Introduction
Evidence has confirmed that endocrine disrupting
chemicals (EDCs) have potentially deleterious effects on
development, growth, metabolism and reproduction, as they can
interfere with the production, release, transport, metabolism,
binding action or elimination of the natural hormones in the body
(1). The effects of exposure to
multiple EDCs on reproduction is of great concern as various EDCs
are present at high levels in the environment, and how the
combination of the compounds these impacts the reproductive system
is largely unknown. The multiple exposure effects may function in
independent, dose addition or interaction manners (2). However, evaluation of mixture
toxicity is not simple because of the complexity of the mechanisms
of action of EDCs, and uncertain interference under different doses
and exposure durations (3).
The prepubertal male reproductive system is highly
response to sex steroids and how EDC exposure would affect
prepubertal development has been scarcely studied. Exposure to EDCs
during this stage may account for a major change in the total
activity of the hormone involved, resulting in adverse consequences
that may be apparent during puberty or even during adult life due
to interference in the developmental programming (4,5).
From the time of conception through to adulthood, humans are
exposed to countless anthropogenic and naturally-occurring EDCs,
among which di-2-(ethylhexyl) phthalate (DEHP) is the most widely
used plasticizer in polyvinylchloride (PVC) plastics, which is
prevalent in cosmetics, personal care products and medical devices,
accounting for ~80% of the total phthalates consumption worldwide
(6). As DEHP is not chemically
bound to PVC, it easily leaches, migrates and evaporates into
indoor air and the atmosphere, foodstuff and other materials. The
mechanism by which DEHP exerts toxic effects in the male
reproductive system has not been fully elucidated (7). Previous evidence revealed that DEHP
exerts its anti-androgen effect by suppressing fetal testosterone
biosynthesis via activation of peroxisome proliferator-activated
receptors (PPARs) (8), and
subsequent inhibition of anti-oxidant enzymes, leading to free
radical production and oxidative stress, which contributed to
oxidative DNA damage (9).
Following exposure to DEHP during prepuberty and puberty,
significant decreases in glutathione (GSH)/glutathione disulfide
(GSSG) redox ratio and a marked increase in the levels of
thiobarbituric acid reactive substances (TBARS) were observed
(10). Epidemiological analysis
also demonstrated that urinary oxidative stress marker
malondialdehyde (MDA) concentrations were significantly associated
with DEHP metabolite levels in prepubertal children (11).
Genistein (GEN), a weak estrogenic phytoestrogen, is
widely present in the Asian diet (12) and is considered to be a potent
anti-oxidant. Notably, GEN may enhance fertility at lower doses by
promoting the acrosome reaction, however, GEN potentially
suppresses male fertility via inhibiting the acrosome reaction at
higher doses, with no significant effect on sperm morphology
(13). Furthermore, isoflavones
can alleviate the oxidative stress induced by other EDCs, cadmium
and tetradecanoylphorbol acetate, via modulating the activity of
anti-oxidative defenses in different systems (14,15), however, the estrogenic and
anti-oxidative effects seem largely dependent on its concentration
(16).
Previous studies have predominantly focused on the
reproductive effects following exposure to a single EDC in
vitro, ex vivo and in vivo (17–19); however, few studies have examined
how multiple EDCs alter mammalian reproductive development,
particularly for those that act via different mechanisms. Our most
recent study demonstrated that GEN normalized reactive oxygen
species-induced neonatal effects of DEHP through an anti-oxidant
action, and also revealed that co-administration of the two EDCs
did not follow classical dose-response effects, which highlighted
the importance of assessing effects across a range of doses and
ages (20).
Oxidative stress is a common pathological process
involved in the mechanism of EDC-induced testicular injury, which
makes oxidative stress monitoring an informative method for
investigating interactions between numerous toxicants and the
reproductive consequences (2,21).
We hypothesized that low-dose GEN exposure would exert its
anti-oxidative role in the reproductive system during prepuberty,
which may alleviate the toxic effects in the reproductive system
induced by different doses of DEHP. The current study examined
reproductive parameters, including testis weight, anogenital
distance (AGD), gene and protein expression associated with
anti-oxidative ability and apoptosis, enzyme activity involved in
the regulation of testicular redox state, to gain an insight into
the early cellular and molecular events that may drive long term
changes caused by EDCs.
Materials and methods
Chemicals, animals and treatment
Di-2-(ethylhexyl) phthalate (DEHP; CAS no. 117-81-7)
was obtained from Tianjin Kermel Chemical Reagent Co., Ltd.
(Tianjin, China); GEN (CAS no. 446-72-0) was obtained from Shaanxi
Huike Botanical Development Co., Ltd. (Xi'an, China). Corn oil was
obtained from Longda Co., Ltd. (Yantai, China).
Prior to study initiation, the experimental protocol
was reviewed and approved by the Committee on Animal Research and
Ethics of Xi'an Jiaotong University (Xi'an, China). Specific
pathogen free Sprague-Dawley rats (21 days old) were obtained
following weaning from the Experimental Animal Center of Xi'an
Jiaotong University and housed in 12-h light/dark cycle at 21±2°C
with relative humidity of 50±5%. Soy-and alfalfa-free diet and
purified water were provided ad libitum. Male rats (n=48)
were housed 3 per cage on arrival. On the subsequent day, all the
rats were treated by daily gavage from postnatal day 22 (PND22) to
PND35 with corn oil (vehicle control), GEN 50 mg/kg body weight
(bw)/day (G50), DEHP50, 150 and 450 mg/kg bw/day (D50, D150 and
D450) and the combination of GEN + DEHP at different doses (G+D50,
G+D150, G+D450), n=6 per group. DEHP and GEN were dissolved in corn
oil and corn oil was administrated at a dose of 2 ml/kg. The dose
of each chemical was calculated daily according to body weight of
each rat prior to dosing.
The dose of GEN was chosen on the basis of previous
reports (22). Serum
concentration of phytoestrogen under a classical Asian diet is
equivalent to that of rat at the dose of 40–50 mg/kg (23,24) and the no observed adverse effect
level of GEN is considered to be 50 mg/kg/day (22). Additionally, 50 mg/kg/day is
considered to be the cut-off for low-dose effects of GEN based on
the weight-of-evidence evaluation of in vivo studies
(25).
Body weight, AGD, testis weight and organ
coefficient
Body weight of each rat was measured on PND36 and
AGD was measured using a vernier caliper by a single investigator
in a blinded manner on the same day. The AGD of each animal was
divided by the cube root of body weight (AGD/body
weight1/3) as the adjusted AGD to avoid errors caused by
differences in body size. On PND36, all the rats were anesthetized
with 400 mg/kg chloral hydrate. The right testis of each rat were
removed and stored in −80°C refrigerator for subsequent analysis of
testicular redox state.
The left testicle of each rat was removed and
weighed separately using an electronic balance and the organ
coefficient was calculated as (organ weight/body weight). The left
testis was immediately placed in Bouin's fixative solution (75 ml
saturated picric acid solution; 25 ml 40% formalin aqueous
solution; 5 ml glacial acetic acid) for 12 h and routinely
processed for histology.
Evaluation of testicular redox state
Testis tissue (200 mg) was cut into small pieces and
homogenized in 1.8 ml ice-cold saline buffer (1:9, wt/v) using an
Ultra-Turrax (T8; IKA®-Werke GmbH & Co., KG, Staufen, Germany)
to obtain testicular homogenates at a concentration of 0.1 g/ml.
Subsequently, testicular homogenates were centrifuged at 3,500 × g
for 5 min at 4°C and the supernatants were collected for further
testicular redox state analysis. Total anti-oxidant capacity
(T-AOC) (26), superoxide
dismutase (SOD) (26), GSH
peroxidase (GSH-PX) (27), total
GSH (28), GSSG (28) and MDA (29) were evaluated using clinical
chemistry assay kits (Nanjing Jiancheng Bioengineering Institute,
Nanjing, China) according to the manufacturer's instructions to
monitor testicular redox state.
T-AOC was determined by the ferric
reducing/anti-oxidant power assay and detected at 520 nm using a
spectrophotometer, the final concentration is expressed as U/mg
protein.
SOD activity was determined by water soluble
tetrazolium salts assay, which monitors the inhibition rate of SOD
to the process of formazan dye formation from tetrazolium salt
mediated by the superoxide anion. The absorbance was scanned at 450
nm using a microplate reader.
GSH-PX activity was detected by determination of the
reduction of GSH. The GSH reacts with
5,5-dithiobis-(2-ni-trobenzoic acid) and produces yellow colored
compounds detected at 412 nm using a spectrophotometer and the
final result are presented as U/mg protein.
T-GSH and GSSG content were measured using
dithionitrobenzoic acid reagent and the absorbance was scanned at
405 nm using microplate reader, GSH content was calculated as
T-GSH-2X GSSG, the final results are expressed as the ratio of
GSH/GSSH.
MDA was analyzed using the TBARS method and the
absorbance was measured with the ultraviolet spectrometer at 532 nm
against blanks prepared with distilled water. The result is
expressed as nmol/mg protein.
Testicular histology
Following fixation in Bouin's fixative solution for
12 h at 4°C, testes collected on PND36 were transferred to 75%
ethanol, embedded in paraffin and cut to 5 μm sections.
Sections were stained with 0.2% hematoxylin for 2 min and 0.5%
eosin for 10 min and evaluated under light microscopy. Evaluations
were performed by an experienced investigator blind to the
treatment groups.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Testis RNA was extracted using total RNA extraction
kit according to the manufacturer's instruction (Fastagen Biotech
Co., Ltd., Shanghai, China; http://www.fastagen.cn/aboutus/lxwm.htm) (30). cDNA was synthesized from isolated
RNA using RevertAid™ First Strand cDNA synthesis kit (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) (31). Total RNA (5 μl) and
oligo(dT)18 (0.5 μg) were added together and incubated at
70°C for 5 min, and then 4 μl 5X Reaction Buffer, 0.5
μl RiboLock RNase Inhibitor, 2 μl dNTP Mix and 2
μl RevertAid Reverse Transcriptase were added and incubated
at 42°C for 60 min.The reaction was terminated at 70°C for 10 min.
qPCR was performed using the Bio-Rad Real-Time PCR system (IQ5;
Bio-Rad Laboratories, Inc., Hercules, CA, USA). The cycling
conditions were as follows: Hot-start DNA Polymerase activation at
95°C for 10 min; PCR (40 cycles) at 95°C for 15 sec, 60°C for 30
sec and 72°C for 30 sec; melt curve (1 cycle) at 95°C for 15 sec,
60°C for 60 sec and 95°C for 15 sec. β-actin was used as an
endogenous control and for normalization of gene targets. The
relative gene expression was analyzed using the 2−∆∆Cq
algorithm (32). The genes and
primer sequences are listed in Table
I.
 | Table IPrimer sets used for reverse
transcription-quantitative polymerase chain reaction. |
Table I
Primer sets used for reverse
transcription-quantitative polymerase chain reaction.
| Gene name | Accession no. | Forward primer | Reverse primer |
|---|
| β-actin | NM_031144.2 |
5′-CTATCGGCAATGAGCGGTTCC-3′ |
5′-TGTGTTGGCATAGAGGTCTTTACG-3′ |
| Nrf2 | NM_031789.2 |
5′-ACGGTGGAGTTCAATGAC-3′ |
5′-TGTTGGCTGTGCTTTAGG-3′ |
| HO-1 | NM_012580.2 |
5′-GAAGAGGAGATAGAGCGAAAC-3′ |
5′-TGTGGCTGGTGTGTAAGG-3′ |
| Caspase-3 | NM_012922.2 |
5′-TGGAACGAACGGACCTGTG-3′ |
5′-CGGGTGCGGTAGAGTAAGC-3′ |
Western blot analysis
Total protein was extracted using a Total Protein
Extraction kit (Beijing Solarbio Science & Technology Co.,
Ltd., Beijing, China) (33).
Protein concentration was determined using NanoDrop2000c
microvolume spectrophotometer (Thermo Fisher Scientific, Inc.)
Proteins (loaded sample, 50 μl at 1 μg/μl)
were separated on a 12% polyacrylamide gel and then transferred to
a polyvinylidene fluoride membrane. Following blocking at room
temperature with 5% milk in 1X Tween-20-PBS (PBST), the membranes
were incubated with specific primary antibodies against rat NF-E2
related factor 2 (Nrf2; 1:200; BS1258; Bioworld Technology, Inc.,
St. Louis Park, MN, USA), heme oxygenase-1 (HO-1; 1:500; ab13248;
Abcam, Cambridge, UK) and cleaved caspase-3 (1:300; 9661; Cell
Signaling Technology, Inc., Danvers, MA, USA) and β-actin
(1:10,000; HC201-01; Beijing Transgen Biotech Co., Ltd., Beijing,
China) diluted in PBST overnight at 4°C, followed by incubation
with horseradish peroxidase (HRP)-conjugated anti-rabbit IgG
(1:2,000; HS101-01) or HRP-conjugated anti-mouse IgG (1:10,000;
HS201-01; Beijing Transgen Biotech Co., Ltd.) for 1 h at room
temperature. Substrate Chemiluminescence kit (EMD Millipore,
Billerica, MA, USA) was used to detect proteins. Images were
captured using the Alpha FluorChem E gel documentation system
(ProteinSimple, San Jose, CA, USA). the density of the lanes was
measured using Bio-Rad QuantityOne software (version 4.6.2; Bio-Rad
Laboratories, Inc.).
Statistical analyses
Data were expressed as the mean ± standard error and
analyzed using SPSS 15.0 (SPSS Inc., Chicago, IL, USA). Normality
and homogeneity of variances were evaluated prior to statistical
analysis. Data were analyzed by one-way analysis of variance and
multiple comparison were done between combined exposure groups and
control, and single exposure groups by least significance
difference tests when equal variances were assumed, otherwise
followed by Games-Howell test. P<0.05 was considered to indicate
a statistically significant difference.
Results
Body weight, AGD, testis weight and organ
coefficient
Body weight, AGD, testis weight and organ
coefficient of each rat on PND36 are presented in Fig. 1. No significant changes of body
weight were observed among the groups. Examination of PND36 body
weight, AGD and adjusted AGD revealed no significant differences
between control and treated animals. Exposure to 150 and 450
mg/kg/day DEHP caused a significant decrease in testis weight and
the testis organ coefficient compared with the control group
(P<0.05), and the combined exposure of GEN + 150 or 450
mg/kg/day increased the testis weight and testis organ coefficient
compared with the corresponding DEHP single exposure groups
(P<0.05), thus, although not completely normalized, GEN may have
a protective effect in prepubertal testis development.
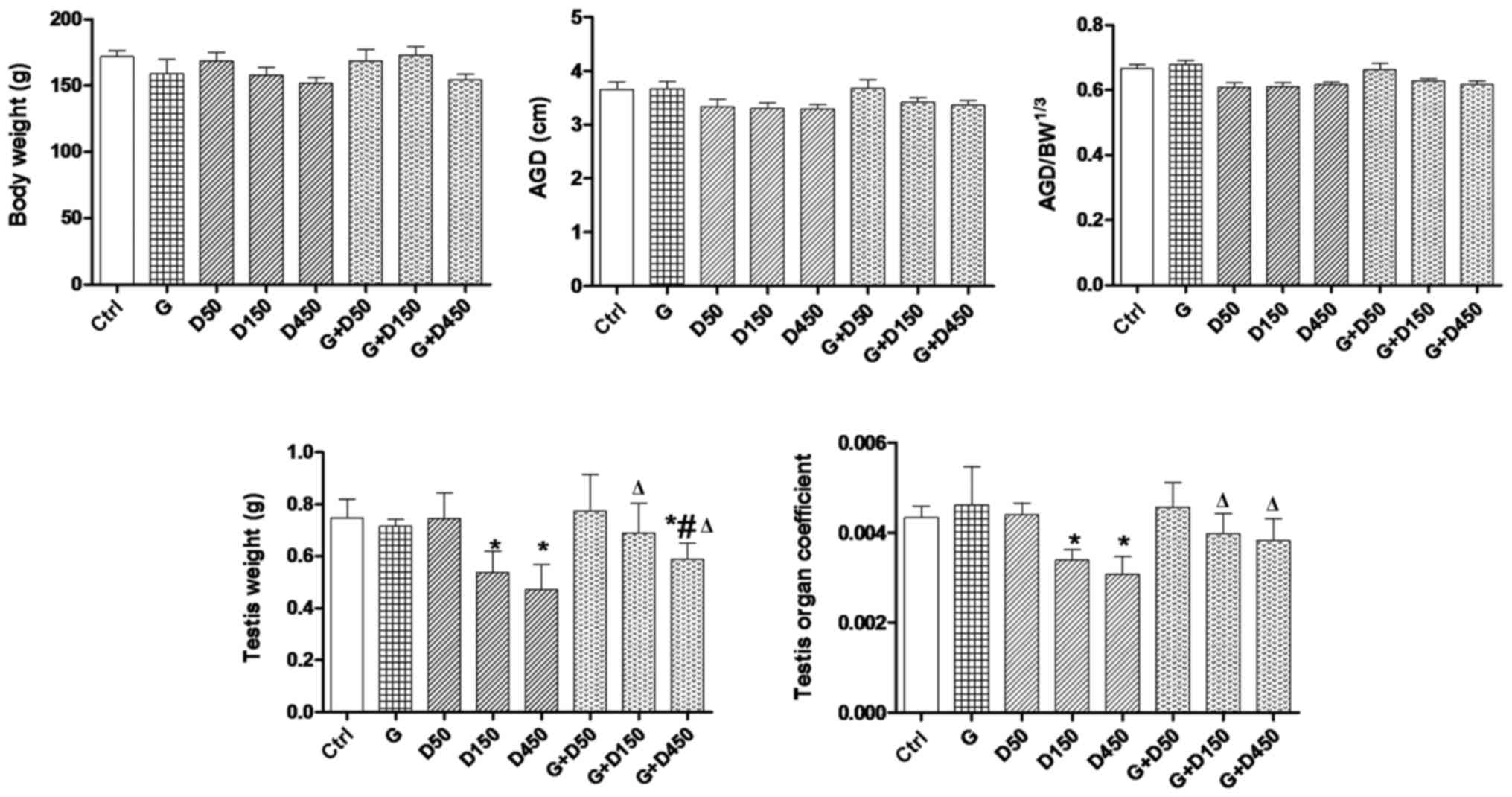 | Figure 1Body weight, AGD, adjusted AGD,
testis weight and organ coefficient comparison between groups on
postnatal day 36. Rats were treated with G (50 mg/kg), D (50, 150,
450 mg/kg) or combined treatment. *P<0.05 vs.
control; #P<0.05 vs. G; ΔP<0.05 vs.
corresponding D group. AGD, anogenital distance; BW, body weight;
G, genistein; D, di-2-(ethylhexyl) phthalate. |
Testicular redox state
The testicular redox state in each group at PND36 is
presented in Fig. 2. The
consecutive DEHP treatment resulted in a significant reduction of
intratesticular T-AOC, SOD activity and GSH-PX level, and the ratio
of GSH/GSSG compared with the control group (P<0.05), whereas
combined treatment with GEN and DEHP significantly increased these
parameters compared with corresponding single DEHP exposure groups
(P<0.05), exhibiting similar trends to the changes in testis
weight and testis organ coefficient. This indicated that the
anti-oxidative role of GEN and the enhancement of testicular
anti-oxidative ability may contribute to the recovery of testis
weight. By contrast, the MDA level in each DEHP-treated group was
increased significantly compared with the control group
(P<0.05). Co-administration of GEN and DEHP resulted in a
significant decrease in the MDA level compared with the
corresponding single DEHP exposure groups (P<0.05), which may be
largely dependent on the increased anti-oxidative capacity
following co-administration with GEN.
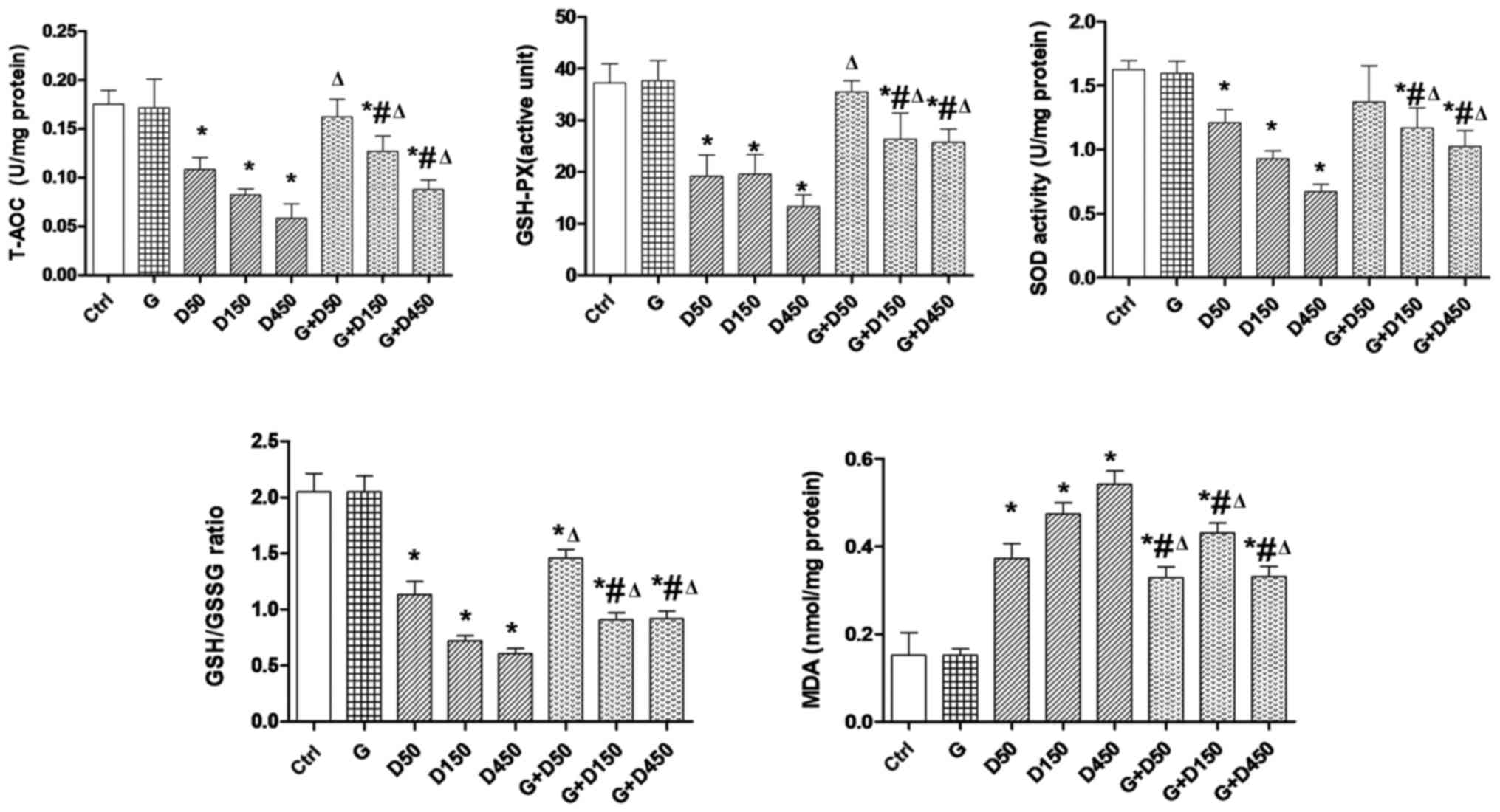 | Figure 2Testicular redox state comparison
between groups at postnatal day 36. Rats were treated with G (50
mg/kg), D (50, 150, 450 mg/kg) or combined treatment.
*P<0.05 vs. control; #P<0.05 vs. G;
ΔP<0.05 vs. corresponding D group. T-AOC, total
anti-oxidant capacity; AGD, anogenital distance; BW, body weight;
G, genistein; D, di-2-(ethylhexyl) phthalate; GSH-PX, gluathione
peroxidase; SOD, superoxide dismutase; GSH/GSSG,
gluathione/glutathione disul-fide; MDA, malondialdehyde. |
Testicular histology
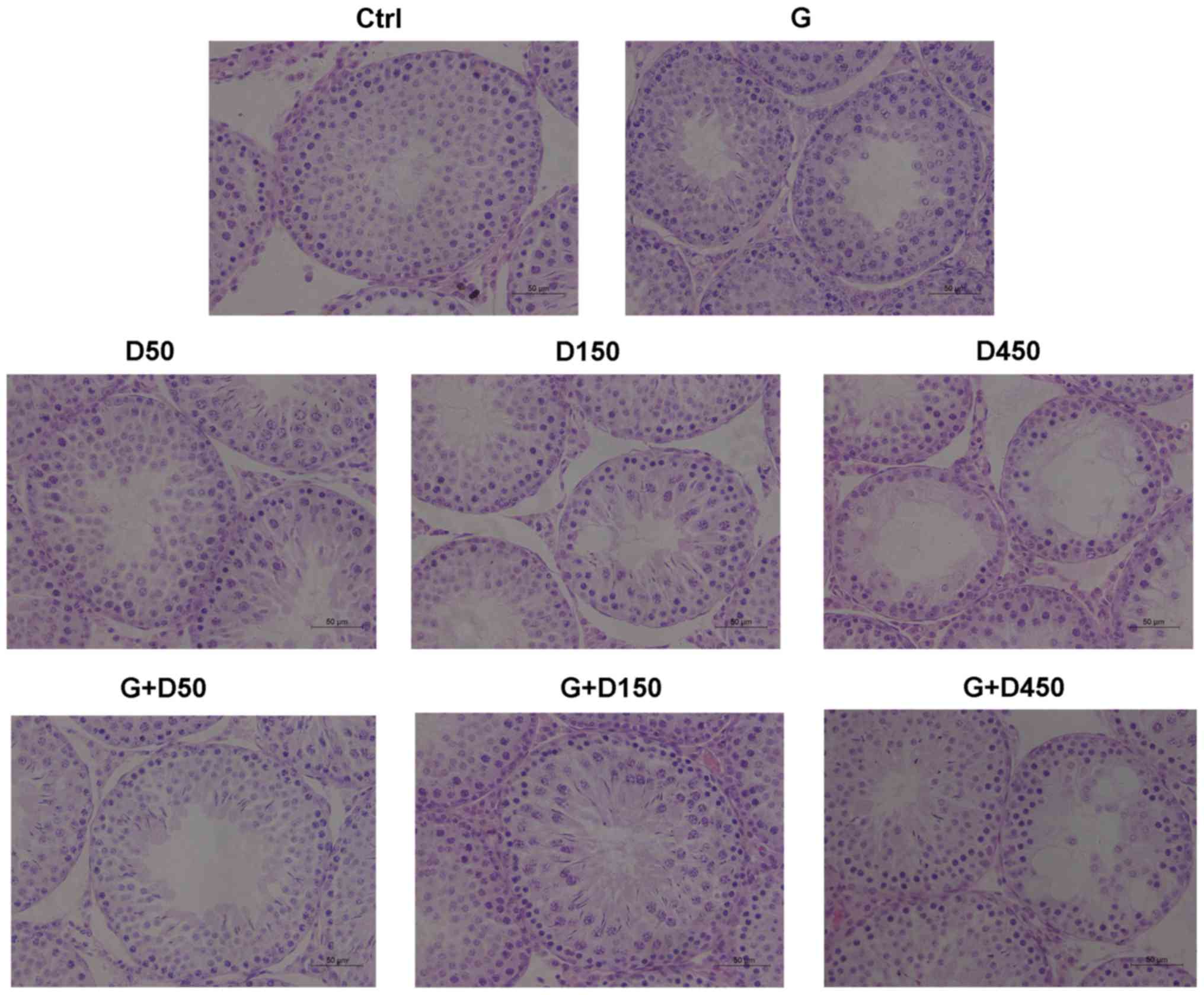
Testicular sections from each group at PND36 are
presented in Fig. 3. No obvious
histological damage was observed following treatment with GEN, D50,
or combined GEN and D50; the tubule diameter and formation of
tubule lumen were normal, and the number of cell layers in each
seminiferous tubule was >5. However, exposure to DEHP at 150
mg/kg/day induced a decrease in the thickness of seminiferous
epithelium and a decrease of the number of cell layers, which was
3–4 layers; single vacuolization was also presented in part of the
seminiferous tubules. The combined treatment with GEN + DEHP at 150
mg/kg/day increased the cell layer numbers compared with D150
single exposure group and no obvious tubular vacuolization was
observed.
The D450 group exhibited shorter tubule diameter and
loosely arranged germ cells, severe tubular vacuolization was
visible in part of the seminiferous tubules and in those tubules no
spermatids were observed, indicating prepubertal exposure to high
dose of DEHP delayed tubule development of the testis during
puberty and had deleterious effects on testis development, which
may lead to adult spermatogenesis arrest. Combined exposure in the
G + D450 group increased the cell layer number and the tubule
diameter compared with the D450 group; vacuolization was still
present in several tubules, which demonstrated that prepubertal
coexposure to GEN partially alleviated DEHP-induced testicular
development disruption, however further testicular damage may not
be reversible.
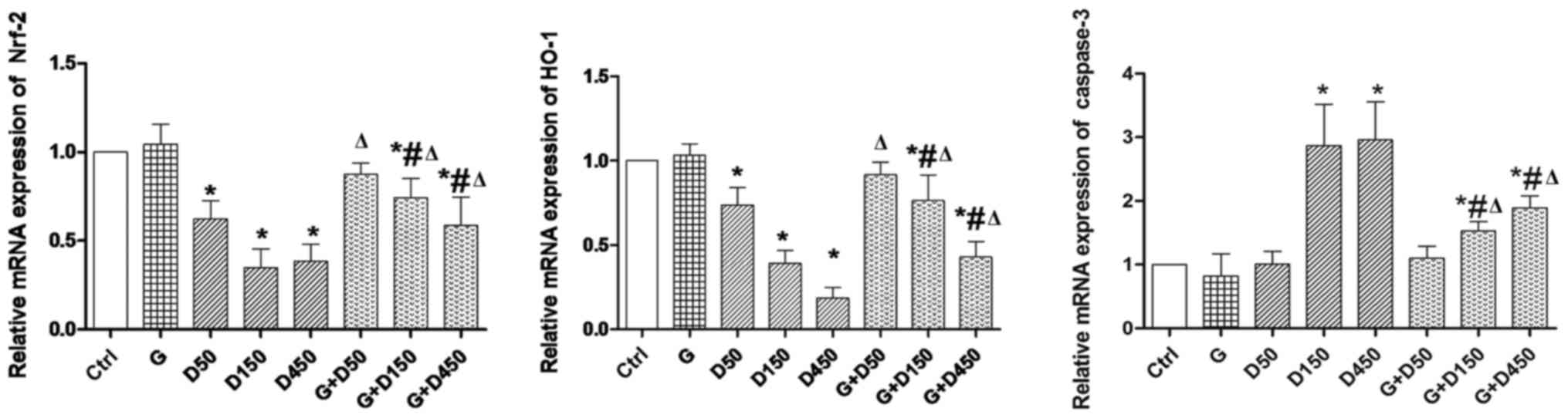
Gene expression of Nrf2, HO-1 and
caspase-3
Gene expression of Nrf2, HO-1 and caspase-3 of each
group on PND36 is presented in Fig.
4. No significant changes were observed between control and
group G. Exposure to 50, 150 and 450 mg/kg/day DEHP caused
significant decrease in Nrf2 and HO-1 expression compared with
control (P<0.05), while the combined exposure of G + D50, G +
D150 and G + D450 increased Nrf2 and HO-1 expression compared with
the corresponding DEHP single exposure groups (P<0.05), which
indicated that although not completely normalized, GEN may exert
its protective effects in prepubertal testis development by
increasing Nrf-2 and HO-1 expression. Caspase-3, the marker of cell
apoptosis, was significantly increased in D150 and D450 groups
compared with the control group (P<0.05), while the combination
of G + D150 and G + D450 decreased caspase 3 mRNA compared with
DEHP single exposure although the expression remained higher than
the control (P<0.05).
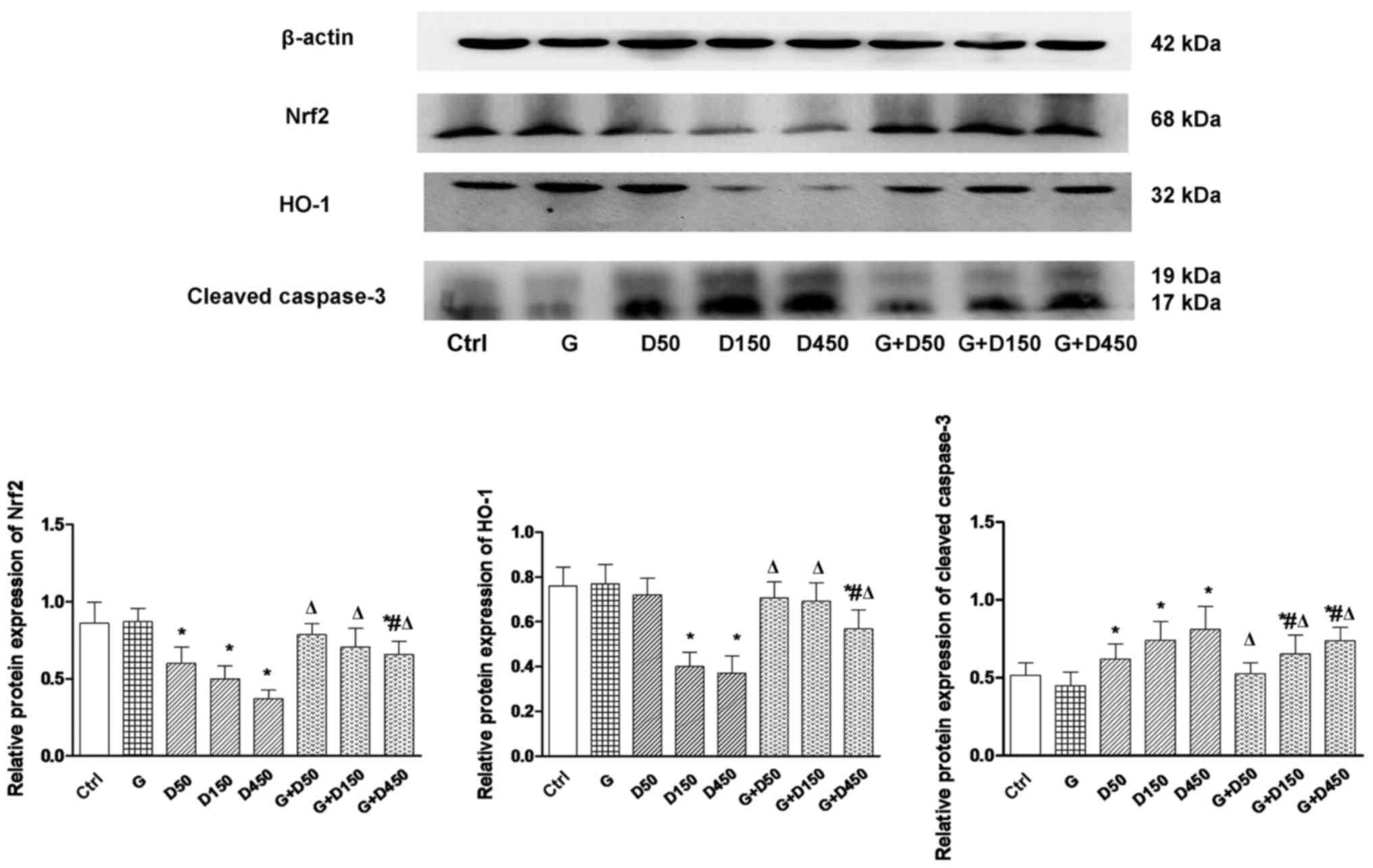
Protein expression of Nrf2, HO-1 and
cleaved caspase-3
Alterations in Nrf2, HO-1 and caspase-3 mRNA at
PND36 were further validated at the protein level by western blot
analysis and subsequent densitometry image analysis (Fig. 5). Consistent with gene expression,
protein expression of Nrf2 and HO-1 exhibited similar trends. No
significant changes were observed between the control and group G.
With the increase in DEHP doses, a significant decrease in Nrf2 and
HO-1 expression relative to control was observed (P<0.05), while
the G + D50, G + D150 and G + D450 groups exhibited an increase in
Nrf2 and HO-1 expression compared with the corresponding DEHP
single exposure groups (P<0.05).
Cleaved caspase-3, the activated form of caspase-3,
was elevated significantly in the three DEHP-treated groups
(P<0.05), while the combination with GEN significantly reduced
cleaved caspase-3 expression, although the level remained increased
compared with the control group (P<0.05), which further
demonstrated that may GEN partially attenuate testis apoptosis in
prepubertal testis, and that Nrf-2 and downstream HO-1 upregulation
may have a vital role in alleviating DEHP-induced oxidative
stress.
Discussion
Prepuberty is a critical period for male
reproductive system development; during this stage the process of
testicular spermatogenesis and steroidogenesis is highly responsive
to EDC-induced endocrine disorders (5), which may result in disturbed
spermatogenesis and higher incidence of testicular germ cell cancer
(34). The acute effects
following prepubertal EDCs exposure include compromised development
of androgen-dependent sex organs due to impaired testosterone
production, ultimately contributing to decreased sperm motility and
fertilizing ability at adulthood (10,35). However, the relative scarcity of
studies on this developmental stage and the imminent concern of
mixed EDCs exposure mean it is important to investigate the
potential acute effects on testis development and other
reproductive parameters (5).
It is well established that DEHP causes endocrine
disruption in males, through its action as an androgen antagonist,
and may have lasting effects on reproductive function, following
childhood and adult exposure. Several toxic effects have been
identified, including activation of PPAR-α gene and oxidative
damage (8,9). Similar to various other isoflavones,
GEN acts as an anti-oxidant, and thus may alleviate the damaging
effects of free radicals in tissues (14,15). GEN also influences multiple
biochemical functions in living cells, including activation of
PPARs, inhibition of several tyrosine kinases, activation of Nrf2
anti-oxidative response and stimulation of autophagy (19,28). Theoretically, we can predict the
effects of exposure to multiple EDCs and the mechanisms involved
based on previous studies, however the co-administration multiple
EDCs may not follow classical dose-responses and the health effects
may differ from what would be expected by simply adding or
subtracting the effects of individual components. Thus, it is
necessary to elucidate the combined effects following exposure to
EDCs, particularly for the most widely used plasticizer and the
most common phytoestrogen.
The present study demonstrated that single exposure
to the most common plasticizer, DEHP, particularly at high doses,
decreased testis weight, and altered testis histology and
development, of which the most significant histological abnormality
was the tubular vacuolation. Typically, the tubular vacuoles are
within or between Sertoli cells. In the prepubertal period Sertoli
cells are relatively quiescent and seminiferous tubules grow
slowly. Vacuolation of Sertoli cells is believed to be a common
early feature of morphological injury to Sertoli cells prior to any
germ cell degeneration, indicative of a breakdown in Sertoli-germ
cell junctions (36).
Alternatively, vacuoles often accompany generalized germinal cell
degeneration, where they probably represent spaces left by the
missing germ cells (37). All
those effects indicate that prepubertal exposure to a high dose of
DEHP may significantly delay tubule development of the testis
during puberty, of which the Sertoli cell may be the main target of
EDC-induced injuries. As Sertoli cell are capable of supporting a
finite number of germ cells through cell-cell contact and
maintaining the integrity of the epithelium, the decreased Sertoli
cell number and functional deterioration established during
prepuberty will inevitably lead to disruption of germinal cells
differentiation or even spermatogenesis arrest. Notably, combined
exposure to GEN and DEHP significantly increased the testis weight
compared with DEHP-single exposure, testis cross sections also
exhibited minor abnormality in the combined exposure, however
tubular vacuolation was still observed in the G + D450 group; it is
reasonable to speculate that GEN may exert protective effects when
in combination with DEHP, which acts differently from the classical
dose-responsive effect, highlighting the importance of assessing
impacts across a range of doses, ages and mixtures.
AGD or adjusted AGD is a non-invasive index of
masculinization and it has been confirmed that in humans AGD is
positively correlated with testis size, sperm count/fertility,
penis length and testosterone levels, consistent with experimental
data from rats (38), which was
associated with EDCs-induced male reproductive disruption during
the masculinization programming window (39,40). However, the majority of published
studies exclusively focused on EDCs exposure during gestation, as
conditions associated with testicular dysgenesis syndrome are
considered to initiate from aberrancies during fetal development.
Other studies revealed that prepubertal rats exposed to phthalates
at 0.4–2.2 g/kg/day exhibited altered seminiferous epithelium and
delayed spermatogenesis (41,42); however no changes in AGD were
reported in the previous reports, even with the highest dose of
DEHP and its combination with GEN. In the current study, there was
no significant alternation in AGD and adjusted AGD among the
different treatment groups; a potential explanation is that the
effects of DEHP, single exposure or in combination with GEN, were
age-specific in this species. Additionally AGD or adjusted AGD may
not be a sensitive parameter to reflect the prepubertal or even
subsequent masculinization process.
It has been confirmed that controlled and low levels
of oxidative stress are essential for normal testicular function,
which is generated by spermatogenesis and steroidogenesis that are
high energy-demanding processes. In a normal physiological state,
testes are equipped with a potent anti-oxidant system that protects
it against damage caused by reactive oxygen species (ROS) (43). Previous studies revealed that
exposure to EDCs caused an imbalance in pro-oxidant/anti-oxidant
levels and thus, promotes the generation of ROS (44,45). DEHP has been reported to have
deleterious effects on the male reproductive system by inducing
dramatic changes in germ cells, Sertoli cells and Leydig cells
(46,47). However, the underlying mechanism
by which DEHP exerts toxic effects on the reproductive system has
not yet been fully elucidated. The results of the current study
demonstrated that exposure to three doses of DEHP caused impairment
of testicular anti-oxidative enzyme activities, alteration of the
ratio of GSH/GSSG and an increase of MDA. The testicular
anti-oxidative enzyme activities were associated with the dose of
DEHP, which revealed aggravation of oxidative stress as the doses
increased. Previous studies reported that DEHP exerted
anti-androgenic potential critically mediated by suppressing fetal
testosterone biosynthesis via PPARs activation (8), however, more recently DEHP was
reported to induce oxidative stress in DEHP-mediated testicular
dysfunction (7,10), in which alterations in the
testicular enzymatic and nonenzymatic cellular anti-oxidants were
involved, and accompanied by elevated level of ROS production and
DNA damage. Kasahara et al (48) reported that DEHP enhanced the
generation of ROS and selectively decreased GSH and ascorbic acid
in the testis, thus inducing apoptosis of spermatocytes to cause
atrophy in testes. In a study conducted to examine sperm function
in adult rats following exposure to low-dose DEHP during
adolescence, Hsu et al (49) reported a significant increase in
hydrogen peroxide generation in the sperm following exposure to
1,000 μg/kg DEHP, accompanied a higher percentage of sperm
with tail abnormalities and increased sperm DNA fragmentation
index.
For combined or mixed exposures, the health effects
may differ from what would be expected from simply adding or
subtracting the effects of individual components, which leads to
concern that combined exposures may exhibit aberrant effects on the
male reproductive system, particularly for those possessing
'low-dose effect' (2,25). The combined exposure of GEN and
DEHP resulted in upregulated activity of anti-oxidative enzymes and
reduced MDA production compared with the DEHP single treatment
group. Even though GEN appears to help testis recover from
DEHP-induced testicular injuries, the enzyme activities were still
reduced and the MDA level increased in the G + D150, G + D450
groups compared with the control group, suggesting that prepubertal
GEN exposure partially attenuated DEHP-induced acute alterations in
prepubertal testes and enhanced testicular anti-oxidative ability.
Dietary intake of isoflavones has an important role in various
physiological processes in the body. It is well established that
GEN has both estrogenic (50) and
anti-oxidative effects (51). A
recent study revealed that GEN improved T-AOC, and decreased
protein carbonyl and MDA levels in nephrotic rats (52). Similar findings were also reported
in other in vitro and in vivo studies (53,54). Although other reported have
investigated the combined effects of GEN with other EDCs in early
studies, the information on the combination effects of DEHP and GEN
is limited. Gong et al (14) revealed that GEN exerted
anti-oxidant and cytoprotective effects in a neurotoxic animal
model induced by another EDC, cadmium. Our most recent study [a
collaboration with Martine Culty's group (20)] examined testicular effects
following gestational DEHP exposure at a relative low dose, and
revealed that the redox markers [NAD(P)H quinone dehydrogenase 1,
SOD2, SOD3, thioredoxin, glutathione S-transferase and catalase]
were altered significantly at PND3, whereas these effects were
attenuated when combined with GEN, demonstrating the involvement of
cellular stress in the short-term effects of DEHP and the
protective effect of GEN.
The expression of genes and proteins involved in the
process of testicular anti-oxidative defense and apoptosis was also
examined in the present study. Exposure to DEHP, particularly at
high doses, induced the downregulation of Nrf2 and HO-1 expression,
whereas caspase-3 was upregulated. By contrast, cotreatment with
GEN attenuated these effects. The kelch like ECH associated protein
1 (Keap1)-Nrf2-anti-oxidant responsive element (ARE) pathway is one
of the most important defense system against oxidative stress, in
which Nrf2 is constitutively controlled by repressor protein Keap1.
In the event of oxidative stress, stress-sensing cysteine in
cytoplasmic Keap1 changes conformation and subsequently dissociates
from Nrf2, followed by Nrf2 translocation to the nucleus where it
heterodimerizes with small Maf bZIP transcription factor protein
and binds to AREs, resulting in the transcriptional regulation of
target genes (55). Of all the
target genes, HO-1 has been reported to be exclusively expressed in
the Sertoli cells in rat testes and have a role in normal
spermatogenesis (56). Different
from the other isomers, including HO-2 and HO-3, HO-1 is an
inducible type of HO and is detectable under normal conditions.
Furthermore, HO-1 is reported to have the most AREs on its
promoter, making it a highly protective anti-oxidant enzyme by
degradation of its pro-oxidant substrate, heme, and enhancing the
production of the anti-oxidants, biliverdin and bilirubin. The
results of the current study suggest that GEN functions as an
anti-oxidant in the testis and suppresses apoptosis by ROS
scavenging via activation of Nrf2 and HO-1 expression, exerting
protection against DEHP-induced testicular toxicity, which is
consistent with the morphological and histological changes in the
PND36 rats.
The current study demonstrated that the natural
phytoestrogen GEN partially normalizes ROS-induced acute effects of
DEHP when administrated prepubertally. Yousef et al
(57) previously reported the
beneficial effects of isoflavones in reducing the negative effects
of cypermethrin on the reproductive characteristics of mature male
New Zealand white rabbits when administered every other day for 12
weeks. However, redox state evaluation was not a focus in previous
studies. In the current study, redox state alteration was evaluated
following administration of three increasing doses of DEHP and
their combination with GEN. The results suggested that prepubertal
exposure to GEN and DEHP may lead to acute alterations, which were
different from their individual effects. Thus, assessing
reproductive risk based on the effects of individual chemicals may
not accurately represent the true outcome of combined exposure
during critical periods of male reproductive development. Future
experiments will involve detailed analysis of cellular and
molecular events contributing to acute effects on testis
development, and epigenetic aberrations that may exert long-term
perturbations in gene expression.
In conclusion, GEN partially attenuated DEHP-induced
male reproductive system damage through the anti-oxidative activity
following acute prepubertal exposure to DEHP. Treatment with GEN to
reduce endocrine-disrupting side effects may have promising future
for attenuating other EDC-induced reproductive disorders.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant no. 81272846).
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Nassouri AS, Archambeaud F and Desailloud
R: Endocrine disruptors: Echoes of congress of endocrinology in
2012. Ann Endocrinol (Paris). 73(Suppl 1): S36–S44. 2012.In French.
View Article : Google Scholar
|
|
2
|
Silins I and Högberg J: Combined toxic
exposures and human health: Biomarkers of exposure and effect. Int
J Environ Res Public Health. 8:629–647. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Spurgeon DJ, Jones OA, Dorne JL, Svendsen
C, Swain S and Stürzenbaum SR: Systems toxicology approaches for
understanding the joint effects of environmental chemical mixtures.
Sci Total Environ. 408:3725–3734. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aksglaede L, Juul A, Leffers H, Skakkebaek
NE and Andersson AM: The sensitivity of the child to sex steroids:
Possible impact of exogenous estrogens. Hum Reprod Update.
12:341–349. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Perobelli JE: The male peripubertal phase
as a developmental window for reproductive toxicology studies. Curr
Pharm Des. 20:5398–5415. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kamrin MA: Phthalate risks, phthalate
regulation, and public health: A review. J Toxicol Environ Health B
Crit Rev. 12:157–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Erkekoglu P, Rachidi W, Yuzugullu OG,
Giray B, Favier A, Ozturk M and Hincal F: Evaluation of
cytotoxicity and oxidative DNA damaging effects of
di(2-ethylhexyl)-phthalate (DEHP) and mono(2-ethylhexyl)-phthalate
(MEHP) on MA-10 Leydig cells and protection by selenium. Toxicol
Appl Pharmacol. 248:52–62. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Culty M, Thuillier R, Li W, Wang Y,
Martinez-Arguelles DB, Benjamin CG, Triantafilou KM, Zirkin BR and
Papadopoulos V: In utero exposure to di-(2-ethylhexyl) phthalate
exerts both short-term and long-lasting suppressive effects on
testosterone production in the rat. Biol Reprod. 78:1018–1028.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
O'Brien ML, Spear BT and Glauert HP: Role
of oxidative stress in peroxisome proliferator-mediated
carcinogenesis. Crit Rev Toxicol. 35:61–88. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Erkekoglu P, Giray B, Rachidi W,
Hininger-Favier I, Roussel AM, Favier A and Hincal F: Effects of
di(2-ethylhexyl)phthalate on testicular oxidant/antioxidant status
in selenium-deficient and selenium-supplemented rats. Environ
Toxicol. 29:98–107. 2014. View Article : Google Scholar
|
|
11
|
Kim S, Kang S, Lee G, Lee S, Jo A, Kwak K,
Kim D, Koh D, Kho YL, Kim S, et al: Urinary phthalate metabolites
among elementary school children of Korea: Sources, risks, and
their association with oxidative stress marker. Sci Total Environ.
472:49–55. 2014. View Article : Google Scholar
|
|
12
|
Duncan AM, Phipps WR and Kurzer MS:
Phyto-oestrogens. Best Pract Res Clin Endocrinol Metab. 17:253–271.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kumi-Diaka J, Nguyen V and Butler A:
Cytotoxic potential of the phytochemical genistein isoflavone
(4′,5′,7-trihydroxyisoflavone) and certain environmental chemical
compounds on testicular cells. Biol Cell. 91:515–523. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gong DK, Liu BH and Tan XH: Genistein
prevents cadmium-induced neurotoxic effects through its antioxidant
mechanisms. Drug Res (Stuttg). 65:65–69. 2015.
|
|
15
|
Georgetti SR, Casagrande R, Vicentini FT,
Baracat MM, Verri WA Jr and Fonseca MJ: Protective effect of
fermented soybean dried extracts against TPA-induced oxidative
stress in hairless mice skin. BioMed Res Int. 2013:3406262013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Utrera M and Estévez M: Impact of trolox,
quercetin, genistein and gallic acid on the oxidative damage to
myofibrillar proteins: The carbonylation pathway. Food Chem.
141:4000–4009. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roberts D, Veeramachaneni DN, Schlaff WD
and Awoniyi CA: Effects of chronic dietary exposure to genistein, a
phytoestrogen, during various stages of development on reproductive
hormones and spermatogenesis in rats. Endocrine. 13:281–286. 2000.
View Article : Google Scholar
|
|
18
|
Christiansen S, Boberg J, Axelstad M,
Dalgaard M, Vinggaard AM, Metzdorff SB and Hass U: Low-dose
perinatal exposure to di(2-ethylhexyl) phthalate induces
anti-androgenic effects in male rats. Reprod Toxicol. 30:313–321.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kma L: Plant extracts and plant-derived
compounds: Promising players in a countermeasure strategy against
radiological exposure. Asian Pac J Cancer Prev. 15:2405–2425. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jones S, Boisvert A, Francois S, Zhang L
and Culty M: In utero exposure to di-(2-ethylhexyl) phthalate
induces testicular effects in neonatal rats that are antagonized by
genistein cotreatment. Biol Reprod. 93:922015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aitken RJ, Smith TB, Jobling MS, Baker MA
and De Iuliis GN: Oxidative stress and male reproductive health.
Asian J Androl. 16:31–38. 2014. View Article : Google Scholar :
|
|
22
|
Michael McClain R, Wolz E, Davidovich A,
Pfannkuch F, Edwards JA and Bausch J: Acute, subchronic and chronic
safety studies with genistein in rats. Food Chem Toxicol. 44:56–80.
2006. View Article : Google Scholar
|
|
23
|
Weber KS, Setchell KD, Stocco DM and
Lephart ED: Dietary soy-phytoestrogens decrease testosterone levels
and prostate weight without altering LH, prostate 5alpha-reductase
or testicular steroidogenic acute regulatory peptide levels in
adult male Sprague-Dawley rats. J Endocrinol. 170:591–599. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Adlercreutz H, Markkanen H and Watanabe S:
Plasma concentrations of phyto-oestrogens in Japanese men. Lancet.
342:1209–1210. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vandenberg LN, Colborn T, Hayes TB,
Heindel JJ, Jacobs DR Jr, Lee DH, Shioda T, Soto AM, vom Saal FS,
Welshons WV, et al: Hormones and endocrine-disrupting chemicals:
Low-dose effects and nonmonotonic dose responses. Endocr Rev.
33:378–455. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei M, Wu Y, Chen D and Gu Y: Changes of
free radicals and digestive enzymes in saliva in cases with
deficiency in spleen-yin syndrome. J Biomed Res. 24:250–255. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang M, Feng L, Gu J, Ma L, Qin D, Wu C
and Jia X: The attenuation of Moutan Cortex on oxidative stress for
renal injury in AGEs-induced mesangial cell dysfunction and
strep-tozotocin-induced diabetic nephropathy rats. Oxid Med Cell
Longev. 2014:4638152014. View Article : Google Scholar
|
|
28
|
Zhang LD, Li HC, Chong T, Gao M, Yin J, Fu
DL, Deng Q and Wang ZM: Prepubertal exposure to genistein
alleviates di-(2-ethylhexyl) phthalate induced testicular oxidative
stress in adult rats. BioMed Res Int. 2014:5986302014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mao GX, Zheng LD, Cao YB, Chen ZM, Lv YD,
Wang YZ, Hu XL, Wang GF and Yan J: Antiaging effect of pine pollen
in human diploid fibroblasts and in a mouse model induced by
D-galactose. Oxid Med Cell Longev. 2012:7509632012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liang C, Yang L and Guo S: All-trans
retinoic acid inhibits migration, invasion and proliferation, and
promotes apoptosis in glioma cell in vitro. Oncol Lett.
9:2833–2838. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dornan MH, Krishnan R, Macklin AM, Selman
M, El Sayes N, Son HH, Davis C, Chen A, Keillor K, Le PJ, et al:
First-in-class small molecule potentiators of cancer virotherapy.
Sci Rep. 6:267862016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
33
|
Shen K, Sun J, Cao X, Zhou D and Li J:
Comparison of different buffers for protein extraction from
formalin-fixed and paraffin-embedded tissue specimens. PLoS One.
10:e01426502015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Skakkebaek NE, Rajpert-De Meyts E, Buck
Louis GM, Toppari J, Andersson AM, Eisenberg ML, Jensen TK,
Jørgensen N, Swan SH, Sapra KJ, et al: Male reproductive disorders
and fertility trends: Influences of environment and genetic
susceptibility. Physiol Rev. 96:55–97. 2016. View Article : Google Scholar :
|
|
35
|
Perobelli JE, Alves TR, de Toledo FC,
Fernandez CD, Anselmo-Franci JA, Klinefelter GR and Kempinas WG:
Impairment on sperm quality and fertility of adult rats after
antiandrogen exposure during prepuberty. Reprod Toxicol.
33:308–315. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xie BG, Li J and Zhu WJ: Pathological
changes of testicular tissue in normal adult mice: A retrospective
analysis. Exp Ther Med. 7:654–656. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schlatt S and Ehmcke J: Regulation of
spermatogenesis: An evolutionary biologist's perspective. Semin
Cell Dev Biol. 29:2–16. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
van den Driesche S, Scott HM, MacLeod DJ,
Fisken M, Walker M and Sharpe RM: Relative importance of prenatal
and postnatal androgen action in determining growth of the penis
and anogenital distance in the rat before, during and after
puberty. Int J Androl. 34:e578-e5862011. View Article : Google Scholar
|
|
39
|
Hsieh MH, Breyer BN, Eisenberg ML and
Baskin LS: Associations among hypospadias, cryptorchidism,
anogenital distance, and endocrine disruption. Curr Urol Rep.
9:137–142. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dean A and Sharpe RM: Clinical review:
Anogenital distance or digit length ratio as measures of fetal
androgen exposure: Relationship to male reproductive development
and its disorders. J Clin Endocrinol Metab. 98:2230–2238. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Albert O and Jégou B: A critical
assessment of the endocrine susceptibility of the human testis to
phthalates from fetal life to adulthood. Hum Reprod Update.
20:231–249. 2014. View Article : Google Scholar
|
|
42
|
Alam MS, Andrina BB, Tay TW, Tsunekawa N,
Kanai Y and Kurohmaru M: Single administration of di(n-butyl)
phthalate delays spermatogenesis in prepubertal rats. Tissue Cell.
42:129–135. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mathur PP and D'Cruz SC: The effect of
environmental contaminants on testicular function. Asian J Androl.
13:585–591. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Koriem KM, Arbid MS and Emam KR:
Therapeutic effect of pectin on octylphenol induced kidney
dysfunction, oxidative stress and apoptosis in rats. Environ
Toxicol Pharmacol. 38:14–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wu HJ, Liu C, Duan WX, Xu SC, He MD, Chen
CH, Wang Y, Zhou Z, Yu ZP, Zhang L, et al: Melatonin ameliorates
bisphenol A-induced DNA damage in the germ cells of adult male
rats. Mutat Res. 752:57–67. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jones S, Boisvert A, Duong TB, Francois S,
Thrane P and Culty M: Disruption of rat testis development
following combined in utero exposure to the phytoestrogen genistein
and antiandrogenic plasticizer di-(2-ethylhexyl) phthalate. Biol
Reprod. 91:642014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang LD, Deng Q, Wang ZM, Gao M, Wang L,
Chong T and Li HC: Disruption of reproductive development in male
rat offspring following gestational and lactational exposure to
di-(2-ethylhexyl) phthalate and genistein. Biol Res. 46:139–146.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kasahara E, Sato EF, Miyoshi M, Konaka R,
Hiramoto K, Sasaki J, Tokuda M, Nakano Y and Inoue M: Role of
oxidative stress in germ cell apoptosis induced by di(2-ethylhexyl)
phthalate. Biochem J. 365:849–856. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hsu PC, Kuo YT, Leon Guo Y, Chen JR, Tsai
SS, Chao HR, Teng YN and Pan MH: The adverse effects of low-dose
exposure to Di(2-ethylhexyl) phthalate during adolescence on sperm
function in adult rats. Environ Toxicol. 31:706–712. 2016.
View Article : Google Scholar
|
|
50
|
Cederroth CR, Auger J, Zimmermann C,
Eustache F and Nef S: Soy, phyto-oestrogens and male reproductive
function: A review. Int J Androl. 33:304–316. 2010. View Article : Google Scholar
|
|
51
|
Qian Y, Guan T, Huang M, Cao L, Li Y,
Cheng H, Jin H and Yu D: Neuroprotection by the soy isoflavone,
genistein, via inhibition of mitochondria-dependent apoptosis
pathways and reactive oxygen induced-NF-κB activation in a cerebral
ischemia mouse model. Neurochem Int. 60:759–767. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Javanbakht MH, Sadria R, Djalali M,
Derakhshanian H, Hosse-inzadeh P, Zarei M, Azizi G, Sedaghat R and
Mirshafiey A: Soy protein and genistein improves renal antioxidant
status in experimental nephrotic syndrome. Nefrologia. 34:483–490.
2014.PubMed/NCBI
|
|
53
|
Boadi WY and Johnson D: Effects of low
doses of quercetin and genistein on oxidation and carbonylation in
hemoglobin and myoglobin. J Diet Suppl. 11:272–287. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Banz W, Hauck S, Gename B, Winters T and
Bartke A: Soy isoflavones modify liver free radical scavenger
systems and liver parameters in Sprague-Dawley rats. J Med Food.
7:477–481. 2004. View Article : Google Scholar
|
|
55
|
Lu MC, Ji JA, Jiang ZY and You QD: The
Keap1-Nrf2-ARE pathway as a potential preventive and therapeutic
target: An update. Med Res Rev. 36:924–963. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Maines MD and Ewing JF: Stress response of
the rat testis: In situ hydridization and immunohistochemical
analysis of heme oxygenase-1 (HSP32) induction by hyperthermia.
Biol Reprod. 54:1070–1079. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yousef MI, El-Demerdash FM and Al-Salhen
KS: Protective role of isoflavones against the toxic effect of
cypermethrin on semen quality and testosterone levels of rabbits. J
Environ Sci Health B. 38:463–478. 2003. View Article : Google Scholar : PubMed/NCBI
|