Introduction
The incidence of lung cancer is increasing each
year, this is largely due to increased levels of pollution and bad
habits, such as smoking (1). In
total, 80–85% of lung cancer cases are non-small cell lung cancer
(NSCLC), and >70% of patients are at a locally advanced or late
stage of the disease when they are diagnosed (2). Erlotinib is a small molecule that
acts as an epidermal growth factor receptor (EGFR) tyrosine kinase
inhibitor (3). Erlotinib is a
drug used for the second-line treatment of patients with locally
advanced or metastatic NSCLC (4).
Erlotinib binds to EGFR with high specificity (5). A previous study has revealed that
erlotinib may hinder the growth of retinoblastoma by inhibiting the
tyrosine kinase activity of the EGFR intracellular domain, thereby
inhibiting cell proliferation and angiogenesis, and inducing the
apoptosis of tumor cells (6).
There are various side effects of erlotinib, the most common are a
rash, abdominal pain, nausea, vomiting and headache (7). Previous studies have also reported
that one of the side effects of erlotinib is dry eyes (8). A study by Fraunfelder et al
(9) proposed that erlotinib may
cause or aggravate dry eyes. Johnson et al (10) reported non-healing of corneal
erosion and infectious keratitis cases caused by the use of
erlotinib for the treatment of lung tumors. Morishige et al
(11) also reported a case of
diffuse water deficiency dry eye following treatment with
erlotinib. Patients have also been reported to exhibit corneal
dissolution and perforation following treatment with erlotinib
(12). In the present study, it
was revealed that erlotinib may cause many of the symptoms of dry
eyes following its topical administration in mice.
Materials and methods
Animal preparation
A total of 60 male specific pathogen‑free BALB/c
mice (age, 6–8 weeks; weight, 18–20 g) were purchased from the
Laboratory Animal Center of Xi'an Jiao Tong University College of
Medicine (Xi'an, China) and used in the present study. All mice had
free asses to food and water. No abnormalities were identified in
the anterior segment or fundus of the eyes when they underwent
slit-lamp microscopy and fundus examination. The Schirmer I test
results were ≥10 mm/5 min (13).
The mice were kept in a standard housing environment throughout the
study, with a room temperature of ~25±1°C, relative humidity
~60±10% and an alternating 12-h light/dark cycle, as previously
described (14). All procedures
were performed in accordance with the Association for Research in
Vision and Ophthalmology Statement for the Use of Animals in
Ophthalmic and Vision Research and the present study was approved
by the Medical and Animal Ethics Committee of The First Affiliated
Hospital of Nanchang University (Nanchang, China).
Preparation of erlotinib eye drops
To prepare the eye drops, erlotinib
(Tarceva®; Roche Diagnostics, Indianapolis, IN, USA) was
diluted in sterile PBS to a concentration of 20 μM and
homogenized by ultrasound vortex (37°C, 100 W, 10 min). The
preservative benzyl bromide (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) was added to the two groups (with a concentration
controlled at 0.005%); one group was a control group using PBS eye
drops, and the second group was the experimental group that
received erlotinib eye drops. Eye drops were stored at 4°C prior to
their use.
Animal experimental procedure
The 60 mice were divided into two groups. Group 1
(n=30) received PBS treatment and group 2 (n=30) received erlotinib
treatment. All mice received 5 μl of the respective eye
drops in their right eye, four times daily. Prior to treatment, all
mice were free from ocular diseases. Prior to treatment and at 1-,
2- and 3-weeks post treatment, a Schirmer test, fluorescein
staining, break‑up time (BUT) of tear film, Lissamine™ green
staining and hematoxylin and eosin (H&E) staining were
performed on each group. Following 3 weeks of treatment, the
eyeballs were enucleated and processed for light and electron
microscopy analyses of the structural changes to the corneal
epithelial cells. Periodic acid-Schiff (PAS) staining was performed
to visualize changes to conjunctival goblet cells. Histological
sections were used for the detection of keratin (K)10 and apoptotic
cells. Tumor necrosis factor (TNF)-α, phosphorylated (p)-EGFR and
EGFR protein were detected by western blot analysis.
Fluorescein and BUT
A total of 1 μl 0.1% liquid sodium
fluorescein was applied onto the conjunctival sac. Following three
blinks, BUTs were recorded in sec. After 90 sec, the corneal
epithelial damage was graded using a cobalt blue filter under a
slit-lamp microscope (Chongqing Kanghua Ruiming S&T Co., Ltd.,
Chongqing, China) with a reticule calibrated for ×16 magnification.
The cornea was divided into four quadrants and they were scored
individually. The fluorescein score was analyzed as previously
described (15) with essential
modifications as follows: Absent, 0; slightly punctate staining ≤30
spots, 1; punctate staining >30 spots but not diffuse, 2; severe
diffuse staining but no positive plaque, 3; positive fluorescein
plaque, 4. The scores of each quadrant were added together to give
a final score (maximum, 16 points).
Corneal dye staining
To evaluate changes in the corneal epithelial cells,
one drop of 3% Lissamine Green B (Sigma-Aldrich; Merck KGaA) was
applied onto the inferior lateral conjunctival sac. The corneal
surface was observed using a slit-lamp microscope with a reticule
calibrated for ×16 magnification and the staining of the cornea was
scored in a blinded manner as follows: Score 0 for no punctuate
staining; score 1 when <1/3 of the cornea was stained; score 2
when ≤2/3 was stained; and score 3 when >2/3 was stained
(16).
PAS staining
The whole eyeball, including the superior and
inferior forniceal conjunctiva, was excised and fixed in 4%
formalin for 12 h at 4°C. The tissue was then cut into 4‑μm
thick sections through the superior and inferior conjunctival
fornices and stained with PAS (Sigma-Aldrich; Merck KGaA) at room
temperature. Briefly, 4‑μm slices were treated with 0.5%
periodic acid for 10 min, and then saturated in dimedone aqueous
solution for 10 min. The slices were then incubated with Schiff's
reagent for 8 min at room temperature. The nuclei were stained with
hematoxylin for 30 sec. The number of PAS-stained cells was counted
per 100 mm2 in four sections of the eye from each mouse,
the average count was determined in each eye as the goblet cell
density. The count was performed by the same observer each time, as
previously described (15).
Corneal tissues were stained with H&E at room temperature. All
images were captured by a light microscope (Carl Zeiss AG,
Oberkochen, Germany) with a magnification of ×20.
Evaluation of inflammation
The inflammatory response was evaluated by slit-lamp
microscopy with a reticule calibrated for ×16 magnification at 9:00
am on the first day of weeks 0, 1, 2 and 3. The inflammatory index
was analyzed as previously described (16). Briefly, the inflammatory index was
scored based on the following parameters: Ciliary hyperemia
(absent, 0; present but <1 mm, 1; present 1–2 mm, 2; and present
>2 mm, 3); central corneal edema (absent, 0; present with
visible iris details, 1; present without visible iris details, 2;
and present without visible pupil, 3); and peripheral corneal edema
(absent, 0; present with visible iris details, 1; present without
visible iris details, 2; and present with no visible iris, 3). The
final inflammatory index result was obtained by totaling the scores
for the different parameters and dividing them by a factor of
nine.
Terminal deoxynucleotidyl transferase
mediated dUTP biotin nick end labeling (TUNEL) assay
A TUNEL assay was performed according to a
previously published method, with some modifications (17). Eyeballs were fixed in 4% buffered
formalin for 24 h at 4°C and then paraffin‑embedded. Sections (5
μm thick) were pre-coated in Histogrip (Zymed; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) in acetone. The dilution of
Histogrip was 1:50. Subsequently, the sections were permeabilized
with 4 μg/ml proteinase K (Merck KGaA) for 10 min at room
temperature. Following this, sections were incubated with terminal
deoxynucleotidyl transferase in buffer containing cobalt chloride,
potassium cacodylate, Tris-HCl, bovine serum albumin (BSA),
biotinylated deoxyuridine triphosphate (dUTP), and deoxy-adenosine
triphosphate (Boehringer-Mannheim GmbH, Mannheim, Germany) for 60
min at 37°C. The reaction was terminated by incubation with sodium
chloride/sodium citrate buffer for 15 min at room temperature, in
PBS for 1 min, and in PBS containing FCS and Triton X-100
(Sigma-Aldrich; Merck KGaA) for 30 min at room temperature.
Sections were washed in PBS for 10 min, and then incubated with
horseradish peroxidase-conjugated streptavidin (1:300; P0397; Dako;
Agilent Technologies, Inc., Santa Clara, CA, USA) in PBS for 1 h at
room temperature. Negative control sections were stained
identically, but with omission of biotinylated dUTP from the nick
end labelling mixture (17).
Apoptotic cells in tissue were counted in a 1-mm2 area
of epithelium in each section, three sections from each sample were
counted by light microscopy (TE-200-OU; Nikon Corporation, Tokyo,
Japan).
Immunofluorescent staining of K10
Immunodetection of K10 was performed as described
previously (15).
Immunofluorescent staining was performed in cryosections
(6-μm thick) of the eyeballs. Sections were fixed in 99.5%
acetone (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) at
‑20°C for 10 min, 2% BSA for blocking, and then incubated at 4°C
overnight with K10 antibody. Mouse anti-human K10 antibodies
(ab16667; Abcam, Cambridge, UK) were used at a dilution of 1:150 as
the primary antibodies, followed by Alexa Fluor®
secondary goat anti-mouse immunoglobulin (Ig)G (1:300; A‑11001;
Invitrogen; Thermo Fisher Scientific Inc.) incubation at room
temperature for 1 h. Nuclei were counterstained with 0.5 g/ml
Hoechst 33342 dye (Thermo Fisher Scientific Inc.). Subsequently,
the specimens were observed under a fluorescent microscope with a
magnification of ×20 (Zeiss GmbH, Jena, Germany).
Scanning electron microscopy
On day 21, the corneas were fixed in 2.5%
glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) for 24 h at 4°C.
Specimens were subsequently post‑fixed with 1% osmium tetroxide in
0.1 M phosphate buffer for 2 h and dehydrated in a graded series of
ethanol solutions at 4°C. Following dehydration, the fixed
specimens were critical‑point dried, gold coated with platinum, and
examined with a scanning electronic microscope with a magnification
of ×10,000 (JSM-6330F; JEOL, Ltd., Tokyo, Japan).
Transmission electron microscopy
(TEM)
On day 21 following erlotinib treatment, the right
corneas were harvested and fixed for 2 h in 4% glutaraldehyde
(pH=7.4) at 37°C, washed with 1/15M phosphate buffer, post‑fixed in
osmium tetroxide for 2 h at room temperature, washed again, and
dehydrated in an acetone series. The specimens were embedded using
epoxy resin in accordance with the standard method. The embedding
blocks were sliced to 50-nm sections. Following baking and dyeing
using uranyl acetate-lead citrate staining for 1 h at
room temperature, the ultrastructure of the corneal epithelial
layer was examined and captured using TEM with a magnification of
×10,000 (model no. H7650; Hitachi, Tokyo, Japan). Microvilli in
epithelium tissue were counted in each photo, and three photos from
each sample were counted.
Western blotting
The cornea and conjunctiva were lysed with cold
radioimmunoprecipitation buffer (1% Triton X-100, 1% sodium
deoxycholate. 0.1% sodium dodecyl sulfate, 0.15 M NaCl, 0.05 M
Tris-HCl). Protein concentrations were measured using a BCA kit
(MicroBCA; Pierce; Thermo Fisher Scientific, Inc.). Equal amounts
(20 μl) of proteins were subjected to electrophoresis on 8%
SDS-PAGE and then transferred into polyvinylidene difluoride
membranes. The membranes were blocked with 2% BSA at room
temperature for 1 h and then incubated with primary antibodies
directed against EGFR (1:1,000; ab52894), p-EGFR (1:1,000; ab40815)
or TNF-α (1:400; ab183218) (all from Abcam, Cambridge, MA, USA) and
β-actin (1:10,000; A5441; Sigma-Aldrich; Merck KGaA) as a loading
control at 4°C overnight, as previously described (15). The membranes were subsequently
incubated with horseradish peroxidase-conjugated goat anti-rabbit
IgG (1:10,000; 1706515; Bio-Rad Laboratories, Inc., Hercules, CA,
USA) secondary antibodies at room temperature for 2 h. Signals were
developed using enhanced chemiluminescence reagents (Xiamen Lulong
Biotech Co., Ltd., Xiamen, China) and captured on film.
Statistical analysis
Data were presented as the mean ± standard
deviation. Statistical analyses were performed using SPSS version
16.0.0 (SPSS, Inc., Chicago, IL, USA). One-way analysis of variance
and Bonferroni's post hoc tests were applied in all comparisons
between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Metabolic conditions of treatment
mice
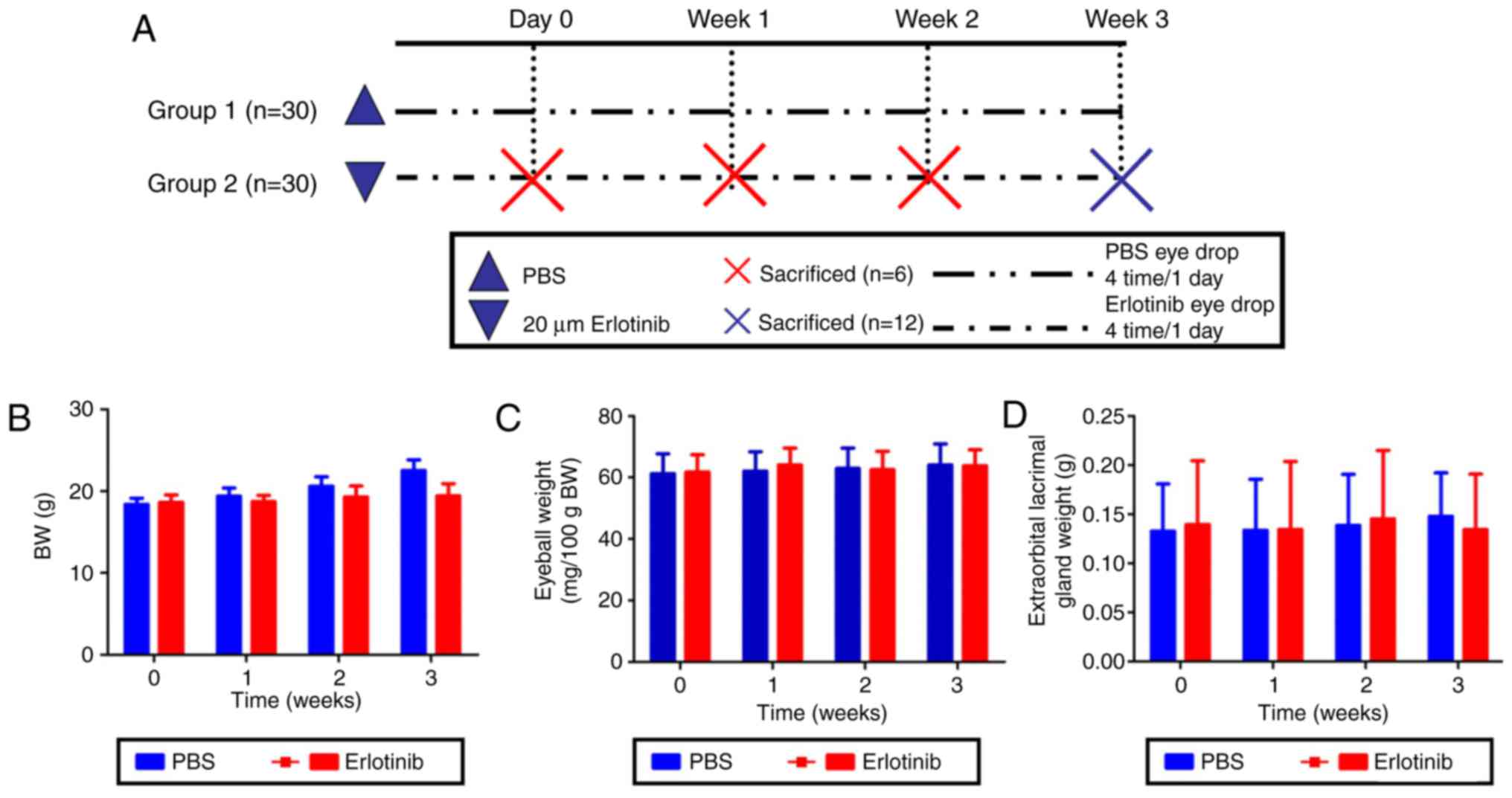
Body, eyeball and extraorbital lacrimal gland
weights were measured in each group at 0, 1, 2, and 3 weeks
following the start of treatment with either 20 μM erlotinib
or PBS (Fig. 1A). No significant
differences were identified in body, eyeball or lacrimal gland
weights between the experimental erlotinib group and the PBS
control group (Fig. 1B–D,
respectively).
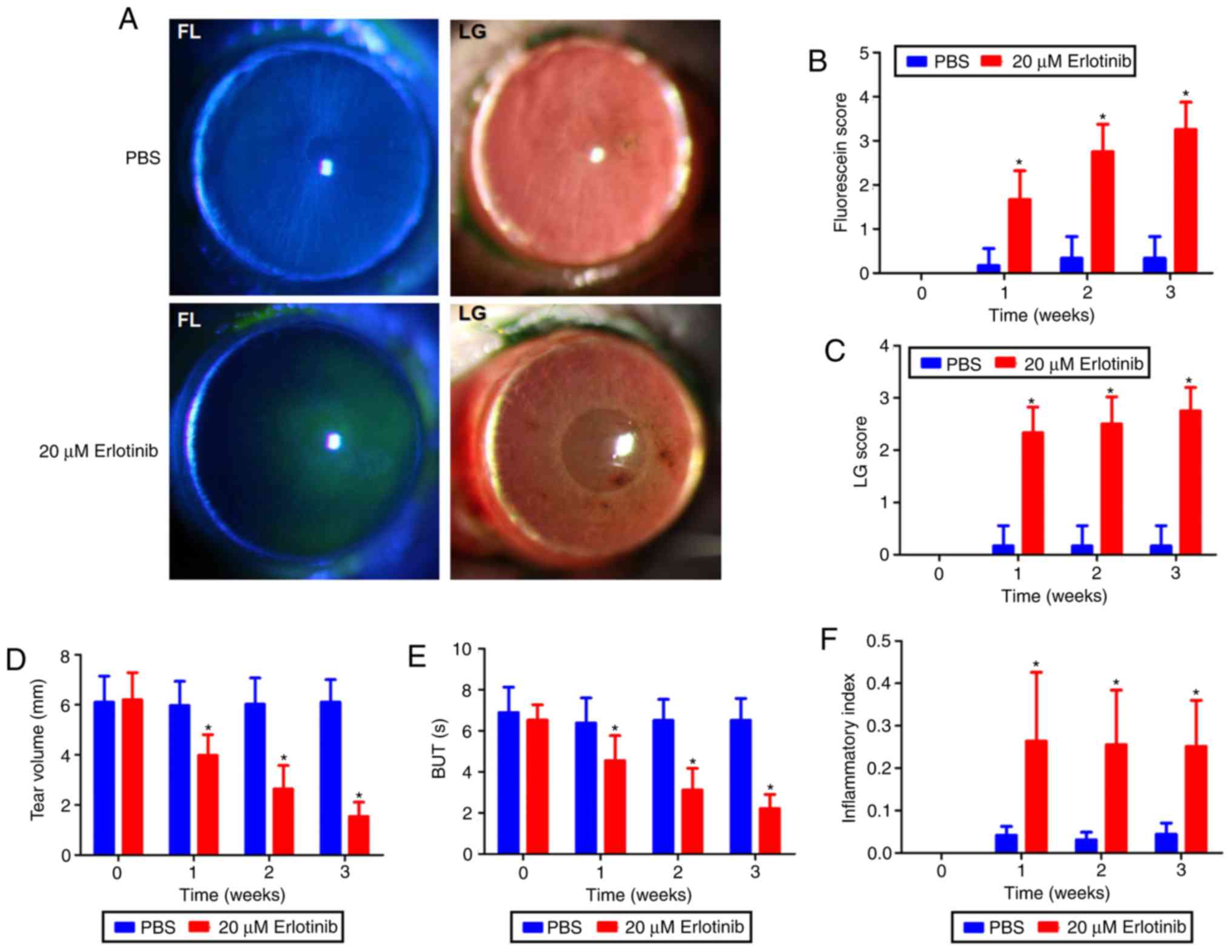
Erlotinib treatment causes tear film
damage and significantly decreases BUT and tear volume
At 3 weeks following the commencement of treatment,
tear film and epithelium damage were observed in the erlotinib
group (Fig. 2A). This was
possibly due to the toxicity of the erlotinib. Following 3 weeks of
treatment, PBS-treated cornea did not have fluorescein sodium and
Lissamine green staining. The fluorescein sodium scores (Fig. 2B) and Lissamine green staining
(Fig. 2C) were significantly
increased in the erlotinib group at all time points following
treatment compared with that in the PBS group (P<0.05). Prior to
treatment, no significant differences were identified in BUTs, tear
volume or scores of tear film, or epithelium damage between the two
groups. At 1, 2 and 3 weeks following treatment, the erlotinib
group demonstrated significantly decreased BUTs and tear volume
compared with that observed in the PBS group (P<0.05; Fig. 2D and E).
Erlotinib treatment increases the
inflammatory index
There was a significant increase in the inflammatory
index at all time points following the commencement of treatment in
the erlotinib-treated group compared with that observed in the
PBS-treated group (P<0.05; Fig.
2F).
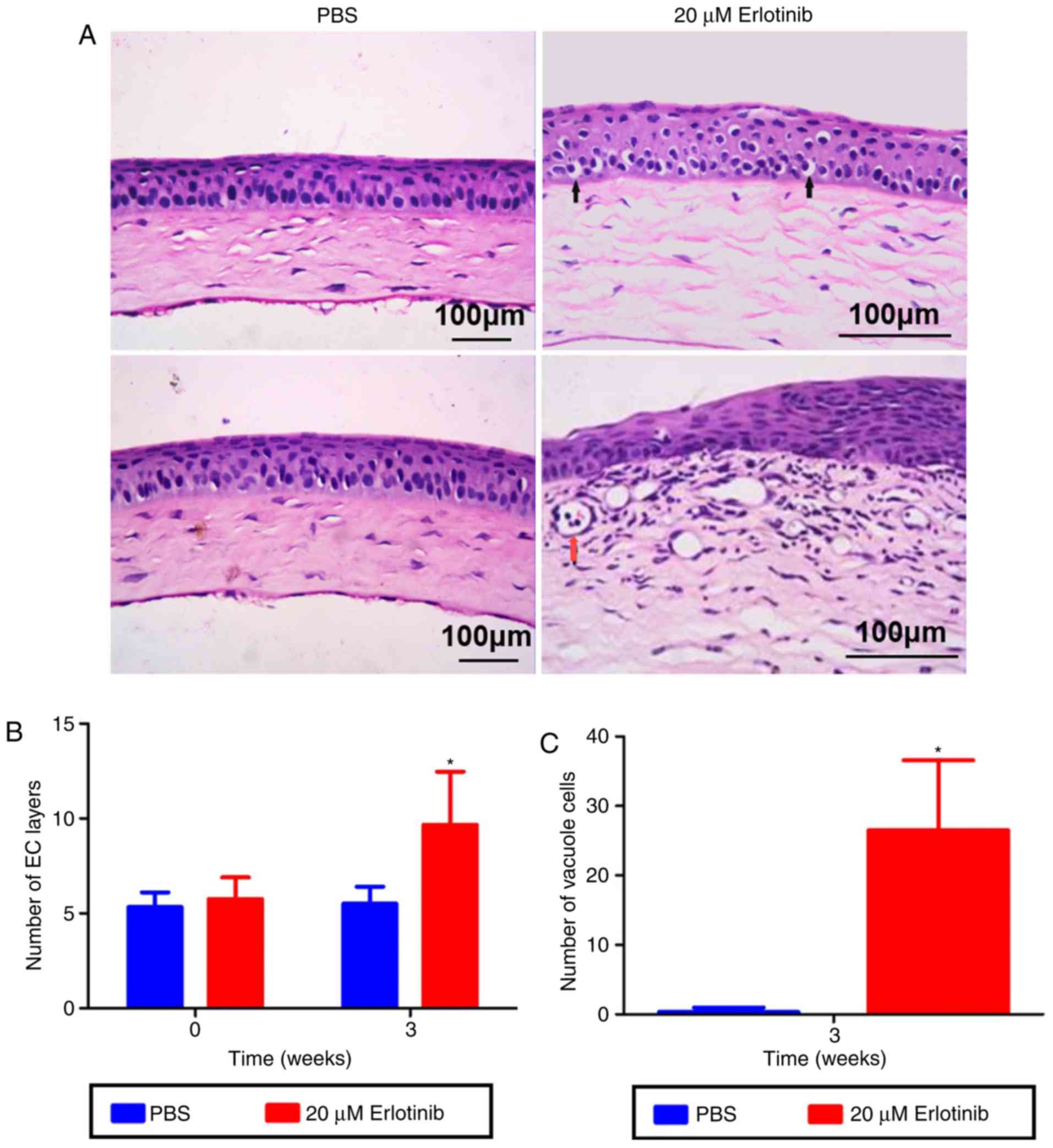
Erlotinib treatment leads to structural
changes in the epithelium
The number of epithelial layers in the central
cornea was significantly increased in the erlotinib group compared
with the number in the PBS group following 3 weeks of treatment
(Fig. 3A and B; P<0.05). A
significant increase in the number of corneal vacuole cells was
also observed in the erlotinib group compared with the number in
the PBS group (P<0.05; Fig.
3C).
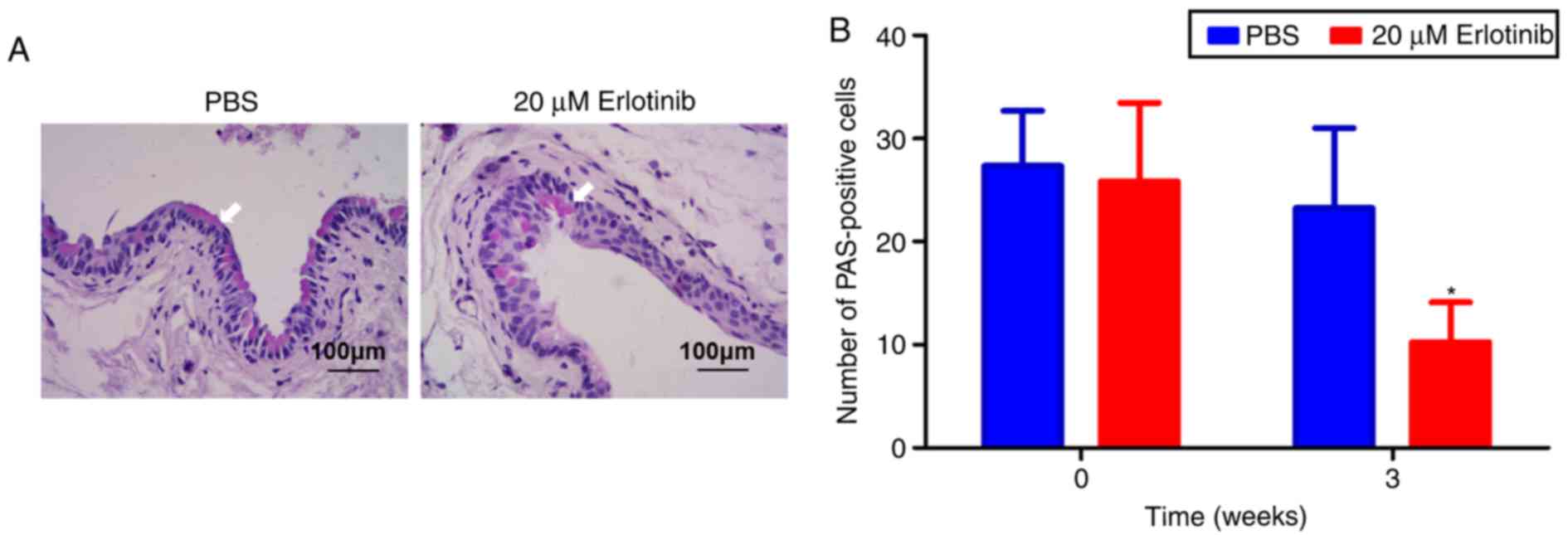
Erlotinib treatment causes a reduction in
goblet cell number
PAS staining was used to examine the effect of
erlotinib on goblet cells in the conjunctiva (Fig. 4A). The results revealed that the
PAS‑positive cell number was significantly decreased in the
erlotinib group at 3 weeks compared with the number in the PBS
group (P<0.05; Fig. 4B). No
notable difference was observed in the number of goblet cells in
the PBS group at 3 weeks compared with the beginning of
treatment.
Erlotinib treatment causes a significant
increase in apoptosis
Compared with the PBS group, immunostaining of K10
revealed a notable increase in K10-positive cells in the central
cornea of the erlotinib group following 3 weeks of treatment
(Fig. 5A). A TUNEL assay revealed
that apoptosis was induced in the corneal superficial but not in
the stroma of the erlotinib-treated group. At 3 weeks following the
commencement of treatment, the number of apoptotic cells and level
of K10 were significantly increased in the corneal epithelium of
the erlotinib-treated group compared with the level in the
PBS-treated group (P<0.05; Fig.
5B).
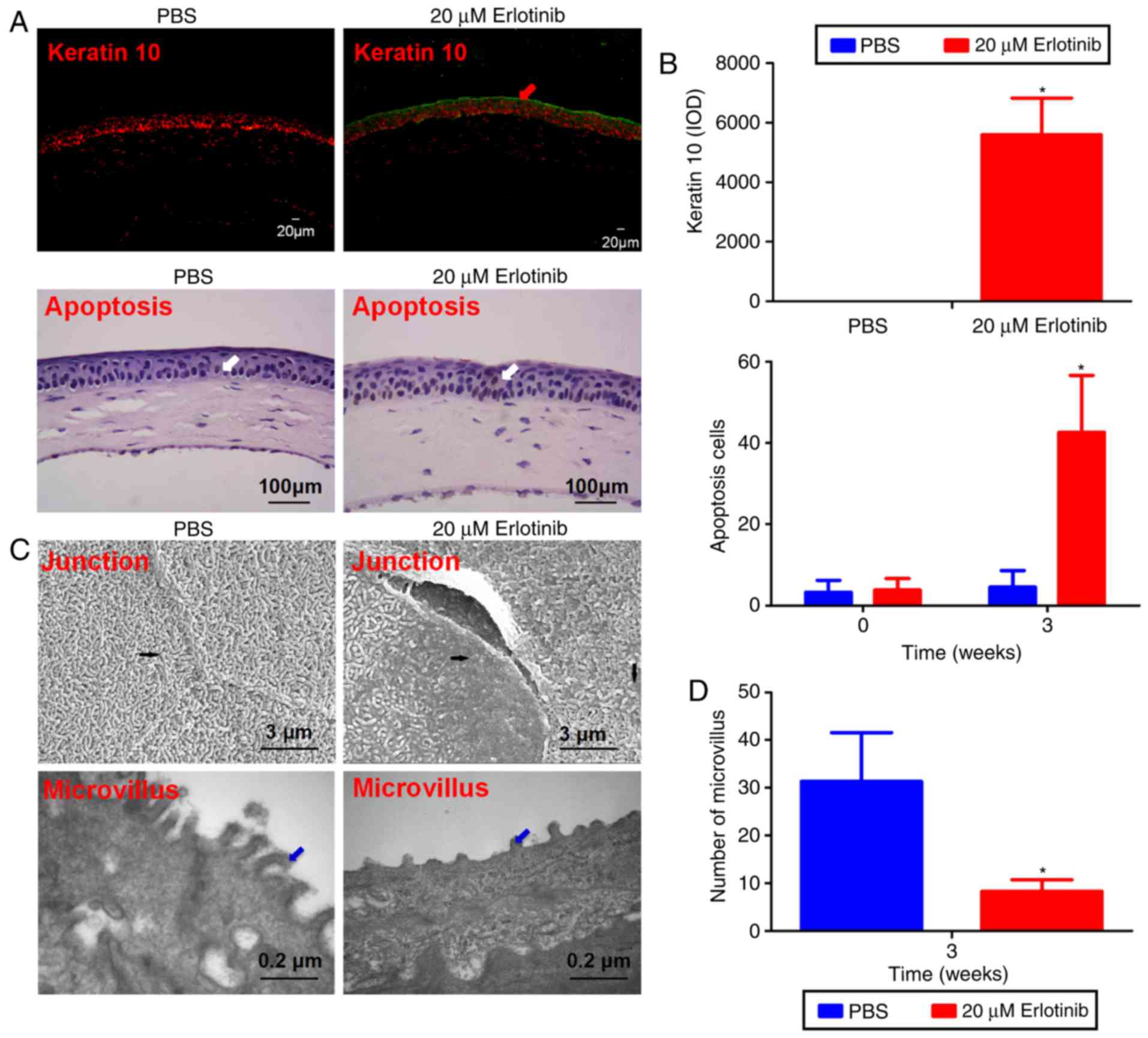 | Figure 5Corneal epithelial squamous
metaplasia ultrastructure. (A) Representative images from a keratin
10 (green line and red arrow) and a terminal deoxynucleotidyl
transferase mediated dUTP biotin nick end labeling assay of the
cornea epithelium following 3 weeks of treatment with either PBS or
erlotinib. White arrows indicate apoptosis‑positive cells. (B) The
level of apoptosis and keratin 10 was quantified. Few apoptotic
cells were observed in the superficial layer of the corneal
epithelium in the PBS group, while significantly more apoptosis was
recorded in the corneal superficial and basal epithelium following
treatment with erlotinib. Compared with the PBS group, there was a
significant upregulation of keratin 10‑positive cells in the
central cornea of the erlotinib group following 3 weeks of
treatment. (C) In the PBS group, an integrated junction between
epithelial cells was observed by scanning electron microscopy
(upper left image, black arrow), whereas a destroyed junction
(upper right image, black arrow) was observed in the erlotinib
group. Following treatment with PBS for 3 weeks, the corneal
epithelial microvilli were extended as digitations and arranged
neatly (lower left image, blue arrow). Following treatment with
erlotinib for 3 weeks, the corneal epithelial microvilli were
shorter and disordered (lower right image, blue arrow). (D) The
number of microvilli observed in the superficial layer of the
corneal epithelium was calculated for each group. Data are
presented as the mean + standard deviation. *P<0.05
vs. the PBS group at the same time point. IOD, integrated optical
density. |
Erlotinib treatment causes
ultrastructural changes to the corneal epithelium
The cornea epithelium was intact and well organized
in the PBS group following 3 weeks of treatment (Fig. 5C). In contrast, the epithelial
cells in the cornea of the erlotinib group were deformed following
3 weeks of treatment. TEM revealed enriched regularly arranged
microvilli and desmosomes extending from surface epithelial cells
in the PBS group following 3 weeks of treatment; whereas, in the
erlotinib group, the majority of microvilli were much shorter and
disorganized, and the morphology of microvilli was also clearly
different from the PBS group. The number of corneal epithelial
microvilli was significantly reduced in the erlotinib group
compared to the number in the PBS group following 3 weeks of
treatment (P<0.05; Fig.
5D).
Inflammatory changes of the ocular
surface
To investigate the mechanisms underlying
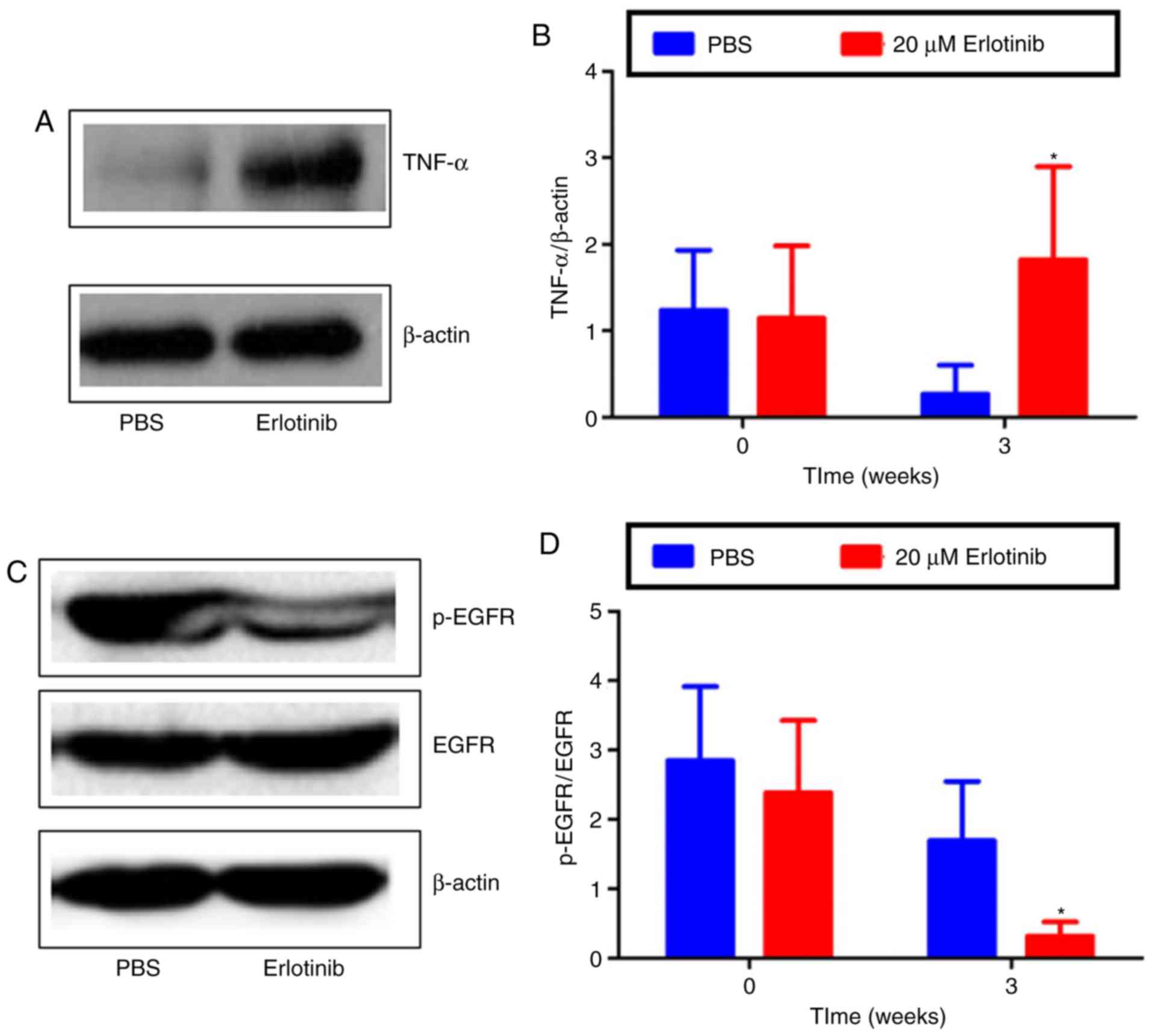
erlotinib-induced damage, the expression of TNF-α, p-EGFR and EGFR
was examined by western blot analysis. The results revealed that,
compared with the PBS group, TNF-α protein levels were
significantly higher in the erlotinib-treated group at 3 weeks
(Fig. 6A and B; P<0.05). The
expression of EGFR remained similar in the PBS- and
erlotinib-treated groups; however, the expression of p-EGFR was
markedly decreased in the ocular surface following treatment with
erlotinib (Fig. 6C). The level of
p-EGFR/EGFR expression was revealed to be significantly reduced at
3 weeks in the erlotinib group compared with the level in the PBS
group (P<0.05; Fig. 6D).
Together, the results suggest that topical erlotinib reduced EGFR
activation and induced inflammation in the development of dry
eyes.
Discussion
To the best of our knowledge, the present study is
the first to investigate the effects of erlotinib eye drops on the
ocular surface of mice. Erlotinib is a small molecule quinazoline
compound; it is used as an anti-tumor drug that targets protein
tyrosine kinase A. Erlotinib is used for the clinical treatment of
NSCLC where it acts as a competitor for adenosine triphosphate
(ATP) binding sites on the intracellular domain of tyrosine kinase
(18). This consequently
reversibly and selectively inhibits the phosphorylation of EGFR and
the related tyrosine kinase activities, including the downstream
signal transduction pathway to block angiogenesis, cell
proliferation and tumor growth (19). In the present study, it was
revealed that the expression of corneal p-EGFR in the erlotinib
group was significantly lower than in the PBS group, which
indicated that the activation of EGFR was inhibited.
In 2007, the International Dry Eye Working Group
proposed that dry eyes should be defined as 'ocular symptoms caused
by multiple factors, including tear film instability, changes of
visual acuity and potential ocular surface damage, decreased tear
osmolarity, and increased and ocular inflammation' (20). Dry eyes have become a global
epidemic (21). According to
epidemiological data, the worldwide prevalence is ~35% (22). Age, environment, medicine and
other factors have been identified as being associated with dry eye
disease (23). Numerous drugs may
cause dry eye disease, including EFGR inhibitor erlotinib used for
the treatment of NSCLC (24).
Previous papers that have reported the ocular side effects of
erlotinib are listed in Table
I.
 | Table IOcular side effects of erlotinib. |
Table I
Ocular side effects of erlotinib.
| Author, year | Country | Experiment
target | Ocular disease or
symptom | (Refs.) |
|---|
| Carser and Summers,
2006 | United Kingdom | Bronchoalveolar
carcinoma | Trichomegaly | (42) |
| Zhang et al,
2007 | America | Metastatic
small-cell lung carcinoma, bronchoalveolar cell carcinoma | Trichomegaly,
ocular irritation | (43) |
| Lane and Goldstein,
2007 | America | NSCLC | Trichomegaly, eye
pain, ocular irritation, corneal ulcer | (44) |
| Methvin and Gausas,
2007 | America | NSCLC | Conjunctivitis,
lower eyelid ectropion, epiphora | (45) |
| Papadopoulos et
al, 2008 | Greece | Lung
adenocarcinoma | Trichomegaly | (46) |
| Braiteh et
al, 2008 | America | Bronchogenic
adenocarcinoma | Trichomegaly | (47) |
| Gillani et
al, 2008 | Pakistan | NSCLC | Unilateral
blindness | (48) |
| Johnson et
al, 2009 | America | Lung cancer | Corneal
erosion | (10) |
| Saif and Gnanaraj,
2010 | America | Pancreatic
cancer | Trichomegaly | (49) |
| Morishige et
al, 2014 | Japan | Metastatic lung
cancer | Dry eye
disease | (11) |
| Kirkpatrick et
al, 2015 | America | IV squamous cell
cancer | Anterior
uveitis | (50) |
| Salman et
al, 2015 | Turkey | Lung
adenocarcinoma | Dry eye disease;
blepharitis; ectropion of lower eyelids | (51) |
| Ou et al,
2017 | America | NSCLC | Dry eye
disease | (24) |
Epidermal growth factor (EGF) has the ability to
stimulate cell proliferation and differentiation, and promote
tissue growth, development and maturation (25). EGF is a potent cytokine in
mammalian eyes, and may stimulate the proliferation,
chemoattraction and migration of conjunctival and corneal
epithelial cells during wound healing (26). EGF serves an important role in the
self-renewal of corneal epithelial cells and is required to
maintain the stability of the corneal microenvironment (27). EGF binds with the EGFR to activate
a series of signal transduction pathways, which induce protein
phosphorylation and lead to the fast differentiation and
proliferation of cells to accelerate the healing of corneal
injuries (28). EFGR is expressed
in the human cornea (29). EGF is
abundant in tears and it may increase corneal epithelial cell
proliferation (30). Corneal
epithelial damage, inflammation, dry eye treatment drugs and
reduced tear secretion may result in an insufficient level of EGF
to enable corneal wound healing, which aggravates dry eye disease
(31). In the present study,
following 3 weeks of topical erlotinib administration, it was
revealed that the dry eye-associated inflammatory index
significantly increased, as well as sodium fluorescein and
Lissamine green staining. Conversely, BUT and tear volume
significantly decreased. The results demonstrated that erlotinib
may cause ocular surface damage similar to dry eye disease.
Histopathology revealed that the corneal epithelial cells in the
experimental group had a disordered arrangement, the number of cell
layers was increased and cell junctions were not clear.
Additionally, there were cells shedding from the epithelium and a
significantly higher number of vacuolated cells compared with the
control group.
Mucin exists in the aqueous layer in the tear film
to help maintain its stability (32). Cell apoptosis, decreased goblet
cells and changes in mucin expression may cause instability of the
tear film and increased tear osmolarity (33). This may activate a series of
inflammatory responses, which increase the ocular surface
epithelium damage resulting in the formation of a damaging cycle
(33). In addition, previous
studies have reported that EGF may promote the proliferation of
goblet cells, thereby increasing the stability of tear film
(34,35). Through the topical application of
erlotinib eye drops, the present study revealed that erlotinib
reduced the number of goblet cells, which may lead to reduced mucin
secretion in the goblet cells, causing dry eye disease. The
increased expression of TNF-α that was observed in the present
study also suggests the presence of ocular surface
inflammation.
Apoptosis is a basic cellular phenomenon, which
serves an important role in the removal of unnecessary or abnormal
cells (36). Previous studies
have reported that the apoptosis of conjunctival and corneal
epithelial cells has a close association with dry eye disease
(15,37). Erlotinib acts directly on the ATP
binding sites of the tyrosine kinase domain, which interferes with
the binding of ATP with tyrosine kinase, thereby inhibiting the
activity of tyrosine kinase and blocking the signaling pathway.
Consequently, erlotinib inhibits the proliferation of cells and
angiogenesis, resulting in cell apoptosis (25). The results of the present study
also revealed that erlotinib caused an increase in the apoptosis of
corneal epithelial cells.
Microvilli in the corneal and conjunctival
epithelial cells are necessary for the localization of tear film on
the subcellular structure of the ocular surface (38). Normal corneal epithelial cell
surface microvilli and micro-structural folds are conducive for the
corneal epithelial adhesion of the various components of the tear
film, including mucin (MUC1, MUC4, MUC16) secreted by corneal
epithelial cells (39). The
interaction of corneal epithelial microvilli and membrane-bound
mucin evenly coats the surface of the cornea with tears through
blinking, and protects epithelial cells from any damage caused by
the friction of the blink, which maintains non-keratinized
characteristics of corneal epithelial cells (40,41). When epithelial cell microvilli are
abnormal in shape and structure, mucin may not be coated on the
surface of corneal epithelial cells. Erlotinib may therefore
inhibit the repair of corneal epithelial tissue and cell damage.
Using an electron microscope, it was revealed that the number of
corneal epithelial microvilli were significantly decreased in the
erlotinib group compared with the control group, and that the
ultrastructure of microvilli also had notable differences.
Occasionally, digitations of microvilli were observed with the
majority being short and irregularly arranged.
In conclusion, in the present study, the
administration of topical erlotinib eye drops was demonstrated to
cause histopathological and ultrastructural changes to the corneal
epithelial cells, similar to dry eye disease in an animal model
reported in a previous study (34). Whether erlotinib is the cause of
dry eye disease, and the mechanism by which it has an effect,
require further investigation.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81160118, 81460092,
81660158 and 81400372).
References
|
1
|
Gansler T, Ganz PA, Grant M, Greene FL,
Johnstone P, Mahoney M, Newman LA, Oh WK, Thomas CR Jr, Thun MJ, et
al: Sixty years of CA: A cancer journal for clinicians. CA Cancer J
Clin. 60:345–350. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vijayvergia N and Mehra R: Clinical
challenges in targeting anaplastic lymphoma kinase in advanced
non-small cell lung cancer. Cancer Chemother Pharmacol. 74:437–446.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Erlotinib versus
chemotherapy as first-line treatment for patients with advanced
EGFR mutation-positive non-small-cell lung cancer (OPTIMAL,
CTONG-0802): A multicentre, open-label, randomised, phase 3 study.
Lancet Oncol. 12:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shepherd FA, Rodrigues Pereira J, Ciuleanu
T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S,
Smylie M, Martins R, et al: Erlotinib in previously treated
non-small-cell lung cancer. N Engl J Med. 353:123–132. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Verkhivker GM: Exploring
sequence-structure relationships in the tyrosine kinome space:
Functional classification of the binding specificity mechanisms for
cancer therapeutics. Bioinformatics. 23:1919–1926. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yin S, Zhou L, Lin J, Xue L and Zhang C:
Design, synthesis and biological activities of novel
oxazolo[4,5-g]quinazolin-2(1H)-one derivatives as EGFR inhibitors.
Eur J Med Chem. 101:462–475. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xiong X, Liu H, Fu L, Li L, Li J, Luo X
and Mei C: Antitumor activity of a new N-substituted thiourea
derivative, an EGFR signaling-targeted inhibitor against a panel of
human lung cancer cell lines. Chemotherapy. 54:463–474. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dickler MN, Cobleigh MA, Miller KD, Klein
PM and Winer EP: Efficacy and safety of erlotinib in patients with
locally advanced or metastatic breast cancer. Breast Cancer Res
Treat. 115:115–121. 2009. View Article : Google Scholar
|
|
9
|
Fraunfelder FT, Sciubba JJ and Mathers WD:
The role of medications in causing dry eye. J Ophthalmol.
2012:2858512012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Johnson KS, Levin F and Chu DS: Persistent
corneal epithelial defect associated with erlotinib treatment.
Cornea. 28:706–707. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Morishige N, Hatabe N, Morita Y, Yamada N,
Kimura K and Sonoda KH: Spontaneous healing of corneal perforation
after temporary discontinuation of erlotinib treatment. Case Rep
Ophthalmol. 5:6–10. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saint-Jean A, Sainz de la Maza M, Morral
M, Morral M, Torras J, Quintana R, Molina JJ and Molina-Prat N:
Ocular adverse events of systemic inhibitors of the epidermal
growth factor receptor: Report of 5 cases. Ophthalmology.
119:1798–1802. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barabino S, Chen W and Dana MR: Tear film
and ocular surface tests in animal models of dry eye: Uses and
limitations. Exp Eye Res. 79:613–621. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Romay C, Armesto J, Remirez D, González R,
Ledon N and García I: Antioxidant and anti‑inflammatory properties
of C‑phycocyanin from blue-green algae. Inflamm Res. 47:36–41.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Z, Yang WZ, Zhu ZZ, Hu QQ, Chen YF,
He H, Chen YX and Liu ZG: Therapeutic effects of topical
doxycycline in a benzalkonium chloride-induced mouse dry eye model.
Invest Ophthalmol Vis Sci. 55:2963–2974. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lemp MA: Report of the national eye
institute/industry workshop on clinical trials in dry eyes. Clao J.
21:221–232. 1995.PubMed/NCBI
|
|
17
|
Williams KA, Standfield SD, Smith JR and
Coster DJ: Corneal graft rejection occurs despite Fas ligand
expression and apoptosis of infiltrating cells. Br J Ophthalmol.
89:632–638. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carey KD, Garton AJ, Romero MS, Kahler J,
Thomson S, Ross S, Park F, Haley JD, Gibson N and Sliwkowski MX:
Kinetic analysis of epidermal growth factor receptor somatic mutant
proteins shows increased sensitivity to the epidermal growth factor
receptor tyrosine kinase inhibitor, erlotinib. Cancer Res.
66:8163–8171. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Minna JD and Dowell J: Erlotinib
hydrochloride. Nat Rev Drug Discov. (Suppl): S14–S15.
2005.PubMed/NCBI
|
|
20
|
The definition and classification of dry
eye disease: Report of the Definition and Classification
Subcommittee of the International Dry Eye WorkShop (2007). Ocul
Surf. 5:75–92. 2007. View Article : Google Scholar
|
|
21
|
Stapleton F, Alves M, Bunya VY, Jalbert I,
Lekhanont K, Malet F, Na KS, Schaumberg D, Uchino M, Vehof J, et
al: TFOS DEWS II Epidemiology Report. Ocul Surf. 15:334–365. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bose T, Diedrichs-Möhring M and Wildner G:
Dry eye disease and uveitis: A closer look at immune mechanisms in
animal models of two ocular autoimmune diseases. Autoimmun Rev.
15:1181–1192. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu NN, Liu L, Li J and Sun YZ: Prevalence
of and risk factors for dry eye symptom in mainland china: A
systematic review and meta-analysis. J Ophthalmol. 2014:7486542014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ou SI, Govindan R, Eaton KD, Otterson GA,
Gutierrez ME, Mita AC, Argiris A, Brega NM, Usari T, Tan W, et al:
Phase I results from a study of crizotinib in combination with
erlotinib in patients with advanced nonsquamous non-small cell lung
cancer. J Thorac Oncol. 12:145–151. 2017. View Article : Google Scholar
|
|
25
|
Wang L, Wu X, Shi T and Lu L: Epidermal
growth factor (EGF)-induced corneal epithelial wound healing
through nuclear factor κB subtype-regulated CCCTC binding factor
(CTCF) activation. J Biol Chem. 288:24363–24371. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huo YN, Chen W and Zheng XX: ROS, MAPK/ERK
and PKC play distinct roles in EGF-stimulated human corneal cell
proliferation and migration. Cell Mol Biol (Noisy-le-grand).
61:6–11. 2015.
|
|
27
|
Nakamura Y, Sotozono C and Kinoshita S:
The epidermal growth factor receptor (EGFR): Role in corneal wound
healing and homeostasis. Exp Eye Res. 72:511–517. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Du H, Hu Z, Bazzoli A and Zhang Y:
Prediction of inhibitory activity of epidermal growth factor
receptor inhibitors using grid search-projection pursuit regression
method. PLoS One. 6:e223672011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wilson SE, He YG and Lloyd SA: EGF, EGF
receptor, basic FGF, TGF beta-1, and IL-1 alpha mRNA in human
corneal epithelial cells and stromal fibroblasts. Invest Ophthalmol
Vis Sci. 33:1756–1765. 1992.PubMed/NCBI
|
|
30
|
Kinoshita S, Adachi W, Sotozono C, Nishida
K, Yokoi N, Quantock AJ and Okubo K: Characteristics of the human
ocular surface epithelium. Prog Retin Eye Res. 20:639–673. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bron AJ, de Paiva CS, Chauhan SK, Bonini
S, Gabison EE, Jain S, Knop E, Markoulli M, Ogawa Y, Perez V, et
al: TFOS DEWS II pathophysiology report. Ocul Surf. 15:438–510.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ablamowicz AF and Nichols JJ: Ocular
surface membrane-associated mucins. Ocul Surf. 14:331–341. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stephens DN and McNamara NA: Altered mucin
and glycoprotein expression in dry eye disease. Optom Vis Sc.
92:931–938. 2015. View Article : Google Scholar
|
|
34
|
Xiao X, He H, Lin Z, Luo P, He H, Zhou T,
Zhou Y and Liu Z: Therapeutic effects of epidermal growth factor on
benzalkonium chloride-induced dry eye in a mouse model. Invest
Ophthalmol Vis Sci. 53:191–197. 2012. View Article : Google Scholar
|
|
35
|
Horikawa Y, Shatos MA, Hodges RR, Zoukhri
D, Rios JD, Chang EL, Bernardino CR, Rubin PA and Dartt DA:
Activation of mitogen-activated protein kinase by cholinergic
agonists and EGF in human compared with rat cultured conjunctival
goblet cells. Invest Ophthalmol Vis Sci. 44:2535–2544. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wickman G, Julian L and Olson MF: How
apoptotic cells aid in the removal of their own cold dead bodies.
Cell Death Differ. 19:735–742. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang L, Sui W, Li Y, Qi X, Wang Y, Zhou Q
and Gao H: Substance P inhibits hyperosmotic stress-induced
apoptosis in corneal epithelial cells through the mechanism of akt
activation and reactive oxygen species scavenging via the
neurokinin-1 receptor. PLoS One. 11:e01498652016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mackay M, Williamson I and Hastewell J:
(A) Cell biology of epithelia. Adv Drug Deliver Rev. 7:313–338.
1991. View Article : Google Scholar
|
|
39
|
Schrader S, Notara M, Beaconsfield M, Tuft
SJ, Daniels JT and Geerling G: Tissue engineering for conjunctival
reconstruction: Established methods and future outlooks. Curr Eye
Res. 34:913–924. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hori Y, Nishida K, Yamato M, Sugiyama H,
Soma T, Inoue T, Maeda N, Okano T and Tano Y: Differential
expression of MUC16 in human oral mucosal epithelium and cultivated
epithelial sheets. Exp Eye Res. 87:191–196. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yañez-Soto B, Mannis MJ, Schwab IR, Li JY,
Leonard BC, Abbott L and Murphy CJ: Interfacial phenomena and the
ocular surface. Ocul Surf. 12:178–201. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Carser JE and Summers YJ: Trichomegaly of
the eyelashes after treatment with erlotinib in non-small cell lung
cancer. J Thorac Oncol. 1:1040–1041. 2006. View Article : Google Scholar
|
|
43
|
Zhang G, Basti S and Jampol LM: Acquired
trichomegaly and symptomatic external ocular changes in patients
receiving epidermal growth factor receptor inhibitors: case reports
and a review of literature. Cornea. 26:858–860. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lane K and Goldstein SM:
Erlotinib-associated trichomegaly. Ophthal Plast Reconstr Surg.
23:65–66. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Methvin AB and Gausas RE: Newly recognized
ocular side effects of erlotinib. Ophthal Plast Reconstr Surg.
23:63–65. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Papadopoulos R, Chasapi V and Bachariou A:
Trichomegaly induced by erlotinib. Orbit. 27:329–330. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Braiteh F, Kurzrock R and Johnson FM:
Trichomegaly of the eyelashes after lung cancer treatment with the
epidermal growth factor receptor inhibitor erlotinib. J Clin Oncol.
26:3460–3462. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gillani JA, Iqbal M, Nasreen S, Khan A and
Samad A: Unilateral blindness as a presenting symptom of lung
cancer treated with erlotinib. J Coll Physicians Surg Pak.
18:125–126. 2008.PubMed/NCBI
|
|
49
|
Saif MW and Gnanaraj J: Erlotinib-induced
trichomegaly in a male patient with pancreatic cancer. Cutan Ocul
Toxicol. 29:62–66. 2010. View Article : Google Scholar
|
|
50
|
Kirkpatrick CA, Almeida DR, Hornick AL,
Chin EK and Boldt HC: Erlotinib-associated bilateral anterior
uveitis: Resolution with posterior sub-Tenon's triamcinolone
without erlotinib cessation. Can J Ophthalmol. 50:e66–e67. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Salman A, Cerman E, Seckin D and Kanitez
M: Erlotinib induced ectropion following papulopustular rash. J
Dermatol Case Rep. 9:46–48. 2015. View Article : Google Scholar : PubMed/NCBI
|