Introduction
Bone defects are usually caused by severe trauma,
tumor resection and periodontal disease (1,2).
The limited intrinsic regenerative ability of the bone following
destruction remains a notable medical problem and is associated
with a severe reduction in the quality of patients. Therefore, it
is important to develop methods of stimulating bone
regeneration.
Bone marrow mesenchymal stem cells (BMSCs) are stem
cell populations capable of self-renewal and may be maintained in a
multipotent state in vitro (3,4).
They are also one of the most important cells contributing to
musculoskeletal tissue development (1). BMSCs differentiate into multiple
lineages, including osteoblasts, chondrocytes, adipocytes and
myoblasts via different signaling pathways (5,6).
Preserving bone morphology and function relies on maintaining the
delicate balance between bone formation and resorption (1,2).
Osteoblasts, which form the bone, differentiate from BMSCs
(7,8). BMSCs are therefore currently
regarded as the gold standard cell source for tissue engineering
and applications in regenerative medicine (9). Previous studies have successfully
demonstrated the potential of BMSCs in promoting bone regeneration
using in vitro and in vivo models (10,11).
Carbon monoxide (CO), a byproduct of heme catalysis
by hemeoxygenase, has long been regarded as a poisonous gas.
However, previous studies have indicated that CO may be
cytoprotective as it induces vasorelaxation (12,13), inhibits cell apoptosis (14), suppresses inflammation (15) and protects organs against
ischemia/reperfusion injury (16,17). CO releasing molecule (CORMs) are
newly identified transition metal carbonyl-based compounds able to
efficiently regulate the release of CO in vitro and in
vivo under appropriate conditions (18). These molecules may therefore be
used as a novel approach of delivering CO. One such CORM, known as
CORM-3 [tricarbonylchloro (glycinato) ruthenium (II)], is fully
water-soluble and has the ability to rapidly liberate CO when
dissolved in physiological solutions (19). As with CO, CORMs exhibit potent
anti-inflammatory effects (20).
Furthermore, CORMs are able to improve vascular function by
inducing significant vasodilation in rat aortas pre-contracted with
phenylephrine (19).
Although various studies (18–20) have suggested that CORMs induce
beneficial effects, the effect of CORMs on osteogenic
differentiation remains unclear. In the present study, rat BMSCs
were used as an in vitro model to investigate the effect of
CORM-3 on osteogenic differentiation.
Materials and methods
Isolation and culture of rat BMSCs
A total of 6 male Sprague Dawley rats (specific
pathogen-free grade, aged 4–5 weeks old and weighing 100–150 g)
were obtained from the Animal Experimental Center of Shandong
University (Jinan, China). Rats were kept at a temperature of
20–25°C, humidity of 50–65% and 12-h light-dark cycle. Rats had
ad libitum access to food and water. The present study was
approved by the Ethics Committee of the School of Dentistry,
Shandong University. Rats were euthanized by intraperitoneal
injection of buffered and diluted pentobarbital sodium (150 mg/kg;
Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China), then soaked in 75% alcohol at 37°C for 10 min.
Subsequently, the femur and tibia were removed and the bone marrow
cavity was rinsed with α-Minimum Essential Medium (MEM) (Shanghai
Hui Ying Biological technology Co., Ltd., Shanghai, China)
supplemented with 20% fetal bovine serum (FBS) and 100 U/ml
penicillin-streptomycin (HyClone; GE Healthcare Life Sciences,
Logan, UT, USA). Bone marrow fluid was incubated at 37°C in an
atmosphere containing 5% CO2. The medium was changed
every 2–3 days, non-adherent cells were discarded and cells were
observed using the Olympus CKX53 phase-contrast microscope (Olympus
Corporation, Tokyo, Japan). When cells reached 80–90% confluence,
BMSCs were digested with 0.25% Trypsin (HyClone; GE Healthcare Life
Sciences) containing 0.02% EDTA. BMSCs from passages (P)3 to 5 were
used in the present study.
Flow cytometric identification of BMSC
surface antigens
BMSCs were digested with 0.25% Trypsin containing
0.02% EDTA (HyClone; GE Healthcare Life Sciences,), rinsed with
phosphate buffered saline (PBS; Beijing Solarbio Science &
Technology Co., Ltd.) three times, then resuspended in 0.5 ml PBS.
Cell density was 2×105/ml, assessed using a cell
counting plate (Shanghai QIUJING Biochemical Reagent and Instrument
Co., Ltd., Shanghai, China). Anti-cluster of differentiation
(CD)45-fluorescein isothiocyanate (FITC; cat no., ab33916, dilution
1:500), anti-CD34-phycoerythrin (PE; cat. no., ab187284, dilution
1:1000), anti-CD90-FITC (cat. no., ab226, dilution 1:500) and
anti-CD44-PE (cat. no., ab23396, dilution 1:500) monoclonal
antibodies (all from Abcam, Cambridge, MA, USA) were added
separately and were incubated with cells in the dark at 37°C for 20
min. Cells were then rinsed with PBS three times, centrifuged at
4°C and 12,000 × g for 5 min and resuspended in PBS. Subsequently,
labeled BMSCs were analyzed using a FACSCalibur flow cytometer and
assessed using FACSDiva™ Version 6.1.3 (BD Biosciences, Franklin
Lakes, NJ, USA). Unstained cells were used as a control.
Alizarin red staining
P3 BMSCs were seeded in 6-well plates at density of
8×103 cells/cm2 and cultured in osteogenic
medium, consisting of α-MEM medium supplemented with 50
μmol/l ascorbic acid (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany), 10 mmol/l β-glycerophosphate (Sigma-Aldrich; Merck KGaA)
and 10−8 mol/l dexamethasone (Beijing Solarbio Science
& Technology Co., Ltd.) at 37°C. Cells in the control group
were treated with α-MEM supplemented with 10% FBS and 100 U/ml
penicillin-streptomycin (the control medium) and cultured at 37°C.
Ascorbic acid was freshly prepared prior to each medium change.
Following 21 days culture, cells were fixed with 4%
paraformaldehyde at 37°C for 30 min. Following three washes with
PBS, cells were incubated with 0.1% (pH 4.2) Alizarin Red S
(Beijing Solarbio Science & Technology Co., Ltd.) at 37°C for
10 min and subsequently washed with PBS three times to remove any
excess stain. Samples were observed using a phase-contrast
microscope at a magnification of ×100 to verify the presence of
mineralized nodules.
Oil red O staining
P3 BMSCs were seeded as aforementioned and cultured
in adipogenic medium, consisting of α-MEM supplemented with 0.1
mmol/l 3-isobutyl-1-methylxanthine (Sigma-Aldrich; Merck KGaA), 10
mg/l insulin, 0.1 mmol/l indometacin, 1 μmol/l dexamethasone
(all Beijing Solarbio Science & Technology Co., Ltd.) at 37°C.
Cells in the control group were cultured in control medium at 37°C.
Following 21 days culture, cells were fixed in 4% paraformaldehyde
at 37°C for 30 min. Following three washes with PBS, cells were
incubated with Oil Red O (Beijing Solarbio Science & Technology
Co., Ltd.) at 37°C for 30 min and subsequently washed with PBS
three times. Samples were observed using phase-contrast microscopy
at a magnification of ×100.
Cell proliferation assay
P3 BMSCs were seeded in 96-well plates at a density
of 5×103 cells/cm2 and cultured in control
medium for 24 h at 37°C. Subsequently the medium was removed and
cells were cultured in fresh control medium containing 0 (CCK-8
control group), 100, 200, 400 and 800 μM CORM-3. CORM-3 was
freshly prepared prior to the experiment by dissolving the compound
in distilled water. Following 24 h, 10 μl Cell Counting
Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Beijing, China)
was added to each well and cells were incubated for a further 2 h
at 37°C. Subsequently, absorbance at 450 nm [optical density
(OD)450 nm] was measured using a SPECTROstar Nano
ultraviolet spectrophotometer (Spectro Analytical Instruments GmbH,
Kleve, Germany). The experiment was repeated in triplicate.
Effects of CORM-3 on osteogenic
differentiation
Cells were divided into 5 groups. The
CORM-3-osteogenic group received BMSCs that were pretreated with
control medium supplemented with 200 μM CORM-3 at 37°C for
24 h. Subsequently, the medium was completely replaced with
osteogenic medium for 3 days. The osteogenic group received BMSCs
cultured in 37°C osteogenic medium for 3 days. The degassed
CORM-3-osteogenic group received BMSCs that were pretreated with
control medium supplemented with 200 μM degassed CORM-3 at
37°C for 24 h. Following this, the medium was replaced with 37°C
osteogenic medium for 3 days. The CORM-3 group received BMSCs
cultured in control medium supplemented with 200 μM CORM-3
at 37°C for 3 days. The control group received BMSCs that were
cultured in control medium alone at 37°C for 3 days. For the
experiments performed for 5 and 7 days, the medium was changed
every third day in all groups.
CORM-3 was freshly prepared as aforementioned.
Degassed CORM-3 was produced by dissolving CORM-3 in distilled
water and placing the solution in a vacuum device at 37°C for 24 h
prior to the experiments. Degassed CORM-3 was used as a negative
control to assess the direct involvement of CO in the
pharmacological activity of CORM-3 (18). For osteogenic differentiation
experiments, BMSCs were seeded in 6-well plates at a density of
5×104 cells/well and cultured in the indicated medium.
The mRNA and protein expression of osteoblast marker genes,
alkaline phosphatase (ALP) activity and matrix mineralization were
subsequently analyzed.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Following 3, 5 and 7 days culture, total RNA was
isolated using TRIzol reagent (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), according to the manufacturer's protocol. cDNA
was synthesized from 1 μg total RNA using a PrimeScript
Reverse Transcriptase reagent kit (Takara Biotechnology Co., Ltd.,
Dalian, China), following the manufacturer's protocol. cDNAs were
subjected to qPCR with SYBR Green (Takara Biotechnology Co., Ltd.)
on a Light Cycler II Real-time PCR system (Roche Diagnostics,
Basel, Switzerland) to detect mRNA levels of runt-related
transcription factor 2 (Runx2), osteocalcin (OCN) and osteopontin
(OPN). The qPCR thermocycling conditions were as follows: Initial
denaturation at 95°C for 15 min, followed by 40 cycles of
denaturation at 95°C for 10 sec, annealing at 60°C for 20 sec and
extension at 72°C for 30 sec. β-actin served as the housekeeping
gene for normalization. The primer sequences used were as follows:
Runx2, forward 5′-CAGACACAATCCTCCCCACC-3′, and reverse
5′-GCCAGAGGCAGAAGTCAGAG-3′; OCN, forward 5′-ATTGTGACGAGCTAGCGGAC-3′
and reverse 5′-TCGAGTCCTGGAGAGTAGCC-3′; OPN, forward
5′-TCAAGGTCATCCCAGTTGCC-3′ and reverse 5′-GACTCATGGCTGGTCTTCCC-3′;
and β-actin, forward 5′-CTCTGTGTGGATTGGTGGCT-3′ and reverse
5′-CGCAGCTCAGTAACAGTCCG-3′. Each sample was tested in triplicate
and gene expression levels were assessed using the LightCycler 480
Software 1.5 (Roche Diagnostics). Results were quantified using the
2−ΔΔCq method (21).
Western blot analysis
Following 3, 5 and 7 days culture, cells were washed
twice with precooled PBS and treated with radio immunoprecipitation
assay lysis buffer (Beijing Solarbio Science & Technology Co.,
Ltd.) containing 1 mmol/l phenylmethylsulfonyl fluoride protease
inhibitors. The protein concentration of each group was measured
using a BCA protein assay kit (Wuhan Boster Biological Technology
Co., Ltd., Wuhan, China), following the manufacturer's protocol.
Protein samples (20 μg/lane) were resolved using 12%
SDS-PAGE (Wuhan Boster Biological Technology Co., Ltd.) and
electrotransferred to a polyvinylidene difluoride membrane (Pall
Corporation, Port Washington, NY, USA). Following blocking in 5%
skimmed milk in Tris-buffered saline with 0.1% Tween-20 (TBST) at
37°C for 1 h, the membrane was incubated overnight at 4°C with
primary antibodies, including rabbit anti-rat Runx2 (cat no.,
12556s, 1:1,000 dilution; Cell Signaling Technology, Inc., Danvers,
MA, USA), mouse anti-rat OCN (cat no., ab13420, 1:500 dilution) and
rabbit anti-rat OPN (cat no., ab8448, 1:1,000 dilution; both from
Abcam). Following three washes with TBST, membranes were incubated
with horseradish peroxidase-conjugated goat anti-rabbit or goat
anti-mouse secondary antibodies (cat. nos., SA00001-2, SA00011;
both 1:10,000 dilution, Proteintech Group, Inc., Rosemont, IL, USA)
for 1 h at room temperature and washed three times with TBST.
Following this, membranes were visualized using enhanced
chemiluminescence (EMD Millipore, Billerica, MA, USA) and a
ChemiScope Western Blot Imaging System (Clinx Science Instruments
Co., Ltd., Shanghai, China). Loading differences were normalized
using a monoclonal GAPDH antibody (cat. no., CW0100, 1:2,000
dilution; Beijing ComWin Biotech Co., Ltd., Beijing, China).
Protein band densities on scanned films were quantified using
ImageJ 1.48u software (National Institutes of Health, Bethesda, MD,
USA) and compared with the control.
ALP activity assay
Following 3, 5 and 7 days culture, cells were washed
twice with ice-cold PBS, harvested in 1% Triton and subsequently
centrifuged at 4°C for 15 min at 12,000 × g. The supernatant was
mixed with an ALP kit (Nanjing Jiancheng Bioengineering Institute,
Nanjing, China), following the manufacturer's protocol. OD values
were read at 520 nm using an ELISA plate reader. The protein
concentration of each sample was measured using a BCA protein assay
kit. ALP activity was calculated according to the ALP kit protocol
and expressed as King-Armstrong units/g of total cellular
protein.
Analysis of mineralization
Following 21 days culture, mineralization was
detected in all groups using alizarin red staining, as
aforementioned. Staining was dissolved in 100 μM
cetylpyridinium chloride (CPC) for 1 h at 37°C. For further
evaluation, the OD value of the staining dissolved by CPC was
measured at 562 nm using an ELISA plate reader. All experiments
were repeated in triplicate.
Statistical analysis
Data were obtained from at least three independent
experiments. The significance of differences was assessed by
one-way analysis of variance method using GraphPad Prism 5 software
(GraphPad Software, Inc., La Jolla, CA, USA), followed by the
Tukey's post hoc test. Data are presented as the mean ± standard
deviation and P<0.05 was considered to indicate a statistically
significant difference.
Results
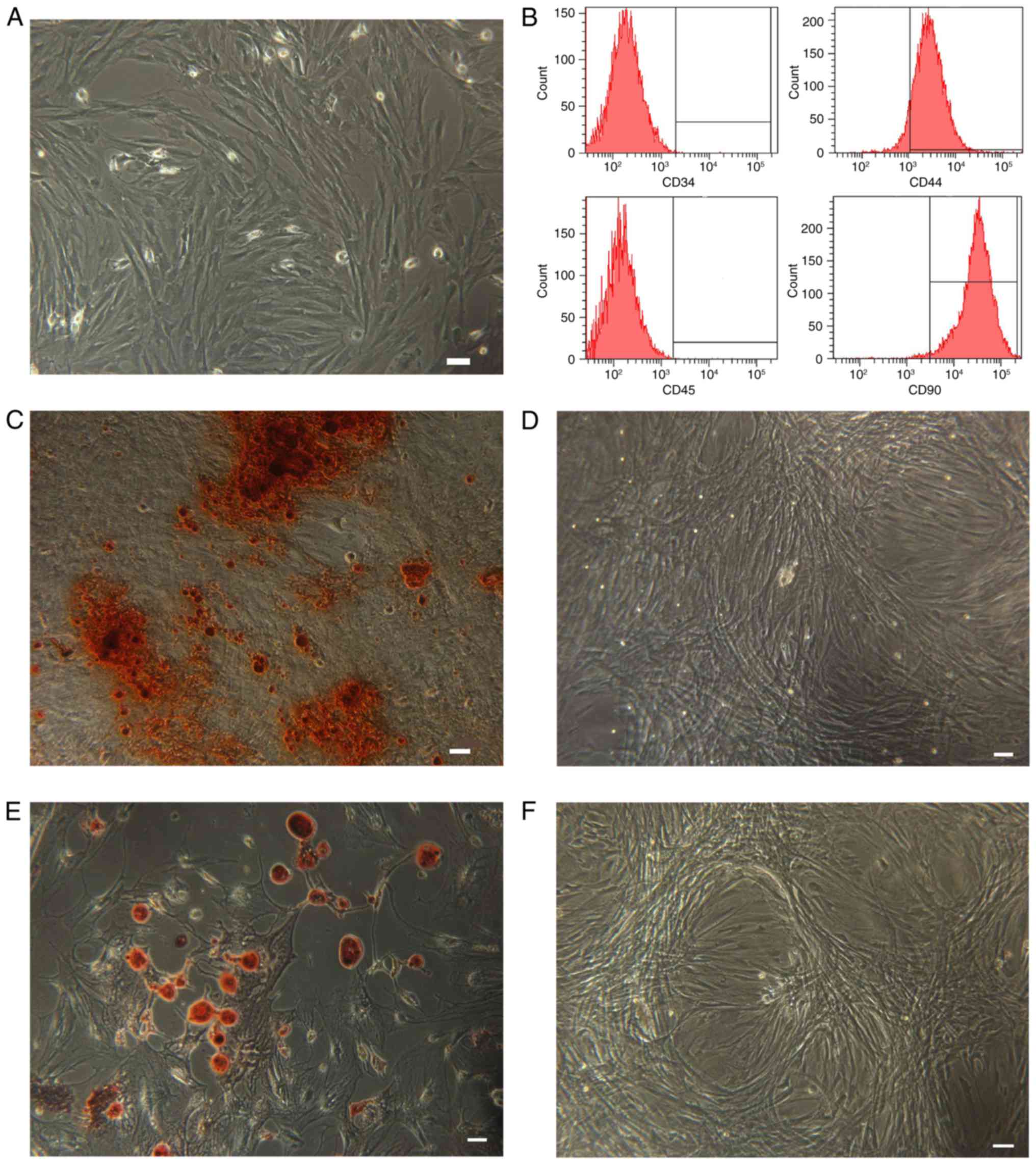
Identification of BMSCs
As indicated in Fig.
1, cultured BMSCs were identified by assessing their morphology
using different techniques. BMSCs presented with fibroblast-like
morphological features (Fig. 1A).
Flow cytometric analysis demonstrated that expression of the MSC
surface markers CD90 (98.4%) and CD44 (92.1%) was high in BMSCs,
whereas expression of the hematopoietic surface markers CD45 (0.2%)
and CD34 (0.1%) was low (Fig.
1B). Furthermore, the multilineage differentiation potential of
BMSCs was evaluated. BMSCs were induced to differentiate into
osteoblasts or adipocytes. The mineralized matrix was visualized
using alizarin red staining (Fig.
1C) and lipid droplets were identified using oil red O staining
(Fig. 1E). Control cell cultures
were negative for alizarin red (Fig.
1D) and oil red O staining (Fig.
1F).
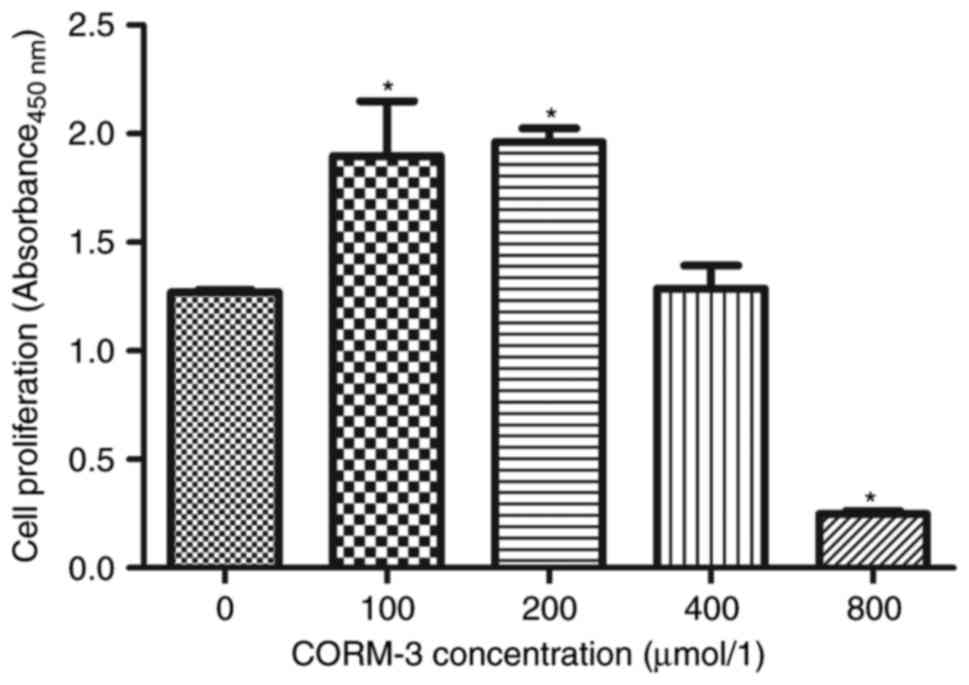
Effect of CORM-3 on BMSC
proliferation
BMSCs were cultured with different concentrations of
CORM-3. The proliferative ability of the cells was assessed using a
CCK-8 kit. As indicated in Fig.
2, 100 and 200 μM CORM-3 significantly promoted the
proliferation of BMSCs, whereas 400 μM CORM-3 had no
significant effect on cell proliferation compared with the control
(P<0.05). Notably, 800 μM CORM-3 significantly inhibited
cell proliferation compared with the control (P<0.05). Based on
these results, 200 μM CORM-3 was selected for the following
experiments.
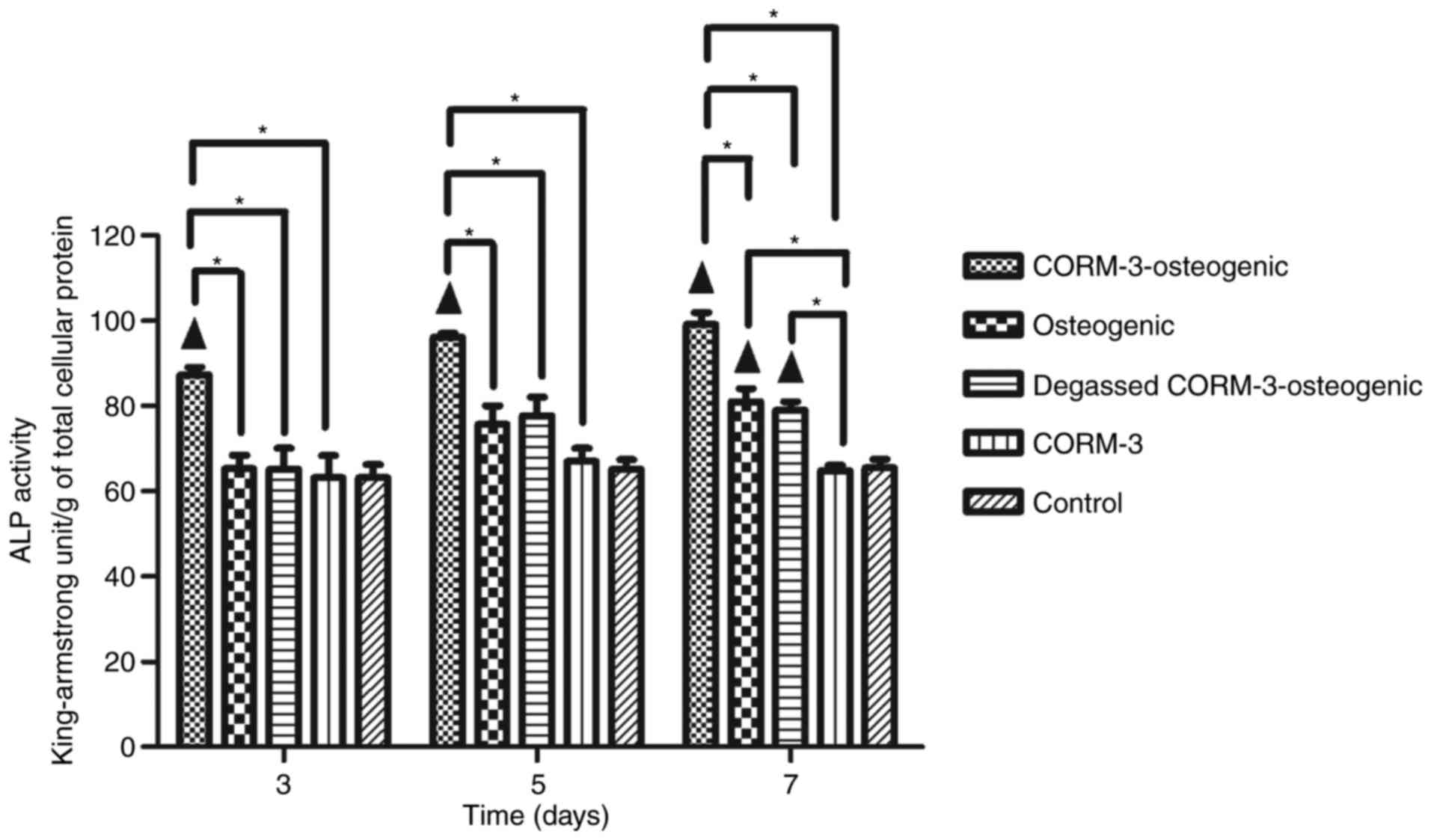
Effect of CORM-3 on ALP activity during
osteogenic differentiation
ALP activity was assessed on days 3, 5 and 7
(Fig. 3). ALP activity in the
CORM-3-osteogenic group increased rapidly from day 3 and peaked on
day 7; it was significantly higher compared with the other groups
on days 3, 5 and 7 (P<0.05). ALP activity was only significantly
increased in the osteogenic group on day 7 compared with the
control group (P<0.05). Notably, there were no significant
differences in ALP activity in the CORM-3 group compared with the
control group. The ALP activity in the degassed CORM-3-osteogenic
group was not increased compared with the osteogenic group on days
3, 5 and 7, suggesting that the influence of CORM-3 on ALP activity
was mediated by CO release (Fig.
3).
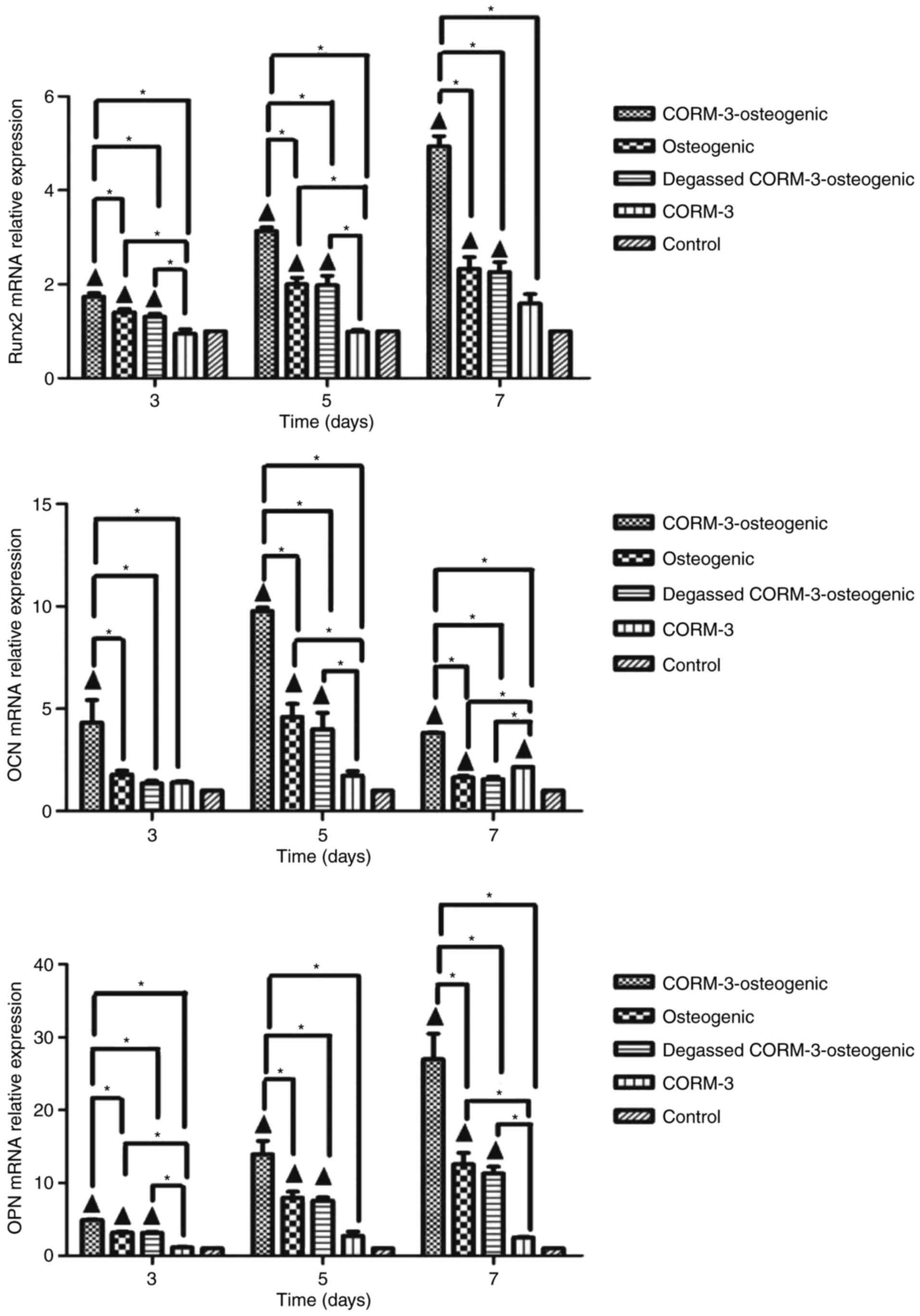
Effects of CORM-3 on Runx2, OCN and OPN
mRNA levels during osteogenic differentiation
To determine whether CORM-3 promotes the expression
of osteo-specific genes, BMSCs were subjected to osteogenic
differentiation with or without CORM-3 pretreatment. mRNA levels of
the osteoblast key transcription factor Runx2 and the osteoblast
marker genes OCN and OPN, were assessed using RT-qPCR at different
time points during osteogenic differentiation. Degassed CORM-3 was
used to determine the involvement of CO on the effect of CORM-3. As
indicated in Fig. 4, levels of
Runx2 mRNA in cells cultured in osteogenic medium and pretreated
with CORM-3 were significantly increased compared with all other
groups on days 3, 5 and 7 (P<0.05). By day 7, CORM-3
pretreatment in the CORM-3-osteogenic group had increased the
expression of Runx2 mRNA by 4.9-fold compared with the control
group (P<0.05), and 2.2-fold compared with the osteogenic group
(P<0.05). CORM-3 without osteogenic solution had no significant
effect on the expression of Runx2 mRNA compared with the control
group. These results in CORM-3-osteogenic, osteogenic and degassed
CORM-3-osteogenic groups suggest that the effect of CORM-3 on the
expression of Runx2 mRNA was mediated by the release of CO, as
degassed CORM-3 was ineffective compared with osteogenic group.
Similar effects were also observed regarding the expression of OPN
mRNA. Levels of OCN mRNA peaked on day 5 in the CORM-3-osteogenic
group and were significantly higher in the CORM-3-osteogenic group
compared with all other groups at each time point (P<0.05).
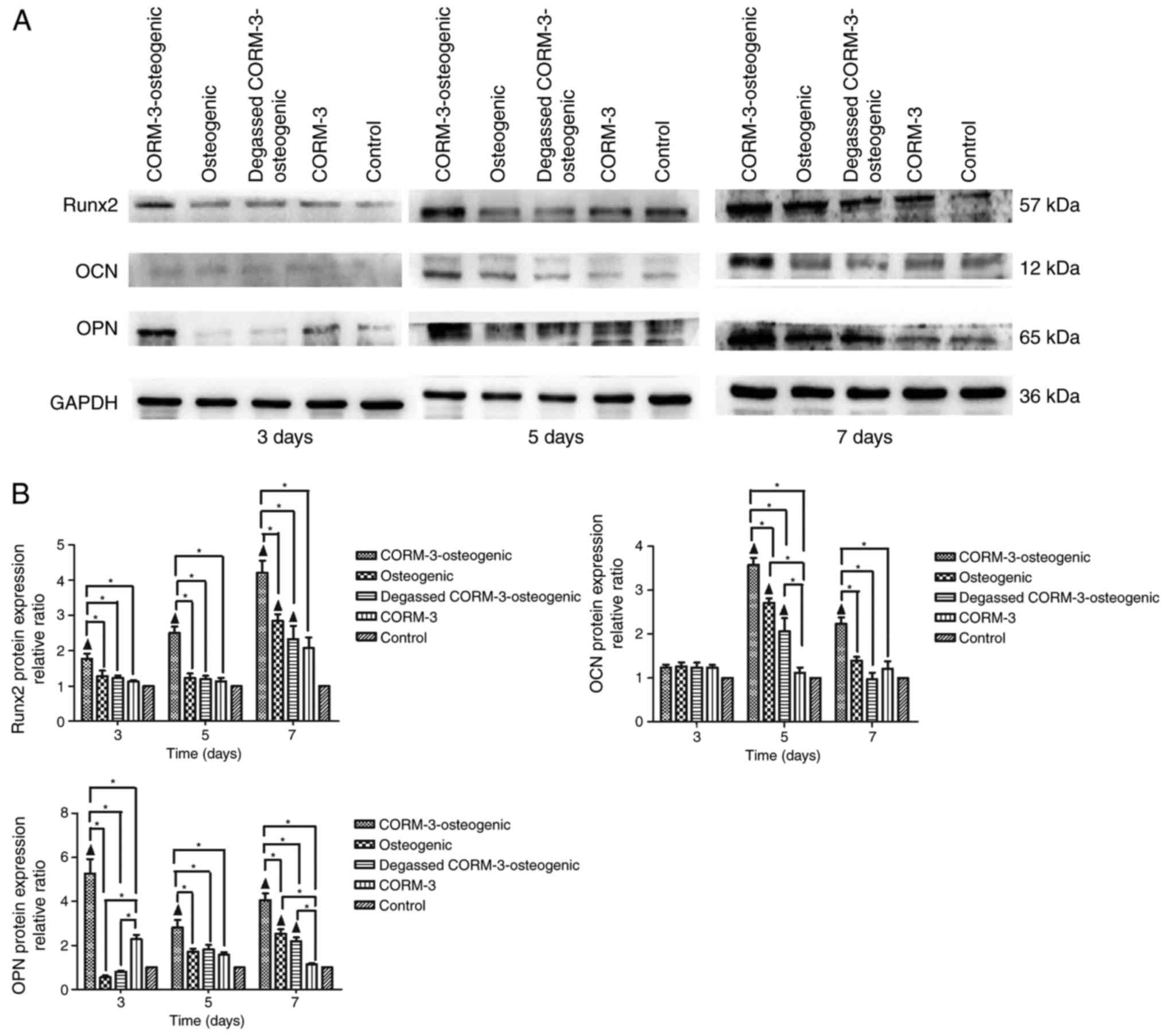
Effects of CORM-3 on Runx2, OCN and OPN
protein expression during osteogenic differentiation
The protein expression of Runx2 and OPN in the
CORM-3-osteogenic group was significantly increased compared with
all other groups at all time points (P<0.05; Fig. 5). The protein expression of Runx2
in the CORM-3-osteogenic group was increased by 2.5-fold compared
with the control group, 2.08-fold compared with the osteogenic
group on day 5, and 4.1-fold compared with the control group and
1.52-fold compared with osteogenic group on day 7 (P<0.05). The
expression of OCN in the CORM-3-osteogenic group peaked on day 5
and then declined on day 7. However, OCN expression remained
significantly increased in the CORM-3-osteogenic group compared
with all other groups on days 5 and 7 (P<0.05). CORM-3 alone had
no significant effect on the protein expressions of Runx2, OCN and
OPN compared with the control group. These results from the
CORM-3-osteogenic, osteogenic and degassed CORM-3-osteogenic groups
suggest that the influence of CORM-3 on the protein expression of
these factors was mediated by CO release, as the degassed solution
of CORM-3 did not significantly increase the expression of any
proteins tested, compared with osteogenic group (Fig. 5).
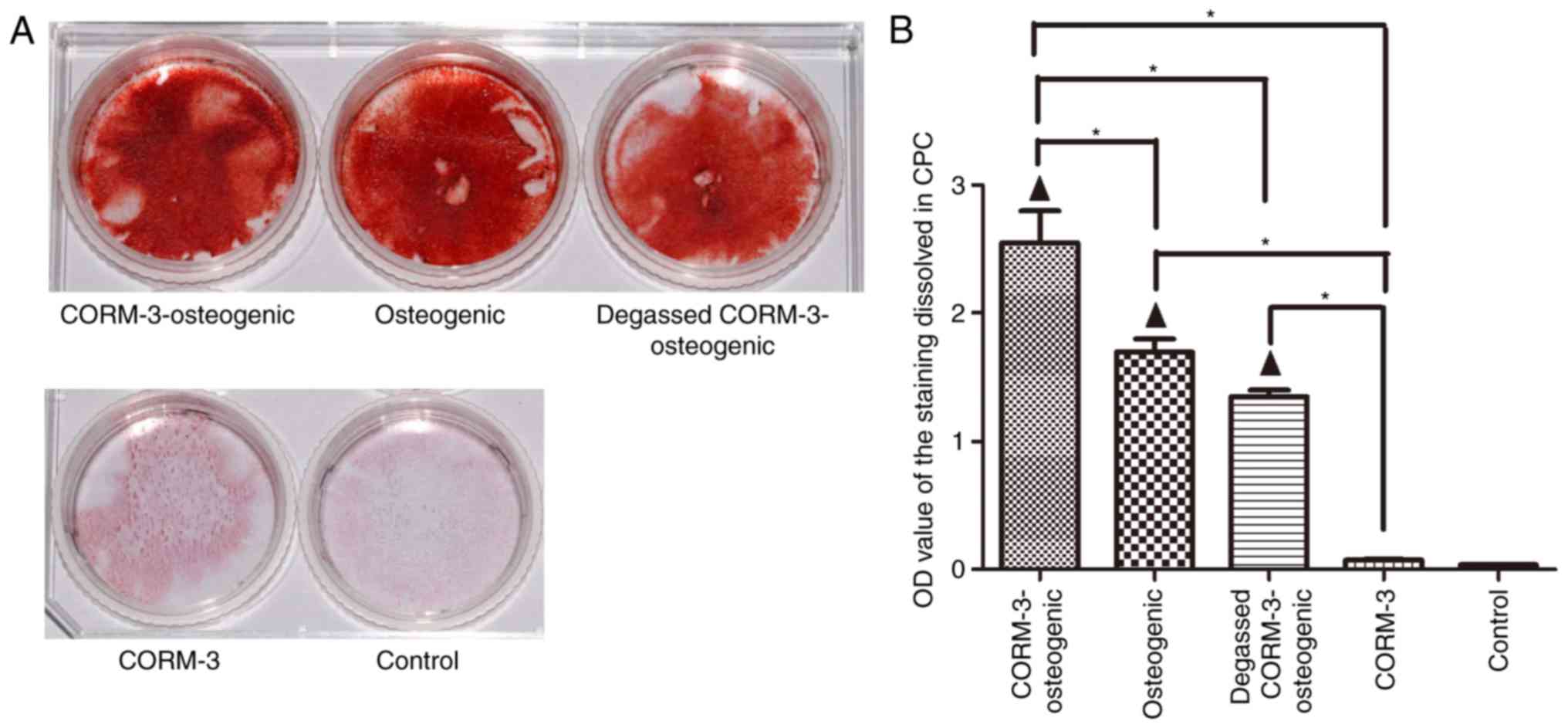
Effects of CORM-3 on mineralization
The results from the alizarin red staining
demonstrated that mineralization in the CORM-3-osteogenic group was
markedly enhanced compared with the osteogenic group after 21 days
culture (Fig. 6A). To evaluate
the mineralization in a quantitative manner, the OD value of the
staining dissolved by CPC was analyzed. As indicated in Fig. 6B, alizarin red staining in the
CORM-3-osteogenic group was significantly higher than in the
osteogenic groups (P<0.05). The osteogenic promotion effect of
CORM-3 was mediated by releasing CO as the degassed CORM-3 did not
affect osteogenesis compared with osteogenic group.
Discussion
Previous studies have identified the beneficial
effects of CO and CORMs; however, little is known regarding the
influence of CORMs on osteogenesis. In the present study, rat BMSCs
were used as an in vitro model to investigate the effects of
CORM-3 on the osteogenic differentiation of BMSCs. The results
suggested that 100 and 200 μM CORM-3 significantly promotes
the proliferation of BMSCs. Additionally, pretreatment with CORM-3
upregulated the mRNA and protein expression of the osteo-specific
markers Runx2, OCN and OPN. Notably, CORM-3 pretreatment also
increased ALP activity and enhanced cell mineralization. The
results of the present study suggest that these effects were
mediated by the release of CO.
Various diseases, including cancer, infection and
degenerative diseases, may cause bone defects (1,2).
Stem cell-based bone engineering has emerged as a promising and
effective alternative approach of treating these diseases (22). BMSCs are presently regarded as the
gold standard cell source for bone tissue engineering, due to their
great potential for self-renewal and their ability to differentiate
into cell lineages characteristic of bone (23). The predominant objective of bone
tissue engineering is to stimulate effective and sufficient bone
regeneration, which is typically based on a successful
osteoinduction. Multiple strategies have been implemented for
osteoinduction, including the application of selected growth
factors (24). Cytokines that
promote osteogenesis include bone morphogenetic proteins (BMPs),
platelet-derived growth factor and fibroblast growth factor
(25). The osteogenic properties
of BMPs have been determined in a number of studies, and
recombinant human (rh)BMP2 and rhBMP7 have been approved by the US
Food and Drug Administration for specific clinical applications
(26,27). However, recent investigations have
reported notable side effects associated with rhBMP-2, including
renal complications, wound complications, increased inflammation
and an increased risk of cancer (25–29). Therefore, novel approaches to
induce effective osteoinduction are required.
CORMs are a novel group of compounds that are
carriers of CO and reproduce its biological actions (18). The anti-inflammatory properties of
CORMs have previously been identified in vitro and in
vivo (15,20). It has been reported that CORM-3
inhibits the expression of adhesion molecules on human gingival
fibroblasts concurrently stimulated by tumor necrosis factor-α and
interleukin-1β (30). It has also
been identified that the systemic administration of CORM-2 reduces
periodontal inflammation and alveolar bone loss in rats with
experimental periodontitis (31).
To further outline the cause for reduced bone loss, it was
hypothesized that CORMs may not only inhibit bone loss by
suppressing inflammation, but may also directly enhance
osteogenesis and/or suppress osteoclastogenesis. Subsequently, the
present study aimed to investigate the effect of CORM-3 on
osteogenesis.
It is well known that Runx2, which belongs to the
runt-domain gene family, is a critical transcription factor for the
differentiation of BMSCs and regulates a number of downstream genes
associated with differentiation (32). Furthermore, Runx2 is a marker for
early osteogenic differentiation (33). When the expression of Runx2 was
inhibited in fetal mice, the skeletal systems of these mice
subsequently exhibited a complete lack of bone formation (34). OCN and OPN are markers of
osteoblasts (35). The results of
the present study indicated that pretreatment with CORM-3
significantly upregulated the expression of Runx2, OCN and OPN
during osteogenic induction from day 3. On day 7, CORM-3
pretreatment enhanced levels of Runx2 mRNA by 4.9-fold compared
with the control group and 2.2-fold compared with osteogenic group.
These results suggest that CORM-3 may promote osteogenic
differentiation. Notably, the present results also suggest that
this effect may be mediated by the release of CO.
ALP, which is considered to be an early marker of
osteogenic differentiation, serves an important role in regulating
cell differentiation. ALP produces inorganic phosphate from
pyro-phosphate (36). Phosphate
is further crystallized with calcium and accelerates
mineralization. The increased activity of ALP provides favorable
conditions for the mineralization process and promotes the
differentiation of cells into osteoblasts (37). Based on our unpublished data, the
expression of heme oxygenase-1 (HO-1) mRNA and protein in BMSCs is
significantly increased 24 h following treatment with CORM-3.
Several studies have suggested that the change in HO-1 expression
in response to different stimuli is consistent with that of ALP
activity and demonstrated that the overexpression of HO-1 increases
ALP activity (38,39). In the present study, CORM-3
pretreatment significantly increased ALP activity after 3 days
culture and these levels were maintained following 7 days culture.
The formation of mineralized nodules is another essential marker of
osteogenesis and in the current study, CORM-3 pretreatment
significantly enhanced cell mineralization on day 21.
Despite the known toxicity of CO at high
concentrations, previous studies have revealed that low
concentrations of CO may exhibit vasoregulatory properties
(13) and modulate inflammation
(15). A clinical study by
Bathoorn et al (40)
demonstrated the feasibility of administering inhaled CO to humans
with chronic obstructive pulmonary disease. Notably, an advantage
of CORMs is that they deliver CO to tissues that exhibit lower
levels of carboxyhemoglobin build-up, which is typical of inhaled
CO (18). Although CORMs have
potential to be used in treatment, further pharmacokinetic and
toxicological response analyses of CORMs are required prior to
their clinical application.
In conclusion, the results of the present study
indicate that CORM-3 promotes the osteogenic differentiation of
BMSCs by releasing CO, suggesting CORM-3 may be developed as a
method of stimulating bone regeneration. However, the molecular
mechanisms of its action require further elucidation. CO is a
well-known secondary messenger involved in a range of physiological
and pathological responses via interaction with specific receptors
(41). The primary signaling
pathways of CO include the HO-1, mitogen-activated protein kinase
and glutathione signaling pathways (14,15,42). Further studies are required to
assess the mechanism by which CORM-3 promotes the osteogenic
differentiation of BMSCs.
Acknowledgments
The present study was supported by Shandong Province
Natural Science Foundation (grant no. ZR2015HM019), Jinan College
and University Science and Technology Innovation Program (grant no.
201401259) and Special Funds for Education and Awards of Shandong
Province [grant no. Lu Cai Jiao Zhi (2014) 94].
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Rauh J, Milan F, Günther KP and Stiehler
M: Bioreactor systems for bone tissue engineering. Tissue Eng Part
B, Rev. 17:263–280. 2011. View Article : Google Scholar
|
|
2
|
Loesche WJ and Grossman NS: Periodontal
disease as a specific, albeit chronic, infection: Diagnosis and
treatment. Clin Microbiol Rev. 14:727–752, table of contents. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Orlic D, Kajstura J, Chimenti S, Jakoniuk
I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM,
et al: Bone marrow cells regenerate infarcted myocardium. Nature.
410:701–705. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
In't Anker PS, Scherjon SA, Kleijburg-van
der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE and Kanhai HH:
Isolation of mesenchymal stem cells of fetal or maternal origin
from human placenta. Stem Cells. 22:1338–1345. 2004. View Article : Google Scholar
|
|
6
|
Murphy MB, Moncivais K and Caplan AI:
Mesenchymal stem cells: Environmentally responsive therapeutics for
regenerative medicine. Exp Mol Med. 45:e542013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aubin JE: Regulation of osteoblast
formation and function. Rev Endoc Metab Disord. 2:81–94. 2001.
View Article : Google Scholar
|
|
8
|
Long F: Building strong bones: Molecular
regulation of the osteoblast lineage. Nat Rev Mol Cell Biol.
13:27–38. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zomorodian E and Baghaban Eslaminejad M:
Mesenchymal stem cells as a potent cell source for bone
regeneration. Stem Cells Int. 2012:9803532012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jones E and Yang X: Mesenchymal stem cells
and bone regeneration: Current status. Injury. 42:562–568. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiao Y, Mareddy S and Crawford R: Clonal
characterization of bone marrow derived stem cells and their
application for bone regeneration. Int J Oral Sci. 2:127–135.
2010.PubMed/NCBI
|
|
12
|
Motterlini R, Gonzales A, Foresti R, Clark
JE, Green CJ and Winslow RM: Heme oxygenase-1-derived carbon
monoxide contributes to the suppression of acute hypertensive
responses in vivo. Circ Res. 83:568–577. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sammut IA, Foresti R, Clark JE, Exon DJ,
Vesely MJ, Sarathchandra P, Green CJ and Motterlini R: Carbon
monoxide is a major contributor to the regulation of vascular tone
in aortas expressing high levels of haeme oxygenase-1. Br J
Pharmacol. 125:1437–1444. 1998. View Article : Google Scholar
|
|
14
|
Zhang X, Shan P, Otterbein LE, Alam J,
Flavell RA, Davis RJ, Choi AM and Lee PJ: Carbon monoxide
inhibition of apoptosis during ischemia-reperfusion lung injury is
dependent on the p38 mitogen-activated protein kinase pathway and
involves caspase 3. J Biol Chem. 278:1248–1258. 2003. View Article : Google Scholar
|
|
15
|
Otterbein LE, Bach FH, Alam J, Soares M,
Tao Lu H, Wysk M, Davis RJ, Flavell RA and Choi AM: Carbon monoxide
has anti-inflammatory effects involving the mitogen-activated
protein kinase pathway. Nat Med. 6:422–428. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matsumoto M, Makino Y, Tanaka T, Tanaka H,
Ishizaka N, Noiri E, Fujita T and Nangaku M: Induction of
renoprotective gene expression by cobalt ameliorates ischemic
injury of the kidney in rats. J Am Soc Nephrol. 14:1825–1832. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sikorski EM, Hock T, Hill-Kapturczak N and
Agarwal A: The story so far: Molecular regulation of the heme
oxygenase-1 gene in renal injury. Am J Physiol Renal Physiol.
286:F425–F441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Motterlini R, Mann BE, Johnson TR, Clark
JE, Foresti R and Green CJ: Bioactivity and pharmacological actions
of carbon monoxide-releasing molecules. Curr Pharm Des.
9:2525–2539. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Foresti R, Hammad J, Clark JE, Johnson TR,
Mann BE, Friebe A, Green CJ and Motterlini R: Vasoactive properties
of CORM-3, a novel water-soluble carbon monoxide-releasing
molecule. Br J Pharmacol. 142:453–460. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang L, Fei D, Gong R, Yang W, Yu W, Pan
S and Zhao M and Zhao M: CORM-2 inhibits TXNIP/NLRP3 inflammasome
pathway in LPS-induced acute lung injury. Inflamm Res. 65:905–915.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods.
25:402–408. 2001. View Article : Google Scholar
|
|
22
|
Seo BM, Miura M, Gronthos S, Bartold PM,
Batouli S, Brahim J, Young M, Robey PG, Wang CY and Shi S:
Investigation of multipotent postnatal stem cells from human
periodontal ligament. Lancet. 364:149–155. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsai TL and Li WJ: Identification of bone
marrow-derived soluble factors regulating human mesenchymal stem
cells for bone regeneration. Stem cell Rep. 8:387–400. 2017.
View Article : Google Scholar
|
|
24
|
Vahabi S, Torshabi M and Mohammadi M:
Osteoinductive activity of DFDBA materials versus growth factors on
gene expression of MG-63 cells: An in vitro study. J Long Term Eff
Med Implants. 26:133–142. 2016. View Article : Google Scholar
|
|
25
|
Gothard D, Smith EL, Kanczler JM, Rashidi
H, Qutachi O, Henstock J, Rotherham M, El Haj A, Shakesheff KM and
Oreffo RO: Tissue engineered bone using select growth factors: A
comprehensive review of animal studies and clinical translation
studies in man. Eur Cells Mater. 28:166–208. 2014. View Article : Google Scholar
|
|
26
|
Bessa PC, Casal M and Reis RL: Bone
morphogenetic proteins in tissue engineering: The road from the
laboratory to the clinic, part I (basic concepts). J Tissue Eng
Regen Med. 2:1–13. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bessa PC, Casal M and Reis RL: Bone
morphogenetic proteins in tissue engineering: The road from
laboratory to clinic, part II (BMP delivery). J of Tissue Eng Regen
Med. 2:81–96. 2008. View
Article : Google Scholar
|
|
28
|
Fu R, Selph S, McDonagh M, Peterson K,
Tiwari A, Chou R and Helfand M: Effectiveness and harms of
recombinant human bone morphogenetic protein-2 in spine fusion: A
systematic review and meta-analysis. Ann Intern Med. 158:890–902.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mesfin A, Buchowski JM, Zebala LP, Bakhsh
WR, Aronson AB, Fogelson JL, Hershman S, Kim HJ, Ahmad A and
Bridwell KH: High-dose rhBMP-2 for adults: Major and minor
complications: A study of 502 spine cases. J Bone Joint Surg Am.
95:1546–1553. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao HQ, Hou M, Wei LL, Mu P, Song H and
Yang P: Mechanism of carbon monoxide affecting the expression of
cellular adhesion molecule under stimulation of inflammatory
cytokines to human gingival fibroblasts. Hua Xi Kou Qiang Yi Xue Za
Zhi. 31:420–424. 2013.(In Chinese).PubMed/NCBI
|
|
31
|
Wei L, Hou M, Wang P and Song H: Effect of
carbon monoxide releasing molecule on experimental periodontitis in
rats. Hua Xi Kou Qiang Yi Xue Za Zhi. 32:23–26. 2014.(In
Chinese).PubMed/NCBI
|
|
32
|
Deng Y, Wu S, Zhou H, Bi X, Wang Y, Hu Y,
Gu P and Fan X: Effects of a miR-31, Runx2, and Satb2 regulatory
loop on the osteogenic differentiation of bone mesenchymal stem
cells. Stem Cells Dev. 22:2278–2286. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schroeder TM, Jensen ED and Westendorf JJ:
Runx2: A master organizer of gene transcription in developing and
maturing osteoblasts. Birth Defects Res C Embryo Today. 75:213–215.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Komori T, Yagi H, Nomura S, Yamaguchi A,
Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, et al:
Targeted disruption of Cbfa1 results in a complete lack of bone
formation owing to maturational arrest of osteoblasts. Cell.
89:755–764. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ching HS, Luddin N, Rahman IA and Ponnuraj
KT: Expression of odontogenic and osteogenic markers in DPSCs and
SHED: A review. Curr Stem Cell Res Ther. 12:71–79. 2017. View Article : Google Scholar
|
|
36
|
Terkeltaub RA: Inorganic pyrophosphate
generation and disposition in pathophysiology. Am J Physiol Cell
Physiol. 281:C1–C11. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wennberg C, Hessle L, Lundberg P, Mauro S,
Narisawa S, Lerner UH and Millán JL: Functional characterization of
osteoblasts and osteoclasts from alkaline phosphatase knockout
mice. J Bone Miner Res. 15:1879–1888. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vanella L, Kim DH, Asprinio D, Peterson
SJ, Barbagallo I, Vanella A, Goldstein D, Ikehara S, Kappas A and
Abraham NG: HO-1 expression increases mesenchymal stem cell-derived
osteoblasts but decreases adipocyte lineage. Bone. 46:236–243.
2010. View Article : Google Scholar
|
|
39
|
Barbagallo I, Vanella A, Peterson SJ, Kim
DH, Tibullo D, Giallongo C, Vanella L, Parrinello N, Palumbo GA, Di
Raimondo F, et al: Overexpression of heme oxygenase-1 increases
human osteoblast stem cell differentiation. J Bone Miner Metab.
28:276–288. 2010. View Article : Google Scholar
|
|
40
|
Bathoorn E, Slebos DJ, Postma DS, Koeter
GH, van Oosterhout AJ, van der Toorn M, Boezen HM and Kerstjens HA:
Anti-inflammatory effects of inhaled carbon monoxide in patients
with COPD: A pilot study. Eur Respir J. 30:1131–1137. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Levitt DG and Levitt MD: Carbon monoxide:
A critical quantitative analysis and review of the extent and
limitations of its second messenger function. Clin Pharmacol.
7:37–56. 2015.PubMed/NCBI
|
|
42
|
Otterbein LE, Foresti R and Motterlini R:
Heme oxygenase-1 and carbon monoxide in the heart: The balancing
act between danger signaling and pro-survival. Circ Res.
118:1940–1959. 2016. View Article : Google Scholar : PubMed/NCBI
|