Introduction
Cardiovascular disease and angiocardiopathy have
high rates of morbidity and mortality, and have attracted increased
attention worldwide due to their associated economic burden, and
deterioration in the quality of life of affected patients and their
families. Emerging evidence indicates that myocardial fibrosis
increases myocardial stiffness, decreases ventricular compliance,
and accelerates the occurrence and progression of heart failure in
response to external stimulation. During the pathogenesis of
ventricular remodeling, nonfunctional fibrous tissue replaces the
normal myocardium to preserve myocardial structure and functional
integrity (1). However, with the
involvement of more intricate neurohumoral factors and pressure or
volume overload, the early stages of beneficial adaptation are
irreversibly converted into a complete decompensatory period,
accompanied by cardiomyocyte necrosis and apoptosis, and the
overdeposition of extracellular matrix in the interstitium. Once
the above pathological processes are activated, the heart is
incapable of returning to its original functional state, even if
the initial cause of the injury is abated (2). Although studies have examined the
relevant molecular mechanisms underlying myocardial fibrosis, the
physiology and pathophysiology remain to be fully elucidated.
Therefore, investigating the mechanisms underlying the development
of cardiac fibrotic conditions is important and beneficial for
clinical diagnosis and management.
Substantial experimental and clinical evidence has
suggested that multifactorial pathophysiological mechanisms are
required for the activation of cardiac fibroblasts and remodeling
of the ventricle, including the renin-angiotensin-aldosterone
system (RAAS), collagen synthesis and degradation, the inflammatory
response and numerous signal transduction pathways, which
ultimately results in severe impairments of myocardial structure
and function. The sustained activation of cardiac β-adrenergic
receptor (β-AR) and the release of catecholamines activate the
sympathoadrenal system, which is involved in the development of
cardiac remodeling. Additionally, the sustained adrenergic
activation of the elevated β-AR sympathetic nervous system
increases cardiac output and impairs systolic function and chamber
dilation. Isoproterenol (ISO) is a non-selective β-AR agonist,
which is used widely applied to establish a cardiac remodeling
model. Increasing evidence suggests that ISO treatment at high
doses supplies short-term increases in myocardial oxidative stress,
resulting in the release of proinflammatory cytokines and
activation of target protein kinases (3,4).
By contrast, studies have shown that various signaling pathways
correlate to the pathogenesis of adaptive and maladaptive cardiac
remodeling. The mitogen-activated protein kinase (MAPK) signaling
pathway is a notable example, which is involved in fibrotic
remodeling of the heart, either directly by promoting oxidative
stress and mitochondrial dysfunction or indirectly by secreting
fibrogenic mediators, which lead to myocardial hypertrophy and
fibrosis (5–7). As MAPK family members, activation of
the MAPK kinase (MEK)1/2-extracellular signal-regulated kinase
(ERK)1/2 signaling pathway involves significant modifications in
the emergence and development of the myocardial fibrotic process,
by interacting with and phosphorylating protein kinases and
transcription factors. It is known that protein phosphorylation is
a critical process consisting of complex signaling cascades, which
mediate protein structure and folding (8,9)
and exert a profound effect on pathophysiological cardiac
conditions.
Pin1, as a unique prolyl isomerase, specifically
recognizes and transforms phosphorylated serine or threonine
immediately to proline (pSer/Thr-Pro). It regulates the catalytic
activities of various enzymes by altering their phosphorylation
status and subcellular localization, protein interactions and
stability (10), and it affects
diverse cellular processes at the molecular level (11–13). Previous studies have suggested
that Pin1 is associated with the progression of several diseases,
including cancer (14), asthma
(15), aging (16), neurodegenerative diseases
(17) and other autoimmune
diseases (18). Emerging evidence
has shown that Pin1 may be one of the most unique and specific
factors correlating to the pathogenesis of various heart diseases.
A study by Toko et al (19) demonstrated that Pin1-knockout mice
(Pin1-KO) showed alleviated pathological hypertrophy, reserved
cardiac function and prolonged survival rates in an experimental
group, in which mice underwent abdominal aortic constriction,
compared with the sham. Toko et al (19) also demonstrated that Pin1 was
mainly involved in cardiac hypertrophy and injury caused by
pressure overload, and that the inhibition of Pin1 provided
beneficial effects by mediating myocardial regeneration and
antagonizing cellular senescence (20). However, the specific mechanisms by
which Pin1 mediates myocardial fibrosis following exposure to
stress stimuli, including ISO, remains to be fully elucidated. The
aim of the present study was to examine whether Pin1 facilitated
ISO-induced structural remodeling by accelerating oxidative stress
and collagen deposition through the MEK1/2-ERK1/2 signaling pathway
in rats.
Materials and methods
Chemicals and reagents
Juglone was purchased from Macklin Biochemical.
(Shanghai, China); ISO, and malondialdehyde (MDA; A3920) and
superoxide dismutase (SOD; S9693) kits were purchased from Nanjing
Jiancheng Bioengineering Institute (Nanjing, China). The antibodies
used were as follows: Phosphorylated (p)ERK (4370), pMEK (9154;
Cell Signaling Technology, Inc., Danvers, MA, Germany), α-smooth
muscle actin (α-SMA; TDY210; TDY Biotech Co., Beijing, China),
glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (ab37168; Abcam,
Cambridge, MA, USA) and Pin1 (SAB4504052; Sigma-Aldrich, Merck
Millipore), goat anti-rabbit immunoglobulin
G-horseradish-peroxidase (IgG-HRP) and goat anti-mouse IgG-HRP
(074-1506, 074-1806) were from Kirkegaard and Perry Laboratories,
Inc., (Gaithersburg, MD, USA), collagen I and III (ab34710, ab6310,
respectively) were from Abcam. Goat anti-rabbit fluorescein
isothiocyanate (FITC; AS-1110), goat anti-mouse FITC (AS-1112),
goat anti-rabbit CY3 (AS-1109), goat anti-mouse CY3 (AS-1111) and
donkey anti-mouse CY3 (AS-1113) were from Aspen Biological (Wuhan,
China). 5-Hydroxy-1,4-naphthoquinone (H47002) was from
Sigma-Aldrich (Merck Millipore).
Animals
Male Sprague-Dawley rats of 7–8 weeks age, weighing
180–200 g, were purchased from the Center for Disease Control and
Prevention of Hubei Province (Hubei, China). The rats were
maintained at 22–25°C under a 12-h light/dark cycle with a relative
humidity of 40–70%. The animals had free access to standard
laboratory chow and drinking water. All experiments were approved
by the Animal Care and Use Committee of Renmin Hospital of Wuhan
University (Wuhan, China) and conformed to the Guidelines for the
Care and Use of Laboratory Animals prepared by the National Academy
of Sciences and published by the National Institutes of Health
(21).
Experimental design
A total of 36 rats were divided randomly into three
groups (12 in each group): In the control group (vehicle control),
the rats did not receive ISO or juglone; in the ISO group, the rats
received an intraperitoneal injection of ISO (5 mg/kg) without
juglone every day for 3 weeks; in the ISO+juglone group, rats
received intraperitoneal injection of ISO and juglone at 5 and 3
mg/kg, respectively. The ISO and juglone were dissolved in saline.
The rats in the control group were handled similarly, but with an
intraperitoneal injection of the same volume of saline. At the end
of the experiment, the rats were sacrificed, and blood samples were
collected and centrifuged (4,390 × g, 15 min at 37°C) to separate
the serum, which was stored at −20°C for subsequent biochemical
assays. The hearts were dissected and weighed to calculate the
heart weight/body weight (HW/BW; mg/g), and the left ventricle of
the heart tissues was snap-frozen in liquid nitrogen and stored at
−80°C until biochemical analysis or fixed in paraformaldehyde for
histological analysis.
Echocardiography
Transthoracic echocardiography measurements (vivid4;
GE Healthcare Life Sciences, Chalfont, UK) were performed under
anesthesia with intraperitoneal administration of chloral hydrate
(30 mg/kg). At the level of the papillary muscles, using M-mode
echocardiography, morphometric and functional parameters, including
left ventricular wall thickness (LVPW), left ventricular septum
thickness (IVS), left ventricular ejection fraction (EF%) and left
ventricular fractional shortening (FS%), were obtained in the
short-axis view. All echocardiographic parameters were determined
based on the average of three consecutive cardiac cycles.
Histopathological analysis
The heart tissues were washed with saline solution
(10092-18; Jinuo Co, Hangzhou, China), fixed in 4% paraformaldehyde
(As1018; Aspen Biological; ≥24 h at 37°C), and then processed using
analytical grade ethanol and xylene. The paraffinized sections (4–5
µm thick) were stained with H&E (hematoxylin, 3–8 min at
37°C and eosin, 1–3 min at 37°C) to visualize inflammatory cell
infiltration, cardiomyocyte necrosis and apoptosis, and fibrosis in
myocardial tissue. As an efficacious histochemical stain for
collagen detection in tissue sections, Picrosirius red (PSR;
AS1067; Aspen Biological was used to differentiate the collagen
types. In addition, using Masson's trichrome staining (1–3 min at
37°C), the collagen volume fraction (CVF), and the interstitial
collagen volume fraction were examined to quantify the degree of
fibrosis. Cardiomyocytes were stained red and fibrous tissues were
stained in blue by Masson's Trichrome staining. The sections were
then examined by light microscopy and images were captured at ×40
and ×200 magnification. The percentages of fibrosis in the heart
were analyzed using Image-Pro Plus software (version 6.0; Media
Cybernetics, Inc., Rockville, MD, USA) and the captured images and
their average values were assessed.
Immunofluorescence assay
The experimental steps were as follows: Sections of
myocardial tissue were fixed with 4% paraformaldehyde (4 µm)
for ≥24 h at 37°C, followed by washing with phosphate-buffered
saline (PBS) for 30 min, and then blocked for 1 h with PBS (37°C).
Following incubation with the primary antibody (anti-Pin1 antibody,
1:25) overnight at 4°C, the cardiac tissues were incubated with
secondary antibody (goat anti-mouse CY3, 1:50) for 1 h at room
temperature. The nuclei were visualized with DAPI (10 min, 37°C;
blue). Images were captured by fluorescence microscopy (550–750 nm;
IX51; Olympus Corp., Tokyo, Japan) using a 200X objective.
To further evaluate the collagen types, the
paraffinized sections were stained using standard
immunofluorescence staining techniques. First, the sections were
prepared, dried, dewaxed, hydrated and repaired. Second, the
sections were washed in PBS, sealed with 10% sheep serum (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 1 h at 37°C
and incubated with primary antibodies (collagen I 1:150; collagen
III 1:200) at 4°C overnight. The sections were washed again with
PBS and incubated with the corresponding secondary antibodies (goat
anti-rabbit CY3 1:50; goat anti-mouse FITC, 1:50) for 1 h at 37°C.
The cells were immunostained with antibodies against collagen type
I (red) and collagen type III (green), and the nuclei were labeled
with DAPI (10 min, 37°C; blue).
Fluorogenic probe dihydroethidium (DHE)
assay and biochemical estimations
Considering the important function of oxidative
stress in ventricular remodeling, the present study examined the
contribution of Pin1 to ISO-mediated oxidative stress using a DHE
assay and biochemical analyses. The fluorogenic probe solution was
diluted 1:10,000 according to the manufacturer's protocols and
protected from the light. The heart tissues were isolated, washed
in PBS and frozen at −80°C. Following sectioning with a
Microtome-Cryostat (CM1900; Leica Microsystems, Inc., Buffalo
Grove, IL, USA), the frozen heart sections were incubated with DHE
solution for 30 min at room temperature to allow incorporation of
the DHE into the membrane. Oxidative stress was indicated by red
staining under a fluorescence microscope (550–750 nm; IX51; Olympus
Corp.). A total of 10 fluorescent images per section of DHE
staining were captured using a confocal microscope (magnification,
×40 and ×200). Additionally, the activity of SOD and levels of MDA
were quantified in the serum using detection kits according to the
manufacturer's protocols.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from left ventricle tissues
with TRIzol reagent (15596-026; Invitrogen; Thermo Fisher
Scientific, Inc.) and reverse-transcribed to cDNA according to the
manufacturer's protocols. The synthesis of the first cDNA was
carried out in two stages: The first reaction solution (5 g DNA
eraser buffer 2.0 µl, gDNA eraser 1.0 µl, RNA 1.0
µg, RNase Free dH2O up to 10.0 µl) was
prepared on ice to remove genome DNA; Primescript RT enzyme mix Ⅰ
(1.0 µl), RT primer mix (1.0 µl), 5*Primescript
buffer 2 (4.0 µl) and RNase Free dH2O (4.0
µl) was then added to the first reaction solution for
reverse transcription PCR. RT-qPCR analysis of the mRNA levels of
Pin1, collagen type I and III was performed using the Toyobo First
Strand cDNA synthesis kit (ReverTra Ace-α-; FSK-100; Toyobo Co.,
Osaka, Japan). The qPCR pre-denaturation was performed at 95°C for
1 min; followed by 40 cycles at 95°C for 15 sec, 58°C for 20 sec
and 72°C for 45 sec. The melting curve was performed at a
temperature ranging between 60 and 95°C (1°C every 20 sec) using
the SYBR® Premix Ex Taq™ kit (RR420A; Takara Bio, Inc.,
Otsu, Japan) on a StepOne™ Real-Time PCR apparatus (Thermo Fisher
Scientific, Inc.). Each experiment was performed in triplicate. The
following gene primers (Invitrogen; Thermo Fisher Scientific, Inc.)
were used: Pin1, forward, 5′-CTGGTGAAGCACACCAATCT-3′ and reverse,
5′-GATGCCTGAATCCGTGAACAC-3′; collagen I, forward,
5′-CCGTGACCTCAAGATGTGCC-3′ and reverse, 5′-GAACCTTCGCTTCCACTCG-3′;
collagen III, forward 5′-TGTCCACAGCCTTCTACACCT-3′ and reverse,
5′-TAGCCACCCATTCCTCCG-3′; GAPDH, forward,
5′-CGCTAACATCAAATGGGGTG-3′ and reverse,
5′-TTGCTGACAATCTTGAGGGAG-3′. GAPDH was used as the invariant
control (22).
Western blot analysis
Western blot analysis was performed to assess the
protein expression levels of Pin1, α-SMA, pMEK1/2 and pERK1/2 in
the myocardium in the different experimental groups. Left ventricle
myocardial tissue was homogenized in an appropriate volume of
ice-cold radioimmunoprecipiation lysis buffer (AS1004; Aspen
Biological). Subsequently, the supernatant was collected following
centrifugation for 3,600 × g for 5 min at 4°C. The protein
concentrations of the samples were determined using the
Bicinchoninic Acid protein assay kit (AS1086; Aspen Biological).
Equal quantities of samples (40 µg) were loaded onto gels
for sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) (10% for GAPDH, α-SMA, pMEK1/2 and pERK1/2; 12% for
Pin1) and blotted onto polyvinylidene fluoride membranes, which
were blocked with 5% skim milk for 1 h at 37°C to reduce any
nonspecific binding. The membranes were then incubated with various
primary antibodies against α-SMA (1:5,000), Pin1 (1:1,000), pMEK1/2
(1:1,500) and pERK1/2 (1:1,500) overnight at 4°C. Following washing
for three times with Tris-buffered saline containing 0.1% Tween-20,
the membranes were treated with either HRP-conjugated goat
anti-rabbit or goat anti-mouse secondary antibodies at 1:10,000
dilutions for 1 h at room temperature to detect immunoreactive
bands. The optical density was determined with AlphaEaseFC software
v4.00 (Alpha Innotech, San Leandro, CA, USA) and an enhanced
chemiluminescence kit (AS1059; Aspen Biological) was used to
analyze the chemiluminescence of the blots. GAPDH was used as the
loading control.
Statistical analysis
All experiments were repeated at least three times
with consistent results and SPSS 17.0 (SPSS, Inc., Chicago, IL,
USA) was used for statistical analyses. The data are presented as
the mean ± standard deviation. One-way analysis of variance was
used to compare multiple groups, followed by Tukey's post hoc
analysis. Independent sample t-tests were used to compare two
groups as appropriate. P<0.05 was considered to indicate a
statistically significant difference.
Results
Pin1 aggravates histopathological changes
in cardiac fibrosis induced by ISO in vivo
In the present study, ISO was selected to establish
cardiac remodeling. ISO is a non-selective β-AR agonist used to
pharmacologically activate the actions of cardiac myocytes and for
treatment of chronic heart failure, and is widely used to establish
cardiac remodeling models by altering signal transduction pathways
and activating β-AR. The subcutaneous administration of ISO, which
mimics β-AR activity, can produce myocardial necrosis (23). Continuous sympathetic activation
in the myocardium promotes the increase of left ventricular mass
and cardiac noradrenaline. Catecholamines activate β-AR through the
classic Smads signaling pathway and promote the transfer of nuclear
factor-κB to the cells, which accelerates the progression of
myocardial fibrosis. Increasing evidences suggests that ISO
treatment at high doses increases myocardial oxidative stress and
pro-inflammatory cytokine synthesis, and stimulates MAPKs (3). Cardiac fibrosis induced by ISO is a
reliable, consistent and well-characterized prototype, correlated
with arrhythmias, myocyte loss and fibrosis with advancement to
heart failure. Therefore, it is possible to use this method to
establish a model of fibrotic remodeling in rats.
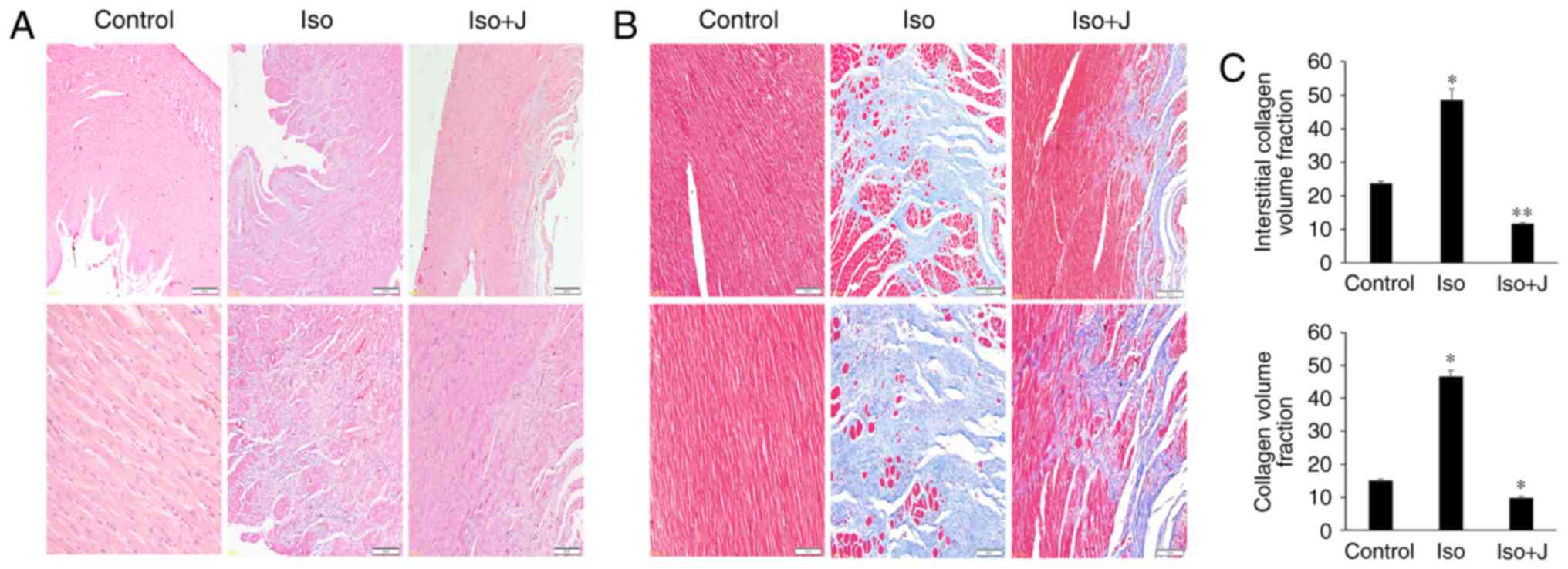
To investigate the variations in histopathological
and morphologic alterations in the experiment intervention groups,
H&E and Masson's trichrome staining were applied to evaluate
cellular inflammatory infiltration, cardiomyocyte necrosis, and
apoptosis and interstitial fibrosis in the different groups. As
shown in Fig. 1A, substantial
inflammatory cellular infiltrate in the interstitium and
disorganized cardiac muscle fibers were observed in the ISO group,
compared with the control group. However, these effects were
reduced in the group treated with juglone following ISO. In
addition, compared with the control group, the myocardial tissue
was arranged irregularly and was congested with large quantities
and disordered collagen fibers in the ISO group (Fig. 1B). A marked decrease in fibrotic
connective tissue was also noted in the ISO+juglone group, which
was consistent with the previous H&E staining results. To
accurately differentiate the differences in the degree of fibrosis
in the myocardium in response to ISO, the percentages of fibrotic
tissue was determined in the captured images using Image-Pro plus
software (version 6.0). The CVF and interstitial collagen volume
fraction were significantly increased in the ISO group (P<0.01).
However, juglone significantly reduced the CVF (P<0.01) and
interstitial collagen volume fraction (P<0.05) following ISO
injection in rats (Fig. 1C).
Pin1 accelerates cardiac dysfunction
caused by ISO in vivo
To probe the underlying association between Pin1 and
cardiac function, echocardiography was used to evaluate changes in
the size, shape and function of the heart. Compared with the
control group, the group of rats treated with ISO suffered marked
injury, as indicated by the sharp decrease in LVPW, IVS, EF%, FS%
and the ratio of HW/BW (Fig. 2A and
B). The macroscopic image shown in Fig. 2A approximately reflects the
significant dilation of the left ventricle in the ISO group.
Compared with the control group, the ratio of HW/BW was lower in
the ISO-treated rats (P<0.01). No statistically significant
differences were found in HW/BW between the wild-type (WT) controls
and rats that treated with juglone following ISO injection, with
the exception of a marginal decrease. According to the statistics
presented in Fig. 2B, the EF% in
the control group was maintained at 84.97±0.47%, but decreased to
64.32±1.54% in the ISO rats and increased to almost 75.54±0.99% in
the ISO+juglone group (P<0.05); IVS was significantly lower in
the ISO group (2.7±0.2 mm), compared with the other experimental
groups. By contrast, FS% decreased from 48.45±0.49% in the control
group to ~31.03±1.08% in the ISO group (P<0.01), and then
increased again to ~38.21±0.66% in the ISO+juglone group.
Similarly, the overall tendency observed for LVPW mimicked the
fractional shortening in the bar graph (Fig. 2B). Based on the aforementioned
results, it was concluded that the subcutaneous injection of ISO in
rats successfully induced myocardial injury and that juglone
improved the impaired left ventricular structure induced by ISO
in vivo.
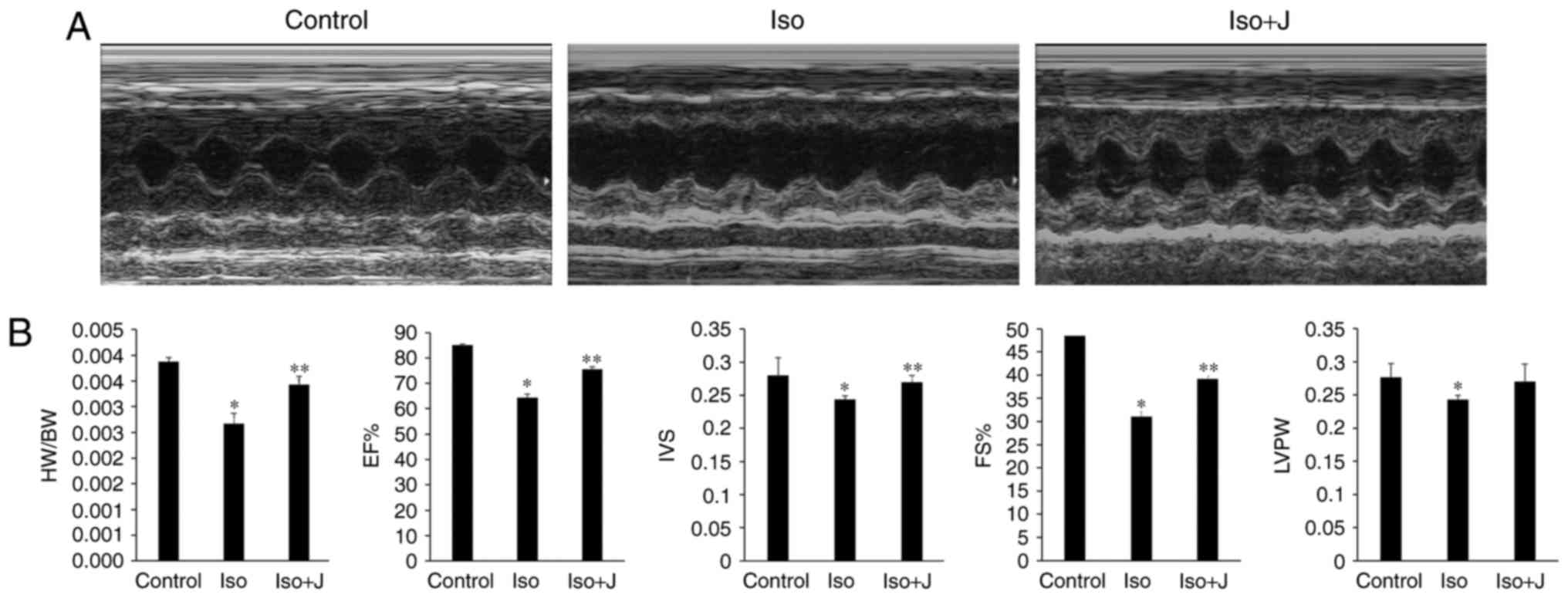 | Figure 2Assessment of cardiac structure and
function. (A) Representative M-mode echocardiograms in the control,
ISO and ISO+J groups. (B) Bar charts from the left show the HW/BW
ratio, EF%, IVS, FS%, and LVPW. *P<0.01 ISO group vs.
control group; **P<0.05 ISO group vs. ISO+J group for
EF%, IVS and FS%; *P<0.05 ISO group vs. control group
for LVPW. Control, normal rat. ISO, rat intraperitoneally injected
with ISO (5 mg/kg); ISO+J, ISO rat intraperitoneally injected with
juglone (3 mg/kg). Data are presented as the mean ± standard error
of the mean from at least three different independent experiments.
ISO, isoprenaline; J, juglone; EF%, left ventricular ejection
fraction; IVS, interventricular septal thickness; FS%, fractional
shortening; LVPW, left ventricular posterior wall thickness. |
Effect of Pin1 on the deposition and mRNA
expression of collagen I and III in ISO-induced rats
Fibrogenesis is characterized by the excessive
accumulation and deposition of extracellular matrix (ECM) proteins.
The quality and organization of collagen fibers significantly
affects the pathogenesis of cardiac fibrosis and the tensile
strength of interstitial tissue. ECM proteins, including collagen I
and collagen III, which are the most abundant collagen types in
cardiac tissue and predominantly contribute to interstitial
fibrosis, respond to various pathological stimuli. Therefore, PSR
staining, immunofluorescence assays and RT-qPCR techniques are used
to detect the deposition and mRNA transcription of collagen I and
III, reflecting the association between Pin1 and alterations in
collagen organization. As shown in Fig. 3A, regular and thin collagen fibers
distributed in the myocardium of normal rats were stained red in
the PSR-stained sections. Compared with the vehicle group, the
ISO-treated rat tissues showed excessive interstitial and
perivascular collagen deposition, and fragmented and disordered
myocardial muscle bundles (Fig.
3A), whereas juglone treatment significantly attenuated the
deposition of collagen (P<0.05). Additionally, an
immunofluorescence technique devised to further differentiate
collagen types among the different groups (Fig. 3B) showed significantly higher
expression levels of collagen I than III in the rats treated with
ISO+juglone, compared with those in rats treated with ISO only.
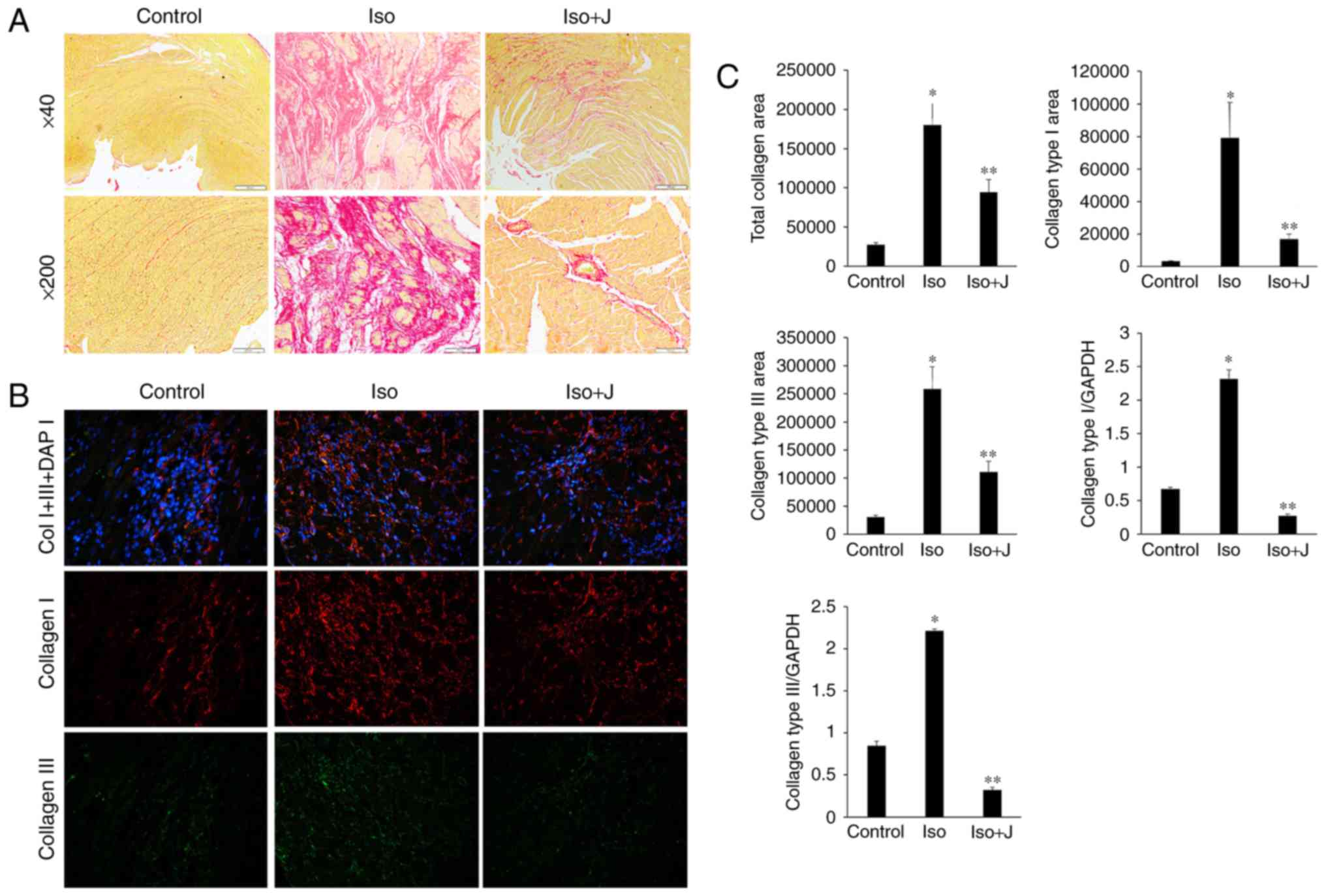 | Figure 3Qualitative and quantitative analysis
of collagen accumulation. (A) Picrosirius red staining of
histological sections of the left ventricle to assess collagen
deposition in different groups (magnification, ×40, top panels and
×200, bottom panels). (B) Immunofluorescent staining of collagen I,
collagen III and total collagen proteins. Collagen I is stained
red, collagen III is stained green, nuclei are stained blue with
4,6-diamidino-2-phenylindole (magnification, ×200). (C) Bar graphs
shows the results of the quantitative analysis of the expression of
collagen I, collagen III, total collagen proteins, collagen
deposition, and mRNA levels of collagen type I and collagen type
III, which were measured using an image analysis system.
*P<0.01 ISO group vs. control group;
**P<0.01 ISO group vs. ISO+J group control, normal
rat. ISO, rat intraperitoneally injected with ISO (5 mg/kg). ISO+J:
ISO rat intraperitoneally injected with juglone (3 mg/kg). Data are
presented as the mean ± standard error of the mean from at least
three independent experiments. ISO, isoprenaline; J, juglone. |
In the present study, the percentage of the collagen
fibers in the ISO group increased, compared with that in the
control group (P<0.01). By contrast, the percentage of fibrous
tissue, including collagen I and III, and total collagen in the
ISO+juglone group was significantly lower, compared with that in
the ISO group (P<0.01). In addition, the varying trends in the
mRNA expression levels of collagen I and III, as determined using
RT-qPCR analysis, were consistent with the immunofluorescence
results (P<0.01 vs. control group; P<0.01 vs. ISO+juglone
group; Fig. 3C). Taken together,
these results suggested that excessive generation and deposition of
collagen were associated with increased mRNA and protein levels of
Pin1, whereas treatment with juglone decreased the expression of
collagen I and III via the inactivation of Pin1.
Effect of Pin1 on oxidative stress and
antioxidant parameters in ISO-treated rats
Oxidative stress is one of the mechanisms underlying
myocardial remodeling, which regulates the ECM by activating matrix
metalloproteinase (MMP) and stimulating excess production of
reactive oxygen species (ROS), ultimately resulting in collagen
synthesis and the inflammatory response (24). In the present study, it was
hypothesized that Pin1 is involved in ISO-induced cardiac fibrosis
by regulating oxidative stress. To confirm this hypothesis,
oxidative stress and antioxidant parameters were assessed in plasma
and tissue samples using the DHE assay and biochemical analyses.
Previous studies have shown that several bioactive substances are
involved in the pathophysiological process of oxidative stress,
among which ROS, reactive nitrogen species and lipid peroxides are
crucial mediators. Conversely, MDA has been attributed to lipid
peroxidation and indicates the degree of plasma membrane damage
(25,26). Therefore, ROS, MDA and SOD were
selected for the subsequent determination of oxidative damage.
As shown in Fig.
4A, minimal ROS immunofluorescence was detected in the control
group, however, its immunofluorescence was marked in the ISO group.
In the ISO+juglone group, the immunofluorescence of ROS in the
nuclei of proliferating cells in the myocardium was weak, compared
with that in the ISO group. To further confirm the association
between the expression of Pin1 and the degree of oxidative stress,
the levels of MDA and SOD in the plasma and heart were evaluated.
The ISO-treated rats showed significantly increased activity of
SOD, an antioxidant enzyme (P<0.01) and increased levels of the
lipid peroxidation product, MDA (P<0.01) in cardiac tissue,
compared with the levels in the normal rats. In addition, the
administration of juglone following ISO injection promoted the
reduction in SOD activity (P<0.01) and downregulated the levels
of MDA (P<0.01) and ROS in the heart, compared with the
observations in to the ISO-treated group (Fig. 4B). Accordingly, these results
confirmed that the protein level of Pin1 was also associated with
oxidative stress and antioxidant substance expression during the
development and progression of cardiac fibrosis, whereas the
suppression of Pin1 markedly weakened the oxidative damage and
occurred synchronously with heart injury. Of note, the experimental
results of the biochemical analyses were consistent with the
functional parameters observed on echocardiography (Fig. 2) and the statistical analysis of
collagen deposition (Fig. 3),
which also confirmed that Pin1 significantly accelerated the
production of ROS and the cardiac dysfunction induced by ISO.
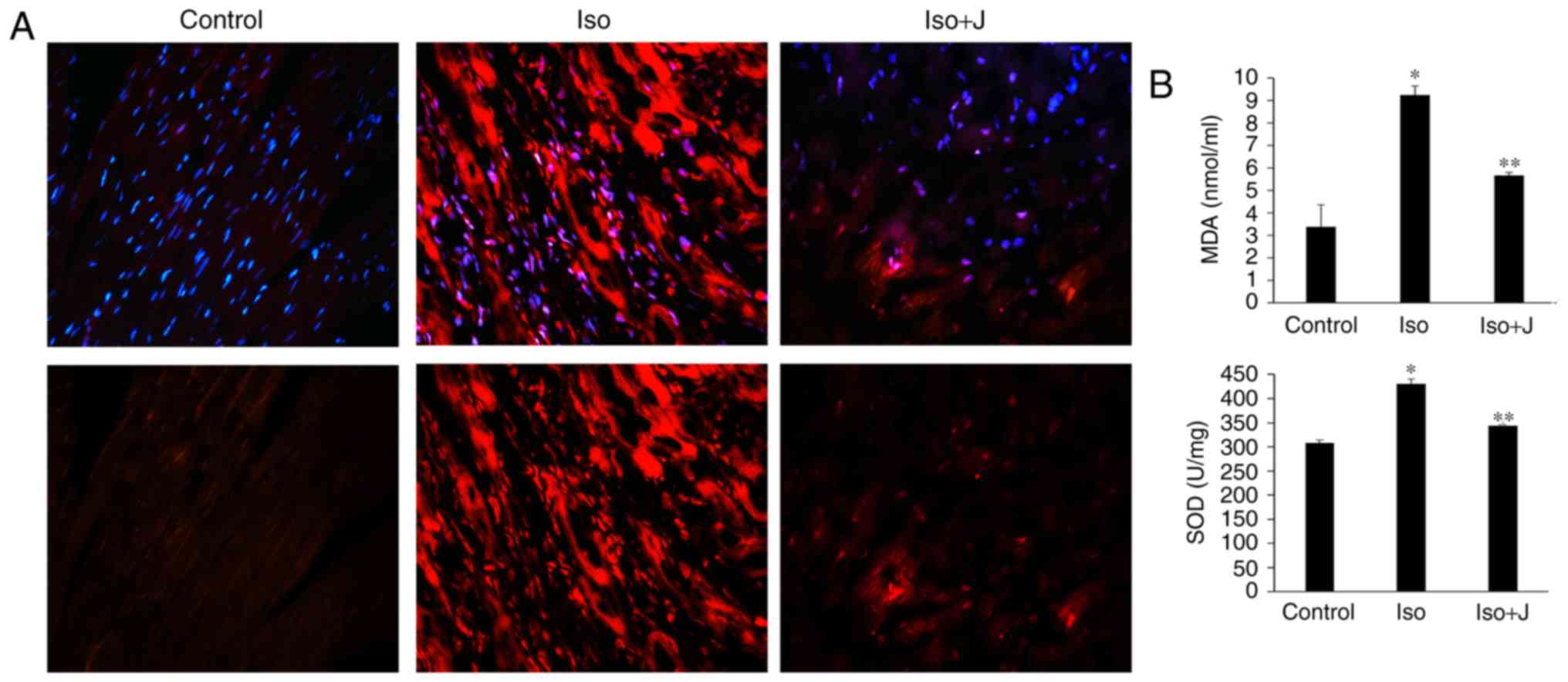 | Figure 4Oxidative stress and measurements of
MDA and SOD. (A) Oxidative stress was detected using the
immunofluorescent probe-DHE (magnification, ×40 and ×200 above and
below, respectively). Reactive oxygen species were stained red and
nuclei were stained blue with 4,6-diamidino-2-phenylindole
(magnification, ×200). (B) Expression of MDA in serum and SOD in
heart tissue. *P<0.01 ISO group vs. control group;
**P<0.01 ISO group vs. ISO+J group. Data are
presented as the mean ± standard error of the mean from at least
three independent experiments. Control, normal rat; ISO, rat
intraperitone-ally injected with ISO (5 mg/kg); ISO+J, ISO rat
intraperitoneally injected with juglone (3 mg/kg). ISO,
isoprenaline; J, juglone; MDA, malondialdehvde; SOD, superoxide
dismutase. |
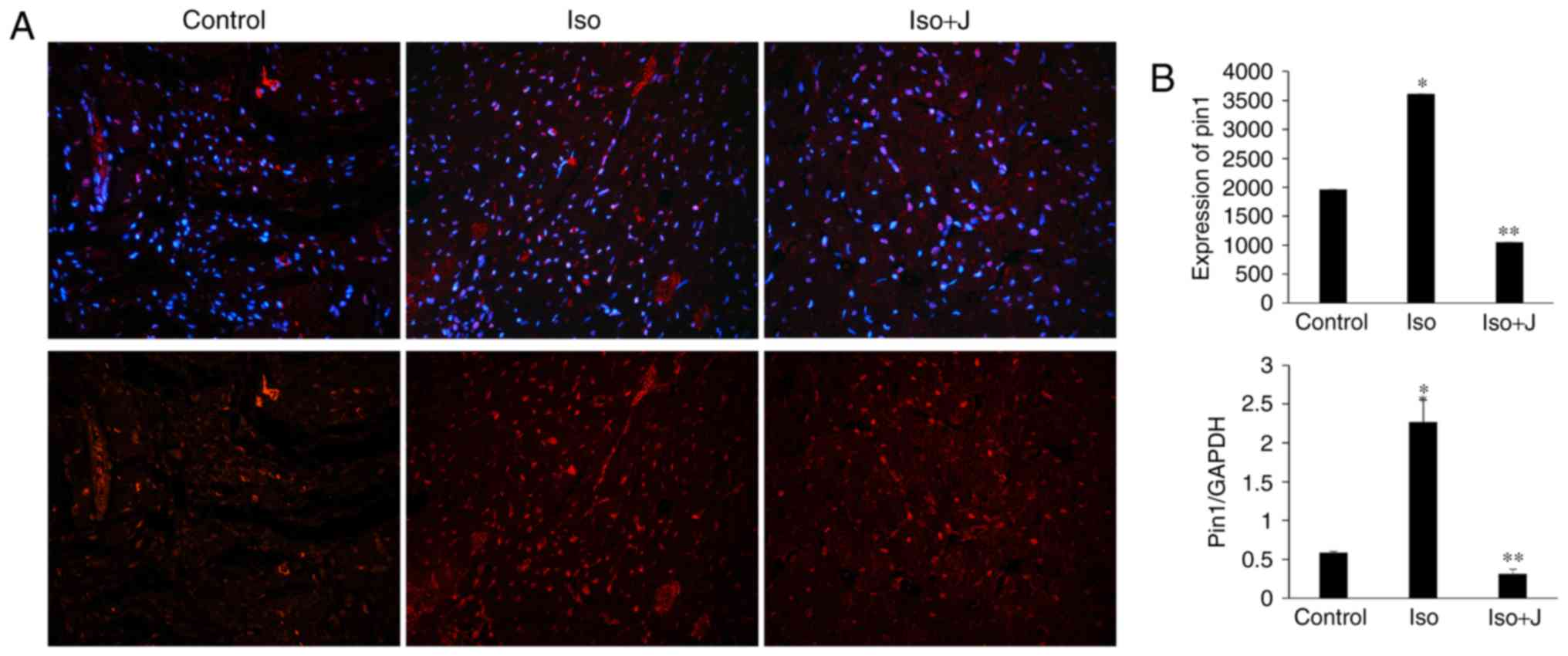
Pin1 protein translation and activation
of MEK/ERK signaling in ISO-treated rats
To examine the association among Pin1, cardiac
fibrosis and dysfunction in the present study, the expression of
Pin1 was evaluated in the different groups. The animal models in
the ISO groups showed significantly elevated mRNA expression of
Pin1 (Fig. 5) and phosphorylated
protein levels of Pin1 (Fig. 6),
compared with the control group. In the group treated with ISO and
juglone, the protein level of pPin1 was downregulated in the heart
(Fig. 6). Additionally, the
increased mRNA expression of Pin1 in the ISO group (P<0.01) was
reduced in the ISO+juglone group (P<0.01) (Fig. 5).
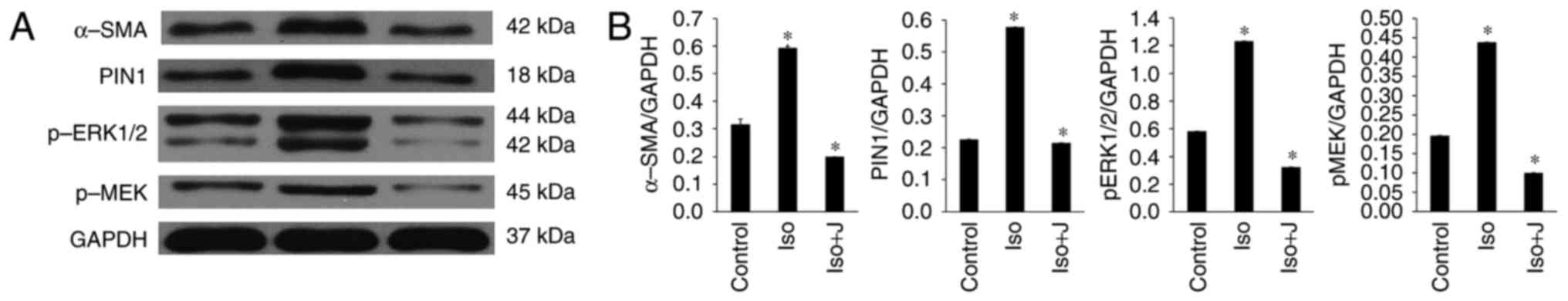 | Figure 6Expression of MEK1/2-ERK1/2 signal
transduction pathway-related proteins. (A) Western blot analysis
results showing the protein expression levels of α-SMA, Pin1,
pERK1/2 and pMEK. (B) Ratio of α-SMA, Pin1, pMEK1/2 and pERK1/2 to
GAPDH. *P<0.01. Control, normal rat; ISO, rat
intraperitoneally injected with ISO (5 mg/kg); ISO+J, ISO rat
intraperitoneally injected with juglone (3 mg/kg). Data are
presented the mean ± standard error of the mean derived from at
least three independent experiments. ISO, isoprenaline; J, juglone;
MEK1/2, mitogen-activated protein kinase kinase 1/2; ERK1/2,
extracellular-signal regulated protein kinase 1/2; pMEK1/2,
phosphorylated MEK1/2; pERK1/2, phosphorylated ERK1/2; GAPDH,
glyceraldehyde phosphate dehydrogenase. |
According to the results of the western blot
analysis, ISO promoted the activation of pERK1/2 and protein
transcription of Pin1 in response to pressure stress, and these
effects were efficiently suppressed by the Pin1-specific inhibitor
juglone. It was also demonstrated that ISO stimulated the
expression of pMEK1/2 in vivo, which is a kinase functioning
upstream from ERK1/2. Additionally, the protein expression of
α-SMA, a specialized type of protein observed in activated cardiac
fibroblasts and serving as a biomarker for cardiac fibrosis, was
markedly upregulated in the group of rats treated with ISO.
Discussion
Myocardial remodeling represents one of the most
relevant cardiac adaptability responses to pathological
hyperplasia, consisting of structural and electrical remodeling.
However, microstructural analysis has shown that the structural
remodeling is dependent on cardiac hypertrophy and interstitial
fibrosis (27). During the
initial stage of myocardial fibrosis and dysfunction, activation of
the RAAS system promotes the production of catecholamines and
activates the cardiac sympathetic ganglion to meet physical
requirements during rest or exertion (28). However, the compensatory phase of
this beneficial adaptation is irreversibly translated to the
cardiac functional decompensatory period, ultimately leading to
end-stage organ failure and even sudden death (29). Substantial injury and necrosis of
cardiomyocytes and nonfunctional fibrous tissue increase myocardial
stiffness, reduce diastolic function, and thereby affect the right
and left ventricles (30).
Collectively, an improved understanding of the potential mechanism
underlying myocardial fibrosis is likely to enable the exploitation
of novel therapeutic targets for cardiovascular diseases following
various forms of damage.
Peptidyl-prolyl cis/trans isomerase is generally
present in prokaryotic and eukaryotic organisms, modulating the
three-dimensional conformation of phosphoproteins, and it is
classified into three subfamilies: Cyclophilin, FK binding protein
and parvulin. As a member of the evolutionarily conserved
peptidyl-prolyl isomerase family, Pin1 was first identified and
cloned from a yeast-two hybrid system in 1996. The expression of
Pin1 is mainly localized at high levels in the nucleus of the
neonatal heart; following maturity, it is transferred to the
cytoplasm with reduced expression. Pin1 has emerged as a novel
molecule, which is involved in several complex cellular processes,
including cell cycle progression, neuronal differentiation and
survival, and genotoxic and cellular stress responses, and in the
pathogenesis of cancer, Alzheimer's disease, Parkinson's disease
and rheumatoid arthritis in humans (31). As a member of the parvulin
subfamily, Pin1 has attracted interest in cardiovascular research
as it catalyzes the conversion of specific pSer/Thr-Pro motifs
between two completely different conformations in a subset of
proteins. The functional protein acts as a signal to recruit other
proteins into signaling networks or to drive substrate close to
their catalytic sites, triggering numerous synergistic signaling
cascades in the pathophysiology of cardiac tissue. As reported by
Sakai et al (32), the
activation of Pin1 is involved in endothelin-1 (ET-1)-induced
cardiomyocyte hypertrophy by upregulating the transcriptional
activity of the c-Jun N-terminal kinase signaling pathway, and by
promoting hypertrophic protein synthesis, cardiomyocyte growth and
cytoskeletal reorganization. However, the activity of Pin1 during
cardiac fibrosis of maladaptive remodeling has not been
investigated. In the present study, marked inflammatory cell
infiltration, significant interstitial fibrosis and cardiac
dysfunction were observed following ISO stimulation, as
demonstrated by pathological examination, echocardiography and
immunofluorescence, together with an increase in the protein
expression of pPin1. These results suggested that the activation of
Pin1 was involved in the fibrotic responses, and contributed to the
structural and functional abnormalities observed in the ISO-treated
rats. Following intervention with juglone, a specific inhibitor of
Pin1, the formation of fibrous tissue and systolic and diastolic
dysfunction were alleviated. Pin1 inhibitors, including the natural
product juglone, the small molecule polyisobutylene and others,
synchronously inhibit multiple signaling pathways and subsequently
prevent the activation of specific kinases or phosphatases. Juglone
is an active ingredient in the traditional Chinese herb Sophora
japonica Ait, and possesses anti-inflammatory, antioxidant and
antifibrotic activities (33).
The present study also demonstrated that the protein expression of
α-SMA manifested a trend consistent with that of Pin1. Taken
together, these findings suggested that the activation of Pin1 had
a positive effect on the cardiac fibrosis process following cardiac
injury and that juglone exerted cardioprotective effects subsequent
to the inactivation of Pin1.
Accumulating evidence has shown that cardiac
remodeling is triggered and affected by the simultaneous action of
diverse and cross-linked signaling cascades. Pin1 is a small
protein, which contains a unique N-terminal WW domain and
C-terminal catalytic peptidyl prolyl isomerase domain. As the only
known enzyme to specifically recognize pSer/pThr-Pro motifs, Pin1
affects the transcriptional, translational and post-translational
protein conformations associated with various cell signaling
pathways (32). Transforming
growth factor-β (TGF-β), a component of a well-known profibrogenic
signaling cascade, stimulates myofibroblast proliferation,
migration and differentiation, and facilitates ECM synthesis, which
has significant consequences for the pathogenesis of cardiac
fibrotic alterations. Previous studies have shown that Pin1
activates Smad-dependent pathways to mediate ECM deposition in
bleomycin-induced pulmonary fibrosis, based on the interaction with
Smad3 and Smad6. Notably, Pin1-KO mice exhibit apparently blunted
ECM production and deposition in vitro and in vivo.
These observations suggest that Pin1 exacerbates collagen
accumulation and aggravates airway remodeling in an animal model of
lung fibrosis, which is most commonly induced by activating the
Smad pathway (34). In addition,
previous experimental evidence supports the hypothesis that
upregulated Pin1 contributes to the advancement of cardiac
remodeling and fibrosis in diabetic mice. Consistent with the
experimental results, the complicated pathogenesis of Pin1 in
myocardial fibrosis and dysfunction caused by streptozotocin was
found to lie principally in the phosphorylation of
phosphatidylinositol 3 kinase/protein kinase B pathways, the
TGF-β1/Smad pathway, MMP balance and the expression of
α-SMA (35). Therefore, multiple
molecular signals and the complexity of their interactions are
implicated in the fibrotic response. Although several studies have
examined the association between Pin1 and cardiovascular disease,
the precise mechanism underlying the involvement of Pin1 in cardiac
fibrosis through non-Smad pathways has not been elucidated.
Therefore, the present study concentrated on other transduction
pathways in heart remodeling, with a focus on the MEK1/2-ERK1/2
signaling pathway. During activation of the Ras/Raf/MEK/ERK
signaling pathway, Ras GTPase recruits and stimulates Raf kinase
and then phosphorylates MEK1/2, accompanied by activated
MEK1/2-induced phosphorylation of the downstream kinases ERK1/2.
Once released and activated, ERK1/2 is involved in the
phosphorylation of numerous cytoplasmic targets and activates
multiple transcription factors in the nucleus, ultimately resulting
in the reprogramming of cardiac gene expression (36). Zheng et al (37) indicated that high expression
levels of constitutively active Ras activate downstream molecules
in the MEK-ERK1/2 pathway, inducing disarrangement of the
myocardium and interstitial fibrosis in hypertrophic
cardiomyopathy. By contrast, certain studies have indicated that
different mutations promote ERK1/2 pathway activation, inducing
cardiac pathologies in patients with genetic disorders (38,39). However, less is known about the
mechanisms of Pin1 and the MEK1/2-ERK1/2 signaling pathways in
cardiac fibrosis in response to ISO. The present study provides the
first detailed description, to the best of our knowledge, of the
specific mechanism underlying this phenomenon.
Fibrogenesis is characterized by the excessive
accumulation and deposition of ECM. The proper functioning of
mesenchymal components is essential for cardiovascular health,
predominantly due to its antiproliferative, anti-overstretch, and
antideformative properties. ECM proteins are a family of complex
macromolecules, which are involved in the structural and functional
dynamic equilibrium in the heart with respect to myocardial
compliance, tissue differentiation and angiogenesis. Fibrillar
collagens are the most abundant collagen types in the myocardium
(40), exerting essential
physiological control on reparative and fibrotic pathways. These
fibers are expressed at relatively high levels during the
pathogenesis of myocardial remodeling. Generally, there are five
types of collagen subtypes in the myocardium, of which types I and
III account for >90% of the total collagen content of the heart
and are predominantly responsible for interstitial fibrosis
(41). However, disturbance of
the regulated balance of the secretion, synthesis and degradation
of collagen metabolism leads to structural and functional
impairments in remodeling hearts. A study by Shen et al
(42) examined the role of Pin1
in an animal model of renal fibrosis based on a high phosphate diet
(HPD) for 8–12 weeks. It was found that individual ECM genes, which
are known to be involved in tissue fibrosis, including collagens
I/III/V, fibronectin-1 and TGF-β1, were significantly increased at
the mRNA level in the HPD kidney, whereas low expression levels and
reduced accumulation in the ECM were observed in WT and Pin1-KO
mouse kidneys. These findings also showed that Pin1 was required
for macrophage recruitment and chemokine expression during the
inflammatory response following HPD, excluding ECM production.
These results suggest that Pin1 modulates HPD-induced renal
fibrosis by regulating calcium deposition, proinflammatory cytokine
expression, macrophage infiltration and ECM accumulation. Similar
to previous findings, the present study revealed excessive
interstitial and perivascular collagen deposition in an ISO-induced
model of fibrosis. Compared with the ISO group, the CVF and
interstitial collagen volume fraction were significantly decreased
in the ISO+juglone group, and no abnormalities were observed in the
WT controls. Additionally, the expression and distribution of type
III collagen was more prominent, compared with that in type I
collagen in the fibrotic heart, as revealed by immunofluorescence
and RT-qPCR analysis, and this effect was completely alleviated
following the administration of juglone. These results are in
agreement with previous studies, and demonstrated that Pin1 was
required for collagen accumulation following ISO-induced cardiac
dysfunction and fibrosis. Additionally, the suppression of Pin1
effectively improved the synthesis and deposition of collagen
protein.
One of the most important pathological mechanisms of
cardiac fibrosis is the imbalance between pro-oxidant and
antioxidant defense, also known as oxidative stress, which is a
crucial regulator of various heart diseases and contributes to the
pathophysiology of congestive heart failure. ROS are important
components of fibrotic remodeling through directly modulating the
expression of ECM, disrupting the balance of substrate metabolism,
and transferring the quantity and quality of the interstitial ECM.
A study by Frantz et al showed that the upregulation of ROS
promoted the formation of disulfide linkages, altered protein
conformation and activated multiple cellular processes involved in
apoptosis, necrosis and senescence (43). Given the observed data, the
appropriate management of ROS is imperative for regulating
oxidative stress (44).
Additionally, animal experiments have shown that mitochondrial
dysfunction and oxidative stress are involved in the initiation and
development of left ventricular remodeling and heart failure
following myocardial infarction (45). These findings suggest that
oxidative damage and fibrosis interact with each other, and
accelerate structural alterations and ventricular dysfunction
during deteriorating cardiac remodeling. In another study, Tanaka
et al attributed the ET-1 or phenylephrine-mediated
overexpression of ROS in cardiomyocytes to a reactive sympathetic
nervous system. Under the same stimulating conditions, ERK1/2
activity was also increased. Antioxidant treatment of the
cardiomyocytes effectively prevented the increased production of
ROS and inhibited the activation of ERK1/2. These experimental
results suggest that ROS can be inhibited by inactivation of the
ERK1/2 pathway (46). Xu et
al showed that ERK1/2 signaling pathway activation is
responsible for diabetic myocardial pathologic changes, including
oxidative stress, the inflammatory response, apoptosis and
remodeling (7). However, it has
been demonstrated that Pin1 regulates the mitochondrial import of
the 66-kDa isoform of the growth factor adaptor Shc (p66Shc) and
promotes ROS release in mitochondria, which are involved in the
regulation of oxidative damage (47). However, in the phosphorylation of
signaling and disease (11), the
mechanism by which Pin1 coordinates the cardiac fibrosis remains to
be fully elucidated. Based on the aforementioned findings, the
results of the present study indicated that SOD, an important
antioxidant, and MDA, acting as a biomarker for lipid peroxidation
injury, were significantly increased in the remodeling heart
treated with ISO, compared with those in the control and
ISO+juglone groups. The rats with ISO-induced myocardial fibrosis
showed enhanced ROS production based on immunofluorescence, which
was consistent with the changes in the expression of different
oxidative stress markers, compared with the control rats.
Conversely, oxidative stress marker activity was decreased in rat
plasma and tissues exposed to ISO+juglone. In addition, the
phosphorylated protein levels of Pin1, MEK1/2, ERK1/2 and α-SMA
were significantly elevated in association with excessive ECM
deposition and abnormal cardiac function in ISO-treated rats. Taken
together, these results led to the following conclusions: Pin1
regulated diverse functional domains underlying the cardiac
remodeling process, including collagen formation and degradation,
molecular pathways and stress responses. The administration of
juglone significantly suppressed the overexpression of ROS,
effectively attenuating myocardial collagen deposition in the
interstitium and improving fibrotic myocardial remodeling.
In conclusion, the results of the present study
suggested that the activation of Pin1 promoted cardiac
extracellular matrix deposition and oxidative stress damage by
regulating the phosphorylation of the MEK1/2-ERK1/2 signal
transduction pathway and the expression of α-SMA during the
fibrotic process. By contrast, restriction of the high expression
levels of Pin1 by treatment with juglone, a specific inhibitor of
Pin1, alleviated cardiac injury and failure in the experimental
models, and prevented the subsequent cardiac fibrosis and negative
effects induced by these stimuli. These findings indicated that the
inhibition of Pin1 exerted cardioprotective effects and attenuated
ISO-induced cardiac fibrosis, which may be an effective therapeutic
option for cardiovascular diseases and heart failure.
Acknowledgments
The present study was supported by the National
Nature Science Foundation of China (grant no. 81170085).
References
|
1
|
Grimaldi V, De Pascale MR, Zullo A,
Soricelli A, Infante T, Mancini FP and Napoli C: Evidence of
epigenetic tags in cardiac fibrosis. J Cardiol. 69:401–408. 2017.
View Article : Google Scholar
|
|
2
|
Barallobre-Barreiro J, Didangelos A,
Schoendube FA, Drozdov I, Yin X, Fernán dez-Caggiano M, Willeit P,
Puntmann VO, Aldama-López G, Shah AM, et al: Proteomics analysis of
cardiac extracellular matrix remodeling in a porcine model of
ischemia/reperfusion injury. Circulation. 125:789–802. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Davel AP, Brum PC and Rossoni LV:
Isoproterenol induces vascular oxidative stress and endothelial
dysfunction via a Gi α-coupled β2-adrenoceptor signaling path way.
PLoS One. 9:e918772014. View Article : Google Scholar
|
|
4
|
Shin E, Ko KS, Rhee BD, Han J and Kim N:
Different effects of prolonged β-adren ergic stimulation on heart
and cerebral artery. Integr Med Res. 3:204–210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gallo S, Sala V, Gatti S and Crepaldi T:
Cellular and molecular mechanisms of HG F/MET in the cardiovascular
system. Clin Sci (Lond). 129:1173–1193. 2015. View Article : Google Scholar
|
|
6
|
Wang S, Luo M, Zhang Z, Gu J, Chen J,
Payne KM, Tan Y, Wang Y, Yin X, Zhang X, et al: Zinc deficiency
exacerbat es while zinc supplement attenuates cardiac hypertrophy
in high-fat diet-induced obese mice through modulating p38
MAPK-dependent signaling. Toxicol Lett. 258:134–146. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu Z, Sun J, Tong Q, Lin Q, Qian L, Park Y
and Zheng Y: The role of ERK1/2 in the development of diabetic
Cardiomyopathy. Int J Mol Sci. 17:E20012016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao X, Ji J, Yu LR, Veenstra T and Wang
XW: Cell cycle-dependent phosphorylati on of nucleophosmin and its
potential regulation by peptidyl-prolyl cis/trans iso merase. J Mol
Biochem. 4:95–103. 2015.
|
|
9
|
Rogals MJ, Greenwood AI, Kwon J, Lu KP and
Nicholson LK: Neighboring phosphoSer-Pro motifs in the undefined
domain of IRAK1 impart bivalent advantage for Pin1 binding. FEBS J.
283:4528–4548. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shah M, Smolko CM, Kinicki S, Chapman ZD,
Brautigan DL and Janes KA: Profiling subcellular protein
phosphatase responses to coxsackievirus B3 infection of
cardiomyocytes. Mol Cell Proteomics. 16(4 suppl 1): S244–S262.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu KP and Zhou XZ: The prolyl isomerase
IN1: A pivotal new twist in phosphorylation signalling and disease.
Nat Rev Mol Cell Biol. 8:904–916. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wulf G, Finn G, Suizu F and Lu KP:
Phosphorylation-specific prolyl isomerization: Is there an
underlying theme. Nat Cell Biol. 7:435–441. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hariharan N and Sussman MA: Pin1: A
Molecular Orchestrator in the Heart. Trends Cardiovasc Med.
24:256–262. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liao XH, Zhang AL, Zheng M, Li MQ, Chen
CP, Xu H, Chu QS, Yang D, Lu W, Tsai TF, et al: Chemical or genetic
Pin1 inhibition exerts potent anticancer activity against
hepatocellular carcinoma by blocking multiple cancer-driving
pathways. Sci Rep. 7:436392017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shen ZJ, Esnault S, Rosenthal LA, Szakaly
RJ, Sorkness RL, Westmark PR, Sandor M and Malter JS: Pin1
regulates TGF-beta1 production by activated human and murine
eosinophils and contribute stoallergic lung fibrosis. J Clin
Invest. 118:479–490. 2008.PubMed/NCBI
|
|
16
|
Driver JA, Zhou XZ and Lu KP: Pin1
dysregulation helps to explain the inverse association between
cancer and Alzheimer's disease. Biochim Biophys Acta.
1850:2069–2076. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liou YC, Sun A, Ryo A, Zhou XZ, Yu ZX,
Huang HK, Uchida T, Bronson R, Bing G, Li X, et al: Role of the
prolyl isomerase Pin1 in protecting against age-dependent
neurodegeneration. Nature. 424:556–561. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liou YC, Zhou XZ and Lu KP: Prolyl
isomerase Pin1 as a molecular switch to det ermine the fate of
phosphoproteins. Trends Biochem Sci. 36:501–514. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Toko H, Konstandin MH, Doroudgar S,
Ormachea L, Joyo E, Joyo AY, Din S, Gude NA, Collins B, Völkers M,
et al: Regulation of cardiac hypertrophic signaling by prolyl
isomerase Pin1. Circ Res. 112:1244–1252. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Toko H, Hariharan N, Konstandin MH,
Ormachea L, McGregor M, Gude NA, Sundararaman B, Joyo E, Joyo AY,
Collins B, et al: Differential regulation of cellular senescence
and differentiation by pro lyl isomerase pin1 in cardiac progenitor
cells. J Biol Chem. 289:5348–5356. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Carbone L: Pain management standards in
the eighth edition of the Guide for the Care and Use of Laboratory
Animals. J Am Assoc Lab Anim Sci. 51:322–328. 2012.PubMed/NCBI
|
|
22
|
Cho YS, Lee SY, Kim KH and Nam YK:
Differential modulations of two glyceralde hyde 3-phosphate
dehydrogenase mRNAs in response to bacterial and viral cha llenges
in a marine teleost Oplegnathus fasciatus (Perciformes). Fish
Shellfish Immunol. 25:472–476. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gauthaman KK, Saleem MT, Thanislas PT,
Prabhu VV, Krishnamoorthy KK, Devaraj NS and Somasundaram JS:
Cardioprotective effect of the Hibiscus rosa sinensis flo wers in
an oxidative stress model of myocardial ischemic reperfusion injury
in rat. BMC Complement Altern Med. 6:322006. View Article : Google Scholar
|
|
24
|
Horn MA and Trafford AW: Aging and the
cardiac collagen matrix: Novel mediators of fibrotic remodelling. J
Mol Cell Cardiol. 93:175–185. 2016. View Article : Google Scholar :
|
|
25
|
Keune WJ, Jones DR and Divecha N: PtdIns5P
and Pin1 in oxidative stress signaling. Adv Biol Regul. 53:179–189.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Paneni F, Costantino S, Castello L,
Battista R, Capretti G, Chiandotto S, D'Amario D, Scavone G,
Villano A, Rustighi A, et al: Targeting prolyl-isomerase Pin1
prevents mitochondrial oxidative stress and vascular dysfunction:
Insights in patients with diabetes. Eur Heart J. 36:817–828. 2015.
View Article : Google Scholar
|
|
27
|
Zhou S, Sun W, Zhang Z and Zheng Y: The
role of Nrf2-mediated pathway in cardiac remodeling and heart
failure. Oxid Med Cell Longev. 2014:2604292014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
D'Elia E, Vaduganathan M, Gori M, Gavazzi
A, Butler J and Senni M: Role of biomarkers in cardiac structure
phenotyping in heart failure with preserved ejection fraction:
Critical appraisal and practical use. Eur J Heart Fail.
17:1231–1239. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wencker D, Chandra M, Nguyen K, Miao W,
Garantziotis S, Factor SM, Shirani J, Armstrong RC and Kitsis RN: A
mechanistic role for cardiac myocyte apoptosis in heart failure. J
Clin Invest. 111:1497–1504. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hynes RO: The extracellular matrix: Not
just pretty fibrils. Science. 27;326:1216–1219. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ranganathan R, Lu KP, Hunter T and Noel
JP: Structural and functional analysis of the mitotic rotamase Pin1
suggests substrate recognition is phosphorylation dependent. Cell.
89:875–886. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sakai S, Shimojo N, Kimura T, Tajiri K,
Maruyama H, Homma S, Kuga K, Mizutani T, Aonuma K and Miyauchi T:
Involvement of peptidyl-prolyl isomerase Pin1 in the inhibito ry
effect of fluvastatin on endothelin-1-induced cardiomyocyte
hypertrophy. Life Sci. 102:98–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Van Raamsdonk JM and Hekimi S: Deletion of
the mitochondrial superoxide dismu tase sod-2 extends lifespan in
Caenorhabditis elegans. Plos Genet. 5:e10003612009. View Article : Google Scholar
|
|
34
|
Shen ZJ, Braun RK, Hu J, Xie Q, Chu H,
Love RB, Stodola LA, Rosenthal LA, Szakaly RJ, Sorkness RL and
Malter JS: Pin1 protein regulates Smad protein signaling and
pulmonary fibrosis. J Biol Chem. 287:23294–23305. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu X, Liang E, Song X, Du Z, Zhang Y and
Zhao Y: Inhibition of Pin1 alleviates myocardial fibrosis and
dysfunction in STZ-induced diabetic mice. Biochem Biophys Res
Commun. 479:109–115. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tarone G, Sbroggiò M and Brancaccio M: Key
role of ERK1/2 molecular scaffolds in heart pathology. Cell Mol
Life Sci. 70:4047–4054. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zheng M, Dilly K, Dos Santos Cruz J, Li M,
Gu Y, Ursitti JA, Chen J, Ross J Jr, Chien KR, Lederer JW and Wang
Y: Sarcoplasmic reticulum calcium defect in Ras-induced
hypertrophic cardiomyopathy heart. Am J Physiol Heart Circ Physiol.
286:H424–H433. 2004. View Article : Google Scholar
|
|
38
|
Purcell NH, Wilkins BJ, York A,
Saba-El-Leil MK, Meloche S, Robbins J and Molkentin JD: Genetic
inhibition of cardiac ERK1/2 promotes stress-induced apopto sis and
heart failure but has no effect on hypertrophy in vivo. Proc Natl
Acad Sci USA. 104:14074–14079. 2007. View Article : Google Scholar
|
|
39
|
Aoki Y, Niihori T, Narumi Y, Kure S and
Matsubara Y: The RAS/MAPK syndromes: Novel roles of the RAS pathway
in human genetic disorders. Hum Mutat. 29:992–1006. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Creemers EE and Pinto YM: Molecular
mechanisms that control interstitial fibrosis in the
pressure-overloaded heart. Cardiovasc Res. 89:265–272. 2011.
View Article : Google Scholar
|
|
41
|
Reichert K, Pereira do Carmo HR, Galluce
Torina A, Diógenes de Carvalho D, Carvalho Sposito A, de Souza
Vilarinho KA, da Mota Silveira-Filho L, Martins de Oliveira PP and
Petrucci O: Atorvastatin improves ventricular remodeling after
myocardial infarction by interfering with collagen metabolism. PLoS
One. 11:e01668452016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shen ZJ, Hu J, Shiizaki K, Kuro-o M and
Malter JS: Phosphate-induced renal fibrosis requires the Prolyl
isomerase Pin1. PLoS One. 11:e01500932016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Frantz S, Kelly RA and Bourcier T: Role of
TLR-2 in the activation of nuclear factor kappaB by oxidative
stress in cardiac myocytes. J Biol Chem. 276:5197–5203. 2001.
View Article : Google Scholar
|
|
44
|
Matsushima S, Ide T, Yamato M, Matsusaka
H, Hattori F, Ikeuchi M, Kubota T, Sunagawa K, Hasegawa Y, Kurihara
T, et al: Over expression of mitochondrial peroxiredoxin-3 prevents
left ventricular remo deling and failure after myocardial
infarction in mice. Circulation. 113:1779–1786. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Baudino TA, Carver W, Giles W and Borg TK:
Cardiac fibroblasts: Friend or foe. Am J Physiol Heart Circ
Physiol. 291:H1015–H1026. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tanaka K, Honda M and Takabatake T: Redox
regulation of MAPK pathways and cardiac hypertrophy in adult rat
cardiac myocyte. J Am Coll Cardiol. 37:676–685. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pinton P, Rimessi A, Marchi S, Orsini F,
Migliaccio E, Giorgio M, Contursi C, Minucci S, Mantovani F,
Wieckowski MR, et al: Protein kinase C beta and prolyl isomerase 1
regulate mitochondrial effects of the life-span determinant p66Shc.
Science. 315:659–663. 2007. View Article : Google Scholar : PubMed/NCBI
|