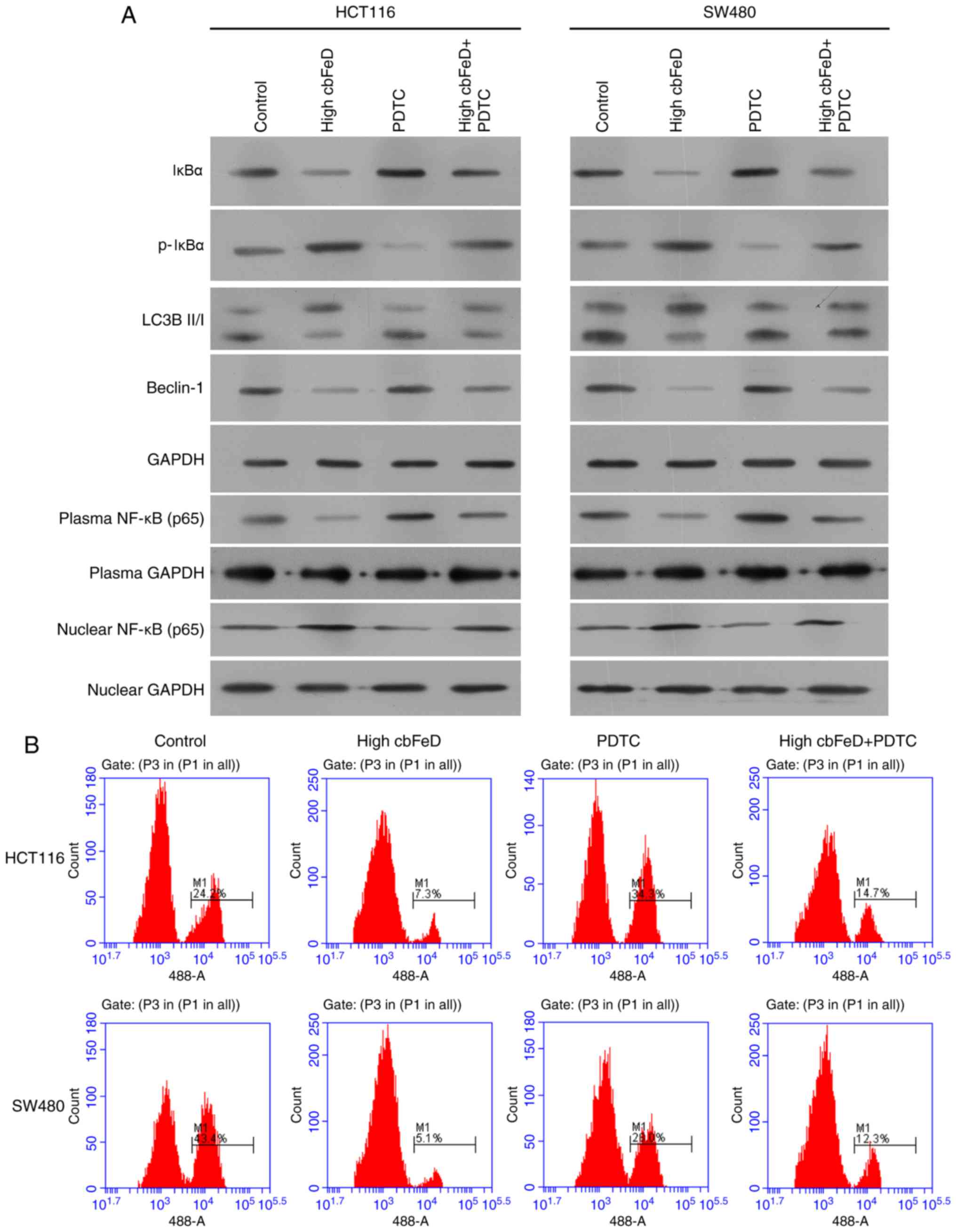 | Figure 7Effects of PDTC and a high dose of
cbFeD on expression levels of p65, IκBα, p-IκBα, LC3B-I,
LC3B-II and Beclin-1, and on autophagic vacuoles in HCT116 and
SW480 cells. Cells were treated with the p65 inhibitor PDTC, with
or without, cbFeD-containing serum solutions prepared from
mice treated by gastrogavage with saline (control), or 20 (high)
g/kg of cbFeD. (A) Expression levels of p65, IκBα, p-IκBα,
LC3B-I, LC3B-II and Beclin-1 were examined using western blot
analysis and (B) detection of autophagic vacuoles was performed
using AO staining. cbFeD, Codonopis bulleynana Forest
ex Diels; PDTC, pyrrolidine dithiocarbamate; NF-κB, nuclear
factor-κB; IκB, inhibitor of NF-κB; p-, phosphorylated; LC3,
microtubule-associated protein 1 light chain 3; AO, acridine
orange. |
cbFeD suppresses tumorigenicity in
vivo
To confirm the above findings, particularly the
results of the CCK-8 assay (Fig.
1A), and due to the fact that SW480 cells have been used in the
establishment of xenograft tumors in previous studies (29,30), SW480 cell were used to establish a
nude-mouse transplanted tumor model in the present study. A high
dose of cbFeD or oxaliplatin were administered to nude mice
by gastrogavage and, 6 weeks following intragastric administration,
these two groups exhibited significantly smaller tumors, compared
with those in the normal saline group (Fig. 8A and B). The H&E staining
showed that the cbFeD induced a higher level of inflammatory
cell infiltration (Fig. 8C). The
IHC staining of LC3B and Beclin-1 showed that the numbers of LC3B-
and Beclin-1-positive cells were decreased, suggesting cbFeD
inhibited autophagy (Fig. 8D).
The results of the IHC staining of p65 showed that the number of
p65-positive cells was increased, suggesting that cbFeD
induced the activation of the NF-κB signaling pathway. The
oxaliplatin control produced the same results as the tumor model.
There results confirmed that cbFeD inhibited autophagy via
activation of the NF-κB signaling pathway.
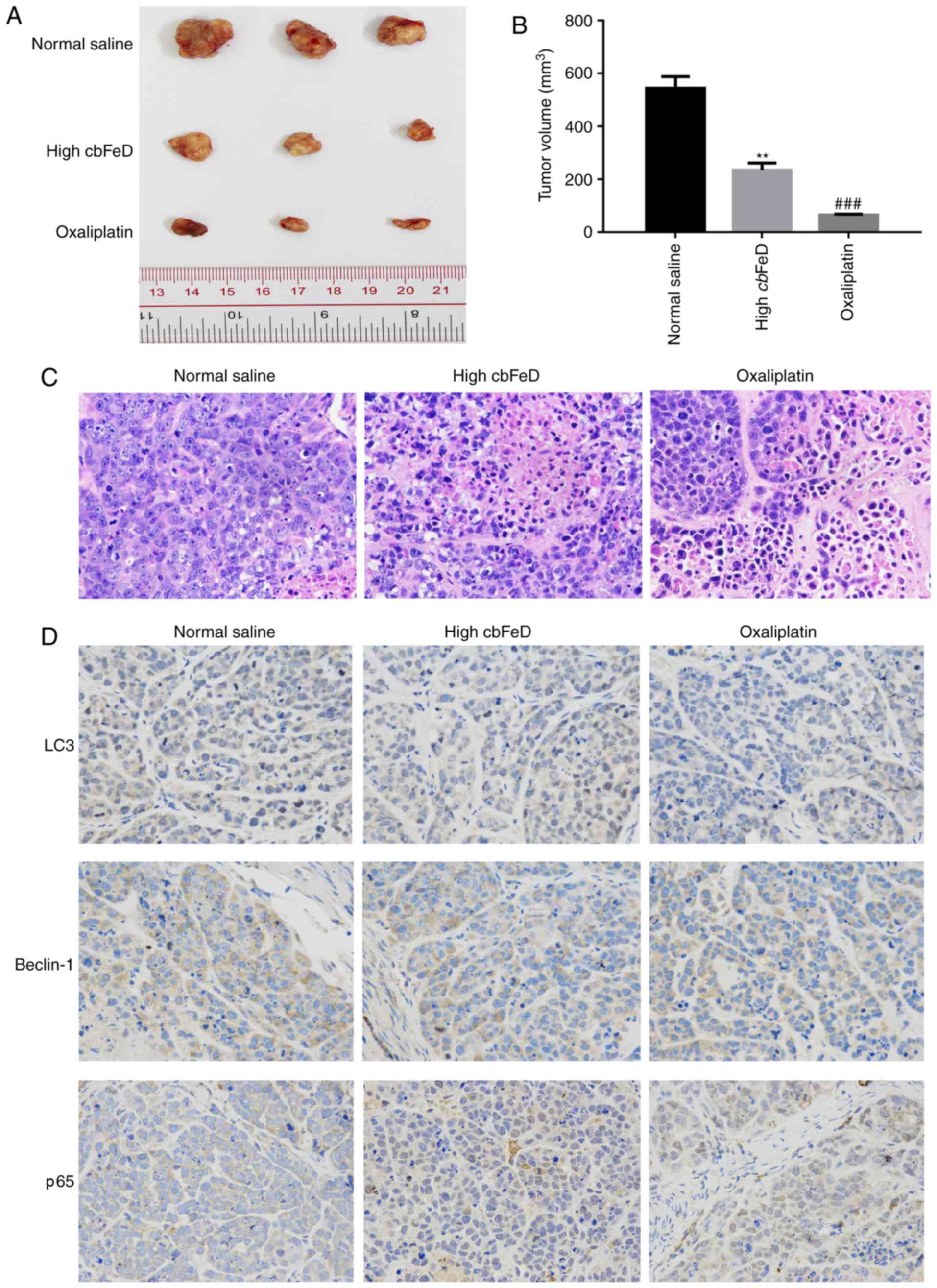 | Figure 8Effects of cbFeD and
oxaliplatin on growth of xenograft tumors. Nude mice were
subcutaneously injected in the right flank with 2.0×106
SW480 cells in 0.1 ml PBS. Once tumors had formed, tumor volume was
measured. The mice were then randomly divided into three groups
(n=5): Normal control group, mice treated with normal saline via
gavage; high cbFeD group, mice treated with a high dose (20
g/kg) of cbFeD via gavage; oxaliplatin group, mice with
colon cancer treated with oxaliplatin (5 mg/kg) via gavage. After 6
weeks, the dissected tumors were collected and H&E and IHC
staining were performed. (A) Tumor size. (B) tumor volume. (C)
H&E staining of xenograft tumors. Magnification, ×400. (D) IHC
staining of p65, LC3B-II and Beclin-1. Magnification, ×400.
**P<0.01 and ###P<0.001 vs. control.
cbFeD, Codonopis bulleynana Forest ex Diels; LC3,
microtubule-associated protein 1 light chain 3; IHC,
immunohistochemistry. |
Discussion
In the present study, the antitumor effects and
mechanism of cbFeD on human colon cancer cells were
investigated in vitro and in vivo using HCT116 and
SW480 colon cancer cells. The effect of oxaliplatin was also
examined in colon cancer cells, which was used as a positive
control.
It has been demonstrated that autophagy is involved
in resistance to chemotherapy and radiotherapy (14,15). Through autophagic cells, damaged
proteins or organelles are removed, which may paradoxically promote
the survival of irradiated cells (18). The characteristics of the fresh
root of cbFeD render it a unique national medicinal herb,
which is used in a range of investigations in China. cbFeD
has wide range of pharmacological effects, including anticancer
effects. The anticancer role of cbFeD was confirmed in the
present study. cbFeD inhibited cell proliferation, increased
the proportion of cells in the S phase, and promoted the apoptosis
of HCT116 and SW480 cells. The inhibition of cell proliferation,
and promotion of cell cycle arrest and cell apoptosis in the HCT116
and SW480 cells by cbFeD were found to occur in a
dose-dependent manner. The inhibition of cbFeD in autophagy
was also confirmed by various methods. Western blot analysis of the
ratio of LC3B-II and LC3B-I, and the expression of Beclin-1 showed
that the ratio of LC3B-II and LC3B-I and the expression of Beclin-1
were significantly decreased. The results of the AO staining showed
that the numbers of autophagic cells in the HCT116 and SW480 cells
were gradually reduced with increased cbFeD dose. Fewer
autophagic vesicles were observed in the cells exposed to a higher
dose of cbFeD, determined using electron microscopy. The
inhibition of autophagy by cbFeD may contribute to the
inhibition of cell proliferation and promotion of cell death. The
results suggested that cbFeD is promising in sensitizing
colon cancer cells to chemotherapy or radiotherapy by inducing
autophagy.
It has also been suggested that cbFeD has a
certain positive effect on chemotherapy by reducing toxicity and
enhancing immune function. cbFeD can significantly improve
the increase of hemoglobin in elderly individuals with a relatively
high degree of safety (10).
Previous pharmacodynamic and acute toxicity investigations of
cbFeD have shown that it can enhance gastrointestinal
peristalsis, improve tolerance to fatigue and hypoxia, and can
promote the recovery of Hb, RBCs, IgG, and the immunosuppressive
effect in hemorrhagic blood-deficient mice (11,12). cbFeD can enhance the immune
function of mice with xenograft tumors, and enhance the phagocytic
functions of the reticuloendothelial system (13). The NF-κB signaling pathway serves
as an important regulator of immune function through the regulation
of cell death and autophagy (31).
In the present study, it was found that cbFeD
inhibited the expression of IκBα but enhanced the expression of
p-IκBα. The level of p65 in plasma was decreased, however, the
level in the nucleus was increased. Following treatment with a low
dose of cbFeD, p65 entered the nuclei of HCT116 and SW480
cells. These results suggested that cbFeD activated the
NF-κB signaling pathway. PDTC inhibits the p65-dependent activation
of NF-κB (32). Following
treatment of cells in the present study with PDTC, inactivation of
the NF-κB signaling pathway was observed. The ratio of LC3B-II and
LC3B-I, and the expression of Beclin-1 increased, the production of
autophagic vacuoles in the HCT116 and SW480 cells was also
increased. The role of cbFeD in colon cancer was further
examined in vivo in the present study. It was found that
cbFeD suppressed tumorigenicity in vivo. By treating
cancer cells with cbFeD, cell apoptosis was increased,
autophagy was inhibited and the NF-κB signaling pathway was
activated. Taken together, the results of the present study showed
that cbFeD inhibited autophagy via activation of the NF-κB
signaling pathway in colon cancer cells. Therefore, cbFeD
may be a promising Chinese herbal compound for development for use
in cancer therapy.
Acknowledgments
The study was supported by the National Natural
Science Foundation of China (grant no. 61363061).
References
|
1
|
Arber N and Levin B: Chemoprevention of
colorectal neoplasia: The potential for personalized medicine.
Gastroenterology. 134:1224–1237. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang J, Du XL, Li ST, Wang BY, Wu YY, Chen
ZL, Lv M, Shen YW, Wang X, Dong DF, et al: Characteristics of
differently located colorectal cancers support proximal and distal
classification: A population-based study of 57,847 patients. PLoS
One. 11:e01675402016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Katz ML, Young GS, Zimmermann BJ, Tatum CM
and Paskett ED: Assessing colorectal cancer screening barriers by
two methods. J Cancer Educ. Dec 8–2016.Epub ahead of print.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alajez NM: Large-scale analysis of gene
expression data reveals a novel gene expression signature
associated with colorectal cancer distant recurrence. PLoS One.
11:e01674552016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rocha R, Marinho R, Aparicio D, Fragoso M,
Sousa M, Gomes A, Leichsenring C, Carneiro C, Geraldes V and Nunes
V: Impact of bowel resection margins in node negative colon cancer.
Springerplus. 5:19592016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Panteleimonitis S, Ahmed J, Harper M and
Parvaiz A: Critical analysis of the literature investigating
urogenital function preservation following robotic rectal cancer
surgery. World J Gastrointest Surg. 8:744–754. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sommerer C and Zeier M: Clinical
manifestation and management of ADPKD in Western countries. Kidney
Dis (Basel). 2:120–127. 2016. View Article : Google Scholar
|
|
8
|
Liu D and Liang XC: New developments in
the pharmacodynamics and pharmacokinetics of combination of Chinese
medicine and Western medicine. Chin J Integr Med. 23:312–319. 2017.
View Article : Google Scholar
|
|
9
|
Pinhua L, Yarong J, Mingyan L, Shirui L
and Yaguan Z: Total flavonoids and antioxidant activity of aerial
parts of Codonopsis bulleyana. Southwest China J Agricultural Sci.
27:1894–1898. 2014.
|
|
10
|
Chen Z, Li Y, Lu L, Lu B, Zhou J and Hu Y:
Pharmacodynamic study on Qi-Blood-Enriching effects of Codonopis
bulleynana Fores tex Diels. Shanghai Zhong Yi Xue Yuan. 43:68–71.
2009.
|
|
11
|
Chen Z, Li Y, Zhou J, Wang Y, Lu B, Lu Z
and Yuan J: Chou can bu tong bu wei ti qu wu dui qi xu yu xue xu
dong wu mo xing de ying xiang. J Chin Med Materials. 11:1731–1733.
2009.In Chinese.
|
|
12
|
Dong LD, Qian ZG, Yang Z and Cheng YX:
Study on the anti-hyperlipidemia, anti-fatigue and anti-anoxia
effects of Codonopsis foetens Hook. Yunnan Chin Med J. 36:66–68.
2015.In Chinese.
|
|
13
|
Chen ZJ, Li YS, Wei QH, Chen SL and Chen
DX: Effects of Codonopis bulleynana Forest ex Diels on enhancing
sensitivity, reducing toxicity of chemotherapy and regulating
immune function in Sarcoma 180 tumor-bearing mice. Chin Traditional
Patent Med. 34:1848–1851. 2012.In Chinese.
|
|
14
|
Whitehurst C, Pantelides ML, Moore JV,
Brooman PJ and Blacklock NJ: In vivo laser light distribution in
human prostatic carcinoma. J Urol. 151:1411–1415. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiong L, Liu Z, Ouyang G, Lin L, Huang H,
Kang H, Chen W, Miao X and Wen Y: Autophagy inhibition enhances
photocytotoxicity of Photosan-II in human colorectal cancer cells.
Oncotarget. 8:6419–6432. 2017.
|
|
16
|
Shintani T and Klionsky DJ: Autophagy in
health and disease: A double-edged sword. Science. 306:990–995.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rubinsztein DC, Gestwicki JE, Murphy LO
and Klionsky DJ: Potential therapeutic applications of autophagy.
Nat Rev Drug Discov. 6:304–312. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Panganiban RA, Snow AL and Day RM:
Mechanisms of radiation toxicity in transformed and non-transformed
cells. Int J Mol Sci. 14:15931–15958. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen ST, Lee TY, Tsai TH, Lin YC, Lin CP,
Shieh HR, Hsu ML, Chi CW, Lee MC, Chang HH and Chen YJ: The
Traditional Chinese Medicine DangguiBuxue tang sensitizes
colorectal cancer cells to chemoradiotherapy. Molecules.
21:pii:E1677. 2016. View Article : Google Scholar
|
|
20
|
Wang M, Tan W, Zhou J, Leow J, Go M, Lee
HS and Casey PJ: A small molecule inhibitor of isoprenylcysteine
carboxymethyltransferase induces autophagic cell death in PC3
prostate cancer cells. J Biol Chem. 283:18678–18684. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang B, Qiao L, Shi Y, Feng X, Chen D and
Guo H: ASPP2 inhibits oxaliplatin-induced autophagy and promotes
apoptosis of colon cancer cells. Xi Bao Yu Fen Zi Mian Yi Xue Za
Zhi. 31:898–904. 2015.In Chinese. PubMed/NCBI
|
|
22
|
Tan S, Peng X, Peng W, Zhao Y and Wei Y:
Enhancement of oxaliplatin-induced cell apoptosis and tumor
suppression by 3-methyladenine in colon cancer. Oncol Lett.
9:2056–2062. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
O’Donovan TR, Rajendran S, O’Reilly S,
O’Sullivan GC and McKenna SL: Lithium modulates autophagy in
esophageal and colorectal cancer cells and enhances the efficacy of
therapeutic agents in vitro and in vivo. PLoS One. 10:e01346762015.
View Article : Google Scholar
|
|
24
|
Shan J, Xuan Y, Zhang Q, Zhu C, Liu Z and
Zhang S: Ursolic acid synergistically enhances the therapeutic
effects of oxaliplatin in colorectal cancer. Protein Cell.
7:571–585. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shan J, Xuan Y, Zhang Q, Zhu C, Liu Z,
Zhang S, et al: Inhibition of the NF-κB pathway by nafamostat
mesilate suppresses colorectal cancer growth and metastasis. Cancer
Lett. 380:87–97. 2016. View Article : Google Scholar
|
|
26
|
Zeng Y, Liu XH, Tarbell J and Fu B:
Sphingosine 1-phosphate induced synthesis of glycocalyx on
endothelial cells. Exp Cell Res. 339:90–95. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Paglin S, Hollister T, Delohery T, Hackett
N, McMahill M, Sphicas E, Domingo D and Yahalom J: A novel response
of cancer cells to radiation involves autophagy and formation of
acidic vesicles. Cancer Res. 61:439–444. 2001.PubMed/NCBI
|
|
28
|
Dutta D, Chakraborty B, Sarkar A,
Chowdhury C and Das P: A potent betulinic acid analogue ascertains
an antagonistic mechanism between autophagy and proteasomal
degradation pathway in HT-29 cells. BMC Cancer. 16:232016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen SL, Cai SR, Zhang XH, Li WF, Zhai ET,
Peng JJ, Wu H, Chen CQ, Ma JP, Wang Z and He YL: Targeting CRMP-4
by lentivirus-mediated RNA interference inhibits SW480 cell
proliferation and colorectal cancer growth. Exp Ther Med.
12:2003–2008. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang H, Ju H, Zhang L, Lu H and Jie K:
microRNA-577 suppresses tumor growth and enhances chemosensitivity
in colorectal cancer. J Biochem Mol Toxicol. Feb 2–2017.Epub ahead
of print. View Article : Google Scholar
|
|
31
|
Baldwin AS: Regulation of cell death and
autophagy by IKK and NF-kappaB: Critical mechanisms in immune
function and cancer. Immunol Rev. 246:327–345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Samuel T, Fadlalla K, Gales DN, Putcha BD
and Manne U: Variable NF-κB pathway responses in colon cancer cells
treated with chemotherapeutic drugs. BMC Cancer. 14:5992014.
View Article : Google Scholar
|